Submitted:
07 December 2023
Posted:
08 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
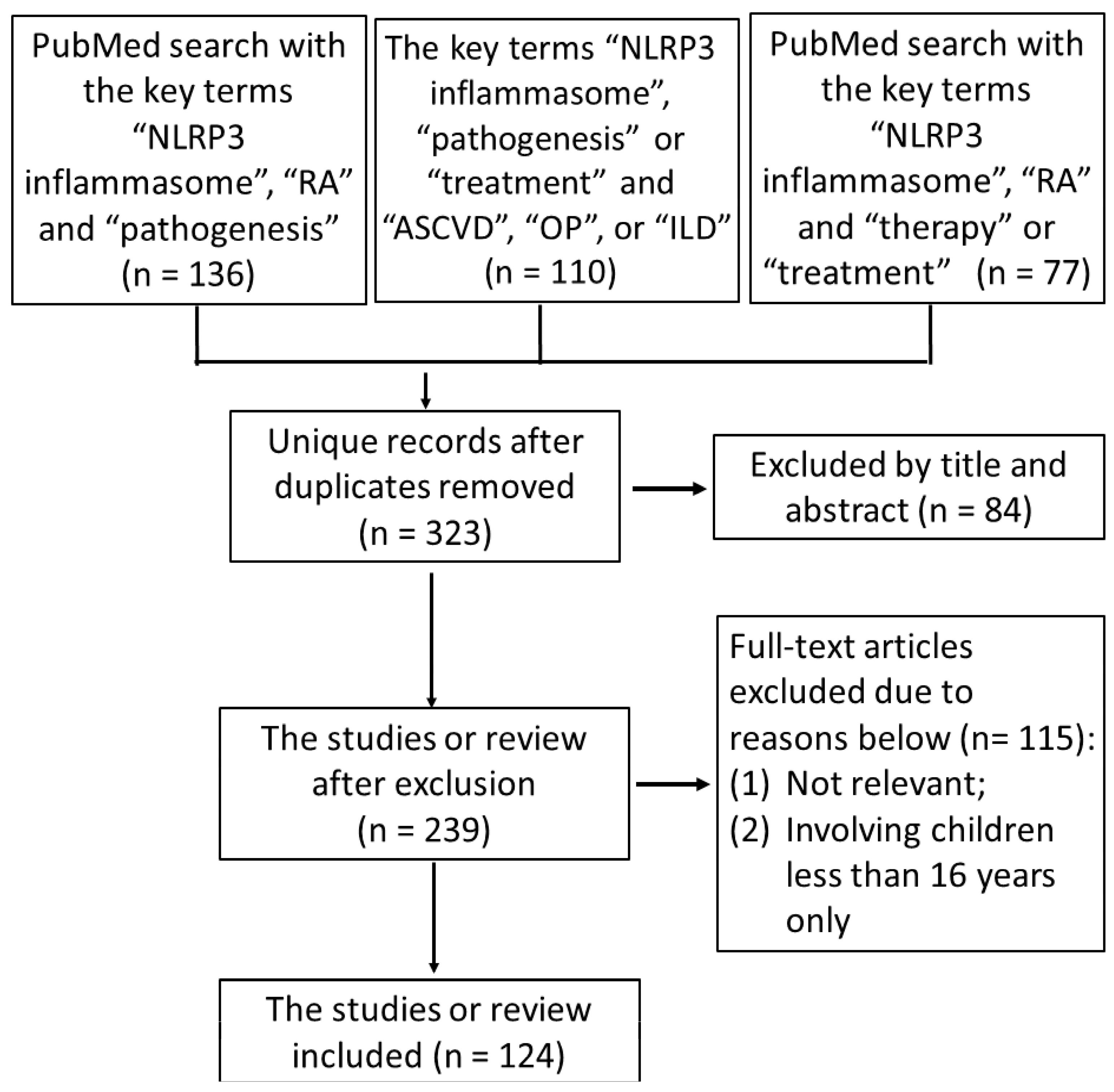
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
3. Etiopathogenesis of RA and Its Comorbidities
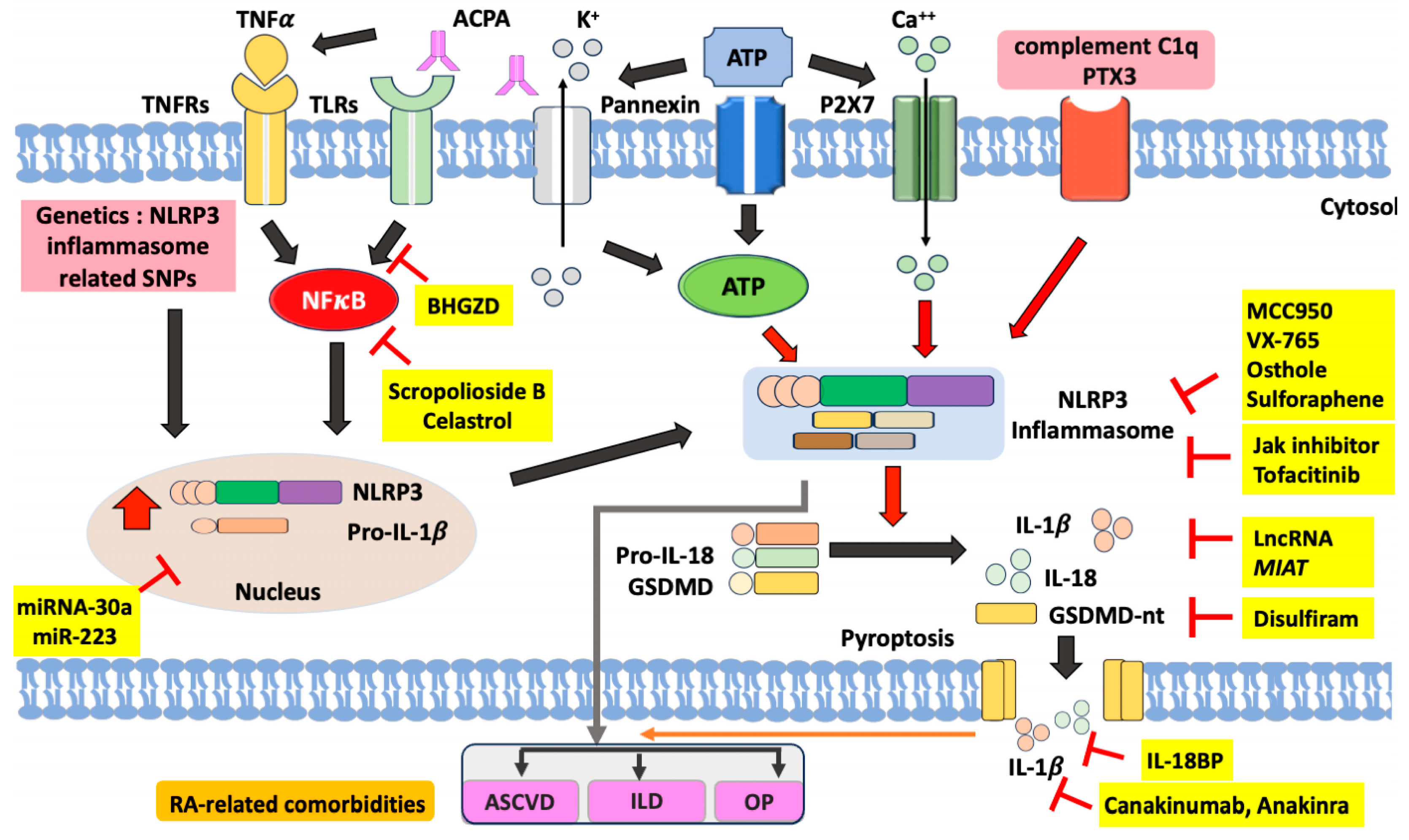
4. NLRP3-Inflammasome
4.1. NLRP3-Inflammasome Signaling in Immune Responses and Inflammation
4.2. NLRP3-Inflammasome Activation and Regulation in RA Pathogenesis.
4.3. The Genetic Predisposition of NLRP3 Inflammasome in RA.
4.4. The Involvement of NLRP3 Inflammasome Activation in RA-Related Comorbidities
5. Therapeutic Potential by Targeting NLRP3 Inflammasome
5.1. Small Molecule Inhibitors
5.2. Natural Products
5.3. Disease-Modifying Anti-Rheumatic Drugs (DMARDs)
5.4. Janus Kinase Inhibitors (JAKi)
5.5. microRNAs (miRNAs) and Stem Cells
6. Conclusions
Authors’ Contributions
Data Availability
Conflicts of Interest Statement
Funding
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet. 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid arthritis: common origins, divergent mechanisms. N Engl J Med. 2023, 388, 529–542. [Google Scholar] [CrossRef]
- Lai, C.H.; Lai, M.S.; Lai, K.L.; Chen, H.H.; Chiu, Y.M. Nationwide population-based epidemiologic study of rheumatoid arthritis in Taiwan. Clin Exp Rheumatol. 2012, 30, 358–363. [Google Scholar]
- Kurko, J.; Besenyei, T.; Laki, J.; Glant, T.T.; Mikecz, K.; Szekanecz, Z. Genetics of rheumatoid arthritis - a comprehensive review. Clin Rev Allergy Immunol. 2013, 45, 170–179. [Google Scholar] [CrossRef]
- Viatte, S.; Plant, D.; Raychaudhuri, S. Genetics and epigenetics of rheumatoid arthritis. Nat Rev Rheumatol. 2013, 9, 141–153. [Google Scholar] [CrossRef]
- Furst, D.E.; Emery, P. Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology (Oxford) 2014, 53, 1560–1569. [Google Scholar] [CrossRef]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheuamtoid arthritis: from pathogenesis to novel therapeutic opportunisties. Front Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef]
- Szekanecz, Z.; McInnes, I.B.; Schett, G.; Szamosi, S.; Benkő, S.; Szűcs, G. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat Rev Rheumatol. 2021, 17, 585–595. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.; Li, W.; Zhao, Y. NLRP3 inflammasome: checkpoint connecting innate and adaptive immunity in autoimmune diseases. Front Immunol. 2021, 12, 732933. [Google Scholar] [CrossRef]
- Mitrović, J.; Hrkač, S.; Tečer, J.; Golob, M.; Ljilja, Posavec, A.; Kolar, Mitrović, H.; Grgurević, L. Pathogenesis of extraarticular manifestations in rheumatoid arthritis-a comprehensive review. Biomedicines 2023, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Hajjaj-Hassouni, N.; Harigai, M.; Luo, S.F.; Kurucz, R.; Maciel, G.; Mola, E.M.; Montecucco, C.M.; McInnes, I.; Radner, H.; et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014, 73, 62–68. [Google Scholar]
- Gabriel, S.E.; Michaud, K. Epidemiological studies in incidence, prevalence, mortality and comorbidity of the rheumatic diseases. Arthritis Res Thera. 2009, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Symmons, D.P.; Gabriel, S.E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011, 7, 399–408. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Rho, Y.H.; Chung, C.P.; Oeser, A.; Solus, J.; Asanuma, Y.; Sokka, T.; Pincus, T.; Raggi, P.; Gebretsadik, T.; Shintani, A.; et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum 2009, 61, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Haugeberg, G.; Uhlig, T.; Falch, J.A.; Halse, J.I.; Kvien, T.K. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis. Arthritis Rheum. 2000, 43, 522–530. [Google Scholar] [CrossRef]
- Kim, S.Y.; Schneeweiss, S.; Liu, J.; Daniel, G.W.; Chang, C.L.; Garneau, K.; Solomon, D.H. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res Thera. 2010, 12, R154. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Collins, B.F.; Ho, L.A.; Raghu, G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev. 2015, 24, 1–16. [Google Scholar] [CrossRef]
- Ng, K.H.; Chen, D.Y.; Lin, C.H.; Chao, W.C.; Chen, Y.M.; Chen, Y.H.; Huang, W.N.; Hsieh, T.Y.; Lai, K.L.; Tang, K.T.; et al. Risk of interstitial lung disease in patients with newly diagnosed systemic autoimmune rheumatic disease: a nationwide, population-based cohort study. Semin Arthritis Rheum. 2020, 50, 840–845. [Google Scholar] [CrossRef]
