Submitted:
11 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
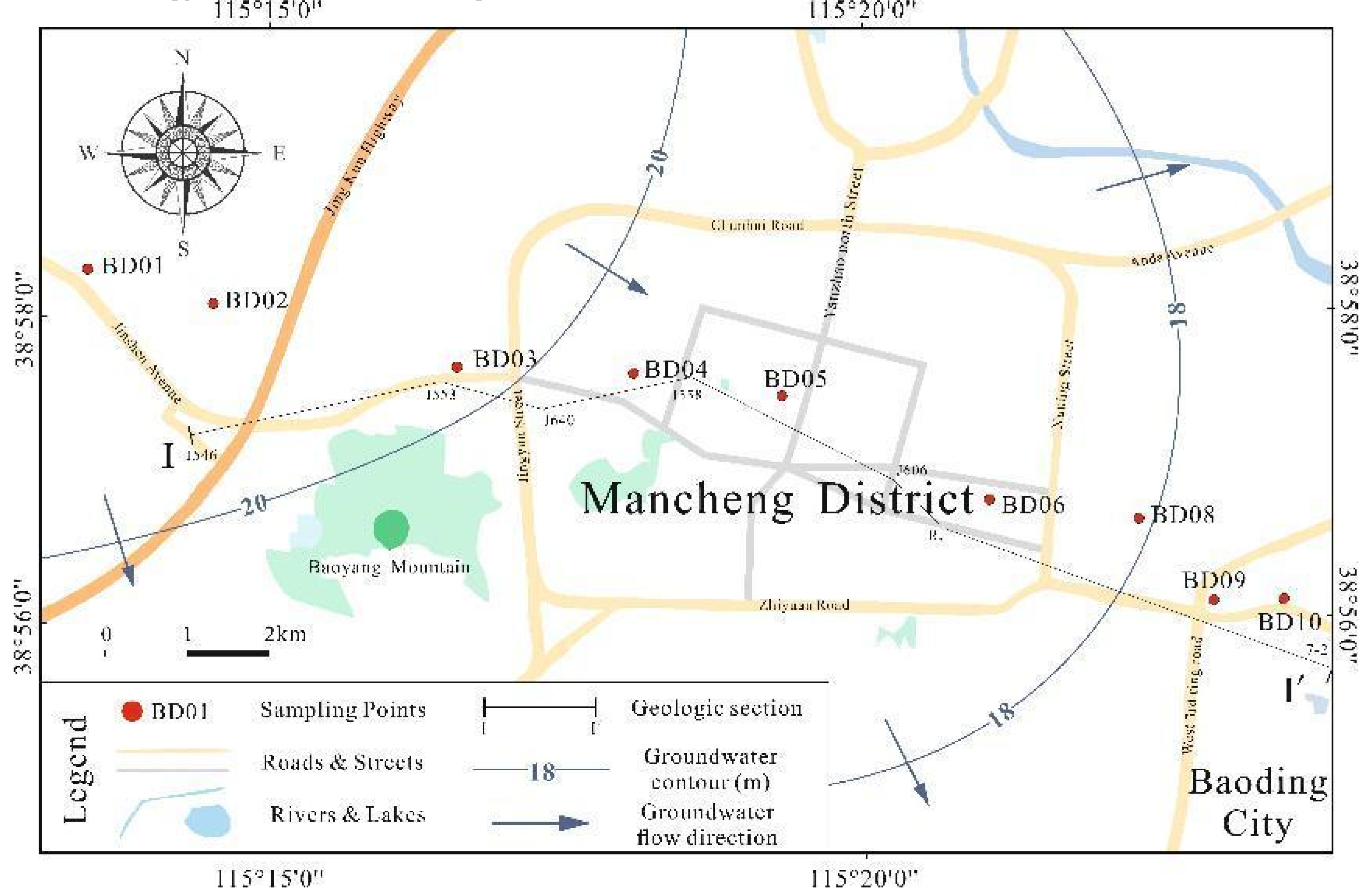
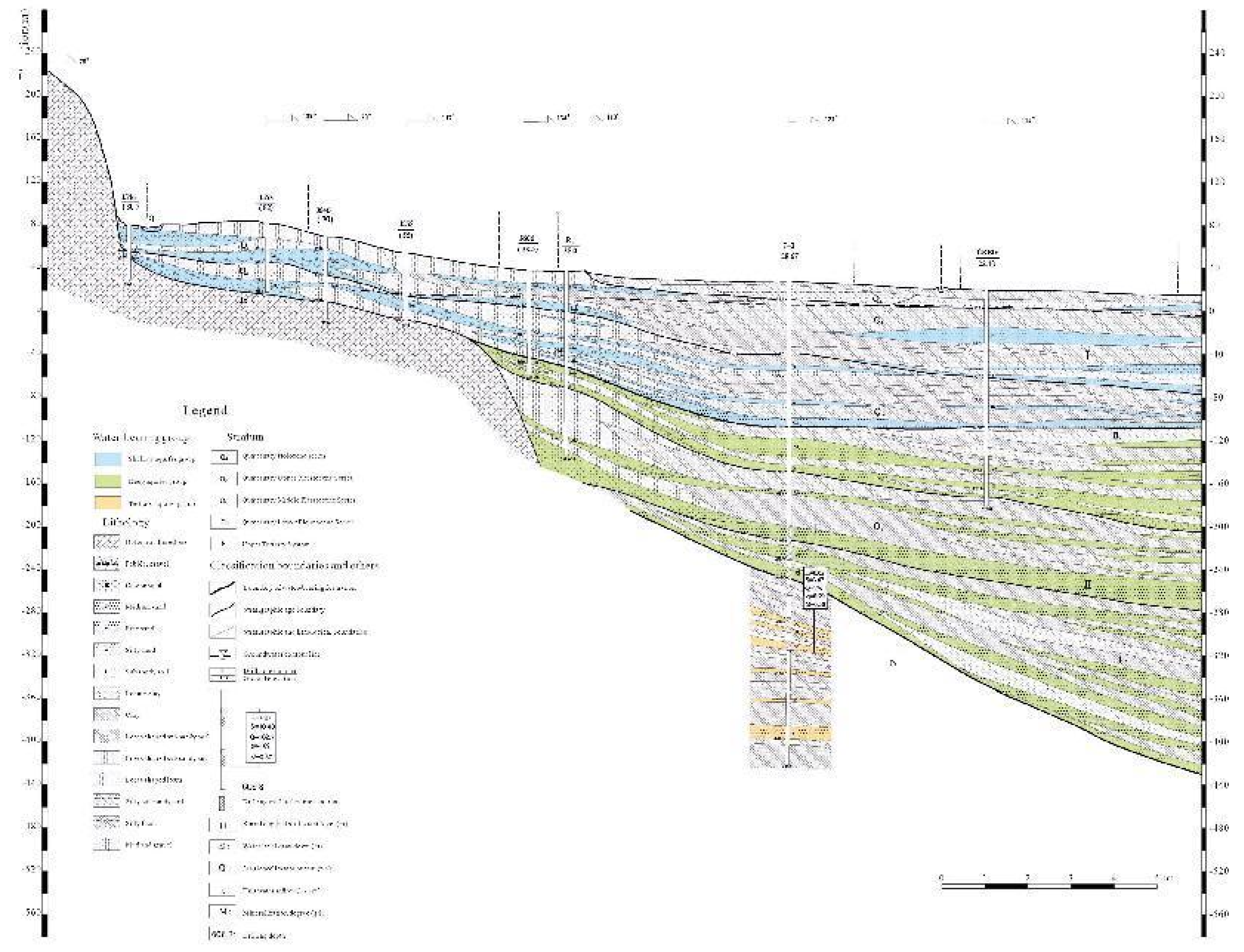
2.1. Study Area Overview
2.2. Sample Collection and Testing
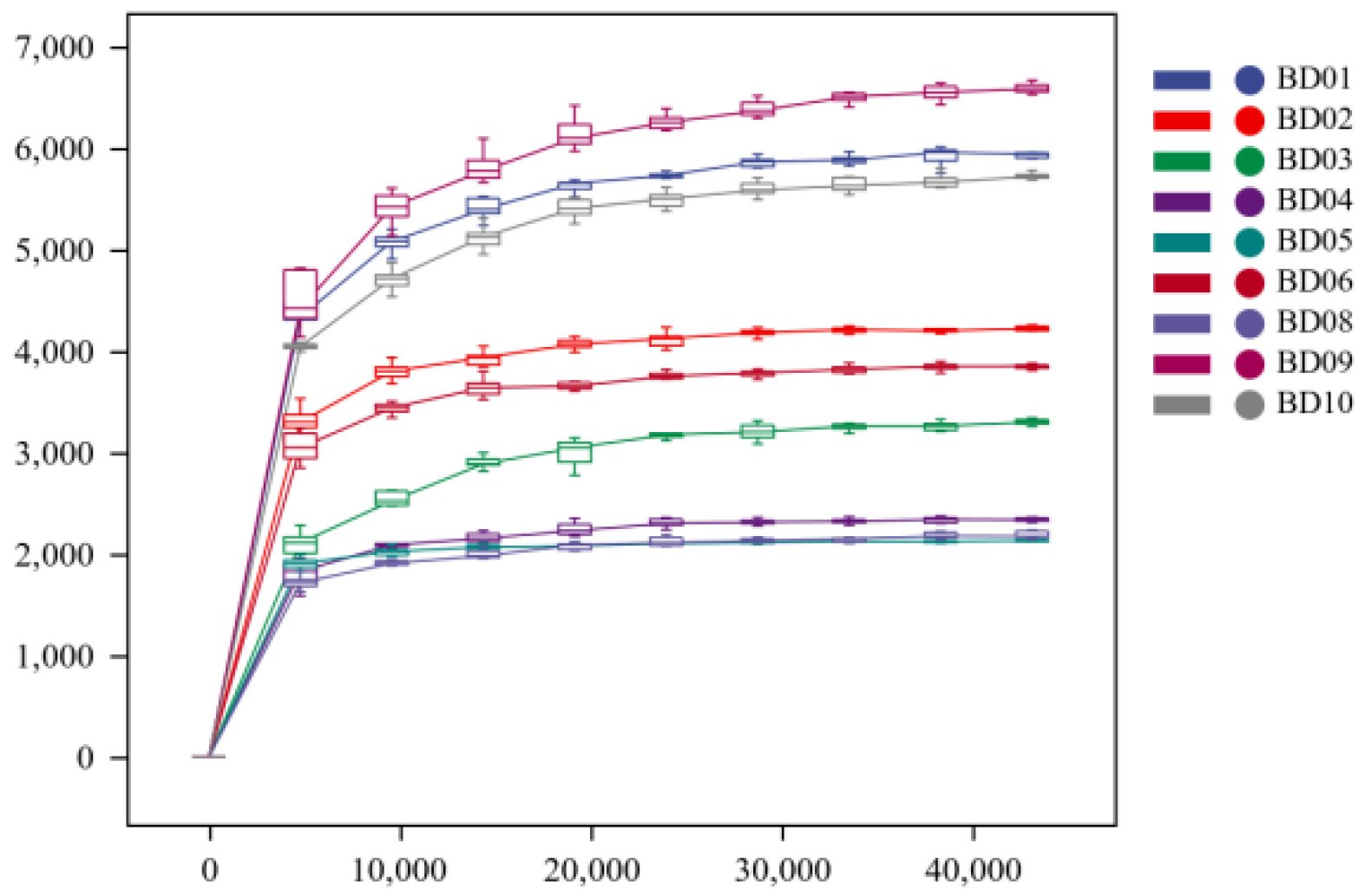
2.3. Sequence and Data Analysis
3. Results and Analysis
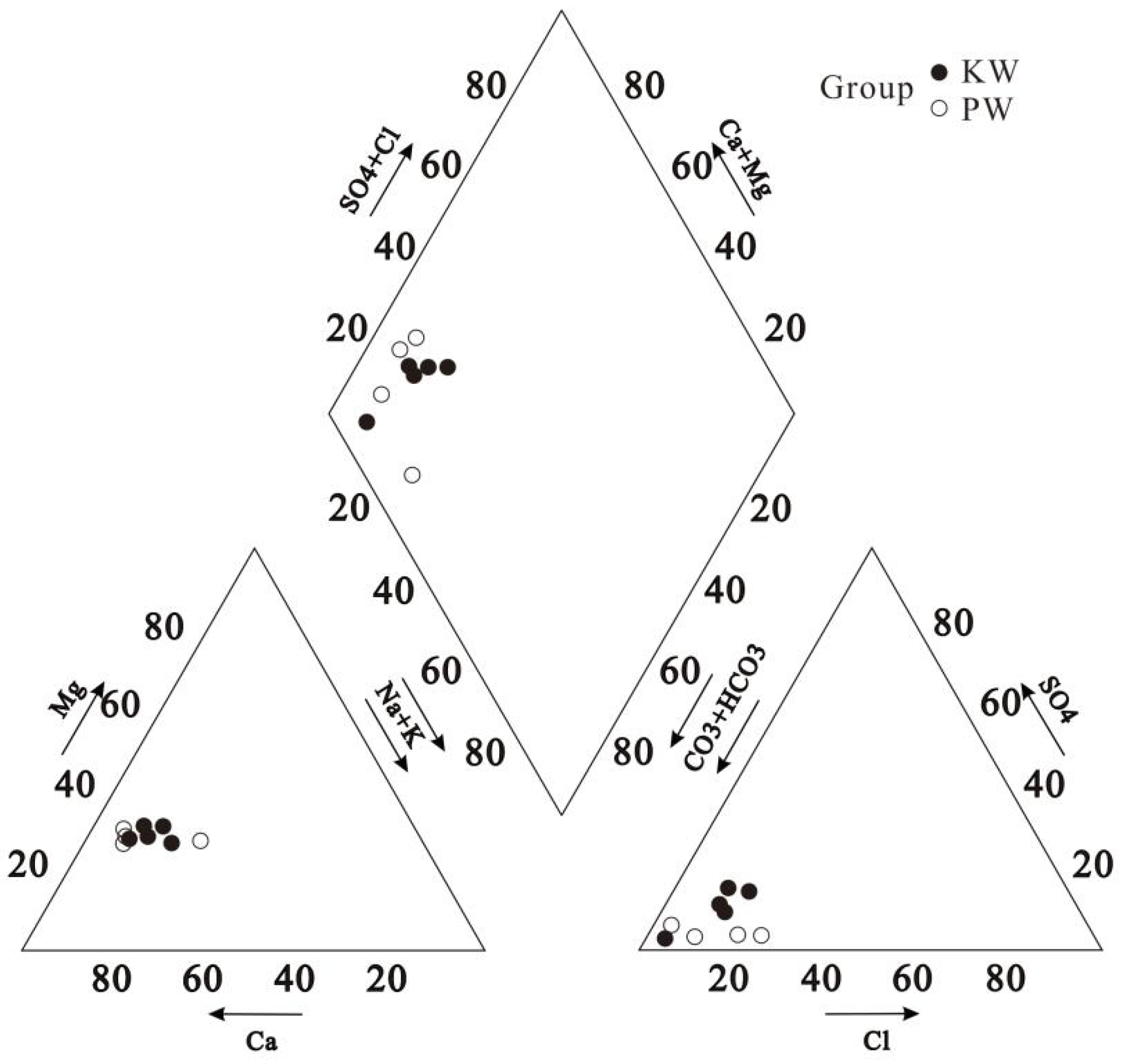
3.1. Hydrochemical Characteristics
3.2. Microbial Community Structure
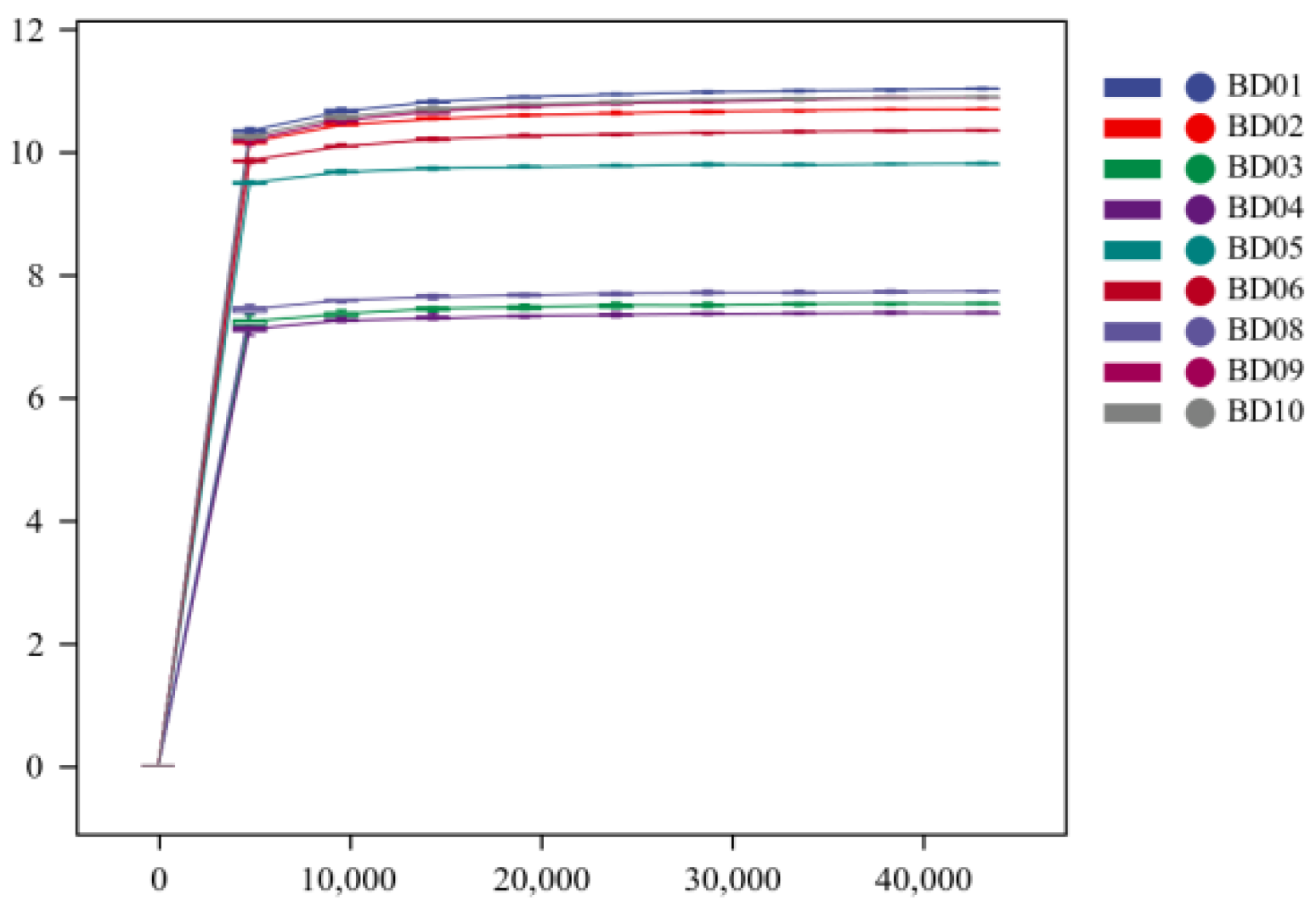
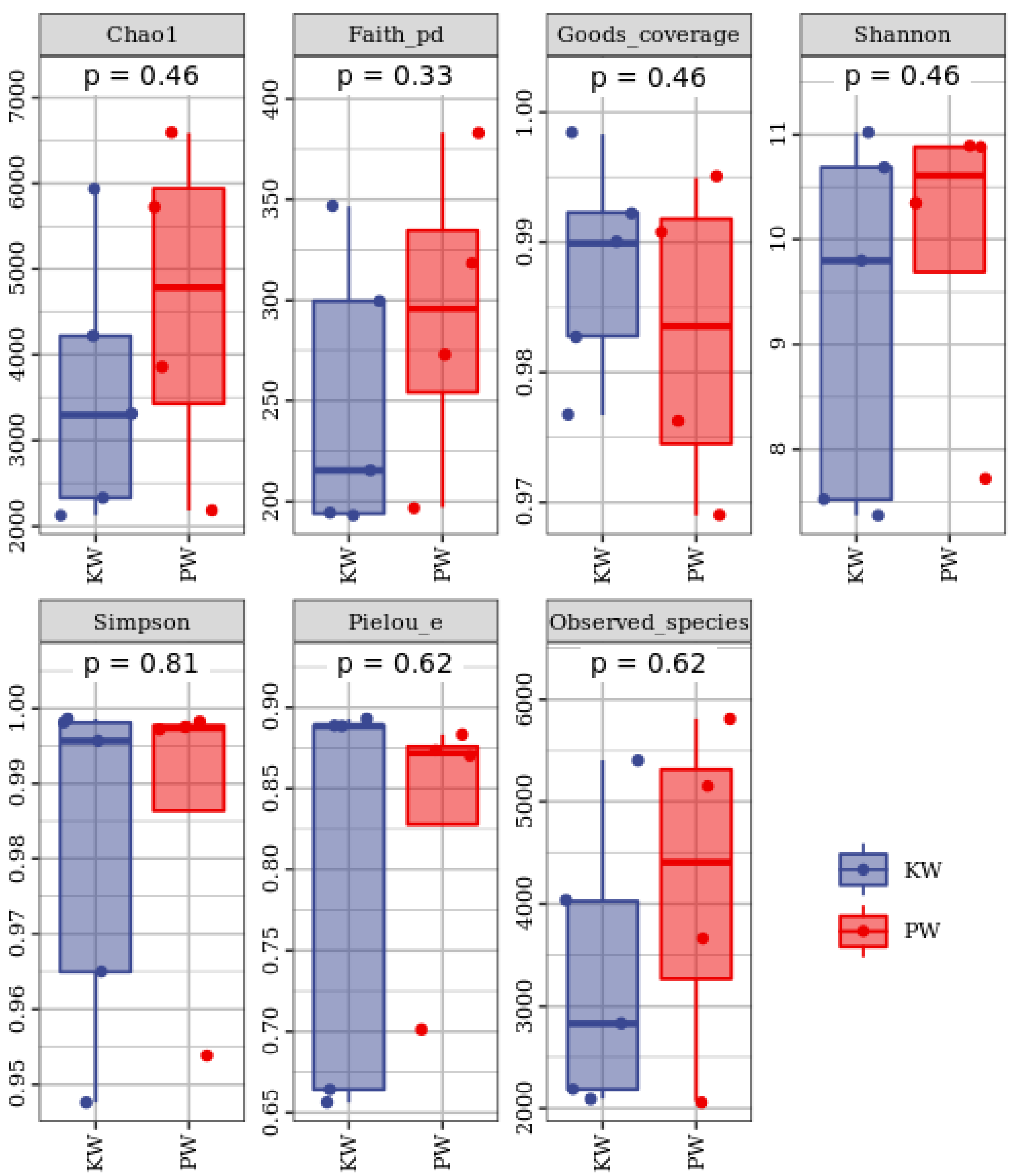
3.2.1. Diversity Analysis
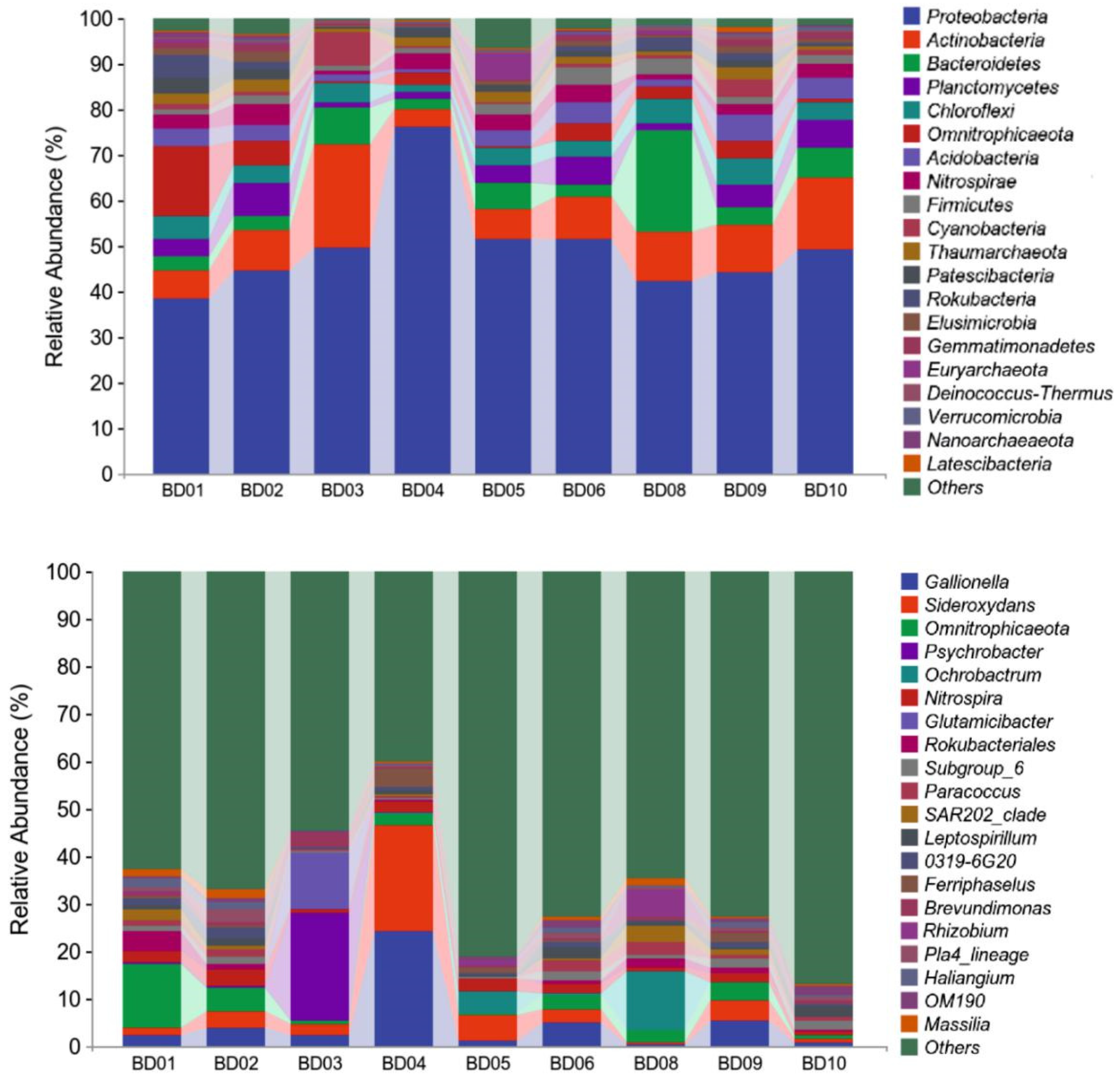
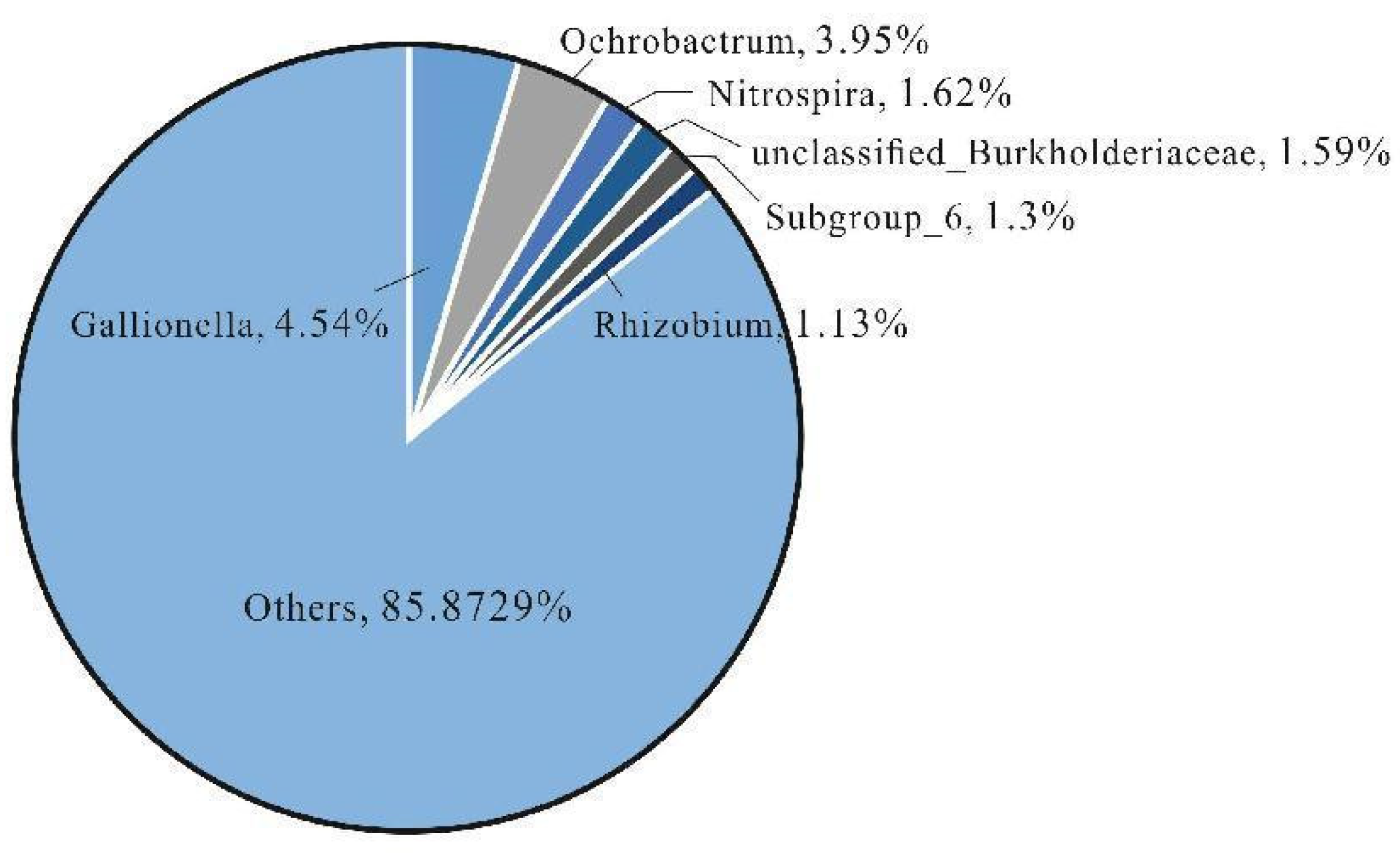
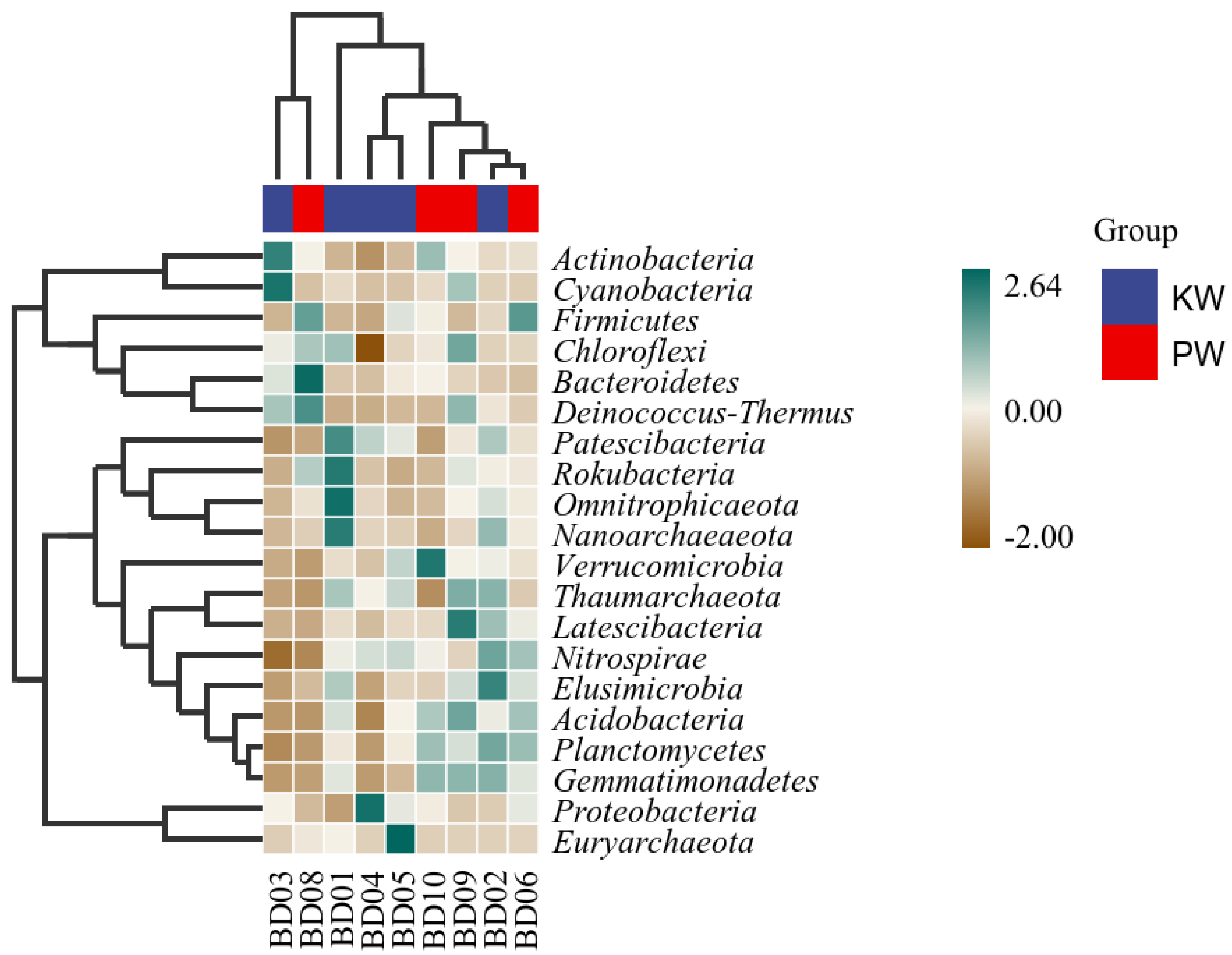
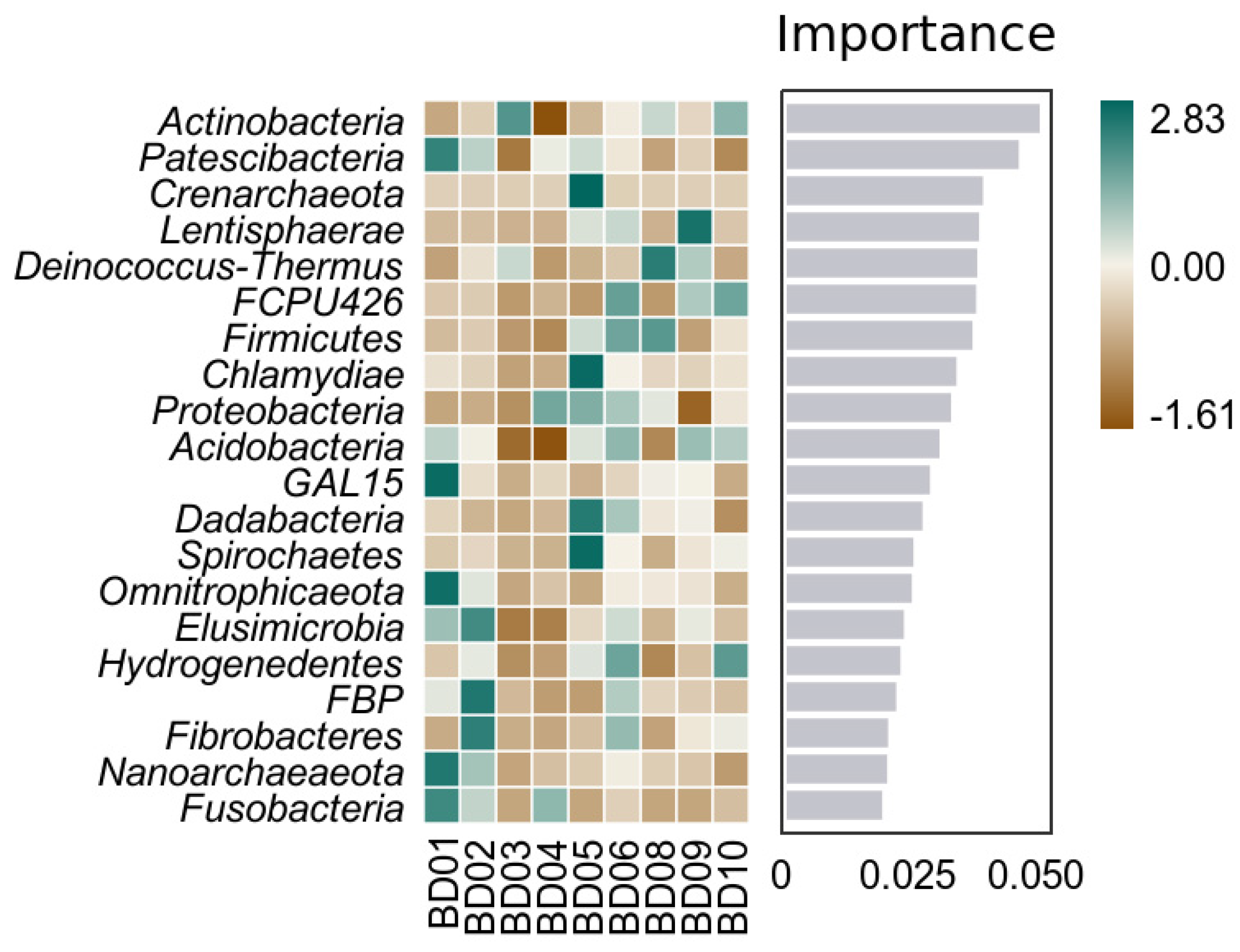
