1. Introduction
Coffee is a beverage that has been integrated into modern society, becoming an integral part of the daily routine of millions of people around the world, were there are approximately 1 billion coffee drinkers worldwide and over 2 billion cups of coffee are consumed daily [
1]. Coffee occupies a significant position in today’s industry and has a far-reaching impact on economies around the world. With an estimated annual production of more than 169.50 million bags (10 million Tons) [
2] coffee has become one of the most traded commodities on the planet [
3], supporting the livelihoods of countless farmers, traders and businesses along its complex supply chain. But its importance transcends mere economic value; coffee represents culture, community and connection, fostering social interactions and driving innovation [
4,
5].
Even though coffee is such an important commodity, its industry produces over 23 million tons of trash every year, from the pulp of fresh coffee cherries to the packing of roasted beans, according to sustainability researcher Gunter Pauli. Brazil, Vietnam and Colombia are the main producers of coffee worldwide [
6], according to the FNC in 2021 the top producer of mild washed Arabica coffee in the world, Colombia, produced 12.6 million bags of green coffee weighing 60 kg [
7]. Colombian coffee agroindustry uses only 9.5% of the fruit for beverage preparation, with 90.5 percent being by-products dumped into water bodies. This pollution reduces ecosystem life and soil quality. Around two million tons of pulp and 420000 tons of mucilage are dumped in open fields, affecting the value chain and environment. Efforts are being made to utilize these materials in pig and livestock industries, beverages, vinegar, biogas, caffeine, pectins, peptic enzymes, proteins, and fertilizers [
8,
9,
10,
11]. There are several ways of coffee waste management such as oxidation ponds [
12], Modular anaerobic treatment systems [
13], Anaerobic Upflow Filters [
14], Worm farming [
15], Composting [
16], and hydrothermal valorization [
17,
18,
19,
20,
21].
This paper will mainly focus on hydrothermal carbonization (HTC) and Liquid Hot Water Extraction (LHW). Hydrothermal carbonization (HTC) is a promising thermal conversion technology that transforms biomass into hydrochar through the application of heat and pressure in a water-rich environment. This process mimics the natural coal formation process, ranging from 180-260 °C, resulting in a carbon-rich product with improved fuel properties and potential applications in bioenergy, carbon sequestration, and soil improvement [
22]. Liquid Hot Water Extraction (LHW) involves increasing the water temperature under high pressure. This enhances the solubility and extraction efficiency of target compounds, making LHW a valuable technique for obtaining bioactive compounds, essential oils, and other valuable substances from various natural sources [
23,
24]. LHW (120-180 °C) is considered an environmentally friendly and cost-effective alternative to traditional solvent extraction methods, as it eliminates the need for harmful organic solvents. The extracted bioactive compounds find applications in the food, pharmaceutical, and nutraceutical industries due to their potential health benefits.
Previous research has been done regarding the use of coffee (Spent coffee grounds and silverskin) and different hydrothermal processes to obtain platform molecules such as biochar [
25], polysaccharides (Cellobiose, Glucose, Xylose, Galactose, Arabinose and Maltose) [
26], and activated carbon [
20] using HTC; via LHW polyphenols, hydroxymethylfurfural, feruloylquinic acid and epicatechin were obtained [
27] as well as antioxidants (DPPH, ABTS, HPLC) [
28]. It’s worth noticing that little to no information was found on the valorization of coffee berry waste.
The scope of this paper is to analyze the different platform chemicals that are produced in LHW and HTC using residual waste from coffee cherry. This will be achieved through making experiments modifying time, temperature and the implementation of catalysts. The quantification with analytic methods (HPLC-IR), characterization (IR-Spectroscopy, elemental analysis), purification, kinetic analysis and the optimization of variables based on each platform chemical are some of the goals of this research.
2. Materials and Methods
2.1. Biomass characterization
2.1.1. Proximal Essay
2.1.1.1. Moisture
For the determination of moisture in the coffee cherry waste sample, the technical report NREL/TP-510-42621 was used as a guide. One mass of 1,000 gram of both wet and air-dried biomass (milled) was weighted, put in a crucible, and inserted in a Memmert Oven at 105 °C for 24 hours. Afterwards the moisture of the samples was calculated using equation 1.
2.1.1.2. Ash
For the determination of ash in the coffee berry waste sample, the laboratory analytical report NREL/TP-510-42622 was followed. 1,000 gram of both wet and air-dried (milled) biomass was weighted, put in a crucible, and inserted in a muffle furnace with the following program: Hold at 105 °C for 12 minutes, ramp to 250 °C at 10°C / minute, hold at 250 °C for 30 minutes, ramp to 575 °C at 20 °C / minute, hold at 575 °C for 180 minutes. Then the temperature was allowed to drop to 105 °C and it was weighted. This was done in triplicates and calculated with equation 2 and 3.
2.1.1.3. Volatile Matter
For the determination of volatile matter in the coffee berry waste sample, the laboratory analytical report ISO-18123-2015 was followed. A sample of 1 g of dried (milled) biomass was taken and put in a crucible. Afterward the muffle furnace was set at 900 °C, and the sample was introduced for 7 minutes, time during which the volatile substances are released as gases or vapors. The weight loss experienced by the sample during this process represents the volatile matter content and is calculated with the equation 4.
2.1.1.4. Fixed Carbon
The determination of the fixed carbon is not a laboratory procedure, it’s a calculated value involving all the characterizations previously done. According to ASTM D 3172—89, the fixed carbon corresponds to equation 5.
2.1.2. Ultimate essay
A Thermo Scientific FLASH 2000 Series Organic Elemental Analyzers was used and the Technical Report NREL/TP-510-42620 was followed.
2.1.3. Chemical composition
The lignin, cellulose and hemicellulose were measured as Neutral Detergent Fiber, Acid Detergent Fiber and lignin. The procedure in Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition was carried out, the following relations should be kept in mind:
Fiber in neutral detergent: Composed of cellulose, hemicellulose, and lignin.
Fiber in acid detergent: Composed of cellulose and lignin.
2.2. Hydrothermal experiments:
Experiments were done in a 100 mL batch reactor at different temperatures 120-180 °C (LHW) with autogenous pressure, for 1 hour with 1:10 biomass-water ratio (5 grams biomass, 45 grams water). To analyze the kinetics of the processes, a set of experiments were done at 180 °C modifying the reaction time from 1 to 5 hours.
To test another hydrothermal range, reactions were done at different temperatures from 200 to 260 °C (HTC) with autogenous pressure, for 1 hour with a 1:10 biomass-water ratio (10 grams biomass, 90 grams water). To analyze the kinetics of the processes, a set of experiments were done at 200ºC modifying the reaction time from 1 to 5 hours.
In all cases, the solid and liquid fractions were separated through vacuum filtration, and they were measured to keep a mass balance. The solid was recovered and washed with water, ethanol, and acetone and dried at 105 °C until constant weight. In order to characterize the solid, Infrared spectroscopy was done with a Nicolet iS10 Spectrometer Thermo Fisher Scientific, elemental analysis was taken with a Thermo Flash 2000 following the NREL/TP-510-42620 as well as SEM images. To monitor the liquid fraction, pH and conductivity were measured and the platform chemicals (PC) were quantified using HPLC-IR and HPLC-MS.
2.3. Analytical methods
For the quantification of PC a method was developed for HPLC-IR using standards of xylose, glucose, formic acid, levulinic acid, HMF and Furfural. Said method was obtained with a Hitachi Elite LaChrom with a Hitachi L-2490 refraction index detector at 40ºC, a SHODEX Sugar SH1821 column at 60 °C, mobile phase of 0.005 M H2SO4 and flow of 0,5 mL/min. The validation of the method is presented in the supplementary material.
2.4. Homogeneous catalysts
In order to improve the yields obtained in the hydrothermal reactions previously made, a set of acid and basic homogeneous catalysts were used to make the reactions more efficient and specific. H2SO4 0,02M, CH3COOH 0,2M, KOH 0,02M and NaHCO3 0,2M solutions were prepared and reactions at 180ºC for different times were done using the catalysts solutions instead of water. The obtained fractions were characterized as previously done with the other experiments.
2.5. Purification
After optimizing the conditions and obtaining the best yielding conditions, tests of purification were done using techniques such as distillation (at 100 °C), liquid-liquid partition (octanol-water), C-18 column chromatography (water and ethanol as mobile phases) and ionic interchange chromatography (DOWEX-50W, AMBERLITE IRA 400 and DOWEX* MARATHON* MR-3 with Buffers of pH 4.3, 7 and 12 as mobile phases). All of the obtained extracts were characterized via HPLC-IR in order to test the efficiency of the separation.
3. Results
3.1. Biomass Characterization
3.1.1. Proximal essay
Table 1 presents the characterization of coffee biomass obtained in this study and other types of coffee biomass from previous articles. The chemical composition of the biomass is influenced by moisture content, ash content, fixed carbon, volatile matter, and geographical location. Moisture content is crucial for accurate and repeatable results, as it helps to start from conditions with similar moisture content. Ash content is influenced by the mineral content of the biomass source and corresponds to inorganic products specific to the biomass. Fixed carbon is related to the organic matter in the biomass and can be influenced by the type of biomass, maturity stage, and carbon/hydrogen ratio. Volatile matter, consisting of combustible gases and compounds, is affected by factors such as biomass decomposition, volatile organic compounds, and processing methods. Geographical location, harvesting and processing methods, contaminants, age and maturity of the biomass source, and species and variety within a biomass type also affect the results of proximate analysis. These factors are important for comparing the results with reports found in literature, as they may not be consistent in different types of coffee residues used in valorization processes.
The biomass of coffee cherries is similar to that of coffee pulp, as it contains pulp, husk, and mucilage. The moisture of the biomass after kiln drying and grinding is calculated as BHP moisture. The initial biomass moisture corresponds to the characterisation of coffee cherries without pre-treatment processes. Reducing the moisture from 80.79 to 10.94 (7 times) was possible. However, due to its high moisture percentage, hydrothermal techniques can be used by calculating the biomass-water ratio without having to add external water. The biomass has a low amount of volatile matter compared to husks and SCG, indicating a low amount of organic components that release gases when heated. It also has a low, but relevant, amount of volatile matter when compared to husks and SCG, implying that it consists of a low amount of organic components that release gases when the biomass is heated, but that will also be the organic groups that will later be extracted in the aqueous phase, so that, perhaps for recovery processes, parts of the coffee waste with a higher percentage of volatiles could be of interest.
3.1.2. Ultimate essay
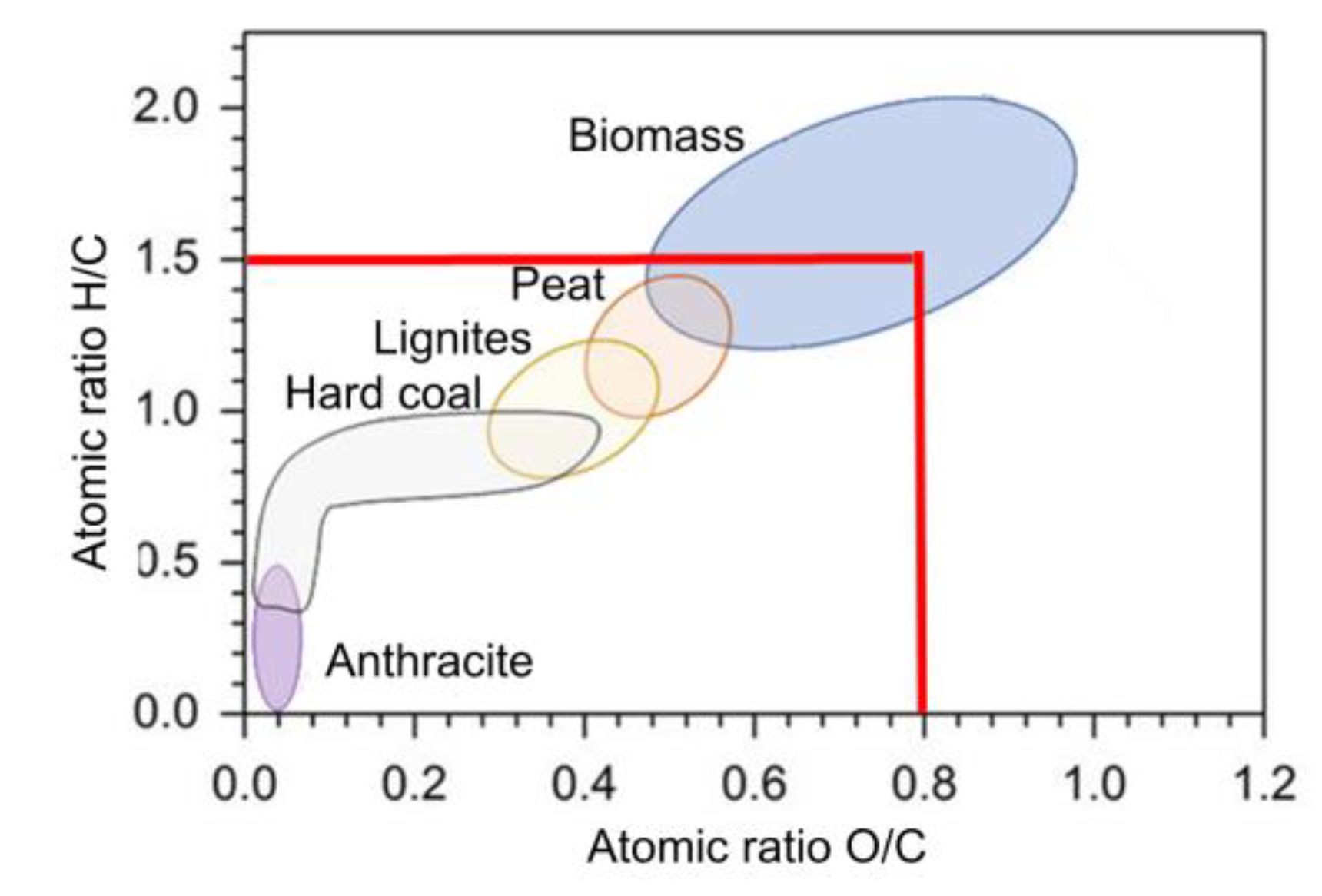
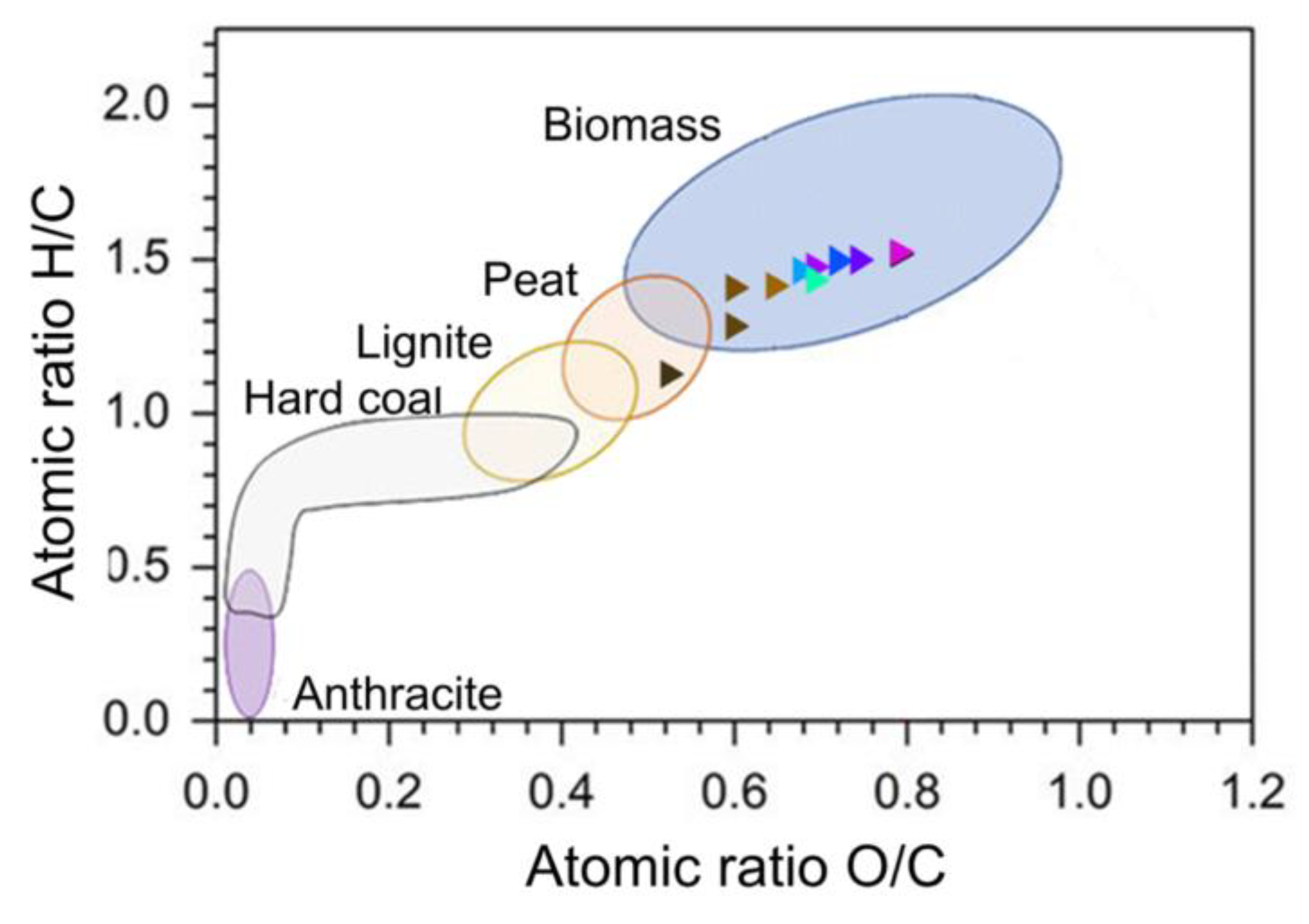
The ultimate assay is of great importance as it provides accurate information on the elemental composition of biomass, which is essential for understanding its potential and applications. It provides information on the amount of carbon, which is directly related to the energy content of the biomass and its suitability as a renewable energy source. The most relevant factors are the O/C, H/C and C/N ratios, where said ratios classify the biomass within the van Krevelen diagram (
Figure 1) and has been used widely to describe the change of biomass composition during the thermochemical decomposition. On the other hand, the C/N ratio shows how relevant and feasible a biomass valorization process by means of microbial decomposition can be. When the C/N ratio is over 24/1 de rate of relative descomposition is slower, then it is equal to 24/1 it’s the ideal microbial diet, and when its under 24/1 the rate is faster.
