Preprint
Brief Report
Challenging Standard Protocols: A New Perspective on MMR Deficiency from a Longitudinal Regional Study
Altmetrics
Downloads
100
Views
26
Comments
0
This version is not peer-reviewed
Submitted:
07 December 2023
Posted:
12 December 2023
You are already at the latest version
Alerts
Abstract
The role of DNA mismatch repair (MMR) tumor testing in colon and endometrial carcinomas is well-established, yet the universal application of this testing faces challenges due to limited data on its efficacy in diverse populations. This retrospective study at a community hospital in Northern Indiana examined MMR immunohistochemistry (IHC) results across 549 cases. Our analysis found intact MMR expression in 469 cases, while 80 demonstrated MMR deficiency, translating to MMR deficiency rates of 10% in colon cancers and 22% in uterine carcinomas. Notably, only 16 patients (2.9%) were categorized as high microsatellite instability (MSI-H), a rate significantly lower than the 3-5-fold higher incidences reported in other Midwestern populations, including Ohio. These findings suggest that the benefits of universal cascade methylation testing, particularly in MLH1/PMS2 IHC-negative tumors, may vary significantly based on regional demographics. Given the notably lower MSI-H incidence in this study, re-evaluating reflex testing protocols in low-resource settings could lead to more targeted, cost-effective approaches without compromising diagnostic accuracy. This study challenges the one-size-fits-all approach to MMR testing, highlighting the need for tailored strategies that consider regional variations in cancer genetics.
Keywords:
Subject: Medicine and Pharmacology - Pathology and Pathobiology
1. Introduction
Lynch syndrome (LS), also known as hereditary nonpolyposis colorectal cancer (HNPCC), is an autosomal dominant disorder. It is characterized by mutations in the DNA mismatch repair (MMR) system. These mutations predispose individuals to various tumors. The most notable of these tumors are colorectal and endometrial cancers. Central to LS are the MMR genes: MLH1, MSH2, MSH6, and PMS2. Reflex testing for MMR proteins is widely advocated in all new cases of colon and endometrial cancers. This testing facilitates the early detection of LS. It also aids in the proactive management of associated malignancies. Additionally, it enables genetic counseling for affected families.
Despite the consensus on universal tumor screening for LS using microsatellite instability (MSI) and MMR immunohistochemistry (IHC), the prevalence and spectrum of MMR deficiencies exhibit considerable variation, influenced by ethnic and geographic factors [1,2,3]. Yet there is a gap in understanding these variations, especially in community hospital settings, which are crucial for developing tailored screening protocols.
Addressing this gap, our study embarked on a retrospective analysis of MMR IHC results from a community hospital in Northern Indiana. This region-specific study aims to shed light on the incidence of MMR deficiencies in a specific demographic, questioning the uniform application of current testing protocols and highlighting the need for regionally adapted strategies, particularly in settings with limited resources. By focusing on regional variations, our research seeks to contribute to a more efficient and cost-effective approach in the management and surveillance of LS and associated cancers.
2. Materials and Methods
We reviewed and categorized all cases in which MMR IHCs were performed and examined at this institution from 2015 to 2021 based on tumor type and location. Immunohistochemical studies (IHC) were performed on surgical specimens for a four-panel of proteins, including MLH1, PMS2, MSH2, and MSH6. A positive result was defined as the loss of one or more of the four MMR proteins by immunohistochemical staining. Board-certified pathologists made the reading and interpretation. (Figure 1) In cases with dual loss of MLH1/PMS2 expression, the PCR-based MLH 1 promotor methylation assay was utilized to distinguish between sporadic epigenetic silencing of MLH1 by methylation versus loss of MLH1 without methylation, suggesting genetic mutations such as those involved in Lynch Syndrome. Relevant clinical information was extracted from the pathology report for all cases with MMR IHC.
3. Results
A total of 549 MMR IHC tests were conducted during the reviewed period. Information on tumor type, organ, and primary or metastatic characteristics is provided in Table 1. Intact MMR expression was identified in 469 cases, while MMR deficiency was identified in 80 cases, consisting of 28 out of 272 colon cancers (10%) and 52 out of 241 uterine carcinomas (22%). A summary of abnormal MMR IHCs can be seen in Table 2. Dual loss of MLH1/PMS2 was observed in 66 cases (82.5%), loss of MSH6 in 2 cases (2.5%), loss of PMS2 in 6 cases (7.5%), and dual loss of MSH2/MSH6 in 6 cases (7.5%). Colon and uterine cancers demonstrated different rates of abnormal MMR staining. Among 272 colon cancers, 28 (10%) exhibited abnormal MMR IHC, whereas 52 out of 241 (22%) uterine cancers exhibited loss of nuclear stains. Dual loss of MLH1/PMS2 was observed in 21 out of 28 (75%) abnormal MMR cases in colon tumors and 45 out of 52 (87%) in uterine cancers.
