Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
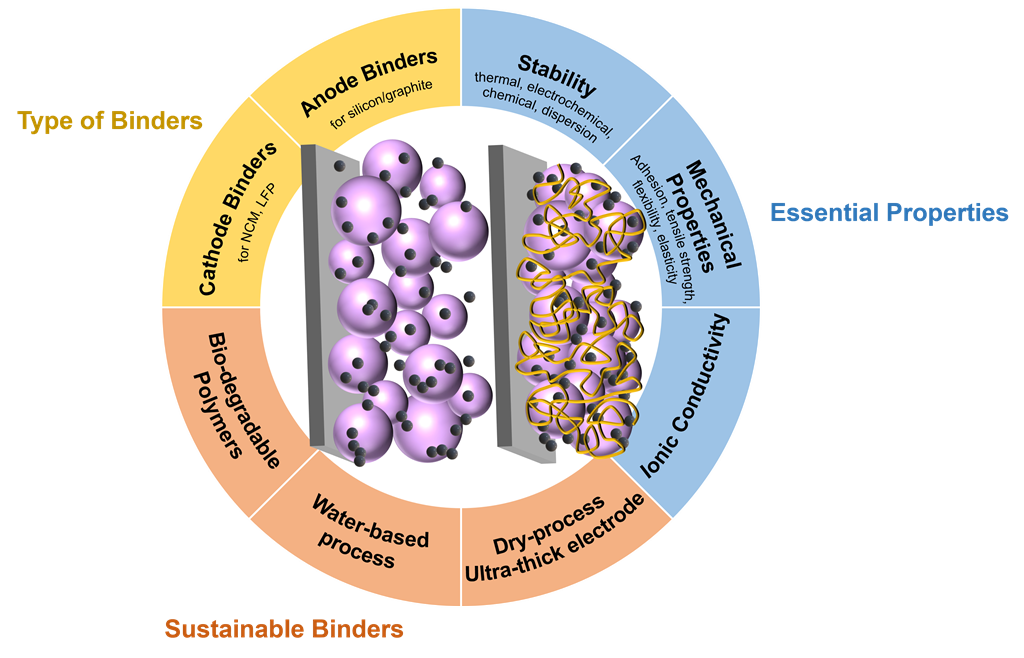
Keywords:
1. Introduction
2. Essential properties of binders
2.1. Stability
2.1.1. Thermal Stability
2.1.2. Electrochemical Stability
2.1.3. Chemical stability
2.1.4. Dispersion Stability
2.2. Mechanical Properties
2.2.1. Adhesion
2.2.2. Tensile strength
2.2.3. Flexibility and elasticity
2.3. Ionic Conductivity
3. Typical Binders
3.1. Anode Binders
3.1.1. PVdF
3.1.2. PAA
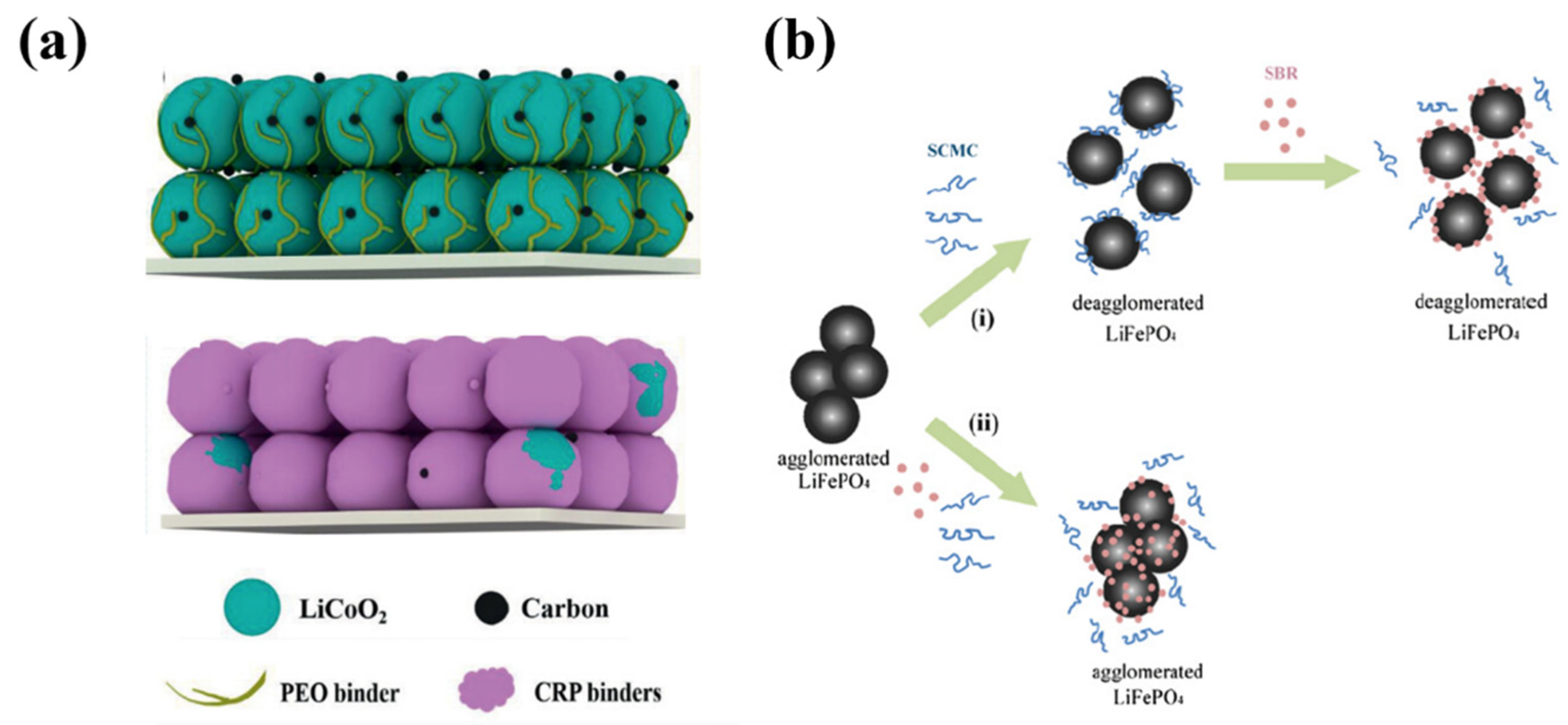
3.1.3. CMC/SBR
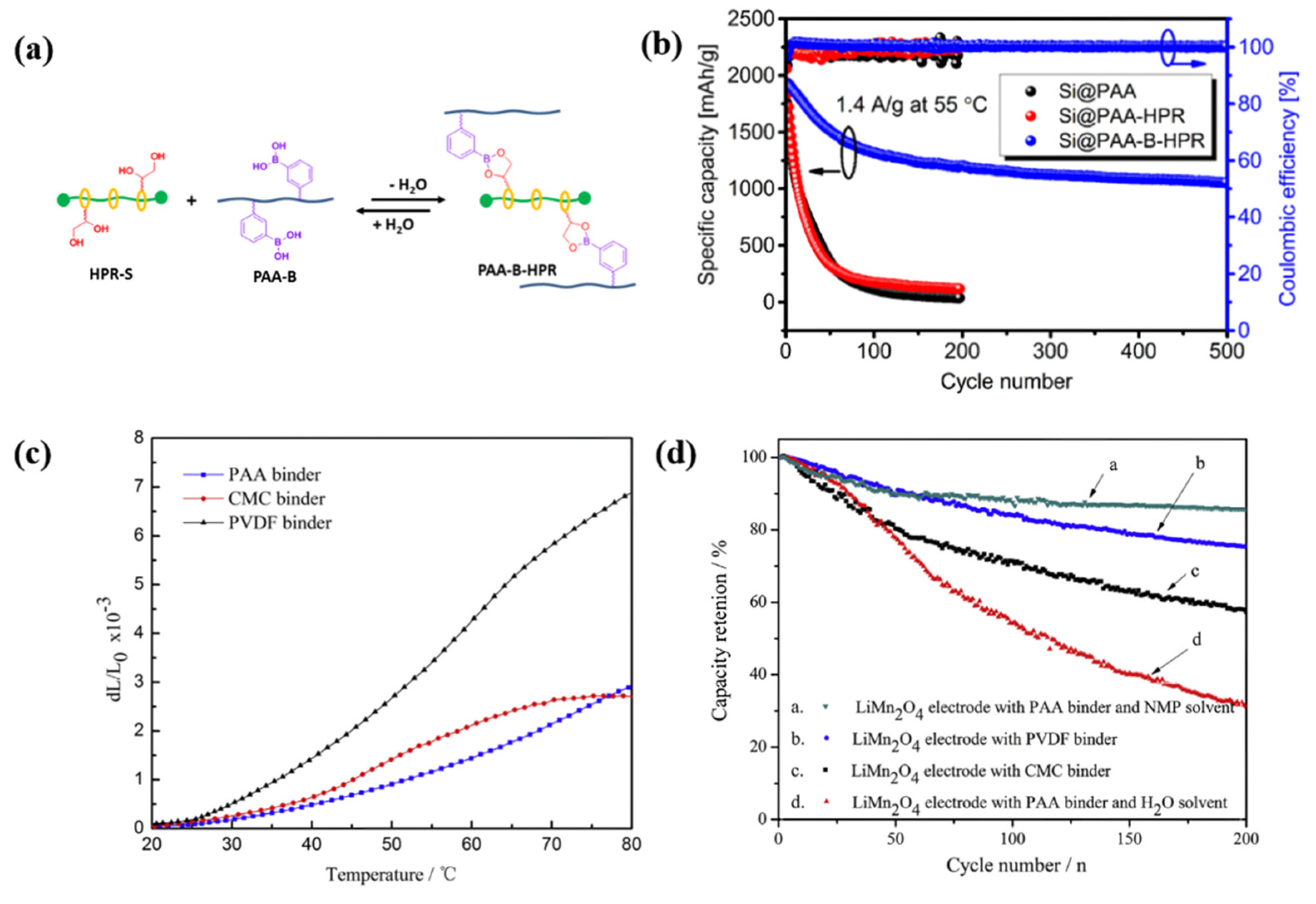
3.1.4. Binders for Si/Graphite (Si/G) Anodes
3.2. Cathode binders
3.2.1. Binders for NCM
3.2.2. Binders for LFP
4. Sustainable binders for LIBs
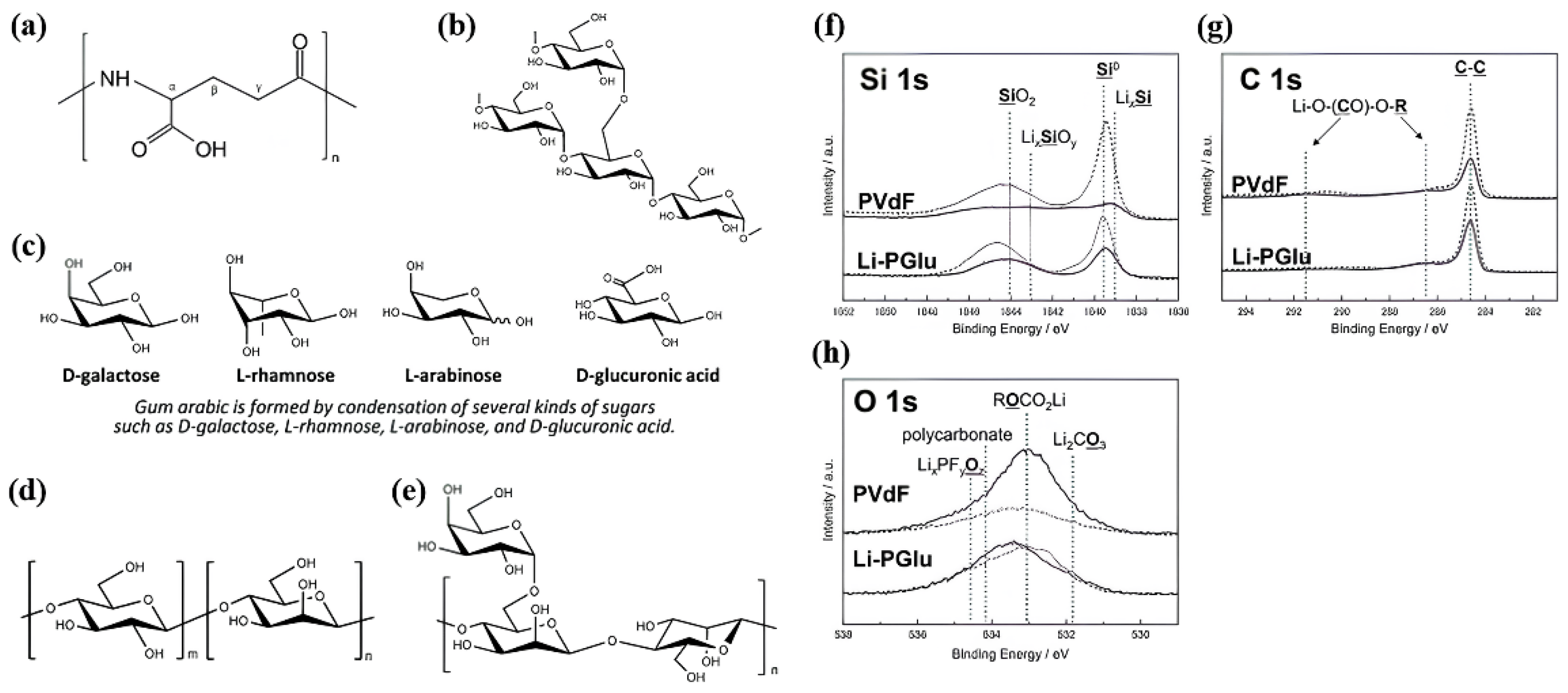
4.1. Bio-based Eco-friendly binder
4.2. Water-based process
4.3. Dry process and ultra-thick electrode
5. Conclusions and outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Whittingham, M. S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 4244. [Google Scholar] [CrossRef]
- Sun, X.; Hao, H.; Zhao, F.; Liu, Z. Tracing global lithium flow: A trade-linked material flow analysis. Resources, Conservation & Recycling 2017, 124, 50. [Google Scholar]
- Cheng, Z.; Jiang, H.; Zhang, X.; Cheng, F.; Wu, M.; Zhang, H. Fundamental Understanding and Facing Challenges in Structural Design of Porous Si-Based Anodes for Lithium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2301109. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Sun, Y.-K. Recent Progress of Advanced Binders for Li-S Batteries. J. Power Source 2018, 396, 19–32. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H. Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring Chemical, Mechanical, and Electrical Functionalities of Binders for Advanced Energy-Storage Devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Wang, Y.; Cheng, Y.-T. The Influence of Polyvinylidene Fluoride (PVDF) Binder Properties on LiNi0.33Co0.33Mn0.33O2 (NMC) Electrodes Made by a Dry-Powder-Coating Process. J. Electrochem. Soc. 2019, 166, A2151–A2157. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.; Ju, Z.; Wang, L.; Hui, Z.; Mayilvahanan, K.; Takeuchi, K. J.; Marschilok, A. C.; West, A. C.; Takeuchi, E. S.; Yu, G. From Fundamental Understanding to Engineering Design of High-Performance Thick Electrodes for Scalable Energy-Storage Systems. Adv. Mater. 2021, 33, 2101275. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Zhu, Y.; Zhang, X.; Lutz, D. M.; Fang, Z.; Takeuchi, K. J.; Takeuchi, E. S.; Marschilok, A. C.; Yu, G. Understanding Thickness-Dependent Transport Kinetics in Nanosheet-Based Battery Electrodes. Chem. Mater. 2020, 32, 1684–1962. [Google Scholar] [CrossRef]
- Cheng, H.-M.; Li, F. Charge delivery goes the distance. Science 2017, 356, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Li, X.; Haregewoin, A. M.; Sarang, K.; Lutkenhaus, J.; Kostecki, R.; Verduzco, R. A Comprehensive Study of Hydrolyzed Polyacrylamide as a Binder for Silicon Anodes. ACS Appl. Mater. Interfaces. 2019, 11, 44090–44100. [Google Scholar] [CrossRef]
- Woo, H.; Park, K.; Kim, J.; Yun, A. J.; Nam, S.; Park, B. 3D Meshlike Polyacrylamide Hydrogel as a Novel Binder System via in Situ Polymerization for High-Performance Si-Based Electrode. Adv. Mater. Interfaces. 2020, 7, 1901475. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Kim, H.-M.; Yoo, B.-I.; Yi, J.-W.; Choi, M.-J. Solvent-Free Fabrication of Thick Electrodes in Thermoplastic Binders for High Energy Density Lithium Ion Batteries. Nanomaterials. 2022, 12, 3320. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Danner, T.; Singh, M.; Hein, S.; Kaiser, J.; Hahn, H.; Latz, A. Thick electrodes for Li-ion batteries: A model based analysis, J. Power Sources 2016, 334, 191–201. [Google Scholar] [CrossRef]
- Park, K.Y.; Park, J.W.; Seong, W.; Yoon, K.; Hwang, T.H.; Ko, K.H.; Han, J.H.; Yang, J.; Kang, K. Understanding capacity fading mechanism of thick electrodes for lithium-ion rechargeable batteries, J. Power Sources 2020, 468, 228369. [Google Scholar] [CrossRef]
- Preman, A.; Lee, H.; Yoo, J.; Kim, I.; Saito, T.; Ahn, S.-K. Progress of 3D Network Binders in Silicon Anodes for Lithium Ion Batteries. J. Mater. Chem. A. 2012, 00, 1–3. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Zhang, G.; Yang, Z.; Chen, Y.; Deng, Y.; Yang, Y.; Wang, C. Water-based dual-network conductive polymer binders for high-performance Li–S batteries. Electrochim. Acta. 2021, 371, 137822. [Google Scholar] [CrossRef]