Regarding the characterization of coffee cherries by final test, the percentages of the different elements are quite similar to those reported for coffee husks (
Table 2), since, again, this type of residue is included in coffee cherries. No information was reported for the characterization of either the pulp or the cherry. Considering the O/C and H/C ratios and their location in the Van Krevelen diagram (
Figure 1), the sample obtained can be effectively classified as a biomass type fuel, with a higher amount of oxygen and hydrogen than traditional fossil fuels, and therefore has acceptable conditions to be implemented as a biofuel.
In the same way, the C/N ratio corresponding to 19.98 allows the biomass to be classified according to its possibility of being valorized or treated with microorganisms. In the present case, the ratio favors rapid decomposition of the biomass by the microorganisms (20:1), which is why one of the most common uses of coffee residues is as a soil fertiliser.
3.1.3. Structural essay
Depending on the plant species and developmental circumstances, the structures and chemical composition of these biopolymers differ significantly. Hemicellulose and lignin are linked to cellulose by covalent bonds, while cellulose is linked to hemicellulose and lignin by hydrogen bonds. Their direct utilisation is a challenge due to the complicated and dense spatial structure, so treatment, in this case hydrothermal, is required to enable efficient conversion. It is important to identify the composition of the biomass in order to know the possible platform chemicals that will be produced after valorization. Considering the results obtained in
Table 3, a relevant percentage of all fractions (lignin, cellulose and hemicellulose) and therefore different types of reactions and products will be obtained. For this work, the main focus was on monitoring the valorization of hemicellulose and cellulose (hydrolysable fraction) in order to focus on the production of sugars (glucose and xylose), organic acids (levulinic, formic, acetic) and furfural derivatives (furfural and hydroxymethylfurfural (HMF)). It is worth stressing the importance of this characterisation process as it allows us to know the physicochemical routes and processes that the biomass and its possible valorization products will take.
The study compares cherries and other types of coffee waste, revealing greater similarity to SCG but a decrease in hemicellulose compared to pulp and husk. Understanding the percentages of each structure in the biomass can help predict the type of platform chemicals and hydrothermal process to be implemented. In pulp, low hemicellulose makes it unlikely to obtain xylose and derivatives, requiring the use of lignin through an HTC process. In husk, cellulose is abundant but little hemicellulose and lignin, making it suitable for glucose extraction through LHW and fermentation processes. Lignin, which is 13.7% higher, than hemicellulose, acts as a structural component in the biomass cell wall, enveloping and protecting hemicellulose. This hinders the breaking process with water, decreasing its decomposition and valorization efficiency. Pre-treatment at scaling up or initial fractionation of cellulose, hemicellulose, and lignin could be beneficial, but would require more time, energy, and expenses. It’s important to note that the sum of lignin, hemicellulose, and cellulose does not equal 100% due to the non-consideration of proteins, fats, fibers, and carbohydrates in the characterisation process.
3.2. Hydrothermal valorization via LHW
Hydrothermal processes were carried out from 120 to 180 °C for one hour and at 180 °C for 5 hours, starting in all cases from 3 grams of biomass and 27 grams of water. Solid, liquid and gas phase results were obtained as shown in
Table 4.
It can then be observed that the ratio of solid fraction, liquid fraction and gas fraction remain constant in all tests performed at one hour time, indicating that there are not a large number of reactions taking place and that the physical characteristics of the solid phase are remaining relatively constant. On the other hand, with increasing time at a constant temperature, a variation in both the solid and liquid phases and an increase in the gaseous fraction are observed. This may be due to dehydration processes in the medium leading to the removal of water in gaseous form, decarboxylation leading to the removal of CO2 and changes in the structures in both the aqueous and solid phase.
3.2.1. Preliminary follows up of liquid fraction
A preliminary follow-up was conducted on the obtaining of species of interest by measuring pH and conductivity in liquid fractions obtained after hydrothermal experiments. Conductivity in acids is related to the capacity of an acid to conduct electric current, and it is directly proportional to the concentration of ions in the acid solution. When an acid dissolves in water, some covalent bonds in the acid molecules are broken, releasing hydrogen ions (H⁺) into solution. These ions are electric charge carriers, moving towards the opposite electrode when an electric potential is applied across the solution. pH is a logarithmic scale that measures the concentration of hydrogen ions (H⁺) in an aqueous solution. In acidic solutions, the concentrations of H⁺ ions are higher, making the pH lower (less than 7 on the pH scale). A neutral solution, with a pH of 7 and equal concentrations of H⁺ and OH- ions, is the case in sugars. The variation of pH in the aqueous fraction may indicate the presence of acidic or basic species being produced in the medium.
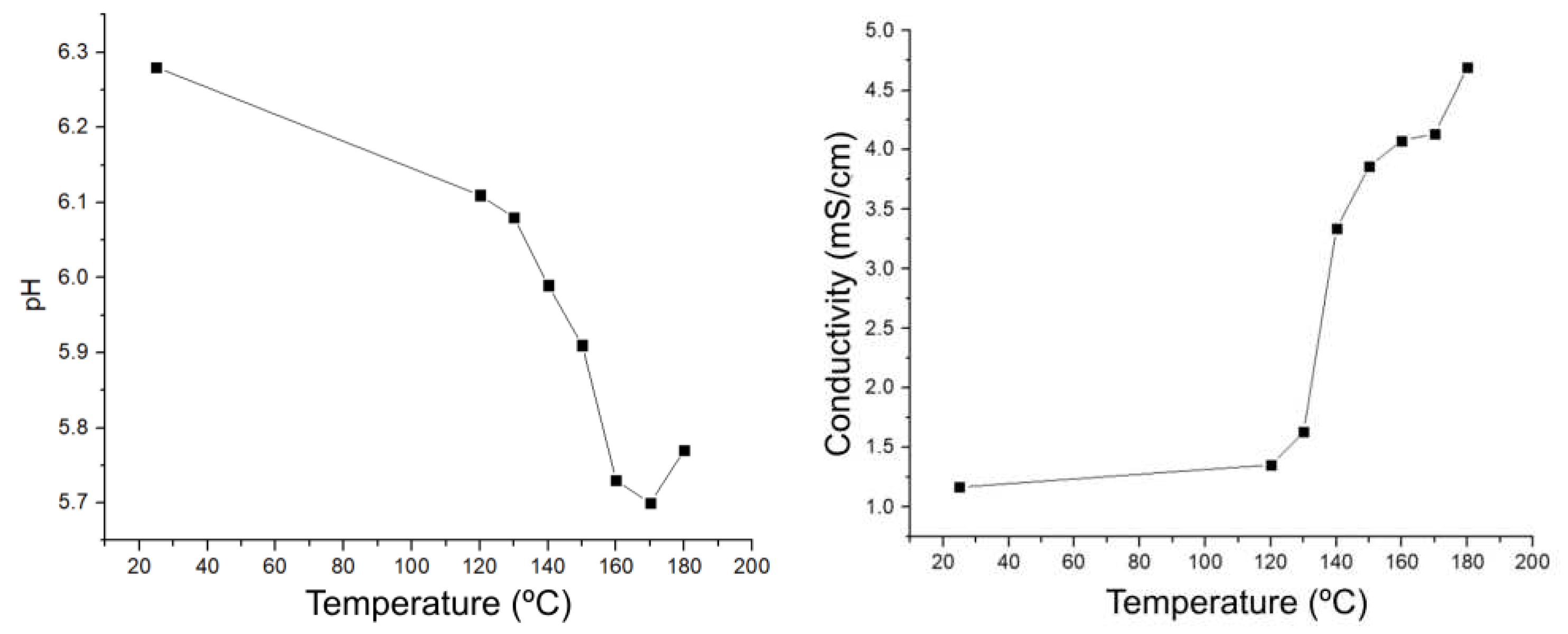
Figure 2 shows the change in pH (decrease) and conductivity (increase) in the liquid fraction at different temperatures (120-180 °C) with a constant time (1 hour). A change of 3.336 mS/cm of conductivity is observed, in general, and when applying the criterion of the first derivative, it is observed that the point of greatest change is at 140 °C, so it can be concluded that from this temperature onwards, charged species start to be produced, and not only the presence of sugars. As for the pH, there is a change of 0.51 pH units and the greatest change in the acidity of the medium occurs at 150 °C, so it can be concluded that below 150 °C there are few acidic species in the system and there are no relevant changes in the liquid fraction.
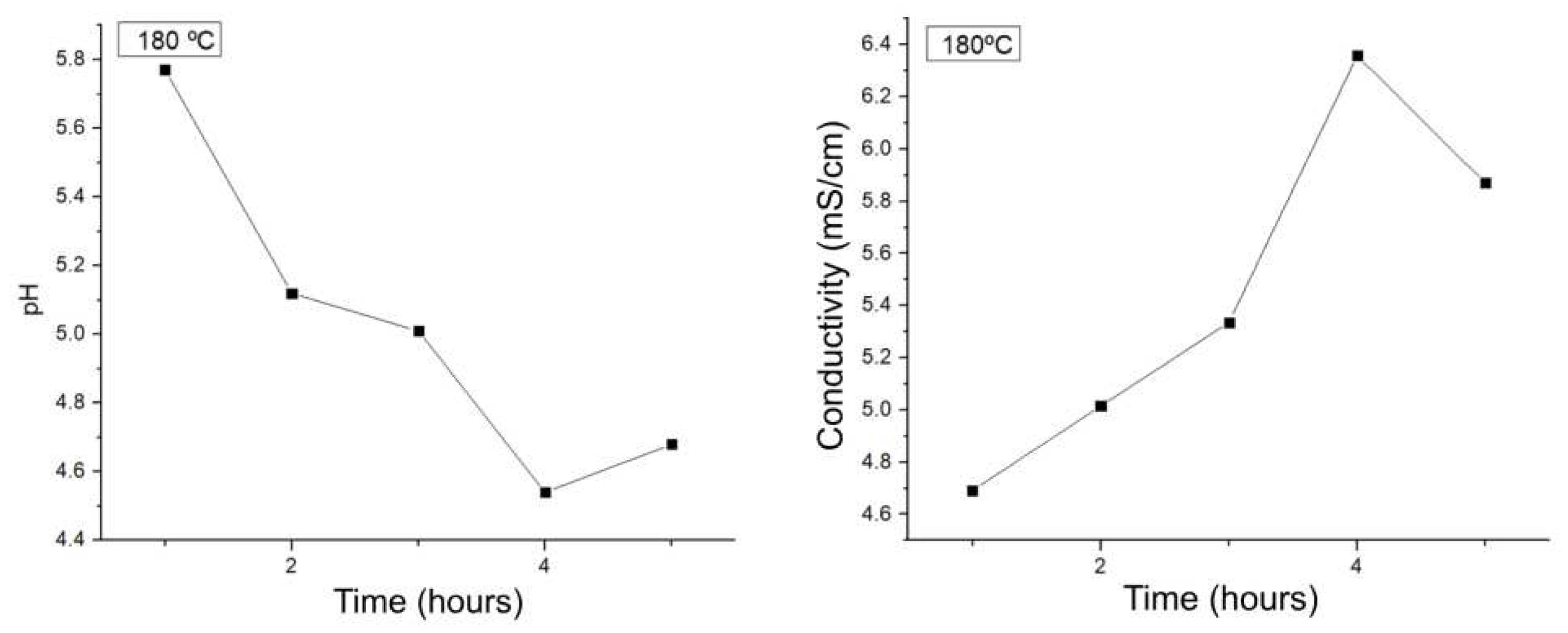
The same procedure was repeated by monitoring the pH and conductivity of the liquid fraction at 180 for 5 hours, obtaining
Figure 3. In both cases, in both conductivity and pH, it is observed that after 4 hours both values decay, which can be explained by a possible degradation of the products previously obtained and, therefore, the decrease of the species of importance for this project. Analyzing the variations from 1-4 hours, there is a variation of 1.74 pH units and 5.002 conductivity units, values higher than those obtained only by modifying the temperature. Therefore, it can be preliminarily concluded that the LHW process carried out at 180ºC for 4 hours will be the one with the highest production of platform chemicals of interest or, at least, the one with the best transformation of biomass to the aqueous phase in the form of organics.
3.2.2. Characterization of solid fraction
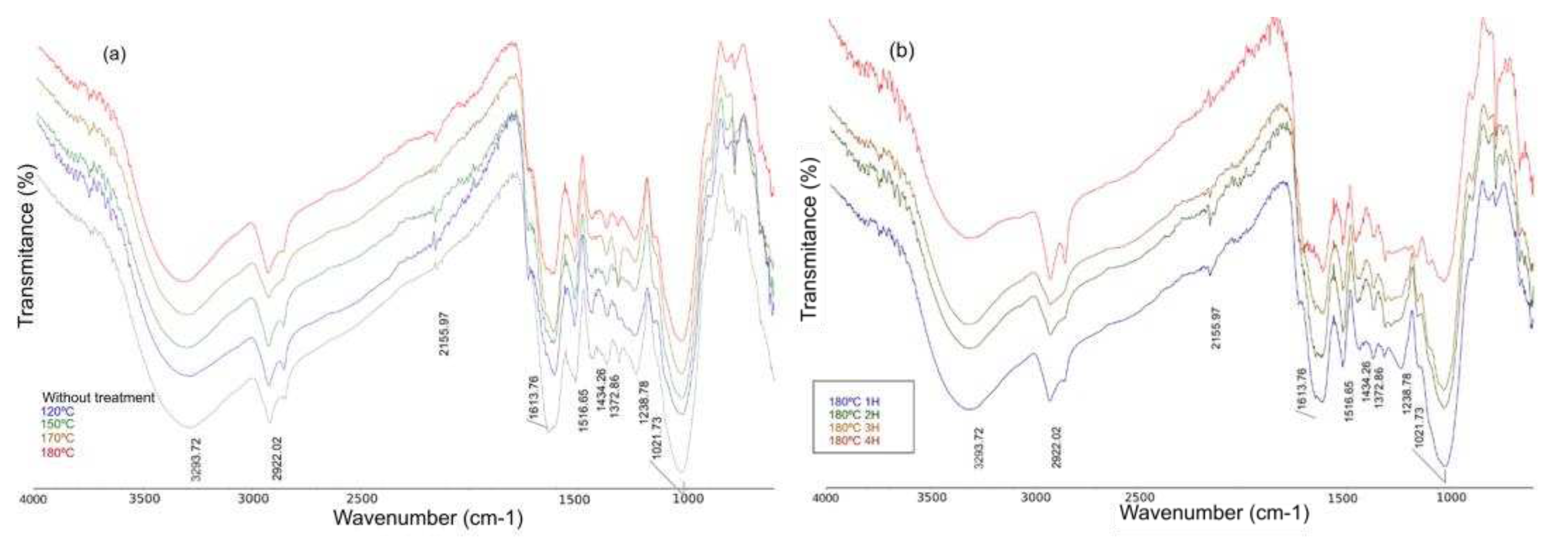
Figure 4 shows the infrared spectra obtained for the solid fraction before the hydrothermal treatment and with the hydrothermal processes at 120, 150, 170 and 180 °C. It is important to highlight that the main bands (
Table 5) are those of O-H stretching (3284–3308 cm
-1), C-H stretching (2919-2923 cm
-1), C=C stretching (1608-1622 cm
-1), and those present around 1500 cm
-1 (C=C stretching of lignin and aromatic C), 1200 cm
-1 (C-O-C in cellulose and hemicellulose) and finally around 1000 cm
-1 (corresponding to cellulose and hemicellulose as well). With the last 3 signals, it is possible to follow the transformation of the initial biomass and the breakdown of the primary structures (lignin, cellulose, and hemicellulose) and thus the disappearance of their functional groups in the solid fraction by hydrolysis to proceed to be extracted in the aqueous fraction.
In the case of
Figure 4a there is no relevant variation in the previously mentioned signals, so no transformation of biomass to biochar is taking place yet, nor are processes of breakdown of the primary lignocellulosic structures.
