Submitted:
07 December 2023
Posted:
08 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
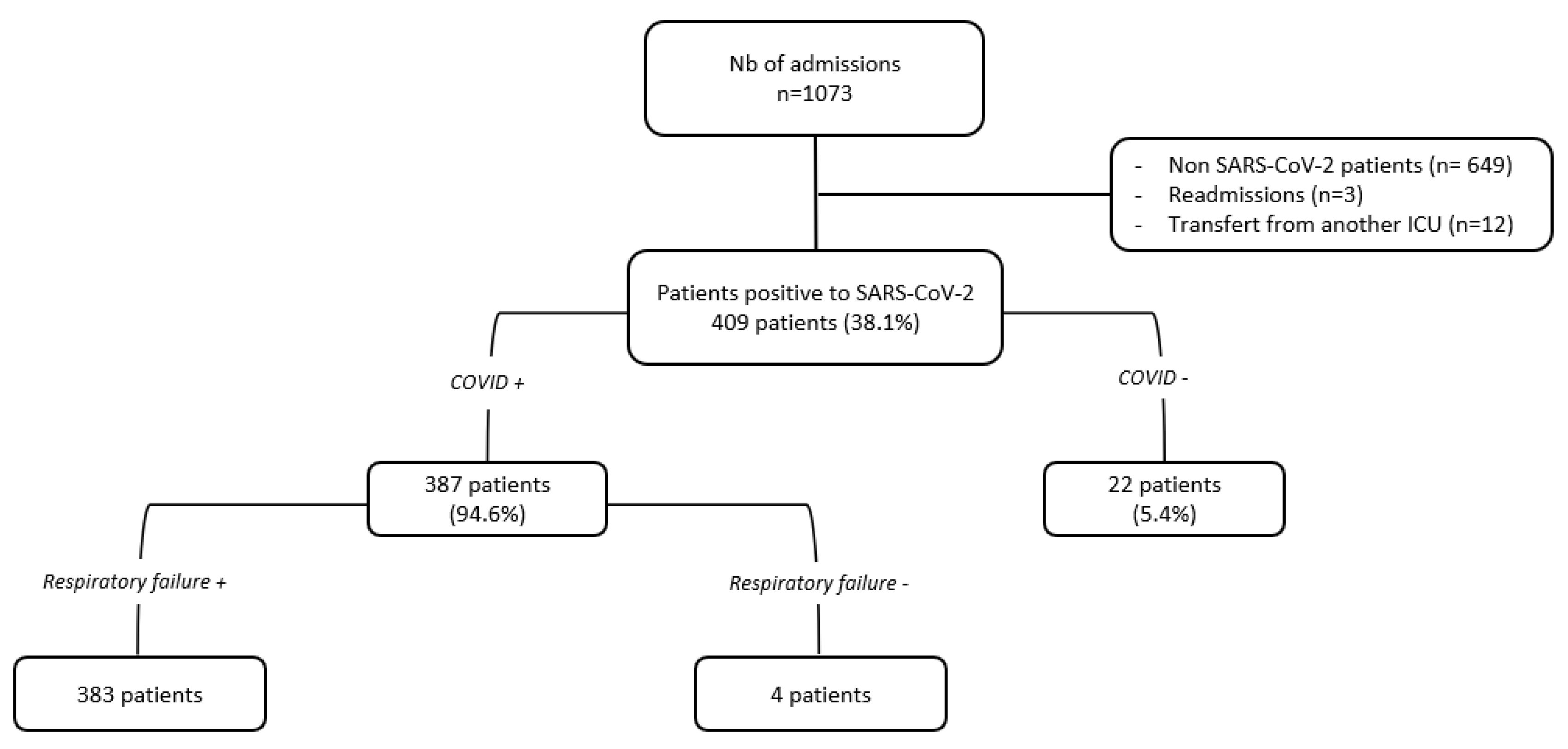
2. Materials and Methods
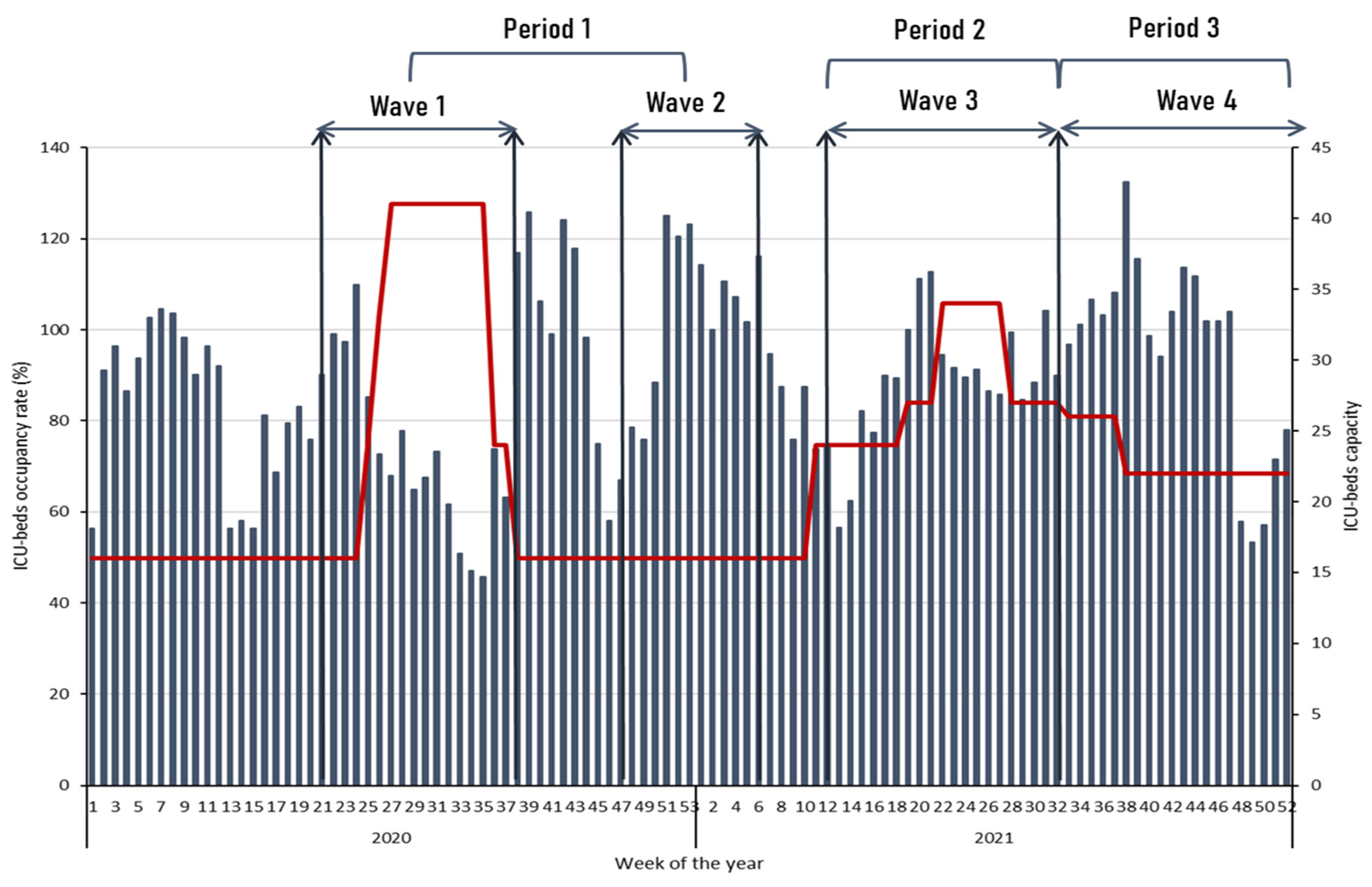
- − Period 1 (Waves 1 and 2): from May to September 2020, and from November 2020 to February 2021 caused by the original SARS-CoV-2 strain.
- − Period 2 (Wave 3): from March to July 2021, caused by gamma (P.1) and alpha (B.1.1.7) variants circulation (88% and 12%, respectively).
