Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
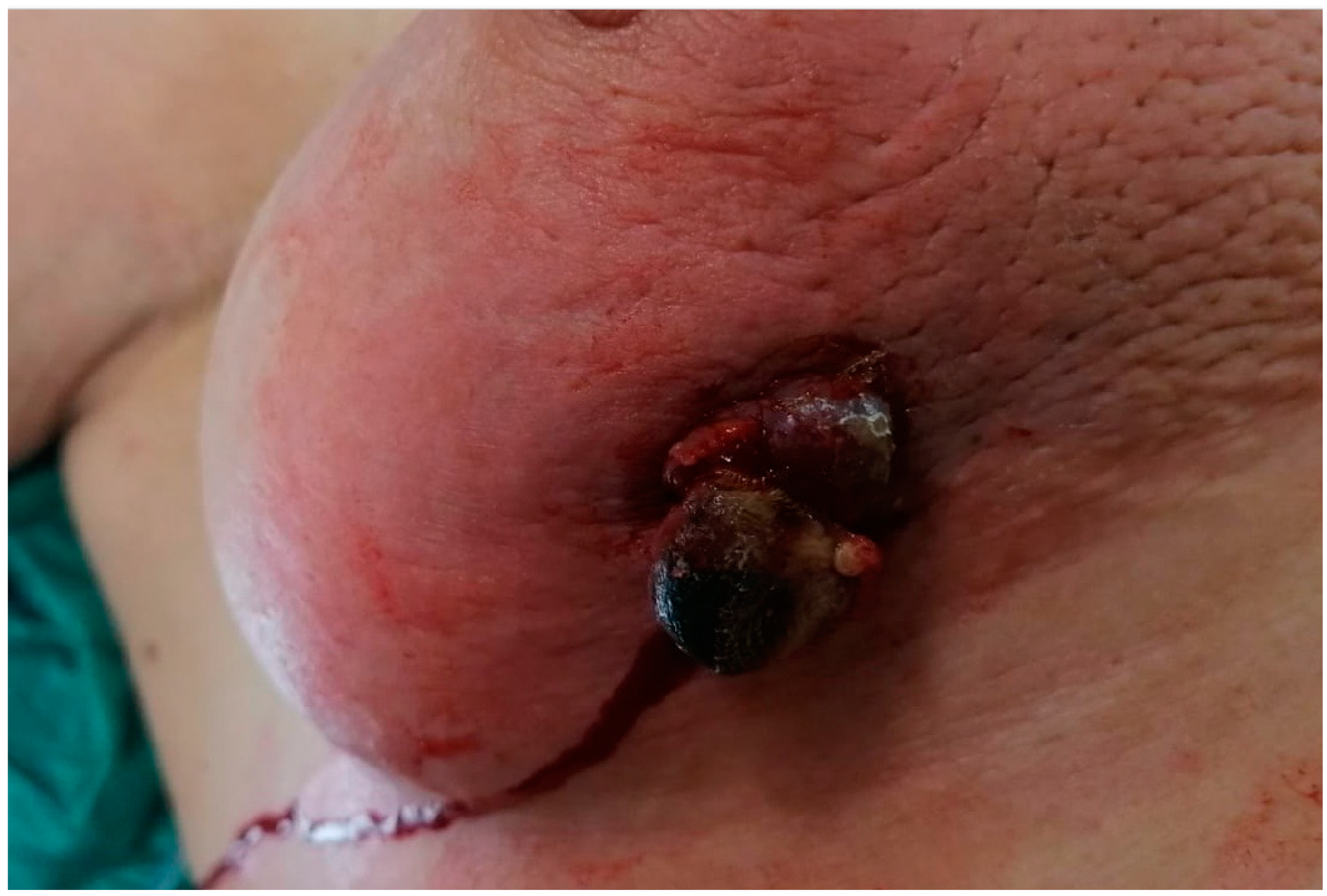
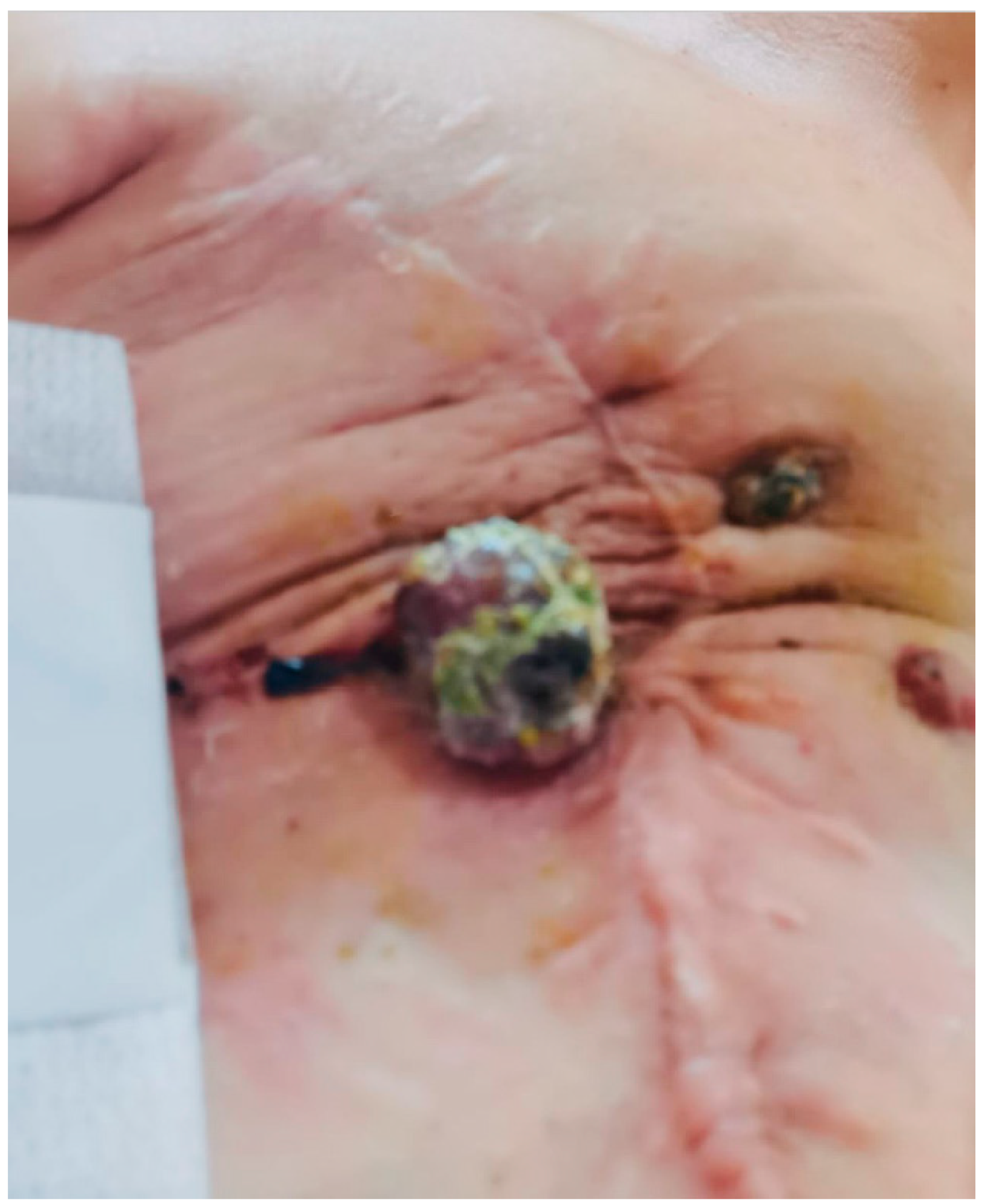
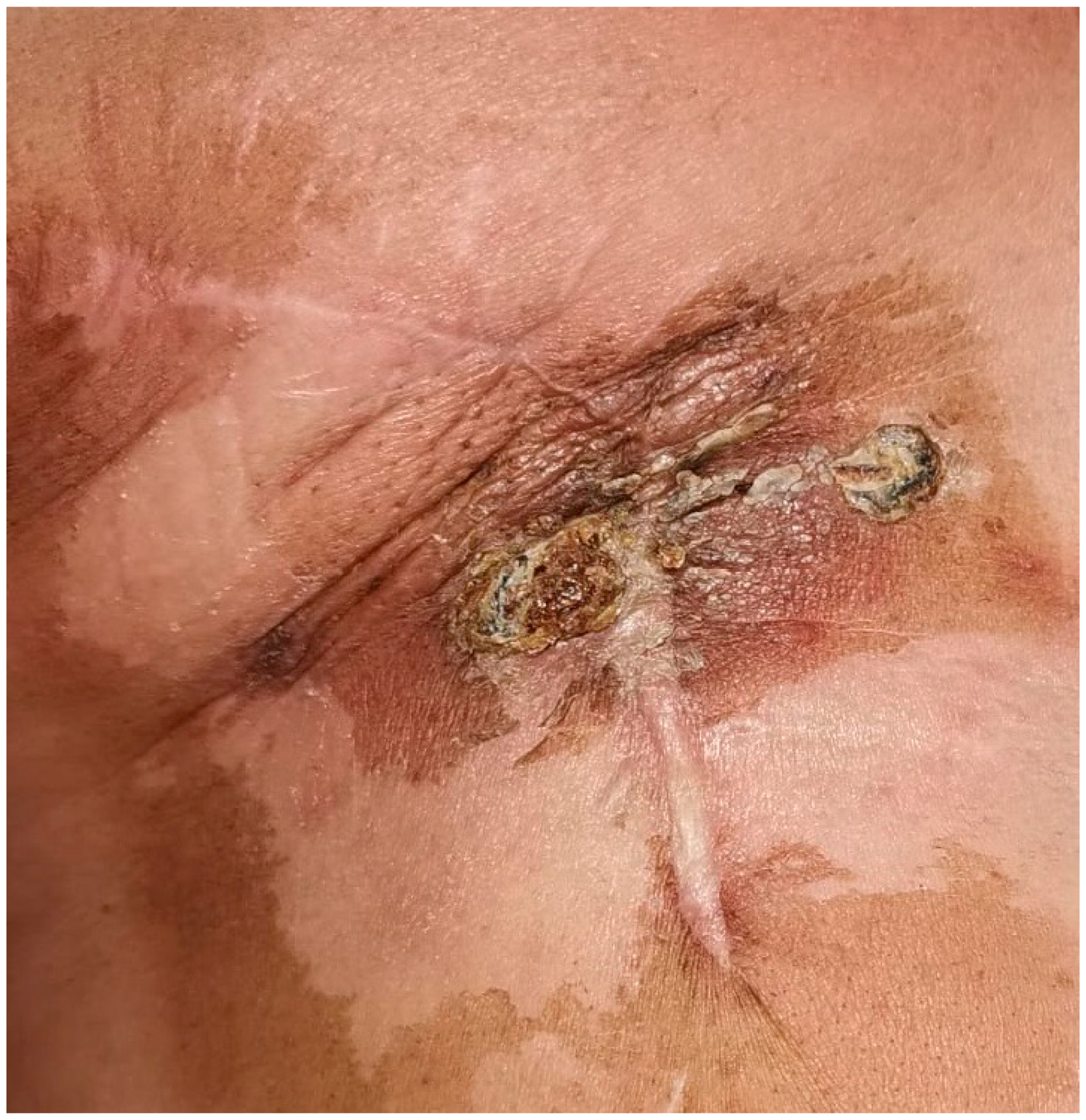
Case Presentation
Literature Review
Discussion
Conclusion
Conflicts of Interest
Ethical Statement
References
- Shah S, Rosa M. Radiation-associated angiosarcoma of the breast. Clinical and pathologic features. Arch Pathol Lab Med. 2016 May;140((5)):477–81. [CrossRef]
- Seo IS, Min K-W. Postirradiation epithelioid angiosarcoma of the breast. A case report with immunohistochemical and electron microscopic study. Ultrastruct Pathol. 2003 Jun; 27((3)):197–203. [CrossRef]
