1. Introduction
Municipal biowaste (MBW) has been proposed as sustainable feedstock for the production of value added water soluble biobased products (SBPs) to use in different sectors of the chemical industry and agriculture in place of synthetic products obtained from fossil sources [
1]. To this end, the chemical hydrolysis and ozonisation of as collected [
2] or fermented MBW under anaerobic and aerobic conditions [
1] have been investigated to assess their environmental and economic sustainability and social acceptability. The results of these studies have shown that reaching these objective is possible, pending on optimisation of the SBPs performance in some applications.
A typical example needing optimisation is the SBP product obtained by hydrolysis from the MBW anaerobic digestate. This product contains organic and mineral matter. The organic matter is constituted by a pool of molecules with widely different molecular weight, ranging from 5 to over 750 kDa, and with different chemical compositions. They are made by aliphatic and aromatic C moieties functionalised with acid and basic groups of different strength, which are bonded to a wide range of mineral elements. These features are memories of the chemical moieties and macro-molecularity of the pristine MBW from which the SBP is derived. Thanks to their chemical composition, the SBPs have been found to exhibit a number of properties as soil fertilisers and plant growth biostimulants, surfactants and biopolymers to manufacture plastic articles. Studies to assess the performance of the SBPs in real operational conditions have shown that, while they are very efficient as agrochemicals, their use for the manufactures of detergents presents some draw backs due to their black colour. Also, their performance for the manufacture of plastic articles is severely limited by their lack of film forming properties and poor mechanical properties. All these drawbacks stem from the content of aromatic lignin-like moieties. Further studies have attempted to reduce the content of aromatic C in the SBPs by ozonisation. The results have shown that this is possible, but ozonisation causes severe depolymerisation. The reaction yields 30 % products with molecular weight ≥ 100 kDa and 70 % of products with molecular weight < 5 kDa [
2], the latter ones characterised by the complete loss of the surfactant properties of the pristine SBPs.
While looking for milder oxidation conditions, the authors of the present work have conceptualised the MBW autocatalytic chemical reactions. According to the authors vision, the oxidation reactions of MBW might occur at room temperature in water solution without added chemical reagents and/or catalysts. Indeed, the SBPs, thanks to their acid and basic functional groups, have been shown to bond Fe ions, to perform as photosensitiser in advanced photocatalytic oxidation processes [
3] and to promote the oxidation of many organic pollutants present in industrial waste waters [
1] under irradiation with simulated solar light. Also, depending on the concentration in water, the SBPs have been found to promote their auto-oxidation. The same products have been proven to promote the oxidation of ammonia to nitrogen [
4], even in the absence of irradiation. These findings proposed that, in addition to their photosensitising properties, the SBPs could perform also as catalysts for their mild auto-oxidation under proper experimental conditions. A somewhat similar approach is reported in a doctoral thesis [
5] describing functionalised products derived from agro-industrial biomass waste to catalyse a number of reactions applied to biomass for the production of platform chemicals.
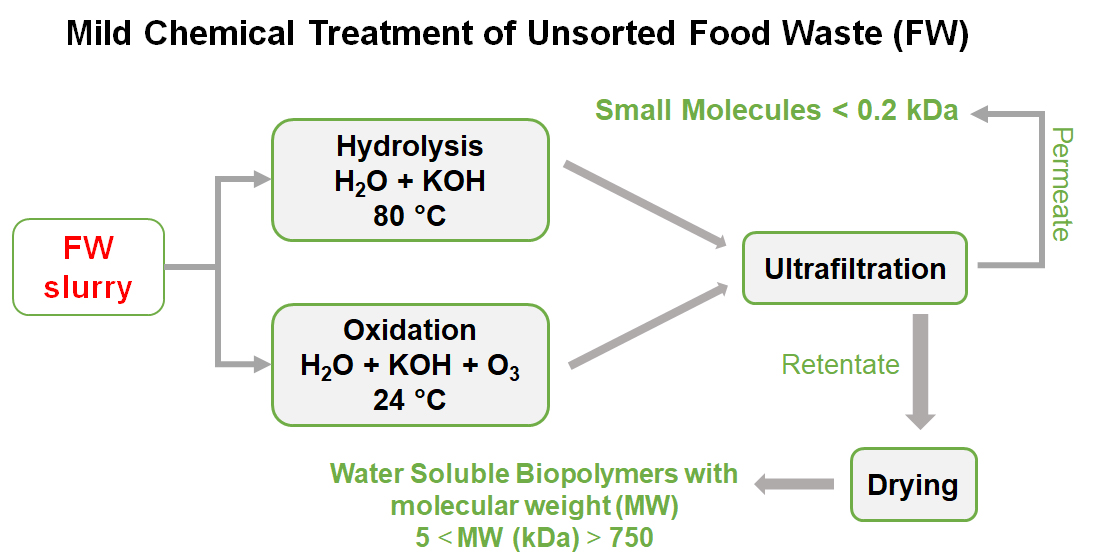
The present work describes the behaviour of the aqueous solutions containing the SBP derived from MBW anaerobic digestate. The behaviour of the SBP solution without added reagents and/or catalysts, irradiate and not irradiated with simulated solar light, was investigated in comparison with the SBP solution containing added alkali and/or hydrogen peroxide. On one hand, the complex chemical composition of the SBP offered ground supporting the foreseen autocatalysis. On the other hand, it made the demonstration of the autocatalysis concept quite challenging. The SBPs, as well as the sourcing MBW chemical compositions are not well known, unlike the case of reactions performed on hydrocarbons from fossil sources and the synthetic products derived from them. Therefore, for MBW and their derived SBPs, an analytical protocol was specifically developed [
1], which included fractionation of the tested SBP by sequential ultrafiltration with membranes having different cut offs in the 0.2-750 kDa range, determination of the content of organic and inorganic matter, and of elemental C and N, characterization of functional groups by solid state NMR spectroscopy and potentiometric titration. The results reported here in after will show how this analytical protocol is particularly necessary in the case of the present work aiming to assess the autocatalytic effect of the tested SBP derived from the MBW anaerobic digestate.
2. Results
2.1. Treatments of SBP and recoveries of C in crude soluble products.
Table 1 reports the experimental conditions under which the SBP solution at 33 g/L concentration was treated and the soluble carbon as mol/mol % of the total C in the SBP before the treatments, which was recovered from the solution at the end of the treatments. During the treatment, some insoluble materials formed. This was separated from the soluble phase by centrifugation. The recovered insoluble material accounted for 3-9 % of the total C in the SBP before the treatments (
Table S1). The formation of insoluble matter in the oxidation of lignocellulose materials has been reported and discussed previously [
1]. In the present work aiming to develop water soluble products with improved surfactant properties or workability for the manufacture of plastic materials, the insoluble co-product was an undesirable. Values of the recovered C mol/mol % for the recovered soluble material (
Table 1) and for the insoluble material were calculated from the respective recovered dry mass weights and C contents reported in
Table S1.
The data in
Table 1 for the treatment No. 1-D0 and No. 4-L0, respectively performed without and with light irradiation, in the absence of added H
2O
2 show that the pH of the starting SBP solution in water decreases after 14 days from 10 to 9. For the treatments No. 2-D2, 3-D3, 5-L2, 6-L3, performed in the presence of added H
2O
2 at 2-3 H
2O
2/C mole ratio, without and with light irradiation, the pH decreases to about 5. According to previous work [
1,
2] for the ozonisation of SBPs, the pH decrease reported in
Table 1 is consistent with the oxidation of SBPs organic matter, and formation of carboxylic functional groups and/or CO
2. The same treatments were replicated adding KOH to keep the pH 10 of the pristine SBP solution constant during the treatments performed under the same above conditions. The data for the treatments No. 7-12 in
Table 1 show that the amounts of added KOH to maintain pH 10 is equivalent to the formation of 0.24 acid equivalents produced per mole of starting organic (H
+/C eq/mol) in the case of No.7-D0 and No.10-L0 treatments, of 0.34 H
+/C eq/mol in the case of No. 8-D2 and No. 11-L2, and 0.40 H
+/C eq/mol in the case of No.9-D3 and No.12-L3 treatments. The data confirm that the production of organic functional COOH groups and/or CO
2 occurs also for the SBP solutions containing no added H
2O
2, and that the produced H
+/C eq/mol amount depends on the amount of added H
2O
2.
Parallel to the pH decrease,
Table 1 shows that the recovered soluble C at the end of the treatments No.1-6 decreases along the decrease of pH of the recovered solution. Similar trends were observed for the weight of the recovered soluble matter and for the C/N mol/mol ratio in the dried recovered soluble matter (
Table S1).
Evaluation of data for the recovered soluble C in
Table 1 should account for the variability of the measured values, due to handling the recovery of the reaction medium, separating the soluble and insoluble phases, drying, weighing and analysing the products for their C and N content (see section 4). To this purpose,
Table 2 reports the mean values and standard deviations calculated over the values for treatments No. 1-6 and No.7-12, separately. Compared to treatments No.7-12, treatments No. 1-6 are characterised by significantly lower mean values and also much higher standard deviations values. The large differences of mean and standard deviation values between the two groups stem mainly from the production of CO
2 and its fate. While in treatments No.7-12 at constant pH 10, the produced CO
2 remains in the recovered material in form of carbonate, in treatments No. 1-6 it is lost in the gas phase. On one hand the 5.9 standard deviation for treatments No.7-12 is likely to represent largely the variability due to handling, separating and analysing the crude matter at the end of the treatments. On the other, the comparison of the data for the two groups of treatments should potentially allow assessing the effect of pH on the soluble organic matter recovered in the different treatments. This poses the issue of understanding how much of the recovered soluble C in treatments No.7-12 is due to the formation of CO
2 and of organic COOH functional groups.
To assess the relative contributions of the CO
2 and organic C, samples of the products obtained in
Table 1 treatments were further treated with HCl in order to obtain CO
2 free samples (see section 4).
