Submitted:
08 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. mRNA Vaccines and Tumor Immunity
2.1. Principle and Characteristics of mRNA Vaccines in Tumor Immunotherapy
2.1.1. The Basic Working Principle of mRNA Vaccines
2.1.2. Characteristics and Advantages of mRNA Vaccines
2.2. Current Status and Challenges of mRNA Vaccines in Tumor Therapy
2.2.1. Existing Clinical Application Cases of mRNA Vaccines
2.2.2. The Challenges of mRNA Vaccines
2.3. Tumor Immune Mechanism Induced by mRNA Vaccines
2.3.1. Immunogenicity and Immune Memory
2.3.2. Immune Cells and Tumor Antigens
2.4. Development and Future Prospects of mRNA Vaccines in Tumor Immunotherapy
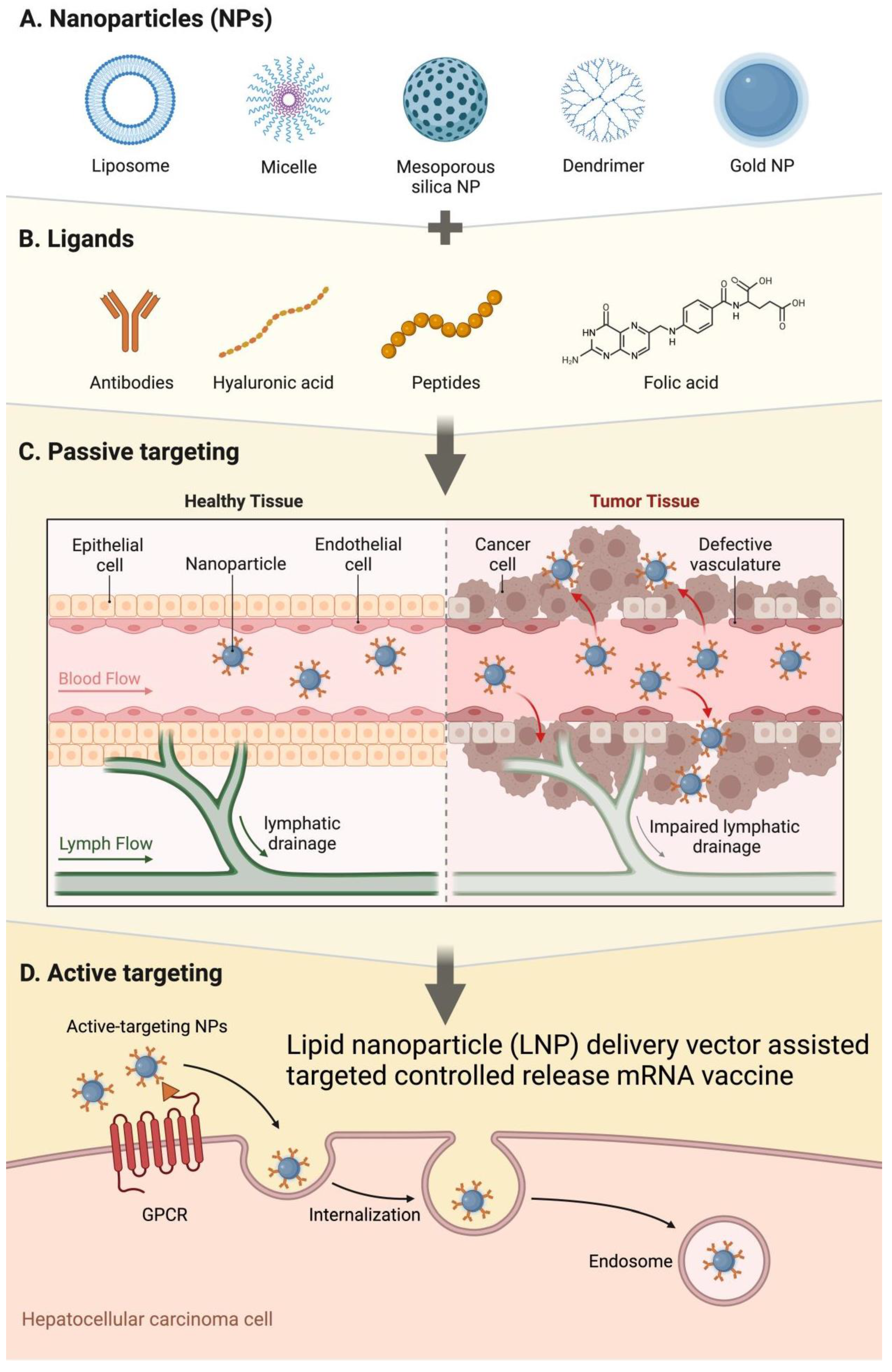
3. The Role of Lipid Nanoparticles (LNPs) in mRNA Vaccine Delivery
3.1. Structure and Characteristics of LNPs
| Nanoparticle Carrier Type | Nanomaterial Properties | Related Research | Targeted Tumor | Types of Nanomedicine |
| Liposomes | Lipid bilayer structure, high encapsulation ability | Doxil, Onivyde | Ovarian cancer, Pancreatic cancer | Chemotherapeutic drug delivery |
| Polymeric Nanoparticles | Tunable release properties | Abraxane, Genexol-PM | Breast cancer, Gastric cancer | Chemotherapeutic drug delivery |
| Gold Nanoparticles | Biocompatibility, surface-enhanced Raman scattering | - | Lung cancer, Breast cancer | Tumor photothermal therapy |
| Iron Oxide Magnetic Nanoparticles | Magnetic properties, imaging functionality | Ferumoxytol | Brain tumors, Breast cancer | Magnetic resonance imaging |
| Metal-Organic Frameworks (MOFs) | High drug-loading capacity, controlled release | - | Lung cancer, Colorectal cancer | Drug delivery, Imaging |
| Graphene Oxide | Large surface area, drug-loading capability | - | Lung cancer, Breast cancer | Drug delivery |
| Carbon Nanotubes | High drug-loading capacity, biocompatibility | - | Lung cancer, Breast cancer | Drug delivery, Photothermal therapy |
| Protein Nanoparticles | Biocompatibility, specific targeting | Abraxane | Pancreatic cancer, Ovarian cancer | Protein drug delivery |
| Lipid Nanoparticles | Biocompatibility, high drug-loading capacity | Pfizer-BioNTech mRNA vaccine | Breast cancer, Colorectal cancer | mRNA vaccines |
| Iron Oxide Nanoparticles | Magnetic properties, imaging functionality | - | Liver cancer, Breast cancer | Magnetic resonance imaging |
| PLGA Nanoparticles | Biodegradability, controlled release | - | Lung cancer, Breast cancer | Drug delivery |
| Protein Polymer Nanocomplexes | Targeted, biocompatible | - | Gastric cancer, Colorectal cancer | Protein drug delivery |
| Phospholipid Nanoparticles | Biocompatibility, stability | - | Gastric cancer, Liver cancer | Drug delivery |
| Silica Nanoparticles | Tunable morphology, drug-loading capability | - | Liver cancer, Breast cancer | Drug delivery |
| Polymer Micelles | High drug-loading capacity, solubility | - | Lung cancer, Pancreatic cancer | Chemotherapeutic drug delivery |
| Nanoemulsions | Drug-carrying capacity, stability | - | Pancreatic cancer, Colorectal cancer | Drug delivery, Treatment |
3.2. Delivery Mechanism of LNPs as mRNA Vaccine Carriers
4. Application of LNP-Assisted mRNA Vaccines in Tumor Immunotherapy
4.1. Progress of Experimental Research
4.3. Other Potential Application Areas
5. Future Prospects and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bevers S, Kooijmans SAA, Van de Velde E, et al. mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Mol Ther. 2022 Sep 7;30(9):3078-3094. [CrossRef]
- Ramos da Silva J, Bitencourt Rodrigues K, Formoso Pelegrin G, et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci Transl Med. 2023 Mar 8;15(686):eabn3464. [CrossRef]
- Sittplangkoon C, Alameh MG, Weissman D, et al. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front Immunol. 2022 Oct 14;13:983000. [CrossRef]
- Zhang R, Shao S, Piao Y, et al. Esterase-Labile Quaternium Lipidoid Enabling Improved mRNA-LNP Stability and Spleen-Selective mRNA Transfection. Adv Mater. 2023 Nov;35(46):e2303614. [CrossRef]
- Li F, Zhang XQ, Ho W, et al. mRNA lipid nanoparticle-mediated pyroptosis sensitizes immunologically cold tumors to checkpoint immunotherapy. Nat Commun. 2023 Jul 15;14(1):4223. [CrossRef]
- Liu W, Alameh MG, Yang JF, et al. Lipid Nanoparticles Delivering Constitutively Active STING mRNA to Stimulate Antitumor Immunity. Int J Mol Sci. 2022 Nov 22;23(23):14504. [CrossRef]
- Kitte R, Rabel M, Geczy R, et al. Lipid nanoparticles outperform electroporation in mRNA-based CAR T cell engineering. Mol Ther Methods Clin Dev. 2023 Oct 18;31:101139. [CrossRef]
- Qiu K, Duan X, Mao M, et al. mRNA-LNP vaccination-based immunotherapy augments CD8+ T cell responses against HPV-positive oropharyngeal cancer. NPJ Vaccines. 2023 Sep 29;8(1):144. [CrossRef]
- Golubovskaya V, Sienkiewicz J, Sun J, et al. CAR-NK Cells Generated with mRNA-LNPs Kill Tumor Target Cells In Vitro and In Vivo. Int J Mol Sci. 2023 Aug 29;24(17):13364. [CrossRef]
- Kiaie SH, Majidi Zolbanin N, Ahmadi A, et al. Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects. J Nanobiotechnology. 2022 Jun 14;20(1):276. [CrossRef]
- Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. 2021 May 15;601:120586. [CrossRef]
- Ndeupen S, Qin Z, Jacobsen S, et al. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021 Dec 17;24(12):103479. [CrossRef]
- Alameh MG, Tombácz I, Bettini E, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021 Dec 14;54(12):2877-2892.e7. [CrossRef]
- Zhang NN, Li XF, Deng YQ, et al. A Thermostable mRNA Vaccine against COVID-19. Cell. 2020 Sep 3;182(5):1271-1283.e16. [CrossRef]
- Kon E, Elia U, Peer D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr Opin Biotechnol. 2022 Feb;73:329-336. [CrossRef]
- Hassett KJ, Higgins J, Woods A, et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J Control Release. 2021 Jul 10;335:237-246. [CrossRef]
- Zong Y, Lin Y, Wei T, et al. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv Mater. 2023 May 17:e2303261. [CrossRef]
- Verbeke R, Hogan MJ, Loré K, et al. Innate immune mechanisms of mRNA vaccines. Immunity. 2022 Nov 8;55(11):1993-2005. [CrossRef]
- Muramatsu H, Lam K, Bajusz C, et al. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol Ther. 2022 May 4;30(5):1941-1951. [CrossRef]
- Wollner CJ, Richner M, Hassert MA, et al. A Dengue Virus Serotype 1 mRNA-LNP Vaccine Elicits Protective Immune Responses. J Virol. 2021 May 24;95(12):e02482-20. [CrossRef]
- Oude Blenke E, Örnskov E, Schöneich C, et al. The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case. J Pharm Sci. 2023 Feb;112(2):386-403. [CrossRef]
- Ripoll M, Bernard MC, Vaure C, et al. An imidazole modified lipid confers enhanced mRNA-LNP stability and strong immunization properties in mice and non-human primates. Biomaterials. 2022 Jul;286:121570. [CrossRef]
- Hayashi CTH, Cao Y, Clark LC, et al. mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum. NPJ Vaccines. 2022 Dec 1;7(1):155. [CrossRef]
