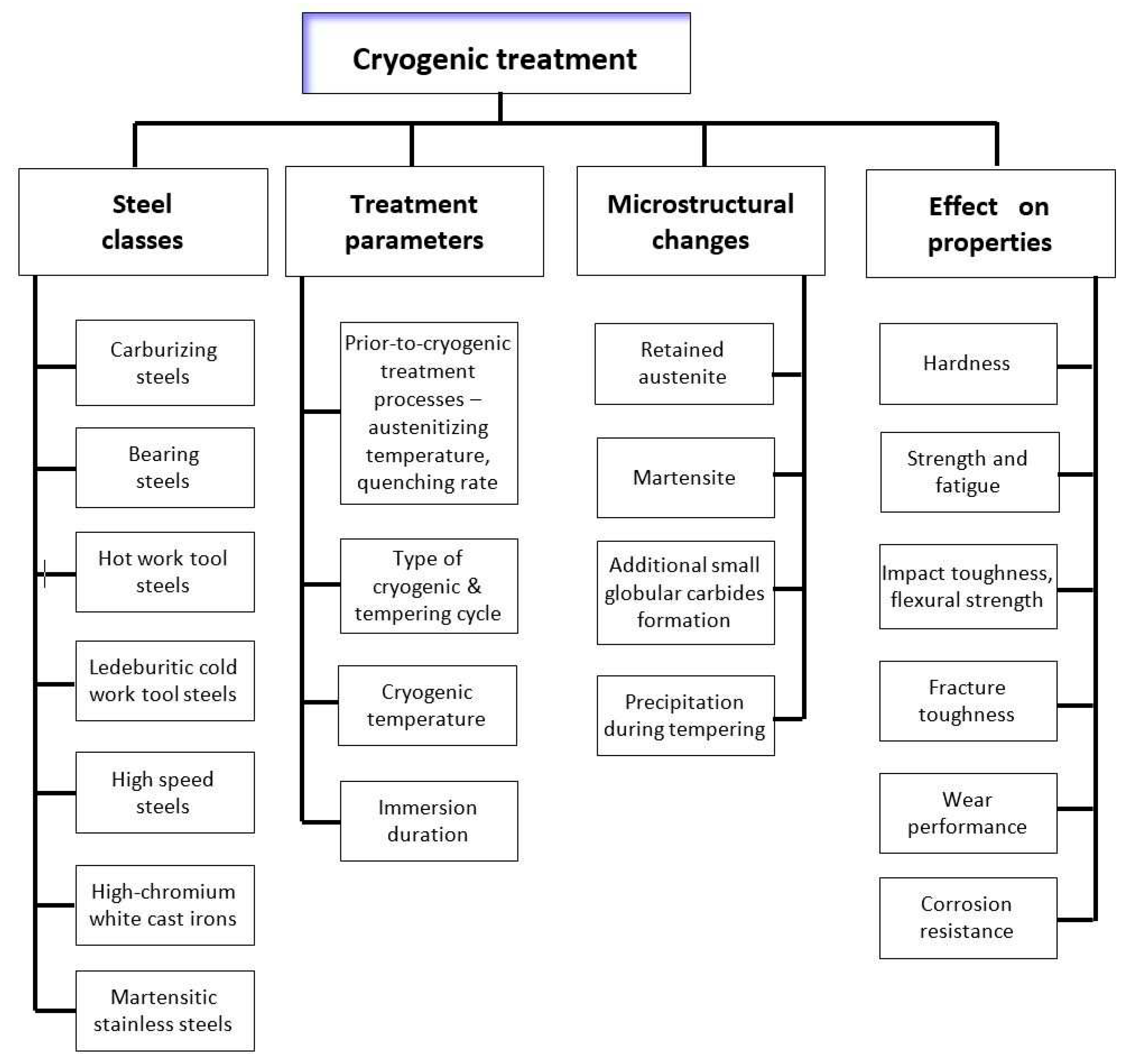
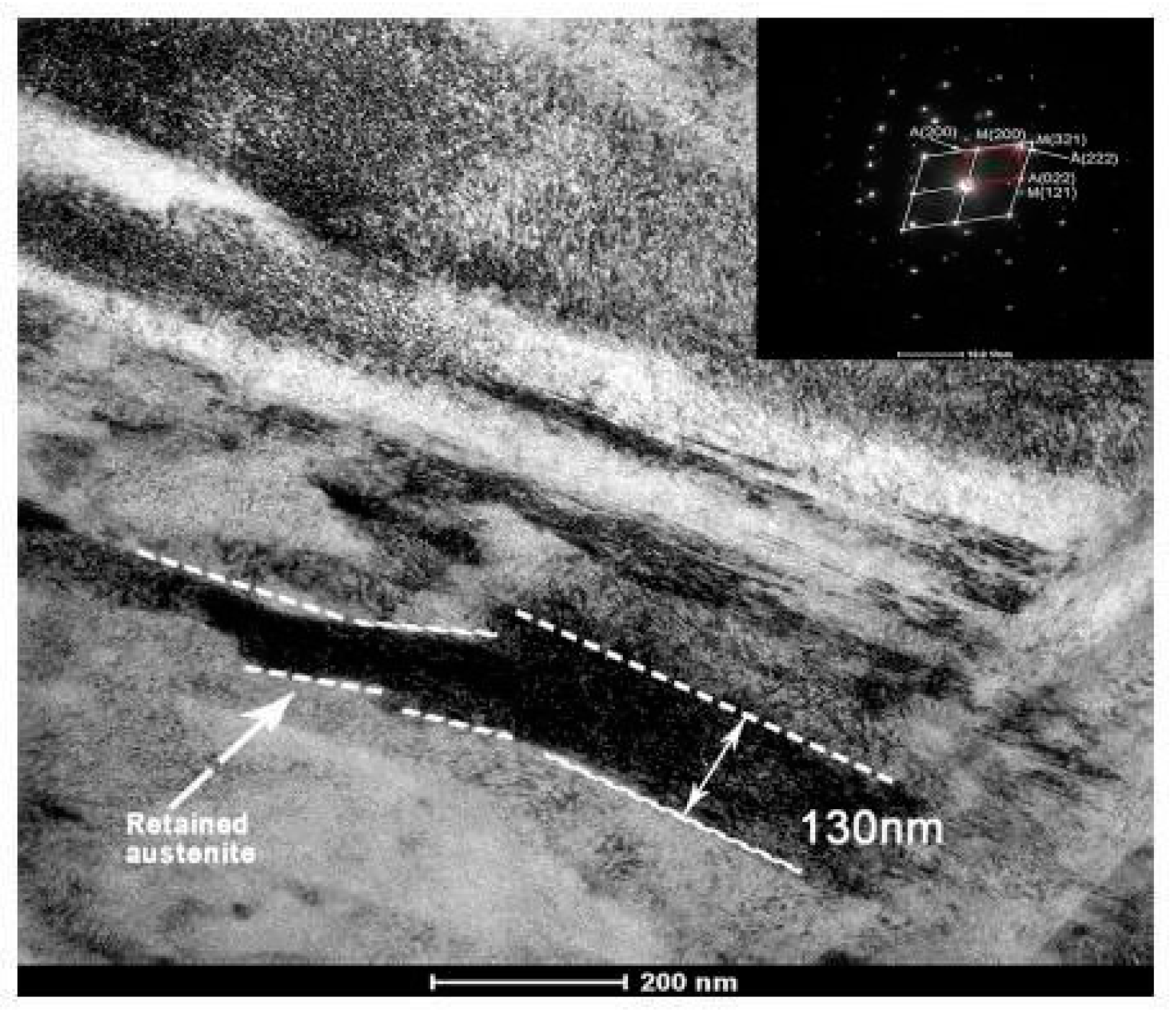
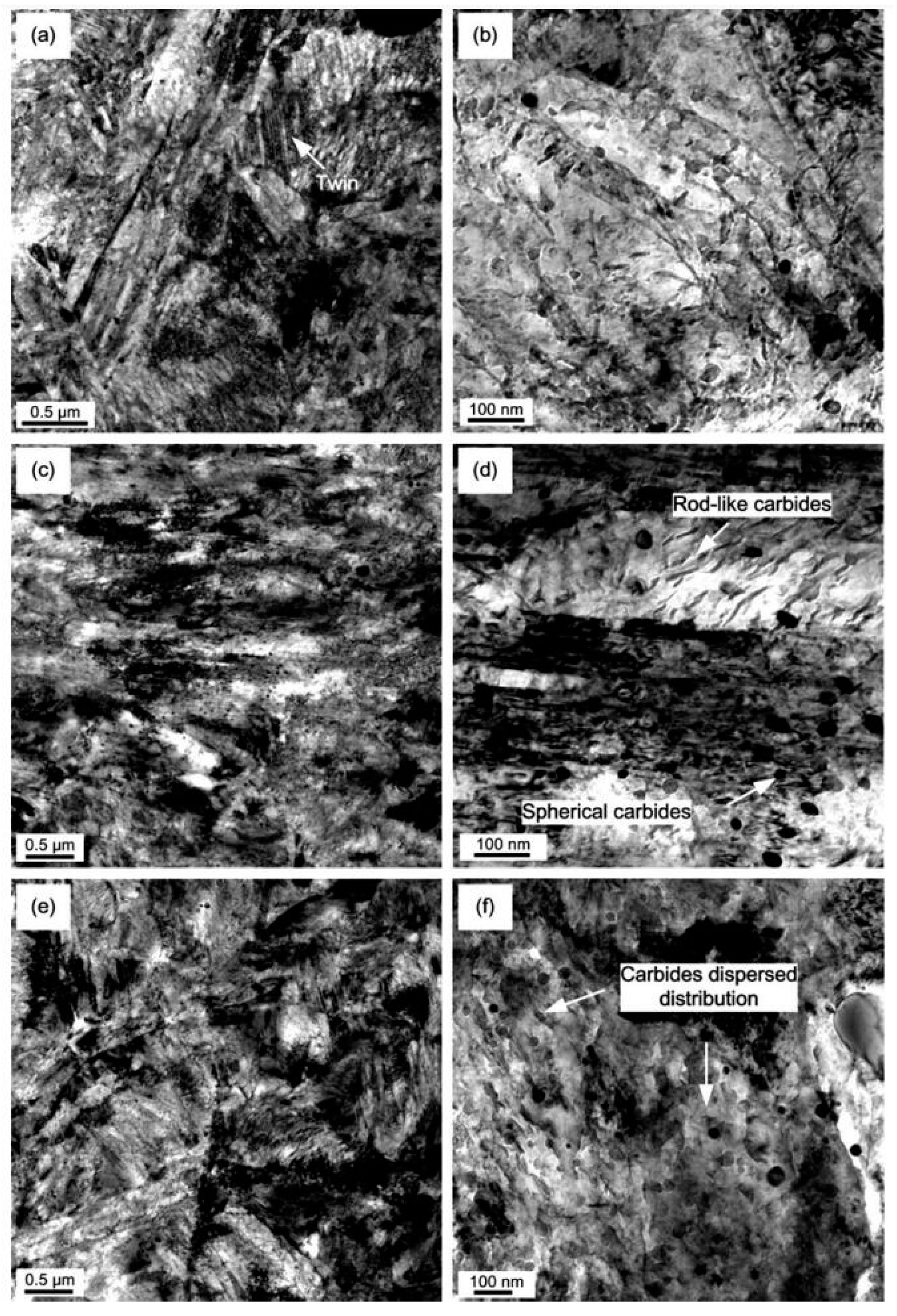
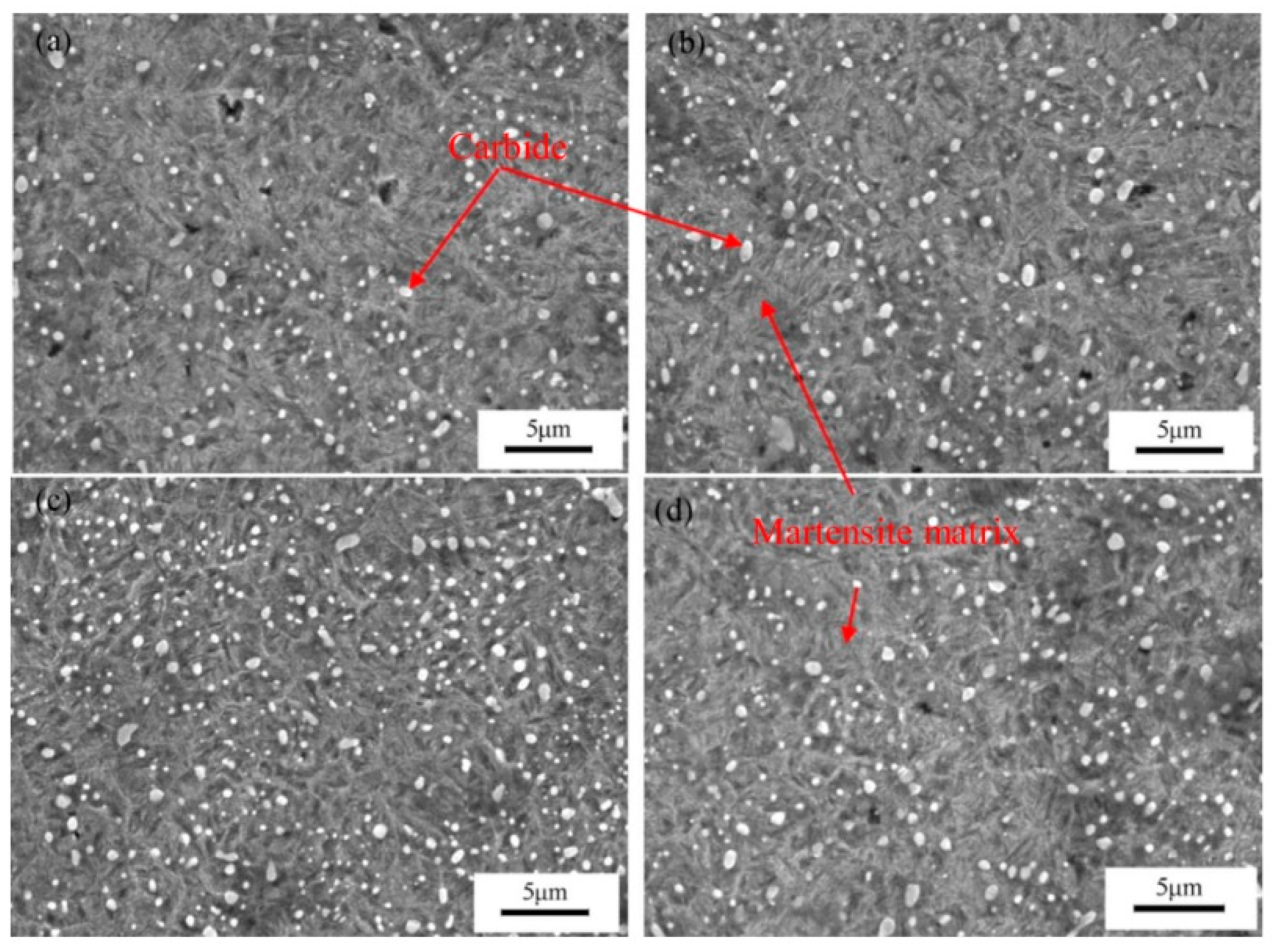
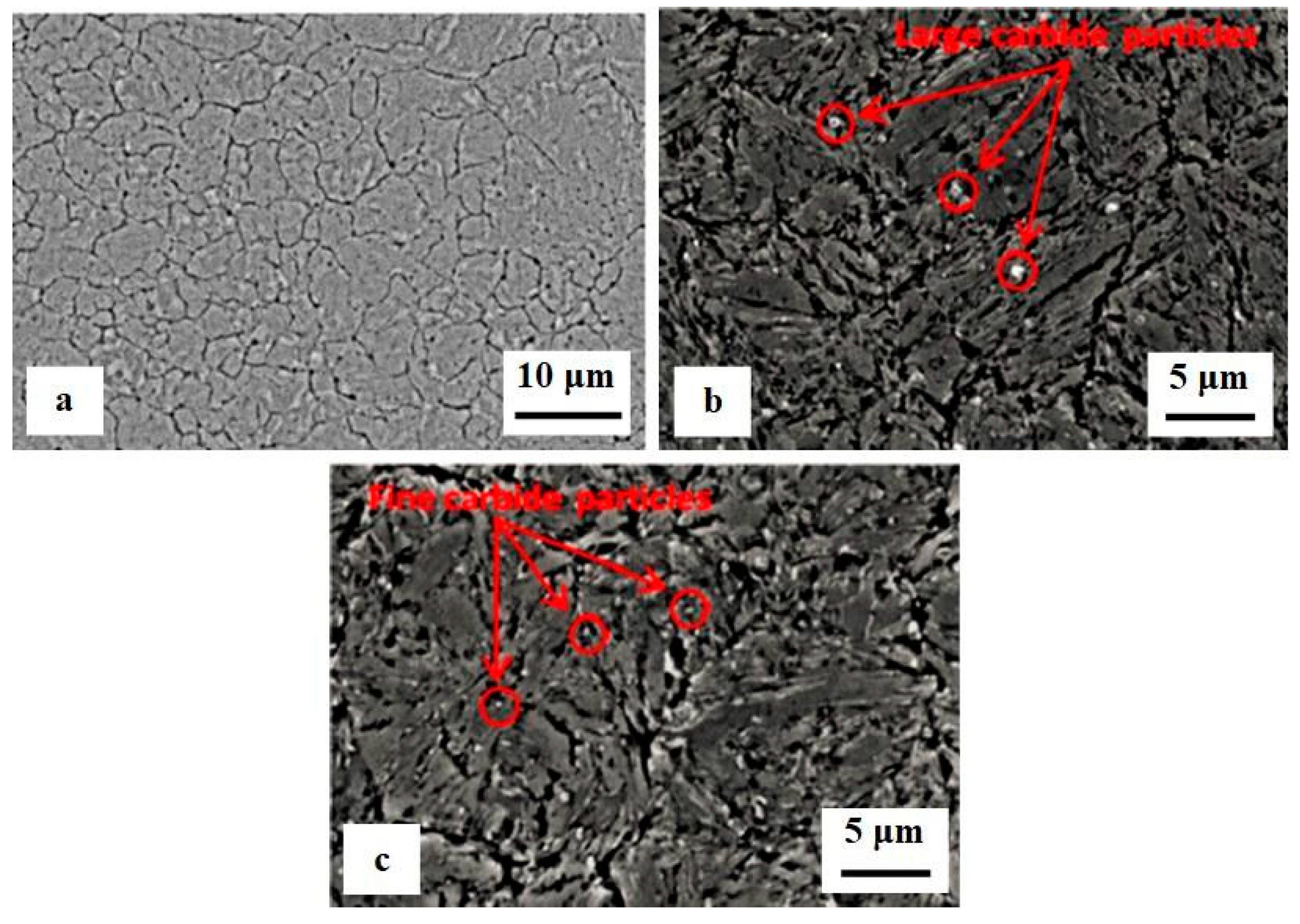
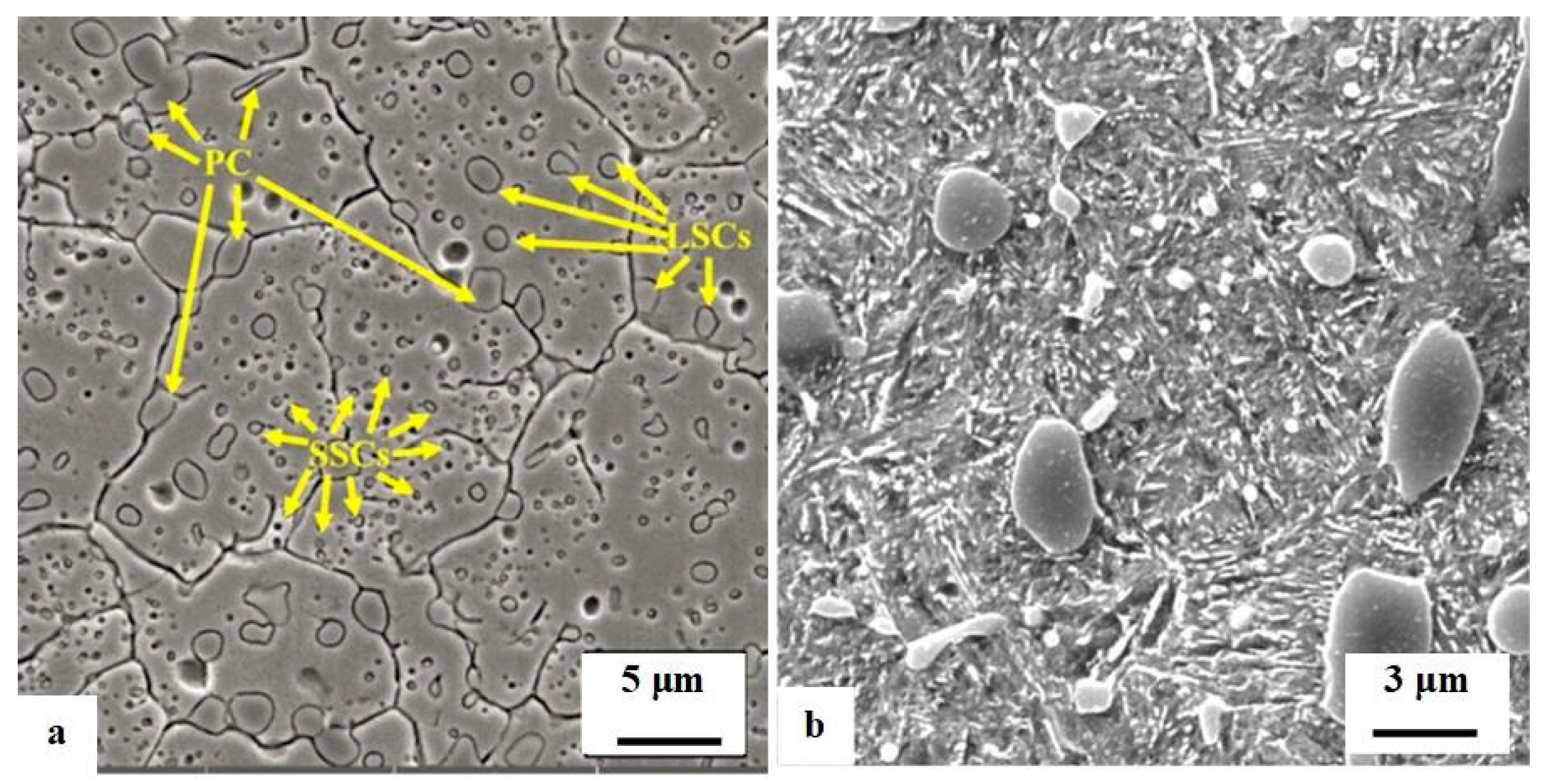
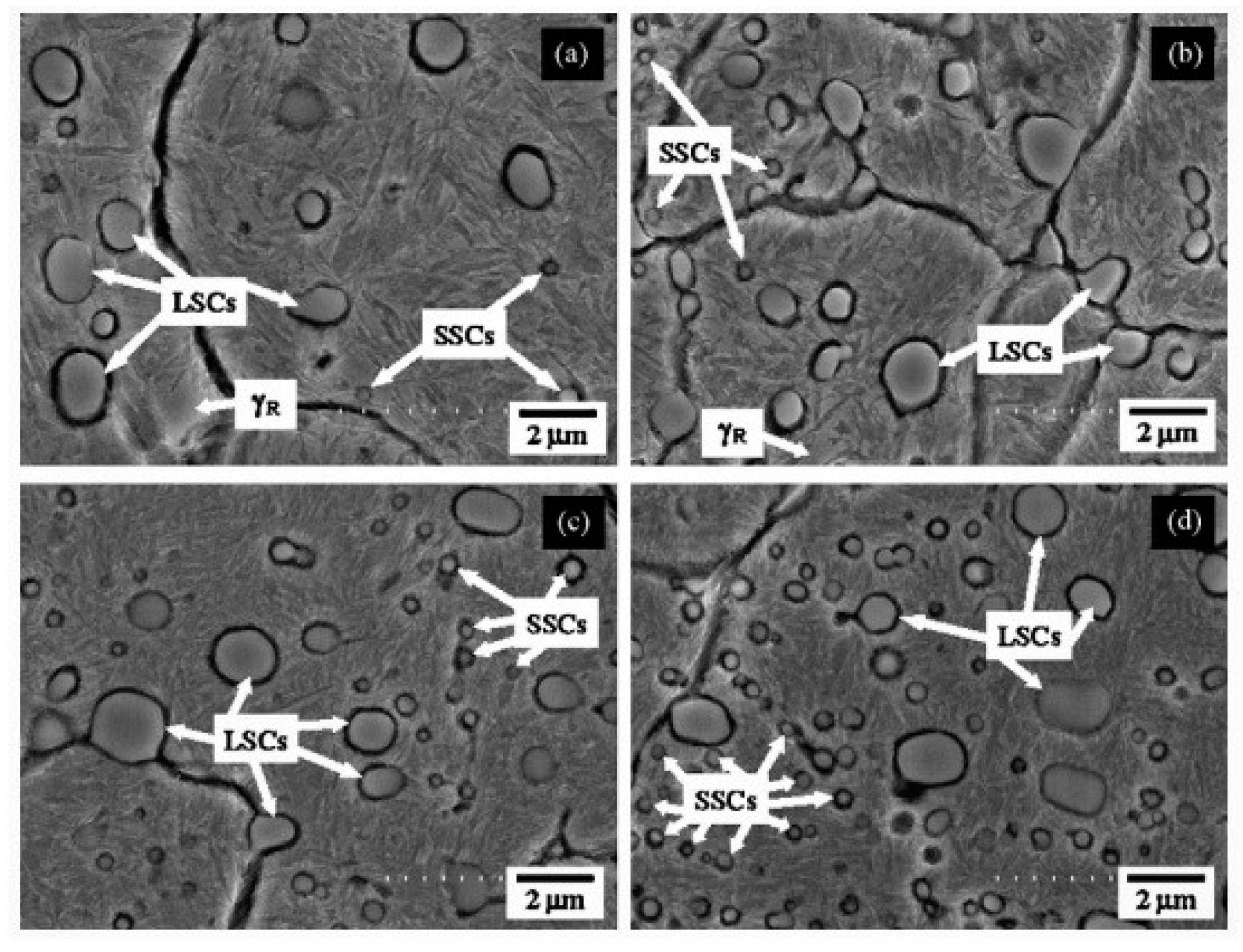
