1. Introduction
The main titanium minerals are ilmenite (FeTiO3), rutile (TiO2) and anatase (TiO2) that is a rutile polymorph. Ilmenite and rutile are the main Ti source in the production of pure titanium dioxide as well as metallic titanium. However, with the increasing demand for titanium, the deposits of these minerals are gradually exhausting and anatase has been paid great attention as a possible option to replace them (Gao et al., 2019). The global reserves of anatase are abundant, and Brazil is the country with the largest anatase reserve (440 Mt) containing an average of 17.7 wt% TiO2 (Filho and Neto, 2019; Baltar et al., 2009). In the State of Minas Gerais, 200 Mt of anatase containing 12% to 15% of TiO2, is an sterile of the phosphate exploration, which is stored in piles. The residue remains without defined use because a commercial process to produce high pure TiO2 that proved to be technically and economically feasible has not been developed.
Considered as a “strategic mineral”, the search for potential sources of titanium minerals is paramount. However, many difficulties exist in the use of anatase as a raw material, one is its low chemical reactivity, which leads to sulphation efficiency significantly low and the other is the existence of a complex mineralogy assemblage that directly influences the downstream purification steps.
The great majority of the studies about the production of high-grade TiO2 focus on ilmenite which is so far much more reactive and, consequently, easily solubilized than anatase. Other forms of TiO2 such as rutile and leucoxene, whose acid digestion is also difficult, need very fine grains and thermal reduction to enable the dissolution of Ti. Because of the low reactivity of anatase, since 1980 the studies have focused on the upgrade of anatase concentrate in order to eliminate the impurities and raise the content of TiO2. Different pyrometallurgical and/or hydrometallurgical treatments were extensively investigated, mainly alkaline or reductive roasting followed by leaching, but the direct sulfuric digestion was scarcely evaluated (Barnard et al., 2019; Lakshmanan et al., 2014; Trindade and Teixeira, 1988). However, according to Xue et al., 2009 the process still accounts for 40% of the total TiO2 pigment produced in the world (Gasquez et al., 2014). The main advantages of the sulphate process is the low capital costs and the flexibility as the process allows the use of low grade titanium raw materials such as the residues from the phosphate exploration.
The sulfuric acid digestion is the first stage of the sulphate process and consists of the digestion of the raw material (fine grain size 90% < 44 μm) with concentrated sulfuric acid (80-98%), at temperatures between 170 and 220 °C, and reaction times that vary from 2 to 6 hours. After the reaction time, the solid is leached with water to promote the solubilization of sulphates and to obtain a liquor containing the metal of interest (Haynes, 2014 apud Gontijo et al., 2020; Lakshmanan et al., 2014; Freitas e Brocchi, 1995). In the case of titanium, the raw material is converted to solid titanyl sulphate (TiOSO4), which is soluble in water and can be leached into a titanyl sulphate rich liquor. That is followed by hydrolysis to produce hydrated titania.
According to Jablonski and Tylutka (2016), the concentration of sulfuric acid is directly related to the sulphation reaction rate, since the amount of elements leached is dependent on this factor. In addition, the maximum temperature reached in the process and the time spent to reach its maximum are dependent on the acid concentration. At low concentrations, the reaction is unstable, which may indicate incomplete sulphation of the material (Jablonski and Tylutka, 2016). In order to implement the sulphate process, the ideal is that the minerals of interest have high solubility in sulfuric acid and exothermicity that allows the reaction to be sustained after reaching the ignition temperature. However, this is not always the case of all titanium minerals and anatase is an example (Freitas and Brocchi, 1995).
Table 1.
Examples of different processes to produce pureTiO2 from anatase ore concentrate.
Table 1.
Examples of different processes to produce pureTiO2 from anatase ore concentrate.
| Authors |
TiO2 (%) in the raw material |
Processes |
| Chao and Senkler (1991) |
74,5 |
Upgrade: chloride leaching, alkaline leaching, chloride leaching |
| Chao et al. (1993) |
62,9 |
Upgrade: a reducing roasting, magnetic separation, pressure chloride leaching |
| Freitas et al. (2007) |
53,8 |
Upgrade: calcination, reduction in H2, magnetic separation, chloride leaching |
| Freitas and Gracioso (1985) |
70,0 |
Chloride leaching, alkaline digestion, sulphuric digestion, hydrolysis- precipitation |
| Jha and Tathavadkar (2005) |
57,8 |
Alkaline digestion, leaching with water, |
| Patent: Mineração Vale do Paranaíba (1980) |
78,1 |
Upgrade: Acid digestion under pressure, chloride leaching, magnetic separation |
| Paixão and Mendonça (1979) |
75,6 |
Upgrade: roasting, magnetic separation, HCl chloride leaching, NaOH neutralization |
| Smith Jr and Castro Sheldon (2007) |
52,9 |
Upgrade: leaching in autoclave with H2SO4 e FeSO4, drying with NaCl addition, calcination, chloride leaching |
| Trindade e Teixeira (1988) |
76,5 |
Upgrade: chloride leaching in 4 stages |
However, up to now there is no definition of one technological route, economically and technically viable, for the production of pure TiO2 from anatase ore. The present study investigated the chemical dissolution of an anatase concentrate containing 56.5% of TiO2 by means of a sulphation in order to present a feasible technical alternative to produce high grade TiO2 using residues from the mining industry.
