1. Introduction
In flowering plants, the male gametophyte, or pollen grain, is released after the second pollen mitosis. Wheat pollen grains, when mature, are tricellular, possess high moisture content, and exhibit a short lifespan [
1,
2]. This limited viability is attributed to the respiratory activity in tricellular pollen, necessitating successful pollination within a brief 30 to 40-min window post-pollen shedding for seed production (Fritz and Lukaszewski, 1989). The success of pollination in crop plants hinges on the vigor and viability of pollen grains, with the collection stage playing a crucial role. Pollen from closed flowers, due to its immaturity and reduced susceptibility to contamination, is considered ideal for maintaining high viability (He et al., 2017).
Pollen viability is evaluated through stainability, germinability, and fertilization capability (Dafni and Firmage, 2000). In genetic improvement programs, in vitro germination serves as a common viability assay, but specific protocols and culture media are required for each species (Machado et al., 2014; Zambon et al., 2014). Alternatively, cytological observations using vital fluorescent dyes offer indirect methods for assessing pollen viability (Impe et al., 2020). Various factors, such as pollen vigor, developmental stage, temperature, and moisture content, contribute to the duration of pollen viability, leading to variability among crops and even genotypes within a species (Liu et al., 2023; Patel and Mankad, 2014; Koubouris, 2009).
In breeding programs, understanding pollen viability and germination is crucial for controlled pollination. Preserving pollen viability is essential for overcoming barriers to hybridization, especially when dealing with plants or species with differing flowering times or growing in distinct regions (Yoshiji & Shiokawa, 1992). The viability of pollen varies between species; for instance, Agrostis stolonifera L. pollen loses viability rapidly, while maize pollen becomes non-viable within two hours in field conditions. Buckwheat, on the other hand, prefers low temperatures and high humidity for preserving pollen viability (Fei and Nelson, 2003; Luna et al., 2001; Adhikari and Campbell, 1998).
In wheat breeding programs, a significant challenge arises from genetic variation in flowering time among elite parents. Storing pollen until the desired pollination time can help overcome these differences. While it is commonly believed that wheat pollen has a short lifespan, there is limited information on optimal storage conditions (Fritz & Lukaszewski, 1989). Previous studies emphasize the importance of in vitro pollen germination and staining for assessing stored pollen, correlating well with fertile seed yield (Sedgley et al., 1992).
Storage conditions, particularly temperature, profoundly impact pollen viability (Deng and Harbaugh, 2004). Ultra-low temperature (cryo) preservation, requiring moderate dehydration, is effective in preventing damage to cell membranes caused by ice crystal formation. However, achieving the right moisture level and thawing method is complex, making identification of the optimal preservation temperature essential (Sauve et al., 2000; Jia et al., 2022; Liu et al., 2023; Yuan et al., 2018). Limited data exist on wheat pollen storage at low temperatures, with one study reporting preservation at 5 ºC for one day (Baninasab et al., 2017). Evaluating pollen viability for numerous wheat lines is challenging due to the short lifespan post-shedding and variations in flowering periods.
To address these challenges, this study aimed to investigate suitable temperature conditions and storage durations for preserving pollen grains from 50 spring wheat genotypes, intending to contribute to the development of effective pollen preservation methods for breeding purposes.
2. Results
2.1. Assessment of Pollen Viability and Germination Across Genotypes:
The assessment of pollen viability and in vitro pollen germination revealed substantial variability among different genotypes in relation to storage time (ST) and temperature conditions, as depicted in
Figure 1 and
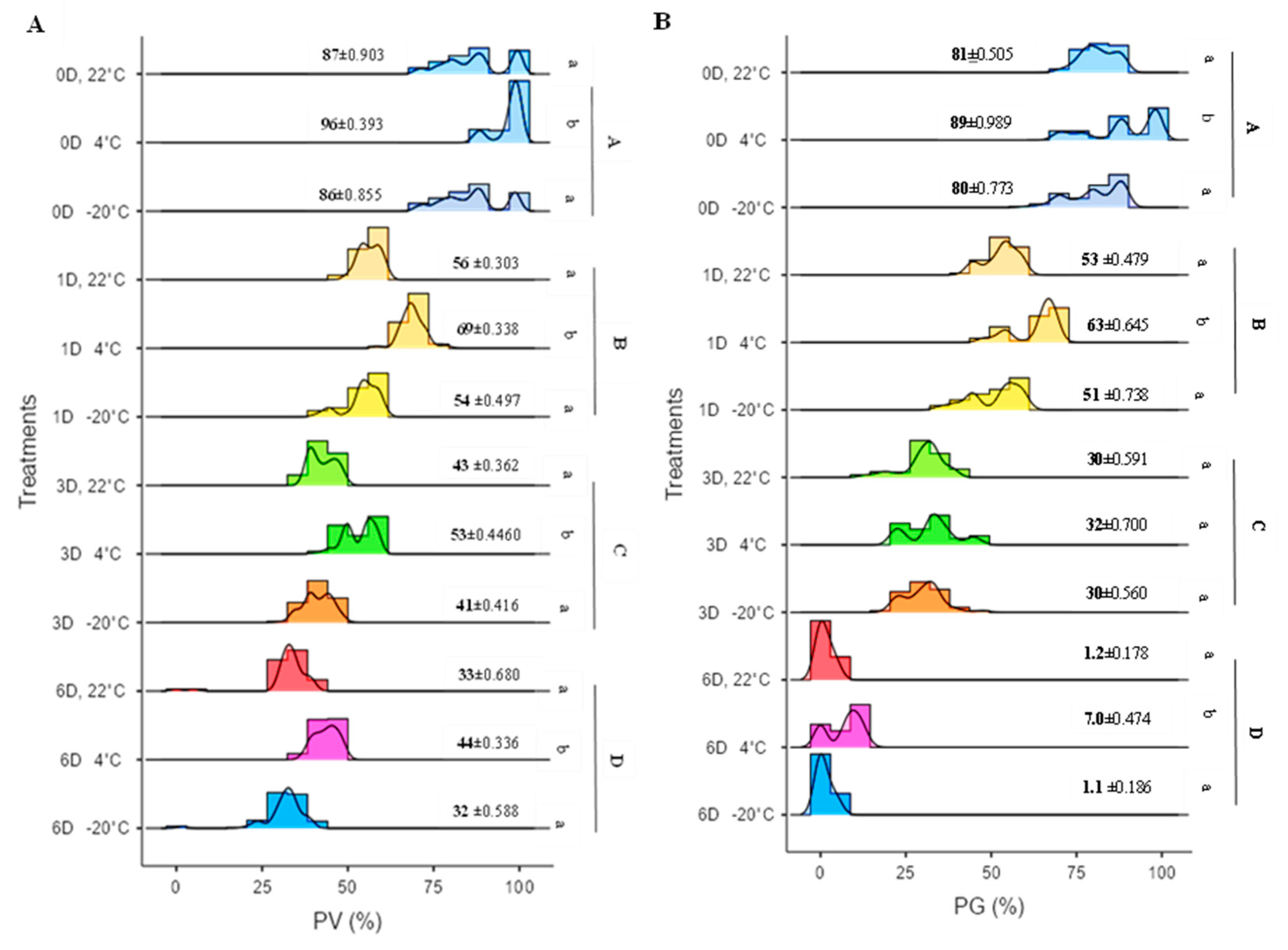
Figure 2. Histograms illustrating pollen viability exhibited pronounced variation regarding storage duration. Freshly collected pollen (with no storage, 0 days) exhibited the highest pollen viability percentages across all 50 spring wheat genotypes. The pollen viability percentages ranged from 71% to 100% for these fresh samples (
Figure 3A,
Table S1). Similarly, in vitro pollen germination rates ranged from 63% to 98.6% for the same fresh pollen samples (
Figure 3B,
Table S2). Notably, for all genotypes and under all storage temperature conditions, both in vitro pollen germination and pollen viability exhibited significant decreases as storage time elapsed (
Figure 3,
Tables S1 and S2).
