1. Introduction
COVID-19 is a newly discovered infectious respiratory illness that was first documented in December 2019 in Wuhan, China. It was brought on by SARS-CoV-2 or the new coronavirus (nCoV-2019). The World Health Organization (WHO) designated COVID-19 as a pandemic on March 11, 2020, with a death rate of 3.4 % (WHO, 2020). It belongs to a novel class of coronaviruses that may infect the lungs, upper neck, sinuses, and nose. It belongs to the order Nidovirales' Coronaviridae family's coronavirus genus. Alpha, beta, gamma, and delta coronaviruses are subgroups of the family Coronaviridae. Scientists identified this new virus as a member of the family on January 7, 2020. (Madabhavi et al., 2020).
1.1. Structure of the Coronavirus
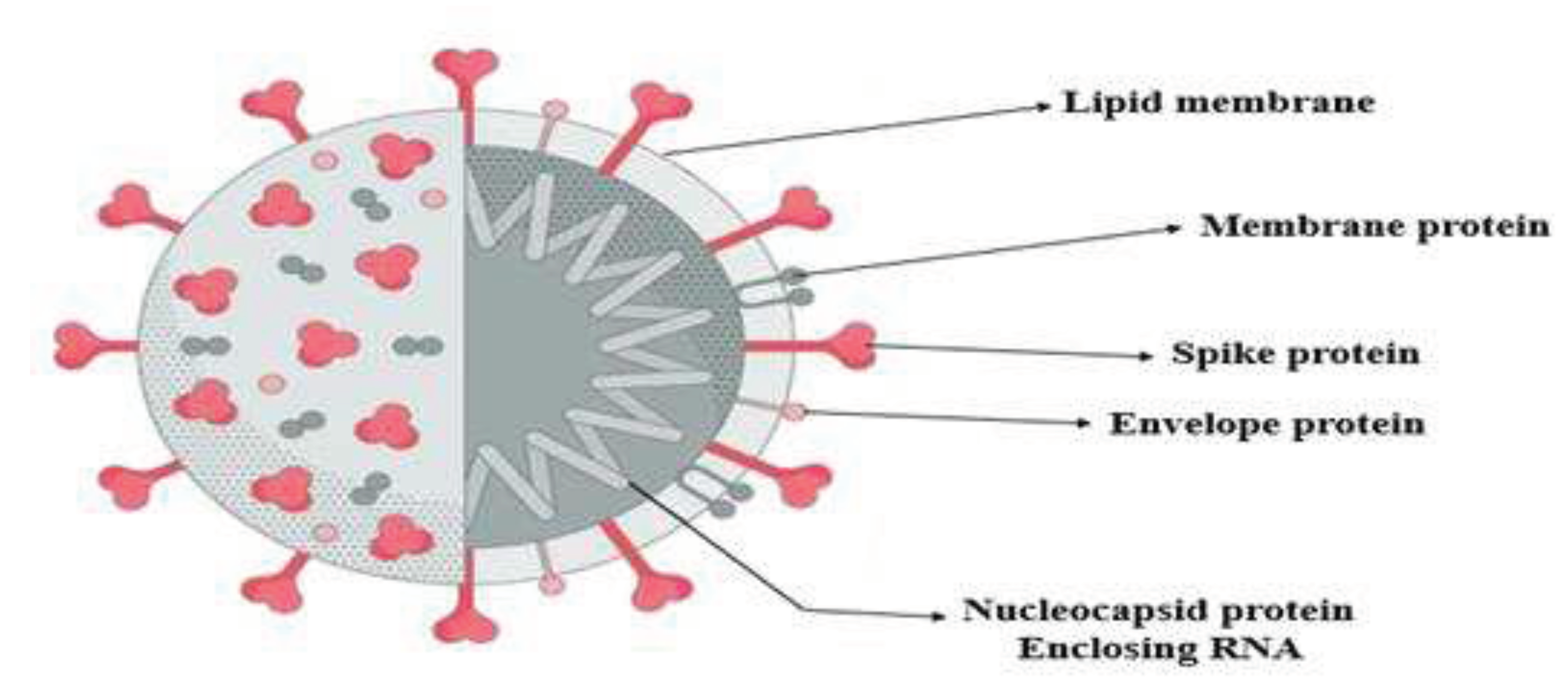
It is a ssRNA virus and has crown-shaped peplomer spikes on the outer surface. Woo et al., (2010) stated that the size of coronavirus is 80-160 nm in diameter and 27-32 kBs in length. This virus has structural similarities with bat coronavirus and SARS-coronavirus, as shown in
Figure 1.
According to Madabhavi et al., (2020), transcription mistakes, RNA-dependent RNA polymerase (RdRP) jumps, and rapid mutation rates all contribute to the high recombination rate of coronavirus. It has never been reported that this COVID-19 strain has infected people before (Li et al., 2020). The genetic sequence information for SARS-CoV-2 was provided by the Global Initiative on Sharing All Influenza Data (GISAID) on January 10, 2020. The virus was given the designation nCoV-2019 by the WHO and the International Committee on Taxonomy of Viruses (ICTV) on January 12, 2020. These organizations designated the illness as COVID-19 on February 11, 2020.
Initial research on the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) was conducted in Saudi Arabia at the beginning of 2012. This virus was shown to be responsible for acute respiratory distress syndrome (ARDS), pneumonia, and kidney failure. It was phylogenetically distinct from the human coronavirus (Rahman & Sarkar, 2019). According to Yin & Wunderink (2018), the virus causes infections in the respiratory, hepatic, neurological, and gastrointestinal systems of the body. Elderly people with lung, heart, and/or diabetic conditions were more susceptible to COVID-19 sickness (Huang et al., 2020).
Compared to copper and cardboard, SARS-CoV-2 was more stable on plastic and stainless steel (Van Doremalen et al., 2020). It was said to be able to endure on a variety of surfaces for many hours or days, including:
-
(i)
Copper (pennies, tea kettles, and cookware): up to 4 hours
-
(ii)
Boxes for shipment made of cardboard: up to 24 hours
-
(iii)
Plastic (milk jugs, detergent bottles, elevator buttons, and bus seats): 2 to 3 days
-
(iv)
Stainless steel (refrigerators, pots and pans, sinks, and some water bottles): 2 to 3 days
1.2. Virology and Pathogenesis
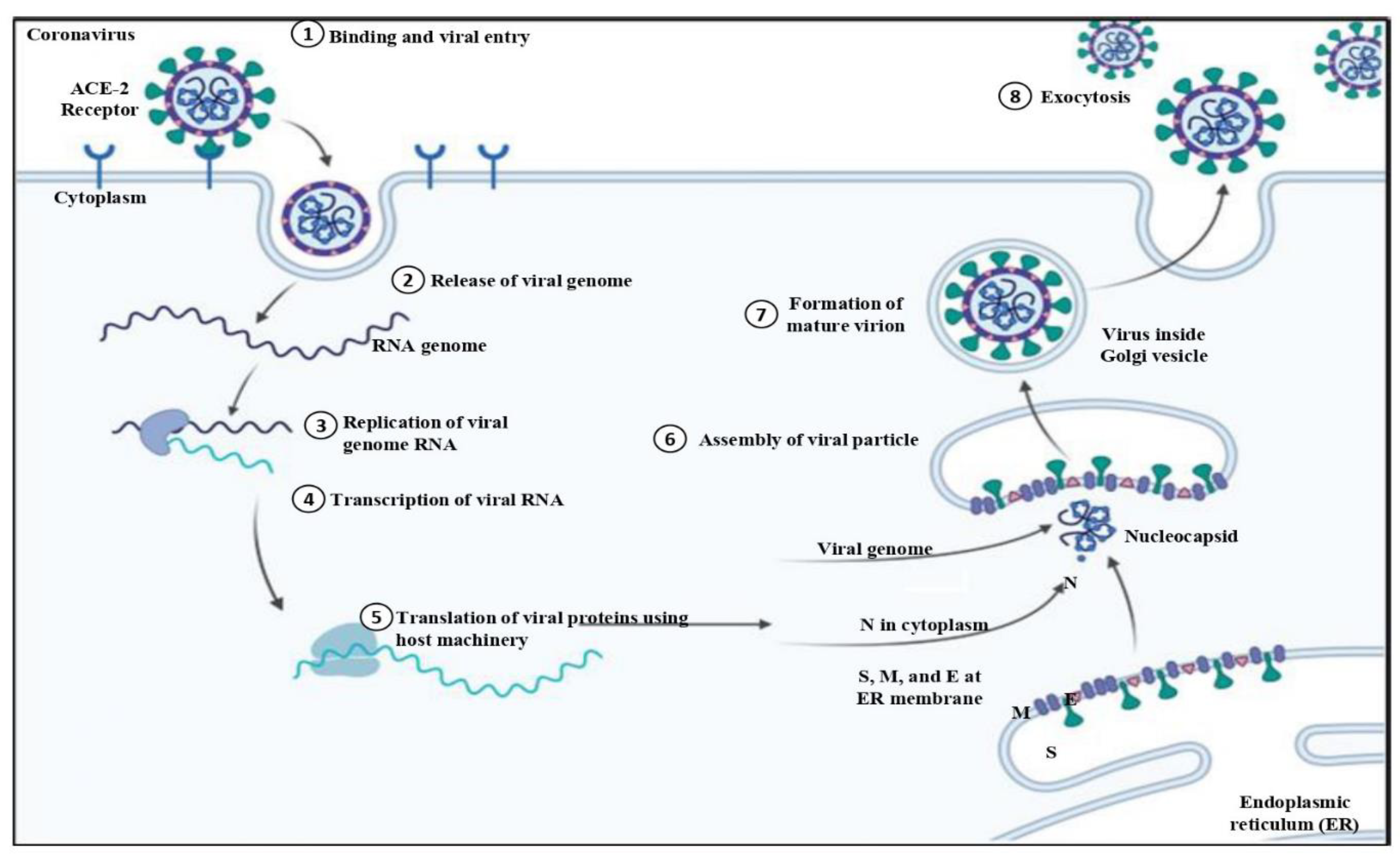
Understanding the virology and pathogenesis of COVID-19 is crucial. Understanding how the virus functions at a cellular level and how it causes sickness in people is important for properly combating this worldwide health issue (Pollard et al., 2020). The angiotensin-converting enzyme 2 (ACE 2) receptor is found on human cells of the respiratory tract, where the spike protein of SARS-CoV-2 is situated (Jackson et al., 2022). This connection makes it easier for the virus to enter host cells and start an infection. The virus then reproduces and creates viral particles, which cause the release of fresh infectious virions and the appearance of symptoms, as shown in
Figure 2 (King et al., 2020).