Figure 4b shows a decrease in the OH stretching band, as well as a decrease in the intensity of the hemicellulose, cellulose and lignin bands in the zone between 1000 and 1600 cm
-1, which allows us to conclude that an effective process of hydrolysis and obtaining organic species in the liquid fraction of the hydrothermal process has been carried out, an aspect that goes hand in hand with the results previously obtained from the monitoring of pH and conductivity. It is also important to note the appearance of a band at around 780 cm
-1, typical of alcohols and phenols that may have been produced after the hydrothermal reactions.
On the other hand, in order to follow up and partially characterise the solid fraction, the elemental analysis of the solids obtained in each of the hydrothermal valorization processes was carried out (
Table 6). It can be observed that there are no major changes in the H/C ratio under the temperature of 180 °C, where the ratio starts to decrease due to an increase in the percentage of carbon in the solid fraction. The percentage of oxygen decreases in a more relevant way in the experiments at 180 °C with longer time, causing the O/C ratio to decrease as well.
Considering these changes, the different experiments were placed on the Van Krevelen diagram (
Figure 5) to characterize the fuel type in which the solid fraction is classified. The colored signs represent the arrangement of the 120 -180 °C tests for one hour, while the brown signs represent the experiments at 180 °C for different hours. It is observed that, at higher reaction times and temperatures, the residual biomass from the hydrothermal process starts to assimilate more to other types of fuels, in this case classifying the 180 °C -5h test as a peat-type fuel, which has applications for cleaning oil spills and for water purification filters, as well as a fuel.
The C/N ratio is also modified by the hydrothermal processes, reaching 31:1. This increase in the ratio, as was shown in the initial characterization of the biomass, is above the 24:1 ratio, which allows us to classify the relative decomposition process as a slow speed one, contrasting with what we had initially, and suggesting that the biochar obtained would not be easily degraded by microorganisms.
3.2.3. Quantification of platform chemicals
The quantification of the platform chemicals (PC) was done using the validated method for HPLC-IR described previously, which can be seen more in dept in the supplementary material, and an external calibration from 0,1 to 10 g/L of each PC (Xylose, glucose, formic acid, levulinic acid, HMF and furfural).
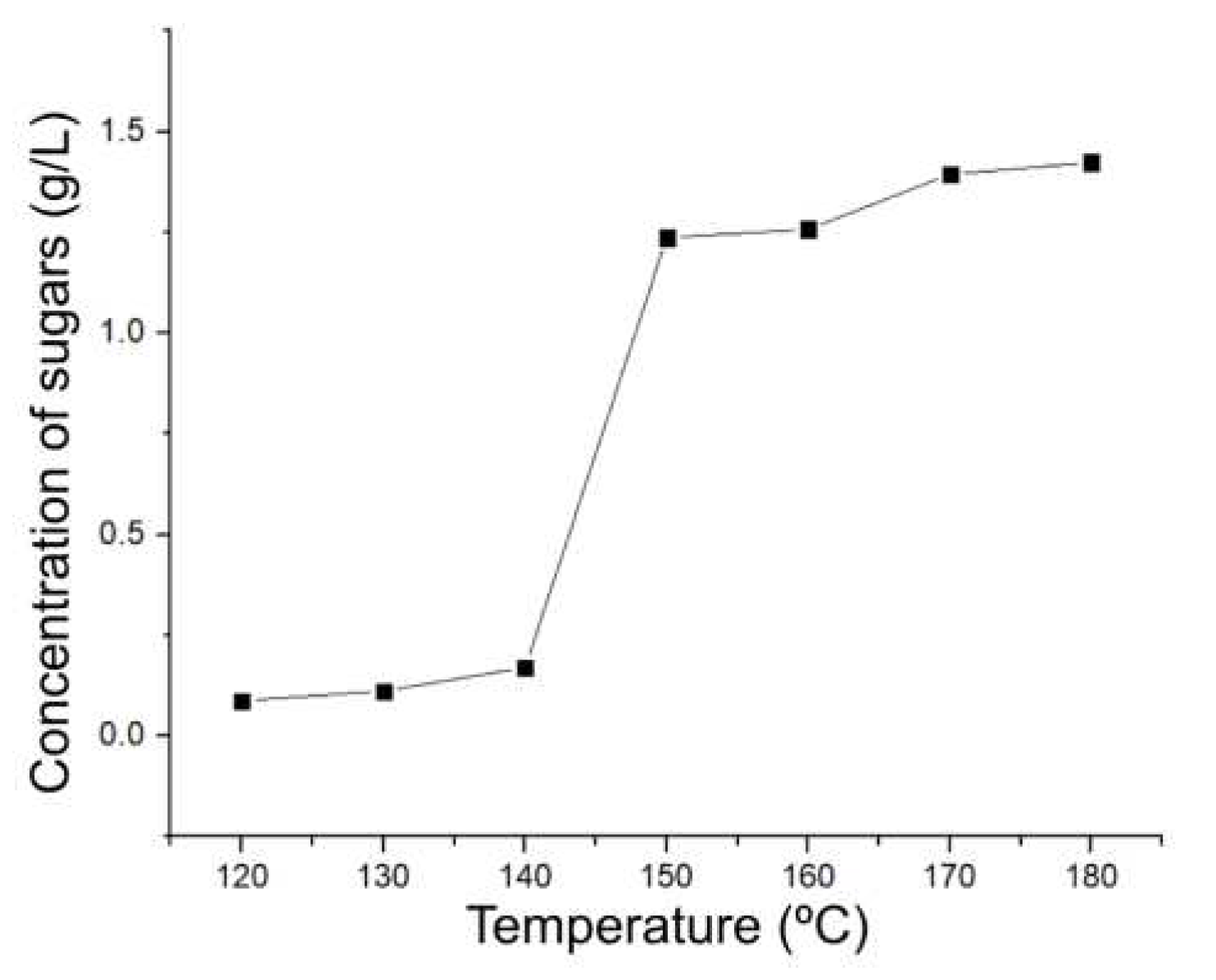
It was observed that in those experiments corresponding to temperatures of 120, 130 and 140 °C there is no relevant products obtained, an aspect that goes hand in hand with what was found by monitoring the pH and conductivity of the liquid fractions. Subsequently, from 150 to 180 °C, an extraction of sugars and other compounds possibly with characteristics of cellu-oligosaccharides is observed. Then, quantified products in the LHW range with one-hour experiments are shown in
Table 7 and
Figure 6.
It is observed that the results obtained correlate with those found in the monitoring by conductivity and pH, where there is an increase in the concentration of sugars at the temperature of 150 °C where the process of hydrolysis of the hemicellulose/cellulose begins to take place. After this, hydrolysis continues to take place, but in a uniform manner as the temperature is raised to 180 °C. It is worth noting that no other chemical platform compounds were produced at these one-hour temperatures due to the inability to favour reaching products requiring further chemical processes such as hydrogenation, dehydrogenation, decarboxylations and aldol condensations.
The yield of sugars production from two aspects was calculated in the same way, initially from the initially weighed biomass, in this case 3 grams, obtaining a maximum of 1.086%w sugars. The same process was also used, but taking the percentage of the biomass that corresponds to the lignocellulosic structure from which the sugars come (cellulose and hemicellulose) as the initial quantity for the process, as other components such as lignin, lipids, pigments and others do not contribute and could even inhibit the hydrolysis process. With this ratio, a yield of 2.709%w was obtained, which can also be maximized. It is worth mentioning that in the LHW range and with only one hour, it was not possible to obtain more PC, only the extraction of sugars. Subsequently, the same PC quantification and monitoring process was carried out for the experiments performed at constant temperature (180 °C) and modifying the reaction time, obtaining the results presented in
Table 8, and their respective graph (
Figure 7).
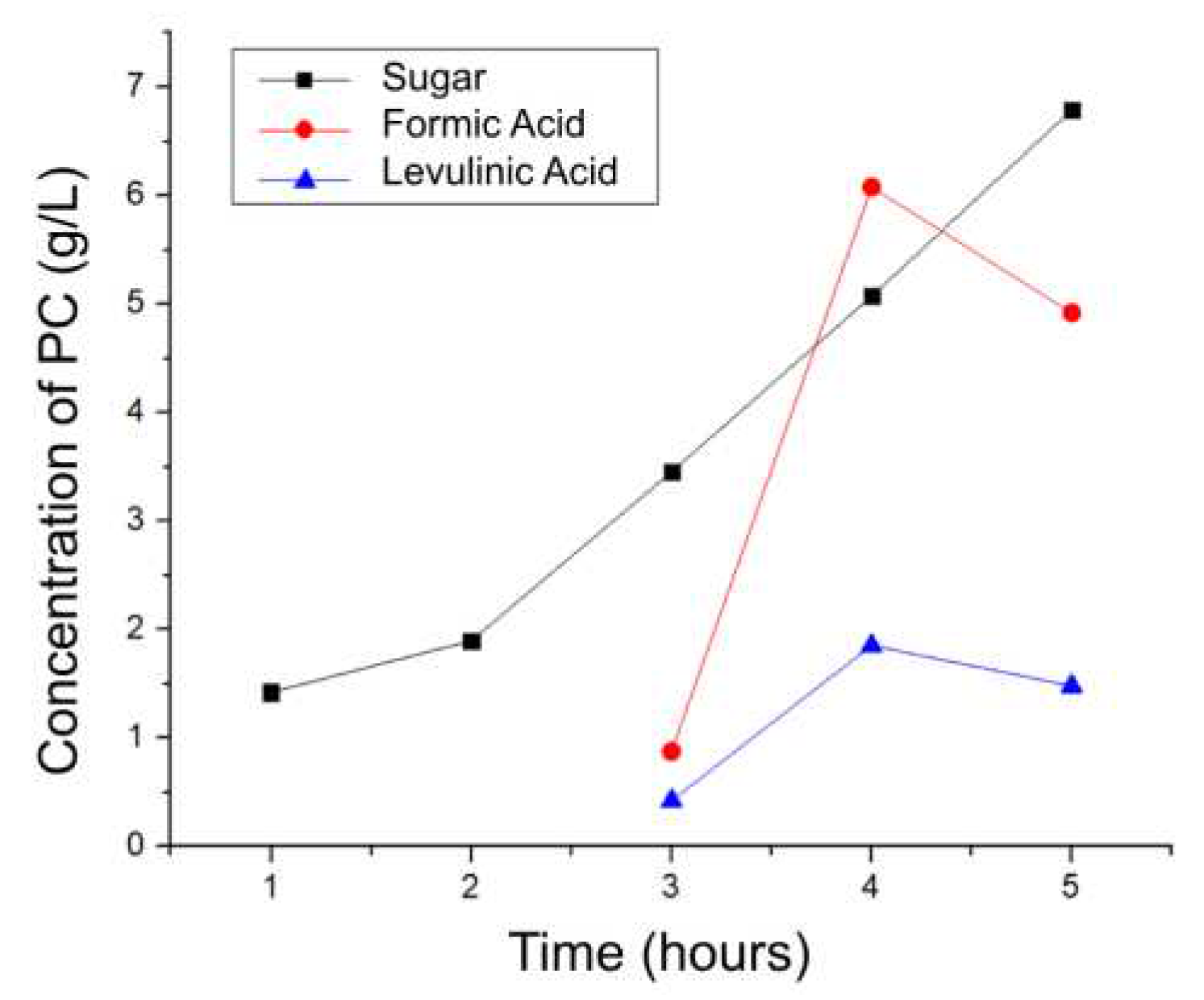
IIt is observed that as the reaction time increases, the processes of hydrolysis, dehydration, rehydration, and condensation are favored (
Figure 7), which leads to the production of PC. After 3 hours, formic and levulinic acid starts to be extracted and after 4 hours HMF and furfural are obtained. After this, at 5 hours, the efficiency of all products except sugars decreases, due to possible degradation to smaller molecules and the instability of some of the previously quantified molecules.
On the other hand,
Table 8 shows the yield of the process based on the amount of initial biomass and its corresponding lignocellulosic structures. It is concluded that the best condition to valorize coffee without the implementation of catalysts and in the LHW range is the temperature of 180 °C for 4 hours, achieving a valorization of about 25%w. As previously mentioned, continuing with the process for more hours would decrease the yield of the reaction and therefore HMF and furfural would not be obtained and the acids would start to break down into ketones, smaller acids and would undergo decarboxylation eliminating CO
2.
3.3. Catalysts implementation
Table 9 compares the total yields and concentrations as a function of reaction time and catalyst used. It is then observed that in all the hours the total yield of all the catalysts exceeds the yield of the reaction without catalyst, an aspect that demonstrates the efficiency of the catalysis. On the other hand, it can be observed how the different catalysts lead to the majority production of different PCs, in the case of sulphuric acid, it favours the formation of sugars and formic acid, besides being the only catalyst that allows reaching HMF and furfural, for acetic acid, the reaction leads to the production of mostly levulinic acid.
In the case of basic catalysts, both potassium hydroxide and sodium bicarbonate favor the production of formic acid in addition to producing biochar with lignite characteristics and sodium in its structure, characteristics that are not favored by implementing acid catalysts. These conclusions go hand in hand with what has been reported in the literature where acid catalysts decrease the quality of the biochar or biocrude obtained but improve the extraction of organic compounds [
35]. It is also observed that the acidic medium increases the production of levulinic acid, HMF and furfural, benefiting the dehydration reactions [
36] as they form Lewis’s acid sites that can effectively catalyze the dehydration reactions. In the context of HMF, furfural and levulinic acid production, these catalysts can promote the dehydration of precursor compounds, facilitating the formation of the desired products. Lewis’s acids can also activate the carbonyl groups of the reactants; in the case of HMF and levulinic acid synthesis, this activation is essential to initiate key reactions, such as the dehydration of sugars or the formation of intermediates leading to levulinic acid. Finally, Lewis’s acid sites can promote isomerization reactions, which may be involved in the conversion of intermediates into final products. This may improve the selectivity and increase the yield of HMF and levulinic acid [
37].