Cases with dual MLH1/PMS2 loss were followed up with cascade somatic hypermethylation analysis of the MLH1 promoter locus. Sixty-one of the 66 patients (92.4%) tested positive for MLH1 promoter hypermethylation. Two cases were negative for hypermethylation, while the remaining three cases had insufficient tissue for additional testing. Among all tested patients, 16 (2.9%) were categorized as MSI-H. Dual MLH1/PMS2 loss represented 82.5% of all MMR deficiency, while only 3% of patients were not hypermethylated in MLH1/PMS2 IHC loss cases.
4. Discussion
MMR testing for colon and endometrial carcinomas is widely adopted. However, comprehensive data on the prevalence and range of MMR deficiencies in cancer patients is still limited. Our study, conducted in Northern Indiana, USA, offers crucial insights. It reveals the variability of MMR deficiency in cancer, which goes beyond the complexities of conventional genetic analysis. This variability raises important questions. It makes us consider the underlying biological mechanisms of MMR deficiency. It also brings into focus broader genetic and environmental factors. These factors influence MMR deficiency rates in different populations. Our research focused on a cohort with a significant Amish demographic. This group is known for its unique genetic lineage and lifestyle. These characteristics suggest that our findings could have broader implications. They require examination in diverse genetic and environmental settings. The work of Lynch et al. [4] and others underscores the importance of considering distinct genetic backgrounds in understanding cancer risks and treatment responses. Comparing our findings with studies from other U.S. regions and internationally is crucial. It helps us understand the full spectrum of MMR deficiency. These comparisons provide insights. They show how lifestyle, dietary habits, and environmental exposures interact with genetic predispositions. This interaction influences the prevalence and manifestations of MMR deficiency. For instance, Campbell’s research sheds light on the complexity of MMR-related cancer risks. It highlights the significant interplay between MLH1 polymorphisms and lifestyle factors. Factors like smoking and a Western diet notably modify the risk of colon cancer [5]. These findings highlight an intricate relationship. It’s between genetic variations and environmental factors in the development of colon cancer. This aligns with our study’s focus on diverse genetic backgrounds and lifestyle factors.
To our knowledge, this is the first report on MMR analysis from a community hospital in Indiana. Our study population exhibited an MSI-H rate of 2.9%, markedly lower than previously reported rates and about half of those reported in Ohio [6,7,8,9]. This demographic, including a substantial Amish population, may show significant variances from the general population, which is noteworthy. The unique genetic pool of the Amish population in our study might contribute to the observed variations in MMR deficiency rates. Research by Caldes et al. [1] and Nyström-Lahti et al. [2] has highlighted the role of genetic diversity in cancer susceptibility. A more comprehensive genetic analysis of our cohort could reveal specific mutations or patterns prevalent in this group, providing insights into their association with MMR deficiency rates. This includes investigating the prevalence of known high-risk mutations in genes such as MLH1, MSH2, and MSH6, and identifying any novel mutations or variants unique to this population.
Our research aimed to determine the frequency and patterns of MMR deficiency in a significant cohort of colon and uterine cancer cases, using IHC testing. We detected MMR deficiency in 80 cases (14.6%), including 28 of 272 colon cancers (10%) and 52 of 241 uterine carcinomas (22%), each showing loss of nuclear staining. The concurrent loss of MLH1/PMS2, observed in 66 cases (82.5%), was the most frequent abnormality, aligning with previous studies that reported a high prevalence of MLH1/PMS2 loss in both colon and uterine cancers.
The different rates of abnormal MMR IHC between colon and uterine cancers highlight the importance of organ-specific evaluations when assessing MMR deficiencies. Uterine cancers showed a higher rate of MMR deficiency compared to colon cancers, which might be due to a greater prevalence of Lynch syndrome among women with uterine cancer or other genetic factors that predispose women to developing MMR-deficient tumors.
