Submitted:
08 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Vegetative Growth Parameters

2.2. Plants Productivity Parameters.
2.3. Nutritional Composition of Tomato Fruit.
3. Discussion
4. Materials and Methods
4.1. Experimental Site and Greenhouse Management.
4.2. Biological Material and Procedure.
4.3. Experimental Design and Statistical Analysis.
4.4. Accidental Observation
4.5. Data Collection
4.5.1. Growth Measurements.
4.5.1.1. Plant Height (cm):
4.5.1.2. Leaf Expansion Rate (m²):
4.5.1.3. Leaf Area:
4.5.1.4. Total Leaf Count per Observation Date:
4.5.1.5. Leaf Fresh and Dry Weight:
4.5.1.6. Stem Diameter:
4.5.1.7. Average Distance between Two Leaves and Two Inflorescence:
4.5.2. Productivity and Chemical Parametors:
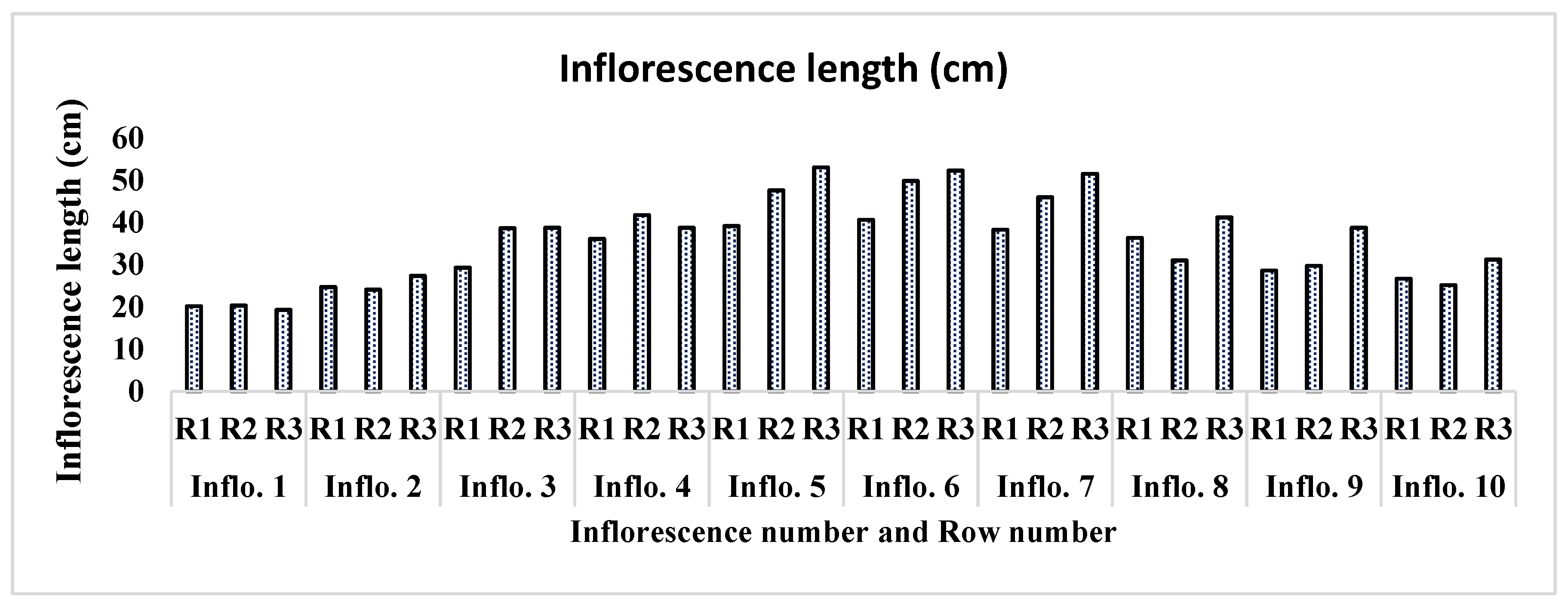
4.5.2.1. Inflorescence Length and Inflorescence Type:
4.5.2.2. Fruit Keeping Quality:
4.5.2.3. Fruit Size and Mass of Fruit/Inflorescence:
4.5.2.4. Fruit Firmness ((kg /cm²).
4.5.2.5. Fruit Fresh and Dry Matter:
4.5.2.6. Nitrate Contents (mg / kg) and Sugar contents (Brix %)
4.5.2.7. Titratable Acidity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamshiri, Shamshiri, R. R.; Jones, J.W.; Thorp, K.R.; Ahmad, D.; Man, H.C.; Taheri, S. Review of optimum temperature, humidity, and vapour pressure deficit for microclimate evaluation and control in greenhouse cultivation of tomato: A review. Int. Agrophysics 2018, 32, 287–302. [Google Scholar] [CrossRef]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bueno, R.P.; Romero-Gonzalez, R.; Gonzalez-Fernandes, M.J.; Guil-Guerrero, J.L. Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J. Sci. Food Agric. 2017, 97, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Bansal, R.; Rana, N.; Kumawat, S.; Bhatt, V.; Jadhav, P.; Deshmukh, R. Unexplored nutritive potential of tomato to combat global malnutrition. Crit. Rev. Food Sci. Nutr. 2022, 62, 1003–1034. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Cmaj 2000, 163, 739–744. [Google Scholar] [PubMed]
- Costa, J.M.; Heuvelink, E. Today's worldwide tomato production. Fruit Veg Tech 2007, 2007, 14–16. [Google Scholar]
- Branthôme, F.X. Worldwide (total fresh) tomato production exceeds 187 million tonnes in 2020. Tomato news. 2022.
