1. Introduction
Genetic engineering has significantly contributed to the fundamental and practical understanding of plant biology and genetics over the past 45 years. Notably, potato was one of the first plant species to successfully undergo genetic transformation [
1]. Since then numerous publications have detailed the generation of diverse transgenic potato plants carrying of a wide array of engineered traits [
2,
3,
4]. Potato is an important food crop, which is cultivated not only for direct human consumption, but also as animal feed and industrial crop for the production of alcohol, biofuel, starch, and other substances. It stands as the 4th major staple crop globally, following maize, wheat, and rice, and is cultivated in over 150 countries. Faced with various production challenges, breeders are constantly making efforts to develop new varieties adapted to local agricultural needs in order to increase productivity while reducing costs [
5]. The breeding of potato is traditionally achieved through sexual hybridization between related species. However, the limiting factor in potato breeding is the long generation period, which makes it difficult to effectively introduce new traits into commercial varieties using traditional approaches to crossing. For this reason, potato genetic engineering is an important area of research, focusing on improving traits that are not easily modified by conventional breeding [
2,
3].
The generation of transgenic plants in any species hinges on the efficient selection of transgenic tissues, typically facilitated by various marker genes that confer resistance to selective substances, such as antibiotics or herbicides. However, the presence of antibiotic or herbicide resistance genes in transgenic plants intended for commercial use has raised significant consumer concerns regarding health risks and biosafety. This complicates the regulatory processes overseeing the release of biotech crops [
6]. Thus, the development of marker-free transgenic plants stands is one of the options to mitigate perceived risks and reduce public concerns
In addressing the issue of marker gene presence, various strategies have emerged over the past decades. These include approaches that involve the direct removal of selective sequences from the transformation process or the controlled elimination of marker genes from the plant genome after the marker-assistant selection of transgenic plants is completed. The methods of removal include co-transformation, site-specific recombination, transposition, and the utilization of double right border-binary vectors or multi auto-transformation vectors [
7,
8,
9,
10]. In co-transformation, vectors are carried two separate T-DNAs: one containing the gene of interest and the other encoding a selection marker gene, both of which are introduced into the plant genome simultaneously. If insertions occur at unlinked genomic loci, they can independently segregate in subsequent generations, yielding marker-free segregants [
10]. Similarly, segregation is necessary when transgenic plants are transformed with the double right border twin T-DNA vector, featuring an additional copy of the right border sequence inserted between the marker gene and the gene of interest [
11]. Segregation-based strategies are extensively applied for various seed-propagated crops [
9,
10] but are impractical for vegetatively propagated species, such as potato.
Approaches directly applied to primary transformants, such as the site-specific recombination or systems involving transposon-based and multi auto-transformation vectors are better suited for potato. Various self-excision site-specific recombination systems, including Cre-lox, FLP-FRT, and R/RS, have already been applied to varying degrees for vegetatively propagated horticulture crops, including apricot [
12], tomato [
13], blood orange [
14], strawberries [
15], apples [
16,
17,
18] and pears [
19]. The Cre-lox and R/RS systems also have also demonstrated effectiveness in generating marker-free transgenic plants of potato. The heat shock inducible Cre-lox systems achieved a 71% excision efficiency of the
NPTII antibiotic resistance marker gene in potato cv. ‘Desire’ [
20]; while the R/RS recombination tool exhibited lower efficacy, producing only 29% marker-free transgenic plants from regenerated shoots of the same cultivar [
21]. The ipt(isopentenyl transferase)-type multi-auto-transformation vector has also been employed to generate marker-free disease-resistant transgenic potato plants [
22]. While these approaches have been successful, they do carry certain limitations. These include the need for designing sophisticated constructs, resulting in decreased transformation efficiency; the requirement for additional time for manipulations to re-regenerate marker-free plants after induced excision; the inability to achieve 100% excision efficiency; and unwanted chromosomal rearrangements in the plant genome, as a result of the expression of recombinase systems, leading to genetic and phenotypic changes.
An ideal approach to overcome these limitations involves a marker-free transformation procedure, where a simple expression cassette exclusively encoding the gene of interest is transferred into the plant genome via
Agrobacterium or gun-mediated transformation. In practice only a limited number of crops, such as alfalfa [
23], peanut [
24], tobacco [
25] and wheat [
26], have been transformed using this approach, with an efficacy ramging from 0.1 to 50%. This straightforward procedure
relies on the high regenerative capability of cultivated
in vitro tissue to produce plants with a high frequency. Given that potato is among the highly regenerative species, marker-less genetic transformation has been successfully applied to generate of late blight resistant [
27,
28], virus resistant [
29,
30] and oxidative stress tolerant transgenic potato lines [
31]. The primary challenge associated with marker-less transformation is the necessity for molecular screening of several hundreds of regenerated plant to discover a few transgenic events. Since regenerated plants need to be maintained
in vitro, until the results of molecular tests are obtained, transformation without selective genes becomes a time consuming and expensive procedure.
In our current study, we have analyzed the possibility of generation of transgenic potato plants without using selective substances. We employed an expression construct encoding GFP to monitor the transformation process by cultivating internodal explants on both selective and non-selective media. This approach facilitated the analysis of the efficiency of chimeric and transgenic plantlet formation at various time intervals after co-cultivation with Agrobacterium. We also studied the capacity of five potato cultivars to produce transgenic plants without selective pressure and developed a regeneration-specific timeline, aiming to streamline the marker-less genetic transformation by reducing the number of manipulations needed.
