1. Introduction
The genus
Babesia are members of the Babesiidae family order Piroplasmida [
1,
2]. Members of the Piroplasmida are common infectious agents for both humans, and domestic and wild animals. The species implicated in human disease can be classified into distinct clades [
1,
3,
4,
5].
B. microti, the primary cause of human babesiosis in the US is a member of the Babesia microti-like clade that is also sometimes referred to as
“small” Babesia. Small
Babesia are primariliy transmitted by
Ixodes scapularis ticks, the same tick species that transmits
Borrelia burgderferi, the causative agent of Lyme disease.
B. duncani and
B. duncani-type organisms are members of the
Babesia sensu lato Western clade, an emerging disease of humans particularly along the US west coast. Another clad are phlogenetically related to large
Babesia such as
B. divergens in Western Europe,
B. bovis,
B. bigemina and
B. canis. The immune response, virulence characteristics and pathophysiology of the diverse clades and species remain understudied and their similarity and differences largely unknown. There is evidence that severity of infection, immunity and drug efficacy may differ depending on the clade of
Babesia as well as the species. [
2,
6,
7,
8]
Babesia microti (
B. microti) is an Apicomplexan protozoan parasite endemic to the Northeastern and upper Mid-Western United States [
8,
9,
10].
B. microti is the primary cause of human babesiosis in the US. It is transmitted to humans through the bite of infected
Ixodes scapularis ticks, transfusion of contaminated blood and congenital infection. Because
B. microti is transmitted by the same species of tick that transmits
Borrelia burgdorferi, co-infections of
Borrelia burgdorferi and
B. microti from a single tick bite are becoming increasingly common [
11,
12,
13].
B. microti invades red blood cells and forms a transient parasitophorous vacuole (PV) from which it immediately escapes to reside and replicate by binary fission in the erythrocyte cytosol. Unlike
Plasmodium species,
B. microti is not known to have an extra-erythrocytic stage and appears to remain in the circulation for the duration of infection. The parasite can survive blood banking procedures resulting in its becoming an important pathogen in transfusion medicine in addition to tick-transmitted infection [
14,
15,
16]. Disease severity due to
B. microti ranges from severe infections requiring hospitalization, that can result in death, to asymptomatic [
8,
17,
18]. Known factors that increase susceptibility to severe disease are an age greater than 50, general immune suppression, and lack of a spleen [
8,
19]. Individuals treated with Rituximab, a drug that depletes B cells, are often highly susceptible to babesiosis, may fail to clear parasitemia and often result in treatment failures including drug resistant parasites.
B. microti parasitemia can persist for years even with treatment suggesting an important role for B cells in control of
B. microti infection [
19,
20,
21,
22]. In summary, the immunopathology of babesiosis remains poorly defined, and as such, risk factors and other determinants for severe disease remain unknown.
In a prior study we evaluated
B. microti infection in C3H, BALB/C and C57BL6 mice to examine how the differences in genetic background of the mice impacted peak parasitemia, and resolution of infection as defined by clearance of parasitemia [
23]. Peak parsitemia was lowest in C57BL6 mice and highest in C3H mice (range of 35-60% parasitemia depending on mouse strain) and occurred 7-10 days post-infection. However, all three mouse strains rapidly resolved paraistemia over a similar time course resulting in parasite clearance to undetectable levels by day 14-15 post-infection in immunologically intact animals. We used targetted gene deletion mouse strains in the C57BL6 mouse background to examine the importance of CD4 T cells, B. cells and other cytokines in the regulation of peak parasitemia and parasite clearance (resolution of infection). We demonstrated that CD4 T cells are important for resolution of infection as CD4-/- mice did not clear the parasite and developed a persistent parasitemia. In contrast, the absence of CD4 T cells did not impact levels of peak parasitemia. Mice deficient in the IFN-γ receptor and mice that lack mature B cells exhibited extended parasitemia, but ultimately did resolve infection. Mice deficient in MyD88, the adaptor protein for many TLRs, IL-18 and IL-1, developed peak parasitemia levels and cleared parasites similar to wild type (WT) mice suggesting that toll receptor signaling, IL-18 and IL-1 do not play a essential role in the primary response to infection with
B. microti. We also examined whether phagocyte production of nitric oxide contributed to control of
B. microti using inducible nitric oxide synthase (iNOS) mice and demonstrated iNOS was not critical for regulating parasitemia levels or resolving infection.
Limited studies in humans suggest that resolution of human
B. microti infection may not inevitably result in protection against re-infection [
24]. In the present study, we evaluated if WT mice developed protective immunity to
B. microti following the initial clearance of the parasite and whether CD4 T cells, B cells, IFNγ receptors, iNOS and MyD88 contributed to protection against rechallenge. In our reinfection model, we reinfected WT mice or specific gene-deficient mice 30-36 days after an initial primary challenge with
B. microti, and evaluated the development of parasitemia. We hypothesized that mice that developed an adaptive immune response that controlled or eliminated infection with
B. microti would not be susceptible to reinfection with the same strain of
B microti. We also tested the hypothesis that elements of the adaptive immune response including CD4 T cells, B cells and IFN-γ would have a greater role in protection against rechallenge than observed in primary infection when the innate immune response is also predicted to be an important factor in control of parsitemia [
25].
2. Materials and Methods
Mice
The following mouse strains purchased from Jackson Laboratories (Bar Harbor, ME) were used for experiments: C57BL6J, B6.129S2-Cd4tm1Mak/J, B6.129P2 (SJL)-Myd88tm.1.1Defr/J, B6.129S7-Ifngr1tm1Agt/J, B6.129P2-Nos2tmaLau/J, B6.129S2-Ighmtm1Cgn/J, All mice were females and purchased between 6–10 wks of age and all targeted gene deleted mice (KO mice) were in a C57BL/6J background. All animal studies were approved by the New York Medical College Institutional Animal Care and Use Committee (IACUC). The studies were approved in the NYMC IACUC protocol number 13958.
