2. Case Description
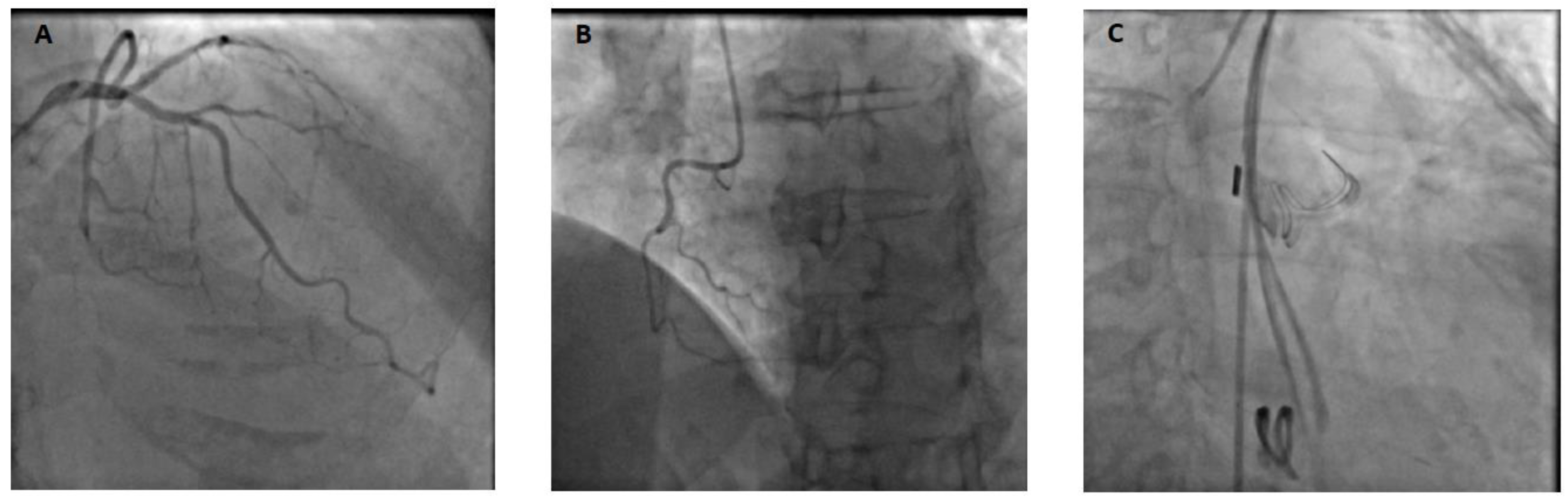
A 69-year-old patient without any relevant past medical history underwent aortic valve replacement in the context of a bicuspid valve and severe aortic stenosis. The surgery was uneventful, and the patient was weaned from cardiopulmonary bypass successfully with a minimal amount of vasopressor support and under transesophageal echocardiography (TEE) guidance. However, while surgical hemostasis was performed, the patient became progressively unstable and required increased doses of vasopressors and inotropes. After sternal closure, another TEE examination was urgently performed and showed severe dilation and global akinesia of the right ventricle. The surgical and anesthesiology team decided to proceed to a percutaneous coronary intervention (PCI). Angiography showed complete occlusion of the right coronary artery (
Figure 1); however, the artery was not technically amenable to percutaneous treatment. The patient was transferred to our tertiary ECMO center in critical condition. Indeed, on arrival, the patient presented with profound CS refractory to optimal medical management (SCAI E). VA-ECMO was immediately initiated, complicated by a brief cardiac arrest during cannulation (bilateral femoral cannulation: arterial cannula was inserted on the left and venous cannula on the right side). Due to an ischemic time of more than 8 hours, the small caliber of the right coronary artery and left coronary dominance (
Figure 1), it was decided by a multidisciplinary team (cardiac critical care, cardiology and cardiac surgery consultants) to not proceed with emergent coronary artery bypass graft surgery in a patient presenting with multiple organ failure (MOF). Anticoagulation was withhold during the initial 48-hour period. This decision was made because the patient presented rapidly after admission a tamponade along with a hemorrhagic shock resulting from bleeding at the aortotomy site. Subsequently, a conventional anticoagulation regimen with unfractionated heparin was initiated and gradually increased towards therapeutic anticoagulation levels (Anti-Xa 0.3-0.5 IU/ml). The patient's anticoagulation status was closely monitored through Anti-Xa assay measurement.