The pathogenesis of COVID-19 involves a complex interplay of immune responses. The immune system is combated by the virus, and individuals remain asymptomatic or experience mild symptoms (Goldsmith et al., 2004). However, in severe cases, an excessive immune response or cytokine storm can lead to widespread inflammation, lung damage, and other systemic complications (Jackson et al., 2022). This chapter aimed to explore the causes, signs, symptoms, spread, management, and suggestions of the COVID-19 illness.
2. Symptoms
nCoV-2019 has varying effects on various individuals. From minor symptoms to serious sickness, a broad variety of symptoms have been observed. The virus may stay latent for up to 14 days, although it typically takes 5–6 days for symptoms to appear in an infected individual. Individual differences in symptoms and intensity exist.
-
(a)
Fever, a dry cough, and fatigue are the most typical symptoms.
-
(b)
Less common signs and symptoms include a rash on the skin, discoloration of the fingers or toes, headaches, conjunctivitis, aches and pains, sore throats, diarrhoea, nausea, or vomiting.
-
(c)
Breathing problems, chest discomfort, loss of speech, and immobility are serious signs.
3. Transmission of COVID-19
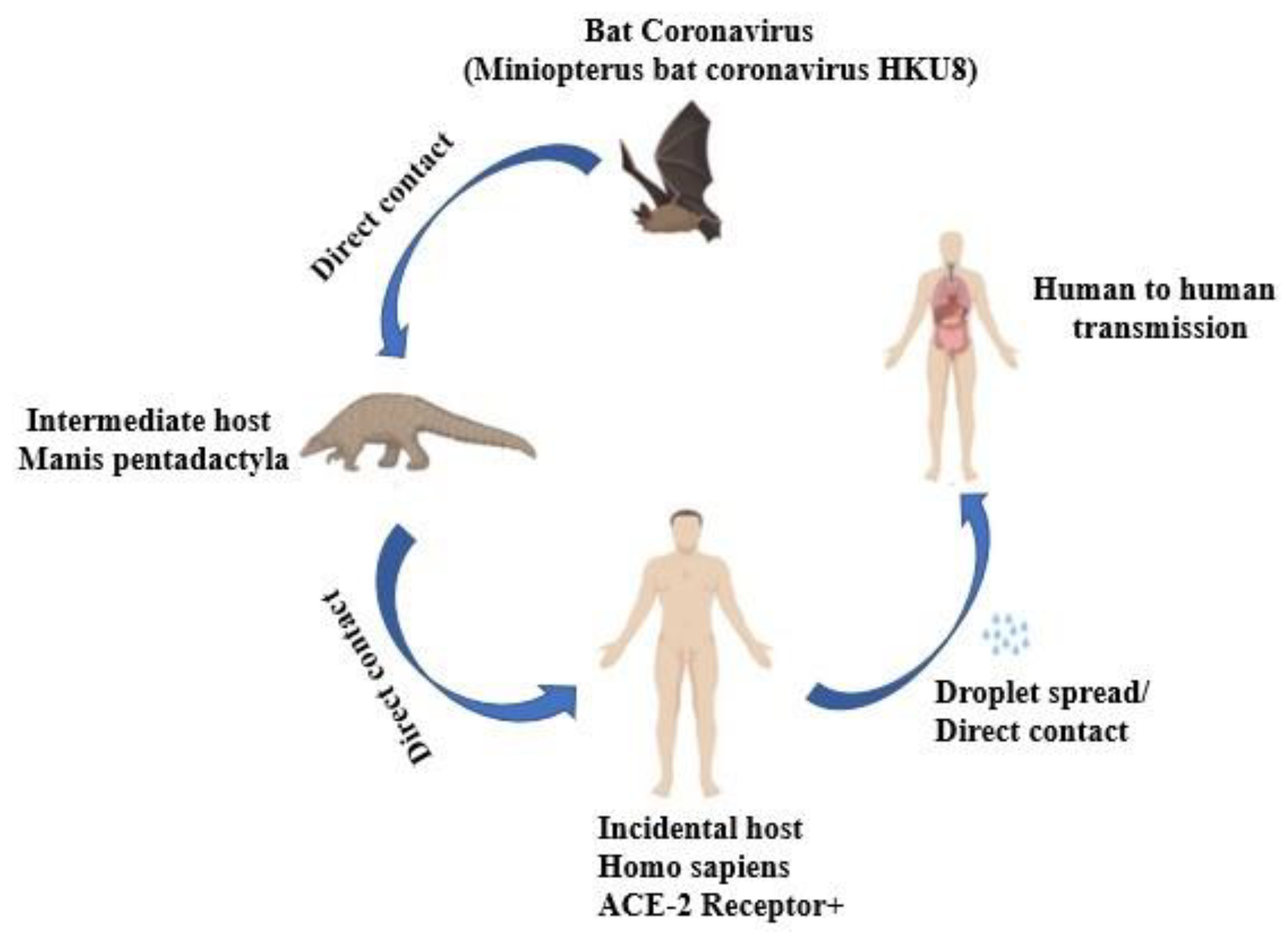
The SARS pandemic outbreak in Guangdong, China, caused by the SARS-CoV-2 was reported in 2002, contrary to the scientific belief that coronavirus disease transmission exclusively occurred in animals. The virus spread through many ways such as airborne, fomite, contact, and droplet transmissions (Ayouni et al., 2021). SARS-CoV-2 transmission is shown diagrammatically in
Figure 3.
3.1. Contact and Droplet Transmission
When an infected individual coughs or sneezes aerosol droplets, and a healthy person breathes or swallows them, the virus may be transmitted from one person to another through direct, indirect, or close contact (Ghinai et al., 2020). Aerosols are defined as droplets with a diameter of less than 5 μm, while respiratory droplets are defined as droplets with a diameter of greater than 5–10 μm. Up to 6 feet out, or perhaps beyond, the sick individual may spray the aerosol droplets.
