Submitted:
10 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Historical threat
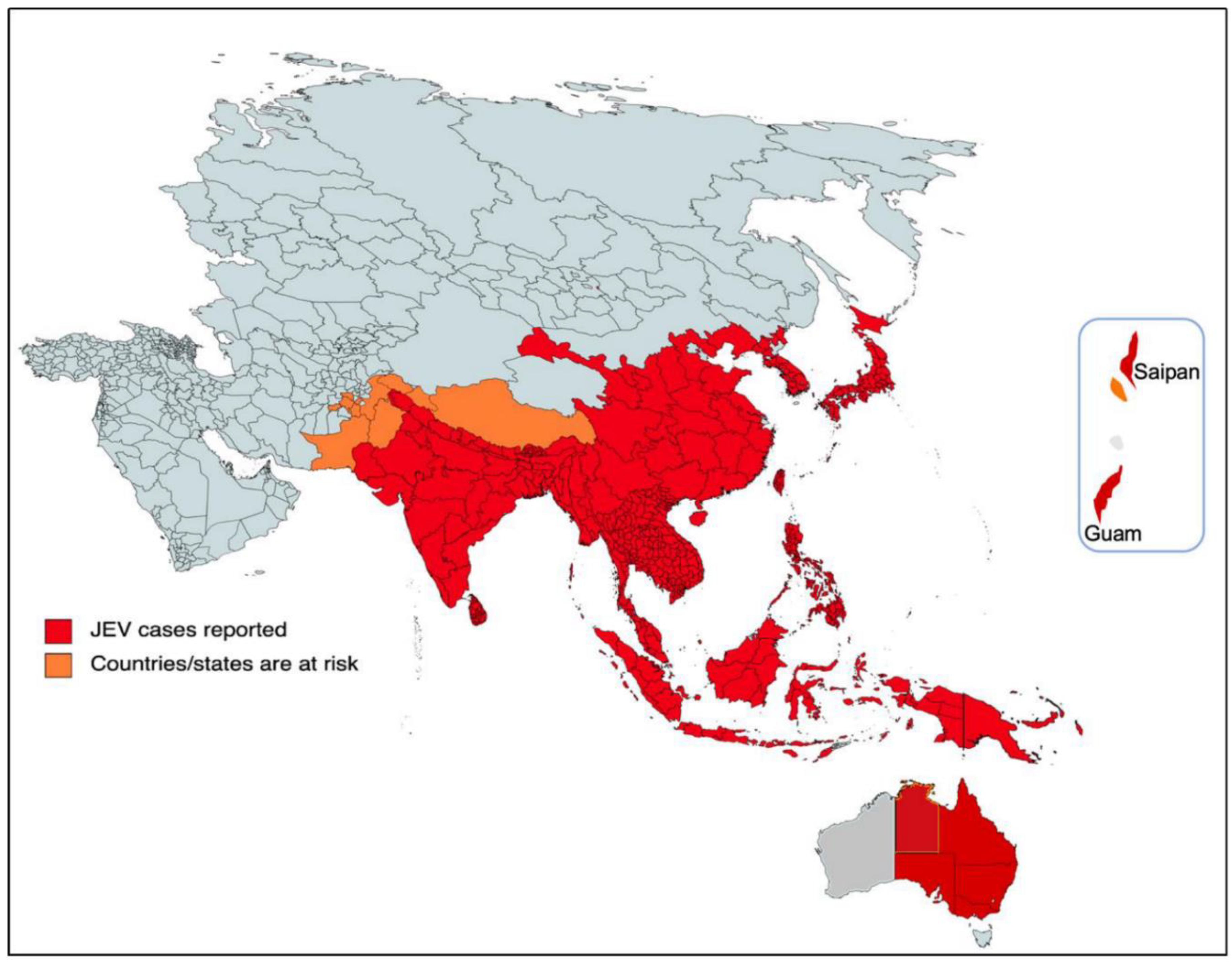
The current threat
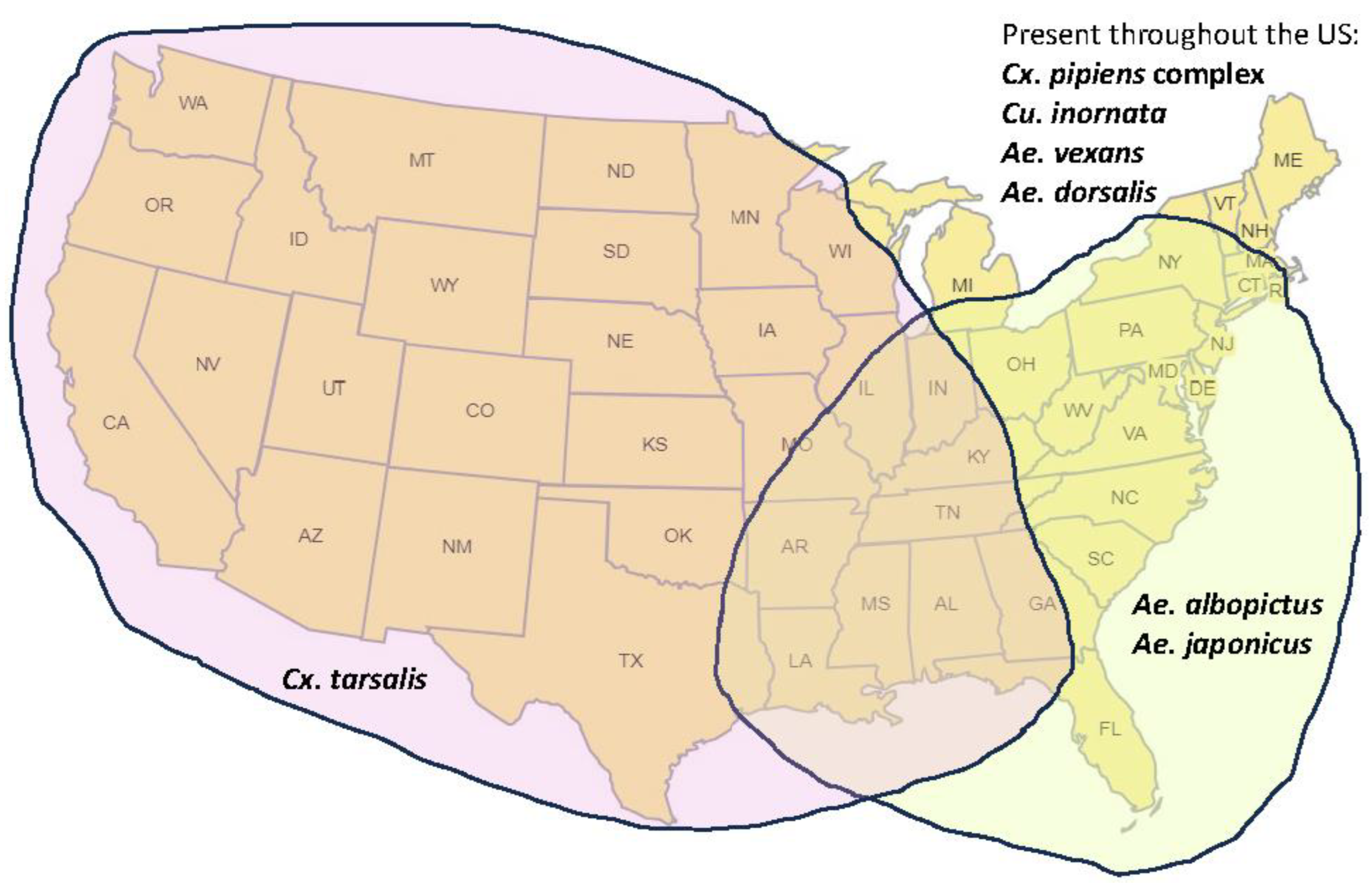
Mechanisms of potential introduction and spread of JEV
Consequences of and response to the introduction of JEV in North America
Vaccines as a countermeasure
Conclusions
References
- Quan, T.M.; Thao, T.T.N.; Duy, N.M.; Nhat, T.M.; Clapham, H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000-2015. Elife 2020, 9, e51027. [Google Scholar] [CrossRef]
- Vannice, K.S.; Hills, S.L.; Schwartz, L.M.; Barrett, A.D.; Heffelfinger, J.; Hombach, J.; Letson, G.W.; Solomon, T.; Marfin, A.A.; Anderson, K.; et al. The future of Japanese encephalitis vaccination: expert recommendations for achieving and maintaining optimal JE control. npj Vaccines 2021, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, Present, and Future of Japanese Encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, G. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther. Clin. Risk Manag. 2015, 11, 435–448. [Google Scholar] [PubMed]
- Auerswald, H.; Maquart, P.-O.; Chevalier, V.; Boyer, S. Mosquito Vector Competence for Japanese Encephalitis Virus. Viruses 2021, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.R.; Strathe, E.; Etcheverry, L.; Cohnstaedt, L.W.; McVey, D.S.; Piaggio, J.; Cernicchiaro, N. Assessment of data on vector and host competence for Japanese encephalitis virus: A systematic review of the literature. Prev. Veter- Med. 2018, 154, 71–89. [Google Scholar] [CrossRef]
- Nemeth, N.; Oesterle, P.; Bosco-Lauth, A.; Bowen, R.; Kohler, D. North American Birds as Potential Amplifying Hosts of Japanese Encephalitis Virus. Am. J. Trop. Med. Hyg. 2012, 87, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L. The Natural History of Japanese Encephalitis Virus. Annu. Rev. Microbiol. 1986, 40, 395–414. [Google Scholar] [CrossRef]