When the patient stabilized and organ function was restored, sedation was stopped, allowing neurological evaluation. Awakening occurred on postoperative Day 4, without any neurological sequelae, and the patient was extubated. Levosimendan was initiated on Day 10, and fluid depletion was achieved through diuretic administration. Nonetheless, the patient could not be weaned off VA-ECMO due to an akinetic and nonrecruitable right ventricle, despite inotropic support. It was decided to wait further on MCS. The patient was conscious, calm, and cooperative but remained bedridden because of the femoro-femoral VA-ECMO. After two weeks, the patient still could not be weaned off VA-ECMO. Indeed, below two liters per minute of blood flow, the patient showed both macrocirculatory and microcirculatory signs of ARF. The mean arterial pressure dropped, the central venous pressure (CVP) increased up to 25 mmHg, the SvO
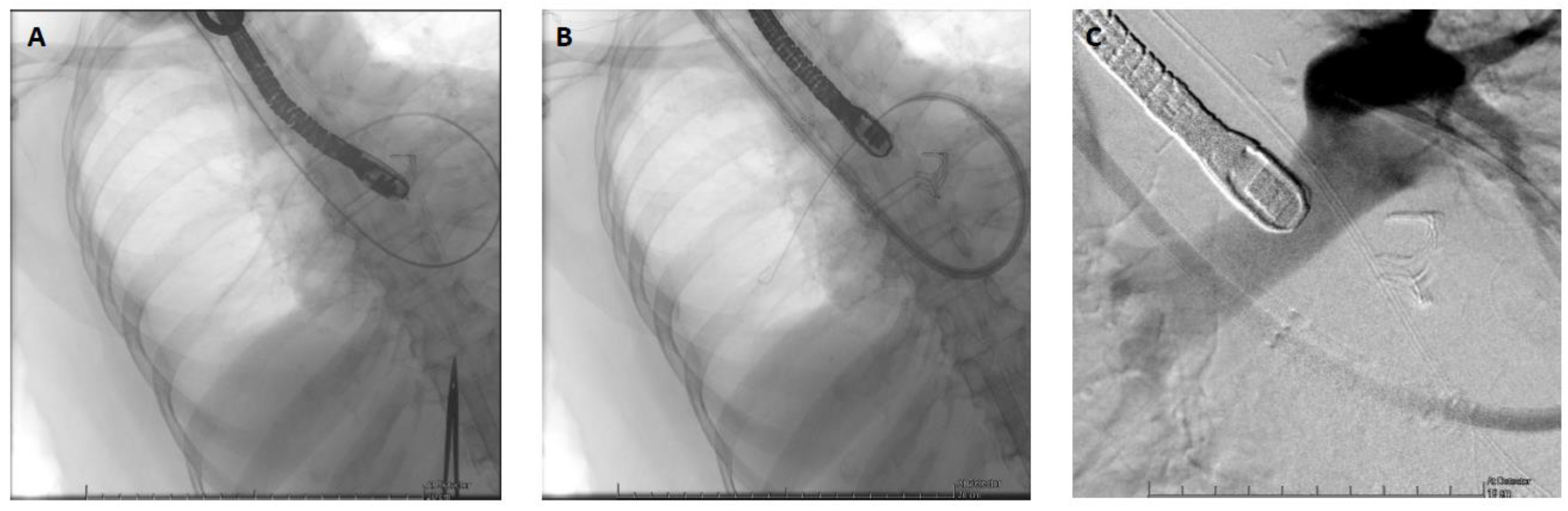
2 dropped from 60 to 30%, the lactate level increased up to 4 mmol/l, and the patient became oliguric. The TOE exam showed a severely dilated and akinetic RV (TAPSE = 4mm, S’ VD 2.3 cm/s, FAC RV = 10%), a small LV size with a preserved function and a LVOT VTI 10 cm/s. Due to her age and an INTERMACS 1 profile, the patient was not eligible, in our country, for a heart transplant or a definitive RVAD implantation (Heartmate III, modified insertion for the right ventricle). Faced with the prospect of prolonged weaning and no other therapeutic alternatives, VA-ECMO was switched to a temporary percutaneous RVAD by implanting a ProtekDuo cannula through the right internal jugular route (
Figure 2). A CentriMag
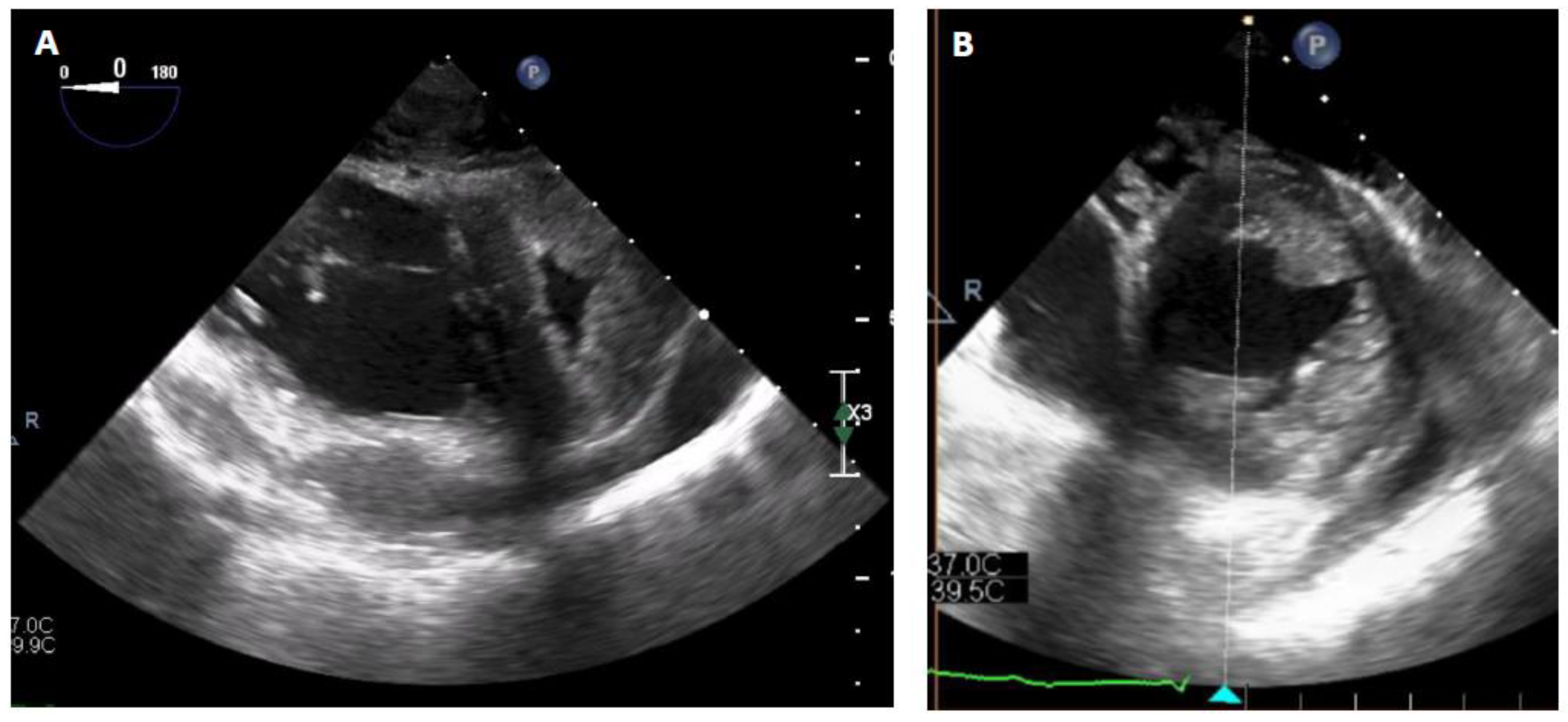
TM centrifugal pump (Abbott, Chicago, USA) without an oxygenator was connected to the cannula with an initial flow rate of 4 liters per minute. After a few minutes, the VA-ECMO components could be removed without any vasopressor support. After the initiation of the RVAD, intraoperative TEE showed a reconfiguration of the ventricles. The right ventricle was suitable unloaded by the RVAD, while the left ventricle resumed a circular shape with complete regression of the D-shaped sign (
Figure 3). The hemodynamic tolerance of the RVAD was very good (lactate levels < 2 mmol/L, SvO
