1. Introduction
Global warming is a pressing environmental issue caused primarily by the release of greenhouse gases, such as carbon (C), nitrogen (N), and phosphorus (P) compounds into the atmosphere [
1]. Carbon dioxide (CO
2), methane (CH
4), and nitrous oxide (N
2O) are the main contributors to greenhouse gas emissions, with CO
2 accounting for 80%, CH
4 for 11%, and N
2O for 6% [
2].
Intensive agriculture is one of the major contributors to greenhouse gas emissions, accounting for 18.4% of total emissions [
3]. It negatively impacts the ecosystem by reducing soil biodiversity, impairing ecosystem functioning, lowering productivity, increasing resistance of weed pests to chemical products, causing soil degradation, and water contamination, all of which contribute to climate change [
4]. In contrast, soil organic carbon (SOC) sequestration refers to the removal of carbon from the atmosphere through photosynthesis, organic matter, and residue decomposition, and it provides a way to store atmospheric carbon [
5].
The Latin American and Caribbean region contributes 8.1% of GHG emissions, with Argentina, Brazil, Mexico, and Venezuela contributing 5.7% [
6]. However, the region also holds one of the most significant carbon reserves globally, with the Amazon basin containing approximately 57% of the world’s remaining primary forests, storing around 104 Gt ha
−1 of C, and harboring up to 50% of the world’s biodiversity and one-third of all plant species [
7].
Ecuador is a country in the Latin American and Caribbean region with a wide variety of woody species, which release leaves as part of their physiological development [
8]. In cacao (
Theobroma cacao L.) agroforestry systems, C sequestration is significant when planted at high densities [
9]. Cocoa is considered an important commodity for the country, with annual exports in the world cocoa market growing at about 4.5% annually and a current planting area of 543,547 ha
−1 [
10]. Ecuador is the third-largest exporter of organic cocoa, with 10% of cocoa production grown in the Amazon region, primarily under conventional and organic agroforestry systems [
11].
Previous studies indicate that cocoa crops can absorb CO
2 from 80,000 ha
−1, releasing 63,000 CO
2 ha
−1 and producing about 730,000 t ha
−1, roughly 14% of the world’s cocoa harvest, to be converted into carbohydrates [
12]. Cocoa presents an alternative for the accumulation of OC, and there is potential for C sequestration as a means of rural development and environmental conservation [
13]. Studies have been conducted on OC sequestration in the Carrizal Chone river basin with the aim of implementing appropriate technologies in cocoa-producing countries and comparing values in cumulative CO sequestration per hectare [
14,
15].
Different cocoa plantation species can have varying levels of soil organic carbon sequestration [
13]. Soil organic carbon sequestration refers to the process of capturing carbon dioxide from the atmosphere and storing it in the soil in the form of organic matter. Research has demonstrated that the type of cocoa plantation species can affect the amount of organic matter produced and the rate at which it decomposes, both of which can do. This leads to the following question. Does the species of cocoa (
Theobroma cacao L.) positively affect the capture in different level of organic carbon in the soil?
2. Materials and Methods
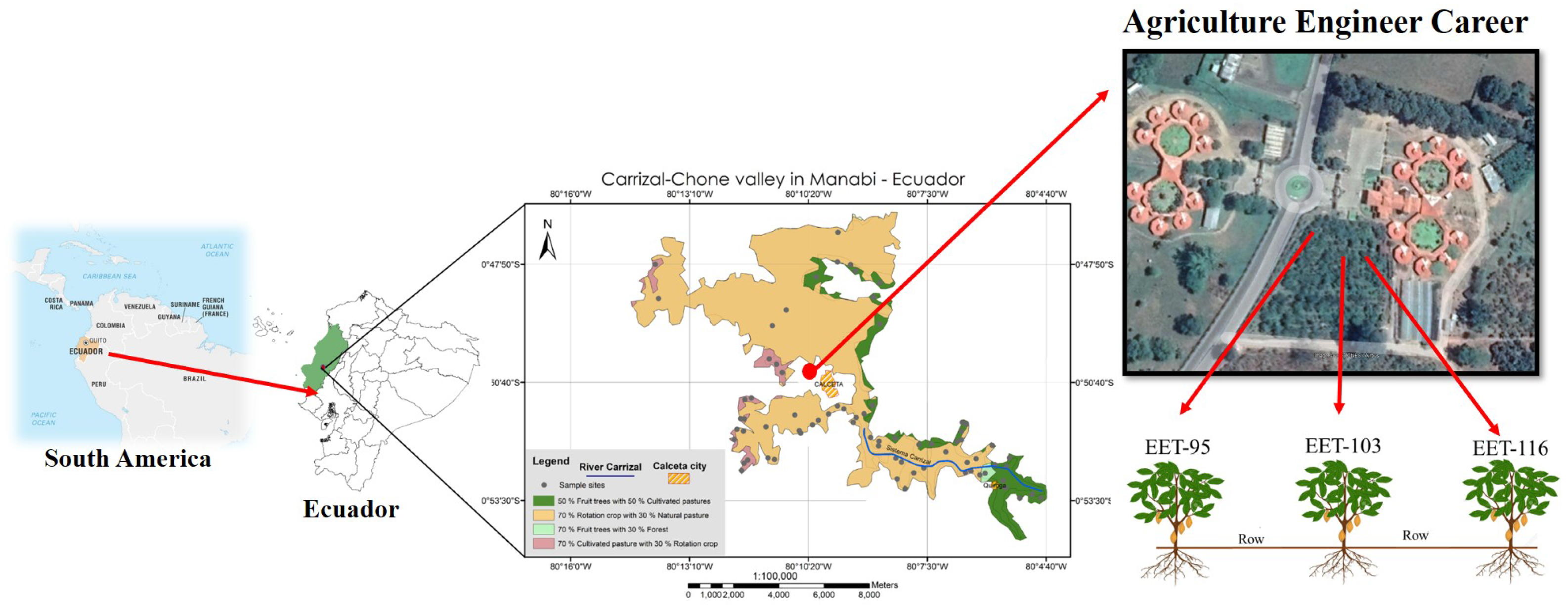
The study area is situated at 0°51”46” S, 80°80’61” W, and between 19 and 80 m.a.s.l. (
Figure 1). The plots were located at the Polytechnic Campus of Agricultural Engender career of ESPAM MFL, in the area where soil samples were taken in the three species of cocoa (
Theobroma cacao L.) cultivations. The average annual temperature of the area is 25.6°C, the average annual potential evapotranspiration is 1365.2 mm, and the average annual precipitation is 838.7 mm, with a dry period occurring from June to December, and a rainy period from January to May.