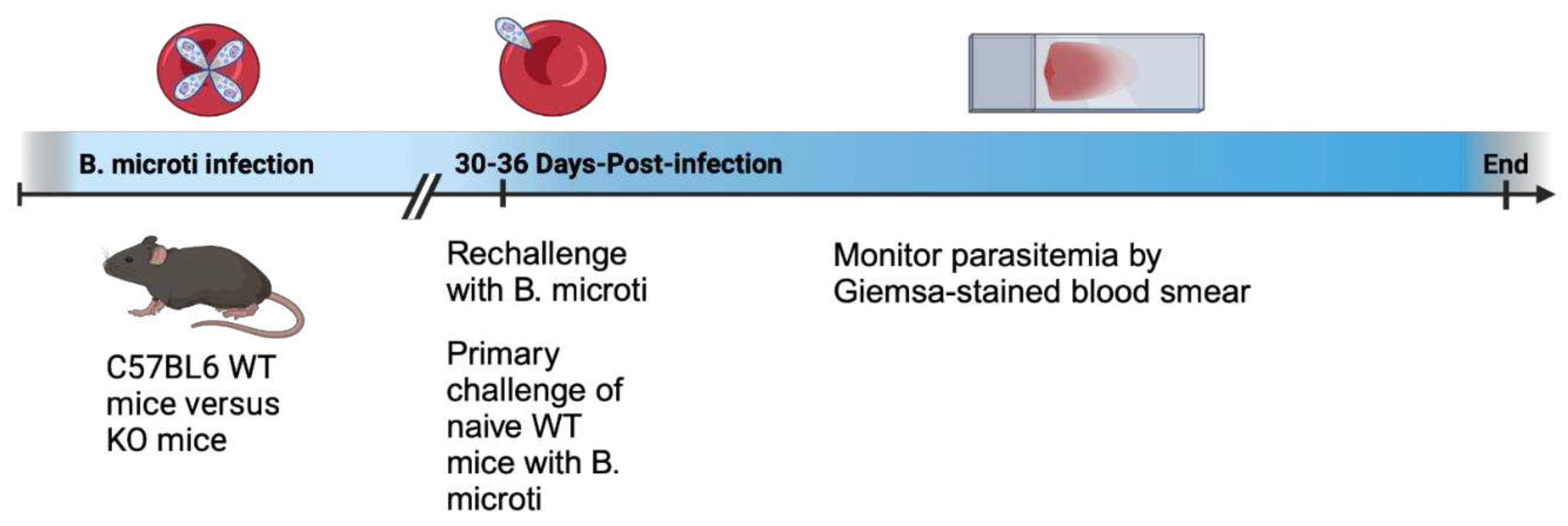
Parasite and infection model
The
B. microti Gray strain, originally isolated from a human infection on Nantucket Island, Massachusetts in 1970, was originally obtained from the American Type Culture Collection (ATCC 30221) and was used for all of the studies [
26]. Parasites were maintained by serial passage in mice after being rapidly thawed from storage in liquid nitrogen and injected intraperitoneally (IP) into two mice to allow parasites to recover and replicate to sufficient numbers for serial passage in mice.
B. microti currently can not be propagated long-term in vitro in red blood cell culture but must be propagated in mice or Golden Hamsters. Parasites were passaged twice through mice before being used for experimentation. For experiments, 4-5 mice per group were infected by IP injection of 100 μl of blood pooled blood collected from infected mice 5 days post-infection when parasitemia was roughly 30%. Blood was pooled prior to infection to ensure all mice within an experiment received the same dose of parasites in the same inoculate at the same time. Mice were infected with approximately 1 x 10
7 infected red blood cells. During the primary infection, blood was collected by tail snip to create a thin film blood smear on a microscope slide daily or every other day for the first 14 days post-infection then approximately every 5 days for 25-36 days post-infection. For reinfection, WT and targeted gene deletion mice were rechallenged IP with
B. microti using a similar protocol as for primary infection except blood was collected only for the first 3-10 days to monitor protection against reinfection. Naïve WT mice were challenged at the same time as mice were reinfected with the same parasite inoculate to compare parasitemia during rechallenge compared to primary infection in each experiment. Rechallenge with
B. microti was performed 30-36 days PI and the days post primary challenge with
B. microti are listed in the figure legend for each experiment.
Hematology
Blood smears on glass slides were fixed for 2 minutes in ice cold methanol and stained with Giemsa Stain (Richard-Allan Scientific, Kalamazoo, MI), as per the manufacturer’s instructions. Parasitemia was evaluated by at least 3 counts of 100 red blood cells per slide per mouse using a 100X oil objective and bright field microscopy using a Nikon Eclipse TiE inverted microscope.
Statistics
The mean, standard deviation and standard error from mouse groups was determined. Analysis of Variance (ANOVA) was used to compare parasitemia between multiple groups using GraphPad Prism. An unpaired Student’s t-test was used to compare single time points from two groups of mice.