- Desbiens C, Hogue JC, Lévesque Y. Primary breast angiosarcoma: avoiding a common trap. Case Rep Oncol Med. 2011; Jul 2011: 517047. [CrossRef]
- Arora TK, Terracina KP, Soong J, Idowu MO, Takabe K. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014 Feb;3((1)):28-34. [CrossRef]
- Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006 Jan;48((1)):106-14. [CrossRef]
- Laé M, Lebel A, Hamel-Viard F, Asselain B, Trassard M, Sastre X, Kirova YM. Can c-myc amplification reliably discriminate post-radiation from primary angiosarcoma of the breast? Cancer Radiother. 2015 May;19((3)):168–74. [CrossRef]
- Singh R, Chufal KS, Pahuja AK, Suresh T, Chowdhary RL, Ahmad I. Primary angiosarcoma of the breast: a radiation oncologist's perspective with a concise review of the literature. BMJ Case Rep. 2019 Jul 18;12((7)):e227036. [CrossRef]
- Bousquet G, Confavreux C, Magné N, de Lara CT, Poortmans P, Senkus E, de Lafontan B, Bolla M, Largillier R, Lagneau E, Kadish S, Lemanski C, Ozsahin M, Belkacémi Y. Outcome and prognostic factors in breast sarcoma: a multicenter study from the rare cancer network. Radiother Oncol. 2007 Dec;85((3)):355-61. [CrossRef]
- Tozon N, Sersa G, Cemazar M. Electrochemotherapy: potentiation of local antitumor effectiveness of cisplatin in dogs and cats. Anticancer Res. 2001 Jul-Aug;21((4A)):2483-8.
