Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Hemoglobins hierarhy
2.2. Cysteine residues in HbA and HbB sequences
2.2.1. HbA
2.2.2. HbB
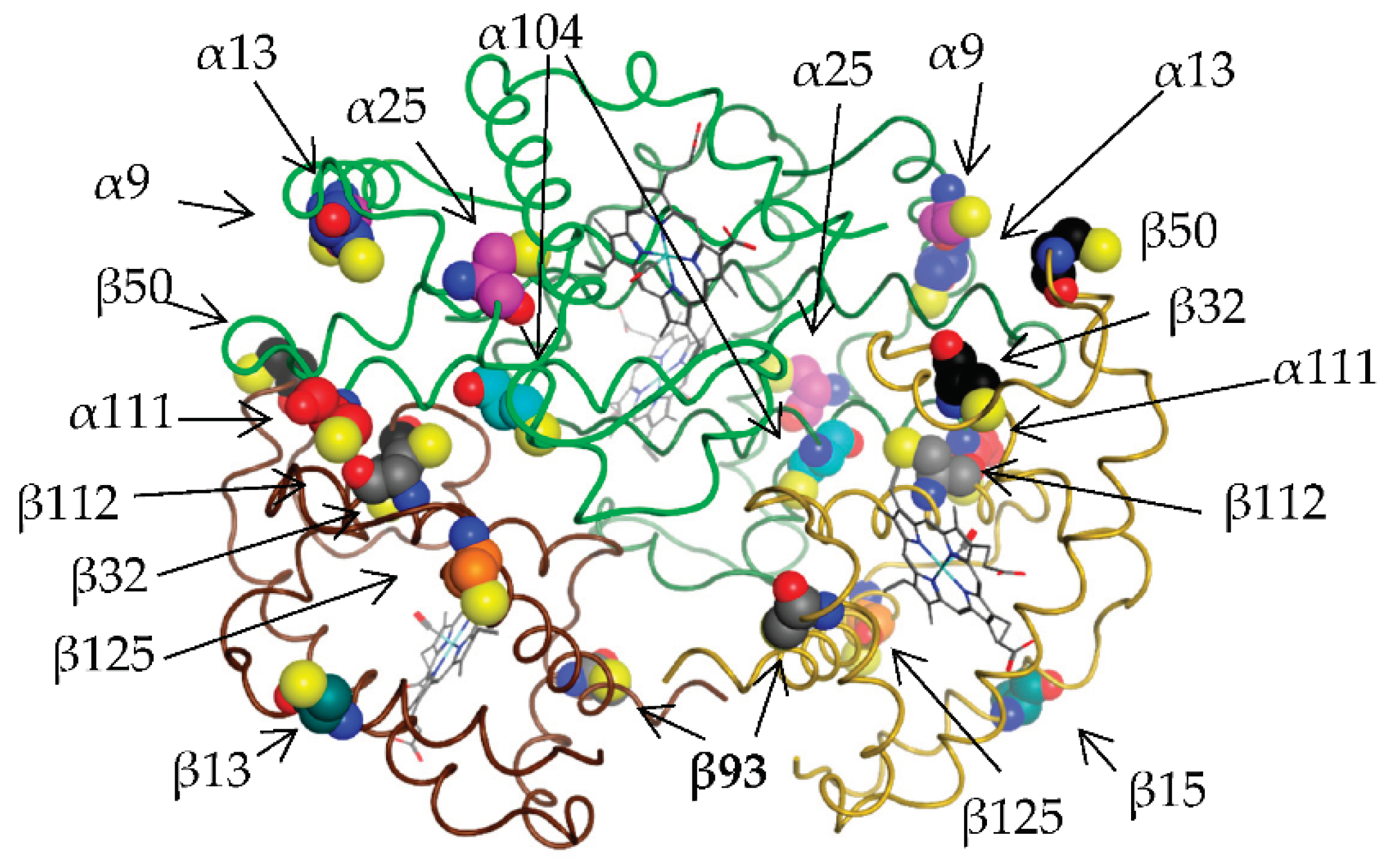
2.3. GSH binding pockets conservativity
3. Discussion
4. Materials and Methods
4.1. Hemoglobin sequences dataset
4.1.1. Alpha hemoglobins
4.1.2. Beta hemoglobins
4.1.3. Mu hemoglobins
4.2. Hemoglobin ASA measurement
4.3. Phylogenetic analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciaccio, C.; Coletta, A.; Coletta, M. Role of Hemoglobin Structural-Functional Relationships in Oxygen Transport. Mol. Aspects Med. 2022, 84, 101022. [Google Scholar] [CrossRef]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P. A Single–Cell Type Transcriptomics Map of Human Tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Wood, W.G. Haemoglobin Synthesis during Human Fetal Development. Br. Med. Bull. 1976, 32, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Latypova, L.; Puzenko, A.; Poluektov, Y.; Anashkina, A.; Petrushanko, I.; Bogdanova, A.; Feldman, Y. Hydration of Methemoglobin Studied by in Silico Modeling and Dielectric Spectroscopy. J. Chem. Phys. 2021, 155, 015101. [Google Scholar] [CrossRef] [PubMed]
- Reeder, B.J.; Wilson, M.T. Hemoglobin and Myoglobin Associated Oxidative Stress: From Molecular Mechanisms to Disease States. Curr. Med. Chem. 2005, 12, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Pamenter, M.E. Adaptations to a Hypoxic Lifestyle in Naked Mole-Rats. J. Exp. Biol. 2022, 225, jeb196725. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.; Lykkeboe, G.; Weber, R.E.; Maloiy, G.M.O. Blood Respiratory Properties in the Naked Mole Rat Heterocephalus Glaber, a Mammal of Low Body Temperature. Respir. Physiol. 1976, 28, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qiao, Z.; Duan, Q.; Nevo, E. Adaptation of Mammals to Hypoxia. Anim. Models Exp. Med. 2021, 4, 311–318. [Google Scholar] [CrossRef]
- Shams, I.; Avivi, A.; Nevo, E. Oxygen and Carbon Dioxide Fluctuations in Burrows of Subterranean Blind Mole Rats Indicate Tolerance to Hypoxic–Hypercapnic Stresses. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2005, 142, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M. Andean, Tibetan, and Ethiopian Patterns of Adaptation to High-Altitude Hypoxia. Integr. Comp. Biol. 2006, 46, 18–24. [Google Scholar] [CrossRef]
- Arieli, R.; Heth, G.; Nevo, E.; Hoch, D. Hematocrit and Hemoglobin Concentration in Four Chromosomal Species and Some Isolated Populations of Actively Speciating Subterranean Mole Rats in Israel. Experientia 1986, 42, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Shams, I.; Avivi, A.; Nevo, E.; Ivanitskaya, E. Assignment of Erythropoietin (EPO) to Blind Subterranean Mole Rat Chromosome 1q by in Situ Hybridization. Cytogenet. Genome Res. 2005, 108, 362. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M. Two Routes to Functional Adaptation: Tibetan and Andean High-Altitude Natives. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 8655–8660. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M.; Strohl, K.P.; Blangero, J.; Williams-Blangero, S.; Almasy, L.A.; Decker, M.J.; Worthman, C.M.; Goldstein, M.C.; Vargas, E.; Villena, M.; et al. Ventilation and Hypoxic Ventilatory Response of Tibetan and Aymara High Altitude Natives. Am. J. Phys. Anthropol. 1997, 104, 427–447. [Google Scholar] [CrossRef]