2. Materials and experimental procedure
2.1. Material
A Brazilian anatase ore, physically concentrated and containing around 57% TiO2, was used in this investigation. The grain size after milling and classification was 99,3%< 62 µm.
2.2. Chemical and mineralogical characterization
The Brazilian anatase concentrate and the residues from the processes samples were chemically characterised by Wavelength- dispersive X-ray spectroscopy (WDXS) model Primus II, by Rigaku, with rhodium tube, vacuum system, LiF1, PET, RX25, Ge, RX35 crystals, scintillation detector and gas flow proportional counters.
The mineralogical characterization was carried out FEG- Quanta 200 FEI Scanning Electron Microscope (SEM) equipped with a secondary electron detector, backscattered electron detector, transmitted electron detector (STEM), integrated detector Pegasus: EDS (Energy-dispersive X-ray spectroscopy) and EBSD (Electron backscatter diffraction), operating between 200V and 30 kV voltage, beam current greater than 100 nA, resolution of 1.6 nm at 30 kV in high vacuum and ESEMTM mode, 3.5 nm at 3 kV at low vacuum, focal length: 3 mm to 99 mm, 12x magnification (at longest working distance) at 1,000,000x at high and low vacuum. X-ray diffraction was also employed and the analysis was conducted in a Rigaku equipment, D/Max Ultima Plus model, using Cu (K∝) radiation with wavelength of 1.5418 Å, tension of 40 kV and current of 30 mA. The samples was prepared by powder method, the analyze was made in a 2Ө angle range from 4° to 80° with scan step size of 0.02°s-1. The qualitative analysis of the mineral phases was performed by comparing the diffractograms with crystallographic reference standards available in the JCPDS-ICDD database (Joint