3. Results
In a previous study we examined primary infection with
B. microti including the contribution of different cells and cytokines to regulation of peak parastemia and resolution of infection using mice with targeted gene deletions. In the current study we extend these findings by evaluating whether infected mice that clear or suppress
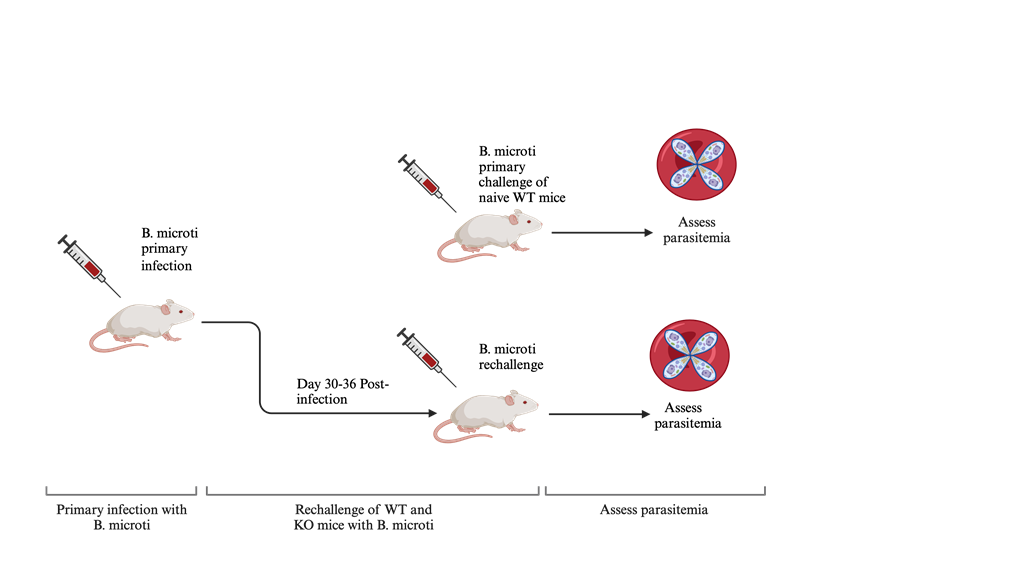
B. microti infection are protected from rechallenge with the parasite. A diagram of the experimental mouse model is shown in
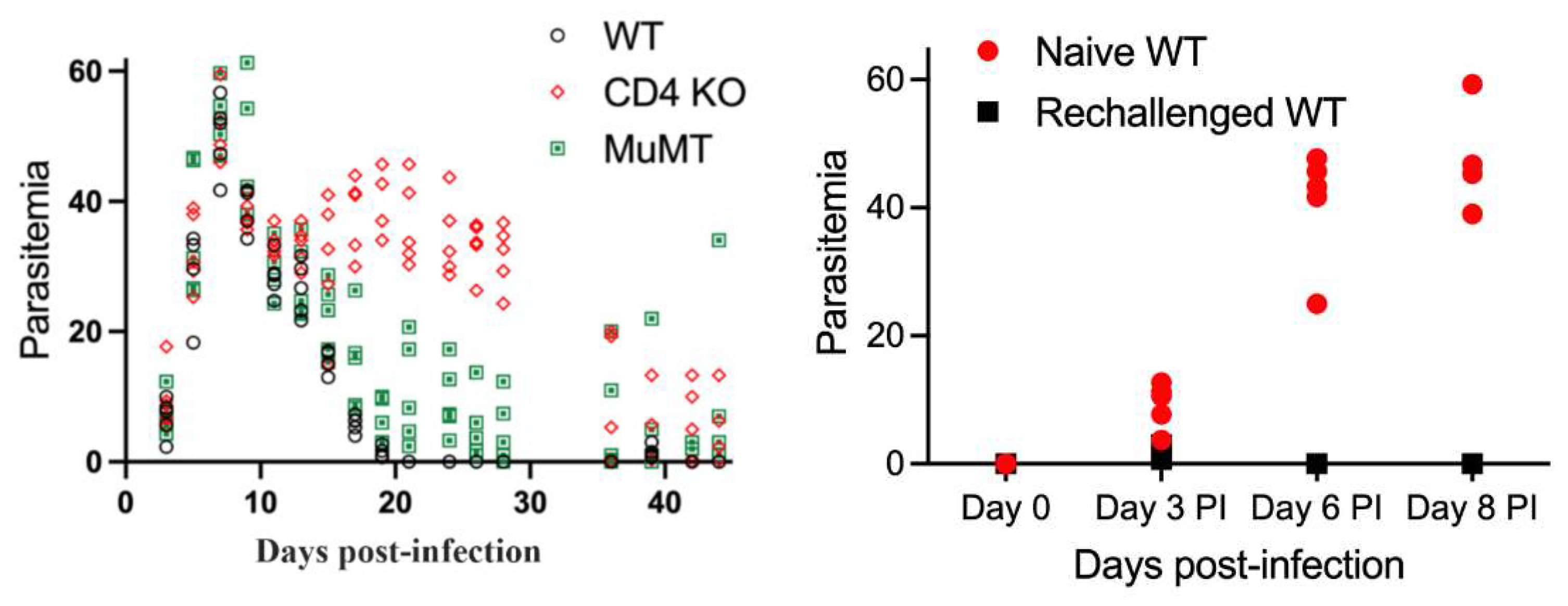
Figure 1. In
Figure 2 we show parasitemia during primary infection of naïve WT mice longitudinally for 36 days post-infection in comparision to infected CD4-/- mice and MuMT mice deficient in mature B cells. WT, CD4-/- and MuMT mice exhibited similar parasitema levels that peaked at approxiamtely 45% parasitemia on day 8 post-infection. Parasitemia levels decreased rapidly thereafter and were no longer detectable in WT mice in blood smears by day 20 post-infection. In contrast, both CD4-/- and MuMT mice were impaired in their abilty to resolve parasitemia and maintained parasitemia levels above 20% for CD4-/- mice and 1-10% for MuMT mice out to day 36 post-infection (pi). While CD4-/- mice did not clear infection, there was still a significant reduction of parasitemia observed during the primary response in naïve animals, indicating that CD4-/- mice were able to partially control the infection over time. MuMT mice were more effective than CD4-/- mice in their ability to resolve parasitemia but less effective than WT mice. Thus it is clear that CD4 T cells contribute to resolution of
B. microti infection in the C57BL6 mouse background while the contribution of B cells to resolution of infection was less pronounced but still evident.
WT mice that resolved a primary infection with
B. microti infection were rechallenged with
B. microti 36 days post initiation of primary infection to evalaute whether mice were protected against rechallenge. Parasitemia levels in response to rechallenge of WT mice was compared to primary challenge of naïve WT mice to assess the degree of protection conferred by previous infection. WT mice that had previously cleared a primary infection with
B. microti were resistant to rechallenge (
Figure 2). Although parasites could be detected in blood smears on day 3 post-rechallenge, parasitemia remained below 1% and parasites were no longer detectable by day 6 or 8 post-rechallenge. These results indicate that mice that previous cleared a
B. microti infection developed an effective adaptive immune response that protected against rechallenge with the same strain of parasite.
