Submitted:
01 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Work and Instruments
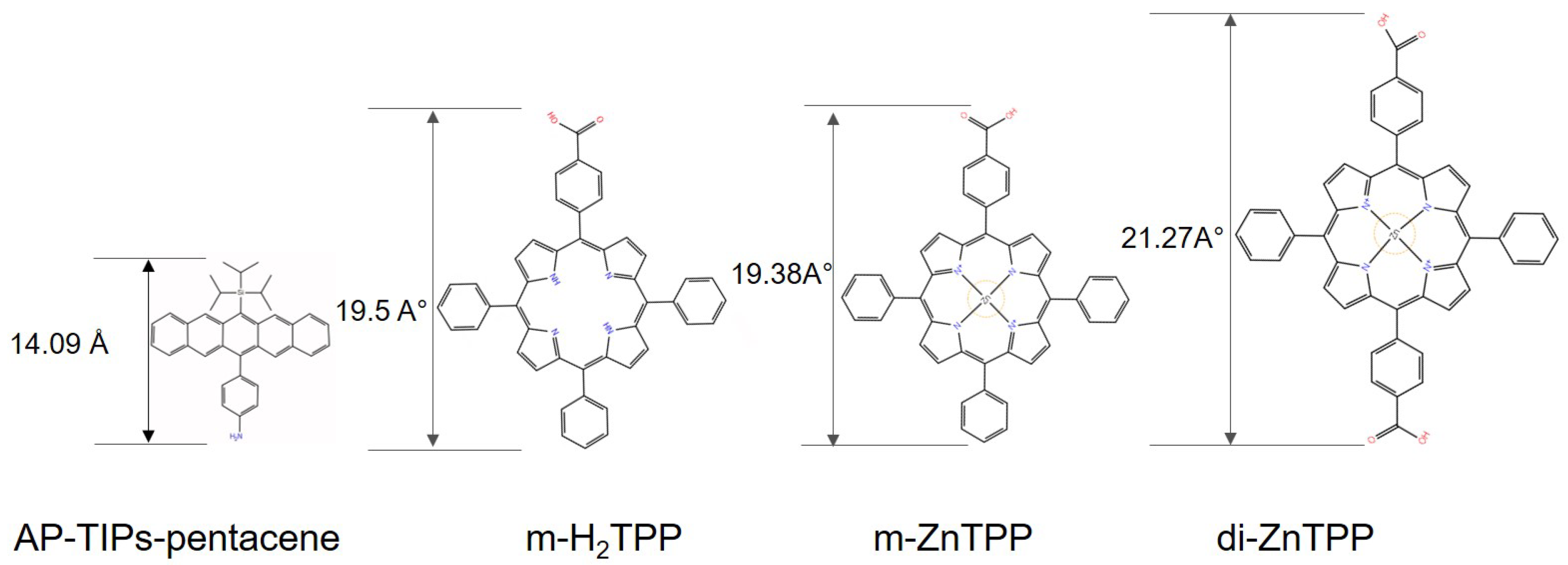
2.1. Materials
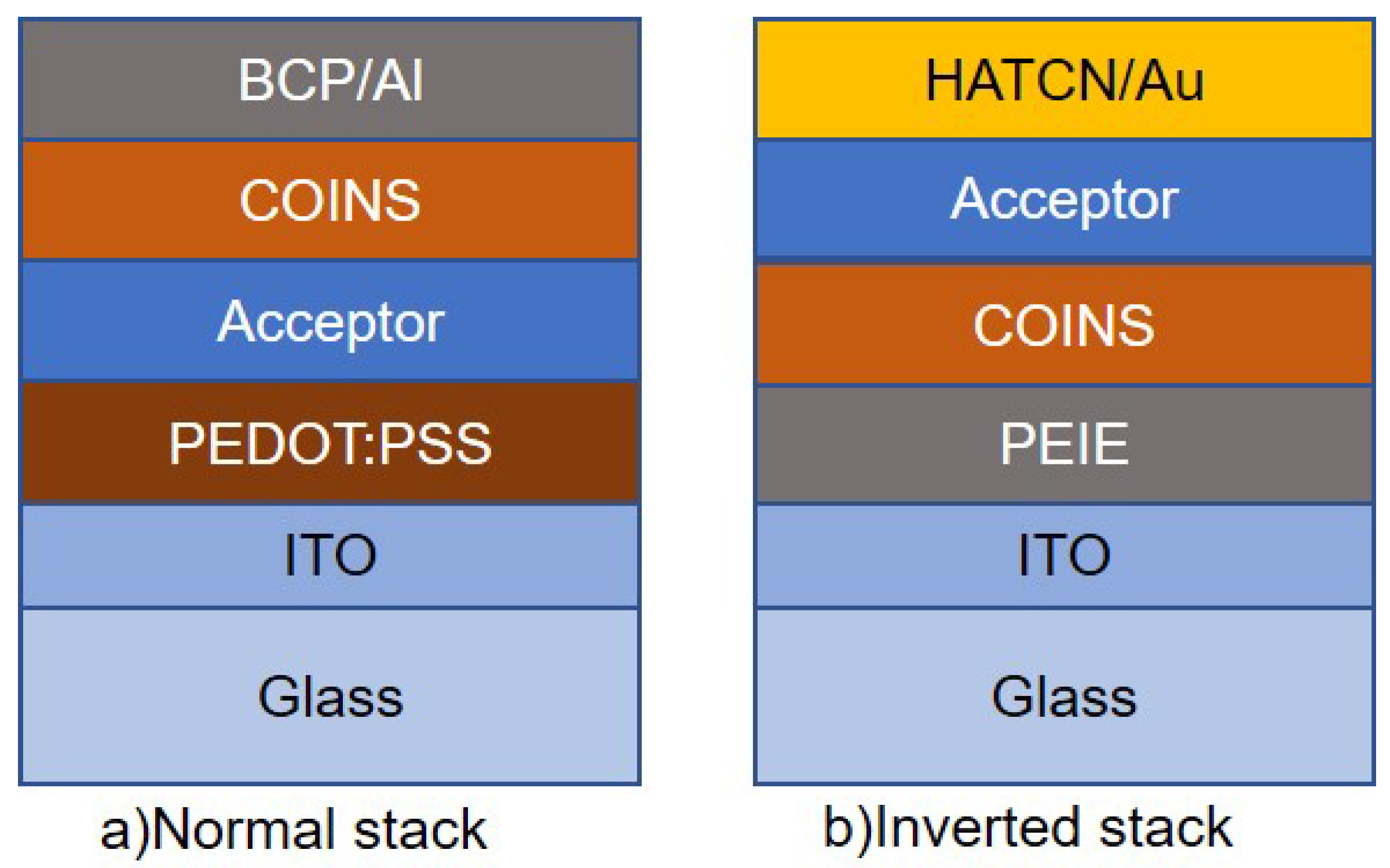
2.2. Device Preparation
2.3. Experimental Methods
3. Results
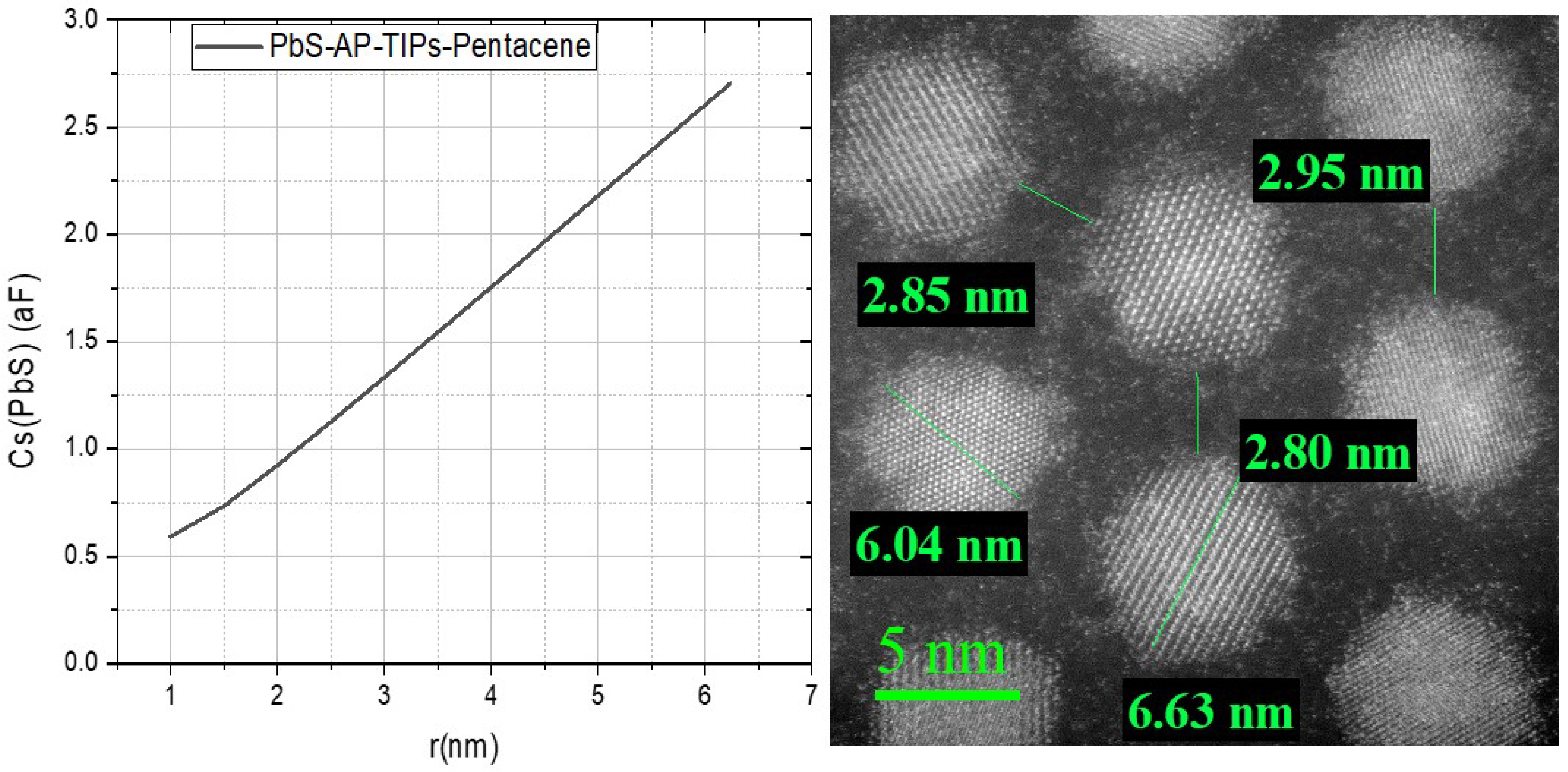
3.1. Transmission Electron Microscope (TEM)
3.2. Atomic Force Microscope (AFM)
4. Electrical Characteristics
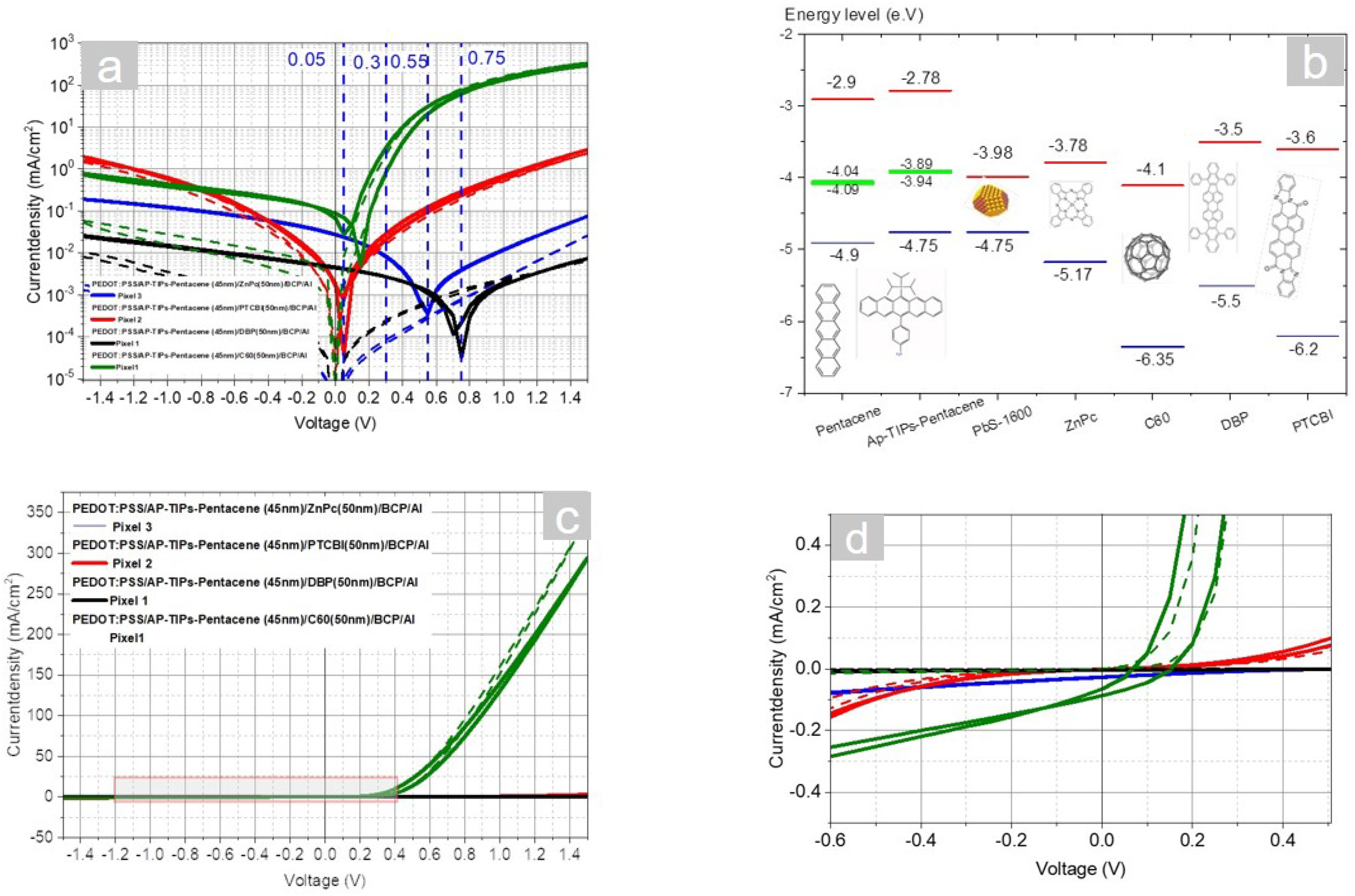
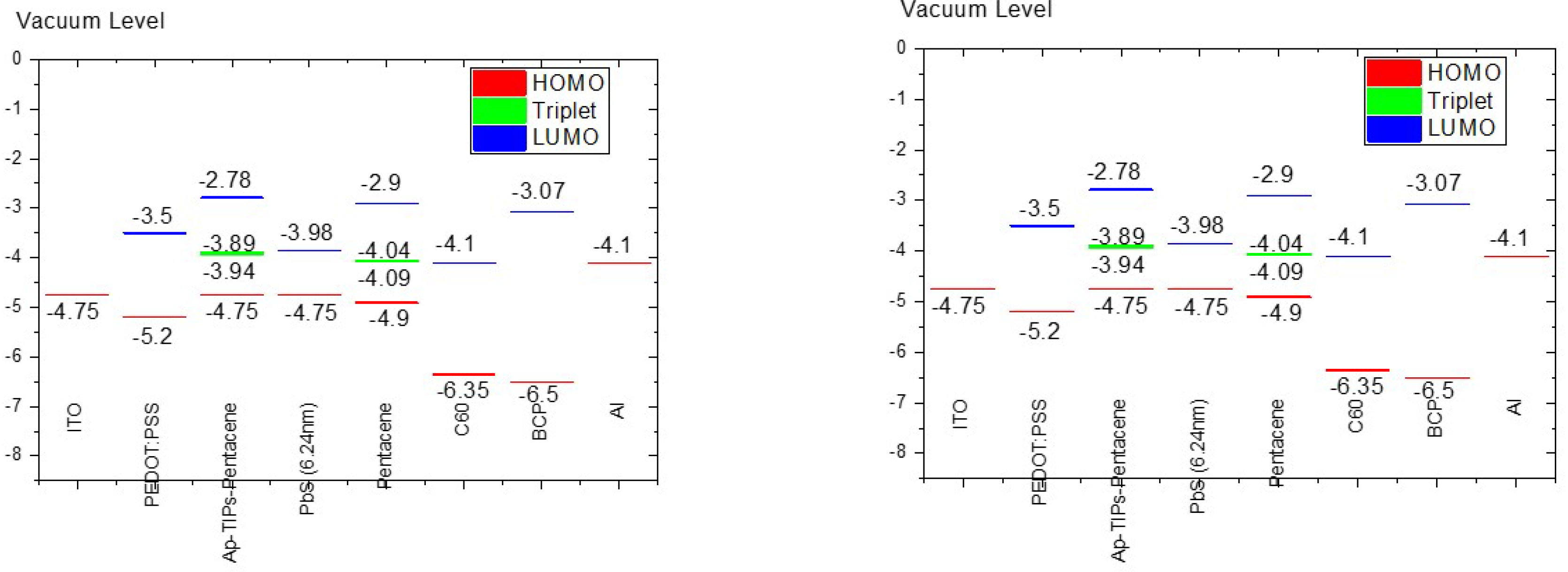
4.1. AP-TIPS-Pentacene
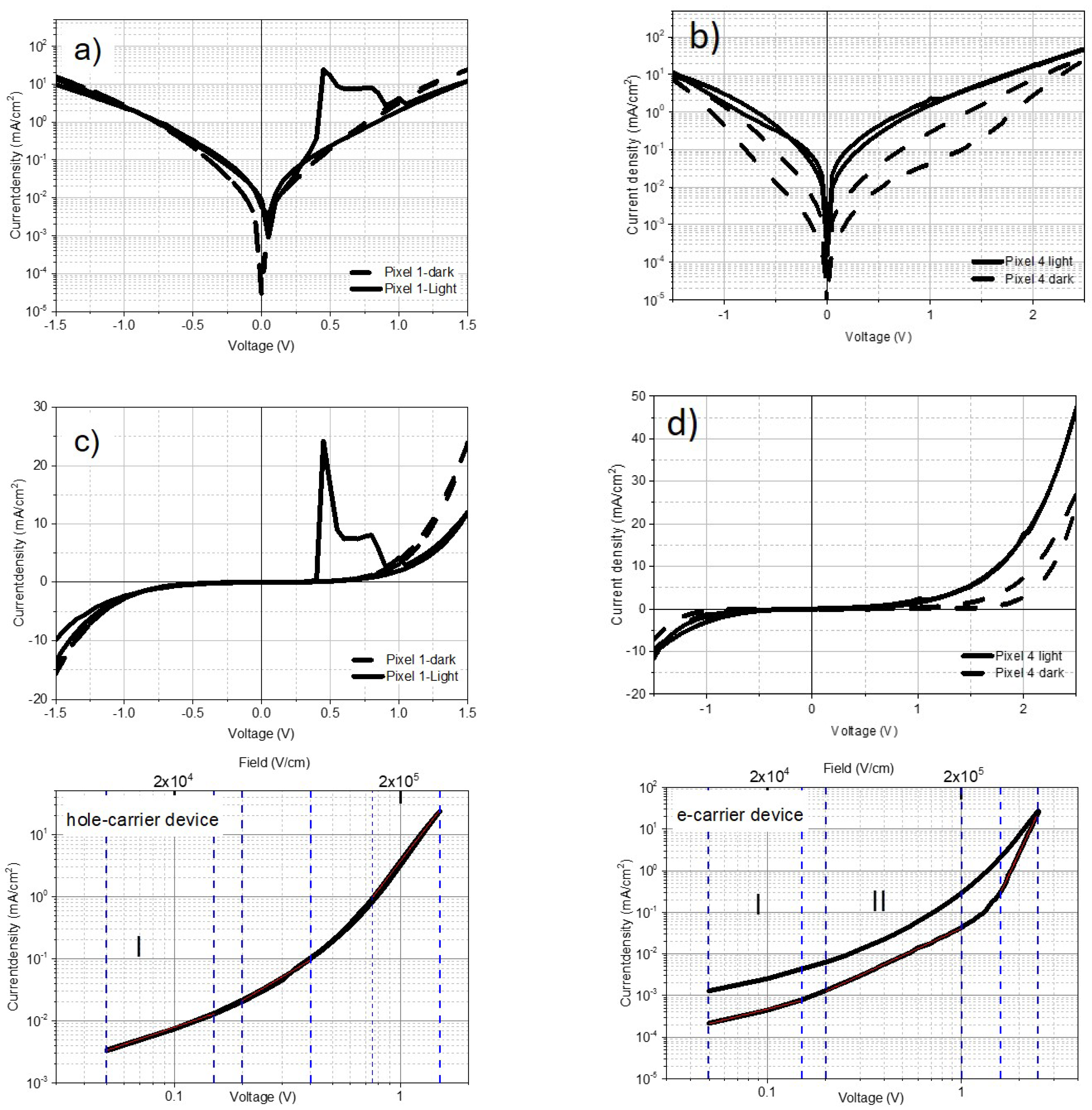
4.2. AP-TIPS-pentacene- Single Carrier Devices
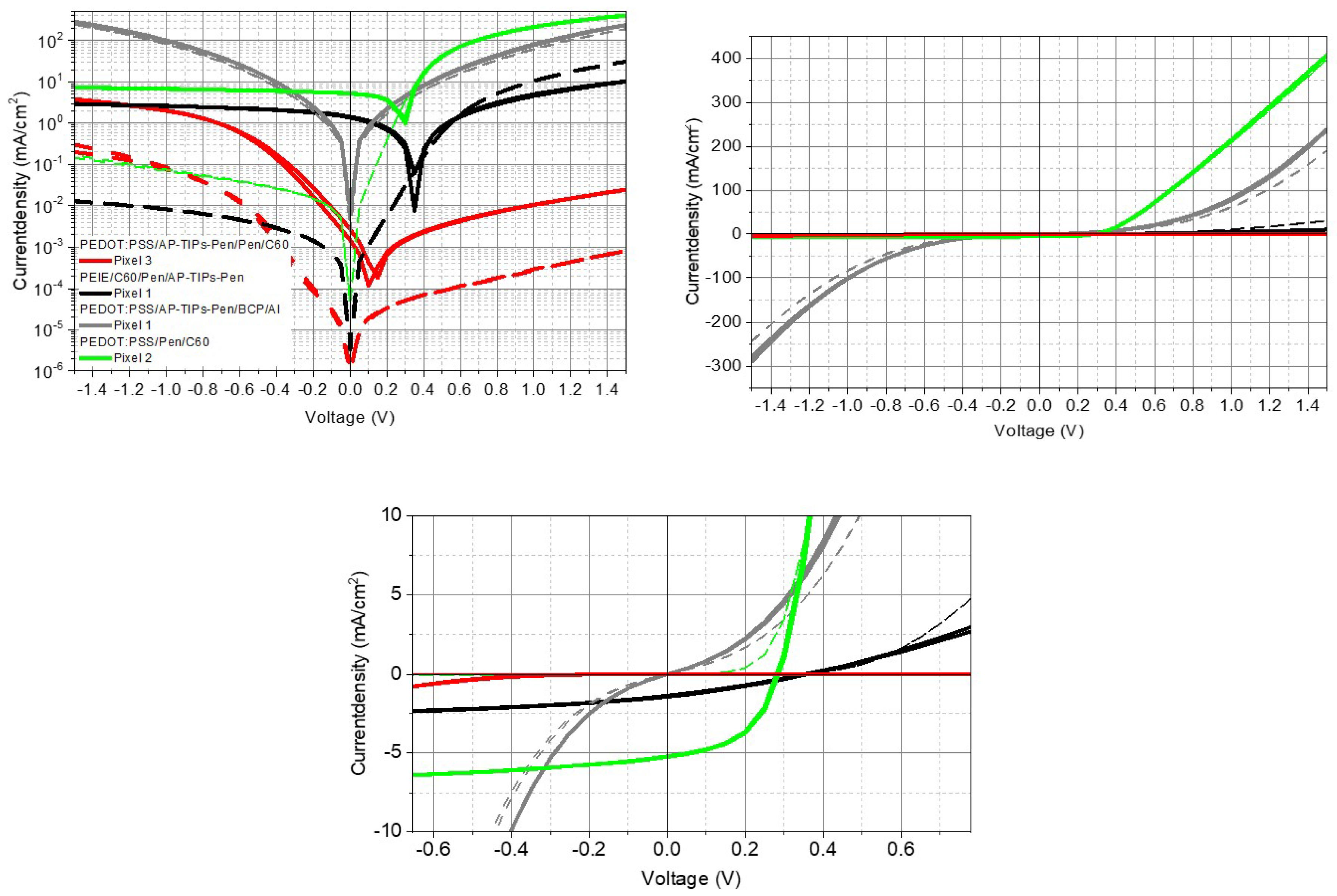
4.3. Difference between Normal and Inverted stack
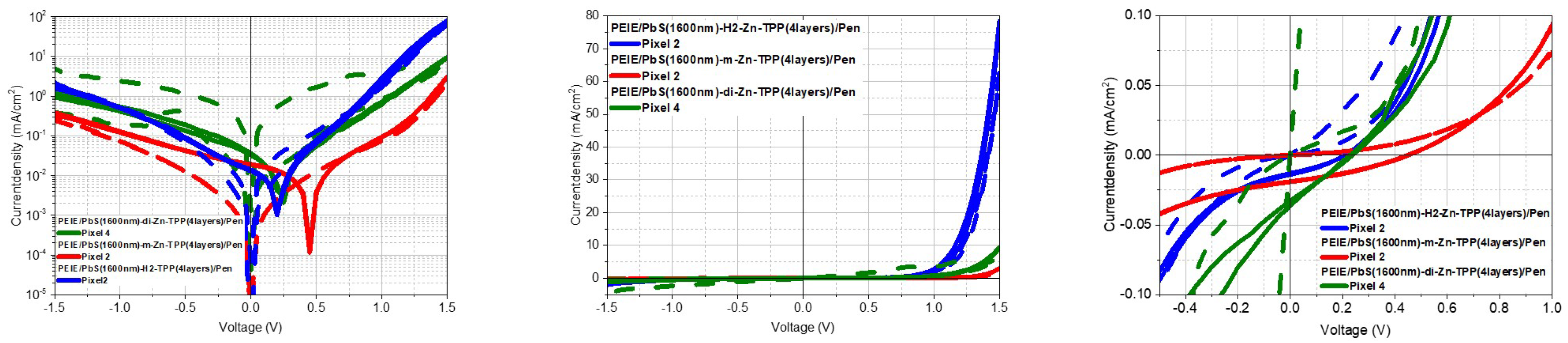
4.4. Tetraphenylporphyrin Ligands-TPP
| Sample | Pixel | FF | Efficiency | ||
| - | - | mA/cm2 | V | - | Percent |
| m-Zn-TPP | 2 | -0.01621 | 0.45009 | 0.32815 | 0.00281 |
| di-Zn-TPP | 4 | -0.02813 | 0.24074 | 0.24811 | 0.00198 |