Committee on Powder Diffraction Standards—International Center for Diffraction Data). Textural characterization of the mineralogy was achieved using scanning electron microscopy (SEM), performed on an FEI Quanta 200 field emission gun (FEG) scanning electron microscope with energy-dispersive X-ray spectrometry (EDS) and back-scatter electron imaging capabilities.
2.3. Sulphuric digestion
The sulphuric digestion process was carried out with sulphuric acid (98%), temperatures (190 °C, 200 °C, 210 °C and 220 °C), anatase:sulphuric acid ratios (w/w) (1:1.3 and 1:2) and time (3, 4 and 5 hours). The concentrate and sulphuric acid were manually homogenized, and heated according to previously determined conditions. After sulphuric digestion, the system was leached with Milli-Q water or dilute sulphuric acid at 70 °C. After leaching, the slurry was filtered under vacuum and the solid was washed with a volume of Milli-Q water, dried and sent to be analysed. All the experiments were performed in duplicate. The metallurgical recovery was calculated using Equation (1):
where m
r is the mass of residue, c
r is the TiO
2 concentration in the residue, m
c is the mass of concentrate and c
c is the TiO
2 concentration in the concentrate.
3. Results and discussion
3.1. Chemical and mineralogical characterization of the anatase concentrate
The chemical analysis (
Table 2) indicated the anatase ore concentrate has a content of 56.5% of TiO
2 and the main impurity is iron oxide, 15%. The concentrate has silica, aluminum, phosphorus, calcium and Rare Earth Elements (REE) between 1.61 and 6.01%. The concentrate also contains other impurities in smaller proportions such as ZrO
2, Nb
2O
5.
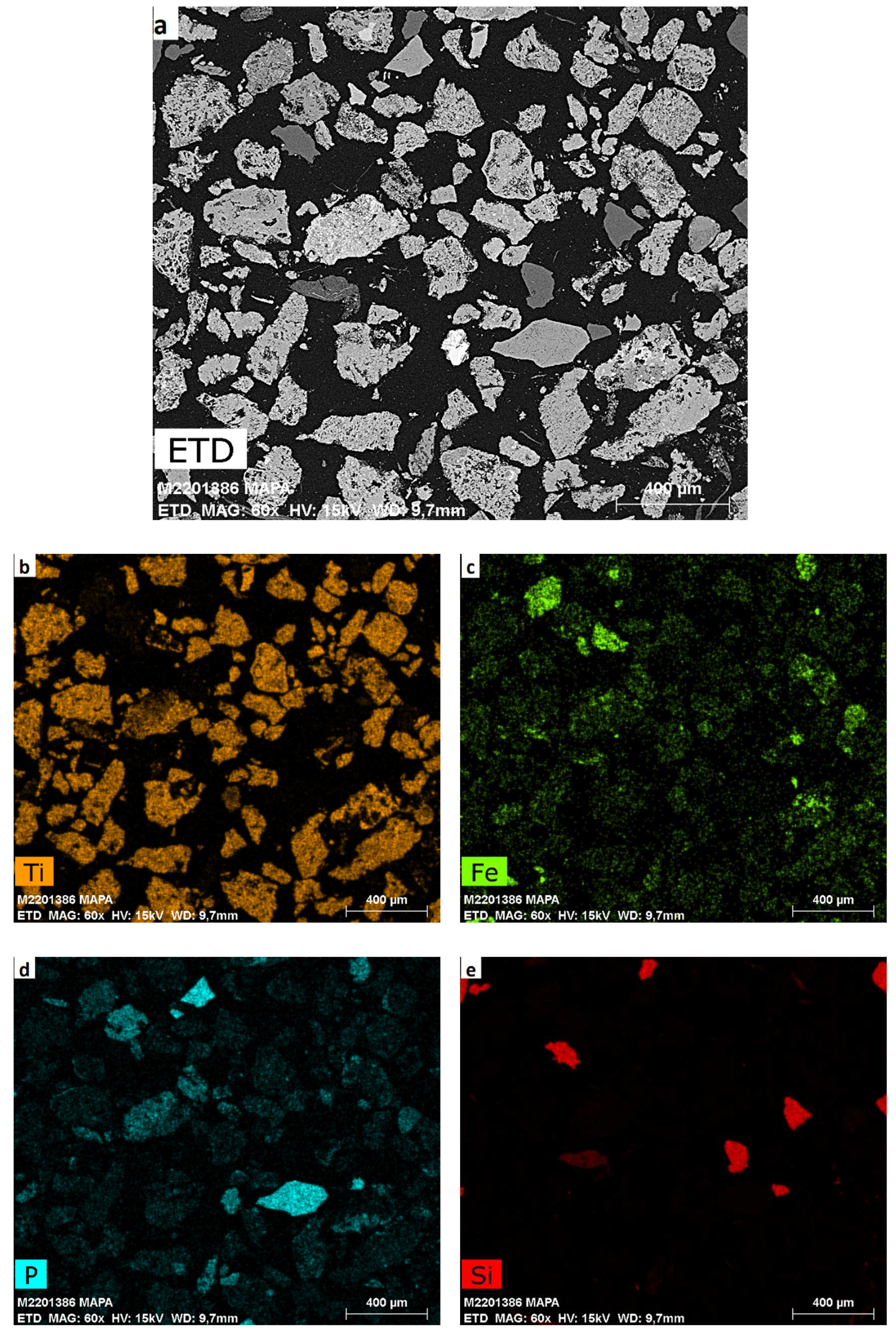
