1. Introduction
Cell culture is currently a widely used biotechnology platform, and cell lines derived from various species and origins have become essential tools in the study of human metabolism and physiology [
1]. Mammalian cell lines, such as Chinese hamster ovary cells, hybridoma cells, human embryonic kidney cells, and young hamster kidney cells, are commonly used for the development of antibodies and other drugs [
2]. Additionally, insect-derived cell lines are becoming increasingly important in gene therapy research because they are useful for the production of recombinant proteins, viruses, and viral components [
3]. As biological research continues to advance, the risks increase that cells will be misidentified or contaminated with other cell types or exogenous factors [
4,
5], including viruses, and such contamination can be difficult to detect and remediate. Although some viral infections result in morphological changes, such as cytopathic effects detectable by microscopy, other viral infections are associated with no visible changes in cellular appearance or alterations that occur slowly and are not easily observed. Viral contamination of biological cell cultures can be costly. For example, in 2009, Genzyme was required to pay
$1.75 billion in fines due to viral contamination, in addition to reporting
$1–3 billion in lost product sales [
6]. Additionally, contamination impacts experimental results, potentially affecting experimental reproducibility and leading to wasted human and material resources. More importantly, viral contamination is a safety concern that poses a threat to human health. Vaccine products have been found to have been inadvertently contaminated with harmful viruses during production [
7], and hemophiliacs treated with virus-infected plasma developed autoimmune deficiency virus infections [
8], which eventually led to thousands of deaths in the 1980s and 1990s. Therefore, developing effective methods for detecting viral contamination in cells is essential.
Epstein-Barr virus (EBV) is a member of the human herpesvirus family, which includes eight viruses categorized into three subfamilies (α, β, and γ). EBV, also known as human herpesvirus type IV, belongs to the γ subfamily, genus Lymphocryptovirus [
9]. EBV is a DNA virus with a genome size of approximately 170 kb that contains more than 100 open-reading frames. EBV was first identified in 1964 in tissue samples from children suffering from African Hodgkin'’s disease [
10]. EBV is also recognized as a tumor-associated virus and was declared a class I carcinogen by the International Agency for Research on Cancer and the World Health Organization in the late 1990s [
11]. EBV is transmitted primarily through direct contact with saliva, although aerosol-mediated transmission also occurs, and the virus infects lymphocytes and oropharyngeal epithelial cells. Saliva from first-time infected individuals present with very high levels of EBV DNA, which can persist for several months. Although EBV DNA levels subside over time following new infections, EBV can be periodically released into the oral secretions of carriers [
12]. During the early stages of infection, the virus proliferates in the lymphocytes of the pharynx, after which the virus enters the bloodstream and spreads to the lymphatic system. EBV displays prolonged latency in lymphocytes, interfering with immune functions and potentially inducing cell proliferation and transformation. EBV infection involves many organ systems and is often misdiagnosed or underdiagnosed. Therefore, early diagnosis and rational treatment are extremely important.
Cell lines that contain EBV genes can be broadly classified into two categories. One category includes cell lines that are transformed by latent EBV infection, such as the
in vitro transformation of resting B cells into immortalized lymphoblastoid cell lines. In 1973, a study reported that B95-8 cells transformed from marmoset blood leukocytes regularly released high EBV titers and displayed transforming activity [
13]. The second category includes cell lines contaminated with EBV. During latent EBV infection, the virus is detectable in the nucleus in a ring form, linked to the chromatin of the host genome by the Epstein–Barr nuclear antigen 1 (EBNA1) protein [
14]; genes expressed during latent EBV infections are referred to as latent genes. Multiple reports in the literature have described the detection of EBV infection in various cell types maintained in cell culture banks [
15,
16]; these infections can be attributed to the presence of an existing EBV infection during the initial process of cell line establishment, EBV contamination of culturing materials, or improper manipulation by the experimental staff.