- Lannes, N.; Summerfield, A.; Filgueira, L. Regulation of inflammation in Japanese encephalitis. J. Neuroinflammation 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.S.A.; Gibbons, R.V.; Perng, G.C.; Solomon, T. Unravelling the neuropathogenesis of Japanese encephalitis. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 955–956. [Google Scholar] [CrossRef]
- Chapagain, S.; Singh, P.P.; Le, K.; Safronetz, D.; Wood, H.; Karniychuk, U. Japanese encephalitis virus persists in the human reproductive epithelium and porcine reproductive tissues. PLOS Neglected Trop. Dis. 2022, 16, e0010656. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Shankar, S.K.; Ravi, V.; Chandramuki, A.; Gourie-Devi, M. Japanese encephalitis virus antigen in the human brain and its topographic distribution. Acta Neuropathol. 1995, 89, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Japanese encephalitis vaccines: current vaccines and future prospects. Curr. Top. Microbiol. Immunol. 2002, 267, 105–138. [Google Scholar] [PubMed]
- Hegde, N.R.; Gore, M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccines Immunother. 2017, 13, 1320–1337. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Xu, C.; Doi, S.A.; Clark, J.; Wangdi, K.; Mills, D.J.; Lau, C.L. Comparison of immunogenicity and safety of licensed Japanese encephalitis vaccines: A systematic review and network meta-analysis. Vaccine 2021, 39, 4429–4436. [Google Scholar] [CrossRef] [PubMed]
- Appaiahgari, M.B.; Vrati, S. Clinical development of IMOJEV®—a recombinant Japanese encephalitis chimeric vaccine (JE-CV). Expert Opin. Biol. Ther. 2012, 12, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, T. Ixiaro: a new vaccine against Japanese encephalitis. Expert Rev Vaccines 2009, 8, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol Infect. 2019, 147, e232. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Ritchie, S.A.; Phillips, D.A.; Lee, J.M.; Hills, S.; van den Hurk, A.F.; Pyke, A.; Johansen, C.A.; Mackenzie, J.S. Japanese encephalitis in north Queensland, Australia, 1998. Med. J. Aust. 1999, 170, 533–536. [Google Scholar] [CrossRef]
- Hurk, A.F.v.D.; Skinner, E.; Ritchie, S.A.; Mackenzie, J.S. The Emergence of Japanese Encephalitis Virus in Australia in 2022: Existing Knowledge of Mosquito Vectors. Viruses 2022, 14, 1208. [Google Scholar] [CrossRef]