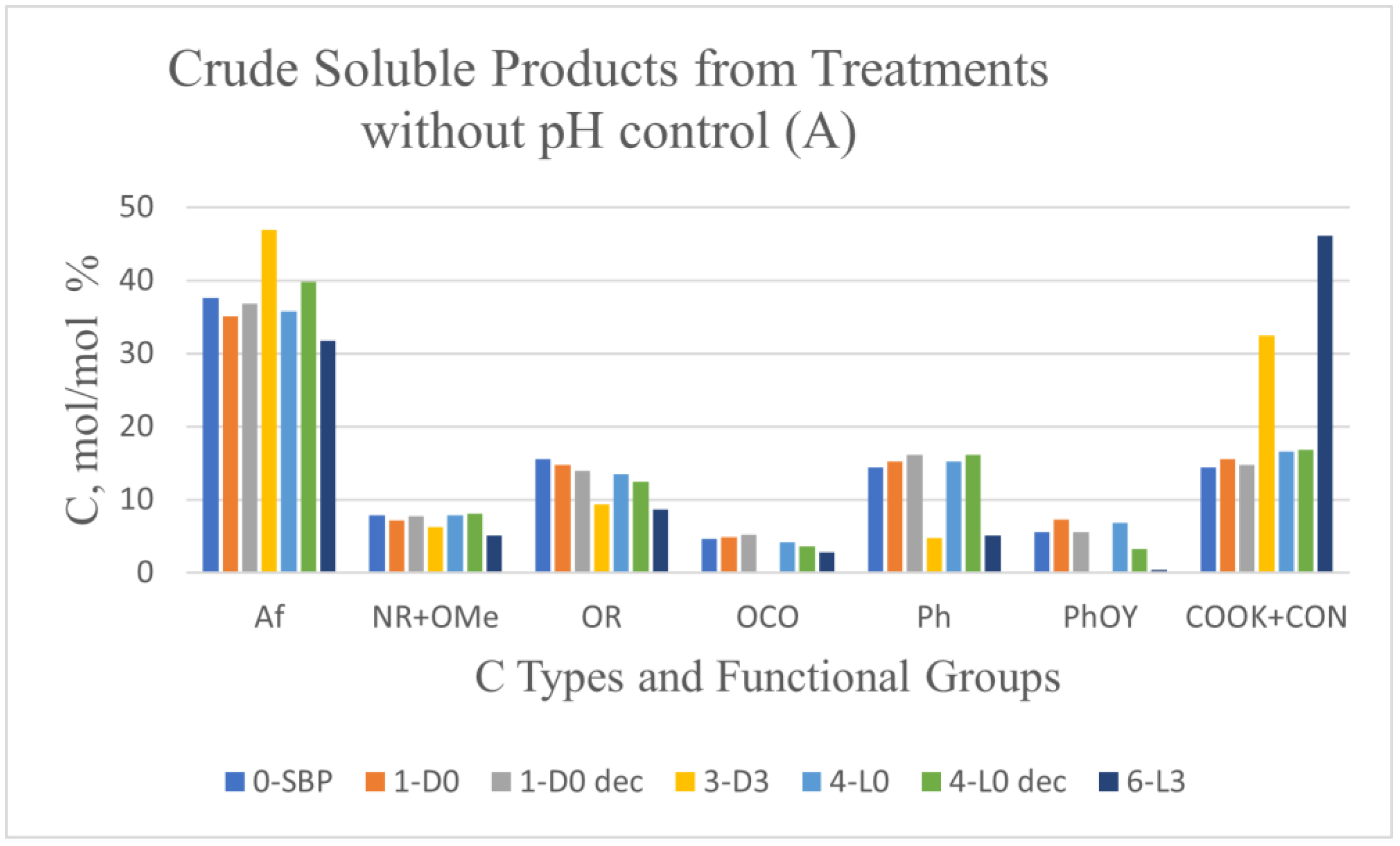
Figure 1 reports the C types and functional groups composition of the products determined by 13 C NMR solid state spectroscopy.
The content of the C types and functional groups listed in
Figure 1 was estimated from the measured areas of the 13C NMR resonance band covering the following chemical shift (δ, ppm) ranges: 0–53 for aliphatic (Af) C; 53–63 ppm for amine (NR) and methoxy (OMe) C; 63–95 ppm for alkoxy (OR) C; 95–110 ppm for anomeric (OCO) C; 110–140 ppm for aromatic (Ph) C; 140-160 ppm for phenol/phenoxy C (PhOY, Y = H, R); 160–185 ppm for carboxylate and amide (COX: X = OM, NR; M = metal, R = H, alkyl and/or aryl) C. The total integrated band area over the 0-185 ppm range was assumed to represent the total C moles in the analysed sample (see
Section 4).
For the crude soluble products listed in
Figure 1A and 1B, the COX resonance signal was generally very broad, covering all the 160-185 ppm resonance range. Some spectra exhibited a high intensity sharp signal overriding the broad band the 160-185 ppm range. In these cases, the measured band area for the COX resonance accounted for 40-60 % of the total integrated band area over the 0-185 ppm range. This occurred particularly for the products obtained in the treatments at controlled pH 10 (
Figure 1B), which were expected to contain potassium carbonate, formed as a consequence of the mineralisation of the SBP organic matter in the aqueous alkaline medium. Potassium carbonate is characterised by a sharp intense 13C resonance single at 170.3 ppm [
6]. To determine the content of potassium carbonate, some selected samples of the crude soluble products obtained at constant pH 10 were treated by HCl (see
Section 4) and the expected CO
2 free products were analysed by 13C NMR spectroscopy. The same HCl treatment and spectroscopic analysis was performed on selected samples of the crude soluble products obtained at acid pH. This allowed assessing possible effects of the HCl treatments, other than decarbonation, on the CO
2 free organic matter of the pristine crude soluble products.
2.2. Recoveries of soluble organic C and CO2 for treatments in absence of added H2O2.
For the scope of the present work, it was of primary importance comparing the crude soluble products obtained in the treatments carried out in absence of added H
2O
2 with their corresponding samples treated by HCl. According to the data in
Figure 1, the 1-D0 and 4-L0 do not show any significant composition difference compared to the corresponding samples 1-D0dec and 4-L0dec subjected to the HCl decarbonation treatment. For each C type and functional group,
Table 3 reports the mean and standard deviation calculated over the 1-D0 and 1-D0dec, and over the 4-L0 and 4-L0dec samples, separately.
It may be observed that the relative standard deviation values range from 2.8 % to 11 %, except in the case of the PhOY functional group, which show rather large 19 % and 51 % values. This poses doubts about the significance of the 13C signals measured in the PhOY 140-160 ppm. In most cases the broad PhOY resonance band could hardly be distinguished from the background noise.
On the contrary, the data in
Figure 1 evidence large significant composition differences for the 7-D0 and 10-L0 compared to the corresponding samples 7-D0dec and 10-L0dec subjected to the HCl decarbonation treatment. In these cases, the relative standard deviations were found to range from 16 to 58 % of the mean values calculated for all C types and functional groups. The data in
Figure 1 evidence that the COX content, much lower in the decarbonated samples than in the pristine crude soluble 7-D0 and 10-L0 samples, is primarily responsible for the large composition differences measured for these samples’ couples. These results prove that the measured area of the broad COX resonance band in the 7-D0 and 10-L0 products includes contributions of the resonance signal of CO
2 C (in form of potassium carbonate) and of organic carboxyl C (COXorg), which were calculated according to the following equations (1) and (2),
where (
Figure 1): A and C are the values of COX and Af, respectively, for 7-D0dec or 10-L0dec; B is the value of Af for 7-D0 or 7- L0; D is the value of COX for 7-D0 or 7- L0.
Table 4 reports the calculated values of COXorg and CO
2 (mol/mol %) carbon contained in the products obtained in the 1-D0, 4-L0, 7D0 and 10-L0 treatments listed in
Table 1, and in
Figure 1A and 1B. As expected, the data confirm that in the products obtained in the treatments 7-D0 and 10-L0 at constant pH 10, the produced CO
2 is retained in form of potassium carbonate in the strong alkaline water phase. On the contrary, no potassium carbonate is found in the products obtained in the treatments 1-D0 and 4-L0 without pH control. The amount of CO
2 calculated from the 13C spectroscopic data for 7-D0 and 10-L0 products corresponds to the values calculated from the consumption of alkali to keep pH 10 constant during the treatments (see
Table 1, column H
+/C).
Table 4.
Content of COXorg and CO2 carbon (mol/mol %) in samples 1-D0, 4-L0, 7D0 and 10-L0.
Table 4.
Content of COXorg and CO2 carbon (mol/mol %) in samples 1-D0, 4-L0, 7D0 and 10-L0.