2. Materials and Methods
2.1. Plant Material and General Conditions
The plant materials used in this study consisted of various potato germplasms generously provided by the Doka-Gene Technology Ltd, Russia. These included five commercial cultivars: ‘Chicago’, ‘La Strada’, ‘Lion Heart’, ‘Manhattan’ and ‘Pirol’, along with five breeding accessions, specifically #10-9-3, #10-10-10, #12-22-129, #12-36-42 and ‘Indigo’ (formerly #10-17-10). In vitro stock plants of potato were multiplicated in plastic vessels using phytohormone-free Murashige and Skoog (MS) basal medium supplemented with 3% sucrose, solidified with 7 g L−1 agar, and adjusted to a pH 5.8-5.9 before autoclaving. Vessels were maintained in a culture room at 21 ± 2 °C, subjected to a 16 h light / 8 h dark cycle under artificial light (40 μmol m–2 s–1) provided by OSRAM cool white and fluora fluorescent lamps. In all experiments internodal segments of 0.5-1 cm in length, excised from three to four-week-old in vitro plants, were used as explants. Explants were cultured in 100 mm × 20 mm glass Petri dishes containing 25 ml of regeneration medium.
2.2. Potato Regeneration Media
All regeneration media were consisted of MS mineral salts and vitamins, supplemented with 30 g L−1 sucrose and 100 mg L−1 myo-inositol, solidified with 7 g L−1 agar, and adjusted to a pH range of 5.8-5.9. Four combinations of phytogormones were used to induce adventitious regeneration, comprising: zeatin (Zea) 3 mg L−1, indoleacetic acid (IAA) 2 mg L−1, gibberellic acid (GA) 1 mg L−1; zeatin-riboside (Zea-R) 3 mg L−1, IAA 2 mg L−1, GA 1 mg L−1; Zea 3 mg L−1, IAA 0.5 mg L−1; Zea-R 3 mg L−1, IAA 0.5 mg L−1. The preferred combinations of phytohormones were determined through preliminary experiments (data not shown).
2.3. Agrobacterium-mediated Genetic Transformation of Potato With and Without Antibiotic Selection
In these experiments
A. tumefaciens strain AGL0 harboring pBIN-mGFP5-ER binary vector was used [
32]. T-DNA of this construct consists of the
NPTII selectable marker gene under
nos promoter and the modified
GFP gene driven by
CaMV 35S promoter along with a leader sequence for expression localization in the endoplasmic reticulum of the cells. Prior to transformation, colonies of bacteria were cultured overnight in liquid LB medium supplemented with 100 mg L
−1 kanamycin at 28 ºC on an orbital shaker. Shortly prior to explants isolation, the agrobacterial suspension was diluted with phytohormon-free MS medium to a final density of OD600 = 0.2. Approximately 50-75 freshly cut intermodal segments were immersed in 15 ml of
Agrobacterium suspension and inoculated in a 100 ml glass for 25-30 minutes at room temperature. Following this, the explants were placed onto sterilized paper and blotted dry for 2–3 min under a laminar flow. After removing the excess of bacteria, the explants were transferred to Petri dishes containing the regeneration medium covered with sterilized filter paper, and maintained for 3 days in the dark at 25 ± 1 °C. Post-co-cultivation, groups of 10-12 explants were transferred to 100 mm × 20 mm glass Petri dishes containing 25 ml of fresh regeneration medium supplemented with 500 mg L