3.2.2. Microbial Composition Analysis
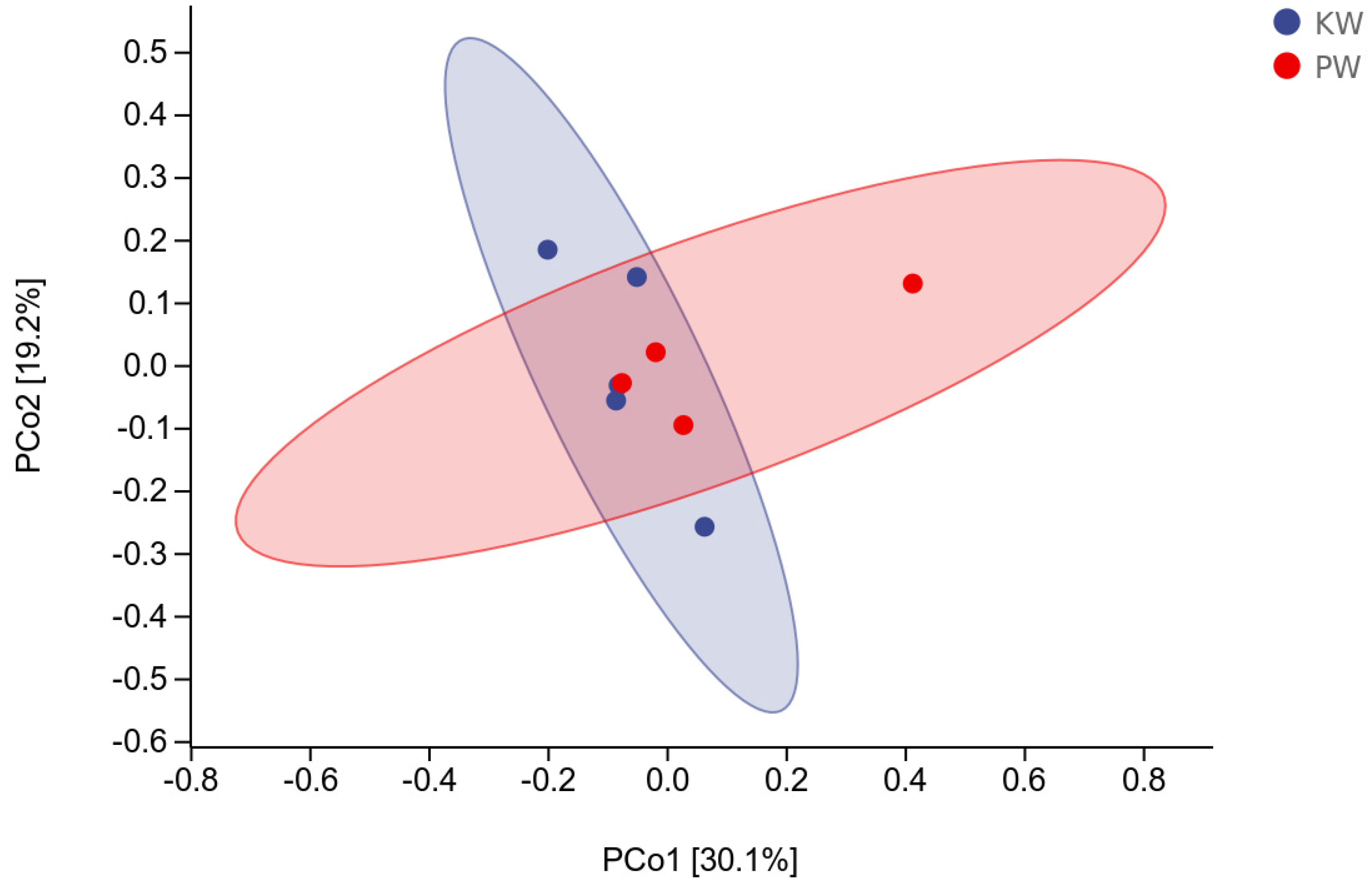
3.2.3. Analysis of Differences in Microbial Community Composition
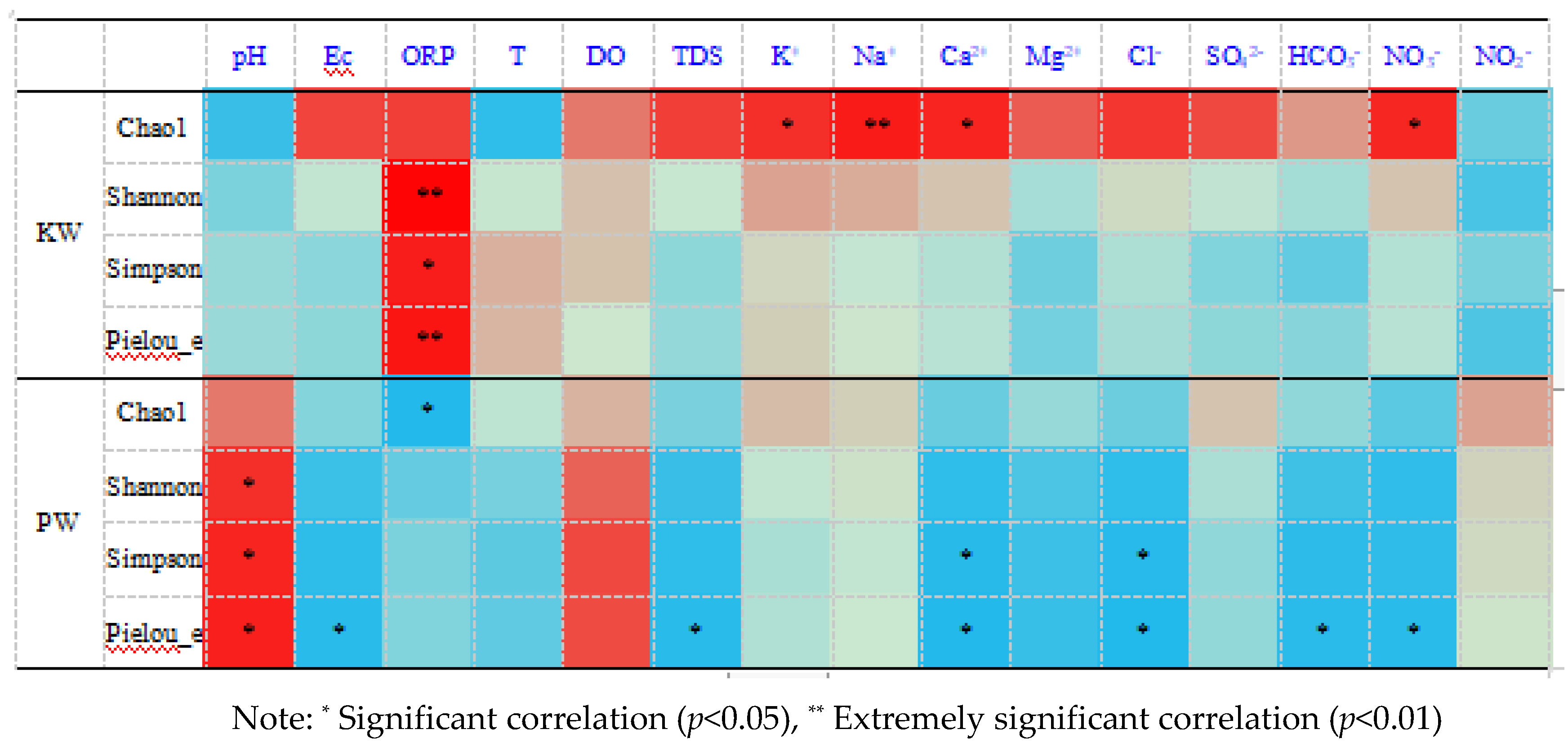
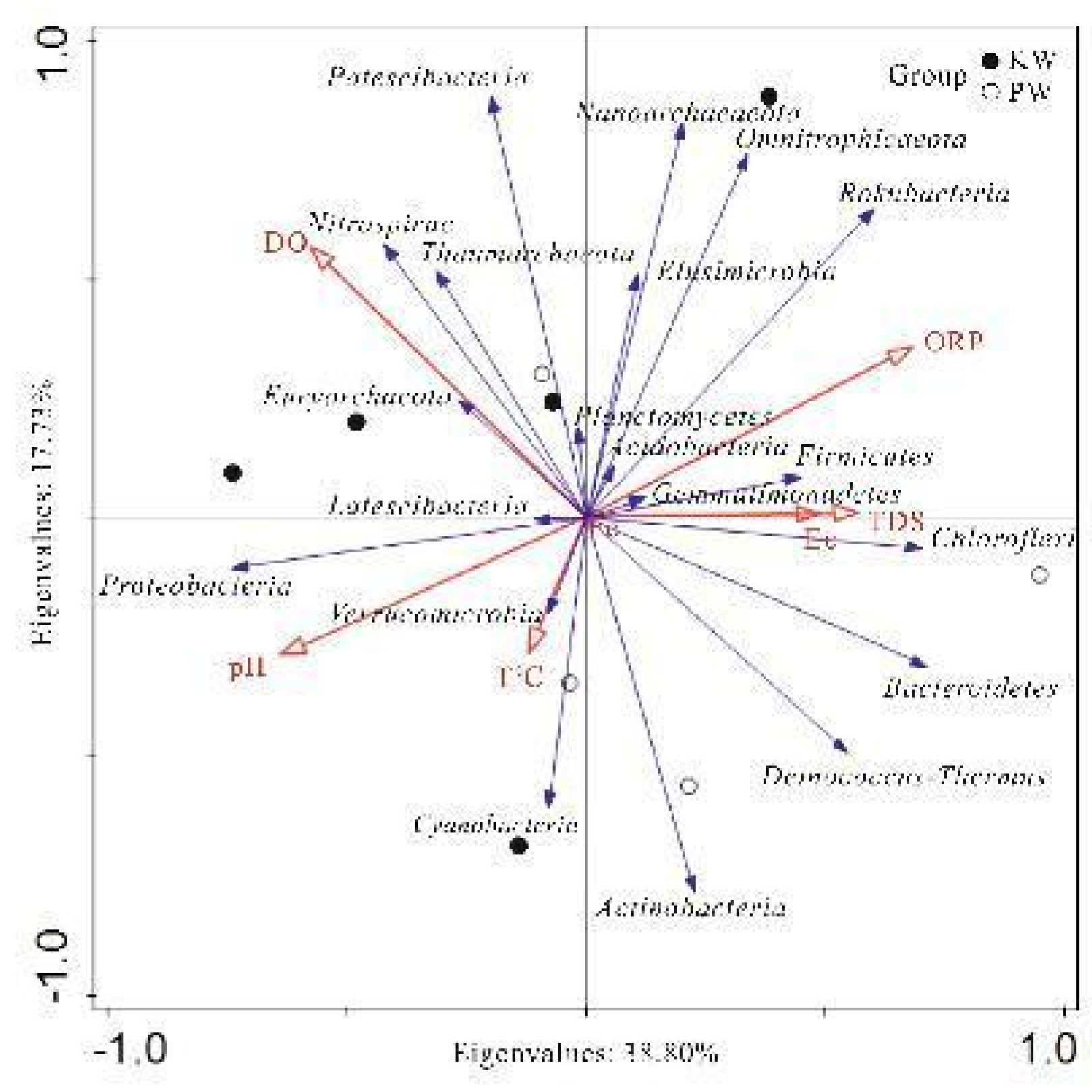
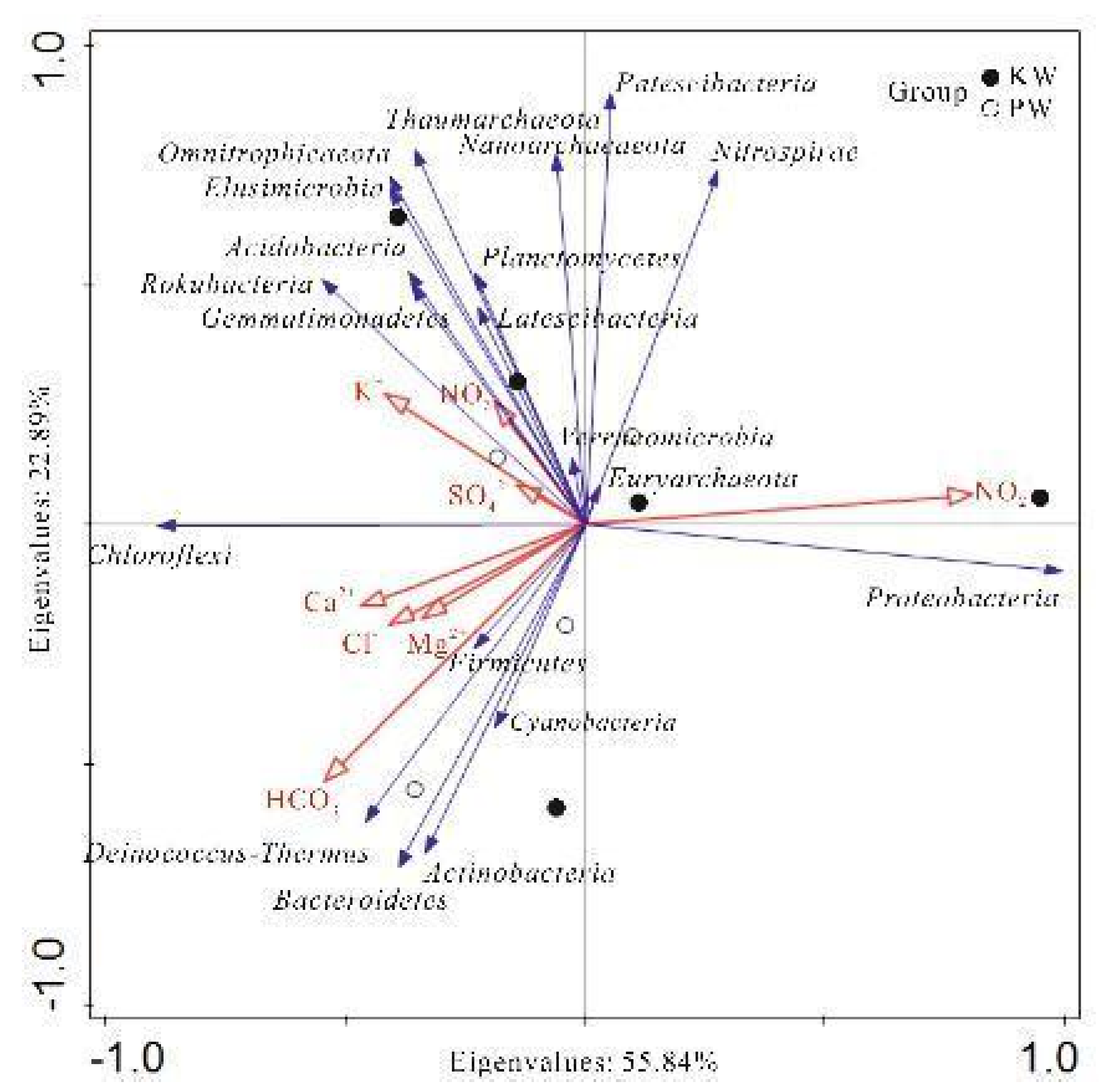
3.3. Correlation Analysis of Microbial Community and Environmental Characteristics
4. Conclusions
References
- Retter, C. Karwautz, and C. Griebler, "Groundwater Microbial Communities in Times of Climate Change," Current Issues in Molecular Biology, vol. 41, no. 1, pp. 509-538, 2021. [CrossRef]
- A. Lozupone, R. Knight, "Global patterns in bacterial diversity," Proceedings of the National Academy of Sciences of the United States of America, vol. 104, no. 27, 2007.