3.2. Airborne Transmission
When held in the air for a long period of time and across great distances, an infectious agent brought on by the transmission of aerosols that still carry the virus may propagate (WHO, 2014; Bhardwaj and Vikram, 2023; Bhardwaj and Singh, 2023). During medical procedure aerosols generate and viruses may be spread through the air (WHO, 2020). In the absence of aerosol-generating methods, the virus might potentially spread by aerosols, especially in enclosed spaces with inadequate ventilation. When creating aerosols of infectious samples using powerful jet nebulizers in a controlled laboratory environment, researchers have observed that the SARS-CoV-2 virus was present in air samples for up to 3 hours and up to 16 hours (Fears et al., 2020). In their investigations that were carried out in hospitals where COVID-19 patients were hospitalized, a number of researchers reported the presence of SARS-CoV-2 RNA in air samples (Guo et al., 2020).
3.3. Formite/Fomite Transmission
The respiratory secretion of the sick person may contaminate the items and surfaces. There have been reports of greater concentrations of the SARS-CoV-2 virus in healthcare facilities where COVID-19 patients were being treated, depending on the temperature, humidity of the environment, and the kind of surfaces (Ding et al., 2020). So, by touching contaminated surfaces and then contacting your mouth, nose, or eyes with infected hands, the virus might be indirectly spread. Since persons who touch potentially contaminated surfaces often also come into close contact with the ill person, it may be difficult to tell the difference between respiratory droplet and fomite transmission. Nevertheless, considering that other respiratory viruses may also spread by fomite, this method is thought to be a plausible means of transmission for SARS-CoV-2.
3.4. Other Modes of Transmission
Even while asymptomatic, some virus carriers may still propagate the disease. SARS-CoV-2 has been found in a variety of biological samples, including plasma, serum, urine, and faeces (Pan et al., 2020; Wang et al., 2020a). In a recent scientific brief on breastfeeding, WHO recommended that mothers with suspected or confirmed COVID-19 be encouraged to commence or maintain breastfeeding (WHO, 2020). SARS-CoV-2 may spread to other animals, including dogs, cats, and farmed minks (Newman et al., 2020; Oreshkova et al., 2020).
4. Impact on Healthcare Systems and Infection Control MEASURES taken
Worldwide healthcare systems were severely impacted by the COVID-19 pandemic, needing modifications to infection control procedures in order to manage and alleviate the disaster. The increase in COVID-19 cases overburdened several healthcare systems (Filip et al., 2022).
Healthcare personnel experienced extended work hours, elevated stress levels, heightened exposure risks, frequent use of personal protective equipment (PPE) kits, etc. (Molento, 2021). There was a lack of ventilators, ICU beds, and other necessities at hospitals. Non-emergency medical treatments and elective surgery were postponed. While the use of telemedicine and online consultations were increased (Haward et al., 2023).
Governments quickly implemented emergency procedures to improve and reorganise infection control plans everywhere (Filip et al., 2022). Cross-border cooperation made it easier to share knowledge, resources, and vital assistance to handle the enormous demand. The fast spread of the virus demanded that efficient containment measures be implemented right once. The usage of masks, physical distance, better ventilation systems in public areas and medical institutions, hand hygiene practices, and vaccine programs were included in the measure to reduce COVID-19 transmission (Chiu et al., 2020).
It is essential to comprehend immunological processes in order to create vaccines and specialized treatments. In order to develop herd immunity and lessen the strain on healthcare systems, mass vaccination programs were launched (Liu et al., 2021). Reliable data collection and network, and surveillance systems were set up to track cases, identify hotspots, and monitor the transmission of viruses. Testing and contact tracing were essential for quickly identifying and isolating patients (Rodrigues & Plotkin, 2020). The continuing fight against COVID-19 was greatly helped by continued research and surveillance. The epidemic encouraged quick research and development in vaccine, therapeutic, and diagnostic fields (Majid et al., 2021).
5. Antibacterial drug Resistance: An Overview
Another powerful adversary, the silent but steadily worsening threat of antibacterial drug resistance, was lurking in the shadows among the COVID-19 epidemic that had brought the entire world to its knees (WHO, 2020). In addition to the novel coronavirus's imminent threat to humanity, scientists and medical experts were keenly aware that the fight against bacterial illnesses was also nearing a turning point (Ventola, 2015). Researchers have explored the use of antibiotics as potential co-therapies for COVID-19 to address secondary bacterial infections or to modulate the immune response. However, this approach must be carefully studied to minimize the risk of antibiotic resistance (Chedid et al., 2021).
Antibacterial drug resistance, often known as antibiotic resistance, describes how well bacteria are able to survive the effects of medications intended to kill them or stop their growth (Ventola, 2015). Antibiotic drugs are used to both prevent and cure bacterial infections. Antibiotic resistance arises when bacteria develop a resistance to antibiotic treatment (Uddin et al., 2021). Antibiotic resistance is caused by bacteria, not by people or other animals. These microorganisms have the potential to infect both humans and animals, and their illnesses are more difficult to treat than those brought on by non-resistant bacteria (Prestinaci et al., 2015). The immune response to COVID-19 can also play a role in antibacterial drug resistance.
It has been noted that antibiotic resistance increases mortality, lengthens hospital stays, and raises medical costs. This resistance results from bacteria's capacity for adaptation, which significantly reduces the effectiveness of current antibiotics (WHO, 2020). COVID-19 patients often require hospitalization, and healthcare settings can serve as reservoirs for antibiotic-resistant bacteria. The presence of COVID-19 patients in hospitals can facilitate the transmission of both SARS-CoV-2 and antibiotic-resistant bacteria. A study showed the predominance of gram-negative bacteria in hospitalized Indian COVID-19 patients in India was resistant to higher generation of antimicrobials (Seethalakshmi et al., 2022).
5.1. The Mechanisms behind Resistance
The COVID-19 pandemic has brought to the forefront the importance of understanding the mechanisms underlying the disease and its interplay with antibacterial drug resistance (Adebisi et al., 2021). While COVID-19 is primarily a viral infection, its impact extends beyond the virus itself and intersects with antibacterial drug resistance in several ways (Knight et al., 2021). COVID-19 can weaken the immune system, making patients more susceptible to secondary bacterial infections. Due to this, COVID-19 patients are using more antibiotics, which may have an impact on the development and spread of antibacterial drug resistance (Sahu et al., 2022). Antibacterial drug resistance can arise through several mechanisms, often working in concert:
-
(i)
Genetic mutations: Bacteria have the capacity to acquire genetic mutations, which enable them to evolve resistance to antibiotics. These mutations can influence either the drug's intended target site or the bacteria's capability to expel the drug from their cells (CDC, 2019).
-
(ii)
Horizontal gene transfer: Bacteria are remarkably skilled at swapping genetic material with one another. This can include the transfer of plasmids, small pieces of DNA that may contain antibiotic resistance genes. This enables the rapid spread of resistance within bacterial populations (O’Neill, 2016).
-
(iii)
Overuse and misuse of antibiotics: In the early stages of the COVID-19 pandemic, there was uncertainty about the appropriate treatment. As a result, antibiotics were often prescribed as precautionary measures. The extensive and sometimes indiscriminate utilization of antibiotics in healthcare, agriculture, and even household products has hastened the emergence of resistance. When antibiotics are employed excessively or inappropriately, it provides bacteria with greater opportunities to cultivate resistance (CDC, 2021). This overuse of antibiotics can drive the development of antibiotic-resistant bacteria (Haldane et al., 2021).
