1. Introduction
History. Indigo, the blue natural vat dye, was extracted from plants in ancient times, since 3000-4000 BCE, in Asia, Africa and Europe, as well as in America. It got its name Indigo, because it reached Europe from Indus Valley in India. At that time indigo was used mainly for dyeing clothes [
1]. It was also used to synthesize the “Mayan Indigo pigments” by various civilizations to paint frescoes and decorate pottery, statues, masks [
2]. Faced by high prices charged by the British traders for natural indigo dye, the German Adolf Baeyer succeeded in synthesizing a cheap indigo dye in 1882. This explained why natural plant- produced- indigo dye and indigo crop became a part of history [
1].
Present situation Blue indigo is a vat dye that is insoluble in water in its oxidized form, and hence allows for good color fastness to washing. It is known for its excellent wash fastness and quite good resistance to light. The dyeing process is carried out in an alkaline medium with a pH between 10 and 12, using sodium hydroxide and sodium dithionite as a reducing agent. The reduced form of indigo, called the leuco -indigo, is yellow and water-soluble, and it diffuses inside the cellulosic fiber. The dye is then oxidized back to the blue insoluble indigo dye. The annual synthetic indigo production is estimated to 70,000 tons [
3]. Indigo is the main dye used in the production of the 4 billion denim pieces, which corresponds to a market volume of 90 billion dollars [
4]. The dyeing process of cotton into denim with synthetic indigo dyes presents three main ecological problems: 1.
Mutagenic contaminants, with up 0.6% aniline and 0.4% N-methylaniline by weight are present as in synthesized indigo dyes [
5]; 2. The indigo reduction into leuco-species using
sodium dithionite leads to the formation of substantial quantities of
waste water; 3.
Huge amounts of water, approximately 20 liters per meter of fabric, are required for dyeing denim pants, as the cotton warp yarns are immersed in 10 to 15 successive dye baths with intermittent exposure to the air to fix the indigo properly [
4].
Demand for more sustainable dyes and dyeing processes. During the past decade, developments in natural indigo dyeing technologies have been made to improve the environmental impact of the dyeing process. Bio-based reducing agent [
6], such as glucose, fructose, are being used to replace the sodium dithionite, while cationization of cellulosic fibers allows for improved dye absorption and exhaustion [
7]. Ecotechnologies such as Smart-Indigo™ utilizes electrochemical reduction [
8] to optimize denim dyeing and reduce environmental impacts. Recently, a renowned denim brand Wrangler, along with a Spanish based textile mill Tejidos Royo has invested in a foam dyeing technique [
9] using technology of Gaston Systems Inc., a US-based textile firm, to produce its first line of denims that will be foam dyed.
Natural Blue Indigo dyes from Plant species. The interest in natural indigo dye is increasing as the demand for organic clothes and natural dyes is growing [
3]. In countries such as Japan and the United States, natural indigo is being used for both jean dyeing or luxury silk batik printing. In India, as far back as 2000 BC, indigo was extracted from the
Indigofera tinctoria shrub. In Europe, indigo was extracted from the woad plant, or
Isatis tinctoria, which contains isatane B, and indigo from
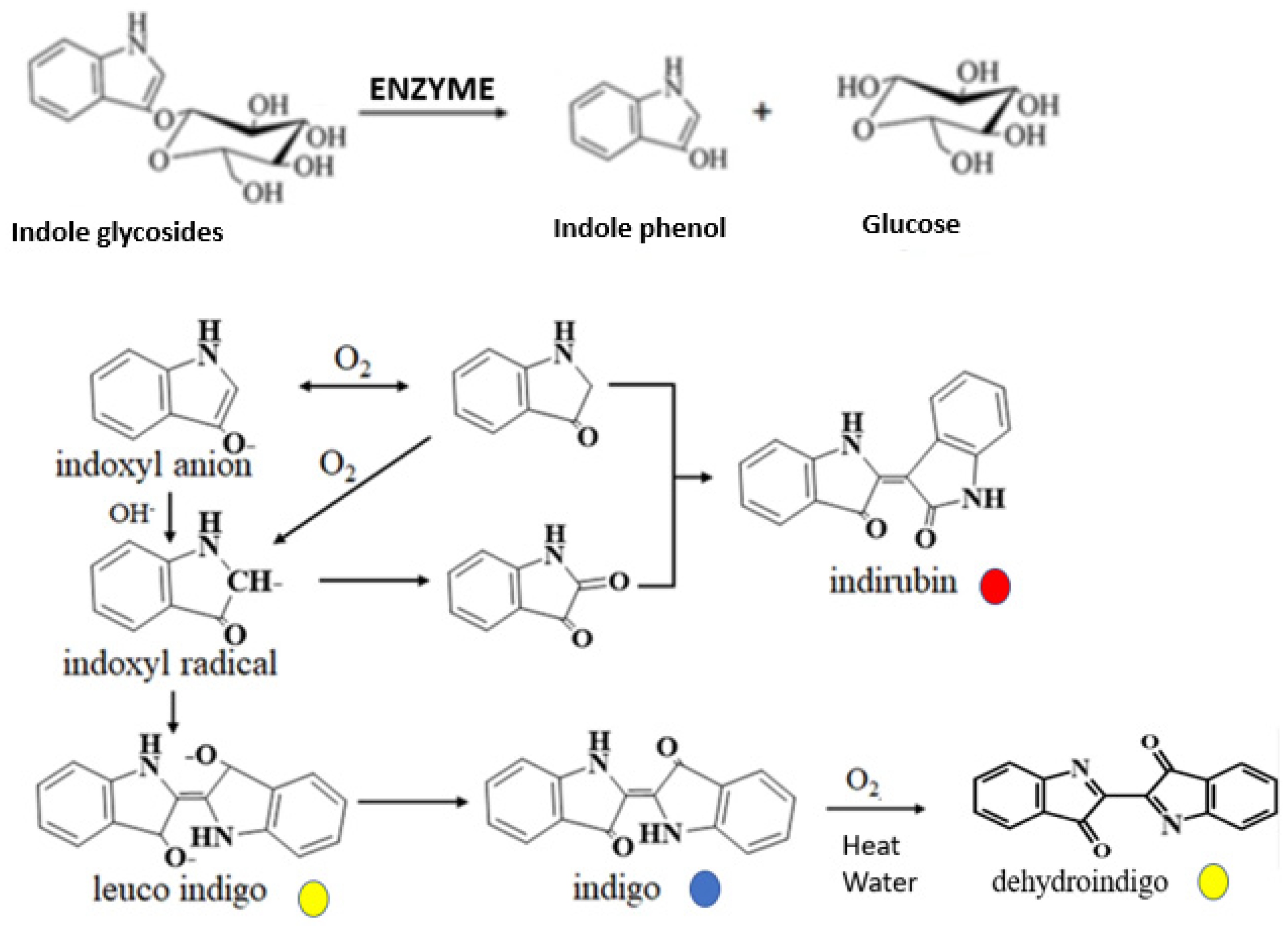
Persicaria tinctoria was extracted in Japan. The first step involves grinding the indigo leaves and then macerating them to rupture the vacuoles and chloroplasts of the leaves, which contain indole and an enzyme, respectively. Indole (colorless), when in contact with the enzyme, transforms into indoxyl. Subsequently, indoxyl dimerizes into leuco indigo (yellow), which oxidizes to form blue indigo (insoluble) in the presence of oxygen. Under certain conditions (pH, temperature, oxygen), indirubin, a red dye, can also be formed from the isomerization of these molecules [
10,
11,
12] (Figure 8). Only the blue dye indigo is extracted using appropriate solvent, and then sold for dyeing textiles such as blue denim.
Dyeing using indigo leaves Leaves are composed of lots of organic compounds including pharmaceuticals, antimicrobials, amino acids, vitamins and dyes [
10,
12]. Dyeing textiles directly with crushed indigo leaves is possible in an artisanal manner. Ancient practices used it to dye hair, and today there is a resurgence of this technique. By varying pH and temperature, and using reducing agents, different shades can be obtained on silk fabric, from blue green to purple and yellowish shades [
13].