Specifically, at 0 days of storage (fresh pollen), the pollen viability was high at 90.03%, accompanied by an in vitro pollen germination rate of 83.40%. However, after the first day of storage, these values dropped substantially to 59.75% for pollen viability and 55.65% for in vitro pollen germination. Furthermore, after three days of storage, pollen viability decreased to 45.65%, while in vitro pollen germination plummeted to 30.93%. Finally, after six days of storage, both pollen viability and in vitro pollen germination reached their lowest levels at 35.87% and 3.10%, respectively (Table 2,
Figure 3). These findings underscore that, at each storage duration, in vitro pollen germination was consistently lower than pollen viability.
To assess variations among genotypes, storage time (ST), storage temperature (T), as well as interactions between genotypes (G) and ST, G and T, and T×ST, an analysis of variation (ANOVA) was conducted. The analysis revealed statistically significant differences (
p < 0.001) in pollen viability and in vitro pollen germination among genotypes, pollen storage temperatures, and storage duration (
Table 1). Importantly, the ANOVA also indicated significant impacts (
p < 0.001) of genotypes on both pollen viability and in vitro pollen germination rates, as well as significant interactions with storage time (ST) and temperature (T) (
Table 1).
2.2. Influence of Storage Duration and Temperature on Pollen Viability and Germination:
The impact of storage duration and temperature on both pollen viability (PV) and in vitro pollen germination (PG) was conspicuous, with more pronounced declines observed at 22 °C and −20 °C. At ambient temperature (22 °C), one day of storage resulted in approximately 56.46% pollen viability and 53.19% in vitro pollen germination. Similarly, at −20 °C, after one day of storage, pollen exhibited viability of approximately 53.94% and in vitro pollen germination of 50.67%. Subsequently, after three and six days of storage, pollen viability dropped to 41.04% and 31.59%, and in vitro pollen germination dwindled to 30.16% and 1.07%, respectively (
Figure 3,
Tables S1 and S2).
In contrast, when pollen was stored at 4 °C compared to storage at 22 °C and −20 °C, it significantly preserved both PV and PG across all storage durations (1D, 3D, and 6D). After 6 days of storage at 4 °C, pollen from 36 out of 50 spring wheat genotypes still maintained germination rates ranging from 6% to 14% (
Table S2) (
Figure 5). Conversely, when stored at 22 °C and −20 °C, only 16 and 12 genotypes retained germination rates of about 3–5% and 2–5%, respectively (
Table S2). Least significant difference (LSD) analysis conducted among different storage temperatures at each storage duration for both pollen viability and germination indicated that the 4 °C storage temperature exhibited significant variation compared to 22 °C and −20 °C (
Figure 3). Pollen viability and in vitro pollen germination from all 50 spring wheat genotype samples stored at room temperature and −20 °C showed nearly identical trends (
Tables S1 and S2).