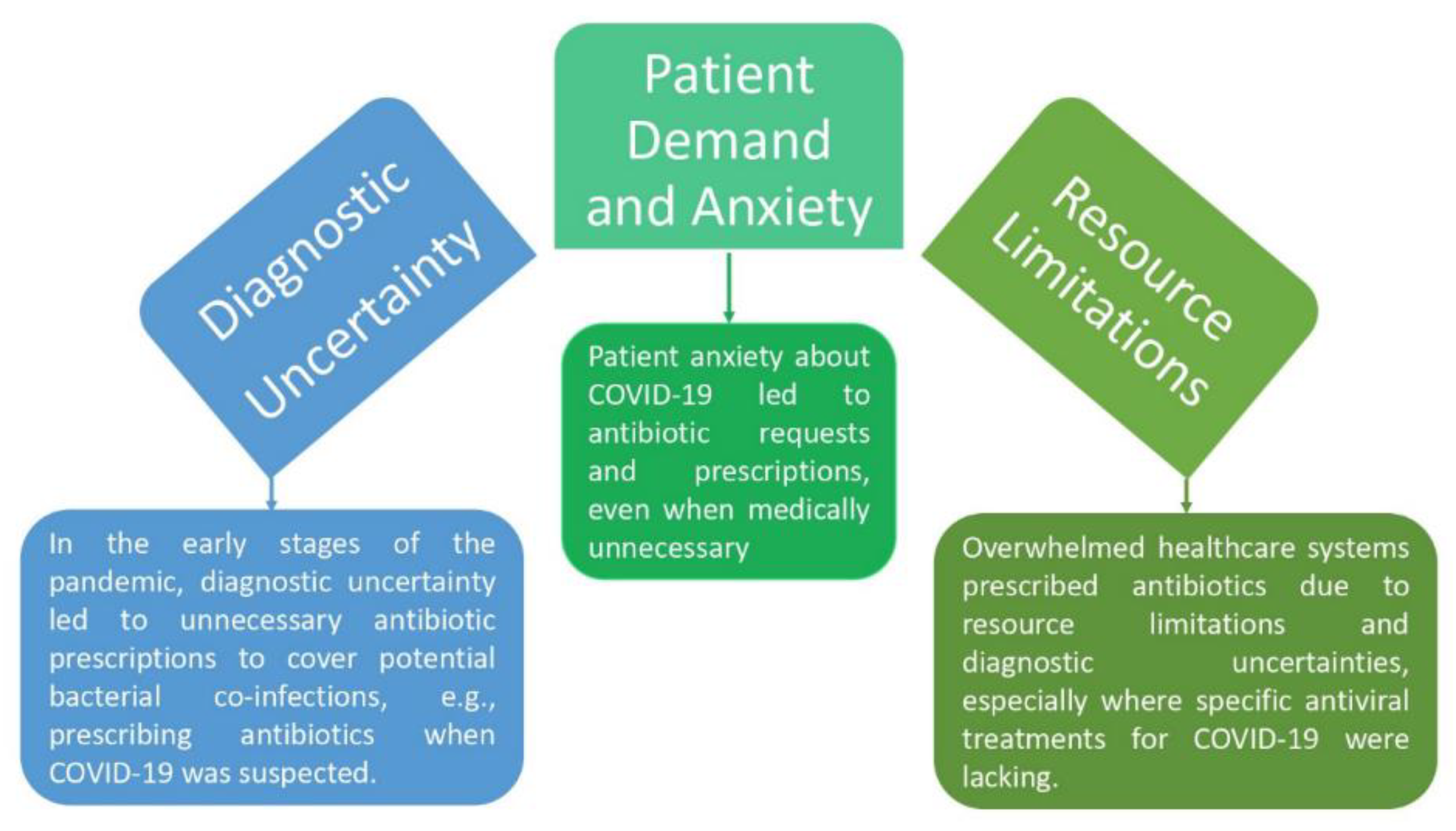
Antibiotics were overused during the COVID-19 epidemic, despite the fact that they are useless against viral illnesses like COVID-19 (Malik & Mundra, 2022). Antibiotic overprescription was caused by a number of circumstances (
Figure 4). First of all, the early phases of the pandemic were marked by severe diagnostic ambiguity. When COVID-19 was suspected, medical professionals often turned to giving antibiotics as a preventative step. In circumstances where the cause of the sickness was not evident, this was largely motivated by the need to cover suspected bacterial co-infections (Ghosh et al., 2021).
However, it resulted in the overuse of antibiotics, increasing worries about the rapid development of antibiotic resistance. The demand from patients and their worry contributed to the misuse of antibiotics. Some individuals asked their doctors for antibiotics as a result of their worry and dread about COVID-19. Antibiotics have sometimes been provided by medical professionals to allay patient worries despite the absence of a medical need. Although this procedure was intended to ease patient anxiety, it also led to the misuse of antibiotics (Akram et al., 2022).
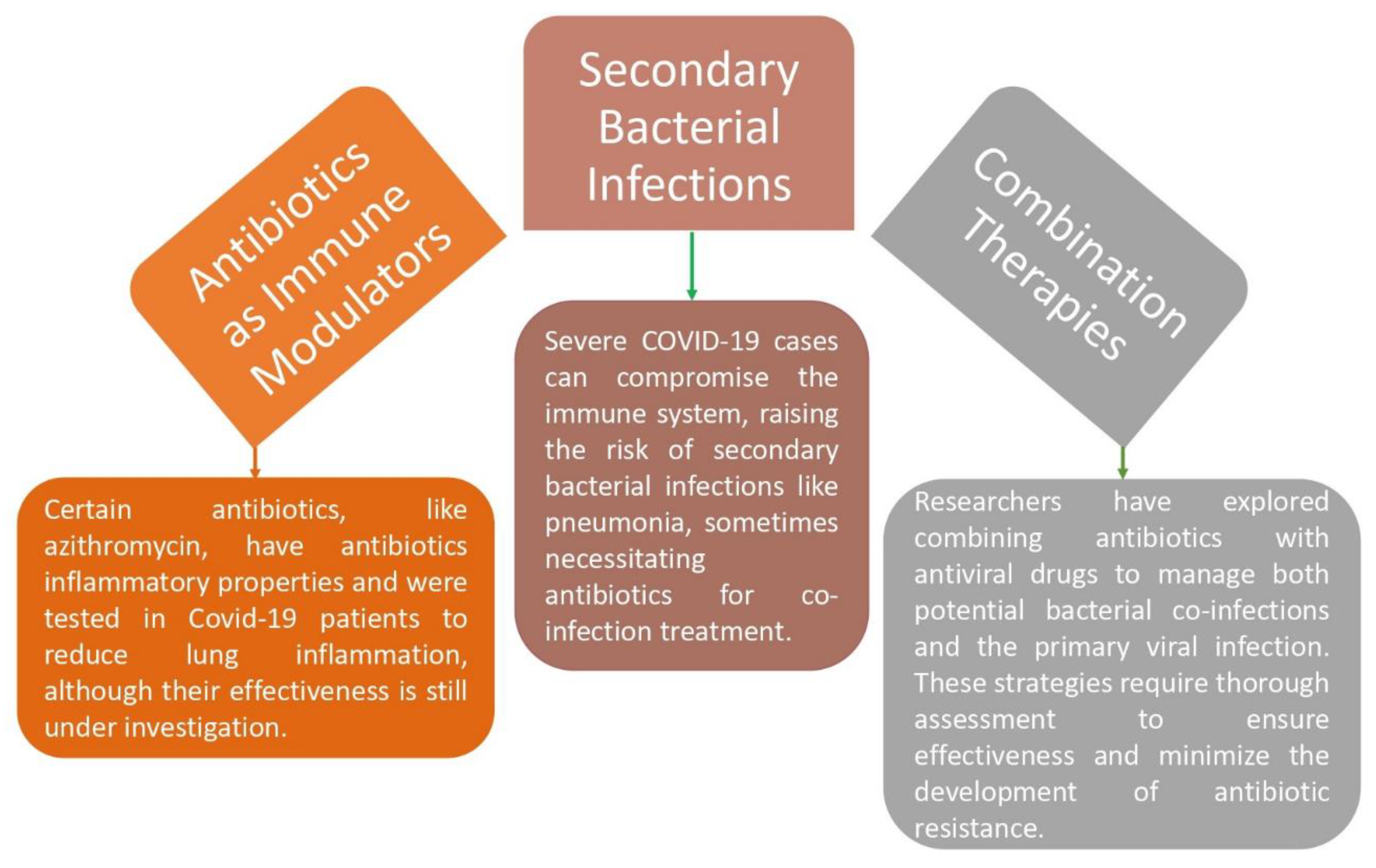
Antibiotic usage was also influenced by resource shortages in the pandemic's overburdened healthcare systems. Given the unavailability of specific antiviral therapies for COVID-19, clinicians sometimes recommend antibiotics to alleviate diagnostic ambiguity in areas with low resources. This choice was made as a precaution to address the possibility of bacterial co-infections, but it unintentionally increased antibiotic resistance (Malik & Mundra, 2022). Antibiotics may indirectly affect viral infections, like COVID-19, even though their primary purpose is to treat bacterial infections. For instance, several antibiotics, such as macrolides like azithromycin, have been investigated as possible therapies for COVID-19 because they have anti-inflammatory effects. They are believed to control the immunological response and lessen inflammation in the lungs. Their effectiveness is still being investigated (Malik & Mundra, 2022).