- Wu, C.-J.; Wu, S.-Y. The species of mosquitoes transmitting Japanese B type encephalitis in Fukien. Acta Microbiol. Sin 1957, 5, 27–32. (In Chinese) [Google Scholar]
- Weng, M.H.; Lien, J.C.; Wang, Y.M.; Lin, C.C.; Lin, H.C.; Chin, C. Isolation of Japanese encephalitis virus from mosquitoes collected in Northern Taiwan between 1995 and 1996. J. Microbiol. Immunol. Infect. 1999, 32, 9–13. [Google Scholar] [PubMed]
- Moore, C.G.; Francy, D.B.; Eliason, D.A.; Monath, T.P. Aedes albopictus in the United States: rapid spread of a potential disease vector. J. Am. Mosq. Control. Assoc. 1988, 4, 356–361. [Google Scholar] [PubMed]
- Rosen, L.; Tesh, R.B.; Lien, J.C.; Cross, J.H. Transovarial Transmission of Japanese Encephalitis Virus by Mosquitoes. Science 1978, 199, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Nanfack-Minkeu, F.; Delong, A.; Luri, M.; Poelstra, J.W. Invasive Aedes japonicus Mosquitoes Dominate the Aedes Fauna Collected with Gravid Traps in Wooster, Northeastern Ohio, USA. Insects 2023, 6, 56. [Google Scholar] [CrossRef]
- Oliveira, A.R.S.; Piaggio, J.; Cohnstaedt, L.W.; McVey, D.S.; Cernicchiaro, N. Introduction of the Japanese encephalitis virus (JEV) in the United States – A qualitative risk assessment. Transbound. Emerg. Dis. 2019, 66, 1558–1574. [Google Scholar] [CrossRef] [PubMed]
- Nett, R.; Campbell, G.; Reisen, W. Potential for the Emergence of Japanese Encephalitis Virus in California. Vector-Borne Zoonotic Dis. 2009, 9, 511–517. [Google Scholar] [CrossRef]
- https://www.sacbee.com/news/california/article282384908.html.
- https://jevisn.org/; https://www.swinehealth.org/potential-for-jev-in-us-leads-to-usda-statement-on-preparedness-and-testing/.
- McGuinness, S.L.; Lau, C.L.; Leder, K. The evolving Japanese encephalitis situation in Australia and implications for travel medicine. J. Travel Med. 2023, 30. [Google Scholar] [CrossRef]