2.3. Correlation of Pollen Viability and Germination with Storage Duration:
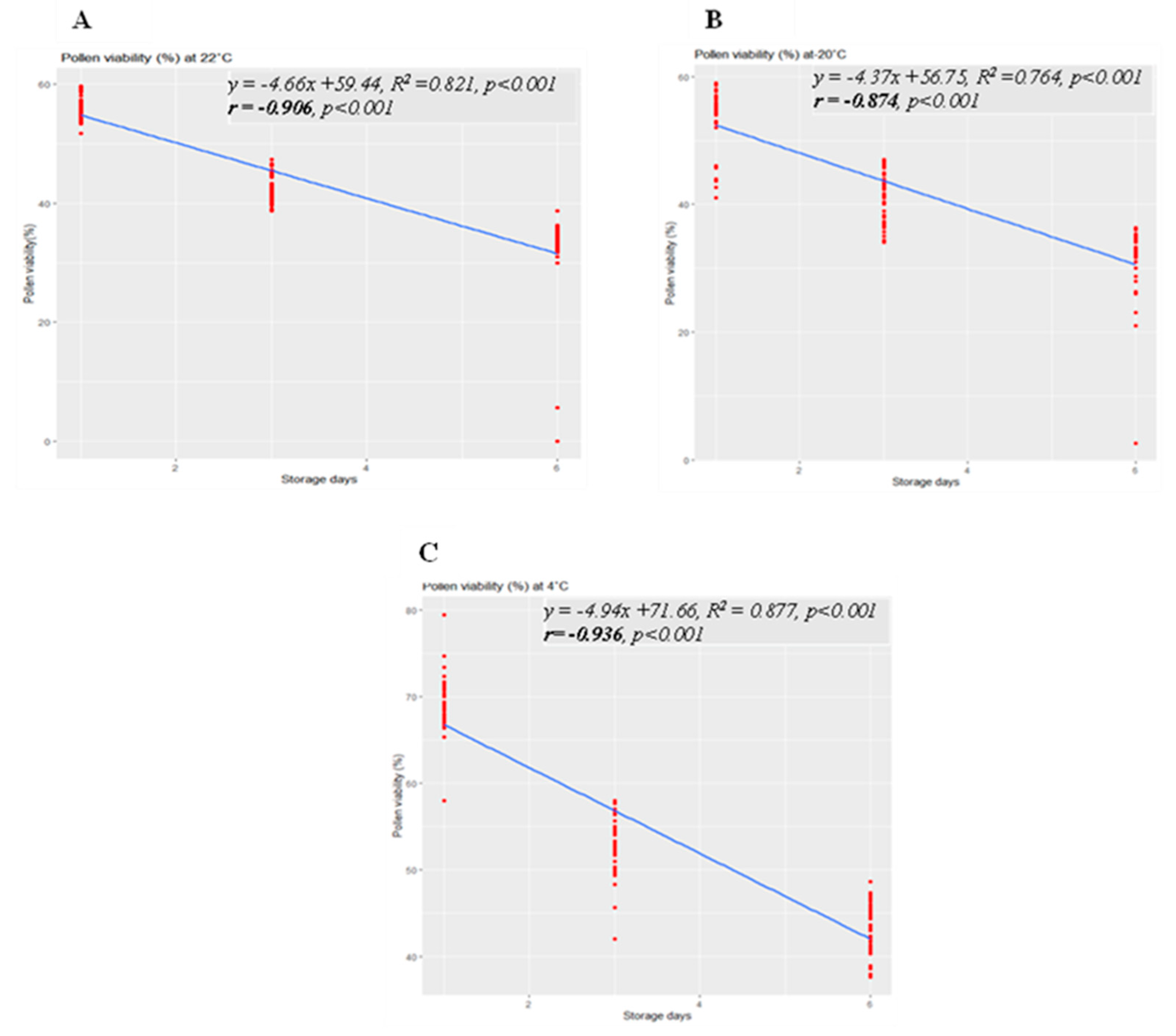
Linear regression analysis revealed significant linear regressions between pollen viability and storage day at various storage temperatures, namely 22 °C, −20 °C, and 4 °C (
Figure 4A-C). Simple linear regression analysis demonstrated notably strong correlations, with R2 values of 0.821 (
p < 0.001) at 22 °C, 0.764 (
p < 0.001) at −20 °C, and 0.877 (
p < 0.001) at 4 °C. Negative regression results indicated that with an increase in storage time, pollen viability declined. The maximum pollen viability on the first day of storage was 55%, 52%, and 66% at 22 °C, −20 °C, and 4 °C, respectively. After 3 days of storage, pollen germination decreased to 47%, 44%, and 58% at 22 °C, −20 °C, and 4 °C, respectively. Subsequently, on the 6th day of storage, germination rates reduced to 31.5%, 31%, and 43% at 22 °C, −20 °C, and 4 °C, respectively. These results underscore that pollen germination was notably higher at the 4 °C storage temperature compared to 22 °C and −20 °C across the different storage durations.
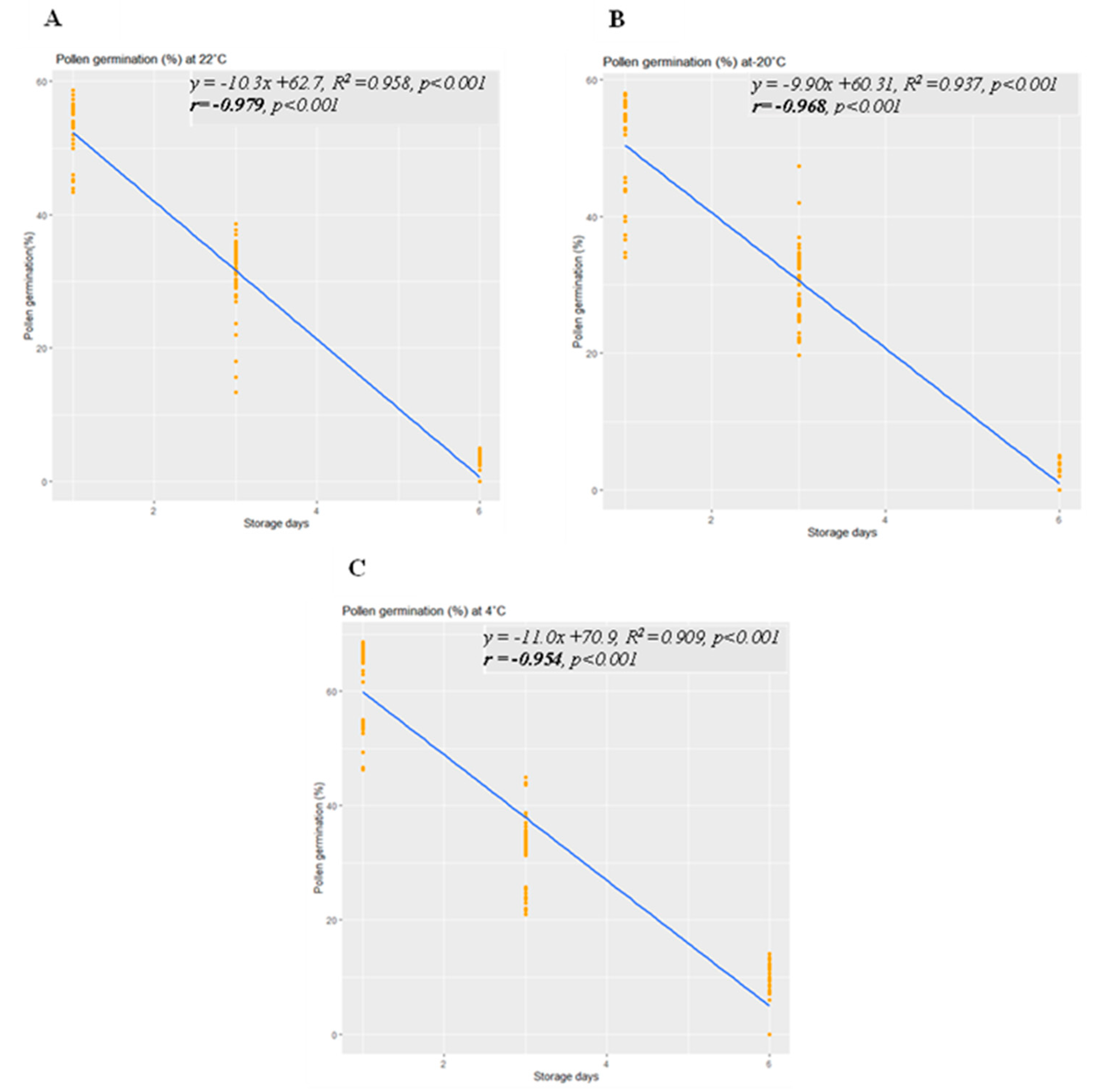
Likewise, linear regression analysis demonstrated significant linear regressions between pollen germination and storage day at 22 °C, −20 °C, and 4 °C (
Figure 5A-C). Simple linear regression analysis revealed strong correlations, with R2 values of 0.958 (
p < 0.001) at 22 °C, 0.937 (
p < 0.001) at −20 °C, and 0.909 (
p < 0.001) at 4 °C. Negative regression results indicated that with an increase in storage time, pollen germination declined. The maximum pollen germination on the first day of storage was 52%, 50%, and 60% at 22 °C, −20 °C, and 4 °C, respectively. After 3 days of storage, pollen germination decreased to 32%, 31%, and 38% at 22 °C, −20 °C, and 4 °C, respectively. Subsequently, on the 6th day of storage, germination rates plummeted to 1%, 1.3%, and 5.2% at 22 °C, −20 °C, and 4 °C, respectively. These findings emphasize that pollen germination exhibited significantly higher rates when stored at 4 °C compared to 22 °C and −20 °C across various storage duration.
3. Discussion
Since there are no reports of 4˚C temperature influence on pollen longevity of wheat under 6 days storage conditions, the present report aimed at providing information on the best storage conditions of wheat pollen to be further used for raising the fertilization potentials of selected wheat genotypes. Wheat is recognized as a self-pollinating crop (Shewry, 2009; Griffin, 2012) and is distinguished by its relatively high moisture content and limited shelf life (McCormick, 2004). Therefore, it is imperative to conduct pollination within a tight timeframe of 30–40 min following pollen shedding to ensure successful seed set (Fritz and Lukaszewski, 1989). Pollen viability plays a pivotal role in fertilization (Fernando et al., 2005), embryonic development (Hosoo et al., 2005), and seed quality (Kormutak et al., 2013). The longevity and viability of plant pollen vary significantly among species and are influenced by environmental factors (Dafni and Firmage, 2000). The capacity of pollen to maintain viability over time and under different storage conditions hinges on both its genetic characteristics and environmental factors (Souza et al., 2015; Du et al., 2007; Fernando et al., 2006). Optimal storage conditions for pollen also differ across species and cultivars (Naik et al., 2013).
