Submitted:
08 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Brake Dynamometer Test and Airborne Brake-wear Particle Measurement
2.2. X-ray Absorption Fine Structure Analysis
3. Results and Discussion
3.1. Charactorization of Brake-wear Particles
3.1.1. Brake Pad and Disc Wear
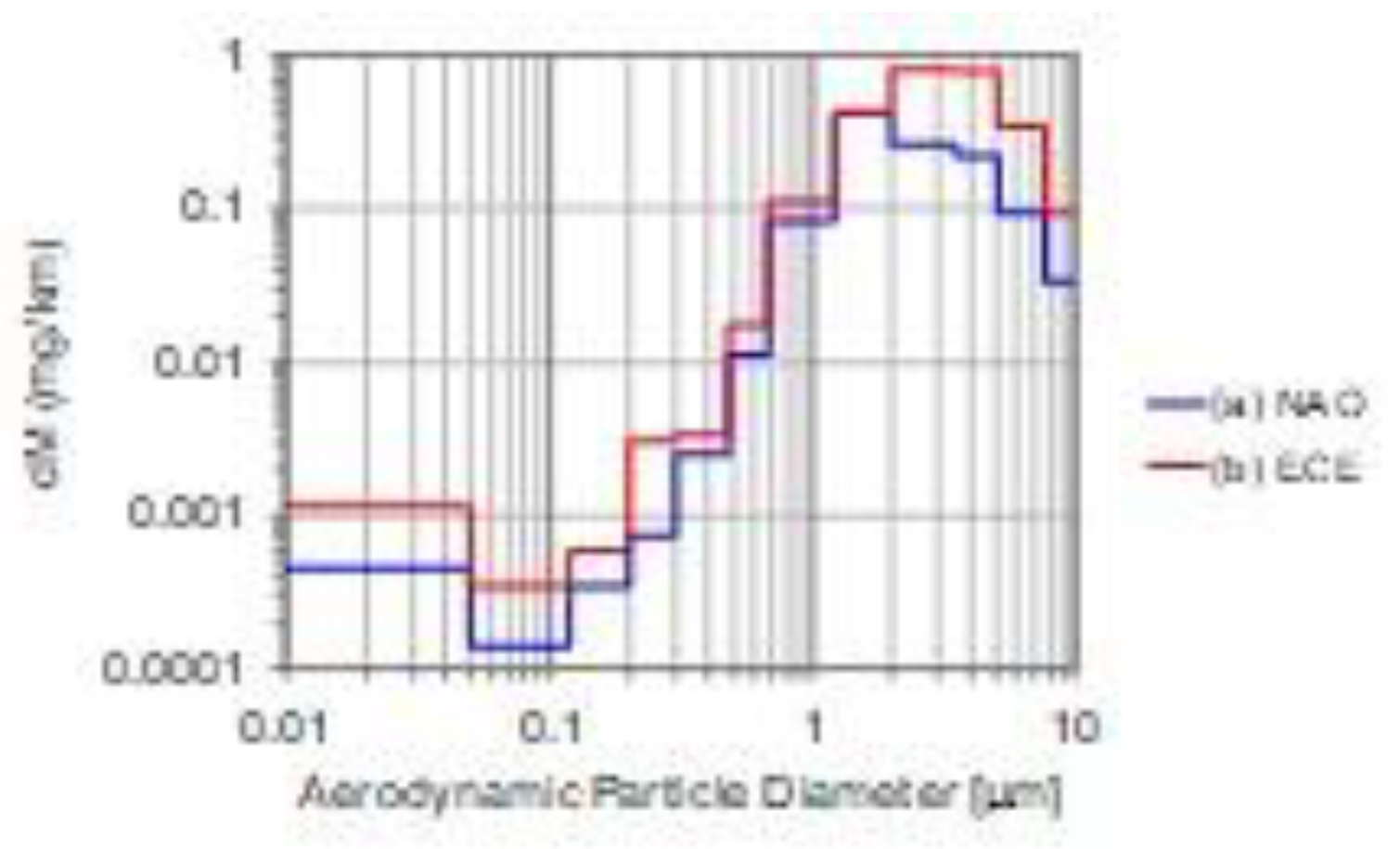
3.1.2. Brake-wear Particle Emissions
3.1.3. Chemical Characterization
3.2. Iron Speciation
3.2.1. XAFS Spectra for Reference Materials
3.2.2. XAFS Spectra for Brake-wear Particles
3.2.3. Iron Speciation