Antibacterial activity. Several papers show that some of the indigo powders/extracts have an antibacterial activity on certain bacteria such as
Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Proteus vulgaris, Candida albicans [
10,
14].
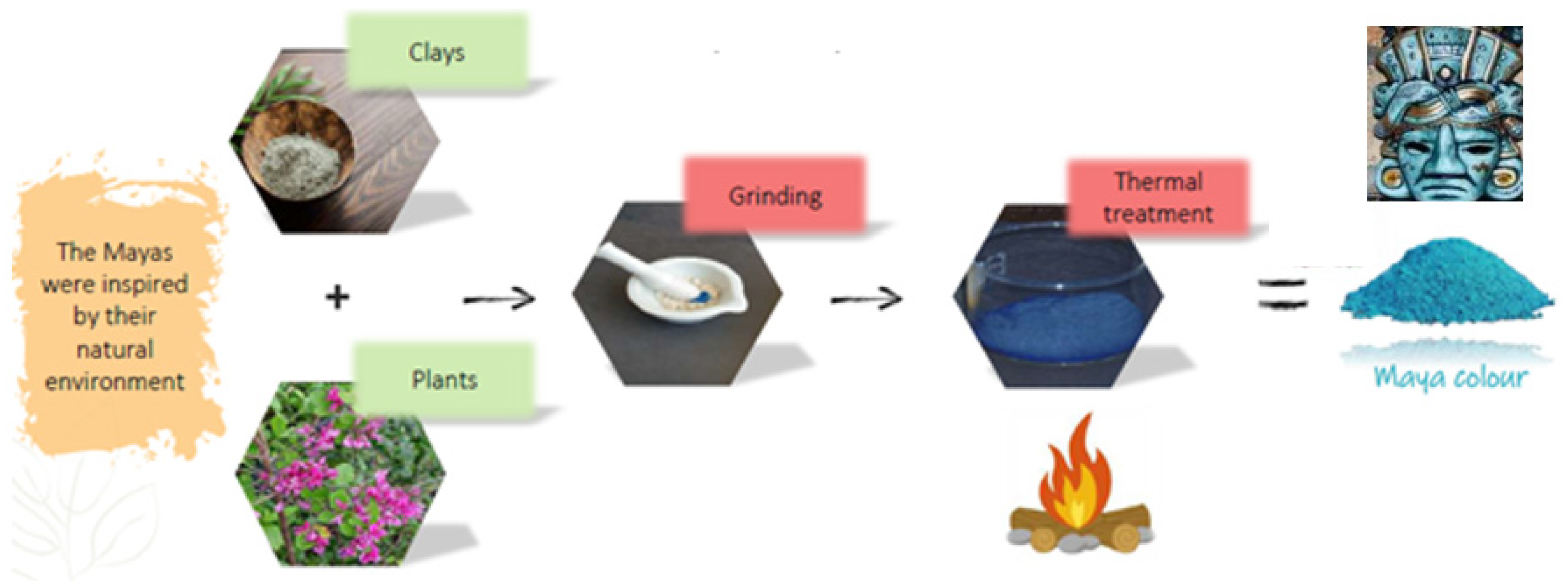
Mayan indigo pigments. Natural Indigo pigments were synthesized by various civilizations to paint frescoes and decorate pottery, statues, masks, and more (
Figure 1). These colors have endured through the centuries, retaining their brilliance. The remarkable stability of Maya blue can be attributed to the confinement of indigo molecules within the channels of a clay matrix, achieved through a thermal treatment [
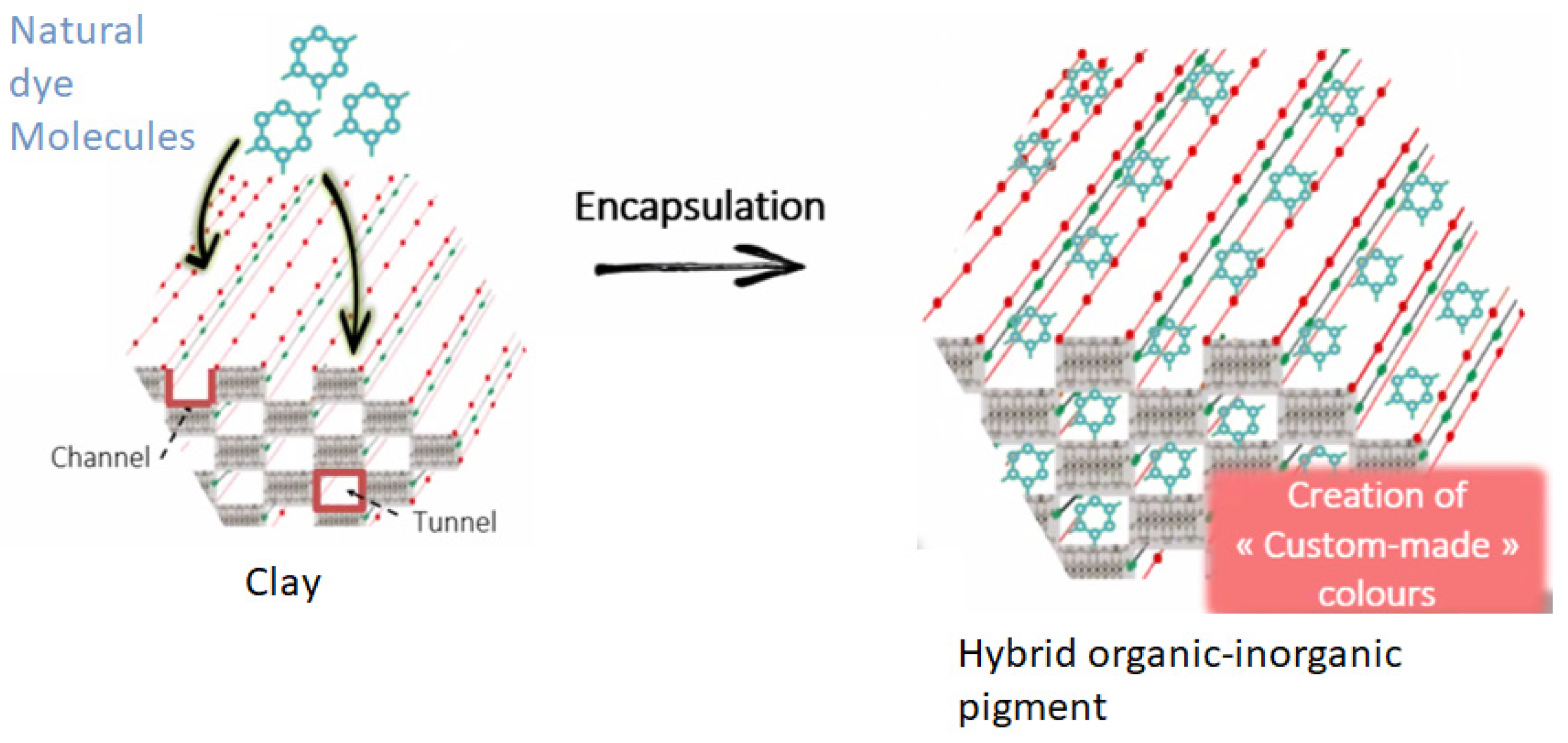
2]. Spectroscopic techniques have revealed that this pigment consists of three ingredients: a plant-based dye, clay, and resin. The dye is indigo, extracted from the leaves of the Indigofera suffruticosa plant. The clay used is palygorskite, a relatively rare magnesium and aluminum silicate that, unlike most clays, features channels where indigo molecules can be incorporated [
15] (
Figure 2). In Mexico, this clay is extracted from the soils of the Maya kingdom. The third ingredient is copal resin, traditionally used as incense in religious rituals, which hardens to form amber. Maya blue can thus be seen as a precursor to the materials we now call hybrids, composed of both organic and inorganic compounds [
2]. Several French researchers carried their on the Mayan pigment nanotechnology [
16,
17], and co-founded the PIGM’Azur company, to develop pigments inspired from Mayan paintings. Using 90% Sepiolite clay [Mg
8Si
12O
30(OH)
4(OH
2)
4.8H
20] and 10%
Indigofera Tinctoria Extract (patent FR0708777 (17/12/2007), together with grinding and heating process, the Mayan indigo pigment NC63 Blue has been produced. The blue indigo dye molecules (guests) are protected from external aggression by incorporation in the nano-channels (hosts) of sepiolite. Molecular interactions between the sepiolite and indigo through the formation of organic-inorganic complexes confer stability to indigo dyes [
16,
17]. These hybrid organic/inorganic pigments have a high degree of naturalness (100% for NC63 Blue according to ISO 16125), saturation, and stability to acids, bases and UV light.