The material was also analyzed by SEM-EDS, and
Figure 1a presents the general morphology of the sample where we can see the heterogeneity of mineral phases, and chemical composition as well as a large dispersion in particle size. The SEM image also shows particles of a few hundred nanometers aggregated to micrometric particles of phyllosilicates.
Figure 1b–e shows the main elements present in the concentrate.
Figure 1b–e shows the expressive presence of Ti, P, Fe and Si. The minor elements can replace Ti into the crystal lattice and it depends, in part, on the ionic charge and radius of the element. Cations with higher charge and/or smaller radius than Ti
4+ (Fe
3+, Cr
3+, Mo
4+, Mn
4+, Nb
5+, Ta
5+, Sb
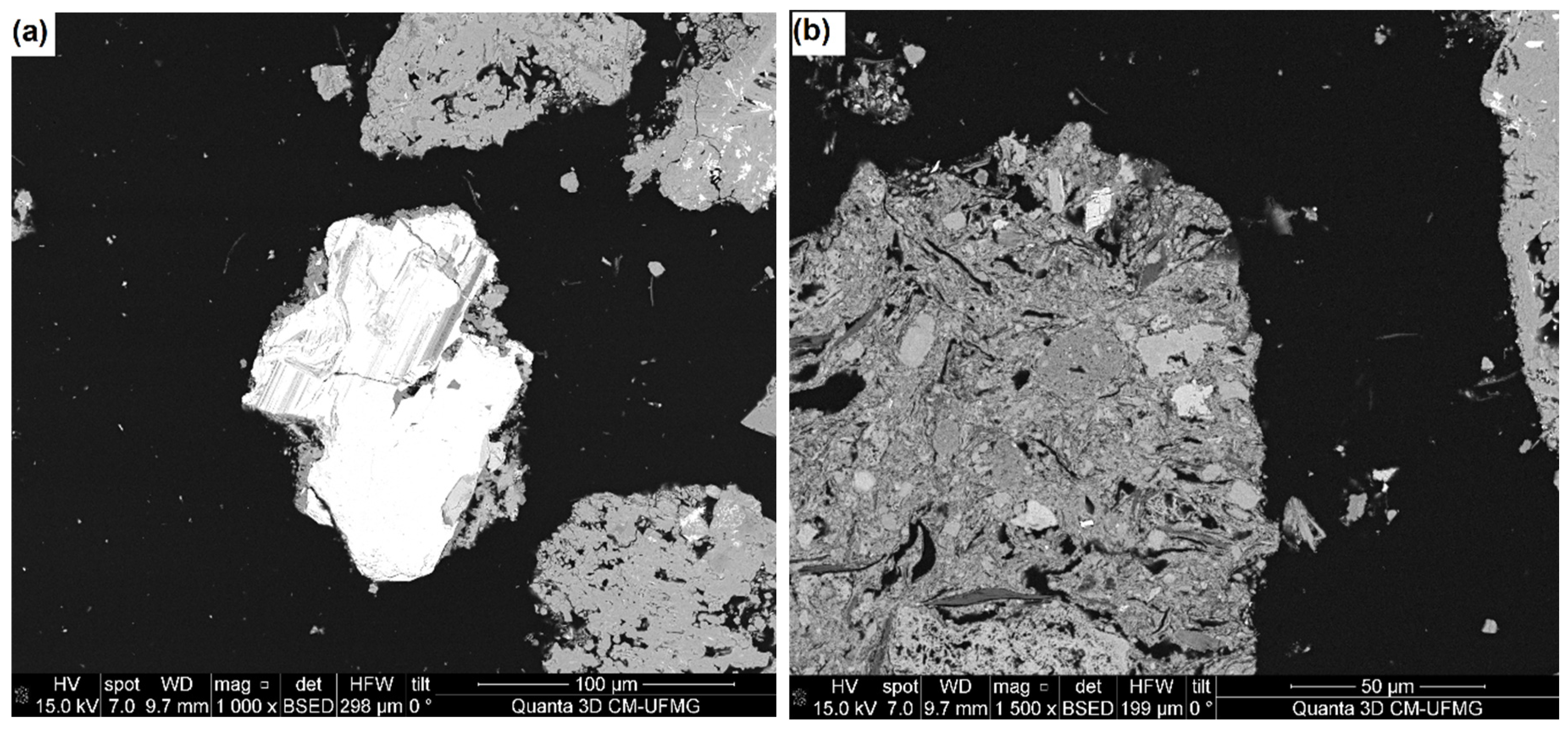
3+) are preferentially accepted. Those impurity elements may also be distributed in several different minerals, with significant intergrowths between them. It is the case of niobium and zirconium that, although they are present in smaller amounts compared to other impurities, they were found as inclusions in TiO
2 grains substituting titanium in the lattices of the anatase crystal (
Figure 2). The presence of these metals prevents obtaining products with a high TiO
2 content (Barnard et al., 2019) making it difficult to achieve acceptable impurity levels.
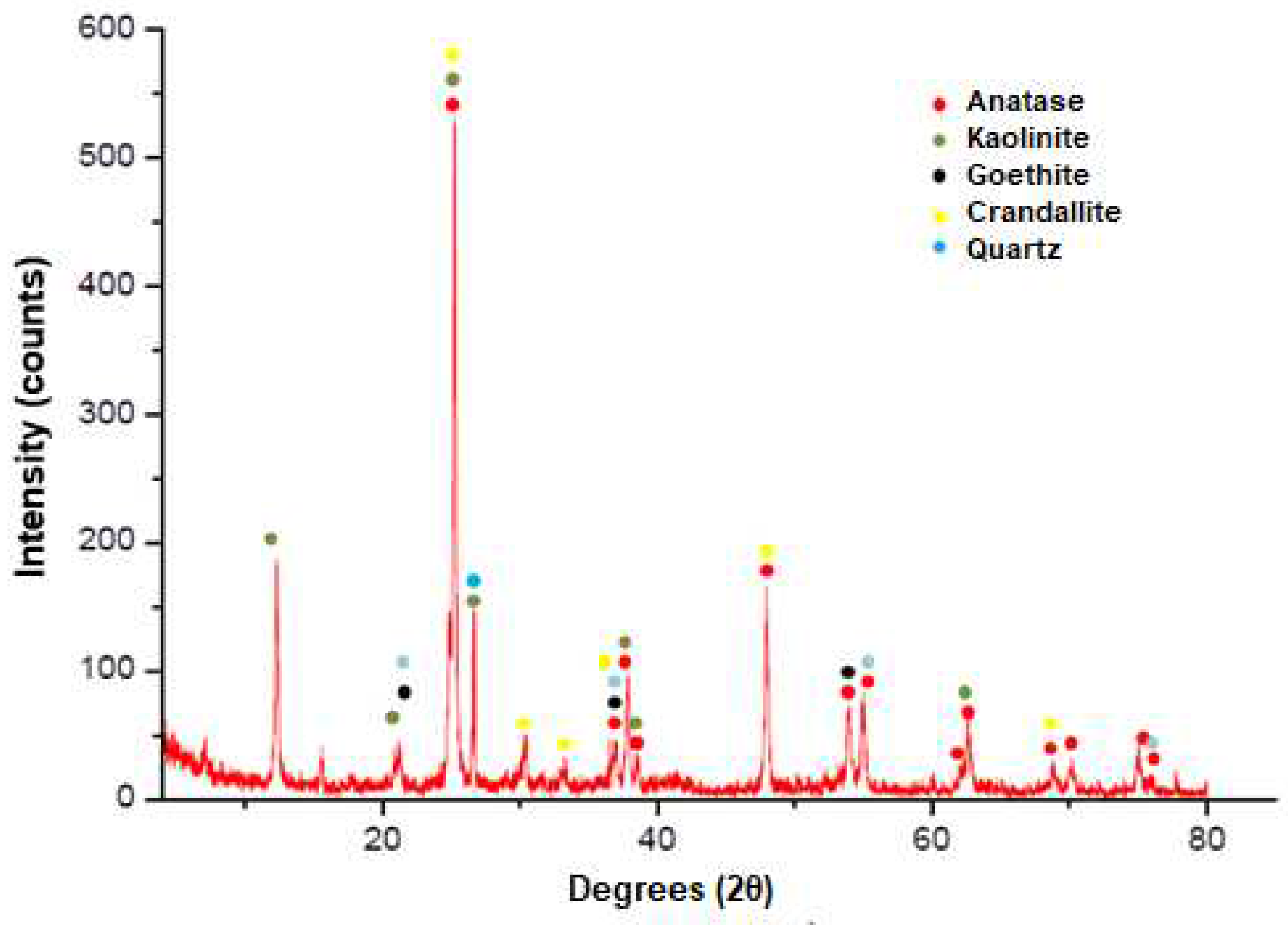
The X-ray diffraction diagram (
Figure 3) indicates the mineralogical phases of the anatase concentrate, making it possible to predict the reactivity of each phase during the acid digestion process. The peak of highest intensity refers to anatase (TiO
2), and goethite (FeOOH), kaolinite (Al
2Si
2O
5(OH)
4), crandallite (CaAl
3(PO
4)
2(OH)
5.H
2O) and quartz (SiO
2) were also identified.