- So, Y.; Bae, H.-S.; Kang, Y.; Chung, J.; Park, N.; Kim, J.; Jung, H.-T.; Won, J.; Ryou, M.-H.; Kim, Y. Eco-Friendly Water-Processable Polyimide Binders with High Adhesion to Silicon Anodes for Lithium-Ion Batteries. Nanomaterials 2021, 11, 3164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, L.; Li, L. Ion exchange membranes as electrolyte to improve high temperature capacity retention of LiMn2O4 cathode Lithium Ion Batteries. Chem. Commun. 2012, 48, 9858–9860. [Google Scholar] [CrossRef] [PubMed]
- Komoda, Y.; Ishibashi, K.; Kuratani, K.; Suzuki, K.; Ohmura, N.; Kobayashi, H. Effects of drying rate and slurry microstructure on the formation process of LIB cathode and electrochemical properties. J. Power Sources 2023, 568, 232983. [Google Scholar] [CrossRef]
- Zhang, H.X.; Min, Z.R.; Ming, Q.Z. Dynamically Cross-Linked Polymeric Binder-Made Durable Silicon Anode of a Wide Operating Temperature Li-Ion Battery. ACS Appl. Mater. Interfaces. 2021, 13, 28737–28748. [Google Scholar]
- Zhang, Z.; Zeng, T.; Lai, Y.; Jia, M.; Li, J. A comparative study of different binders and their effects on electrochemical properties of LiMn2O4 cathode in lithium ion batteries. J. Power Sources 2014, 247, 1–8. [Google Scholar] [CrossRef]
- Pham, H.-P.; Kim, G.; Jung, H.-M.; Song, S.-W. Fluorinated Polyimide as a Novel High-Voltage Binder for High-Capacity Cathode of Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1704690. [Google Scholar] [CrossRef]
- Li, J.-T.; Wu, Z.Y.; Lu, Y.-Q.; Zhou, Y.; Huang, Q.-S.; Sun, S.-G. Water Soluble Binder, an Electrochemical Performance Booster for Electrode Materials with High Energy Density. Adv. Energy Mater. 2017, 7, 1701185. [Google Scholar] [CrossRef]
- Nguyen, V.; Kuss, C. Review—Conducting Polymer-Based Binders for Lithium-Ion Batteries and Beyond. J. Electrochem. Soc. 2020, 167, 065501. [Google Scholar] [CrossRef]
- Pasquier, A. D.; Disma, F.; Bowmer, T.; Gozdz, A. S.; Amatucci, G.; Tarascon, J.-M. Differential Scanning Calorimetry Study of the Reactivity of Carbon Anodes in Plastic Li-Ion Batteries. J. Electrochem. Soc. 1998, 145, 472–477. [Google Scholar] [CrossRef]
- Maleki, H.; Deng, G.; Kerzhner-Haller, I.; Anani, A.; Howard, J. N. Thermal Stability Studies of Binder Materials in Anodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 4470. [Google Scholar] [CrossRef]
- Liang, J.; Chen, D.; Adair, K.; Sun, Q.; Holmes, N. G.; Zhao Y.; Sun,Y.; Luo J.; Li, R; Zhang, Li. Zhao, S.; Lu, S.; Huang, H.; Zhang, X.; Singh, C. V.; Sun, X. Insight into Prolonged Cycling Life of 4 V All-Solid-State Polymer Batteries by a High-Voltage Stable Binder. Adv. Energy Mater. 2021, 11, 2002455.
- Zhang, L.; Wu, X.; Qian, W.; Pan, K.; Zhang, X.; Li, L.; Jia, M.; Zhang, S. Exploring More Functions in Binders for Lithium Batteries. Electrochem. Energy Rev. 2023, 6, 36. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, D.; Sun, M.; Liu, L.; Hu, W.; Jiang, B.; Chu, L.; Li, M. Polyethylene Oxide as a Multifunctional Binder for High-Performance Ternary Layered Cathodes. Polymers 2021, 13, 3992. [Google Scholar] [CrossRef]
- Pieczonka, N. P. W.; Borgel, V.; Ziv, B.; Leifer, N.; Dargel, V.; Aurbach, D.; Kim, J.-H.; Liu, Z.; Huang, X.; Krachkovskiy, S. A. Lithium Polyacrylate (LiPAA) as an Advanced Binder and a Passivating Agent for High-Voltage Li-Ion Batteries. Adv. Energy Mater. 2015, 5, 1501008. [Google Scholar] [CrossRef]
- Li, H.; Guan, C.; Zhang, J.; Cheng, K.; Chen, Q.; He, L.; Ge, X.; Lai, Y.; Sun, H.; Zhang, Z. Robust Artificial Interphases Constructed by a Versatile Protein-Based Binder for High-Voltage Na-Ion Battery Cathodes. Adv.Mater. 2022, 34, 2202624. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Deng, J.; Li, W.; Malyi, O.; Zhang, Y.; Zhou, X.; Pan, S.; Wei, J.; Cai, Y.; Chen, Z.; Chen, X. Water-Soluble Sericin Protein Enabling Stable Solid–Electrolyte Interphase for Fast Charging High Voltage Battery Electrode. Adv.Mater. 2017, 29, 1701828. [Google Scholar] [CrossRef]
- Luntz, A. C.; McCloskey, B. D. Nonaqueous Li–Air Batteries: A Status Report. Chem. Rev. 2014, 114, 11721–11750. [Google Scholar] [CrossRef]
- Younesi, R.; Hahlin, M.; Treskow, M.; Scheers, J.; Johansson, P.; Edström, K. Ether Based Electrolyte, LiB(CN)4 Salt and Binder Degradation in the Li–O2 Battery Studied by Hard X-Ray Photoelectron Spectroscopy (HAXPES). J. Phys. Chem. C. 2012, 116, 18597–18604. [Google Scholar] [CrossRef]