−1 of the antibiotic cefotaxime. Dishes were kept in the culture room at 21 ± 2 °C under a 16 h light / 8 h dark cycle, with subculturing carried out every 2 weeks. All subsequent media plates contained the same cefotaxime concentration. From the third week after inoculation, plantlets 1.5-3.0 cm in length, regenerated by the explants under-non selective conditions, were cut and transferred to culture vessels (ten plantlets per vessel) containing 100 ml of phytohormone–free MS medium supplemented with 150 mg L
−1 cefotaxime. Once the plantlets grew to 5-10 сm in length, shoot tips with one to two expanded leaves were moved into phytohormone–free MS medium containing 100 mg L
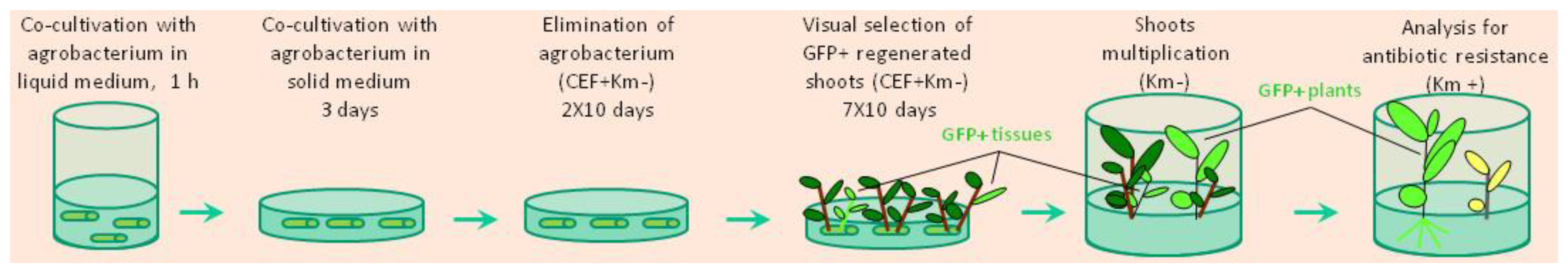
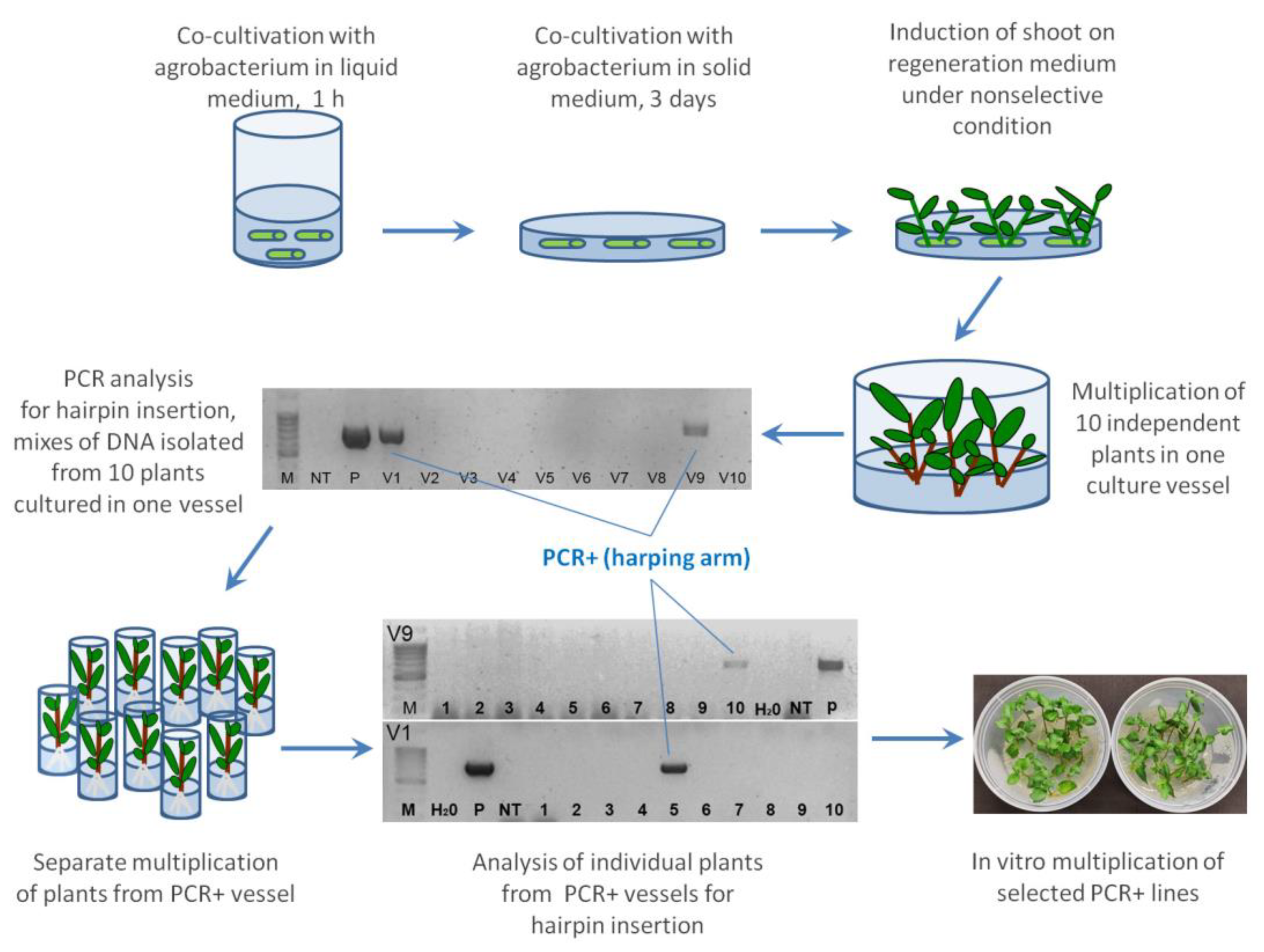
−1 kanamycin for the indentication of transgenic events through rooting. The step-by-step scheme of non-selective genetic transformation of potato is presented in
Figure 1.
Concurrently, the antibiotic-assisted genetic transformation was carried out. After co-cultivation with Agrobacterium in dark for 3 days, a portion of intermodal segments was transferred to the corresponding regeneration medium supplemented with kanamycin (50 mg L
−1). Shoot regeneration and transgenic plant selection were then performed as described [
32]. To avoid duplication of transgenic lines, only one kanamycin-resistant shoot was collected from each explant.
2.3. GFP Monitoring
Visual screening for transient and stable T-DNA incorporation was conducted using a ZEISS SteREO Discovery.V12 microscope equipped with a PentaFluar S 120 vertical illuminator. In the observations, two commercially available filter sets, namely 38 GFP BP (EX BP 470/40, BS FT 495, EM BP 525/50) and 57 GFP BP (EX BP 470/40, BS FT 495, EM LP 550) from Carl Zeiss MicroImaging GmbH (Germany) were used.
2.4. Generation of Marker-Free Potato Plants for the Silencing of the SteIF4E1 and SteIF4E2 Genes
The agrobacterial strain AGL0 harbouring the pWS-E1-Lhca3 vector designed for the post-transcriptional silencing of the
SteIF4E1 and
SteIF4E2 genes of potato was used [
30]. The expression cassette included the hpRNAi structure, consisted of inverted fragments of
SteIF4E1 gene separated by the
Strbsc1 intron and controlled by the
StLhca3 promoter and terminator. Notably, the T-DNA region of pWS-E1-Lhca3 was not included any selective genes. The agrobacterium-mediated transformation of cv. ‘La Strada’ was carried out as specified in section 2.2. The regeneration media for plantlet development was supplemented with 3 mg L
−1 Zea-R, 2 mg L
−1 IAA and 1 mg L
−1 GA. Plantlets that within the initial 40 days after inoculation were discarded. Two weeks later, all plantlets developed on the explants were cut and transferred to culture vessels (ten plantlets per vessel) containing phytohormone–free MS medium supplemented with 250 mg L
−1 cefotaxime. The explants were discarded. Upon the regeneration of plants to a length of 5-8 cm, the two upper leaves of each plant grown in a single vessel were harvested and pooled together for isolation of genomic DNA. The presence of hairpin constructs in the DNA mix was validated using PCR with the primers and conditions described previously [
30]. In cases where specific band amplification was observed, each plant from a positive vessel was individually cultivated, sampled for total DNA extraction, and subjected to PCR to identify transgenic events.
