Introduction
Cerebrotendinous xanthomatosis (CTX) is a rare disease attributed to partial or complete loss of the enzyme sterol-27-hydroxylase leading to chenodeoxycholic acid and cholic acid production deficiency, and accumulation of sterol intermediates, mainly cholestanol, in plasma and in several other tissues. The clinical phenotype of CTX greatly varies among patients, and include tendon xanthomas, gallbladder stones, diarrhea, cataract, and neurological abnormalities 1. The diagnostic identification typically is characterized by an elevated level of plasma cholestanol and identification of CYP27A1 gene variants. However, there is a great diversity of phenotypes of this pathology so it is often difficult to identify the disease, and underdiagnosis is frequent, but the treatment with chenodeoxycholic acid can prevent the unfavorable evolution of the disease 2. In his regard, a case of brain damage with absent tendon xanthomas has been described 3. We report here an opposite case, that is, alteration of the CYP27A1 gene characteristic of CTX, without elevation of cholestanol in plasma and tendon, with extremely large Achilles tendon lesions but absence of other clinical manifestations typical of the disease.
Case description
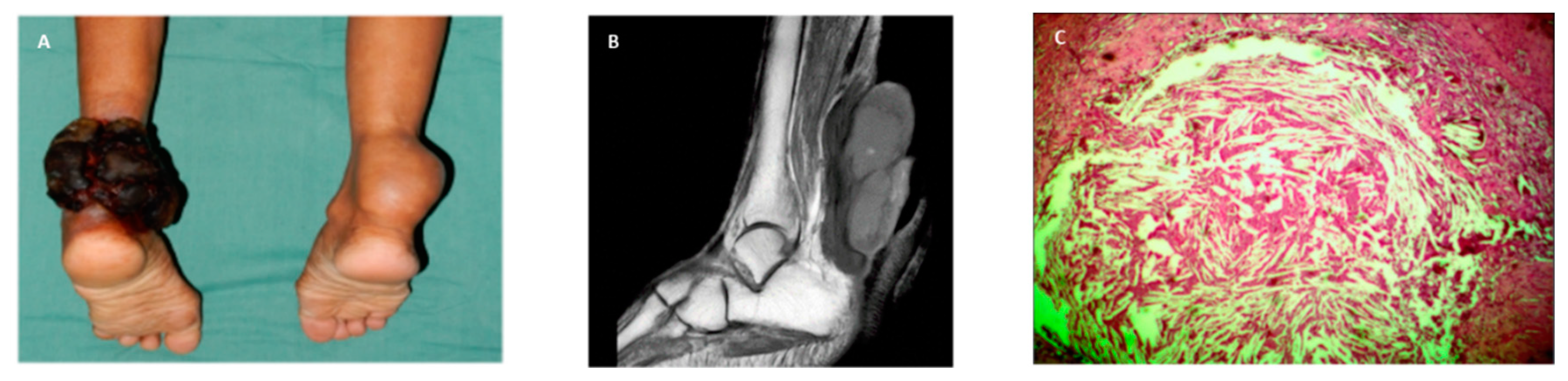
A 68-year-old female patient, mulatto, rural worker from the countryside of the state of São Paulo, Brazil, since childhood presenting bulging in both Achilles’ tendons. She was a smoker until 20 years ago. In the last 8 months, an ulcer appeared in the left Achilles tendon with significant local bleeding, which required blood transfusion. Biopsies of the left tendon lesion showed xanthogranuloma with giant cells, abundant amounts of apparently cholesterol crystals surrounded by fibrous proliferation, and permeated squamous cells of the superficial layer of the epidermis without neoplastic cells, but containing keratinous material (
Figure 1). Due to its size, the xanthogranuloma was removed, which resulted in the impossibility of walking.
Her BMI was 23.73 kg/m². Presence of bilateral cataract and in the retinal examination nonspecific atrophy of retinal pigment epithelium. Doppler examination of the lower limbs shows calcified atheroma without significant hemodynamic repercussions. Abdominal ultrasound shows aortoiliac atheromas and gallbladder presenting multiple images compatible with polyps. There are no gastrointestinal or neurological complaints or alterations in clinical examination. Carotid ultrasound shows atheroma in the bifurcation of the right and left internal and external carotid arteries, but all below 50%. Intima-media thickness is 0.15 cm. Myocardial scintigraphy does not show significant changes.
Plasma and Achilles tendon sterol analyses by gas-liquid chromatography coupled to mass spectrometry (Shimadzu GCM-QP2010 Plus, Kyoto, Japan) are shown for the present case, healthy controls and for our two unpublished typical CTX patients characterized by the elevated plasma cholestanol (
Table 1). Other authors have considered plasma cholestanol concentrations as normal below 5.0 µg/mL
4, elevated as 7.7 and 11.0 µg/mL in two CTX cases
5, or varying from 5.5 to 54.8 in several CTX cases
6.
Our case has no elevation of cholestanol in plasma and tendon, but the latter is rich in cholesterol crystals. Sitosterolemia was excluded by the very low concentrations of campesterol and sitosterol 7.
Two genetic variants in compound heterozygosity were identified in the CYP27A1 gene. One variant found in exon5, c.C1016T:p.T339M (rs121908102) has been related in several individuals with clinical signs of CTX, being considered a pathogenic variant 8,9,10. The other variant was found in intron 4, c.844+4A>G (rs1016174396) not directly altering the amino acid sequence of the CYP27A1 protein and described as uncertain significance.