- A. Nickerson, C. M. Ott, S. J. Mister, B. J. Morrow, L. Burns-Keliher, D. L. Pierson, "Microbial responses to microgravity and other low-shear environments," Microbiology and Molecular Biology Reviews, vol. 68, no. 2, pp. 345-361, 2004.
- E. Wegner, M. Gaspar, P. Geesink, et al., "Biogeochemical Regimes in Shallow Aquifers Reflect the Metabolic Coupling of the Elements Nitrogen, Sulfur, and Carbon," Applied and Environmental Microbiology, vol. 85, no. 9, 2019. [CrossRef]
- C. J. Castelle, C. T. Brown, B. C. Thomas, et al., "Unusual respiratory capacity and nitrogen metabolism in a Parcubacterium (OD1) of the Candidate Phyla Radiation," Scientific Reports, vol. 7, 40101, 2017. [CrossRef]
- C. J. Castelle, K. C. Wrighton, B. C. Thomas, et al., "Genomic expansion of domain archaea highlights roles for organisms from new phyla in anaerobic carbon cycling," Current Biology, vol. 25, no. 6, pp. 690-701, 2015. [CrossRef]
- C. Q. Qin, H. Ding, S. L. Li, et al., "Hydrogeochemical Dynamics and Response of Karst Catchment to Rainstorms in a Critical Zone Observatory (CZO), Southwest China," Frontiers in Water, vol. 2, 577511, 2020. [CrossRef]
- C. Rinke, F. Rubino, L. F. Messer, et al., "A phylogenomic and ecological analysis of the globally abundant Marine Group II archaea (Ca. Poseidoniales ord. nov.)," The ISME Journal, vol. 13, 2018. [CrossRef]