- Kon E, Levy Y, Elia U, et al. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium. Sci Adv. 2023 Mar 10;9(10):eadg1036. [CrossRef]
- Qin Z, Bouteau A, Herbst C, et al. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. PLoS Pathog. 2022 Sep 2;18(9):e1010830. [CrossRef]
- McMahon M, O'Dell G, Tan J, et al. Assessment of a quadrivalent nucleoside-modified mRNA vaccine that protects against group 2 influenza viruses. Proc Natl Acad Sci U S A. 2022 Nov 8;119(45):e2206333119. [CrossRef]
- Monslow MA, Elbashir S, Sullivan NL, et al. Immunogenicity generated by mRNA vaccine encoding VZV gE antigen is comparable to adjuvanted subunit vaccine and better than live attenuated vaccine in nonhuman primates. Vaccine. 2020 Aug 10;38(36):5793-5802. [CrossRef]
- Sáez-Llorens X, Lanata C, Aranguren E, et al. Safety and immunogenicity of mRNA-LNP COVID-19 vaccine CVnCoV in Latin American adults: A phase 2 randomized study. Vaccine X. 2022 Aug;11:100189. [CrossRef]
- Hoffmann MAG, Yang Z, Huey-Tubman KE, et al. ESCRT recruitment to SARS-CoV-2 spike induces virus-like particles that improve mRNA vaccines. Cell. 2023 May 25;186(11):2380-2391.e9. [CrossRef]
- Pardi N, Hogan MJ, Naradikian MS, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018 Jun 4;215(6):1571-1588. [CrossRef]
- Laczkó D, Hogan MJ, Toulmin SA, et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity. 2020 Oct 13;53(4):724-732.e7. [CrossRef]
- Valentin A, Bergamaschi C, Rosati M, et al. Comparative immunogenicity of an mRNA/LNP and a DNA vaccine targeting HIV gag conserved elements in macaques. Front Immunol. 2022 Jul 22;13:945706. [CrossRef]
- Pardi N, Carreño JM, O'Dell G, et al. Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses. Nat Commun. 2022 Aug 9;13(1):4677. [CrossRef]
- Aldrich C, Leroux-Roels I, Huang KB, et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine. 2021 Feb 22;39(8):1310-1318. [CrossRef]
- Pilkington EH, Suys EJA, Trevaskis NL, et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021 Sep 1;131:16-40. [CrossRef]
- Naderi Sohi A, Kiani J, Arefian E, et al. Development of an mRNA-LNP Vaccine against SARS-CoV-2: Evaluation of Immune Response in Mouse and Rhesus Macaque. Vaccines (Basel). 2021 Sep 10;9(9):1007. [CrossRef]
- Pardi N, Hogan MJ, Pelc RS, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017 Mar 9;543(7644):248-251. [CrossRef]
- Liang Q, Wang Y, Zhang S, et al. RBD trimer mRNA vaccine elicits broad and protective immune responses against SARS-CoV-2 variants. iScience. 2022 Apr 15;25(4):104043. [CrossRef]
- Bavli Y, Chen BM, Gross G, et al. Anti-PEG antibodies before and after a first dose of Comirnaty (mRNA-LNP-based SARS-CoV-2 vaccine). J Control Release. 2023 Feb;354:316-322. [CrossRef]
- Liu T, Tian Y, Zheng A, et al. Design Strategies for and Stability of mRNA-Lipid Nanoparticle COVID-19 Vaccines. Polymers (Basel). 2022 Oct 6;14(19):4195. [CrossRef]
- Qin Z, Bouteau A, Herbst C, et al. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. bioRxiv [Preprint]. 2022 Aug 20:2022.03.16.484616.
- He L, Sun W, Yang L, et al. A multiple-target mRNA-LNP vaccine induces protective immunity against experimental multi-serotype DENV in mice. Virol Sin. 2022 Oct;37(5):746-757. [CrossRef]
- Lederer K, Castaño D, Gómez Atria D, et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity. 2020 Dec 15;53(6):1281-1295.e5. [CrossRef]
- Ndeupen S, Qin Z, Jacobsen S, et al. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. bioRxiv [Preprint]. 2021 Jul 23:2021.03.04.430128.
- Chen T, Zhu S, Wei N, et al. Protective Immune Responses Induced by an mRNA-LNP Vaccine Encoding prM-E Proteins against Japanese Encephalitis Virus Infection. Viruses. 2022 May 24;14(6):1121. [CrossRef]
- Guéguen C, Ben Chimol T, Briand M, et al. Evaluating how cationic lipid affects mRNA-LNP physical properties and biodistribution. Eur J Pharm Biopharm. 2023 Aug 12:S0939-6411(23)00205-9. [CrossRef]
- Hayashi CTH, Cao Y, Clark LC, et al. Author Correction: mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum. NPJ Vaccines. 2023 Aug 11;8(1):115. [CrossRef]
- Jansen EM, Frijlink HW, Hinrichs WL, et al. Are inhaled mRNA vaccines safe and effective? A review of preclinical studies. Expert Opin Drug Deliv. 2022 Nov;19(11):1471-1485. [CrossRef]
- Egan KP, Awasthi S, Tebaldi G, et al. A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1. Viruses. 2023 Jun 30;15(7):1483. [CrossRef]
- Granados-Riveron JT, Aquino-Jarquin G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed Pharmacother. 2021 Oct;142:111953. [CrossRef]
- Ndeupen S, Bouteau A, Herbst C, et al. Langerhans cells and cDC1s play redundant roles in mRNA-LNP induced protective anti-influenza and anti-SARS-CoV-2 immune responses. PLoS Pathog. 2022 Jan 24;18(1):e1010255. [CrossRef]
- Zhang Y, Li S, Chu H, et al. A novel mRNA vaccine, TGGT1_278620 mRNA-LNP, prolongs the survival time in BALB/c mice with acute toxoplasmosis. Microbiol Spectr. 2023 Dec 1:e0286623. [CrossRef]
- Zhang Y, Li D, Shen Y, et al. Immunization with a novel mRNA vaccine, TGGT1_216200 mRNA-LNP, prolongs survival time in BALB/c mice against acute toxoplasmosis. Front Immunol. 2023 Apr 14;14:1161507. [CrossRef]
- Lamoot A, Lammens J, De Lombaerde E, et al. Successful batch and continuous lyophilization of mRNA LNP formulations depend on cryoprotectants and ionizable lipids. Biomater Sci. 2023 Jun 13;11(12):4327-4334. [CrossRef]
- Ndeupen S, Bouteau A, Herbst C, et al. Langerhans cells and cDC1s play redundant roles in mRNA-LNP induced protective anti-influenza and anti-SARS-CoV-2 responses. bioRxiv [Preprint]. 2021 Aug 2:2021.08.01.454662.
- Zhang L, More KR, Ojha A, et al. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. NPJ Vaccines. 2023 Oct 11;8(1):156. [CrossRef]
- Ge N, Sun J, Liu Z, et al. An mRNA vaccine encoding Chikungunya virus E2-E1 protein elicits robust neutralizing antibody responses and CTL immune responses. Virol Sin. 2022 Apr;37(2):266-276. [CrossRef]
- Carter B, Huang P, Liu G, et al. A pan-variant mRNA-LNP T cell vaccine protects HLA transgenic mice from mortality after infection with SARS-CoV-2 Beta. Front Immunol. 2023 Mar 9;14:1135815. [CrossRef]
- Sáez-Llorens X, Lanata C, Aranguren E, et al. Corrigendum to "Safety and immunogenicity of mRNA-LNP COVID-19 vaccine CVnCoV in Latin American adults: A phase 2 randomized study" [Vaccine: X 11 (2022) 100189]. Vaccine X. 2023 Aug;14:100307. [CrossRef]
- Baldeon Vaca G, Meyer M, Cadete A, et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. Sci Adv. 2023 Sep 22;9(38):eadh1655. [CrossRef]
- Patel N, Davis Z, Hofmann C, et al. Development and Characterization of an In Vitro Cell-Based Assay to Predict Potency of mRNA-LNP-Based Vaccines. Vaccines (Basel). 2023 Jul 10;11(7):1224. [CrossRef]
- Rizvi F, Lee YR, Diaz-Aragon R, et al. VEGFA mRNA-LNP promotes biliary epithelial cell-to-hepatocyte conversion in acute and chronic liver diseases and reverses steatosis and fibrosis. bioRxiv [Preprint]. 2023 Apr 18:2023.04.17.537186.
