Submitted:
08 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
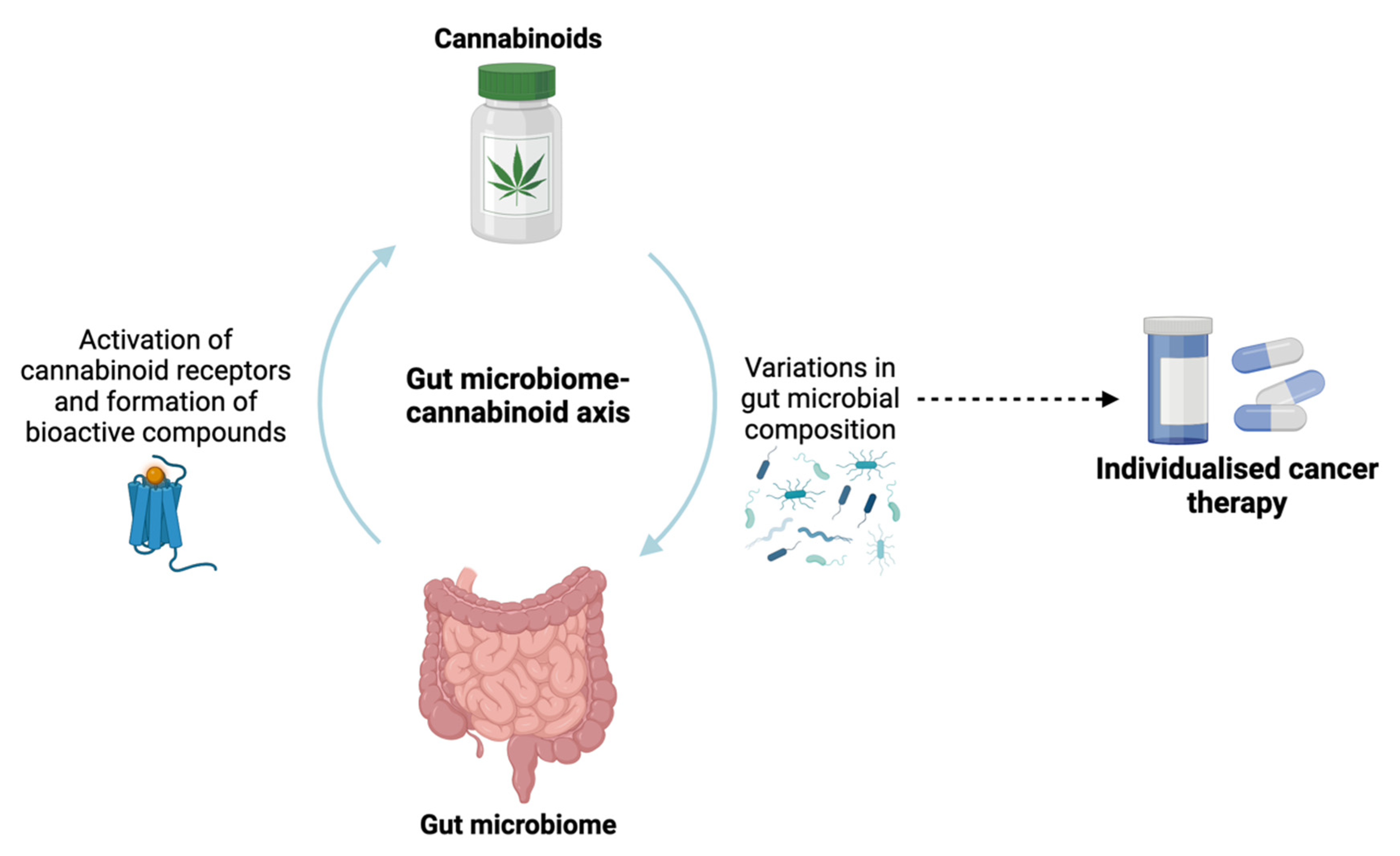
1.1. Cancer and gut microbiota
1.2. Cannabis Overview
2. Cannabis in cancer research
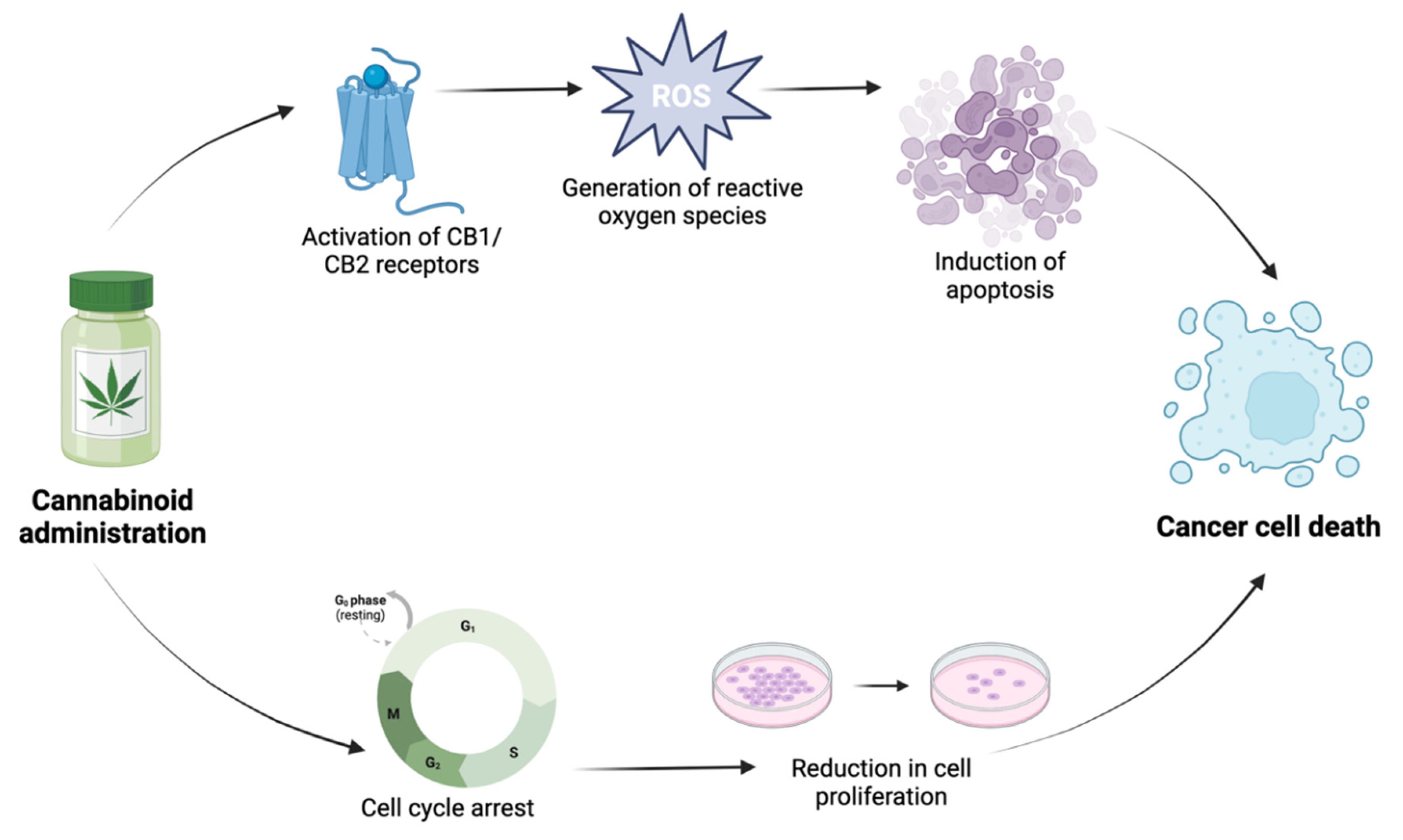
2.1. Anticancer properties of cannabinoids
2.1.1. Induction of apoptosis
2.1.2. Suppression of Angiogenesis
2.1.3. Prevention of metastasis
2.2. Potential role of gut microbiota in modulating the anticancer effects of cannabis
2.3. Implications for Cancer Treatment
3. Gut Microbiome and cannabis interactions
3.1. The ECS and Gut Microbiota
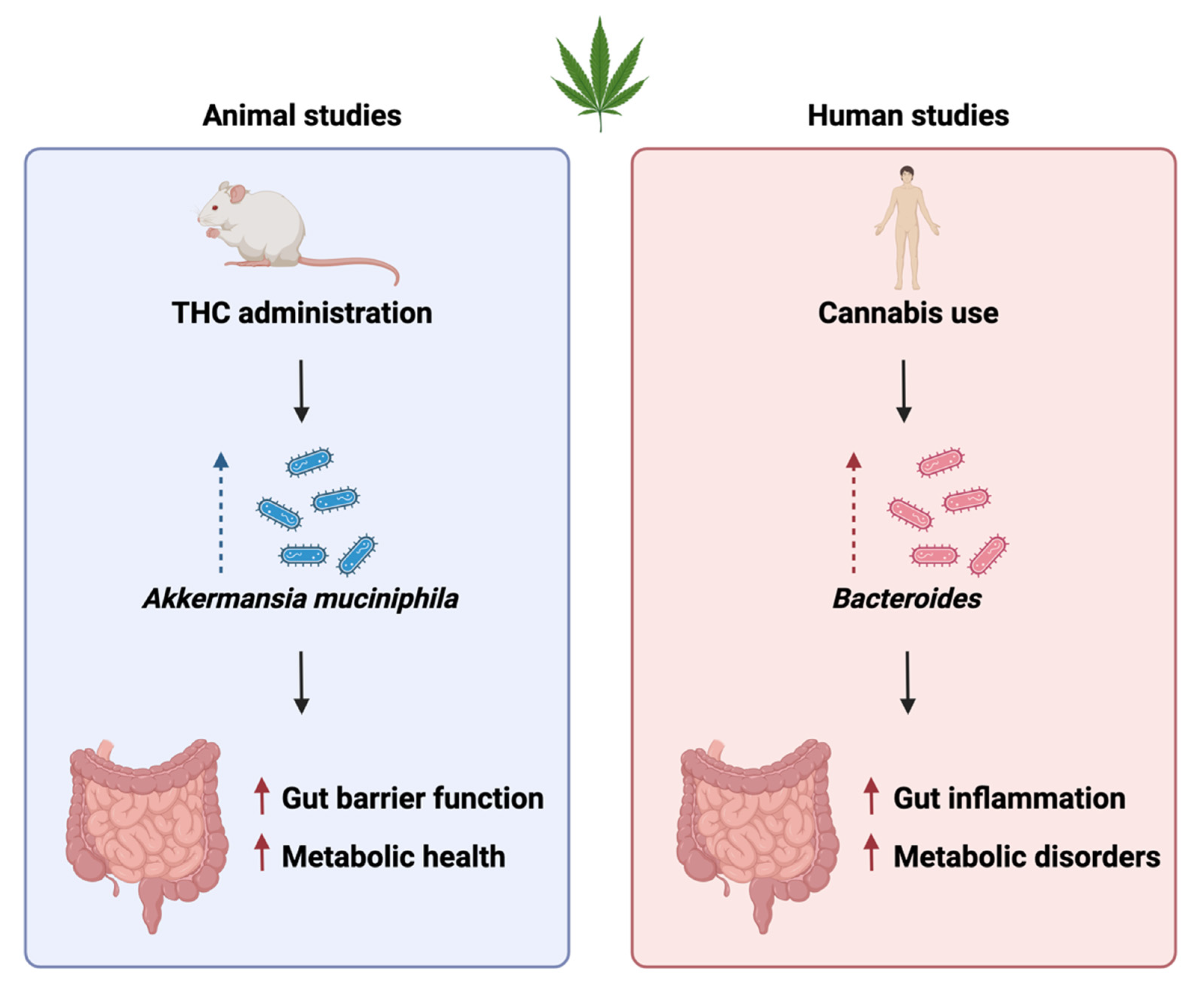
3.2. Impact of Cannabis administration on gut microbiota composition
3.3. Gut microbiota-mediated metabolism of cannabinoids
3.4. Influence of gut microbiota on cannabis pharmacology
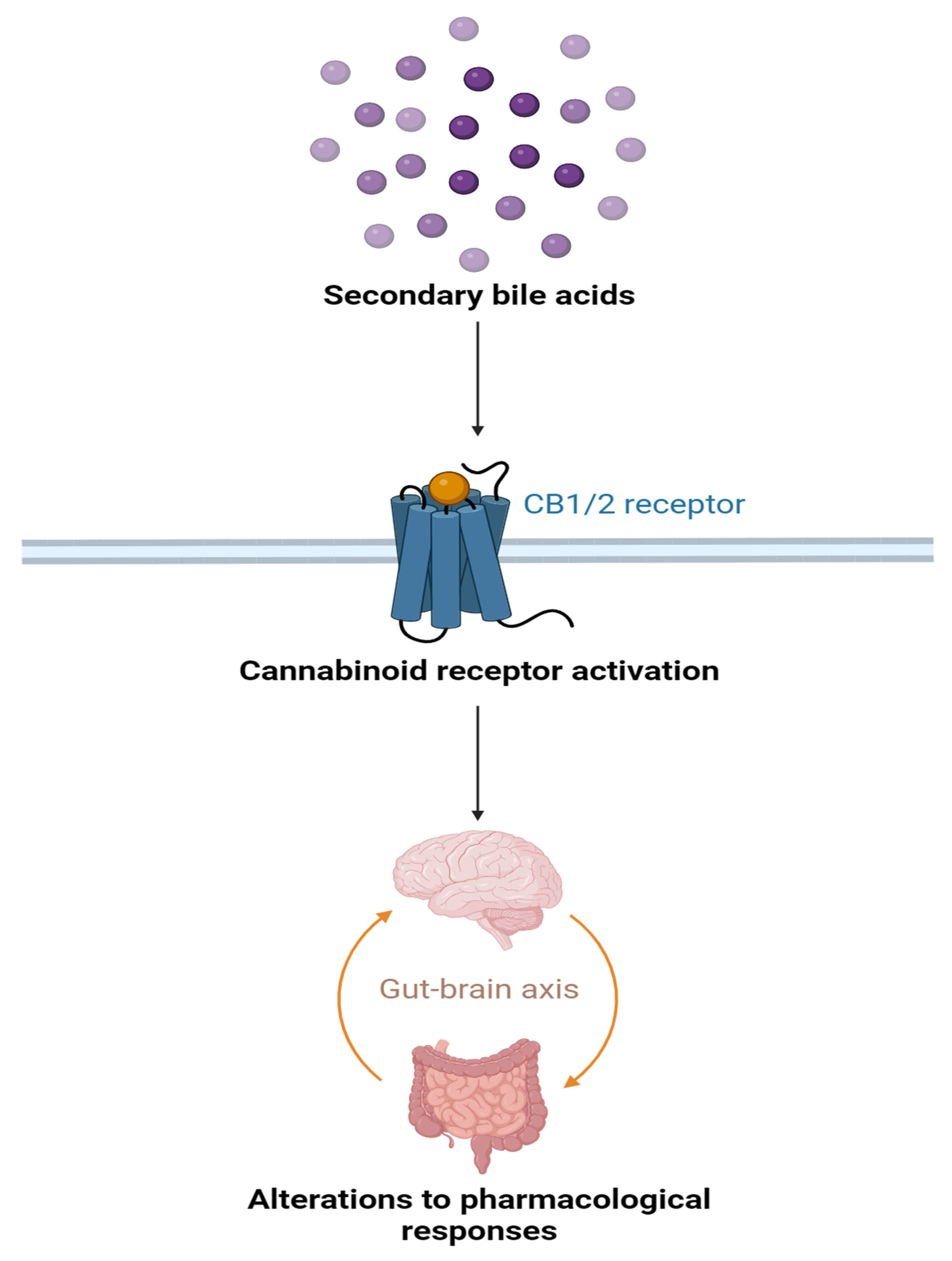
3.5. Modulation of Cannabinoid Receptor Activation
3.6. Gut Microbiota and Cannabinoid-Induced Gut Motility
4. Gut Microbiome, Cannabis, and Cancer: Potential Interplay
4.1. Gut Microbiome-Mediated Modulation of Immune Responses
4.2. The Bidirectional Relationship: Cannabis, Gut Microbiota, and Cancer