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef]
- Ohashi, Y.; Murai, M.; Ishigami, Y.; Goto, E. Light-Intercepting Characteristics and Growth of Tomatoes Cultivated in a Greenhouse Using a Movable Bench System. Horticulturae 2022, 8, 60. [Google Scholar] [CrossRef]
- Viuda-Martos, M. , Sanchez-Zapata, E., Sayas-Barberá, E., Sendra, E., Pérez-Álvarez, J.A., Fernández-López, J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef]
- Omid, M.; Shafaei, A. Temperature and relative humidity changes inside greenhouse. Int. Agrophysics 2005, 19. [Google Scholar]
- Morison, J.I.L.; Lawlor, D.W. Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell & Environment, 1999, 22, 659–682. [Google Scholar]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.E.; Gallet-Budynek, A.; Hofmockel, K.S.; Bernhardt, E.S.; Billings, S.A.; Jackson, R.B.; Finzi, A.C. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 2011, 14, 349–357. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production product quality–a review, J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Mamatha, H.; Srinivasa Rao, N.K.; Laxman, R.H.; Shivashankara, K.S.; Bhatt, R.M.; Pavithra, K.C. Impact of elevated CO 2 on growth, physiology, yield, and quality of tomato (Lycopersicon esculentum Mill) cv. Arka Ashish. Photosynthetica 2014, 52, 519–528. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave CR, J. Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Qian, T.; Dieleman, J.A.; Elings, A.; De Gelder, A.; Marcelis, L.F.M. Response of tomato crop growth and development to a vertical temperature gradient in a semi-closed greenhouse. J. Hortic. Sci. Biotech. 2015, 90, 578–584. [Google Scholar] [CrossRef]
- Pearce, B.D.; Grange, R.I.; Hardwick, K. The growth of young tomato fruit, I. Effects of temperature and irradiance on fruit grown in controlled environments. J. Hortic. Sci. 1993, 68, 1–11. [Google Scholar] [CrossRef]
- Ayankojo, I.T.; Morgan, K.T. Increasing air temperatures and its effects on growth and productivity of tomato in South Florida. Plants 2020, 9, 1245. [Google Scholar] [CrossRef] [PubMed]
- Blom, T.J.; Ingratta, F.J. The effect of high pressure sodium lighting on the production of tomatoes, cucumbers and roses. III Int. Symp. Energy Prot. Cultiv. 1983, 148, 905–914. [Google Scholar] [CrossRef]
- Steinger, T.; Roy, B.A.; Stanton, M.L. Evolution in stressful environments II: Adaptive value and costs of plasticity in response to low light in Sinapis arvensis. J. Evol. Biol. 2003, 16, 313–323. [Google Scholar] [CrossRef]
- Fierro, A.; Gosselin, A.; Tremblay, N. Supplemental carbon dioxide and light improved tomato and pepper seedling growth and yield. HortScience 1994, 29, 152–154. [Google Scholar] [CrossRef]
- Suzuki, M.; Umeda, H.; Matsuo, S.; Kawasaki, Y.; Ahn, D.; Hamamoto, H.; Iwasaki, Y. Effects of relative humidity and nutrient supply on growth and nutrient uptake in greenhouse tomato production. Sci. Hortic. 2015, 187, 44–49. [Google Scholar] [CrossRef]
- Leonardi, C.; Guichard, S.; Bertin, N. High vapour pressure deficit influences growth, transpiration and quality of tomato fruits. Sci. Hortic. 2000, 84, 285–296. [Google Scholar] [CrossRef]
- Guichard, S.; Gary, C.; Leonardi, C.; Bertin, N. Analysis of growth and water relations of tomato fruits in relation to air vapor pressure deficit and plant fruit load. J. Plant Growth Regul. 2005, 24, 201–213. [Google Scholar] [CrossRef]
- Bakker, J.C. Analysis of humidity effects on growth and production of glasshouse fruit vegetables; Wageningen University and Research: 1991.
- Mitova, I.; Patamanska, G.; Gigova, A. analysis of vegetative and reproductive growth of greenhouse tomatoes cultivated under drip irrigation and fertigation with increasing fertilizer rates. Scientific Papers. Series E. Land Reclamation, 2021. [Google Scholar]
- Hicklenton, P.R.; Jolliffe, P.A. Effects of greenhouse CO2 enrichment on the yield and photosynthetic physiology of tomato plants. Can. J. Plant Sci. 1978, 58, 801–817. [Google Scholar] [CrossRef]
- Frantz, J.M.; Joly, R.J.; Mitchell, C.A. Intracanopy lighting influences radiation capture, productivity, and leaf senescence in cowpea canopies. J. Am. Soc. Hortic. Sci. 2000, 125, 694–701. [Google Scholar] [CrossRef]
- Verkerk, K. Temperature, Light and the Tomato. Meded. Van Landbouwhogesch. Te Wagening. /Nederl. 1955, 55, 175–224. [Google Scholar]
- Demers, D.A.; Dorais, M.; Wien, C.H.; Gosselin, A. Effects of supplemental light duration on greenhouse tomato (Lycopersicon esculentum Mill.) plants and fruit yields. Sci. Hortic. 1998, 74, 295–306. [Google Scholar] [CrossRef]
- Demers, D.A.; Gosselin, A. Growing greenhouse tomato and sweet pepper under supplemental lighting: Optimal photoperiod, negative effects of long photoperiod and their causes. IV Int. ISHS Symp. Artif. Light. 2000, 580, 83–88. [Google Scholar] [CrossRef]
- Hurd, R.G. Long-day effects on growth and flower initiation of tomato plants in low light. Ann. Appl. Biol. 1973, 73, 221–228. [Google Scholar] [CrossRef]
- Suyanto, H.; Rupiasih, N.N.; Handayani, D. Influence of light wavelengths on growth of tomato. J. Bumi Lestari (J. Environ.) 2012, 12, 338–344. [Google Scholar]
- Logendra, S.; Putman, J.D.; Janes, H.W. The influence of light period on carbon partitioning, translocation and growth in tomato. Sci. Hortic. 1990, 42, 75–83. [Google Scholar] [CrossRef]
- Cherie, E. The complete guide to growing tomatoes: A complete step-by-step guide including heirloom tomatoes (back-to-basics gardening); Atlantic Publishin g Group Inc. 2010. [Google Scholar]
- Anwarzai, N.; Kattegoudar, J.; Anjanappa, M.; Sood, M.; Kumar BA RS, M. Evaluation of Cherry Tomato (Solanum Lycopersicum L. var. cerasiforme) Genotypes for Growth and Yield Parameters. J. Homepage: Http://Www. Ijcmas. Com 2020, 9, 2020. [Google Scholar] [CrossRef]
- Kittas, C.; Karamanis, M.; Katsoulas, N. Air temperature regime in a forced ventilated greenhouse with rose crop. Energy Build. 2005, 37, 807–812. [Google Scholar] [CrossRef]
- Alani, M.A.; Elkaaby, E.A.; Majeed, W.A.A.; Al-Mandelawy, F.N.; Almaliky, A.K.Q.; Raad, J.M.; Shukor, K. Evaluation of Growth, Yield and some Qualities Characteristics of Two Cherry Tomato (Solanum lycopersicum var. cerasiforme) Cultivars under Plastichouse Conditions. IOP Conf. Ser. : Earth Environ. Sci. 2023, 1225, 012026. [Google Scholar] [CrossRef]
- Jo, W.J.; Shin, J.H. Effect of leaf-area management on tomato plant growth in greenhouses. Horticulture, Environment, and Biotechnology, 2020, 61, 981–988. [Google Scholar] [CrossRef]
- Renuka, D.M.; Sadashiva, A.T.; Kavita, B.T.; Vijendrakumar, R.C.; Hanumanthiah, M.R. Evaluation of cherry tomato lines (Solanum lycopersicum var. cerasiforme) for growth, yield and quality traits. Plant Archives 2014, 14, 151–154. [Google Scholar]
- Pallardy, S. Physiology of woody plants Third edition. 2008. [Google Scholar]
- Wilson, K.B. Baldocchi, D.D., Hanson, P.J. Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol. 2000, 20, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, T.C.; Sridhara, S.; Ramesh, N.; Gopakkali, P.; El-Ansary, D.O.; Mahmoud, E.A.; Abdel-Hamid, A.M. Assessing the impact of higher levels of CO2 and temperature and their interactions on tomato (Solanum lycopersicum L.). Plants 2021, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Wei, P. Increase the productivity of tomato by changing the greenhouse environment. Second version. Research Skill Literature Report. 2017.