- Kuleshova, I.D.; Zaripov, P.I.; Poluektov, Y.M.; Anashkina, A.A.; Kaluzhny, D.N.; Parshina, E.Y.; Maksimov, G.V.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Changes in Hemoglobin Properties in Complex with Glutathione and after Glutathionylation. Int. J. Mol. Sci. 2023, 24, 13557. [Google Scholar] [CrossRef] [PubMed]
- Fenk, S.; Melnikova, E.V.; Anashkina, A.A.; Poluektov, Y.M.; Zaripov, P.I.; Mitkevich, V.A.; Tkachev, Y.V.; Kaestner, L.; Minetti, G.; Mairbäurl, H.; et al. Hemoglobin Is an Oxygen-Dependent Glutathione Buffer Adapting the Intracellular Reduced Glutathione Levels to Oxygen Availability. Redox Biol. 2022, 58, 102535. [Google Scholar] [CrossRef]
- Craescu, C.T.; Poyart, C.; Schaeffer, C.; Garel, M.C.; Kister, J.; Beuzard, Y. Covalent Binding of Glutathione to Hemoglobin. II. Functional Consequences and Structural Changes Reflected in NMR Spectra. J. Biol. Chem. 1986, 261, 14710–14716. [Google Scholar] [CrossRef]
- Mieyal, J.J.; Gallogly, M.M.; Qanungo, S.; Sabens, E.A.; Shelton, M.D. Molecular Mechanisms and Clinical Implications of Reversible Protein S-Glutathionylation. Antioxid. Redox Signal. 2008, 10, 1941–1988. [Google Scholar] [CrossRef]
- D, G.; I, D.-D.; A, M.; D, B.; A, S.; R, R. Membrane Skeletal Protein S-Glutathionylation in Human Red Blood Cells as Index of Oxidative Stress. Chem. Res. Toxicol. 2019, 32. [Google Scholar] [CrossRef]
- Jensen, F.B. Red Blood Cell pH, the Bohr Effect, and Other Oxygenation-Linked Phenomena in Blood O2 and CO2 Transport. Acta Physiol. Scand. 2004, 182, 215–227. [Google Scholar] [CrossRef]
- Jensen, F.B.; Kolind, R.A.H.; Jensen, N.S.; Montesanti, G.; Wang, T. Interspecific Variation and Plasticity in Hemoglobin Nitrite Reductase Activity and Its Correlation with Oxygen Affinity in Vertebrates. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2017, 206, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hempe, J.M.; Ory-Ascani, J.; Hsia, D. Genetic Variation in Mouse Beta Globin Cysteine Content Modifies Glutathione Metabolism: Implications for the Use of Mouse Models. Exp. Biol. Med. 2007, 232, 437–444. [Google Scholar] [CrossRef]
- Lu, M.; Wang, H.; Li, X.-F.; Arnold, L.L.; Cohen, S.M.; Le, X.C. Binding of Dimethylarsinous Acid to Cys-13α of Rat Hemoglobin Is Responsible for the Retention of Arsenic in Rat Blood. Chem. Res. Toxicol. 2007, 20, 27–37. [Google Scholar] [CrossRef]
- Balagopalakrishna, C.; Abugo, O.O.; Horsky, J.; Manoharan, P.T.; Nagababu, E.; Rifkind, J.M. Superoxide Produced in the Heme Pocket of the Beta-Chain of Hemoglobin Reacts with the Beta-93 Cysteine to Produce a Thiyl Radical. Biochemistry 1998, 37, 13194–13202. [Google Scholar] [CrossRef]