| m-TPP | 2 | -0.01153 | 0.20961 | 0.31217 | 8.795E-4 |
References
- Scheele, M.; Hanifi, D.; Zherebetskyy, D.; Chourou, S.T.; Axnanda, S.; Rancatore, B.J.; Thorkelsson, K.; Xu, T.; Liu, Z.; Wang, L.W.; et al. PbS nanoparticles capped with tetrathiafulvalenetetracarboxylate: utilizing energy level alignment for efficient carrier transport. ACS nano 2014, 8, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Ehrler, B.; Wilson, M.W.B.; Rao, A.; Friend, R.H.; Greenham, N.C. Singlet exciton fission-sensitized infrared quantum dot solar cells. Nano letters 2012, 12, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B.; Michl, J. Singlet fission. Chemical reviews 2010, 110, 6891–6936. [Google Scholar] [CrossRef] [PubMed]
- Papa, C.M.; Garakyaraghi, S.; Granger, D.B.; Anthony, J.E.; Castellano, F.N. TIPS-pentacene triplet exciton generation on PbS quantum dots results from indirect sensitization. Chemical science 2020, 11, 5690–5696. [Google Scholar] [CrossRef] [PubMed]
- Garakyaraghi, S.; Mongin, C.; Granger, D.B.; Anthony, J.E.; Castellano, F.N. Delayed molecular triplet generation from energized lead sulfide quantum dots. The journal of physical chemistry letters 2017, 8, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Pun, A.B.; Asadpoordarvish, A.; Kumarasamy, E.; Tayebjee, M.J.Y.; Niesner, D.; McCamey, D.R.; Sanders, S.N.; Campos, L.M.; Sfeir, M.Y. Ultra-fast intramolecular singlet fission to persistent multiexcitons by molecular design. Nature chemistry 2019, 11, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.A.; Houtepen, A.J.; Crisp, R.W. Quantum dot solar cells: Small beginnings have large impacts. Applied Sciences 2018, 8, 1867. [Google Scholar] [CrossRef]
- Hu, J.; Xu, K.; Shen, L.; Wu, Q.; He, G.; Wang, J.Y.; Pei, J.; Xia, J.; Sfeir, M.Y. New insights into the design of conjugated polymers for intramolecular singlet fission. Nature communications 