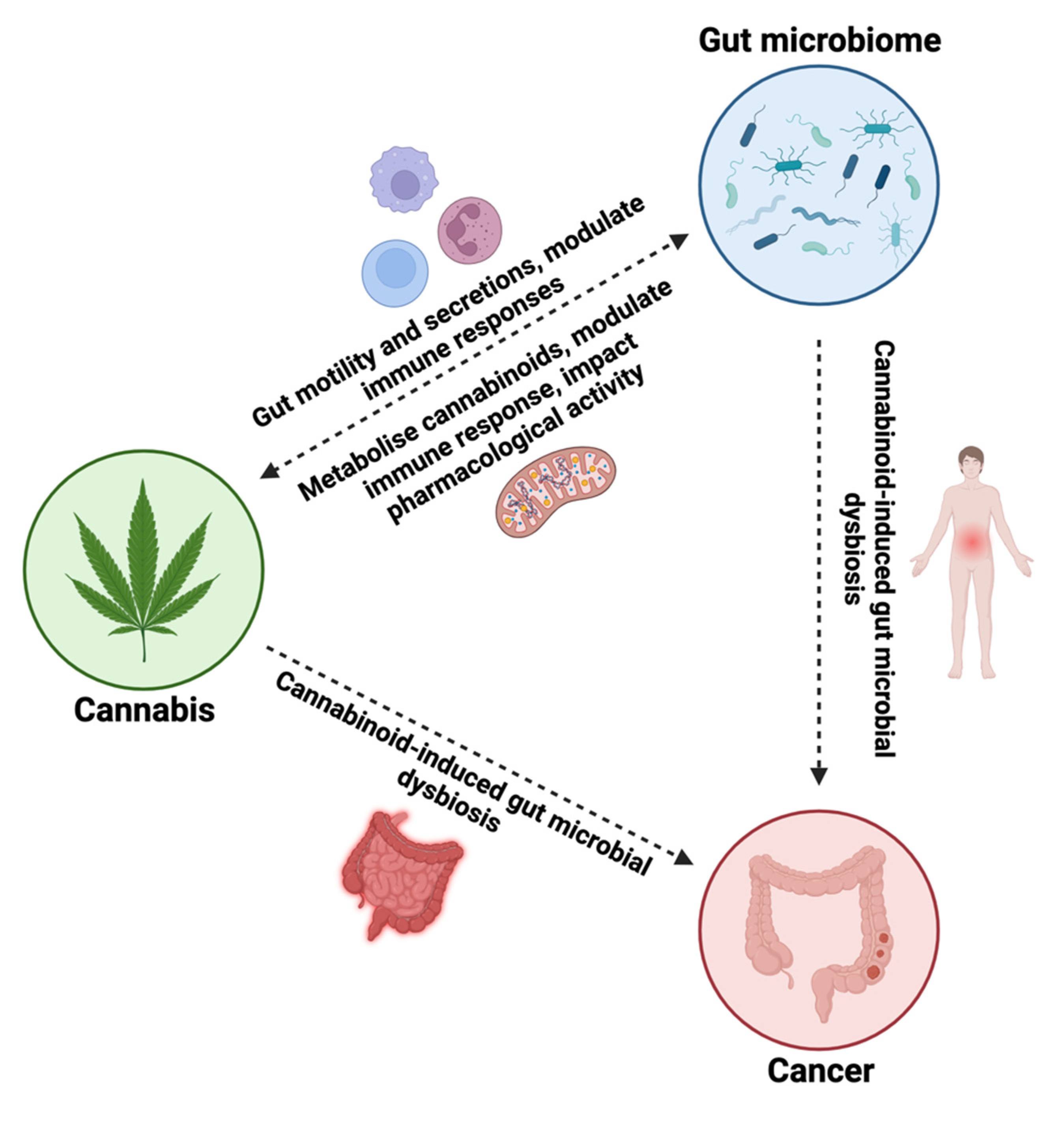
4.3. Modulation of the gut microbiota to potentially enhance the effects of cannabis in cancer therapy
4.3.1. Cancer pain and chemotherapy side effects management
5. Safety considerations and clinical trials
5.1. Personalized medicine and gut microbiota profiling
6. Conclusions and Future Directions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abrams, D. I., & Guzman, M. (2015). Cannabis in cancer care. Clinical Pharmacology & Therapeutics, 97(6), 575-586.
- Adeyeye, S. A. O., & Ashaolu, T. J. (2021). Heterocyclic amine formation and mitigation in processed meat and meat products: A Mini-Review. Journal of Food Protection, 84(11), 1868-1877. [PubMed]
- Ahmad Kamal, N. H., Selamat, J., & Sanny, M. (2018). Simultaneous formation of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines (HCAs) in gas-grilled beef satay at different temperatures. Food Additives & Contaminants: Part A, 35(5), 848-869. [CrossRef]