- Moyles IR, Korosec CS, Heffernan JM. Determination of significant immunological timescales from mRNA-LNP-based vaccines in humans. J Math Biol. 2023 Apr 30;86(5):86. [CrossRef]
- Li D, Zhang Y, Li S, et al. A novel Toxoplasma gondii TGGT1_316290 mRNA-LNP vaccine elicits protective immune response against toxoplasmosis in mice. Front Microbiol. 2023 Mar 21;14:1145114. [CrossRef]
- Zhang M, Sun J, Li M, et al. Modified mRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol Ther Methods Clin Dev. 2020 Jul 21;18:702-712. [CrossRef]
- Zhang X, Jozic A, Song P, et al. mRNA vaccine against fibroblast activation protein ameliorates murine models of inflammatory arthritis. Rheumatol Immunol Res. 2023 Jul 22;4(2):90-97. [CrossRef]
- Hsu FF, Liang KH, Kumari M, et al. An efficient approach for SARS-CoV-2 monoclonal antibody production via modified mRNA-LNP immunization. Int J Pharm. 2022 Nov 5;627:122256. [CrossRef]
- Hermosilla J, Alonso-García A, Salmerón-García A, et al. Analysing the In-Use Stability of mRNA-LNP COVID-19 Vaccines Comirnaty™ (Pfizer) and Spikevax™ (Moderna): A Comparative Study of the Particulate. Vaccines (Basel). 2023 Oct 25;11(11):1635. [CrossRef]
- Egan KP, Hook LM, Naughton A, et al. An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins gC, gD, and gE protects mice against HSV-1 genital lesions and latent infection. PLoS Pathog. 2020 Jul 27;16(7):e1008795. [CrossRef]
- Nag K, Chandra Baray J, Rahman Khan M, et al. An mRNA-based vaccine candidate against SARS-CoV-2 elicits stable immuno-response with single dose. Vaccine. 2021 Jun 23;39(28):3745-3755. [CrossRef]
- Kim D, Lai CJ, Cha I, et al. SFTSV Gn-Head mRNA vaccine confers efficient protection against lethal viral challenge. J Med Virol. 2023 Nov;95(11):e29203. [CrossRef]
- Xia H, He YR, Zhan XY, et al. Mpox virus mRNA-lipid nanoparticle vaccine candidates evoke antibody responses and drive protection against the Vaccinia virus challenge in mice. Antiviral Res. 2023 Aug;216:105668. [CrossRef]
- Hoffmann MAG, Yang Z, Huey-Tubman KE, et al. ESCRT recruitment to mRNA-encoded SARS-CoV-2 spike induces virus-like particles and enhanced antibody responses. bioRxiv [Preprint]. 2022 Dec 27:2022.12.26.521940.
- Ndeupen S, Qin Z, Igyártó BZ. Single-cell suspension preparation from murine organs following in vivo mRNA-LNP exposure. STAR Protoc. 2022 May 18;3(2):101350. [CrossRef]
- Zamani P, Mashreghi M, Rezazade Bazaz M, et al. Characterization of stability, safety and immunogenicity of the mRNA lipid nanoparticle vaccine Iribovax against COVID-19 in nonhuman primates. J Control Release. 2023 Aug;360:316-334. [CrossRef]
- Nelson CS, Jenks JA, Pardi N, et al. Human Cytomegalovirus Glycoprotein B Nucleoside-Modified mRNA Vaccine Elicits Antibody Responses with Greater Durability and Breadth than MF59-Adjuvanted gB Protein Immunization. J Virol. 2020 Apr 16;94(9):e00186-20. [CrossRef]
- Lelis F, Byk LA, Pustylnikov S, et al. Safety, immunogenicity and efficacy of an mRNA-based COVID-19 vaccine, GLB-COV2-043, in preclinical animal models. Sci Rep. 2023 Dec 1;13(1):21172. [CrossRef]
- Narayanan E, Falcone S, Elbashir SM, et al. Rational Design and In Vivo Characterization of mRNA-Encoded Broadly Neutralizing Antibody Combinations against HIV-1. Antibodies (Basel). 2022 Oct 24;11(4):67. [CrossRef]
- Mao S, Li S, Zhang Y, et al. A highly efficient needle-free-injection delivery system for mRNA-LNP vaccination against SARS-CoV-2. Nano Today. 2023 Feb;48:101730. [CrossRef]
- Chivukula S, Plitnik T, Tibbitts T, et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines. 2021 Dec 16;6(1):153. [CrossRef]
- Rizvi F, Lee YR, Diaz-Aragon R, et al. VEGFA mRNA-LNP promotes biliary epithelial cell-to-hepatocyte conversion in acute and chronic liver diseases and reverses steatosis and fibrosis. Cell Stem Cell. 2023 Nov 20:S1934-5909(23)00392-2. [CrossRef]
- Chuang YM, Alameh MG, Abouneameh S, et al. A mosquito AgTRIO mRNA vaccine contributes to immunity against malaria. NPJ Vaccines. 2023 Jun 7;8(1):88. [CrossRef]
- Pardi N, LaBranche CC, Ferrari G, et al. Characterization of HIV-1 Nucleoside-Modified mRNA Vaccines in Rabbits and Rhesus Macaques. Mol Ther Nucleic Acids. 2019 Apr 15;15:36-47. [CrossRef]
- Gouma S, Furey C, Santos JJS, et al. Nucleoside-Modified mRNA-Based Influenza Vaccines Circumvent Problems Associated with H3N2 Vaccine Strain Egg Adaptation. J Virol. 2023 Jan 31;97(1):e0172322. [CrossRef]
- John S, Yuzhakov O, Woods A, et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine. 2018 Mar 14;36(12):1689-1699. [CrossRef]
- Patra T, Meyer K, Haga Y, et al. Hepatitis C virus E1 and modified E2 delivered from an mRNA vaccine induces protective immunity. NPJ Vaccines. 2023 Mar 18;8(1):42. [CrossRef]
- Zhao H, Wang TC, Li XF, et al. Long-term stability and protection efficacy of the RBD-targeting COVID-19 mRNA vaccine in nonhuman primates. Signal Transduct Target Ther. 2021 Dec 24;6(1):438. [CrossRef]
- Medjmedj A, Ngalle-Loth A, Clemençon R, et al. In Cellulo and In Vivo Comparison of Cholesterol, Beta-Sitosterol and Dioleylphosphatidylethanolamine for Lipid Nanoparticle Formulation of mRNA. Nanomaterials (Basel). 2022 Jul 17;12(14):2446. [CrossRef]
- El-Mayta R, Padilla MS, Billingsley MM, Testing the In Vitr et al.o and In Vivo Efficiency of mRNA-Lipid Nanoparticles Formulated by Microfluidic Mixing. J Vis Exp. 2023 Jan 20;(191). [CrossRef]
- Xu S, Zhang B, Yao J, et al. A new H9 influenza virus mRNA vaccine elicits robust protective immunity against infection. Vaccine. 2023 May 2;41(18):2905-2913. [CrossRef]
- Hook LM, Awasthi S, Cairns TM, et al. Antibodies to Crucial Epitopes on HSV-2 Glycoprotein D as a Guide to Dosing an mRNA Genital Herpes Vaccine. Viruses. 2022 Mar 5;14(3):540. [CrossRef]
- Ci L, Hard M, Zhang H, et al. Biodistribution of Lipid 5, mRNA, and Its Translated Protein Following Intravenous Administration of mRNA-Encapsulated Lipid Nanoparticles in Rats. Drug Metab Dispos. 2023 Jul;51(7):813-823. [CrossRef]
- Maharjan R, Hada S, Lee JE, et al. Comparative study of lipid nanoparticle-based mRNA vaccine bioprocess with machine learning and combinatorial artificial neural network-design of experiment approach. Int J Pharm. 2023 Jun 10;640:123012. [CrossRef]
- Ma Q, Li R, Guo J, et al. Immunization with a Prefusion SARS-CoV-2 Spike Protein Vaccine (RBMRNA-176) Protects against Viral Challenge in Mice and Nonhuman Primates. Vaccines (Basel). 2022 Oct 11;10(10):1698. [CrossRef]
- Ma N, Xia ZW, Zhang ZG, et al. Development of an mRNA vaccine against a panel of heterologous H1N1 seasonal influenza viruses using a consensus hemagglutinin sequence. Emerg Microbes Infect. 2023 Dec;12(1):2202278. [CrossRef]
- Cui L, Hunter MR, Sonzini S, et al. Mechanistic Studies of an Automated Lipid Nanoparticle Reveal Critical Pharmaceutical Properties Associated with Enhanced mRNA Functional Delivery In Vitro and In Vivo. Small. 2022 Mar;18(9):e2105832. [CrossRef]
- Wilhelmy C, Keil IS, Uebbing L, et al. Polysarcosine-Functionalized mRNA Lipid Nanoparticles Tailored for Immunotherapy. Pharmaceutics. 2023 Aug 1;15(8):2068. [CrossRef]
- Dézsi L, Mészáros T, Kozma G, et al. A naturally hypersensitive porcine model may help understand the mechanism of COVID-19 mRNA vaccine-induced rare (pseudo) allergic reactions: complement activation as a possible contributing factor. Geroscience. 2022 Apr;44(2):597-618. [CrossRef]
- Bai S, Yang T, Zhu C, et al. A single vaccination of nucleoside-modified Rabies mRNA vaccine induces prolonged highly protective immune responses in mice. Front Immunol. 2023 Jan 17;13:1099991. [CrossRef]
- Appelberg S, John L, Pardi N, et al. Nucleoside-Modified mRNA Vaccines Protect IFNAR-/- Mice against Crimean-Congo Hemorrhagic Fever Virus Infection. J Virol. 2022 Feb 9;96(3):e0156821. [CrossRef]
- LaTourette PC 2nd, Awasthi S, Desmond A, et al. Protection against herpes simplex virus type 2 infection in a neonatal murine model using a trivalent nucleoside-modified mRNA in lipid nanoparticle vaccine. Vaccine. 2020 Nov 3;38(47):7409-7413. [CrossRef]
- Ma Y, Fenton OS. An Efficacy and Mechanism Driven Study on the Impact of Hypoxia on Lipid Nanoparticle Mediated mRNA Delivery. J Am Chem Soc. 2023 May 24;145(20):11375-11386. [CrossRef]
- Reinhart AG, Osterwald A, Ringler P, et al. Investigations into mRNA Lipid Nanoparticles Shelf-Life Stability under Nonfrozen Conditions. Mol Pharm. 2023 Dec 4;20(12):6492-6503. [CrossRef]
- Szebeni J, Storm G, Ljubimova JY, et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat Nanotechnol. 2022 Apr;17(4):337-346. [CrossRef]
- Hajiaghapour Asr M, Dayani F, Saedi Segherloo F, et al. Lipid Nanoparticles as Promising Carriers for mRNA Vaccines for Viral Lung Infections. Pharmaceutics. 2023 Apr 3;15(4):1127. [CrossRef]
- Rohde CM, Lindemann C, Giovanelli M, et al. Toxicological Assessments of a Pandemic COVID-19 Vaccine-Demonstrating the Suitability of a Platform Approach for mRNA Vaccines. Vaccines (Basel). 2023 Feb 11;11(2):417. [CrossRef]
- Zhu W, Wei L, Dong C, et al. cGAMP-adjuvanted multivalent influenza mRNA vaccines induce broadly protective immunity through cutaneous vaccination in mice. Mol Ther Nucleic Acids. 2022 Nov 9;30:421-437. [CrossRef]
- Austin LA, Smith JS, Nahas DD, et al. Split-Dose Administration Enhances Immune Responses Elicited by a mRNA/Lipid Nanoparticle Vaccine Expressing Respiratory Syncytial Virus F Protein. Mol Pharm. 2023 Jan 2;20(1):279-289. [CrossRef]
- Thaller A, Schmauder L, Frieß W, et al. SV-AUC as a stability-indicating method for the characterization of mRNA-LNPs. Eur J Pharm Biopharm. 2023 Jan;182:152-156. [CrossRef]
- Mu Z, Wiehe K, Saunders KO, et al. Ability of nucleoside-modified mRNA to encode HIV-1 envelope trimer nanoparticles. bioRxiv [Preprint]. 2021 Aug 9:2021.08.09.455714.