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Yadavalli, S.K.; Chen, M.; Abbaspourtamijani, A.; Qi, Y.; Padture, N.P. Interfacial toughening with self-assembled monolayers enhances perovskite solar cell reliability. Science 2021, 372, 618–622. [Google Scholar] [CrossRef]
- Brown, P.; Kim, D.; Lunt, R.; Bawendi, M.; Grossman, J.; Bulovic, V. Energy level modification in lead sulfide quantum dot photovoltaics through ligand exchange. APS 2014, 2014, L24–002. [Google Scholar] [CrossRef]
- Tabachnyk, M.; Ehrler, B.; Gélinas, S.; Böhm, M.L.; Walker, B.J.; Musselman, K.P.; Greenham, N.C.; Friend, R.H.; Rao, A. Resonant energy transfer of triplet excitons from pentacene to PbSe nanocrystals. Nature materials 2014, 13, 1033–1038. [Google Scholar] [CrossRef]
- Davis, N.J.L.K.; Allardice, J.R.; Xiao, J.; Petty, A.J.; Greenham, N.C.; Anthony, J.E.; Rao, A. Singlet fission and triplet transfer to PbS quantum dots in TIPS-tetracene carboxylic acid ligands. The journal of physical chemistry letters 2018, 9, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Mandelis, A.; Yang, Z.; Guo, X.; Lan, X.; Liu, M.; Walters, G.; Melnikov, A.; Sargent, E.H. Temperature-and ligand-dependent carrier transport dynamics in photovoltaic PbS colloidal quantum dot thin films using diffusion-wave methods. Solar Energy Materials and Solar Cells 2017, 164, 135–145. [Google Scholar] [CrossRef]
- Liu, Y.; Gibbs, M.; Puthussery, J.; Gaik, S.; Ihly, R.; W. Hillhouse, H.; Law, M. Dependence of Carrier Mobility on Nanocrystal Size and Ligand Length in PbSe Nanocrystal Solids. Nano Lett 2010, 10, 1960–1969. [CrossRef] [PubMed]
- Scheele, M.; Brütting, W.; Schreiber, F. Coupled organic-inorganic nanostructures (COIN). Physical Chemistry Chemical Physics 2015, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ko, J.H.; Kim, Y.H.; Jeong, S. Steric-hindrance-driven shape transition in PbS quantum dots: understanding size-dependent stability. Journal of the American Chemical Society 2013, 135, 5278–5281. [Google Scholar] [CrossRef] [PubMed]