- Tymms, K.; Zochling, J.; Scott, J.; Bird, P.; Burnet, S.; de Jager, J.; Griffiths, H.; Nicholls, D.; Roberts, L.; Arnold, M.; et al. Barriers to optimal disease control for rheumatoid arthritis patients with moderate and high disease activity. Arthritis Care Res 2014, 66, 190–196. [Google Scholar] [CrossRef]
- Scott, I.C.; Machin, A.; Mallen, C.D.; Hider, S.L. The extra-articular impacts of rheumatoid arthritis: moving towards holistic care. BMC Rheumatology 2018, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Muruve, D.A.; Petrilli, V.; Zaiss, A.K.; White, L.R.; Clark, S.A.; Ross, P.J.; Tschopp, J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 2008, 452, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Gaide, O.; Petrilli, V.; Mayor, A.; Tschopp, J. NALP inflammasome: a central role in innate immunity. Semin Immunopathol. 2007, 29, 29,213–229. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.J.; McDermott, M.F.; Kanneganti, T.D. Inflammasomes and autoimmunity. Trends Mol Med. 2011, 17, 57–64. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019, 9, 477–489. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 inflammasome: activation and regulation. Trends Biochemical Sciences 2023, 48, 331–344. [Google Scholar] [CrossRef]
- Kim, M.L.; Chae, J.J.; Park, Y.H.; De Nardo, D.; Stirzaker, R.A.; Ko, H.J.; Tye, H.; Cengia, L.; DiRago, L.; Metcalf, D.; et al. Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1β. J Exp Med. 2015; 212, 927–938. [Google Scholar]
- Hsieh, C.W.; Chen, Y.M.; Lin, C.C.; Tang, K.T.; Chen, H.H.; Hung, W.T.; Lai, K.L.; Chen, D.Y. Elevated Expression of the NLRP3 Inflammasome and Its Correlation with Disease Activity in Adult-onset Still Disease. J Rheumatol. 2017, 44, 1142–1150. [Google Scholar] [CrossRef]
- Gattorno, M.; Martini, A. Beyond the NLRP3 inflammasome: autoinflammatory diseases reach adolescence. Arthritis Rheum 2013, 65, 1137–1147. [Google Scholar] [CrossRef]
- Moltrasio, C.; Romagnuolo, M.; Marzano, A.V. NLRP3 inflammasome and NLRP3-related autoinflammatory diseases: from cryopyrin function to targeted therapies. Front Immunol 2022, 13, 1007705. [Google Scholar] [CrossRef] [PubMed]
- Kolly, L.; Busso, N.; Palmer, G.; Talabot-Ayer, D.; Chobaz, V.; So, A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2010, 129, 178–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Li, H. NLRP3 inflammasome plays an important role in the pathogenesis of collagen-induced arthritis. Mediators Inflamm. 2016, 2016, 9656270. [Google Scholar] [CrossRef] [PubMed]
- Unterberger, S.; Davies, K.A.; Rambhatla, S.B.; Sacre, S. Contribution of Toll-like receptors and the NLRP3 inflammasome in rheumatoid arthritis pathophysiology. Immuno Targets and Therapy 2021, 10, 285–298. [Google Scholar] [CrossRef]
- Yin, H.; Liu, N.; Sigdel, K.R.; Duan, L. Role of NLRP3 inflammasome in rheumatoid arthritis. Front Immunol. 2022, 13, 931690. [Google Scholar] [CrossRef]
- Murakami, T.; Nakaminami, Y.; Takahata, Y.; Hata, K.; Nishimura, R. Activation and function of NLRP3 inflammasome in bone and joint-related diseases. Int J Mol Sci. 2022, 23, 5365. [Google Scholar] [CrossRef]
- Rosengren, S.; Hoffman, H.M.; Bugbee, W.; Boyle, D.L. Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann Rheum Dis. 2005, 64, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Fu, R.; Wang, S.; Huang, Y.; Li, X.; Zhou, M.; Zhao, J.; Yang, N. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clinical and Experimental Immunology. 2018, 194, 231–243. [Google Scholar] [CrossRef]
- Wu, X.Y.; Li, K.T.; Yang, H.X.; Yang, B.; Lu, X.; Zhao, L.D.; et al. Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J Autoimmun. 2020, 106, 102336. [Google Scholar] [CrossRef]
- Dong, X.; Zhengl, Z.; Linl, P.; Fu, X.; Li, F.; Jiang, J.; Zhu, P. ACPAs promote IL-1β production in rheumatoid arthritis by activating the NLRP3 inflammasome. Cell Mol Immunol. 2020, 17, 261–271. [Google Scholar] [CrossRef]
- Werner, L.E.; Wagner, U. Calcium-sensing receptor-mediated NLRP3 inflammasome activation in rheumatoid arthritis and autoinflammation. Front Physiol. 2023, 13, 1078569. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef]