2.5. Molecular Analysis
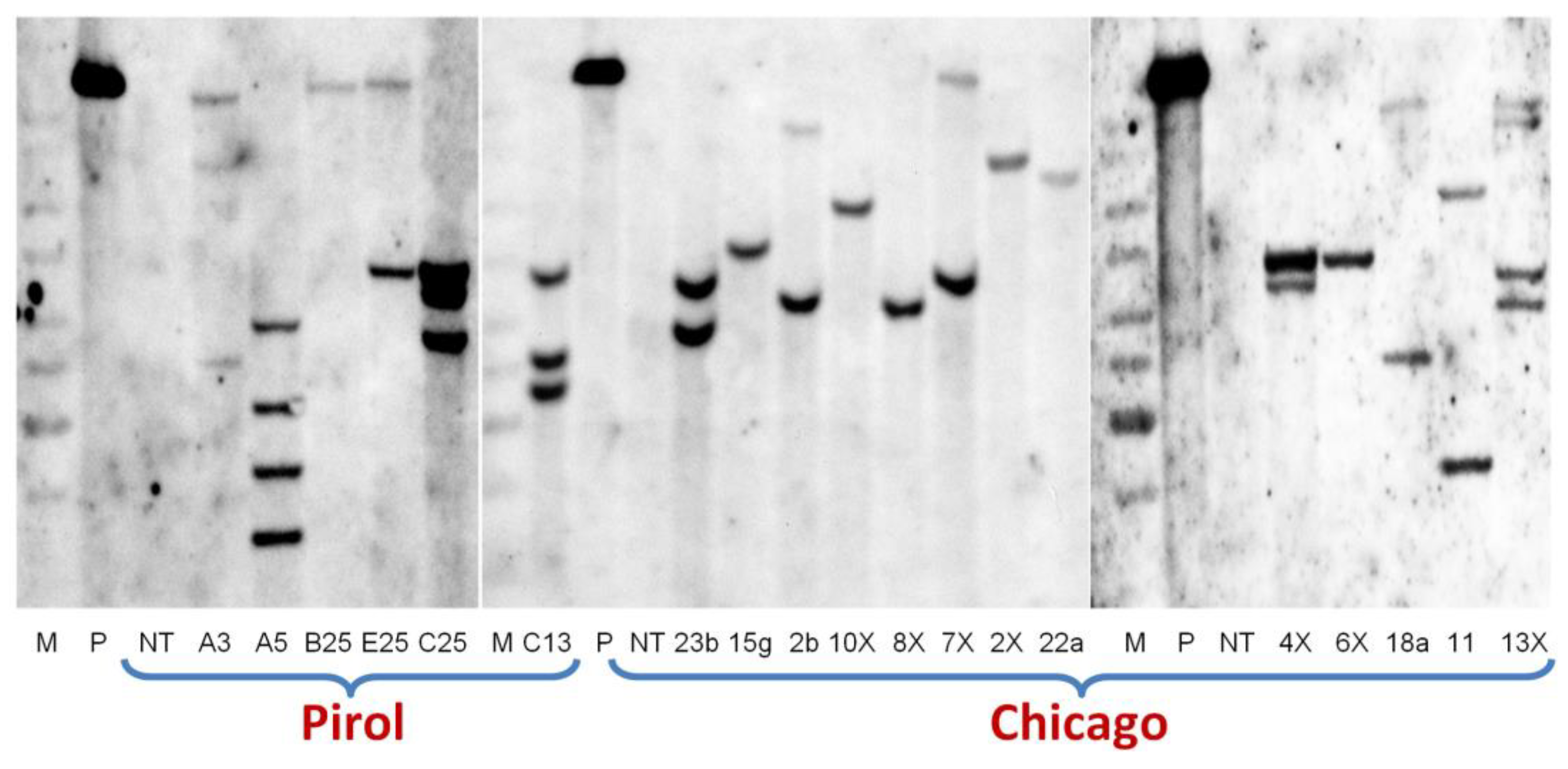
To analyze integrated T-DNA copies, Southern hybridization was conducted.
For the blot analysis, 30 μg of potato genomic DNA was digested overnight at 37°C with 60U
HindIII which cleaves the T-DNA of pBIN-mGFP5-ER at a single position (5′ end of the
CaMV 35S promoter). Subsequently, the DNA was separated through gel electrophoresis
in 0.9%
agarose, and then transferred and immobilized onto Hybond N+ membrane (GE Healthcare, Amersham Bioscience, Amersham, UK). Membranes were
probed with alkaline phosphatase-labeled probes
and detected using CDP-Star detection reagent (Amersham CDP-Star Detection reagent, GE Healthcare) as previuously described [
30].
Hybridizing bands were visualized on X-ray film (Retina XBE blue sensitive, Carestream Health INC., NY, United States) at room temperature for 24 h. The DNA probe (711bp) for hybridization
was synthesized via PCR using specific primers for the coding region of the GFP gene (forward, 5’-AGTAAAGGAGAAGAACTTTTCACTGGAGTT-3’; reverse, 5’-TTTGTATAGTTCATCCATGCCATGTGT-3’) and labbeled with the alkaline phosphatase using Amersham Gene Images AlkPhos Direct Labelling and Detection System (GE Healthcare, Amersham Bioscience, Amersham, UK).
For qRT-PCR analysis, total RNA was extracted from
in vitro grown plants as described [
34]. First-strand cDNA was synthesized from 5 μg of each DNase-treated RNA sample using RevertAid Reverse Transcriptase (Thermo Scientific). Gene expression analysis was conducted using Quantitative real-time reverse-transcription PCR (qRT-PCR) with QuantStudio™ 5 Real-Time PCR Cycler (Thermo Fisher Scientific), as previously described [
30]. The normalization of the
the SteIF4E1 and SteIF4E2 values was performed using the expression levels of the
ef1α, a housekeeping potato gene. All primer pairs and analysis conditions were detailed in the previous publication [
29]. The reported values represent the mean ± standard deviations (SD) of four biological replicates with three technical replicates.