3.2.4. Phase Transformation
3.2.5. Tribo-Reduction
3.2.6. Tribo-Oxidations
4. Conclusions
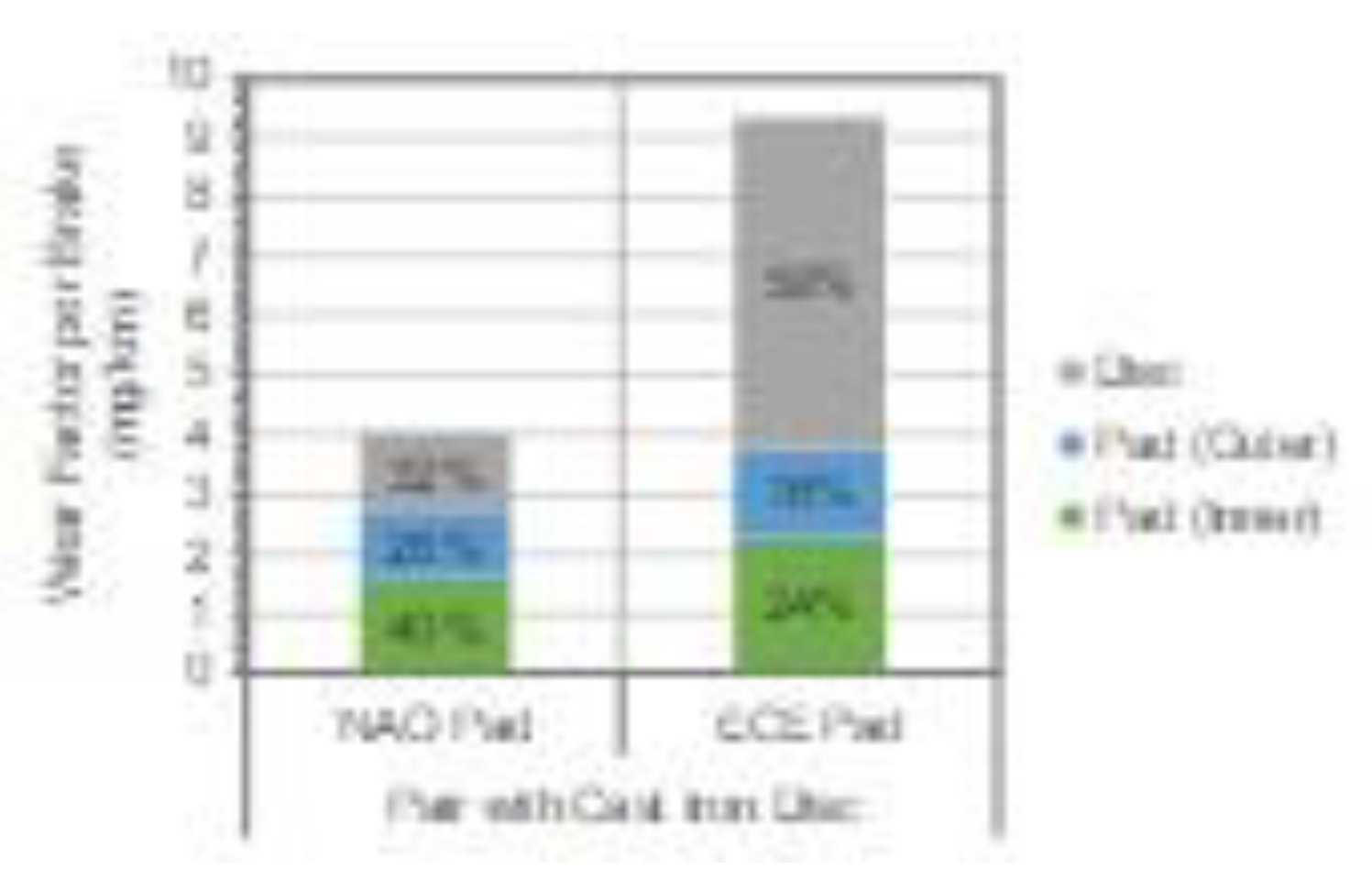
- Significant differences in wear coefficients and PM10 emissions were observed between NAO and ECE brake pads. Mass losses as wear factors were found to be 4.02 mg/km per brake for the NAO pads and 9.31 mg/km per brake for the ECE pads. Emissions of brake-wear particles were 1.43 ± 0.29 mg/km per brake for PM10 with NAO and in 3.73 ± 0.19 mg/km per brake for PM10 with ECE.