| Sample |
COXorg |
CO2
|
| 1-D0 |
15.1±0.64 |
none |
| 4-L0 |
16.7 ± 0.14 |
none |
| 7-D0 |
11.1 |
25.1 |
| 10-L0 |
12.4 |
25.0 |
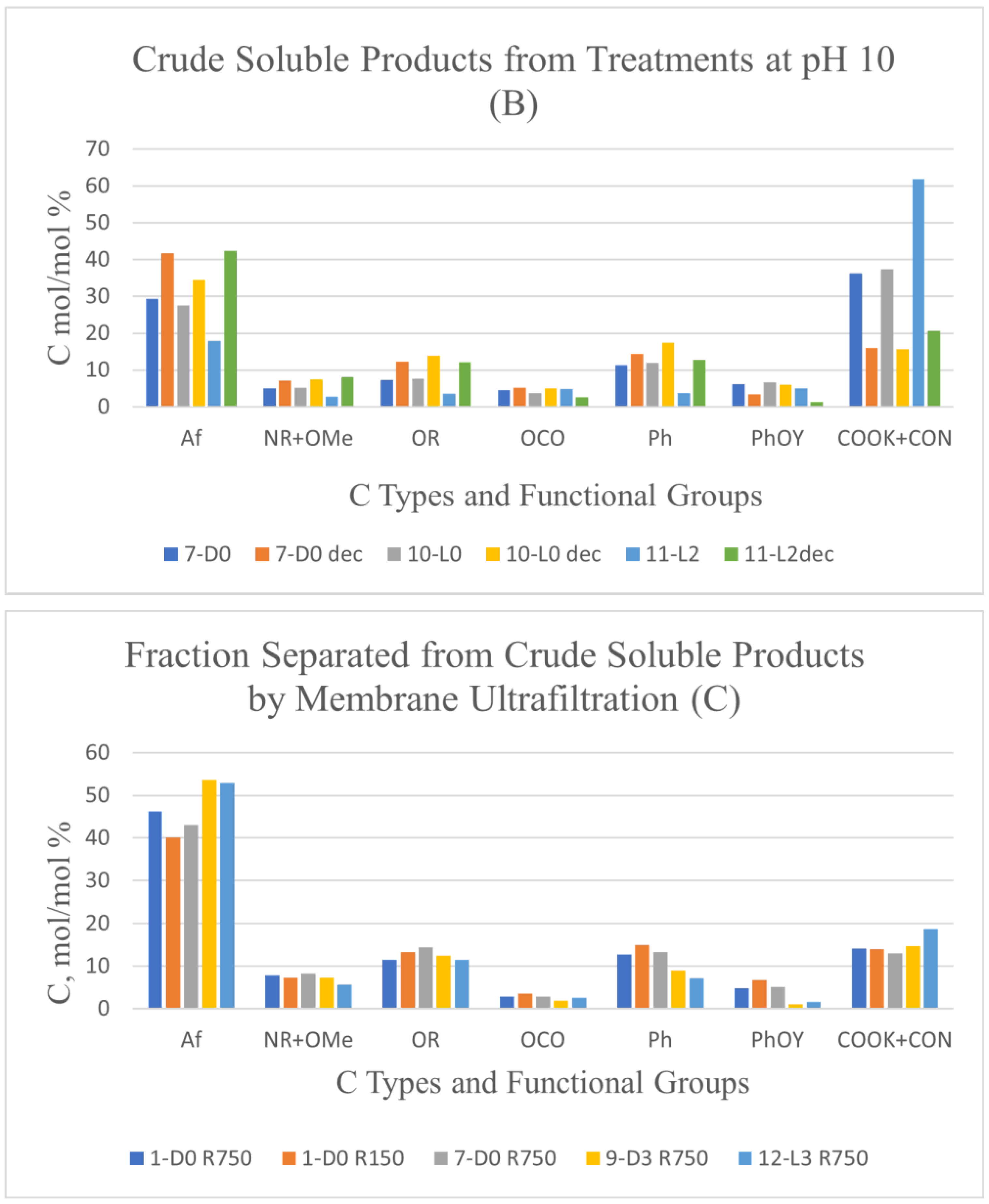
Similar calculations by equations 1 and 2 were applied to the data reported in
Figure 1B for the crude soluble product obtained in the treatments 11-L2 and 11-L2dec at constant pH in the presence of hydrogen peroxide at 2 H
2O
2/C mol/mol ratio. The calculated amount of CO
2 C in the 11-L2 sample was 53.2 mol/mol %, against 8.7 mol/mol % for organic COX C. These results indicated that organic C mineralization in the presence of hydrogen peroxide is more than double than that (Table 4) calculated for the 7-D0 and 10 L-0 samples of the crude soluble product obtained in the treatments at constant pH 10 in the absence of hydrogen peroxide without and with light irradiation, respectively.
2.3. Molecular weight distribution in pristine SBP and crude soluble products obtained in absence of added H2O2.
Further information on the effects of the 1-D0, 4-L0, 7-D0 and 10-L0 treatments of SBP in absence of added H
2O
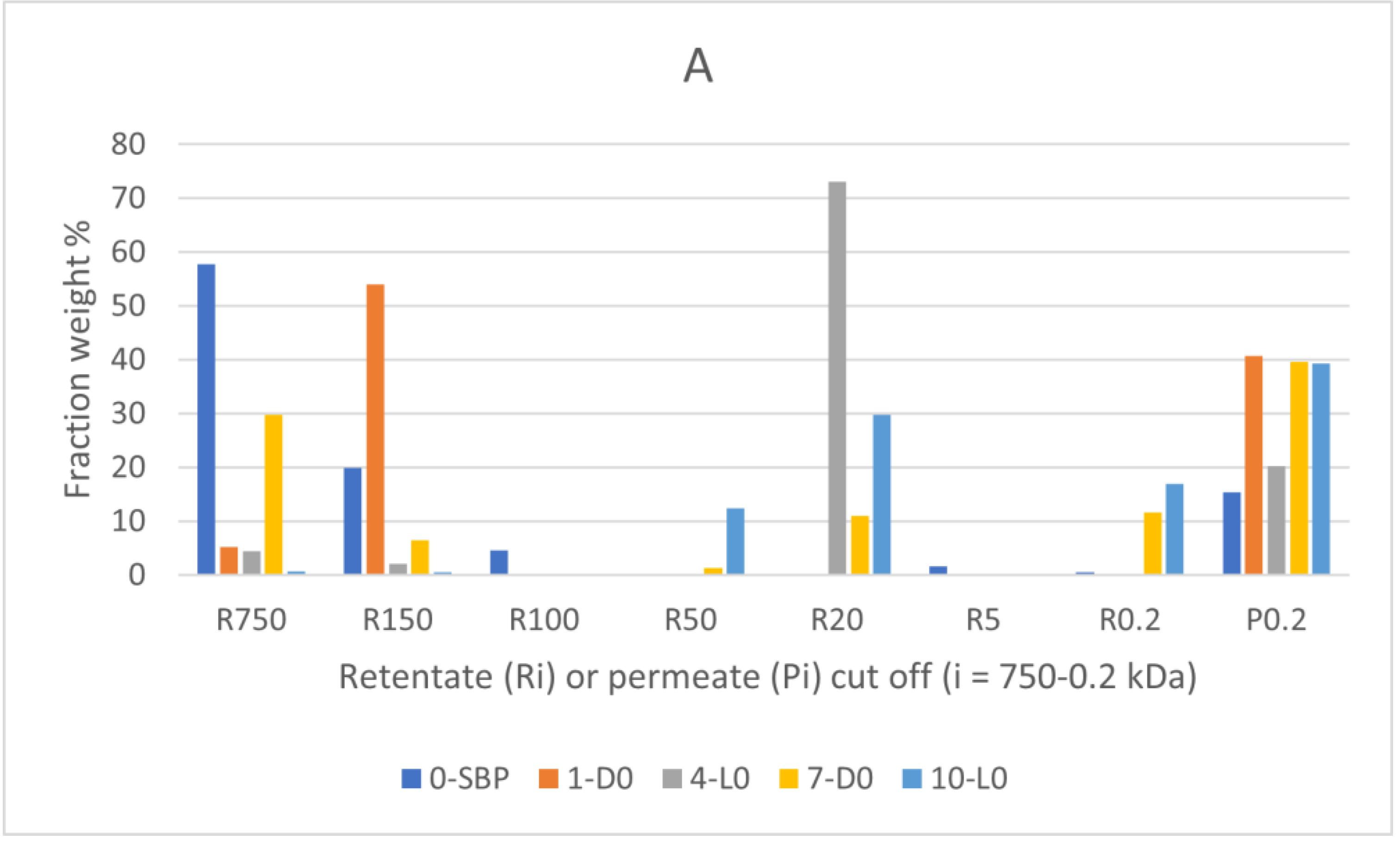
2 was obtained by fractionating the recovered crude soluble materials through sequential membrane ultrafiltration. To this end, the pristine SBP and the recovered soluble products were fed to polysulphone membranes with decreasing molecular cut off at 750, 150, 100, 50, 20, 5, 0.2 kDa, and the collected retentates at each step and the final permeate through the 0.2 kDa membrane were collected, weighed and analysed for their C content.
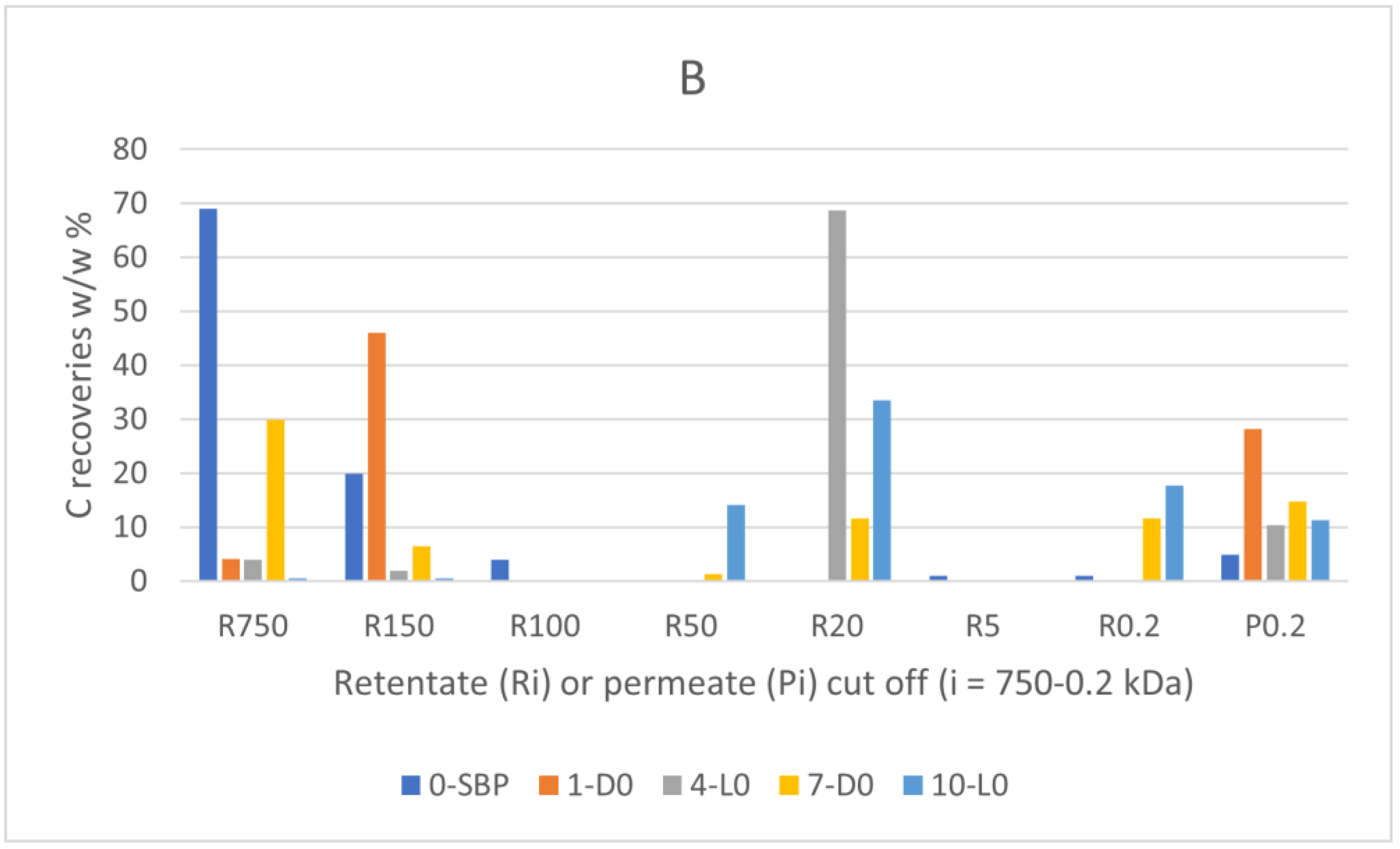
Figure 2 reports the results of the fractionation process.