- Demers, D.A.; Gosselin, A. Growing greenhouse tomato and sweet pepper under supplemental lighting: Optimal photoperiod, negative effects of long photoperiod and their causes. In IV Int. ISHS Symp. Artif. Light. 2000, 580, 83–88. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Lee, K.; Jeong, H.B.; Cho, M.C.; Nam, C.W.; Yang, E.Y. Physiological traits of thirty-five tomato accessions in response to low temperature. Agriculture 2021, 11, 792. [Google Scholar] [CrossRef]
- Kirschbaum, M.U. Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef]
- Singh, G.; Singh, N.; Dixit, P.S.; Singh, A.; Singh, R.P.; Vishen, G.S.; Verma SR, K. Effect of integrated nutrient management on growth, yield and quality of tomato (Solanum lycopersicum L. ) var. Kashi amrit. International Journal of Chemical Studies 2021, 9, 262–269. [Google Scholar] [CrossRef]
- Guodaar, L. Effects of climate variability on tomato crop production in the Offinso North District of Ashanti region. Doctoral Dissertation. 2015.
- Abdul-Baki, A.A. Tolerance of tomato cultivars and selected germplasm to heat stress. J. Am. Soc. Hortic. Sci. 1991, 116, 1113–1116. [Google Scholar] [CrossRef]