As impurities are present in anatase grains as inclusions, it is difficult to obtain a high purity concentrate using only physical processing as the tightly intergrown crystals of minerals may affect liberation (Lane et al., 2008). SEM analysis (
Figure 1) confirmed these intergrown crystals of minerals, since it is observed that several elements are present in the same grain.
3.2. Sulphuric acid digestion
Generally, sulphuric acid digestion consists of heating the ore along with concentrated sulfuric acid (80-98%) at temperatures of up to 300 °C for periods of 2 to 6 hours. The process is a pre-treatment to produce metal sulphates soluble in water. The dissolution in water produces a liquor containing various metals, not only the one of interest. This technique aims to transform a difficult-to-leach metal oxide to a more readily leachable metal sulphate (Lakshmanan et al., 2014; Freitas and Brocchi, 1995; Haynes, 2014 apud Gontijo, 2020; Demol et al., 2018; Sadri et al., 2017; Wang et al., 2010; Sukla et al., 1986). The sulphuric digestion of titanium ore followed by leaching with water, produces a liquor rich in soluble titanyl sulphate (TiOSO
4), however, it also contains some impurities. The process can be described according to the following reactions.
According to Aguiar (2021), during dissolution, the titanium usually presents an oxidation number of +4 and the metal is commonly linked to oxygen, forming the TiO
2+. The interaction of this species with SO
42- results in TiOSO
4 (Equation (3)), which in turn can lead to the formation of TiO(SO
4)
2 (Equation (4)).
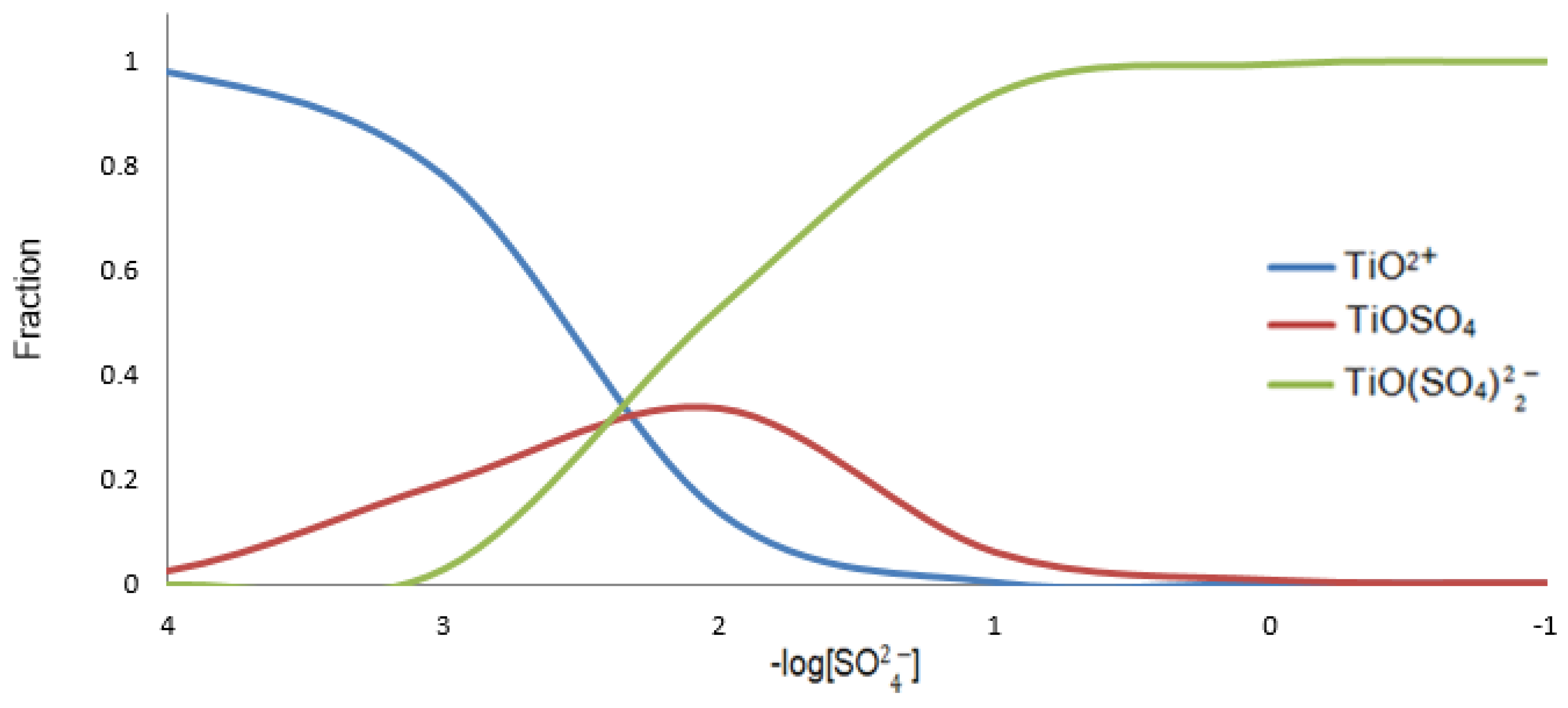
Figure 4 shows the distribution diagram for titanium species in sulfuric medium considering the TiO
2+, o TiOSO
4 e o TiO(SO
4)
2.