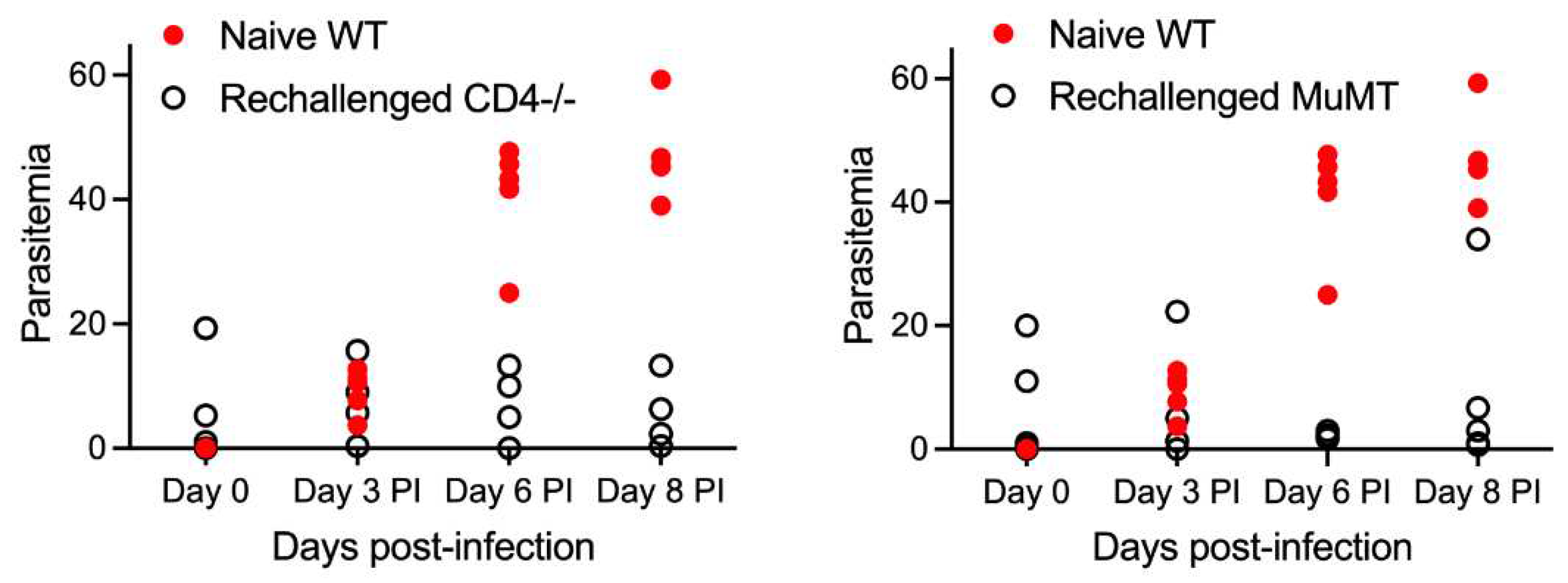
Having established that primary challenge of WT mice resulted in protection of mice from rechallenge with
B. microti, we examined the importance of CD4 and B cells to protection against rechallenge using CD4-/- mice and MuMT mice respectively. Both CD4-/- and MuMT maintained a low level of parasitemia after re-challenge simialr to the residual parasitemia 36 days pi after primary challenge. Upon rechallenge with
B. microti, CD4-/- mice demonstrated significantly reduced parasitemia (less than 20%) compared to naïve WT mice (43-60%), indicating that some level of protection developed in these animals. MuMT mice developed a low level of parasitemia (<5%) with the exception of one rechallenged MuMT mouse that reached parastemia levels similar to naïve WT mice (
Figure 2). In summary our results show that both CD4 T cells and B cells contribute to protection of mice against rechallenge. However, neither CD4 T cells or B cells appear to be absolutely required for protection against rechallenge since both CD4-/- and MuMT mice were generally far more resistant to reinfection compared to naïve WT mice. This suggests that antibody does play a role in limiting reinfection with the parasite, and could be playing a role in clearance and moderate infection control in CD4-/- mice.