- Gehl J, Sersa G, Matthiessen LW, Muir T, Soden D, Occhini A, Quaglino P, Curatolo P, Campana LG, Kunte C, Clover AJP, Bertino G, Farricha V, Odili J, Dahlstrom K, Benazzo M, Mir LM. Updated standard operating procedures for electrochemotherapy of cutaneous tumors and skin metastases. Acta Oncol. 2018 Jul;57((7)):874-82. [CrossRef]
- Cemazar M, Sersa G. Recent Advances in Electrochemotherapy. Bioelectricity. 2019 Dec 1;1((4)):204-213. [CrossRef]
- Campana LG, Kis E, Bottyán K, Orlando A, de Terlizzi F, Mitsala G, Careri R, Curatolo P, Snoj M, Sersa G, Valpione S, Quaglino P, Mowatt D, Brizio M, Schepler H. Electrochemotherapy for advanced cutaneous angiosarcoma: A European register-based cohort study from the International Network for Sharing Practices of electrochemotherapy (InspECT). Int J Surg. 2019 Dec;72:34-42. [CrossRef]
- Coindre JM, Trojani M, Contesso G, David M, Rouesse J, Bui NB, Bodaert A, De Mascarel I, De Mascarel A, Goussot JF. Reproducibility of a histopathologic grading system for adult soft tissue sarcoma. Cancer. 1986 Jul 15;58((2)):306-9. [CrossRef]
- Cencelj-Arnez R, Novak J, Klevisar Ivancic A, Bosnjak M, Cemazar M, Snoj M.Radiotherapy-associated angiosarcoma in the breast reconstructed by autologous Free-flap and treated with electrochemotherapy. Radiol Oncol. 2020 Dec 29;55((1)):77-81. [CrossRef]
- Zhou G, Mei Z. Electrochemotherapy for advanced cutaneous angiosarcoma: A european register-based cohort study from the international network for sharing practices of electrochemotherapy (InspECT)-An invited commentary. Int J Surg. 2019 Dec;72:232-3. [CrossRef]
- Alagkiozidis I. A Commentary on "Electrochemotherapy for advanced cutaneous angiosarcoma: A European register-based cohort study from the International Network for Sharing Practices of Electrochemotherapy (InspECT)" Int J Surg 2019 Dec;72:196-7. [CrossRef]
- Campana LG, Kis E, Bottyán K, Orlando A, de Terlizzi F, Mitsala G, Careri R, Curatolo P, Snoj M, Sersa G, Valpione S, Quaglino P, Mowatt D, Brizio M,Schepler H. Electrochemotherapy for advanced cutaneous angiosarcoma: A European register-based cohort study from the International Network for Sharing Practices of electrochemotherapy (InspECT). Int J Surg. 2019 Dec;72:34-42. [CrossRef]
- Benevento R, Carafa F, Di Nardo D, Pellino G, Letizia A, Taddeo M, Gambardella A, Canonico S, Santoriello A. Angiosarcoma of the breast: a new therapeutic approach? Int J Surg Case Rep. 2015;13:30-2. [CrossRef]
- Guida M, Ruggieri E, Fucci L, Ressa M, D'Aluisio L, Fanelli G, Strippoli S. Image Gallery: A case of cutaneous giant angiosarcoma treated successfully with electrochemotherapy. Br J Dermatol. 2017 Aug;177(2):e27. [CrossRef]
- Guida M, Campana LG, Curatolo P, Strippoli S, Bonadies A, Grilz G, Cabula C, Rotunno R, Bucher S, Solari N, Santoriello A, Valpione S, Rossi CR. Local treatment with electrochemotherapy of superficial angiosarcomas: Efficacy and safety results from a multi-institutional retrospective study. J Surg Oncol. 2016 Aug;114(2):246-53. [CrossRef]
- Borgognoni L, Pescitelli L, Gerlini G, Brandani P, Gelli R, Giannotti V, Bellucci F, Sestini S. Efficacy of Electrochemotherapy in the Treatment of Cutaneous Melanoma Metastases and Rare Non-melanoma Skin Cancer. Anticancer Res. 2020 Nov;40((11)):6485-6492. [CrossRef]
- Tozon N, Sersa G, Cemazar M. Electrochemotherapy: potentiation of local antitumour effectiveness of cisplatin in dogs and cats. Anticancer Res. 2001 Jul-Aug;21((4A)):2483-8.