Existing tests to detect EBV can be divided into three categories: nucleic acid assays, serological assays, and
in situ hybridization assays. Nucleic acid assays include gene-specific amplification techniques and whole-genome sequencing. Gene-specific amplification techniques include polymerase chain reaction (PCR) and isothermal amplification. Currently the most widely used assay, PCR is a mature and reliable molecular method, and quantitative PCR (qPCR) can be used to monitor disease progression or treatment efficacy by detecting changes in the EBV load in blood [
17]. Isothermal amplification techniques, such as recombinant enzyme-mediated isothermal amplification and loop-mediated isothermal amplification (LAMP), are easy to perform, have limited equipment requirements, return rapid responses, and display high sensitivity. Wang et al. utilized a recombinase-aided amplification (RAA) method to detect EBV in whole blood specimens and serum [
18]. Iwata et al. used the LAMP technique to detect EBV in serum and pharyngeal swabs [
19]; however, designing primers for use in the LAMP technique is complicated and can be difficult to replicate. Although whole-genome sequencing can be used to accurately evaluate the full EBV sequence [
20], this method is time-consuming, expensive, and not conducive to high-volume clinical screening. Serological assays are based on the detection of relevant antibodies produced in response to EBV infection. Commonly used serological assays utilize techniques such as immunofluorescence staining, enzyme immunoassays, chemiluminescence immunoassays, western blotting, and immunofluorescence reactions [
21].
In situ hybridization assays to detect Epstein-Barr early RNA(EBER), a small EBV-encoded RNA that is continuously transcribed and expressed after infection, are the current gold standard for detecting EBV infection in clinic. However, this technique can only be applied to tissue samples and is generally limited to the clinical diagnosis of EBV-related diseases, such as cancer and lymphoma. Therefore, a simple system that allows for the rapid detection of EBV in multiple contexts, including both cell culture and tissue samples, remains necessary.
Recombinase polymerase amplification (RPA) is a highly sensitive and selective isothermal amplification technique that can be performed at 37–42°C and can be used to amplify a large number of samples in a short period of time. RPA can be performed on small sample volumes and can be applied to a variety of sample types, including microorganisms, body fluids, surgical biopsies, organ tissues, and plant and animal products [
22]. RPA, first introduced in 2006 by Niall Armes of ASM Scientific Ltd., UK [
23], relies on modified homologous recombination mechanisms. In addition to reaction cofactors, such as DNA polymerase and energy-generating molecules, the standard RPA reaction reagent contains three key proteins: T4 uvsX recombinase, T4 uvsY recombinase loading factor, and T4 gp32 single-strand binding protein [
23]. RPA reagents are commercially available, and the basic RPA reaction kit can be augmented by additional commercially available kits that use different probes: exo, fpg, and nfo [
24]. The exo and fpg probes are typically used for real-time detection, whereas the nfo probe is typically used for detection systems that use lateral flow dipsticks. RPA technology is well-suited for on-site detection in low-resource environments and represents a promising platform for the development of amplification-based detection systems.
In this study, we combined RPA technology with a lateral flow assay (LFA) to develop an RPA-LFA detection system for application to the rapid and bulk screening of EBV contamination in cell lines stored by cell banks and as a means to conduct regular and daily inspection of cell lines used in biological experiments, ensuring the quality of cell lines and the safety of experimental personnel. This system also demonstrates high potential for clinical adaptation to improve EBV detection in blood and tissue samples.
4. Discussion
Cell culture is the most fundamental step in biological experiments, but human error and contamination carried by the cells themselves can have a dramatic effect on experimental research and the cellular products produced. Several cell lines have been shown to be persistently infected by EBV; for example, EBV can infect B lymphocytes and convert them into a continuously proliferating lymphoblastoid-like cell line [
29]. Additionally, EBV, a herpesvirus that widely circulates in society, may also be introduced into cells by operators who carry EBV themselves due to inadvertent manipulation, resulting in cellular virus contamination. Existing PCR-based methods for EBV detection have drawbacks, such as long detection periods, complicated operations, and limited applications. Therefore, we used an RPA method combined with an LFA to establish a rapid EBV detection system. The operating procedure of the system is shown in
Figure 7. The RPA-LFA system consists of three parts: sample preparation, nucleic acid amplification, and use of LFA test strips to visualize the results. Cellular DNA is first extracted as the sample type for the assay and then amplified by RPA for 5–15 min at 39 °C. The nfo probe is introduced during amplification to bind to the test strip for rapid visualization of the results.