2 > 60%, normal renal and hepatic function). The day after the implantation, the patient was out of bed, placed in a chair, and began rehabilitation. Slow and gradual weaning was instituted. In fact, during the following three weeks, a gradual reduction in the flow rate of the RVAD was carried out at a rate of approximately one liter per week. Interestingly, the serial TTE exam did not show a significant improvement in RV contractile function, but as the pulmonary pressure, pulmonary resistance and LVEDP remained low, the patient’s hemodynamics adapted and showed physiological similarities with a Fontan circulation. During gradual weaning, the renal and hepatic function was monitored daily (creatinine, BUN, AST, ALT, Bilirubine and Factor V levels) and we did not observe any biological signs of congestion or organ dysfunction. Finally, the patient could undergo explantation after 3 weeks of RVAD support. Closed monitoring for 5 additional days was carried out in the ICU. Right ventricular systolic function remained poor (three-dimensional RV ejection fraction (RVEF) = 26%, RV fractional area change (FAC) = 21%, severely reduced longitudinal function with global akinesia except for the apical segments of the anterior and lateral wall) but was well tolerated. CVP remained below 15 mmHg, SvO
2 was above 60% with normal lactate levels, and renal and hepatic functions remained stable. Anticoagulation therapy was empirically initiated to prevent thromboembolic events, as the patient would likely not survive any abrupt increase in RV afterload. Diuretics and antihypertensive treatments were continued to maintain a low LVEDP. To be noted, during mechanical circulatory support, the patient did develop only minor ECMO-related complications. Minor bleeding at the canula site were noted after canulation and stopped rapidly after optimization of the coagulation profile. Femoral venous thrombosis (at the site of the cannula) was diagnosed after explantation and treated accordingly. We did not observe any Protekduo-related complications.
The patient was transferred to the medical ward after 5 weeks in the ICU, stayed 5 months in an acute medical setting, went to a rehabilitation center afterward and returned home 6 months after the initial surgery. In total, she remained on MCS for 39 days (21 on VA-ECMO and 18 on ProtekDuo).