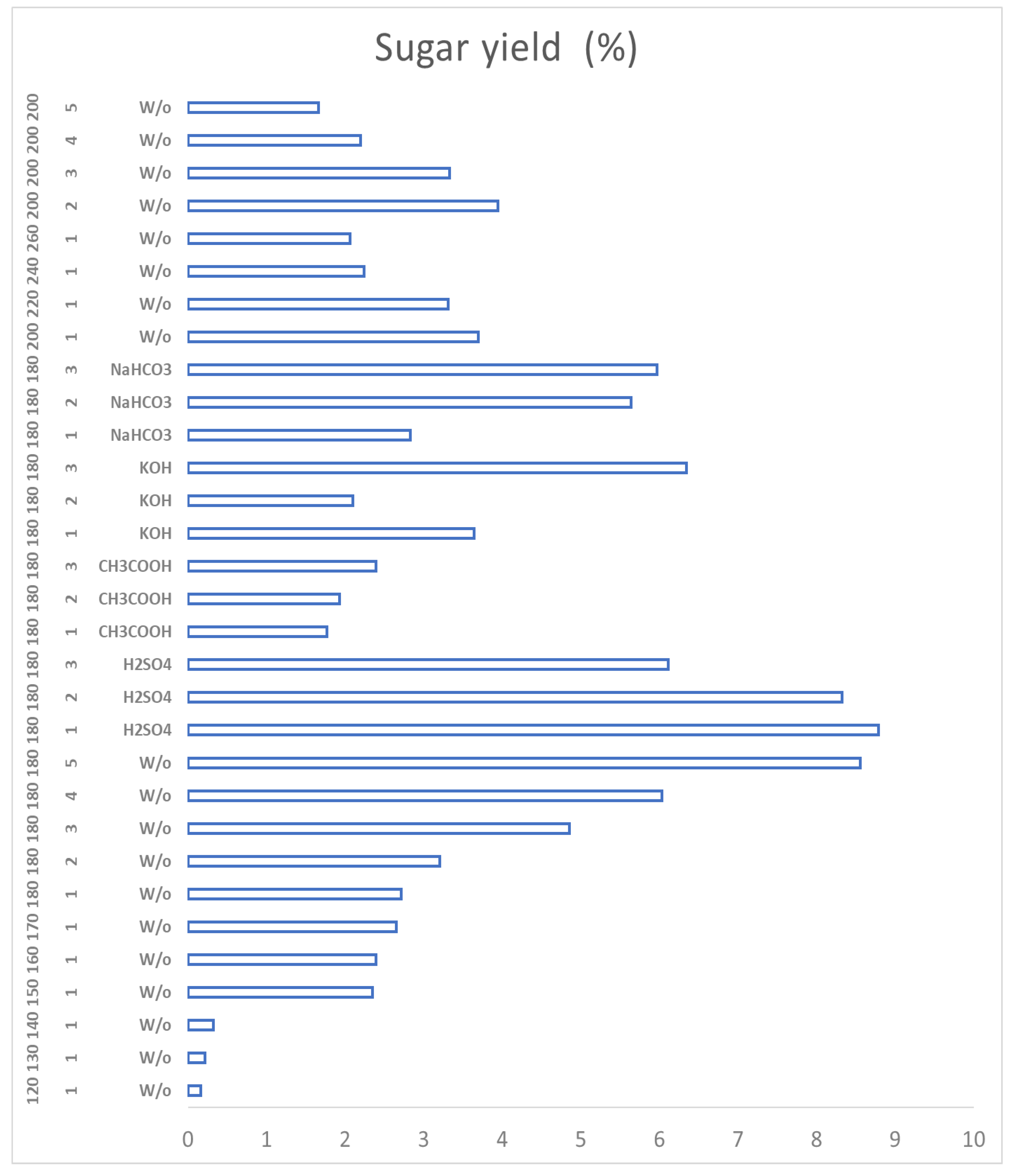
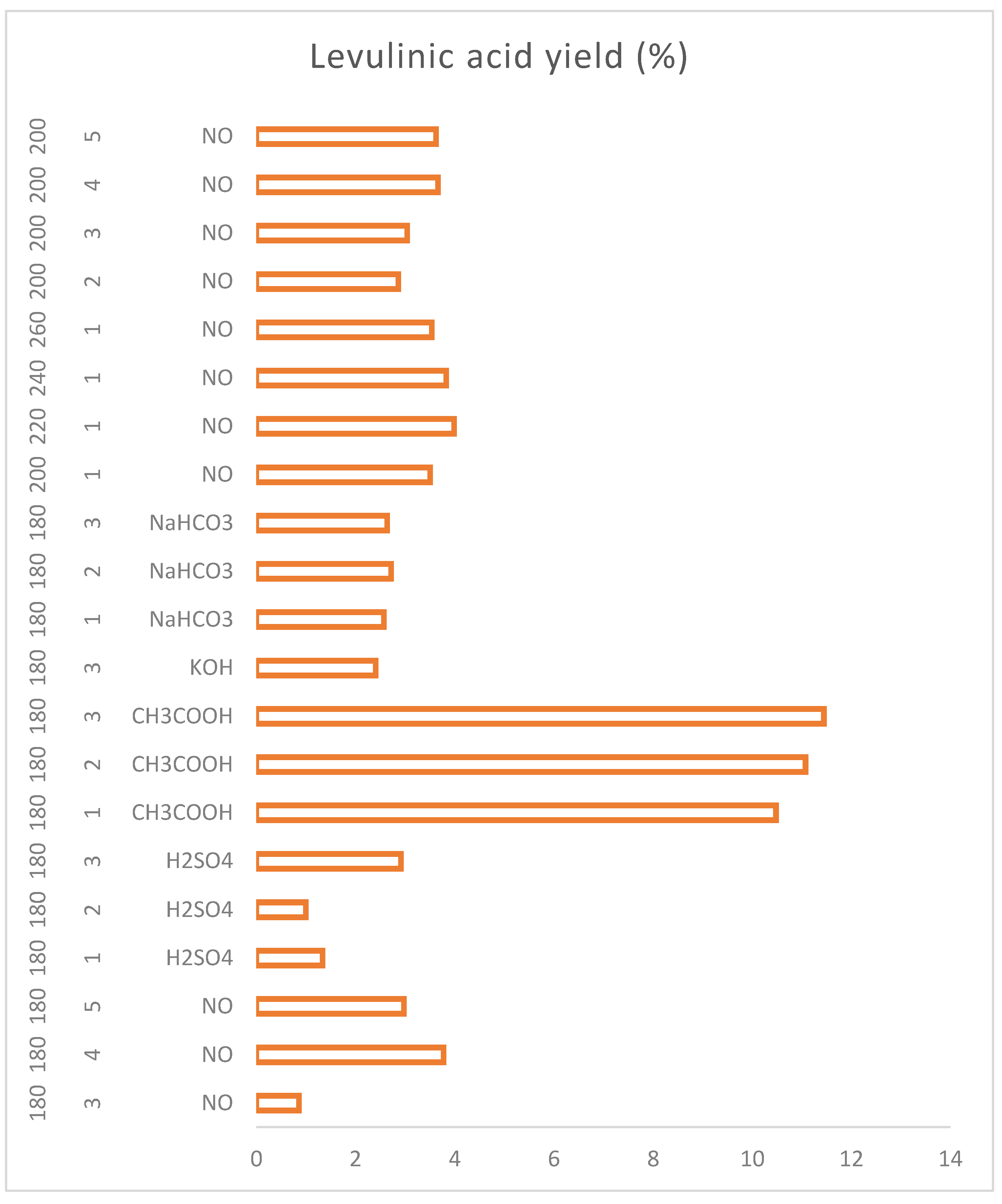
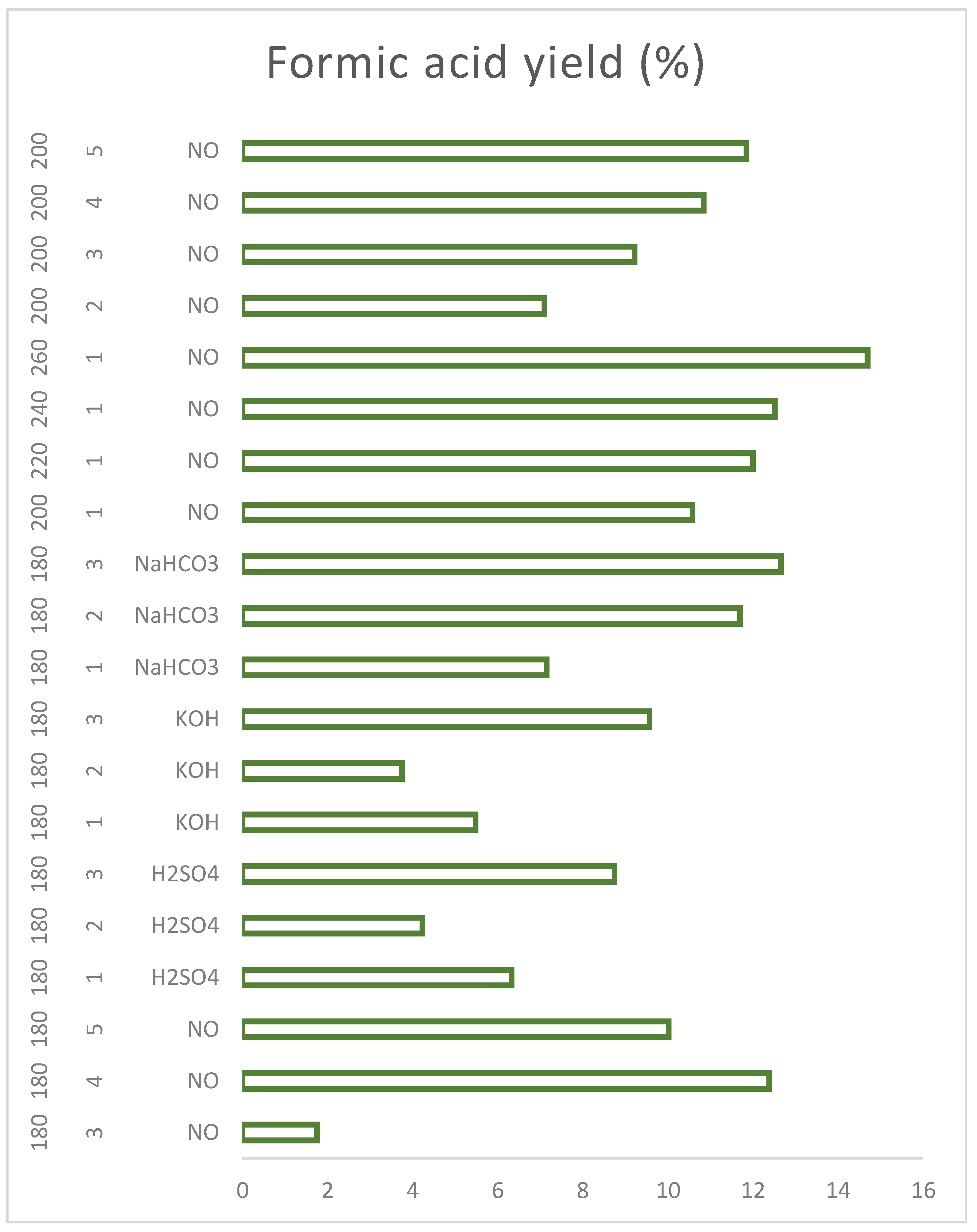
Figure 8 shows a graph with the specific behaviour of the coffee residues that allows to know the functioning of the catalysts and their selectivity in order to select the reaction conditions depending on the desired PCs.
In the case of sulphuric acid, the presence of a variety of platform chemicals can be observed and how they decrease the concentration of sugars (by means of hydrolysis) to proceed to produce organic acids, HMF and furfural. Acetic acid is, on the contrary, a very selective catalyst for levulinic acid, obtaining mostly levulinic acid and non-hydrolysed sugars, and it is also the catalyst with the highest yield against levulinic acid from the first hour. The basic catalysts produce mostly formic acid and levulinic acid, but, unlike the acid catalysts, the concentration of sugars does not decrease over time and more sugars continue to be produced, This may be because the acid medium favours the hydrolysis of cellulose and hemicellulose species more efficiently and rapidly breaking the glycosidic bonds and obtaining sugars from shorter times; on the other hand, the base does not favor these initial breaks in the biomass, so hydrolysis is slower and takes place little by little.
3.4. Hydrothermal valorization via HTC
To cover another area within the hydrothermal processes (HTC), reactions were carried out between 200 and 260 °C for one hour and at 200 °C for 5 hours, starting in all cases from 10 grams of biomass and 90 grams of water. Solid, liquid and gas phase results were obtained as shown in
Table 10.
It is observed that when the temperature is modified between the 200 and 260 °C tests, there is an increase in the amount of liquid fraction and a significant decrease in the gaseous fraction. This can be attributed to the fact that the reaction is mainly going towards the production of biochar, an aspect that is denoted by the decrease in the weight of the solid fraction due to the loss of oxygen and hydrogen molecules from the initial biomass. By preferring the production of biochar, no significant extraction processes are carried out in the liquid fraction (which increases its quantity) and there are no dehydration, decarboxylation or hydration processes that lead to the expulsion of gases into the reaction medium.
On the other hand, for the experiments carried out at constant temperature for different times, there is an increase in the solid fraction and the liquid fraction remains relatively constant, the opposite behavior to that presented by the higher HTC valorization temperatures, and which may imply that the biochar is not yet being fully favoured and the organic species are maintained in the liquid phase.
3.4.1. Preliminary follows up of liquid fraction.
The liquid fraction was monitored in the same way as in the LHW process, using conductivity and pH properties to understand the behavior of dissociated, acidic, basic and neutral species in the medium.
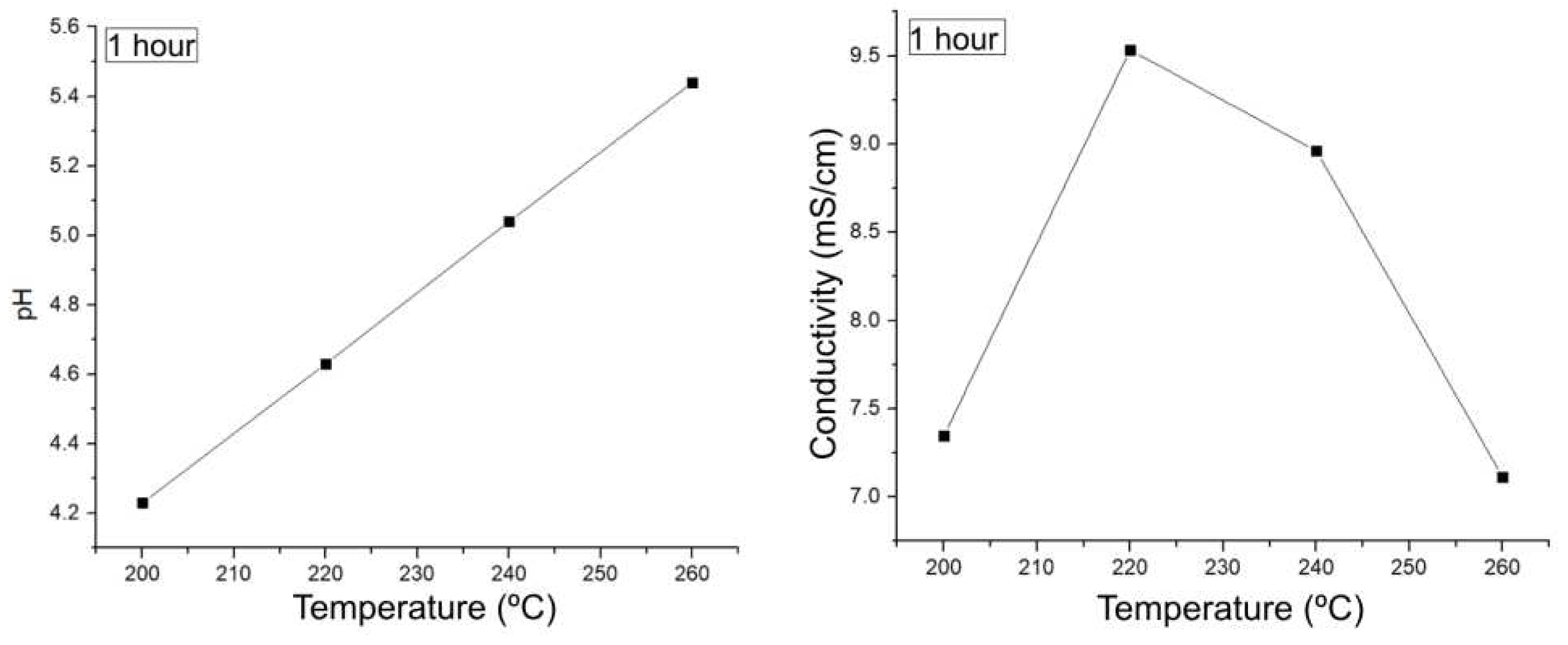
Figure 9 shows how the pH behavior in HTC is opposite to that found in LHW. As the temperature increases in HTC, the pH increases and becomes more basic, this implies a decrease in the acidic species in the medium. As for the conductivity, a maximum occurs at 220 °C, where the highest concentration of a species that dissociates in the medium probably occurs, and then the conductivity decreases following the behavior of the pH, allowing us to conclude that the species of interest produced are decreasing. This goes hand in hand with the analysis in
Table 5-1, where it can be assumed that higher temperatures favor the production of biochar and decrease some of the organic species of interest in the medium.
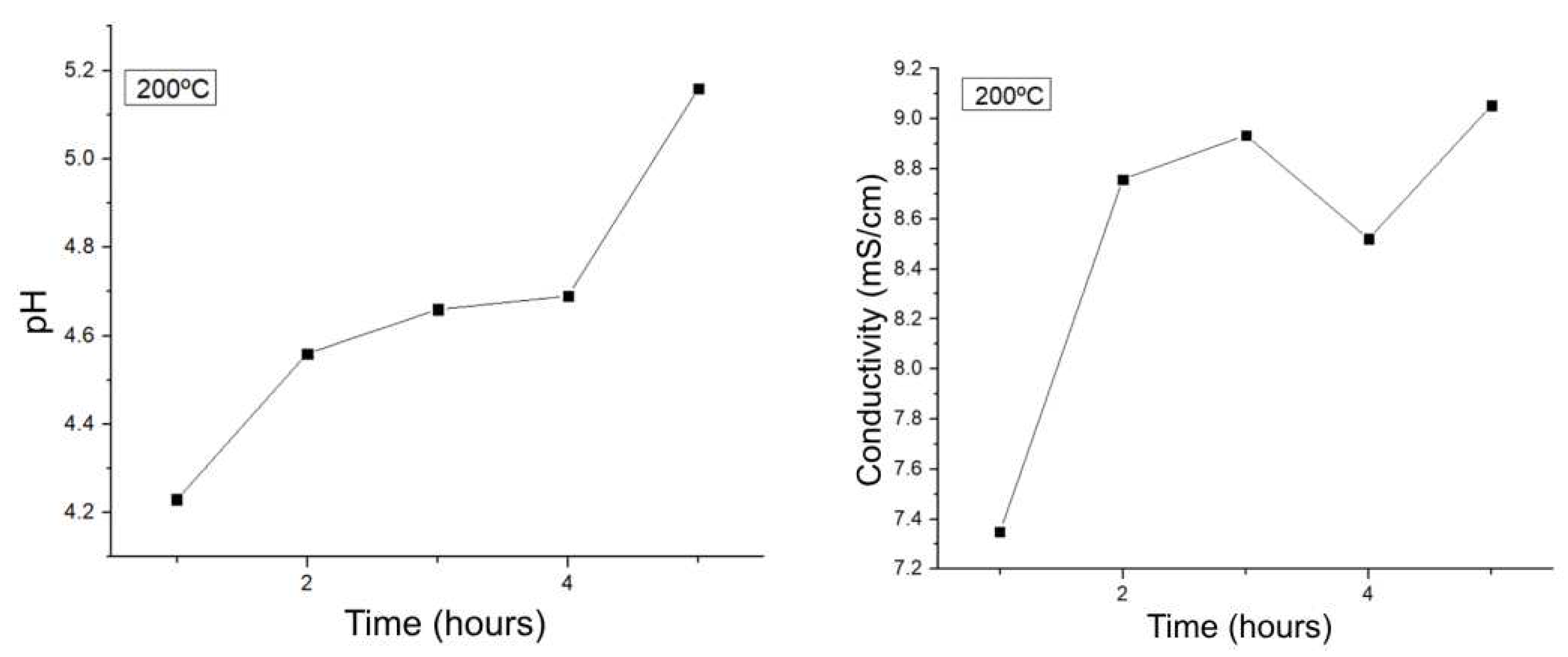
A similar behavior is presented in
Figure 10, where the reaction at 200 °C is followed. There is a minimum in pH in the one-hour reaction, implying that most of the acidic species are present at this time, and then decrease as the reaction continues until 5 hours. As for the conductivity, it keeps oscillating after 2 hours of reaction, which can happen due to the equilibrium present in the system, but it does not decay as it happens from 240 °C onwards.
