Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract

Keywords:
Introduction
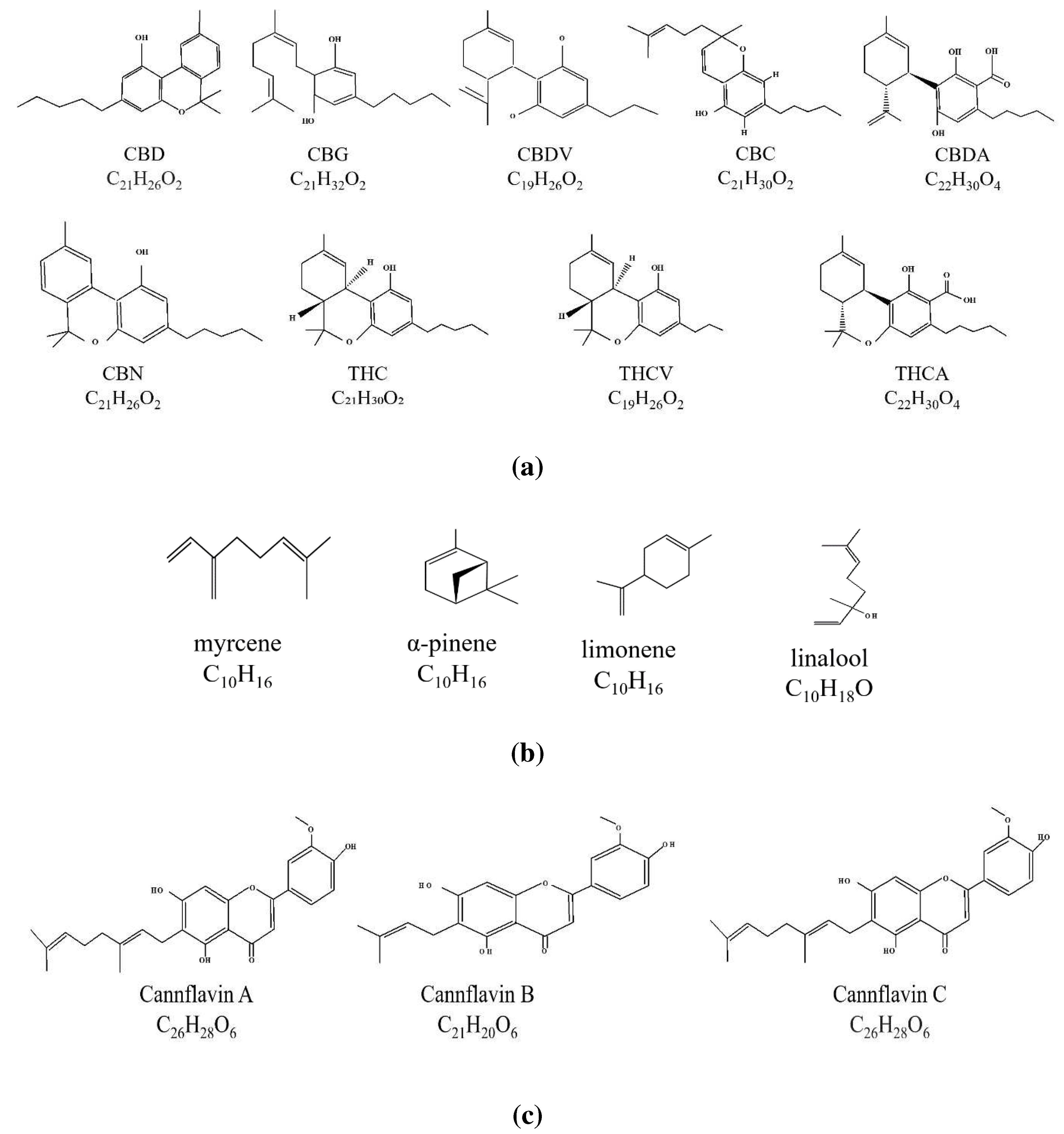
2. Phytochemicals in medicinal cannabis
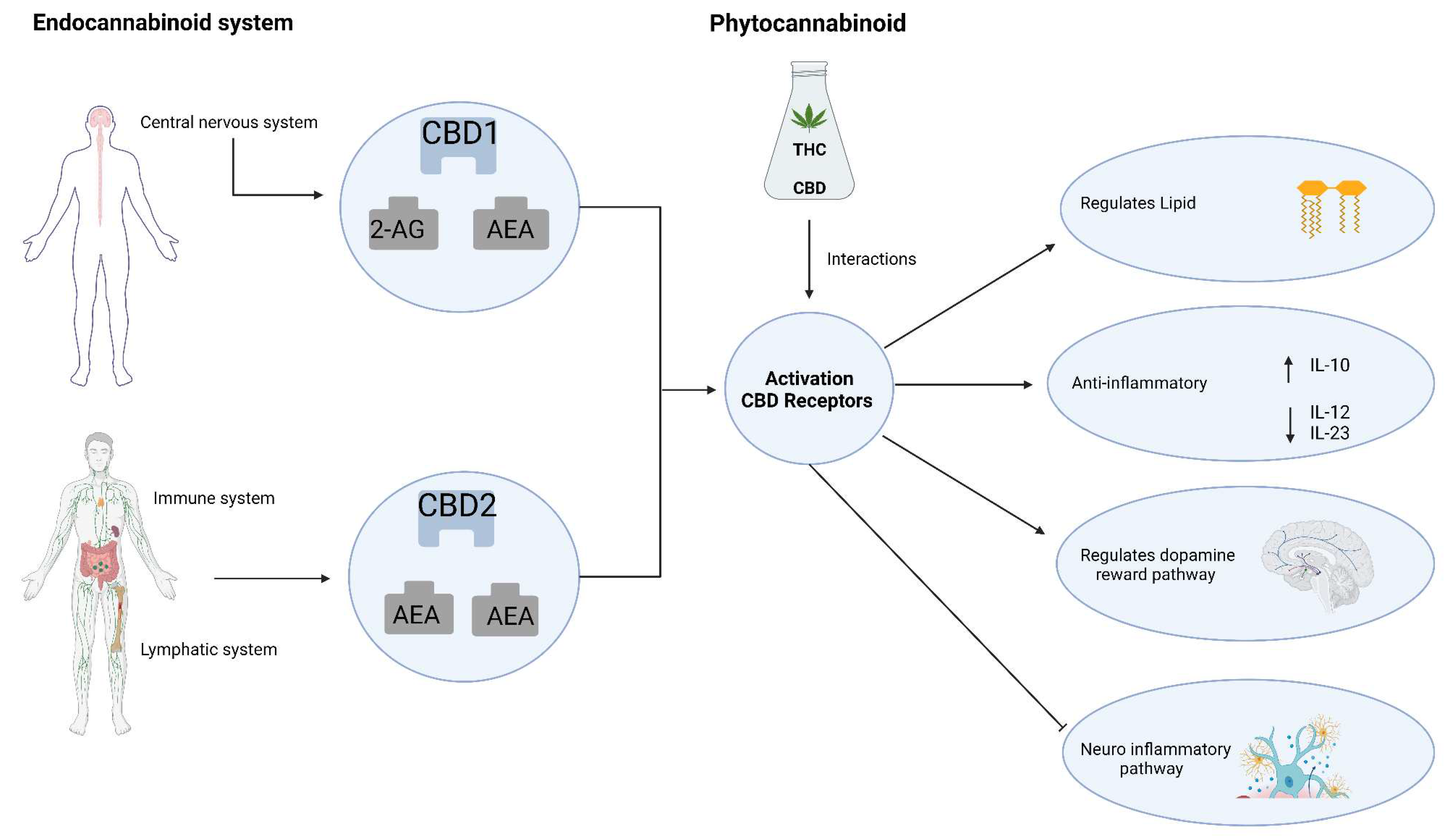
3. The endocannabinoid system and neuroinflammation
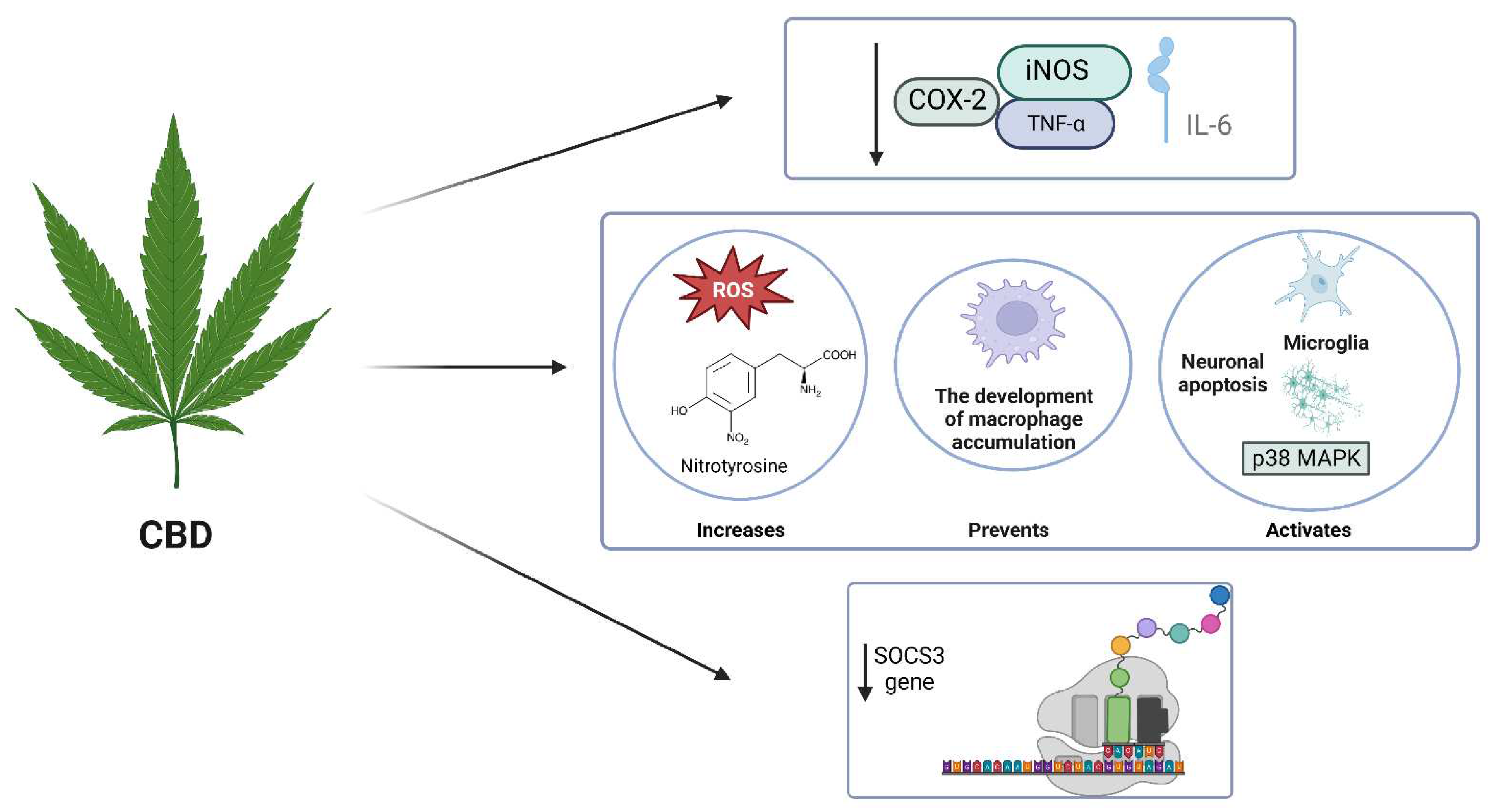
4. Anti-neuroinflammatory activity of phytochemicals in C. Sativa
4.1. CBD
4.2. THC

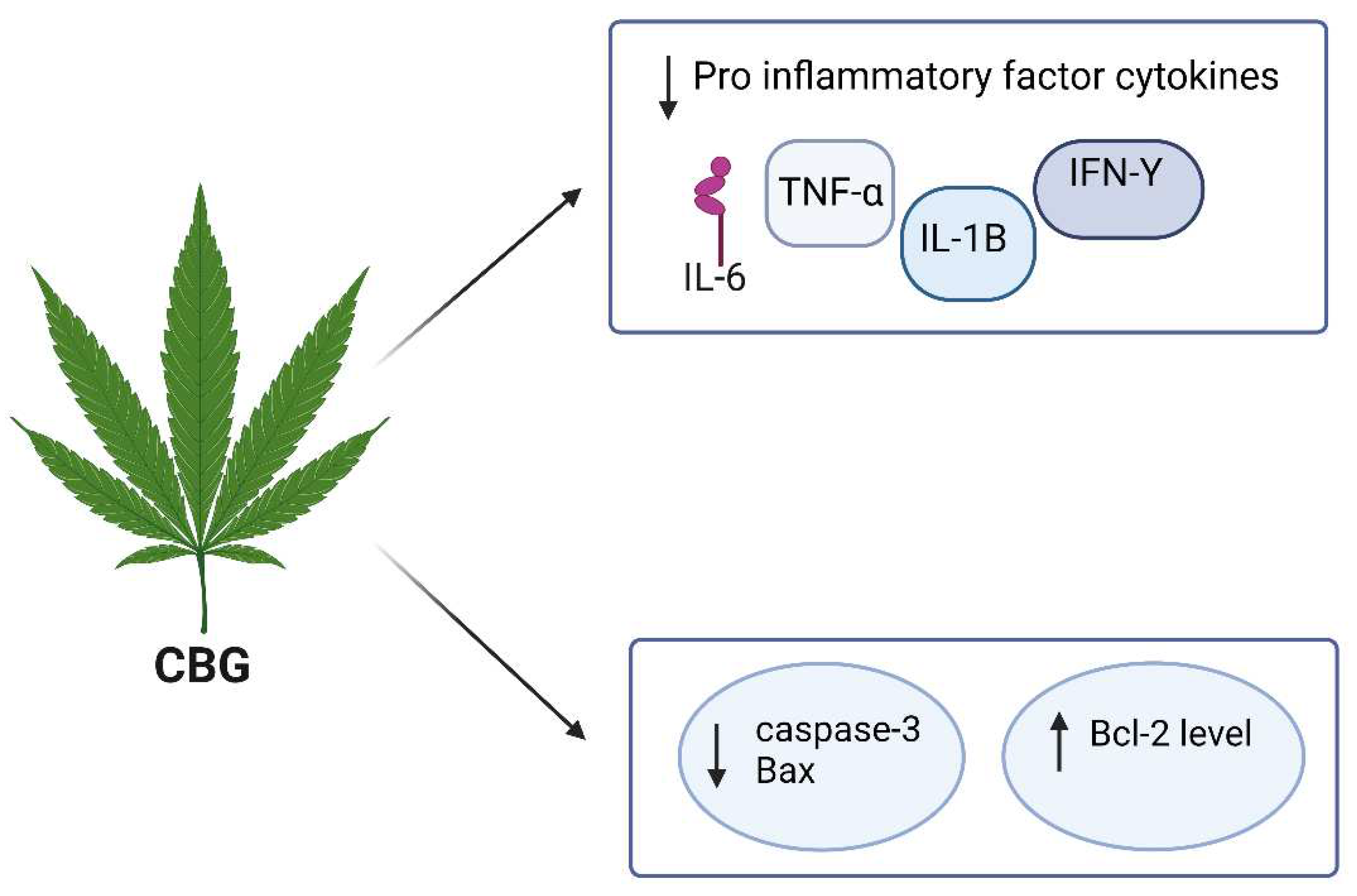
4.3. CBG
| Compound | Model | Concentration/ Dose |
indicated neurodegenerative diseases | Outcome | References |
|---|---|---|---|---|---|
| CBD | in vitro glutamate neuronal toxicity model | N/A | N/A | CBD was shown to be more protective than either α-tocopherol or vitamin C and comparable to butylated hydroxytoluene (BHT). | (Saito et al., 2012; Yousaf et al., 2022) |
| THC | in vivo in hemiparkinsonian rats | N/A | PD | neuroprotective effect | (Lastres-Becker et al., 2005) |
| CBD | in vivo in hemiparkinsonian rats | 3 mg/kg | PD | exhibited a potent neuroprotective effect in this rat model | (Lastres-Becker et al., 2005) |
| CBD | N/A | < 1 μM | N/A | inhibit activated microglial cell migration by antagonising the abnormal-cannabidiol (Abn-CBD)-sensitive receptor | (Walter et al., 2003) |
| CBD |
in vitro PC12 cells |
N/A | AD | Neuroprotective against the neuronal damage induced by the β-amyloid peptide (Aβ) inhibits Aβ-induced neurotoxicity. | (Esposito et al., 2006) |
| CBD |
In vivo mouse model |
N/A | AD | Attenuated the expression of several glial pro-inflammatory proteins, including glial fibrillary acidic protein, inducible nitric oxide synthase (iNOS) and interleukin 1β (IL-1β), which are major contributors to the propagation of neuroinflammation and oxidative stress. | (Esposito et al., 2007) |
| CBD |
in vivo mouse model |
100-200 mg/kg | Dravet Syndrome | It has beneficial effects on seizures and social deficits | (Kaplan et al., 2017) |
| CBD |
in vivo mouse model |
10 mg/kg twice- daily |
schizophrenia | improves the social and cognitive dysfunctions | (Osborne et al., 2017) |
| CBDV | Clinical trial | Single oral dose | ASD | It modulates glutamatergic but not γ-aminobutyric acid (GABA) neurotransmission in adult male patients, although the biological response may differ between autistic individuals. | (Pretzsch et al., 2019) |