- − Period 3 (Wave 4): from August to December 2021, caused by delta (B.1.617) and gamma (P.1) variants circulation (78% and 21%, respectively).
3. Results
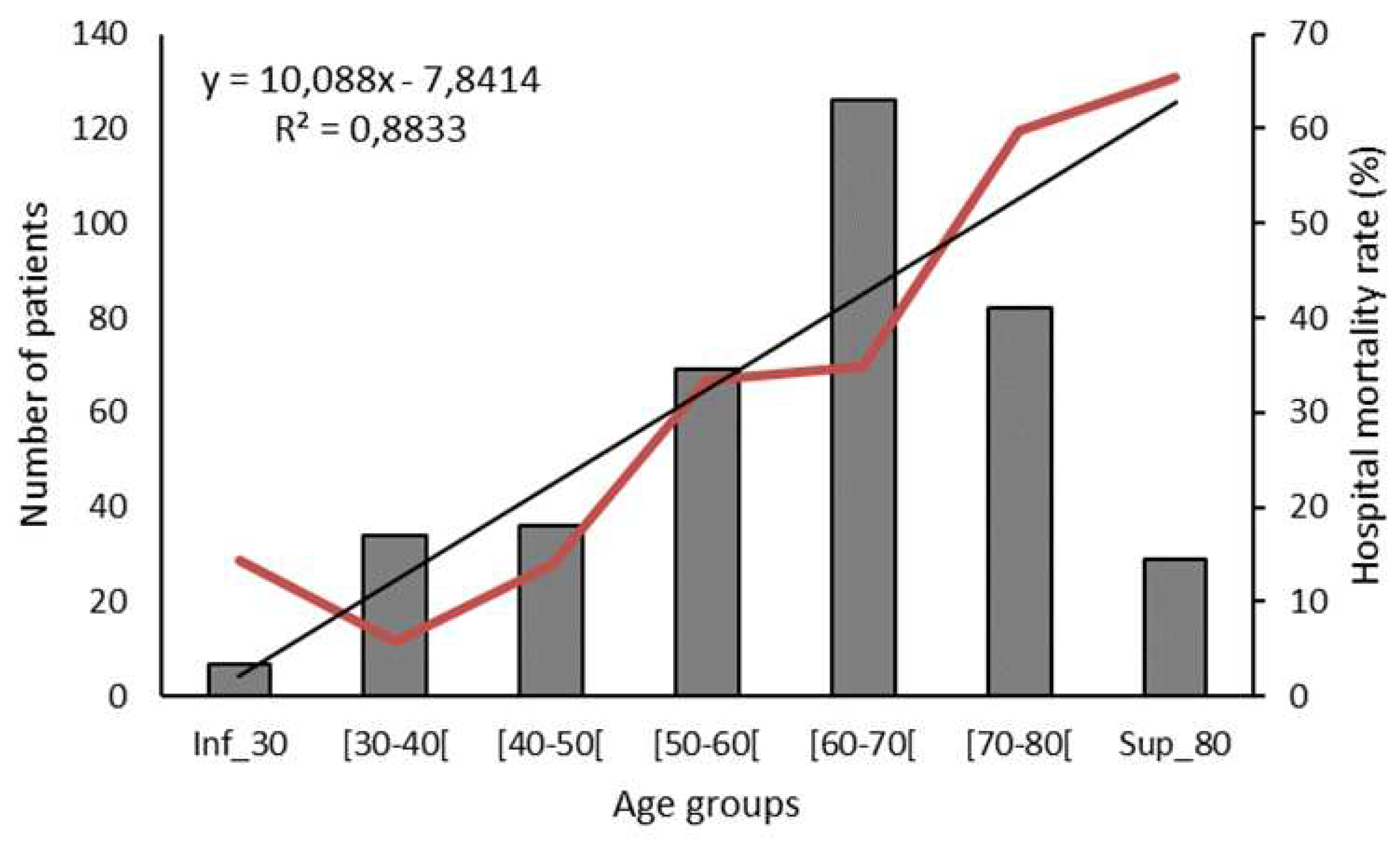
3.1. Demographic characteristics and comorbidities
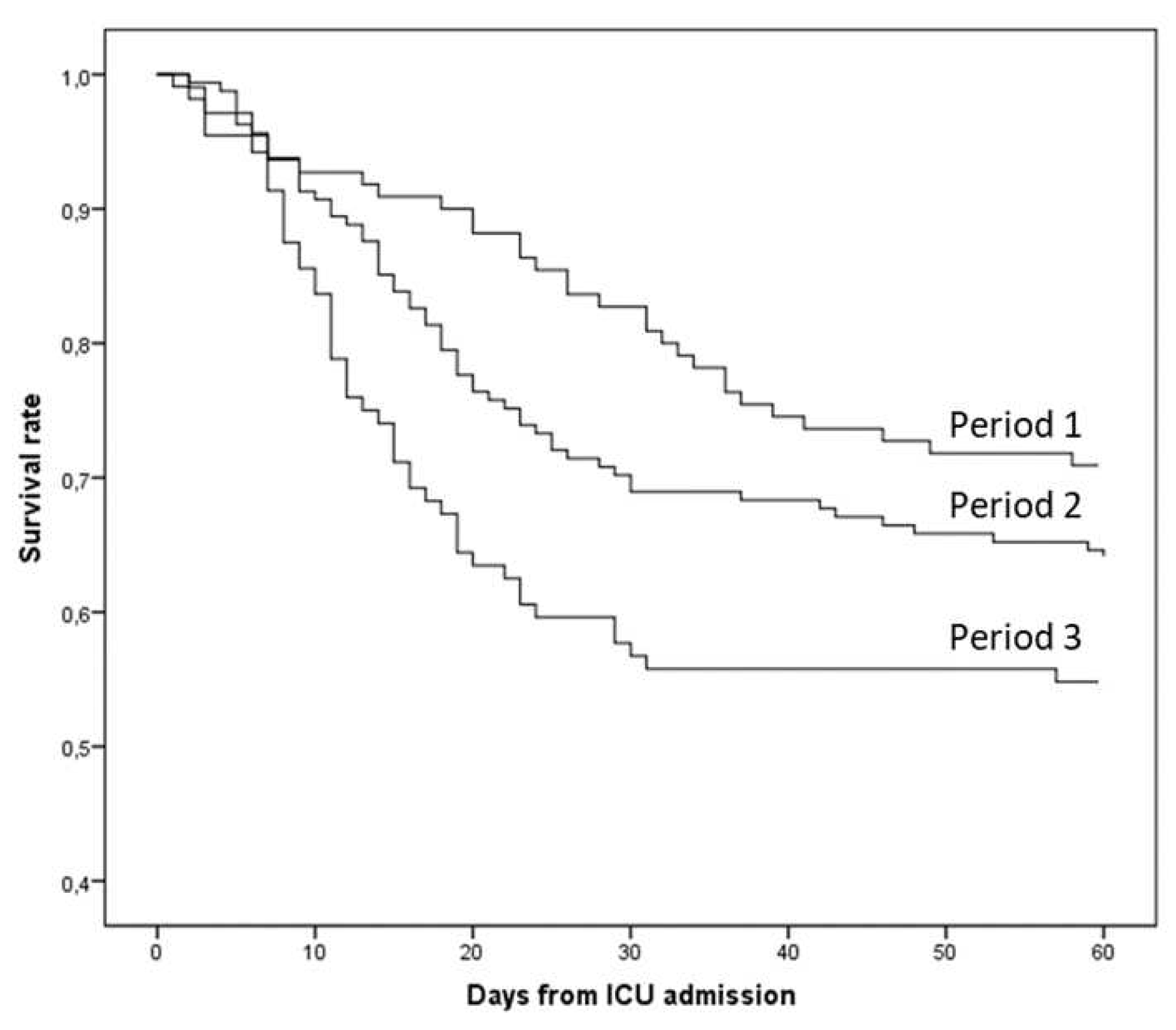
3.2. Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miliu, A.; Lavergne, A.; Succo, T.; Laizé, C.; Andrieu, A.; Enfissi, A.; Enouf, V.; Van der Werf, S.; Blanchet, D.; Demar, M.; et al. Dynamics of SARS-CoV-2 Lineages in French Guiana in 2020–2021: 4 Epidemic Waves with Cross-Influences from Europe and South America. Infect Genet Evol 2022, 105, 105370. [Google Scholar] [CrossRef]
- Chang, R.; Elhusseiny, K.M.; Yeh, Y.-C.; Sun, W.-Z. COVID-19 ICU and Mechanical Ventilation Patient Characteristics and Outcomes-A Systematic Review and Meta-Analysis. PLoS One 2021, 16, e0246318. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F.; et al. Clinical, Laboratory and Imaging Features of COVID-19: A Systematic Review and Meta-Analysis. Travel Med Infect Dis 2020, 34, 101623. [Google Scholar] [CrossRef]
- Zhang, J.J.Y.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin Infect Dis 2020, 71, 2199–2206. [Google Scholar] [CrossRef]
- Jung, C.; Fjølner, J.; Bruno, R.R.; Wernly, B.; Artigas, A.; Bollen Pinto, B.; Schefold, J.C.; Wolff, G.; Kelm, M.; Beil, M.; et al. Differences in Mortality in Critically Ill Elderly Patients during the Second COVID-19 Surge in Europe. Crit Care 2021, 25, 344. [Google Scholar] [CrossRef]
- Jung, C.; Flaatten, H.; Fjølner, J.; Bruno, R.R.; Wernly, B.; Artigas, A.; Bollen Pinto, B.; Schefold, J.C.; Wolff, G.; Kelm, M.; et al. The Impact of Frailty on Survival in Elderly Intensive Care Patients with COVID-19: The COVIP Study. Crit Care 2021, 25, 149. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Yang, L.; Zou, X.; Wang, Y.; Wu, Y.; Zhou, T.; Yuan, Y.; Qi, H.; Fu, S.; et al. Clinical Course and Predictors of 60-Day Mortality in 239 Critically Ill Patients with COVID-19: A Multicenter Retrospective Study from Wuhan, China. Crit Care 2020, 24, 394. [Google Scholar] [CrossRef]