The possibility of secondary bacterial infections is a component of COVID-19's antibacterial action (
Figure 5). In addition to damaging lung tissue and lowering immunity, severe COVID-19 infections raise the risk of subsequent bacterial infections including bacterial pneumonia. In order to treat both probable bacterial co-infections and the original viral infection, researchers have also looked at combination medicines that include antibiotics and antiviral medications. To assure their effectiveness and reduce the chance of spreading antibiotic resistance, a rising global health problem, such combination medicines must first undergo a comprehensive study before being developed and used (Malik & Mundra, 2022).
As a result, the COVID-19 pandemic's excessive usage of antibiotics was influenced by diagnostic uncertainty, patient anxiety, and resource constraints. Although antibiotics are ineffective against viruses, they may indirectly affect viral infections by altering the immune system and treating bacterial illnesses that arise as a result of viral infections. However, it is crucial to use antibiotics sparingly to reduce the chance of fostering antibiotic resistance, which represents a serious danger to the general public's health (Founou et al., 2021).
5.2. Global Trends in Antibacterial Resistance
Antibacterial resistance is a global concern. As per the update in September 2021, several trends were particularly concerning:
Rising resistance rates: Resistance to common antibiotics, such as penicillin and erythromycin, has been steadily increasing. Bacteria once easily treated with these drugs are becoming difficult to combat (CDC, 2019).
Emergence of superbugs: Superbugs are bacteria that have developed resistance to a variety of medicines, making them very difficult to treat. Methicillin-Resistant Staphylococcus Aureus (MRSA) and Carbapenem-Resistant Enterobacteriaceae (CRE) are two notable examples (CDC, 2019).
Limited new antibiotics: The flow of newly developed antibiotics has been diminishing, as pharmaceutical companies have encountered reduced incentives to invest in the synthesis and formulation of novel drugs compared to other medical domains (O'Neill, 2016).
5.3. Implications for the COVID-19 Pandemic
The interplay between COVID-19 and antibacterial resistance is a complex one. While COVID-19 is a viral infection, bacterial co-infections are not uncommon in severe cases. The misuse of antibiotics in the treatment of COVID-19, when bacterial infections are not present, can exacerbate antibacterial resistance issues (WHO, 2020). Additionally, the strain on healthcare systems during the pandemic may indirectly contribute to the spread of resistant bacteria. In crowded hospitals and ICUs, infection control measures can be compromised, facilitating the transmission of both COVID-19 and drug-resistant bacteria (Ventola, 2015). The use of antibiotics during previous epidemics stands out as a subject of high importance and scrutiny among these lessons.
H1N1 influenza pandemic (2009): The H1N1 influenza pandemic, sometimes known as the swine flu, was a worldwide health emergency that occurred more than ten years ago. During this outbreak, antibiotics were crucial, especially in the treatment of secondary bacterial infections (Al Hajjar & Mclntosh, 2010). Pneumonia cases increased during the pandemic, and antibiotics were crucial in treating these bacterial co-infections. It also brought attention to the dangers of antibiotic abuse and the chance that antibiotic resistance could worsen an already terrible situation (Smith et al., 2015).
Ebola outbreaks: Ebola virus disease outbreaks in West Africa, particularly the 2014-2016 epidemic, posed unique challenges. While antibiotics were ineffective against viruses like Ebola, they were crucial for treating concurrent bacterial infections. In the chaotic and resource-constrained environments of Ebola treatment centers, antibiotics became essential tools to combat secondary infections and improve patient outcomes (WHO, 2014).
6. Impact of Bacterial Co-Infections during Viral Pandemics
6.1. The Silent Threat Within Bacterial Co-Infections Amid Viral Pandemics
Viral pandemics, like the COVID-19 catastrophe, have captured the public's attention because of their swift spread and catastrophic effects. But beneath the surface, bacterial co-infections pose a less obvious but no less important threat (Rawson et al., 2021). In the context of a viral pandemic, the viral pathogen is frequently the main emphasis. However, the complex interactions between bacteria and viruses greatly affect the intensity and course of the illness. When someone is already facing a viral illness, bacterial co-infections can take advantage of their compromised immune systems, which can lead to higher rates of morbidity and death. When a virus is present, the host's immune system is weakened, which makes the environment more favourable for subsequent bacterial infections.
The primary focus of the immune system is on eliminating the viral danger, which may leave room for bacterial infections to establish a foothold and worsen the clinical state of the patient (Chiu et al., 2020). It can be difficult to distinguish between bacterial and viral infections based just on clinical symptoms, particularly in the early stages. The intricacy of diagnosis becomes even more pronounced during a pandemic, underscoring the pressing requirement for enhanced diagnostic instruments and monitoring frameworks to quickly distinguish between bacterial and viral causes (Liu et al., 2021). Concerns over the indiscriminate use of antibiotics are raised by the possibility of bacterial co-infections.
Healthcare professionals may use broad-spectrum antibiotics in the lack of specific diagnostic information, which might lead to an increase in antimicrobial resistance. To lessen the long-term effects of antibiotic resistance, it is crucial to strike a balance between prompt treatment and prudent antibiotic usage (Rodrigues et al., 2020). The latent hazard of co-infections with bacteria requires a multimodal strategy to address. In order to detect and monitor the incidence of bacterial co-infections during viral pandemics, more sophisticated surveillance techniques are needed.
Furthermore, developing diagnostic technologies is essential for quick and precise co-infection diagnosis, which enables focused and efficient therapy. It is essential to promote ethical antibiotic usage through guidelines and education in order to stop the spread of antibiotic resistance. Moreover, it is crucial to create thorough immunisation regimens that address possible risks of bacterial co-infection in addition to the main viral pathogen. The complex interaction between viruses and bacteria highlights the significance of identifying and treating bacterial co-infections in the context of viral pandemics (Majid et al., 2021). Because of the hidden threat they offer, public health strategies that combine better diagnoses, cautious antibiotic usage, and preventive interventions are needed to protect people from the combined effects of bacterial and viral diseases.
6.2. The Coexistence of Viruses and Bacteria
The human body turns into a battlefield for both viruses and bacteria during viral pandemics. Viruses like SARS-CoV-2, enter host cells and impair the immune system. In the meantime, bacteria are opportunistic pathogens that take advantage of every opportunity to infect people who are already debilitated from viral diseases. There could be serious, even lethal, repercussions from their combinatorial effect (Martin-Loeches et al., 2011).
Several bacterial pathogens commonly exploit the conditions created by viral infections:
-
(i)
Streptococcus pneumoniae: Known for causing pneumonia, this bacterium often finds an entry point in the damaged lung tissue of individuals with viral respiratory illnesses (Song et al., 2013).
-
(ii)
Staphylococcus aureus: This adaptable bacterium may result in a variety of infections, including those of the skin and soft tissues, which may develop as a consequence of viral skin lesions or weakened immunity (Miller et al., 2005).
-
(iii)
Haemophilus influenza: Often responsible for secondary respiratory infections, this bacterium thrives in the aftermath of viral damage to the respiratory tract (Avalos et al., 2018).
-
(iv)
Escherichia coli: Particularly in cases of severe viral gastroenteritis, it exacerbates symptoms and lead to severe dehydration (Nataro & Kaper, 1998).
6.3. The Consequences of Bacterial Co-Infections
Bacterial co-infections can significantly worsen the prognosis of viral illnesses. During viral pandemics, patients who have co-infections with bacteria may experience prolonged illness, increased hospitalization rates, and a higher risk of complications. The study conducted by Rawson et al., (2021), highlights how the coexistence of bacterial pathogens and viral infections can cause extended sickness and make recovery more difficult for those afflicted. The complex interactions between bacteria and viruses frequently have a synergistic effect that increases the disease's overall severity.
Healthcare systems are under more stress because of this increased demand for hospital resources, which makes it harder for them to efficiently handle the inflow of patients during a pandemic. The necessity of paying close attention to co-management measures is further highlighted by the increased risk of consequences linked to bacterial co-infections. Research indicates that co-infected persons are more vulnerable to serious sequelae, such as organ failure, respiratory distress, or secondary infections (Rawson et al., 2021).
To address these issues, patient treatment must take a comprehensive and integrated strategy that includes both antibacterial and antiviral therapies. The research conducted by Rawson et al., (2021) stresses the significance of readiness and preemptive actions to lessen the consequences of bacterial co-infections on the healthcare system as a whole. To create efficient management and preventive plans during viral pandemics and to guarantee a more adaptable and robust healthcare system, it is imperative to recognise these consequences.