- Beygi, H.; Sajjadi, S.A.; Babakhani, A.; Young, J.F.; van Veggel, F.C.J.M. Surface chemistry of as-synthesized and air-oxidized PbS quantum dots. Applied Surface Science 2018, 457, 1–10. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chemical reviews 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Giansante, C.; Infante, I. Surface traps in colloidal quantum dots: a combined experimental and theoretical perspective. The journal of physical chemistry letters 2017, 8, 5209–5215. [Google Scholar] [CrossRef]
- Tom, A.E.; Thomas, A.; Ison, V.V. Novel post-synthesis purification strategies and the ligand exchange processes in simplifying the fabrication of PbS quantum dot solar cells. RSC Advances 2020, 10, 30707–30715. [Google Scholar] [CrossRef]
- Falkenberg, C.; Olthof, S.; Rieger, R.; Baumgarten, M.; Muellen, K.; Leo, K.; Riede, M. The role of energy level matching in organic solar cells—Hexaazatriphenylene hexacarbonitrile as transparent electron transport material. Solar Energy Materials and Solar Cells 2011, 95, 927–932. [Google Scholar] [CrossRef]
- Brütting, W.; Riel, H.; Beierlein, T.; Riess, W. Influence of trapped and interfacial charges in organic multilayer light-emitting devices. Journal of Applied Physics 2001, 89, 1704–1712. [Google Scholar] [CrossRef]
- Laikhtman, B.; Wolf, E.L. Tunneling time and effective capacitance for single electron tunneling. Physics Letters A 1989, 139, 257–260. [Google Scholar] [CrossRef]
- Liao, M.S.; Scheiner, S. Electronic structure and bonding in metal porphyrins, metal= Fe, Co, Ni, Cu, Zn. The Journal of chemical physics 2002, 117, 205–219. [Google Scholar] [CrossRef]

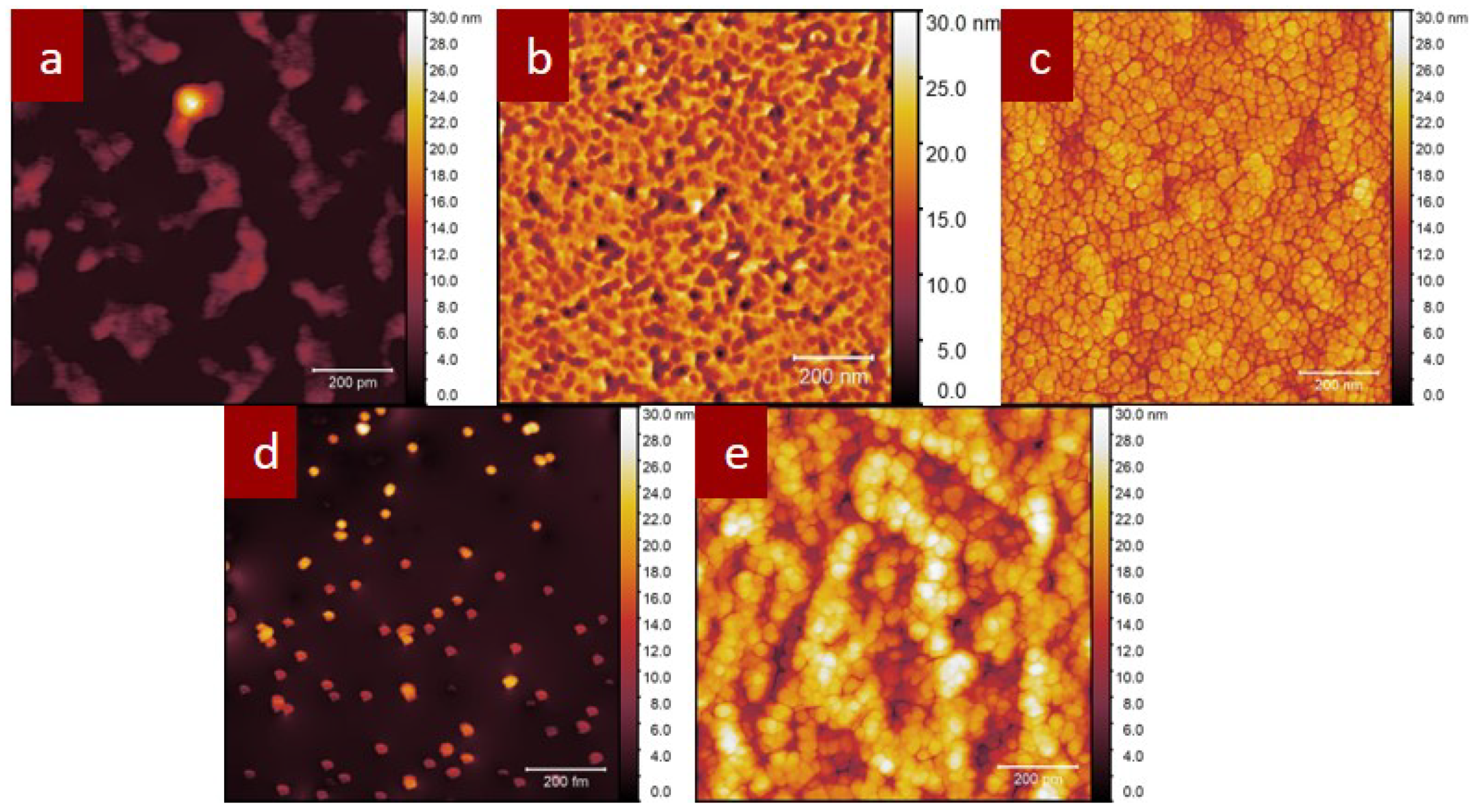
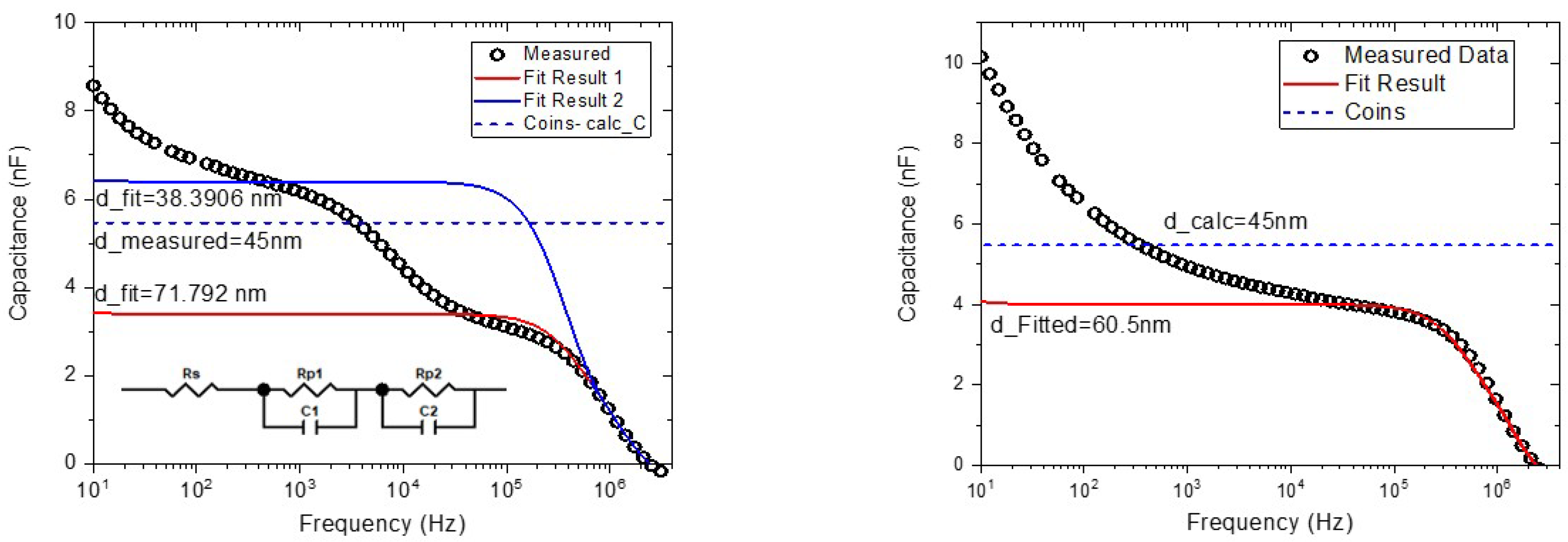
| COINs | Thickness (nm) | Roughness (nm) |
| PbS-AP-TIPS-pentacene | 45 | 1.582 |
| 28 | 3.77 | |
| PbS-mZnTPP-carboxylate | 3 | 0.6 |
| PbS-diZnTPP-carboxylate | 26 | 5.239 |