The addition of probes to the system is crucial. Initially, we only replaced the normal forward and reverse primers during RPA amplification with FITC-labeled forward primers and biotin-labeled reverse primers, but the negative and blank controls consistently showed false positives when applied to the lateral flow dipstick. By varying the primer content, we observed that the false-positive bands were proportional to the primer content within a certain range. Therefore, the primers were determined to be the main factor causing this phenomenon. The initial speculation was that some of the forward and reverse primers formed primer dimers, and such primer dimers with two markers at the same time were eventually titrated on the test strip and captured to form positive bands. The same false-positive phenomenon was also observed in a study of avian influenza virus detection using nfo probes [
30], in which Wang et al. introduced probes and base substitutions to eliminate the false-positive phenomenon. Therefore, we introduced a labeled probe in addition to the normal RPA assay, and only the reverse primer was labeled. Nfo was added to cut off the C3-Spacer of the 3′ end of the probe to obtain a new target fragment with both labels and eliminate the interference of the primer dimer. Most studies introducing nfo probes for RPA have used the TwistAmp® nfo kit from TwistDx. For example, Greeshma et al. used this kit for rapid detection of black pepper infestation by pepper yellow mottle virus [
31], and Velasco et al. used the kit for quality control of seafood [
32]. However, the TwistAmp® nfo kit is expensive and not easy to purchase, and the long procurement period did not meet the requirements of the assay. To reduce the assay cost and optimize the procedure, we built our own amplification system containing nfo. At first, we added the designed probe, nfo, and its buffer directly to the RPA amplification system according to the recommendation in the instruction manual, but little amplification was observed on the agarose gel after adding these reagents. We then created a two-step method for EBV detection, i.e., amplification with labeled reverse primer according to the normal RPA system, followed by re-amplification with the addition of the probe, nfo, and its buffer, which effectively eliminated false positives. We also determined the minimum reaction time and found that a positive test could still be detected when the total time of the two-step reaction was shortened to 10 min.
Another interesting finding of this study is that nine of the 10 EBV-positive cell lines have been associated with EBV in the literature, but an association has not been reported for A-431. For example, Uphoff et al. screened cells for EBV, and their positive test results included six of the EBV-positive cell lines in this study: B95-8, ARH-77, Daudi, JVM-2, NK-92, and Raji [
15]. Farage, CCRF-SB, and MC/CAR are listed as EBV transformants in the NCBI database. Nevertheless, no mention of A-431 cells containing EBV fragments within their genes has been reported in the published literature to date. Subsequent examinations of other A-431 cell batches showed that only one batch was positive for EBV according to both the PCR and RPA methods; the remaining batches were negative (
Supplementary Figure S3C–D). We hypothesized that the previous batch of A-431 cells may have been contaminated by the external environment during late culture, resulting in EBV positivity, or that the human-derived cells may have been infected with EBV when the cell line was initially established. In either case, EBV contamination of cells presents an obstacle to the health of the operator and the conduct of biological experiments, further suggesting that cellular EBV testing is imperative.
Although EBV detection methods in the field using isothermal amplification technology exist, the detection system established in this study still has certain advantages. The use of LAMP for the detection of EBV in serum and pharyngeal swabs [
19] requires a complex primer design, whereas RPA primer design is simple and easy to perform. Isothermal amplification of nucleic acids using RAA has been applied in a manner similar to RPA, including in a study by Yuan Gao et al. [
33] to detect the sensitivity and specificity of RAA for detecting EBV in extracted nucleic acids. Additionally, Jing-yi Li et al. used the RAA method in combination with magnetic beads enriched with recombinant human mannan-binding lectin to detect low-carbon-load EBVs in blood [
34]. However, the RPA method was developed first and is more mature and stable than the RAA method, which does have some advantages. Furthermore, the RPA-LFA detection system that we established has only been shown to be feasible at the cellular quality control level, and further applications for clinical testing can be explored using different types of clinical samples, such as blood and pharyngeal swabs. Moreover, more possibilities for rapid binding of target fragments in this amplification system can be explored to reduce the time required to extract DNA and simplify the overall process. For example, microfluidic biochips based on microelectromechanical systems technology have been reported to enable rapid cell lysis, resulting in the acquisition of amplifiable genomic DNA in less than 20 minutes. [
35] Consequently, the establishment of the RPA-LFA rapid EBV detection system has great significance in the pharmaceutical field, including for cell quality control and clinical testing.