- Bilinski, A.; Emanuel, E.J. COVID-19 and Excess All-Cause Mortality in the US and 18 Comparison Countries. JAMA 2020, 324, 2100–2102. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical Characteristics and Day-90 Outcomes of 4244 Critically Ill Adults with COVID-19: A Prospective Cohort Study. Intensive Care Med 2021, 47, 60–73. [CrossRef]
- Dongelmans, D.A.; Termorshuizen, F.; Brinkman, S.; Bakhshi-Raiez, F.; Arbous, M.S.; de Lange, D.W.; van Bussel, B.C.T.; de Keizer, N.F. ; Dutch COVID-19 Research Consortium Characteristics and Outcome of COVID-19 Patients Admitted to the ICU: A Nationwide Cohort Study on the Comparison between the First and the Consecutive Upsurges of the Second Wave of the COVID-19 Pandemic in the Netherlands. Ann Intensive Care 2022, 12, 5. [Google Scholar] [CrossRef]
- Kumar A K, A.; Mishra, N. Mortality during the COVID-19 Pandemic: The Blind Spots in Statistics. Lancet Infect Dis 2022, 22, 428–429. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Zhang, S.; Huang, N.; Zhao, T.; Lu, Q.-B.; Cui, F. Differences in Incidence and Fatality of COVID-19 by SARS-CoV-2 Omicron Variant versus Delta Variant in Relation to Vaccine Coverage: A World-Wide Review. J Med Virol 2023, 95, e28118. [Google Scholar] [CrossRef]
- Huang, C.; Yang, L.; Pan, J.; Xu, X.; Peng, R. Correlation between Vaccine Coverage and the COVID-19 Pandemic throughout the World: Based on Real-World Data. Journal of Medical Virology 2022, 94, 2181–2187. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 1–12. [Google Scholar] [CrossRef]
- Nacher, M.; Vignier, N.; Rousseau, C.; Adenis, A.; Douine, M.; Basurko, C.; de Toffol, B.; Elenga, N.; Kallel, H.; Pujot, J.; et al. The Burden of COVID-19 in French Guiana: Vaccine-Averted Deaths, Hospitalizations and Costs. Vaccine X 2023, 13, 100271. [Google Scholar] [CrossRef]
- Kallel, H.; Resiere, D.; Houcke, S.; Hommel, D.; Pujo, J.M.; Martino, F.; Carles, M.; Mehdaoui, H. ; Antilles-Guyane Association of Critical Care Medicine Critical Care Medicine in the French Territories in the Americas: Current Situation and Prospects. Rev. Panam. Salud Pública 2021, 45, e46. [Google Scholar] [CrossRef]
- Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. New England Journal of Medicine 2021, 385, 777–789. [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone Treatment for the Acute Respiratory Distress Syndrome: A Multicentre, Randomised Controlled Trial. Lancet Respir Med 2020, 8, 267–276. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021, 384, 693–704. [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Sablerolles, R.S.G.; Lafeber, M.; van Kempen, J.A.L.; van de Loo, B.P.A.; Boersma, E.; Rietdijk, W.J.R.; Polinder-Bos, H.A.; Mooijaart, S.P.; van der Kuy, H.; Versmissen, J.; et al. Association between Clinical Frailty Scale Score and Hospital Mortality in Adult Patients with COVID-19 (COMET): An International, Multicentre, Retrospective, Observational Cohort Study. Lancet Healthy Longev 2021, 2, e163–e170. [Google Scholar] [CrossRef]
- Kucewicz-Czech, E.; Damps, M. Triage during the COVID-19 Pandemic. Anaesthesiol Intensive Ther 2020, 52, 312–315. [Google Scholar] [CrossRef]
- Azoulay, É.; Beloucif, S.; Guidet, B.; Pateron, D.; Vivien, B.; Le Dorze, M. Admission Decisions to Intensive Care Units in the Context of the Major COVID-19 Outbreak: Local Guidance from the COVID-19 Paris-Region Area. Crit Care 2020, 24, 293. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Myatra, S.N.; Lobo, S.M. Surging ICU during COVID-19 Pandemic: An Overview. Curr Opin Crit Care 2022, 28, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Guidet, B.; Jung, C.; Flaatten, H.; Fjølner, J.; Artigas, A.; Pinto, B.B.; Schefold, J.C.; Beil, M.; Sigal, S.; van Heerden, P.V.; et al. Increased 30-Day Mortality in Very Old ICU Patients with COVID-19 Compared to Patients with Respiratory Failure without COVID-19. Intensive Care Med 2022, 48, 435–447. [Google Scholar] [CrossRef]
- Subramaniam, A.; Shekar, K.; Afroz, A.; Ashwin, S.; Billah, B.; Brown, H.; Kundi, H.; Lim, Z.J.; Ponnapa Reddy, M.; Curtis, J.R. Frailty and Mortality Associations in Patients with COVID-19: A Systematic Review and Meta-Analysis. Intern Med J 2022. [Google Scholar] [CrossRef]
- Dres, M.; Hajage, D.; Lebbah, S.; Kimmoun, A.; Pham, T.; Béduneau, G.; Combes, A.; Mercat, A.; Guidet, B.; Demoule, A.; et al. Characteristics, Management, and Prognosis of Elderly Patients with COVID-19 Admitted in the ICU during the First Wave: Insights from the COVID-ICU Study : Prognosis of COVID-19 Elderly Critically Ill Patients