One frequent role of CD4 T cells during infection is the production of IFN-γ that often serves to activate macrophages as well as interferon-stimulated genes (ISGs) many of which have antimicrobial functions in a wide variety of diverse cell types. In a previous study we showed that IFN-γ receptor deficient (IFN-γR) mice infected with
B. microti developed peak parasitemia levels similar to WT mice but had only a moderate delay in resolving parasitemia [
23]. This suggested that the protective function of CD4 T cells during primary
B. microti infection was in large part independent of IFN-γ. In the current study we tested the hypothesis that IFNγ, was more important for resistance of mice to reinfection with
B. microti compared to primary
B. microti infection when innate immunity was also likely to be important for regulating parasitemia levels. As shown in
Figure 3 and
Figure 4, IFN-γR-/- had a few days delay in parasite clarance compared to WT mice during primary
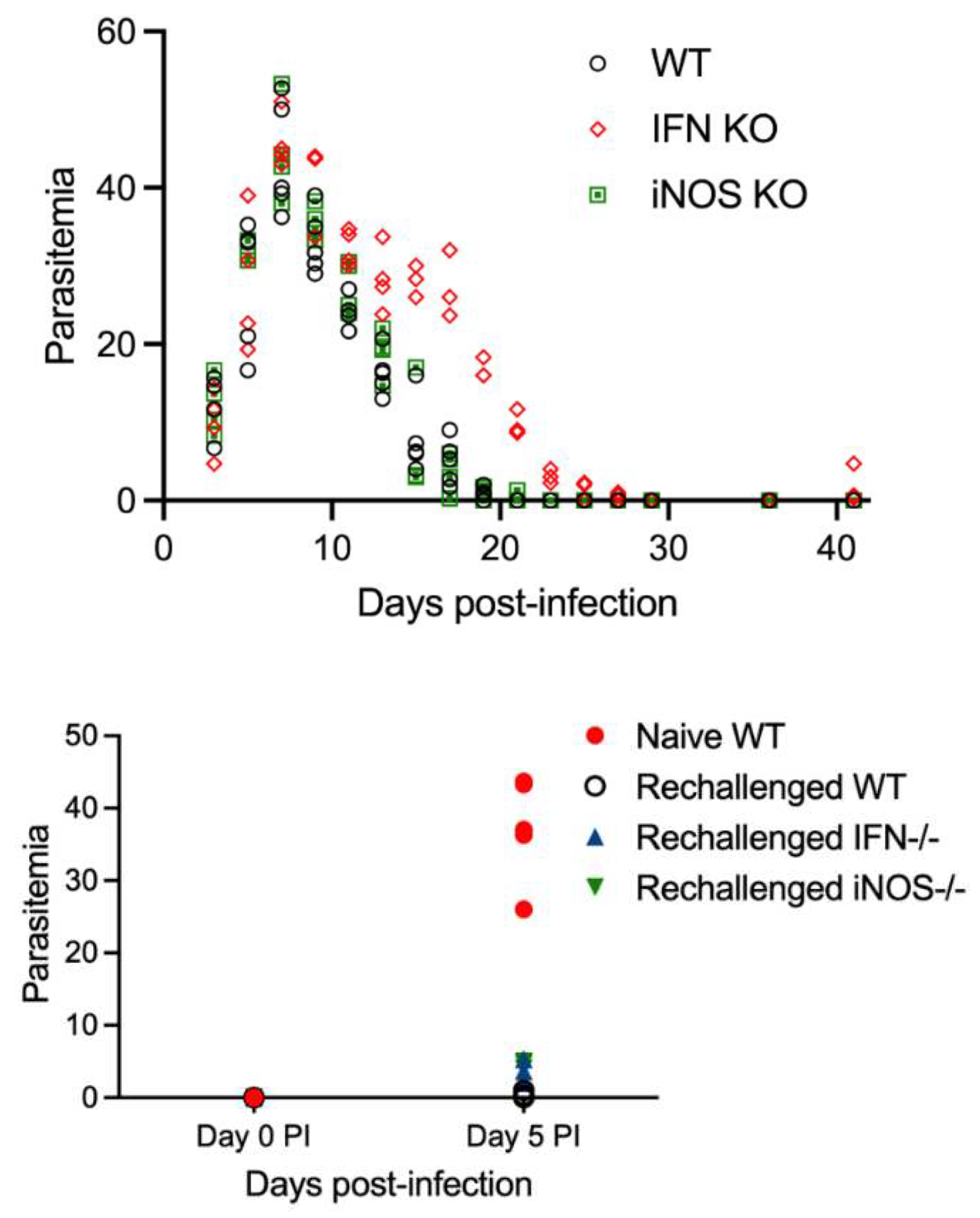
B. microti infection. IFN-γR-/- mice were as resistant as WT mice to rechallenge with
B. microti except for one IFN-γR-/- mouse that developed detectable parasitemia below 5%. This suggests that CD4 T cells are not primarily mediating protection against rechallenge by the production of IFN-γ including IFN-γ-induced activation of monocytes/macrophages. We also evalauted the importance of phagocyte production of nitric oxide using inducible nitric oxide synthase (iNOS) using iNOS-/- mice. As shown in
Figure 3, parasitemia levels following primary challenge and rechallenge of iNOS-/- mice was similar to WT mice. These results suggest that neither IFN-γR or iNOS plays a critical role in resistance of mice to reinfection with
B. microti. We also repeated our
B. microti primary and rechallenge experiment in IFN-γR-/- mice simultaneously with MyD88-/- mice. Consistent with the experiment shown in
Figure 3, IFN-γR-/- mice had a slight delay in parasite clearance during primary infection with