- Al-Ghezi, Z. Z., Miranda, K., Nagarkatti, M., & Nagarkatti, P. S. (2019). Combination of cannabinoids, Δ9-tetrahydrocannabinol and cannabidiol, ameliorates experimental multiple sclerosis by suppressing neuroinflammation through regulation of miRNA-mediated signaling pathways. Frontiers in immunology, 10, 1921.
- Alhouayek, M., Lambert, D. M., Delzenne, N. M., Cani, P. D., & Muccioli, G. G. (2011). Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. The FASEB journal, 25(8), 2711-2721. [CrossRef]
- Alsherbiny, M. A., Bhuyan, D. J., Low, M. N., Chang, D., & Li, C. G. (2021). Synergistic Interactions of Cannabidiol with Chemotherapeutic Drugs in MCF7 Cells: Mode of Interaction and Proteomics Analysis of Mechanisms. International Journal of Molecular Sciences, 22(18), 10103. [CrossRef]
- Andre, C. M., Hausman, J.-F., & Guerriero, G. (2016). Cannabis sativa: the plant of the thousand and one molecules. Frontiers in plant science, 19. [CrossRef]
- Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A., & Gordon, J. I. (2005). Host-bacterial mutualism in the human intestine. Science, 307(5717), 1915-1920. [CrossRef]