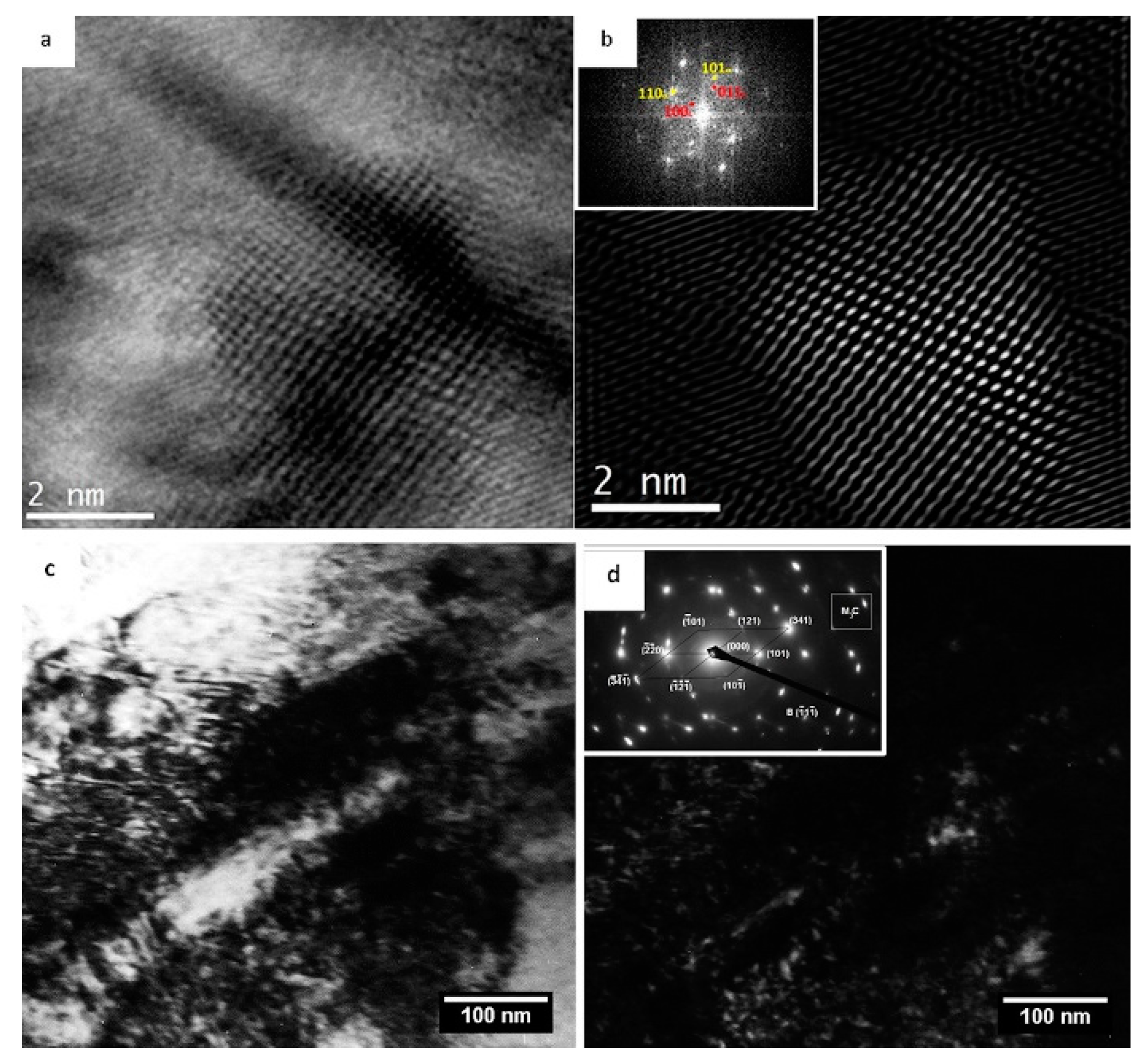
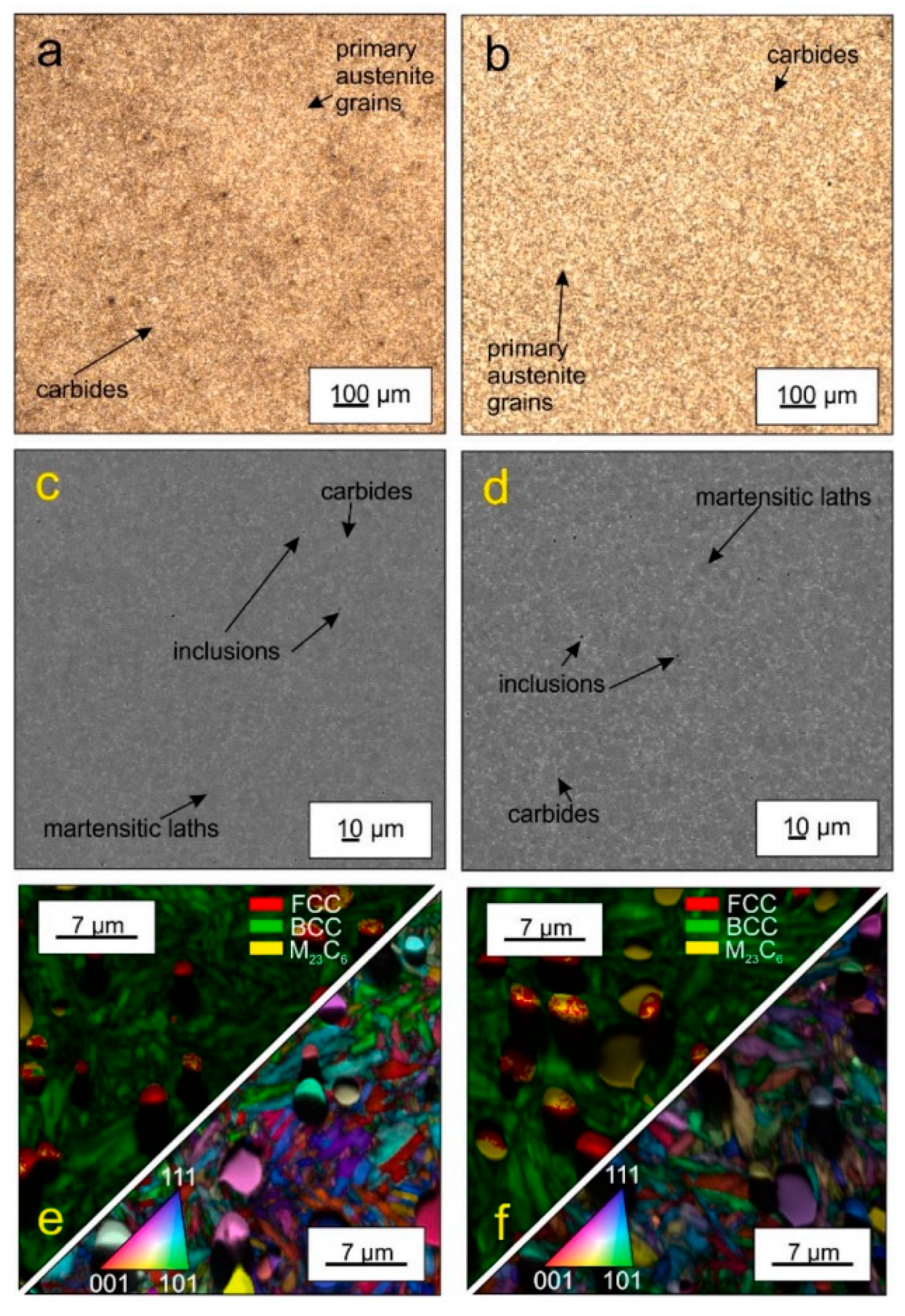
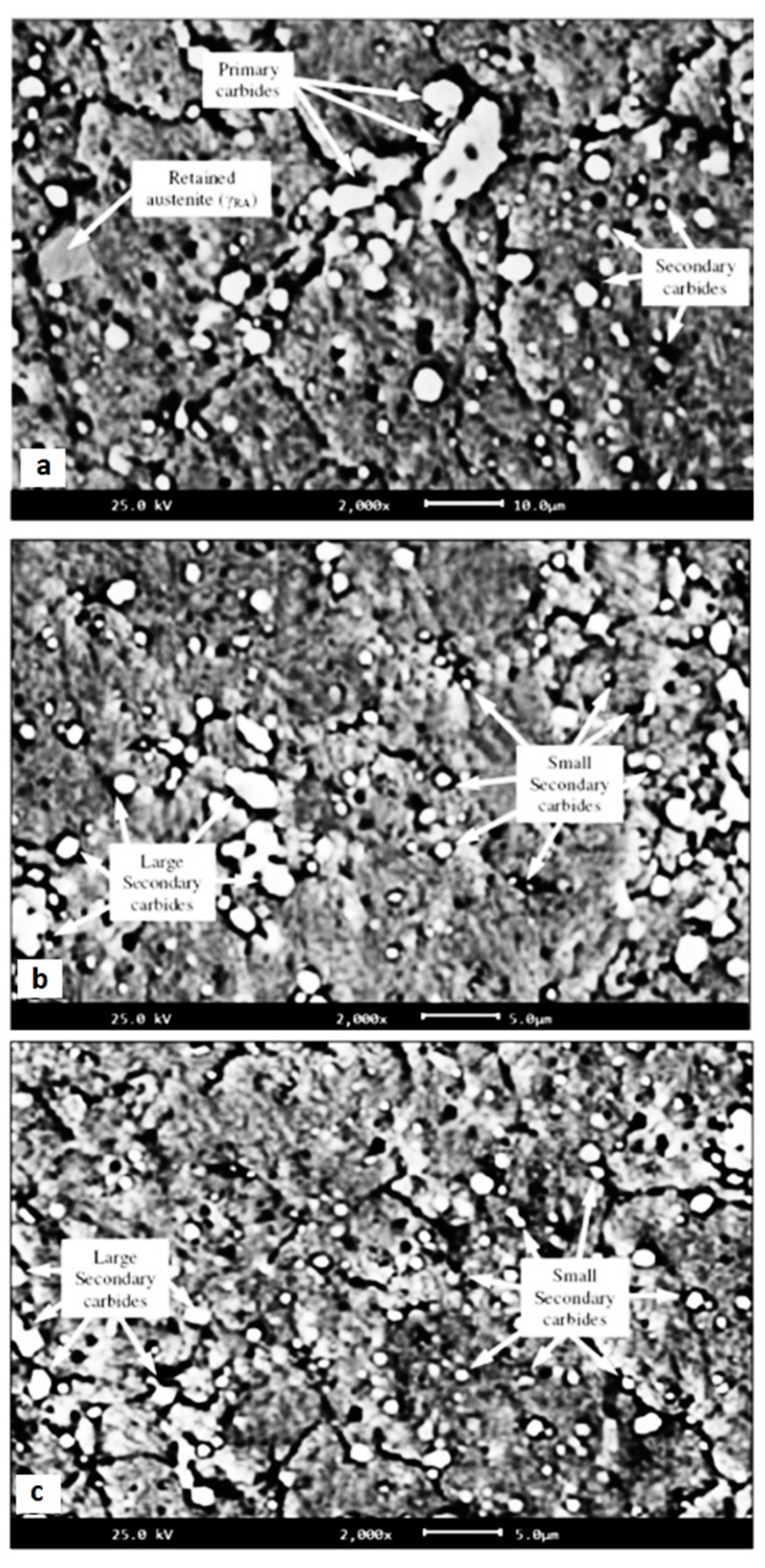
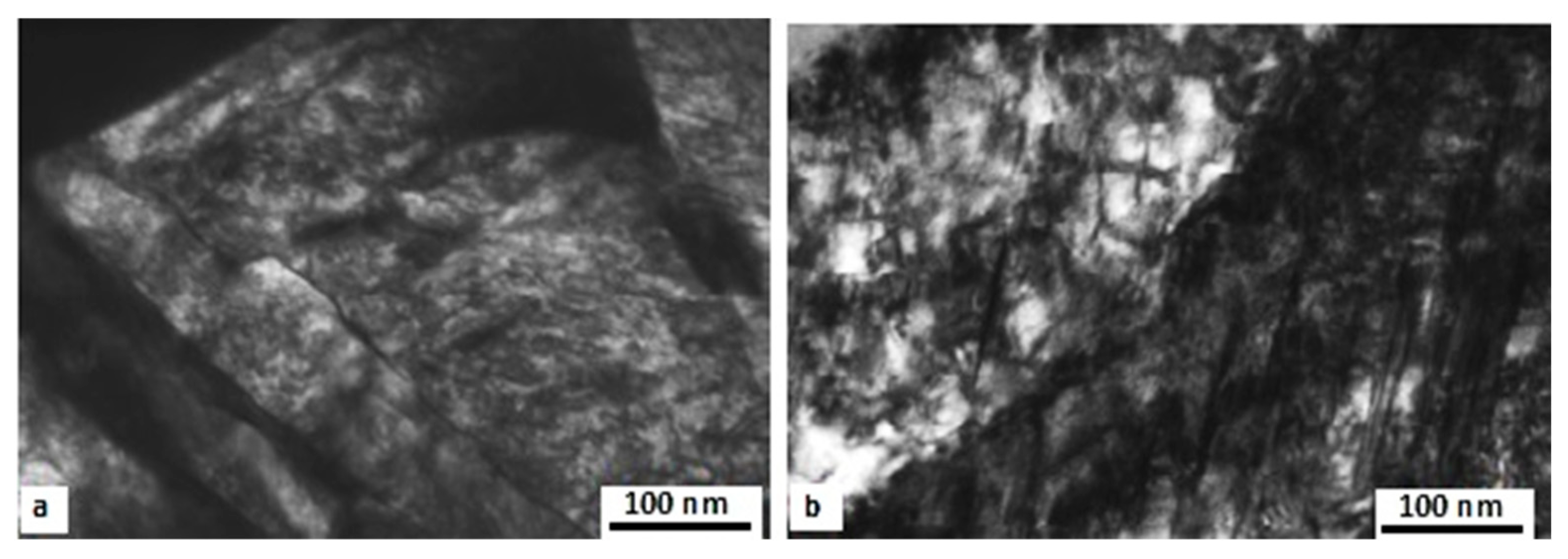
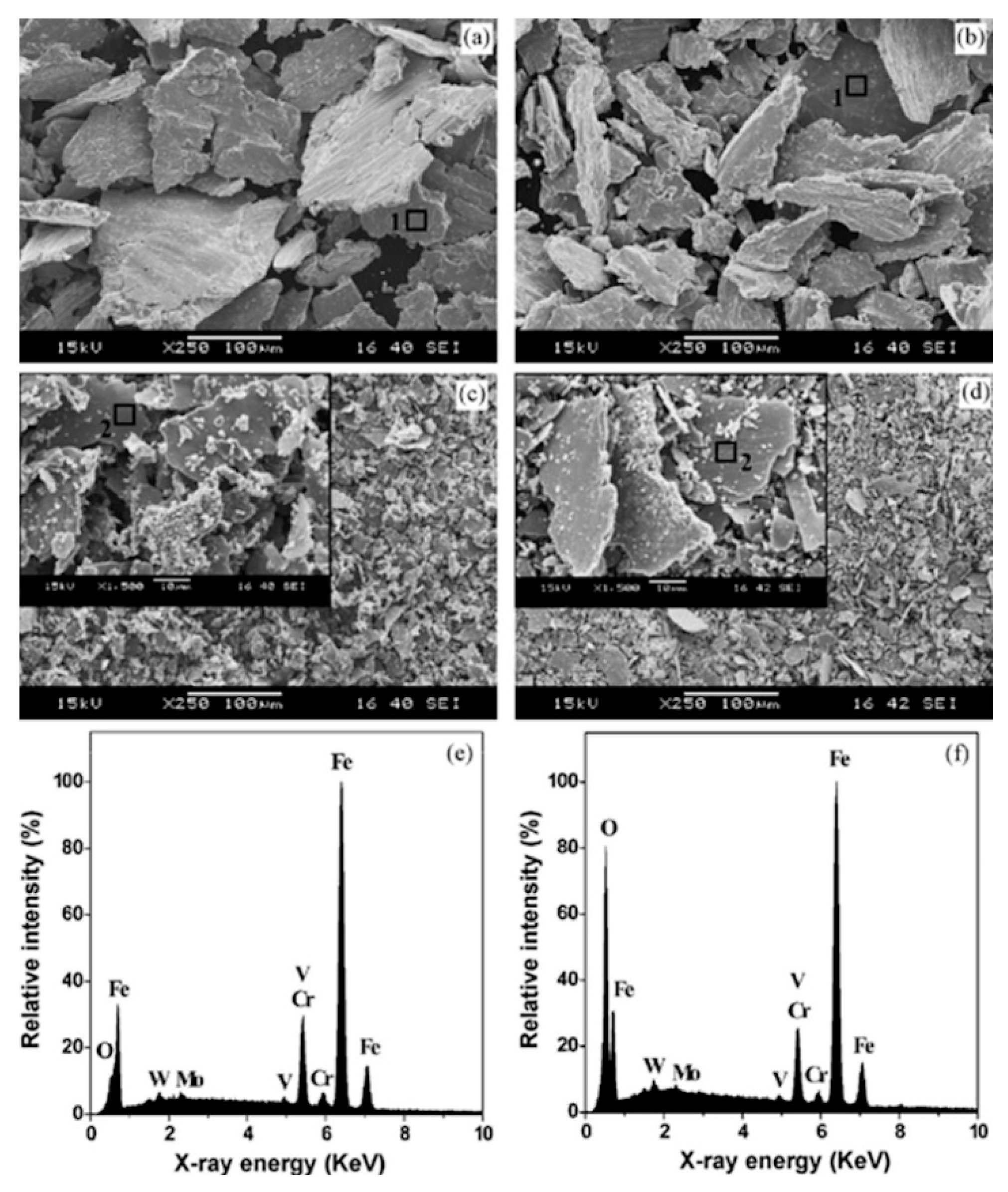
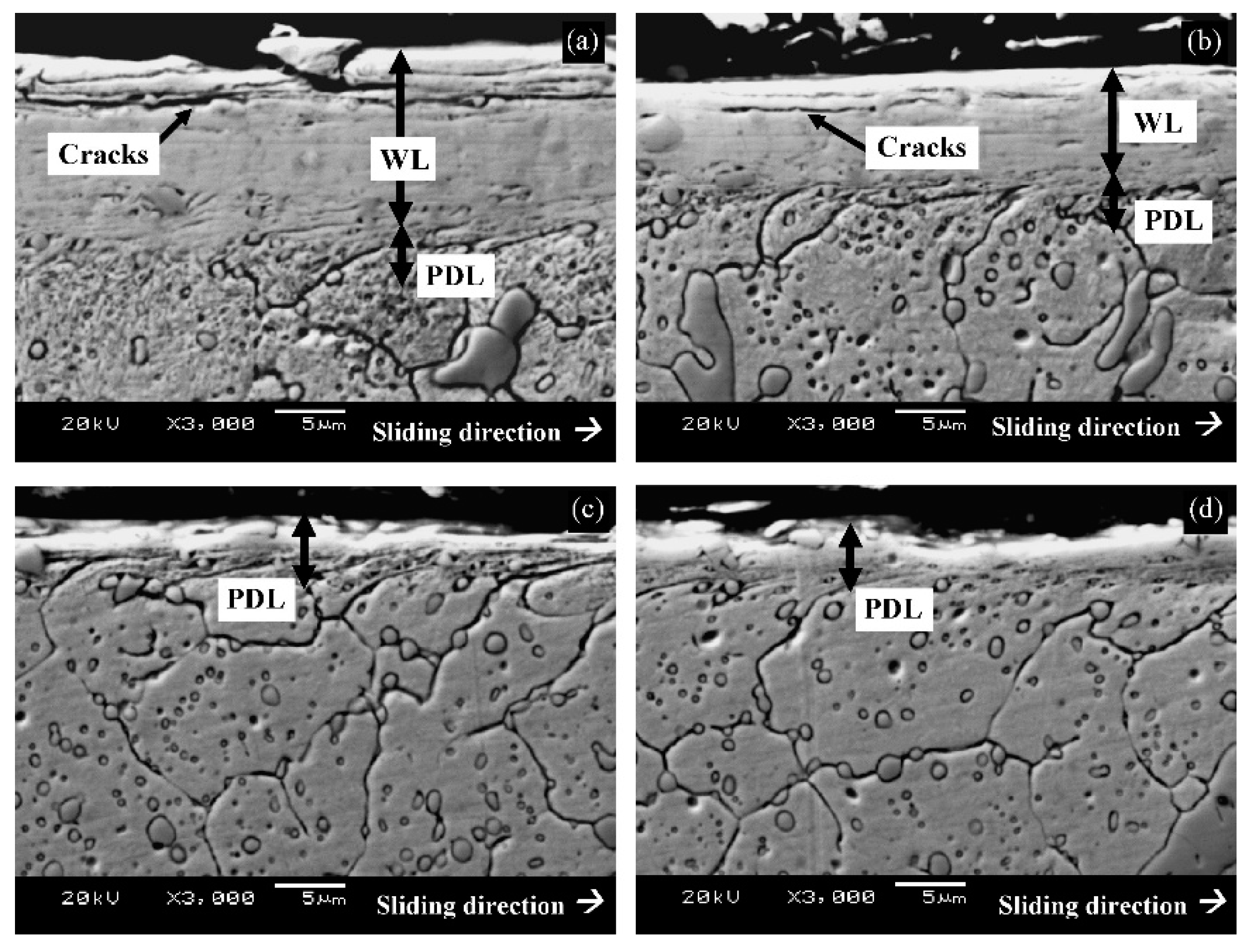
The impact of CT parameters and microstructural changes on the mechanical properties, wear and corrosion resistance, and properties important for manufacturing is analysed in the following sub-sections.
4.1. Carburised steels
In carburised steels,
Table 1, after final quenching and low-temperature tempering (normally not exceeding 200 °C to prevent a substantial hardness decrease on the surface), the surface layers exhibit microstructural states similar to those of bulk martensitic steels. Cryogenic treatment results in a useful improvement in hardness and wear resistance.
