Submitted:
08 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Hydrogen blending in the gas pipelines
1.2. Gas mixture properties
1.3. Tunisian context
3. Results and discussion
3.1. Preliminary analysis
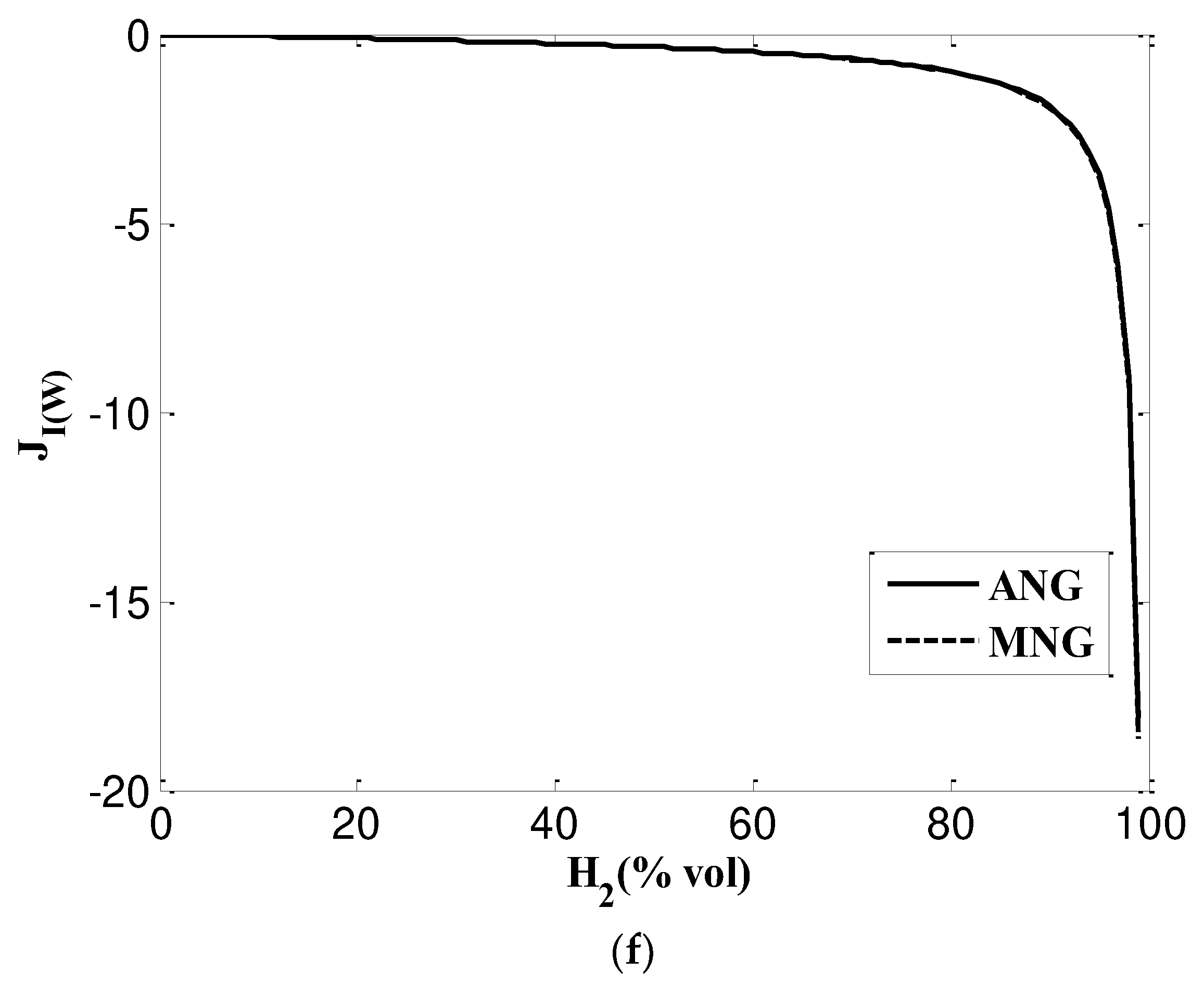
3.2. Analysis of interchangeability criteria