Objective of this paper: Hence the aim of this study was to test the efficiency of Mayan blue pigments produced by PIGM’Azur for the dyeing/printing of textiles, and compare their color fastness to existing textiles dyed/printed with natural indigo dyes or extracts. Direct printing using flat or rotary screens with natural blue indigo vat dye is not easy to perform as the leuco-species oxidizes easily. In general batik and resist dyeing or discharge printing are used to achieve printed designs with vat blue indigo dyes. A comparative experimental study is carried on a various range of textile fibers to show the potential use of indigo-based colored products. Formulations with Mayan indigo pigment are optimized to achieve pigment dyeing and direct printing. The possibility of using 100% natural PIGM’Azur—Mayan inspired piments on textile fabrics, for direct printing through the use of bio-based binders, as a breakthrough technology, will be presented.
2. Materials and Methods
2.1. Materials
2.1.1. Fabrics
A Multifiber fabric, with the following fiber yarns in weft direction (actetate, acrylics, triacetate, cotton, polyester PET1, and PET2, polyamide 6, silk, polypropylene, viscose, wool, (see Figure 5a), was used for the dyeing experiments. For dyeing and printing, textile fabrics (woven, knitted and lace) having specific grammage between 80- 100g/m² were used. Twill woven polyester, cotton, wool fabrics were used, knitted jersey of acetate and polyamide 6, as well as a lace of polyamide 6, were used. All fabrics were scoured and bleached (except wool). Samples were conditioned under 65% ± 2% relative humidity and 20 ± 2 °C for at least 24 h prior to use.
2.1.2. Dyes, pigments and leaves used
Three Blue indigo dyes from three different origins were purchased from GREENING (FRANCE):
− Indigo 1: Indigofera suffruticosa. Crops under organic conditions in Central America. Artisanal and fair trade production.
− Indigo 2: Indigofera Tinctoria L species. Artisanal production in India.
− Indigo 3: Persicaria tinctoria (Aiton) Spach species = Polygonum tinctorium Aiton. Indigo of Renouée des teinturiers. Organic crops and artisanal production in Provence by Laura and Amandine (Le Champ des Couleurs).
2.1.3.
Powered leaves of Indigofera Tinctoria were purchased from AROMA ZONE (France) and Blue hybrid organic-inorganic pigment NC63 Blue was provided by PIGM’Azur, both in powdered form and in the form of paste (90% bio-based glycerin and 10% pigment). PIGM’Azur uses Sepiolite (90%) and Indigofera Tinctoria Extract (10%) to produce the NC63 Blue, which is thus 100% Natural, COSMOS & ECOCERT certifiable, and NON TOXIC as per toys, food contact and cosmetic regulation.
Figure 3.
Indigo-based coloring agents used for dyeing and printing of textiles.
Figure 3.
Indigo-based coloring agents used for dyeing and printing of textiles.
2.1.4. Other chemicals used
All chemicals, fructose, quick lime and ethanol were also purchased from GREENING for dyeing process.
For pigment dyeing the four following binders were tested: Dicrylan (a soft polyurethane from Hunstman chemicals), two synthetic self-crosslinking acrylic copolymers (APPRETAN N96100 and APPRETAN N92111), and a bio-based aqueous styrene-acrylic type polymer dispersion, with 40% renewable material-APPRETAN NTR@6553. More than 40% of Appretan®’s active content is based on a polysaccharide from renewable raw materials designed for food filtration articles, and tea bags. It is Formaldehyde-free. The Appretan® NTR grades are Bluesign® registered and compliant to the GOTS 6.0 requirements and to Oekotex Standard 100.
For printing, bio-based sodium alginate and glycerin thickeners from Sigma Aldrich were used.
2.2. Methods for dyeing and printing textiles with dyes, powdered leaves and pigments
2.2.1. Dyeing, Batik-dyeing and resist-dyeing with bleu indigo dyes from GREENING
2.5 g of indigo was dispersed in 4 ml of ethanol to obtain a homogeneous paste, and 6 ml of very hot water was added. 7.5 g of fructose was separately dissolved in 10 ml of hot water. In a 500 ml of stainless steel beaker, 480 ml of hot water (70 °C) was added, then while stirring, the prepared indigo paste followed by the fructose syrup, then quick lime (5g) were added. The beaker was tightly closed, and placed in a water bath at 50 °C for 20 minutes. As the beaker is opened, a greenish yellow solution formed, indicates the formation of the water-soluble indigo leuco species. Various fabrics, including tied fabrics (for batik dyeing), multifiber fabric and polyamide lace were dyed by making the number of immersions in the dye bath followed by rinsing and oxidising in air, necessary to obtain the shade required. A final rinse in water with acetic acid (or pH5), was performed to eliminate the alkalinity. In some cases, textiles were dyed in one step using this exhaustion dyeing, by using closed beakers.
2.2.2. Dyeing with powdered green leaves from Indigofera Tinctoria (from AROMA ZONE)
Three sets of five dyebaths were prepared, by pouring 200 ml of water (at pH 3, 5, 7, 9, 12 respectively) in a 500 ml stainless beaker and adding 5g of green indigo leaf powder in each dyebath. 2 g of multifibre fabric or other fabrics were immersed in each dyebath. The temperature of the three sets of dyebaths were set at 50°and 70 °C for 30 minutes, and at room temperature (25 °C) for 2 hours, repectively. Textiles were dyed in one step using this exhaustion dyeing, by using closed beakers.