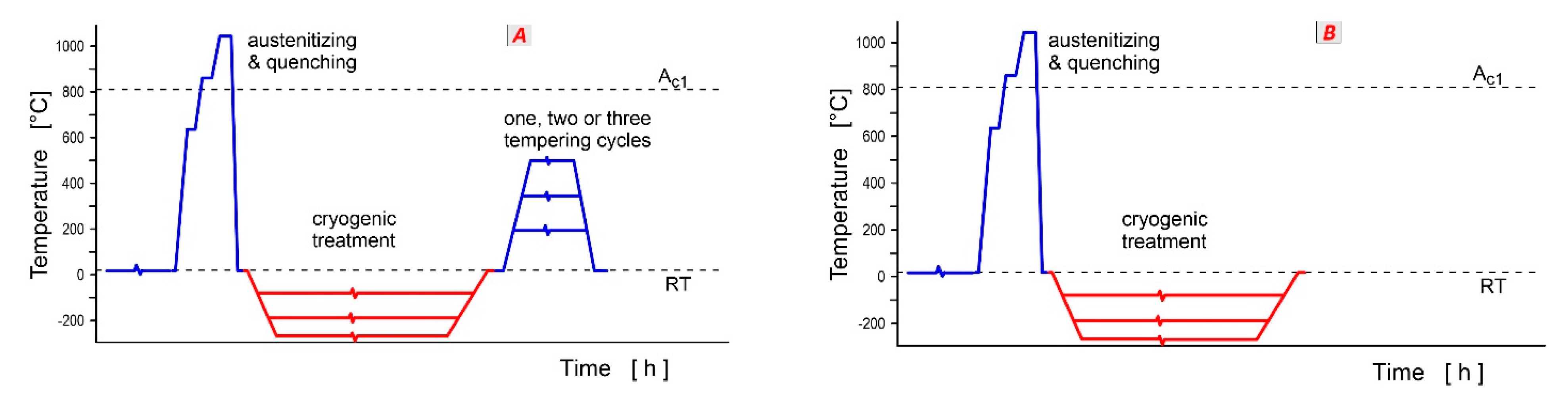
Cryogenic treatment via Sequence
A increased the hardness of carburised steels without Ni (En353, DIN 1.7131, IS 2062 and 20MnCr5) by 25–100 HV [
18,
33,
46]. The extent of the hardness increase was only marginally affected by CT temperature and duration. However, CT of IS 2062 steel at -77 °C progressively increased microhardness, depending on the duration, by 50 HV0.1 after 3 h treatment and up to 100 HV0.1 after 24 h treatment [
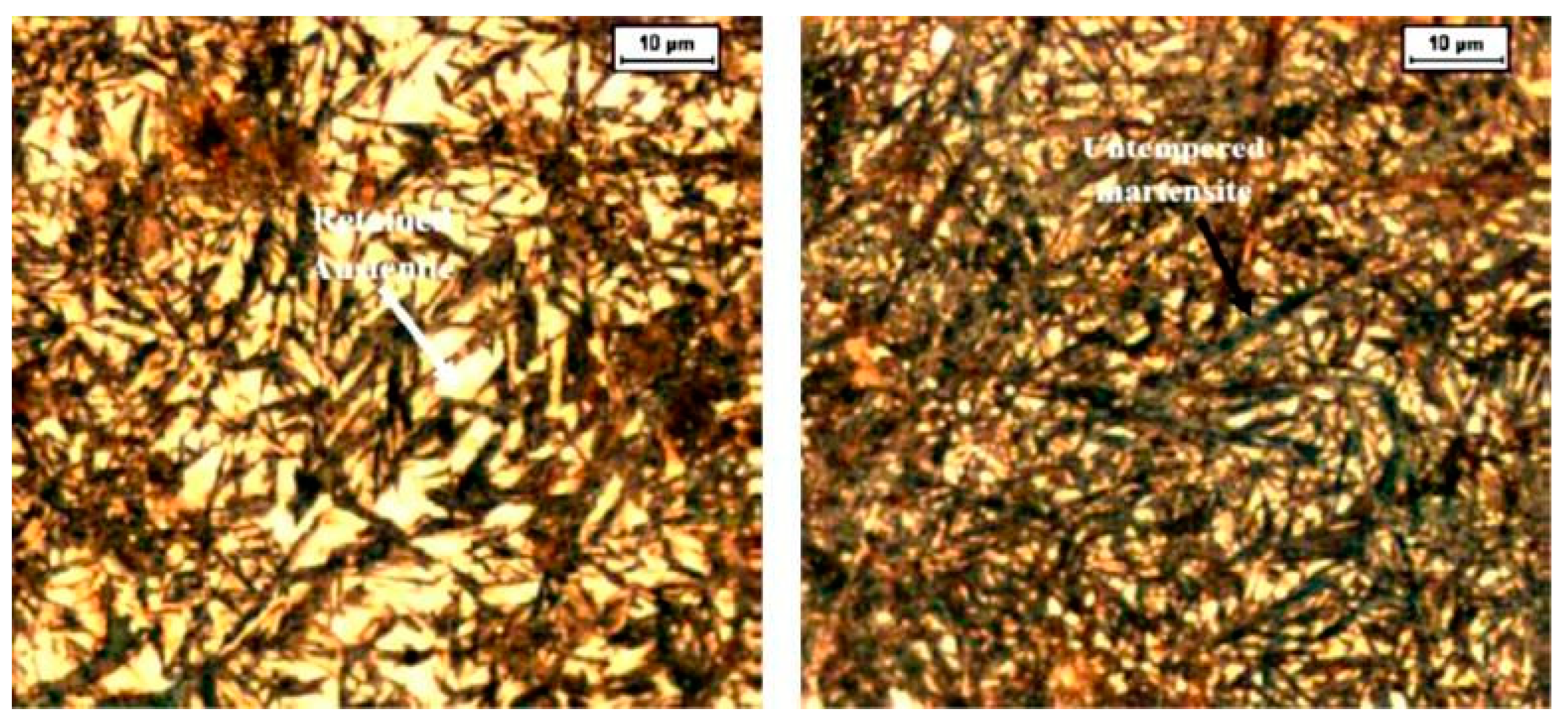
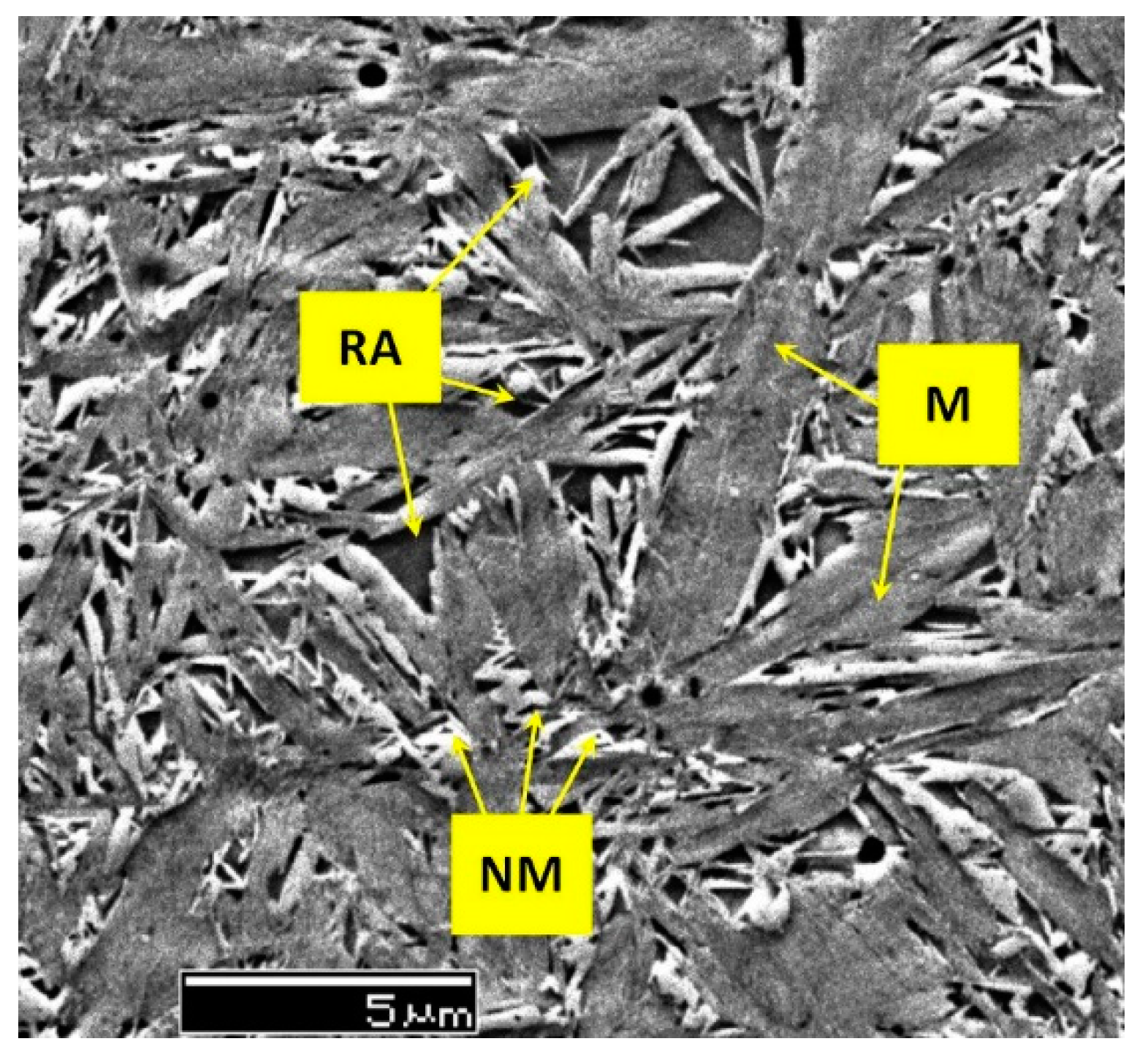
273]. A lower CT temperature (-196 °C) led to a microhardness increase greater than 300 HV0.1. This result is consistent with the microstructural observation that CT reduces the retained austenite (soft phase) and that the extent of retained austenite reduction increases with decreasing the cryogenic temperature and/or longer treatment duration.