- Heuvelink, E. Growth, development and yield of a tomato crop: Periodic destructive measurements in a greenhouse. Sci. Hortic. 1995, 61, 77–99. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Lin, L.J.; Luther, G.C.; Hanson, P. Raising healthy tomato seedlings. AVRDC–The World Vegetable Center publication.
- Alsadon, A.A. , Al-Helal, I. M., Ibrahim, A.A., Shady, M.R., Al-Selwey, W.A. (, August). Growth analysis of tomato plants in controlled greenhouses. In XXX International Horticultural Congress IHC2018: III International Symposium on Innovation and New Technologies in Protected 2018, 1271, 177–184. [Google Scholar]
- Demers, D.A.; Dorais, M.; Wien, C.H.; Gosselin, A. Effects of supplemental light duration on greenhouse tomato (Lycopersicon esculentum Mill.) plants and fruit yields. Sci. Hortic. 1998, 74, 295–306. [Google Scholar] [CrossRef]
- Chen, J.M.; Rich, P.M.; Gower, S.T.; Norman, J.M.; Plummer, S. Leaf area index of boreal forests: Theory, techniques, and measurements. J. Geophys. Res. Atmos. 1997, 102, 29429–29443. [Google Scholar] [CrossRef]
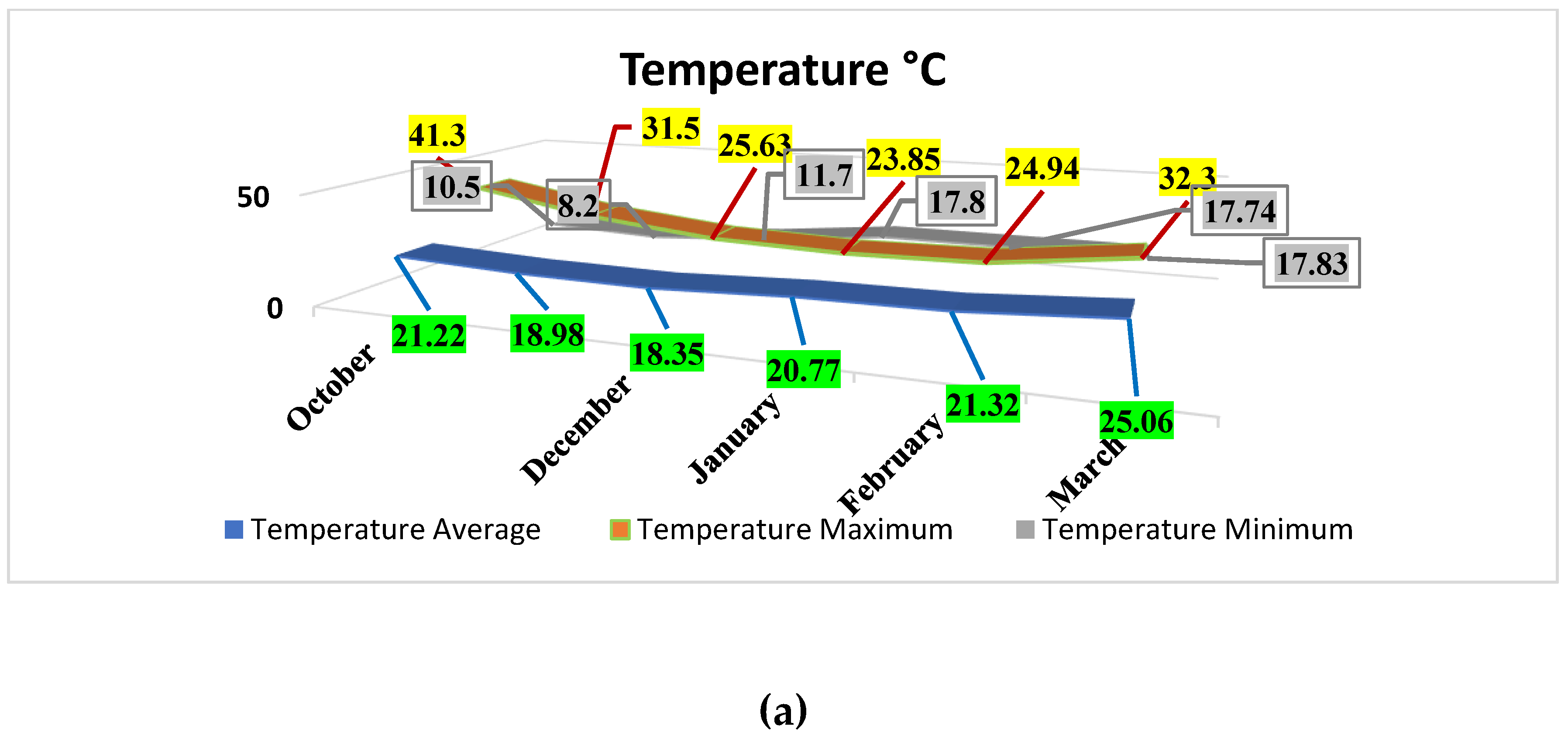
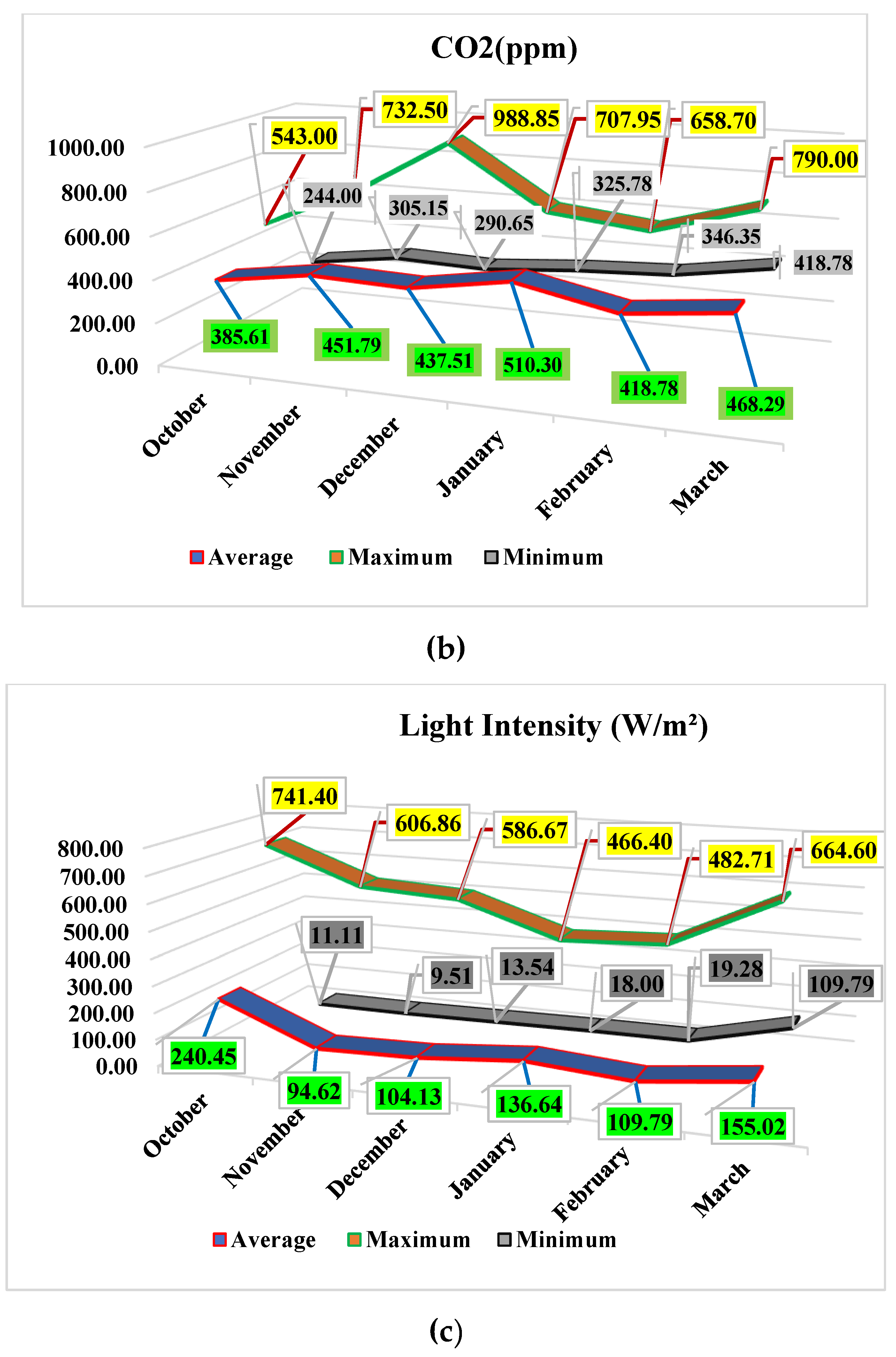