The data in
Figure 1A show that the R750, R150 and R100 in order of decreasing weight abundance are the major high molecular weight fractions of the pristine SBP solution (0-SBP in
Figure 1) ultra-filtered readily after its preparation at pH 10. However, keeping the SBP in the alkaline aqueous solution for 14 days (treatment No. 1-D0 in
Table 1) yields the product (1-D0 in
Figure 1A) exhibiting a drastic compositional change with the R750 fraction reduced at 5.2 % level and the R150 and P0.2 fraction increased up to 54 and 40.8 %, respectively, compared to the composition of the pristine SBP. For the SBP solution irradiated with simulated solar light for the same time, the crude soluble product (4-L0 in
Figure 1A) exhibits further reduction of the fractions’ molecular weight with the R750 and R150 accounting for 6.5 %, and the R20 and P0.2 fractions accounting for over 89.1 % of the total recovered material. As shown in
Table 1, during the 14 day treatments, the pH of the two 1-D0 and 4-L0 solutions decreased to 9. For the products obtained in the treatments carried out at constant pH 10 (i.e., 7-D0 and 10-L0 in
Figure 1A), the reductions of molecular weight seemed less, compared to the 1-D0 and 4-L0 products in
Figure 1A. For 7-D0 (in
Figure 1A) obtained without irradiation of the pH 10 reaction medium, the R750 fraction accounted for 30 % and the R150 for 6.5 %, as compared to 5 % for R750 and 54 % for R150 in the 1-D0 product (in
Figure 1A) obtained without pH control. For 10-L0 (in
Figure 1A) obtained from the irradiated reaction medium, the R50 accounted for 14.2 % against 0 % for the 4-L0 product, while the lower molecular weight fractions R20, R 0.2 and P0.2 fractions all together were 86.2 % in the 10-L0 product, lower than 93.3 % in the 4-L0 product (
Figure 1A) obtained without pH control of the reaction medium. For the 4-D0 and 10-L0 products, the P0.2 fraction is supposed to contain potassium carbonate, the product of the mineralisation of organic C (see
Section 2.2).
Figure 1B reports the total C distribution over each of the above materials. For the pristine SBP (0-SBP in
Figure 1B), most of the total carbon (69 %) is accounted by the R750 fraction. For the crude soluble products obtained in each treatment, the carbon recoveries are given as w/w % relative to the carbon in the pristine SBP. The C recovery values are calculated based on the mass data given in
Figure 1A and the C content measured for each fraction. It may be observed that the pattern of C recovery distribution changes significantly depending upon the type of treatment. The carbon recovered with the R750 fractions of the crude soluble products ranges from 0.5 % for 10-L0 to 30 % for 7-D0, while most of the remaining C (46-81 %) is recovered with the lower molecular weight fractions.
Both the mass and C recovery data in
Figure 1 indicate that all treatments cause depolymerisation of the pristine SBP organic matter and that the effect is stronger in the treatments carried out by irradiation and/or without pH control of the reaction medium. These findings offer a highly relevant information, which coupled to the results reported in
Section 2.1 and
Section 2.2, undoubtedly demonstrate the autocatalytic properties of SBP to react with water and lead to the depolymerisation and mineralisation of its own organic matter.
2.4. Products obtained in the presence of added H2O2.
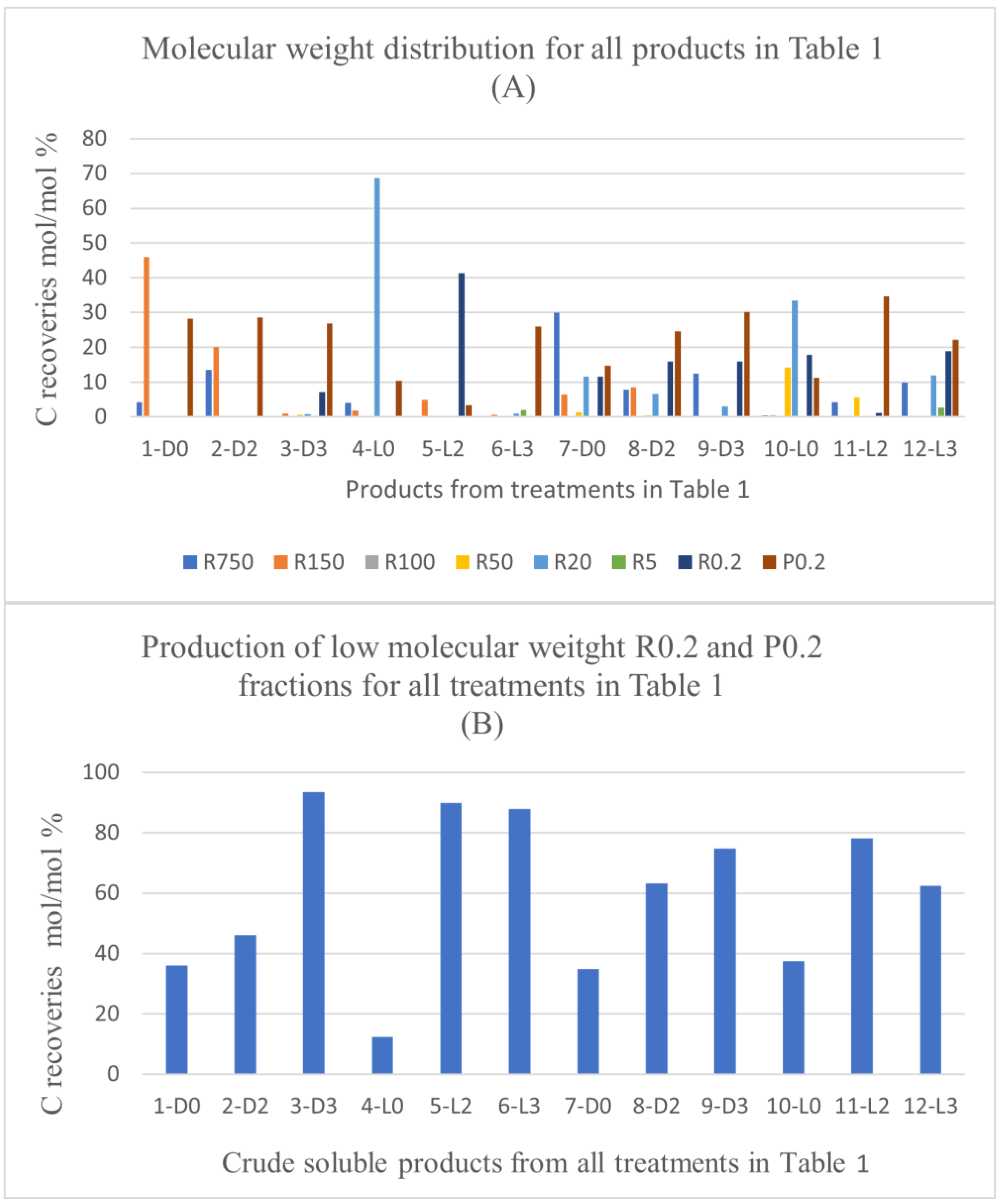
Figure 3A reports the C recoveries, as mol/mol % relative to the carbon of the pristine SBP, with each fraction isolated from each crude soluble product obtained in all SBP treatments listed in
Table 1. The C recoveries are calculated from the weight and carbon content of the fraction isolated through the sequential ultrafiltration with the same membranes listed in
Figure 2. The different colours of the histogram columns identify the different retentates and permeates obtained in the ultrafiltration.
The data evidence the effect of hydrogen peroxide on the molecular weight distribution of the crude soluble products. For the treatments carried out at constant pH 10, without (7-D0, 8-D2, 9-D3) and with irradiation (10-L0, 11-L2, 12-L3), the data show the increase of the lowest molecular weight fractions R0.2 and/or P0.2 occurring in the presence of added H
2O
2, compared to the reaction in absence of H
2O
2. This is readily evidenced in
Figure 3B, which reports the total C recovered with the retentate (R0.2) and permeate (P0.2) fractions as % of the total C recovered with all fractions.