The application of cryogenic treatment (Sequence
A) led to a greater hardness increase in carburised steels in cases when there was a higher retained austenite content present in their microstructures due to supersaturation of the surface. This is particularly relevant for Ni-containing steels. In SNCM 415, a hardness increase of almost 200 HV was observed due to CT at -85 °C for 24 h [
225]. For 17Cr2Ni2MoVNb, 21NiCrMo2, and 20Cr2Ni4A steels, there was a hardness increase of almost 40, 80, and 120 HV1, respectively, after CT at -80, -150, or -196 °C (each of them for 1 h) [
229,
230] or after CT at -120 °C for 2 h [
274]. The hardness increase due to cryogenic treatments is a result of 30 to 50 % retained austenite reduction as well as more extensive precipitation of fine carbides due to cryogenic treatment [
229]. For Ni-containing steels, the hardness increase showed clear cryo-temperature dependence for 20CrNi2MoV steel [
180] and time dependence for 18NiCrMo5 steel [
275]. For example, there was a temperature-dependent hardness increase of 36 and 74 HV for 20CrNi2MoV steel subjected to 4 h treatment at -80 and -196 °C, respectively (
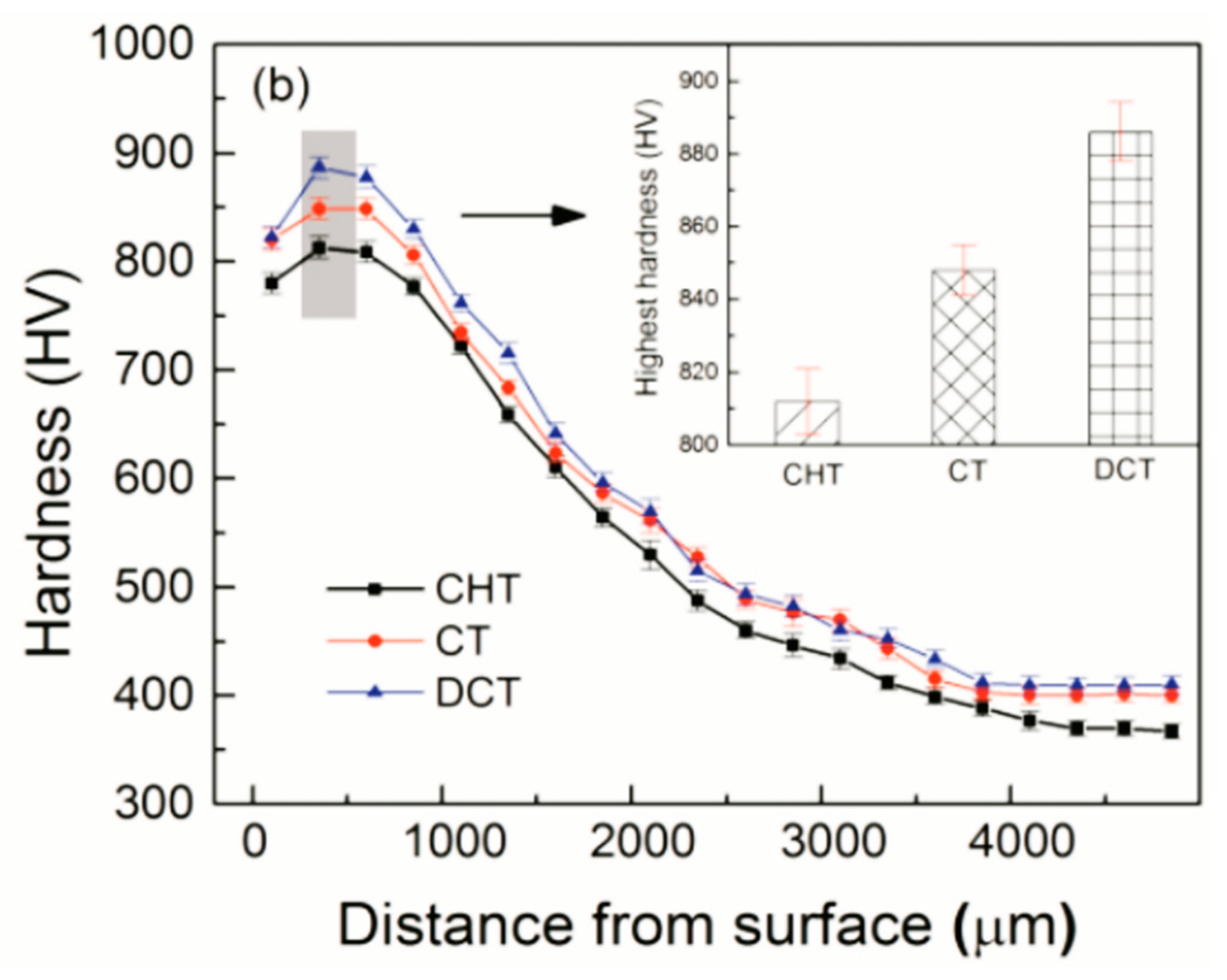
Figure 27) [
180]. Furthermore, not only is the hardness increase in the carburised case due to CT evident, but the hardness, in this case, is also higher at a greater depth below the surface, as can be seen from a comparison of the hardness depth profiles in
Figure 27.
Tempering prior to cryogenic treatment (Sequence
E or
F) provides only marginal hardness increments. CT increased hardness by 0.6 HRC for 18NiCrMo5 steel [
275], but there was almost no effect for gear wheels made of 18CrNiMo7-6 steel treated at -30, -40, -80, or -196 °C [
226]. There were also negligible hardness increments observed for AISI 8620 steel (CT at -40 °C for 1 h) [
231] and for 17Cr2Ni2MoVNb and 20Cr2Ni4A steels (CT at -196 °C for 1 h) [
230].
The principal explanation for the CT-controlled hardness increase is that cryogenic treatment reduces the retained austenite amount. This reduction is greater for Ni-containing steels [
226,
229,
230] than for steels without Ni [
225]. Tempering prior to CT thermally stabilises retained austenite [
126,
276]. Hence, Sequences
E and
F act less effectively in RA reduction, and a minimal hardness increment due to CT is a logical consequence. A minor contribution to the overall hardness increase of cryogenically treated carburised cases could be expected from greater precipitation of transient carbides. However, this eventuality requires further clarification through careful and systematic investigations.
Carburised steels are low carbon and low alloy steels; hence, their bulk toughness is usually very high. However, carburising results in the formation of a hard high carbon–containing carburised case on their surfaces, a factor that negatively affects their toughness [
277]. Carburised 20CrNi2MoV steel (with a 2 mm case depth) was subjected to cryogenic treatment at either -80 or -196 °C for 4 h (following Sequence
A), and there was a 9.8% reduction in Charpy V-notch (CVN) impact energy [
278]. For IS 2062 steel carburised to a depth of 0.5 mm, CT at -77 or
-196 °C for 3–24 h (Sequence
F) slightly (for -77 °C) or substantially (for -196 °C) worsened CVN impact energy [
273]. The toughness deterioration was dependent on the CT duration, that is, the longer the CT, the more remarkable the reduction in toughness. The observed decrease in the toughness of CT steels can generally be attributed to the decrease in RA. Since the RA decrease depends on the CT duration, the decrease in toughness should also be time-dependent. Retained austenite is a face-centred cubic (FCC) structure with a high strain hardening exponent [
279,
280]. Consequently, austenite is a tougher phase and has a higher strain hardening rate. Both the strain hardening rate and toughness of the austenite phase increase with increasing carbon content [
281] in the austenite. Therefore, as the austenite volume fraction increases, the fracture toughness (K
IC) of the material should also increase [
282].
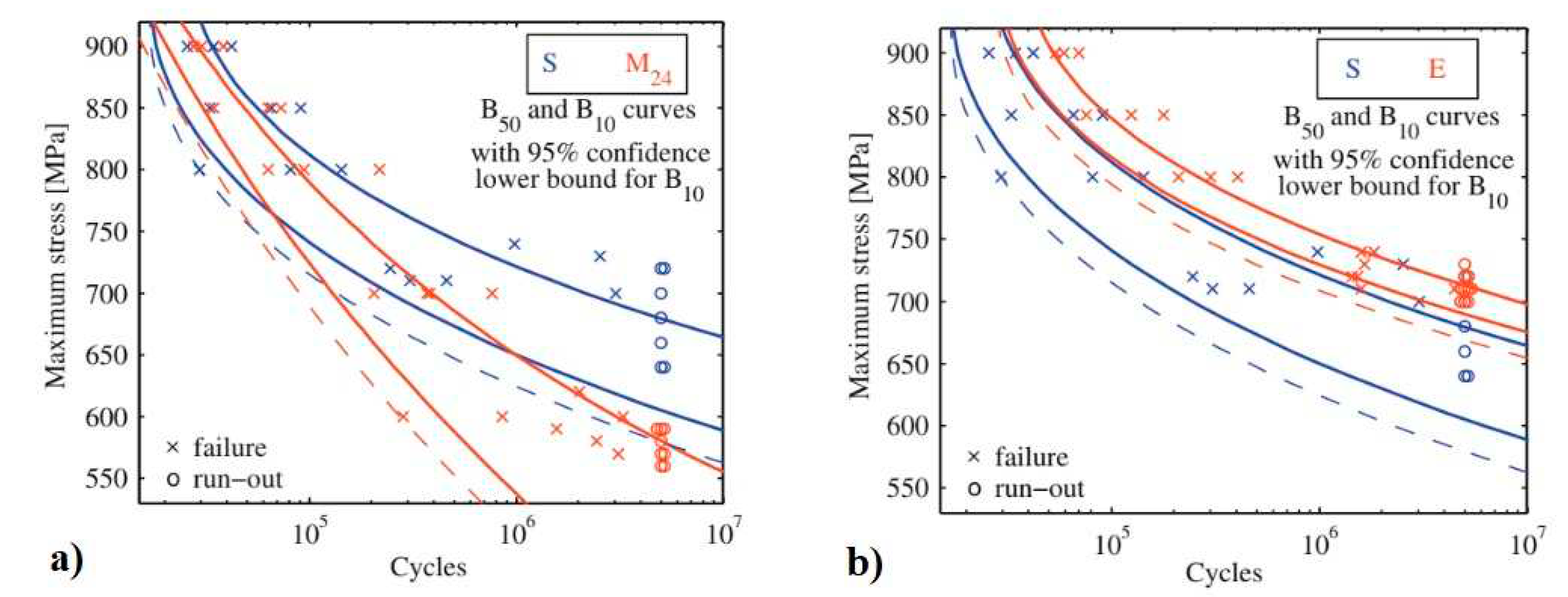
Cryogenic treatment of carburised steels leads to variations in their fatigue performance. For 18NiCrMo5 steel, CT at -185 °C for 1 or 24 h, according to Sequence
A, reduced fatigue performance, while Sequence
E improved this property up to 25% [
283]. This is illustrated in
Figure 28. From the comparison of the SN curves slopes it is evident that the specimens cryogenically treated by Sequence
E shows a fatigue behaviour similar to conventionally treated ones, although transposed horizontally (enhanced fatigue limit) and with a ~82% reduced in scatter. While, the specimens treated by using the Sequence
A behave in a definitely different way. The fatigue life at higher stress levels appears to be comparable to conventionally treated steel while negative impact of cryogenic treatment (Sequence
A) becomes evident at lower stress levels.
Two Ni-containing carburised steels (SAE-4320 and SAE-9310) were treated at -73 or -196 °C following Sequence
A [
284]. The bending fatigue endurance limits for the SAE-4320 specimens were 1310 MPa for the carburised condition, 1170 MPa for CT at -73 °C, and 1280 MPa for the -196 °C condition. The endurance limits for the SAE-9310 specimens were 1170 and 1070 MPa for the carburised and cryogenically treated at -73 °C, respectively. To explain the variations in fatigue performance of differently treated carburised steels in should be noted that the retained fraction of ductile austenite can act as a crack arrestor in the fatigue crack propagation stage. Therefore, retained austenite reduction [
18,
285] should have a detrimental effect on the final stage of fatigue life. The application of treatment via Sequence
A reduces RA more significantly than Sequence
E; this is logically reflected in the reduced bending fatigue endurance limits of cryogenically treated steels. Furthermore, the whole fatigue process, from nucleation to propagation, is strongly influenced by the presence of residual stresses in the material. These stresses are compressive in a carburised case [
286,
287], but they are more significantly reduced when tempering is included in the final heat treatment step (Sequence
A) than when Sequence
E is applied. Last but not least, the role of minor but expected precipitation of dispersed nano-carbides [
288] should be considered, although this point deserves further careful investigation.
Cryogenic treatment following Sequence
A improves the wear resistance of almost all carburised steels. The extent of the improvement depends on both the CT temperature and the duration. The maximum wear resistance for En353, 20CrNi2MoV, 16MnCr5, 20MnCr5, and 17Cr2Ni2MoVNb steels was obtained by treatments at -196 °C (or close to this temperature) [
18,
33,
46,
180,
229,
289], even though the use of higher temperatures (e.g., between -80 and -103 °C) can also significantly improve this property [
181,
225,
228,
289]. Two diagrams in
Figure 29 clearly delineate enhanced wear performance of cryogenically treated 20CrNi2MoV carburised steel as compared with the same steel without applying the cryogenic treatment. It is also seen that cryogenic treatment at -196 °C (here denoted as "DCT") provides the steel with better wear performance than what can be obtained by cryogenic treatment at -80 °C (denoted as "CT"). Treatment at the boiling point of helium (-269 °C) had a positive effect on the wear resistance of 20MnCr5 steel, but this improvement was smaller compared to treatment at -196 °C [
33]. Alternatively, CT at -40 °C (following Sequence
A or
F) had no effect on the wear performance of AISI 8620 steel [
231]. As for CT duration, immersion in a cryogenic medium for 24 h resulted in maximum wear performance in most cases [
18,
33,
46,
289]. The improvement is commonly attributed to the higher degree of RA-to-martensite transformation (thus higher hardness) by cryogenic treatment [
18,
33,
46]. However, the role of the still unclear ‘refinement and better distribution’ of carbides [
46,
289] should also be considered and investigated systematically.