2.2.3. Dyeing and printing with PIGM’Azur Indigo-based pigments in powdered form, or in paste form with glycerin
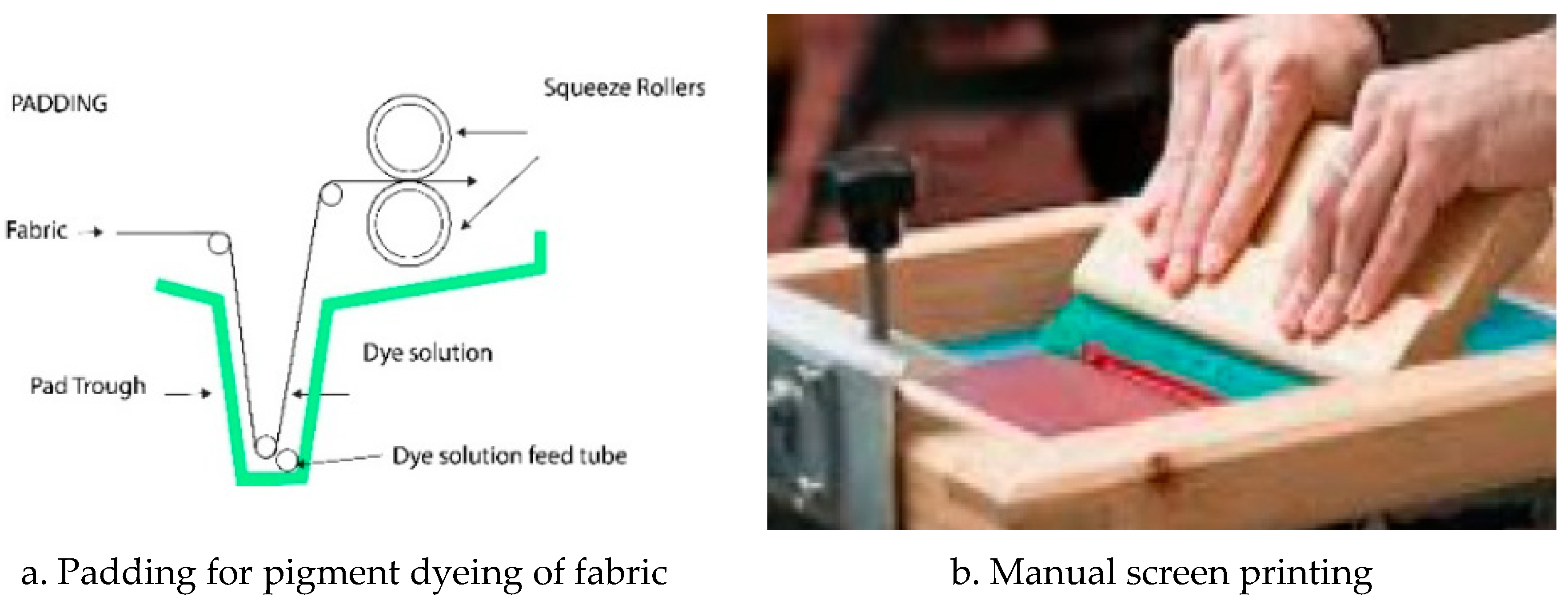
Pigment dyeing was performed on cotton fabrics using pad-dry cure process with an aqueous formulation with very low viscosity (20 mPa sec), containing the binder and the pigments. The fabrics were padded with the pigment formulation with a horizontal padding machine (Weriner Mathis AG, Oberhasli, Switzerland) for an uptake of 100%.
Screen Printing: Printing formulation were prepared to reach 30 000 mPa sec, suitable for manual screen printing before application on A4-format fabric samples.
First a sodium alginate thickener paste was prepared using 40 g of low molecular sodium alginate powder mixed with 500 ml of distilled water acidified to pH 5 using acetic acid. The thickener paste was kept aside for 24 hours before use. The printing paste was prepared by mixing the indigo pigment powder or the indigo pigment/glycerin paste with the APPRETAN NTR@6553 binder and with the alginate paste or glycerin thickener, using a motorized high speed stirrer.
2.3. Characterization of dyed and printed fabrics
2.3.1. Washing Test
For the washing test, the SL-F09 Rotawash Washing Fastness Testing machine was used. Washing was carried at 40 °C for 30 minutes using 5g/L of Ultravon detergent, 50 ml of water and 1 g of textile. For evaluation of colour degradation, a scale from 1 (the lowest washing fastness) to 5 (the highest washing fastness) was used, according to ISO 6330:2012.
2.3.2.
Rub test was done using a crockmeter. A sample was placed on the metal arm. In case the size of the sample was smaller than the size of the base, masking tape had to be used to fix the sample. Then a metal weight was placed over the sample to hold it in place. On the upper arm of the device a piece of cotton fabric was placed. The rub test was done by moving the upper arm in such a way that the surface of the printed sample was rubbed by the small piece of cotton fabric forward and back for 10 times. The test was done twice, first with a dry reference cotton piece and second with a wet reference cotton piece, according to the standard ISO 105-X12:2016.
2.3.3. Light fastness
To test the UV light resistance of the fabric samples, Xenotest 440 was used according to standard ISO 105-B02:2014. The samples were attached to small frames, half of the sample was covered using a hard paper and the rest was exposed to UV light. The exposure lasted for 16 hours. Then the resistance was evaluated using a wool standard and scale of numbers from 1 (the lowest fastness) to 7 (the highest fastness).
2.3.4.
Antibacterial tests in natural dye powders and on dyed fabrics using Agar diffusion test (ISO20645:2004). The level of antibacterial activity is assessed by examining the extent of bacterial growth in the contact zone between the agar and the fabric specimen and, if present, the extent of the inhibition zone around the specimen. 10 (±1) mL of nutritive agar medium were poured on Petri dishes. Inoculums of bacteria (0.5 ± 0.1) mL with a bacterial culture of 1–5 × 108 CFU/mL were then poured on the agar media. 1 mg of natural indigo powder or squared indo-dyed textile sample of 9 cm² were then placed on the surface. To maintain good contact, if necessary, a sterilized inox ring was placed on the surface of the textile sample to guarantee good contact between the fabric and the agar. Immediately after placing textile samples on the agar, petri dishes were placed in incubation for 24 h at 37 (±1) °C. The inhibition zone was measured. The halo is the zone free from bacteria near the sample edges. Contact zone under the tested textile sample was analyzed visually to check whether bacteria growth occurred or not.
3. Results
3.1. Dyeing with blue Indigo dyes
3.1.1. Spectral analysis of reduced blue Indigo dyes
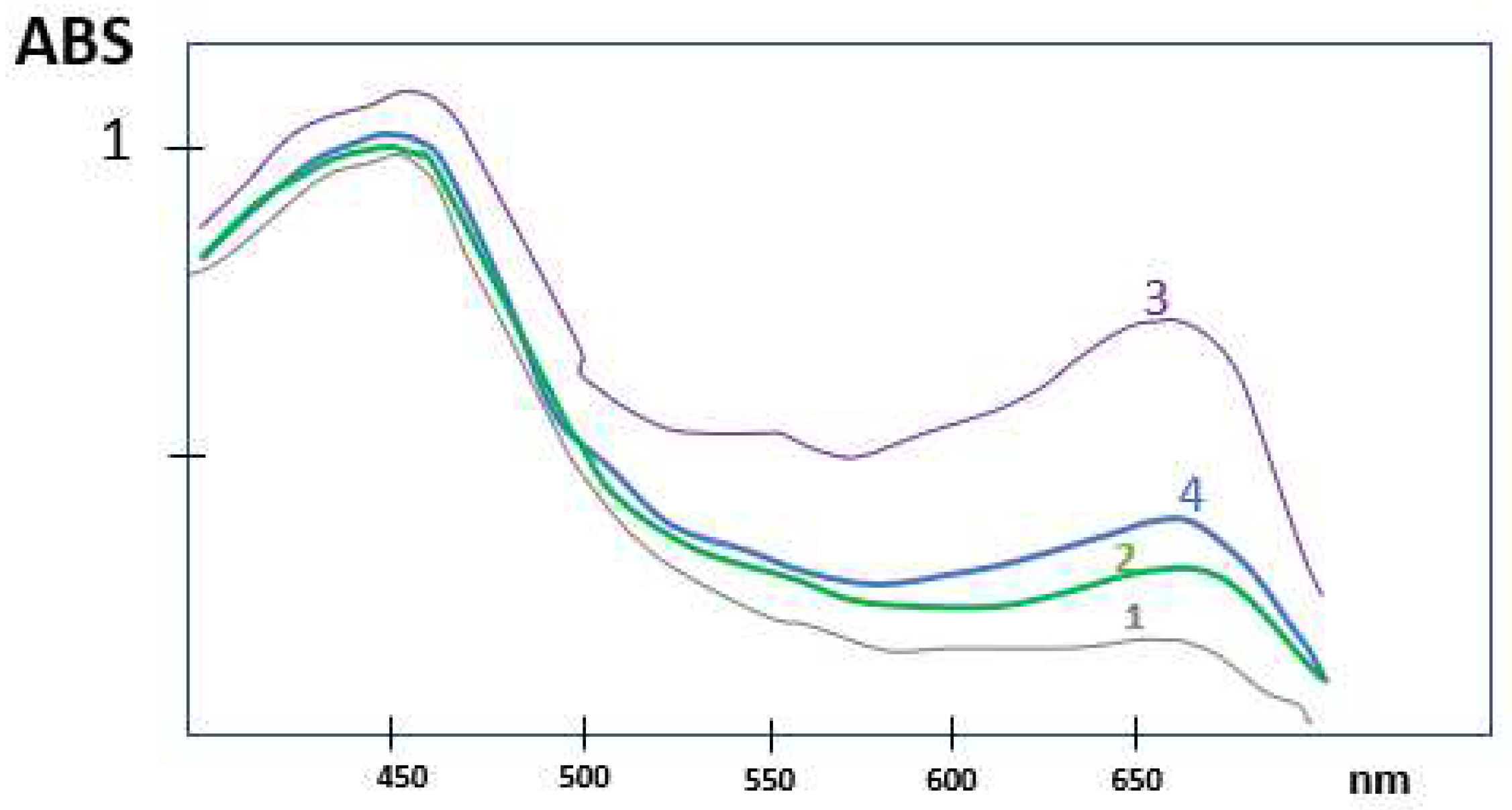
Figure 4.