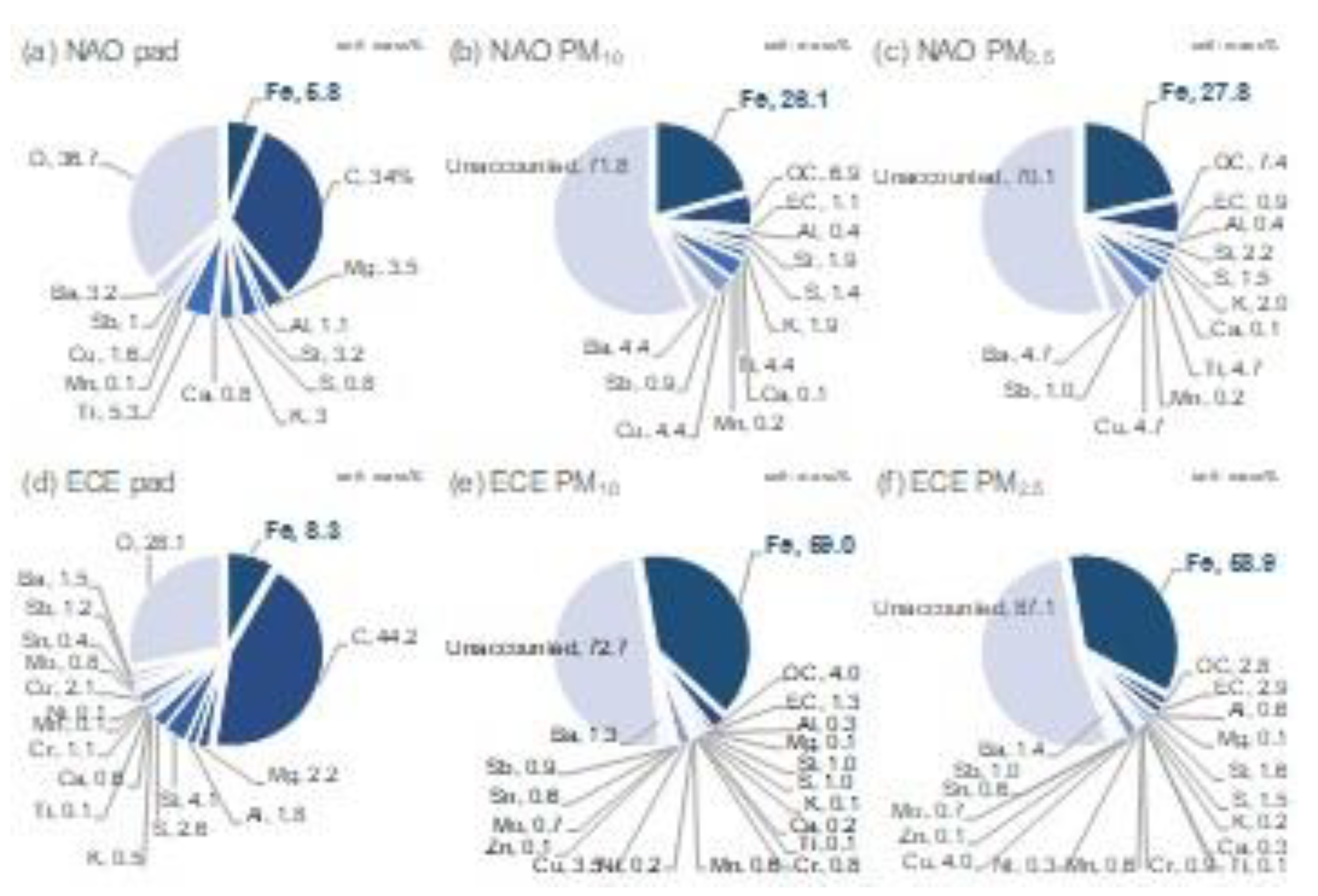
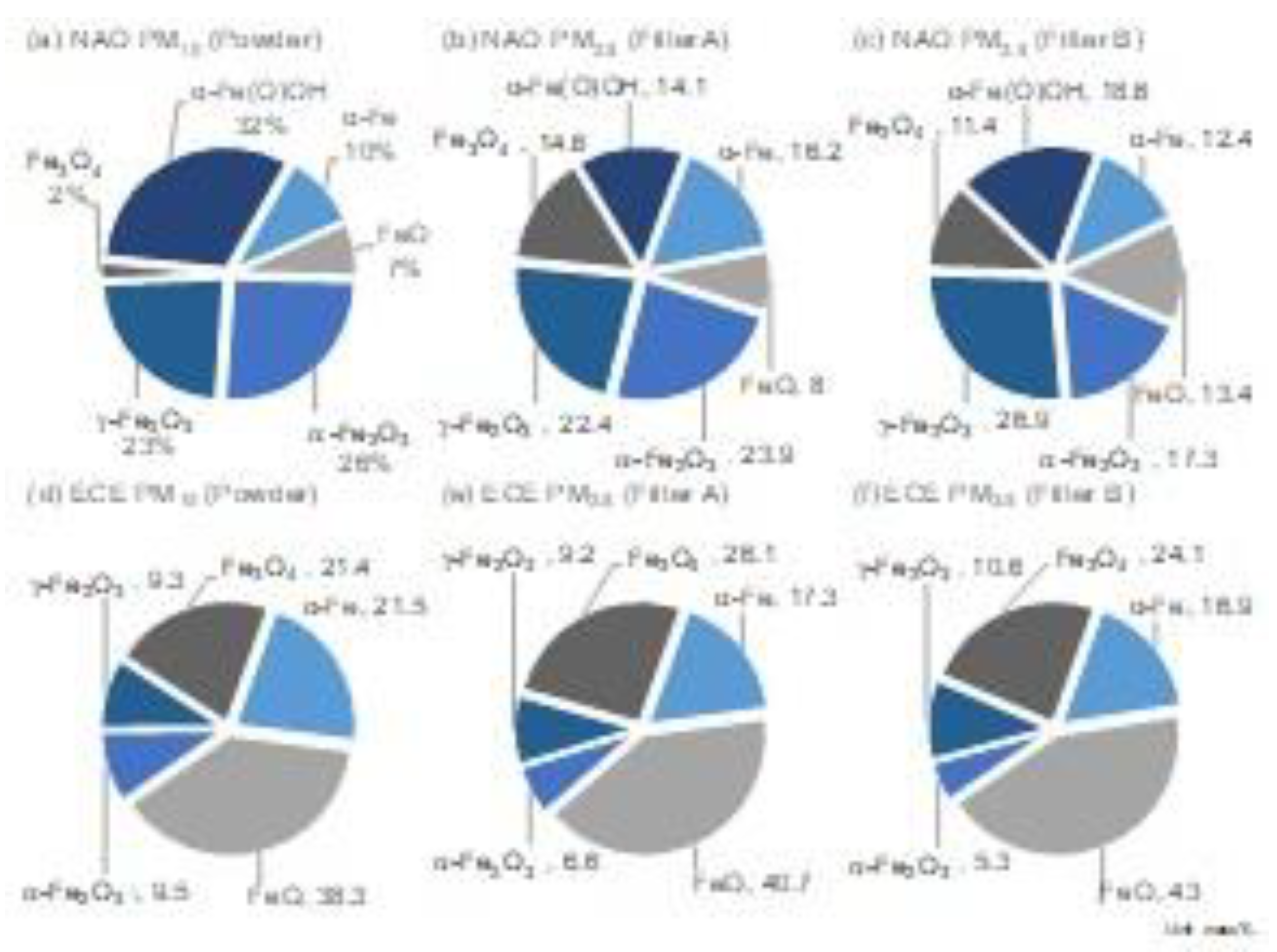
- The dominant contribution to PM10 and PM2.5 brake-wear particles was Fe for both NAO and ECE. The iron concentration ratio in the particle mass were comparable to the disc to pads ratio measured by wear mass.