- Yakob, L.; Hu, W.; Frentiu, F.D.; Gyawali, N.; Hugo, L.E.; Johnson, B.; Lau, C.; Furuya-Kanamori, L.; Magalhaes, R.S.; Devine, G. Japanese Encephalitis Emergence in Australia: The Potential Population at Risk. Clin. Infect. Dis. 2022, 76, 335–337. [Google Scholar] [CrossRef]
- Kramer, L.D.; Ciota, A.T.; Kilpatrick, A.M. Introduction, Spread, and Establishment of West Nile Virus in the Americas. J. Med Èntomol. 2019, 56, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.; Brito, A.F.; Swetnam, D.M.; Vogels, C.B.F.; Tokarz, R.E.; Andersen, K.G.; Smith, R.C.; Bedford, T.; Grubaugh, N.D. Twenty years of West Nile virus spread and evolution in the Americas visualized by Nextstrain. PLOS Pathog. 2019, 15, e1008042. [Google Scholar] [CrossRef] [PubMed]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease? PLoS Negl Trop Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Vaughn, D.; Khanh, V.T. Japanese encephalitis. J Neurol Neurosurg Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, C.; Sohier, C.; Matthis, S.; de Regge, N. Japanese encephalitis virus interaction with mosquitoes: A review of vector competence, vector capacity and mosquito immunity. Pathogens 2022, 11, 317. [Google Scholar] [CrossRef]
- Scherer, W.F.; Buescher, E.L. Ecologic studies of Japanese encephalitis in Japan. I. Introduction. Am. J. Trop. Med. Hyg. 1959, 8, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.S.; Gurley, E.S.; Pulliam, J.R. Rethinking Japanese Encephalitis Virus Transmission: A Framework for Implicating Host and Vector Species. PLoS Negl. Trop. Dis. 2015, 9, e0004074. [Google Scholar] [CrossRef]
- Maharaj, P.D.; Bosco-Lauth, A.M.; Langevin, S.A.; Anishchenko, M.; Bowen, R.A.; Reisen, W.K.; Brault, A.C. West Nile and St. Louis encephalitis viral genetic determinants of avian host competence. PLOS Neglected Trop. Dis. 2018, 12, e0006302. [Google Scholar] [CrossRef] [PubMed]
- García-Nicolás, O.; Braun, R.O.; Milona, P.; Lewandowska, M.; Dijkman, R.; Alves, M.P.; Summerfield, A. Targeting of the Nasal Mucosa by Japanese Encephalitis Virus for Non-Vector-Borne Transmission. J. Virol. 2018, 92, e01091–18. [Google Scholar] [CrossRef]