Spectral curves of the Reduced Leuco-indigo dye using the same concentration of blue indigo dyes at 50 °C, (1) Indigofera Tinctoria (India), (2) Indigofera Persicaria tinctoria (France), (3) Indigofera suffruticosa (Central America) and (4) Synthetic indigo (from BASF).
Figure 4.
Spectral curves of the Reduced Leuco-indigo dye using the same concentration of blue indigo dyes at 50 °C, (1) Indigofera Tinctoria (India), (2) Indigofera Persicaria tinctoria (France), (3) Indigofera suffruticosa (Central America) and (4) Synthetic indigo (from BASF).
Yellow Leuco species of blue indigo dyed were produced by alkaline reduction, and analyzed by spectrophotometric analysis. 0.025 g of each three blue natural dye was mixed with 1.5 ml of ethanol, and 3 ml of hot purified water. 3ml of Caustic Soda (30%) and a chemical reducing agent was used, and the dye solution was maintained at 50 °C during 20 minutes before spectral analysis. These were compared to leuco species formed with a synthetic Indigo dye form BASF (270 9726) Badische Anilin & Soda-Fabrik AG.
3.1.2. Results of textile dyeing with blue indigo dye from GREENING at 50 °C
Blue indigo dyes from the three different plant origins give similar results. The color intensity (K/S) of the dyed fabrics were slightly higher for the Indigofera suffruticosa (Central America).
Figure 5a, shows the dyed multifiber fabric, and knitted acetate and woven cotton (dyed using Batik technique) and the dyed polyamide 6 lace, for dyeing using only one immersion for 20 minutes in the dyebath, at 50 °C, using blue indigo dyes from
Indigofera Persicaria tinctoria. Proteinic fibers (wool, silk), cellulosic fibers (cotton and viscose) as well as synthetic polyamide and acetate fibers were dyed. The yellowish-blue color shade of dyed silk and wool can be explained by the yellowish shade of unbleached scoured silk and wool respectively (see
Figure 5a). Dye color intensity is higher on synthetic polyamide 6.6 and acetate rayon. None of the acrylic fibers (Creslan 61, and Orlan 75) was dyed, most probably because these fibers are in general negatively charged which repel the negatively charged leuco-indigo species. The two polyester-PET fibers: Dacron 54 and Dacron 64 were not dyed at this temperature as their glass transition temperature “Tg = 80 °C” is well above the dyeing temperature used (50 °C). The non mobility of the amorphous PET chains at lower temperature (50 °C) does not allow for dye diffusion inside the fiber.
Figure 5.
Dyeing of fabrics with blue indigo dyes from Indigofera Persicaria tinctoria, at 50 °C.
Figure 5.
Dyeing of fabrics with blue indigo dyes from Indigofera Persicaria tinctoria, at 50 °C.
3.1.3. Antibacterial inhibition activity
The three blue indigo dye powders were tested against Staphylococcus Epidermis and Escherichia Coli according to method described in section (2.3.d).
Indigofera Tinctoria (India) did not show any antibacterial activity against these two bacteria. Indigofera suffruticosa (Central America) showed antibacterial activity against Escherichia Coli only, and Indigofera Persicaria tinctoria powder had antimicrobial activity against the two tested bacteria.
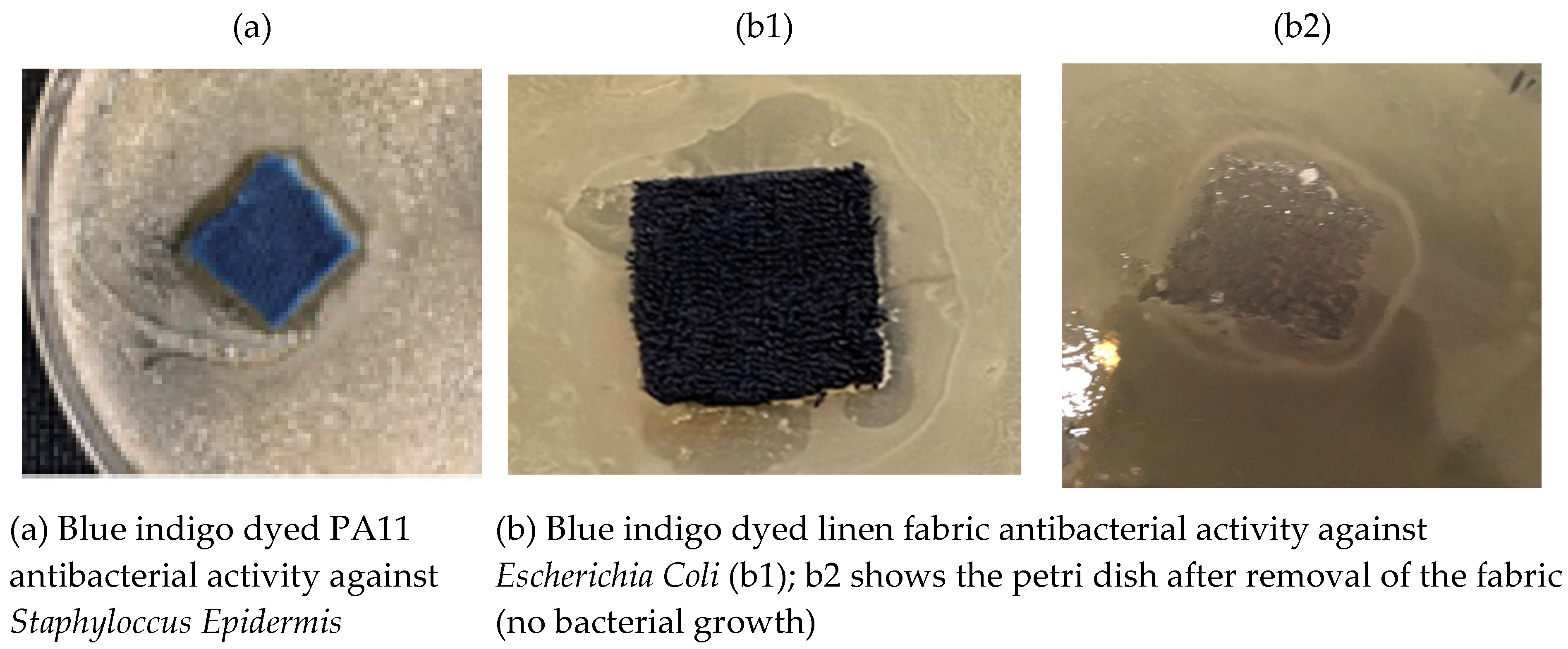
Figure 6 shows that an inhibition zone for polyamide (PA11-
Figure 6a) and linen (cellulose-
Figure 6a) fabrics dyed with blue indigo dye from
Indigofera Persicaria tinctoria, confirming the antibacterial activity of dyed fabrics.