In our current investigation, we observed substantial variations in the longevity of wheat pollen among different genotypes and storage temperatures. These findings are consistent with earlier research (Dafni and Firmage, 2000; Lora et al., 2006; Masum-Akond et al., 2012; Dutta et al., 2013; Novara et al., 2017; Yuan et al., 2018). However, studies focusing on the short-term storage of spring wheat pollen at various temperatures are relatively scarce (Baninasab et al., 2017). Proper storage temperatures are crucial for preserving pollen viability (Alburquerque et al., 2007). Hence, the primary objective of our study was to establish the optimal temperature range for storing spring wheat pollen and to determine the duration for which wheat pollen can be stored under diverse conditions without compromising viability.
The longevity of pollen in rice, wheat, and maize can vary from mere minutes to several hours (Luna et al., 2001). Our study highlighted genotype-specific variations in pollen viability and longevity among all observed genotypes, which aligns with findings from previous research (Fernando et al., 2006; Souza et al., 2015; Du et al., 2007; Bryhan and Serdar, 2009). Our assessments of pollen viability and germination at temperatures of 22 °C, −20 °C, and 4 °C demonstrated that the highest levels of pollen viability (43.42%) and germination (7.05%) occurred at 4 °C after six days of storage. Lower temperatures are typically utilized for long-term pollen preservation due to reduced pollen respiration and decreased consumption of soluble sugars and organic acids (Akihama et al., 1979; Yin and Zhao, 2005). However, we observed a decline in in vitro pollen germination at −20 °C, which may be attributed to freezing and thawing of the pollen grains. According to Bhat et al. (2012), reduced pollen germination during storage can be linked to intracellular ice accumulation, cell death, and subzero temperatures.
Numerous factors can influence pollen viability, including pollen handling during collection, the maturity stage of flowering, and environmental conditions such as air temperature and moisture content (Martins et al., 2017; Jumrani et al., 2018; Du et al., 2007). Our findings indicated a consistent decrease in the percentage of pollen viability and in vitro pollen germination for all genotypes across all storage temperatures over time. Initially, fresh pollen exhibited high pollen viability (90.03%) and in vitro pollen germination (83.40%). However, after one and three days, these values significantly declined to 59.75% and 55.65% for pollen viability and 45.65% and 30.93% for in vitro pollen germination, respectively. Subsequently, after six days of storage, pollen viability reached 35.87%, and in vitro pollen germination plummeted to 3.10%. These results are consistent with the findings of Parzies et al. (2005), who reported a decrease in pollen viability of barley genotypes originating from semi-arid regions to less than 50% within 24 h after anthesis. Our results are also in agreement with studies on other cereal species, which have shown that pollen grains from plants like sorghum and maize have exceptionally short lifespans, ranging from a few minutes to several hours (Luna et al., 2001; Tuinstra and Wedel, 2000).