- Bar-Sela, G., Vorobeichik, M., Drawsheh, S., Omer, A., Goldberg, V., & Muller, E. (2013). The Medical Necessity for Medicinal Cannabis: Prospective, Observational Study Evaluating the Treatment in Cancer Patients on Supportive or Palliative Care. Evidence-Based Complementary and Alternative Medicine, 2013, 510392. [CrossRef]
- Blázquez, C., Casanova, M. L., Planas, A., Gómez del Pulgar, T., Villanueva, C., Fernández-Aceñero, M. J., Aragonés, J., Huffman, J. W., Jorcano, J. L., & Guzmán, M. (2003). Inhibition of tumor angiogenesis by cannabinoids. The FASEB journal, 17(3), 1-16. [CrossRef]
- Bonnie, R. J., Kesselheim, A. S., & Clark, D. J. (2017). Both urgency and balance needed in addressing opioid epidemic: a report from the National Academies of Sciences, Engineering, and Medicine. Jama, 318(5), 423-424.
- Borrelli, F., Pagano, E., Romano, B., Panzera, S., Maiello, F., Coppola, D., De Petrocellis, L., Buono, L., Orlando, P., & Izzo, A. A. (2014). Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis, 35(12), 2787-2797. [CrossRef]
- Cameron, C., Watson, D., & Robinson, J. (2014). Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder–related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. Journal of clinical psychopharmacology, 34(5), 559.