| THCV |
in vivo mouse model |
<3 mg/kg | PD | alleviates motor inhibition in 6-OHDA-lesioned rodents by blocking CB1 receptors at low doses | (Espadas et al., 2020) |
| THC | N/A | N/A | PD | It reduced levodopa-induced dyskinesia | (Cristino et al., 2020) |
| CBN |
in vitro C6 glioma cells |
0.3–30000 nM EC50: 700 nM |
N/A | It inhibited NO production and iNOS expression | (Esposito et al., 2001) |
| CBN | N/A | N/A | MS | It may antagonise the 2-AG-induced recruitment of microglial cells and produces minimal palliative. | (Walter et al., 2003) |
| THC |
in vitro BV-2 murine microglial cell line |
10 μM | N/A | It decreases the production and release of proinflammatory cytokines, including interleukin-1β, interleukin-6, and interferon (IFN)β, from LPS-activated microglial cells. | (Kozela et al., 2010) |
| CBG | in vitro murine microglial cell line |
25 μM | MS | It has inhibited the microglia-driven inflammatory response, protected neurons from toxic insults in vitro, and restored motor function impairment by inhibiting the synthesis of IL-1β, IL-6, TNF-α, the chemokine, MIP-1α and prostaglandin E2 (PGE2). | (Carrillo-Salinas et al., 2014; Granja et al., 2012) |
| CBG |
in vitro NSC-34 motor neurons |
7.5 µM | N/A | CBG pre-treatment has REDUCED IL-1β, TNF-α, IFN-γ and PPARγ protein levels and reduced nitrotyrosine, SOD1 and iNOS protein levels and restored Nrf-2 levels. | (Gugliandolo et al., 2018) |
| CBG |
In vivo and in vitro |
N/A | PD | It shows a neuroprotective against inflammation-driven neuronal damage, acting through the activation of the canonic binding site in PPARγ receptors. |
(García et al., 2018) |
| CBG |
In vivo and In vitro neuroblastoma Neuro-2a (N2a) |
2 g/ 6.319 mM | HD | It has improved motor deficits, reactive astrogliosis and microglial activation, inhibiting the upregulation of proinflammatory markers and improving antioxidant defences in the brain. | (Díaz-Alonso et al., 2016) |
| CBDA |
In vitro Neuro-2a (N2a) cells |
25 μM | HD | CBDA shows potent neuroprotective activity by activating PPARγ with higher potency than their decarboxylated products | (Nadal et al., 2017) |