- Tanaka, Y. Recent progress in treatments of rheumatoid arthritis: an overview of developments in biologics and small molecules, and remaining unmet needs. Rheumatology 2021, 60, vi12–vi20. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease modifying antirheumatic drugs:2022 update. Ann Rheum Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Kerschbaumer, A.; Dörner, T.; Dougados, M.; Fleischmann, R.M.; Geissler, K.; McInnes, I.; Pope, J.E.; van der Heijde, D.; Stoffer-Marx, M.; et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021, 80, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaumer, A.; Sepriano, A.; Bergstra, S.A.; Smolen, J.S.; van der Heijde, D.; Caporali, R.; Edwards, C.J.; Verschueren, P.; de, Souza, S.; Pope, J.E.; et al. Efficacy of synthetic and biological DMARDs: a systemic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2023, 82, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.B.; Hasler, C.; Popp, F.; Mattow, F.; Durmisi, M.; Souza, A.; Hasler, P.; Rubbert-Roth, A.; Schulze-Koops, H.; Kempis, J.V. Effectiveness, tolerability, and safety of tofacitinib in rheumatoid arthritis: A Retrospective Analysis of Real-World Data from the St. Gallen and Aarau Cohorts. J Clin Med. 2019, 8, 1548. [Google Scholar] [CrossRef]
- Lee, E.B.; Fleischmann, R.; Hall, S.; Wilkinson, B.; Bradley, J.D.; Gruben, D.; Koncz, T.; Krishnaswami, S.; Wallenstein, G.V.; Zang, C.; et al. A. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014, 370, 2377–2386. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Burmester, G.; Nash, P.; Zerbini, C.A.; Soma, K.; Kwok, K.; Hendrikx, T.; Bananis, E.; Fleischmann, R. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2016, 75, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Mekni, N.; De Rosa, M.; Cipollina, C.; Gulotta, M.R.; De, Simone, G.; Lombino, J.; Padova, A.; Perricone, U. In silico insights towards the identification of NLRP3 druggable hot spots. Int J Mol Sci. 2019, 20, 4974. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.; Yang, Y.; Tao, J. Therapeutic potential of targeting the NLRP3 inflammasome in rheumatod arthritis. Inflammation 2023, 46, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, R.; Tang, M.; Zhao, M.; Jiang, X.; Cai, X.; Ye, N.; Su, K.; Peng, J.; Zhang, X.; et al. Recent progress and prospects of small molecules for NLRP3 inflammasome inhibition. J Med Chem. 2023, 66, 14447–14473. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Terao, C. The impact of cigarette smoking on risk of rheumatoid arthritis: a narrative review. Cells. 2020, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Viapiana, O.; Rossini, M.; Orsolini, G.; Bertoldo, E.; Giollo, A.; Gatti, D.; Fassio, A. Association between environmental air pollution and rheumatoid arthritis flares. Rheumatology (Oxford) 2021, 60, 4591–4597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, Y.; Jin, K.; Wen, Z.; Cao, W.; Wu, B.; Wen, R.; Tian, L.; Berry, G.J.; Goronzy, J.J.; et al. The DNA Repair Nuclease MRE11A Functions as a Mitochondrial Protector and Prevents T Cell Pyroptosis and Tissue Inflammation. Cell Metab. 2019, 30, 477–492e6. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Kremers, H.M.; Crowson, C.S.; Therneau, T.M.; Roger, V.L.; Gabriel, S.E. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients. Arthritis Rheumatol. 2008, 58, 2268–2274. [Google Scholar] [CrossRef]
- Aviňa-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008, 59, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.F.; Gourraud, P.A.; Cantagrel, A.; Davignon, J.L.; Constantin, A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint bone spine 2011, 78, 179–183. [Google Scholar] [CrossRef]
- Im, C.H.; Kim, N.R.; Kang, J.W.; Kim, J.H.; Kang, J.Y.; Bae, G.B.; Nam, E.J.; Kang, Y.M. Inflammatory burden interacts with conventional cardiovascular risk factors for carotid plaque formation in rheumatoid arthritis. Rheumatology (Oxford) 2015, 54, 808–815. [Google Scholar] [CrossRef]
- Chester, Wasko, M.; Dasgupta, A.; Ilse, Sears, G.; Fries, J.F.; Ward, M.M. Prednisone use and risk of mortality in patients with rheumatoid arthritis: moderation by use of disease-modifying antirheumatic drugs. Arthritis Care Res. 2016, 68, 706–710. [CrossRef] [PubMed]
- Danelich, I.M.; Wright, S.S.; Lose, J.M.; Tefft, B.J.; Cicci, J.D.; Reed, B,N. Safety of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease. Pharmacotherapy 2015, 35, 520–535. [CrossRef] [PubMed]
- Maehlen, M.T.; Provan, S.A.; de Rooy, D.P.; van der Helm-van, A.H.; Krabben, A.; Saxne, T.; Lindqvist, E.; Semb, A.G.; Uhlig, T.; van, der, Heijde, D.; et al. Associations between APOE Genotypes and Disease Susceptibility, Joint Damage and Lipid Levels in Patients with Rheumatoid Arthritis. PLoS ONE 2013, 8, e60970.