- Hernandez, C. R.; Etiemble, A.; Douillard, T.; Mazouzi, D.; Karkar, Z.; Maire, E.; Guyomard, D.; Lestriez, B.; RouéA, L. Facile and Very Effective Method to Enhance the Mechanical Strength and the Cyclability of Si-Based Electrodes for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701787. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Xu, Y.; Meng, Q.; Chou, S.-L.; Ma, J.; Kang, Y.-M.; Liu, H.-K. Chemically Bonded Sn Nanoparticles Using the Crosslinked Epoxy Binder for High Energy-Density Li Ion Battery. Adv. Mater. Interfaces. 2016, 3, 1600662. [Google Scholar] [CrossRef]
- Liu, Z.; Han, S.; Xu, C.; Luo, Y.; Peng, N.; Qin, C.; Zhou, M.; Wang, W.; Chen, L.; Okada, S. In Situ Crosslinked PVA–PEI Polymer Binder for Long-Cycle Silicon Anodes in Li-Ion Batteries. RSC Adv. 2016, 6, 68371–68378. [Google Scholar] [CrossRef]
- Liu, W.-R.; Yang, M.-H.; Wu, H.-C.; Chiao, S. M.; Wu, N.-L. Enhanced Cycle Life of Si Anode for Li-Ion Batteries by Using Modified Elastomeric Binder. Electrochem. Solid-State Lett. 2004, 8, A100. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.; Kim, S.-H.; Jang, B.; Kim, J.; Oh, S.; Kim, J.-Y.; Choi, N.-S.; Lee, K.; Kim, B.-S. A photo-cross-linkable polymeric binder for silicon anodes in lithium ion batteries. RSC Adv. 2013, 3, 12625–12630. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, L.; Peng, X.; Liu, T.; Jiang, Y.; Qin, F.; Hu, L.; Chu, P.; Huo, K.; Zhou, Y. Enhanced Ion Conductivity in Conducting Polymer Binder for High-Performance Silicon Anodes in Advanced lithium-ion Batteries. Adv. Energy Mater. 2018, 8, 1702314. [Google Scholar] [CrossRef]
- Hitomi, S.; Kubota, K.; Horiba, T.; Hida, K.; Matsuyama, T.; Oji, H.; Yasuno, S.; Komaba, S. Application of acrylic-rubber-based latex binder to high-voltage spinel electrodes of Lithium-Ion Batteries. ChemElectroChem. 2019, 6, 5070–5079. [Google Scholar] [CrossRef]
- Jin, B.; Wang, D.; Song, L.; Cai, Y.; Ali, A.; Hou, Y.; Chen, J.; Zhang, Q.; Zhan, X. Biomass-derived fluorinated corn starch emulsion as binder for silicon and silicon oxide based anodes in lithium-ion Batteries. Electrochim. Acta. 2021, 365, 137359. [Google Scholar] [CrossRef]
- Gordon, R.; Kassar, M.; Willenbacher, N. Effect of Polymeric Binders on Dispersion of Active Particles in Aqueous LiFePO4-Based Cathode Slurries as well as on Mechanical and Electrical Properties of Corresponding Dry Layers. ACS Omega. 2020, 5, 11455–11465. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Lin, Y.-S. Interactions between organic additives and active powders in water-based lithium iron phosphate electrode slurries. J. Power Sources 2012, 220, 413–421. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Conveying Advanced Li-ion Battery Materials into Practice The Impact of Electrode Slurry Preparation Skills. Adv. Energy Mater. 2016, 6, 1600655. [Google Scholar] [CrossRef]
- Ransil, A.; Belcher, A. Structural ceramic batteries using an earth-abundant inorganic waterglass binder. Nat. Commun. 2021, 12, 6494. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, J.; Gao, Y.; Wang, X.; Zhang, Y.; Zhang, S. A water-soluble, adhesive and 3D cross-linked polyelectrolyte binder for high-performance lithium–sulfur batteries. J. Mater. Chem. A. 2021, 9, 2375–2384. [Google Scholar] [CrossRef]
- Vogl, U. S.; Das, P. K.; Weber, A. Z.; Winter, M.; Kostecki, R.; Lux, S.F. Mechanism of Interactions between CMC Binder and Si Single Crystal Facets. Langmuir. 2014, 30, 10299–10307. [Google Scholar] [CrossRef]
- Shi, Q.; Wong, S.-C.; Ye, W.; Hou, J.; Zhao, J.; Yin, J. Mechanism of Adhesion between Polymer Fibers at Nanoscale Contacts. Langmuir. 2012, 28, 4663–4671. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Frank, C.; Mori, S. Interaction of Poly(vinylidene fluoride) with Graphite Particles. Surface Morphology of a Composite Film and Its Relation to Processing Parameters. Chem. Mater. 2003, 15, 850–861. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Tan, Q.; Wan, Q.; Han, K.; Liu, Z.; Li, P.; An, F.; Qu, X. A synergetic strategy for an advanced electrode with Fe3O4 embedded in a 3D N-doped porous graphene framework and a strong adhesive binder for lithium/potassium ion batteries with an ultralong cycle lifespan. J. Mater. Chem. A. 2019, 7, 19430–19441. [Google Scholar] [CrossRef]
- Yao, D.; Feng, J.; Wang, J.; Deng, Y.; Wang, C. Synthesis of silicon anode binders with ultra-high content of catechol groups and the effect of molecular weight on battery performance. J. Power Sources 2020, 463, 228188. [Google Scholar] [CrossRef]