| CBDA | in vivo | 10 and 30 mg/kg | Dravet syndrome | It has an anticonvulsant against pentylenetetrazol-induced seizures and hyperthermia-induced seizures. | (Anderson et al., 2019) |
| CBDV |
in vivo mouse model |
CBDV | Rett syndrome (RTT), a rare neurological disorder affecting predominantly females | It improves behavioural and functional deficits. | (Hagberg et al., 2002; Ricceri et al., 2013; Vigli et al., 2018; Zamberletti et al., 2019) |
| CBC |
In vitro |
1 μM | N/A | CBC exert potential actions on brain health through effects on adult neural stem cells using whole brain-derived neural stem progenitor cells (NSPCs). | (Shinjyo & Di Marzo, 2013) |
| THC |
In vitro |
10 μM | N/A | THC reduces IL-1β, IL-6, and TNFα production in LPS-stimulated rat microglial cells. | (Puffenbarger et al., 2000) |
| THC |
In vitro |
0 – 15 μM | AD | It inhibits the enzyme acetylcholinesterase (AChE) and prevents AChE-induced amyloid β-peptide (Aβ) aggregation, which is considered the key pathological marker of Alzheimer's disease. | (Eubanks et al., 2006) |
| THC |
in vivo R6/1 mouse model |
10 mg/kg | HD | It inhibits acetylcholine esterase (AchE)-induced aggregation of Aβ and attenuates the motor coordination deficits of R6/1 mice. | (Dowie et al., 2010) |
| THCA |
In vitro N2a cells |
10 μM IC50 of 0.47 μM |
HD | It has neuroprotective activity by activate PPARγ transcriptional activity. | (Nadal et al., 2017) |
4.4. Terpenes
4.5. Flavonoids
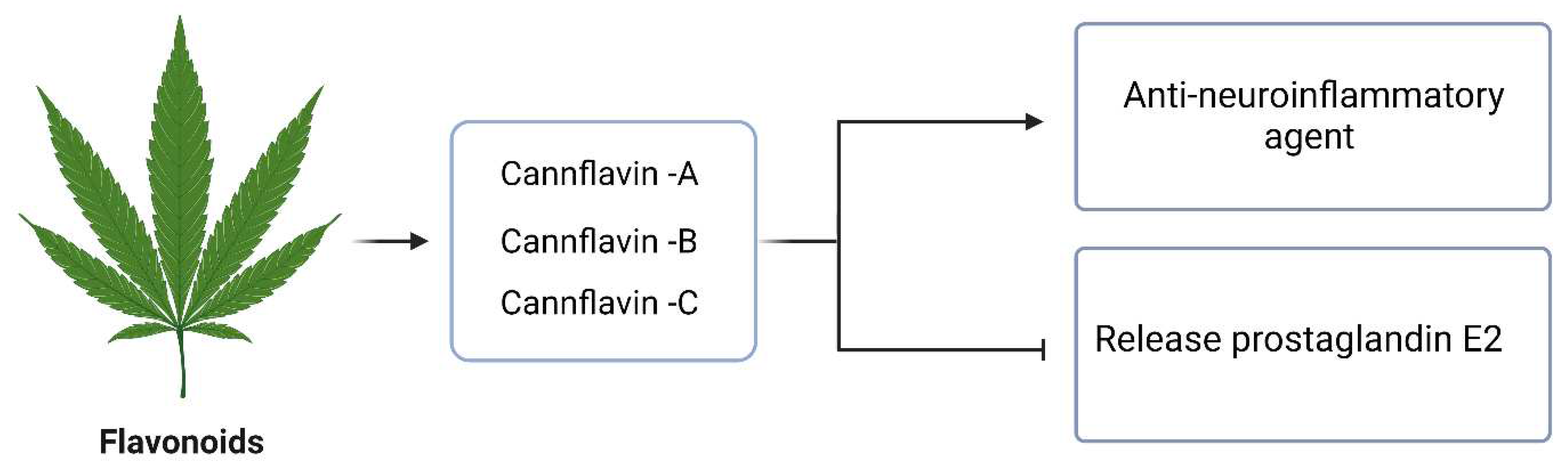
5. Entourage effects among the phytochemicals in C. Sativa
5.1. The preclinical and clinical evidence
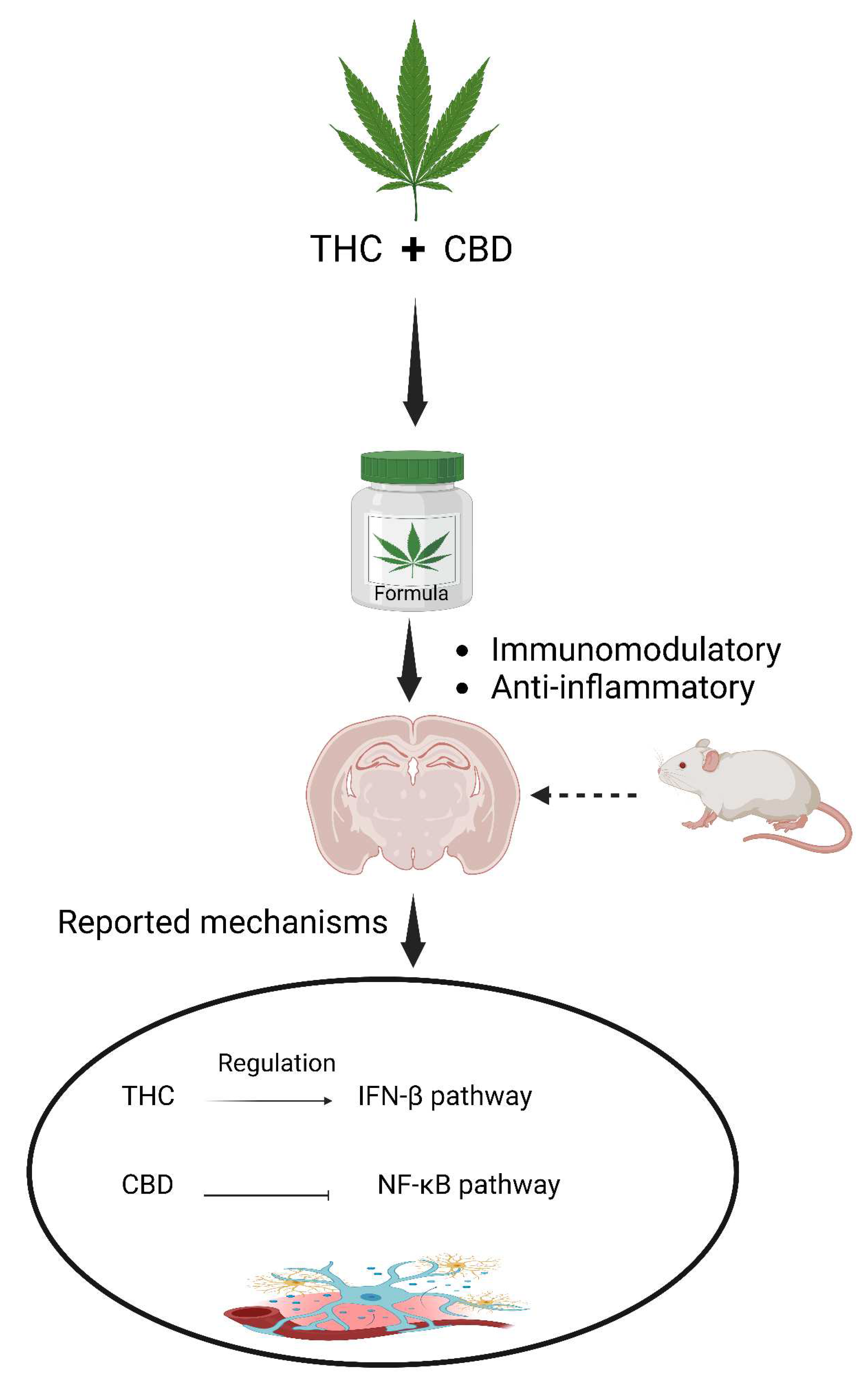
5.2. The entourage effects in the context of neuroinflammation
5.3. The mechanisms underpin the entourage effects
6. Conclusion
References
- Abd Rashed, A., Abd Rahman, A. Z., & Rathi, D. N. G. (2021). Essential oils as a potential neuroprotective remedy for age-related neurodegenerative diseases: A review. Molecules, 26(4), 1107. [CrossRef]
- Agostinho, P., A Cunha, R., & Oliveira, C. (2010). Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Current pharmaceutical design, 16(25), 2766-2778.