- China Water Resources Bulletin. (2021). Beijing: China Water & Power Press.
- C. White, D. B. Ringelberg, R. J. Palmer, "Quantitative Comparison of the in situ Microbial Communities in Different Biomes," 1995.
- D. L. Kirchman, "The ecology of Cytophaga-Flavobacteria in aquatic environments," FEMS Microbiology Ecology, vol. 39, no. 2, pp. 91-100, 2002.
- D. M. Han, M. J. Currell, H. Guo, "Controls on distributions of sulphate, fluoride, and salinity in aquitard porewater from the North China Plain: Long-term implications for groundwater quality," Journal of Hydrology, 2021.
- D. P. Herlemann, M. Labrenz, K. Jürgens, et al., "Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea," The ISME Journal, vol. 5, no. 10, pp. 1571-1579, 2011.
- V. Wehncke, N. A. Mariano, "Groundwater and Its Role in Maintaining the Ecological Functions of Ecosystems—A Review," [Online]. Available: 10.1007/978-3-030-65443-6_4, 2021.
- E. Hutchinson, "Concluding remarks - cold spring harbor symposia on quantitative biology. Reprinted in 1991: classics in theoretical biology," Bulletin of Mathematical Biology, vol. 53, no. 1507, pp. 193-213, 1957.
- M. Zaitsev, I. V. Tsitko, F. A. Rainey, et al., "New aerobic ammonium-dependent obligately oxalotrophic bacteria: description of Ammoniphilus oxalaticus gen. nov. sp. nov. and Ammoniphilus oxalivorans gen. nov. sp. nov.," International Journal of Systematic Bacteriology, vol. 48, pt. 1, no. 1, pp. 151, 1998. [CrossRef]
- W. Paerl, N. S. Hall, and E. S. Calandrino, "Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change," Science of the Total Environment, vol. 409, no. 10, pp. 1739-1745, 2011. [CrossRef]
- A. Vorholt, "Microbial life in the phyllosphere," Nature Reviews Microbiology, vol. 10, no. 12, pp. 828-840, 2012. [CrossRef]
- Wang, C. Jenkins, R. I. Webb, and J. A. Fuerst, "Isolation of Gemmata-like and Isosphaera-like planctomycete bacteria from soil and freshwater," Applied and Environmental Microbiology, vol. 68, no. 1, pp. 417-422, 2002. [CrossRef]
- Faust, J. Raes, "Microbial interactions: from networks to models," Nature Reviews Microbiology, vol. 10, no. 8, pp. 538-550, 2012.