3.2.1. Knoy index
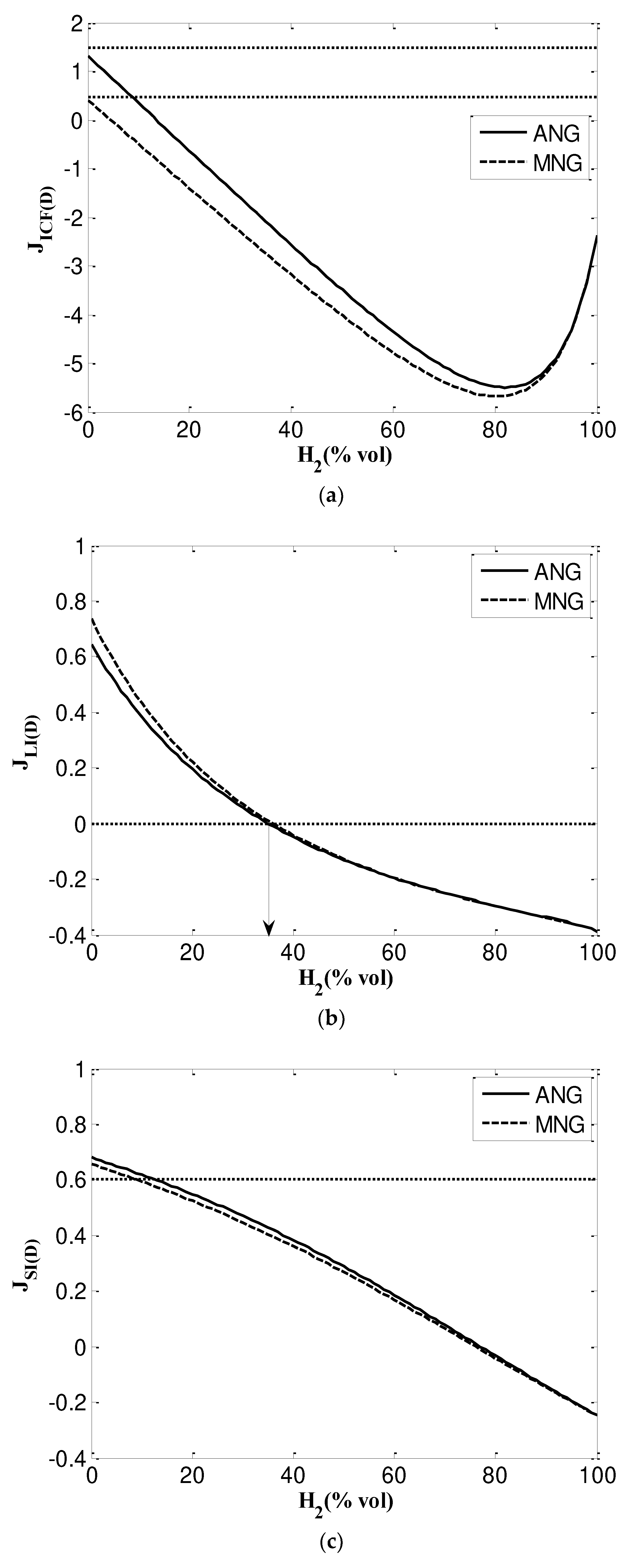
3.2.2. Dutton factors
- a)
- Incomplete combustion factor (
- b)
- Lift Index ()
- c)
- Soot Index ()
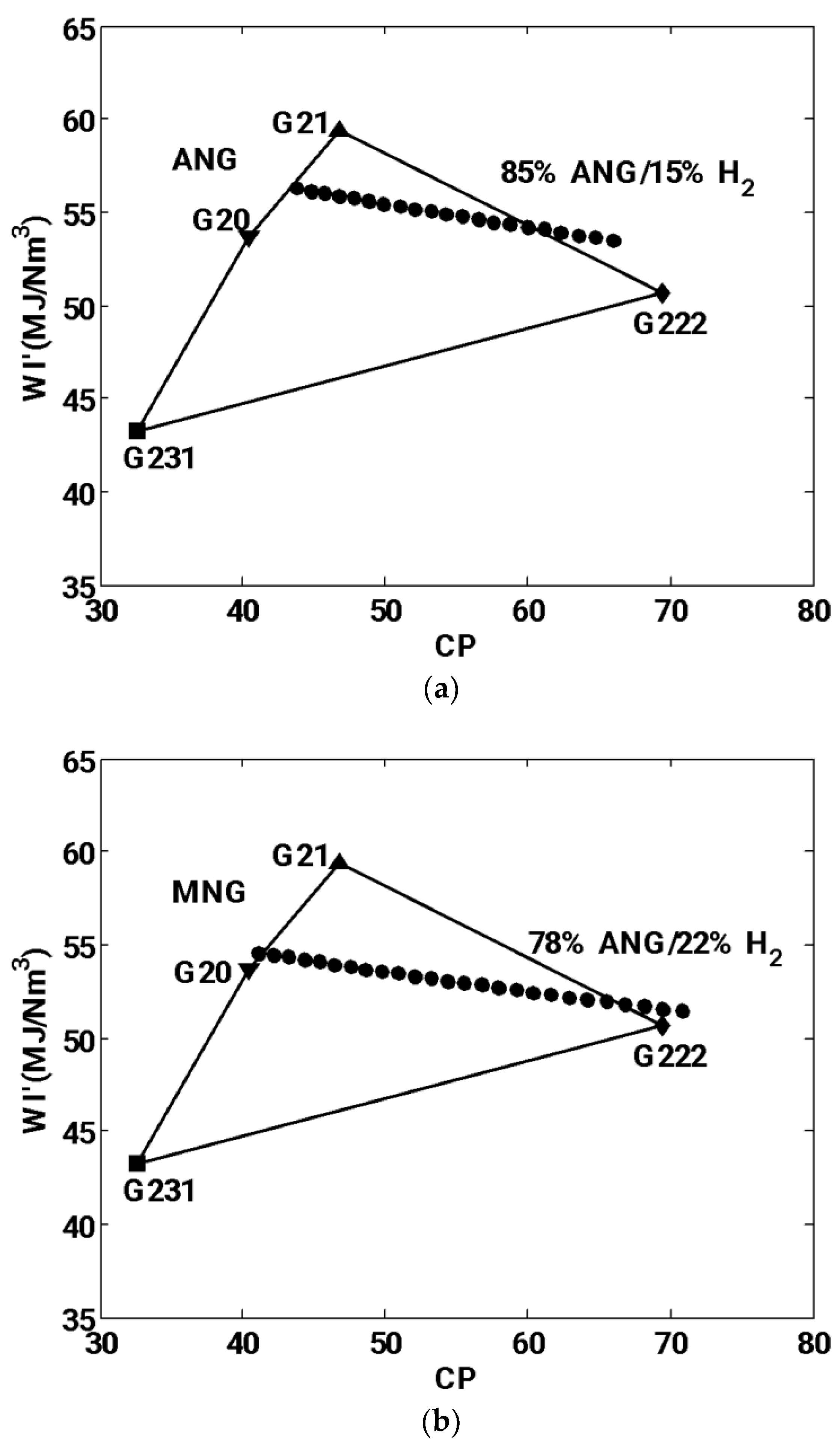
3.2.3. Delbourg method (used in Europe)