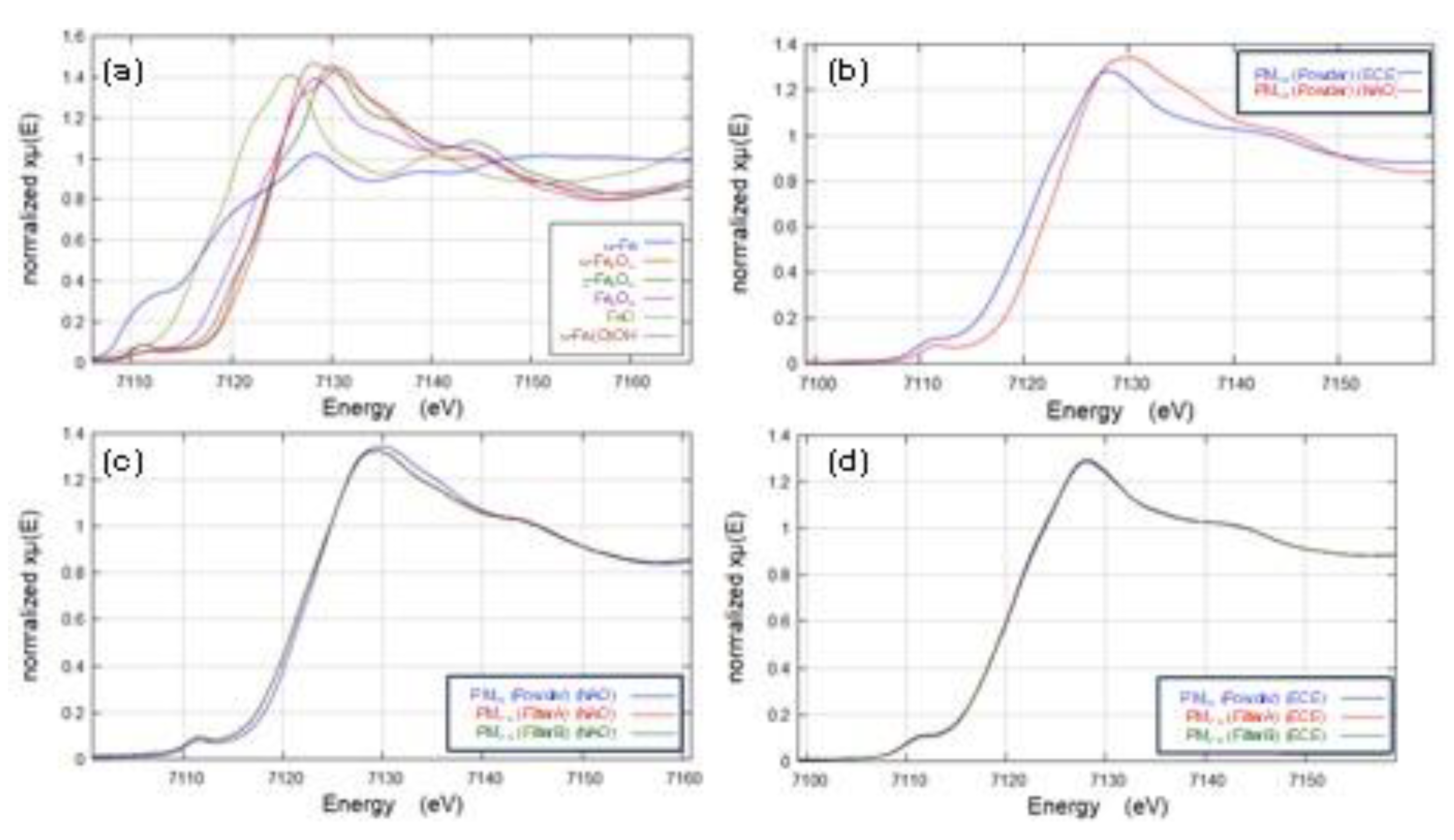
- Differences in the consistency of iron oxides and hydroxides were observed between NAO and ECE brake pads.
- The hydroxide goethite (α-Fe(O)OH, Fe (III)) was detected only in the NAO pad. It is difficult to fully explain the mechanism based on only the bulk temperature, but a high goethite (α-Fe(O)OH, Fe (III)) contribution in the NOA brake was suggested because of the phase transformation from goethite (α-Fe(O)OH, Fe (III)) to iron oxides Fe2O3 (hematite (α-Fe2O3, Fe (III)) and Maghemite (γ-Fe2O3, Fe (III))) in this study.
- Metallic iron (α -Fe, Fe (0)) was generated not only from abrasive wear but also from the tribo-chemical reduction with magnetite (Fe3O4, Fe (II, III)) as the starting material.