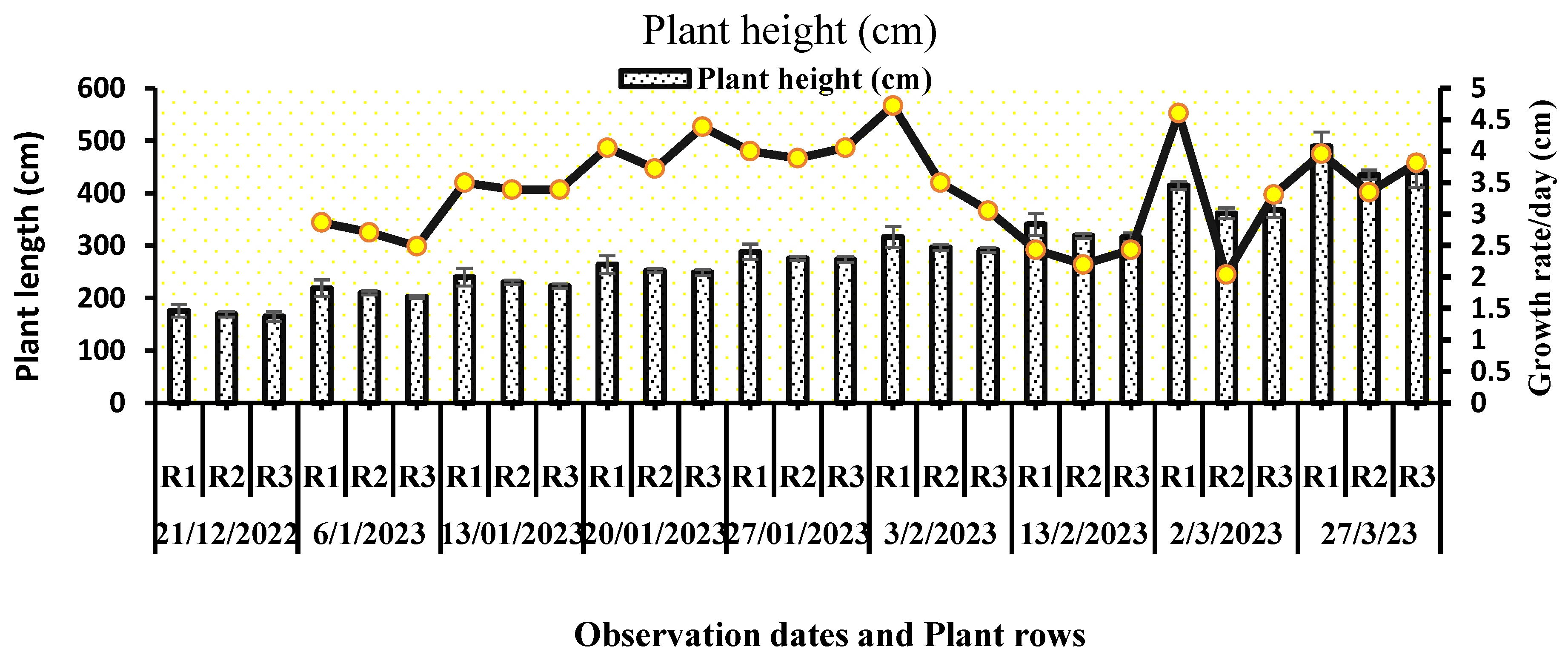
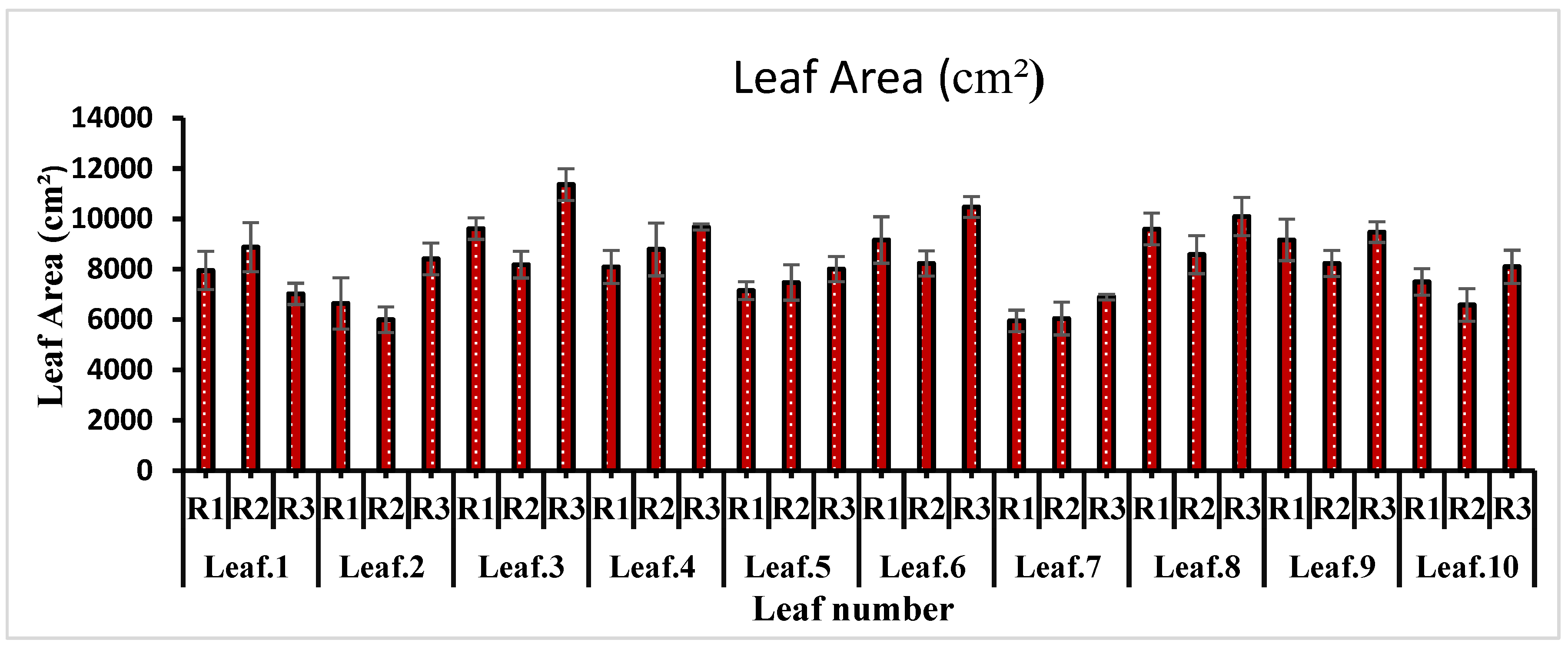
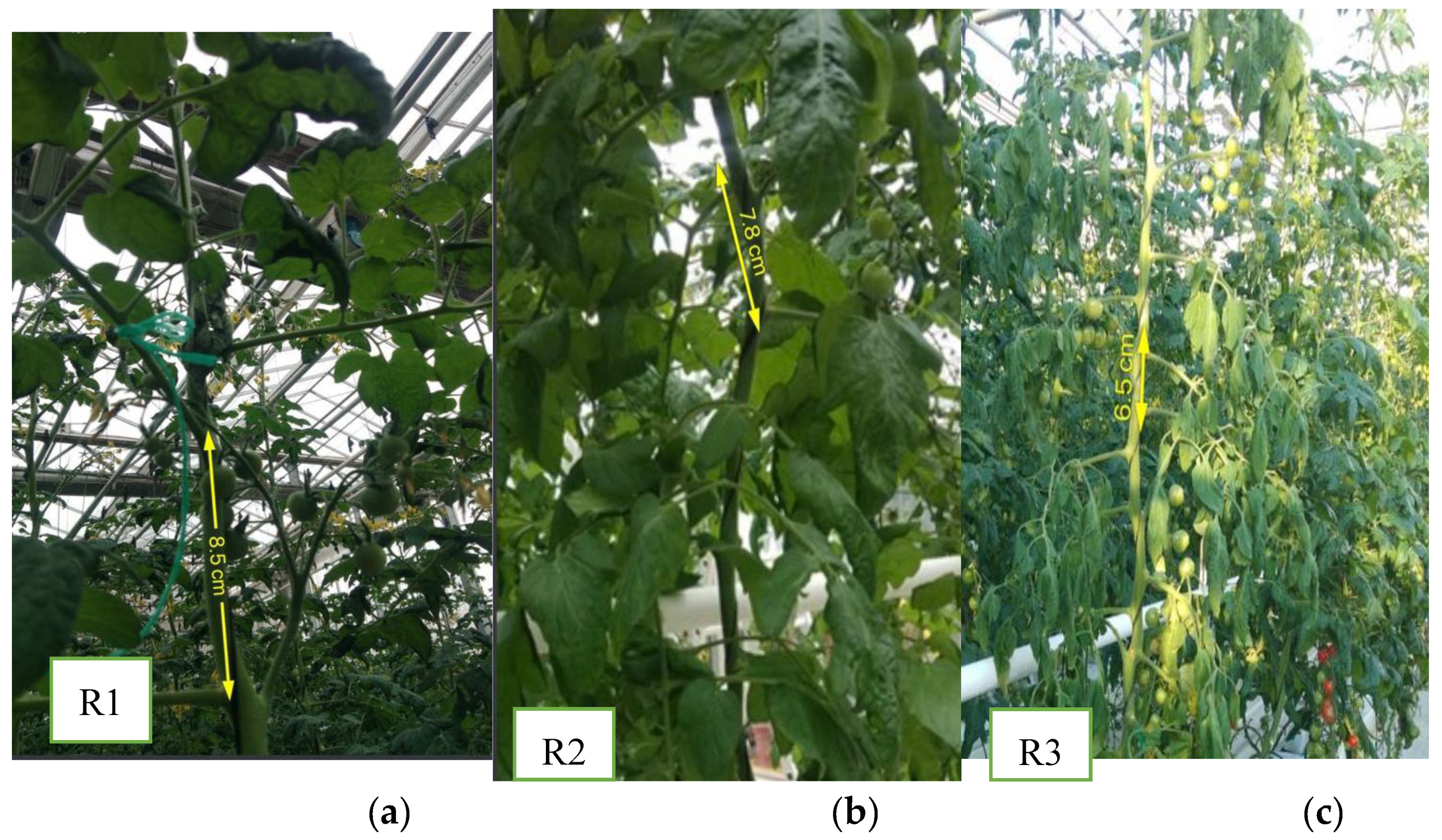

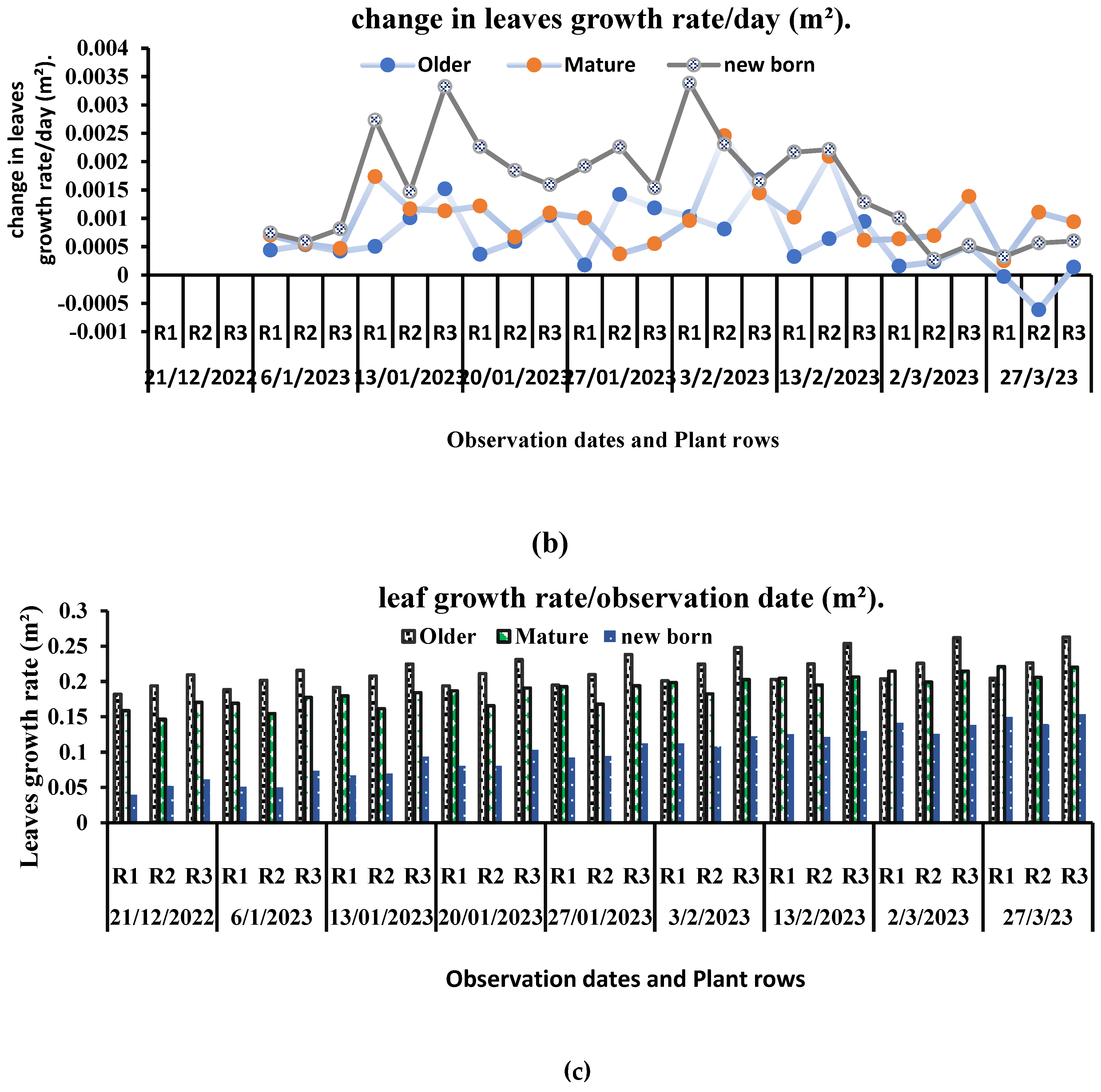
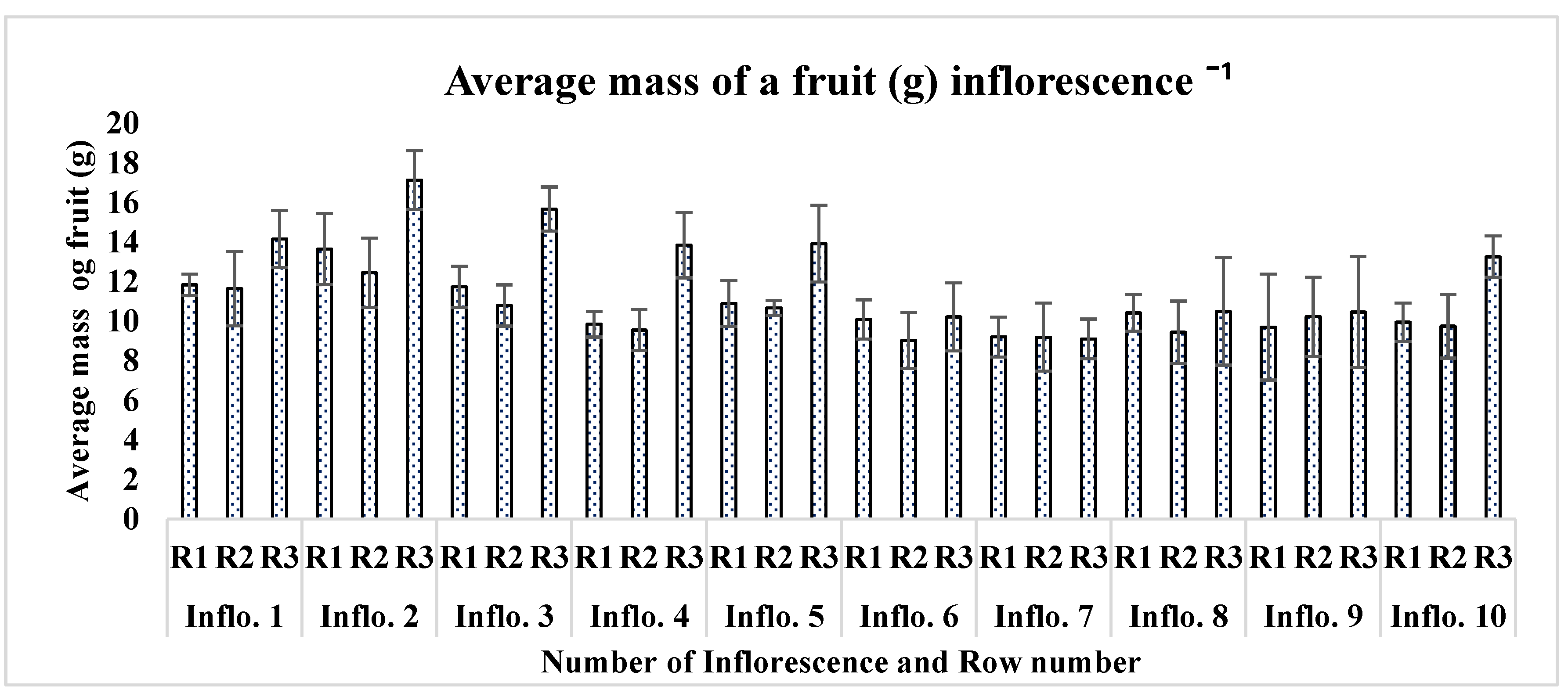

| Observation Dates | Older leaf growth rate (m²) | Mature leaf growth rate (m²) | Nascent leaf growth rate (m²) | Stem diameter growth rate at base (mm) |
Stem diameter growth rate in middle (mm) | Stem diameter growth rate at top (mm) |
Total height of plant | Total number of leaves plants¯¹ |
|---|---|---|---|---|---|---|---|---|
| 21-12-2022 | 0.194 ±0.013 | 0.158 ±0.011 | 0.051 ±0.011 | 10.07±0.867 | 11.46±0.99 | 5.83±0.328 | 169.88 ±5.38 | 32.13±1.52 |
| 06-01-2023 | 0.201 ±0.013 | 0.166 ±0.011 | 0.058 ±0.013 | 10.64±0.637 | 12.24±0.62 | 6.58±0.580 | 210.22 ±8.18 | 36.11±0.83 |
| 13-01-2023 | 0.207 ±0.016 | 0.174 ±0.011 | 0.077 ±0.014 | 11.06±0.622 | 12.89±0.94 | 7.29±0.638 | 230.77 ±8.52 | 39.44±0.50 |
| 20-01-2023 | 0.211 ±0.018 | 0.180 ±0.013 | 0.088 ±0.013 | 11.46±0.428 | 13.45±1.13 | 7.75±0.401 | 255.11 ±7.87 | 42.77±1.07 |
| 27-01-2023 | 0.214 ±0.022 | 0.184 ±0.014 | 0.099 ±0.011 | 11.86±0.378 | 13.98±1.16 | 8.20±0.364 | 279.00 ±7.88 | 46.44±0.50 |