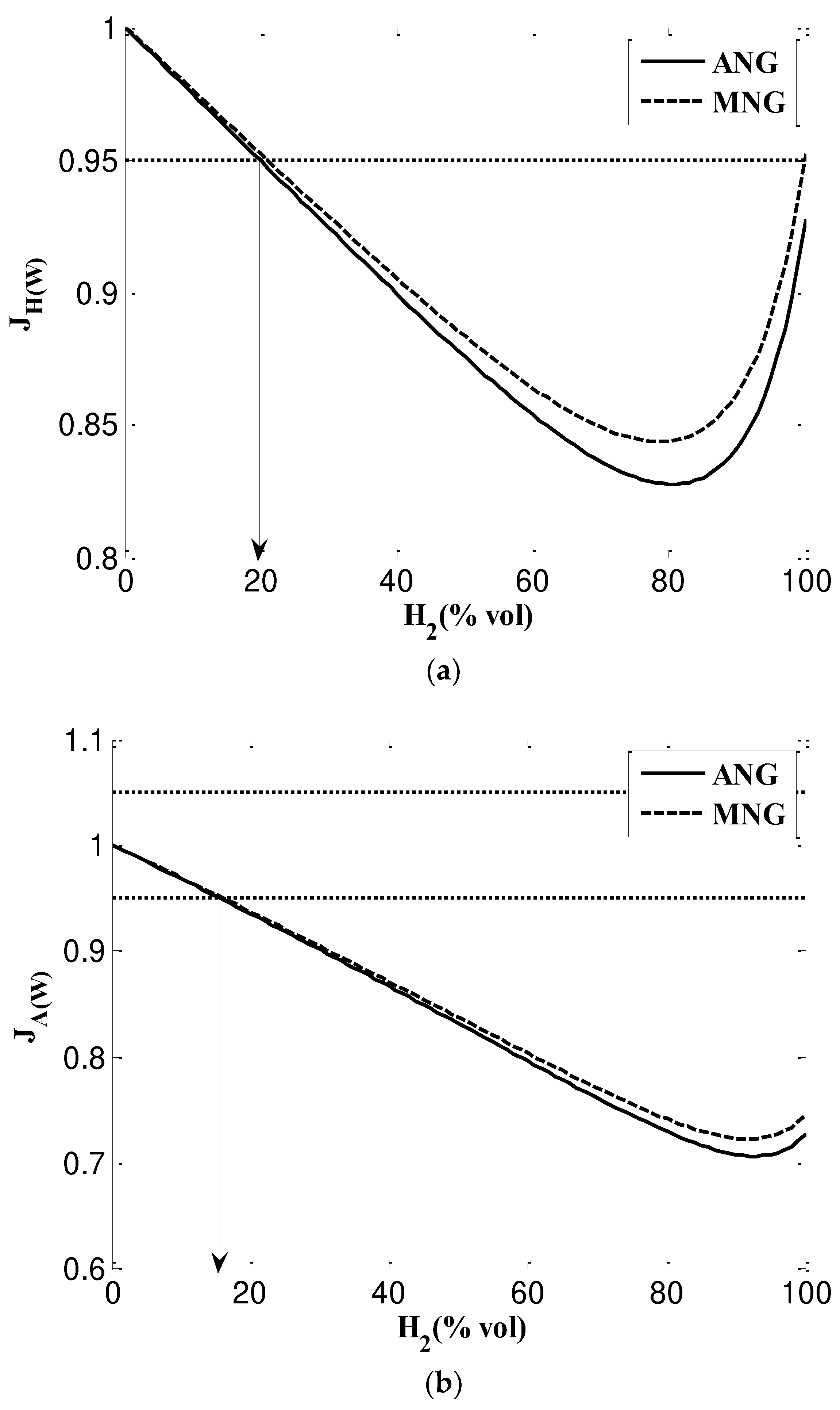
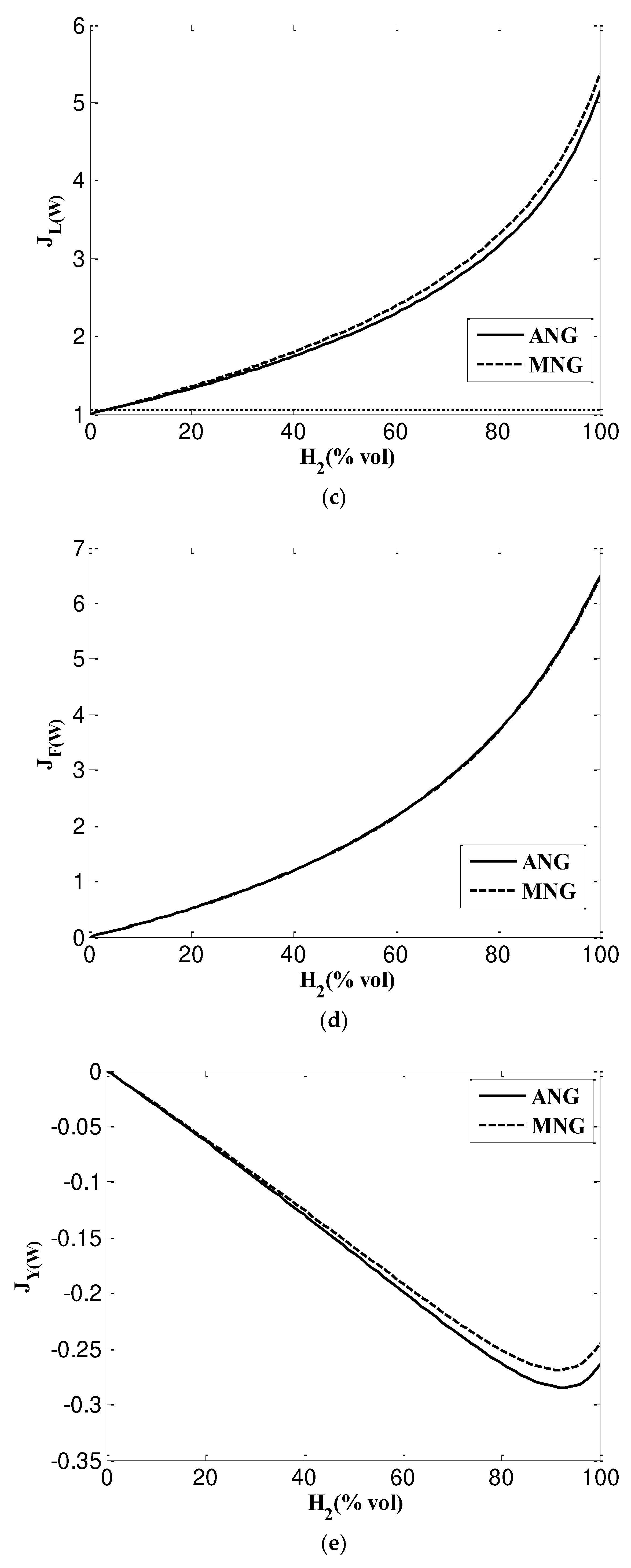
3.2.4. Weaver method (used in USA)
Conclusions
- 1)
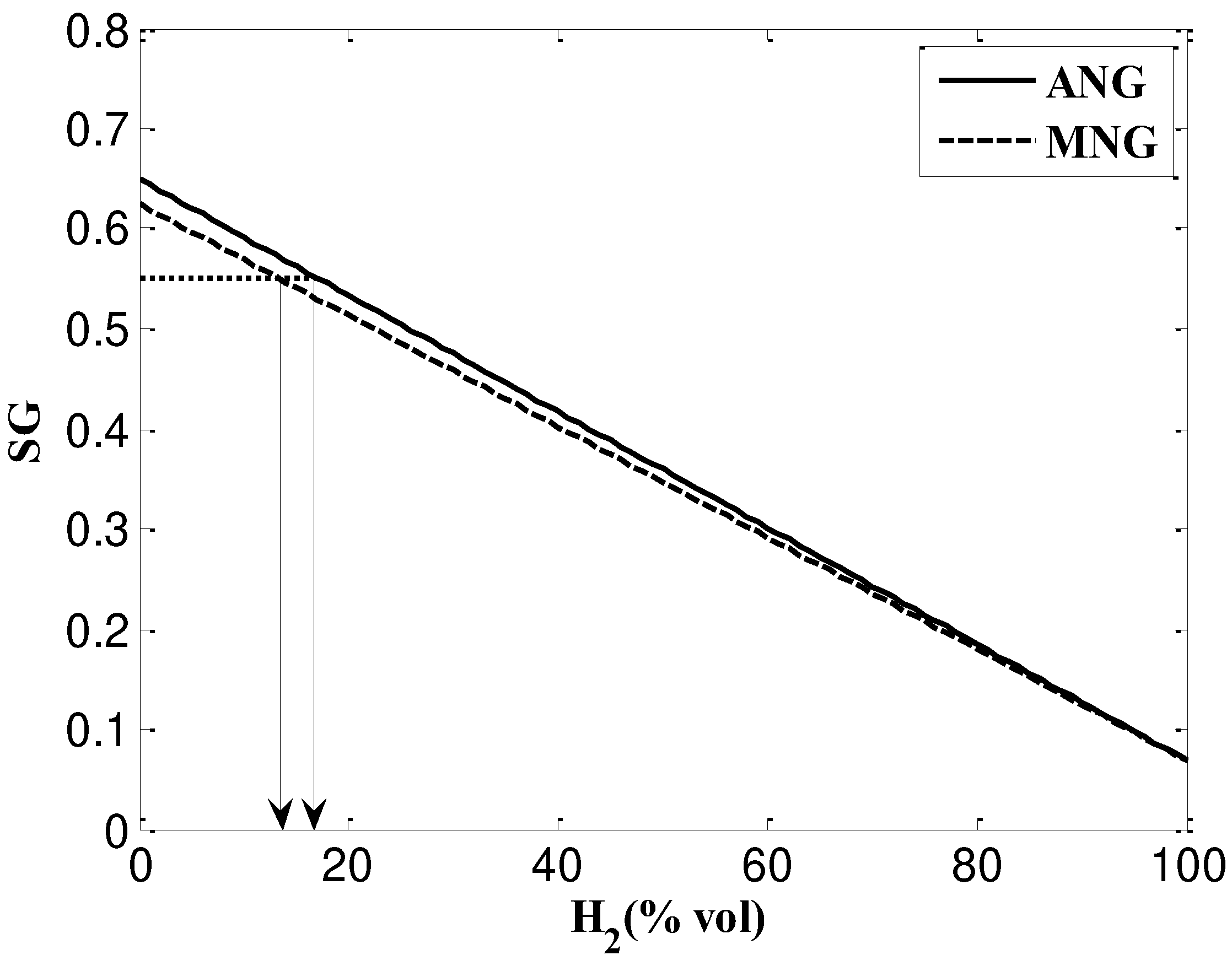
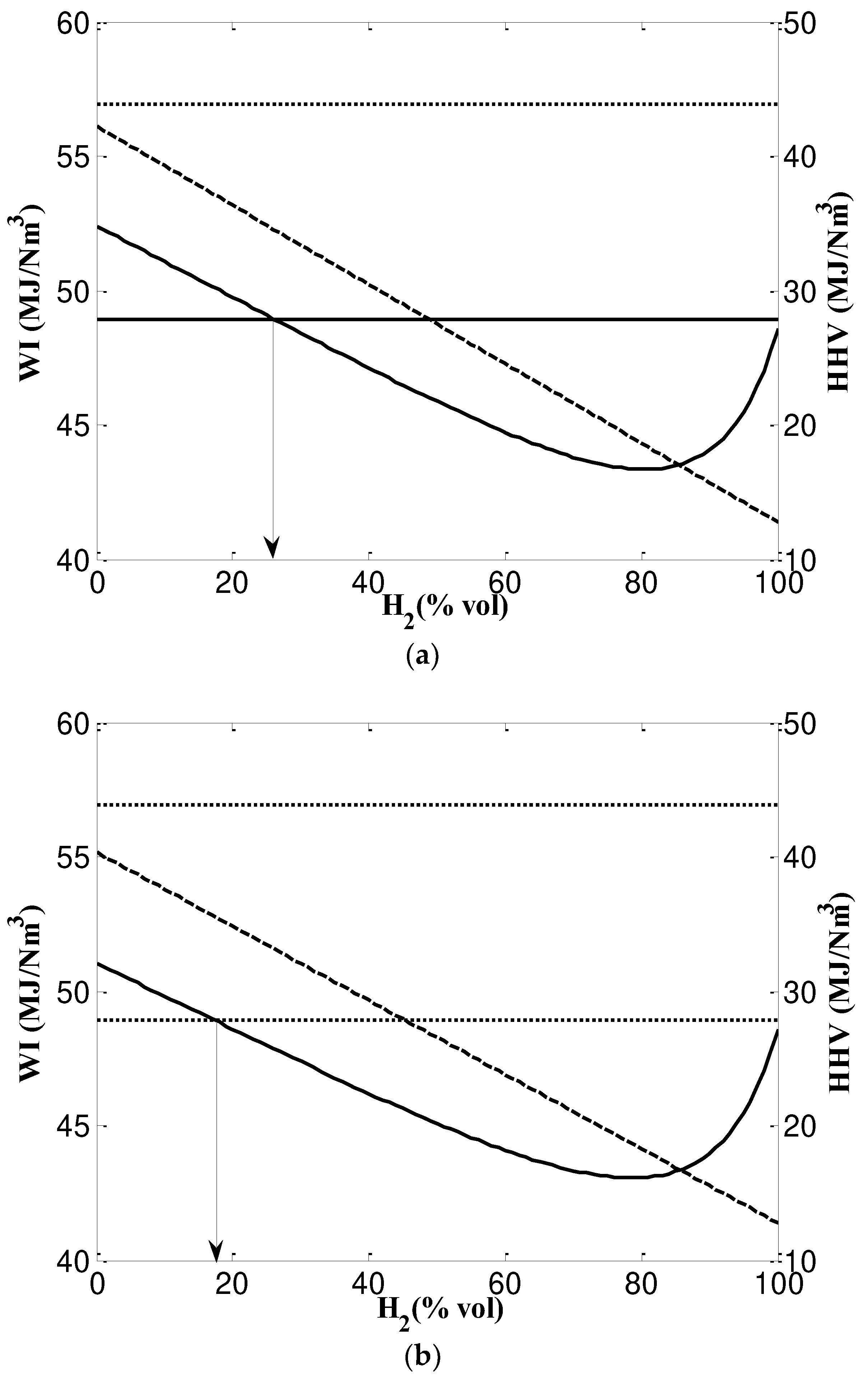
- Based on SG, NG and NG/H2 mixtures are interchangeable and the