Submitted:
06 December 2023
Posted:
08 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
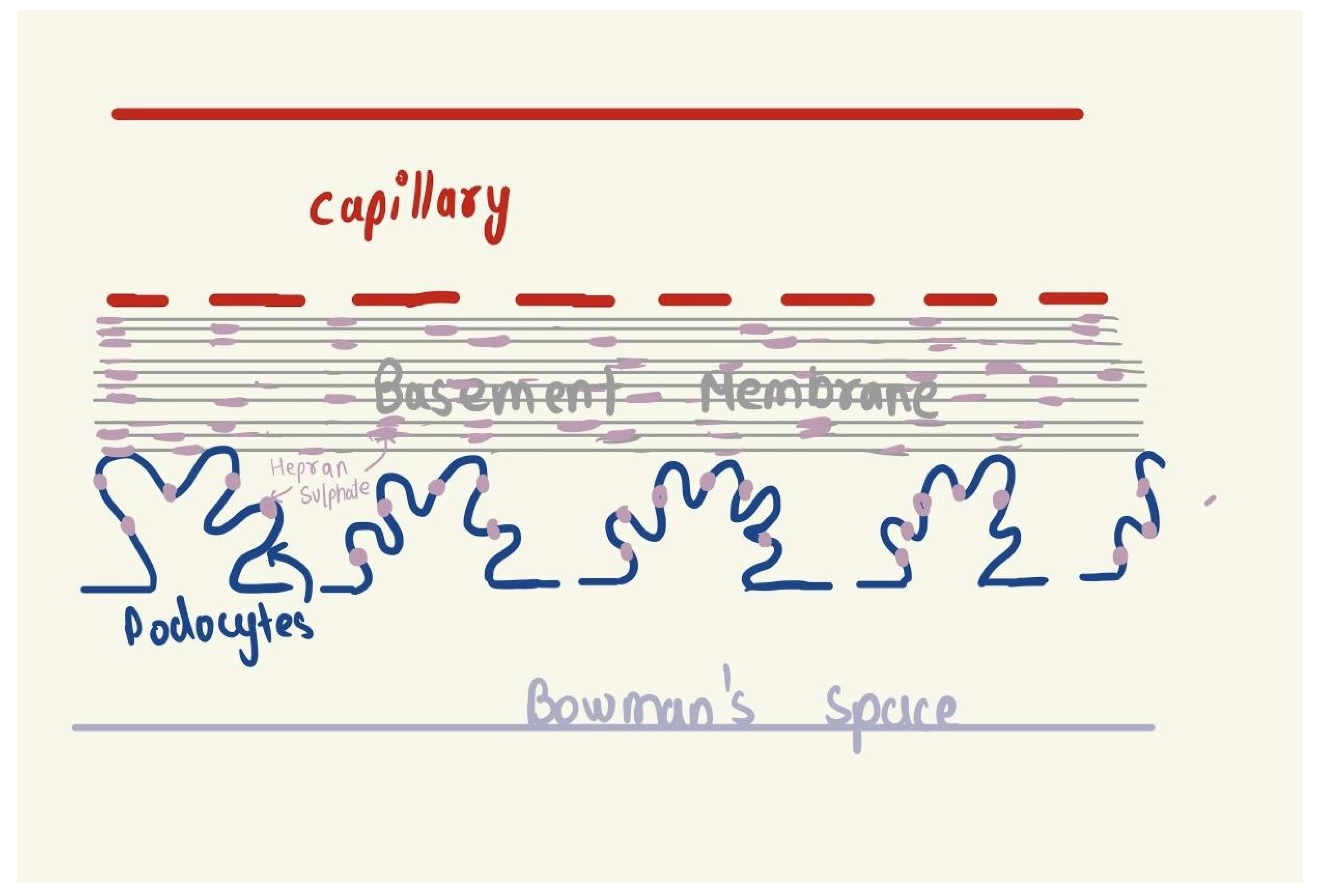
- Etiologic factors
- Mechanisms of injury
- Response to that injury
Etiologic factors
Categories of GN
- Linear antibody deposition: Anti-GBM disease,
- Granular immune complex-mediated injury,
- pauci-immune narcotising and crescentic GN [https://www.ncbi.nlm.nih.gov/books/NBK557430/]
[A] Linear antibody deposition: Anti-GBM disease
[B] Granular immune ccomplex deposition
- Post-infectious GN, especially after a Streptococcus infection (complex deposition mainly sub-epithelial)
- Collagen vascular disease
- Lupus nephritis (complex deposition mainly sub-endothelial)
- Henoch-Schönlein purpura - there are immunoglobulin A deposits and associated systemic vasculitis
- Immunoglobulin A nephropathy without vasculitis
- Mixed cryoglobulinemia
- Membranoproliferative glomerulonephritis
- Fibrillary glomerulonephritis
[C] Pauci-immune crescentic GN
- Granulomatosis with polyangiitis {GPA} (previously known as Wegner’s disease)
- Microscopic polyangiitis
- Eosinophilic granulomatosis with polyangiitis {EPGA} (previously known as Chrug-Strauss syndrome)
- The release of toxic substances from neutrophils leads to damage to the small blood vessel walls.