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Shen, M.Y.; Lee, A.S.; Wang, C.C.; Chen, W.Y.; Chang, C.M.; Chang, K.C.; Stancel, N.; Chen, C.H. Electronegative low-density lipoprotein increases the risk of ischemic lower-extremity peripheral artery disease in uremia patients on maintenance hemodialysis. Sci. Rep. 2017, 7, 4654. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Chen, C.H.; Chen, Y.M.; Hsieh, T.Y.; Li, J.P.; Shen, M.Y.; Lan, J.L.; Chen, D.Y. Association between Negatively Charged Low-Density Lipoprotein L5 and Subclinical Atherosclerosis in Rheumatoid Arthritis Patients. J. Clin. Med. 2019, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Lu, J.; Walterscheid, J.P.; Chen, H.H.; Engler, D.A.; Sawamura, T.; Chang, P.Y.; Safi, H.J.; Yang, C.Y.; Chen, C.H. Electronegative LDL circulating in smokers impairs endothelial progenitor cell differentiation by inhibiting Akt phosphorylation via LOX-1. J. Lipid Res. 2008, 49, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Chen, P.K.; Lan, J.L.; Chang, S.H.; Hsieh, T.Y.; Liao, P.J.; Chen, C.H.; Chen, D.Y. Association of Electronegative LDL with Macrophage Foam Cell Formation and CD11c Expression in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2020, 21, 5883. [Google Scholar] [CrossRef] [PubMed]
- Van, Staa, T.P.; Geusens, P.; Bijlsma, J.W.; Leufkens, H.G.; Cooper, C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006, 54, 3104–3112. [CrossRef]
- Yu, X.H.; Yang, Y.Q.; Cao, R.R.; Cai, M.K.; Zhang, L.; Deng, F.Y.; Lei, S.F. Rheumatoid arthritis and osteoporosis: shared genetic effect, pleiotropy and causality. Human Molecular Genetics. 2021, 30, 1932–1940. [Google Scholar] [CrossRef]
- Hecht, C.; Englbrecht, M.; Rech, J.; Schmidt, S.; Araujo, E.; Engelke, K.; Finzel, S.; Schett, G. Additive Effect of Anti-Citrullinated Protein Antibodies and Rheumatoid Factor on Bone Erosions in Patients with RA. Ann. Rheum. Dis. 2015, 74, 2151. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Narla, R.; Baker, J.F.; Wysham, K.D. Risk factors for osteoporosis and fractures in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2022, 36, 101773. [Google Scholar] [CrossRef] [PubMed]
- Kadura, S.; Raghu, G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur. Respir. Rev. 2021, 30, 210011. [Google Scholar] [CrossRef] [PubMed]
- Castelino, F.V.; Varga, J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Thera. 2010, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Takayanagi, N.; Sugiura, H.; Miyahara, Y.; Tokunaga, D.; Kawabata, Y.; Sugita, Y. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J. 2011, 37, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Paulin, F.; Doyle, T.J.; Fletcher, E.A.; Ascherman, D.P.; Rosas, I.O. Rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis: shared mechanistic and phenotypic traits suggest overlapping disease mechanisms. Rev Investing Clin. 2015, 67, 280–286. [Google Scholar]
- Johnson, C. Recent advances in the pathogenesis, prediction, and management of rheumatoid arthritis-associated interstitial lung disease. Curr Opin Rheumatol. 2017, 29, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, J.; Lau, J.; Wang, S.; Taneja, V.; Matteson, E.L.; Vassallo, R. Mechanisms of lung disease development in rheumatoid arthritis. Nat Rev Rheumatol. 2019, 15, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Paulin, F.; Doyle, T.J.; Fletcher, E.A.; Ascherman, D.P.; Rosas, I.O. Rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis: shared mechanistic and phenotypic traits suggest overlapping disease mechanisms. Rev Invest Clin. 2015, 67, 280–286. [Google Scholar]
- Oka, S.; Furukawa, H.; Shimada, K.; Sugii, S.; Hashimoto, A.; Komiya, A.; Fukui, N.; Suda, A.; Tsunoda, S.; Ito, S.; et al. Association of Human Leukocyte Antigen Alleles with Chronic Lung Diseases in Rheumatoid Arthritis. Rheumatology 2016, 55, 1301–1307. [Google Scholar] [CrossRef]
- Juge, P.A.; Borie, R.; Kannengiesser, C.; Gazal, S.; Revy, P.; Wemeau-Stervinou, L.; Debray, M.P.; Ottaviani, S.; Marchand-Adam, S.; Nathan, N.; et al. AB0007 Shared Genetic Predisposition in Rheumatoid Arthritis–Interstitial Lung Disease and Familial Pulmonary Fibrosis. Ann. Rheum. Dis. 2017, 76, 1049. [Google Scholar]