| 03-02-2023 | 0.224 ±0.023 | 0.194 ±0.010 | 0.114 ±0.007 | 12.48±0.546 | 14.39±1.13 | 8.58±0.289 | 301.55 ±13.03 | 51.88±1.01 |
| 13-02-2023 | 0.227 ±0.025 | 0.201±0.006 | 0.125 ±0.004 | 12.84±0.405 | 14.92±1.08 | 8.90±0.301 | 325.22± 13.53 | 56.00±2.60 |
| 02-03-2022 | 0.230 ±0.029 | 0.209 ±0.008 | 0.135 ±0.008 | 13.26±0.541 | 15.50±1.30 | 9.36±0.442 | 381.22 ±29.13 | 68.11±2.52 |
| 27-03-2022 | 0.231 ±0.029 | 0.215 ±0.008 | 0.147 ±0.006 | 12.85±0.552 | 15.84±1.00 | 9.86±0.512 | 454.88 ±29.94 | 77.00±2.64 |
| F | 0.793 | 6.748 | 22.493 | 6.053 | 4.458 | 19.879 | 78.638 | 131.875 |
| P | 0.626 | 0.009 | <.001 | 0.012 | 0.028 | <.001 | <.001 | <.001 |
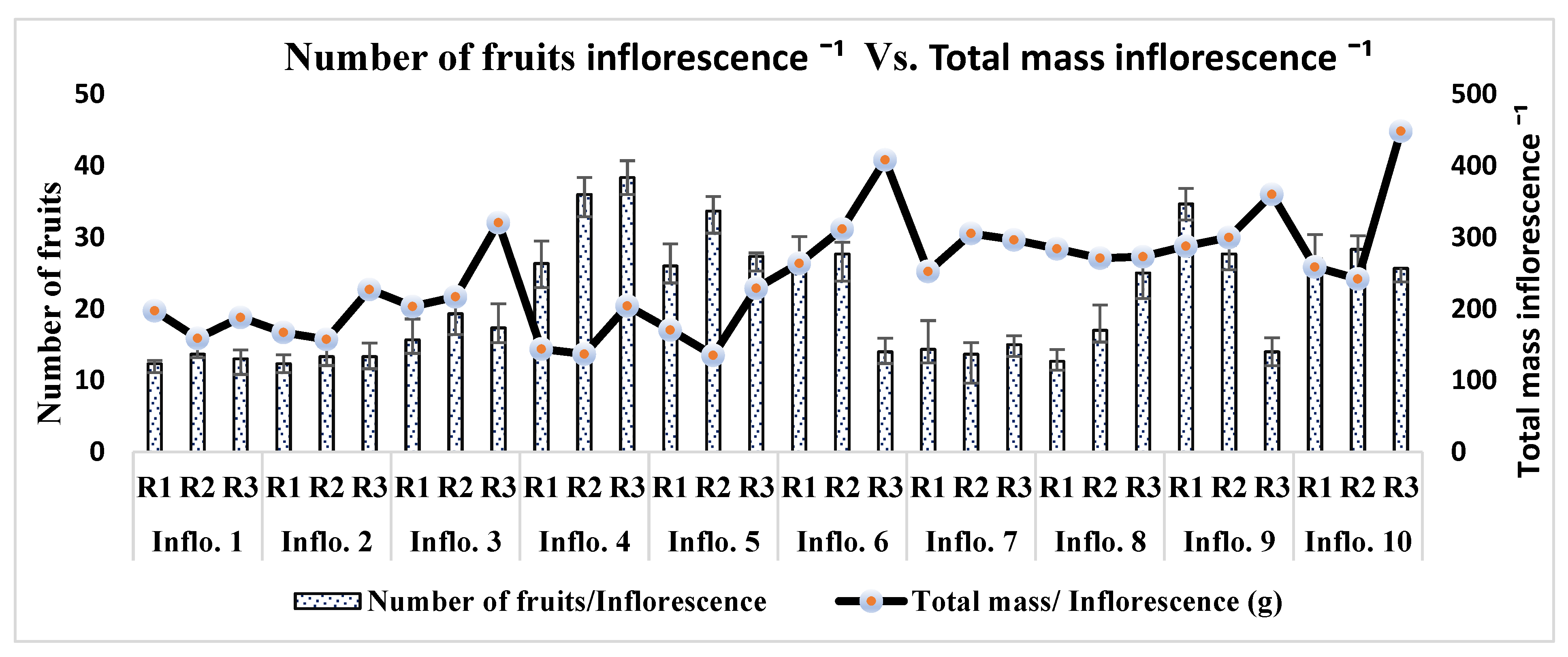
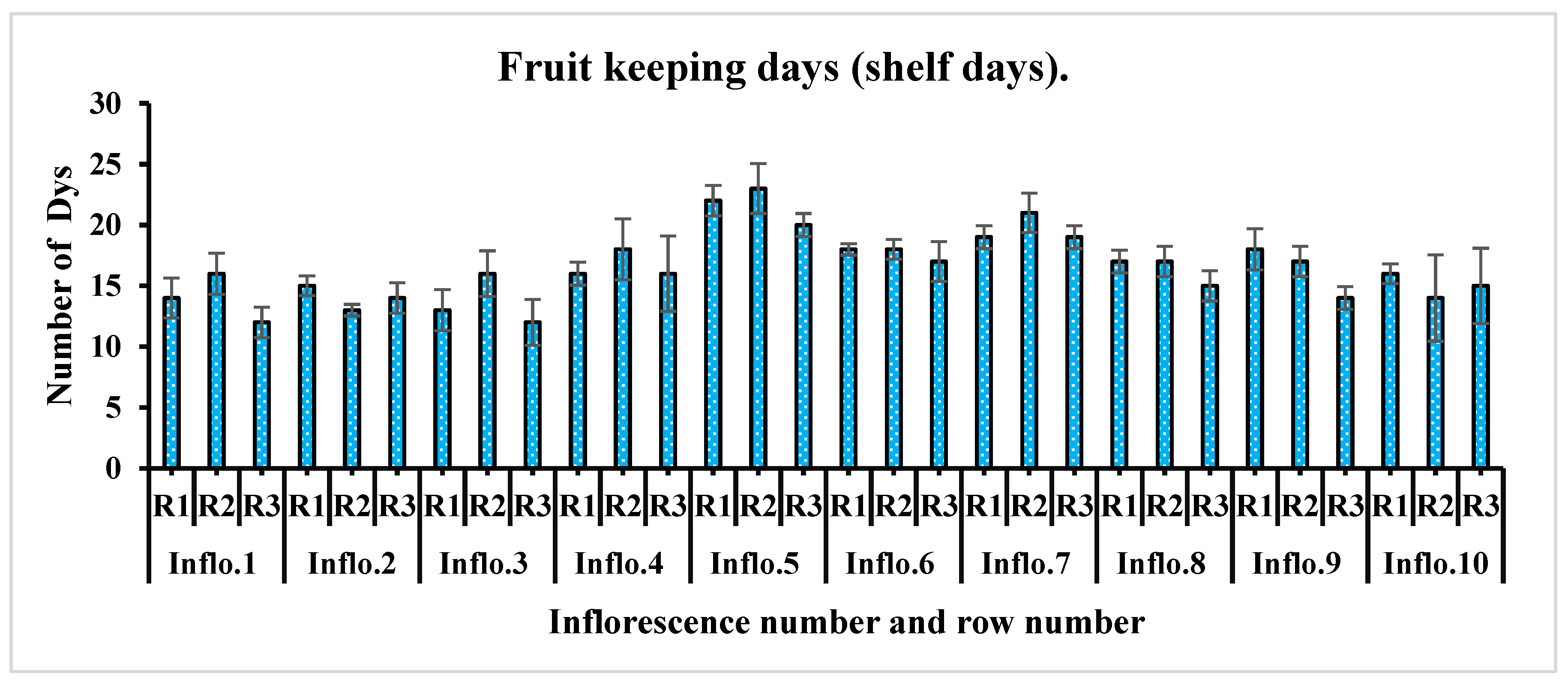
| Inflorescence number. | Inflorescence Length. (cm) | Number of fruits. | Total Fruit mass (g) | Average fruit mass(g) | Fruit Dry matter (%) | Fruit diameter (mm) | Shelf days |
|---|---|---|---|---|---|---|---|
| 1 | 19.90±0.55 | 13.00±0.66 | 181.46±20.06 | 12.54±1.39 | 3.16±0.08 | 28.97±1.8868 | 14.00±2.00 |
| 2 | 25.36±1.72 | 13.00±0.57 | 183.84±37.80 | 14.40±2.43 | 3.69±0.61 | 30.23±1.2818 | 14.00±1.00 |