- Park, S.L.; Huang, Y.-J.S.; Vanlandingham, D.L. Re-Examining the Importance of Pigs in the Transmission of Japanese Encephalitis Virus. Pathogens 2022, 11, 575. [Google Scholar] [CrossRef]
- Lyons, A.C.; Huang, Y.-J.S.; Park, S.L.; Ayers, V.B.; Hettenbach, S.M.; Higgs, S.; McVey, D.S.; Noronha, L.; Hsu, W.-W.; Vanlandingham, D.L. Shedding of Japanese Encephalitis Virus in Oral Fluid of Infected Swine. Vector-Borne Zoonotic Dis. 2018, 18, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, M.E.; García-Nicolás, O.; Brechbühl, D.; Python, S.; Zumkehr, B.; Nougairede, A.; Charrel, R.N.; Posthaus, H.; Oevermann, A.; Summerfield, A. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat. Commun. 2016, 7, 10832. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Palinski, R.; Xu, Y.; Wang, Q.; Cao, S.; Geng, Y.; Zhao, Q.; Wen, Y.; Huang, X.; Yan, Q.; et al. Aerosol and Contact Transmission Following Intranasal Infection of Mice with Japanese Encephalitis Virus. Viruses 2019, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- https://www.nass.usda.gov/Newsroom/2022/03-30-2022.php.
- https://nppc.org/the-pork-industry/.
- Pires, A.F.A.; Peterson, A.; Baron, J.N.; Adams, R.; Martínez-López, B.; Moore, D. Small-scale and backyard livestock owners needs assessment in the western United States. PLOS ONE 2019, 14, e0212372. [Google Scholar] [CrossRef] [PubMed]
- Nidaira, M.; Taira, K.; Itokazu, K.; Kudaka, J.; Nakamura, M.; Ohno, A.; Takasaki, T. Survey of the Antibody against Japanese Encephalitis Virus in Ryukyu Wild Boars (Sus scrofa riukiuanus) in Okinawa, Japan. Jpn. J. Infect. Dis. 2007, 60, 309–311. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.L.; Burdett, C.L.; Farnsworth, M.L.; Lutman, M.W.; Theobald, D.M.; Riggs, P.D.; Grear, D.A.; Miller, R.S. Modeling and Mapping the Probability of Occurrence of Invasive Wild Pigs across the Contiguous United States. PLOS ONE 2015, 10, e0133771. [Google Scholar] [CrossRef] [PubMed]
- https://www.theguardian.com/environment/2021/nov/24/pig-patrol-amsterdam-airports-innovative-approach-to-flight-safety.