- Aguado, T., Palazuelos, J., Monory, K., Stella, N., Cravatt, B., Lutz, B., Marsicano, G., Kokaia, Z., Guzmán, M., & Galve-Roperh, I. (2006). The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. Journal of Neuroscience, 26(5), 1551-1561. [CrossRef]
- Al Mansouri, S., Ojha, S., Al Maamari, E., Al Ameri, M., Nurulain, S. M., & Bahi, A. (2014). The cannabinoid receptor 2 agonist, β-caryophyllene, reduced voluntary alcohol intake and attenuated ethanol-induced place preference and sensitivity in mice. Pharmacology Biochemistry and Behavior, 124, 260-268.
- Aloisi, F. (2001). Immune function of microglia. Glia, 36(2), 165-179. [CrossRef]
- Amar, M. B. (2006). Cannabinoids in medicine: A review of their therapeutic potential. Journal of ethnopharmacology, 105(1-2), 1-25.
- Ambrose, T., & Simmons, A. (2019). Cannabis, cannabinoids, and the endocannabinoid system—is there therapeutic potential for inflammatory bowel disease? Journal of Crohn's and Colitis, 13(4), 525-535.
- Anderson, L. L., Low, I. K., Banister, S. D., McGregor, I. S., & Arnold, J. C. (2019). Pharmacokinetics of Phytocannabinoid Acids and Anticonvulsant Effect of Cannabidiolic Acid in a Mouse Model of Dravet Syndrome. Journal of Natural Products, 82(11), 3047-3055. [CrossRef]
- Andre, C. M., Hausman, J.-F., & Guerriero, G. (2016). Cannabis sativa: the plant of the thousand and one molecules. Frontiers in plant science, 19. [CrossRef]
- Aso, E., & Ferrer, I. (2014). Cannabinoids for treatment of Alzheimer’s disease: moving toward the clinic. Frontiers in pharmacology, 5, 37.
- Aso, E., & Ferrer, I. (2016). CB2 cannabinoid receptor as potential target against Alzheimer's disease. Frontiers in neuroscience, 10, 243.
- Baker, D., Jackson, S., & Pryce, G. (2007). Cannabinoid control of neuroinflammation related to multiple sclerosis. British journal of pharmacology, 152(5), 649-654. [CrossRef]
- Bamberger, M. E., Harris, M. E., McDonald, D. R., Husemann, J., & Landreth, G. E. (2003). A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. Journal of Neuroscience, 23(7), 2665-2674. [CrossRef]
- Banister, S. D., Arnold, J. C., Connor, M., Glass, M., & McGregor, I. S. (2019). Dark classics in chemical neuroscience: Δ9-tetrahydrocannabinol. ACS Chemical Neuroscience, 10(5), 2160-2175.
- Baron, E. P. (2018). Medicinal properties of cannabinoids, terpenes, and flavonoids in cannabis, and benefits in migraine, headache, and pain: an update on current evidence and cannabis science. Headache: The Journal of Head and Face Pain, 58(7), 1139-1186. [CrossRef]
- Ben-Shabat, S., Fride, E., Sheskin, T., Tamiri, T., Rhee, M.-H., Vogel, Z., Bisogno, T., De Petrocellis, L., Di Marzo, V., & Mechoulam, R. (1998). An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. European journal of pharmacology, 353(1), 23-31. [CrossRef]
- Berman, P., Futoran, K., Lewitus, G. M., Mukha, D., Benami, M., Shlomi, T., & Meiri, D. (2018). A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Scientific Reports, 8(1), 14280. [CrossRef]
- Bilkei-Gorzo, A. (2012). The endocannabinoid system in normal and pathological brain ageing. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1607), 3326-3341.
- Biringer, R. G. (2021). Endocannabinoid signaling pathways: Beyond CB1R and CB2R. Journal of cell communication and signaling, 15(3), 335-360. [CrossRef]
- Blasco-Benito, S., Seijo-Vila, M., Caro-Villalobos, M., Tundidor, I., Andradas, C., García-Taboada, E., Wade, J., Smith, S., Guzmán, M., & Pérez-Gómez, E. (2018). Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochemical pharmacology, 157, 285-293. [CrossRef]
- Blazquez, C., Chiarlone, A., Sagredo, O., Aguado, T., Pazos, M. R., Resel, E., Palazuelos, J., Julien, B., Salazar, M., & Boerner, C. (2011). Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain, 134(1), 119-136. [CrossRef]
- Bonini, S. A., Premoli, M., Tambaro, S., Kumar, A., Maccarinelli, G., Memo, M., & Mastinu, A. (2018). Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. Journal of ethnopharmacology, 227, 300-315. [CrossRef]
- Bradshaw, E. M., Chibnik, L. B., Keenan, B. T., Ottoboni, L., Raj, T., Tang, A., Rosenkrantz, L. L., Imboywa, S., Lee, M., & Von Korff, A. (2013). CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nature neuroscience, 16(7), 848-850.