2.1. Management of the cocoa plantation
The cocoa crop was planted in March 1990 with the varieties Tropical Experimental Station its acronym in Spanish (EET), EET 95, EET 103 and EET 116. With a planted area of 0.84 ha, the cocoa trees are established at a distance of 4 meters between plants and 4 meters between rows with a total of 205 plants. The presence of trees within the cocoa crop, the only shade that is present is from a guachapéele tree (Albizia guachapele).
Weed control is done mechanically with a scythe every 8 to 15 days; the weeds found in the cocoa crop are broad-leaved and thin. Phytosanitary control is done by pruning, which consists of eliminating diseased fruit, sucker shoots and branches, and pruning is done twice a year at the beginning of the rainy season and at the end of the rainy season.
Insect management is controlled every 8 days with traps and applications are made according to the insect damage threshold or preventive traps are placed. Harvest management is done manually. Fertilization is carried out in a complete edaphic way, which consists of direct applications to the soil. Complete edaphic fertilization + foliar application of biostimulants: Fertilizers: MicroEssentials SZ 150 kg ha−1, Korn Kali 500 kg ha−1, Yesolina 333 kg ha−1, Urea 722 kg ha−1.
Irrigation was carried out using the sprinkler method, with a frequency of twice a week, with a time of two hours and an irrigation laminal amine of 17.6 mm. In the months of January, February and March, the crop is not irrigated because the rainfall in these months meets the water needs of the crop. The residues of the husks are left in the crop and the pruning residues are cut and left in the same row between plants which is 0.48 cm while the residues of leaves are 0.20 cm.
2.2. Soil sampling and laboratory analysis
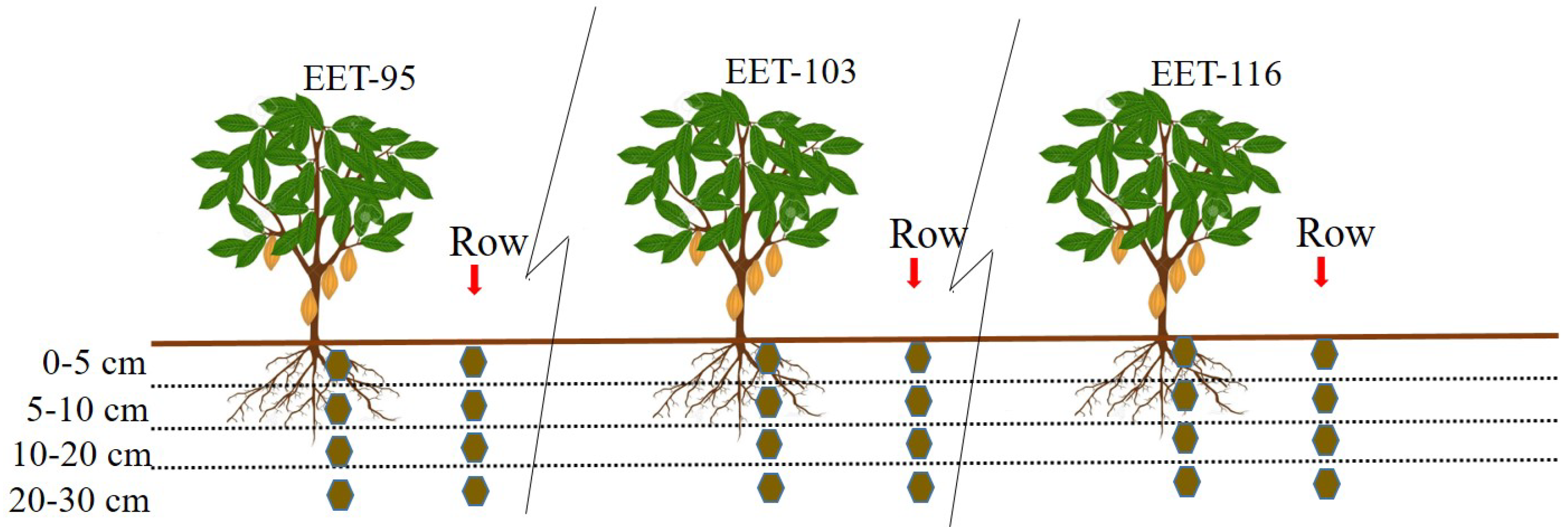
Soil sampling was carried out between July and August 2022 in different soil-management types and also in different species of cocoa crop. Soil samples were selected in a design which allowed to determine if the tree influence has a direct effect on the soil, and in turn a comparison between species. In each area EET 95, EET193 and EET 116 crop, below tree and outside the plant (row), 10 sampling points were selected. Therefore, a total of 20 sampling points per area were sampled. Samples were taken at 4 different depth intervals (0–5 cm, 5–10 cm, 10–20 cm and 20–30 cm) at each sampling point, having previously removed the grass and mulch surface. Soil samples were taken a manual soil sampler. Overall a total of 240 soil samples were taken (area × sampling points × depth).