The RPA-LFA system requires further improvement. First, the two-step method established in this study has the potential to be integrated into a one-step amplification method. TwistDx published a study on RPA technology in 2018 [
36] that contains detailed kit components and can provide a reference for subsequent experimental component adjustment so that the RPA amplification phase and nfo probe digestion phase do not interfere with each other and react simultaneously. Second, the use of different types of reaction equipment can be explored to reduce the assay cost. Instead of using a dedicated instrument to maintain isothermal amplification of reactions, other more cost-effective instruments, such as constant temperature metal baths and boiling water baths, can be used, and human-generated heat has been demonstrated for device-free amplification of HIV [
37]. In addition, the introduction of other types of probes can also enrich the detection capabilities of the reaction system. Currently, only qualitative and rough quantitative detection of EBV is possible, but the lowest detection limit of 16 copies of the monkeypox virus was recently reported by introducing exo probes [
38]. Therefore, the introduction of other types of probes and the addition of common qPCR for more accurate quantitative detection can be considered in the future. Finally, the sensitivity of the RPA-LFA system can be improved. In this study, a single reaction system (50 μL) could detect 1×10
3 copies of EBV, but in a similar study of RPA combined with an LFA for virus detection, the detection sensitivity was as low as 200 copies [
39]. We previously demonstrated that changing the probe amount does not change the reaction sensitivity; therefore, other conditions need to be explored to improve the detection sensitivity of the RPA-LFA system. When customizing the lateral flow strips, AuNPs with larger particle sizes were selected as labeling materials to improve sensitivity. Choi et al. used two different sizes of AuNPs to achieve the sensitive detection of cardiac troponin I (cTnI) by LFA, and the difference in sensitivity between different sizes of AuNPs was obvious [
40]. On the other hand, combinations with other methods can also be considered, such as those used in a study by Jonathan et al., in which gold nanoparticles were used as amplicons in combination with electrochemical methods, presenting higher detection sensitivity through specific binding of amplicons [
41].
The RPA-LFA rapid EBV detection system established in this study is simple to operate, does not require expensive equipment, and allows rapid visualization, which is important for quality management of cell banks. It also provides a new method for EBV detection in clinical samples and other applications.
Figure 1.
Primer screening and sample type selection. (A) Agarose gel electrophoresis results after PCR amplification with different primers. #1, #2, #3, #4, #5: five different primer pairs with target fragment sizes of 265, 231, 134, 159, and 95 bp, respectively; D: ddH2O; M: DL2000 marker; (B) Agarose gel electrophoresis results after PCR amplification of different sample types. A: cellular DNA; B: cell supernatant; C: cell precipitate; D: ddH2O; M: DL2000 marker.
Figure 1.
Primer screening and sample type selection. (A) Agarose gel electrophoresis results after PCR amplification with different primers. #1, #2, #3, #4, #5: five different primer pairs with target fragment sizes of 265, 231, 134, 159, and 95 bp, respectively; D: ddH2O; M: DL2000 marker; (B) Agarose gel electrophoresis results after PCR amplification of different sample types. A: cellular DNA; B: cell supernatant; C: cell precipitate; D: ddH2O; M: DL2000 marker.
Figure 2.
Establishment of the RPA-LFA detection system for EBV. (A) Validation of the RPA system. 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; (B) RPA-LFA validation (forward primer labeled with FITC, reverse primer labeled with biotin). a: ddH2O; b: HeLa cell DNA; c: B95-8 cell DNA; (C) RPA-LFA validation (reverse primer labeled with biotin and addition of nfo, nfo buffer, and the probe). 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; (D) Addition of different reagents to the common RPA system. 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; 4: B95-8 cell DNA with probe added; 5: B95-8 cell DNA with nfo added; 6: B95-8 cell DNA with nfo buffer added; 7: B95-8 cell DNA and addition of nfo and nfo buffer; (E) RPA system validation (reverse primer labeled with biotin and nfo and probe were added). 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; (F) RPA-LFA system validation (reverse primer labeled with biotin and nfo and probe were added). (G) RPA system validation (two-step method). 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; (H) RPA-LFA system validation (two-step method). a: ddH2O; b: HeLa cell DNA; c: B95-8 cell DNA; M: DL2000 marker. Note: The upper line of the lateral flow dipstick is the quality control line, and the lower line is the detection line, i.e., the presence of only the quality control line indicates a negative result for EBV, and the presence of both lines indicates a positive result.