- Caesar, L. K., & Cech, N. B. (2019). Synergy and antagonism in natural product extracts: when 1+ 1 does not equal 2. Natural product reports, 36(6), 869-888. [CrossRef]
- Callaway, J. (2004). Hempseed as a nutritional resource: An overview. Euphytica, 140, 65-72. [CrossRef]
- Cardona, A. E., Huang, D., Sasse, M. E., & Ransohoff, R. M. (2006). Isolation of murine microglial cells for RNA analysis or flow cytometry. Nature protocols, 1(4), 1947-1951. [CrossRef]
- Carow, B., & Rottenberg, M. E. (2014). SOCS3, a Major Regulator of Infection and Inflammation. Front Immunol, 5, 58. [CrossRef]
- Carrier, E. J., Auchampach, J. A., & Hillard, C. J. (2006). Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proceedings of the National Academy of Sciences, 103(20), 7895-7900. [CrossRef]
- Carrillo-Salinas, F. J., Navarrete, C., Mecha, M., Feliú, A., Collado, J. A., Cantarero, I., Bellido, M. L., Muñoz, E., & Guaza, C. (2014). A cannabigerol derivative suppresses immune responses and protects mice from experimental autoimmune encephalomyelitis. PloS one, 9(4), e94733. [CrossRef]
- Casarejos, M. J., Perucho, J., Gomez, A., Munoz, M. P., Fernandez-Estevez, M., Sagredo, O., Fernandez Ruiz, J., Guzman, M., de Yebenes, J. G., & Mena, M. A. (2013). Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy. Journal of Alzheimer's Disease, 35(3), 525-539. [CrossRef]
- Castillo, A., Tolón, M., Fernández-Ruiz, J., Romero, J., & Martinez-Orgado, J. (2010). The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic–ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiology of disease, 37(2), 434-440. [CrossRef]
- Chacon, F. T., Raup-Konsavage, W. M., Vrana, K. E., & Kellogg, J. J. (2022). Secondary Terpenes in Cannabis sativa L.: Synthesis and Synergy. Biomedicines, 10(12). [CrossRef]
- Cheng, Y., Dong, Z., & Liu, S. (2014). β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology, 94(1-2), 1-12. [CrossRef]
- Cherniakov, I., Izgelov, D., Domb, A. J., & Hoffman, A. (2017). The effect of Pro NanoLipospheres (PNL) formulation containing natural absorption enhancers on the oral bioavailability of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a rat model. European Journal of Pharmaceutical Sciences, 109, 21-30. [CrossRef]
- Chiurchiù, V., Leuti, A., & Maccarrone, M. (2015). Cannabinoid Signaling and Neuroinflammatory Diseases: A Melting pot for the Regulation of Brain Immune Responses. J Neuroimmune Pharmacol, 10(2), 268-280. [CrossRef]
- Cogan, P. S. (2020). The ‘entourage effect’ or ‘hodge-podge hashish’: the questionable rebranding, marketing, and expectations of cannabis polypharmacy. Expert Review of Clinical Pharmacology, 13(8), 835-845. [CrossRef]
- Correa, F., Docagne, F., Mestre, L., Clemente, D., Hernangómez, M., Loría, F., & Guaza, C. (2009). A role for CB2 receptors in anandamide signalling pathways involved in the regulation of IL-12 and IL-23 in microglial cells. Biochemical pharmacology, 77(1), 86-100. [CrossRef]
- Correa, F., Hernangómez, M., Mestre, L., Loría, F., Spagnolo, A., Docagne, F., Di Marzo, V., & Guaza, C. (2010). Anandamide enhances IL-10 production in activated microglia by targeting CB2 receptors: Roles of ERK1/2, JNK, and NF-κB. Glia, 58(2), 135-147.
- Costa, L., Garrick, J., Roquè, P., & Pellacani, C. (2016). Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev 2016: 2986796. In: Epub 2016/02/24. [CrossRef]
- Cox-Georgian, D., Ramadoss, N., Dona, C., & Basu, C. (2019). Therapeutic and medicinal uses of terpenes. Medicinal plants: from farm to pharmacy, 333-359.
- Cristino, L., Bisogno, T., & Di Marzo, V. (2020). Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature Reviews Neurology, 16(1), 9-29. [CrossRef]
- De-Oliveira, A., Ribeiro-Pinto, L., Otto, S., Gonçalves, A., & Paumgartten, F. (1997). Induction of liver monooxygenases by β-myrcene. Toxicology, 124(2), 135-140. [CrossRef]
- De Petrocellis, L., Ligresti, A., Moriello, A. S., Allarà, M., Bisogno, T., Petrosino, S., Stott, C. G., & Di Marzo, V. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British journal of pharmacology, 163(7), 1479-1494. [CrossRef]
- De Vries, H. E., Blom-Roosemalen, M. C., Van Oosten, M., De Boer, A. G., Van Berkel, T. J., Breimer, D. D., & Kuiper, J. (1996). The influence of cytokines on the integrity of the blood-brain barrier in vitro. Journal of neuroimmunology, 64(1), 37-43.
- Di Marzo, V., & Piscitelli, F. (2015). The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics, 12, 692-698.
- Díaz-Alonso, J., Paraíso-Luna, J., Navarrete, C., del Río, C., Cantarero, I., Palomares, B., Aguareles, J., Fernández-Ruiz, J., Bellido, M. L., Pollastro, F., Appendino, G., Calzado, M. A., Galve-Roperh, I., & Muñoz, E. (2016). VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Scientific Reports, 6(1), 29789. [CrossRef]
- Dowie, M., Howard, M., Nicholson, L., Faull, R., Hannan, A., & Glass, M. (2010). Behavioural and molecular consequences of chronic cannabinoid treatment in Huntington's disease transgenic mice. Neuroscience, 170(1), 324-336.
- Downer, E. J. (2020). Anti-inflammatory Potential of Terpenes Present in Cannabis sativa L. ACS Chemical Neuroscience, 11(5), 659-662. [CrossRef]
- El-Remessy, A. B., Tang, Y., Zhu, G., Matragoon, S., Khalifa, Y., Liu, E., Liu, J., Hanson, E., Mian, S., & Fatteh, N. (2008). Neuroprotective effects of cannabidiol in endotoxin-induced uveitis: critical role of p38 MAPK activation. Molecular vision, 14, 2190.
- El Khoury, J. B., Moore, K. J., Means, T. K., Leung, J., Terada, K., Toft, M., Freeman, M. W., & Luster, A. D. (2003). CD36 mediates the innate host response to β-amyloid. The Journal of experimental medicine, 197(12), 1657-1666. [CrossRef]
- El Maghraby, G., Williams, A. C., & Barry, B. (2004). Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. International journal of pharmaceutics, 276(1-2), 143-161. [CrossRef]
- Eljaschewitsch, E., Witting, A., Mawrin, C., Lee, T., Schmidt, P. M., Wolf, S., Hoertnagl, H., Raine, C. S., Schneider-Stock, R., & Nitsch, R. (2006). The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron, 49(1), 67-79. [CrossRef]
- ElSohly, M. A., Radwan, M. M., Gul, W., Chandra, S., & Galal, A. (2017). Phytochemistry of Cannabis sativa L. Phytocannabinoids: unraveling the complex chemistry and pharmacology of Cannabis sativa, 1-36.
- Erridge, S., Mangal, N., Salazar, O., Pacchetti, B., & Sodergren, M. H. (2020). Cannflavins – From plant to patient: A scoping review. Fitoterapia, 146, 104712. [CrossRef]
- Espadas, I., Keifman, E., Palomo-Garo, C., Burgaz, S., García, C., Fernández-Ruiz, J., & Moratalla, R. (2020). Beneficial effects of the phytocannabinoid Δ9-THCV in L-DOPA-induced dyskinesia in Parkinson's disease. Neurobiology of disease, 141, 104892. [CrossRef]
- Esposito, G., De Filippis, D., Carnuccio, R., Izzo, A. A., & Iuvone, T. (2006). The marijuana component cannabidiol inhibits β-amyloid-induced tau protein hyperphosphorylation through Wnt/β-catenin pathway rescue in PC12 cells. Journal of molecular medicine, 84, 253-258. [CrossRef]
- Esposito, G., Scuderi, C., Savani, C., Steardo Jr, L., De Filippis, D., Cottone, P., Iuvone, T., Cuomo, V., & Steardo, L. (2007). Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. British journal of pharmacology, 151(8), 1272-1279.
- Eubanks, L. M., Rogers, C. J., Beuscher IV, A. E., Koob, G. F., Olson, A. J., Dickerson, T. J., & Janda, K. D. (2006). A molecular link between the active component of marijuana and Alzheimer's disease pathology. Molecular pharmaceutics, 3(6), 773-777.
- Fassbender, K., Walter, S., Kühl, S., Landmann, R., Ishii, K., Bertsch, T., Stalder, A., Muehlhauser, F., Liu, Y., & Ulmer, A. (2004). The LPS receptor (CD14) links innate immunity with Alzheimer's disease. The FASEB Journal, 18(1), 203-205.
- Feliú, A., Moreno-Martet, M., Mecha, M., Carrillo-Salinas, F., De Lago, E., Fernández-Ruiz, J., & Guaza, C. (2015). AS ativex®-like combination of phytocannabinoids as a disease-modifying therapy in a viral model of multiple sclerosis. British journal of pharmacology, 172(14), 3579-3595.
- Fidyt, K., Fiedorowicz, A., Strządała, L., & Szumny, A. (2016). β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer medicine, 5(10), 3007-3017.