The average concentration of
in the liquor after leaching reported in the literature is 2M (Tian, 2018; Calçada, et al., 2009 apud Barnard et al., 2019). In
Figure 4, at 2 M of sulphate the species present in the liquor is 100% TiO(SO
4). However, Jablonski e Tylutka (2016), Zhang et al. (2011) and Freitas and Brocchi (1995), among other authors, mention only the presence of TiOSO
4 in titanium liquors and do not refer to the species TiO(SO
4).
3.2.1. Effect of Temperature on sulphuric digestion of the concentrate
Temperature is an important variable of the sulphuric digestion as it accelerates the decomposition of the minerals and the conversion of the metals into soluble sulphates. Generally, the temperature of sulphuric digestion in the metallurgy of titanium varies from room temperature to 330 °C.
Table 3 shows the TiO
2 content for the residues generated from sulphuric digestion followed by water leaching and the metallurgical recoveries calculated by Equation (1).
Table 3 shows the increase in metallurgical recovery from 67 to 86% with increasing temperature, as the heat favors the process. These results are promising, since the literature, although quite scarce, reports yields of sulfuric digestion of concentrates < 50% (Freitas and Brocchi, 1995). The titanium metallurgical recoveries increase with the temperature in the following order: 190 °C = 200 °C < 210 °C< 220 °C. When the temperatures are 190 °C and 200 °C, a small difference is observed between the metallurgical recoveries and the titanium extraction rates are close. A significant rise in metallurgical recovery was observed for the experiments at 220 °C. Different from the ilmenite ore, the reaction of anatase with sulphuric acid is not exothermic, which is one great disadvantage as it will be necessary to add heat during the entire reaction time to sustain the process (Bekker and Dutton, 2004). The significant levels of TiO
2 in the residues compared to the anatase concentrate indicate that there was a significant mass reduction, which in this case was between 69 and 78% (
Table 3). The increase in the contents of SiO
2, K
2O, SrO e BaO indicate that sulphates that formed are insoluble or the mineral did not react with the acid and remained in the residue.
Regarding other chemical elements, such as Fe and P, their dissolution was considerably high, which will need a further process of purification. The metallurgical recovery of these elements ranges from 76.9 to 88.0%. Related to iron, the metallurgical recoveries are similar for the temperatures investigated, while for phosphorus, the recoveries are relatively higher at lower temperatures. Moreover, Zr as well as Nb were considerably dissolved, mainly at 220 °C, which is in disagreement with the literature (Barnard et al., 2019) that reports poor dissolution of these elements in acid leach.
3.2.2. Effect of the anatase:sulphuric acid ratio in the digestion
The minimum amount of acid necessary to convert the main minerals into soluble sulphate was calculated based on the stoichiometric of the chemical reaction between the anatase concentrate and the sulphuric acid. It took into account the content of the following elements present in the minerals: Ca, Ti, Fe, Al, Mn, V, Nb and Zr. The stoichiometric is equivalent to 1000g of anatase concentrate for 1097g of sulphuric acid, which implies in anatase:sulphuric acid ratio of 1:1.1. Bekker and Dutton (2004) suggested the use of anatase:sulphuric acid ratio equal to 1:1.3 (w/w) when investigating the dissolution of titanium. Therefore, in order to ensure an effective sulphation it was used in the present study an amount of acid, slightly above the stoichiometric, which corresponds to the ratio 1:1.3 (w/w).
Table 4 shows the relationship between anatase anatase:sulphuric acid ratio, the TiO
2 content in the residue and the metallurgical recoveries.