Figure 2.
Undisturbed soil samples were taken with a hand soil sampler to determine bulk density (BD). The samples were taken at the five pits, distinguishing between pit zone below tree and row at four depths (0-10 cm, 10–20 cm and 20–30 cm) with three repetitions, in totaling 45 samples. The samples were oven-dried at 105 °C for 72 h to a constant mass. The bulk density of the soil was calculated by dividing the dry mass of soil by the volume of the bulk density sampler (98.2 cm
−3), according to Hao at al., (2008). At the same time subsamples until constant weight to obtain soil sample moisture, while the rest of the soil in each sample was air dried. The samples were passed through a 2 mm sieve and homogenized, and stoniness was determined as % in mass. The samples were dried to a constant mass at 40°C for 72 h. SOC concentration was determined in accordance with [
16]., Equation (
1). The stock of soil organic carbon for each soil-depth interval (SOC) and for the whole soil profile were calculated in accordance with according to [
17]. Equation
1:
where SOCstocki is total soil organic carbon in a given layer (t ha
−1). SOCi is organic carbon concentration (g g
−1), BDi is bulk density (T m
−3), d is the thickness of the depth interval (m),
is the fraction (0 - 1) of gravel larger than 2 mm in the soil, and n is the number of soil layers. Thus, Equation
2 gives the total soil organic carbon, SOCstock (t ha
−1) in the whole soil profile. BD values for upper soil depths not sampled were interpolated using mass-conserving splines.
2.3. Statistical data analysis
Differences between average values of SOC and SOCstock in the three cocoa crop species by depth and location were evaluated using a parametric ANOVA test. A PCA analysis and general linear model (GLM) was used to investigate the effect of the soil depth, location and species on soil characteristics such as humidity and carbon stock. All the statistical analyses were performed with InfoSTAT 2018.
3. Results and discussion
3.1. Soil moisture
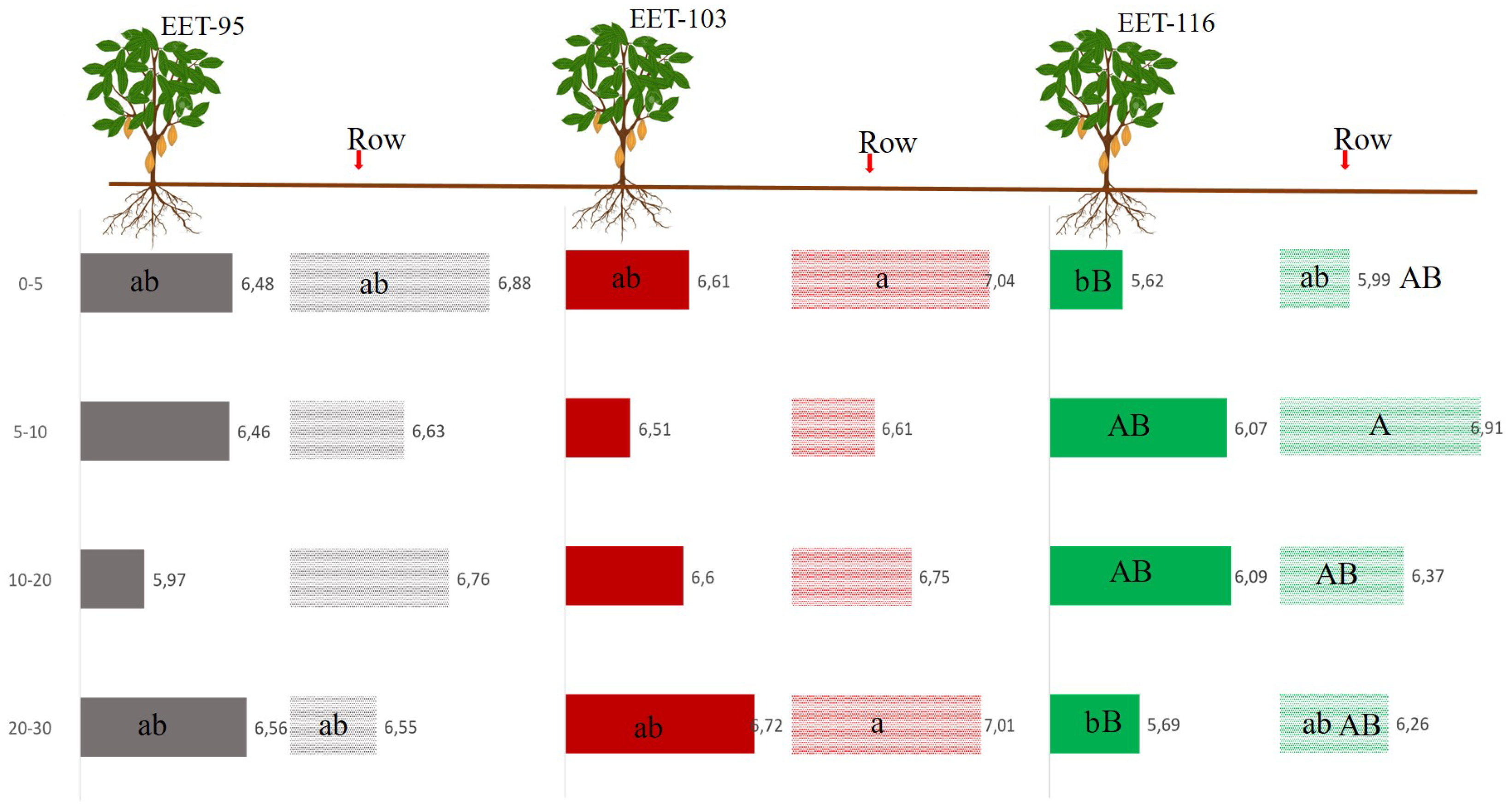