- Szebeni J, Kiss B, Bozó T, et al. Insights into the Structure of Comirnaty Covid-19 Vaccine: A Theory on Soft, Partially Bilayer-Covered Nanoparticles with Hydrogen Bond-Stabilized mRNA-Lipid Complexes. ACS Nano. 2023 Jul 25;17(14):13147-13157. [CrossRef]
- Messerian KO, Zverev A, Kramarczyk JF, et al. Pressure-dependent fouling behavior during sterile filtration of mRNA-containing lipid nanoparticles. Biotechnol Bioeng. 2022 Nov;119(11):3221-3229. [CrossRef]
- Shepherd SJ, Han X, Mukalel AJ, et al. Throughput-scalable manufacturing of SARS-CoV-2 mRNA lipid nanoparticle vaccines. Proc Natl Acad Sci U S A. 2023 Aug 15;120(33):e2303567120. [CrossRef]
- Lazaros G, Klein AL, Hatziantoniou S, et al. The Novel Platform of mRNA COVID-19 Vaccines and Myocarditis: Clues into the Potential Underlying Mechanism. Vaccine. 2021 Aug 16;39(35):4925-4927. [CrossRef]
- Han X, Alameh MG, Butowska K, et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat Nanotechnol. 2023 Sep;18(9):1105-1114. [CrossRef]
- Wang MM, Wappelhorst CN, Jensen EL, et al. Elucidation of lipid nanoparticle surface structure in mRNA vaccines. Sci Rep. 2023 Oct 5;13(1):16744. [CrossRef]
- Saunders KO, Pardi N, Parks R, et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. bioRxiv [Preprint]. 2020 Dec 31:2020.12.30.424745.
- Knudson CJ, Alves-Peixoto P, Muramatsu H, et al. Lipid-nanoparticle-encapsulated mRNA vaccines induce protective memory CD8 T cells against a lethal viral infection. Mol Ther. 2021 Sep 1;29(9):2769-2781. [CrossRef]
- Mu Z, Wiehe K, Saunders KO, et al. mRNA-encoded HIV-1 Env trimer ferritin nanoparticles induce monoclonal antibodies that neutralize heterologous HIV-1 isolates in mice. Cell Rep. 2022 Mar 15;38(11):110514. [CrossRef]
- Li Z, Zhang XQ, Ho W, et al. Enzyme-Catalyzed One-Step Synthesis of Ionizable Cationic Lipids for Lipid Nanoparticle-Based mRNA COVID-19 Vaccines. ACS Nano. 2022 Nov 22;16(11):18936-18950. [CrossRef]
- Saunders KO, Pardi N, Parks R, et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines. 2021 Apr 9;6(1):50. [CrossRef]
- Willis E, Pardi N, Parkhouse K, et al. Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci Transl Med. 2020 Jan 8;12(525):eaav5701. [CrossRef]
- Melzi E, Willis JR, Ma KM, et al. Membrane-bound mRNA immunogens lower the threshold to activate HIV Env V2 apex-directed broadly neutralizing B cell precursors in humanized mice. Immunity. 2022 Nov 8;55(11):2168-2186.e6. [CrossRef]
- Ma Y, Fenton OS. A Unified Strategy to Improve Lipid Nanoparticle Mediated mRNA Delivery Using Adenosine Triphosphate. J Am Chem Soc. 2023 Sep 13;145(36):19800-19811. [CrossRef]
- Everton E, Rizvi F, Smith AR, et al. Transient yet Robust Expression of Proteins in the Mouse Liver via Intravenous Injection of Lipid Nanoparticle-encapsulated Nucleoside-modified mRNA. Bio Protoc. 2021 Oct 5;11(19):e4184. [CrossRef]
- Lindgren G, Ols S, Liang F, et al. Induction of Robust B Cell Responses after Influenza mRNA Vaccination Is Accompanied by Circulating Hemagglutinin-Specific ICOS+ PD-1+ CXCR3+ T Follicular Helper Cells. Front Immunol. 2017 Nov 13;8:1539. [CrossRef]
- Shirane D, Tanaka H, Sakurai Y, et al. Development of an Alcohol Dilution-Lyophilization Method for the Preparation of mRNA-LNPs with Improved Storage Stability. Pharmaceutics. 2023 Jun 26;15(7):1819. [CrossRef]
- Kiaie SH, Majidi Zolbanin N, Ahmadi A, et al. Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects. J Nanobiotechnology. 2022 Jun 14;20(1):276.
- Ndeupen S, Qin Z, Jacobsen S, et al. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021 Dec 17;24(12):103479. [CrossRef]
- Zong Y, Lin Y, Wei T, et al. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv Mater. 2023 May 17:e2303261. [CrossRef]
- Ramos da Silva J, Bitencourt Rodrigues K, Formoso Pelegrin G, et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci Transl Med. 2023 Mar 8;15(686):eabn3464. [CrossRef]
- Ogawa K, Kato N, Yoshida M, et al. Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain. J Control Release. 2022 Aug;348:34-41. [CrossRef]
- Wollner CJ, Richner M, Hassert MA, et al. A Dengue Virus Serotype 1 mRNA-LNP Vaccine Elicits Protective Immune Responses. J Virol.2021 May 24;95(12):e02482-20. [CrossRef]
- Ripoll M, Bernard MC, Vaure C, et al. An imidazole modified lipid confers enhanced mRNA-LNP stability and strong immunization properties in mice and non-human primates. Biomaterials. 2022 Jul;286:121570. [CrossRef]
- Kon E, Levy Y, Elia U, et al. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium. Sci Adv. 2023 Mar 10;9(10):eadg1036. [CrossRef]
- Granados-Riveron JT, Aquino-Jarquin G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed Pharmacother. 2021 Oct;142:111953. [CrossRef]
- Qin Z, Bouteau A, Herbst C, et al. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. bioRxiv [Preprint]. 2022 Aug 20:2022.03.16.484616.
- Qin Z, Bouteau A, Herbst C, et al. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. PLoS Pathog. 2022 Sep 2;18(9):e1010830. [CrossRef]
- Baldeon Vaca G, Meyer M, Cadete A, et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. Sci Adv. 2023 Sep 22;9(38):eadh1655. [CrossRef]
- Hayashi CTH, Cao Y, Clark LC, et al. mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum. NPJ Vaccines. 2022 Dec 1;7(1):155. [CrossRef]
- Qiu K, Duan X, Mao M, et al. mRNA-LNP vaccination-based immunotherapy augments CD8+ T cell responses against HPV-positive oropharyngeal cancer. NPJ Vaccines. 2023 Sep 29;8(1):144. [CrossRef]
- Valentin A, Bergamaschi C, Rosati M, et al. Comparative immunogenicity of an mRNA/LNP and a DNA vaccine targeting HIV gag conserved elements in macaques. Front Immunol. 2022 Jul 22;13:945706. [CrossRef]
- Aldrich C, Leroux-Roels I, Huang KB, et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine. 2021 Feb 22;39(8):1310-1318. [CrossRef]
- Daly O, Mahiny AJ, Majeski S, et al. ASL mRNA-LNP Therapeutic for the Treatment of Argininosuccinic Aciduria Enables Survival Benefit in a Mouse Model. Biomedicines. 2023 Jun 16;11(6):1735. [CrossRef]
- Zhang M, Sun J, Li M, et al. Modified mRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol Ther Methods Clin Dev. 2020 Jul 21;18:702-712. [CrossRef]
- Zhang R, Shao S, Piao Y, et al. Esterase-Labile Quaternium Lipidoid Enabling Improved mRNA-LNP Stability and Spleen-Selective mRNA Transfection. Adv Mater. 2023 Nov;35(46):e2303614. [CrossRef]
- Naderi Sohi A, Kiani J, Arefian E, et al. Development of an mRNA-LNP Vaccine against SARS-CoV-2: Evaluation of Immune Response in Mouse and Rhesus Macaque. Vaccines (Basel). 2021 Sep 10;9(9):1007. [CrossRef]
- Guéguen C, Ben Chimol T, Briand M, et al. Evaluating how cationic lipid affects mRNA-LNP physical properties and biodistribution. Eur J Pharm Biopharm. 2023 Aug 12:S0939-6411(23)00205-9. [CrossRef]
- He L, Sun W, Yang L, et al. A multiple-target mRNA-LNP vaccine induces protective immunity against experimental multi-serotype DENV in mice. Virol Sin. 2022 Oct;37(5):746-757. [CrossRef]
- Hsu FF, Liang KH, Kumari M, et al. An efficient approach for SARS-CoV-2 monoclonal antibody production via modified mRNA-LNP immunization. Int J Pharm. 2022 Nov 5;627:122256. [CrossRef]
- Zhang Y, Li D, Shen Y, et al. Immunization with a novel mRNA vaccine, TGGT1_216200 mRNA-LNP, prolongs survival time in BALB/c mice against acute toxoplasmosis. Front Immunol. 2023 Apr 14;14:1161507. [CrossRef]
- Zhang L, More KR, Ojha A, et al. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. NPJ Vaccines. 2023 Oct 11;8(1):156. [CrossRef]
- Chen T, Zhu S, Wei N, et al. Protective Immune Responses Induced by an mRNA-LNP Vaccine Encoding prM-E Proteins against Japanese Encephalitis Virus Infection. Viruses. 2022 May 24;14(6):1121. [CrossRef]
- Mao S, Li S, Zhang Y, et al. A highly efficient needle-free-injection delivery system for mRNA-LNP vaccination against SARS-CoV-2. Nano Today. 2023 Feb;48:101730. [CrossRef]
- Ndeupen S, Qin Z, Igyártó BZ. Single-cell suspension preparation from murine organs following in vivo mRNA-LNP exposure. STAR Protoc. 2022 May 18;3(2):101350. [CrossRef]
- Golubovskaya V, Sienkiewicz J, Sun J, et al. mRNA-Lipid Nanoparticle (LNP) Delivery of Humanized EpCAM-CD3 Bispecific Antibody Significantly Blocks Colorectal Cancer Tumor Growth. Cancers (Basel). 2023 May 22;15(10):2860. [CrossRef]
- Patel N, Davis Z, Hofmann C, et al. Development and Characterization of an In Vitro Cell-Based Assay to Predict Potency of mRNA-LNP-Based Vaccines. Vaccines (Basel). 2023 Jul 10;11(7):1224. [CrossRef]
- Rizvi F, Lee YR, Diaz-Aragon R, et al. VEGFA mRNA-LNP promotes biliary epithelial cell-to-hepatocyte conversion in acute and chronic liver diseases and reverses steatosis and fibrosis. Cell Stem Cell. 2023 Nov 20:S1934-5909(23)00392-2. [CrossRef]
- Moyles IR, Korosec CS, Heffernan JM. Determination of significant immunological timescales from mRNA-LNP-based vaccines in humans. J Math Biol. 2023 Apr 30;86(5):86. [CrossRef]
- Lamoot A, Lammens J, De Lombaerde E, et al. Successful batch and continuous lyophilization of mRNA LNP formulations depend on cryoprotectants and ionizable lipids. Biomater Sci. 2023 Jun 13;11(12):4327-4334. [CrossRef]
- Li D, Zhang Y, Li S, et al. A novel Toxoplasma gondii TGGT1_316290 mRNA-LNP vaccine elicits protective immune response against toxoplasmosis in mice. Front Microbiol. 2023 Mar 21;14:1145114. [CrossRef]
- Sáez-Llorens X, Lanata C, Aranguren E, et al. Safety and immunogenicity of mRNA-LNP COVID-19 vaccine CVnCoV in Latin American adults: A phase 2 randomized study. Vaccine X. 2022 Aug;11:100189. [CrossRef]
- Carter B, Huang P, Liu G, et al. A pan-variant mRNA-LNP T cell vaccine protects HLA transgenic mice from mortality after infection with SARS-CoV-2 Beta. Front Immunol. 2023 Mar 9;14:1135815. [CrossRef]
- Ndeupen S, Bouteau A, Herbst C, et al. Langerhans cells and cDC1s play redundant roles in mRNA-LNP induced protective anti-influenza and anti-SARS-CoV-2 responses. bioRxiv [Preprint]. 2021 Aug 2:2021.08.01.454662.