maximum mixing percentage of hydrogen content to ANG is 17% and to MNG is 13%. However, when considering WI these values grow up to 24% and 17% for ANG and MNG respectively. According to SG, NG and NG/H2 mixtures can be used interchangeably with a maximum hydrogen concentration of 17% for ANG and 13% for MNG. However, when WI is taken into account, ANG and MNG's values increase to 24% and 17%, respectively.
- 2)
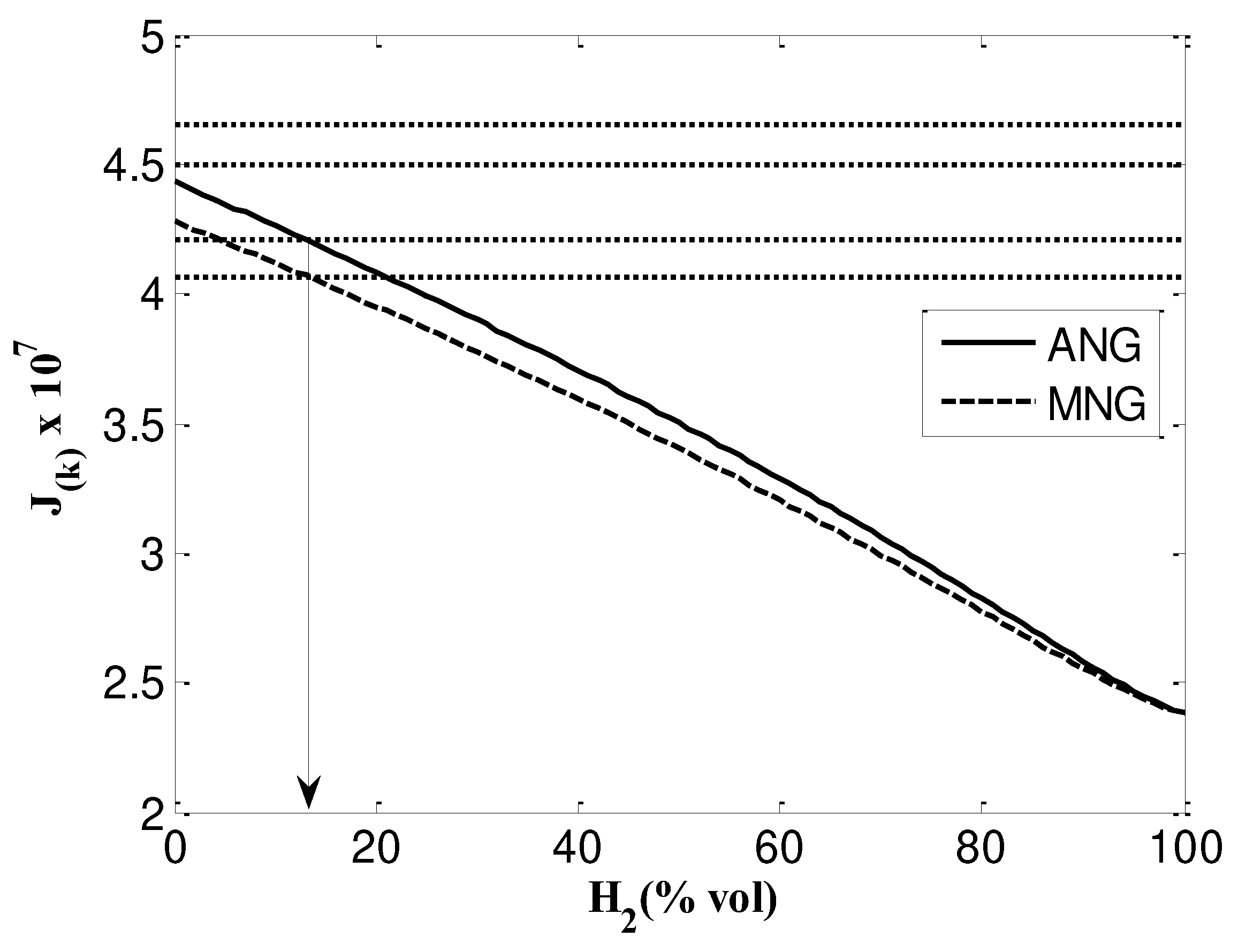
- According to the Knoy method, the maximum allowable mixing content of hydrogen is 14%.