- Filiano, A. J., Gadani, S. P., & Kipnis, J. (2015). Interactions of innate and adaptive immunity in brain development and function. Brain research, 1617, 18-27. [CrossRef]
- Finlay, D. B., Sircombe, K. J., Nimick, M., Jones, C., & Glass, M. (2020). Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Frontiers in pharmacology, 11, 359. [CrossRef]
- Frassinetti, S., Moccia, E., Caltavuturo, L., Gabriele, M., Longo, V., Bellani, L., Giorgi, G., & Giorgetti, L. (2018). Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food chemistry, 262, 56-66. [CrossRef]
- Galiègue, S., Mary, S., Marchand, J., Dussossoy, D., Carrière, D., Carayon, P., Bouaboula, M., Shire, D., LE Fur, G., & Casellas, P. (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European journal of biochemistry, 232(1), 54-61. [CrossRef]
- García, C., Gómez-Cañas, M., Burgaz, S., Palomares, B., Gómez-Gálvez, Y., Palomo-Garo, C., Campo, S., Ferrer-Hernández, J., Pavicic, C., Navarrete, C., Luz Bellido, M., García-Arencibia, M., Ruth Pazos, M., Muñoz, E., & Fernández-Ruiz, J. (2018). Benefits of VCE-003.2, a cannabigerol quinone derivative, against inflammation-driven neuronal deterioration in experimental Parkinson’s disease: possible involvement of different binding sites at the PPARγ receptor. Journal of Neuroinflammation, 15(1), 19. [CrossRef]
- Gaston, T. E., & Friedman, D. (2017). Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy & Behavior, 70, 313-318. [CrossRef]
- Gertsch, J., Leonti, M., Raduner, S., Racz, I., Chen, J.-Z., Xie, X.-Q., Altmann, K.-H., Karsak, M., & Zimmer, A. (2008). Beta-caryophyllene is a dietary cannabinoid. Proceedings of the National Academy of Sciences, 105(26), 9099-9104. [CrossRef]
- Giacobbe, J., Marrocu, A., Di Benedetto, M. G., Pariante, C. M., & Borsini, A. (2021). A systematic, integrative review of the effects of the endocannabinoid system on inflammation and neurogenesis in animal models of affective disorders. Brain, behavior, and immunity, 93, 353-367. [CrossRef]
- Goulle, J., Saussereau, E., & Lacroix, C. (2008). Delta-9-tetrahydrocannabinol pharmacokinetics. Annales pharmaceutiques francaises.
- Granja, A. G., Carrillo-Salinas, F., Pagani, A., Gómez-Cañas, M., Negri, R., Navarrete, C., Mecha, M., Mestre, L., Fiebich, B. L., & Cantarero, I. (2012). A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. Journal of neuroimmune pharmacology, 7(4), 1002-1016. [CrossRef]
- Griciuc, A., Serrano-Pozo, A., Parrado, A. R., Lesinski, A. N., Asselin, C. N., Mullin, K., Hooli, B., Choi, S. H., Hyman, B. T., & Tanzi, R. E. (2013). Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron, 78(4), 631-643. [CrossRef]
- Guerreiro, R., Wojtas, A., Bras, J., Carrasquillo, M., Rogaeva, E., Majounie, E., Cruchaga, C., Sassi, C., Kauwe, J. S., & Younkin, S. (2013). TREM2 variants in Alzheimer's disease. New England Journal of Medicine, 368(2), 117-127.
- Gugliandolo, A., Pollastro, F., Grassi, G., Bramanti, P., & Mazzon, E. (2018). In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. International Journal of Molecular Sciences, 19(7), 1992. https://www.mdpi.com/1422-0067/19/7/1992. [CrossRef]
- Gülck, T., Booth, J., Carvalho, Â., Khakimov, B., Crocoll, C., Motawia, M., Møller, B., Bohlmann, J., & Gallage, N. (2020). Synthetic biology of cannabinoids and cannabinoid glucosides in Nicotiana benthamiana and Saccharomyces cerevisiae. Journal of Natural Products, 83(10), 2877-2893. [CrossRef]
- Hagberg, B., Hanefeld, F., Percy, A., & Skjeldal, O. (2002). An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett syndrome clinical criteria consensus panel satellite to European Paediatric neurology society meeting, Baden Baden, Germany, 11 September 2001. European journal of paediatric neurology: EJPN: official journal of the European Paediatric Neurology Society, 6(5), 293-297. [CrossRef]
- Hanuš, L. O., Meyer, S. M., Muñoz, E., Taglialatela-Scafati, O., & Appendino, G. (2016). Phytocannabinoids: a unified critical inventory. Natural product reports, 33(12), 1357-1392. [CrossRef]
- Hartsel, J. A., Eades, J., Hickory, B., & Makriyannis, A. (2016). Cannabis sativa and Hemp. In Nutraceuticals (pp. 735-754). Elsevier.
- Hazzah, T., Andre, C., Richter, G., McGrath, S., & Collins, F. (2020). Cannabis in veterinary medicine: a critical review. AHVMA, 61, 25.
- He, B., Chen, Y., Yu, S., Hao, Y., Wang, F., & Qu, L. (2022). Food plant extracts for sleep-related skin health: mechanisms and prospects. Food Bioscience, 101951. [CrossRef]
- Heneka, M. T., Kummer, M. P., Stutz, A., Delekate, A., Schwartz, S., Vieira-Saecker, A., Griep, A., Axt, D., Remus, A., & Tzeng, T.-C. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature, 493(7434), 674-678. [CrossRef]
- Hickman, S. E., Allison, E. K., & El Khoury, J. (2008). Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer's disease mice. Journal of Neuroscience, 28(33), 8354-8360.
- Hill, A. J., Weston, S. E., Jones, N. A., Smith, I., Bevan, S. A., Williamson, E. M., Stephens, G. J., Williams, C. M., & Whalley, B. J. (2010). Δ9-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia, 51(8), 1522-1532.
- Hillard, C. J. (2018). Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology, 43(1), 155-172.
- Howlett, A. C., Evans, D., & Houston, D. (2019). The cannabinoid receptor. Marijuana/cannabinoids, 35-72.
- Jean-Gilles, L., Gran, B., & Constantinescu, C. S. (2010). Interaction between cytokines, cannabinoids and the nervous system. Immunobiology, 215(8), 606-610. [CrossRef]
- Ji, K., Akgul, G., Wollmuth, L. P., & Tsirka, S. E. (2013). Microglia actively regulate the number of functional synapses. PloS one, 8(2), e56293. [CrossRef]
- Jin, D., Dai, K., Xie, Z., & Chen, J. (2020). Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Scientific Reports, 10(1), 1-14. [CrossRef]
- Johnson, J. R., Burnell-Nugent, M., Lossignol, D., Ganae-Motan, E. D., Potts, R., & Fallon, M. T. (2010). Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. Journal of pain and symptom management, 39(2), 167-179.
- Kagan, J. C., & Horng, T. (2013). NLRP3 inflammasome activation: CD36 serves double duty. Nature immunology, 14(8), 772-774. [CrossRef]
- Kaplan, J. S., Stella, N., Catterall, W. A., & Westenbroek, R. E. (2017). Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proceedings of the National Academy of Sciences, 114(42), 11229-11234. [CrossRef]
- Kennedy, D. O., Dodd, F. L., Robertson, B. C., Okello, E. J., Reay, J. L., Scholey, A. B., & Haskell, C. F. (2011). Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. Journal of Psychopharmacology, 25(8), 1088-1100. [CrossRef]
- Keren-Shaul, H., Spinrad, A., Weiner, A., Matcovitch-Natan, O., Dvir-Szternfeld, R., Ulland, T. K., David, E., Baruch, K., Lara-Astaiso, D., & Toth, B. (2017). A unique microglia type associated with restricting development of Alzheimer’s disease. Cell, 169(7), 1276-1290. e1217. [CrossRef]
- Kettenmann, H., Hanisch, U.-K., Noda, M., & Verkhratsky, A. (2011). Physiology of microglia. Physiological reviews, 91(2), 461-553. [CrossRef]
- Killestein, J., Hoogervorst, E., Reif, M., Blauw, B., Smits, M., Uitdehaag, B., Nagelkerken, L., & Polman, C. (2003). Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. Journal of neuroimmunology, 137(1-2), 140-143.
- Kitazawa, M., Oddo, S., Yamasaki, T. R., Green, K. N., & LaFerla, F. M. (2005). Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. Journal of Neuroscience, 25(39), 8843-8853.
- Koltai, H., & Namdar, D. (2020). Cannabis Phytomolecule 'Entourage': From Domestication to Medical Use. Trends in Plant Science, 25(10), 976-984. [CrossRef]
- Konsman, J. P. (2022). Cytokines in the Brain and Neuroinflammation: We Didn’t Starve the Fire! Pharmaceuticals, 15(2), 140. https://www.mdpi.com/1424-8247/15/2/140. [CrossRef]
- Kozela, E., Pietr, M., Juknat, A., Rimmerman, N., Levy, R., & Vogel, Z. (2010). Cannabinoids Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-activated NF-κB and Interferon-β/STAT Proinflammatory Pathways in BV-2 Microglial Cells*. Journal of Biological Chemistry, 285(3), 1616-1626. [CrossRef]
- Laflamme, N., & Rivest, S. (1999). Effects of systemic immunogenic insults and circulating proinflammatory cytokines on the transcription of the inhibitory factor κBα within specific cellular populations of the rat brain. Journal of neurochemistry, 73(1), 309-321. [CrossRef]
- Lastres-Becker, I., Bizat, N., Boyer, F., Hantraye, P., Brouillet, E., & Fernández-Ruiz, J. (2003). Effects of cannabinoids in the rat model of Huntington's disease generated by an intrastriatal injection of malonate. Neuroreport, 14(6), 813-816. [CrossRef]
- Lastres-Becker, I., Molina-Holgado, F., Ramos, J. A., Mechoulam, R., & Fernández-Ruiz, J. (2005). Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiology of disease, 19(1-2), 96-107.