3. Comments
The present case report highlights that during an isolated RV infarction leading to refractory CS, switching from VA-ECMO to an isolated RVAD via a ProtekDuo cannula is a feasible strategy in the context of prolonged weaning from mechanical circulatory support (MCS). This approach allowed awakening, extubation and mobilization of the patient while enabling the right ventricle to progressively adapt to new loading conditions.
RVF due to acute myocardial infarction (MI) is rare but is associated with high morbidity and mortality. In post-MI RVF, RV contractile function becomes severely impaired, resulting in a decrease in RV systolic pressure, an increase in end-diastolic volume, and an increase in diastolic pressure. Due to the pericardial constraint, the pressure and volume overload lead to a shift of the interventricular septum to the left, decreasing the LV compliance and LV filling and therefore leading to a low end-diastolic volume. With the reduction in diastolic function and dilation, the volume overload of the right ventricle causes an increase in right atrial pressure and a decrease in venous return and systemic venous congestion, which can lead to acute kidney injury (AKI), ischemic hepatitis, or mesenteric ischemia. Altogether, these combined effects can lead to decreased left heart filling, reduced systemic cardiac output and ultimately to CS with multiple organ failure [
1].
Management strategies for CS in post-MI RVF include early hemodynamic and ultrasound recognition, recommendation for PCI within 90 minutes (class 1), careful fluid management, administration of vasoactive/inotropic drugs to maintain end-organ perfusion and optimization of RV afterload [
2]. In this patient, PCI was not technically feasible, and surgical revascularization was not performed because of the long ischemic time, the presence of a dominant left coronary network and a right coronary artery too small to be bypassed, all in the context of a patient with multiorgan failure.
In cases of CS refractory to optimal medical management, early initiation of short-term MCS should be considered [
2]. Current options for percutaneous MCS for isolated RVF include the Impella RP, TandemHeart RV assist device, ProtekDuo dual lumen cannula, and VA-ECMO [
3].