Figure 2.
Establishment of the RPA-LFA detection system for EBV. (A) Validation of the RPA system. 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; (B) RPA-LFA validation (forward primer labeled with FITC, reverse primer labeled with biotin). a: ddH2O; b: HeLa cell DNA; c: B95-8 cell DNA; (C) RPA-LFA validation (reverse primer labeled with biotin and addition of nfo, nfo buffer, and the probe). 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; (D) Addition of different reagents to the common RPA system. 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; 4: B95-8 cell DNA with probe added; 5: B95-8 cell DNA with nfo added; 6: B95-8 cell DNA with nfo buffer added; 7: B95-8 cell DNA and addition of nfo and nfo buffer; (E) RPA system validation (reverse primer labeled with biotin and nfo and probe were added). 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; (F) RPA-LFA system validation (reverse primer labeled with biotin and nfo and probe were added). (G) RPA system validation (two-step method). 1: ddH2O; 2: HeLa cell DNA; 3: B95-8 cell DNA; (H) RPA-LFA system validation (two-step method). a: ddH2O; b: HeLa cell DNA; c: B95-8 cell DNA; M: DL2000 marker. Note: The upper line of the lateral flow dipstick is the quality control line, and the lower line is the detection line, i.e., the presence of only the quality control line indicates a negative result for EBV, and the presence of both lines indicates a positive result.
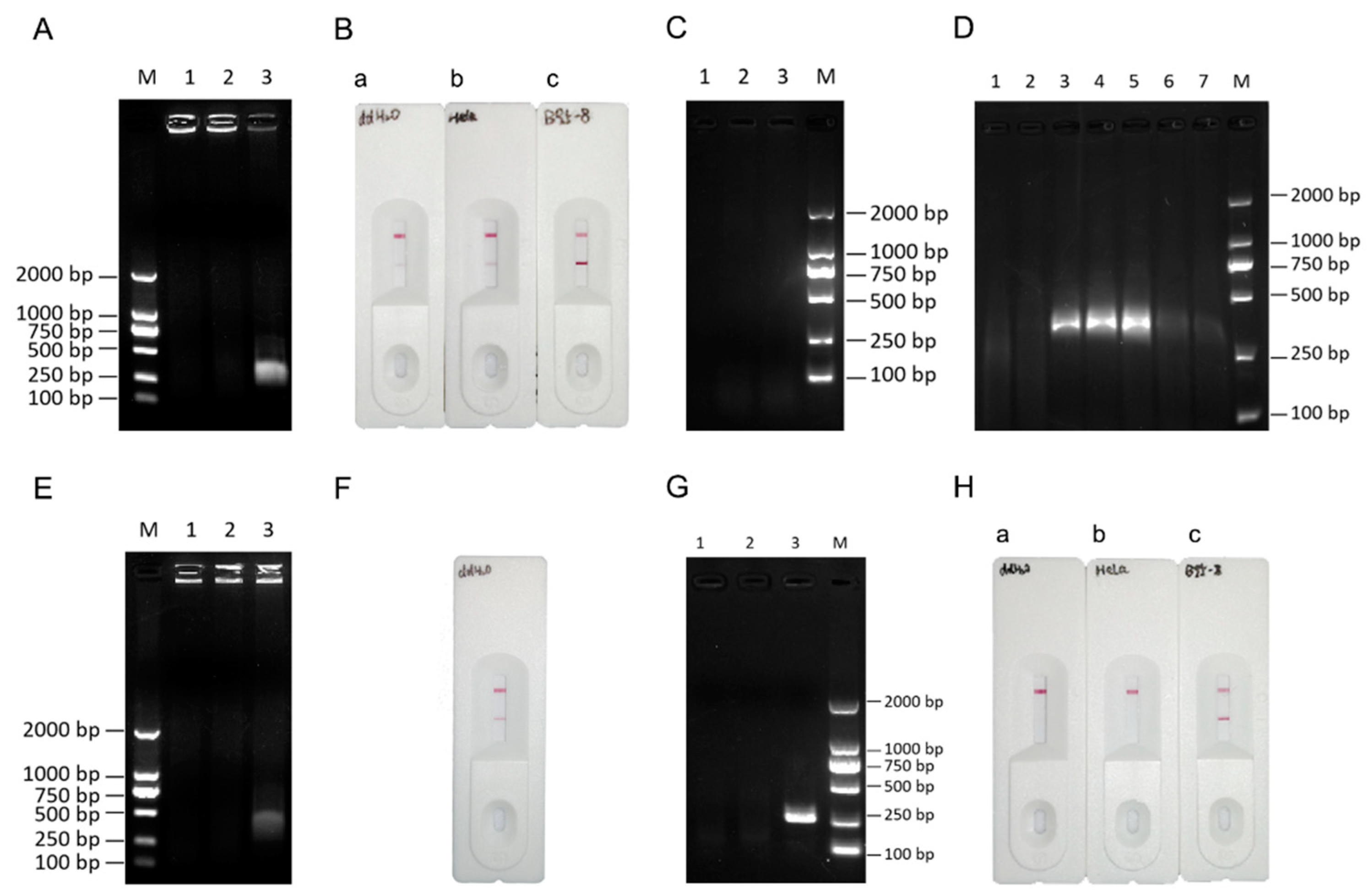
Figure 3.