According to
Table 4, by increasing the anatase:sulphuric acid ratio from 1:1.3 to 1:2, the metallurgical recovery increased from 71.6% to 86.0% and the titanium contained in the residue decreased from 51.9% to 35.8%. The greater amount of sulfuric acid for the 1:2 ratio favours the formation of all kinds of soluble sulphates, and consequently, the dissolution of a large part of the contaminants occurred, which ranges from 76.1% to 88.4%. The dissolution of niobium was the most affected by the increase of the concentrate:acid ratio, whose metallurgical recovery increased from 50.4% to 76.4%, which implies a higher concentration of niobium in the liquor. In a study carried out by Freitas and Brocchi (1995) investigating a sample of anatase concentrate, used an anatase:acid ratio of 1:1.55 and the metallurgical recovery was only 48.4%. Bekker and Dutton (2004) in a study of the digestion of steelmaking slags stated that the amount of sulphuric acid is the limiting factor for the occurrence of the reactions.
3.2.3. Effect of time in sulphuric digestion
It is important to ensure that the digestion time is sufficient for the effective sulphation of the titanium. Generally, 2 to 6 hours are sufficient to promote the conversion of the anatase into soluble sulphate (Demol et al., 2018; Freitas and Brocchi, 1995).
Table 5 shows the percentage of each element present in the residues of the digestion and metallurgical recoveries for different times.
According to
Table 5, for 3 hours of sulfuric digestion, the metallurgical recovery of TiO
2 was 82.3%, a value close to that using 4 hours of digestion, 86.0%. Increasing the time of sulfuric digestion to 5h the metallurgical recovery was reduced. Time does not seem to be a decisive variable when it comes to the dissolution of iron, phosphorus and zirconium, since the metallurgical recoveries of these species, although high, 77.4% to 88.8%, remained stable. Niobium, in turn, showed a considerable increase in solubilization for the 4 hours of digestion, reaching 76% of dissolution, while for the other times of digestion the metallurgical recoveries are close to 61%.
3.2.4. Effect of using dilute sulphuric acid as a leaching agent
In addition to leaching using water, another alternative to dissolve the titanium sulphate after the sulphuric acid digestion, is to leach the material with dilute sulphuric acid. Therefore, H2SO4 5% was used as a leaching agent in an attempt to improve the process.
In general, H2SO4 is considered a suitable leaching agent, however, the results indicated that H2SO4 5% has the same efficiency of water. When the water is used the process efficiency is 86.0%, and for H2SO4 the efficiency is 81.2%. Both leaching agents are not selective and the dissolution of other elements such as niobium, zirconium and iron also occurs in similar extension. Therefore, water is a better option to solubilize titanium after the sulphuric digestion.
4. Conclusion
Anatase is a non reactive mineral which makes the acid sulfuric digestion much more difficult than the digestion of other titanium minerals such as ilmenite. This meant that for many years the processes that used anatase as a source of titanium were focused on the removal of the impurities (upgrade) and not on the digestion of the ore. However, by applying the experimental conditions proposed in this investigation—anatase:sulfuric acid ratio equal to 1:2, temperature 220ºC, time of reaction 3 hours, water as leaching agent—the process of sulphation was greatly enhanced and the viability of producing TiO2 from mining residues or ores by sulfuric digestion can turn into reality.
Acknowledgments
The authors would like to thank CNPq, FAPEMIG, FINEP and RenovaMin Project for financial support.
References
- Aguiar, E.M.M.M. Recuperação de titânio e vanádio de fonte secundária. 2021. Dissertation (Master’s in Chemical Engineering)—USP (Escola Politécnica da Universidade de São Paulo), São Paulo, 2021. [CrossRef]
- Barnard, K.R.; Mcdonald, R.G.; Pownceby, M.I.; Sparrow, G.J.; Zhang, W. Processing anatase ores for pigment production. Hydrometallurgy, v. 185, p. 226-237, 2019. [CrossRef]
- Baltar, C.A.M.; Sampaio, J.A.; Andrade, M.C.; Pinto, D.C. Minerais de Titânio. In: LUZ, A.B.; LINS, F.A.F. (org.). Rochas & Minerais Industriais. 2 ed.Rio de Janeiro: CETEM/MCT, v. 1 c.37, p. 841-864, 2009.
- Bekker, J.H.; Dutton, D.F., 2004. Recovery of titanium dioxide from titanium oxide bearing materials like steelmaking slags. PCT Patent US2004/0136899 A1. Date of filing: December 12, 2000; Date of publication: July 15, 2004.
- Chao, N.T.; G.H.; Senkler, G.J. 1991. Method for purifying TiO2 ore. US Patent 5011666; Date of filing: July 28, 1988. Date of publication: April 30, 1991.
- Chao, T., Kremer, W.L., Fonseca Mourão, M.J., Jardim Paixão, J.M., 1993. Process for purifying anatase TiO2 ore. PCT Patent WO93/22465. Date of filing: May 01, 1992; Date of publication: November 11, 1993.
- Chaves, N. 1978. Processo de utilização de concentrado de anatásio como matéria prima para fabricação de pigmento de titânio pelo processo sulfato. PI 7605001. Date of filing: July 30, 1976; Date of publication: February 14, 1978.