2.6. Statistical Analysis
The data presented are the mean ± SD. Significant differences were determined via t-test with a threshold of p<0.05. Intra-experimental data were analyzed with Statistica10 software (©StatSoft Inc) using analysis of variance (ANOVA), followed by Tukey's HSD test.
4. Discussion
The successful genetic transformation of potato hinges on efficient plant regeneration protocols and optimized methodologies for recognizing transgenic tissues. An increasing number of scientific reports on genetically engineered potato published since the late 1980s, alongside the worldwide commercial releases of GM varieties, indicate that potato is a relevant crop for improvement through biotechnology [
2,
3,
4,
5]. Given the increased likelihood of obtaining transgenic events when more cells are capable of differentiating into shoots, we initially screened ten potato cultivars for their regeneration potential to select the most suitable ones for antibiotic-free transformations. Typically, a two-stage regeneration protocol is used in potato, where the stage of shoots regeneration follows the stage of callus induction [
4]. Cytokinins play a paramount role in initiating morphogenesis in potato. Although BAP (6-benzylaminopurine), kinetin and TDZ (thidiazuron) are reported to stimulate regeneration in potato, zeatin currently is known as the most popular cytokinin for generating transgenic potato plants. Most transformation protocols involve supplementing the regeneration medium with 1-5 mg L
−1 of zeatin along with low level of auxin.
In the present study various zeatin-containing variant of the regeneration medium combined with different auxins were used, including twin variants where zeatin was replaced with zeatin-ribozide, which has been reported to exhibit superior regeneration frequency and even enable one-step transformation of potato [
34]. The tested potato cultivars responded positively to various plant phytohormone combinations, with two variants proving to be more effective in practice. The combination of 3 mg L
−1 Zea and 0.5 mg IAA L
−1 was found better for five cultivars, while the combination of the same level of zeatin along with a significantly higher concentration of IAA (2 mg L
−1) and the additional incorporation of gibberellic acid (1 mg L
−1) to support the enhanced outgrowth of morphogenic buds was essential for successful regeneration in ‘Chicago’, ‘Lion Heart’, and #10-9-3. Moreover, while the replacement by the more expensive zeatin-ribozide generally had no significant effect in listed above cultivars, it proved more successful in two other cultivars ('Indigo' and 'La Strada'). Nonetheless zeatin-ribozide was more successful in two cultivars (‘Indigo’, ‘La Strada’). This observation aligns with the early notion that application of zeatin-ribozide serves as viable alternative for specific cultivars to achieve higher efficiency of regeneration, an important prerequisite for successful genetic transformation [
34].
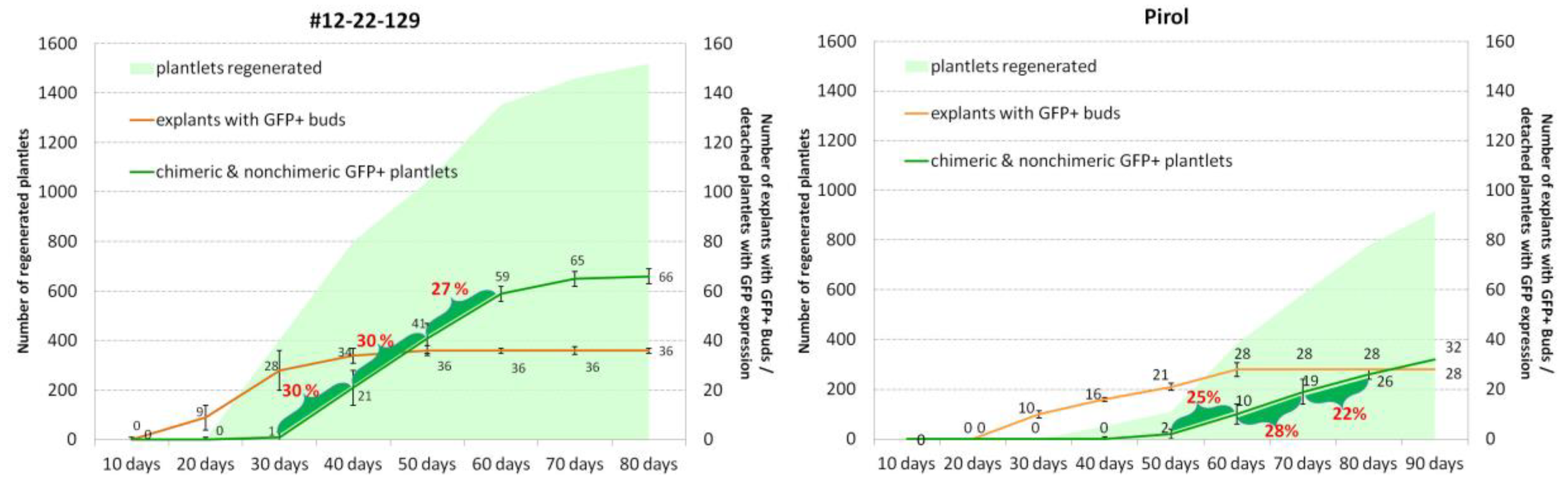
The experiments revealed the pivotal importance of the timeline of plantlets regeneration from internodal explants. We observed substantial disparities between genotypes in the speed of adventitious shoot formation. Certain cultivars, such as ‘Chicago’ and #12-22-129, initiated the development of plantlets much earlier than others. In contrast, the cultivars characterized by a late regeneration, such as Pirol’, #10-9-3, ‘Indigo’ and ‘Lion Heart’ started the development of plantlets when the cultivars characterized by an early regeneration had already produced a maximum number of shoots. This genotype-specific property can be taken into account when potato varieties with different types of regeneration periods undergo marker-free transformation. Our results suggest that ‘early regenerative’ cultivars produced the majority of transgenic plants (approximately 75%) between 30 and 60 days post inoculation with
Agrobacterium, whereas the ‘late’ cultivars preferably generated transgenic events during the 50-80 days of cultivation. Similarly, cultivars with an intermediate regeneration timeline predominantly generate transgenic plants between 40 and 70 days after co-cultivation with
Agrobacterium. These findings could be leveraged to potentially reduce the number of analysed regenerated plantlets, given that numerous manipulations such as DNA extraction, PCR analysis, and prolonged in vitro cultivation of each regenerated plant are necessary to detect transgenics. Our data are consistent with those reported by Jo et al. 2014 [
28], who, under non-selective conditions, also observed differences between three potato genotypes in the number of PCR positive plants over the number of tested regenerated shoots. In their study, shoots were only collected at three-week intervals, and only a portion of the generated shoots were analyzed. However, the timeline-dependant effect was clearly observed, as a higher portion of PCR+ plants of ‘Potae9’ and ‘Bintje’ was found between 31-50 and 31-70 days, respectively, while explants of ‘Atlantic’ produced a significant portion of transgenic plants during the 50-90 days of culture [
28].