3.4.2. Characterization of solid fraction
The same technique as in LHW was implemented to make a preliminary follow-up to the solid phase.
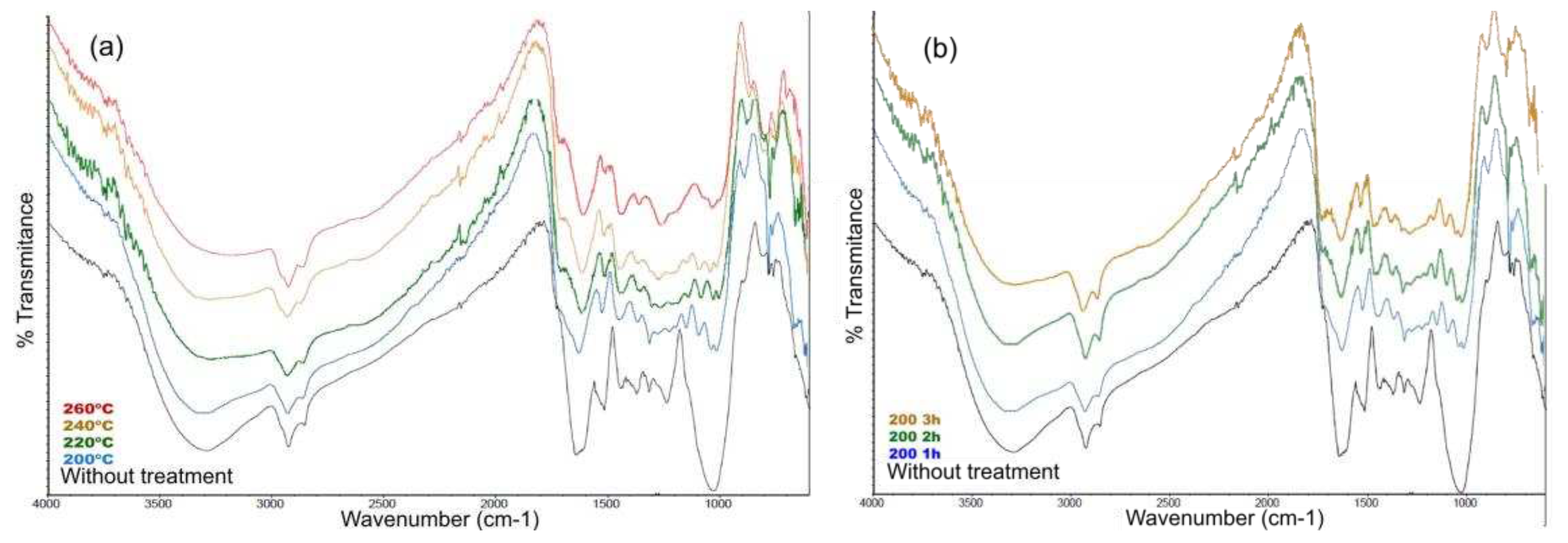
Figure 11 shows the spectra of the hydrothermal processes carried out between 200 and 260 °C, and the signal of the initial biomass.
Table 11 shows the signals that are maintained, although changing their intensity in the biochar spectra. Initially, it can be observed that there is a decrease or disappearance of the signal at 3200 cm
-1 characteristic of the OH vibration, there is also a significant decrease in the signals of the aromatic components, and C=O (ketone and acid), as well as a decrease in the signals between 1500-1350 cm
-1 of amorphous polysaccharides. Finally, and the most significant change, the decrease of the signal at 1030 cm
-1 corresponding to cellulose, hemicellulose and lignin vibrations, showing then that significant hydrolysis processes of the fundamental structures of the lignocellulosic biomass are being carried out in order to proceed to be transformed into bio-char or extracted to the aqueous fraction.
The same monitoring procedure was repeated for the experiments performed at 200 ºC for different times, resulting in
Figure 11b. In this figure, the same changes described above can be seen with respect to the initial biomass and, unlike the LHW process, there are no major changes between the spectra at different times, which suggests that the greatest change in the solid is found between the temperatures of 180-200 ºC where the lignocellulosic structures of the biomass are broken.
In order to monitor and partially characterize the solid fraction, elemental analysis of the solid obtained in each of the hydrothermal valorization processes was carried out, obtaining the
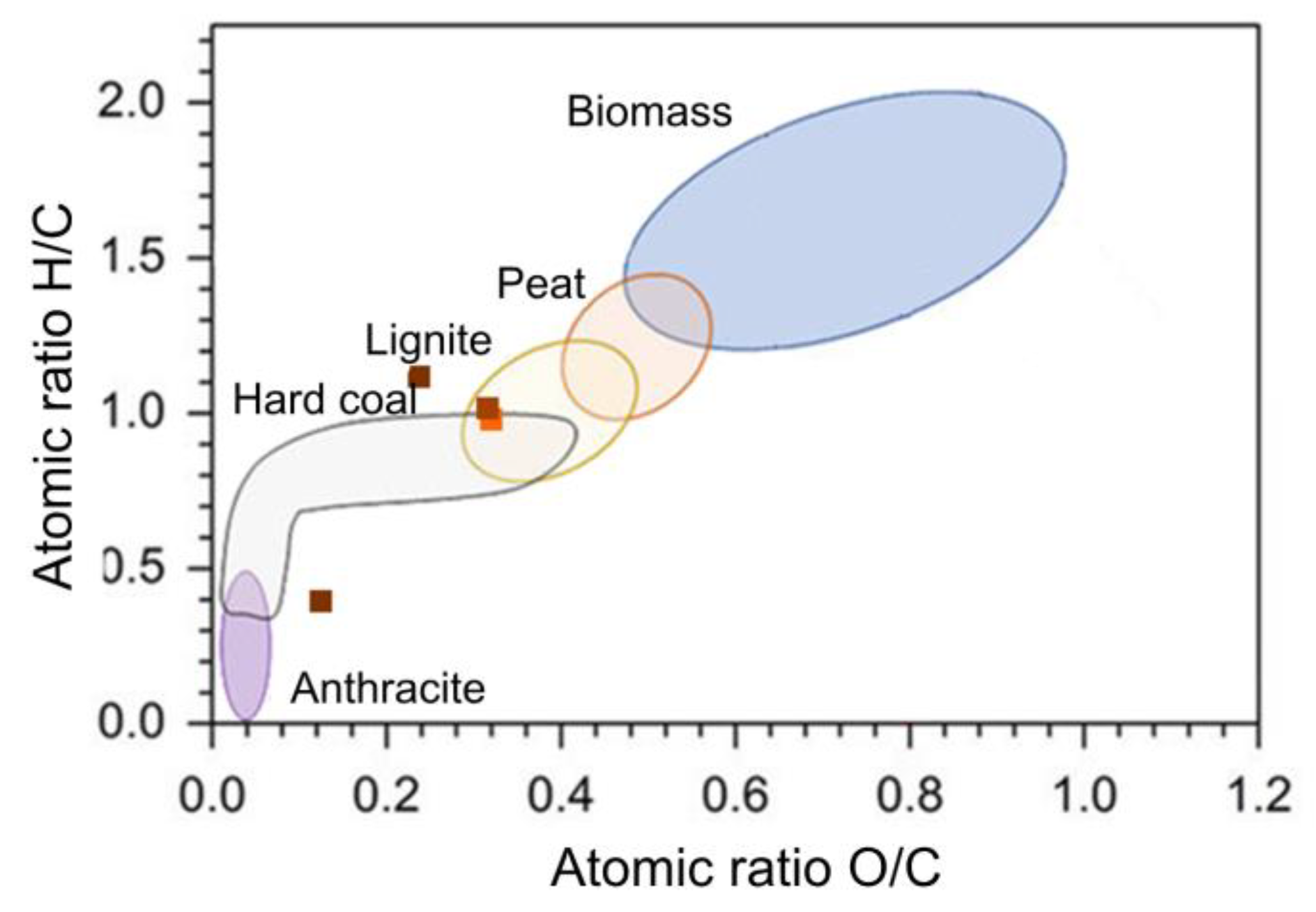
Table 12.
Figure 12 shows the location of the H/C and O/C ratios in the Van Krevelen diagram where the different fractions were characterized as lignite, hard coal and anthracite, with the highest temperature achieving a significant biomass transformation due to the loss of oxygen and hydrogen. Anthracite can be used as a fuel and hard coal can be used as a coating for paints and binders. On the other hand, as the temperature increases, the organic characteristics of the biomass are lost, leaving mostly inorganic components that affect the C/N ratio and make these biochars unsuitable for implementation in processes related to micro-organism treatments. Comparing
Table 12 with that previously obtained for LHW, it is found that there is a greater change in the percentages of carbon, hydrogen, and nitrogen, and therefore, that processes above 200ºC lead to carbonization of the biomass and obtaining new characteristics in the biomass.
3.4.3. Quantification of platform chemicals
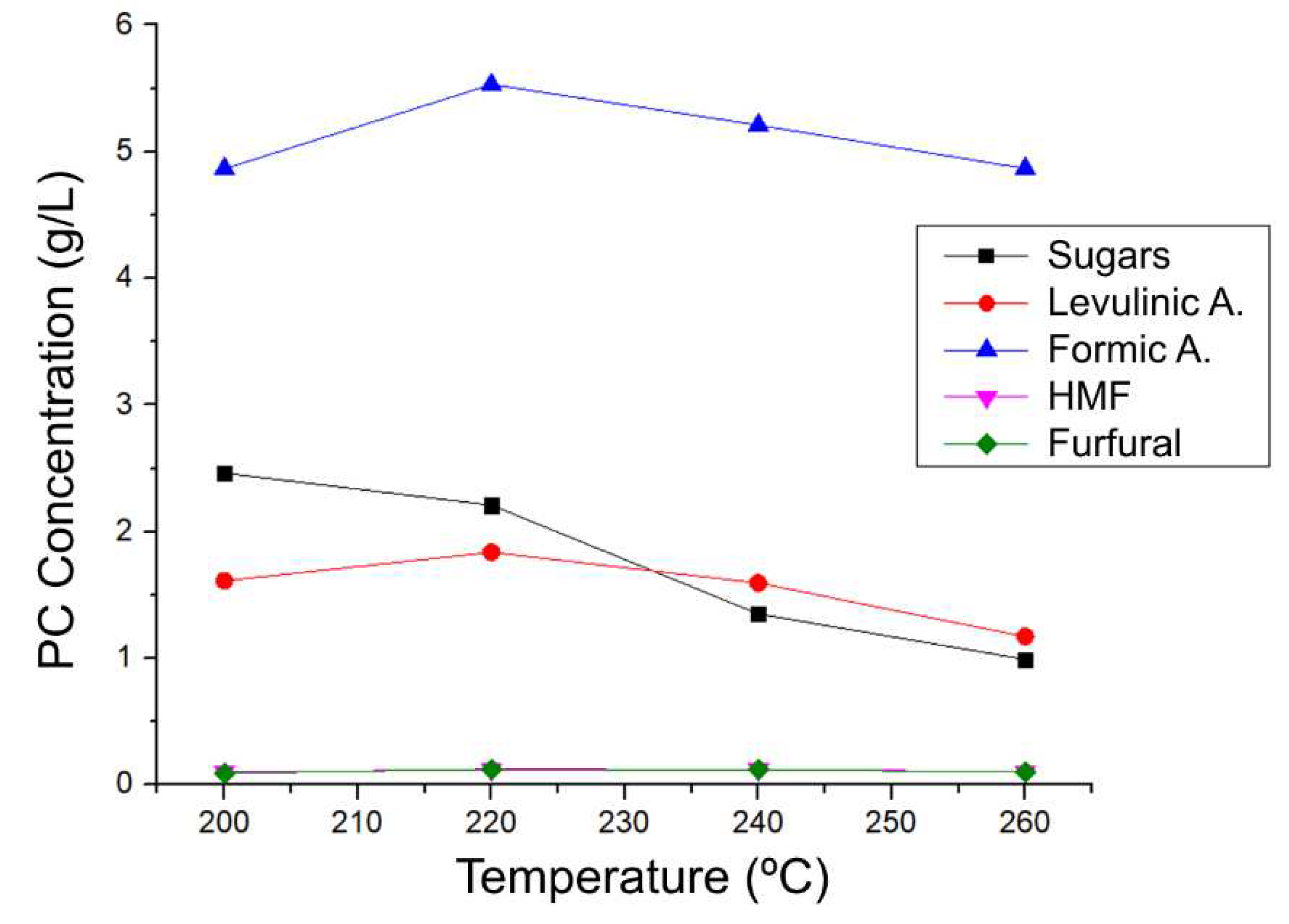
For the quantification process, undiluted liquid fractions were injected into the chromatograph using the previously validated method to qualitatively monitor the production of platform chemicals of interest through hydrothermal HTC processes from 200 ºC to 260 ºC for one hour, resulting in
Table 13 and
Figure 13.
Figure 13 shows that the highest concentrations were obtained at 220 ºC (for acids) and at 200ºC for sugars, while HMF and Furfural remained relatively constant, this information goes hand in hand with the results previously obtained for the conductivity of these experiments, where a maximum of conductivity was presented at 220ºC caused by the increase in acidic species. From that temperature onwards, the concentrations start to decrease for all compounds except HMF and Furfural, due to degradations of the PCs. On the other hand, when observing
Table 13, the yield (calculated based on the corresponding lignocellulosic structure) of all the processes is very close, but 260ºC is the one with the highest yield, although the concentration of PCs is low compared to the others. This is because the final volume of the reaction at 260ºC (83.312 mL) is greater than that of the other temperatures (62 mL on average), and because the way of evaluating the yield is (g of PC/g of lignocellulosic structure) *100%, having a greater final volume, the grams in these mL are greater than those of other reactions with higher concentrations, but smaller volumes. It is also worth noting that HTC processes with coffee residues lead mostly to the formation of formic acid and the concentration of sugars decreases compared to LHW processes.
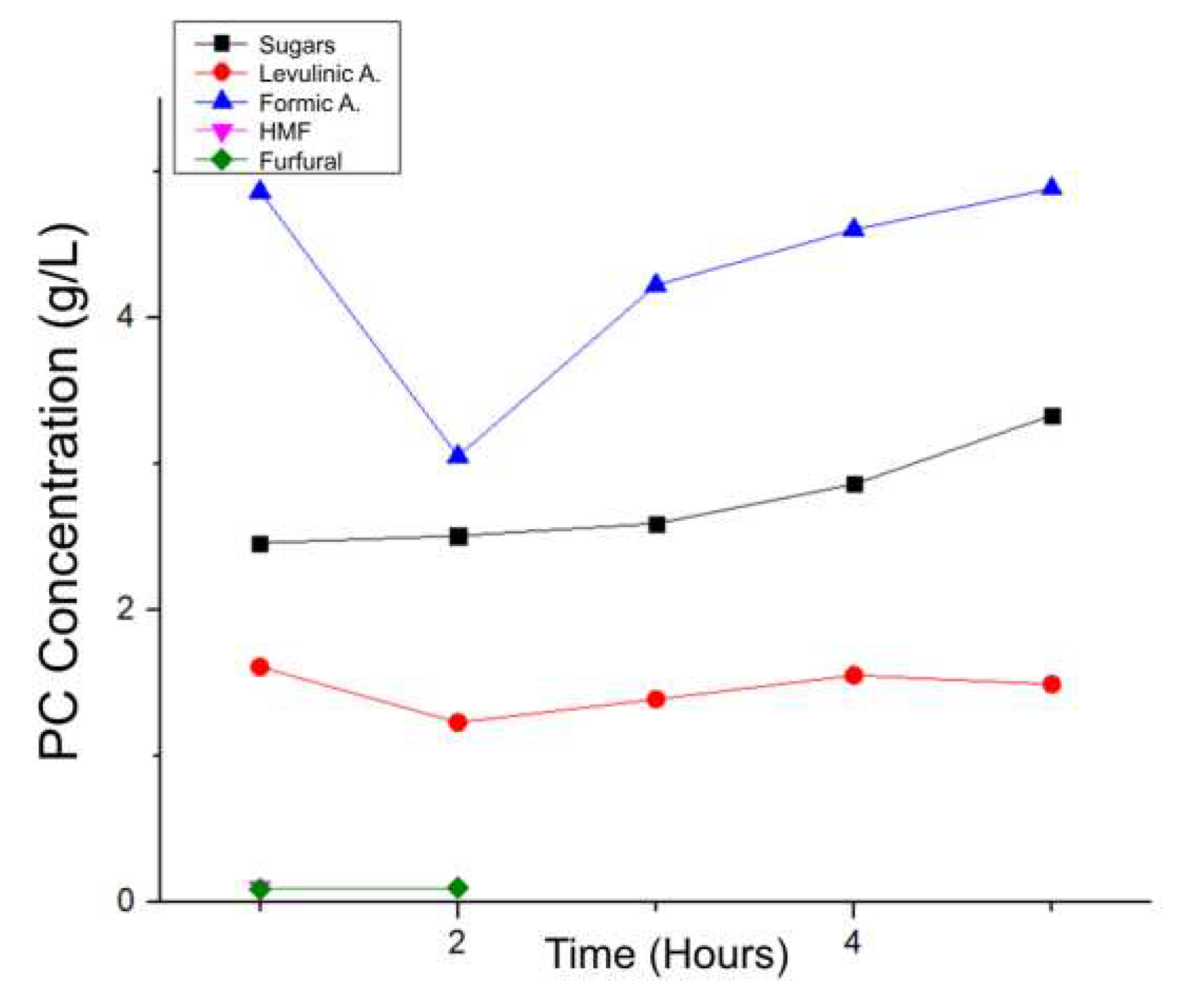
Table 14 and
Figure 14 show the PCs results for the hydrothermal processes at constant temperature and varying time:
In the case of sugars and organic acids, an increase is observed as the reaction hours go by, while on the other hand, HMF concentrations decrease to the point of being below the calibration curve and not being relevant compared to the other species produced. A maximum yield of 21.049%w was achieved, a value similar to that obtained for the process at 260 ºC for 1 hour but obtaining a greater quantity of sugars instead of products such as HMF and furfural.