- 3)
- Dutton indices predict that the maximum hydrogen content is more than 36%.
- 4)
- Graphical Delbourg diagrams illustrates maximum blending ratio up to 15% and 22% into ANG and MNG respectively.
- 5)
- Weaver index method have been testing American gas appliances, which impose stricter requirements on WI and CP. Maximum hydrogen content mixing percentages for ANG and MNG are 20% and 17%, respectively, according to calculation results. However, when hydrogen content increases, there is a greater risk of flame lift and light back phenomena. Equipment for flame stabilization will then be needed.
Funding
References
- Price, A. (2015). The exploitation of renewable sources of energy for power generation. In Electrochemical energy storage for renewable sources and grid balancing (pp. 3-12). Elsevier.
- Tang, D. , Tan, G. L., Li, G. W., Liang, J. G., Ahmad, S. M., Bahadur, A.,... & Bououdina, M. (2023). State-of-the-art hydrogen generation techniques and storage methods: A critical review. Journal of Energy Storage, 64, 107196.
- Taibi, E., Miranda, R., Vanhoudt, W., Winkel, T., Lanoix, J. C., & Barth, F. (2018). Hydrogen from renewable power: Technology outlook for the energy transition.
- EU Commission. A Hydrogen Strategy for a Climate Neutral Europe. 2020. Available online: https://ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf (accessed on 16 February 2023).
- Cheli, L., Guzzo, G., Adolfo, D., & Carcasci, C. (2021). Steady-state analysis of a natural gas distribution network with hydrogen injection to absorb excess renewable electricity. international journal of hydrogen energy, 46(50), 25562-25577.
- Vidas, L., Castro, R., & Pires, A. (2022). A review of the impact of hydrogen integration in natural gas distribution networks and electric smart grids. Energies, 15(9), 3160.
- Gaz, R. D. F. G., Storengy, F., Reseau, G. D. S., & Syndicat, P. D. E. G. N. (2019). Technical and economic conditions for injecting hydrogen into natural gas networks-Final report June 2019.
- Shirvill, L. C. , Roberts, T. A., Royle, M., Willoughby, D. B., & Sathiah, P. (2019). Experimental study of hydrogen explosion in repeated pipe congestion–Part 2: Effects of increase in hydrogen concentration in hydrogen-methane-air mixture. International Journal of Hydrogen Energy, 44(5), 3264-3276.
- Raju, A. S. , Martinez-Moralez, A., Lever, O., & Asiedu-Akrofi, L. (2022). Hydrogen blending impacts study. California Public Utilities Commission.
- Gondal, I. A. (2019). Hydrogen integration in power-to-gas networks. International journal of hydrogen energy, 44(3), 1803-1815.
- Guzzo, G. , Cheli, L., & Carcasci, C. (2022). Hydrogen blending in the Italian scenario: Effects on a real distribution network considering natural gas origin. Journal of Cleaner Production, 379, 134682.
- Melaina, M. W. , Antonia, O., & Penev, M. (2013). Blending hydrogen into natural gas pipeline networks: a review of key issues.
- Topolski, K. , Reznicek, E. P., Erdener, B. C., San Marchi, C. W., Ronevich, J. A., Fring, L.,... & Chung, M. (2022). Hydrogen blending into natural gas pipeline infrastructure: review of the State of technology.
- https://www.iee.fraunhofer.de/content/dam/iee/energiesystemtechnik/en/documents/Studies-Reports/FINAL_FraunhoferIEE_ShortStudy_H2_Blending_EU_ECF_Jan22.
- Cavana, M., Mazza, A., Chicco, G., & Leone, P. (2021). Electrical and gas networks coupling through hydrogen blending under increasing distributed photovoltaic generation. Applied Energy, 290, 116764.
- Vaccariello, E., Trinchero, R., Stievano, I. S., & Leone, P. (2021). A statistical assessment of blending hydrogen into gas networks. Energies, 14(16), 5055.
- HyDeploy (2020). Gas Network Innovation Competition. Available at https://hydeploy.co.uk/.
- Hall, J. E. , Hooker, P., & Jeffrey, K. E. (2021). Gas detection of hydrogen/natural gas blends in the gas industry. International Journal of Hydrogen Energy, 46(23), 12555-12565.
- Edwards, R. L. , Font-Palma, C., & Howe, J. (2021). The status of hydrogen technologies in the UK: A multi-disciplinary review. Sustainable Energy Technologies and Assessments, 43, 100901.
- Storage, E. P2G, 2018. GRHYD project inaugurates first P2G demonstrator in France, 9-10.