| 3 | 35.54±5.43 | 17.44±1.83 | 235.41±56.50 | 12.86±2.81 | 7.78±0.16 | 28.87±2.7486 | 13.67±2.08 |
| 4 | 38.83±2.83 | 34.67±4.48 | 161.63±37.02 | 11.08±2.39 | 10.31±3.88 | 28.74±1.7164 | 16.67±1.15 |
| 5 | 46.56±7.00 | 31.22±2.21 | 178.17±47.30 | 11.83±1.81 | 13.89±4.24 | 27.30±0.7007 | 21.67±1.52 |
| 6 | 47.54±6.13 | 26.00±2.81 | 240.27±36.76 | 9.79±0.85 | 11.49±3.44 | 28.35±0.6140 | 17.67±0.57 |
| 7 | 45.23±6.65 | 14.33±0.66 | 226.80±19.27 | 9.71±1.10 | 11.83±0.9 | 28.47±2.0001 | 19.67±1.15 |
| 8 | 36.17±5.08 | 18.22±6.25 | 213.19±11.75 | 9.95± 0.71 | 9.85±0.791 | 26.82±1.2453 | 16.33±1.15 |
| 9 | 32.33±5.57 | 28.78±9.91 | 220.76±10.83 | 10.26±0.36 | 10.18±0.8 | 26.57±1.6044 | 16.32±2.08 |
| 10 | 27.67±3.14 | 27.00±1.33 | 243.22±81.81 | 11.72±3.43 | 9.47±0.304 | 26.87±1.9358 | 15.00±1.00 |
| F | 22.700 | 36.078 | 1.479 | 1.604 | 210.726 | 1.401 | 0.998 |
| p | <.001 | <.001 | 0.296 | 0.260 | <.001 | 0.322 | 0.475 |
| Inflorescence number. | Brix (%) | Nitrate contents. (mg/kg) | Fruit acidity | Fruit Firmness (kg /cm²) |
Leaf Area (cm²) |
|---|---|---|---|---|---|
| 1 | 7.21±0.2536 | 136.67±5.77 | 12.76±0.399 | 6.43±0.38 | 7183±16 |