- Ndeupen S, Bouteau A, Herbst C, et al. Langerhans cells and cDC1s play redundant roles in mRNA-LNP induced protective anti-influenza and anti-SARS-CoV-2 immune responses. PLoS Pathog. 2022 Jan 24;18(1):e1010255. [CrossRef]
- Hayashi CTH, Cao Y, Clark LC, et al. Author Correction: mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum. NPJ Vaccines. 2023 Aug 11;8(1):115. [CrossRef]
- Bavli Y, Chen BM, Gross G, Anti-PEG antibodies before and after a first doseet al. of Comirnaty (mRNA-LNP-based SARS-CoV-2 vaccine). J Control Release. 2023 Feb;354:316-322. [CrossRef]
- Hermosilla J, Alonso-García A, Salmerón-García A, et al. Analysing the In-Use Stability of mRNA-LNP COVID-19 Vaccines Comirnaty™ (Pfizer) and Spikevax™ (Moderna): A Comparative Study of the Particulate. Vaccines (Basel). 2023 Oct 25;11(11):1635. [CrossRef]
- Egan KP, Awasthi S, Tebaldi G, et al. A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1. Viruses. 2023 Jun 30;15(7):1483. [CrossRef]
- Zhang Y, Li S, Chu H, et al. A novel mRNA vaccine, TGGT1_278620 mRNA-LNP, prolongs the survival time in BALB/c mice with acute toxoplasmosis. Microbiol Spectr. 2023 Dec 1:e0286623. [CrossRef]
- Sáez-Llorens X, Lanata C, Aranguren E, et al. Corrigendum to "Safety and immunogenicity of mRNA-LNP COVID-19 vaccine CVnCoV in Latin American adults: A phase 2 randomized study" [Vaccine: X 11 (2022) 100189]. Vaccine X. 2023 Aug;14:100307. [CrossRef]
- Vlatkovic I. Non-Immunotherapy Application of LNP-mRNA: Maximizing Efficacy and Safety. Biomedicines. 2021 May 10;9(5):530. [CrossRef]
- Maugeri M, Nawaz M, Papadimitriou A, et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat Commun. 2019 Sep 24;10(1):4333. [CrossRef]
- Kenjo E, Hozumi H, Makita Y, et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat Commun. 2021 Dec 8;12(1):7101. [CrossRef]
- Álvarez-Benedicto E, Farbiak L, Márquez Ramírez M, et al. Optimization of phospholipid chemistry for improved lipid nanoparticle (LNP) delivery of messenger RNA (mRNA). Biomater Sci. 2022 Jan 18;10(2):549-559. [CrossRef]
- Peng L, Fang Z, Renauer PA, et al. Multiplexed LNP-mRNA vaccination against pathogenic coronavirus species. Cell Rep. 2022 Aug 2;40(5):111160. [CrossRef]
- Tsiambas E, Chrysovergis A, Papanikolaou V, et al. Impact of Ribosome Activity on SARS-CoV-2 LNP - Based mRNA Vaccines. Front Mol Biosci. 2021 Apr 20;8:654866. [CrossRef]
- Bahl K, Senn JJ, Yuzhakov O, et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol Ther. 2017 Jun 7;25(6):1316-1327. [CrossRef]
- Patel SK, Billingsley MM, Frazee C, et al. Hydroxycholesterol substitution in ionizable lipid nanoparticles for mRNA delivery to T cells. J Control Release. 2022 Jul;347:521-532. [CrossRef]
- Gao K, Li J, Song H, et al. In utero delivery of mRNA to the heart, diaphragm and muscle with lipid nanoparticles. Bioact Mater. 2023 Feb 17;25:387-398. [CrossRef]
- Ball RL, Hajj KA, Vizelman J, et al. Lipid Nanoparticle Formulations for Enhanced Co-delivery of siRNA and mRNA. Nano Lett. 2018 Jun 13;18(6):3814-3822. [CrossRef]
- Bogaert B, Sauvage F, Guagliardo R, et al. A lipid nanoparticle platform for mRNA delivery through repurposing of cationic amphiphilic drugs. J Control Release. 2022 Oct;350:256-270. [CrossRef]
- Huayamares SG, Lokugamage MP, Rab R, et al. High-throughput screens identify a lipid nanoparticle that preferentially delivers mRNA to human tumors in vivo. J Control Release. 2023 May;357:394-403. [CrossRef]
- Da Silva Sanchez AJ, Zhao K, Huayamares SG, et al. Substituting racemic ionizable lipids with stereopure ionizable lipids can increase mRNA delivery. J Control Release. 2023 Jan;353:270-277. [CrossRef]
- Swingle KL, Safford HC, Geisler HC, et al. Ionizable Lipid Nanoparticles for In Vivo mRNA Delivery to the Placenta during Pregnancy. J Am Chem Soc. 2023 Mar 1;145(8):4691-4706. [CrossRef]
- Attarwala H, Lumley M, Liang M, et al. Translational Pharmacokinetic/Pharmacodynamic Model for mRNA-3927, an Investigational Therapeutic for the Treatment of Propionic Acidemia. Nucleic Acid Ther. 2023 Apr;33(2):141-147. [CrossRef]
- Miao L, Lin J, Huang Y, et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat Commun. 2020 May 15;11(1):2424. [CrossRef]
- Liang F, Lindgren G, Lin A, et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol Ther. 2017 Dec 6;25(12):2635-2647. [CrossRef]
- Ryals RC, Patel S, Acosta C, et al. The effects of PEGylation on LNP based mRNA delivery to the eye. PLoS One. 2020 Oct 29;15(10):e0241006. [CrossRef]
- Zhuang X, Qi Y, Wang M, et al. mRNA Vaccines Encoding the HA Protein of Influenza A H1N1 Virus Delivered by Cationic Lipid Nanoparticles Induce Protective Immune Responses in Mice. Vaccines (Basel). 2020 Mar 10;8(1):123. [CrossRef]
- Sinegra AJ, Evangelopoulos M, Park J, et al. Lipid Nanoparticle Spherical Nucleic Acids for Intracellular DNA and RNA Delivery. Nano Lett. 2021 Aug 11;21(15):6584-6591. [CrossRef]
- Zhang Y, Yan J, Hou X, et al. STING Agonist-Derived LNP-mRNA Vaccine Enhances Protective Immunity Against SARS-CoV-2. Nano Lett. 2023 Apr 12;23(7):2593-2600. [CrossRef]
- Dobrowolski C, Paunovska K, Schrader Echeverri E, et al. Nanoparticle single-cell multiomic readouts reveal that cell heterogeneity influences lipid nanoparticle-mediated messenger RNA delivery. Nat Nanotechnol. 2022 Aug;17(8):871-879. [CrossRef]
- Ickenstein LM, Garidel P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin Drug Deliv. 2019 Nov;16(11):1205-1226. [CrossRef]
- Semple SC, Leone R, Barbosa CJ, et al. Lipid Nanoparticle Delivery Systems to Enable mRNA-Based Therapeutics. Pharmaceutics. 2022 Feb 11;14(2):398. [CrossRef]
- Tanaka H, Hagiwara S, Shirane D, et al. Ready-to-Use-Type Lyophilized Lipid Nanoparticle Formulation for the Postencapsulation of Messenger RNA. ACS Nano. 2023 Feb 14;17(3):2588-2601. [CrossRef]
- Tam A, Kulkarni J, An K, et al. Lipid nanoparticle formulations for optimal RNA-based topical delivery to murine airways. Eur J Pharm Sci. 2022 Sep 1;176:106234. [CrossRef]
- Hamilton AG, Swingle KL, Joseph RA, et al. Ionizable Lipid Nanoparticles with Integrated Immune Checkpoint Inhibition for mRNA CAR T Cell Engineering. Adv Healthc Mater. 2023 Dec;12(30):e2301515. [CrossRef]
- Shi D, Toyonaga S, Anderson DG. In Vivo RNA Delivery to Hematopoietic Stem and Progenitor Cells via Targeted Lipid Nanoparticles. Nano Lett. 2023 Apr 12;23(7):2938-2944. [CrossRef]
- Fang Z, Peng L, Filler R, et al. Omicron-specific mRNA vaccination alone and as a heterologous booster against SARS-CoV-2. bioRxiv [Preprint]. 2022 Feb 28:2022.02.14.480449.