We also assessed the MLH1 promoter hypermethylation status in cases with simultaneous MLH1/PMS2 loss. The majority of these cases (92.4%) showed MLH1 promoter hypermethylation, indicating that most cases of dual MLH1/PMS2 loss likely result from sporadic MLH1 inactivation rather than germline mutations. This finding is consistent with previous studies reporting high rates of MLH1 promoter hypermethylation in colorectal and endometrial cancers with MLH1/PMS2 loss.
Interestingly, 3% of patients with dual MLH1/PMS2 loss did not exhibit hypermethylation. This subgroup may carry germline mutations in the MLH1 gene, necessitating further genetic testing to identify potential Lynch syndrome cases. Detecting such cases is crucial for implementing appropriate surveillance strategies and reducing Lynch syndrome-associated morbidity and mortality. However, our cohort may not fully represent the broader population. Second, we did not perform MSI testing or germline mutation analysis for all patients with MMR deficiency. Therefore, we cannot conclusively determine the cause of MMR deficiency in these cases. Nonetheless, our study offers significant insights into the frequency and patterns of MMR deficiency in colon and uterine cancers, emphasizing the need for organ-specific considerations in evaluating MMR status.
The varied rates of MMR deficiency observed in our study have important implications for Lynch Syndrome screening protocols. Research by Tannergård et al. [3] suggests that a universal approach to Lynch Syndrome screening may not be effective, especially in populations with unique genetic backgrounds. Developing tailored screening strategies based on specific regional genetic and demographic data could lead to more effective identification and management of Lynch Syndrome. This could involve creating specific genetic panels optimized for regional populations, considering the local prevalence of particular MMR gene mutations.
The economic aspects of MMR testing protocols, particularly in resource-limited settings, are a significant concern. Studies like Uson et al. [8] have started addressing the cost implications of genetic testing. Our study contributes to this discussion by highlighting the need for cost-effective testing strategies tailored to specific regional requirements, ensuring efficient resource allocation in healthcare systems. This might include a comprehensive cost-benefit analysis of different testing strategies, such as universal screening versus targeted screening based on family history or other risk factors, considering the cost of testing, the potential for early cancer detection, and the overall impact on patient outcomes.
Technological advancements in genetic testing, as discussed by Edwards and Monahan [9], are poised to transform the field of cancer diagnostics. Our findings pave the way for new research avenues. Future studies incorporating MMR deficiency testing and genetic counseling are needed to fully understand the clinical implications of MMR deficiencies. These studies could offer valuable insights into the natural history of MMR-deficient tumors, the efficacy of different screening and prevention strategies, and the impact of genetic counseling on patient outcomes. Moreover, research into integrating genetic counseling into routine cancer care, especially in populations with high rates of MMR deficiency, could enhance patient understanding of their genetic risk, inform treatment decisions, and potentially improve adherence to recommended surveillance and prevention strategies.
While our study provides valuable insights, it’s important to acknowledge its limitations, including its retrospective nature and focus on a specific demographic. However, the strength of our research lies in its detailed examination of a large cohort, allowing for a focused exploration of MMR deficiencies in a specific population. This approach contributes to our understanding of regional variations in Lynch Syndrome and related cancers and underscores the importance of personalized approaches to cancer screening and management.
The findings of our study extend beyond the immediate clinical implications for Lynch Syndrome screening. They highlight the necessity of moving towards more personalized diagnostic strategies in cancer care. Tailoring diagnostic approaches to specific regional genetic and demographic factors can lead to more effective and personalized cancer care, ultimately contributing to better patient outcomes and more efficient healthcare systems. Our study not only adds to the existing body of knowledge on MMR deficiency in colon and endometrial cancers but also opens up new paths for future exploration. The integration of regional genetic data into screening protocols, the adoption of advanced diagnostic technologies, and the consideration of economic aspects in testing protocols are all critical areas warranting further investigation. As the field of cancer diagnostics evolves, a nuanced understanding of MMR deficiency across diverse populations will be pivotal in enhancing cancer diagnostics and treatment strategies, ultimately contributing to better patient outcomes and healthcare efficiency.
5. Conclusions
This study reveals notable regional variations in MMR deficiency, with a higher prevalence in uterine versus colon cancers in Northern Indiana. The findings, particularly the lower incidence of high microsatellite instability (MSI-H), suggest the need for re-evaluated, region-specific MMR testing protocols, especially in resource-limited settings. Our research emphasizes the importance of tailoring cancer diagnostic strategies to regional genetic variations, aiming for more efficient and cost-effective healthcare outcomes.