The decline in pollen germination may be attributed to the inactivation of crucial germination enzymes and substrates, as well as the reduced ability of pollen grains to germinate when stored at room temperature. Moreover, Gandadikusumah et al. (2017) reported that the loss of pollen viability during storage is linked to enzymatic activity that diminishes the availability of respiratory substrates.
In our study, in vitro pollen germination percentages consistently lagged behind pollen viability test results for all 50 spring wheat genotypes examined. This discrepancy may be attributed to various uncontrollable variables, including pollen density, the choice of the most suitable growth medium, and the specific environmental requirements of each genotype (Hechmi et al., 2015). Consistent with our findings, Cheng and McComb (1992) reported low and variable germination rates of wheat pollen grains under in vitro conditions, with a maximum germination rate of 6.8%. Devrnja et al. (2012) also noted that trinucleate pollen germinates more rapidly but has a shorter lifespan than binucleate pollen. Furthermore, some species with trinucleate pollen may encounter difficulties in developing pollen tubes in vitro, as indicated by Mulcahy and Mulcahy (1988). Variations in pollen longevity among plant species can be attributed to differences in pollen desiccation tolerance (Song and Tachibana, 2007).
Our study highlighted that pollen viability and in vitro pollen germination were notably higher at 4 °C than at −20 °C and 22 °C across all selected spring wheat genotypes. In a prior study, spring wheat genotypes stored at 5 °C exhibited approximately 1.64% pollen germination after 24 h of storage but experienced a complete loss of viability, with 0.00% germination after 48 and 72 h (Baninasab et al., 2017). In contrast, our study found that spring wheat genotypes maintained 6–14% germination at 4 °C after six days of storage. The data analysis emphasized the significant impact of genotypes on pollen viability and germination rates, as well as their interactions with storage temperatures and durations, corroborating earlier findings (Baninasab et al., 2017).
Our results demonstrated a significant negative correlation and linear regression between both pollen viability and in vitro pollen germination with storage duration (days) at 22 °C, −20 °C, and 4 °C. These findings illustrate that an increase in storage duration led to a reduction in the viability and longevity of wheat pollen. This decrease in pollen viability and longevity with prolonged storage days has been documented in previous studies (Parzies et al., 2005; Luna et al., Tuinstra and Wedel, 2000; Martins et al., 2017; Jumrani et al., 2018; Du et al., 2007).
4. Materials and Methods
Plant Materials and Growth Conditions: We collected pure seeds of 50 diverse spring wheat genotypes, including landraces, pre-green revolution, post-green revolution varieties, recent cultivars, and advanced lines. The wheat genotypes were cultivated using a randomized complete block design (RCBD) with three replications at the National Institute for Genomics and Advanced Biotechnology (NIGAB) in Islamabad, Pakistan. The seeds were sown using a wheat planter in 1.2 m x 3 m plots, with each plot consisting of six rows spaced 20 cm apart. Standard agronomic practices were used throughout the experiment.
Pollen Collection and Storage: During the flowering stage, we collected spikes with yellow anthers to extract anthers for sampling. To extract the anthers, we carefully opened the glume and lemma using forceps. Subsequently, we stored the sampled anthers in tightly sealed plastic vials for future use. For each wheat genotype, anthers were stored at three different temperatures: ambient temperature (22 °C), refrigeration (4 °C), and deep freezing (−20 °C). Pollen viability and in vitro pollen germination were evaluated after storage periods of 1, 3, and 6 days at each of these storage temperatures.
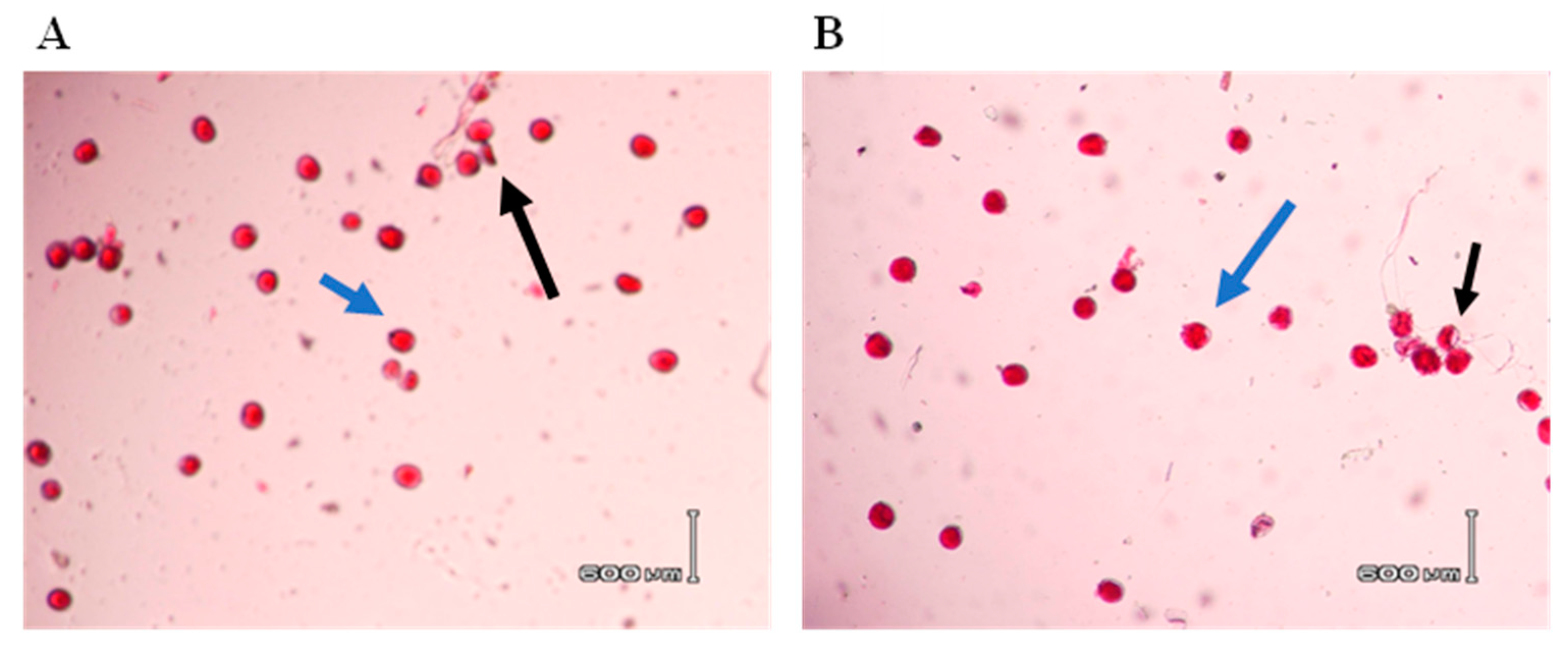
Pollen Viability: To determine the optimal conditions for preserving pollen viability, we tested three different storage temperatures (22 °C, 4 °C, and −20 °C) over four storage durations (0-day, 1st day, 3rd day, and 6th day). Pollen grains collected from 50 genotypes and stored under these conditions were placed on slides with one to two drops of ALEXANDER’s solution and covered with cover-slips (following Dafni and Firmage, 2000). A compound microscope (Olympus) with 5X magnification was used to assess the level of pollen staining. Pollen grains that stained fully and darkly (magenta-red or red) were classified as viable, those with light staining (magenta-red or red) were considered semi-viable, and those stained blue-green, blue, or not stained at all (lacking color) were deemed non-viable (adapted from Mert, 2009). Pollen viability was quantified as the percentage of stained pollen grains out of the total.