- Joo, Y.B.; Ahn, S.M.; Bang, S.Y.; Park, Y.; Hong, S.J.; Lee, Y.; Cho, S.K.; Choi, C.B.; Sung, Y.K.; Kim, T.H.; et al. MUC5B promoter variant rs35705950, rare but significant susceptibility locus in rheumatoid arthritis-interstitial lung disease with usual interstitial pneumonia in Asian populations. RMD Open 2022, 8, e002790. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Honda, S.; Ikari, K.; Kanai, M.; Takeda, Y.; Kamatani, Y.; Morisaki, T.; Tanaka, E.; Kumanogoh, A.; Harigai, M.; et al. Association of the RPA3-UMAD1 locus with interstitial lung diseases complicated with rheumatoid arthritis in Japanese. Ann Rheum Dis. 2020, 79, 1305–1309. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, D.; Wang, L.; Wang, S.; Roden, A.C.; Zhao, H.; Li, X.; Prakash, Y.S.; Matteson, E.L.; Tschumperlin, D.J.; et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2019, 316, L487–L497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Al-Aly, Z.; Zheng, B.; van, Donkelaar, A.; Martin, R.V.; Pineau, C.A.; Bernatsky, S. Fine particles matter components and interstitial lung disease in rheumatoid arthritis. Eur Respir J. 2022, 60, 2102149. [CrossRef]
- Dolasia, K.; Bisht, M.K.; Pradhan, G.; Udgata, A.; Mukhopadhyay, S. TLRs/NLRs: shaping the landscape of host immunity. Int Rev Immunol. 2018, 37, 3–19. [Google Scholar] [CrossRef]
- Jung, J.Y.; Suh, C.H.; Kim, H.A. The role of damage-associated molecular pattern for pathogenesis and biomarkers in adult-onset Still’s disease. Expert Rev Mol Diagn. 2019, 19, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Heilig, R.; Dick, M.S.; Sborgi, L.; Meunier, E.; Hiller, S.; Broz, P. The gasdermin-D pore acts a conduit for IL-1β secretion in mice. Eur J Immunol. 2018, 48, 584–592. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009, 7, 99–109. [CrossRef]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewicz, M.; Sand, J.; Beer, H.D. Caspase-1: the inflammasome and beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Vande, Walle, L.; Lamkanfi, M. Pyroptosis. Curr Biol. 2016, 26, R568–R72.
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomedicine Pharmacotherapy 2020, 130, 110542. [CrossRef]
- Wu, X.; Ren, G.; Zhou, R.; Ge, J.; Chen, F.H. The role of Ca (2+) in acid-sensing ion channel 1a-mediated chondrocyte pyroptosis in rat adjuvant arthritis. Lab Invest. 2019, 99, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.E.; Wagner, U. Calcium-sensing receptor-mediated NLRP3 inflammasome activation in rheumatoid arthritis and autoinflammation. Front Physiol. 2023, 13, 1078569. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, X.; Xu, X.; Hu, L.; Zhou, G.; Liu, R.; Yang, G.; Cui, D. Inflammasomes in rheumatoid arthritis: a pilot study. BMC Rheumatol. 2023, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Kastbom, A.; Verma, D.; Eriksson, P.; Skogh, T.; Wingren, G.; Söderkvist, P. Genetic variations in proteins of the cryopyrine inflammasome influences the susceptibility and severity of rheumatoid arthritis (the Swedish TIRA project). Rheumatology (Oxford) 2008, 47, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Jenko, B.; Praprotnik, S.; Tomsic, M.; Dolzan, V. NLRP3 and CARD8 polymorphisms influence higher disease activity in rheumatoid arthritis. J Med Biochem. 2016, 35, 319–323. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, J.; Yang, Q.; Zhang, Y.; Han, L. NLRP3 p. Q705K and CARD8 p.C10X single nucleotide polymorphisms are not associated with susceptibility to rheumatoid arthritis: a meta-analysis. Int J Rheum Dis. 2017, 20, 1481–1491. [CrossRef]
- Li, R.N.; Ou, T.T.; Lin, C.H.; Lin, Y.Z.; Fang, T.J.; Chen, Y.J.; Tseng, C.C.; Sung, W.Y.; Wu, C.C.; Yen, J.H. NLRP3 gene polymorphisms in rheumatoid arthritis and primary Sjögren’s syndrome patients. Diagnostics (Basel). 2023, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Sode, J.; Vogel, U.; Bank, S.; Andersen, P.S.; Thomsen, M.K.; Hetland, M.L.; Locht, H.; Heegaard, N.H.; Andersen, V. Anti-TNF treatment response in rheumatoid arthritis patients is associated with genetic variation in the NLRP3-inflammasome. PLOS ONE. 2014, 9, e10036. [Google Scholar] [CrossRef] [PubMed]
- Mathews, R.J.; Robinson, J.I.; Battellino, M.; Wong, C.; Taylor, J.C; Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate (BRAGGSS).; Eyre, S.; Churchman, S.M.; Wilson, A.G.; Isaacs, J.D, et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis. 2014, 73, 1202–1210.