- Libao, C.; Xiaohua, X.; Jingying, X.; Ke, W.; Jun, Y. Binder effect on cycling performance of silicon/carbon composite anodes for lithium ion batteries. J. Appl. Electrochem. 2006, 36, 1099–1104. [Google Scholar]
- Jeong, Y.; Choi, J. Mussel-inspired self-healing metallopolymers for silicon nanoparticle anodes. ACS Nano, 2019, 13, 8364–8373. [Google Scholar] [CrossRef] [PubMed]
- Yuca, N.; Cetintasoglu, M.; Dogdu, M.; Akbulut, H.; Tabanli, S.; Colak, U.; Taskin, O. Highly efficient poly(fluorene phenylene) copolymer as a new class of binder for high-capacity silicon anode in LIBs. Int J Energy Res. 2018, 42, 1148–1157. [Google Scholar] [CrossRef]
- Liu, T.; Chu, Q.; Yan, C.; Zhang, S.; Lin, Z.; Lu, J. Interweaving 3D network binder for high-areal-capacity Si anode through combined hard and soft polymers. Adv. Energy Mater. 2019, 9, 1802645. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.-T.; Liu, J.; Deng, Y.-P.; Wu, Z.-G.; Yin, Z.-W.; Guo, D.; Huang, L.; Sun, S.-G. Suppressing the voltage-fading of layered lithium-rich cathode materials via an aqueous binder for Li-ion batteries. Chem. Commun. 2016, 52, 4683–4686. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.; Jeong, J.; Cho, K. Y.; Ryou, M.-H.; Lee, Y. Highly Adhesive and Soluble Copolyimide Binder: Improving the Long-Term Cycle Life of Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces. 2015, 7, 14851–14858. [Google Scholar] [CrossRef] [PubMed]
- Saal, A.; Hagemann, T.; Schubert, U. S. Polymers for Battery Applications—Active Materials, Membranes, and Binders. Adv. Energy Mater. 2021, 11, 2001984. [Google Scholar] [CrossRef]
- Gupta, B. S.; Reiniati, I.; Laborie, M.-P. G. Surface Properties and Adhesion of Wood Fiber Reinforced Thermoplastic Composites. Colloids Surf. Physicochem. Eng. Asp. 2007, 302, 388–395. [Google Scholar] [CrossRef]
- Shin, D.; Park, H.; Paik, U. Cross-Linked Poly(Acrylic Acid)-Carboxymethyl Cellulose and Styrene-Butadiene Rubber as an Efficient Binder System and Its Physicochemical Effects on a High Energy Density Graphite Anode for Li-Ion Batteries. Electrochem. Commun. 2017, 77, 103–106. [Google Scholar] [CrossRef]
- Liu, J.; Galpaya, D. G. D.; Yan, L.; Sun, M.; Lin, Z.; Yan, C.; Liang, C.; Zhang, S. Exploiting a Robust Biopolymer Network Binder for an Ultrahigh-Areal-Capacity Li–S Battery. Energy Environ. Sci. 2017, 10, 750–755. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Z.; Kang, Y.; Zhou, Y.; Li, Y.; He, X.; Wang, L.; Mai, W.; Wang, X.; Zhou, G.; Wang, J.; Li, J.; Tavajohi, N.; Li, B. Rational design of functional binder systems for high-energy lithium-based rechargeable batteries. Energy Storage Mater. 2021, 35, 353–377. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, Y.; Wang, H.; Aboalhassan, A.; Li, G.; Xu, G.; Xue, C.; Yu, J.; Yan, J.; Ding, B. Highly Elastic Block Copolymer Binders for Silicon Anodes in lithium-Ion Batteries. ACS Appl. Mater. Interfaces. 2020, 12, 38132–38139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.; Jin, B.; Peng, R. A new linear heptafluoro glycidyl ether binder: synthesis, characterization, and mechanical properties. Macromol. Res. 2023, 31, 699–709. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science. 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Kim, J. S.; Choi, W.; Cho, K. Y.; Byun, D.; Lim, J.; Lee, J. K. Effect of Polyimide Binder on Electrochemical Characteristics of Surface-Modified Silicon Anode for lithium ion Batteries. J. Power Sources 2013, 244, 521–526. [Google Scholar] [CrossRef]
- Yao, D.; Yang, Y.; Deng, Y.; Wang, C. Flexible Polyimides through One-Pot Synthesis as Water-Soluble Binders for Silicon Anodes in Lithium Ion Batteries. J. Power Sources 2018, 379, 26–32. [Google Scholar]
- Ma, L.; Meng, J.-Q.; Cheng, Y.-J.; Ji, Q.; Zuo, X.; Wang, X.; Zhu, J.; Xia, Y. Poly(Siloxane Imide) Binder for Silicon-Based Lithium-Ion Battery Anodes via Rigidness/Softness Coupling. Chem. – Asian J. 2020, 15, 2674–2680. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Y.; Gong, C.-R.; Zheng, Y.-Q.; Zhou, Z.-X.; Yu, L.-M. An ultraviolet curable silicon/graphite electrode binder for long-cycling lithium ion batteries. J. Power Sources 2021, 485, 229348. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, S.; Xie, M.; Zheng, Y.; Xu, G.; Gao, S.; Li, Jian.; Liu, Z.; Liu, X.; Liu, J.; Yan, B.; Yan, W.; Zhang, Z.; Cui, G. A delicately designed functional binder enabling in situ construction of 3D cross-linking robust network for high-performance Si/graphite composite anode. J. Polym. Sci. 2022, 60, 1835–1844.