7. Influence of Cytokine Storms on Bacterial Susceptibility
Cytokine storms, characterized by an overwhelming release of immune-signalling molecules, represent a dramatic and often life-threatening response by the immune system to various triggers, including viral infections like COVID-19 (Mehta et al., 2020). Cytokine storms (hypercytokinemia) occur when the immune system launches an exaggerated response to an infection (Mehta et al., 2020). As a result, pro-inflammatory cytokines including interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α) are released in excessive amounts. Viral infections, like SARS-CoV-2, are notorious triggers for cytokine storms (Montazersaheb et al., 2022). In severe cases of COVID-19, a cytokine storm can occur. This can lead to tissue damage and increase the risk of secondary bacterial infections (Alcock & Masters, 2021).
8. Clinical Implications and Challenges
The therapeutic relevance of bacterial susceptibility during cytokine storms has been brought to light by the COVID-19 pandemic (Langford et al., 2020). Due to cytokine-induced immunosuppression, patients with severe COVID-19 are more vulnerable to bacterial co-infections, especially in the lungs. The management of bacterial co-infections in the context of cytokine storms presents challenges. Antibiotics may be necessary, but their use must be judicious to prevent the development of antibiotic resistance, which is already a global concern (Langford et al., 2020). A rapidly expanding area of study is the dynamics of cytokine-induced immunosuppression and bacterial susceptibility (Hotchkiss et al., 2013). It has the potential to advance care plans for patients who are affected by cytokine storms, not just in the context of viral pandemics but also in sepsis and other illnesses.
9. Potential Interactions between COVID-19 and Antibacterial Resistance
Antibacterial resistance, a major worldwide health issue, interacts indirectly but significantly with the COVID-19 pandemic, which is largely brought on by the viral infection SARS-CoV-2. The misuse of antibiotics in the early phases of the epidemic has been one of the main problems. Despite their ineffectiveness against viral infections, antibiotics were often administered to COVID-19 patients with severe respiratory symptoms. The overuse of antibiotics makes it more difficult to treat bacterial infections in the future and may hasten the development of antibiotic resistance (Knight et al., 2021). Additionally, COVID-19 might impair breathing and weaken the immune system, increasing a person's susceptibility to secondary bacterial infections.
Antibiotics may be required in such circumstances, but cautious selection is essential to prevent the development of resistance. Additionally, the epidemic has caused healthcare systems to divert resources (Ukuhor, 2021). The excessive emphasis paid to COVID-19 has sometimes led to a lack of focus on bacterial infections, which might be a factor in the development of resistance. Nosocomial infections, especially in critical care units, have increased throughout the pandemic and are often linked to the use of antibiotics and the threat of the spread of bacteria that are resistant to such antibiotics. Given the interconnection of human, animal, and environmental health, these interactions highlight the need for a comprehensive "One Health" approach to health challenges. To stop the spread of antibiotic resistance during pandemics, it is crucial to balance the response to both viral and bacterial threats (Perez de la Lastra et al., 2022).
10. Immunosuppression and Secondary Bacterial Infections in COVID-19 Patients
SARS-CoV-2 may reduce the body's immune response in this interaction, which is noted as immunosuppression. Due to this immunosuppression, COVID-19 patients may become more susceptible to secondary bacterial infections, which might affect how their clinical course and course of therapy develop (De Bruyn et al., 2022). Pro-inflammatory cytokines may be overproduced when the immune system reacts to a virus aggressively, which can result in an overzealous immunological response. Ironically, this hyper-inflammatory condition might make the immune system less effective at warding off bacterial infections. In severe instances of COVID-19, this immunosuppression may develop, and the use of immune-modulatory therapies may make it worse (Moreno-Torres et al., 2022).
Patients with COVID-19 have weakened their immune systems. Pneumonia is a frequent consequence that may affect different organs and is caused by these illnesses. Respiratory discomfort may be made worse by bacterial pneumonia, which is often brought on by organisms like Streptococcus pneumoniae or Staphylococcus aureus. This increases the risk of serious consequences. COVID-19 patients have also been documented to experience bacterial urinary tract infections (UTIs) and bloodstream infections (Manohar et al., 2020). Bacterial UTIs can arise due to various bacteria entering the urinary tract, causing symptoms such as painful urination, frequent urination, and fever. These infections can complicate the clinical course of COVID-19, requiring specific antibiotic treatment to manage both the viral and bacterial components of the illness. Bloodstream infections (bacteremia) and UTIs have both been documented in COVID-19 patients. Bacteremia develops when germs enter the circulation and might result in sepsis, a potentially fatal illness (Timsit et al., 2020).
According to the presence or absence of subsequent bacterial infections in the COVID-19 and influenza groups, the percentage of death has been calculated (Shafran et al., 2021) and a comparable analysis was done. Bacterial infections significantly decreased the survival rates of both groups. In comparison to 33 % of patients with one infection and 61 % with two or more, 13.2 % of patients with no illnesses died. While 27.7 % of patients with early infection and 51.9 % of patients with late infection died. Patients with the COVID-19 infection were shown to have a greater chance of dying when compared to those with influenza.
17.6 % of patients with a single illness and 25 % of patients with multiple illnesses per patient in the influenza group died. The COVID-19 group, on the other hand, had a mortality rate for patients with a single infection of 48.1 % and a death rate for patients with multiple infections of 75.9 %. 5.9 % of patients in the influenza group who had no illness expired. A total of 21.7 % of COVID-19 patients who had no bacterial infection died. The clinical care of COVID-19 patients is complicated by the prevalence of secondary bacterial infections. Doctors often have to strike a compromise between administering antibiotics to treat secondary bacterial illnesses and treating the initial viral infection. This may be difficult since using antibiotics improperly or excessively might lead to the development of antibiotic resistance. Therefore, it is essential to get an accurate diagnosis and utilize antibiotics wisely (Manohar et al., 2020).
11. Treatment of COVID-19
WHO recommends home isolation or quarantine for 14 days for confirmed or suspected cases of mild illness. If any person has serious symptoms, they should seek immediate medical attention. As per the guidelines of the WHO, everybody should wear a face mask and clean their hands with hand sanitizer every 15-20 minutes. A universal disinfectant like sodium hypochlorite (NaOCl), or hydrogen peroxide (H2O2) is used for the sanitization of the area and any surface. Monoclonal antibodies (mAbs) and human antibodies are used for the treatment of the infection from the virus. Beigel et al., (2018) have reported that there are several techniques that are used for the creation of human antibodies and mAbs such as plasma from recovered individuals, and animal preparations such as by genetically engineered cows.
Ritonavir, Lopinavir, and Protease Inhibitors, or a combination of these antiviral medications plus Ribavirin, are used to treat SARS, and they may also be effective against COVID-19 (NIAID, 2020). Remdesivir, a medication that was first created to treat the Ebola virus, is also effective for the treatment of MERS-CoV, SARS-CoV, and SARS-CoV-2 whether used alone or in combination with Interferon-beta or chloroquine (Agostini et al., 2018; Sheahan et al., 2020). According to many researchers, COVID-19 is being tested against Nafamostat, Ivermectin, Favipiravir, Darunavir, Baricitinib, Cobicistat, Arbidol, Oseltamivir, Nitazoxanide, and Penciclovir (Wang et al., 2020b; Richardson et al., 2020).
In accordance with Shrotri et al., (2021), the national regulatory authority has approved the use of 18 vaccines, including two protein subunit vaccines (RBD-Dimer and EpiVacCorona), two RNA vaccines (Moderna and Pfizer-BioNTech), five viral vector vaccines (Johnson & Johnson, Sputnik V, Sputnik Light, Convidecia, and Oxford-AstraZeneca), and nine conventional inactivated vaccine These shots gave the recipient lifetime protection against the coronavirus. Dead viruses are included in inactivated vaccines, which are created using Whole-Virion Inactivated Vero Cell technology. Although it never infects individuals, it may cause the immune system to produce antibodies to fight infection. It is the vaccination technique that has been around the longest. Utilizing Whole-Virion Inactivated Vero Cell technology, several vaccines are being created for illnesses including Influenza, Polio, Pertussis, Rabies, and Japanese Encephalitis.
12. Conclusions and Recommendations
Understanding the modes of transmission of COVID-19 is pivotal to implementing effective public health measures. Since its emergence, researchers and healthcare professionals have gathered substantial knowledge about how the virus is transmitted. As infected individuals can transmit the virus during the asymptomatic phase, this makes it challenging to identify and isolate cases solely based on symptoms. The outbreak of the coronavirus severely disrupted the global economy. Almost all nations struggled to control the transmission of the disease by quarantining suspected persons, testing and treating patients, restricting large assemblies, maintaining complete or partial lockdowns, etc.