- Awni, A.A.; Hamed, Z.O.; Abdul-Hassan, Abbas, A.; Abdulamir, A.S. Effect of NLRP3 inflammasome genes polymorphisms on disease susceptibility and response to TNF-α inhibitors in Iraqi patients with rheumatoid arthritis. Heliyon 2023, 9, e16814. [CrossRef] [PubMed]
- Karasawa, T.; Takahashi, M. Role of NLRP3 inflammasome in atherosclerosis. J Atheroscler Thromb. 2017, 24, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Baragetti, A.; Catapano, A.L.; Magni, P. Multifactorial activation of NLRP3 inflammasome: relevance for a precision approach to atherosclerotic cardiovascular risk and disease. Int. J. Mol. Sci. 2020, 21, 4459. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.P.; Lukens, J.R.; Wilhelm, A.J.; Moore, J.L.; Mendez-Fernandez, Y.; Kanneganti, T.D.; Major, A.S. Oxidized Low-Density Lipoprotein Immune Complex Priming of the Nlrp3 Inflammasome Involves TLR and FcγR Cooperation and Is Dependent on CARD9. J Immunol. 2017, 198, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Varghese, G.P.; Fransén, K.; Hurtig-Wennlöf, A.; Bengtsson, T.; Jansson, J.H.; Sirsjö, A. Q705K variant in NLRP3 gene confers protection against myocardial infarction in female individuals. Biomed. Rep. 2013, 1, 879–882. [Google Scholar] [CrossRef]
- Paramel, G.V.; Folkersen, L.; Strawbridge, R.J.; Elmabsout, A.A.; Särndahl, E.; Lundman, P.; Jansson, J.H.; Hansson, G.K.; Sirsjö, A.; Fransén, K. CARD8 gene encoding a protein of innate immunity is expressed in human atherosclerosis and associated with markers of inflammation. Clin Sci. 2013, 125, 401–407. [Google Scholar] [CrossRef]
- Bai, Y.; Nie, S.; Jiang, G.; Zhou, Y.; Zhou, M.; Zhao, Y.; Li, S.; Wang, F.; Lv, Q.; Huang, Y.; et al. Regulation of CARD8 expression by ANRIL and association of CARD8 single nucleotide polymorphism rs2043211 (p.C10X) with ischemic stroke. Stroke 2014, 45, 383–388. [Google Scholar] [CrossRef]
- García-Bermúdez, M.; López-Mejías, R.; González-Juanatey, C.; Corrales, A.; Castañeda, S.; Ortiz, A.M.; Miranda-Filloy, J.A.; Gómez-Vaquero, C.; Fernández-Gutiérrez, B.; Balsa, A.; et al. CARD8 rs2043211 (p.C10X) polymorphism is not associated with disease susceptibility or cardiovascular events in Spanish rheumatoid arthritis patients. DNA Cell Biol. 2013, 32, 28–33. [Google Scholar] [CrossRef]
- Murakami, T.; Nakaminami, Y.; Takahata, Y.; Hata, K.; Nishimura, R. Activation and function of NLRP3 inflammasome in bone and joint-related diseases. Int J Mol Sci. 2022, 23, 5365. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, C.; Kuang, Z.; Zheng, Q. The Role of NLRP3 Inflammasome Activities in Bone Diseases and Vascular Calcification. Inflammation 2021, 44, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, L.; Yu, X.; Li, P.; Wang, Q.; Li, C. Effects of irisin on osteoblast apoptosis and osteoporosis in postmenopausal osteoporosis rats through upregulating Nrf2 and inhibiting NLRP3 inflammasome. Exp Ther Med. 2020, 19, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Feng, C.; Li, C.; Li, Z.; He, J.; Tu, C. The regulatory role and therapeutic application of pyroptosis in musculoskeletal diseases. Cell Death Discovery 2022, 8, 492. [Google Scholar] [CrossRef]
- Lei, L.; Sun, J.; Han, J.; Jiang, X.; Wang, Z.; Chen, L. Interleukin-17 induces pyroptosis in osteoblasts through the NLRP3 inflammasome pathway in vitro. Int Immunopharmacol. 2021, 96, 107781. [Google Scholar] [CrossRef]
- Polzer, K.; Joosten, L.; Gasser, J.; Distler, J.H.; Ruiz, G.; Baum, W.; Redlich, K.; Bobacz, K.; Smolen, J.S.; van, den, Berg, W.; et al. Interleukin-1 is essential for systemic inflammatory bone loss. Ann Rheum Dis. 2010, 69, 284–290.
- He, Z.; Sun, Y.; Wu, J.; Xiong, Z.; Zhang, S.; Liu, J.; Liu, Y.; Li, H.; Jin, T.; Yang, Y.; et al. Evaluation of genetic variants in IL-1B and its interaction with the predisposition of osteoporosis in the northwestern Chinese Han population. J Gene Med. 2020, 22, e3214. [Google Scholar] [CrossRef]
- Zhang, W.; Cong, X.L.; Qin, Y.H.; He, Z.W.; He, D.Y.; Dai, S.M. IL-18 upregulates the production of key regulators of osteoclastogenesis from fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation 2013, 36, 103–109. [Google Scholar] [CrossRef]
- Lasithiotaki, I.; Giannarakis, I.; Tsitoura, E.; Samara, K.D.; Margaritopoulos, G.A.; Choulaki, C.; Vasarmidi, E.; Tzanakis, N.; Voloudaki, A.; Sidiropoulos, P.; et al. NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. Eur Respir J. 2016, 47, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Artlett, C. M., Sassi-Gaha, S., Rieger, J. L., Boesteanu, A. C., Feghali-Bostwick, C. A., and Katsikis, P. D. The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum. 2011, 63, 3563–3574. [CrossRef] [PubMed]
- Woo, S.; Gandhi, S.; Ghincea, A.; Saber, T; Lee, C.J.; Ryu, C. Targeting the NLRP3 inflammasome and associated cytokines in scleroderma associated interstitial lung disease. Front Cell Dev Biol. 2023, 11, 1254904.
- Ramos-Martinez, E.; Vega-Sánchez, A.E.; Pérez-Rubio, G.; Mejia, M.; Buendía-Roldán, I.; González-Pérez, M.I.; Mateos-Toledo H,N.; Andrade, W.A.; Falfán-Valencia, R.; Rojas-Serrano, J. Enhanced activity of the NLRP3 inflammasome in the lung of patients with anti-synthetase syndrome. Cells 2023, 12, 60.
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nature Reviews Drug Discovery 2018, 17, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Hill, J.R.; Day, C.J.; Zamoshnikova, A.; Boucher, D.; Massey, N.L.; Chitty, J.L.; Fraser, J.A.; Jennings, M.P.; Robertson, A.A.B.; et al. MCC950 directly targets the NLRP3 ATP- hydrolysis motif for inflammasome inhibition. Nature Chemical Biology 2019, 15, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, Y. Effects and mechanisms of potent caspase-1 inhibitor VX765 treatment on collagen induced arthritis in mice. Clinical and Experimental Rheumatology 2016, 34, 111–118. [Google Scholar] [PubMed]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nature Immunology 2020, 21, 736–745. [Google Scholar] [CrossRef]
- You, R.; He, X.; Zeng, Z.; Zhan, Y.; Xiao, Y.; Xiao, R. Pyroptosis and its role in autoimmune diseases: a potential therapeutic target. Front Immunol. 2022, 13, 841732. [Google Scholar] [CrossRef]
- van, der, Heijden, T.; Kritikou, E.; Venema, W.; van, Duijn, J.; van, Santbrink, P.J.; Slütter, B.; Foks, A.C.; Bot, I.; Kuiper, J. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1457–1461.