- Jin, B.; Wang, D.; Song, L.; Cai, Y.; Ali, A.; Hou, Y.; Chen, J.; Zhang, Q.; Zhan, X. Biomass-derived fluorinated corn starch emulsion as binder for silicon and silicon oxide based anodes in lithium-ion batteries. Electrochim. Acta. 2021, 365, 137359. [Google Scholar] [CrossRef]
- Cao, P.-F.; Naguib, M.; Du, Z.; Stacy, E.; Li, B.; Hong, T.; Xing, K.; Voylov, D.; Li, J.; Wood III, D.; Sokolov, A.; Nanda, J.; Saito, T. Effect of Binder Architecture on the Performance of Silicon/Graphite Composite Anodes for Lithium Ion Batteries. ACS Appl. Mater. Interfaces. 2018, 10, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, F.; Xie, Y.; Xu, X.; Li, F.; Han, X.; Yao, X.; Wang, D.; Hou, Y.; Gao, X.; He, Q.; Lu, J.; Zhan, X.; Zhang, Q. In situ interlocked gradient adaptive network binder with robust adhesion and cycle performance for silicon anodes. J. Power Sources 2023, 580, 233267. [Google Scholar] [CrossRef]
- Ahn, J.; Im, H.-G.; Lee, Y.; Lee, D.; Jang, H.; Oh, Y.; Chung, K.; Park, T.; Um, M.-K.; Yi, J.; Kim, J.; Kang, D.; Yoo, J.-K. A novel organosilicon-type binder for LiCoO2 cathode in Li-ion batteries. Energy Storage Mater. 2022, 49, 58–66. [Google Scholar] [CrossRef]
- Jenkins, C.; Coles, S.; Loveridge, M. J. Investigation into Durable Polymers with Enhanced Toughness and Elasticity for Application in Flexible Li-Ion Batteries. ACS Appl. Polym. Energy Mater. 2020, 3, 12494–12505. [Google Scholar] [CrossRef]
- Yim, T.; Choi, S. J.; Park, J.-H.; Cho, W.; Jo, Y. N.; Kim, T.-H.; Kim, Y.-J. The Effect of an Elastic Functional Group in a Rigid Binder Framework of Silicon–Graphite Composites on Their Electrochemical Performance. Phys. Chem. Chem. Phys. 2014, 17, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Verdier, N.; El Khakani, S.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M.; Rochefort, D. Polyacrylonitrile-Based Rubber (HNBR) as a New Potential Elastomeric Binder for Lithium-Ion Battery Electrodes. J. Power Sources 2019, 440, 227111. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Chen, Y.; Huang, J.; Zhang, T.; Zeng, H.; Wang, C.; Liu, G.; Deng, Y. A Quadruple-Hydrogen-Bonded Supramolecular Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. Small. 2018, 14, 1801189. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Jeong, Y. K.; Deniz, E.; AlQaradawi, S. Y.; Choi, J. W.; Coskun, A. Dynamic Cross-Linking of Polymeric Binders Based on Host–Guest Interactions for Silicon Anodes in Lithium Ion Batteries. ACS Nano. 2015, 9(11), 11317–11324. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, J.-S.; Noh, J.; Lee, I.; Kim, H. J.; Choi, S.; Seo, J.; Jeon, S.; Kim, T.-S.; Lee, J.-Y.; Choi, J. W. Wearable Textile Battery Rechargeable by Solar Energy. Nano Lett. 2013, 13, 5753–5761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Chai, L.; Xue, P.; Hao, W.; Zheng, H. A Coordinatively Cross-Linked Polymeric Network as a Functional Binder for High-Performance Silicon Submicro-Particle Anodes in Lithium-ion Batteries. J. Mater. Chem. A 2014, 2, 19036–19045. [Google Scholar] [CrossRef]
- Song, J.; Zhou, M.; Yi, R.; Xu, T.; Gordin, M. L.; Tang, D.; Yu, Z.; Regula, M.; Wang, D. Interpenetrated Gel Polymer Binder for High-Performance Silicon Anodes in Lithium-ion Batteries. Adv. Funct. Mater. 2014, 24, 5904–5910. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Zhang, T.; Nuli, Y.; Wang, J.; Hirano, S. Silicon Microparticle Anodes with Self-Healing Multiple Network Binder. Joule. 2018, 2, 950–961. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, S.; Zhu, G.; Yuan, Y.; Qu, Q.; Wang, Y.; Zheng, H. The Effects of Cross-Linking Cations on the Electrochemical Behavior of Silicon Anodes with Alginate Binder. Electrochimica Acta. 2018, 269, 405–414. [Google Scholar] [CrossRef]
- Kim, S.; Han, D.-Y.; Song, G.; Lee, J.; Park, T.; Park, S. Resilient binder network with enhanced ionic conductivity for High-Areal-Capacity Si-based anodes in Lithium-Ion batteries. Chem. Eng. J. 2023, 473, 145441. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Huang, Y.; He, X.; Zhuo, H.; Chen, S. Rational Design of Effective Binders for LiFePO4 Cathodes. Polymers. 2021, 13, 3146. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Yin, J.; Wang, H.; Yuan, X.; Li, Q. Effect of Different Binders on Electrochemical Properties of LiFePO4/C Cathode Material in lithium ion Batteries. Chem. Eng. J. 2014, 237, 497–502. [Google Scholar] [CrossRef]
- Olmedo-Martínez, J. L.; Meabe, L.; Basterretxea, A.; Mecerreyes, D.; Müller, A. J. Effect of Chemical Structure and Salt Concentration on the Crystallization and Ionic Conductivity of Aliphatic Polyethers. Polymers. 2019, 11, 452. [Google Scholar] [CrossRef]
- Shetty, S. K.; Ismayil; Noor, I. M. Effect of New Crystalline Phase on the Ionic Conduction Properties of Sodium Perchlorate Salt Doped Carboxymethyl Cellulose Biopolymer Electrolyte Films. J. Polym. Res. 2021, 28, 415. [CrossRef]
- He, R.; Kyu, T. Effect of Plasticization on Ionic Conductivity Enhancement in Relation to Glass Transition Temperature of Crosslinked Polymer Electrolyte Membranes. Macromolecules. 2016, 49, 5637–5648. [Google Scholar] [CrossRef]
- Han, L.; Lehmann, M. L.; Zhu, J.; Liu, T.; Zhou, Z.; Tang, X.; Heish, C.-T.; Sokolov, A. P.; Cao, P.; Chen, X. C.; Saito, T. Recent Developments and Challenges in Hybrid Solid Electrolytes for Lithium-ion Batteries. Front. Energy Res. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Nam, J.; Kim, E.; K.K, R.; Kim, Y.; Kim, T.-H. A conductive self healing polymeric binder using hydrogen bonding for Si anodes in lithium ion batteries. Sci Rep 2020, 10, 14966. [CrossRef] [PubMed]
- Koo, B.; Kim, H.; Cho, Y.; Lee, K. T.; Choi, N.-S.; Cho, J. A Highly Cross-Linked Polymeric Binder for High-Performance Silicon Negative Electrodes in Lithium Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 8762–8767. [Google Scholar] [CrossRef]
- Salem, D. N.; Lavrisa, M.; Abu-Lebdeh, D. Y. Ionically-Functionalized Poly(thiophene) Conductive Polymers as Binders for Silicon and Graphite Anodes for Li-Ion Batteries. Energy Technol. 2016, 4, 331–340. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.; Fuller, T.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: polyacrylic acid. ACS Appl. Mater. Interfaces. 2010, 2, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Aoki, S.; Horiba, T.; Schulz-Dobrick, M.; Han, Z.-J.; Fukuyama, S.; Oji, H.; Yasuno, S.; Komaba, S. “Natto” Binder of Poly-γ-glutamate Enabling to Enhance Silicon/Graphite Composite Electrode Performance for Lithium-ion Batteries. ACS Sustainable Chem. Eng. 2017, 5, 6343–6355. [Google Scholar] [CrossRef]