3.5. Kinetics
3.5.1. Kinetics of LHW
In order to analyze the behavior of hydrothermal reactions, the reason why they are carried out must be understood. Water is implemented as a reactant in these reactions due to its capacity to self-ionize, generating hydronium and hydroxyl ions that are free in the aqueous phase. As the temperature of the water increases, the ratio of hydronium and hydroxyl ions is maintained, but the concentration of these ions’ changes, causing both the Kw and the pH of the water to change as the temperature increases. The higher the temperature, the higher the concentration of free ions because the dissociation of water requires energy, this being an endothermic reaction, and the higher the concentration of hydronium ions, the more hydrolysis processes are favored by generating a positive charge on the oxygen of the β-1-4 glycosidic bonds and favoring the breaking of the bonds.
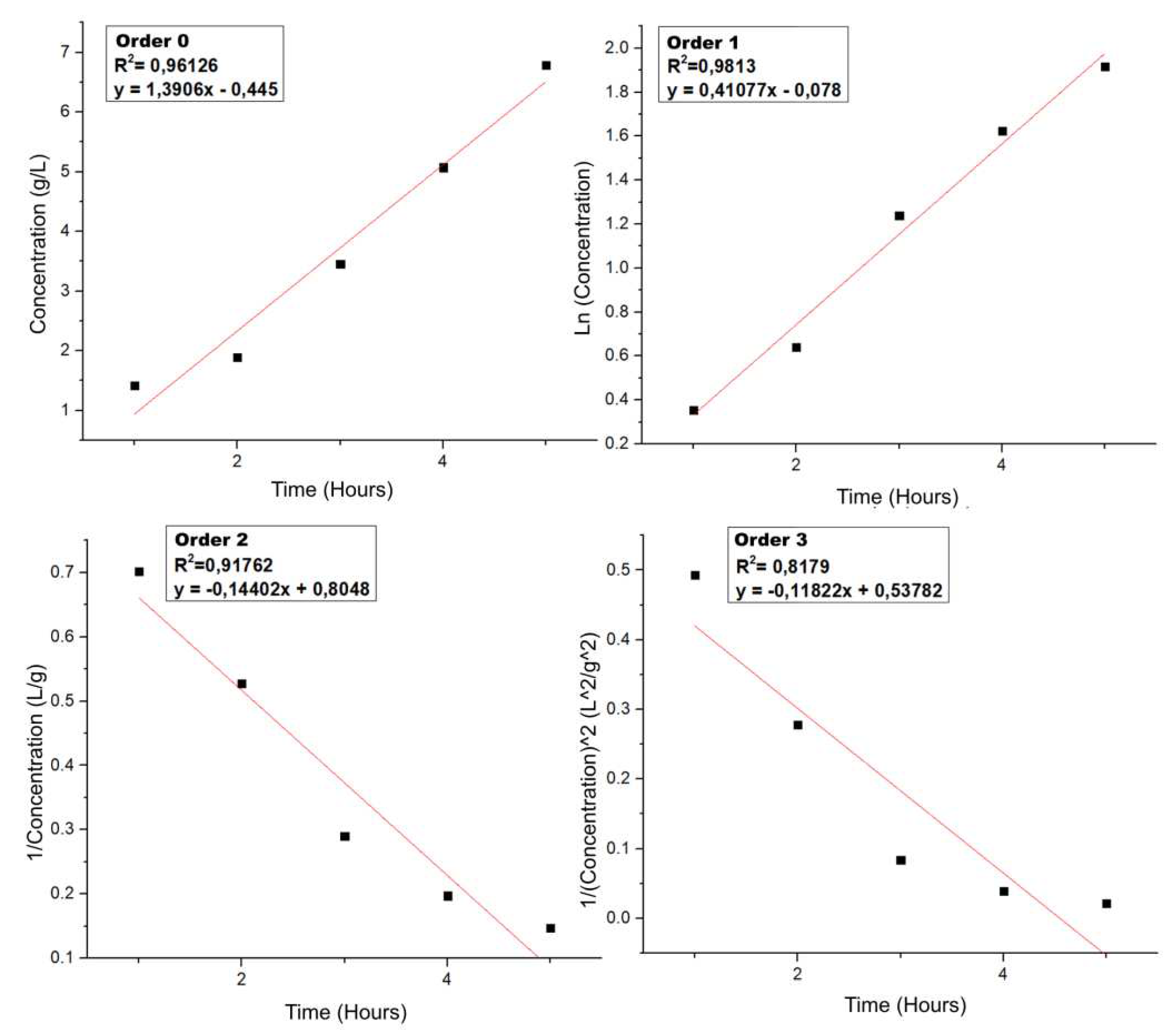
To analyze the kinetics of the initial hydrolysis of the lignocellulosic biomass to sugars, the concentrations obtained were taken as a function of time at 180 ºC by HPLC-IR and the respective mathematical treatments were made at kinetics of order 0, 1, 2 and 3 to obtain
Figure 15.
When comparing the correlation with the linear regression of each order, it is observed that the best R2 is that of order 1, which allows us to say that it is a pseudo-first order reaction, as it behaves like a first order reaction because the rate equation of the reaction is expressed as a first order rate equation. The reaction is higher order because it involves more than one reactant (water and lignocellulosic biomass), because one of the reactants is present in excess, its concentration remains apparently unchanged throughout the reaction. Thus, the reaction, when dependent on the concentration of the other reactant, then the result apparently follows a first order rate equation.
The fact that it is a pseudo first order reaction means that the rate of reaction (hydrolysis to sugars) is directly proportional to the concentration of the lignocellulosic biomass, therefore, the equation governing the process of hydrolysis of biomass to sugars in LHW is equation 6 and the rate constant is the corresponding slope in
Figure 15 order 1 (0.41077 s
-1).
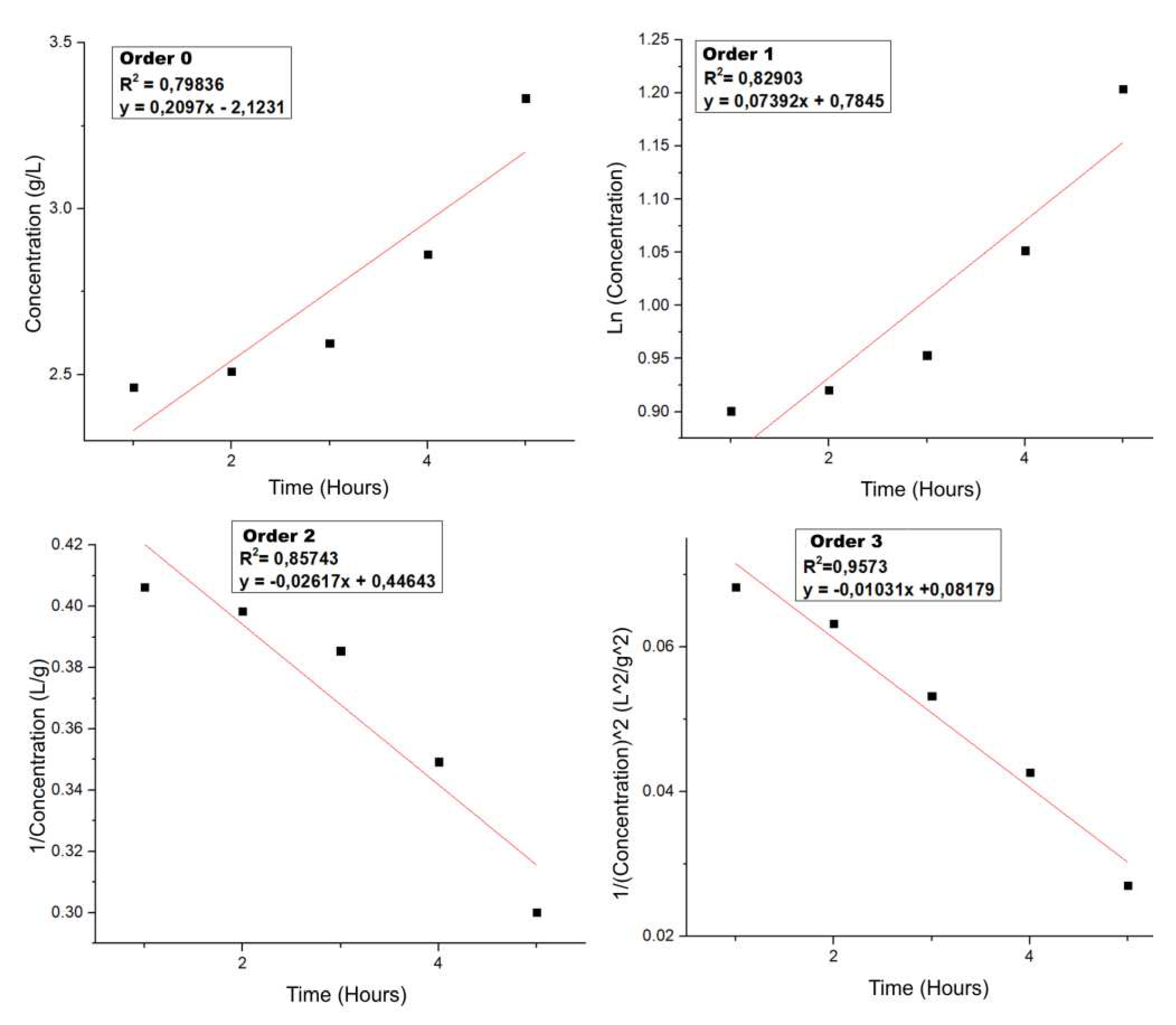
3.5.2. Kinetics of HTC
To describe the kinetics of the HTC process, the same technique previously introduced for LHW was implemented, at a constant temperature of 200 ºC and at different times, the relevant mathematical treatments were carried out and the
Figure 16 was obtained.
In the case of HTC, it is observed that the behavior is similar to that of a higher order kinetics, in these reactions more than two species are involved, an aspect that can be explained due to the fact that the higher temperature makes hydrolysis more effective, but in the same way it does not stop at the sugars, as it did in LHW, but the reaction continues until levulinic and formic acid and then other compounds that derive from the degradation of these. It is also important to note that at the working temperature biochar is also being produced, this being another species that affects the kinetics of the reaction.
The rate constant corresponds to the slope of the higher order graph (0.0103 L/g.s) and the equation (7) governing the process is presented below:
This shows that the reactions carried out in HTC are more complex than those in LHW, the hydrolysis process is accompanied by other species and does not stop at the sugars but continues the process up to the other PCs. The production of acids, furfural, HMF, gases, biochar and other products make it impossible to follow the kinetics of specific reactions, as the chemical processes of hydrothermal valorization are simultaneous and can be favored/inhibited by the species present in the medium, making the kinetics and their description more complex.
3.6. Optimization based on platform chemicals.
Considering the experiments carried out, the mechanism and the yields based on each platform chemical, the optimal conditions to favor the specific obtaining of each PC are reported.
For sugars,
Figure 17 shows that the use of H
2SO
4 favors the production of sugars at 180ºC in less time and more selectively, but that similar concentrations can be reached with reactions at 180ºC, but for a longer time (5 hours), because these processes allow the hydrolysis of the lignocellulosic structures to take place, but the energy provided to the system is not enough for the subsequent reactions to take place. It is important to emphasize that the energy expenditure to carry out the reaction for 5 hours is higher than that involved in a one-hour reaction. Weak basic catalysts also act favorably for the process and could be successfully implemented. On the other hand, the temperature increases above 220°C and the weak acid catalyst act as inhibitors of the sugar production process and favor the production of other products.
The production of levulinic acid is also not favoured at low temperatures as there is not enough energy for the reactions characteristic of hydrothermal processes to take place.
Figure 18 shows how the process of obtaining levulinic acid requires the use of a catalyst that increases the yield and displaces the hydrothermal reactions to obtain it. With the results of the present work, the use of weak acid catalysts such as acetic acid is suggested, since, as previously presented, strong acid catalysts lead to the majority production of sugars and not the breakdown of these to levulinic acid.
The increase of reaction time and temperature are actors that do not drastically change the yield of levulinic acid and therefore a catalyst is highly recommended.
As for formic acid,
Figure 19 shows that the process is not favored at low temperatures and short reaction times, which is why it is not produced below 180ºC-3h, because although there is enough energy to hydrolyze even sugars, the condensation and hydration reactions that form formic acid are not fully favored. The best condition for obtaining this PQP is the implementation of HTC especially at temperatures above 240°C, although significant concentrations can also be obtained by increasing the reaction time at temperatures not as high as 180 and 200°C. In the same way, the implementation of weak basic catalysts leads to the formation of formic acid in less time and under LHW conditions.
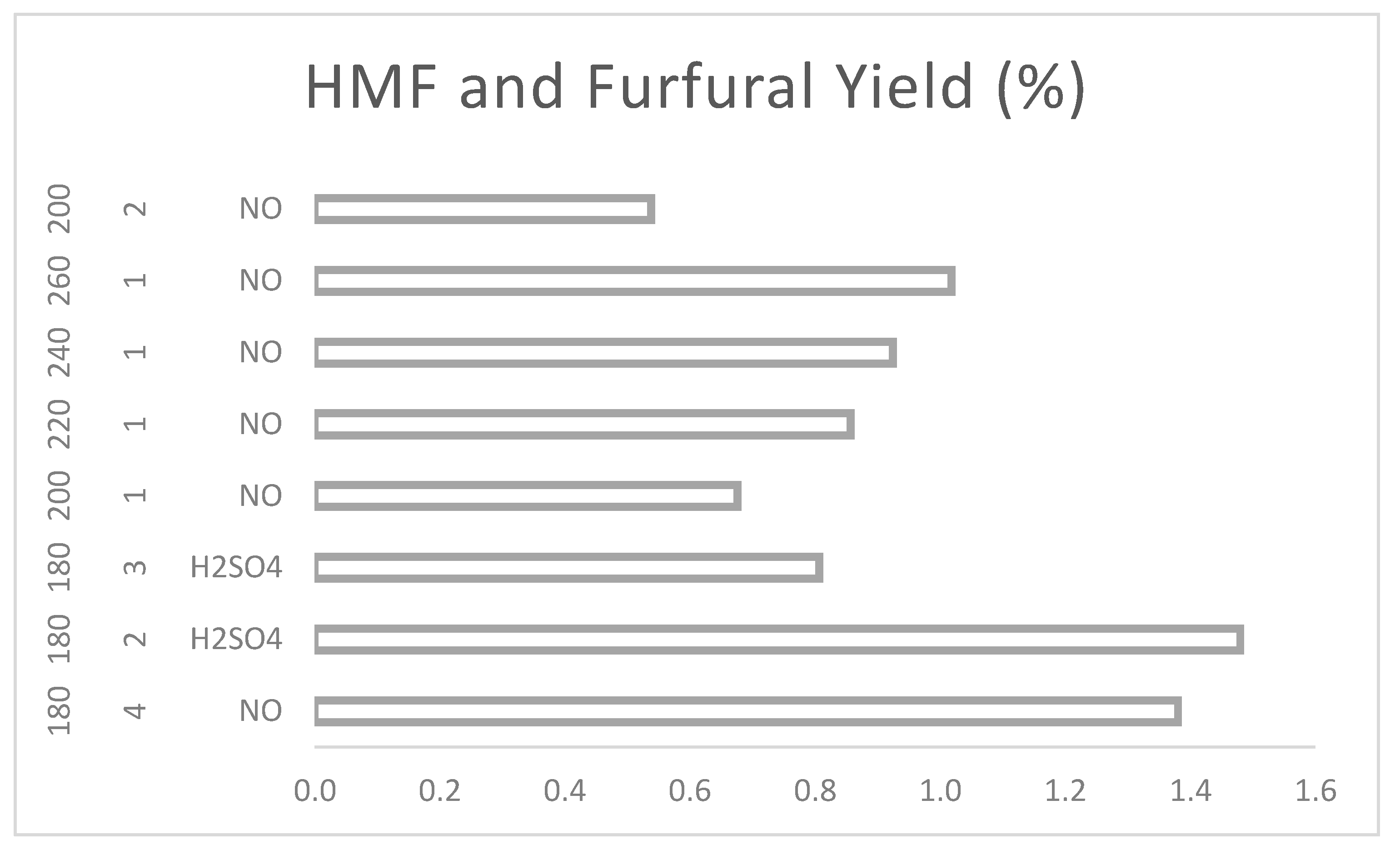
HMF and furfural are the minority products of the hydrothermal reactions carried out with coffee residues, achieving, as shown in
Figure 20, a maximum yield of 1.480%w based on the corresponding lignocellulosic structures. In order to obtain these products, temperatures above 180 ºC must be used, supported either by a longer reaction time or by strong acid catalysts. Reactions above 200 °C and for more than one hour start to cause degradation of the products and are therefore not suggested and it is desired to stop the reaction on these specific products.