Determination of the RPA-LFA reaction temperature. (A)Determination of the optimum RPA temperature for different cell types. M: DL2000 marker; (B) Determination of the optimal RPA temperature for B95-8 cell DNA. (C) RPA-LFA validation (two-step method). a: amplification at 35°C; b: 37°C; c: 39°C; d: 41°C; and e: 43°C.
Figure 3.
Determination of the RPA-LFA reaction temperature. (A)Determination of the optimum RPA temperature for different cell types. M: DL2000 marker; (B) Determination of the optimal RPA temperature for B95-8 cell DNA. (C) RPA-LFA validation (two-step method). a: amplification at 35°C; b: 37°C; c: 39°C; d: 41°C; and e: 43°C.
Figure 4.
Determination of the RPA-LFA reaction time. (A) RPA-conjugated agarose gel electrophoresis to assess amplification using different reaction times. 1: 5 min; 2: 10 min; 3: 15 min; 4: 20 min; (B) RPA-conjugated agarose gel electrophoresis to assess amplification using different reaction times (first step). 1: 5 min; 2: 10 min; 3: 15 min; 4: 20 min; (C) RPA-conjugated agarose gel electrophoresis to assess amplification using different reaction times (second step). 1: 5 min; 2: 10 min; 3: 15 min; (D) RPA-conjugated lateral flow chromatography strips used to assess assays with different reaction times (first step). a: 5 min; b: 10 min; c: 15 min; d: 20 min; (E) RPA combined with lateral flow chromatography strips used to assess results for assays with different reaction times (second step). a: 5 min; b: 10 min; c: 15 min; M: DL2000 marker.
Figure 4.
Determination of the RPA-LFA reaction time. (A) RPA-conjugated agarose gel electrophoresis to assess amplification using different reaction times. 1: 5 min; 2: 10 min; 3: 15 min; 4: 20 min; (B) RPA-conjugated agarose gel electrophoresis to assess amplification using different reaction times (first step). 1: 5 min; 2: 10 min; 3: 15 min; 4: 20 min; (C) RPA-conjugated agarose gel electrophoresis to assess amplification using different reaction times (second step). 1: 5 min; 2: 10 min; 3: 15 min; (D) RPA-conjugated lateral flow chromatography strips used to assess assays with different reaction times (first step). a: 5 min; b: 10 min; c: 15 min; d: 20 min; (E) RPA combined with lateral flow chromatography strips used to assess results for assays with different reaction times (second step). a: 5 min; b: 10 min; c: 15 min; M: DL2000 marker.
Figure 5.
Comparison of the sensitivity of the PCR and RPA-LFA methods for EBV detection. (A) EBV standard curve. (B) Sensitivity of EBV detection by the PCR method and RPA method. M: DL2000 marker; 1: 1×106 copies/μL; 2: 1×105 copies/μL; 3: 1×104 copies/μL; 4: 1×103 copies/μL; 5: 1×102 copies/μL; 6: 1×101 copies/μL; 7: 1×100 copies/μL; (C) Sensitivity of EBV detection by the RPA-LFA method. a: 1×106 copies/μL; b: 1×105 copies/μL; c: 1×104 copies/μL; d: 1×103 copies/μL; e: 1×102 copies/μL; f: 1×101 copies/μL; g: 1×100 copies/μL.