Figure 6(b2) shows no bacterial growth under the dyed fabric, confirming the bioactive property of the dyed fabrics.
Figure 6.
Antibacterial activity of PA11 and linen dyed with bleu indigo from Indigofera Persicaria tinctoria (Inhibition zone confirms antibacterial activity).
Figure 6.
Antibacterial activity of PA11 and linen dyed with bleu indigo from Indigofera Persicaria tinctoria (Inhibition zone confirms antibacterial activity).
3.1.4. Wash, Rub and light fastness of the dyed samples
All dyed samples were washed once, before subjecting to rub, wash and light fastness tests described in
Section 2.3. All fiber fabrics have good wash fastness 5/5 whatever the fiber nature, however the rub fastness, specially the wet rubfastness was not good (3/5) specially for hydrophilic fibers such as cotton. Indeed, the insoluble blue dye molecules are immobilized inside the fiber and there is no chemical bond between the cellulosic chains and the indigo dyes. Also, when dark shade color is targeting through several dips (each dip followed by air oxidation), rub fastness is not very good (2/3). Dyeing for a longer time in a close beaker (45 minutes) improves the wet rub fastness (3-4/5) of the blue indigo dyed cotton fabric, most probably because the leuco species can diffuse deeper into the fiber inner core and thus the dyes are not easily rub out.
Table 1.
Wah/Rub/light fastness of textiles dyed with blue indigo dyes from Indigofera Persicaria tinctoria and with the indigo leaves from Indigofera Tinctoria.
3.2. Dyeing fabrics with indigo leaves
Dyeing of the multifiber fabric with the indigo leaves, was carried without the need of a reducing agent. Only the pH was varied using drops of acetic acid and sodium hydroxide. Dyeing of the multifiber fabric with the indigo leaves, can be carried without the need of a reducing agent. Only the pH was varied using drops of acetic acid and sodium hydroxide.
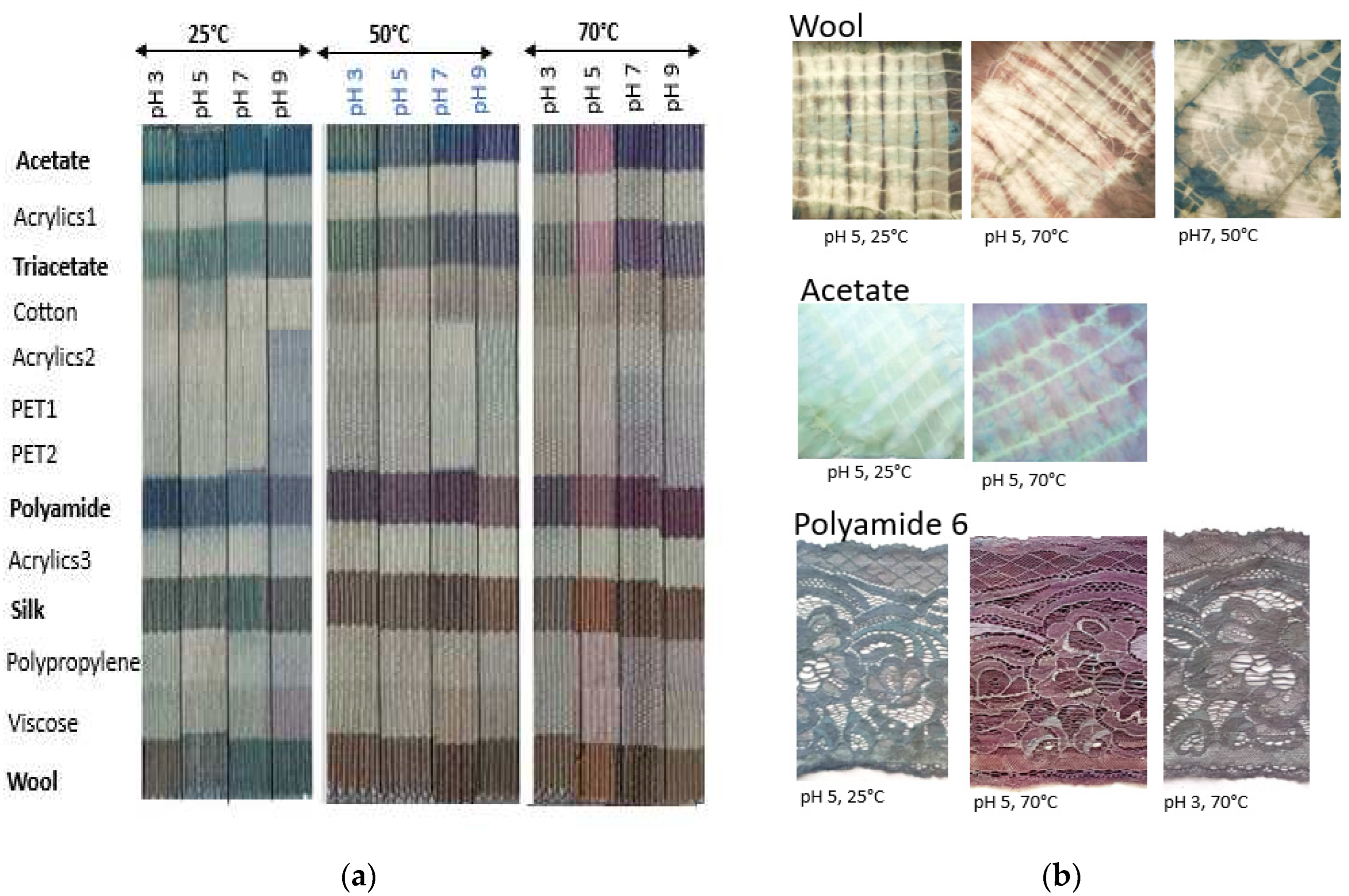
Figure 7a shows the various color variations for dyeing at different temperatures (25 °C, 50 °C and 70 °C) and various pH (3, 5, 7, 9). Wide range of colors was obtained, confirming the dichromic and trichromic nature of dyes produced from indigo leaves (in contact with water). Greenish-blue, violet, pink, brown, reddish brown, yellowish brown, grey, etc… shades were obtained, depending on the fiber nature, temperature and pH. Compared to blue indigo dyes, with indigo leaves, very little dyeing took place for the cellulosic (cotton and viscose) fibers, while the triacetate rayon was dyed with the indigo leaves but not with the blue indigo dyes. Surprisingly, all fabrics gave good washfastness (4/5), and rub fastness was around (4/5), most probably explained by the dyeing held in an closed beaker, which enhances dye diffusion in the inner core of fibers (as explained above).
Figure 7.
(a) Multifiber fabric dyed with powdered indigo leaves. (b) Batik and lace dyeing with powdered indigo leaves.
Figure 7.
(a) Multifiber fabric dyed with powdered indigo leaves. (b) Batik and lace dyeing with powdered indigo leaves.
Figure 8.
Possible pathways for dye components production.
Figure 8.
Possible pathways for dye components production.
Proteinic fibres (wool and silk), polyamides, acetate and triacetate can be dyed with the indigo leaves. Blueish green shades were observed at 25 °C; and more reddish shades at 50 °C and 70 °C. Formation of blue indigo dye (λ
max = 612 nm) and red indiburin (λ
max = 547 nm) [
11] and their diffusion into fibers can explain the different shades observed. At 25 °C, for wool and silk, grey and brownish shades can also be observed, as a function of pH, most probably because of the pale yellowish color of the undyed silk and wool. However, yellowish flavonoids [
10] present in the indigo leaves may also diffuse inside fibers to give the more greenish (at 25 °C) and brownish shades at higher temperatures. It is also possible that yellowish dehydroindigo (absorbing at 455nm), may be formed when the blue indigo is subjected to high temperature in presence of oxygen and water as demonstrated by Rondao et al. [
18].