It may be observed in
Figure 3B that, in the case of the treatments carried without light irradiation and with no pH control (1-D0, 2-D2 and 3-D3), the total production of R0.2 and P0.2 increases upon increasing the added content of H
2O
2 from 2 to 3 moles per SBP C mole. In the other cases, no effect or significant trend appears evident by increasing the content of H
2O
2 above 2 moles per SBP C mole. A somewhat similar trend is observed due to light irradiation. Compared to the 2-D2, the 5-L2 treatment caused a strong increases of the total production of R0.2 and P0.2. In all other cases, no definite effect or trend may be picked out as being caused by light irradiation.
The data in
Figure 3 show that depolymerisation and/or mineralisation of SBP organic matter is particularly evident in treatments 3-D3, 5-L2, 6-L3, where the sum of R0.2 and P0.2 % values in
Figure 3B accounts for 74-93 % of the values reported in
Table 1 for the total C recovered with 3-D3, 5-L2, 6-L3 treatments. Considering the data in
Table 1 and
Figure 1-3, it appears evident that the SBP treatments in the presence of hydrogen peroxide at 2 and 3 H
2O
2/C ratio are too drastic, due to the enhancement of depolymerisation and mineralisation by oxidation of the SBP organic matter. Similar results were obtained for the SBP treatments at controlled pH 10 and 0.1-0.5 H
2O
2/C mole ratio not reported here. Under these conditions, 0.06-0.15 H
+/C eq./mole were produced. This indicated that the oxidation of the SBP organic C to organic COOH functional groups and/or CO
2 was quite less than in the treatments No. 7-12 at 2-3 H
2O
2/C mole ratio reported in
Table 1.
2.5. Chemical composition and properties of the molecular weight fractions isolated from the crude soluble products obtained in all treatments performed in the present work.
Figure 4 and 5 report data related to the chemical composition and surface activity properties of the fractions obtained from the crude soluble products obtained in all treatments carried out in the present work. More detailed data are given in
Table S3.
For reactions involving products of complex chemical composition such as SBP, the ratio of the carbon to nitrogen (C/N) content in the product has been used as index of the effect of a chemical or a biochemical reaction on the product chemical nature. For example, for the chemical hydrolysis of a wide variety of municipal biowaste composts, the following equation 3 has been proposed [
1]:
where Z is the ratio of the measured total aliphatic, aromatic, carboxylic, phenol, phenoxide, methoxy, amine, amide and ketonic carbon over the sum of amine, amide and carboxylate functional groups. A similar significant linear relationship has been confirmed between the C/N values of different composts with the C/N values of the derived SBPs products [
4]. The relationship allows predicting the chemical composition of SBPs obtained from different composts. According to the authors [
4], it constitutes a valuable tool to assist the industrialisation of the SBPs production process.
The data in
Figure 4 show that C/N values vary over a very wide range, from a minimum 3.3 value for the P0.2 fraction of the crude soluble product obtained in 3-D3 treatment listed in
Table 1 to 49 for P0.2 of the crude soluble product obtained in the 15-L0 treatment of the pristine SBP solution carried out at controlled pH 10, under irradiation and in the absence of added H
2O
2. Most of the fractions in
Figure 4 have C/N values different from the 8.7 value for the pristine SBP reported in
Table S1. The variability of the C/N and molecular weight values of the fractions composing the crude soluble products obtained in the different treatments of the pristine SBP reflects the complexity of the supply chain that includes the pristine MBW and the SBP anaerobic digestate, from which the products are derived. In essence, each of the fractions in
Figure 4, obtained by ultrafiltration of the crude soluble products described in
Section 2.1-2.4, are mixture of molecules having not only different molecular weights, but also different chemical composition. With specific references to the P0.2 fractions, the C/N values higher than 8.7 (measured for pristine SBP) are likely contributed mostly by the content of potassium carbonate CO
2. This occurs for most of the P0.2 fractions isolated from the crude soluble products obtained in the treatments at constant pH 10, as anticipated by the data for the crude soluble products obtained at constant pH 10 (see
Section 2.2). On the contrary, low C/N values, as for example C/N 3.3 and 4.4 for P0.2 isolated from the 3-D3 and 6-L3 crude soluble products, indicate the presence of small molecules containing N, as organic oxymes isolated in the ozonisation of SBP [
7].
Table 4 reports mean C/N values calculated from the single C/N values (
Figure 4 and
Table S6) of the fractions isolated as retentates or permeates through membranes with the same cut off values.
Table 4.
Mean C/N value of fractions with the same molecular weight.
Table 4.
Mean C/N value of fractions with the same molecular weight.
| Fractions |
Mean |
StDa |
STDrb
|
Data pointsc |
| R750 |
8.5 |
2.7 |
32 |
14 |
| R150 |
7.3 |
1.4 |
20 |
14 |
| R50 |
7.4 |
1.5 |
21 |
8 |
| R20 |
9.5 |
5.2 |
55 |
13 |
| R5 |
6.7 |
1.1 |
16 |
5 |
| R0.2 |
9.9 |
7.4 |
75 |
13 |
| P0.2 |
14.4 |
11.0 |
76 |
19 |
Mean C/N values have relatively high relative standard deviations, which do not allow confirming that the differences between average values are statistically significant. Many factors contribute to the high standard deviations. For the complex molecular mixtures dealt with in the present work, major factors are the different reaction conditions adopted, e.g. the applied H
2O
2/C mole ratio, together with the replicability of the course of the reaction under the same experimental conditions and the change of the solution conformation of the molecular pool constituting the crude soluble products from which the corresponding fractions in
Figure 4 are isolated. The polymeric molecules in the crude soluble products, through their basic and acid functional groups, may establish intermolecular H-bonds. Depending on their concentration in water, these molecules form aggregates of different sizes trough the H-bond network established between macromolecules of different composition. In dilute solutions, the size of these aggregates is likely to decrease and therefore the composition of retentates through a membrane with a specific cut off may change depending on the concentration of the solution fed to the membrane. Yet, within the limitations posed by the relatively high variability of the C/N parameter, the data in Table 4 show some apparent differences that may help to rationalise the nature of the crude soluble products. For example, the lowest molecular weight P0.2 fractions have the highest C/N average (14.4) and standard deviation (11.0) corresponding to STDr 76 %. These results are most likely due to the fact that the P0.2 fractions contain variable amounts of carbonates, as anticipated in the
Section 2.2. The same may be true for the R0.2 fractions. By comparison, all other fractions exhibit lower average C/N values in the 6.7-9.5 range and lower STDr in the 20-55 % range. Excluding the P0.2 and R0.2 fractions, the apparent order of average C/N values is R20 > R750 > R150 = R50 > R50 > R5. The C/N data therefore indicate that, based on equation 3 and on the molecular weight, the R750 and R5 have, respectively, the lowest and the highest relative content of amino carboxylic and peptide functional groups plausibly organised in protein like-moieties with different molecular weights.
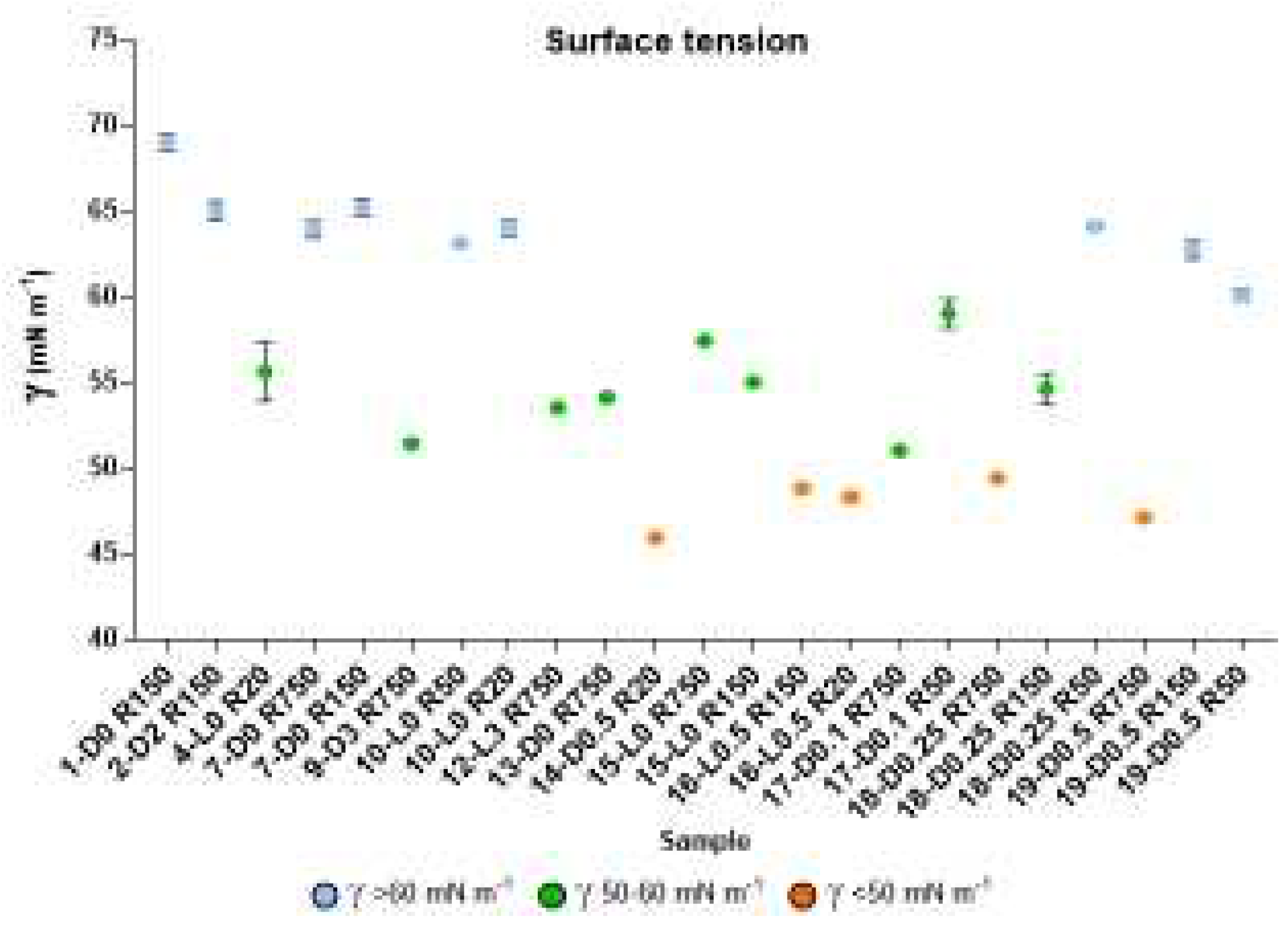
Selected samples of the products listed in
Figure 4 were investigated for their capacity to lower the surface tension of water. The results are reported in
Figure 5 and also in
Table S3. The graphical representation of the measured surface tension (γ) values in different colours (
Figure 4) allows observing readily that the experimental γ data may be divided in three groups: i.e. γ < 50; 50 ≤ γ < 60; γ ≥ 60.