- Sivaraj, P.; Abhilash, K. P.; Nalini, B.; Perumal, P.; Selvin, P. C. Performance Enhancement of PVDF/LiClO4 Based Nanocomposite Solid Polymer Electrolytes via Incorporation of Li0.5La0.5TiO3 Nano Filler for All-Solid-State Batteries. Macromol. Res. 2020, 28, 739–750. [Google Scholar] [CrossRef]
- He, J.; Zhong, H.; Wang, J.; Zhang, L. Investigation on xanthan gum as novel water soluble binder for LiFePO4 cathode in Lithium-ion Batteries. J. Alloy. Compd. 2017, 714, 409–418. [Google Scholar] [CrossRef]
- Virya, A.; Lian, K. Lithium polyacrylate-polyacrylamide blend as polymer electrolytes for solid- state electrochemical capacitors. Electrochem. Commun. 2018, 97, 77–81. [Google Scholar] [CrossRef]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; D’Souza, M.; Doux, J.-M.; Wu, E. A.; Trieu, O. Y.; Gong, Y.; Zhou, Q.; Snyder, K.; Meng, Y. Role of Polyacrylic Acid (PAA) Binder on the Solid Electrolyte Interphase in Silicon Anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Lux, S. F.; Schappacher, F.; Balducci, A.; Passerini, S.; Winter, M. Low Cost, Environmentally Benign Binders for Lithium-ion Batteries. J. Electrochem. Soc. 2010, 157, A320. [Google Scholar] [CrossRef]
- Su, T.-T.; Ren, W.-F.; Wang, K.; Yuan, J.-M.; Shao, C.-Y.; Ma, J.-L.; Chen, X.-H.; Xiao, L.-P.; Sun, R.-C. Bifunctional Hydrogen-Bonding Cross-Linked Polymeric Binders for Silicon Anodes of Lithium-ion Batteries. Electrochim. Acta. 2022, 402, 139552. [Google Scholar] [CrossRef]
- Lee, K.; Lim, S.; Go, N.; Kim, J.; Mun, J.; Kim, T.-H. Dopamine-grafted heparin as an additive to the commercialized carboxymethyl cellulose/styrene-butadiene rubber binder for practical use of SiOx/graphite composite anode. Sci Rep. 2018, 8, 11322. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yuan, L.-X.; Zhang, W.-X.; Hu, X.-L.; Huang, Y.-H. Enhanced Cyclability for Sulfur Cathode Achieved by a Water-Soluble Binder. J. Phys. Chem. C. 2011, 115, 15703–15709. [Google Scholar] [CrossRef]
- Kim, J.; Ma, H.; Cha, H.; Lee, H.; Sung, J.; Seo, M.; Oh, P.; Park, M.; Cho, J. A highly stabilized nickel-rich cathode material by nanoscale epitaxy control for high-energy Lithium-ion Batteries. Energy Environ. Sci. 2018, 11, 1449–1459. [Google Scholar] [CrossRef]
- Colomer, M.; Roa, C.; Ortiz, A.; Ballesteros, L.; Molina, P. Influence of Nd3+ Doping on the Structure, Thermal Evolution and Photoluminescence Properties of Nanoparticulate TiO2 Xerogels. J. Alloy. Compd. 2020, 819, 152972. [Google Scholar] [CrossRef]
- Hong, C.; Leng, Q.; Zhu, J.; Zheng, S.; He, S.; Li, Y.; Liu, R.; Wan, J.; Yang, Y. Revealing the correlation between structural evolution and Li+ diffusion kinetics of nickel-rich cathode materials in Li-ion batteries. J. Mater. Chem. A 2020, 8, 8540–8547. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, T.; Li, D.; Wang, K.; Wei, X.; Liu, S. High-Performance Aqueous Sodium-Ion Battery Based on Graphene-Doped Na2MnFe(CN)6−Zinc with a Highly Stable Discharge Platform and Wide Electrochemical Stability. Energy Fuels. 2021, 35, 10860–10868. [Google Scholar] [CrossRef]
- Ha, S.; Yoon, H.; Jung, J.; Kim, H.; Won, S.; Kwak, J.; Lim, H.; Jin, H.-J.; Wie, J.; Yun, Y. 3D-structured organic-inorganic hybrid solid-electrolyte-interface layers for Lithium metal anode. Energy Storage Mater. 2021, 37, 567–575. [Google Scholar] [CrossRef]
- Moon, S.; Kim, D.; Kwak, J.; Lee, S.; Lim, H.; Kang, K.; Jin, H.-J.; Yun, Y. Unveiling the pseudocapacitive effects of ultramesopores on nanoporous carbon. Appl. Surf. Sci. 2021, 537, 148037. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, M.; Kim, N.; Yang, S.; Jin, H.-J.; Yun, Y. Hierarchically nanoporous pyropolymer nanofibers for surface-induced sodium-ion storage. Electrochim. Acta. 2017, 242, 38–46. [Google Scholar] [CrossRef]
- Taylor, M.; Clarkson, D.; Greenbaum, S.; Panzer, M. Examining the Impact of Polyzwitterion Chemistry on Lithium Ion Transport in Ionogel Electrolytes. ACS Appl. Polym. Mater. 2021, 3, 2635–2645. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-ion Batteries. Angew. Chem.-Int. Edit. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Sethuraman, V. A.; Nguyen, A.; Chon, M. J.; Nadimpalli, S. P. V.; Wang, H.; Abraham, D. P.; Bower, A. F.; Shenoy, V. B.; Guduru, P. R. Stress Evolution in Composite Silicon Electrodes during Lithiation/Delithiation. J. Electrochem. Soc. 2013, 160, A739–A746. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.; Sun, Y. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Silicon-Graphite Composite for Lithium-Ion Batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Choi, S.; Kang, J.; Ryu, J.; Park, S. Revisiting Classical Rocking Chair Lithium-Ion Battery. Macromol. Res. 2020, 28, 1175–1191. [Google Scholar] [CrossRef]
- Heubner, C.; Langklotz, U.; Michaelis, A. Theoretical optimization of electrode design parameters of Si based anodes for lithium-ion batteries. J. Energy Storage. 2018, 15, 181–190. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.; Wu, Ji. Silicon-Based Nanomaterials for Lithium-ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy. 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Du, Z.; Dunlap, R.; Obrovac, M. High Energy Density Calendered Si Alloy/Graphite Anodes. J. Electrochem. Soc. 2014, 161, A1698–A1705. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, Y.; Wang, Y.; Lee, H.; Choi, J. A “Sticky” Mucin-Inspired DNA-Polysaccharide Binder for Silicon and Silicon–Graphite Blended Anodes in Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1707594. [Google Scholar] [CrossRef] [PubMed]
- Gendensuren, B.; Oh, E. Dual-crosslinked network binder of alginate with polyacrylamide for silicon/graphite anodes of lithium ion battery. J. Power Sources 2018, 384, 379–386. [Google Scholar] [CrossRef]
- Gendensure, B.; Sugartseren, N.; Kim, M.; Oh, E.-S. Incorporation of aniline tetramer into alginate-grafted-polyacrylamide as polymeric binder for high-capacity silicon/graphite anodes. Chem. Eng. J. 2022, 433, 133553. [Google Scholar] [CrossRef]
- Fang, C.; Xiao, H.; Zheng, T.; Bai, H.; Liu, G. Organic Solvent Free Process to Fabricate High Performance Silicon/Graphite Composite Anode. J. Compos. Sci. 2021, 5, 188. [Google Scholar] [CrossRef]