Figure 3 shows the behavior of moisture in each of the sections of the soil profile. From 0-5 cm the variety EET103 showed in the row a higher percentage of moisture with 7.04%, being significantly different only from EET116 below tree with 5.62% moisture. From this behavior of moisture on the surface, it can be understood that the effect of crop residues and pruning in each of the species directly affects moisture retention on a different scale compared to the samples under the tree. Traditional management in cocoa plantations leads to not having residues or vegetation cover under the tree [
17].
This management has an opposite effect to what we see in the rows where better moisture retention is noted [
18]. In sections 5-10 and 10-20 cm, there were no significant differences between varieties of cocoa plants, neither below tree and the rows. In these sections of the soil profile, humidity already has a more standardized behavior since there is no difference in any locality and the values that are appreciated would be related to the texture of the soil. In these sections there is largely a sandy-loam soil, which means that at the time the soil samples were taken (August 2022) it was a dry season. Nevertheless, the plantations have sprinkler irrigation, maintaining the soils with their respective humidity. However, in section 20-30 cm showed a similar behavior as in the first section, being the variety EET103 in the row with the highest percentage of moisture of 7.01% and being significantly different only from EET116 below tree with 5.69%. There were no significant differences in the other sampling locations. The increase in humidity in the last section can be assumed to be due to the slight change in texture that exists in the area [
19]. A small percentage increase in clay content has had a notable effect on moisture in the 20-30 cm section.
Moisture was also analyzed vertically to see the behavior in each of the varieties of cocoa plants. The results showed that only the variety EET116 showed significant differences between the row and below cocoa plant. The section 5-10 cm in the row with 6.91% and below plant at 0-5 and 20-30 cm with 5.62% and 5.69%, respectively.
Figure 3.