VA-ECMO involves a rapidly implantable percutaneous device that not only unloads the right ventricle and reduces venous congestion but also supports end-organ perfusion. This is the most widely used and easily accessible short-term MCS in refractory CS [
4]. Nonetheless, when organ functions are restored and only RVF persists, VA-ECMO can be considered a limitation, particularly regarding mobilization and rehabilitation.
A more right ventricle-specific MCS system exists and could therefore be considered. The Impella RP (right peripheral; Abiomed Inc., Danvers, MA) is a percutaneously implantable microaxial 22 French pump. Blood is aspirated from the right atrium and ejects into the pulmonary artery (PA), bypassing the right ventricle. A reduced RA pressure, increased PA pressure, and increased LV preload characterize the hemodynamic effects of the Impella RP. The main disadvantage of this device, at least in Europe at present, is that it can only be placed through femoral access, which limits patient mobility and therefore rehabilitation. Compared to other MCS systems, it does not provide any respiratory support due to the absence of an oxygenator. In addition, rotating at 33,000 RPM for a maximum flow of 4 L/min, the pump could generate clinically significant hemolysis and therefore increase morbidity. Finally, it requires anticoagulation therapy, leading to potential bleeding complications [
5]. Successful use of the Impella RP has been reported in post-MI RVF, in massive pulmonary embolism [
6], in postcardiotomy syndrome [
7], after LVAD implantation [
8], and in primary graft dysfunction after orthotopic heart transplantation [
9]. However, even if these studies have shown encouraging results, they only included small and heterogeneous patient cohorts, with a small proportion presenting post-MI RVF. Furthermore, some results have been the subject of postapproval study assessment by the US Food and Drug Administration (FDA). Therefore, the Impella RP may be used in RVF refractory to medical therapy as a bridge to recovery but requires further analysis regarding long-term benefits.
The TandemHeart RVAD is a dual-access extracorporeal centrifugal pump that can deliver blood flow at a rate of up to 4 L/min. Classically, it uses two 21 F cannulas implanted via the femoral veins: one is placed in the right atrium (inflow), and the other is placed in the PA (outflow) [
10]. The TandemHeart RVAD can be used with a circuit-coupled oxygenator and can be used to manage concomitant hypoxic respiratory failure [
11]. The hemodynamic profile of the TandemHeart RVAD is similar to that of the Impella RP, with a reduced RA pressure, increased PA pressure and increased LV preload [
7]. Due to the required femoral access, it shares the same disadvantages as the VA-ECMO and IMPELLA RP devices regarding patient mobilization and rehabilitation.
The ProtekDuo cannula is a 29 F or 31 F double-lumen cannula that is inserted percutaneously via the right internal jugular vein [
12]. The inflow is positioned in the right atrium and the outflow in the PA. These 2 ports are connected to a centrifugal pump, with or without an oxygenator, which, depending on the size, delivers blood flow at a rate of up to 4 L/min. The major advantage of the ProtekDuo cannula is the elimination of femoral access, thus allowing patient mobilization [
13]. In addition, the blood flow generated by the device is close to that of a definitive RVAD and therefore permits direct loading and assessment of the left ventricle. It could be an interesting step in patients in whom a definite RVAD approach is limited by the evaluation of LV valve physiology and diastolic and systolic functions during VA-ECMO support. It is this MCS system that was used in our patient.
The use of the ProtekDuo® cannula coupled with a centrifugal pump for isolated acute RV failure has been the subject of several case series. In a series of 13 patients with acute RV failure treated with the ProtekDuo®, including four who had acute RV myocardial infarction, Nicolais et al. reported a median duration of support of 6 days and a survival rate of 54% to device explantation [
14]. In another retrospective study of 10 patients with acute myocardial infarction complicated by acute RV failure who underwent ProtekDuo® implantation for RVAD support, Kremer et al. reported a mean duration of RVAD support of 10 ± 7.4 days and a 30-day and 1-year survival rate of 60% [
15]. Finally, in a retrospective cohort study of 40 patients with acute RV failure, Badu et al. reported an overall rate of survival to discharge of 68%. To our knowledge, none of those studies mentioned or proposed strategies to improve rehabilitation, including switching from VA-ECMO to ProtekDuo®/RVAD support. The ProtekDuo® cannula may offer additional benefits, including nonsurgical, single-site access in the upper body, which may allow for earlier extubation and/or mobilization, as in our patient [
16].