- Zeng, W.; Wu, D.; Sun, Y.; Suo, Y.; Yu, Q.; Zeng, M.; Gao, Q.; Yu, B.; Jiang, X.; Wang, Y. The selective NLRP3 inhibitor MCC950 hinders atherosclerosis development by attenuating inflammation and pyroptosis in macrophages. Sci. Rep. 2021, 11, 19305. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, X.; Xu, H.; Li, Q.; Meng, L.; He, M.; Zhang, J.; Zhang, Z.; Zhang, Z. VX-765 attenuates atherosclerosis in ApoE deficient mice by modulating VSMCs pyroptosis. Exp. Cell Res. 2020, 389, 111847. [Google Scholar] [CrossRef]
- Mansoori, M.N.; Shukla, P.; Kakaji, M.; Tyagi, A.M.; Srivastava, K.; Shukla, M.; Dixit, M.; Kureel, J.; Gupta, S.; Singh, D. IL-18BP is decreased in osteoporotic women: prevents inflammasome mediated IL-18 activation and reduces Th17 differentiation. Sci Rep. 2016, 6, 33680. [Google Scholar] [CrossRef]
- Huang, C.L.; Chen, D.Y.; Tzang, C.C.; Lin, J.W.; Tzang, B.S.; Hsu, T.C. Celastrol attenuates human parvovirus B19 NS1-induced NLRP3 inflammasome activation in macrophages. Mol Med Rep. 2023, 28, 193. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Yang, J.; Zhang, L.; Liu, J.; Xu, S.; Wang, M.; Zhang, L.; Sun, Y.; Yan, W.; Hou, G.; et al. Celastrol inhibits rheumatoid arthritis through the ROS-NF-κB-NLRP3 inflammasome axis. Int Immunopharmacol. 2021, 98, 107879. [Google Scholar] [CrossRef]
- Wu, D. Efficacy of Baihu-Guizhi Decoction Combined With Western Medicine in Treating Rheumatoid Arthritis. Chin J Urban Rural Enterp Hyg. 2021, 36, 158–160. [Google Scholar]
- Li, W.; Wang, K.; Liu, Y.; Wu, H.; He, Y.; Li, C.; Wang, Q.; Su, X.; Yan, S.; Su, W.; et al. A novel drug combination of mangiferin and cinnamic acid alleviates rheumatoid arthritis by inhibiting TLR4/NF-κB/NLRP3 activation-induced pyroptosis. Front Immunol. 2022, 13, 912933. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, T.; Shen, J.; Shi, X.; Luo, F.; Ren, Y. Sulforaphene targets NLRP3 inflammasome to suppress M1 polarization of macrophages and inflammatory response in rheumatoid arthritis. J Biochem Mol Toxicol. 2023, 37, e23362. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Liu, Z.; Hou, J.; Huang, T.; Yang, M. Osthole improves colllagen-induced arthritis in a rat model through inhibiting inflammation and cellular stress. Cell Mol Biol Lett. 2018, 23, 19. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Z.; Zhang, Q.; Yu, J.; Han, D.; Liu, J.; Li, P.; Li, F. Osthole: a potential AMPK agonist that inhibits NLRP3 inflammasome by regulating mitochondrial homeostasis for combating rheumatoid arthritis. Phytomemdicine 2023, 110, 154640. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, L.; Ling, S.; Duan, J.; Qian, F.; Li, Y.; Xu, J.W. Scropolioside B inhibits IL-1β and cytokines expression through NF-kB and inflammasome NLRP3 pathways. Mediators Inflammation 2014, 2014, 819053. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Ning, Y.; Ningm, X.; Zhang, H. Wedelolactone ameliorate synovial inflammation and cardiac complications in a murine model of collagen-induced arthritis by inhibiting NF-κB/NLRP3 inflammasome activation. Folia Histochemica et Cytobiologica 2022, 60, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Eugenia, Schroeder, M.; Russo, S.; Costa, C.; Hori, J.; Tiscornia, I.; Bollati-Fogolín, M, Zamboni DS, Ferreira G, Cairoli E, Hill M. Pro-inflammatory Ca++-activated K+ channels are inhibited by hydroxychloroquine. Sci Rep. 2017, 7, 1892.
- Cui, J.; Hong, P.; Li, Z.; Lin, J.; Wu, X.; Nie, K.; Zhang, X.; Wan, J. Chloroquine inhibits NLRP3 inflammasomes activation and alleviates renal fibrosis in mouse model of hyperuricemic nephropathy with aggravation by a high-fat-diet. Int Immunopharmacol. 2023, 120, 110353. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.; Singh, J.A. Anakinra for rheumatoid arthritis. Cochrane Database Systematic Review. 2009, 21, CD005121. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhan, N.; Jin, Y.; Ling, H.; Xiao, C.; Xie, Z.; Zhong, H.; Yu, X.; Tang, R.; Ma, J.; et al. Tofacitinib restores the balance of γδTreg/γδT17 cells in rheumatoid arthritis by inhibiting the NLRP3 inflammasome. Theranostics 2021, 11, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, G.; Martynova, E.V.; Gilazieva, Z.E.; McIntyre, A.; Rizvanov, A.A.; Khaiboullina, S.F. MicroRNA post-transcriptional regulation of the NLRP3 inflammasome in immunopathologies. Front Pharm. 2019, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Xi, Q.; Liu, H.; Guo, X.; Zhang, J.; Zhang, Z.; Li, Y.; Yang, G.; Zhou, D.; Yang, H.; et al. miR-21 promotes NLRP3 inflammasome activation to mediate pyroptosis and endotoxic shock. Cell Death Dis. 2019, 10, 461. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function, and decay. Nat Rev Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Wei, B.; Pei, G. MicroRNAs: critical regulators in Th17 cells and players in diseases. Cell Mol Immunol. 2010, 7, 175–181. [Google Scholar] [CrossRef]
- Xie, Q.; Wei, M.; Zhang, B.; Kang, X.; Liu, D.; Zheng, W.; Pan, X.; Quan, Y.; Liao, D.; Shen, J. MicroRNA-33 regulates the NLRP3 inflammasome signaling pathway in macrophages. Mol Med Rep 2018, 17, 3318–3327. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, W.; Chen, Y.; Chen, Y.; Shi, J.; Qin, R.; Wang, H.; Wang, R.; Yuan, H.; Sun, W. RelA/MicroRNA-30a/NLRP3 signal axis is involved in rheumatoid arthritis via regulating NLRP3 inflammsome in macrophages. Cell Death and Disease 2021, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, D.; Ma, W.; Liu, J.; Ning, Q.; Tang, F.; Li, L. miR-223 in exosomes from bone marrow mesenchymal stem cells ameliorates rheumatoid arthritis via downregulation of NLRP3 expression in macrophages. Molecular Immunology 2022, 143, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.L.; Chen, Y.M.; Tang, K.T.; Chen, P.K.; Liu, H.J.; Chen, D.Y. MicroRNA-223 inhibits IL-18-mediated neutrophil extracellular trap through regulating calcium influx in adult-onset Still’s disease. Sci Rep. 2021, 11, 15676. [Google Scholar] [CrossRef]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009, 458, 223–227. [Google Scholar] [CrossRef]