References
- Caldes, T.; Godino, J.; De La Hoya, M.; Garcia Carbonero, I.; Perez Segura, P.; Eng, C.; et al. Germline mutations of MLH1 and MSH2 in hereditary nonpolyposis colorectal cancer families from Spain: A prevalence study. Int J Cancer. 2002, 98, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Nyström-Lahti, M.; Wu, Y.; Moisio, A.-L.; Hofstra, R.M.W.; Osinga, J.; Mecklin, J.-P.; Järvinen, H.J.; Leisti, J.; Buys, C.H.C.M.; de la Chapelle, A.; et al. DNA mismatch repair gene mutations in 55 kindreds with verified or putative hereditary non-polyposis colorectal cancer. Hum. Mol. Genet. 1996, 5, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Tannergård, P.; Lipford, J.R.; Kolodner, R.; Frödin, J.E.; Nordenskjöld, M.; Lindblom, A. Screening for hMLH1 gene mutations in Swedish hereditary nonpolyposis colon cancer families. Cancer Res. 1995, 55, 6092–6096. [Google Scholar] [PubMed]
- Lynch, H.; Lynch, P.; Lanspa, S.; Snyder, C.; Lynch, J.; Boland, C. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin. Genet. 2009, 76, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Curtin, K.; Ulrich, C.M.; Samowitz, W.S.; Bigler, J.; Velicer, C.M.; Caan, B.; Potter, J.D.; Slattery, M.L. Mismatch repair polymorphisms and risk of colon cancer, tumour microsatellite instability and interactions with lifestyle factors. Gut 2008, 58, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, R.; Frankel, W.L.; Swanson, B.J.; Jones, D.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; et al. Prospective Statewide Study of Universal Screening for Hereditary Colorectal Cancer: The Ohio Colorectal Cancer Prevention Initiative. JCO Precis. Oncol. 2021, 5, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, F.; Ulivi, P.; Tedaldi, G. Genetic predisposition to colorectal cancer: A review of genes to test. Int. J. Mol. Sci. 2023, 24, 2137. [Google Scholar] [CrossRef] [PubMed]