On the other hand, bulk density did not show any difference in depth or sampling location. The average for the EET95 variety was 1.12 g/cm−3 below tree and 1.15 g/cm−3 in the row. Likewise, EET103 and EET 116 with 1.14, 1.18, 1.22 and 1.17 (g/cm−3), respectively. The apparent density after 15 years of management in cocoa plantations, the values have been standardized and without significant differences, it is not a determining factor of discrimination for the comparison of organic carbon concentration in the soil.
3.2. Effect of tree present vs management plantation on SOC and SOCstock
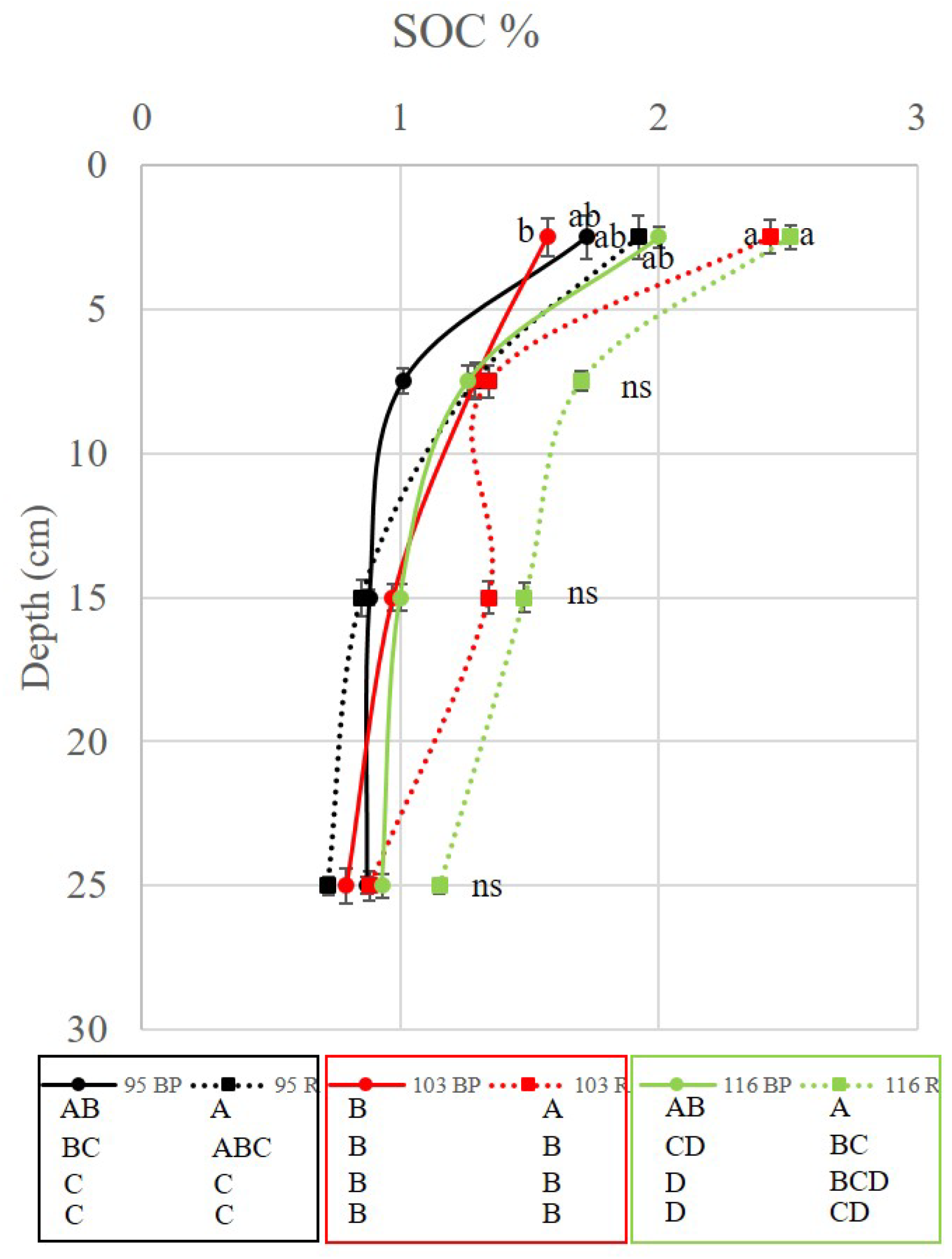
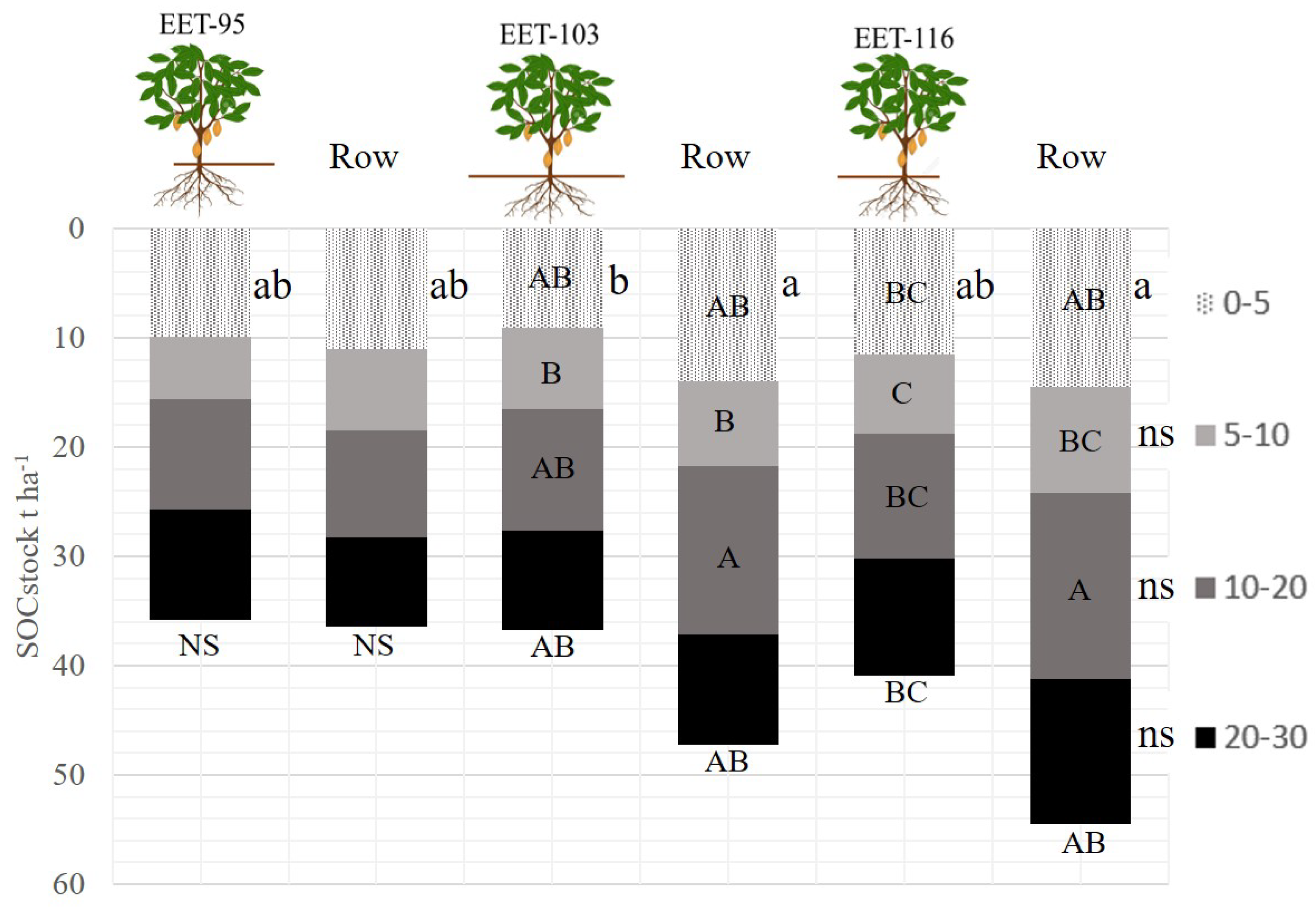
The results obtained in the concentration of soil organic carbon in the different species of cocoa plantations, showed an average on the surface (0-5 cm) of 2% of carbon concentration. The highest being the row in the species EET 116 with 2.51% and the lowest located under the tree EET 103 with 1.57% being significantly different between them. In the other locations there were no differences in the concentrations of organic carbon. Likewise, no significant differences were found in the other depths of the soil profile. However, the soil organic carbon concentration decreased as the depth increased, averaging 1.3%, 1.0% and 0.89% in each of the depths, respectively. [
5] affirms that the concentration in the upper layer was up to 4 times higher than in the lower layer from 0 to 100 cm, in comparison in our work of 0 to 30 cm the differences reached almost 3 times more in the upper layer vs second lower layer. An important behavior on surface carbon dynamics is that waste management has played an important role in these systems [
13]. Pruning, and the shells of the cocoa pod are accumulated in the rows between the plants. This residue has been incorporated and decomposed during the 15 years that the plantations are old. The accumulation of these residues makes a fundamental contribution to the superficial layer of the soil where the most active microbial activity is found [
20]. On the contrary, as seen in
Figure 4, below the cacao tree the organic carbon concentrations were lower than in the rows. This explains the importance of the incorporation of the organic residues in the rows for their incorporation through decomposition in time [