- Platonov, A.E.; Rossi, G.; Karan, L.S.; Mironov, K.O.; Busani, L.; Rezza, G. Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy. Eurosurveillance 2012, 17, 20241. [Google Scholar] [CrossRef]
- Ravanini, P.; Huhtamo, E.; Ilaria, V.; Crobu, M.G.; Nicosia, A.M.; Servino, L.; Rivasi, F.; Allegrini, S.; Miglio, U.; Magri, A.; et al. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Eurosurveillance 2012, 17, 20221. [Google Scholar] [CrossRef]
- https://www.farmbiosecurity.com.au/wp-content/uploads/2023/05/IntegratedMosquitoManagementPrinciplesforPiggeries_v3.pdf.
- Work, T.H. On the Japanese B—West Nile Virus Complex or an Arbovirus Problem of Six Continents *. Am. J. Trop. Med. Hyg. 1971, 20, 169–186. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.; Mason, G.; Bowen, R. Pathogenesis of Japanese Encephalitis Virus Infection in a Golden Hamster Model and Evaluation of Flavivirus Cross-Protective Immunity. Am. J. Trop. Med. Hyg. 2011, 84, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Goverdhan, M.K.; Kulkarni, A.B.; Gupta, A.K.; Tupe, C.D.; Rodrigues, J.J. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. . 1992, 36, 277–283. [Google Scholar]
- Nemeth, N.M.; Bosco-Lauth, A.M.; Bowen, R.A. Cross-protection between West Nile and Japanese encephalitis viruses in red-winged blackbirds (Agelaius phoeniceus). Avian Dis. 2009, 53, 421–425. [Google Scholar] [CrossRef]
- Reisen, W.K.; Lothrop, H.D.; Wheeler, S.S.; Kennsington, M.; Gutierrez, A.; Fang, Y.; Garcia, S.; Lothrop, B. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003-2006. J Med Entomol. 2008, 45, 494–508. [Google Scholar]
- Bae, W.; Kim, J.H.; Kim, J.; Lee, J.; Hwang, E.S. Changes of epidemiological characteristics of Japanese encephalitis viral infection and birds as a potential viral transmitter in Korea. J. Korean Med. Sci. 2018, 33, e70. [Google Scholar] [CrossRef] [PubMed]
- Diptyanusa, A.; Herini, E.S.; Indarjulianto, S.; Satoto, T.B.T. Estimation of Japanese encephalitis virus infection prevalence in mosquitoes and bats through nationwide sentinel surveillance in Indonesia. PLOS ONE 2022, 17, e0275647. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Guidelines for surveillance, prevention, and control of West Nile virus infection--United States. MMWR Morb Mortal Wkly Rep. 2000, 49, 25–28. [Google Scholar]
- Lindsey, N.P.; Brown, J.A.; Kightlinger, L.; Rosenberg, L.; Fischer, M.; The ArboNET Evaluation Working Group State Health Department Perceived Utility of and Satisfaction with ArboNET, the U. S. National Arboviral Surveillance System. Public Heal. Rep. 2012, 127, 383–390. [Google Scholar] [CrossRef]
- Fan, Y.-C.; Chen, Y.-Y.; Chen, J.-M.; Huang, C.; Huang, M.; Chiou, S.-S. Effectiveness of Live-Attenuated Genotype III Japanese Encephalitis Viral Vaccine against Circulating Genotype I Viruses in Swine. Viruses 2022, 14, 114. [Google Scholar] [CrossRef]
- Nah, J.-J.; Yang, D.-K.; Kim, H.-H.; Song, J.-Y. The present and future of veterinary vaccines for Japanese encephalitis in Korea. Clin. Exp. Vaccine Res. 2015, 4, 130–136. [Google Scholar] [CrossRef]
- Bandrick, M.; Ariza-Nieto, C.; Baidoo, S.K.; Molitor, T.W. Colostral antibody-mediated and cell-mediated immunity contributes to innate and antigen-specific immunity in piglets. Dev. Comp. Immunol. 2014, 43, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Markoff, L. Points to consider in the development of a surrogate for efficacy of novel Japanese encephalitis virus vaccines. Vaccine 2000, 18, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Van Gessel, Y.; Klade, C.S.; Putnak, R.; Formica, A.; Krasaesub, S.; Spruth, M.; Cena, B.; Tungtaeng, A.; Gettayacamin, M.; Dewasthaly, S. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO®) induced neutralizing antibody titers. Vaccine 2011, 29, 5925–5931. [Google Scholar] [CrossRef] [PubMed]
- Hombach, J.; Solomon, T.; Kurane, I.; Jacobson, J.; Wood, D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine 2005, 23, 5205–5211. [Google Scholar] [CrossRef]
- Food & Drug Administration. Summary Basis of Regulatory Action, IXIARO, 12 April, 2018.