- Alayash, A.I. βCysteine 93 in Human Hemoglobin: A Gateway to Oxidative Stability in Health and Disease. Lab. Invest. 2021, 101, 4–11. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Shumaev, K.B.; Novikova, N.N.; Topunov, A.F. Effect of Iron–Nitric Oxide Complexes on the Reactivity of Hemoglobin Cysteines. Appl. Biochem. Microbiol. 2020, 56, 512–520. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Reddy, A.B. Circadian Clocks in Human Red Blood Cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bhatt, V.S.; Sun, G.; Wang, P.G.; Palmer, A.F. Site-Selective Glycosylation of Hemoglobin on Cys Β93. Bioconjug. Chem. 2008, 19, 2221–2230. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, T.-J.; Simplaceanu, V.; Ho, C. Ligand Binding Properties and Structural Studies of Recombinant and Chemically Modified Hemoglobins Altered at Beta 93 Cysteine. Biochemistry 2002, 41, 11901–11913. [Google Scholar] [CrossRef]
- T, N. Protein Glutathionylation and Oxidative Stress. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2007, 855. [Google Scholar] [CrossRef]
- Mitra, K.; Ubarretxena-Belandia, I.; Taguchi, T.; Warren, G.; Engelman, D.M. Modulation of the Bilayer Thickness of Exocytic Pathway Membranes by Membrane Proteins Rather than Cholesterol. Proc. Natl. Acad. Sci. 2004, 101, 4083–4088. [Google Scholar] [CrossRef]
- Hu, Y.; Thapa, A.; Fan, H.; Ma, T.; Wu, Q.; Ma, S.; Zhang, D.; Wang, B.; Li, M.; Yan, L.; et al. Genomic Evidence for Two Phylogenetic Species and Long-Term Population Bottlenecks in Red Pandas. Sci. Adv. 2020, 6, eaax5751. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The Yak Genome and Adaptation to Life at High Altitude. Nat. Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef]
- Werhahn, G.; Senn, H.; Ghazali, M.; Karmacharya, D.; Sherchan, A.M.; Joshi, J.; Kusi, N.; López-Bao, J.V.; Rosen, T.; Kachel, S.; et al. The Unique Genetic Adaptation of the Himalayan Wolf to High-Altitudes and Consequences for Conservation. Glob. Ecol. Conserv. 2018, 16, e00455. [Google Scholar] [CrossRef]
- Signore, A.V.; Storz, J.F. Biochemical Pedomorphosis and Genetic Assimilation in the Hypoxia Adaptation of Tibetan Antelope. Sci. Adv. 2020, 6, eabb5447. [Google Scholar] [CrossRef] [PubMed]
- Pairet, B.; Jaenicke, E. Structure of the Altitude Adapted Hemoglobin of Guinea Pig in the R2-State. PloS One 2010, 5, e12389. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.R.; Powell, F.L. Common Themes of Adaptation to Hypoxia: Insights from Comparative Physiology. Hypoxia Genes Bedside 2001, 153–167. [Google Scholar]
- Yu, L.; Wang, X.; Ting, N.; Zhang, Y. Mitogenomic Analysis of Chinese Snub-Nosed Monkeys: Evidence of Positive Selection in NADH Dehydrogenase Genes in High-Altitude Adaptation. Mitochondrion 2011, 11, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Tomasco, I.H.; Boullosa, N.; Hoffmann, F.G.; Lessa, E.P. Molecular Adaptive Convergence in the α-Globin Gene in Subterranean Octodontid Rodents. Gene 2017, 628, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Pejo, M.; Tomasco, I.H. Adaptive Evolution of β-Globin Gene in Subterranean in South America Octodontid Rodents. Gene 2021, 772, 145352. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F. Hemoglobin Function and Physiological Adaptation to Hypoxia in High-Altitude Mammals. J. Mammal. 2007, 88, 24–31. [Google Scholar] [CrossRef]