In Vitro Pollen Germination Test: To assess pollen germination across 50 wheat genotypes under various storage conditions and durations, an in vitro pollen germination test was conducted. A liquid pollen germination medium was prepared, consisting of dissolved H3BO3 (0.05 g), Ca(NO3)2.4H2O (0.03 g), BK Salts (including MgSO4.7H2O (0.2 g), KNO3 (0.1 g), and 19% maltose), and polyethylene glycol (PEG6000, 13%) adjusted to a pH of 6, as described by Jayaprakash et al. (2015). Using a light compound microscope (Olympus BX41 with DP12 camera), we counted pollen grains and germinated pollen grains from randomly selected microscopic fields to determine in vitro pollen germination. Pollen grains were considered germinated when the length of the pollen tube exceeded the diameter of the pollen grain, following the criteria of Prasad et al. (2006).
Statistical Analysis: Descriptive statistics were performed in Excel and GraphPad Prism. Analysis of variance (ANOVA) was conducted in SPSS (v16.0) to assess variations among genotypes (G), storage time (ST), storage temperature (T), G×T, G×ST, and T×ST. Simple linear regression was performed using the R function “jamovi” for all 50 spring wheat genotypes to investigate the relationship between pollen viability and in vitro pollen germination with storage days. In this analysis, pollen viability and in vitro pollen germination were treated as dependent variables, while storage days were considered independent variables.
5. Conclusions
The results of this study indicate that wheat pollen viability and germination are influenced by factors such as variety, storage duration, and temperature. The study revealed that pollen from different spring wheat genotypes displayed varying levels of viability and germination capacity. Notably, even after six days of storage at a temperature of 4 °C, 36 spring wheat genotypes still exhibited germination rates ranging from 6% to 14%, indicating that they remained viable.
In light of these findings, it is advisable to limit the storage of pollen to a maximum of six days at a temperature of 4 °C. Furthermore, the study suggests a correlation between storage duration (in days) and variations in both pollen viability and in vitro pollen germination. This insight could prove valuable in the development of a standardized protocol for pollen storage in breeding programs.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1 and Table S2.
Author Contributions
I.K. performed the experiment, analyzed data and wrote the first draft. M.S. conceptualized the study and wrote first draft. A.S. conceptualized the study. M.S., M.K.N. supervised the experiments. A.S. and M.K.N. improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sichuan Science and Technology Support Program, China (2022ZDZX0014,2021YFYZ0002). Pakistan Science Foundation (PSF-MSRT II/Agr/P-COMSATS-Vehari 11).
Data Availability Statement
All data is presented in supplementary material.
Acknowledgments
All the seed material and equipment necessary for this study were provided by National Institute for Genomics and Advanced Biotechnology (NIGAB), NARC, and Department of Biosciences, COMSATS University Islamabad.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, I.; Wu, J.; Sajjad, M. Pollen viability-based heat susceptibility index (HSIpv): a useful selection criterion for heat-tolerant genotypes in wheat. Front. Plant Sci. 2022, 13, 1064569. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S. Control of male gametophyte development. Plant Cell 2004, 16, S142–S153. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.N.; Campbell, C.G. In vitro germination and viability of buckwheat (Fagopyrum esculentum Moench) pollen. Euphytica 1998, 102, 87–92. [Google Scholar] [CrossRef]
- Akihama, T.; Omura, M.; Kozaki, I. Long-term storage of fruit tree pollen and its application in breeding. Jpn. Agric. Res. Q. 1979, 13, 238–241. [Google Scholar]
- Akond, A.M.; Pounders, C.T.; Blythe, E.K.; Wang, X. Longevity of crapemyrtle pollen stored at different temperatures. Sci. hortic. 2012, 139, 53–57. [Google Scholar] [CrossRef]
- Alburquerque, N.; Montiel, F.; Burgos, L. Influence of storage temperature on the viability of sweet cherry pollen. Span. J. Agric. Res. 2007, 5, 86–90. [Google Scholar] [CrossRef]
- Baninasab, B.; Tabori, M.; Yu, J.; Zhang, Y.; Wang, X.; Deschiffart, I.; Khanizadeh, S.; et al. Low temperature storage and in-vitro pollen germination of selected spring wheat accessions. J. Agric. Sci. 2017, 9, 1–6. [Google Scholar] [CrossRef]
- Bhat, Z.A.; Dhillon, W.S.; Shafi RH, S.; Rather, J.A.; Mir, A.H.; Shafi, W.; et al. Influence of storage temperature on viability in vitro germination capacity of pear (Pyrus spp) pollen, J. Agric. Sci. 2012, 4, 128–135. [Google Scholar] [CrossRef]
- Bryhan, N.; Serdar, U. In vitro pollen germination and tube growth of some European chestnut genotypes (Castanea sativa Mill. ). Fruits 2009, 64, 157–165. [Google Scholar]
- Chatterjee, R.; Sarkar, S.; Rao, G.N. Improvised media for in vitro pollen germination of some species of Apocynaceae. Int. J. Env. 2014, 3, 146–153. [Google Scholar] [CrossRef]
- Cheng, C.; Mcomb, J.A. In vitro germination of wheat pollen on raffinose medium. New phytologist. 1992, 120, 459–462. [Google Scholar] [CrossRef]
- Dafni, A.; Firmage, D. Pollen viability and longevity: practical, ecological and evolutionary implications. Pl. Syst. Evol. 2000, 22, 113–132. [Google Scholar] [CrossRef]
- De Souza, E.H.; Souza, F.V.D.; Rossi, M.L.; Brancalleao, N.; da Silva Ledo, C.A.; Martinelli, A.P.; et al. Viability, storage and ultrastructure analysis of Aechmea bicolor (Bromeliaceae) pollen grains, an endemic species to the Atlantic forest. Euphytica 2015, 204, 13–28. [Google Scholar] [CrossRef]
- Devrnja, N.; Milojević, J.; Tubić, L.; Zdravković-Korać, S.; Cingel, A.; Ćalić, D.; et al. Pollen morphology, viability, and germination of Tanacetum vulgare L. Hort. Sci. 2012, 47, 440–442.