21]. Likewise, microorganisms at depth do not have this access to nutrients, so the difference in carbon concentration is notable [
22].
The accumulation of total organic carbon in the different cocoa species at different sections of the soil profile, it was observed that in the locality of the rows between the species EET 116 and EET 103 values from 0-5 cm of 14.44 and 14 (t ha−1), respectively. The sample located under the tree of the EET 103 species showed to be the one that accumulated less tons of carbon compared to the other species, with a value of 9.04 t ha−1. In the next sections, the accumulation of organic carbon did not show significant differences between the different sampling locations.
Accumulation decreased in the 5-10 cm section by an average of 7.56 t ha
−1. On the other hand, the notable increase in carbon accumulation in the 10-20 cm section was 12.5 t ha
−1 and in the last section (20-30 cm) it decreased to 10.2 t ha
−1. The accumulation of carbon (t ha
−1) in each section of the profile shows the dynamics of the roots. These results are related to the profile moisture values. As the rows of the plantation have a larger layer covered on the surface, whether it is alive or dead vegetation, in this particular case it is residue from pruning and harvesting, an accumulation of organic carbon was found in the surface section (0-5 cm) due to decomposition over time, adding environmental factors such as temperature [
23]. These factors in a tropical climate cause microbial activity to be more active than in the samples located under the tree where no pruning or harvesting residues are found [
23,
24].
On the other hand, there was an increase in accumulation in the 10-20 cm section of the soil profile,
Figure 5. This could be explained by the growth of the roots of the cocoa trees. In times of long summers with sprinkler irrigation, moisture is retained for much longer under the pruning and harvest residues. This has a direct effect on the development of the roots causing them to drift towards the direction of the rows in search of water. This leads to a greater presence of roots in that section. According to studies, the greatest amounts of roots are between 10 to 20 cm deep [
23,
25].