3.7. Purification
To perform the separation of the formic acid, the boiling point difference of the system was used, implementing a simple distillation system at 100 ºC to eliminate both water and formic acid. After the formic acid was collected, it was injected into the HPLC-IR to check the purity of the separated product.
Preliminary separation tests were performed with the remaining mixture from the distillation (mixture of sugars and levulinic acid). The liquid-liquid separation with octanol to extract the levulinic acid in the organic phase and the sugars in the aqueous phase was not successful because levulinic acid has polar characteristics, which means that the partition is not completely carried out and a large amount of the sample remains in the aqueous phase. In the same way, separation was attempted by implementing a column chromatography with a silica gel stationary phase and ethanol mobile phase followed by water due to the affinity of levulinic acid towards ethanol. The process was again unsatisfactory due to the similarity in polarity of the compounds to be separated.
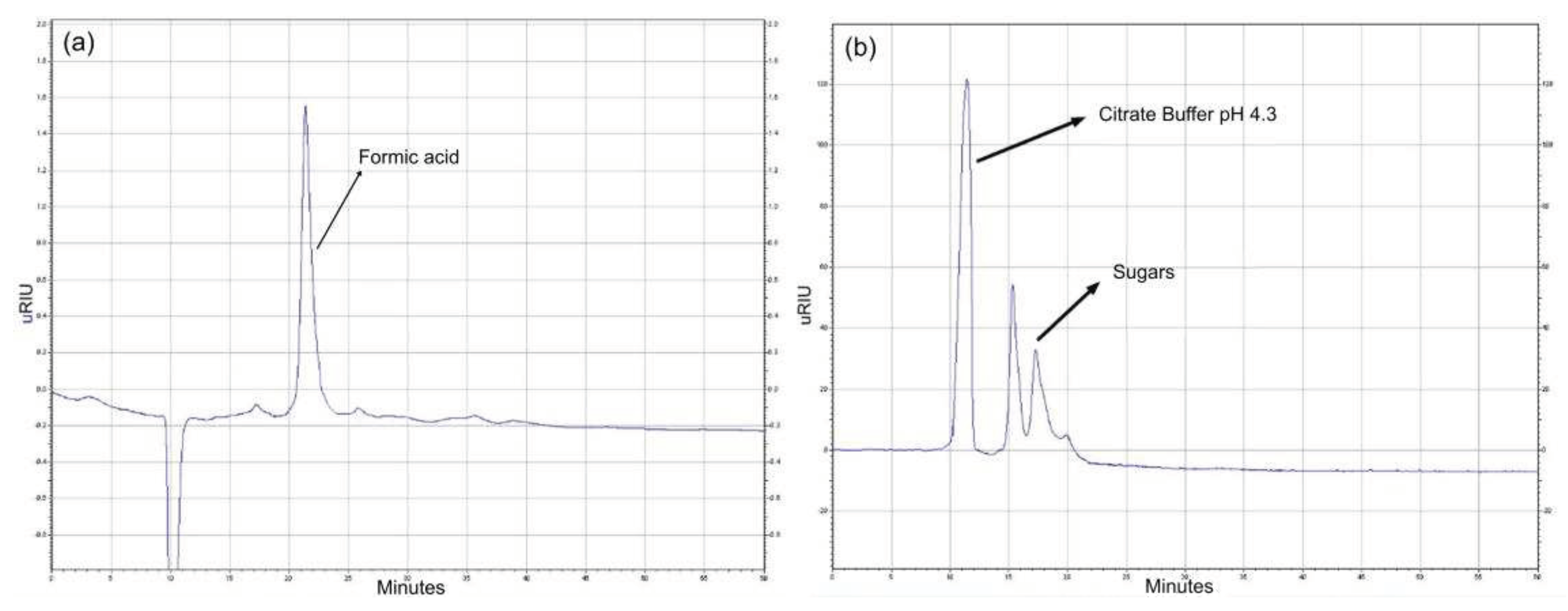
Due to the inability to separate by polarity, ion exchange (AMBERLITE IRA 400), cationic (DOWEX-50W) and anionic:cationic (DOWEX* MARATHON* MR-3) resins were implemented. Considering the behavior of an acid when it is at a pH below its pKa, as is the case of levulinic acid, it would be expected that this would be in its anionic form at the elution pH of the first buffer (4.3), and that therefore the most suitable resin for the separation process would be AMBERLITE IRA 400, which would retain the levulinic acid in its levulinate form while leaving the sugars in the mixture to elute. This process was confirmed by obtaining zero separation of the desired compounds in the case of the DOWEX MARATHON MR-3, while in the case of the DOWEX-50W a separation was achieved, but of some colored compounds that do not absorb in the IR (a brown colored spot was retained), but not of levulinic acid and sugars. Finally, as expected, when implementing the anion exchange column, levulinic acid was retained as levulinate when eluting the column at pH 4.3 while the sugars came out with the buffer (
Figure 21). Subsequently, when the pH of the buffer was changed above the pKa of the levulinic acid, the levulinic acid started to elute without sugars, thus achieving its separation.
It is therefore suggested that separation methods such as liquid-liquid partitioning and silica gel column chromatography are not suitable for the separation of the platform chemicals. On the other hand, distillation followed by anion exchange chromatography can be implemented to separate and purify the desired compounds. It is worth noting that in this process neither HMF nor furfural were separated due to their low concentrations and the loss of these in the distillation process due to possible residues in the assembly.
5. Conclusions
The study characterized coffee waste biomass through ultimate, proximate, and structural tests. It was found to be highly humid (80.79%) with fuel characteristics suitable for biorefinery processes and microorganism valorization processes. The biomass contained similar percentages of hemicellulose (12.5%), lignin (13.7%), and hemicellulose (27.6%).
For the LHW range, reactions below 150 °C did not allow hydrolysis of lignocellulosic structures to sugars. pH and conductivity were used as properties to monitor PQP production. The solid fractions were classified according to fuel type and infrared spectroscopy was used to follow the disappearance of functional groups from lignin, cellulose, and hemicellulose between 1000 and 1500 cm-1 and their relationship with the production of platform chemicals. The best conditions for obtaining platform chemicals were achieved at 180 °C at 4 hours, with a yield of 23.565%w. The efficiency and selectivity of homogeneous catalysts were tested, with acetic acid leading to selective production of levulinic acid, sulphuric acid leading to the highest efficiency of hydrolysis of sugars, potassium hydroxide leading to the production of biochar, formic acid, and sugars, and sodium bicarbonate producing formic acid and levulinic acid.
The kinetics of the hydrolysis of lignocellulosic structures to sugars in the LHW range was evaluated, with orders of 1 with their respective rate constant. Purification of PQPs was achieved using distillation techniques and anion exchange chromatography with AMBERLITE IRA 400 exchange resin. HTC valorization trials were conducted, with the solid phase characterizing as hard coal and anthracites. Optimal conditions for obtaining sugars involved using sulphuric acid as a catalyst or 180 °C for 5 hours, formic acid reactions should be carried out at HTC conditions preferably 260 °C, and levulinic acid requires weak acid catalysts like acetic acid. HMF and furfural were benefited by strong acid catalysts at 180°C or a longer reaction time. In conclusion, coffee cherry waste as a raw material for hydrothermal biorefinery is viable and favored for obtaining glucose, xylose, levulinic acid, and furfural, with lower percentages of HMF and furfural.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Table S1: Retention time for standards used; Figure S1: Standards used for analytic method development. Table S2: Information of calibration curves for platform chemicals (PC); Figure S2: Calibration curves done for every PC.; Figure S3: Residuals of calibration curves; Table S3: Repeatability of the system based on the retention time; Table S4: Repeatability of the system based on the area; Table S5: Results for the repeatability of the method; Table S6: Results for intermediate precision for the method developed; Table S7: Accuracy of the results obtained for the method; Table S8: Detection and quantification limit for the method; Table S9: Results for robustness of the method.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, Lozano-Perez, Alejandra S..; methodology, Lozano-Perez, Alejandra S..; validation, Lozano-Perez, Alejandra S.; formal analysis, Lozano-Perez, Alejandra S.; investigation, Lozano-Perez, Alejandra S.; resources, Guerrero-Fajardo, Carlos A.; data curation, Lozano-Perez, Alejandra S.; writing—original draft preparation, Lozano-Perez, Alejandra S.; writing—review and editing, Guerrero-Fajardo, Carlos A.; supervision, Guerrero-Fajardo, Carlos A.; project administration, Guerrero-Fajardo, Carlos A.; funding acquisition, Lozano-Perez, Alejandra S. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by MINCIENCIAS, grant number Contrato de financiamiento de recuperación contingente No. 80740-101-2022.
Acknowledgments
We thank the Universidad Nacional de Colombia and the Departamento de Química for their support and the possibility of using equipment and techniques that allowed the development of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Word Population Review, “Coffee Consumption by Country 2023,”. Available online: https://worldpopulationreview.com/country-rankings/coffee-consumption-by-country,.
- International Coffee Organization, “Trade Statistics—December 2021. Available online: http://www.ico.org/#:~:text=Total%20production%20for%20coffee%20year,remains%201.2%25%20below%20world%20production.
- D. Apriani, F. Marissa, G. Gustriani, and H. Sinta, “Determinants of Indonesia’s Coffee Commodity with Trading Partner Countries,” Jurnal Ekonomi Pembangunan: Kajian Masalah Ekonomi dan Pembangunan, vol. 23, no. 1, pp. 98–109, Jun. 2022. [CrossRef]
- C. Arruda, V. P. R. Minim, M. A. M. Ferreira, L. A. Minim, N. M. da Silva, and C. F. Soares, “Justificativas e motivações do consumo e não consumo de café,” Ciência e Tecnologia de Alimentos, vol. 29, no. 4, pp. 754–763, Dec. 2009. [CrossRef]
- Folch and J. Planas, “Cooperation, Fair Trade, and the Development of Organic Coffee Growing in Chiapas (1980–2015),” Sustainability, vol. 11, no. 2, p. 357, Jan. 2019. [CrossRef]
- FAO Statistics, “World Coffee Production by Country,” 2019.
- Federación de Cafeteros, “Colombian coffee production closes 2021 at 12.6 million bags,”. Available online: https://federaciondecafeteros.org/wp/listado-noticias/colombian-coffee-production-closes-2021-at-12-6-million-bags/?lang=en.
- D. Peshev, D. Mitev, L. Peeva, and G. Peev, “Valorization of spent coffee grounds—A new approach,” Sep Purif Technol, vol. 192, pp. 271–277, Feb. 2018. [CrossRef]
- P. Mazzafera, “Degradation of caffeine by microorganisms and potential use of decaffeinated coffee husk and pulp in animal feeding,” Sci Agric, vol. 59, no. 4, pp. 815–821, Dec. 2002. [CrossRef]
- R. K. Kasongo, A. Verdoodt, P. Kanyankagote, G. Baert, and E. Van Ranst, “Coffee waste as an alternative fertilizer with soil improving properties for sandy soils in humid tropical environments,” Soil Use Manag, vol. 27, no. 1, pp. 94–102, Mar. 2011. [CrossRef]
- S. Abdeltaif, K. SirElkhatim, and A. Hassan, “Estimation of Phenolic and Flavonoid Compounds and Antioxidant Activity of Spent Coffee and Black Tea (Processing) Waste for Potential Recovery and Reuse in Sudan,” Recycling, vol. 3, no. 2, p. 27, Jun. 2018. [CrossRef]
- N. Rodriguez, “Manejo de Residuos en la Agroindustria Cafetera,". Available online: https://silo.tips/download/manejo-de-residuos-en-la-agroindustria-cafetera.
- Asociación Interamericana de Ingeniería Sanitaria y Ambiental, “Gestión Integral de residuos sólidos urbanos,” 2018.
- D. Gallego, J. Montoya, and J. Valverde, “Funcionamiento hidráulico de un filtro anaerobio de flujo ascendente,” Ingeniería química, 2007.
- L. Mendoza Gomez, “Manual de Lombricultura,” 2008.
- S. C. de Bomfim et al., “Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment,” Waste, vol. 1, no. 1, pp. 2–20, Aug. 2022. [CrossRef]
- S. Vijayalaxmi, S. K. Jayalakshmi, and K. Sreeramulu, “Polyphenols from different agricultural residues: extraction, identification and their antioxidant properties,” J Food Sci Technol, vol. 52, no. 5, pp. 2761–2769, May 2015. 20 May. [CrossRef]
- N. Solomakou, P. Tsafrakidou, and A. M. Goula, “Valorization of SCG through Extraction of Phenolic Compounds and Synthesis of New Biosorbent,” Sustainability, vol. 14, no. 15, p. 9358, Jul. 2022. [CrossRef]
- N. C. Silva et al., “Pretreatment and enzymatic hydrolysis of coffee husk for the production of potentially fermentable sugars,” Journal of Chemical Technology & Biotechnology, vol. 97, no. 3, pp. 676–688, Mar. 2022. [CrossRef]
- K. C. Kemp, S. Bin Baek, W.-G. Lee, M. Meyyappan, and K. S. Kim, “Activated carbon derived from waste coffee grounds for stable methane storage,” Nanotechnology, vol. 26, no. 38, p. 385602, Sep. 2015. [CrossRef]
- D. Kim, K. Lee, D. Bae, and K. Y. Park, “Characterizations of biochar from hydrothermal carbonization of exhausted coffee residue,” J Mater Cycles Waste Manag, vol. 19, no. 3, pp. 1036–1043, Jul. 2017. [CrossRef]
- L.-P. Xiao, Z.-J. Shi, F. Xu, and R.-C. Sun, “Hydrothermal carbonization of lignocellulosic biomass,” Bioresour Technol, vol. 118, pp. 619–623, Aug. 2012. [CrossRef]
- M. Plaza and C. Turner, “Pressurized hot water extraction of bioactives,” TrAC Trends in Analytical Chemistry, vol. 71, pp. 39–54, Sep. 2015. [CrossRef]
- M. Çam et al., “Simultaneous extraction of phenolics and essential oil from peppermint by pressurized hot water extraction,” J Food Sci Technol, vol. 56, no. 1, pp. 200–207, Jan. 2019. [CrossRef]
- J. Massaya, G. Pickens, B. Mills-Lamptey, and C. J. Chuck, “Enhanced Hydrothermal Carbonization of Spent Coffee Grounds for the Efficient Production of Solid Fuel with Lower Nitrogen Content,” Energy & Fuels, vol. 35, no. 11, pp. 9462–9473, Jun. 2021. [CrossRef]
- N. Kumar, R. Weldon, and J. G. Lynam, “Hydrothermal carbonization of coffee silverskins,” Biocatal Agric Biotechnol, vol. 36, p. 102145, Sep. 2021. [CrossRef]
- M. Mariotti-Celis, M. Martínez-Cifuentes, N. Huamán-Castilla, M. Vargas-González, F. Pedreschi, and J. Pérez-Correa, “The Antioxidant and Safety Properties of Spent Coffee Ground Extracts Impacted by the Combined Hot Pressurized Liquid Extraction–Resin Purification Process,” Molecules, vol. 23, no. 1, p. 21, Dec. 2017. [CrossRef]
- J. L. Xu, T. J. Kim, J.-K. Kim, and Y. Choi, “Simultaneous roasting and extraction of green coffee beans by pressurized liquid extraction,” Food Chem, vol. 281, pp. 261–268, May 2019. 20 May. [CrossRef]
- D. V. Phuong, L. P. Tan Quoc, P. Van Tan, and L. N. Doan Duy, “Production of bioethanol from Robusta coffee pulp (Coffea robusta L.) in Vietnam,” Foods and Raw Materials, pp. 10–17, Oct. 2019. [CrossRef]
- R. Manrique, D. Vásquez, C. Ceballos, F. Chejne, and A. Amell, “Evaluation of the Energy Density for Burning Disaggregated and Pelletized Coffee Husks,” ACS Omega, vol. 4, no. 2, pp. 2957–2963, Feb. 2019. [CrossRef]
- J. E. Park, G. B. Lee, C. J. Jeong, H. Kim, and C. G. Kim, “Determination of Relationship between Higher Heating Value and Atomic Ratio of Hydrogen to Carbon in Spent Coffee Grounds by Hydrothermal Carbonization,” Energies (Basel), vol. 14, no. 20, p. 6551, Oct. 2021. [CrossRef]
- R. A. R. Frómeta, J. L. Sánchez, and J. M. R. García, “Evaluation of coffee pulp as substrate for polygalacturonase production in solid state fermentation,” Emir J Food Agric, p. 117, Mar. 2020. [CrossRef]
- Colantoni et al., “Spent coffee ground characterization, pelletization test and emissions assessment in the combustion process,” Sci Rep, vol. 11, no. 1, p. 5119, Mar. 2021. [CrossRef]
- M. Gouvea, C. Torres, A. S. Franca, L. S. Oliveira, and E. S. Oliveira, “Feasibility of ethanol production from coffee husks,” Biotechnol Lett, vol. 31, no. 9, pp. 1315–1319, Sep. 2009. [CrossRef]
- W. Yang, X. Li, S. Liu, and L. Feng, “Direct hydrothermal liquefaction of undried macroalgae Enteromorpha prolifera using acid catalysts,” Energy Convers Manag, vol. 87, pp. 938–945, Nov. 2014. [CrossRef]
- S. Yin and Z. Tan, “Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions,” Appl Energy, vol. 92, pp. 234–239, Apr. 2012. [CrossRef]
- S. Liu, K. Wang, H. Yu, B. Li, and S. Yu, “Catalytic preparation of levulinic acid from cellobiose via Brønsted-Lewis acidic ionic liquids functional catalysts,” Sci Rep, vol. 9, no. 1, p. 1810, Feb. 2019. [CrossRef]
Figure 1.