Figure 5.
Comparison of the sensitivity of the PCR and RPA-LFA methods for EBV detection. (A) EBV standard curve. (B) Sensitivity of EBV detection by the PCR method and RPA method. M: DL2000 marker; 1: 1×106 copies/μL; 2: 1×105 copies/μL; 3: 1×104 copies/μL; 4: 1×103 copies/μL; 5: 1×102 copies/μL; 6: 1×101 copies/μL; 7: 1×100 copies/μL; (C) Sensitivity of EBV detection by the RPA-LFA method. a: 1×106 copies/μL; b: 1×105 copies/μL; c: 1×104 copies/μL; d: 1×103 copies/μL; e: 1×102 copies/μL; f: 1×101 copies/μL; g: 1×100 copies/μL.
Figure 6.
Verification of the specificity of the RPA-LFA system. (A)Detection of cells containing other endogenous viruses using the RPA-LFA system. a: HeLa; b: SiHa; c: Hep-G2/2.2.15; d: RK13; (B) RPA-LFA detection of EBV-negative cell lines. a: A-204; b: H9; c: HTR-8; (C) RPA-LFA detection of EBV-positive cell lines. a: NK-92; b: Daudi; c: ARH-77; d: Raji; e: JVM-2; f: A-431; g: Farage; h: MC/CAR; i: CCRF-SB.
Figure 6.
Verification of the specificity of the RPA-LFA system. (A)Detection of cells containing other endogenous viruses using the RPA-LFA system. a: HeLa; b: SiHa; c: Hep-G2/2.2.15; d: RK13; (B) RPA-LFA detection of EBV-negative cell lines. a: A-204; b: H9; c: HTR-8; (C) RPA-LFA detection of EBV-positive cell lines. a: NK-92; b: Daudi; c: ARH-77; d: Raji; e: JVM-2; f: A-431; g: Farage; h: MC/CAR; i: CCRF-SB.
Figure 7.
Pattern of EBV detection by the PCR, RPA, and RPA-LFA systems. The three steps, sample preparation, nucleic acid amplification, and the LFA, are shown.
Figure 7.
Pattern of EBV detection by the PCR, RPA, and RPA-LFA systems. The three steps, sample preparation, nucleic acid amplification, and the LFA, are shown.
Table 1.
PCR primer sequences.
Table 1.
PCR primer sequences.
| Genebank |
Nucleotide positions |
Primer |
Sequence(5′ - 3′) |
| V01555 |
83520
–83784
82507
-82737
165806
–165904
82339
-82497
4683
-4777 |
PCR-FP1
PCR-RP1
PCR-FP2
PCR-RP2
PCR-FP3
PCR-RP3
PCR-FP4
PCR-RP4
PCR-FP5
PCR-RP5 |
CTTGGAGACAGGCTTAACCAGACTCA
CCATGGCTGCACCGATGAAAGTTAT
GTGCCTCCTCAAATGTTCCAGAAGT
TAAACTGAATCTCCACCTGTGTAACCTCA
CTACCTGTGCCGCATGAAACTGGGCGAGACCGA
CATGTCACAGTAAGGACAGAGAAGTCTGGG
AGTTAGCATTGGCGTCGG
GGAACGGTGATTAGGCACTG
CCTGGTCATCCTTTGCCA
TGCTTCGTTATAGCCGTAGT |
Table 2.
RPA primers and probe sequences.
Table 2.
RPA primers and probe sequences.
| Genebank |
Primer |
Sequence(5′ - 3′) |
| V01555 |
RPA-FP1
RPA-FP2
RPA-RP
RPA-Probe
|
[FITC]CTTGGAGACAGGCTTAACCAGACTCA
CTTGGAGACAGGCTTAACCAGACTCA
[BIOTIN]CCATGGCTGCACCGATGAAAGTTAT
[FITC]TGCCGGCCCCTCGAGATTCTGACCGGGGACC
[THF]CTGGTTGCTCTGTTG [C3-Spacer] |