3.5. PRINTING with MAYAN BLEU PIGMENTS produced by PIGM’Azur
3.5.a. Optimizing water-based binder content for pigment dyeing formulation applied by padding using PIGM’Azur pigment/glycerin paste
Four pigment dyeing formulations for padding process, with a viscosity of 20 mPa sec were prepared using aqueous solutions of four (4) different binders (45 ml with 100g/L of binder) and 5g of PIGM’Azur pigment/glycerin paste).
Table 2 gives more details on the four binders. A4 format of scoured cotton fabrics were padded with pigment dyeing solution (pick-up rate 100%), and dried at 110 °C and fixed at 180 °C for 1 minute.
Figure 9.
Methods used for fabric dyeing and printing using the blue PIGM’Azur pigments.
Figure 9.
Methods used for fabric dyeing and printing using the blue PIGM’Azur pigments.
Table 2 shows that with the soft polyurethane Dicrylan binder, color leveling was not good, as the pigment/glycerin paste was not completely dispersible in the aqueous Dicrylan solution. The three Appretan acrylic -based binders (APPRETAN N96100, APPRETAN N92111 and APPRETAN NTR@6553) allowed a more uniform colored coating on the cotton.
Optimizing water-based biosourced binder APPRETAN NTR@6553 for pigment printing
For further experiments, the biosourced water-based APPRETAN NTR@6553-formulation was optimized for application of PIGM’Azur pigment paste through padding process. 5g of indigo pigment/glycerin paste was mixed with each of the 4 different concentrations (20, 60, 80, 100g/L) of the APPRETAN NTR@6553 binder, and applied by padding, then dried and cross-linked at 180 °C for 60 seconds. Then the padded colored samples were subjected to wash test at 40 °C (for 30 min.) and to dry rub and wet fastness tests.
Table 3 shows the values for wash and rub fastness testing and the 60g/L of APPRETAN NTR@6553 seem adequate for good wash and rub fastness (4 -5), without making the fabric too rigid.
Table 4 shows that similar wash fastness were obtained for this binder cross-linked at 150 °C for 90 seconds.
3.5.b. Screen printing using (a) Mayan indigo pigment/glycerin mix and (b) the indigo Mayan pigment powder provided by PIGM’Azur
Four different printing pastes were prepared:
pastes 1 & 2 using the indigo pigment/glycerin paste and
pastes 3 &4 using indigo pigment powder from PIGM’Azur. For all printing pastes, APPRETAN NTR@6553 was used as binder. Sodium alginate thickener paste (see
Section 2.2) was used for printing pastes 1, 2 &3, while glycerin was used as thickener for paste 4.
Table 5 gives a more detailed description of the quantities of product used for each printing paste. The pastes were applied onto fabric by using laboratory hand screen printing technique, and the printed fabrics dried at 110 °C for 3 minutes and cured at 150 °C for 5 minutes.
Printing with paste 1 gave very good durable results on both polyester and cotton samples: Rub dry and wet fastnesses: 5/5, Wash fastness 4/5; light fastness: 7/7. However, very pale shade prints were obtained.
Printing with paste 2, with increase proportion of pigment/glycerin paste did not allow to increase the color intensity of the prints (see
Table 6b), and for the cotton sample, pigments were washed off during the first wash. However on the synthetic fabrics, polyester and polyamide, good wash fastness (4-5) were maintained, as well as good dry and wet rub dry fastness(4-5/5) and light fastness: (7/7) were maintained after the first wash.
Printing pastes 3 and 4 were prepared using the Mayan Indigo pigment powder with increased quantity of Appretan binder compared to pastes 1 & 2. For paste 3, sodium alginate and for paste 4 glycerin, respectively were used as thickener (see
Table 7a) as also published in a recent paper on textile printing paste [
19]. Using paste 3, darker blue shades (compared to paste 2) with good rub dry and wet fastnesses: 5/5, Wash fastness 4/5; light fastness: 7/7, were obtained for both polyester and cotton. With bio-based glycerin as thickener in paste 4, darker shade was also obtained but for the polyester sample alone, wash fastness was very good (5/5) with the rub dry and wet fastnesses maintained to (5/5), and light fastness to 7. However, the pigment was washed out from the cotton sample.
Glycerin allows proper dispersion of the blue indigo pigment in the printing paste, but does not allow the good color fastness on the cotton fabric. However, on the polyester fabric, darker shades with very good wash/rub/light fastnesses are obtained. As ethylene glycol is one of the monomer for polyester formation, it is probable that better wetting occurs between the printing paste and the PET fabric, resulting in high wash/rub fastnesses [
20,
21,
22].
Vegetable Glycerin or glycerol is a biodegradable thickener [
23], nowadays readily obtained by reactions such as saponification of natural bio-based fatty acids, and used as a sweetener or thickener in nourishments and refreshments [
24], and can be used as a sustainable washable thickener for printing the polyester fabrics with the indigo pigments. In the case of cotton, the alginate thickener yields the highest print resistance.
Table 7.
Printed textile samples with Mayan Indigo pigment powder mixed with alginate or glycerin thickener.
Table 7.
Printed textile samples with Mayan Indigo pigment powder mixed with alginate or glycerin thickener.
 |
 |
| Printing paste 3 |
Printing paste 4 |
4. Discussions and conclusions
Natural indigo dyes and pigments, especially when used with non-toxic, bio-sourced agents, provide a possibility of sustainable dyeing and printing.
It is high time to explore the wide diversity of coloration and prints that can be obtained on various textile materials, using natural indigo leaf extracts, dyes or and Mayan-inspired-hybrid organic/inorganic indigo pigment, perhaps already explored by humanity, centuries ago.
Proteinic fibers (wool, silk), cellulosic fibers (cotton and viscose) as well as synthetic polyamide and acetate fibers can be dyed blue using natural blue indigo dyes from three different plant species, from India, Central America and France, with slightly higher color intensity (K/S) in the case of Indigofera suffruticosa from Central America. Blue indigo dye extract from Indigofera Persicaria tinctoria had antimicrobial activity against the two tested bacteria,
Staphylococcus Epidermis, and
Escherichia Coli. The two other blue indigo dyes did not show similar antibacterial activity contrarily to what is described in literature [
10]. This can be explained by the fact that indigo powders have different compositions depending on the plant from which they are derived, and the extraction process used for obtaining the blue indigo dye powder can have an impact on the contents of the blue indigo powder. Indeed, indigo leaves contain lots of organic products (Indole alkaloids, Nucleosides, sterols, amino acids, tryptanthrin others [
10,
14]), which may be present in the indigo blue dye extract and which have antibacterial properties.
Through literature review, as well as experiments led and presented in this paper, we demonstrated using indigo leaves, that a variety of dyeing shades can be obtained, ranging from pink, violet, blue, green, grey, brown, purple, depending on dyeing conditions (pH, temperature) and textile fiber nature. There is neither the need for dye extraction step from indigo leaves, nor the need for alkaline reducing agent for textile dyeing. Proteinic fibres (wool and silk), polyamides, acetate and triacetate can be dyed with the indigo leaves. Unfortunately, cellulosic fibers and polyester fibers could not be dyed in the dyeing conditions fixed in our experiments (maximum 70 °C). All fabrics dyed with the indigo leaves gave good washfastness (4/5), and rub fastness was around (4/5). In presence of water, enzymatic reaction allows breaking down of indole glycosides present in the leaves, with the production of glucose and indole phenol. Most probably as this indole phenol molecule has low water solubility, it diffuses inside certain fibers only (hydrophobic fibers, or protein fibers containing non polar amino-acids) before its dimerization in presence of oxygen to form blue indigo or red indiburin, or even yellow dehydroindigo in presence of heat. Hydrophilic cellulosic fibers are not dyed, probably because very little leuco-indigo species are formed in presence of in-situ glucose produced by the enzymatic reaction.