- Campana LG, Valpione S, Tosi A, Rastrelli M, Rossi CR, Aliberti C. Angiosarcoma on Lymphedema (Stewart-Treves Syndrome): A 12-Year Follow-up after Isolated Limb Perfusion, Limb Infusion, and Electrochemotherapy. J Vasc Interv Radiol. 2016 Mar;27((3)):444-6.
- Solari N, Spagnolo F, Ponte E, Quaglia A, Lillini R, Battista M, Queirolo P, Cafiero F. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: a series of 39 patients treated with palliative intent. J Surg Oncol. 2014 Mar;109((3)):270-4. [CrossRef]
- Mocerino C, Iannaci G, Sapere P, Luise R, Canonico S, Gambardella A. Multidisciplinary approach to breast angiosarcoma in an elderly patient: Repeated local relapses and significant objective responses. Int J Immunopathol Pharmacol. 2016 Sep;29((3)):537-42. [CrossRef]
- Laurino S, Omer LC, Albano F, Marino G, Bianculli A, Solazzo AP, Sgambato A, Falco G, Russi S, Bochicchio AM. Radiation-induced sarcomas: A single referral cancer center experience and literature review. Front Oncol. 2022 Sep 30;12:986123. [CrossRef]
- di Meo N, Drabeni M, Gatti A, Trevisan G. A Stewart-Treves syndrome of the lower limb. Dermatol Online J. 2012 Jun 15;18((6)):14. [CrossRef]
- Parisi S, Ruggiero R, Gualtieri G, Volpe ML, Rinaldi S, Nesta G, Bogdanovich L, Lucido FS, Tolone S, Parmeggiani D, Gambardella C, Docimo L. Combined LOCalizer™ and Intraoperative Ultrasound Localization: First Experience in Localization of Non-palpable Breast Cancer. In Vivo. 2021 May-Jun;35((3)):1669-76. [CrossRef]
- Parisi S, Gambardella C, Conzo G, Ruggiero R, Tolone S, Lucido FS, Iovino F, Fisone F, Brusciano L, Parmeggiani D, Docimo L. Advanced Localization Technique for Non-Palpable Breast Cancer: Radiofrequency alone VS Combined Technique with Ultrasound. J Clin Med. 2023 Aug 2;12((15)):5076. [CrossRef]
- Parisi S., Gambardella C., Ruggiero R., Tolone S., Lucido F.S., Docimo L. Radiofrequency Identification—RFID using LOCalizer-Tag in Non-palpable Breast Lump. Indian J. Surg. 2022. [CrossRef]
- Dogan A, Kern P, Schultheis B, Häusler G, Rezniczek GA, Tempfer CB. Radiogenic angiosarcoma of the breast: case report and systematic review of the literature. BMC Cancer. 2018 Apr 24;18((1)):463. [CrossRef]
- D'Angelo SP, Munhoz RR, Kuk D, Landa J, Hartley EW, Bonafede M, Dickson MA, Gounder M, Keohan ML, Crago AM, Antonescu CR, Tap WD. Outcomes of systemic therapy for patients with metastatic angiosarcoma. Oncology. 2015;89:205–14. [CrossRef]
- Depla AL, Scharloo-Karels CH, de Jong MAA, Oldenborg S, Kolff MW, Oei SB, van Coevorden F, van Rhoon GC, Baartman EA, Scholten RJ, Crezee J, van Tienhoven G. Treatment and prognostic factors of radiation-associated angiosarcoma (RAAS) after primary breast cancer: a systematic review. Eur J Cancer. 2014 Jul;50((10)):1779-88. [CrossRef]
- Bertino G, Sersa G, De Terlizzi F, Occhini A, Plaschke CC, Groselj A, Langdon C, Grau JJ, McCaul JA, Heuveling D, Cemazar M, Strojan P, de Bree R, Leemans CR, Wessel I, Gehl J, Benazzo M. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur J Cancer. 2016 Aug;63:41-52. [CrossRef]
- Al-Hadithy N, Dehnel A, George A, Kisiel R, Lunt C, Stone C. Patient reported outcomes in prospective cohort study of Electrochemotherapy. Int J Surg. 2018 Apr;52:110-9. [CrossRef]