The data point and the standard deviation bars in
Figure 5, and the statistical analysis given in
Table 5 show that the mean values of the three groups are significantly different one from the other. The SBP treatments at H
2O
2/C 0.5-0.1 mole ratios without pH control, and without or with irradiation yield products with the lowest γ
< 50 values. All others have
γ > 50. All samples obtained by virtue of the autocatalytic properties of SBP in absence of added H
2O
2 seem to exhibit the highest γ
≥ 60 surface tension values. On the other hand, the treatments in the presence of H
2O
2/C 2-3 seem to yield fractions with some improvement of the surface activity properties, but produce high de-polymerization of the pristine SBP organic matter. The treatments at H
2O
2/C 0.5-0.1 mole ratios produce also de-polymerization of the pristine SBPs organic matter, but yield the fractions exhibiting the best surface activity property.
3. Discussion
3.1. The autocatalytic property of SBP.
The SBP depolymerisation (
Figure 2) and mineralisation (
Figure 1) in water, induced by the 1-D0, 4-D0, 7-L0, 10-L0 SBP treatments in absence of added H
2O
2, prove that the SBP auto-catalyses its own oxidation. In the absence of added oxidant reagents, the reaction can only occur through the generation oxygen and/or hydroxyl radicals [
3] catalysed by SBP [
1]. Therefore, under these conditions, water acts as solvent and oxidizing agent. The data also demonstrate that the SBP auto-oxidation occurs both with and without light irradiation of the SBO solution.
The auto-oxidation of a compost derived SBP in water solution at 500 mg L
-1 under irradiation with simulated solar for 0-15 hours has been reported [
1] to yield crude soluble products with increasing content of organic carboxylic functional groups, and to cause decrease of the SBP aromatic chromophores and fluorophores, lighter colour and complete loss of the surfactant properties of the pristine compost derived SBP. Photo-bleaching, as evidenced by the lighter colour of the product, was more marked in the presence of added hydrogen peroxide. The auto-oxidation of SBPs solution without irradiation with simulated solar light, as observed for the SBP tested in the present work, is so far unknown in literature.
Photo-assisted chemical reactions under light and dark conditions are known to occur for systems containing a wide range of mineral ions from groups I through IV and rare-earth elements [
8]. Bio- and chemiluminescent reactions [
9] are examples of reactions occurring in the dark and producing light. These reactions involve complex systems involving condensed heteroaromatic rings, molecular oxygen or hydrogen peroxide, potassium hydroxide and complexed Fe
3+/Fe
2+ ions participating in redox reactions. The SBP investigate in the present work, derived from the MBW anaerobic digestate, and the compost derived SBPs have all organic and mineral components [
1] for chemical reactions to occur in water in the dark.
Thanks to their complex chemical composition, compost derived SBPs have been shown to perform as photosensitisers favouring Photo-Fenton processes under irradiation with simulate solar light [
1]. The Fe ions in SBPs are inherited from the pristine MBW from which the products are obtained. These ions are bonded to the SBPs organic matter. In this fashion, SBPs can maintain Fe(III) in solution at pH values above 4.5–5. Through this mechanism, SBPs have been demonstrated to induce the oxidation of organic pollutants in water with significant mineralisation of organic C and/or their self-oxidation.
The autocatalytic properties of the SBP obtained from the MBW anaerobic digestate, which are disclosed in the present work, are a novelty, valuable for many aspects. The results described in
Section 2.1-2.3 offer scope for further research aiming to increase the scientific knowledge of advanced oxidation processes [
3], biomass derived C catalysts [
5] and photo-assisted chemical reactions under light and dark conditions. At the same time, they open new perspectives for developing no energy and no reagents consumption, clean, mild oxidation processes for the valorisation of MBW as feedstock for the production of new value added chemical specialities.
3.2. Criticalities for the implementation of the SBP catalytic properties.
Aside from the scientific value, the results of the present work indicate a number of criticalities that need to be solved to improve and implement the oxidation process for the valorisation of MBW as feedstock for the production of competitive biowaste-based biosurfactants and materials to industrial and commercial level.
The auto-catalyzed reactions in absence of added H
2O
2 (1-D0, 4-L0, 7-D0 and 10-L0) yield the lowest de-polymerization degree of the pristine SBP. This is readily evident from the data in
Figure 3, showing that the total production of the low molecular weight fractions (R0.2 and P0.2) in the 12-37 % range. Unfortunately, the surface tension values of the high molecular weight fractions (Ri, i = 750-20) are too high (γ = 56−69). The treatments in the presence of hydrogen peroxide at 2-3 H
2O
2/C mol/mol ratio gave products with slightly better surface tension properties, i.e., γ = 52-53 for the products obtained in the 9-D3 and 12-L3 treatments (
Figure 3). However, compared to the treatments in absence of hydrogen peroxide, the de-polymerization degree induced by the treatments in presence of hydrogen peroxide was quite high. For example, the production of the R0.2 and P0.2 fractions (
Figure 3B) amounted to 75 % and 62 % for the 9-D3 and 12-L3 treatments, respectively. The treatments at H
2O
2/C 0.5-0.1 mole ratios produced also high de-polymerization of the pristine SBP organic matter, but yielded high molecular weight products exhibiting the best surface activity properties. The surface tension (
Figure 5) range from 47 to 49 mN m
-1 for the R750, R150 and R20 fractions isolated by membrane filtration of the crude soluble products obtained in the treatment at 0.25-0.5 H
2O
2/C mol/mol ratio. Furthermore, the R750 and R 150 fractions of the crude soluble products obtained from the treatments 19-D0.5 and 16-L0.5, respectively, yielded the 2 g L
-1 solutions exhibiting the lightest yellow colours (
Table S3) coupled to the lowest surface tension (
Figure 5).
Figure 6 shows the example of the colour of the solutions of the R750 fractions obtained from the 7-D0 and 15-L0 treatments, and of the R750 and R150 fractions obtained from the treatments 19-D0.5 and 16-L0.5, respectively. Lighter white colour coupled to lower surface tension 38 mN m
-1 had been observed only for the R150 fraction isolated from the ozonised SBP [
7]. Bleaching and improving surface activity make SBPs more competitive biosurfactants against current commercial products [
2,
7].
Considering the induced depolymerisation degree and the surface tension values of the products, none of the treatments in the presence of H
2O
2 seems competitive with the ozonisation of SBP [
7] reported to yield 30 % of polymers with molecular weights from100 to ≥ 750 kDa, and biosurfactants’ solutions with white colour and surface tension values of 38 mN m
-1 at the product critical micellar concentration of 0.47 g L
-1. Compared to the ozonisation of SBP, the auto-catalyzed reactions in absence of added H
2O
2 (1-D0, 4-L0, 7-D0 and 10-L0) are the only ones producing a definite improvement, although only for the reduced depolymerisation degree, and not for the products surfactants properties.
Undoubtedly, obtaining valuable products by just keeping SBP in water solution is the most desirable lowest cost process, as it would not involve energy and reagents consumption, and process wastes needing secondary treatments for their disposal. The results obtained with the SBP treatments in absence and in the presence of hydrogen peroxide at 0.1-0.5 H2O2/C mol/mol ratio offer scope for investigating the reaction of SBP with water containing hydrogen peroxide in catalytic amounts at H2O2/C μmol/mol level, to perform as precursor of active O and OH radicals. Such system might allow controlling better the rate of the SBP chemical oxidation, in order reduce the depolymerisation degree and improve the surface activity properties of the products, compared to the products obtained in the present work.
3.3. Scopes and perspectives of future improvements
The underlying concept of the previous work [
1] was the realisation of a MBW-based biorefinery, which could compete with fossil-based refineries for the production of commodities, fine chemicals and chemical specialities [
10,
11]. The lignocellulose proximates in MBW constitute in principle potential polymeric raw materials made of aliphatic and aromatic C types, substituted by a variety of oxygenated functional groups. The mild chemical treatments adopted in the previous and present work aimed to recover the MBW organic matter in soluble form, while maintaining as much as possible the functionalised polymeric structure. Contrary to the pristine MBW, the recovered polymeric soluble organic matter could be fractionated into different molecular cuts with different chemical composition, exploiting the different molecular sizes and/or solubilities in acid and alkaline water [
1]. This methodological approach is quite different from the technology used in fossil-based refineries, which encompasses several thermal and/or multistep chemical processes to manufacture organic commodities, fine chemicals and materials [
10,
11].
For the envisioned MBW-based biorefinery to compete with fossil-based refinery, the previous work addressed several issues, such as the site-specific variability of MBW [
4], the processes sustainability, the products replicability with guaranteed specification and performance, and the production flexibility [
2], i.e., the capacity of the biorefinery to obtain different products and to modulate their production according to the market demand. Production flexibility is well practiced in fossil-based refineries [
12,
13,
14,
15,
16].
In this context, the development of the autocatalytic SBP process in the presence of hydrogen peroxide at H
2O
2/C μmol/mol level is a further option offered by the results of the present work, which might contribute more economic sustainability and production flexibility to the aimed MBW-based refinery. The improvement of the yield, quality and performance of the surfactants and polymers described in the present work is a highly worthwhile objective to be achieved, upon considering that the current commercial counterpart products account for large shares of the world chemical market. These commercial products are used for the manufacture of consumer chemicals, auxiliaries for industry and plastics, which constitute about 60 % of the chemicals sales in the European market [
17].