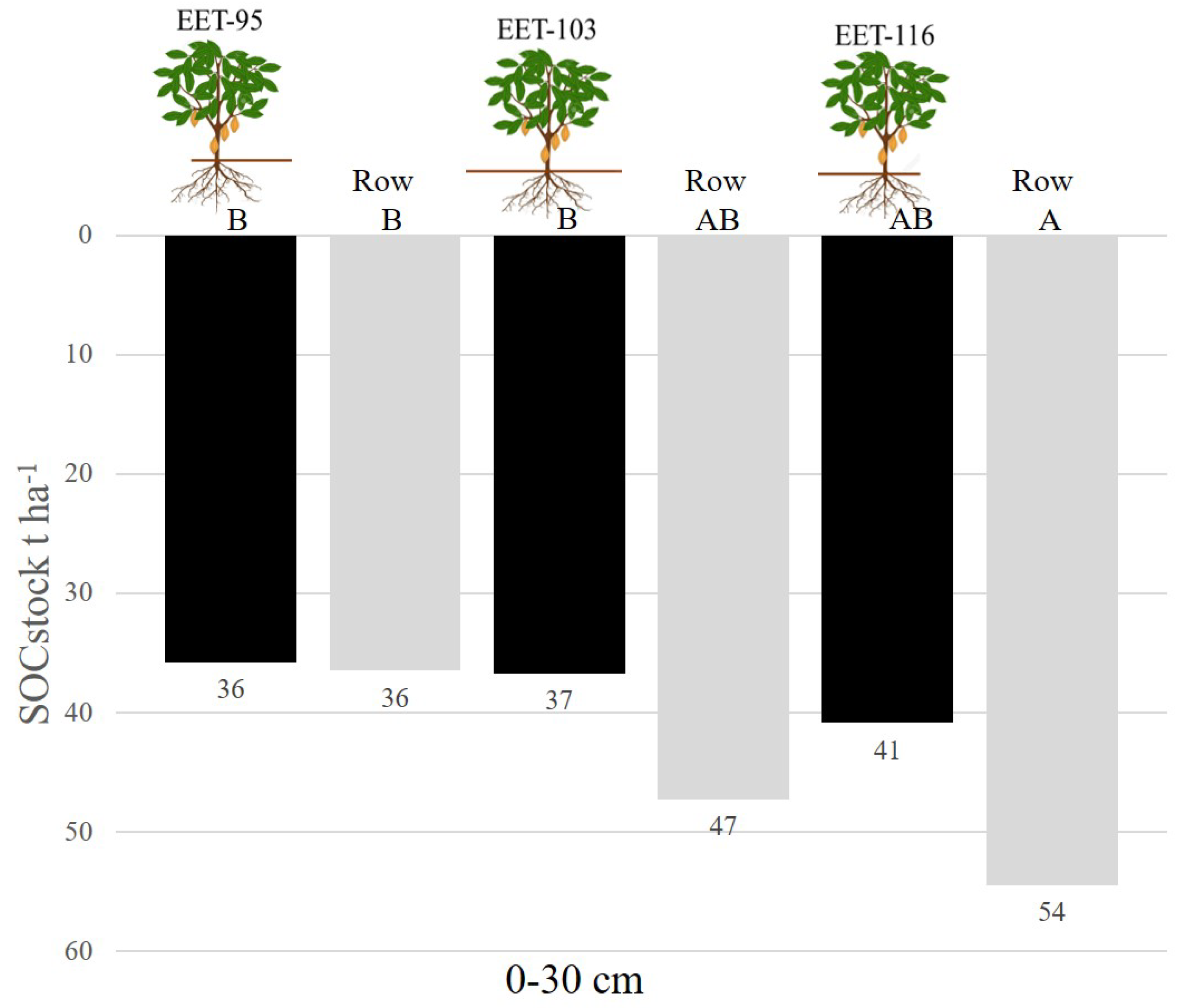
With the analysis of the entire profile analyzed (0-30cm), it was observed that the species EET 116 accumulated 41 t ha
−1 and 54 t ha
−1 below tree and row, respectively. The sample located in the row of the species EET 103 with 47 t ha
−1 did not differ. On the other hand, the lowest values, but without significant differences between the different sampling locations are the species EET95 and EET 103, with 36 t ha
−1, 37 t ha
−1 and 47 t ha
−1, below tree and row, respectively.
Figure 6.
With this experimental design it has been possible to appreciate that the management has a positive impact on the capture of organic carbon in the soil. We can see those organic residues from crops and pruning of the same plantation have significantly increased the accumulation of organic carbon. However, we cannot affirm that all cocoa varieties have this capacity. As can be seen in
Figure 5, the EET-95 variety has shown that it has generated less biomass, since it sequesters between 10 to 15 tons less carbon compared to its competitors EET-103 and EET-116.
4. Conclusions
In conclusion, the species EET116 accumulated more organic carbon due to several factors, possibly a higher decomposing biomass, not only from the tree but also from other trees in the area, which contributes to the incorporation of organic matter throughout the soil profile. The management of a cocoa plantation older than 15 years has had a positive impact on carbon sequestration, regardless of the cocoa variety. Since we in the rows of the plantation is equal to or greater but not less than the tons of carbon in the soil. This makes the agroforestry system of cocoa plantations a major contributor to the organic carbon supply.
Funding
Supported by funding from the ESPAM MFL with the research project. CUP: 91880000.0000.388095 SEMPLADES, Ecuador.
Acknowledgments
To the technical team of the soil laboratory and students ESPAM MFL Ecuador).
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest.” Authors must identify and declare any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. Any role of the funders in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results must be declared in this section. If there is no role, please state “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Fu, H.Z.; Waltman, L. A large-scale bibliometric analysis of global climate change research between 2001 and 2018. Climatic Change 2022, 170. [CrossRef]
- Benbi, D.K. Greenhouse Gas Emissions from Agricultural Soils: Sources and Mitigation Potential. Journal of Crop Improvement 2013, 27, 752–772. [CrossRef]
- Laborde, D.; Mamun, A.; Martin, W.; Piñeiro, V.; Vos, R. Agricultural subsidies and global greenhouse gas emissions. Nature Communications 2021, 12. [CrossRef]
- Brown, J.; Stobart, R.; Hallett, P.; Morris, N.; George, T.; Newton, A.; Valentine, T.; McKenzie, B. Variable impacts of reduced and zero tillage on soil carbon storage across 4–10 years of UK field experiments. Journal of Soils and Sediments 2020, 21, 890–904. [CrossRef]
- Yang, S.; Dong, Y.; Song, X.; Wu, H.; Zhao, X.; Yang, J.; Chen, S.; Smith, J.; Zhang, G.L. Vertical distribution and influencing factors of deep soil organic carbon in a typical subtropical agricultural watershed. Agriculture, Ecosystems & Environment 2022, 339, 108141. [CrossRef]
- Wood, G., .F.J. From Fossil Fuels to Low Carbon Energy Transition: New Regulatory Trends in Latin America; Springer International Publishing, 2022. [CrossRef]
- Leuschner, C.; Homeier, J., Global Forest Biodiversity: Current State, Trends, and Threats. In Progress in Botany Vol. 83; Springer International Publishing, 2022; pp. 125–159. [CrossRef]
- ECLAC. Planning for sustainable territorial development in Latin America and the Caribbean. ECLAC 2019 2019, 1. doi:https://repositorio.cepal.org/bitstream/handle/11362/44732/1/S1900438_en.pdf.