- Armstrong, B.; Hays, K.; Ruther, R.; Hawley, W. B.; Rogers, A.; Greeley, I.; Cavallaro.; Veith, G. M. Role of silicon-graphite homogeneity as promoted by low molecular weight dispersants. J. Power Sources 2022, 517, 230671. [CrossRef]
- Zhao, X.; Yim, C.-H.; Du, N.; Abu-Lebdeh, Y. Shortly Branched, Linear Dextrans as Efficient Binders for Silicon/Graphite Composite Electrodes in Li-Ion Batteries. Ind. Eng. Chem. Res. 2018, 57, 9062–9074. [Google Scholar] [CrossRef]
- Yu, X.; Yang, H.; Meng, H.; Sun, Y.; Zheng, J.; Ma, D.; Xu, X. Three-Dimensional Conductive Gel Network as an Effective Binder for High-Performance Si Electrodes in Lithium-ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 15961–15967. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, Y.; Yang, C.; Sun, H.; Miao, X.; Ji, H.; Yang, G. A flexible network polyimides binder for Si/graphite composite electrode in lithium-ion batteries. Int J Energy Res. 2022, 46, 18100–18108. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Chen, C.; Huang, T.; Yu, A. Natural sesbania gum as an efficient biopolymer binder for high-performance Si-based anodes in lithium-ion batteries. J. Power Sources 2022, 539, 231604. [Google Scholar] [CrossRef]
- Hays, K. A.; Ruther, R. E.; Kukay, A. J.; Cao, P.; Saito, T.; Wood III, D. L.; Li, J. What makes lithium substituted polyacrylic acid a better binder than polyacrylic acid for silicon-graphite composite anodes? J. Power Sources 2018, 384, 136–144. [Google Scholar] [CrossRef]
- Sun, S.; He, D.; Li, P.; Liu, Y.; Wan, Q.; Tan, Q.; Liu, Z.; An, F.; Gong, G.; Qu, X. Improved Adhesion of Cross-Linked Binder and SiO2-Coating Enhances Structural and Cyclic Stability of Silicon Electrodes for Lithium-ion Batteries. J. Power Sources 2020, 454, 227907. [Google Scholar] [CrossRef]
- Hu, B.; Shkrob, I. A.; Zhang, S.; Zhang, L.; Zhang, J.; Li, Y.; Liao, C.; Zhang, Z.; Lu, W.; Zhang, L. The Existence of Optimal Molecular Weight for Poly(Acrylic Acid) Binders in Silicon/Graphite Composite Anode for Lithium-ion Batteries. J. Power Sources 2018, 378, 671–676. [Google Scholar] [CrossRef]
- Li, H.; Peng, L.; Wu, D.; Wu, J.; Zhu, Y.J.; Hu, X. Ultrahigh-capacity and fire-resistant LiFePO4-based composite cathodes for advanced lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1802930. [Google Scholar] [CrossRef]
- Pham, H. Q.; Lee, J.; Jung, H.; Song, S.-W. Non-flammable LiNi0.8Co0.1Mn0.1O2 cathode via functional binder; stabilizing high-voltage interface and performance for safer and high-energy lithium rechargeable batteries. Electrochim. Acta. 2019, 317, 711–721. [Google Scholar] [CrossRef]
- Dong, T.; Mu, P.; Zhang, S.; Zhang, H.; Liu, W.; Cui, G. How Do Polymer Binders Assist Transition Metal Oxide Cathodes to Address the Challenge of High-Voltage Lithium Battery Applications? Electrochem. Energy Rev. 2021, 4, 545–565. [Google Scholar] [CrossRef]
- Kim, N-Y.; Moon, J.; Ryou, M-H.; Kim, S-H.; Kim, J-H.; Kim, J-M.; Bang, J.; Lee, S-Y. Amphiphilic Bottlebrush Polymeric Binders for High-Mass-Loading Cathodes in Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2102109. [CrossRef]
- Geldasa, F. T.; Kebede, M. A.; Shura, M. W.; Hone, F. G. Identifying Surface Degradation, Mechanical Failure, and Thermal Instability Phenomena of High Energy Density Ni-Rich NCM Cathode Materials for Lithium-ion Batteries: A Review. RSC Adv. 2022, 12, 5891–5909. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Danilov, D. L.; Eichel, R. -A.; Notten, P. H. L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005. [CrossRef]
- Guerfi, A.; Kaneko, M.; Petitclerc, M.; Mori, M.; Zaghib, K. LiFePO4 Water-Soluble Binder Electrode for Li-Ion Batteries. J. Power Sources 2007, 163, 1047–1052. [Google Scholar] [CrossRef]
- Bi, H.; Huang, F.; Tang, Y.; Liu, Z.; Lin, T.; Chen, J.; Zhao, W. Study of LiFePO4 cathode modified by graphene sheets for high-performance lithium ion batteries. Electrochim. Acta. 2013, 88, 414–420. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-Based Nanomaterials for Energy Applications. Small. 2017, 13, 1702240. [Google Scholar] [CrossRef]
- Jeong, S.S.; Böckenfeld, N.; Balducci, A.; Winter, M.; Passerini, S. Natural cellulose as binder for lithium battery electrodes. J. Power Sources 2012, 199, 331–335. [Google Scholar] [CrossRef]
- Yoon, J.; Han, G.; Cho, S.; Lee, C.; Lee, E.; Yoon, K.; Jin, H.-J. Microbial-Copolyester-Based Eco-Friendly Binder for Lithium-Ion Battery Electrodes. ACS Appl. Polym. Mater. 2023, 5, 1199–1207. [Google Scholar] [CrossRef]
- Nowak, A.; Trzciński, K.; Zarach, Z.; Li, J.; Roda, D.; Szkoda, M. Poly(hydroxybutyrate-co-hydroxyvalerate) as a biodegradable binder in a negative electrode material for Lithium-Ion Batteries. Appl. Surf. Sci. 2022, 606, 154. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material – Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels. 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Courtel, F. M.; Niketic, S.; Duguay, D.; Abu-Lebdeh, Y.; Davidson, I. J. Water-Soluble Binders for MCMB Carbon Anodes for Lithium-Ion Batteries. J. Power Sources 2011, 196, 2128–2134. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kinoshita, Y.; Misaki, K.; Matsuyama, T.; Komaba, S. Electrochemical Properties of LiCoO2 Electrodes with Latex Binders on High-Voltage Exposure. J. Electrochem. Soc. 2015, 162, A538. [Google Scholar] [CrossRef]
- Jang, W.; K. K., R.; Thorat, G. M.; Kim, S.; Kang, Y.; Kim, T.-H. Lambda Carrageenan as a Water-Soluble Binder for Silicon Anodes in Lithium-ion batteries. ACS Sustain. Chem. Eng. 2022, 10, 12620–12629. [CrossRef]
- Zhang, S.; Ren, S.; Han, D.; Xiao, M.; Wang, S.; Meng, Y. Aqueous Sodium Alginate as Binder: Dramatically Improving the Performance of Dilithium Terephthalate-Based Organic Lithium Ion Batteries. J. Power Sources 2019, 438, 227007. [Google Scholar] [CrossRef]
- Lee, S. H.; Lee, J. H.; Nam, D. H.; Cho, M.; Kim, J.; Chanthad, C.; Lee, Y. Epoxidized Natural Rubber/Chitosan Network Binder for Silicon Anode in Lithium-Ion Battery. ACS Appl. Mater. Interfaces. 2018, 10, 16449–16457. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lee, S. H.; Cho, M.; Kim, J.; Lee, Y. Cross-Linked Chitosan as an Efficient Binder for Si Anode of Li-Ion Batteries. ACS Appl. Mater. Interfaces. 2016, 8, 2658–2665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, T.; Yu, A. A Carboxymethyl Vegetable Gum as a Robust Water Soluble Binder for Silicon Anodes in Lithium-Ion Batteries. J. Power Sources 2021, 489, 229530. [Google Scholar] [CrossRef]