Enhancing surveillance and diagnostic capabilities played a critical role. Rapid and accurate identification of bacterial infections allows for targeted antibiotic therapy and reduces unnecessary antibiotic consumption, especially in cases of epidemics and pandemics. It is essential to consider and wisely utilize the lessons learned from other outbreaks as we traverse the difficulties brought on by the COVID-19 pandemic. Previous outbreaks have highlighted the importance of judicious antibiotic usage. Overuse of antibiotics can lead to the development of antibiotic-resistant bacteria. This can further strain healthcare systems that are already grappling with the primary epidemic or pandemic.
This was evident in the era of COVID-19, where co-infections became a concern. Promoting antibiotic stewardship programs is an ongoing challenge. Healthcare providers must balance the urgent need to treat bacterial infections with the long-term goal of preserving antibiotic effectiveness. Such programs will encourage responsible antibiotic prescribing, reducing the risk of resistance development. The inadequacy of the antibiotic pipeline has been a recurring issue. To prepare for future epidemics and pandemics, investing in the research and development of new antibiotics is paramount to ensure the availability of effective treatments.
Distinguishing between viral and bacterial infections based solely on symptoms can be challenging. Misdiagnosis may lead to inappropriate antibiotic usage, further fuelling the global crisis of antibiotic resistance. Emerging research suggests that viral and bacterial co-infections may have synergistic effects, amplifying the virulence of both pathogens. Understanding these interactions is crucial for developing effective treatment strategies. Due to immunosuppression, certain COVID-19 patients may be more vulnerable to bacterial infections. The entire management of COVID-19 patients must take this susceptibility into account this susceptibility and carefully control the usage of antibiotics in order to minimize the problems and enhance results in this challenging clinical situation.
It is crucial to promote global health security (GHS) and universal health coverage (UHC) in all nations while assisting vulnerable communities via targeted primary healthcare investments through programs like the proposed Pandemic Funds and shared goods for health. Dedicated investments in strengthening health emergency architecture can reduce the gap between GHS and UHC. By reimagining pandemic preparedness and responses, we can reinforce the fundamental underpinnings of global health and safeguard our collective journey toward improved health and well-being.
Recommendations:
Healthcare professionals need to observe and analyze any indications of bacterial co-infections, particularly in patients with severe or protracted COVID-19 disease.
Healthcare providers must exercise caution when prescribing antibiotics during pandemics. Antibiotic stewardship programs can help minimize unnecessary antibiotic usage, reducing the risk of resistance.
Investing in research to better understand viral-bacterial interactions and developing rapid diagnostic tools is essential. This knowledge can inform treatment guidelines and improve pandemic preparedness.
Patients with COVID-19 need to be made aware to prioritise cleanliness standards, early detection of bacterial symptoms, etc.
Effective pandemic planning and response need inclusive governance with diverse stakeholders, as well as legal and regulatory procedures, perhaps including a legally binding pandemic treaty and enforceable International Health Regulations (IHR) changes. These strategies need to cover a variety of health system measures required to stop upcoming medical catastrophes.
Health security programs and response mechanisms, like the ACT Accelerator, should include a broad variety of stakeholders and all elements of the health system in order to effectively avoid future outbreaks.
Ventilators and personal protective equipment (PPE) may be quickly mobilised and distributed through concerted efforts combining cooperation with the public and private sectors. Allocating and storing resources strategically can help prepare healthcare systems for any future emergencies.
Strong training programmes, emphasising effective PPE use, infection control, and stress reduction techniques should be implemented for the special needs of healthcare professionals.
Widespread vaccination campaigns should be initiated and expanded to immunise sizable segments of the populace.
Author’s contributions
All authors have equal contributions.
Conflicts of interest or competing interest
The authors declare that they have no competing financial interests or personal relationship that could have appeared to influence the work reported in this chapter.
Availability of data and materials
Not applicable
Code availability
Not applicable
Funding
This study was not supported by any funding agency.
Acknowledgements
The authors are grateful to Amity University for providing the platform to do this study.
References
- Adebisi, Y.A.; Alaran, A.J.; Okereke, M.; Oke, G.I.; Amos, O.A.; Olaoye, O.C.; Lucero-Prisno, D.E., III. COVID-19 and antimicrobial resistance: a review. Infectious Diseases: Research and Treatment 2021, 14, 11786337211033870. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Denison, M.R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Akram, F.; Imtiaz, M.; ul Haq, I. Emergent crisis of antibiotic resistance: A silent pandemic threat to 21st century. Microbial Pathogenesis 2022, 105923. [Google Scholar] [CrossRef] [PubMed]
- Al Hajjar, S.; McIntosh, K. The first influenza pandemic of the 21st century. Annals of Saudi medicine 2010, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alcock, J.; Masters, A. Cytokine storms, evolution and COVID-19. Evolution, Medicine, and Public Health 2021, 9, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Avalos, M.; van Wezel, G.P.; Raaijmakers, J.M.; Garbeva, P. Health. scents: microbial volatiles as new frontier in antibiotic research? Current opinion in microbiology 2018, 45, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ayouni, I.; Maatoug, J.; Dhouib, W.; Zammit, N.; Fredj, S.B.; Ghammam, R.; Ghannem, H. Effective public health measures to mitigate the spread of COVID-19: a systematic review. BMC public health 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Voell, J.; Kumar, P.; Raviprakash, K.; Wu, H.; Jiao, J.A.; Davey Jr, R.T. A randomized placebo-controlled phase 1 safety and tolerability study of a novel human anti-MERS coronavirus polyclonal intravenous immunoglobulin produced from transchromosomic cattle. The Lancet. Infectious diseases 2018, 18, 410.
Bhardwaj, L.K., & Vikram, V. Air Pollution and Its Effect on Human Health. Preprints 2023, 2023071691. [CrossRef]
- Bhardwaj, L.K.; Singh, V.V.; Dwivedi, K.; Rai, S.A. (2023). Comprehensive Review on Sources, Types, Impact and Challenges of Air Pollution. [CrossRef]
- 10. Centers for Disease Control and Prevention (CDC). (2019). Antibiotic use in the United States, 2018 update: progress and opportunities. US Department of Health and Human Services.
- Centers for Disease Control and Prevention (CDC). (2021). Antibiotic use in the United States, 2018 update: Progress and opportunities. Available online:. Available online: https://www.cdc.gov/antibiotic-use/stewardship-report/outpatient.html.
- Chedid, M.; Waked, R.; Haddad, E.; Chetata, N.; Saliba, G.; Choucair, J. Antibiotics in treatment of COVID-19 complications: a review of frequency, indications, and efficacy. Journal of infection and public health 2021, 14, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.C.; Chi, H.; Tai, Y.L.; Peng, C.C.; Tseng, C.Y.; Chen, C.C.; Lin, C.Y. Impact of wearing masks, hand hygiene, and social distancing on influenza, enterovirus, and all-cause pneumonia during the coronavirus pandemic: retrospective national epidemiological surveillance study. Journal of medical Internet research 2020, 22, e21257. [Google Scholar] [CrossRef]
- De Bruyn, A.; Verellen, S.; Bruckers, L.; Geebelen, L.; Callebaut, I.; De Pauw, I.; Dubois, J. Secondary infection in COVID-19 critically ill patients: a retrospective single-center evaluation. BMC infectious diseases 2022, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Qian, H.; Xu, B.; Huang, Y.; Miao, T.; Yen, H.L.; Li, Y. (2020). Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital (preprint). [CrossRef]
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.S.; Roy, C.J. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerging infectious diseases 2020, 26, 2168. [Google Scholar] [CrossRef] [PubMed]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global challenges to public health care systems during the COVID-19 pandemic: a review of pandemic measures and problems. Journal of Personalized Medicine 2022, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Founou, R.C.; Blocker, A.J.; Noubom, M.; Tsayem, C.; Choukem, S.P.; Dongen, M.V.; Founou, L.L. The COVID-19 pandemic: A threat to antimicrobial resistance containment. Future Science OA 2021, 7, FSO736. [Google Scholar] [CrossRef] [PubMed]
- Ghinai, I.; McPherson, T.D.; Hunter, J.C.; Kirking, H.L.; Christiansen, D.; Joshi, K.; Uyeki, T.M. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. The Lancet 2020, 395, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobia. Resistance Threats in the emerging COVID-19 pandemic: Where do we stand? Journal of infection and public health 2021, 14, 555–560. [Google Scholar] [CrossRef]
- Goldsmith, C.S.; Tatti, K.M.; Ksiazek, T.G.; Rollin, P.E.; Comer, J.A.; Lee, W.W.; Zaki, S.R. Ultrastructura. characterization of SARS coronavirus. Emerging infectious diseases 2004, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.D.; Wang, Z.Y.; Zhang, S.F.; Li, X.; Li, L.; Li, C.; Chen, W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging infectious diseases 2020, 26, 1586. [Google Scholar] [CrossRef] [PubMed]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.S.; Tan, M.; Wu, S.; Legido-Quigley, H. Health systems resilience in managing the COVID-19 pandemic: lessons from 28 countries. Nature Medicine 2021, 27, 964–980. [Google Scholar] [CrossRef]
- Haward, R.; Ridhima, G.; Kalyan, M. The Impact of Personal Protective Equipment on Healthcare Workers on COVID-19 Duty in a Tertiary Care Hospital in South India. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. The Lancet infectious diseases 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nature reviews Molecular cell biology 2022, 23, 3–20. [Google Scholar] [CrossRef]
- King, J.; Kosinski-Collins, M.; Sunderberg, E. (2020). Coronavirus structure, vaccine and therapy development. Biophysical society.