- Du, K.; Shen, B.; Xu, L. Changes of viability of stored poplar pollens and its feasibility for cross breeding. Journal-huazhong agricultural university. 2007, 26, 385. [Google Scholar]
- Dutta, S.K.; Srivastav, M.; Chaudhary, R.; Lal, K.; Patil, P.; Singh, S.K.; Singh, A.K. Low temperature storage of mango (Mangifera indica L.) pollen. Sci. Hortic. 2013, 161, 193–197. [Google Scholar] [CrossRef]
- Fei, S.; Nelson, E. Estimation of pollen viability, shedding pattern, and longevity of creeping bent grass on artificial media. Crop Sci. 2003, 43, 2177–2181. [Google Scholar] [CrossRef]
- Fernando, D.D.; Lazzaro, M.D.; Owens, J.N. Growth and development of conifer pollen tubes. Sex. Plant Repro. 2005, 18, 149–162. [Google Scholar] [CrossRef]
- Fernando, D.D.; Richards, J.L.; Kikkert, J.R. In vitro germination and transient GFP expression of American chestnut (Castanea dentata) pollen. Plant Cell Rep. 2006, 25, 450–456. [Google Scholar] [CrossRef]
- Fritz, S.E.; Lukaszewski, A.J. Pollen longevity in wheat, rye and triticale. Plant breed. 1989, 102, 31–34. [Google Scholar] [CrossRef]
- Gandadikusumah, V.; Wawangningrum, H. ; Rahayu SPollen viability of Aeschyanathus tricolor Hook, J. Trop. Life Sci. 2017, 7, 53–60. [Google Scholar] [CrossRef]
- Griffin, W.B. Outcrossing in New Zealand wheats measured by occurrence of purple grain. N.Z. J. Agric. Res. 2012, 30, 287–290. [Google Scholar] [CrossRef]
- He, W.; Xiao, Q.; Pu, G.; Huang, X.; Li, Y.; Shi, L. Effect of walnut pollen on ‘Shuangzao’ fruit quality and early fruit of several. J. Hunan Agri. Uni. 2017, 43, 266–269. [Google Scholar]
- Hechmi, M.; Mhanna, K.; Feleh, E. In vitro pollen germination of four olive cultivars (Olea europaea L. ): effect of boric acid and storage. Amer. J. Plant Physio. 2015, 10, 55–67. [Google Scholar]
- Heslop-Harrison, J.; Heslop-Harrison, Y.; Shivanna, K.R. The evaluation of pollen quality, and a further appraisal of the fluorochromatic (FCR) test procedure. Theor. Appl. genet. 1984, 67, 367–375. [Google Scholar] [CrossRef]
- Hosoo, Y.; Yoshii, E.; Negishi, K.; Taira, H. A histological comparison of the development of pollen and female gametophytes in fertile and sterile Cryptomeria japonica. Sex. Plant Repro. 2005, 18, 81–89. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Annapoorani, S.; Vikas, V.K.; Sivasamy, M.; Kumar, J.; Saravannan, K.; Sheeba, D.; et al. An improved in vitro germination medium for recalcitrant Bread wheat (Triticum aestivum L) pollen Indian, J. Genet. Plant Breed. 2015, 75, 446–452. [Google Scholar]
- Jia, H.; Liang, X.; Zhang, L.; Zhang, J.; et al. Improving ultra-low temperature preservation technologies of soybean pollen for off-season and off-site hybridization. Front. Plant Sci. 2022, 13, 920522. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Screening soybean genotypes for high temperature tolerance by in vitro pollen germination, pollen tube length reproductive efficiency and seed yield. Indian J. Plant Physiol. 2018, 23, 77–90. [Google Scholar] [CrossRef]
- Kormutak, A.; Vooková, B.; Čamek, V.; Salaj, T.; Galgóci, M.; Maňka, P.; Gömöry, D.; et al. Artificial hybridization of some Abies species. Plant Sys. Evol. 2013, 299, 1175–1184. [Google Scholar] [CrossRef]
- Koubouris, G.C.; Metzidakis, I.T.; Vasilakakis, M.D. Impact of temperature on olive (Olea europaea L. ) pollen performance in relation to relative humidity and genotype. Environ. Exp. Bot. 2009, 67, 209–214. [Google Scholar]
- Liu, X.; Xiao, Y.; Zi, J.; et al. Differential effects of low and high temperature stress on pollen germination and tube length of mango (Mangifera indica L. ) genotypes. Sci Rep 2023, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X. Advances in the study of cryopreservation of fruit tree germplasm. Life Sci. Res. 2001, 5, 227–232. [Google Scholar]
- Lora, J.; De Oteyza, M.P.; Fuentetaja, P.; Hormaza, J.I. Low temperature storage and in vitro germination of cherimoya (Annona cherimola Mill. ) pollen. Sci. Hortic. 2006, 108, 91–94. [Google Scholar] [CrossRef]
- Luna, V.S., M.J., Baltazar, M.B., Gomez, L.R., Townsend, R., Schoper, J.B. et al. Maize pollen longevity and distance isolation requirements for effective pollen control. Crop Sci. 2001, 41, 1551–1557. [CrossRef]
- Martins, E.S.; Davide, L.M.C.; Miranda, G.J.; Barizon, J.D.O.; Souza, F.D.A.; Carvalho, R.P.D.; Gonçalves, M.C.; et al. In vitro pollen viability of maize cultivars at different times of collection. Cienc. Rural 2016, 47, e20151077. [Google Scholar] [CrossRef]
- Mert, C. Temperature responses of pollen germination in walnut (Juglans regia L.). J. Biol. Eviron. Sci. 2009, 3, 37–43. [Google Scholar]