in the ICU. Ann Intensive Care 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, R.; Urgelés, S.; Rodríguez, A.; Bodí, M.; Martín-Loeches, I.; Solé-Violán, J.; Díaz, E.; Gómez, J.; Trefler, S.; Vallverdú, M.; et al. Mortality Comparison between the First and Second/Third Waves among 3,795 Critical COVID-19 Patients with Pneumonia Admitted to the ICU: A Multicentre Retrospective Cohort Study. Lancet Reg Health Eur 2021, 11, 100243. [Google Scholar] [CrossRef]
- Quah, P.; Li, A.; Phua, J. Mortality Rates of Patients with COVID-19 in the Intensive Care Unit: A Systematic Review of the Emerging Literature. Crit Care 2020, 24, 285. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.B.; Mulholland, R.H.; Lone, N.I.; Cheyne, C.P.; De Angelis, D.; Diaz-Ordaz, K.; Donegan, C.; Drake, T.M.; Dunning, J.; Funk, S.; et al. Changes in In-Hospital Mortality in the First Wave of COVID-19: A Multicentre Prospective Observational Cohort Study Using the WHO Clinical Characterisation Protocol UK. Lancet Respir Med 2021, 9, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Emmons-Bell, S.; Alger, H.M.; Bradley, S.M.; Das, S.R.; de Lemos, J.A.; Gakidou, E.; Elkind, M.S.V.; Hay, S.; Hall, J.L.; et al. Trends in Patient Characteristics and COVID-19 In-Hospital Mortality in the United States During the COVID-19 Pandemic. JAMA Netw Open 2021, 4, e218828. [Google Scholar] [CrossRef] [PubMed]
- Doidge, J.C.; Gould, D.W.; Ferrando-Vivas, P.; Mouncey, P.R.; Thomas, K.; Shankar-Hari, M.; Harrison, D.A.; Rowan, K.M. Trends in Intensive Care for Patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med 2021, 203, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.F.F.; Roazzi, A.; da Penha Sobral, A.I.G.; de Oliveira, B.R.B.; Duarte, G.B.; da Silva, J.F.; Nogueira, R.M.T.B.L. A Retrospective Cohort Study of 238,000 COVID-19 Hospitalizations and Deaths in Brazil. Sci Rep 2022, 12, 3629. [Google Scholar] [CrossRef] [PubMed]