Discussion
Due to the extensive necrosis of one of the tendons, our case could have been confounded with an unusual case of aspergilloma reported in the Achilles tendo 11, however, the latter occurred in only one tendon, not in both, as typically described in the metabolic alterations of familial hypercholesterolemia, CTX and sitosterolemia 12.
CTX is often accompanied by several clinical manifestations that include neuropsychiatric, gastrointestinal, premature atherosclerosis and cataract. Nevertheless, the presence of cataracts and atherosclerosis in our patient could be indistinctly attributed to her age as well as to the fact that she was a former cigarette smoker. However, it is unusual for the CTX patient to have large Achilles tendon injuries, apparently sparing other tendons, in addition to massive tendon necrosis and absence of any other manifestations, notably neurological. Cases of necrobiotic xanthogranuloma have been described, but all are characterized by multiple body lesions not limited to the Achilles tendons, nor showing typical genetic alteration of CTX as in our patient 13.
Our case presents an alteration in the CYP27A1 gene characteristic of the cerebrotendinous xanthomatosis (CTX) disease 14. However, it is also noteworthy that the patient, as in other reported cases 6, did not present neurological involvement, or elevation of cholestanol in the plasma and in the Achilles’ tendons. On the other hand, the extreme variability of manifestations in CTX is also exemplified by a recent case of a typical genetic alteration with elevated plasma cholestanol and neurological changes, but no xanthomata 3.
In summary, our case reported here shows that the CTX genetic alteration can occur in the absence of cholestanol elevation but with prominent xanthomata limited to the cholesterol-rich Achilles tendons causing severe inflammatory injury. This case leads us to admit that CTX is not necessarily characterized by increased cholestanol production due to alteration in the sterol 27-hydroxylase gene, but is a more complex pathology that may result from increased uptake or decreased cellular excretion of cholesterol. Molecular characterization of affected CTX families provides early diagnosis and treatment of homozygotes in the presymptomatic state as well as identification of heterozygotes, which is crucial for treatment and genetic counseling and for prenatal diagnosis. The patient is scheduled to be treated with chenodeoxycholic acid.
Author Contributions
R.J.A. was the treating physician; V.S.N carried out sterol analyses; E.R.N. developed genetic analysis and contributed to paper writing; E.C.R.Q. wrote the paper. All authors took part in drafting and revising the paper.
Conflicts of Interest
The authors have no conflict of interest to disclose.
References
- DeBarber AE, Duell PB. Update on cerebrotendinous xanthomatosis. Curr Opin Lipidol. 2021;32(2):123-131. [CrossRef]
- 2. Duell PB, Salen G, Eichler FS, et al. Diagnosis, treatment, and clinical outcomes in 43 cases with cerebrotendinous xanthomatosis. J Clin Lipidol. 2018;12(5):1169-1178. [CrossRef]
- Stenos C, Kalafatakis K, Constantoulakis P, et al. A case of cerebrotendinous xanthomatosis with brain and spinal involvement without tendon xanthomas: Identification of a novel mutation of the CYP27A1 gene. J Clin Lipidol. 2022;16(3):281-285. [CrossRef]
- 4. Guenzel AJ, DeBarber A, Raymond K, Dhamija R. Familial variability of cerebrotendinous xanthomatosis lacking typical biochemical findings. JIMD Rep. 2021;59(1):3-9. [CrossRef]
- Nozue T, Higashikata T, Inazu A, et al. Identification of a novel missense mutation in the sterol 27-hydroxylase gene in two Japanese patients with cerebrotendinous xanthomatosis. Intern Med. 2010;49(12):1127-1131. [CrossRef]
- Stelten BML, Raal FJ, Marais AD, et al. Cerebrotendinous xanthomatosis without neurological involvement. J Intern Med. 2021;290(5):1039-1047. [CrossRef]
- Tada H, Nomura A, Ogura M, et al. Diagnosis and Management of Sitosterolemia 2021. J Atheroscler Thromb. 2021;28(8):791-801. [CrossRef]
- Verrips A, Hoefsloot LH, Steenbergen GC, et al. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 2000;123 ( Pt 5):908-919. [CrossRef]
- Appadurai V, DeBarber A, Chiang P-W, et al. Apparent underdiagnosis of Cerebrotendinous Xanthomatosis revealed by analysis of ~60,000 human exomes. Mol Genet Metab. 2015;116(4):298-304. Mol Genet Metab. [CrossRef]
- Meiner V, Meiner Z, Reshef A, Björkhem I, Leitersdorf E. Cerebrotendinous xanthomatosis: molecular diagnosis enables presymptomatic detection of a treatable disease. Neurology. 1994;44(2):288-290. [CrossRef]
- Park YH, Kim YH, Kim JY, Choi GW, Kim HJ. Aspergilloma clinically mimicking Achilles tendon xanthoma in a non-immunocompromised patient: A case report. Foot ankle Surg Off J Eur Soc Foot Ankle Surg. 2020;26(8):943-945. [CrossRef]
- Zech LAJ, Hoeg JM. Correlating corneal arcus with atherosclerosis in familial hypercholesterolemia. Lipids Health Dis. 2008;7:7. [CrossRef]
- Nelson CA, Zhong CS, Hashemi DA, et al. A Multicenter Cross-Sectional Study and Systematic Review of Necrobiotic Xanthogranuloma With Proposed Diagnostic Criteria. JAMA dermatology. 2020;156(3):270-279. [CrossRef]
- Björkhem I. Cerebrotendinous xanthomatosis. Curr Opin Lipidol. 2013;24(4):283-287. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).