- Fischer, M.; Lindsey, N.; Staples, J.E.; Hills, S.; Centers for Disease Control and Prevention (CDC). Japanese encephalitis vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–27. [Google Scholar]
- CDC. Use of Japanese encephalitis vaccine in children: recommendations of the Advisory Committee on Immunization Practices, 2013. MMWR Morb Mortal Wkly Rep 2013, 62, 898–900. [Google Scholar]
- Putri, W.C.W.S.; Sawitri, A.A.S.; Yuliyatni, P.C.D.; Ariawan, I.M.D.; Meyta, H.; Labiba, S.U.; Suwarba, I.G.N.M.; Sutarsa, I.N. Cost-effectiveness analysis of Japanese Encephalitis (JE) vaccination program in Bali Province, Indonesia. Vaccine 2023, 41, 6930–6940. [Google Scholar] [CrossRef]
- Carias, C.; Hills, S.L.; Kahn, E.B.; Adhikari, B.B.; Fischer, M.; Meltzer, M.I. Comparative economic analysis of strategies for Japanese encephalitis vaccination of U.S. travelers. Vaccine 2020, 38, 3351–3357. [Google Scholar] [CrossRef]
- Amat, C.; Bellanger, A.; Bozon, F.; Léger, R.; Gbaguidi-Haore, H.; Marguet, P. Current practice of French health professionals regarding Japanese encephalitis vaccination. 2019, 49, 602–606. [CrossRef]
- Mills, D.J.; Lau, C.L.; Furuya-Kanamori, L. Low uptake of Japanese encephalitis vaccination among Australian travellers. J. Travel Med. 2020, 28. [Google Scholar] [CrossRef]
- Hills, S.L.; Walter, E.B.; Atmar, R.L.; Fischer, M.; Barnett, E.; Barrett, A.; Bocchini, J.A.; Chen, L.; Deussing, E.; Fink, D.; et al. Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices. MMWR. Recomm. Rep. 2019, 68, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine 2010, 28, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Package-Insert-and-Patient-Information-IXIARO_0PDF (www.fda.gov).
- Jones, T. ChimeriVax-JE. Acambis. Curr Opin Investig Drugs. 2003, 4, 1019–1022. [Google Scholar] [PubMed]
- Appaiahgari, M.B.; Vrati, S. Clinical development of IMOJEV®—a recombinant Japanese encephalitis chimeric vaccine (JE-CV). Expert Opin. Biol. Ther. 2012, 12, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Chokephaibulkit, K.; Houillon, G.; Feroldi, E.; Bouckenooghe, A. Safety and immunogenicity of a live attenuated Japanese encephalitis chimeric virus vaccine (IMOJEV®) in children. Expert Rev. Vaccines 2015, 15, 153–166. [Google Scholar] [CrossRef] [PubMed]
- https://www.substipharm.com/biologics-vaccins/.
- van den Elsen, K.; Chew, B.L.A.; Ho, J.S.; Luo, D. Flavivirus nonstructural proteins and replication complexes as antiviral drug targets. Curr Opin Virol. 2023, 59, 101305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