- Monroe, P.H.M.; Gama-Rodrigues, E.F.; Gama-Rodrigues, A.C.; Marques, J.R.B. Soil carbon stocks and origin under different cacao agroforestry systems in Southern Bahia, Brazil. Agriculture, Ecosystems & Environment 2016, 221, 99–108. [CrossRef]
- Purcell, T.; Martinez-Esguerra, E.; Fernandez, N. The Value of Rents: Global Commodity Chains and Small Cocoa Producers in Ecuador. Antipode 2018, 50, 641–661. [CrossRef]
- Silva, J.; Rojas-Briceño, N.B.; Tineo, D.; Morales, E.; Sopla, J.; Perez, J.; Rodríguez, N.; Fernández, S.; Bautista, R.; Mas, M.; Campos, G.; Gosgot, W.; Juarez, L.; Culqui, L.; Bautista, M.; Castañeda, N.; Lopez, M.; Calderon, M.S.; Bustamante, D.E. Contributions of scientific research to regional development in the Amazonas region, northern Peru. Development Studies Research 2022, 9, 129–141. [CrossRef]
- Ortiz-Rodríguez, O.O.; Villamizar-Gallardo, R.A.; Naranjo-Merino, C.A.; García-Caceres, R.G.; Castañeda-galvís, M.T. Carbon footprint of the colombian cocoa production. Engenharia Agrícola 2016, 36, 260–270. [CrossRef]
- Goñas, M.; Rojas-Briceño, N.B.; Culqui-Gaslac, C.; Arce-Inga, M.; Marlo, G.; Pariente-Mondragón, E.; Oliva-Cruz, M. Carbon Sequestration in Fine Aroma Cocoa Agroforestry Systems in Amazonas, Peru. Sustainability 2022, 14, 9739. [CrossRef]
- Reyna-Bowen, L.; Vera-Montenegro, L.; Reyna, L. Soil-Organic-Carbon Concentration and Storage under Different Land Uses in the Carrizal-Chone Valley in Ecuador. Applied Sciences 2018, 9, 45. [CrossRef]
- Vera, L., H.A.M.F.C.A.G.A.O.K..L.G. Principales suelos y particularidades de su formación del sistema Carrizal-Chone, Manabí, Ecuador. Cultivos Tropicales 2019, 2, 40.
- WALKLEY, A. A CRITICAL EXAMINATION OF A RAPID METHOD FOR DETERMINING ORGANIC CARBON IN SOILS—EFFECT OF VARIATIONS IN DIGESTION CONDITIONS AND OF INORGANIC SOIL CONSTITUENTS. Soil Science 1947, 63, 251–264. [CrossRef]
- Jadán Maza, O.A.; Torres, B.; Selesi, D.; Peña, D.; Rosales, C.; Gunter, S. DIVERSIDAD FLORÍSTICA Y ESTRUCTURA EN CACAOTALES TRADICIONALES Y BOSQUE NATURAL (SUMACO, ECUADOR). Colombia Forestal 2016, 19. [CrossRef]
- Quintana, C.; Girardello, M.; Balslev, H. Balancing plant conservation and agricultural production in the Ecuadorian Dry Inter-Andean Valleys. PeerJ 2019, 7, e6207. [CrossRef]
- Bronick, C.; Lal, R. Soil structure and management: a review. Geoderma 2005, 124, 3–22. [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; Steinbeiss, S.; Thomson, B.C.; Trumbore, S.E.; Gleixner, G. Plant diversity increases soil microbial activity and soil carbon storage. Nature Communications 2015, 6. [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. THE VERTICAL DISTRIBUTION OF SOIL ORGANIC CARBON AND ITS RELATION TO CLIMATE AND VEGETATION. Ecological Applications 2000, 10, 423–436. [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [CrossRef]
- Solly, E.F.; Schöning, I.; Boch, S.; Kandeler, E.; Marhan, S.; Michalzik, B.; Müller, J.; Zscheischler, J.; Trumbore, S.E.; Schrumpf, M. Factors controlling decomposition rates of fine root litter in temperate forests and grasslands. Plant and Soil 2014, 382, 203–218. [CrossRef]
- Jadán, O.; Cifuentes, M.; Torres, B.; Selesi, D.; Veintimilla, D.; Günter, S. INFLUENCE OF TREE COVER ON DIVERSITY, CARBON SEQUESTRATION AND PRODUCTIVITY OF COCOA SYSTEMS IN THE ECUADORIAN AMAZON. BOIS & FORETS DES TROPIQUES 2015, 325, 35. [CrossRef]
- Hayes, D.C.; Seastedt, T.R. Root dynamics of tallgrass prairie in wet and dry years. Canadian Journal of Botany 1987, 65, 787–791. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).