- Demol, J.;Ho, E.; Senanayake, G. Sulfuric acid baking and leaching of rare earth elements, thorium and phosphate from a monazite concentrate: Effect of bake temperature from 200 to 800 °C. Hydrometallurgy, v. 179, p. 254-267, 2018. [CrossRef]
- Freitas, L.R.; Brocchi, E.A. Digestão Sulfúrica de Materiais à base de Titânio. In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E METALURGIA EXTRATIVA, 16, 1995, p. 17–30.
- Freitas, L.R.; Gracioso, J.E. Abertura do anatásio por sulfatação. In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E HIDROMETALURGIA, 11, 1985, Natal, v. II, p. 96- 108.
- Freitas, L.R., Horta, R.M., Tude, J.A.L., 2007. Process for enrichment of anatase mechanical concentrates in order to obtain synthetic rutile with low contents of rare earths and radioactive elements. PCT Patent WO2007/048210 A1. Date of filing: May 03, 2007; Date of publication: November 17, 2008.
- Gao, L.; Rao, B.; Daí, H.; Xie, H.; Wang, P.; Ma, F. Kinetics of sulphuric acid leaching of titanium from refractory anatase under atmospheric pressure. Physicochemical Problems of Mineral Processing, v. 55, n. 2, p. 467–478, 2019. [CrossRef]
- Gazquez, M.J.,; Bolívar, J.P.; Garcia-Tenorio, R.; Vaca, F. A review of the production cycle of Titanium Dioxide Pigment. Materials Sciences and Applications, 2014, 5, 441–458. [CrossRef]
- Gontijo, V.L.; Teixeira, L.A.V.; Ciminelli, V.S.T. The reactivity of iron oxides and hydroxide during low-temperature sulphation. Hydrometallurgy, v. 197, 105452, 2020. [CrossRef]
- Jablonski, M.; Tylutka, S. The influence of initial concentration of sulfuric acid on the degree of leaching of the main elements of ilmenite raw materials. Journal of Thermal Analysis and Calorimetry, v. 124, p. 355- 361, 2016. [CrossRef]
- Jha, A.; Tathavadkar, V.D., 2005. Process for the recovery of titanium dioxide from titanium- containing compositions. PCT Patent WO2005/028369 A1. Date of filing: September 18, 2003; Date of publication: May 19, 2005.
- Lakshmanan, V.I.; Bhowmick, A.; Halim, M.A. Titanium Dioxide: Production, Properties and Applications. In: BROWN, J. Titanium dioxide: chemical properties, applications, and environmental effects. New York: Nova Science Publishers, Inc., 2014, cap. 5, 75-130.
- Lane, G.R.; Martin, C.;Pirard, E. Techniques and applications for predictive metallurgy and ore characterization using optical image analysis. Minerals Engineering, v. 21, p. 568-577, 2008. [CrossRef]
- Mineração Vale Do Paranaíba, S.A., 1980. A method for obtaining higher TiO2 grade anatase concentrates from lower TiO2 grade anatase concentrates. GB Patent 1568333. Date of filing: March 09, 1977; Date of publication: May 29, 1980.
- Paixão, J.M.J., Mendonça, P.A.F., 1979. Process for concentration of titanium containing anatase ore. US Patent 4176159. Date of filing: June 19, 1978; Date of publication: November 27, 1979.
- Queiroz Filho, A.A.; Amorim Neto, A.A. 2019. Titânio. In: Agência Nacional de Mineração, Sumário Mineral 2017. Brasília: ANM, vol 37, 167-169. ISSN: 0101-2053. Available online: https://www.gov.br/anm/pt-br/centrais-de-conteudo/publicacoes/serie-estatisticas-e-economia-mineral/sumario-mineral/sumariomineral_2017.
- Smith Jr., E.M., De Castro Sheldon, A., 2007. Titaniferous ore beneficiation. PCT Patent WO2007/046975 A2. Date of filing: October 18, 2005; Date of publication: April 26, 2007.
- Tian, C. Internal influences of hydrolysis conditions on rutile TiO2 pigment. [CrossRef]
- production via short sulfate process. Materials Research Bulletin, v. 103, p. 83-88, 2018.
- Trindade, R.B.E.; Teixeira, L.A. Beneficiamento de concentrado de titânio (anatásio) por lixiviação oxidante de impurezas. In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E HIDROMETALURGIA, 12, 1988, São Paulo, p. 823- 836.
- Sukla, L.B.; Panda, S.C.; Jena, P.K. Recovery of cobalt, nickel and copper from converter slag through roasting with ammonium sulphate and sulphuric acid. Hydrometallurgy, v. 16, p. 153-165, 1986. [CrossRef]
- Xue, T.; Wang, L.; Qi, T.; Chu, J.; Qu, J.; Liu, C. Decomposition kinectics of titanium slag in sodium hydroxide system. Hydrometallurgy, v. 95, p. 22- 27, 2009. [CrossRef]
- Wang, Y.; Li, J.; Wang, L.; Xue, T., Qi, T. Preparation of Rutile Titanium Dioxide White Pigment via Doping and Calcination of Metatitanic Acid Obtained by the NaOH Molten Salt Method. Industrial & Engineering Chemistry Research, v. 49, p. 7693-7696, 2010. [CrossRef]
- ZHANG, W.; ZHU, Z.; CHENG, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy, v. 108, 177-188, 2011. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).