- Ling, M.; Xu, Y.; Zhao, H.; Gu, X.; Qiu, J.; Li, S.; Wu, M.; Song, X.; Yan, C.; Liu, G.; Zhang, S. Dual-Functional Gum Arabic Binder for Silicon Anodes in lithium ion Batteries. Nano Energy. 2015, 12, 178–185. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Zhang, T.; Li, J.-T.; Huang, L.; Sun, S.-G. A Robust Ion-Conductive Biopolymer as a Binder for Si Anodes of Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 3599–3605. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Nie, Y.; Su, Z.; Long, Y.; Wen, Y.; Su, J. Cross-Linked β-CD-CMC as an Effective Aqueous Binder for Silicon-Based Anodes in Rechargeable Lithium-Ion Batteries. RSC Adv. 2022, 12, 5997–6006. [Google Scholar] [CrossRef]
- Jeong, Y. K.; Kwon, T.; Lee, I.; Kim, T.-S.; Coskun, A.; Choi, J. W. Hyperbranched β-Cyclodextrin Polymer as an Effective Multidimensional Binder for Silicon Anodes in Lithium Rechargeable Batteries. Nano Lett. 2014, 14, 864–870. [Google Scholar] [CrossRef]
- Pejovnik, S.; Dominko, R.; Bele, M.; Gaberscek, M.; Jamnik, J. Electrochemical Binding and Wiring in Battery Materials. J. Power Sources 2008, 184, 593–597. [Google Scholar] [CrossRef]
- Gaberšček, M.; Bele, M.; Drofenik, J.; Dominko, R.; Pejovnik, S. Improved Carbon Anode Properties: Pretreatment of Particles in Polyelectrolyte Solution. J. Power Sources 2001, 97–98, 67–69. [Google Scholar] [CrossRef]
- Dominko, R.; Gaberšček, M.; Bele, M.; Drofenik, J.; Skou, E. M.; Würsig, A.; Novák, P.; Jamnik, J. Understanding the Role of Gelatin as a Pretreating Agent for Use in Li-Ion Batteries. J. Electrochem. Soc. 2004, 151, A1058. [Google Scholar] [CrossRef]
- Luo, C.; Du, L.; Wu, W.; Xu, H.; Zhang, G.; Li, S.; Wang, C.; Lu, Z.; Deng, Y. Novel Lignin-Derived Water-Soluble Binder for Micro Silicon Anode in Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 12621–12629. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, X.; Huang, W.; Wang, Y.; Qu, Q.; Zheng, H. Dynamic Bonded Supramolecular Binder Enables High-Performance Silicon Anodes in Lithium-ion Batteries. J. Power Sources 2020, 463, 228208. [Google Scholar] [CrossRef]
- Yook, S.-H.; Kim, S.-H.; Park, C.-H.; Kim, D.-W. Graphite–Silicon Alloy Composite Anodes Employing Cross-Linked Poly(Vinyl Alcohol) Binders for High-Energy Density Lithium-ion Batteries. RSC Adv. 2016, 6, 83126–83134. [Google Scholar] [CrossRef]
- Liu, N.; He, W.; Liao, H.; Li, Z.; Jiang, J.; Zhang, X.; Dou, H. Polydopamine Grafted Cross-Linked Polyacrylamide as Robust Binder for SiO/C Anode toward High-Stability Lithium-Ion Battery. J. Mater. Sci. 2021, 56, 6337–6348. [Google Scholar] [CrossRef]
- Rolandi, A. C.; Pozo-Gonzalo, C.; Meatza, I.d.; Casado, N.; Mecerreyes, D.; Forsyth, M. Fluorine-Free Poly(ionic Liquid)s Binders for the Aqueous Processing of High-Voltage NMC811 Cathodes. Adv. Energy Sustain. Res. 2023, 4(12), 2300149. [Google Scholar] [CrossRef]
- Xia, Y.; Mathis, T.S.; Zhao, M.-Q.; Anasori, B.; Dang, A.; Zhou, Z.; Cho, H.; Gogotsi, Y.; Yang, S. Thickness-independent capacitance of vertically aligned liquid-crystalline MXenes. Nature 2018, 557, 409–412. [Google Scholar] [CrossRef]
- Shen, J. D.; Ke, S. Microstructural design considerations for Li-ion battery systems. Curr. Opin. Solid State Mat. Sci. 2012, 16, 153–162. [Google Scholar]
- Shi, J.-L.; Xiao, D.-D.; Ge, M.; Yu, X.; Chu, Y.; Huang, X.; Zhang, X.-D.; Yin, Y.-X.; Yang, X.-Q.; Guo, Y.-G.; Gu, L.; Wan, L.-J. High-Capacity Cathode Material with High Voltage for Li-Ion Batteries. Adv. Mater. 2018, 30, 1705575. [Google Scholar] [CrossRef]
- Park, S.-H.; King, P.J.; Tian, R.; Boland, C. S.; Coelho, J.; Zhang, C.; McBean. P.; McEvoy. N.; Kremer. M. P.; Daly. D.; Coleman J. N.; Nicolosi. V. High areal capacity battery electrodes enabled by segregated nanotube networks. Nat. Energy. 2019, 4, 560–567.
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.J.; Erdonmez, C.K.; Halloran, J.W.; Chiang, Y.M. Design of battery electrodes with dual-scale porosity to minimize tortuosity and maximize performance. Adv. Mater. 2013, 25, 1254–1258. [Google Scholar] [CrossRef]
- Wu, X.; Xia, S.; Huang, Y.; Hu, X.; Yuan. B.; Chen. S.; Yu, Y.; Liu, W. High-performance, low-cost, and dense-structure electrodes with high mass loading for lithium-ion batteries. Adv. Funct. Mater. 2019, 29, 1903961. [CrossRef]
- Ryu, M.; Hong, Y.-K.; Lee, S.-Y.; Park, J. Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef]
- Font, F.; Protas, B.; Richardson, G.; Foster, J.M. Binder migration during drying of lithium-ion battery electrodes: Modelling and comparison to experiment. J. Power Sources 2018, 393, 177–185. [Google Scholar] [CrossRef]
- Jaiser, S.; Müller, M.; Baunach, M.; Bauer, W.; Scharfer, P.; Schabel, W. Investigation of film solidification and binder migration during drying of Li-Ion battery anodes. J. Power Sources 2016, 318, 210–219. [Google Scholar] [CrossRef]
- Lombardo, T.; Ngandjong, A.C.; Belhcen, A.; Franco, A.A. Carbon-binder migration: A three-dimensional drying model for lithium-ion battery electrodes. Energy Storage Mater. 2021, 43, 337–347. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci Rep. 2016, 6, 23150. [Google Scholar] [CrossRef]
| Si/G ratio | Binder | Electrolyte | CE (%) | Cycle | Ref. |
|---|---|---|---|---|---|
| Si/G (10:90) | Ur | 1M LiPF6 EC/EMC/DMC (1:1:1) (3wt% FEC) |
99.6 | 400 | [73] |
| Si/G (2.5:97.5) | PAA-VTEO | 1M LiPF6 EC/DMC (1:1) (3wt% FEC) | 89.4 | 477 | [74] |
| Si/G (50:50) | reDNA/ALG | 1M LiPF6 EC/DEC (1:1) (5wt% FEC) |
99.1~99.6 | 300 | [125] |
| Si/G (19:57) | c-Alg-g-PAAm | 1.15M LiPF6 EC/DEC/DMC (3:5:2) (5wt% FEC, 2wt% VC, 5wt% LiBF4) |
72.8 | 100 | [126] |
| Si/G (15:73) | SSC4SA | 1M LiPF6 EC/DEC/FEC (1:1:0.2) | 99 | [45] | |
| 200 | |||||
| Si/G (15:73) | GC-g-LiPAA | 1.2M LiPF6 EC/DMC (3:7) (10wt% FEC) | 90.3 | 100 | [76] |
| Si/G (19:57) | Alg-g-PAMAT | 1.5M LiPF6 EC/DEC/DMC (3:5:2) (5wt% FEC, 2wt% VC, 0.4wt% LiBF4) |
56~62 (capacity retention) |
200 | [127] |
| Si/G (43:43) | PAA | 1M LiPF6 DMC/FEC (7:3) |
88~91 | 40 | [99] |
| Si/G (30:50) | Li-PGlu | 1M LiPF6 EC/DMC (1:1) (2v% FEC) | 73 | 30 | [100] |
| Si/G (15:73) | CMC/SBR=4:6(w/w) | 1.2M LiPF6 EC/DEC (3:7) (30wt% FEC) |
99.8~99.9 | 400 | [128] |
| Si/G (15:73) | LiPAA | 1.2M LiPF6 EC/EMC (3:7) (10wt% FEC) |
91 | 50 | [129] |
| Si/G (20:65) | LiPAA | 1M LiPF6 EC/DEC/FEC(3:6:1) | 79.1 | 50 | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