At present, there are no commercial chemicals made from MBW, except for composts. A typical example is the compost made from food waste anaerobic digestate mixed with urban gardening residues produced by the Italian Acea MBW treatment plant [
18]. This product however has a very low commercial value of 30 €/t [
1,
19]. In the long term vision of the present work, MBW management and biobased economy are intimately connected. The previous work on SBPs [
1] proves that exploitation of MBW as feedstock can generate new relevant benefits for waste management and the biobased economy.
In Europe, about 100 Mt yr
-1 of MBW
1 are generated by 750 million EU citizens and treated by 18000 plants [
20,
21]. About 35 Mt yr
-1 MBW are processed by anaerobic and aerobic fermentations, yielding biogas, anaerobic digestate and/or compost [
22,
23]. The rest is landfilled or incinerated emitting 11000 Mm
3 yr
-1 CO
2, 14000 Mm
3 yr
-1 CH
4, 2-4 kt yr
-1 dust and 154 kt yr
-1 other GHG and toxic organics [
24]. On the other hand, the EU chemical industry produces 330 Mt yr
-1 organic chemicals from fossils to make plastics and chemical products [
25,
26] generating 1037 Mt yr
-1 CO
2 emission. Sale prices [
27,
28,
29,
30,
31] for plastics and high performance surfactants run 1,500-150,000 € t
-1, worth 84-8,400 billion € yr
-1 total market value. By comparison, biobased plastics and chemicals turnover [
25,
26] is 70 billion € yr
-1. In this scenario, the improvements of SBPs yield and quality could in principle allow obtaining new biowaste-based commercial products, which were competitive with the current commercial products and could replace large portions of EU chemicals obtained from fossil feedstock, thus increasing the market output of biobased chemicals and materials.
The realisation of these perspectives would generate considerable environmental, economic and social benefits. For examples, current MBW treatment plants are not cost effective [
1]. The value of the produced biogas and compost is not enough to cover the collection and processing cost of the biowastes. Eighty per cent of it is paid for by citizens’ taxes. The conversion of the MBW treatment plants into biorefineries, producing the improved SBPs competitive with current commercial products, would allow raising the MBW plants’ revenue by two-three order of magnitude. This effect, in turn, would allow obtaining a number of effects: i.e., reducing the citizens’ taxation for waste disposal; establishing a healthier living environment due to reduction of GHG from landfill sites and from the fossil based chemical industry; generating new jobs in the biowaste-based refineries; convincing citizens that wastes are source of economic, social and environmental benefits, rather than cost; promoting social acceptance of the new biorefineries; improving citizens’ attitudes towards waste reuse and recycling; contributing to biobased products standardization/certification; updating EU REACH Chemicals Policy by including SBPs as authorized chemical specialities for the chemical market.
The achievement of above impacts requires the participation of multidisciplinary industrial skills and join ventures (JVs) involving multipurpose industrial actors. For example, the facilities to produce the SBPs should be most suitably located in the MBW operational sites, which traditionally deal with the collection and treatment of the biowaste paid for by the local citizens’ community. On the other hand, chemical companies have assessed competence and experience in the manufacture and marketing of finished consumers’ products and/or materials. The JVs between such actors could more properly be established in form of benefit corporation, a recent new legal tool to create a solid foundation for long term mission alignment and value creation that was established in 2016 in Italy as first European state and second country in the world to adopt it [
32,
33]. The mission of the benefit corporation is perfectly tailored to the achievement of the multiple impacts expected to be achieved by the new waste-based biorefinery. It combines the goal of profit with the purpose of creating a positive impact for society and the environment. For the nature of its mission and skills, the above benefit corporation has all traits to communicate and interact with scientists, technologists, policy makers and citizens.
4. Materials and Methods
4.1. Materials and Treatments.
The SBP was available from previous work [
1]. It was obtained by hydrolysis at pH 13 and 60 ° from the anaerobic digestate of unsorted municipal food waste. The digestate was supplied by Italian Acea Pinerolese MBW treatment plant [
18] located in Pinerolo (TO). In the present work, the SBP (5 g) was dissolved in 150 ml) water at pH 10. The solution was kept at room temperature under the following different conditions: in the absence and presence of added hydrogen peroxide at 0.1-3 H
2O
2/C mole ratio, with and without pH control. In the first case, the pH of the solution was found to decrease from 10 to about 5 over 14 days, and remained constant afterwards. In the second case, the starting pH 10 was kept constant during the 14 days by adding a 0.2 g L
-1 KOH solution in water. The same conditions were applied for the solution kept in a climatic chamber under irradiation with simulated solar light. At the end of the treatments, the solution was centrifuged at 5000 rpm to separate any insoluble material from the soluble phase. The separated insoluble and soluble products were dried at 60 °C.
4.2. Decarbonated CO2-free samples.
The dry soluble products were re-dissolved in water and added with 5 N HC until gas evolution ceased. Afterwards, the solution was centrifuged to separate the precipitated solid from the supernatant liquid phase. The solid was dried first at 60 °C and then in a chemistry lab desiccator over solid NaOH and silica gel.
4.3. Products’ isolation and characterisation.
The separated soluble phase from the treatments described in
Section 4.1 and the decarbonated CO
2-free samples were further processed by sequential membrane ultrafiltration through 8 polysulphone membranes with decreasing molecular cut to collect the retentates at 750 kDa (R750), 150 kDa (R150), 100 kDa (R100), 50 kDa (R50), 20 kDa (R20), 5 kDa (R5), 0.2 kDa (R0.2) and the final permeate at 0.2 kDa (P0.2). The obtained retentate and permeate fractions were dried at 60 °C to constant weight and analysed for their volatile solids, ash, C, N content. The products were characterised for their relative C type and functional composition by 13 C solid state NMR spectroscopy and for their surface tension in water solution at 2 g L
-1 added product concentration.
4.4. Analytical methods.
13C solid state NMR spectra were recorded as previously reported [
1] at 67.9 MHz on a JEOL GSE 270 spectrometer equipped with a Doty probe. The cross-polarization magic angle spinning (CPMAS) technique was employed and for each spectrum about 104 free induction decays were accumulated. The pulse repetition rate was set at 0.5 s, the contact time at 1 ms, the sweep width was 35 KHz and MAS was performed at 5 kHz. Under these conditions, the NMR technique provides quantitative integration values in the different spectral regions. Thus the relative composition of C types and functional groups for each product in
Figure 1 is based on the integration of the band areas in the 13 C NMR spectrum falling in the chemical shift (δ, ppm) ranges: 0-53 for aliphatic (Af) C, 53-63 ppm for amine (NR) and methoxy (OMe) C, 63-95 ppm for alkoxy (OR) C, 95-110 ppm for anomeric (OCO) C, 110-160 ppm for total aromatic (Ph) C and 160-185 ppm for carboxylic and amide (COX, X = OR, OM, NR, R = H, alkyl and/or aryl) C. The total integrated bands area was assumed to represent the total C moles in the analysed sample. All other analytical and product characterisation details were as previously reported [
1].
5. Conclusions
This work shows that the realisation of the long term vision to convert municipal biowaste treatment facilities into cost effective biorefineries producing biofuel, and value added biosurfactants and biopolymers for the manufacture of consumer products and plastic materials, requires improvements of process yields and products’ quality, relative to the current state of art of the research so far carried out [
2]. Process improvements should focus on increasing the yield of the high molecular weight water soluble products by reducing the depolymerisation and/or mineralisation of the pristine material. Product improvements should focus on enhancing the products’ surfactant, workability and mechanical properties.
The data collected in the present work show that, due to the complex chemical composition of the feedstock natural waste materials and the derived products, it is not easy to assess the trade-off between destroying potentially valuable organic matter and gaining value from the residual matter surviving the chemical treatment. Considering the stake in the context depicted in
Section 3.3, investigating the oxidation of SBP assisted by hydrogen peroxide at H
2O
2/C μmol/mol level as function of the reaction time is highly worthwhile.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Table S1: Experimental conditions and products for the oxidation of SBP in water solution at 33 g/L concentration, irradiated and not irradiated with simulated solar light, in the absence and presence of added hydrogen peroxide at 2-3 H
2O
2/C mole ratio, with and without pH control. Data for pristine SBP at day 0 and recovered soluble and insoluble products at day 14: weight (WY, w/w %), and C (CMY) and N (NMY) molar (mol/mol%) yields relative to pristine SBP, and organic C and N w/w % content in recovered materials; Table S2: C mole % recoveries accounted for by molecular weight fractions (MWF) isolated by sequential membrane ultrafiltration in the SBP treatments listed in Table S1; Table S3: Chemical composition and property data for molecular weight fractions of SBPox described in
Table 1 and above
Section 2.1,
Section 2.2 and
Section 2.3: C and N content (w/w %), C/N w/w ratio, and surface tension (γ, mN m
-1) and colour for aqueous solutions containing 2 g L
-1 added products; Table S4: Surface tension values (γ, mN m
-1) in Table 4 and
Figure 2 and mean values for γ < 50, 50 ≤ γ < 60, γ ≥ 60 groups.
Author Contributions
Conceptualisation, E.M.; methodology, E.M.; investigation, E.P., F.C., M.N. and M.F.; resources, A.B.; data curation, E.M., E.P. and M.N.; writing—original draft preparation, E.M.; writing—review and editing, E.M. and E.P.; visualisation, A.B.; supervision, M.N.; project administration, M.N.; funding acquisition, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly co-funded by the European Commission within the LIFE 2019 program, under the LIFE EBP project, grant number LIFE19 ENV/IT/000004.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
To be omitted as the study did not involve humans.