- The GRHYD demonstration project: https://www.engie.com/en/businesses/gas/hydrogen/power-to-gas/the-grhyd-demonstration-project.
- Zhao, Y., McDonell, V., & Samuelsen, S. (2019). Influence of hydrogen addition to pipeline natural gas on the combustion performance of a cooktop burner. International Journal of Hydrogen Energy, 44(23), 12239-12253.
- DESCHAMPS, Y. (1984). Combustibles gazeux. Utilisation et combustibilité des gaz. In Généralités: sciences fondamentales, génie industriel.
- Euro-Asian Council for Standardization, Metrology and Certification (2012). ЕN 437: 2012. Test gases. Test pressures. Appliance categories. Minsk: Euro-Asian Council for Standardization, Metrology and Certification.
- https://www.iso.org/fr/standard/53058.html.
- Honus, S. , Kumagai, S., & Yoshioka, T. (2016). Replacing conventional fuels in USA, Europe, and UK with plastic pyrolysis gases–Part II: Multi-index interchangeability methods. Energy conversion and management, 126, 1128-1145.
- Delbourg, P. , & Lafon, J. (1971). Interchangeabilité des gaz. Energy economics, 731, 44.
- Dutton, B. C. , & Wood, S. W. (1984). Gas interchangeability: prediction of soot deposition on domestic gas appliances with aerated burners. J. Inst. Energy;(United Kingdom), 57(432).
- Weaver, E. R. (1951). Formulas and graphs for representing the interchangeability of fuel gases. Journal of Research of the National Bureau of Standards, 46(3), 213-245.
- NGC+ Interchangeability Work Group. (2005). White paper on natural gas interchangeability and non-combustion end use. Federal Energy Regulatory Commission.
- Knoy, M. F. (1953). Graphic approach to the problem of interchangeability. AGA Proceedings, 938-947.
- GIZ, Study on the opportunities of “Power-to-X” in Tunisia. Available at : https://www.giz.de/en/downloads_els/GIZ%20PtX%20Tunisia%20report-Web.pdf.
- GIZ (2019). Renewable energy projects in Tunisia – Guide summary, available at: http://www.tunisieindustrie.gov.tn/upload/ENR/Guide_Summary_RE_ Tunisia_mai2019.pdf.
- STEG 2022 annual report. Available at : ttps://www.steg.com.tn/fr/institutionnel/publications.html.
- Tunisian Open Data Platform, https://data.gov.tn.
- Related safety issues the injection of hydrogen into natural gas transport and distribution networks: current situation and prospects; Ref.: INERIS-DRA-19-178263-01884B.
- Gaz, R. D. F. G., Storengy, F., Reseau, G. D. S., & Syndicat, P. D. E. G. N. (2019). Technical and economic conditions for injecting hydrogen into natural gas networks-Final report June 2019.
- BRIEF, I. L. (2019). RENEWABLE POWER-TO-HYDROGEN.
- El-Mahallawy, F., & Habik, S. D. (2002). Fundamentals and technology of combustion. Elsevier.
- Turns, S. R. (1996). Introduction to combustion (Vol. 287, p. 569). New York, NY, USA: McGraw-Hill Companies.
- Ren, F., Chu, H., Xiang, L., Han, W., & Gu, M. (2019). Effect of hydrogen addition on the laminar premixed combustion characteristics the main components of natural gas. Journal of the Energy Institute, 92(4), 1178-1190.
- Korobeĭnikov, V. P. (1976). Problems in the theory of point explosion in gases (No. 119). American Mathematical Soc.
- Briggs, T. (2014). The combustion and interchangeability of natural gas on domestic burners. Combustion, 4(3), 67-87.
- Honus, S. , Kumagai, S., & Yoshioka, T. (2016). Replacing conventional fuels in USA, Europe, and UK with plastic pyrolysis gases–Part II: Multi-index interchangeability methods. Energy conversion and management, 126, 1128-1145.
- Alexander, S., Anatolii, K., & Vitalii, T. (2022). Interchangeability and Standardization of the Parameters of Combustible Gases When Using Hydrogen. Architecture and Engineering, 7(1), 33-45.
- Lander, D. (2002). UK situation regarding gas quality. Presentation to Marcogaz Gas Quality WG.
- Union, I. G. (2011). Petroleum B. guidebook to gas interchangeability and gas quality.
- Britain, G. (1996). A Guide to the Gas Safety (Management) Regulations 1996: Guidance on Regulations. HSE Books.
- Fík, J. (1998). Combustion of gaseous fuels and gas burners. GAS Ltd.
- Ortíz, J. M. (2014). Fundamentos de la intercambiabilidad del gas natural. Ciencia, 6-15.
- Alexander, S., Anatolii, K., & Vitalii, T. (2022). Interchangeability and Standardization of the Parameters of Combustible Gases When Using Hydrogen. Architecture and Engineering, 7(1), 33-45.
- Ferguson, D. H. , Straub, D. L., Richards, G. A., & Robey, E. H. (2007). Impact of fuel interchangeability on dynamic instabilities in gas turbine engines (No. DOE/NETL-IR-2007-102). National Energy Technology Laboratory (NETL), Pittsburgh, PA, Morgantown, WV, and Albany, OR (United States).
 demand
demand  national production
national production  royalties ANG [35].