- Radloff K, Gutbier B, Dunne CM, et al. Cationic LNP-formulated mRNA expressing Tie2-agonist in the lung endothelium prevents pulmonary vascular leakage. Mol Ther Nucleic Acids. 2023 Oct 29;34:102068. [CrossRef]
- August A, Brito L, Paris R, et al. Clinical Development of mRNA Vaccines: Challenges and Opportunities. Curr Top Microbiol Immunol. 2022;440:167-186. [CrossRef]
- Fedorowski JJ. Could amantadine interfere with COVID-19 vaccines based on the LNP-mRNA platform? Arch Med Sci. 2021 Mar 28;17(3):827-828. [CrossRef]
- Somiya M, Mine S, Yasukawa K, et al. Sex differences in the incidence of anaphylaxis to LNP-mRNA COVID-19 vaccines. Vaccine. 2021 Jun 8;39(25):3313-3314. [CrossRef]
- Wang W, Feng S, Ye Z, et al. Prediction of lipid nanoparticles for mRNA vaccines by the machine learning algorithm. Acta Pharm Sin B. 2022 Jun;12(6):2950-2962. [CrossRef]
- Leung J, Strong C, Badior KE, et al. Genetically engineered transfusable platelets using mRNA lipid nanoparticles. Sci Adv. 2023 Dec;9(48):eadi0508. [CrossRef]
- Novakowski S, Jiang K, Prakash G, et al. Delivery of mRNA to platelets using lipid nanoparticles. Sci Rep. 2019 Jan 24;9(1):552. [CrossRef]
- Sayers EJ, Peel SE, Schantz A, et al. Endocytic Profiling of Cancer Cell Models Reveals Critical Factors Influencing LNP-Mediated mRNA Delivery and Protein Expression. Mol Ther. 2019 Nov 6;27(11):1950-1962. [CrossRef]
- Wu L, Wang W, Tian J, et al. Engineered mRNA-expressed bispecific antibody prevent intestinal cancer via lipid nanoparticle delivery. Bioengineered. 2021 Dec;12(2):12383-12393. [CrossRef]
- Zeng Y, Escalona-Rayo O, Knol R, et al. Lipid nanoparticle-based mRNA candidates elicit potent T cell responses. Biomater Sci. 2023 Jan 31;11(3):964-974. [CrossRef]
- Wang Y, Si X, Feng Y, et al. Ionizable Lipids with Triazole Moiety from Click Reaction for LNP-Based mRNA Delivery. Molecules. 2023 May 12;28(10):4046. [CrossRef]
- Yeh TF, Lin C, Sung HC. A review of technological developments in lipid nanoparticle application for mRNA vaccination. Hum Vaccin Immunother. 2023 Aug 1;19(2):2256040. [CrossRef]
- Provine NM, Klenerman P. Adenovirus vector and mRNA vaccines: Mechanisms regulating their immunogenicity. Eur J Immunol. 2023 Jun;53(6):e2250022. [CrossRef]
- Qin J, Xue L, Gong N, et al. RGD peptide-based lipids for targeted mRNA delivery and gene editing applications. RSC Adv. 2022 Sep 7;12(39):25397-25404. [CrossRef]
- Goswami R, Chatzikleanthous D, Lou G, et al. Mannosylation of LNP Results in Improved Potency for Self-Amplifying RNA (SAM) Vaccines. ACS Infect Dis. 2019 Sep 13;5(9):1546-1558. [CrossRef]
- Vigil TN, Zhang-Hulsey D, Santos JL, et al. Expediting in vitro characterization of mRNA-based gene therapies via high-content fluorescent imaging. Anal Biochem. 2021 Aug 15;627:114259. [CrossRef]
- Zhang Y, Wang J, Xing H, et al. Enhanced immunogenicity induced by mRNA vaccines with various lipid nanoparticles as carriers for SARS-CoV-2 infection. J Mater Chem B. 2023 Aug 9;11(31):7454-7465. [CrossRef]
- Pine M, Arora G, Hart TM, et al. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Mol Ther. 2023 Sep 6;31(9):2702-2714. [CrossRef]
- Huo H, Cheng X, Xu J, et al. A fluorinated ionizable lipid improves the mRNA delivery efficiency of lipid nanoparticles. J Mater Chem B. 2023 May 17;11(19):4171-4180. [CrossRef]
- Diaz-Trelles R, Perez-Garcia CG. Present and future of lipid nanoparticle-mRNA technology in phenylketonuria disease treatment. Int Rev Cell Mol Biol. 2022;372:159-174. [CrossRef]
- Long J, Yu C, Zhang H, et al. Novel Ionizable Lipid Nanoparticles for SARS-CoV-2 Omicron mRNA Delivery. Adv Healthc Mater. 2023 May;12(13):e2202590. [CrossRef]
- Chang DF, Court KA, Holgate R, et al. Telomerase mRNA Enhances Human Skin Engraftment for Wound Healing. Adv Healthc Mater. 2023 Aug 24:e2302029. [CrossRef]
- VanBlargan LA, Himansu S, Foreman BM, et al. An mRNA Vaccine Protects Mice against Multiple Tick-Transmitted Flavivirus Infections. Cell Rep. 2018 Dec 18;25(12):3382-3392.e3. [CrossRef]
- 탈ak MM, Kaur K, Yoo J, et al. Modified mRNA Formulation and Stability for Cardiac and Skeletal Muscle Delivery. Pharmaceutics. 2023 Aug 22;15(9):2176. [CrossRef]
- Cao W, Xia T. mRNA lipid nanoparticles induce immune tolerance to treat human diseases. Med Rev (Berl). 2023 Apr 14;3(2):180-183. [CrossRef]
- Bähr-Mahmud H, Ellinghaus U, Stadler CR, et al. Preclinical characterization of an mRNA-encoded anti-Claudin 18.2 antibody. Oncoimmunology. 2023 Oct 16;12(1):2255041. [CrossRef]
- Swingle KL, Billingsley MM, Bose SK, et al. Amniotic fluid stabilized lipid nanoparticles for in utero intra-amniotic mRNA delivery. J Control Release. 2022 Jan;341:616-633. [CrossRef]