Van Krevelen diagram por coffee cherry waste.
Figure 1.
Van Krevelen diagram por coffee cherry waste.
Figure 2.
Preliminary follow-up of experiments from 120-180 °C for 1 hour.
Figure 2.
Preliminary follow-up of experiments from 120-180 °C for 1 hour.
Figure 3.
Preliminary follow-up of experiments from 1-5 hours at 180 °C.
Figure 3.
Preliminary follow-up of experiments from 1-5 hours at 180 °C.
Figure 4.
Infrared spectra of biomass (a) from 120 °C to 180 °C for 1 hour and (b) from 1 hour to 4 hours at 180 °C.
Figure 4.
Infrared spectra of biomass (a) from 120 °C to 180 °C for 1 hour and (b) from 1 hour to 4 hours at 180 °C.
Figure 5.
Van Krevelen diagram of LHW experiments.
Figure 5.
Van Krevelen diagram of LHW experiments.
Figure 6.
Concentration of sugars at every LHW experiment for 1 hour.
Figure 6.
Concentration of sugars at every LHW experiment for 1 hour.
Figure 7.
Concentration of sugars at 180 °C for 1-5 hours.
Figure 7.
Concentration of sugars at 180 °C for 1-5 hours.
Figure 8.
Catalysts influence in coffee cherry waste valorization, Autor, 2023.
Figure 8.
Catalysts influence in coffee cherry waste valorization, Autor, 2023.
Figure 9.
Preliminary follow-up of experiments from 200-260 °C for 1 hour.
Figure 9.
Preliminary follow-up of experiments from 200-260 °C for 1 hour.
Figure 10.
Preliminary follow-up of experiments from 1-5 hours at 200 °C.
Figure 10.
Preliminary follow-up of experiments from 1-5 hours at 200 °C.
Figure 11.
Infrared spectra of HTC valorization (a) from 200-260 ºC for 1 hour, (b) from 1-3 hours at 200 ºC.
Figure 11.
Infrared spectra of HTC valorization (a) from 200-260 ºC for 1 hour, (b) from 1-3 hours at 200 ºC.
Figure 12.
Van Krevelen characterization of HTC solid fractions.
Figure 12.
Van Krevelen characterization of HTC solid fractions.
Figure 13.
Platform chemicals production in HTC experiments.
Figure 13.
Platform chemicals production in HTC experiments.
Figure 14.
Platform chemicals production in HTC at different times.
Figure 14.
Platform chemicals production in HTC at different times.
Figure 15.
Mathematical treatments to evaluate the reaction order of kinetics.
Figure 15.
Mathematical treatments to evaluate the reaction order of kinetics.
Figure 16.
Mathematical treatments to evaluate the reaction order of kinetics.
Figure 16.
Mathematical treatments to evaluate the reaction order of kinetics.
Figure 17.
Optimization of reaction conditions for sugar obtention.
Figure 17.
Optimization of reaction conditions for sugar obtention.
Figure 18.
Optimization of reaction conditions for levulinic acid obtention.
Figure 18.
Optimization of reaction conditions for levulinic acid obtention.
Figure 19.
Optimization of reaction conditions for formic acid obtention.
Figure 19.
Optimization of reaction conditions for formic acid obtention.
Figure 20.
Optimization of reaction conditions for HMF and Furfural obtention.
Figure 20.
Optimization of reaction conditions for HMF and Furfural obtention.
Figure 21.
Purification process for (a) Formic acid and (b) sugars).
Figure 21.
Purification process for (a) Formic acid and (b) sugars).
Table 1.
Proximal essay of coffee waste.
Table 1.
Proximal essay of coffee waste.
| Essay |
Coffee Cherry (%)a
|
Pulp (%)b
|
Husk (%)c
|
SCG*(%)d
|
| Moisture (Initial Biomass) |
80.79 |
73.85 |
8.88 |
55.2 |
| Moisture (BHP)** |
10.94 |
- |
- |
- |
| Ashes |
7.79 |
6.29 |
0.79 |
15.3 |
| Volatile matter |
10.06 |
9.80 |
75.85 |
83.3 |
| Fixed carbon |
1.36 |
- |
14.48 |
1.5 |
Table 2.
Ultimate essay of coffee waste.
Table 2.
Ultimate essay of coffee waste.
| Element |
Coffee Cherry (%)a
|
Pulp (%)b
|
Husk (%)c
|
SCG (%)d
|
| Carbon |
45.27 |
|
44.95 |
68.52 |
| Hydrogen |
4.862 |
|
5.34 |
11.04 |
| Nitrogen |
1.471 |
1.5 |
0.60 |
1.40 |
| Sulfur |
0.138 |
|
0.03 |
- |
| Elemental ratio |
| Ratio O/C |
0.792 |
| Ratio H/C |
1.498 |
| Ratio C/N |
19.98 |
Table 3.
Structural essay of coffee waste.
Table 3.
Structural essay of coffee waste.
| Essay |
Coffee Cherry (%)a
|
Pulp (%)b
|
Husk (%)c
|
SCG (%)d
|
| Dry matter (%) |
95.5 |
|
|
|
| Hemicellulose |
12.5 |
3.60 |
7 |
12.1 |
| Lignin |
13.7 |
20.07 |
9 |
17.8 |
| Cellulose |
27.6 |
25.88 |
43 |
23.6 |
Table 4.
Fractions obtained via LHW.
Table 4.
Fractions obtained via LHW.
| Temperature (ºC) |
Time (Hours) |
Solid fraction (g) |
Liquid fraction (g) |
Gas fraction (g) |
| 120 |
1 |
2,784 |
22,920 |
4,296 |
| 130 |
1 |
2,689 |
22,918 |
4,393 |
| 140 |
1 |
2,650 |
22,830 |
4,520 |
| 150 |
1 |
2,633 |
22,835 |
4,532 |
| 160 |
1 |
2,590 |
22,873 |
4,537 |
| 170 |
1 |
2,546 |
22,904 |
4,550 |
| 180 |
1 |
2,498 |
22,890 |
4,502 |
| 180 |
2 |
2,148 |
20,310 |
7,852 |
| 180 |
3 |
1,995 |
16,871 |
12,005 |
| 180 |
4 |
1,895 |
14,281 |
14,105 |
| 180 |
5 |
1,390 |
15,120 |
13,489 |
Table 5.
Characteristic vibrations and functionality of biomass.
Table 5.
Characteristic vibrations and functionality of biomass.
| Wavenumber (cm-1) |
Characteristic vibrations and functionality |
| 3284-3308 |
O-H vibrations of H-bonded hydroxyl groups |
| 2919-2923 y 2853 |
C-H symmetric vibrations of aliphatic CHx. Methyl/Methylene |
| 1705 |
C=O vibrations of ketone, aldehyde and acid groups. |
| 1608-1622 |
C=C vibrations of aromatic compounds, C=O vibrations of conjugated ketones and quinones. |
| 1516 |
C=C-vibrations, indicative of lignin and aromatic C |
| 1500-1350 |
Amorphous polysaccharides |
| 1300-1150 |
Symmetrical C-O-C, C-OH, C-O vibrations of cellulose, hemicellulose, and lignin |
| 1050-1000 |
C-O, C=C, C-C-O vibrations in Cellulose, hemicellulose, and lignin |
| 900 |
O-H vibrations of H-bonded hydroxyl groups |
| 800-600 |
C-H symmetric vibrations of aliphatic CHx. Methyl/Methylene |
Table 6.
Elemental analysis of solid fractions.
Table 6.
Elemental analysis of solid fractions.
| Essay |
Time (Hour) |
C (%) |
H (%) |
N (%) |
O (%) |
O/C |
H/C |
C/N |
| 120 °C 1h |
1 |
44.65 |
5.57 |
2.61 |
47.16 |
0.79 |
1.50 |
19.98 |
| 130ºC 1h |
1 |
46.74 |
5.84 |
1.98 |
45.42 |
0.73 |
1.50 |
27.49 |
| 140ºC 1h |
1 |
46.13 |
5.75 |
2.19 |
45.92 |
0.75 |
1.50 |
24.52 |
| 150ºC 1h |
1 |
47.81 |
5.81 |
2.19 |
44.16 |
0.69 |
1.46 |
25.39 |
| 160ºC 1h |
1 |
44.40 |
5.57 |
2.22 |
47.80 |
0.81 |
1.50 |
23.37 |
| 170ºC 1h |
1 |
48.17 |
5.98 |
2.07 |
43.77 |
0.68 |
1.49 |
27.11 |
| 180ºC 1h |
1 |
48.10 |
5.94 |
2.36 |
43.59 |
0.68 |
1.48 |
23.76 |
| 180ºC 2h |
2 |
46.71 |
5.60 |
2.63 |
45.05 |
0.72 |
1.44 |
20.71 |
| 180ºC 3h |
3 |
49.61 |
5.83 |
2.35 |
42.19 |
0.64 |
1.41 |
24.57 |
| 180ºC 4h |
4 |
51.31 |
5.99 |
2.49 |
40.21 |
0.59 |
1.40 |
24.04 |
| 180ºC 5h |
5 |
52.21 |
5.38 |
2.44 |
39.96 |
0.57 |
1.24 |
24.93 |
Table 7.
Yield of platform chemicals produced from LHW valorization.
Table 7.
Yield of platform chemicals produced from LHW valorization.
| Temperature (°C) |
[ ] of sugars (g/L) |
Yield based on biomass (%) |
Yield based on lignocellulosic structure (%) |
| 120 |
0.087 |
0.066 |
0.167 |
| 130 |
0.111 |
0.085 |
0.213 |
| 140 |
0.170 |
0.129 |
0.323 |
| 150 |
1.237 |
0.941 |
2.347 |
| 160 |
1.258 |
0.959 |
2.391 |
| 170 |
1.394 |
1.064 |
2.654 |
| 180 |
1.424 |
1.086 |
2.709 |
Table 8.
Yields and concentration of platform chemicals produces at 180 °C for 1-5 hours.
Table 8.
Yields and concentration of platform chemicals produces at 180 °C for 1-5 hours.
Time
(H) |
Sugars |
Levulinic acid |
Formic acid |
HMF |
Furfural |
Total Yield (%) |
| |
[ ] g/L |
R (%) |
[ ] g/L |
R (%) |
[ ] g/L |
R (%) |
[ ] g/L |
R (%) |
[ ] g/L |
R (%) |
|
| 1 |
1,424 |
2,716 |
|
|
|
|
|
|
|
|
2,716 |
| 2 |
1,896 |
3,209 |
|
|
|
|
|
|
|
|
3,209 |
| 3 |
3,453 |
4,855 |
0,423 |
0,862 |
0,862 |
1,785 |
|
|
|
|
7,502 |
| 4 |
5,072 |
6,036 |
1,853 |
3,775 |
6,078 |
12,38 |
0,322 |
0,656 |
0,159 |
0,718 |
23,56 |
| 5 |
6,789 |
8,554 |
1,482 |
3,019 |
4,919 |
10,02 |
|
|
|
|
21,59 |
Table 9.
Yields and concentrations of PC using homogeneous catalysts.
Table 9.
Yields and concentrations of PC using homogeneous catalysts.
| Catalyst |
Time
(hours)
|
Total yield (%)* |
Concentration g/L |
| Sugars |
Formic |
Levulinic |
HMF |
Furfural |
| Without |
1 |
2,716 |
1,424 |
|
|
|
|
| H2SO4
|
1 |
16,444 |
6,203 |
3,080 |
0,649 |
|
|
| CH3COOH |
1 |
12,252 |
0,922 |
|
3,774 |
|
|
| KOH |
1 |
9,120 |
2,570 |
2,668 |
|
|
|
| NaHCO3
|
1 |
12,552 |
2,121 |
3,699 |
1,332 |
|
|
| Without |
2 |
3,616 |
1,896 |
|
|
|
|
| H2SO4
|
2 |
15,018 |
6,661 |
2,333 |
0,550 |
0,031 |
0,353 |
| CH3COOH |
2 |
12,754 |
1,007 |
|
4,586 |
|
|
| KOH |
2 |
5,845 |
1,575 |
1,938 |
|
|
|
| NaHCO3
|
2 |
16,044 |
3,384 |
4,842 |
1,125 |
|
|
| Without |
3 |
7,116 |
3,453 |
0,877 |
0,423 |
|
|
| H2SO4
|
3 |
18,576 |
4,073 |
4,023 |
1,341 |
0,289 |
0,373 |
| CH3COOH |
3 |
13,528 |
1,249 |
|
4,739 |
|
|
| KOH |
3 |
18,328 |
3,808 |
3,963 |
0,997 |
|
|
| NaHCO3
|
3 |
17,014 |
3,582 |
5,239 |
1,094 |
|
|
Table 10.
Fractions obtained via HTC.
Table 10.
Fractions obtained via HTC.
| Temperature (ºC) |
Time (hours) |
Solid fraction (g) |
Liquid fraction (g) |
Liquid fraction (g) |
| 200 |
1 |
3,578 |
60,012 |
36,410 |
| 200 |
2 |
4,279 |
64,120 |
31,601 |
| 200 |
3 |
4,531 |
60,234 |
35,235 |
| 200 |
4 |
4,531 |
65,015 |
30,454 |
| 200 |
5 |
4,452 |
66,925 |
28,624 |
| 220 |
1 |
3,407 |
59,901 |
36,692 |
| 240 |
1 |
3,334 |
66,290 |
30,376 |
| 260 |
1 |
2,972 |
83,312 |
13,714 |
Table 11.
Characteristic vibrations and functionality of biomass.
Table 11.
Characteristic vibrations and functionality of biomass.
| Wavenumber (cm-1) |
Characteristic vibrations and functionality |
| 2919-2923 y 2853 |
Symmetrical C-H vibrations of aliphatic CHx. Methyl/Methylene |
| 1608-1622 |
C=C vibrations of aromatic components, C=O vibrations Vibrations of conjugated ketones and quinones. |
| 1516 |
C=C-vibrations, indicative of lignin and aromatic C |
| 1500-1350 |
Amorphous polysaccharides |
| 1300-1150 |
Symmetrical C-O-C, C-OH, C-O vibrations of cellulose, hemicellulose and lignin |
| 1050-1000 |
C-O, C=C, C-C-O vibrations in Cellulose, hemicellulose and lignin |
| 800-600 |
O-H vibrations of alcohols, phenols and hydroxyl groups |
Table 12.
Elemental analysis of HTC experiments.
Table 12.
Elemental analysis of HTC experiments.
| Temperature (ºC) |
Time (Hours) |
C (%) |
H |
N |
O |
O/C |
H/C |
C/N |
| (%) |
(%) |
(%) |
| Initial biomass |
44.65 |
5.57 |
2.61 |
47.16 |
0.79 |
1.50 |
19.98 |
| 200 |
1 |
66.99 |
5.54 |
2.42 |
25.05 |
0.28 |
0.99 |
32.26 |
| 220 |
1 |
67.21 |
6.03 |
2.36 |
24.40 |
0.27 |
1.08 |
33.17 |
| 240 |
1 |
70.42 |
7.06 |
1.59 |
20.94 |
0.22 |
1.20 |
51.56 |
| 260 |
1 |
79.57 |
2.20 |
0.73 |
17.50 |
0.16 |
0.33 |
126.52 |
Table 13.
Quantification of platform chemicals obtained via HTC.
Table 13.
Quantification of platform chemicals obtained via HTC.
| Temperature (ºC) |
Total Yield (%) |
Concentration (g/L) |
| Sugars |
Levulinic A. |
Formic A. |
HMF |
Furfural |
| 200 |
18,486 |
2,461 |
1,613 |
4,866 |
0,104 |
0,093 |
| 220 |
20,151 |
2,209 |
1,837 |
5,531 |
0,119 |
0,124 |
| 240 |
19,499 |
1,350 |
1,594 |
5,210 |
0,120 |
0,119 |
| 260 |
21,311 |
0,992 |
1,172 |
4,868 |
0,106 |
0,104 |
Table 14.
Concentration of platform chemicals obtained via HTC at different times.
Table 14.
Concentration of platform chemicals obtained via HTC at different times.
| Time (hours) |
Total yield (%) |
Concentration (g/L) |
| Sugars |
Levulinic A. |
Formic A. |
HMF |
Furfural |
| 1 |
18,483 |
2,461 |
1,613 |
4,865 |
0,104 |
0,093 |
| 2 |
14,519 |
2,510 |
1,234 |
3,054 |
|
0,104 |
| 3 |
16,165 |
2,594 |
1,393 |
4,224 |
|
|
| 4 |
19,169 |
2,863 |
1,557 |
4,605 |
|
|
| 5 |
21,049 |
3,333 |
1,495 |
4,886 |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).