Data Availability Statement
All the available data are reported in the present and in the previous referenced publications.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Municipal Biowaste (MBW); Soluble Biobased Products (SBPs); Soluble Biobased Product obtained from MBW anaerobic digestate used as pristine material in the treatments reported in the resent work. SBP treatments, 1-D0 (no added H2O2, no pH control, no light irradiation); 2-D2 (added H2O2 at 2 H2O2/C mol/mol, no pH control, no light irradiation); 3-D3 (added H2O2 at 3 H2O2/C mol/mol, no pH control, no light irradiation); 4-L0 (no added H2O2, no pH control, light irradiation); 5-L2 (added H2O2 at 2 H2O2/C mol/mol, no pH control, light irradiation); 6-L3 (added H2O2 at 3 H2O2/C mol/mol, no pH control, light irradiation); 7-D0 (no added H2O2, pH 10 control, no light irradiation); 8-D2 (added H2O2 at 2 H2O2/C mol/mol, pH 10 control, no light irradiation); 9-D3 (added H2O2 at 3 H2O2/C mol/mol, pH 10 control, no light irradiation); 10-L0 and 15-L0 (no added H2O2, pH 10 control, light irradiation); 11-L2 (added H2O2 at 2 H2O2/C mol/mol, pH 10 control, light irradiation); 12-L3 (added H2O2 at 3 H2O2/C mol/mol, pH 10 control, light irradiation); 13-D0 (no added H2O2, pH 10 control, no light irradiation); 14-D0.5 (added H2O2 at 0.5 H2O2/C mol/mol, pH 10 control, no light irradiation); 16-L0.5 (added H2O2 at 0.5 H2O2/C mol/mol, pH 10 control, light irradiation); 17-D0.1 (added H2O2 at 0.1 H2O2/C mol/mol, pH 10 control, no light irradiation); 18-D0.25 (added H2O2 at 0.25 H2O2/C mol/mol, pH 10 control, no light irradiation); 19-D0.5 (added H2O2 at 0.5 H2O2/C mol/mol, pH 10 control, no light irradiation).
References
- Montoneri, E. Municipal waste treatment, technological scale up and commercial exploitation: the case of bio-waste lignin to soluble lignin-like polymers. In Food Waste Reduction and Valorisation; Editor Morone, P., Papendiek, F., Tartiu, V.E.; Springer, Chapter 6, pp 1-42, 2017. [CrossRef]
- Padoan, E.; Montoneri, E.; Baglieri, A.; Francavilla, M.; Negre, M. Mild chemical treatment of unsorted urban food wastes. Molecules 2023, 28, 7670. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P; Kuma, A. Advanced oxidation process: a remediation technique for organic and non-biodegradable pollutant. Results in Surfaces and Interfaces 2023, 11, 100122. [CrossRef]
- Montoneri, E.; Koutinas, M.; Padoan, E.; Negro, V.; Licignano, C.; Leone, S.; Photiou, P.; Kallis, M.; Vyrides, I.; Liendo, F.; Negre, M.; Solaro, S.; Antonini, M.; Mainero, D.; Vlysidis, A.; Konstantinidis, V.; Ladakis, D.; Maina, S.; Koutinas, A. Integrated chemical and biochemical technology to produce biogas with reduced ammonia content from municipal biowaste. Validating lab-scale research in real operational environment. Environ. Sci.: Adv. 2022, 1, 746–768. [Google Scholar] [CrossRef]
- Konwar, L.J. New biomass derived carbon catalysts for biomass valorization Doctoral thesis Åbo Akademi University Turku/Åbo, 2016.. Painosalama Oy – Turku, Finland 2016. ISBN 978-952-12-3396-8.
- SpectraBase. Potassium carbonate. Available online: https://spectrabase.com/spectrum/4DkIM6GVb69 (accessed on 20 November 2023).
- Montoneri, E.; Fabbri, G.; Quagliotto, P.L.; Baglieri, A.; Padoan, E.; Boero, V.; Negre, M. High molecular weight biosurfactants from mild chemical reactions of fermented municipal biowastes. ChemistrySelect 2020, 5, 2564–2576. [Google Scholar] [CrossRef]
- Sakar, M.; Nguyen, C.C.; Vu, M.H.; Do, T.O. Materials and mechanisms of photo-assisted chemical reactions under light and dark conditions: can day–night photocatalysis be achieved? ChemSusChem 2018, 11, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E. What is chemiluminescence? Science in School I Issue 19 : Summer 2011. Available online: https://www.scienceinschool.org/wp-content/uploads/2014/11/issue19_chemiluminescence.pdf (accessed on 2 December 2023).
- Chenier, P.J. Basic organic chemicals. In Survey of Industrial Chemistry; Topics in Applied Chemistry; Springer: Boston, MA, USA, 2002. [Google Scholar]
- Viale, P. The Manufacture of Basic Chemicals. Eurostat Statics in Focus 58/2008. Available online: https://ec.europa.eu/eurostat/documents/3433488/5582672/KS-SF-08-058-EN.PDF/ab295005-e8cb-49ee-bbef-60bd29819c47?version=1.0 (accessed on 12 November 2023).
- Singh, G. Thriving in the New Reality: Why Production Flexibility is Key for Competitive Refineries. 2020. Available online: https://www.shell.com/business-customers/catalysts-technologies/resources-library/thriving-in-the-new-reality-whyproduction-flexibility-is-key-for-competitive-refineries.html#:~:text=Gurminder%20Singh%20on%20Aug%209%2C%202020 (accessed on 12 November 2023).
- Taraphdar, T.; Yadav, P.; Prasa, M.K.E. Natural gas fuels the integration of refining and petrochemicals. 2012. Available online: https://www.digitalrefining.com/article/1000557/natural-gas-fuels-the-integration-of-refining-and-petrochemicals (accessed on 2 December 2023).
- Olsen, T.; Shodowsky, E. Improve refinery flexibility and responsiveness. Hydrocarbon Processing 2015, 87–89. [Google Scholar]
- Shell Global. Thriving in the new reality: Refinery revamp projects FAQ. Shell Catalysts & Technologies 2020. Available online: https://www.shell.com/business-customers/catalysts-technologies/resources-library/refinery-revamp-faq.html (accessed on 2 December 2023).
- Reinartz, S.J.; Schmid, T. Production flexibility, product markets, and capital structure decisions. The Review of Financial Studies 2016, 29, 1501–1548. [Google Scholar] [CrossRef]
- Cefic. 2023. Available online: https://cefic.org/a-pillar-of-the-european-economy/facts-and-figures-of-the-european-chemical-industry/profile/ (accessed on 2 December 2023).
- ACEA. Available online: https://www.aceapinerolese.it (accessed on 10 October 2023).
- Consorzio Italiano Compostatori (CIC). Country Report—The State of the Art of Separate Collection, Composting and AD in Italy. 2017. Available online: https://www.compost.it/wp-content/uploads/2019/08/Rapporto-CIC-2017-Eng-v-2.6-web-version.pdf. (accessed on 17 October 2023).
- Dekker, H.; Decorte, M. European Biogas Association, EBA Statistical Report 2021. Available online: https://www.europeanbiogas.eu/wp-content/uploads/2021/11/EBA-STATISTICAL-REPORT-2021-SHORT-VERSION.pdf (accessed on 2 December 2023).
- Meyer-Kohlstock, D.; Schmitz, T.; Kraft, E. Organic Waste for Compost and Biochar in the EU: Mobilizing the Potential. Resources 2015, 4(3), 457–475. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; VaNoolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient recovery from digestate: Systematic technology review and product classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef]
- Chenier, P.J. Basic organic chemicals. In Survey of Industrial Chemistry; Topics in Applied Chemistry; Springer: Boston, MA, USA, 2002. [Google Scholar]
- Neuwahl & al., Best Available Techniques for Waste Incineration, EU Commission. 2019. Available online: https://op.europa.eu/en/publication-detail/-/publication/075477b7-329a-11ea-ba6e-01aa75ed71a1/language-en (accessed on 2 December 2023).
- Eurostat, Chemicals production and consumption statistics. 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Chemicals_production_and_consumption_statistics (accessed on 2 December 2023).
- Reilly, P.O. Making the Bioeconomy Market: A Review of International Literature May 2017. Available online: https://www.teagasc.ie/media/website/publications/2017/WP2-Making-the-Bioeconomy-Market-DIT.pdf (accessed on 10 October 2023).
- Chen, G.Q. New challenges and opportunities for industrial biotechnology. Microb. Cell Fact. 2012, 11, 111 [CrossRef]. [Google Scholar] [CrossRef]
- Tsagaraki, E.; Karachaliou, E.; Delioglanis, I.; Kouzi, E. D2.1 Bio-Based Products and Applications Potential. 2017. Available online: https://www.bioways.eu/download.php?f=150&l=en&key=441a4e6a27f83a8e828b802c37adc6e1 (accessed on 10 October 2023).
- Isikgor, F.K.; Becer, C.R. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Van Bogaert, I.; Soetaert, W. Sophorolipids. In Biosurfactants, Microbiol. Monogr. 20. Editor Soberón-Chávez, G. Springer-Verlag, Berlin, Germany, Chapter 6, pp 179-210, 2011.
- Connolly, H.E.; Pattanathu, K.S.M.; Banat, I.; Lord, R.A. Resource-recovery and reduction of oily hazardous wastes via biosurfactant washing and bioremediation. Trends in Bioremediation and Phytoremediation, Ulster University 2010, pp 57-172. Available online: https://pure.ulster.ac.uk/en/publications/resource-recovery-and-reduction-of-oily-hazardous-wastes-via-bios-3. https://pure.ulster.ac.uk/ws/portalfiles/portal/11260519/10_Plaza.pdf (accessed on 2 December 2023).
- Nativa Srl. Società Benefit. Available online: https://www.societabenefit.net/english-information/ (accessed on 2 December 2023).
- Nannini, C. A NEW ECONOMY: Società Benefit and B Corps. Dissertation. Università Cattolica Del Sacro Cuore, Faculty of Economics, Undergraduate program in Economics and Management, Academic Year 2020/2021. Available online: https://www.societabenefit.net/wp-content/uploads/2021/07/Nannini-4801146.pdf (accessed on 2 December 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).