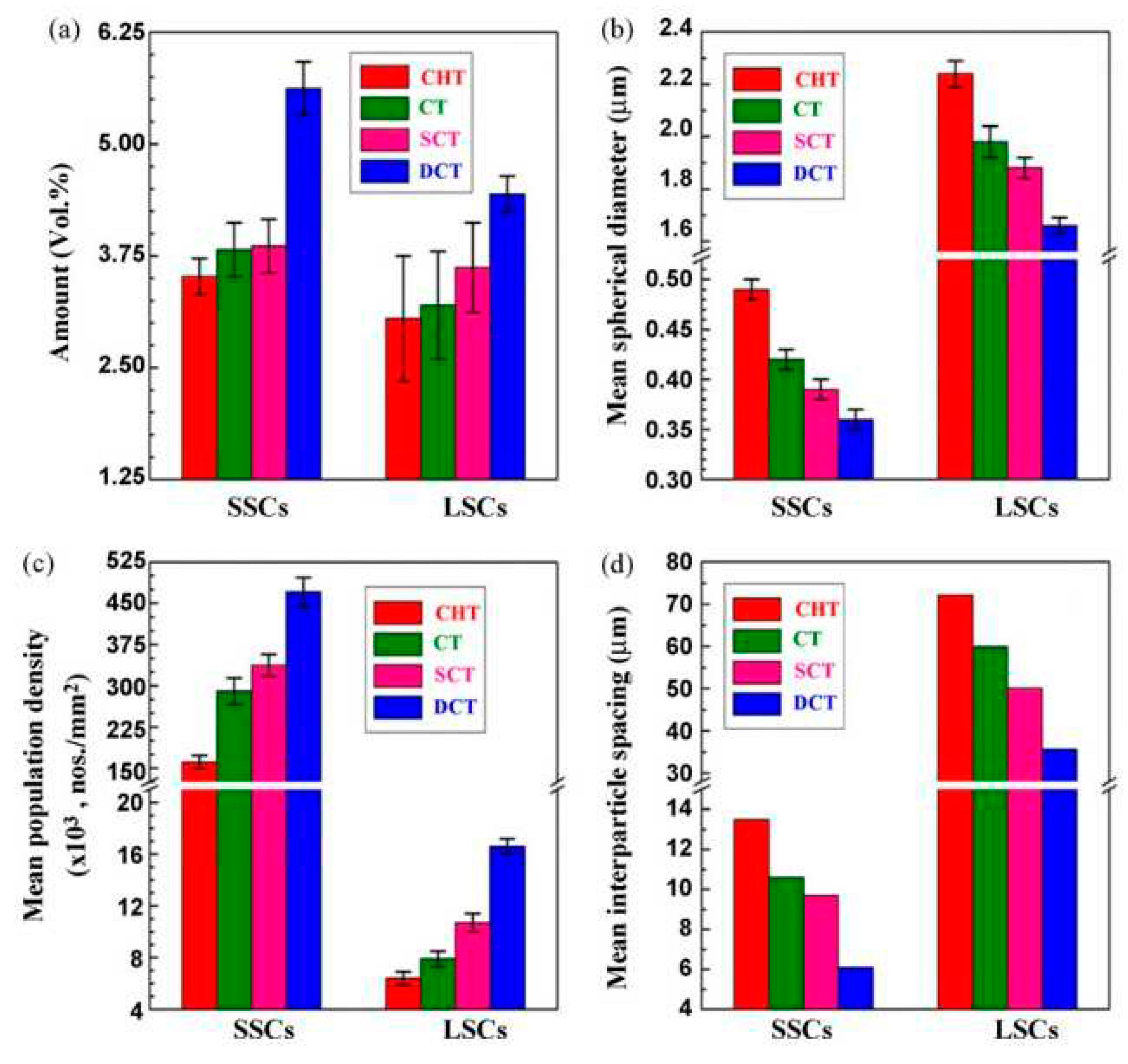
Concluding remarks: Cryogenic treatments increase the hardness of all carburised steels; Sequence A with treatments at or close to -196 °C is more effective than other sequences due to a higher extent of RA-to-martensite. The same applies to the variations in wear resistance. However, an increase in hardness is always accompanied by a decrease in the toughness and fatigue strength of steels. This decrease is small after application of Sequences E or F but more pronounced when Sequence A is applied.
4.2. Ball bearing steels
For ball bearing steels,
Table 2, the retained austenite content and its control are key parameters that determine the final properties and durability of bearing rings and balls. An increase in the retained austenite content contributes to greater fracture resistance of the ring body. In contrast, a decrease in the RA content affects rolling contact fatigue resistance and, thus, the total lifetime (durability) of bearings. Rolling contact fatigue is controlled primarily by the volume fraction of carbides and their distribution characteristics and, at the same time, the inherent toughness of the matrix in terms of the carbon content and martensite morphology.
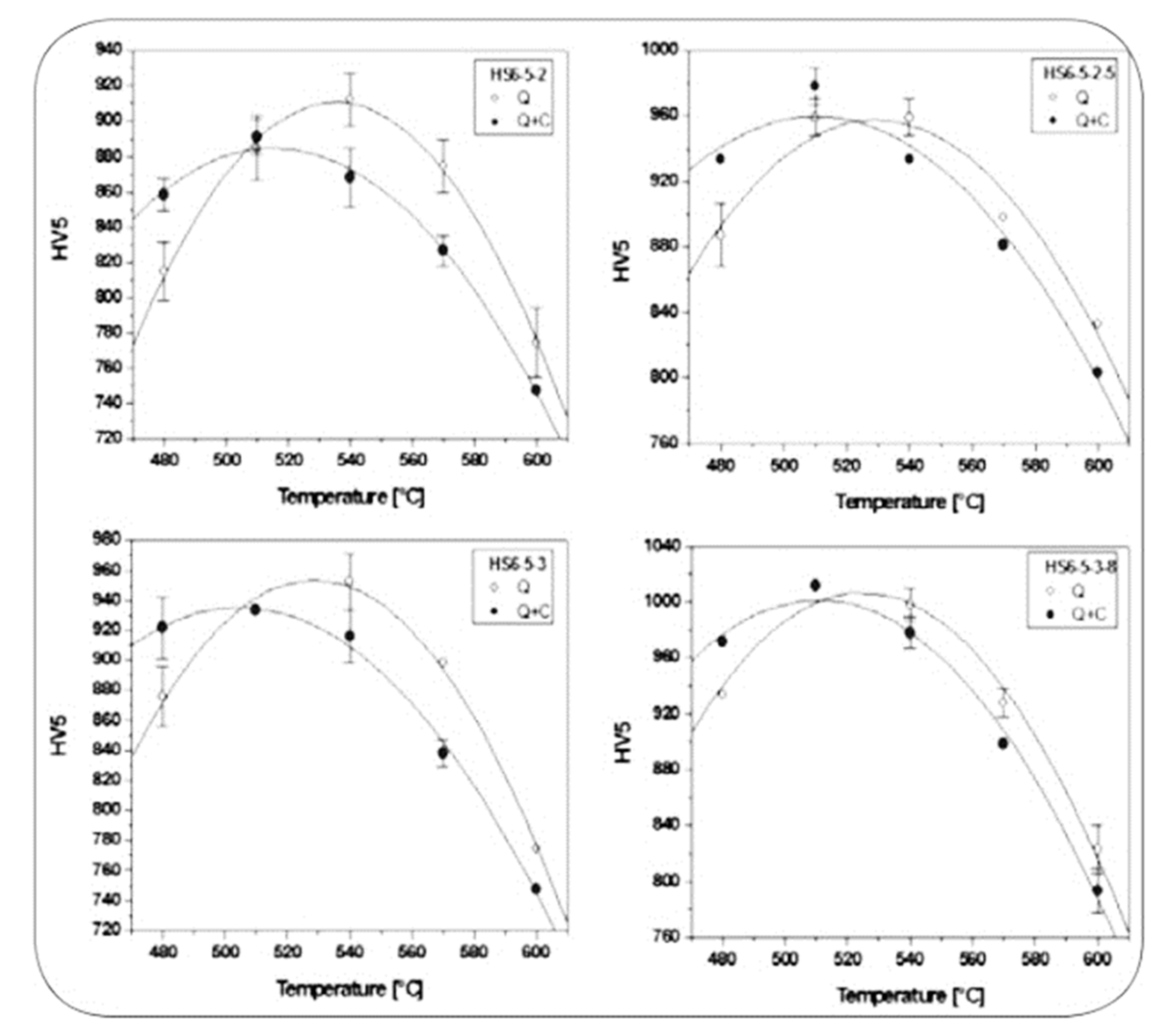
For AISI 52100 steel in the low-temperature tempered condition, cryogenic treatment at -196 °C for 24 and 35 h (Sequence
A) increases the hardness by 60 to 100 HV [
24,