| 2 | 7.50±0.7708 | 125.00±5.00 | 14.12±0.51 | 5.68±0.86 | 7013±12 |
| 3 | 6.91±0.2143 | 135.00±8.66 | 15.81±0.59 | 5.93±0.50 | 10477±13 |
| 4 | 7.15±0.3585 | 131.67±7.63 | 13.77±1.50 | 5.40±0.28 | 8851±79 |
| 5 | 8.07±0.8730 | 133.33±5.77 | 15.63±0.60 | 5.76±0.66 | 7541±43 |
| 6 | 8.85±1.0671 | 145.00±5.00 | 15.64±0.81 | 7.15±0.55 | 9287±11 |
| 7 | 8.22±1.0607 | 124.00±5.29 | 13.73±0.40 | 7.21±0.16 | 6294±52 |
| 8 | 7.79±1.0829 | 125.00±5.00 | 14.73±2.54 | 6.62±0.14 | 9423±77 |
| 9 | 7.70±0.8812 | 133.33±5.77 | 15.19±1.09 | 7.57±0.26 | 8953±64 |
| 10 | 8.03±0.6467 | 150.67±5.03 | 13.21±1.49 | 6.23±0.30 | 7389±76 |
| F | 5.276 | 1.724 | 6.360 | 10.039 | 4.382 |
| P | 0.014 | 0.228 | 0.008 | 0.002 | 0.024 |
| Month | Sunrise (First to last date) | Sunset (First to last date) | Daylength (First to last date) (Time). |
|---|---|---|---|
| January | 7:51am to 7:35am | 16:46pm to 17:22pm | 8:54:29 to 9:47:12 |
| February | 7:34am to 6:55 am | 17:24pm to 18:01pm | 9:49:42 to 11:06:10 |
| March | 6:53am to 6:59 am | 18:02pm to 19:41pm |
11:09:13 to 12:41:43 |
| April | 6:57am to 6:08am | 19:55pm to 20:18pm | 12:44:47 to 14:09:37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