- Legault, J., & Pichette, A. (2007). Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. Journal of Pharmacy and Pharmacology, 59(12), 1643-1647.
- Liu, G., & Jiang, Q. (2016). Alzheimer’s disease CD33 rs3865444 variant does not contribute to cognitive performance. Proceedings of the National Academy of Sciences, 113(12), E1589-E1590. [CrossRef]
- Lorenzetti, B. B., Souza, G. E., Sarti, S. J., Santos Filho, D., & Ferreira, S. H. (1991). Myrcene mimics the peripheral analgesic activity of lemongrass tea. Journal of ethnopharmacology, 34(1), 43-48. [CrossRef]
- Ludwiczuk, A., Skalicka-Woźniak, K., & Georgiev, M. (2017). Terpenoids. In Pharmacognosy (pp. 233-266). Elsevier.
- Lyman, M., Lloyd, D. G., Ji, X., Vizcaychipi, M. P., & Ma, D. (2014). Neuroinflammation: The role and consequences. Neuroscience Research, 79, 1-12. [CrossRef]
- Maccarrone, M., Rossi, S., Bari, M., De Chiara, V., Rapino, C., Musella, A., Bernardi, G., Bagni, C., & Centonze, D. (2010). Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology, 35(7), 1500-1509. [CrossRef]
- Maestroni, G. J. (2004). The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. The FASEB Journal, 18(15), 1914-1916. [CrossRef]
- Marinelli, S., Pacioni, S., Bisogno, T., Di Marzo, V., Prince, D. A., Huguenard, J. R., & Bacci, A. (2008). The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. Journal of Neuroscience, 28(50), 13532-13541. [CrossRef]
- Marquette, C., Van Dam, A.-M., Ceccaldi, P.-E., Weber, P., Haour, F., & Tsiang, H. (1996). Induction of immunoreactive interleukin-1β and tumor necrosis factor-α in the brains of rabies virus infected rats. Journal of neuroimmunology, 68(1-2), 45-51. [CrossRef]
- Marsh, D. T. (2022). Exploring the Bioactivity and Therapeutic Potential of Structurally Diverse Phytochemicals in Neurodegenerative and Gastrointestinal Disease.
- Mawuenyega, K. G., Sigurdson, W., Ovod, V., Munsell, L., Kasten, T., Morris, J. C., Yarasheski, K. E., & Bateman, R. J. (2010). Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science, 330(6012), 1774-1774.
- Mazuz, M., Tiroler, A., Moyal, L., Hodak, E., Nadarajan, S., Vinayaka, A. C., Gorovitz-Haris, B., Lubin, I., Drori, A., & Drori, G. (2020). Synergistic cytotoxic activity of cannabinoids from cannabis sativa against cutaneous T-cell lymphoma (CTCL) in-vitro and ex-vivo. Oncotarget, 11(13), 1141.
- Mechoulam, R., & Ben-Shabat, S. (1999). From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: the ongoing story of cannabis. Natural product reports, 16(2), 131-143. [CrossRef]
- Micheau, O., & Tschopp, J. (2003). Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell, 114(2), 181-190. [CrossRef]
- Moreno-Martet, M., Feliú, A., Espejo-Porras, F., Mecha, M., Carrillo-Salinas, F. J., Fernández-Ruiz, J., Guaza, C., & de Lago, E. (2015). The disease-modifying effects of a Sativex-like combination of phytocannabinoids in mice with experimental autoimmune encephalomyelitis are preferentially due to Δ9-tetrahydrocannabinol acting through CB1 receptors. Multiple sclerosis and related disorders, 4(6), 505-511. [CrossRef]
- Mosley, R. L., Benner, E. J., Kadiu, I., Thomas, M., Boska, M. D., Hasan, K., Laurie, C., & Gendelman, H. E. (2006). Neuroinflammation, oxidative stress, and the pathogenesis of Parkinson’s disease. Clinical neuroscience research, 6(5), 261-281.
- Moulin, D., Boulanger, A., Clark, A., Clarke, H., Dao, T., Finley, G., Furlan, A., Gilron, I., Gordon, A., & Morley-Forster, P. K. (2014). Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Research and Management, 19, 328-335. [CrossRef]
- Nadal, X., del Río, C., Casano, S., Palomares, B., Ferreiro-Vera, C., Navarrete, C., Sánchez-Carnerero, C., Cantarero, I., Bellido, M. L., Meyer, S., Morello, G., Appendino, G., & Muñoz, E. (2017). Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. British journal of pharmacology, 174(23), 4263-4276. [CrossRef]
- Nallathambi, R., Mazuz, M., Namdar, D., Shik, M., Namintzer, D., Vinayaka, A. C., Ion, A., Faigenboim, A., Nasser, A., Laish, I., Konikoff, F. M., & Koltai, H. (2018). Identification of Synergistic Interaction Between Cannabis-Derived Compounds for Cytotoxic Activity in Colorectal Cancer Cell Lines and Colon Polyps That Induces Apoptosis-Related Cell Death and Distinct Gene Expression. Cannabis Cannabinoid Res, 3(1), 120-135. [CrossRef]
- Namdar, D., Voet, H., Ajjampura, V., Nadarajan, S., Mayzlish-Gati, E., Mazuz, M., Shalev, N., & Koltai, H. (2019). Terpenoids and phytocannabinoids co-produced in Cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules, 24(17), 3031. [CrossRef]
- Neves, Â., Rosa, S., Gonçalves, J., Rufino, A., Judas, F., Salgueiro, L., Lopes, M. C., Cavaleiro, C., & Mendes, A. F. (2010). Screening of five essential oils for identification of potential inhibitors of IL-1-induced NF-κB activation and NO production in human chondrocytes: characterization of the inhibitory activity of α-pinene. Planta medica, 76(03), 303-308.
- Noma, Y., Hashimoto, T., Uehara, S., & Asakawa, Y. (2010). Erratum: Microbial transformation of isopinocampheol and caryophyllene oxide. Flavour and fragrance journal, 25(4), 257-266. [CrossRef]
- Osborne, A. L., Solowij, N., Babic, I., Huang, X.-F., & Weston-Green, K. (2017). Improved social interaction, recognition and working memory with cannabidiol treatment in a prenatal infection (poly I: C) rat model. Neuropsychopharmacology, 42(7), 1447-1457.
- Pamplona, F. A., Da Silva, L. R., & Coan, A. C. (2018). Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: observational data meta-analysis. Frontiers in neurology, 9, 759.
- Parkhurst, C. N., Yang, G., Ninan, I., Savas, J. N., Yates III, J. R., Lafaille, J. J., Hempstead, B. L., Littman, D. R., & Gan, W.-B. (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 155(7), 1596-1609.
- Piomelli, D., Beltramo, M., Giuffrida, A., & Stella, N. (1998). Endogenous cannabinoid signaling. Neurobiology of disease, 5(6), 462-473. [CrossRef]
- Pisanti, S., & Bifulco, M. (2019). Medical Cannabis: A plurimillennial history of an evergreen. Journal of cellular physiology, 234(6), 8342-8351. [CrossRef]
- Pretzsch, C. M., Voinescu, B., Lythgoe, D., Horder, J., Mendez, M. A., Wichers, R., Ajram, L., Ivin, G., Heasman, M., & Edden, R. A. (2019). Effects of cannabidivarin (CBDV) on brain excitation and inhibition systems in adults with and without Autism Spectrum Disorder (ASD): a single dose trial during magnetic resonance spectroscopy. Translational psychiatry, 9(1), 313. [CrossRef]
- Puffenbarger, R. A., Boothe, A. C., & Cabral, G. A. (2000). Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia, 29(1), 58-69. [CrossRef]
- Pugazhendhi, A., Suganthy, N., Chau, T. P., Sharma, A., Unpaprom, Y., Ramaraj, R., Karuppusamy, I., & Brindhadevi, K. (2021). Cannabinoids as anticancer and neuroprotective drugs: Structural insights and pharmacological interactions—A review. Process Biochemistry, 111, 9-31. [CrossRef]
- Rahaman, O., & Ganguly, D. (2021). Endocannabinoids in immune regulation and immunopathologies. Immunology, 164(2), 242-252. [CrossRef]
- Rao, R., Nagarkatti, P., & Nagarkatti, M. (2014). Staphylococcal enterotoxin B-induced microRNA-155 targets SOCS1 to promote acute inflammatory lung injury. Infection and immunity, 82(7), 2971-2979. [CrossRef]
- Ricceri, L., De Filippis, B., & Laviola, G. (2013). Rett syndrome treatment in mouse models: searching for effective targets and strategies. Neuropharmacology, 68, 106-115. [CrossRef]
- Rufino, A. T., Ribeiro, M., Judas, F., Salgueiro, L., Lopes, M. C., Cavaleiro, C., & Mendes, A. F. (2014). Anti-inflammatory and chondroprotective activity of (+)-α-pinene: structural and enantiomeric selectivity. Journal of Natural Products, 77(2), 264-269. [CrossRef]
- Russo, E. B. (2019). The case for the entourage effect and conventional breeding of clinical cannabis: no “strain,” no gain. Frontiers in plant science, 9, 1969.