- Serafim, R.B.; Póvoa, P.; Souza-Dantas, V.; Kalil, A.C.; Salluh, J.I.F. Clinical Course and Outcomes of Critically Ill Patients with COVID-19 Infection: A Systematic Review. Clin Microbiol Infect 2021, 27, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Garcia, P.D.; Mas, A.; González-Isern, C.; Ferrer, R.; Máñez, R.; Masclans, J.-R.; Sandoval, E.; Vera, P.; Trenado, J.; Fernández, R.; et al. Non-Invasive Oxygenation Support in Acutely Hypoxemic COVID-19 Patients Admitted to the ICU: A Multicenter Observational Retrospective Study. Critical Care 2022, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Camous, L.; Pommier, J.-D.; Martino, F.; Tressieres, B.; Demoule, A.; Valette, M. Very Late Intubation in COVID-19 Patients: A Forgotten Prognosis Factor? Critical Care 2022, 26, 89. [Google Scholar] [CrossRef]
- Manrique, S.; Claverias, L.; Magret, M.; Masclans, J.R.; Bodi, M.; Trefler, S.; Canadell, L.; Díaz, E.; Sole-Violan, J.; Bisbal-Andrés, E.; et al. Timing of Intubation and ICU Mortality in COVID-19 Patients: A Retrospective Analysis of 4198 Critically Ill Patients during the First and Second Waves. BMC Anesthesiology 2023, 23, 140. [Google Scholar] [CrossRef]
- Naouri, D.; Vuagnat, A.; Beduneau, G.; Dres, M.; Pham, T.; Mercat, A.; Combes, A.; Demoule, A.; Kimmoun, A.; Schmidt, M.; et al. Trends in Clinical Characteristics and Outcomes of All Critically Ill COVID-19 Adult Patients Hospitalized in France between March 2020 and June 2021: A National Database Study. Annals of Intensive Care 2023, 13, 2. [Google Scholar] [CrossRef]
- Rossman, H.; Meir, T.; Somer, J.; Shilo, S.; Gutman, R.; Ben Arie, A.; Segal, E.; Shalit, U.; Gorfine, M. Hospital Load and Increased COVID-19 Related Mortality in Israel. Nat Commun 2021, 12, 1904. [Google Scholar] [CrossRef]
- Bravata, D.M.; Perkins, A.J.; Myers, L.J.; Arling, G.; Zhang, Y.; Zillich, A.J.; Reese, L.; Dysangco, A.; Agarwal, R.; Myers, J.; et al. Association of Intensive Care Unit Patient Load and Demand With Mortality Rates in US Department of Veterans Affairs Hospitals During the COVID-19 Pandemic. JAMA Netw Open 2021, 4, e2034266. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.G.; Breckons, M.; Lee, R.P.; Dotchin, C.; Walker, R. Rationing Care by Frailty during the COVID-19 Pandemic. Age Ageing 2021, 50, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D. da S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and Epidemiology of the P.1 SARS-CoV-2 Lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clinics and Practice 2021, 11, 778–784. [Google Scholar] [CrossRef]