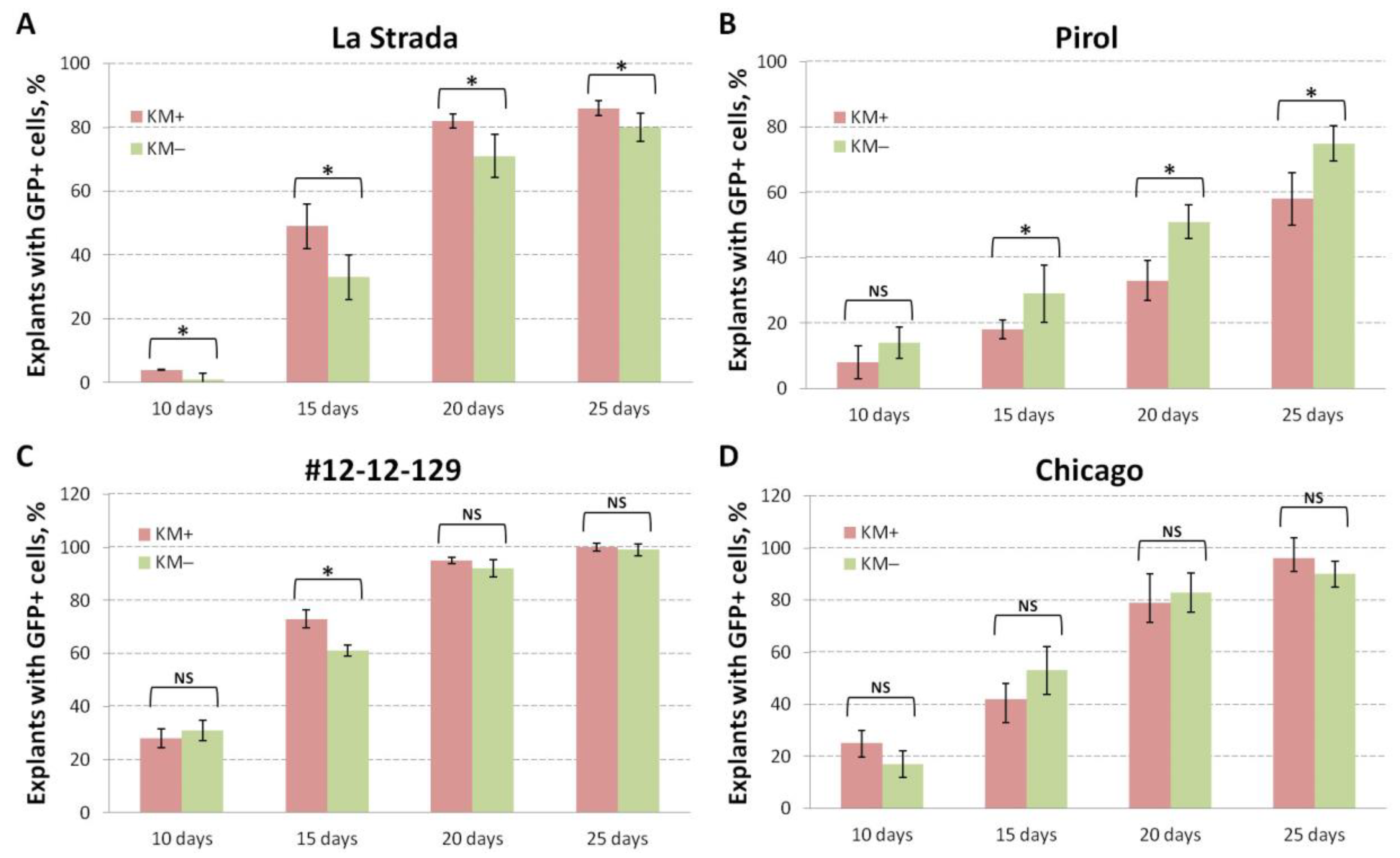
The results we have obtained here align with previous studies, suggesting that antibiotic-free transformation is less dependent on genotype compared to antibiotic-assisted protocols [
26,
28]. In our findings, the efficiency of marker-assisted transformation displayed substantial genotype dependency and varied significantly from 44 to 100% (
Table 2). Conversely, it's challenging to argue for a prominent influence of genotype under non-selective conditions, since the transformation efficiency varied within a narrow range of 0.9 to 2.7%. A similar genotype independent trend was observed in marker-less transformation of three potato cultivars with varying genetic background, where the efficiency varied between 0.6 and 2.4% [
28]. Notably, an average marker-free transformation efficiency of 2.2% was reported for cv. ‘Superior’ in another study [
31], in which leaf explants were used instead of intermodal segments for generation of plantlets. Moreover, in report of Vetten et al. [
27], the highly transformable cultivar 'Carnico' exhibited slightly higher efficacy, achieving 4.5% in marker-less transformation, while the cultivars with lower marker-assisted transformation abilities demonstrated a lower percentage of PCR-positive shoots recovery (0.6 – 2.4%). In the present study, the lowest antibiotic-free transformation efficiency was detected in ‘Chicago’ (0.9%), despite the variety demonstrating an extremely high efficiency in kanamycin-assisted selection (100%). When considering transformation efficiency, it's important to note that marker-free selection is calculated relative to the total number of plants analyzed, whereas antibiotic-assisted transformation is defined as the proportion of explants producing transgenic plants. Due to this distinction, the efficiency of the same varieties when using the two protocols is not directly comparable. Furthermore, the ability to generate more (or fewer) plantlets from the same number of cultured cells may explain why a specific cultivar exhibits reduced marker-free transformation efficiency compared to others.