The 100% natural blue indigo pigment inspired by Mayan civilization and produced by PIGM’Azur has very good light fastness (7/7) compared to the blue indigo dyes (5/7). The aim of the study carried here, was to optimize formulation for fabric pigment dyeing and printing, with durable properties to washing as well as dry and wet rub fastnesses.
Our study confirmed that the use of a water-based APPRETAN NTR@6553, aqueous styrene-acrylic type polymer binder made from 40% renewable material, can allow to produce by padding, the pigment dyeing of cotton and polyester fabrics, yielding, color fastness to wash (4/5) and dry rub (5/5), wet rub (4/5), and light fastness (7/7). As this binder is already used for food-grade packaging, it can potentially yield safe textiles, provided all ecotoxicity and cytotoxicity tests are confirmed.
The aim for printing with the 100% natural indigo pigments was to discover the right combination of ingredients for the printing paste to achieve good print fastness. Such efficient indigo pigment paste for direct screen printing can be achieved by several recipes. Glycerin paste containing indigo pigment from PIGM’Azur yields prints with high wash/rub/light durability in presence bio-binder used, but it does not yield dark shade prints. Higher content of pigment using the powdered indigo pigment form allows to obtain darker shades with very good wash and rub fastness, when combined with the plant-based glycerin thickener for the polyester fabric, while the use of sodium alginate thickener allows to have good print resistance for both cotton and polyester fabrics.
This study confirms the use of indgo -based dyes and pigments to produce durable color properties on textile materials and.
Author Contributions
Conceptualization, Behary N., Volle N., methodology, Behary N.; validation, Behary N., Volle N.; formal analysis, Behary N.; investigation, Behary N.; resources, Behary N., Volle N.; data curation, Behary N., Volle N.X.X.; writing— Behary N.; writing—review and editing, Behary N., Volle N.; visualization, Behary N., Volle N.; supervision, Behary N.; project administration, Behary N.; funding acquisition, Behary N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ENSAIT, University of Lille (First author affiliated university).
Acknowledgments
We acknowledge Huntsmann and Archroma company for having provided samples of chemicals, free of charge. We are grateful to Bachelor, and Master students who carried experiments, during the COVID 19 period, BARDIAUX Claire, HERZ Fanny, PENDOLINO Emma, Eliška Štukavcová, and Protithi Pandit. We are also grateful to the lab technician Claire Pinchon for her technical help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajendra Prasad, Indigo — The Crop that Created History and then Itself Became History, Indian Journal of History of Science, 53.3 (2018) 296-301. [CrossRef]
- et al. Shades of blue: non-invasive spectroscopic investigations of Maya blue pigments. From laboratory mock-ups to Mesoamerican codices, Heritage Science volume 8 (1), (2020).
- Roshan Paul, Richard S. Blackburn, Thomas Bechtold, Indigo and Indigo Colorants, 31 March 2021. [CrossRef]
- Avinash P. Manian, Sophia Mueller, Thomas Bechtold, Tung Pham, Quantification of indigo on denim textiles as basis for jeans recycling, Dyes and Pigments, Volume 216, August 2023, 111327. [CrossRef]
- Michael Cordin, Thomas Bechtold & Tung Pham, Quantification of aniline and N-methylaniline in indigo, Scientific Reports, volume 11, 21135 (2021). [CrossRef]
- Laksanawadee Saikhao, Jantip Setthayanond, Thitinun Karpkird, Thomas Bechtold, Potjanart Suwanruji, Green reducing agents for indigo dyeing on cotton fabrics, Journal of Cleaner Production 197(1), 106-113, (2018). [CrossRef]
- et al. , A promising route to dye cotton by indigo with an ecological exhaustion process: A dyeing process optimization based on a response surface methodology, Industrial Crops and Products, 46, 350-358, (2013). [CrossRef]
- T. Bechtold, E. Burtscher, G. Kühnel, O. Bobleter, Electrochemical reduction processes in indigo dyeing, 2008, Journal of The Society of Dyers and Colourists. [CrossRef]
- Ejajul Hoque et al., Review of foam applications on cotton textiles, Sanjit Acharya et al., Textile research Journal, Volume 93, Issue 1-2, (2023). [CrossRef]
- Yang Qi-yue, Zhang Ting, He Ya-nan, Huang Sheng-jie, Deng Xuan, Han Li & Xie Chun-guang, From natural dye to herbal medicine: a systematic review of chemical constituents, pharmacological effects and clinical applications of indigo naturalis, Chinese Medicine volume 15, Article number: 127 (2020). [CrossRef]
- Ju Z, Sun J, Liu Y. Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies. Molecules. 24(21):38312019. [CrossRef]
- Alagbe, J. O Chemical evaluation of proximate, vitamin and amino acid profile of leaf, stem bark and root of indigofera tinctoria, International Journal on Integrated Education, Volume 3 (1),(2021). [CrossRef]
- Yoo W., Ahn C. Dyeing Behavior of Silk Dyed with Indigo Leaf Powder Using Reduction and Nonreduction Dyeing and Its Relationship with the Amount of Indigotin and Indirubin Adsorbed in Silk. J. Korean Soc. Cloth. Text. 43:753–767 (2019). [CrossRef]
- Mari Kataoka et al., Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils, J Gastroenterol. 36(1):5-9. (2001). [CrossRef]
- M. Sanchez del Rio et al., «The Maya Blue pigment », In: Developments in Palygorskite-Sepiolite Research, Volume 3: A New Outlook on these Nanomaterials, Elsevier, 2011, chapitre 1.
- Sonia Ovarlez, Françoise Giulieri, François Delamare, Nicolas Sbirrazzuoli, Anne-Marie Chaze, Indigo–sepiolite nanohybrids: Temperature-dependent synthesis of two complexes and comparison with indigo–palygorskite system, Microporous and Mesoporous Materials, 142(1), 371-380 (2011). [CrossRef]
- Nicolas Volle, Françoise Giulieri, Alain Burr, Sophie Pagnotta, Anne Marie Chaze, Controlled interactions between silanol groups at the surface of sepiolite and an acrylate matrix: Consequences on the thermal and mechanical properties. Materials Chemistry and Physics, 134(1), 2012, 417-424.
- R. Rondão, et al. « Dehydroindigo, the forgotten indigo and its contribution to the color of Maya Blue. J. Phys. Chem. A, 114, 4, 1699–1708 (2010). [CrossRef]
- A Borisova, Study of print paste composition for natural and synthetic textiles. Part 2: Printing of polyester fabrics 2019 IOP Conf. Ser.: Mater. Sci. Eng. 500 012029. [CrossRef]
- Yang, M.C.; Tsai, H.Y. Ethylene Glycol and Glycerin as the Solvent for Alkaline Treatment of Poly(ethylene Terephthalate) Fabric. Text. Res. J. 1997, 67, 760–766. [Google Scholar] [CrossRef]
- Pawar, S.S.; Maiti, S.; Biranje, S.; Kulkarni, K.; Adivarekar, R.V. A novel green approach for dyeing polyester using glycerine based eutectic solvent as a dyeing medium. Heliyon 2019, 5, e01606. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.C.; Tsai, H.Y. Ethylene Glycol and Glycerin as the Solvent for Alkaline Treatment of Poly(ethylene Terephthalate) Fabric. Text. Res. J. 1997, 67, 760–766. [Google Scholar] [CrossRef]
- Hamid Reza Safaei, Mohsen Shekouhy, Sudabeh Rahmanpura and Athar Shirinfeshana, Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one-pot three component synthesis of 4H-pyrans, Green Chem, 2012, 14, 1696–1704. [CrossRef]
- Chol, C. G., Dhabhai, R., Dalai, A. K., and Reaney, M. Purification of crude glycerol derived from biodiesel production process: experimental studies and techno-economic analyses. Fuel Process. Technol. 178, 78–87. (2018). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).