- K. L. Korbel, P. Greenfield, G. C. Hose, "Agricultural practices linked to shifts in groundwater microbial structure and denitrifying bacteria," The Science of the Total Environment, vol. 807, pt. 2, 150870, 2022.
- K. Manoj, N. Shrivastava, P. Teotia, P. Goyal, A. Varma, S. Sharma, N. Tuteja, V. Kumar, "Omics: Tools for Assessing Environmental Microbial Diversity and Composition," pp. 273-283, 2016.
- K. S. Makarova, L. Aravind, Y. I. Wolf, et al., "Genome of the Extremely Radiation-Resistant Bacterium Deinococcus radiodurans Viewed from the Perspective of Comparative Genomics," Microbiology & Molecular Biology Reviews, vol. 65, no. 1, 2001.
- A. Hug, C. J. Castelle, K. C. Wrighton, et al., "Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling," Microbiome, vol. 1, no. 1, 22, 2013.
- L. Richard, Marchese, et al., "Comparison of the Predictive Performance and Interpretability of Random Forest and Linear Models on Benchmark Data Sets," Journal of Chemical Information and Modeling, vol. 57, no. 8, pp. 1773-1792, 2017. [CrossRef]
- L. Yan, M. Syrie, K. U. Totsche, R. Lehmann, M. Herrmann, K. Küsel, "Groundwater bacterial communities evolve over time in response to recharge," Water Research, vol. 201, 117290, 2021. [CrossRef]
- A. Leibold, "The Niche Concept Revisited: Mechanistic Models and Community Context," Ecology, vol. 76, no. 5, pp. 1371-1382, 1995.
- M. R. Shields, T. S. Bianchi, Y. Gélinas, et al., "Enhanced terrestrial carbon preservation promoted by reactive iron in deltaic sediments," Geophysical Research Letters, vol. 43, 2016. [CrossRef]
- M. W. Hahn, "Broad diversity of viable bacteria in ‘sterile’ (0.2 μm) filtered water," Research in Microbiology, 2004.
- Chesson, "Mechanisms Of Maintenance Of Species Diversity," 2000, vol. 31, no. 1, pp. 343-366. [CrossRef]
- Sharma, A. K. Pandey, S. H. Kim, et al., "Critical review on microbial community during in-situ bioremediation of heavy metals from industrial wastewater," Environmental Technology & Innovation, vol. 21, 101826, 2021. [CrossRef]
- T. Jones, M. S. Robeson, C. L. Lauber, et al., "A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses," The ISME Journal, vol. 3, no. 4, pp. 442-453, 2009.
- Amalfitano, A. Del Bon, A. Zoppini, S. Ghergo, S. Fazi, D. Parrone, P. Casella, F. Stano, and E. Preziosi, "Groundwater geochemistry and microbial community structure in the aquifer transition from volcanic to alluvial areas," Water Research, vol. 65, pp. 384-394, 2014. [CrossRef]
- Louca, S. M. S. Jacques, A. P. F. Pires, J. S. Leal, D. S. Srivastava, L. W. Parfrey, V. F. Farjalla, M. Doebeli, "High taxonomic variability despite stable functional structure across microbial communities," Nature Ecology & Evolution, vol. 1, no. 1, 0015, 2016.
- Maloy, "Brenner's Encyclopedia of Genetics," Brenners Encyclopedia of Genetics, vol. 91, no. 2001, pp. 2-3, 2013.
- M. Flynn, R. A. Sanford, H. Ryu, et al., "Functional microbial diversity explains groundwater chemistry in a pristine aquifer," Bmc Microbiology, vol. 13, no. 1, 146-146, 2013.
- T. M. Lapara, C. H. Nakatsu, L. M. Pantea, "Aerobic Biological Treatment Of a Pharmaceutical Wastewater: Effect Of Temperature On Cod Removal And Bacterial Community Development," Water Research: A Journal of the International Water Association, vol. 35, no. 18, pp. 4417-4425, 2001.
- Chen, "A View on Geomorphologic Zonalization of North China Plain," Geography and Geo-Information Science, 2008.
- G. Fu, Z. Dong, S. Gan, et al., "Groundwater Quality Evaluation for Potable Use and Associated Human Health Risk in Gaobeidian City, North China Plain," Hindawi Limited, 2021.
- N. Pan, Q. Li, Y. Liu, et al., "Hydroclimate variations in the Northern China Plain and their possible socio-cultural influences," [Online]. Available: 10.1007/978-3-658-03097-1_8, [Accessed: 22-Jul-2023].
- Xiao, Q. Hao, et al., "Accessible Phreatic Groundwater Resources in the Central Shijiazhuang of North China Plain: Perspective From the Hydrogeochemical Constraints," Frontiers in Environmental Science, vol. 9, 747097, 2021. [CrossRef]
- Y. Huang, Y. Pan, H. Gong, et al., "Subregional-scale groundwater depletion detected by GRACE for both shallow and deep aquifers in North China Plain," Geophysical Research Letters, vol. 42, no. 6, pp. 1791-1799, 2015.
| pH | Ec (μs/cm) |
ORP (mv) |
DO (mg/L) |
TDS (mg/L) |
K+ (mg/L) |
Na+ (mg/L) |
Ca2+ (mg/L) |
Mg2+ (mg/L) |
Cl- (mg/L) |
SO42- (mg/L) |
HCO3- (mg/L) |
NO3- (mg/L) |
NO2- (mg/L) |
F- (mg/L) |
H2SiO3 (mg/L) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karst Water (n=5) |
Avg | 7.72 | 863.8 | 132.36 | 8.976 | 405.23 | 1.62 | 21.33 | 87.86 | 45.13 | 46.99 | 46.21 | 316.30 | 68.16 | 0.01 | 0.17 | 13.63 |
| Max | 7.83 | 1032 | 163.8 | 9.9 | 487.21 | 3.44 | 35.75 | 106.92 | 54.79 | 74.87 | 71.07 | 344.68 | 109.75 | 0.04 | 0.30 | 15.56 | |
| Min | 7.49 | 550 | 96.2 | 7.46 | 277.03 | 0.84 | 8.58 | 64.10 | 28.40 | 11.73 | 8.96 | 300.23 | 8.64 | 0.00 | 0.11 | 12.35 | |
| Pore Water (n=4) |
Avg | 7.70 | 812.75 | 166.1 | 6.92 | 413.69 | 1.22 | 17.24 | 93.64 | 43.75 | 70.67 | 18.36 | 362.19 | 26.42 | 0.01 | 0.17 | 18.29 |
| Max | 7.88 | 1129 | 190.3 | 9.09 | 572.47 | 1.56 | 29.26 | 135.75 | 56.96 | 139.40 | 22.59 | 418.98 | 41.04 | 0.02 | 0.25 | 23.96 | |
| Min | 7.44 | 581 | 128.8 | 5.18 | 275.33 | 0.86 | 9.95 | 56.36 | 32.68 | 12.66 | 11.93 | 310.58 | 10.65 | 0.00 | 0.14 | 14.85 |
| phylum | Karst water | Pore Water | ||
| Levins niche | Types of commonly used resources | Levins niche | Types of commonly used resources | |
| Omnitrophicaeota | 11.1427 | S5=11.32%,S6=10.62% | 8.6607 | S4=11.65%,S8=13.92%,S9=17.23% |
| Thaumarchaeota | 4.1453 | S1=34.59%,S3=17.67%,S5=25.14% | 6.0497 | S9=32.35% |
| Euryarchaeota | 3.3207 | S3=26.77%,S4=34.46%,S5=32.99% | 9.355 | S4=11.69%,S6=12.36%,S8=12.34% |
| Latescibacteria | 5.1979 | S2=32.32%,S4=18.00%,S8=14.49% | 3.1762 | S3=21.15%,S10=50.18% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