- Uson, P.L.; Riegert-Johnson, D.; Boardman, L.; Kisiel, J.; Mountjoy, L.; Patel, N.; Lizaola-Mayo, B.; Borad, M.J.; Ahn, D.; Sonbol, M.B.; et al. Germline Cancer Susceptibility Gene Testing in Unselected Patients With Colorectal Adenocarcinoma: A Multicenter Prospective Study. Clin. Gastroenterol. Hepatol. 2021, 20, e508–e528. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.; Monahan, K.J. J. Frontline Gastroenterology. 2022, 13, e80–e87. [CrossRef] [PubMed]
Figure 1.
Illustration of MMR IHC loss in colon adenocarcinoma. A. Altered MLH1 expression. B. Deviant expression of PMS2. C. Typical expression of MSH6. D. Standard expression of MSH2.
Figure 1.
Illustration of MMR IHC loss in colon adenocarcinoma. A. Altered MLH1 expression. B. Deviant expression of PMS2. C. Typical expression of MSH6. D. Standard expression of MSH2.
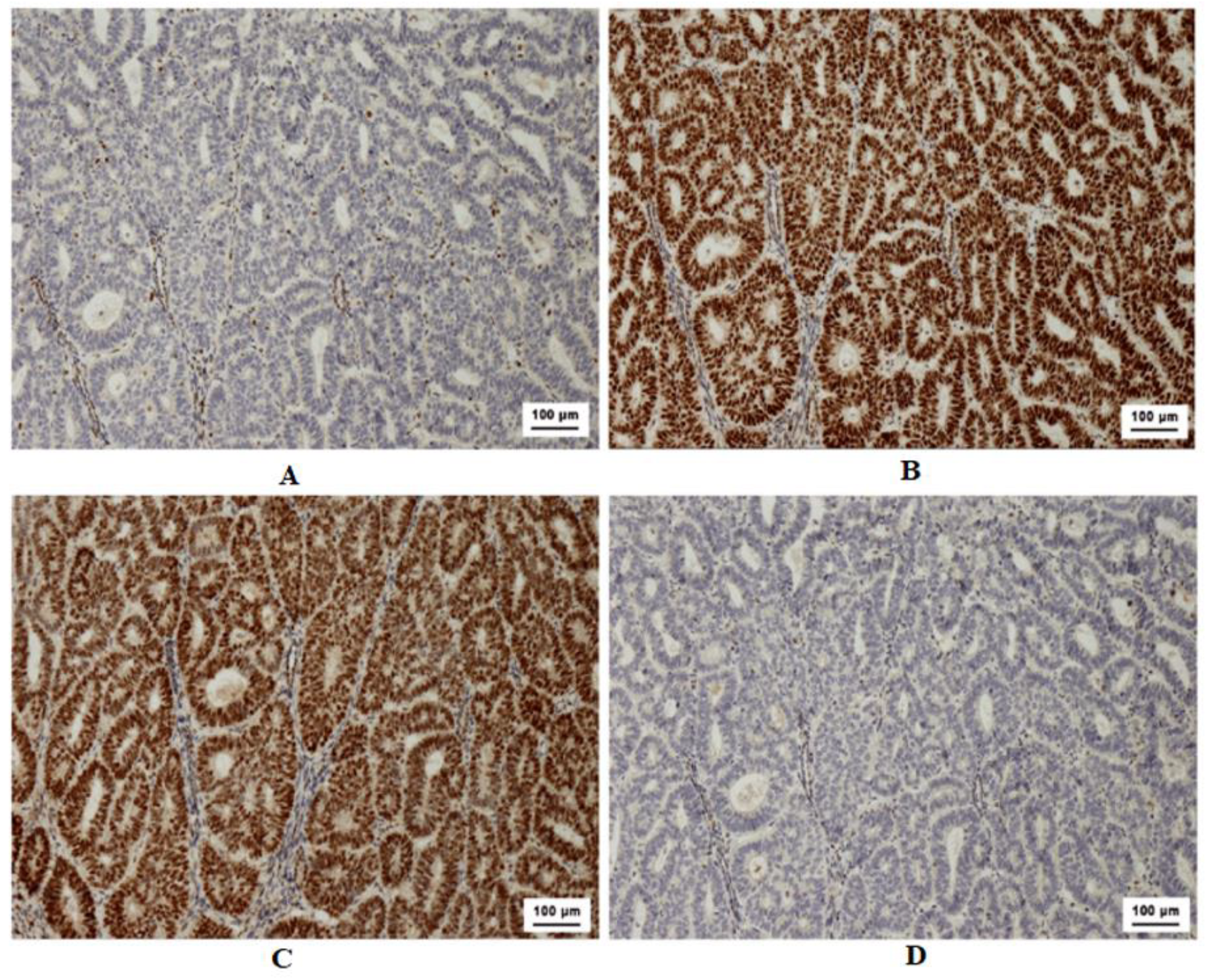
Table 1.
Distribution of MMR Expression Across Cancer Types.
| Cancer Type | Total Cases | MMR Intact Expression | MMR Abnormal Expression |
|---|---|---|---|
| Colon | 225 | 202 | 23 |
| Colon (metastatic) | 47 | 42 | 5 |
| Uterus | 232 | 181 | 51 |
| Uterine (metastatic) | 9 | 8 | 1 |
| Esophagus | 6 | 6 | 0 |
| Pancreas | 4 | 4 | 0 |
| Pancreas (Metastatic) | 1 | 1 | 0 |
| Stomach | 2 | 2 | 0 |
| Appendix | 1 | 1 | 0 |
| Ampulla | 1 | 1 | 0 |
| Ovary | 3 | 3 | 0 |
| Ovary (metastatic) | 3 | 3 | 0 |
| Breast (metastatic) | 1 | 1 | 0 |
| Lymphoma | 4 | 4 | 0 |
| Sarcoma | 1 | 1 | 0 |
| Lung | 1 | 1 | 0 |
| Mesothelioma | 2 | 2 | 0 |
| Skin | 2 | 2 | 0 |
| Bladder | 1 | 1 | 0 |
| Prostate | 1 | 1 | 0 |
| Unknown Primary | 1 | 1 | 0 |
Table 2.
Abnormal MMR Expression in Colon and Uterine Cancers (Total 80).
| Tumor Type | MLH1/PMS2 loss | MSH2/MSH6 loss | MSH6 loss | PMS2 loss | Total Abnormal MMR |
|---|---|---|---|---|---|
| Colon cancer | 21 (75%) | 4 (14.3%) | 1 (3.6%) | 2 (7.1%) | 28 (5.1%) |
| Uterine carcinoma | 45 (87%) | 2 (3.8%) | 1 (1.9%) | 4 (7.7%) | 52 (9.5%) |
| Total | 66 (82.5%) | 6 (7.5%) | 2 (2.5%) | 6 (7.5%) | 80 (14.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