- Magnetite (Fe3O4, Fe (II, III)), which is of interest from the point of view of health effects, was less abundant in NAO than in ECE. The implication is that magnetite (Fe3O4, Fe (II, III)) is less likely to form in NAO due to tribo-oxidations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016; p. 131. [Google Scholar]
- Beddows, D. C. S.; Harrison, R. M. PM10 and PM2.5 Emission Factors for Non-Exhaust Particles from Road Vehicles: Dependence upon Vehicle Mass and Implications for Battery Electric Vehicles. Atmos. Environ. 2021; 244, 117886. [Google Scholar] [CrossRef]
- Pattammattel, A.; Leppert, V. J.; Aronstein, P.; Robinson, M.; Mousavi, A.; Sioutas, C.; Forman, H. J.; O’Day, P. A. Iron speciation in particulate matter (PM2.5) from urban Los Angeles using spectro-microscopy methods. Atmos. Environ. 2021; 245, 117988. [Google Scholar] [CrossRef]
- Kukutschová, J.; Filip, P. Chapter 6—Review of Brake-wear Emissions: a Review of Brake Emission Measurement Studies: Identification of Gaps and Future Needs. Amato, F. (Ed.), Non-Exhaust Emissions, Academic Press, 2018, pp. 123-146. [CrossRef]
- Osterle, W.; Dmitriev, A. I. The role of solid lubricants for brake friction materials. Lubricants 2016, 4, 22. [Google Scholar] [CrossRef]
- Gonet, T.; Maher, B. A. Airborne, Vehicle-Derived Fe-Bearing Nanoparticles in the Urban Environment: A Review. Environ. Sci. Technol. 2019, 53, 9970–9991. [Google Scholar] [CrossRef] [PubMed]
- Gonet, T.; Maher, B.A.; Nyirő-Kósa, I.; Pósfai, M.; Vaculík, M. ; Kukutschová. J. Size-resolved, Quantitative Evaluation of the Magnetic Mineralogy of Airborne Brake-Wear Particulate Emissions. Environ. Pollut 2021, 288, 117808. [Google Scholar] [CrossRef] [PubMed]
- Nyirő-Kósa, I.; Ahmad, F.; Hoffer, A.; Pósfai, M. Nanoscale Physical and Chemical Properties of Individual Airborne Magnetic Particles from Vehicle Emissions. Atmos. Environ. X 2022, 15, 100181. [Google Scholar] [CrossRef]
- Maher, B.A. Airborne Magnetite- and Iron-Rich Pollution Nanoparticles: Potential Neurotoxicants and Environmental Risk Factors for Neurodegenerative Disease, Including Alzheimer’s Disease. J. Alzheim. Dis. 2019, 71, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Gramstat, S.; Mertens, T.; Waninger, R.; Lugovyy, D. Impacts on Brake Particle Emission Testing. Atmosphere 2020, 11, 1132. [Google Scholar] [CrossRef]
- Österle, W.; Deutsch, C.; Gradt, T.; Orts-Gil, G.; Schneider, T.; & Dmitriev, A. I. Tribological screening tests for the selection of raw materials for automtive brake pad formulations. Tribo. Int. 2014, 73, 148–155. [Google Scholar] [CrossRef]
- Filip, P.; Weiss, Z.; Rafaja, D. On Friction Layer Formation in Polymer Matrix Composite Materials for Brake Applications. Wear 2002, 252, 189–198. [Google Scholar] [CrossRef]
- Verma, P. C.; Menapace, L.; Bonfanti, A.; Ciudin, R.; Gialanella, S.; Straffelini, G. Braking Pad-Disc System: Wear Mechanism and Formation of Wear Fragments. Wear 2015, 322–323, 251–258. [Google Scholar] [CrossRef]
- Mathissen, M.; Grochowicz, J.; Schmidt, C.; Vogt, R.; zum Hagen, F.H.F.; Grabiec, T.; Steven, H.; Grigoratos, T. A Novel Real-World Braking Cycle for Studying Brake Wear Particle Emissions. Wear 2018, 414–415, 219–226. [Google Scholar] [CrossRef]
- Jing, W.; Saito, K.; Okamoto, T.; Saito, H.; Sugimoto, K.; Nishita-Hara, C.; Hara, K.; Hayashi, M.; Hasegawa, S.; Okuda, T. Characterization of Elemental Composition and Valence State of Cyclone-collected Aerosol Particles Using EDXRF and XAFS at Three Sites in Japan. Asian J. Atmos. Environ. 2022, 16, 40–58. [Google Scholar] [CrossRef]
- Saito, K.; Okuda, T.; Hasegawa, S.; Nishita-Hara, C.; Hara, K.; Hayashi, M. (2020) Chemical Speciation of Chromium in Atmospheric Particulate Matter Collected with Cyclone by XAFS Method. J. Jpn. Soc. Atmos. Environ. 2020, 55, 27–33, (in Japanese with English Abstract). [Google Scholar] [CrossRef]
- Hagino, H.; Oyama, M.; Sasaki, S. Laboratory Testing of Airborne Brake Wear Particle Emissions using a Dynamometer System under Urban City Driving Cycles. Atmos. Environ. 2016, 131, 269–278. [Google Scholar] [CrossRef]
- JASO C470. Passenger car—Measurement Method for Brake Wear Particle Emissions. 2020, Edition, March 31, 2020.