- Cani, P. D., & Knauf, C. (2016). How gut microbes talk to organs: The role of endocrine and nervous routes. Molecular Metabolism, 5(9), 743-752. [CrossRef] [PubMed]
- Cani, P. D., Possemiers, S., Van de Wiele, T., Guiot, Y., Everard, A., Rottier, O., Geurts, L., Naslain, D., Neyrinck, A., & Lambert, D. M. (2009). Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut, 58(8), 1091-1103. [CrossRef]
- Charitos, I. A., Gagliano-Candela, R., Santacroce, L., & Bottalico, L. (2021). The Cannabis spread throughout the continents and its therapeutic use in history. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders), 21(3), 407-417. [CrossRef]
- Cluny, N. L., Keenan, C. M., Duncan, M., Fox, A., Lutz, B., & Sharkey, K. A. (2010). Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl) methanone (SAB378), a peripherally restricted cannabinoid CB1/CB2 receptor agonist, inhibits gastrointestinal motility but has no effect on experimental colitis in mice. Journal of Pharmacology and Experimental Therapeutics, 334(3), 973-980. [CrossRef]
- Crini, G., Lichtfouse, E., Chanet, G., & Morin-Crini, N. (2020). Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environmental Chemistry Letters, 18(5), 1451-1476. [CrossRef]
- De Filippis, D., Esposito, G., Cirillo, C., Cipriano, M., De Winter, B. Y., Scuderi, C., Sarnelli, G., Cuomo, R., Steardo, L., De Man, J. G., & Iuvone, T. (2011). Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLOS ONE, 6(12), e28159. [CrossRef] [PubMed]
- de Vadder, F., & Mithieux, G. (2018). Gut-brain signaling in energy homeostasis: the unexpected role of microbiota-derived succinate. Journal of Endocrinology, 236(2), R105-R108. [CrossRef]
- Di Marzo, V. (2020). The endocannabinoidome as a substrate for noneuphoric phytocannabinoid action and gut microbiome dysfunction in neuropsychiatric disorders. Dialogues in Clinical Neuroscience, 22(3), 259-269. [CrossRef]
- Di Marzo, V. (2022). The endocannabinoidome as a substrate for noneuphoric phytocannabinoid action and gut microbiome dysfunction in neuropsychiatric disorders. Dialogues in Clinical Neuroscience.
- Di Marzo, V., Ligresti, A., & Cristino, L. (2009). The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. International journal of obesity, 33(2), S18-S24. [CrossRef]
- Dikeocha, I. J., Al-Kabsi, A. M., Miftahussurur, M., & Alshawsh, M. A. (2022). Pharmacomicrobiomics: Influence of gut microbiota on drug and xenobiotic metabolism. The FASEB journal, 36(6), e22350. [CrossRef]
- Donaldson, M. S. (2004). Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutrition journal, 3(1), 1-21. [CrossRef]
- Duvall, C. (2014). Cannabis. Reaktion Books.
- Eladwy, R. A., Vu, H. T., Shah, R., Li, C. G., Chang, D., & Bhuyan, D. J. (2023). The Fight against the Carcinogenic Epstein-Barr Virus: Gut Microbiota, Natural Medicines, and Beyond. International Journal of Molecular Sciences, 24(2), 1716. [CrossRef]
- ElSohly, M., & Gul, W. (2014). Constituents of Cannabis sativa. Handbook of cannabis, 3(1093), 187-188.
- Enright, E. F., Gahan, C. G., Joyce, S. A., & Griffin, B. T. (2016). Focus: microbiome: the impact of the gut microbiota on drug metabolism and clinical outcome. The Yale journal of biology and medicine, 89(3), 375.
- Geller, L. T., Barzily-Rokni, M., Danino, T., Jonas, O. H., Shental, N., Nejman, D., Gavert, N., Zwang, Y., Cooper, Z. A., & Shee, K. (2017). Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science, 357(6356), 1156-1160. [CrossRef]
- Gopalakrishnan, V., Spencer, C. N., Nezi, L., Reuben, A., Andrews, M., Karpinets, T., Prieto, P., Vicente, D., Hoffman, K., & Wei, S. C. (2018). Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science, 359(6371), 97-103. [CrossRef]
- Govari, M., & Pexara, A. (2015). Nitrates and Nitrites in meat products. Journal of the Hellenic veterinary medical society, 66(3), 127-140.
- Guida, F., Turco, F., Iannotta, M., De Gregorio, D., Palumbo, I., Sarnelli, G., Furiano, A., Napolitano, F., Boccella, S., Luongo, L., Mazzitelli, M., Usiello, A., De Filippis, F., Iannotti, F. A., Piscitelli, F., Ercolini, D., de Novellis, V., Di Marzo, V., Cuomo, R., & Maione, S. (2018). Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain, Behavior, and Immunity, 67, 230-245. [CrossRef] [PubMed]
- Hazekamp, A. (2008). Cannabis review. Department of Plant Metobolomics, Leiden University, 2009.
- Hazekamp, A., Fischedick, J. T., Díez, M., Lubbe, A., & Ruhaak, R. L. (2010). 3.24—Chemistry of Cannabis. Comprehensive natural products II, 3, 1033-1084.
- Herrera, B., Carracedo, A., Diez-Zaera, M., del Pulgar, T. G., Guzmán, M., & Velasco, G. (2006). The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Experimental cell research, 312(11), 2121-2131. [CrossRef]
- Holland, J. (2010). The pot book: A complete guide to cannabis. Simon and Schuster.