- Geisler, S.; Coller, J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013, 14, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Mathy, N.W.; Chen, X.M. Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J Biol Chem. 2017, 292, 12375–12382. [Google Scholar] [CrossRef]
- Wang, Z.; Kun, Y.; Lei, Z.; Dawei, W.; Lin, P.; Jibo, W. LncRNA MIAT downregulates IL-1β, TNF-α to suppress macrophage inflammation but is suppressed by ATP-induced NLRP3 inflammasome activation. Cell Cycle 2021, 20, 194–203. [Google Scholar] [CrossRef]
- Sarsenova, M.; Issabekova, A.; Abisheva, S.; Rutskaya-Moroshan, K.; Ogay, V.; Saparov, A. Mesenchymmal stem cell-based therapy for rheumatoid arthritis. Int J Mol Sci. 2021, 22, 11592. [Google Scholar] [CrossRef]
- Shin, T.H.; Kim, H,S.; Kang, T.W.; Lee, B.C.; Lee, H.Y.; Kim, Y.J.; Shin, J.H.; Seo, Y.; Won Choi S.; Lee, S.; et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheuamatoid arthritis. Cell Death and Disease 2016, 7, e2524. [CrossRef] [PubMed]
| Agents | Targets | Experimental model and mechanism | Diseases | References | ||
|---|---|---|---|---|---|---|
| Small molecule inhibitors | ||||||
| MCC950 | The NACHT domain of NLRP3 inflammasome | Block ASC oligomerization, inhibit inflammation (1) Reduce synovitis and cartilage erosion by inhibiting NLRP3 and caspae-1 activation in CIA model. (2) Elevated liver enzymes in phase II clinical trial. | RA, ASCVD, OP, ILD | [126,127,131,132] | ||
| VX-765 | Caspase-1 | Ameliorate the severity and progression of synovitis in CIA murine model. | RA, ASCVD | [128,133] | ||
| Disulfiram | GSDMD | Inhibits pyroptosis and inflammatory cytokine release in both canonical and noncanonical inflammasome pathways. | RA and associated OP | [129] | ||
| IL-18BP | IL-18 binding protein | Reduces Th17 cells with resultant inhibition of osteoclastogenesis and induces osteoblasts formation. | RA and associated OP | [134] | ||
| Natural products | ||||||
| Celastrol (isolated from Tripterygium wilfordii) | Inhibit the ROS-NF-κB-NLRP3 inflammasome axis. |
|
RA | [135,136] | ||
| Baihu-Guizhi decoction (BHGZD) | Inhibit TLR4/NF-κB/NLRP3 activation- induced pyroptosis. | Reduce synovitis as well as bone erosion and alleviate disease activity through inhibiting NF-κB via TLR4/PI3K/AKT signaling to suppress the NLRP3 inflammasome activation and GSDMD-mediated pyroptosis in AIA-modified rat model | RA | [138] | ||
| Sulforaphene (extracted from radish seeds) | NLRP3 | Suppress the M1 polarization of macrophages and reduce synovitis in CIA murine model | RA | [139] | ||
| Osthole (extracted from Angelicae pubescentis radix) | AMPK agonist | Inhibit NLRP3 inflammasome activation by regulating mitochondrial homeostasis in CIA rat model. | RA | [141] | ||
| Scropolioside B (isolated from Scrophularia dentada Royle ex Benth.) | NF-κB and the NLRP3 inflammasome pathway | Inhibit NF-κB activity, reduce NLRP3 expression, and suppress the maturation as well as the release of IL-1β. | RA and associated atherosclerosis | [142] | ||
| Wedelolactone, derived from Eclipta alba | NF-κB and the NLRP3 inflammasome | Ameliorate synovitis and cardiac complications via inhibiting the activation of NF-κB/NLRP3 inflammasome pathway | RA and cardiac complication | [143] | ||
| Disease-modifying anti-rheumatic drugs (DMARDs) | ||||||
| Hydroxychloroquine or chloroquine | The second signal of NLRP3 activation |
|
RA and associated ASCVD | [144,145] | ||
| Anakinra, a biological DMARDs | IL-1β receptor antagonist | Inhibit the NLRP3 inflammasome downstream cytokine, IL-1β, in RA patients. | RA | [146] | ||
| Canakinumab, a biological DMARDs | Monoclonal antibody targeting IL-1β | Reduce the rates of recurrent ASCVD, including myocardial infarction and stroke | RA and associated ASCVD | [147] | ||
| Tofacitinib, a Janus kinase 1/3 inhibitor | NLRP3 inflammasome | Restore the balance of γδTreg/γδT17 cells by inhibiting NLRP3 inflammasome in CIA model | RA | [148] | ||
| Epigenetic regulators | ||||||
| MiRNA-33 inhibitor | NLRP3 and caspase-1 | MiR-33 impairs mitochondrial oxygen consumption rate with increasing ROS, and then upregulates NLRP3 inflammasome expression in macrophages in CIA mice model | RA | [153] | ||
| MiRNA-30a | NLRP3 | MiRNA-30a inhibits the NLRP3 inflammasome activation, reduce synovitis, and bone damage in TNFα-transgenic C57BL/6 mice model. | RA | [154] | ||
| MiRNA-223 | NLRP3 | MiRNA-223 from BMSCs-derived exosomes inhibits NLRP3 activation and the release of IL-β, TNF-α, and IL-18 in RAW264.7 cells by luciferase reporter assay & rescue experiment | RA and associated ASCVD | [155] | ||
| LncRNA MIAT | IL-1β | LncRNA MIAT inhibited the expression of IL-1β, TNF-α and suppressed macrophage inflammation in J774A.1 cell-based assay. | RA | [160] | ||
| Allogenic mesenchymal stem cells | ||||||
| hUCB-MSCs | NLRP3 inflammasome | Downregulate the activation of NLRP3 inflammasome via a paracrine loop of IL-1β signaling in CIA murine model. | RA | [162] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