While marker-less transformation could be perceived as a simpler approach, low transformation percentages are commonly reported [
23,
24,
25,
26,
27,
28,
29,
30], because it is assumed that without the use of selective agents, the proportion of
Agrobacterium-treated cells that can become transgenic is limited. Surprisingly, in our study, the number of explants displayed GFP expression until 30 days after agrobacterial inoculation was either equal to or even higher without the selective pressure in four potato cultivars. Notably, only cv. ‘La Strada’ demonstrated better transient expression when kanamycin-assisted selection was used; however, the efficiency of stable genetic transformation was only 47%, lower than in the other genotypes. These findings suggest that a high level of transient expression does not necessarily lead to high efficiency of transformation in potato. Nevertheless, the presence of explants with high transient expression, regardless of selective pressure, makes the generation of fully transgenic plants in the absence of marker-assisted selection a realistic prospect.
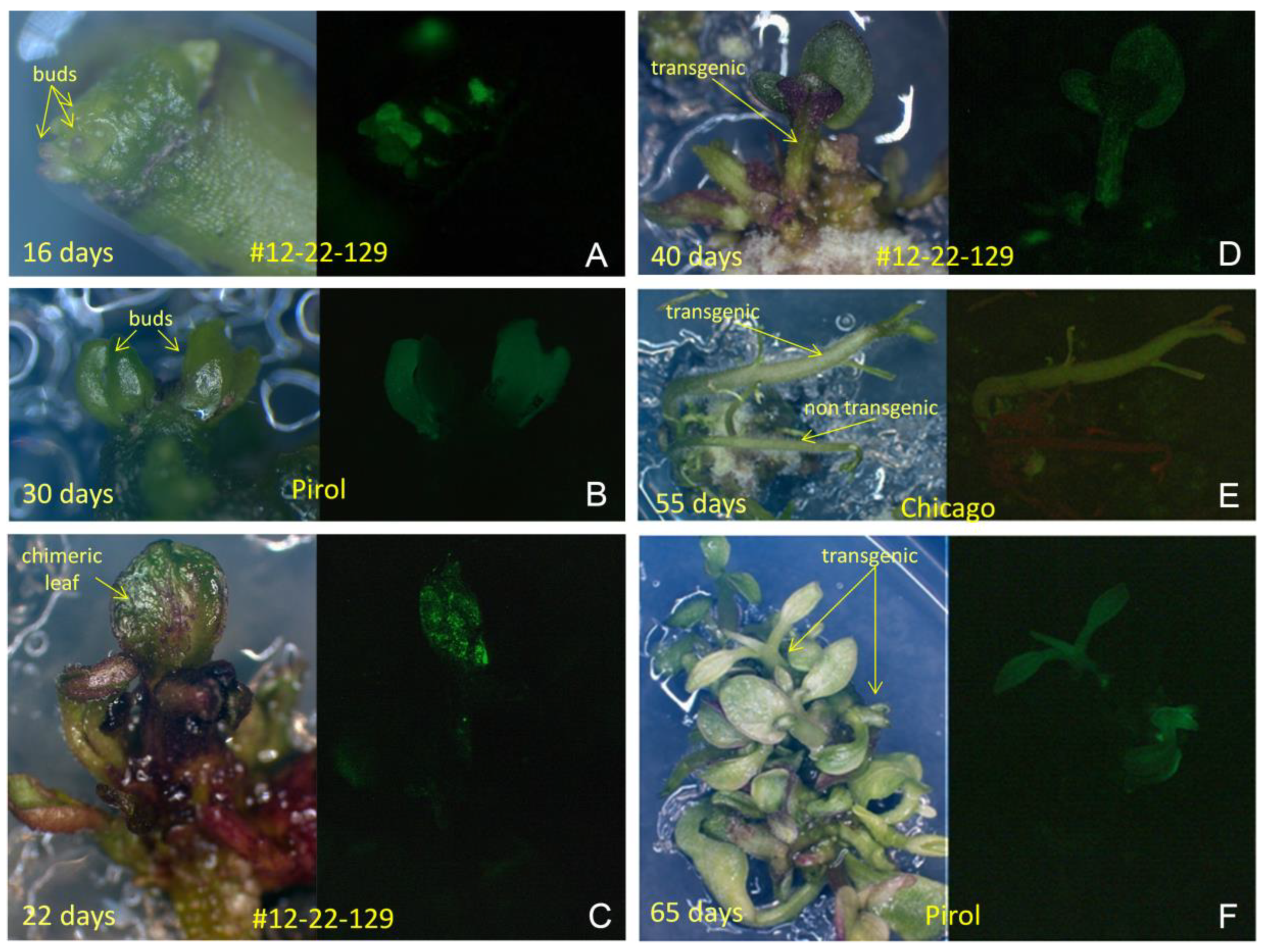
In our experiments, the detection of putative transgenic plants during the stage of kanamycin-free regeneration facilitated by a relatively straightforward screening process based on GFP fluorescence. Given the presence of the NPTII gene in the transferred expression cassette, transgenic events were identified after the finishing of experiments through rooting in the presence of kanamycin.