- Yihunie W, Nibret G, Aschale Y. Recent Advances in Messenger Ribonucleic Acid (mRNA) Vaccines and Their Delivery Systems: A Review. Clin Pharmacol. 2023 Aug 3;15:77-98. [CrossRef]
- Szőke D, Kovács G, Kemecsei É, et al. Nucleoside-modified VEGFC mRNA induces organ-specific lymphatic growth and reverses experimental lymphedema. Nat Commun. 2021 Jun 8;12(1):3460. [CrossRef]
- Pardi N, Weissman D. Nucleoside Modified mRNA Vaccines for Infectious Diseases. Methods Mol Biol. 2017;1499:109-121. [CrossRef]
- Sang Y, Zhang Z, Liu F, et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct Target Ther. 2023 Apr 28;8(1):172. [CrossRef]
- Broudic K, Amberg A, Schaefer M, et al. Nonclinical safety evaluation of a novel ionizable lipid for mRNA delivery. Toxicol Appl Pharmacol. 2022 Sep 15;451:116143. [CrossRef]
- Fekete S, Doneanu C, Addepalli B, et al. Challenges and emerging trends in liquid chromatography-based analyses of mRNA pharmaceuticals. J Pharm Biomed Anal. 2023 Feb 5;224:115174. [CrossRef]
- Sun M, Dang UJ, Yuan Y, et al. Optimization of DOTAP/chol Cationic Lipid Nanoparticles for mRNA, pDNA, and Oligonucleotide Delivery. AAPS PharmSciTech. 2022 May 9;23(5):135. [CrossRef]
- McCrudden CM, Bennie L, Chambers P, et al. Peptide delivery of a multivalent mRNA SARS-CoV-2 vaccine. J Control Release. 2023 Oct;362:536-547. [CrossRef]
- Thran M, Mukherjee J, Pönisch M, et al. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol Med. 2017 Oct;9(10):1434-1447. [CrossRef]
- Zhang J, Shrivastava S, Cleveland RO, et al. Lipid-mRNA Nanoparticle Designed to Enhance Intracellular Delivery Mediated by Shock Waves. ACS Appl Mater Interfaces. 2019 Mar 20;11(11):10481-10491. [CrossRef]
- Nakamura T, Nakade T, Sato Y, et al. Delivering mRNA to a human NK cell line, NK-92 cells, by lipid nanoparticles. Int J Pharm. 2023 Apr 5;636:122810. [CrossRef]
- Huysmans H, Zhong Z, De Temmerman J, et al. Expression Kinetics and Innate Immune Response after Electroporation and LNP-Mediated Delivery of a Self-Amplifying mRNA in the Skin. Mol Ther Nucleic Acids. 2019 Sep 6;17:867-878. [CrossRef]
- Dong S, Wang J, Guo Z, et al. Efficient delivery of VEGFA mRNA for promoting wound healing via ionizable lipid nanoparticles. Bioorg Med Chem. 2023 Jan 15;78:117135. [CrossRef]
- 241. Zhang HL. Current status and patent prospective of lipid nanoparticle for mRNA delivery. Expert Opin Ther Pat. 2023 Feb;33(2):125-131. [CrossRef]
- Yamazaki K, Kubara K, Ishii S, et al. Lipid nanoparticle-targeted mRNA formulation as a treatment for ornithine-transcarbamylase deficiency model mice. Mol Ther Nucleic Acids. 2023 Jul 4;33:210-226. [CrossRef]
- Patel S, Ashwanikumar N, Robinson E, et al. Boosting Intracellular Delivery of Lipid Nanoparticle-Encapsulated mRNA. Nano Lett. 2017 Sep 13;17(9):5711-5718. [CrossRef]
- Olson KE, Namminga KL, Lu Y, et al. Granulocyte-macrophage colony-stimulating factor mRNA and Neuroprotective Immunity in Parkinson's disease. Biomaterials. 2021 May;272:120786. [CrossRef]
- Nawaz M, Heydarkhan-Hagvall S, Tangruksa B, et al. Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions. Adv Sci (Weinh). 2023 Apr;10(12):e2206187. [CrossRef]
- Popowski KD, López de Juan Abad B, George A, et al. Inhalable exosomes outperform liposomes as mRNA and protein drug carriers to the lung. Extracell Vesicle. 2022 Dec;1:100002. [CrossRef]
- Safford HC, Swingle KL, Geisler HC, et al. Orthogonal Design of Experiments for Engineering of Lipid Nanoparticles for mRNA Delivery to the Placenta. Small. 2023 Aug 3:e2303568. [CrossRef]
- Lokugamage MP, Gan Z, Zurla C, et al. Mild Innate Immune Activation Overrides Efficient Nanoparticle-Mediated RNA Delivery. Adv Mater. 2020 Jan;32(1):e1904905. [CrossRef]
- Zhdanov VP. Kinetics of lipid-nanoparticle-mediated intracellular mRNA delivery and function. Phys Rev E. 2017 Oct;96(4-1):042406. [CrossRef]
- Hatit MZC, Dobrowolski CN, Lokugamage MP, et al. Nanoparticle stereochemistry-dependent endocytic processing improves in vivo mRNA delivery. Nat Chem. 2023 Apr;15(4):508-515. [CrossRef]
- Miao H, Huang K, Li Y, et al. Optimization of formulation and atomization of lipid nanoparticles for the inhalation of mRNA. Int J Pharm. 2023 Jun 10;640:123050. [CrossRef]
- Hunter MR, Cui L, Porebski BT, et al. Understanding Intracellular Biology to Improve mRNA Delivery by Lipid Nanoparticles. Small Methods. 2023 Sep;7(9):e2201695. [CrossRef]
- Yu X, Yu C, Wu X, et al. Validation of an HPLC-CAD Method for Determination of Lipid Content in LNP-Encapsulated COVID-19 mRNA Vaccines. Vaccines (Basel). 2023 May 4;11(5):937. [CrossRef]