B. microti (
Figure 4). IFN-γR-/- mice were not more susceptible to
B. microti rechallenge compared to WT mice.
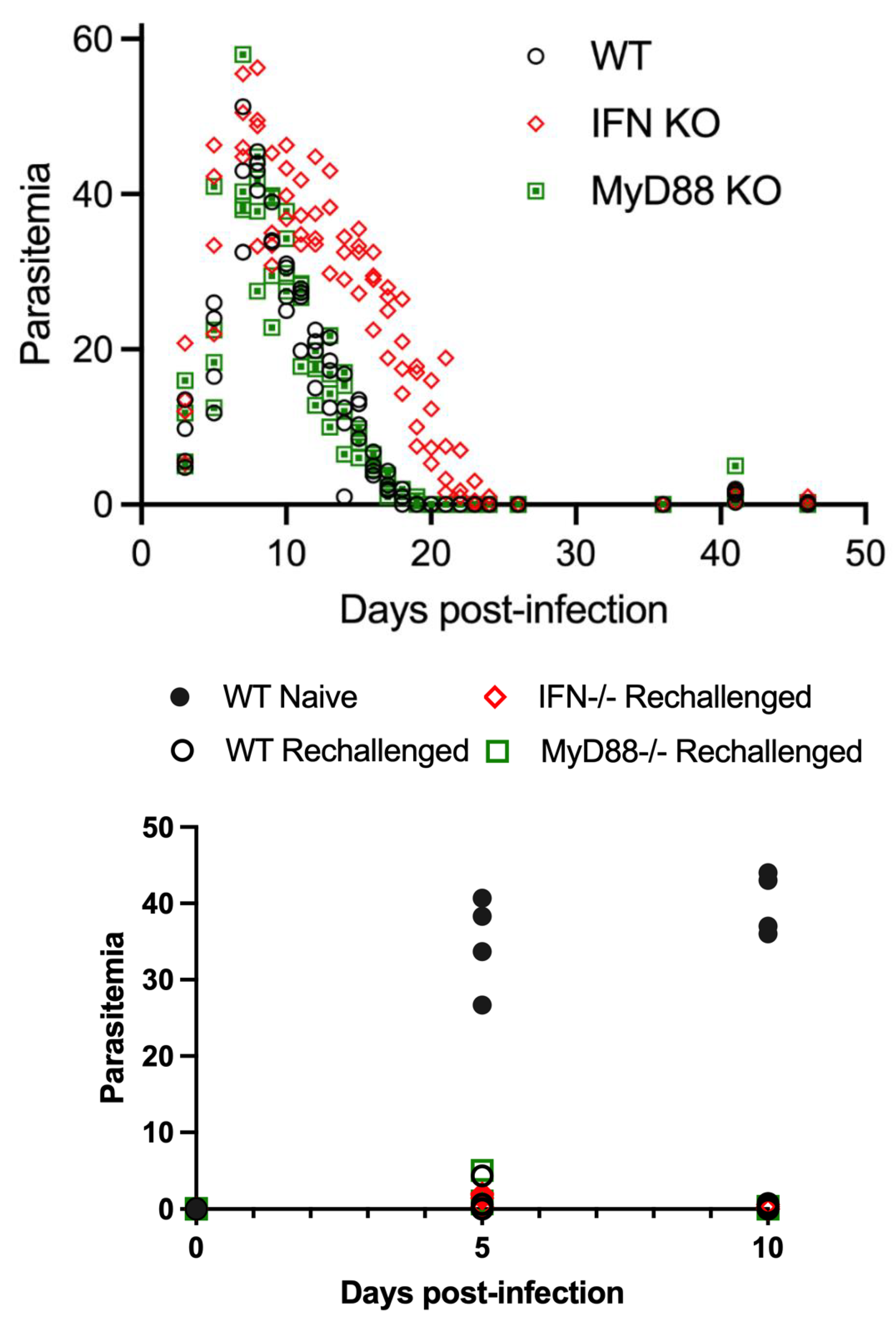
We next evaluated the role of TLRs, IL-1 and IL-18 in protection against rechallenge with
B. microti using MyD88-/- mice. MyD88 is the main adaptor molecule for TLR, IL-1R and IL-18 family members [
27]. As shown in
Figure 4, MyD88-/- mice were similar to WT mice both in their response to both primary infection and rechallenge infection 30 days pi with
B. microti (
Figure 4, and [
23]). These studies confirm that IFN-γR and MyDD88 were not required for resistance to rechallenge with
B. microti.
Discussion
In this study, we sought to identify if there are specific immune factors important for controlling reinfection with B. microti after initial infection. We found that C57BL6 mice were capable of rapidly eliminating parasites following the resolution of primary infection when rechallenged with the same isolate of B. microti 30 days post-infection. Despite an ability to diminish but not clear primary infection, CD4-/- mice did not respond to reinfection with the high level of parasitemia observed in primary infection of naïve mice, indicating that they had developed at least some level of immunity capable of limiting the infection. This protection could be mediated by IgM antibodies, which are plentiful in CD4-/- mice (as they generally lack the ability to class switch antibodies). This possibility is consistent with our observation that at least some MuMT mice did develop parasitemia similar to that observed in naïve animals. Combined these data suggest that antibodies along with CD4 T cells contribute to the control of both primary B. microti infection and reinfection. However, mice are able to suppress parasitemia levels eventhough complete resolution of infection is impaired in the absence of either CD4 T cells or B cells. In contrast, MyD88 signaling of Toll-like receptors, IL-1 and IL-18, and iNOS production of NO species are dispensible for protection against primary infection or rechallenge with B. microti. In our current study IFN-γ deficiency had an impact on the primary response to infection, but not reinfection; thus, it likely has a primary impact on innate immunity prior to the development of adaptive immunity..
Both innate and adaptive immunity have been implicated in control of
B. microti primary infection in mice [
25,
28,
29,
30,
31,
32]. Administration of BCG (Bacillus Calmette-Guerin) has been shown to protect mice from infection with
B. microti, B. rodhaini as well as
Plasmodium berghei and
Plasmodium vinckei [
28]. The study showed BCG protected mice from
B. microti and
Plasmodium species through the production of a non-antibody soluble mediator that killed parasites within infected red blood cells. Severe combined immunodeficient (SCID) mice have a genetic mutation that results in impaired VDJ rearrangement. As a result SCID mice have a functional defect in both mature B cells and T cells. SCID mice deficient in B and T cells, and nude mice deficient in T cells develop a persistent parasitemia in response to
B. microti infection [
25,
29]. However, the ability of SCID mice to control parasitemia has also been shown to depend on the genetic background of the mice [
25]. Another study in BALBC mice showed that CD4 T cells, but not CD8 T cells, were important for resolution of
B. microti primary infection [
33]. This study evaluated the immune response to the
B microti KR-1 strain and found that cell mediated immunity was critical for protection but found little role for B cells using JHD-null mice. Protection by cell mediated immunity did not require IFN-γ as IFN-γ-/- mice were able to clear infection although they did develop higher parasitemia than WT mice.