- Honikel, K.-O. (2008). The use and control of nitrate and nitrite for the processing of meat products. Meat science, 78(1-2), 68-76. [CrossRef]
- Hryhorowicz, S., Kaczmarek-Ryś, M., Zielińska, A., Scott, R. J., Słomski, R., & Pławski, A. (2021). Endocannabinoid system as a promising therapeutic target in inflammatory bowel disease–a systematic review. Frontiers in immunology, 12, 790803. [CrossRef]
- Huang, J., Jiang, Z., Wang, Y., Fan, X., Cai, J., Yao, X., Liu, L., Huang, J., He, J., & Xie, C. (2020). Modulation of gut microbiota to overcome resistance to immune checkpoint blockade in cancer immunotherapy. Current Opinion in Pharmacology, 54, 1-10. [CrossRef]
- Huestis, M. A., Solimini, R., Pichini, S., Pacifici, R., Carlier, J., & Busardò, F. P. (2019). Cannabidiol adverse effects and toxicity. Current neuropharmacology, 17(10), 974-989. [CrossRef]
- Ibrahim, I., Syamala, S., Ayariga, J. A., Xu, J., Robertson, B. K., Meenakshisundaram, S., & Ajayi, O. S. (2022). Modulatory Effect of Gut Microbiota on the Gut-Brain, Gut-Bone Axes, and the Impact of Cannabinoids. Metabolites, 12(12), 1247. https://www.mdpi.com/2218-1989/12/12/1247. [CrossRef]
- Izzo, A. A., Capasso, R., Aviello, G., Borrelli, F., Romano, B., Piscitelli, F., Gallo, L., Capasso, F., Orlando, P., & Di Marzo, V. (2012). Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice [https://doi.org/10.1111/j.1476-5381.2012.01879.x]. British Journal of Pharmacology, 166(4), 1444-1460. [CrossRef] [PubMed]
- Izzo, A. A., & Sharkey, K. A. (2010). Cannabinoids and the gut: New developments and emerging concepts. Pharmacology & therapeutics, 126(1), 21-38. [CrossRef]
- Jasirwan, C. O. M., Lesmana, C. R. A., Hasan, I., Sulaiman, A. S., & Gani, R. A. (2019). The role of gut microbiota in non-alcoholic fatty liver disease: pathways of mechanisms. Bioscience of microbiota, food and health, 38(3), 81-88. [CrossRef]
- Jaye, K., Li, C. G., Chang, D., & Bhuyan, D. J. (2022). The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes, 14(1), 2038865. [CrossRef] [PubMed]
- Jew, S., AbuMweis, S. S., & Jones, P. J. (2009). Evolution of the human diet: linking our ancestral diet to modern functional foods as a means of chronic disease prevention. Journal of medicinal food, 12(5), 925-934. [CrossRef]
- Johnson, J. R., Burnell-Nugent, M., Lossignol, D., Ganae-Motan, E. D., Potts, R., & Fallon, M. T. (2010). Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. Journal of pain and symptom management, 39(2), 167-179.
- Johnson, J. R., Burnell-Nugent, M., Lossignol, D., Ganae-Motan, E. D., Potts, R., & Fallon, M. T. (2010). Multicenter, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study of the Efficacy, Safety, and Tolerability of THC:CBD Extract and THC Extract in Patients with Intractable Cancer-Related Pain. Journal of pain and symptom management, 39(2), 167-179. [CrossRef]
- Karoly, H. C., Mueller, R. L., Bidwell, L. C., & Hutchison, K. E. (2020). Cannabinoids and the microbiota–gut–brain axis: Emerging effects of cannabidiol and potential applications to alcohol use disorders. Alcoholism: Clinical and Experimental Research, 44(2), 340-353. [CrossRef]
- Key, T. J., Schatzkin, A., Willett, W. C., Allen, N. E., Spencer, E. A., & Travis, R. C. (2004). Diet, nutrition and the prevention of cancer. Public health nutrition, 7(1a), 187-200. [CrossRef]
- Klumpers, L. E., & Thacker, D. L. (2019). A brief background on cannabis: From plant to medical indications. Journal of AOAC International, 102(2), 412-420. [CrossRef]
- Koppel, N., Maini Rekdal, V., & Balskus, E. P. (2017). Chemical transformation of xenobiotics by the human gut microbiota. Science, 356(6344), eaag2770. [CrossRef]
- Kushi, L. H., Doyle, C., McCullough, M., Rock, C. L., Demark-Wahnefried, W., Bandera, E. V., Gapstur, S., Patel, A. V., Andrews, K., & Gansler, T. (2012). American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA: a cancer journal for clinicians, 62(1), 30-67.
- Laezza, C., Pagano, C., Navarra, G., Pastorino, O., Proto, M. C., Fiore, D., Piscopo, C., Gazzerro, P., & Bifulco, M. (2020). The endocannabinoid system: A target for cancer treatment. International Journal of Molecular Sciences, 21(3), 747. [CrossRef]
- Laezza, C., Pisanti, S., Crescenzi, E., & Bifulco, M. (2006). Anandamide inhibits Cdk2 and activates Chk1 leading to cell cycle arrest in human breast cancer cells. FEBS Letters, 580(26), 6076-6082. [CrossRef] [PubMed]
- Li, H., He, J., & Jia, W. (2016). The influence of gut microbiota on drug metabolism and toxicity. Expert Opin Drug Metab Toxicol, 12(1), 31-40. [CrossRef] [PubMed]
- Lloyd-Price, J., Abu-Ali, G., & Huttenhower, C. (2016). The healthy human microbiome. Genome medicine, 8(1), 1-11. [CrossRef]
- Lopez-Rodriguez, A. B., Siopi, E., Finn, D. P., Marchand-Leroux, C., Garcia-Segura, L. M., Jafarian-Tehrani, M., & Viveros, M.-P. (2015). CB1 and CB2 Cannabinoid Receptor Antagonists Prevent Minocycline-Induced Neuroprotection Following Traumatic Brain Injury in Mice. Cerebral Cortex, 25(1), 35-45. [CrossRef] [PubMed]
- Lucchetti, M., Kaminska, M., Oluwasegun, A. K., Mosig, A. S., & Wilmes, P. (2021). Emulating the gut–liver axis: Dissecting the microbiome’s effect on drug metabolism using multiorgan-on-chip models. Current Opinion in Endocrine and Metabolic Research, 18, 94-101. [CrossRef] [PubMed]
- Luo, Z., Fitting, S., Robinson, C., Benitez, A., Li, M., Wu, Y., Fu, X., Amato, D., Ning, W., & Funderburg, N. (2021). Chronic cannabis smoking-enriched oral pathobiont drives behavioral changes, macrophage infiltration, and increases β-amyloid protein production in the brain. EBioMedicine, 74. [CrossRef]
- Lyte, M., Vulchanova, L., & Brown, D. R. (2011). Stress at the intestinal surface: catecholamines and mucosa–bacteria interactions. Cell and tissue research, 343, 23-32. [CrossRef]
- Maccarrone, M., Bab, I., Bíró, T., Cabral, G. A., Dey, S. K., Di Marzo, V., Konje, J. C., Kunos, G., Mechoulam, R., & Pacher, P. (2015). Endocannabinoid signaling at the periphery: 50 years after THC. Trends in pharmacological sciences, 36(5), 277-296. [CrossRef]
- Malach, M., Kovalchuk, I., & Kovalchuk, O. (2022). Medical Cannabis in Pediatric Oncology: Friend or Foe? Pharmaceuticals, 15(3), 359.
- Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D., Hirschfield, G. M., Hold, G., Quraishi, M. N., Kinross, J., Smidt, H., & Tuohy, K. M. (2016). The gut microbiota and host health: a new clinical frontier. Gut, 65(2), 330-339. [CrossRef]
- May Soe, T., Thunnicha, O., Szaye Rawicha, H., Tanawin, N., Ananya, J., Nattiya, H., & Krit, P. (2023). Cannabis-Microbiome Interactions in Varied Clinical Contexts: A Comprehensive Systematic Review. medRxiv, 2022.2012.2031.22284080. [CrossRef]
- McDew-White, M., Lee, E., Premadasa, L. S., Alvarez, X., Okeoma, C. M., & Mohan, M. (2023). Cannabinoids modulate the microbiota–gut–brain axis in HIV/SIV infection by reducing neuroinflammation and dysbiosis while concurrently elevating endocannabinoid and indole-3-propionate levels. Journal of Neuroinflammation, 20(1), 62. [CrossRef]
- Minakata, K., Yamagishi, I., Nozawa, H., Hasegawa, K., Suzuki, M., Gonmori, K., Suzuki, O., & Watanabe, K. (2017). Sensitive identification and quantitation of parent forms of six synthetic cannabinoids in urine samples of human cadavers by liquid chromatography–tandem mass spectrometry. Forensic Toxicology, 35, 275-283. [CrossRef]
- Morales, P., & Jagerovic, N. (2021). Cannabinoid-based Anti-cancer Strategies: Slowly Approaching the Bedside. Frontiers in Anti-Cancer Drug Discovery: Volume 12, 12, 1.
- Mou, Y., Du, Y., Zhou, L., Yue, J., Hu, X., Liu, Y., Chen, S., Lin, X., Zhang, G., & Xiao, H. (2022). Gut microbiota interact with the brain through systemic chronic inflammation: Implications on neuroinflammation, neurodegeneration, and aging. Frontiers in immunology, 13, 796288. [CrossRef]
- Muccioli, G. G., Naslain, D., Bäckhed, F., Reigstad, C. S., Lambert, D. M., Delzenne, N. M., & Cani, P. D. (2010). The endocannabinoid system links gut microbiota to adipogenesis. Molecular Systems Biology, 6(1), 392. [CrossRef] [PubMed]
- Neuhouser, M. L. (2019). The importance of healthy dietary patterns in chronic disease prevention. Nutrition Research, 70, 3-6. [CrossRef]
- Nguyen, T. L. A., Vieira-Silva, S., Liston, A., & Raes, J. (2015). How informative is the mouse for human gut microbiota research? Disease models & mechanisms, 8(1), 1-16.
- Pacher, P., Bátkai, S., & Kunos, G. (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological reviews, 58(3), 389-462. [CrossRef]
- Pant, A., Maiti, T. K., Mahajan, D., & Das, B. (2023). Human Gut Microbiota and Drug Metabolism. Microb Ecol, 86(1), 97-111. [CrossRef] [PubMed]
- Pertwee, R. G. (2014). Handbook of cannabis. Oxford University Press, USA.
- Pisanti, S., Malfitano, A. M., Ciaglia, E., Lamberti, A., Ranieri, R., Cuomo, G., Abate, M., Faggiana, G., Proto, M. C., & Fiore, D. (2017). Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacology & therapeutics, 175, 133-150. [CrossRef]
- Platten, M., Nollen, E. A., Röhrig, U. F., Fallarino, F., & Opitz, C. A. (2019). Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nature Reviews Drug Discovery, 18(5), 379-401. [CrossRef]
- Pooja, D., Chinmayi, D., Kajal, G., & Rajesh, S. (2022). Exploring Drug Metabolism by the Gut Microbiota: Modes of Metabolism and Experimental Approaches. Drug Metabolism and Disposition, 50(3), 224. [CrossRef]
- Portenoy, R. K., Ganae-Motan, E. D., Allende, S., Yanagihara, R., Shaiova, L., Weinstein, S., McQuade, R., Wright, S., & Fallon, M. T. (2012). Nabiximols for Opioid-Treated Cancer Patients With Poorly-Controlled Chronic Pain: A Randomized, Placebo-Controlled, Graded-Dose Trial. The Journal of Pain, 13(5), 438-449. [CrossRef]
- Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., Nielsen, T., Pons, N., Levenez, F., & Yamada, T. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. nature, 464(7285), 59-65. [CrossRef]
- Ramer, R., & Hinz, B. (2008). Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. JNCI: Journal of the National Cancer Institute, 100(1), 59-69. [CrossRef]
- Ridlon, J. M., Kang, D.-J., & Hylemon, P. B. (2006). Bile salt biotransformations by human intestinal bacteria. Journal of lipid research, 47(2), 241-259. [CrossRef]
- Roberto, D., Klotz, L. H., & Venkateswaran, V. (2019). Cannabinoid WIN 55,212-2 induces cell cycle arrest and apoptosis, and inhibits proliferation, migration, invasion, and tumor growth in prostate cancer in a cannabinoid-receptor 2 dependent manner. The Prostate, 79(2), 151-159. [CrossRef]
- Round, J. L., & Mazmanian, S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nature reviews immunology, 9(5), 313-323. [CrossRef]
- Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P., Alou, M. T., Daillère, R., Fluckiger, A., Messaoudene, M., Rauber, C., & Roberti, M. P. (2018). Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science, 359(6371), 91-97. [CrossRef]
- Russo, E. (2005). Cannabis in India: ancient lore and modern medicine. In Cannabinoids as therapeutics (pp. 1-22). Springer. [CrossRef]
- Russo, E. B., & Marcu, J. (2017). Cannabis pharmacology: the usual suspects and a few promising leads. Advances in pharmacology, 80, 67-134. [CrossRef]
- Sarfaraz, S., Adhami, V. M., Syed, D. N., Afaq, F., & Mukhtar, H. (2008). Cannabinoids for cancer treatment: progress and promise. Cancer research, 68(2), 339-342. [CrossRef]
- Sarfaraz, S., Afaq, F., Adhami, V. M., Malik, A., & Mukhtar, H. (2006). Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. Journal of Biological Chemistry, 281(51), 39480-39491.