- Storz, J.F. Hemoglobin–Oxygen Affinity in High-Altitude Vertebrates: Is There Evidence for an Adaptive Trend? J. Exp. Biol. 2016, 219, 3190–3203. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gao, W.; Gao, Y.; Tang, S.; Huang, Q.; Tan, X.; Chen, J.; Huang, T. Mitochondrial Genome Analysis of Ochotona Curzoniae and Implication of Cytochrome c Oxidase in Hypoxic Adaptation. Mitochondrion 2008, 8, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Cheviron, Z.A.; McClelland, G.B.; Scott, G.R. Evolution of Physiological Performance Capacities and Environmental Adaptation: Insights from High-Elevation Deer Mice (Peromyscus Maniculatus). J. Mammal. 2019, 100, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.V.; Paijmans, J.L.; Hofreiter, M.; Fago, A.; Weber, R.E.; Springer, M.S.; Campbell, K.L. Emergence of a Chimeric Globin Pseudogene and Increased Hemoglobin Oxygen Affinity Underlie the Evolution of Aquatic Specializations in Sirenia. Mol. Biol. Evol. 2019, 36, 1134–1147. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, G.-D.; Ruan, J.; Chen, Y.-B.; Yang, C.-P.; Cao, X.; Wu, H.; Liu, Y.-H.; Du, Z.-L.; Wang, X.-P. Genomic Analysis of Snub-Nosed Monkeys (Rhinopithecus) Identifies Genes and Processes Related to High-Altitude Adaptation. Nat. Genet. 2016, 48, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W.; Oberthür, W.; Kleinschmidt, T.; Braunitzer, G. Adaptation of Hemoglobin Function to Subterranean Life in the Mole, Talpa Europaea. Respir. Physiol. 1981, 46, 7–16. [Google Scholar] [CrossRef]
- Revsbech, I.G.; Tufts, D.M.; Projecto-Garcia, J.; Moriyama, H.; Weber, R.E.; Storz, J.F.; Fago, A. Hemoglobin Function and Allosteric Regulation in Semi-Fossorial Rodents (Family Sciuridae) with Different Altitudinal Ranges. J. Exp. Biol. 2013, 216, 4264–4271. [Google Scholar] [CrossRef]
- Chiou, K.L.; Janiak, M.C.; Schneider-Crease, I.A.; Sen, S.; Ayele, F.; Chuma, I.S.; Knauf, S.; Lemma, A.; Signore, A.V.; D’Ippolito, A.M. Genomic Signatures of High-Altitude Adaptation and Chromosomal Polymorphism in Geladas. Nat. Ecol. Evol. 2022, 6, 630–643. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, W.; Li, J.; Liu, C.; Hu, M.; Yuan, Y.; Yuan, K.; Zhang, Y.; Song, X.; Han, J.; et al. Draft Genome Assembly for the Tibetan Black Bear (Ursus Thibetanus Thibetanus). Front. Genet. 2020, 11. [Google Scholar] [CrossRef]
- Storz, J.F.; Moriyama, H. Mechanisms of Hemoglobin Adaptation to High Altitude Hypoxia. High Alt. Med. Biol. 2008, 9, 148–157. [Google Scholar] [CrossRef]
- Richter, F.; Meurers, B.H.; Zhu, C.; Medvedeva, V.P.; Chesselet, M.-F. Neurons Express Hemoglobin Alpha- and Beta-Chains in Rat and Human Brains. J. Comp. Neurol. 2009, 515, 538–547. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