Studies to date indicate that host and parasite genotype might play a significant role in the severity as well as resolution of
Babesia spp infections and that the
Babesia strain or clade has a signficant impact on resistance/susceptibily and immune cells and factors that contribute to the outcome of infection. There clearly appears to be an important conribution of mouse genotype to the severity of infection with the WA1
Babesia strain that is related to the
B. duncani-like parasites [
34,
35]. Mice of the C57BL lineage were resistant to the WA1 strain of
Babesia developing parasitemia levels less than 5% and survival greater than 90%. B cell deficient MuMT mice in the C57BL6 genetic background showed only a small increase in parasitemia (increased from 5% to 9%) and all mice survived suggesting a minimal role for B cells in protection. The results for CD4-/- mice in the C57BL6 background challenged with WA1 parasites was similar to that for MuMT mice. Host genetics also impact the whether the absence of T and B cells in mice with the SCID mutation results in increased susceptibilty. The SCID mutation in the C3H mouse background had little impact on the susceptibility of C3H mice to WA1 infection as both WT and SCID mice were susceptible [
25]. The SCID mutation in the C57BL6 mouse background did not impair the resistance of C57BL6 mice to WA1 infection indicating B and T cells were not required for protection against the WA1 parasite strain in C57BL6 mice. In contrast, the SCID mutation in the BALBC background dramatically impaired the ability of the moderately susceptible BALBC mouse strain to control infection. These studies using the WA1
B. duncani-like strain suggest that host genotype may play a critical role in the requirement of innate versus adaptive immunity in the control of
B. duncani-like parasites as well as in the susceptibility and resistance to the parasite.
Studies also suggest that host genotype impacts
B. microti infection but perhaps not to the same degree as the WA1 parasite strain. Our previous study with
B. microti showed that host genotype impacted peak parasitemia levels in C3H, BALB/c and C57BL6 mice but all three mouse strains were able to resolve infection over a similar longitudinal time frame [
23]. An earlier study that examined the impact of mouse genotype on susceptibilty to
B. microti showed that C3H mice reached higher peak parasitemia levels than BALB/C, C57BL6, CBA, and CF1 outbred mice with the other mouse strains showing different degrees of susceptibilty [
36].
Advanced age is strongly associated with disease severity in human babesiosis [
8]{Menis, 2015 #9263;Menis, 2012 #8801. Surprisingly little is known about the mechanisms that underlie age-associated susceptibility to babesiosis. However, a study using a clinical isolate (RM/NS strain) of
B. microt showed DBA/2 mice were highly susceptible to infection compared to C57BL6 and BALB/C mice. The study also showed that age-dependent susceptibility was evident for DBA/2 mice but not C57BL6 or BALB/C mice. Thus more studies are needed to determine the degree to which the increased susceptbility of individuals with increasing age to
B. microti is impacted by host genetics as well as the immune and non-immune mechanisms that underlie the increased susceptibility with age.
Only one previous study to our knowledge directly addressed whether mice that resolved infection with
B. microti are resistant to rechallenge. The previous study addressed rechallenge of BALB/C mice with
B. microti (Munich strain) [
31]. Antibody neutralization of CD4 T cells, CD8 T cells and IFN-γ showed that CD4 T cells and IFN-γ but not CD8 T cells were important for protection against primary infection and rechallenge infection with
B. microti. In contrast, immune sera was not protective suggesting either B cells were not important for protection or that immune sera did not include sufficient levels of anti-
Babesia antibodies to confer protection. In contrast to our current study that showed IFN-γR deficient mice both cleared primary
B. microti infection and were resistant to rechallenge, their study of IFN-γ deficient mice, in the BALB/c genetic background, were unable to clear primary infection and were unable to control rechallenge infection if parasites were killed during primary infection with drug treatment followed by rechallenge with parasites.
Conclusions
Our results suggest that no one aspect of the immune system is solely responsible for adequate protection against reinfection with
B. microti. Our data along with other studies show that CD4 T cells make an important contribution to both primary and rechallenge infection with
B. microti. Our study also suggests that antibody might play an important role in protection against reinfection, while only playing a limiting role in the primary immune response. These data reopen a debate into the importance of B cells and antibody in the control of
B. microti infection especially given the susceptibility of individuals treated with Rituximib and other agents that impair B cell function to persistant babesiosis as well as babesioris refractory to current therapies [
19,
20,
37,
38]. Our studies focused on primary infection and the response to rechallenge infection with
B. microti in C57BL6 mice. Future studies are needed to evaluate the degree to which host genotype impacts the outcome of infection with
B. microti as well as other species such as
B. duncani and
B. duncani-like species. Future studies examining immune and non-immune mechanisms that underlie disease severity in response to
Babesia microti and other
Babesia species are needed to evaluate the potential impact of both host genotics and parasite genetics on the the pathophysiology and immune response during human babesiosis.
Author Contributions
Conceptualization, D.G.M and P.A; methodology, D.G.M and P.A; validation, D.G.M, P.A.; formal analysis, J.C., T.G., S.J.H; investigation, J.C., T.G, S.J.H.; resources, D.G.M, P.A., S.S, A.S; data curation, D.G.M., J.C., T.G., writing—original draft preparation, J.C., T.G., D.G.M., P.A.; writing—review and editing, D.G.M, P.A., S.S, A.S., S.J.H., J.C., T.G.; visualization, D.G.M., J.C., T.G.; supervision, D.G.M.; project administration, D.G.M, P.A.; funding acquisition, D.G.M, P.A., S.S.; A.S.; All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by NIH NIAID, grant number R21AI14523 to D.G.M. and Department of Defense Grant W81XWH-20-1-0518 to D.G.M.
Institutional Review Board Statement
All animal studies were approved by the New York Medical College Institutional Animal Care and Use Committee (IACUC). The studies were approved in the NYMC IACUC protocol number 13958.
Data Availability Statement
The raw data is included in the manuscript as parasitemia is shown in figures for each individual mouse.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lack JB, Reichard MV, Van Den Bussche RA. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int J. Parasitol. 2012;42(4):353-63. [CrossRef]
- Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: A world emerging. Infection, genetics and evolution : Infect Genet Evol. 2012;12(8):1788-809. [CrossRef]
- Goethert HK, Telford SR, 3rd. What is Babesia microti? Parasitology. 2003;127(Pt 4):301-9. [CrossRef]
- Persing DH, Conrad PA. Babesiosis: new insights from phylogenetic analysis. IAD. 1995;4(4):182-95.