- Scheithauer, T. P., Dallinga-Thie, G. M., de Vos, W. M., Nieuwdorp, M., & van Raalte, D. H. (2016). Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Molecular Metabolism, 5(9), 759-770. [CrossRef]
- Schwabe, A. L. (2019). A multifaceted approach to address variation in Cannabis sativa. University of Northern Colorado.
- Schwabe, R. F., & Jobin, C. (2013). The microbiome and cancer. Nature Reviews Cancer, 13(11), 800-812.
- Sender, R., Fuchs, S., & Milo, R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS biology, 14(8), e1002533. [CrossRef]
- Sharon, G., Sampson, T. R., Geschwind, D. H., & Mazmanian, S. K. (2016). The central nervous system and the gut microbiome. Cell, 167(4), 915-932. [CrossRef]
- Solinas, M., Massi, P., Cantelmo, A., Cattaneo, M., Cammarota, R., Bartolini, D., Cinquina, V., Valenti, M., Vicentini, L., & Noonan, D. (2012). Cannabidiol inhibits angiogenesis by multiple mechanisms. British Journal of Pharmacology, 167(6), 1218-1231. [CrossRef]
- Srivastava, R. K., Lutz, B., & Ruiz de Azua, I. (2022). The Microbiome and Gut Endocannabinoid System in the Regulation of Stress Responses and Metabolism. Front Cell Neurosci, 16, 867267. [CrossRef]
- Storr, M. (2008). Yuce B, Andrews CN, Sharkey KA. The role of the endocannabinoid system in the pathophysiology and treatment of irritable bowel syndrome. Neurogastroenterol Motil, 20, 857-868. [CrossRef]
- Tareke, E., Rydberg, P., Karlsson, P., Eriksson, S., & Törnqvist, M. (2002). Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of Agricultural and Food Chemistry, 50(17), 4998-5006.
- Varsha, K. K., Nagarkatti, M., & Nagarkatti, P. (2022). Role of Gut Microbiota in Cannabinoid-Mediated Suppression of Inflammation. Adv Drug Alcohol Res, 2. [CrossRef]
- Velasco, G., Sánchez, C., & Guzmán, M. (2012). Towards the use of cannabinoids as antitumour agents. Nature Reviews Cancer, 12(6), 436-444. [CrossRef]
- Vétizou, M., Pitt, J. M., Daillère, R., Lepage, P., Waldschmitt, N., Flament, C., Rusakiewicz, S., Routy, B., Roberti, M. P., & Duong, C. P. (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science, 350(6264), 1079-1084. [CrossRef]
- Wallace, B. D., Roberts, A. B., Pollet, R. M., Ingle, J. D., Biernat, K. A., Pellock, S. J., Venkatesh, M. K., Guthrie, L., O’Neal, S. K., & Robinson, S. J. (2015). Structure and inhibition of microbiome β-glucuronidases essential to the alleviation of cancer drug toxicity. Chemistry & biology, 22(9), 1238-1249. [CrossRef]
- Wardill, H. R., Wooley, L. T., Bellas, O. M., Cao, K., Cross, C. B., van Dyk, M., Kichenadasse, G., Bowen, J. M., Zannettino, A. C. W., Shakib, S., Crawford, G. B., Boublik, J., Davis, M. M., Smid, S. D., & Price, T. J. (2023). Supporting gut health with medicinal cannabis in people with advanced cancer: potential benefits and challenges. British Journal of Cancer. [CrossRef]
- Watanabe, K., Itokawa, Y., Yamaori, S., Funahashi, T., Kimura, T., Kaji, T., Usami, N., & Yamamoto, I. (2007). Conversion of cannabidiol to Δ 9-tetrahydrocannabinol and related cannabinoids in artificial gastric juice, and their pharmacological effects in mice. Forensic Toxicology, 25, 16-21.
- Williams, M. T., & Hord, N. G. (2005). The role of dietary factors in cancer prevention.
- Yu, X., Wu, Z., Song, Z., Zhang, H., Zhan, J., Yu, H., Huang, H., Yang, B., Xie, L., & Dai, X. (2020). Single-anastomosis duodenal jejunal bypass improve glucose metabolism by regulating gut microbiota and short-chain fatty acids in Goto-Kakisaki rats. Frontiers in Microbiology, 11, 273. [CrossRef]
- Zackular, J., Baxter, N., Iverson, K., Sadler, W., Petrosino, J., Chen, G., & Schloss, P. (2013). The gut microbiome modulates colon tumorigenesis. mBio 4: e00692-13. In. [CrossRef]
- Zhuang, X., Xiong, L., Li, L., Li, M., & Chen, M. (2017). Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. Journal of gastroenterology and hepatology, 32(1), 28-38.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