| Sample | Pixel | FF | Efficiency | ||
| - | - | mA/cm2 | V | - | percent |
| ZnPc | 3 | -0.02355 | 0.53362 | 0.19046 | 0.00278 |
| PTCBI | 2 | -0.00168 | 0.04165 | 0 | 0 |
| DBP | 1 | -0.00419 | 0.73612 | 0.25642 | 8.43E-4 |
| 1 | -0.09152 | 0.11048 | 0.30928 | 0.00293 |
| Sample | (V) | (e.V) | |
| ZnPc | 0.97 | 0.65 | 0.32 |
| PTCBI | 1.15 | 0.35 | 0.8 |
| DBP | 1.25 | 0.9 | 0.35 |
| 0.65 | 0.06-0.15 | 0.59-0.5 |
| Sample | Electron mobility | Hole mobility | |
| - | |||
| PbS-AP-TIPS-pentacene (1600nm) | - | ||
| PbS-AP-TIPS-pentacene (1600nm) | - |
| Sample | Pixel | FF | Efficiency | ||
| - | - | mA/cm2 | V | - | Percent |
| Normal Stack | |||||
| COINs/Pen/ | 3 | -0.00168 | 0.12403 | 0.19263 | 5.035E-5 |
| COINs/BCP/Al | 1 | -0.00674 | 0.001 | 0.00E+00 | 0 |
| Pen/ | 2 | -5.16213 | 0.28292 | 0.50081 | 0.73877 |
| Inverted Stack | |||||
| /Pen/COINs | 1 | -1.10365 | 0.35154 | 0.2856 | 0.13655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