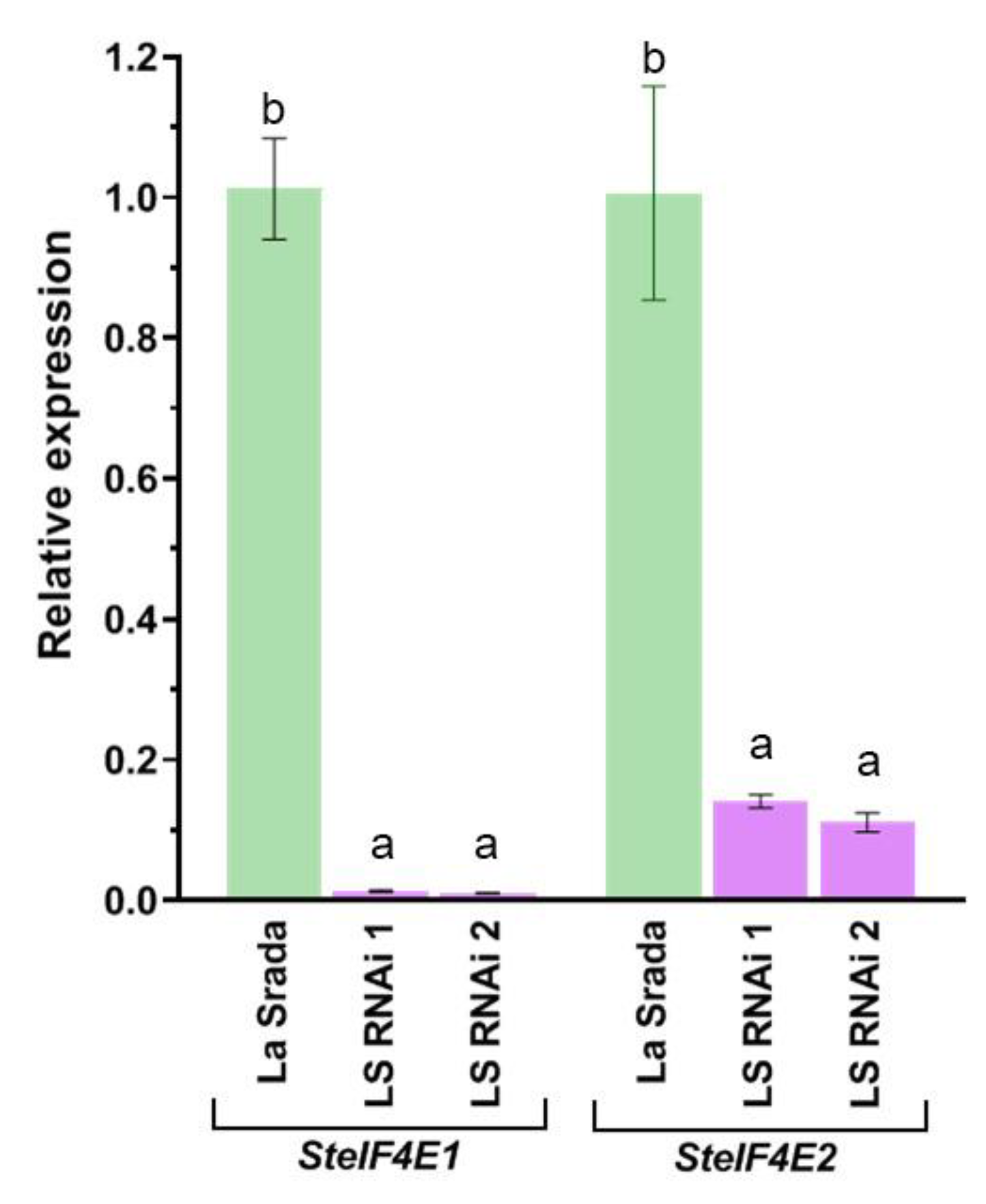
Following the established timeline of transgenic plants emergence, we endeavored to adopt a 'blind' detection approach for ‘La Strada’ when a minimal gene expression cassette was used for producing transgenic plants. The results, based on the PCR and qp-PCR analysis, underscored the reproducibility of the suggested timeline-based transformation approach, as independent marker-free transgenic plants were successfully produced according to the PCR and qp-PCR analyses. Despite a significant reduction in the number of inoculated explants and the one-time collection of plants regenerated between 40 and 55 days, we were able to generate transgenic plants with a frequency of 0.5% (per all analyzed plants) or 1.9% (per inoculated explants). Notably, a similar marker-less transformation efficiency of 0.5% was previously achieved when employing the timeline-based approach to generate intragenic PPV-resistant plants of the cv. 'Pirol' using the same minimal gene expression cassette [
30]. Considering that the transgenic plants of 'La Strada' produced in the present study demonstrated a significantly higher RNAi-mediated silencing of target genes than the resistant intragenic line of 'Pirol', we hope to further co confirm the viral resistance in both new lines during a greenhouse test.
While the ability to generate transgenic elite potato cultivars without using selectable marker genes may not be as effective as marker-assisted selection, we believe it will significantly facilitate the potential commercial release of new biotech potato varieties produced via cisgenic and intragenic strategies of genetic transformation. This novel advancement offers promising prospects for the development and deployment of biotech potato, underlining the ongoing advancements in genetic engineering and crop improvement.