- Jalovecka M, Sojka D, Ascencio M, Schnittger L. Babesia Life Cycle - When Phylogeny Meets Biology. Trends Parasitol. 2019. [CrossRef]
- Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, et al. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006;36(7):779-89. [CrossRef]
- Singh P, Lonardi S, Liang Q, Vydyam P, Khabirova E, Fang T, et al. Babesia duncani multi-omics identifies virulence factors and drug targets. Nat Microbiol. 2023;8(5):845-59. [CrossRef]
- Vannier E, Krause PJ. Human babesiosis. NEJM 2012;366(25):2397-407. [CrossRef]
- Goethert HK, Telford SR, 3rd. Not "out of Nantucket": Babesia microti in southern New England comprises at least two major populations. Parasit Vectors. 2014;7:546. [CrossRef]
- Babesiosis surveillance - 18 States, 2011. MMWR 2012;61(27):505-9.
- Krause PJ, Telford SR, 3rd, Spielman A, Sikand V, Ryan R, Christianson D, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 1996;275(21):1657-60. [CrossRef]
- Knapp KL, Rice NA. Human Coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res. 2015;2015:587131. [CrossRef]
- Parveen N, Bhanot P. Babesia microti-Borrelia Burgdorferi Coinfection. Pathogens. 2019;8(3). Epub 20190731. [CrossRef]
- Leiby, DA. Transfusion-transmitted Babesia spp.: bull's-eye on Babesia microti. Clin Micro Reviews. 2011;24(1):14-28. [CrossRef]
- Lobo CA, Cursino-Santos JR, Alhassan A, Rodrigues M. Babesia: an emerging infectious threat in transfusion medicine. PLoS Pathogens. 2013;9(7):e1003387. [CrossRef]
- Moritz ED, Winton CS, Tonnetti L, Townsend RL, Berardi VP, Hewins ME, et al. Screening for Babesia microti in the U.S. Blood Supply. NEJM 2016;375(23):2236-45. [CrossRef]
- Krause PJ, McKay K, Gadbaw J, Christianson D, Closter L, Lepore T, et al. Increasing health burden of human babesiosis in endemic sites. AJTMH 2003;68(4):431-6. [CrossRef]
- Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis 2001;32(8):1117-25. [CrossRef]
- Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008;46(3):370-6. [CrossRef]
- Wormser GP, Prasad A, Neuhaus E, Joshi S, Nowakowski J, Nelson J, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 2010;50(3):381-6. [CrossRef]
- Krause PJ, Spielman A, Telford SR, 3rd, Sikand VK, McKay K, Christianson D, et al. Persistent parasitemia after acute babesiosis. NEJM 1998;339(3):160-5. [CrossRef]
- Raffalli J, Wormser GP. Persistence of babesiosis for >2 years in a patient on rituximab for rheumatoid arthritis. Diagn Microbiol Infect Dis. 2016;85(2):231-2. [CrossRef]
- Skariah S, Arnaboldi P, Dattwyler RJ, Sultan AA, Gaylets C, Walwyn O, et al. Elimination of Babesia microti Is Dependent on Intraerythrocytic Killing and CD4(+) T Cells. J Immunol. 2017;199(2):633-42. [CrossRef]
- Ho J, Carey E, Carey DE, Krause PJ. Recurrence of Human Babesiosis Caused by Reinfection. Emerg Infect Dis. 2021;27(10):2658-61. [CrossRef]
- Aguilar-Delfin I, Homer MJ, Wettstein PJ, Persing DH. Innate resistance to Babesia infection is influenced by genetic background and gender.Infect Immun. 2001;69(12):7955-8. [CrossRef]
- Gleason NN, Healy GR, Western KA, Benson GD, Schultz MG. The "Gray" strain of Babesia microti from a human case established in laboratory animals. J Parasitol. 1970;56(6):1256-7. [CrossRef]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143-50. [CrossRef]
- Clark IA, Allison AC, Cox FE. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976;259(5541):309-11. [CrossRef]
- Clark IA, Allison AC. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974;252(5481):328-9. [CrossRef]
- Clark IA, Cox FE, Allison AC. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977;74(1):9-18. [CrossRef]
- Igarashi I, Suzuki R, Waki S, Tagawa Y, Seng S, Tum S, et al. Roles of CD4(+) T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect Immun 1999;67(8):4143-8. [CrossRef]
- Vannier E, Borggraefe I, Telford SR, 3rd, Menon S, Brauns T, Spielman A et al. Age-associated decline in resistance to Babesia microti is genetically determined. J Infect Dis. 2004;189(9):1721-8. [CrossRef]
- Clawson ML, Paciorkowski N, Rajan TV, La Vake C, Pope C, La Vake M, et al. Cellular immunity, but not gamma interferon, is essential for resolution of Babesia microti infection in BALB/c mice. Infect Immun 2002;70(9):5304-6. [CrossRef]
- Aguilar-Delfin I, Wettstein PJ, Persing DH. Resistance to acute babesiosis is associated with interleukin-12- and gamma interferon-mediated responses and requires macrophages and natural killer cells. Infect Immun. 2003;71(4):2002-8. [CrossRef]
- Moro MH, David CS, Magera JM, Wettstein PJ, Barthold SW, Persing DH. Differential effects of infection with a Babesia-like piroplasm, WA1, in inbred mice. Infect Immun. 1998;66(2):492-8. [CrossRef]
- Ruebush MJ, Hanson WL. Susceptibility of five strains of mice to Babesia microti of human origin. J Parasitol. 1979;65(3):430-3. [CrossRef]
- Marcos LA, Wormser GP. Relapsing Babesiosis With Molecular Evidence of Resistance to Certain Antimicrobials Commonly Used to Treat Babesia microti Infections. Open Forum Infect Dis. 2023;10(8):ofad391. [CrossRef]
- Marcos LA, Leung A, Kirkman L, Wormser GP. Use of tafenoquine to treat a patient with relapsing babesiosis with clinical and molecular evidence of resistance to azithromycin and atovaquone. IDCases. 2022;27:e01460. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).