- Saito, V. M., Rezende, R. M., & Teixeira, A. L. (2012). Cannabinoid modulation of neuroinflammatory disorders. Curr Neuropharmacol, 10(2), 159-166. [CrossRef]
- Santiago, M., Sachdev, S., Arnold, J. C., McGregor, I. S., & Connor, M. (2019). Absence of entourage: terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis and cannabinoid research, 4(3), 165-176.
- Saresella, M., La Rosa, F., Piancone, F., Zoppis, M., Marventano, I., Calabrese, E., Rainone, V., Nemni, R., Mancuso, R., & Clerici, M. (2016). The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Molecular neurodegeneration, 11(1), 1-14. 10.1186/s13024-016-0088-1.
- Schilling, S., Melzer, R., & McCabe, P. F. (2020). Cannabis sativa. Current Biology, 30(1), R8-R9.
- Schubert, D., Kepchia, D., Liang, Z., Dargusch, R., Goldberg, J., & Maher, P. (2019). Efficacy of cannabinoids in a pre-clinical drug-screening platform for Alzheimer’s disease. Molecular neurobiology, 56, 7719-7730. [CrossRef]
- Sheedy, F. J., Grebe, A., Rayner, K. J., Kalantari, P., Ramkhelawon, B., Carpenter, S. B., Becker, C. E., Ediriweera, H. N., Mullick, A. E., & Golenbock, D. T. (2013). CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology, 14(8), 812-820. [CrossRef]
- Sheng, W. S., Hu, S., Min, X., Cabral, G. A., Lokensgard, J. R., & Peterson, P. K. (2005). Synthetic cannabinoid WIN55, 212-2 inhibits generation of inflammatory mediators by IL-1β-stimulated human astrocytes. Glia, 49(2), 211-219.
- Shinjyo, N., & Di Marzo, V. (2013). The effect of cannabichromene on adult neural stem/progenitor cells. Neurochemistry International, 63(5), 432-437. [CrossRef]
- Sido, J. M., Jackson, A. R., Nagarkatti, P. S., & Nagarkatti, M. (2016). Marijuana-derived Δ-9-tetrahydrocannabinol suppresses Th1/Th17 cell-mediated delayed-type hypersensitivity through microRNA regulation. Journal of molecular medicine, 94(9), 1039-1051.
- Sinha, D., Bonner, T. I., Bhat, N. R., & Matsuda, L. A. (1998). Expression of the CB1 cannabinoid receptor in macrophage-like cells from brain tissue: immunochemical characterization by fusion protein antibodies. Journal of neuroimmunology, 82(1), 13-21. [CrossRef]
- Soundara Rajan, T., Giacoppo, S., Scionti, D., Diomede, F., Grassi, G., Pollastro, F., Piattelli, A., Bramanti, P., Mazzon, E., & Trubiani, O. (2017). Cannabidiol activates neuronal precursor genes in human gingival mesenchymal stromal cells. Journal of cellular biochemistry, 118(6), 1531-1546. [CrossRef]
- Stewart, C. R., Stuart, L. M., Wilkinson, K., Van Gils, J. M., Deng, J., Halle, A., Rayner, K. J., Boyer, L., Zhong, R., & Frazier, W. A. (2010). CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature immunology, 11(2), 155-161. [CrossRef]
- Tahir, M. N., Shahbazi, F., Rondeau-Gagné, S., & Trant, J. F. (2021). The biosynthesis of the cannabinoids. Journal of cannabis research, 3(1), 1-12. [CrossRef]
- Ubeed, H. M. S. A., Bhuyan, D. J., Alsherbiny, M. A., Basu, A., & Vuong, Q. V. (2022). A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules, 27(3). [CrossRef]
- Valdeolivas, S., Navarrete, C., Cantarero, I., Bellido, M. L., Muñoz, E., & Sagredo, O. (2015). Neuroprotective properties of cannabigerol in Huntington’s disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics, 12(1), 185-199.
- Van Cleemput, M., Cattoor, K., De Bosscher, K., Haegeman, G., De Keukeleire, D., & Heyerick, A. (2009). Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. Journal of Natural Products, 72(6), 1220-1230.
- Vigli, D., Cosentino, L., Raggi, C., Laviola, G., Woolley-Roberts, M., & De Filippis, B. (2018). Chronic treatment with the phytocannabinoid Cannabidivarin (CBDV) rescues behavioural alterations and brain atrophy in a mouse model of Rett syndrome. Neuropharmacology, 140, 121-129. [CrossRef]
- Walter, L., Dinh, T., & Stella, N. (2004). ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. Journal of Neuroscience, 24(37), 8068-8074. [CrossRef]
- Walter, L., Franklin, A., Witting, A., Möller, T., & Stella, N. (2002). Astrocytes in culture produce anandamide and other acylethanolamides. Journal of Biological Chemistry, 277(23), 20869-20876. [CrossRef]
- Walter, L., Franklin, A., Witting, A., Wade, C., Xie, Y., Kunos, G., Mackie, K., & Stella, N. (2003). Nonpsychotropic cannabinoid receptors regulate microglial cell migration. Journal of Neuroscience, 23(4), 1398-1405. [CrossRef]
- Wang, Q., Dong, X., Zhang, R., & Zhao, C. (2021). Flavonoids with potential anti-amyloidogenic effects as therapeutic drugs for treating Alzheimer’s disease. Journal of Alzheimer's Disease, 84(2), 505-533. [CrossRef]
- Wang, W., Wu, N., Zu, Y., & Fu, Y. (2008). Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food chemistry, 108(3), 1019-1022. [CrossRef]
- Werz, O., Seegers, J., Schaible, A. M., Weinigel, C., Barz, D., Koeberle, A., Allegrone, G., Pollastro, F., Zampieri, L., & Grassi, G. (2014). Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition, 2(3), 53-60. [CrossRef]
- Wirenfeldt, M., Babcock, A. A., & Vinters, H. V. (2011). Microglia–insights into immune system structure, function, and reactivity in the central nervous system. Histology and histopathology, Vol. 26, nº 4 (2011).
- Wu, W., Zhang, R., McClements, D. J., Chefetz, B., Polubesova, T., & Xing, B. (2018). Transformation and speciation analysis of silver nanoparticles of dietary supplement in simulated human gastrointestinal tract. Environmental science & technology, 52(15), 8792-8800. [CrossRef]
- Xi, Z.-X., Peng, X.-Q., Li, X., Song, R., Zhang, H.-Y., Liu, Q.-R., Yang, H.-J., Bi, G.-H., Li, J., & Gardner, E. L. (2011). Brain cannabinoid CB2 receptors modulate cocaine's actions in mice. Nature neuroscience, 14(9), 1160-1166.
- Yousaf, M., Chang, D., Liu, Y., Liu, T., & Zhou, X. (2022). Neuroprotection of Cannabidiol, Its Synthetic Derivatives and Combination Preparations against Microglia-Mediated Neuroinflammation in Neurological Disorders. Molecules, 27(15), 4961. https://mdpi-res.com/d_attachment/molecules/molecules-27-04961/article_deploy/molecules-27-04961.pdf?version=1659606660. [CrossRef]
- Zamberletti, E., Gabaglio, M., Piscitelli, F., Brodie, J. S., Woolley-Roberts, M., Barbiero, I., Tramarin, M., Binelli, G., Landsberger, N., & Kilstrup-Nielsen, C. (2019). Cannabidivarin completely rescues cognitive deficits and delays neurological and motor defects in male Mecp2 mutant mice. Journal of Psychopharmacology, 33(7), 894-907. [CrossRef]
- Zhang, Z., Yang, C., Dai, X., Ao, Y., & Li, Y. (2017). Inhibitory effect of trans-caryophyllene (TC) on leukocyte-endothelial attachment. Toxicology and Applied Pharmacology, 329, 326-333. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