- Neutrophil migration through blood vessel walls causing inflammation in surrounding tissues.
- Release of signaling factors which in turn attract more neutrophils, perpetuating the inflammation and destruction of small blood vessels.
- Hydralazine
- Levamisole-contaminated cocaine
- Propylthiouracil and methimazole
- Allopurinol
- Sulfasalazine
- Minocycline
- Penicillamine
- Rifampicin
- Aminoguanidine
- Sofosbuvir
- Anti-TNF therapy for rheumatoid arthritis and ankylosing spondylitis (Naik and Shawar 2023)
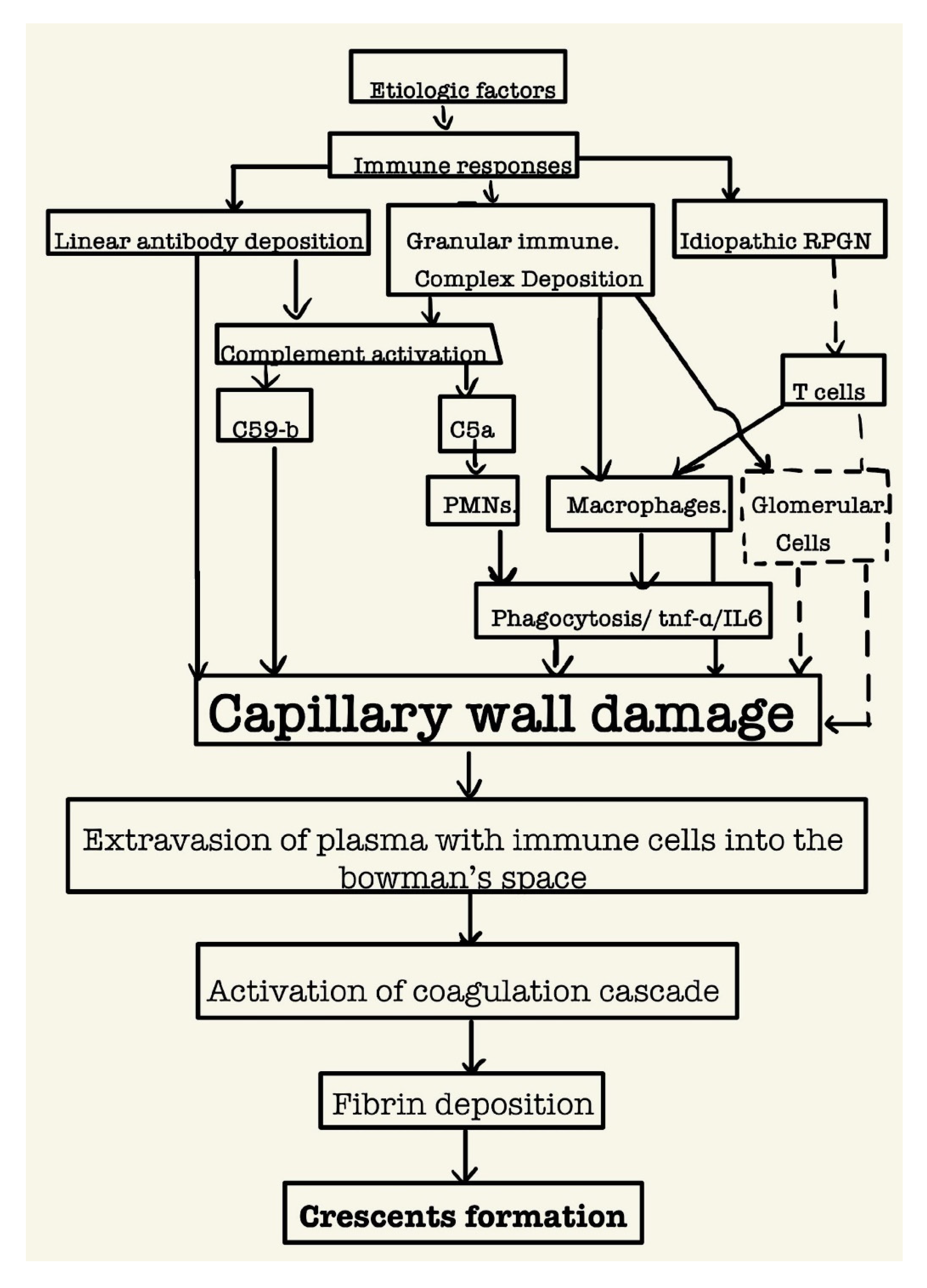
Conclusion
- RPGN, characterized by Rapid loss of renal function (Clinical manifestations include gross hematuria, proteinuria, oliguria, hypertension, and edema.) over a very short period (days to weeks)
- Glomerular crescents are the histologic hallmark of the disease
- They are best diagnosed by renal biopsy electron microscopic studies.