- Fisman, D.N.; Tuite, A.R. Evaluation of the Relative Virulence of Novel SARS-CoV-2 Variants: A Retrospective Cohort Study in Ontario, Canada. CMAJ 2021, 193, E1619–E1625. [Google Scholar] [CrossRef]
- Dyer, O. Covid-19: Unvaccinated Face 11 Times Risk of Death from Delta Variant, CDC Data Show. BMJ 2021, 374, n2282. [Google Scholar] [CrossRef]
- Tabatabai, M.; Juarez, P.D.; Matthews-Juarez, P.; Wilus, D.M.; Ramesh, A.; Alcendor, D.J.; Tabatabai, N.; Singh, K.P. An Analysis of COVID-19 Mortality During the Dominancy of Alpha, Delta, and Omicron in the USA. J Prim Care Community Health 2023, 14, 21501319231170164. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, H.; He, D. Differences in Case-Fatality-Rate of Emerging SARS-CoV-2 Variants. Public Health Pract (Oxf) 2023, 5, 100350. [Google Scholar] [CrossRef] [PubMed]
| Maximal respiratory support | Overall (n = 383) |
Period 1 (n = 110) |
Period 2 (n = 161) |
p * | Period 3 (n = 104) |
p $ |
|---|---|---|---|---|---|---|
| High-flow nasal cannula oxygen | n = 138 (36.0%) | n = 39 (35.5%) | n = 61 (37.9%) | 0.683 | n = 34 (32.7%) | 0.670 |
| Hospital mortality | 18 (13%) | 2 (5.1%) | 10 (16.4%) | 0.091 | 5 (14.7%) | 0.166 |
| ICU LOS (days) | 7 (5–9) | 8 (6–12) | 6 (4–8) | 0.004 | 6 (5–10) | 0.095 |
| Hospital LOS (days) | 16 (12–20) | 19 (16–23) | 14 (11–17) | 0.000 | 15 (11–18) | 0.011 |
| Non-invasive mechanical ventilation | n = 60 (15.7%) | n = 4 (3.6%) | n = 30 (18.6%) | <0.001 | n = 24 (23.1%) | <0.001 |
| Hospital mortality | 16 (26.7%) | 2 (50%) | 9 (30%) | 0.580 | 5 (20.8%) | 0.212 |
| ICU LOS (days) | 10 (7–14) | 13 (7–29) | 9 (5–10) | 0.180 | 11 (7–15) | 0.776 |
| Hospital LOS (days) | 17 (12–26) | 16 (9–48) | 16 (12–25) | 0.979 | 17 (11–23) | 0.975 |
| Invasive mechanical ventilation | n = 185 (48.3%) | n = 67 (60.9%) | n = 70 (43.5%) | 0.005 | n = 46 (44.2%) | 0.015 |
| Hospital mortality | 109 (58.9%) | 30 (44.8%) | 40 (57.1%) | 0.148 | 39 (84.8%) | <0.001 |
| ICU LOS (days) | 18 (10–28) | 21 (9–33) | 19 (11–28) | 0.411 | 13 (7–25) | 0.088 |
| Hospital LOS (days) | 23 (13–39) | 26 (13–42) | 25 (15–41) | 0.973 | 16 (9–29) | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