royalties ANG [35].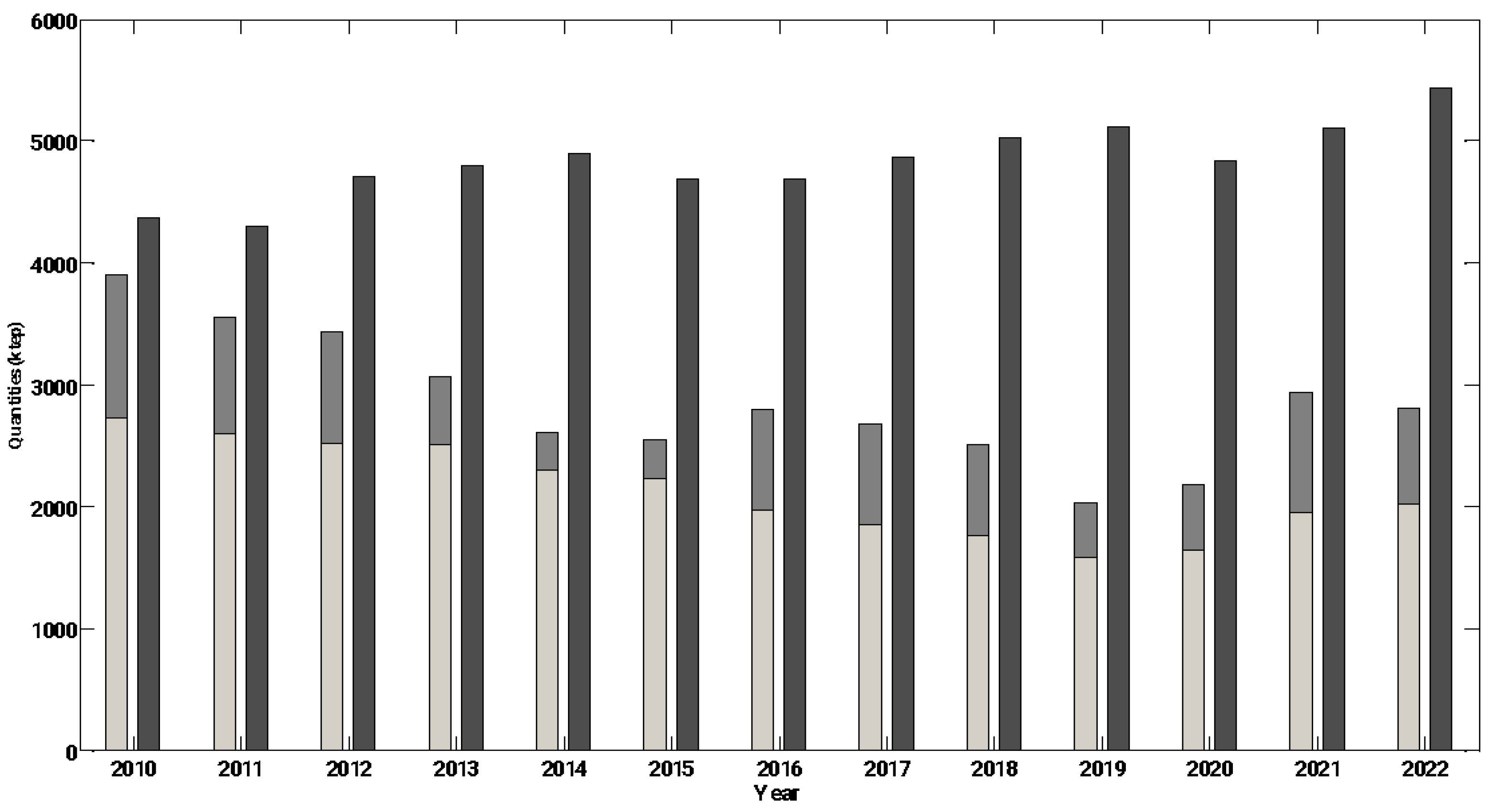
| Method or index | Country | Controlled parameters |
|---|---|---|
| Knoy index | Europe | HHV |
| Delbourg method | Europe | S, Y |
| Dutton’s criteria | UK/AUS | I, L, S |
| AGA method | USA | F, L, Y |
| Weaver method | USA | F, I, L, S, Y, HHV |
| Components | CH4 | C2H6 | C3H8 | nC4H10 | iC4H10 | nC5H12 | iC5H12 | C6H14 | CO2 | N2 | He |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ANG (% vol) | 83.42 | 7.77 | 1.95 | 0.45 | 0.25 | 0.10 | 0.09 | 0.12 | 0.25 | 5.42 | 0.18 |
| MNG (% vol) | 87.04 | 4.70 | 1.23 | 0.27 | 0.29 | 0.06 | 0.07 | 0.04 | 0.02 | 6.28 | 0.00 |
| Combustion parameters | High Heating Value (kJ/Nm3) | Density (kg/m3) |
SG | Wobbe index (kJ/Nm3) | Combustion Potential |
|---|---|---|---|---|---|
| ANG | 42 213 | 0.84 | 0.65 | 52 388 | 43.75 |
| MNG | 40 325 | 0.80 | 0.62 | 50 892 | 41.15 |
| Gas reference | Composition | Wobbe Index (kJ/Nm3) | Combustion Potential | Comment |
|---|---|---|---|---|
| G20 | 100% CH4 | 53685 | 40.37 | Reference’s gas |
| G21 | 13% C3H8 /87%CH4 | 59365 | 46.70 | Limit of incomplete combustion |
| G222 | 23% H2 / 77% CH4 | 50673 | 69.42 | Limit of flame flashback |
| G231 | 15% N2 / 85% CH4 | 43264 | 32.53 | Limit of flame lifting |
| Indices | definition | Calculation Formula | Condition of interchangeability |
|---|---|---|---|
| Heat rate ratio | Ratio between the Wobbe index for the substitute gas (“s” index) and the Wobbe index for the replaced or adjustment gas (“a” index) | ||
| Primary air ratio | Ratio between the theoretical required air for combustion for each gas | = | |
| Lifting index | Includes the flame speed (S) of both gases as well as the volume fraction of oxygen in them | ||
| Flashback index | Includes the flame speed (S) of both gases as well as primary air ratio | ||
| yellow tipping index | Includes the total content of hydrogen atoms in molecule of gas (Nc) and primary air ratio | ||
| Incomplete combustion index | Calculated using the ratio of the number of hydrogen and carbon in molecules (RH/C)of compared gases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