- Mathissen, M.; Grochowicz, J.; Schmidt, C.; Vogt, R.; Zum Hagen, F.H.F.; Grabiec, T.; Steven, H.; Grigoratos, T. A Novel Real-World Braking Cycle for Studying Brake Wear Particle Emissions. Wear 2018, 414–415, 219–226. [Google Scholar] [CrossRef]
- GRPE-2023-4e Rev.V6. Clean-(PMP) Proposal to Amend ECE/TRANS/WP.29/GRPE/2023/4. Proposal for a New UN GTR on Laboratory Measurement of Brake Emissions for Light-Duty Vehicles. Available online: https://wiki.unece.org/download/attachments/172852339/GRPE-2023-4e%20Rev.V6.docx?api=v2 (accessed on 22 November 2022).
- Rule, A.; Geyh, A.; Ramos-Bonilla, J. P.; Mihalic, J. N.; Margulies, J. D.; Polyak, L. M.; Kesavan, J.; Breysse, P. Design and Characterization of a Sequential Cyclone System for the Collection of Bulk Particulate Matter. J. Environ. Monit. 2010, 12, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Isobe, R.; Nagai, Y.; Okahisa, S.; Funato, K. Inoue, K. Development of a High-Volume PM2.5 Particle Sampler Using Impactor and Cyclone Techniques. Aerosol Air Qual. Res. 2015, 15, 759–767. [Google Scholar] [CrossRef]
- Ishihara, N.; Okuda, T.; Hagino, H.; Oguro, A.; Tani, Y.; Okochi, H.; Tokoro, C.; Fujii-Kuriyama, Y. ; Itoh, Vogel, C.F.A.; Ishihara, Y. Involvement of Polycyclic Aromatic Hydrocarbons and Endotoxin in Macrophage Expression of Interleukin-33 Induced by Exposure to Particulate Matter. J. Toxicol. Sci. 2022, 47, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Crow, D.; Lowenthal, D.H.; Merrifield, T. Comparison of IMPROVE and NIOSH Carbon Measurements. Aerosol Sci. Technol. 2001, 34, 23–34. [Google Scholar] [CrossRef]
- Spada, N.J.; Yatkin, S.; Giacomo, J.; Trzepla, K.; Hyslop, N.P. Evaluating IMPROVE PM2.5 Element Measurements. J. Air Waste Manag. Assoc. 2023, 73, 843–852. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Grigoratos, T.; Mathissen, M.; Vedula, R.; Mamakos, A.; Agudelo, C.; Gramstat, S.; Giechaskiel, B. Interlaboratory Study on Brake Particle Emissions—Part I: Particulate Matter Mass Emissions. Atmosphere 2023, 14, 498. [Google Scholar] [CrossRef]
- Storch, L.; Hamatschek, C.; Hesse, D.; Feist, F.; Bachmann, T.; Eichler, P.; Grigoratos, T. Comprehensive Analysis of Current Primary Measures to Mitigate Brake Wear Particle Emissions from Light-Duty Vehicles. Atmosphere 2023, 14, 712. [Google Scholar] [CrossRef]
- Hinds, W.C.; Yifang, Z. Chapter 4—Particle Size Statics: Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 3rd ed. John Wiley & Sons, Inc. 2022, pp. 65-94. ISBN: 978-1-119-49406-5.