- Mainly three types of crescentic GN: Anti GBM antibody disease; immune complex deposition, pauci immune GN
- Diagnostic approaches should include immunofluorescence studies on the biopsy as well as the detection of plasma ANCA.
- Prompt diagnosis is a must to reverse the damage. As final fibrous stages are unlikely to respond to immunosuppressive therapy.
Institutional Review Board Statement
Conflicts of Interest
References
- Satchell, S.C.; Braet, F.; Qureshi, S.H.; Patel, N.N.; Murphy, G.J.; Fu, J.; Lee, K.; Chuang, P.Y.; Liu, Z.; He, J.C.; et al. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Physiol. 2009, 296, F947–F956. [Google Scholar] [CrossRef]
- Immunotactoid Glomerulopathy. Diagnostic Pathology: Kidney Diseases [Internet]. 2016 [cited 2023 Dec 5];266–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780323377072500549.
- discussant P, Charles Jennette J. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164–77. https://doi.org/10.1046/j.1523-1755.2003.00843.x. [CrossRef]
- Moorani KN, Aziz M, Amanullah F. Rapidly progressive glomerulonephritis in children. Pak J Med Sci [Internet]. 2022 [cited 2023 Dec 2];38(2):417. https://doi.org/10.12669/pjms.38.icon-2022.5774. [CrossRef]
- Lionaki S, Liapis G, Boletis JN. Pathogenesis and Management of Acute Kidney Injury in Patients with Nephrotic Syndrome Due to Primary Glomerulopathies. Medicina (B Aires) [Internet]. 2019 [cited 2023 Dec 2];55(7). https://doi.org/10.3390/medicina55070365. [CrossRef]
- Naik RH, Shawar SH. Rapidly Progressive Glomerulonephritis. StatPearls [Internet]. 2023 May 1 [cited 2023 Dec 2].. https://doi.org/10.20944/preprints202312.0614.v1. [CrossRef]
- Naik RH, Shawar SH. Rapidly Progressive Glomerulonephritis. StatPearls [Internet]. 2023 May 1 [cited 2023 Dec 2]. https://doi.org/10.20944/preprints202312.0614.v1. [CrossRef]
- Naik RH, Shawar SH. Rapidly Progressive Glomerulonephritis. StatPearls [Internet]. 2023 May 1 [cited 2023 Dec 2]. https://doi.org/10.20944/preprints202312.0614.v1. [CrossRef]
- Kaartinen K, Safa A, Kotha S, Ratti G, Meri S. Complement dysregulation in glomerulonephritis. 2019 [cited 2023 Dec 2]. https://doi.org/10.1016/j.smim.2019.101331. [CrossRef]
- Kaartinen K, Safa A, Kotha S, Ratti G, Meri S. Complement dysregulation in glomerulonephritis. 2019 [cited 2023 Dec 2]. https://doi.org/10.1016/j.smim.2019.101331. [CrossRef]
- Overview of the classification and treatment of rapidly progressive (crescentic) glomerulonephritis - UpToDate [Internet]. [cited 2023 Dec 2]. Available from: https://www.uptodate.com/contents/overview-of-the-classification-and-treatment-of-rapidly-progressive-crescentic-glomerulonephritis.
- Overview of the classification and treatment of rapidly progressive (crescentic) glomerulonephritis - UpToDate [Internet]. [cited 2023 Dec 2]. Available from: https://www.uptodate.com/contents/overview-of-the-classification-and-treatment-of-rapidly-progressive-crescentic-glomerulonephritis.
- Moorani KN, Aziz M, Amanullah F. Rapidly progressive glomerulonephritis in children. Pak J Med Sci [Internet]. 2022 [cited 2023 Dec 2];38(2):417–25. https://doi.org/10.12669/pjms.38.ICON-2022.5774. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