- Mulcahy, G.B.; Mulcahy, D.L. The effect of supplemented media on the growth in vitro of bi-and tri-nucleate pollen. Plant Sci. 1988, 55, 213–216. [Google Scholar] [CrossRef]
- Naik, S.; Rana, P.; Rana, V. Pollen storage and use for enhancing fruit production in kiwifruit (Actinidia deliciosa A. Chev.). J. Appl. Hortic. 2013, 15, 128–132. [Google Scholar] [CrossRef]
- Novara, C.; Scari, L.; Morgia, V.; Reale, L.; Genre, A.; Siniscalco, C.; et al. Viability and germinability in long term storage of Corylus avellana pollen. Sci. Horti. 2017, 214, 295–303. [Google Scholar] [CrossRef]
- Parzies, H.K.; Schnaithmann, F.; Geiger, H.H. Pollen viability of Hordeum spp genotypes with different flowering characteristics. Euphytica 2005, 145, 229–235. [Google Scholar] [CrossRef]
- Patel, R.G.; Mankad, A.U. In vitro pollen germination-A review. Int. J. Sci. Res. 2014, 3, 304–307. [Google Scholar]
- Prasad, P.V.; Boote, K.J.; Allen Jr, L.H. Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum (Sorghum bicolor (L.) Moench are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric. For. Meteorol. 2006, 139, 237–251. [Google Scholar] [CrossRef]
- Rodriguez-Riano, T.; Dafni, A. A new procedure to asses pollen viability. Sex. Plant Repro. 2000, 12, 241–244. [Google Scholar] [CrossRef]
- Satish, D.; Ravikumar, R.L. Standardization of in vitro pollen germination media in selected varieties of cotton and tomato. Karnataka J. Agric. Sci. 2010, 23, 317–319. [Google Scholar]
- Sauve, R.; Craddock, J.; Reed, S.; Schlarbaum, S. Storage of flowering dogwood (Cornus florida L.) pollen. Hort Sci. 2000, 35, 108–109. [Google Scholar]
- Song, J.; Tachibana, S. Loss of viability of tomato pollen during long-term dry storage is associated with reduced capacity for translating polyamine biosynthetic enzyme genes after rehydration. J. Exp. Bot. 2007, 58, 4235–4244. [Google Scholar] [CrossRef]
- Sulusoglu, M.; Cavusoglu, A. In vitro pollen viability and pollen germination of service tree (Sorbus domestica L.). Int. J. Biosci. 2014, 5, 108–114. [Google Scholar]
- Tuinstra, M.R.; Wedel, J. Estimation of pollen viability in grain sorghum. Crop sci. 2000, 40(4), 968–970. [Google Scholar] [CrossRef]
- Yin, J.L.; Zhao, H.E. Summary of influencial factors on pollen viability and its preservation methods. China Agri. Sci. Bull. 2005, 21, 110–113. [Google Scholar]
- Yuan, S.C.; Chin, S.W.; Lee, C.Y.; Chen, F.C. Phalaenopsis pollinia storage at sub-zero temperature and its pollen viability assessment. Bot. Stud. 2018, 59, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Xin, X.; Yin, G.; He, J.; Huang, B. Cryopreservation of Citrus anthers in the national crop genebank of China. In Vitro Cell Dev. Biol. Plant. 2017, 53, 318–327. [Google Scholar] [CrossRef]
- Mukti, G. Pollen storage and viability. International Journal of Botany and Research, 2014, 4, 1–18. [Google Scholar]
- Machado, C.A.; Moura CR, F.; Lemos EE, P.; Ramos SR, R.; Ribeiro, F.E.; Lédo, A.S. Pollen grain viability of coconut accessions at low temperatures. Acta Sci. Agron. 2014, 36, 227–232. [Google Scholar] [CrossRef]
- Impe, D.; Reitz, J.; Köpnick, C.; Rolletschek, H.; Börner, A.; Senula, A. Assessment of pollen viability for wheat. Front. Plant Sci. 2020, 10, 1588–10. [Google Scholar] [CrossRef]
- Zambon, C.R.; Silva LF, O.; Pio, R.; Figueiredo, M.A.; Silva, K.N. Estabelecimento de meio de cultura e quantificação da germinação de grãos de pólen de cultivares de marmeleiros. Rev. Bras. Frutic. 2014, 36, 400–407. [Google Scholar] [CrossRef]
- Deng, Z.; Harbaugh, B. Technique for in vitro pollen germination and short term pollen storage in caladium. Hortscience 2004, 39, 365–367. [Google Scholar] [CrossRef]
- Athwal, R.S.; Kimber, G. Anther size and pollen longevity in wheat/rye addition lines. Wheat Information Service, Kyoto University, 1970, 30, 30–32. [Google Scholar]
- Razora, O.P.; Zsuffa, L. Pollen viability of some Populous species as indicated by in vitro germination and tetrazolium chloride staining. Canadian Journal of Botany, 1986, 64, 1086–1088. [Google Scholar] [CrossRef]
- Sedgley, M.; Harbard, J.; Smith, R.M.; Wickneswari, R. Development of hybridization techniques for Acacia mangium and Acacia auriculiformis. In L. T. Carron & K. M. Aken (Eds.), Breeding Technologies for Tropical Acacias (Proceedings No. 37, pp. 63–69). Australian Centre for International Agricultural Research 1992.
- Yoshiji, N.; Shiokawa, Y. A study on the storage of Lilium pollen. Journal of the Japanese Society for Horticultural Science, 1992, 61, 399–403. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).