- Aliakbarinodehi N, Gallud A, Mapar M, et al. Interaction Kinetics of Individual mRNA-Containing Lipid Nanoparticles with an Endosomal Membrane Mimic: Dependence on pH, Protein Corona Formation, and Lipoprotein Depletion. ACS Nano. 2022 Dec 27;16(12):20163-20173. [CrossRef]
- Xue L, Gong N, Shepherd SJ, et al. Rational Design of Bisphosphonate Lipid-like Materials for mRNA Delivery to the Bone Microenvironment. J Am Chem Soc. 2022 Jun 8;144(22):9926-9937. [CrossRef]
- van Rijn CJM, Vlaming KE, Bem RA, et al. Low energy nebulization preserves integrity of SARS-CoV-2 mRNA vaccines for respiratory delivery. Sci Rep. 2023 May 31;13(1):8851. [CrossRef]
- Gan Z, Lokugamage MP, Hatit MZC, et al. Nanoparticles containing constrained phospholipids deliver mRNA to liver immune cells in vivo without targeting ligands. Bioeng Transl Med. 2020 May 27;5(3):e10161. [CrossRef]
- Zha W, Wang J, Guo Z, et al. Efficient delivery of VEGF-A mRNA for promoting diabetic wound healing via ionizable lipid nanoparticles. Int J Pharm. 2023 Feb 5;632:122565. [CrossRef]
- Shepherd SJ, Warzecha CC, Yadavali S, et al. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021 Jul 14;21(13):5671-5680. [CrossRef]
- Ye Z, Chen J, Zhao X, et al. In Vitro Engineering Chimeric Antigen Receptor Macrophages and T Cells by Lipid Nanoparticle-Mediated mRNA Delivery. ACS Biomater Sci Eng. 2022 Feb 14;8(2):722-733. [CrossRef]
- Bepperling A, Richter G. Determination of mRNA copy number in degradable lipid nanoparticles via density contrast analytical ultracentrifugation. Eur Biophys J. 2023 Jul;52(4-5):393-400. [CrossRef]
- Sarode A, Patel P, Vargas-Montoya N, et al. Inhalable dry powder product (DPP) of mRNA lipid nanoparticles (LNPs) for pulmonary delivery. Drug Deliv Transl Res. 2023 Aug 1. [CrossRef]
- Huang H, Zhang C, Yang S, et al. The investigation of mRNA vaccines formulated in liposomes administrated in multiple routes against SARS-CoV-2. J Control Release. 2021 Jul 10;335:449-456. [CrossRef]
- Elia U, Ramishetti S, Rosenfeld R, et al. Design of SARS-CoV-2 hFc-Conjugated Receptor-Binding Domain mRNA Vaccine Delivered via Lipid Nanoparticles. ACS Nano. 2021 Jun 22;15(6):9627-9637. [CrossRef]
- Yang D, Song CQ. The Delivery of ABE mRNA to the Adult Murine Liver by Lipid Nanoparticles (LNPs). Methods Mol Biol. 2023;2606:159-170. [CrossRef]
- Nakashima I, Saito S, Akahoshi E, et al. Non-viral inducible caspase 9 mRNA delivery using lipid nanoparticles against breast cancer: An in vitro study. Biochem Biophys Res Commun. 2022 Dec 20;635:144-153. [CrossRef]
- Wang T, Sung TC, Yu T, et al. Next-generation materials for RNA-lipid nanoparticles: lyophilization and targeted transfection. J Mater Chem B. 2023 Jun 14;11(23):5083-5093. [CrossRef]
- Takanashi A, Pouton CW, Al-Wassiti H. Delivery and Expression of mRNA in the Secondary Lymphoid Organs Drive Immune Responses to Lipid Nanoparticle-mRNA Vaccines after Intramuscular Injection. Mol Pharm. 2023 Aug 7;20(8):3876-3885. [CrossRef]
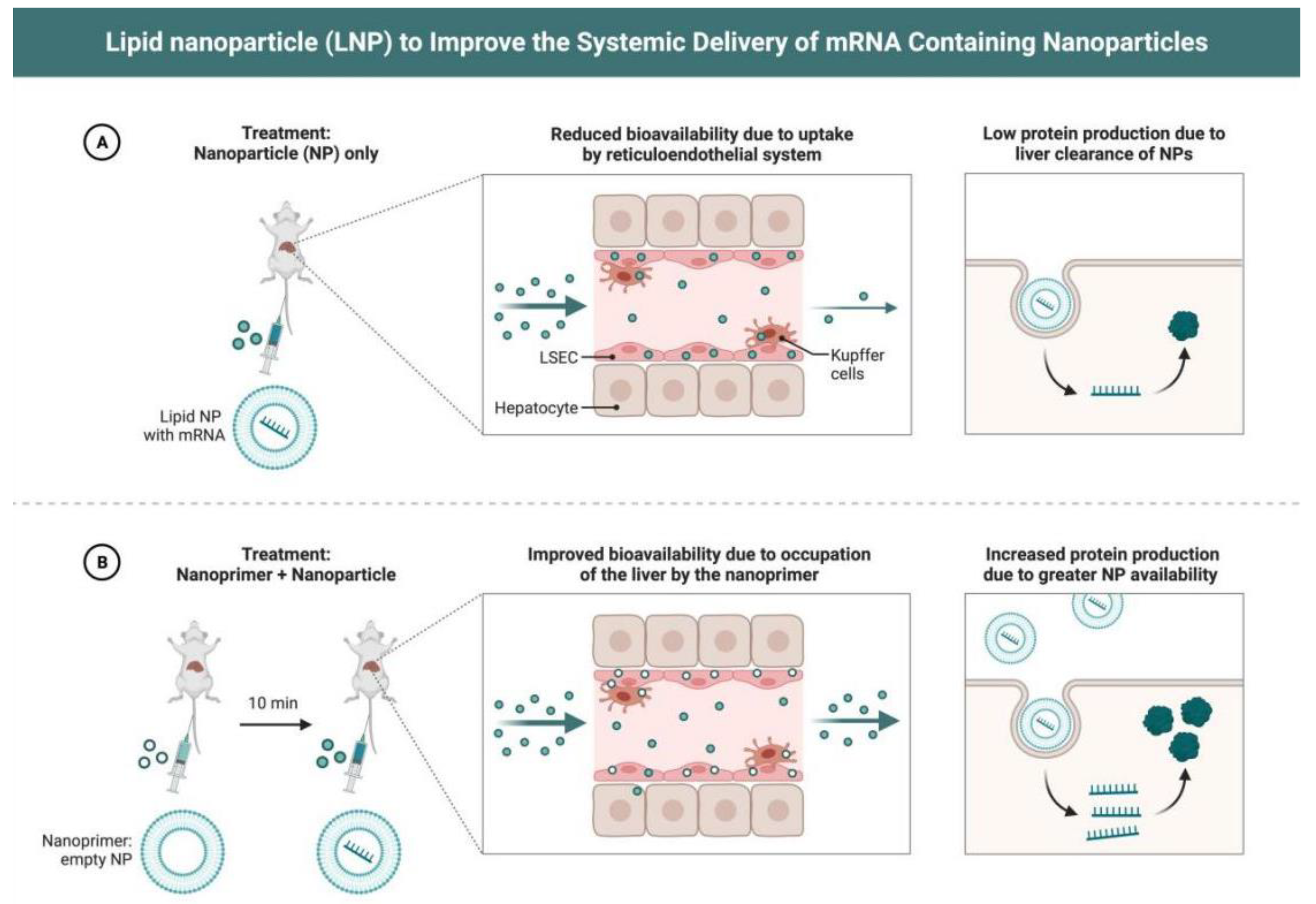
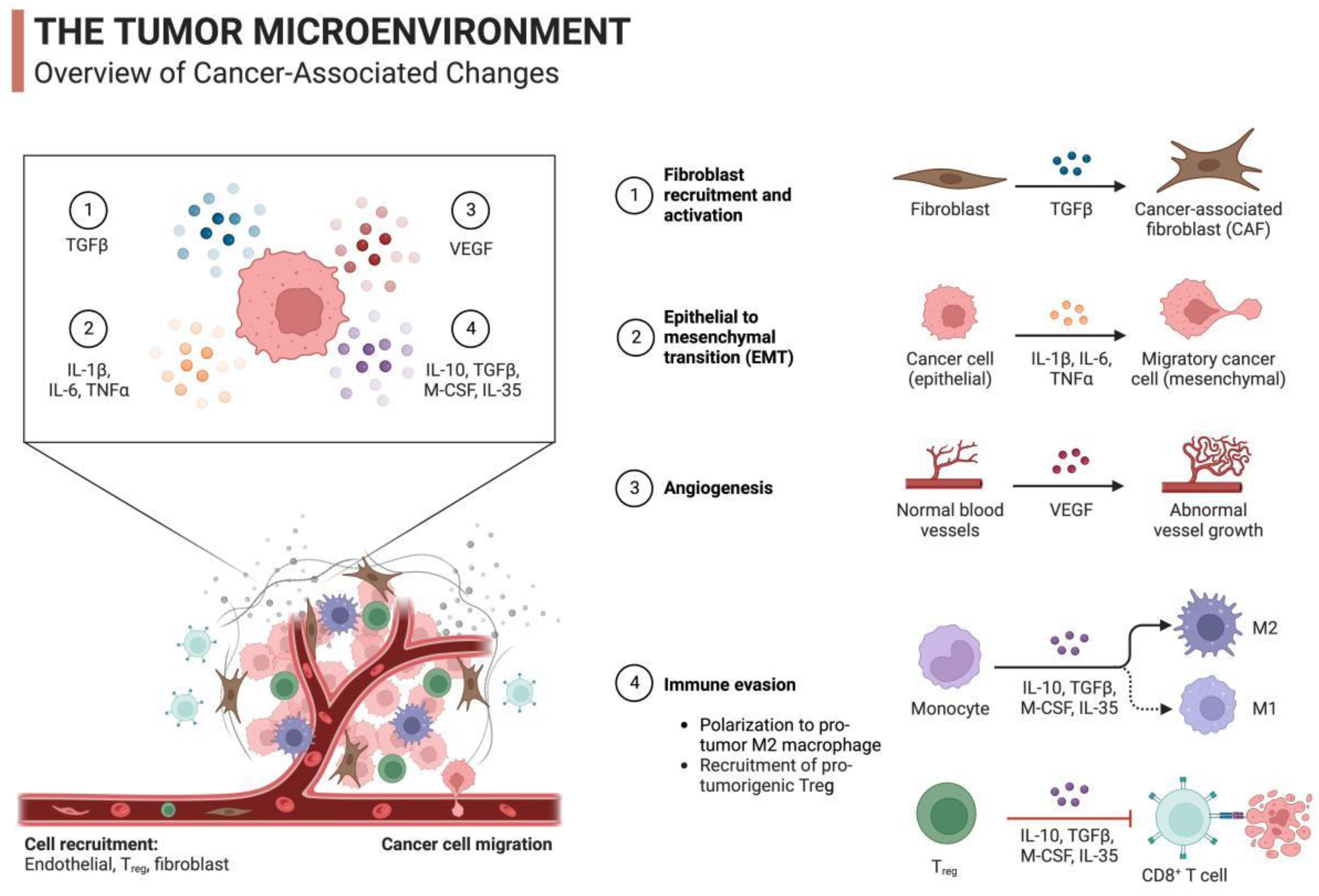
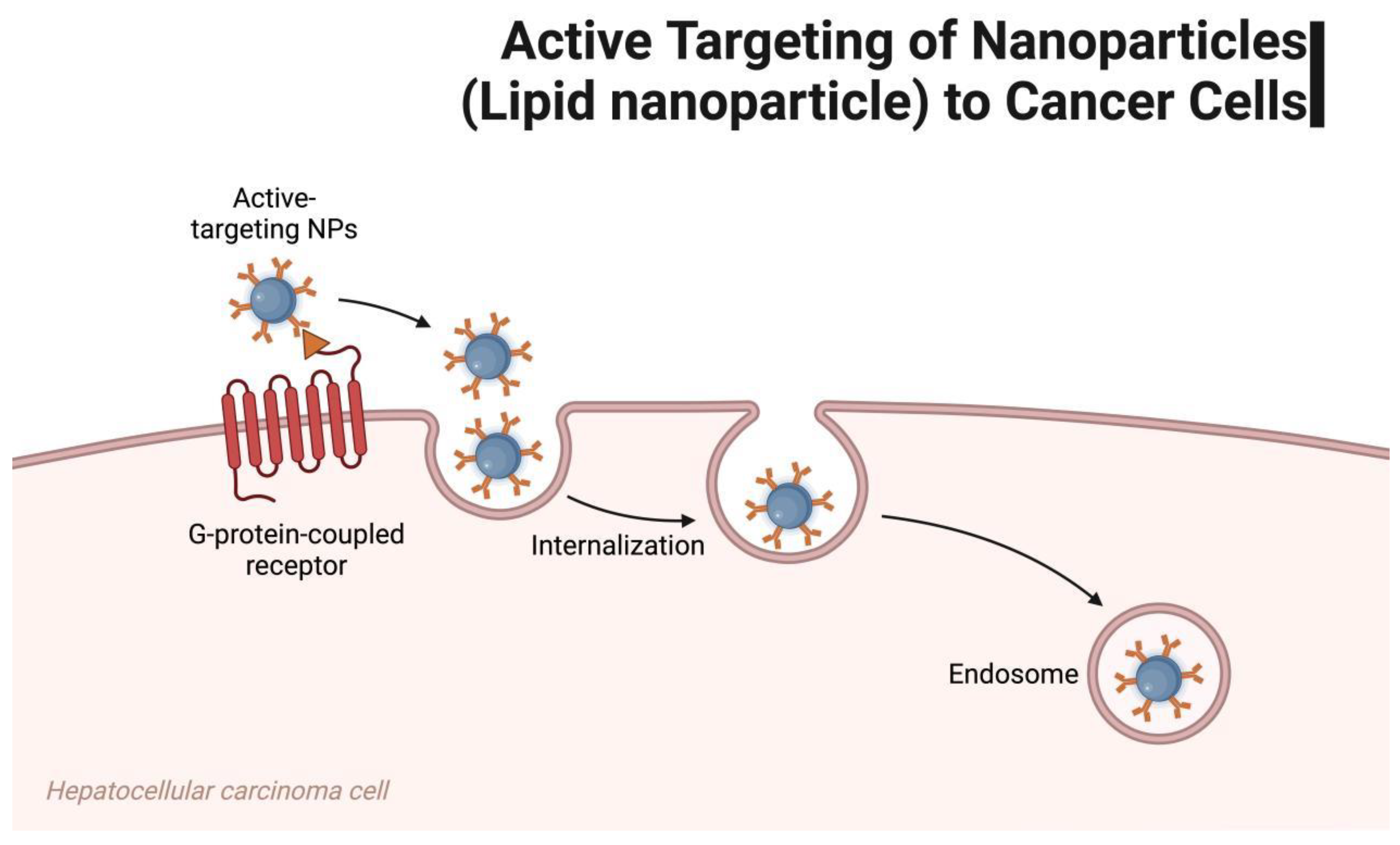
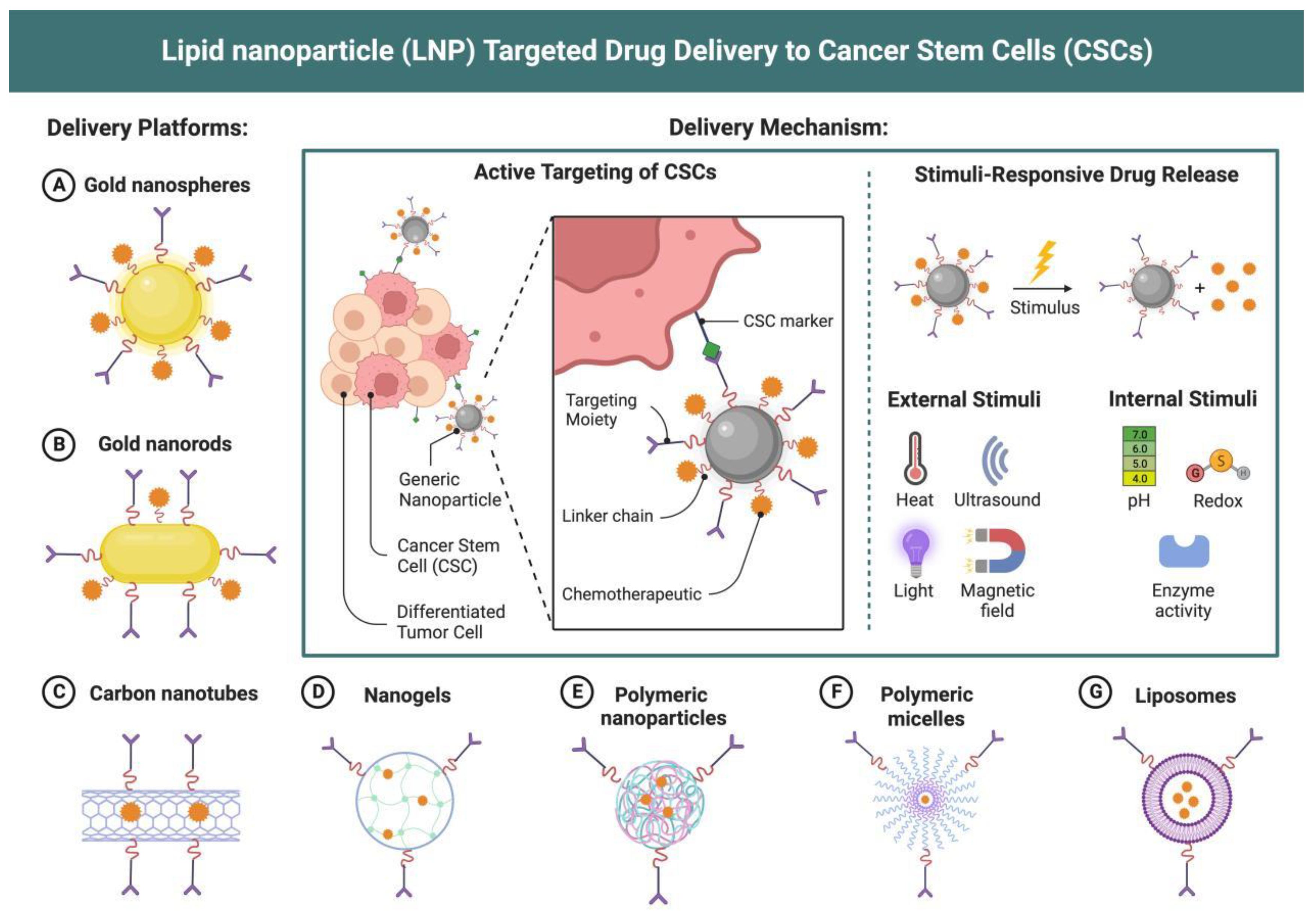
| mRNA Vaccine Type | mRNA Vaccine Carrier Properties | Related Research | Specific Disease Applications | Types of Bionanomaterials used with mRNA Vaccines |
| Lipid Nanoparticles (LNP) | High encapsulation, intracellular delivery | Pfizer-BioNTech, Moderna | COVID-19 | Liposomes, Polymeric Nanoparticles |
| Polymeric Nanoparticles | Tunable release, stability | CureVac | COVID-19, Vaccine Development | Polymers, Liposomes |
| Protein-Polymer Nanocomplexes | Targeted, stability | Arcturus Therapeutics | COVID-19, Vaccine Development | Proteins, Polymers |
| Lipid-Protein Complexes | Efficient transfection, mRNA protection | Acuitas Therapeutics | COVID-19, Other Vaccines | Lipids, Proteins |
| Lipid-Peptide Complexes | Specific targeting, enhanced immunity | Moderna | COVID-19 | Lipids, Peptides |
| Nano-Peptide Particles | Antigen presentation, immune activation | Stanford Research | COVID-19, Cancer Vaccines | Peptides |
| Magnetic Nanoparticles | Imaging-guided, vaccine delivery | Under Research | Cancer, Vaccine Development | Iron Oxide Magnetic Nanoparticles |
| Metal-Organic Frameworks (MOFs) | High drug loading, controlled release | Under Research | Vaccine Development | MOFs, mRNA Vaccines |
| Carbon-Based Nanomaterials | Biocompatibility, delivery efficiency | Under Research | Cancer Immunotherapy | Carbon Nanotubes, Graphene Oxide |
| Gold Nanoparticles | Efficient transport, immune activation | Under Research | Cancer, Vaccine Development | Gold Nanoparticles, mRNA Vaccines |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