- Campana LG, Balestrieri N, Menin N. Adjuvant skin-sparing electrochemotherapy in a breast cancer patient with a prosthetic implant: 5-year follow-up outcomes. J Surg Case Rep. 2022May;5:rjac199. [CrossRef]
- Ferioli M, Perrone AM, Buwenge M, Arcelli A, Vadala' M, Fionda B, Malato MC, De Iaco P, Zamagni C, Cammelli S, Tagliaferri L, Morganti AG. Combination of Electrochemotherapy with Radiotherapy: A Comprehensive, Systematic, PRISMA-Compliant Review of Efficacy and Potential Radiosensitizing Effects in Tumor Control. Curr Oncol. 2023 Nov 13;30(11):9895-9905. PMID: 37999139; PMCID: PMC10670517. [CrossRef]
| STUDY | Type of study | Patients | AGE | History | FIRST TREATMENT | LATENCY PERIOD months |
CLINICAL FEATURES | SKIN REGION | RE-TREATMENT | Histology | C myc amplification |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cencelj-Arnez 2020 [16] |
CR | 1 | 63 | Syncronus bilateral Luminal BC (right breast) |
Mastectomy + 6 cicles x 5-fluorouracil, epirubicin, Cyclophosphamide+ letrozole + RT 25Gy |
60 | Ulcerated red lesion | Lower-medial quadrant In right breast |
Excision + ECT + doxorubicin | High grade RAS | Yes |
| Campana 2019 [19] |
CS | 20 (10 breast RAS) |
/ | / | / | / | / | / | / | / | / |
| Benevento 2015 [20] |
CR | 1 | 76 | Invasive ductal carcinoma Luminal BC (left breast) pT1 pN0 M0 G2 |
BCS + 50 Gy in 25 fractions of 200 cGy/daily with boost of 10 Gy in 5 fractions of 200 cGy/daily + Tamoxifene |
48 | painful, violet, multi-nodular mass |
Left>right breast | Excision + Mastectomy (after 4 years)+ doxorubicin |
grade-II RAS |
/ |
| Guida 2016 [20] |
RS | 19 (6 breast RAS) |
69 | / | / | 96 | / | Scalp (5) Breast (8) Skin (3) Soft tissue (3) |
ECT (19/19)+ Surgery (17/19) + RT (5/19) + CT (3/19) |
RAS | / |
| Mocerino 2015 [26] |
CR | 1 | 77 | invasive ductal carcinoma pT1N0M0 ER +15%; PgR +30%; HER2 IHC 1+ (left breast) |
BCL + 60 Gy in 30 fractions + tamoxifen |
84 | ecchymotic lesion (1.3 cm) |
near the scar |
Excision + left mastectomy (after 1 year)+ right mastectomy (after 2 years) + ECT+ 69 Gy + Doxorubicin |
low-grade RAS |
/ |
| Laurino 2022 [27] |
CR | 1 | 61 | infiltrating ductal carcinoma, pT1cN0, grade G2, ER 98%, PGR 20%, HER2+, left breast |
BCL + 50 Gy in 25 fractions + 10 Gy in 5 fractions by photons+ Adjuvant CT + letrozole |
72 | / | Left breast | Neoadjuvant CT + mastectomy (after 1 year) + ECT+ Re-excision |
high-grade RAS (G3), positive for Factor VIII and CD31, with extensive areas of necrosis and ulceration. | / |
| Laurino 2022 [27] |
CR | 1 | 63 | infiltrating ductal breast cancer pT1cN1(1/18), G2, ER: 90%, PGR: 60%, Ki67 index at 15%, and HER2 negative Left breast |
BCS+ 5-fluorouracil, epidoxorubicin, and cyclophosphamide+ 50 Gy in 25 fractions + 10 Gy in 5 fractions by photons+ letrozole |
108 | ulcerated and bleeding left breast lump, 7 cm in diameter, adherent to the chest wall | Left breast | Radiofrequency termoablation + gemcitabine and docetaxel + ECT + |
RAS | / |
| Parisi 2023 | CR | 1 | 59 | breast invasive ductal Luminal B carcinoma pT1c N0 M0 (right breast) |
BCL + 60 Gy in 30 fractions+ femara |
60 | exophytic lump | near the scar |
Excision + right mastectomy + Paclitaxel (doxorubicin contraindicated) + 40.5 Gy in 15 fractions + ECT |
Grade II RAS | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