- Iijima, A.; Sato, K.; Yano, K.; Kato, M.; Kozawa, K.; Furuta, N. Emission Factor for Antimony in Brake Abrasion Dusts as One of the Major Atmospheric Antimony Sources. Environ. Sci. Technol. 2008, 42, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Fedrizzi, L.; Andreatta, F. Corrosion Stiction in Automotive Braking Systems. Materials 2023, 16, 3710. [Google Scholar] [CrossRef] [PubMed]
- Takada, T. Formation and Physical Properties of Iron Oxides Hydroxides, Review. Denki Kagaku oyobi Kogyo Butsuri Kagaku 1969, 37, 328–335, (in Japanese with English Figures). [Google Scholar] [CrossRef]
- Diamandescu, L.; Mihǎilǎ-Tǎrǎbǎşanu, D.; Popescu-Pogrion, N. Hydrothermal Transformation of α-FeOOH into α-Fe2O3 in the Presence of Silicon Oxide. Materials Lett. 1996, 27, 253–257. [Google Scholar] [CrossRef]
- Grigoratos, T.; Agudelo, C.; Grochowicz, J.; Gramstat, S.; Robere, M.; Perricone, G.; Sin, A.; Paulus, A.; Zessinger, M.; Hortet, A.; et al. Statistical Assessment and Temperature Study from the Interlaboratory Application of the WLTP–Brake Cycle. Atmosphere 2020, 11, 1309. [Google Scholar] [CrossRef]
- Sutter, G.; Ranc, N. Flash Temperature Measurement during Dry Friction Process at High Sliding speed. Wear 2010, 268, 1237–1242. [Google Scholar] [CrossRef]
- Okayama, K.; Kishimoto, H.; Hiratsuka, K. Tribo-Reduction of Metal Oxides by Tribo-Degradation of Phenolic Resin in Brake Pad. Trans. Jpn. Soc. Mech. Eng. C 2013, 79, 2558–2570, (in Japanese with English Figures). [Google Scholar] [CrossRef]
- Lange, J.; Ostermeyer, G. The Effect of Metal Pickup to the Friction Interfaces, SAE Tech. Pap. 2011, 2011-01-2348. [CrossRef]
- Kawamoto, M.; Shintani, S.; Sone, T.; Okabayashi, K. Wear Characteristics of Carbon Steel and 17Cr Stainless Steel in Relation to the Surface Temperature. J. Japan Inst. Met. Mater. 1973, 37, 1236–1242, (in Japanese with English Figures). [Google Scholar] [CrossRef]
- Kawamoto, M.; Shintani, S.; Okabayashi, K. Relation between Wear of Iron and Steel and Sliding Surface Temperature. Tetsu-to-Hagane 1975, 61, 3139–3148, (in Japanese with English Figures). [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Takei, T. Analysis of Metal Pick-Up Formation Process within Automotive Brake Pad. SAE Int. J. Mater. Manf. 2020, 13, 27–43. [Google Scholar] [CrossRef]
- Nukumizu, K.; Kobayashi, T.; Abe, T.; Unno, M. Study of the Formulation Mechanism for Metal Pick-up on the Frictional Surface of a Disc Brake Pad. SAE Tech. Pap. 2008, 2008-01-2541. [CrossRef]
- Funabiki, K.; Nakamura, M.; Tsuriya, M. Carbonization of Phenolic Resins. Jpn. Thermo. Plastic Ind. Assoc. 1981, 1981. 2, 220–235. [Google Scholar] [CrossRef]
- Inoue, M.; Hara, Y.; Sasada, T. ; Degradation of Cured Phenolic Resin for Brake Lining Caused by Shearing Force: 1st Report, Molecular Weight Distribution of Extracts. Trans. Jpn. Soc. Mech. Eng. C. 1990, 56, 222–227. [Google Scholar] [CrossRef]
- Straffelini, G.; Pellizzari, M.; Maines, L. Effect of Sliding Speed and Contact Pressure on The Oxidative Wear of Austempered Ductile Iron. Wear 2011, 270, 714–719. [Google Scholar] [CrossRef]
- 45. Straffelini, G; Molinari, A. Mild Sliding Wear of Fe-0.2%C, Ti-6%Al and Al-7072: a Comparative Study. Tribol. Lett. 2011, 41, 227–238. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