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Chandler, C.I. Antimicrobial resistance and COVID-19: Intersections and implications. Elife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clinical microbiology and infection 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Feng, Z. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England journal of medicine 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Cai, J.; Deng, X.; Peng, C.; Chen, X.; Yu, H. (2021). Herd immunity induced by COVID-19 vaccination programs to suppress epidemics caused by SARS-CoV-2 wild type and variants in China. MedRxiv.
- Madabhavi, I.; Sarkar, M.; Kadakol, N. COVID-19: a review. Monaldi Archives for Chest Disease 2020, 90. [Google Scholar] [CrossRef]
- Majid, S.; Khan, M.S.; Rashid, S.; Niyaz, A.; Farooq, R.; Bhat, S.A.; Qureshi, W. COVID-19: Diagnostics, therapeutic advances, and vaccine development. Current Clinical Microbiology Reports 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.S.; Mundra, S. Increasing consumption of antibiotics during the COVID-19 pandemic: Implications for patient health and emerging anti-microbial resistance. Antibiotics 2022, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P.; Loh, B.; Athira, S.; Nachimuthu, R.; Hua, X.; Welburn, S.C.; Leptihn, S. (2020). Secondary bacterial infections during pulmonary viral disease: phage therapeutics as alternatives to antibiotics? Frontiers in Microbiology, 1434.
- Martín-Loeches, I.; Sanchez-Corral, A.; Diaz, E.; Granada, R.M.; Zaragoza, R.; Villavicencio, C.; H1N1 SEMICYUC Working Group. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A (H1N1) virus. Chest 2011, 139, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Miller, R.A.; Buehner, G.; Chang, Y.; Harper, J.M.; Sigler, R.; Smith-Wheelock, M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging cell 2005, 4, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Molento, M.B. Ivermectin against COVID-19: The unprecedented consequences in Latin America. One Health 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virology Journal 2022, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, V.; de Mendoza, C.; de la Fuente, S.; Sánchez, E.; Martínez-Urbistondo, M.; Herráiz, J.; Cuervas-Mons, V. Bacterial infections in patients hospitalized with COVID-19. Internal and Emergency Medicine 2022, 17, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic escherichia coli. Clinical microbiology reviews 1998, 11, 142–201. [Google Scholar] [CrossRef]
- National Institute of Allergy and Infectious Diseases (NIAID) (US). (2020). Identifier NCT04280705, Adaptive COVID-19 Treatment Trial (ACTT). Available online:. Available online: https://clinicaltrials.gov/ct2/show/NCT04280705.
- Newman, A.; Smith, D.; Ghai, R.R.; Wallace, R.M.; Torchetti, M.K.; Loiacono, C.; Behravesh, C.B. First reported cases of SARS-CoV-2 infection in companion animals—New York, March–April 2020. Morbidity and Mortality Weekly Report 2020, 69, 710. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, J. (2016). Tackling drug-resistant infections globally: final report and recommendations.
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Munnink, B.B.O.; Hakze-van Der Honing, R.W.; Stegeman, A. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance 2020, 25, 2001005. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. The Lancet infectious diseases 2020, 20, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Lastra, J.M.; Anand, U.; González-Acosta, S.; López, M.R.; Dey, A.; Bontempi, E.; Morales delaNuez, A. Antimicrobial resistance in the COVID-19 landscape: is there an opportunity for anti-infective antibodies and antimicrobial peptides? Frontiers in Immunology 2022, 13, 921483. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.A.; Morran, M.P.; Nestor-Kalinoski, A.L. (2020). The COVID-19 pandemic: a global health crisis. Physiological genomics.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: a global multifaceted phenomenon. Pathogens and global health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sarkar, A. Risk factors for fatal middle east respiratory syndrome coronavirus infections in Saudi Arabia: analysis of the WHO Line List, 2013–2018. American journal of public health 2019, 109, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Wilson, R.C.; Holmes, A. Understanding the role of bacterial and fungal infection in COVID-19. Clinical Microbiology and Infection 2021, 27, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.; Plotkin, S.A. Impact of vaccines; health, economic and social perspectives. Frontiers in microbiology 2020, 11, 1526. [Google Scholar] [CrossRef]
- Sahu, C.; Singh, S.; Pathak, A.; Singh, S.; Patel, S.S.; Ghoshal, U.; Garg, A. Bacterial coinfections in COVID: Prevalence, antibiotic sensitivity patterns and clinical outcomes from a tertiary institute of Northern India. Journal of family medicine and primary care 2022, 11, 4473. [Google Scholar] [PubMed]
- Seethalakshmi, P.S.; Charity, O.J.; Giakoumis, T.; Kiran, G.S.; Sriskandan, S.; Voulvoulis, N.; Selvin, J. Delineating the impact of COVID-19 on antimicrobial resistance: An Indian perspective. Science of The Total Environment 2022, 818, 151702. [Google Scholar] [CrossRef]
- Shafran, N.; Shafran, I.; Ben-Zvi, H.; Sofer, S.; Sheena, L.; Krause, I.; Sklan, E.H. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Scientific reports 2021, 11, 12703. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Baric, R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nature communications 2020, 11, 222. [Google Scholar] [CrossRef]
- Shrotri, M.; Swinnen, T.; Kampmann, B.; Parker, E.P. An interactive website tracking COVID-19 vaccine development. The Lancet Global Health 2021, 9, e590–e592. [Google Scholar] [CrossRef]
- Smith, R.A.; M’ikanatha, N.M.; Read, A.F. Antibiotic resistance: a primer and call to action. Health communication 2015, 30, 309–314. [Google Scholar] [CrossRef]
- Song, J.Y.; Nahm, M.H.; Moseley, M.A. Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. Journal of Korean medical science 2013, 28, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Ruppé, E.; Barbier, F.; Tabah, A.; Bassetti, M. Bloodstream infections in critically ill patients: an expert statement. Intensive care medicine 2020, 46, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan BR, M.; Mitra, S.; Emran, T.B.; Koirala, N. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. Journal of infection and public health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Ukuhor, H.O. The interrelationships between antimicrobial resistance, COVID-19, past, and future pandemics. Journal of Infection and Public Health 2021, 14, 53–60. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Munster, V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England journal of medicine 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: part 1: causes and threats. Pharmacy and therapeutics 2015, 40, 277. [Google Scholar]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Peng, Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. jama, 2020; 323, 1061–1069. [Google Scholar]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Huang, Y.; Lau, S.K.; Yuen, K.Y. Coronavirus genomics and bioinformatics analysis. viruses 2010, 2, 1804–1820. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). (2014). Infection Prevention and Control of Epidemic-and Pandemic-prone Acute Respiratory Infections in Health Care. Geneva. Available online: https://apps.who.int/iris/bitstream/handle/10665/112656/9789241507134_eng.pdf;jsessionid=41AA684FB64571CE8D8A453C4F2B2096?sequence=1.
- World Health Organization (WHO). (2020). Transmission of SARS-CoV-2: implications for infection prevention precautions: scientific brief, 09 July 2020 (No. WHO/2019-nCoV/Sci_Brief/Transmission_modes/2020.3).
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2018, 23, 130–137. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).