173]. However, the treatments at higher temperatures can also bring undisputable benefits with respect to the hardness values obtained. Treatment at -100 °C for 210 min (following Sequence
A), for instance, resulted in a 60 HV hardness increase for the given steel grade [
228]. Cryogenic treatment at -120 °C for 2 h increased the hardness by 60 to 70 HV [
274]. For treatment Sequence
A applied to near-eutectoid steel (0.86 wt.% C) at -190 °C for either 12 or 36 h, the longer treatment duration resulted in a greater hardness increase [
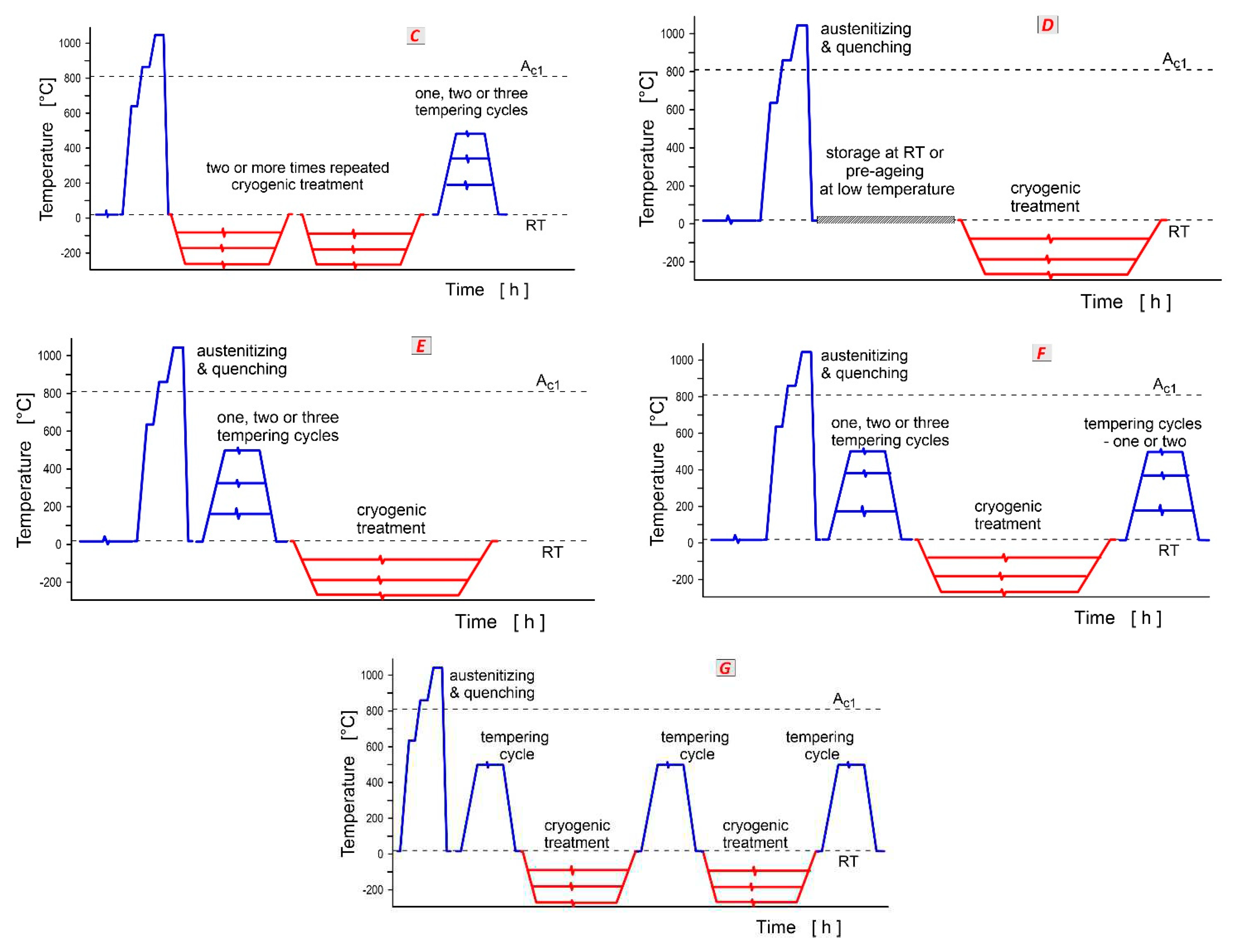
151], mainly due to a more complete RA-to-martensite transformation. On the other hand, repeated CT (cyclic, Sequence
C) was not effective. A maximum hardness value of 64 HRC (CHT resulted in 62.7 HRC) was achieved after the first cycle at -196 °C for 6 h; however, the second and third cycles slightly reduced the hardness [
207]. Sequence
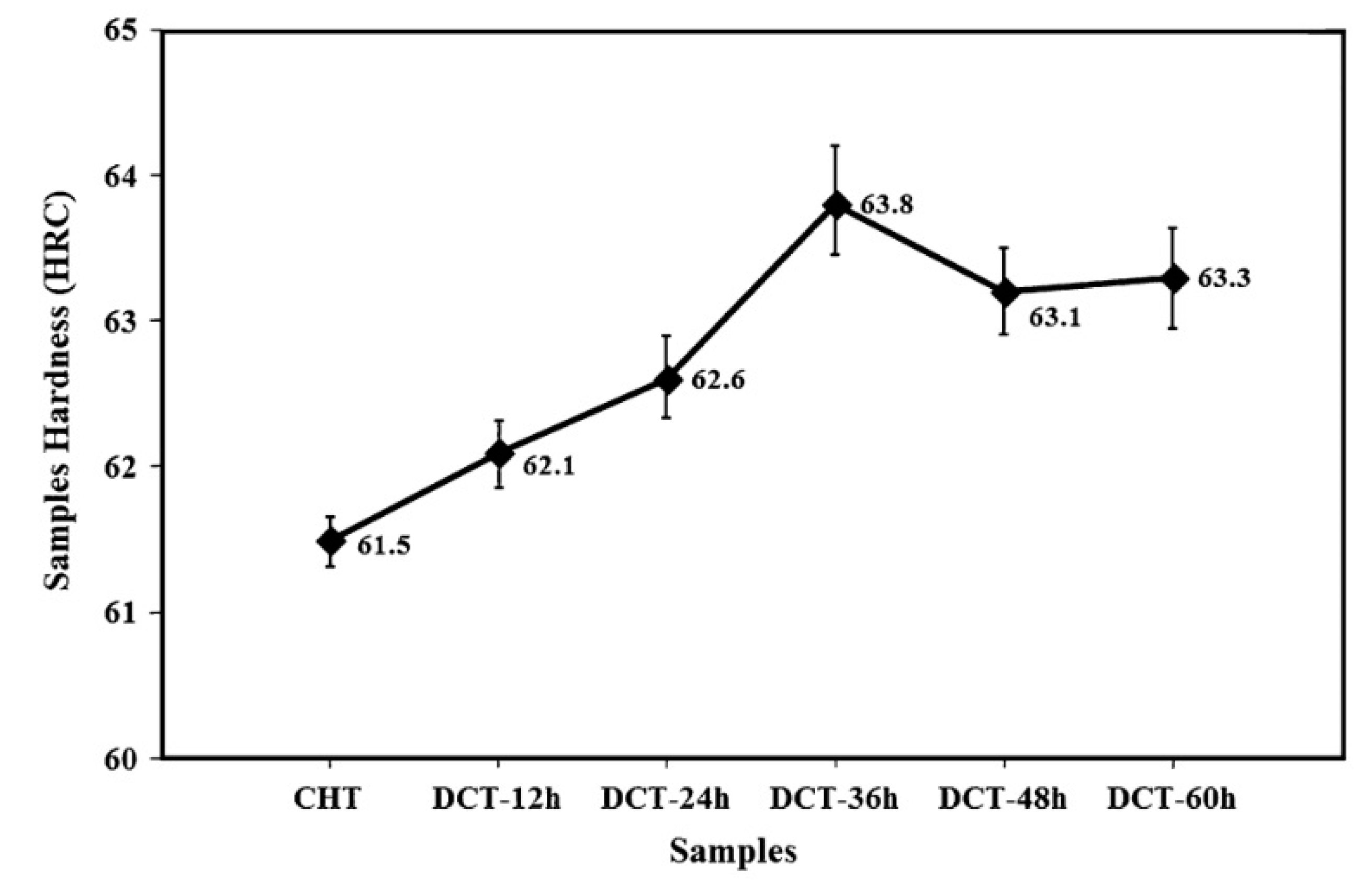
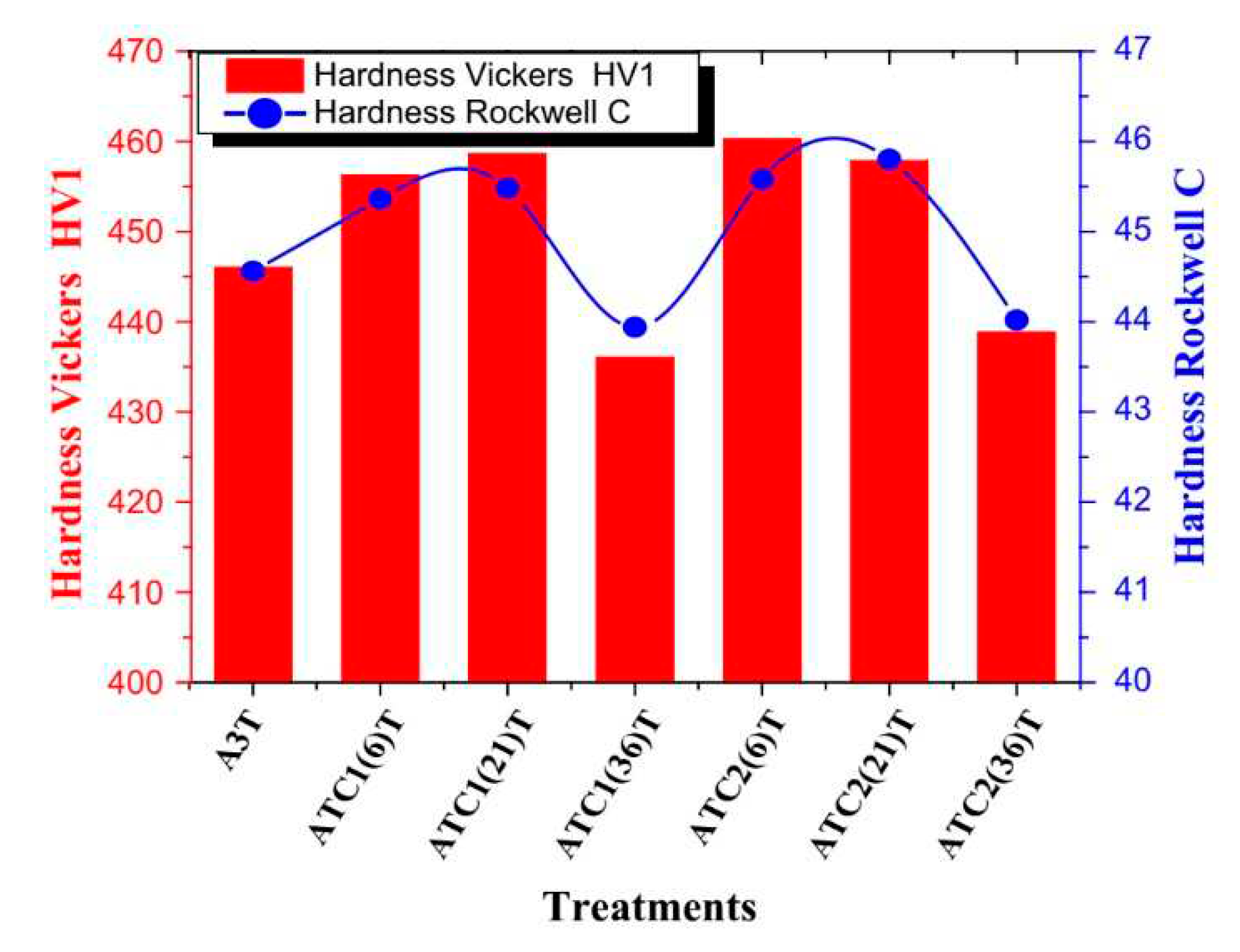
E (with pre-tempering at 180 °C for 2 h before CT at -145 °C for 12–60 h) was less efficient in increasing the hardness of AISI 52100 steel (max. increase 2.3 HRC at CT time of 36 h,
Figure 30) than Sequence
A [
45]. Despite that the hardness increment is remarkable in this case, and can be referred (according to the authors of Ref. [
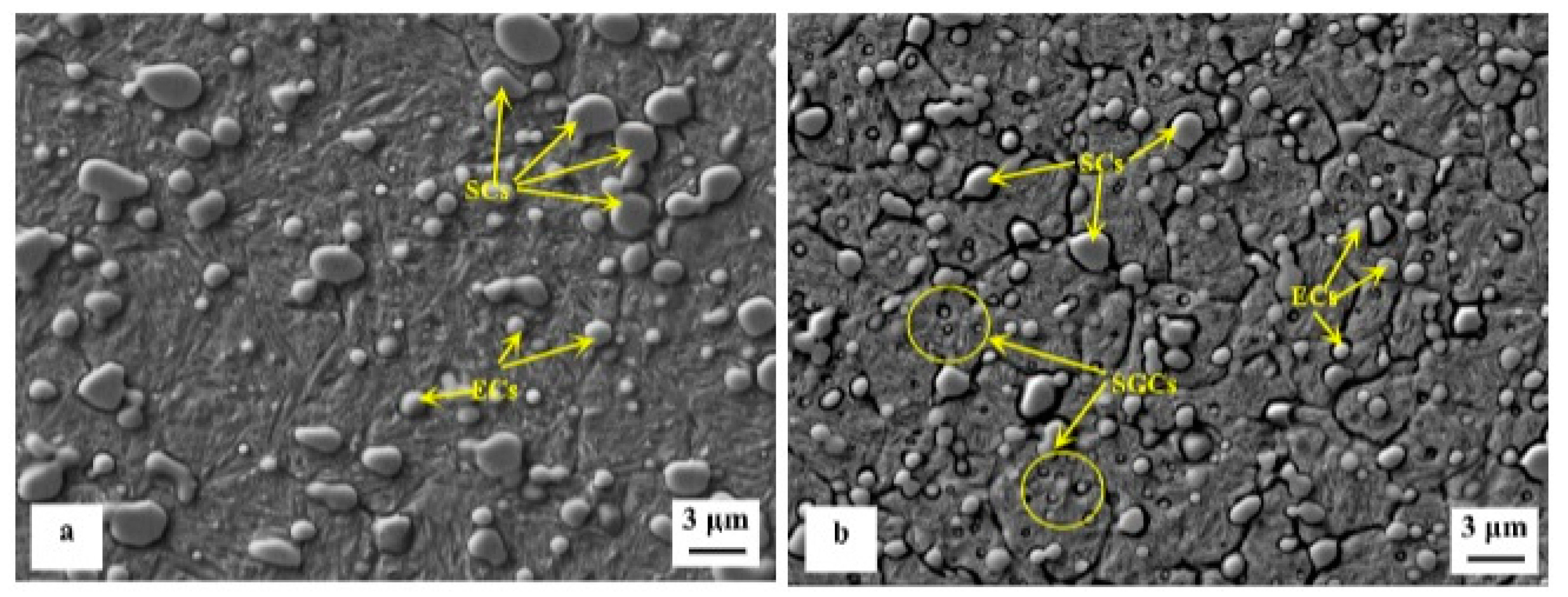
45]) to the more complete martensitic transformation (even though incomplete, due to RA stabilisation by pre-tempering), and to maximum additional SGCs count that occurred at 36 h duration.
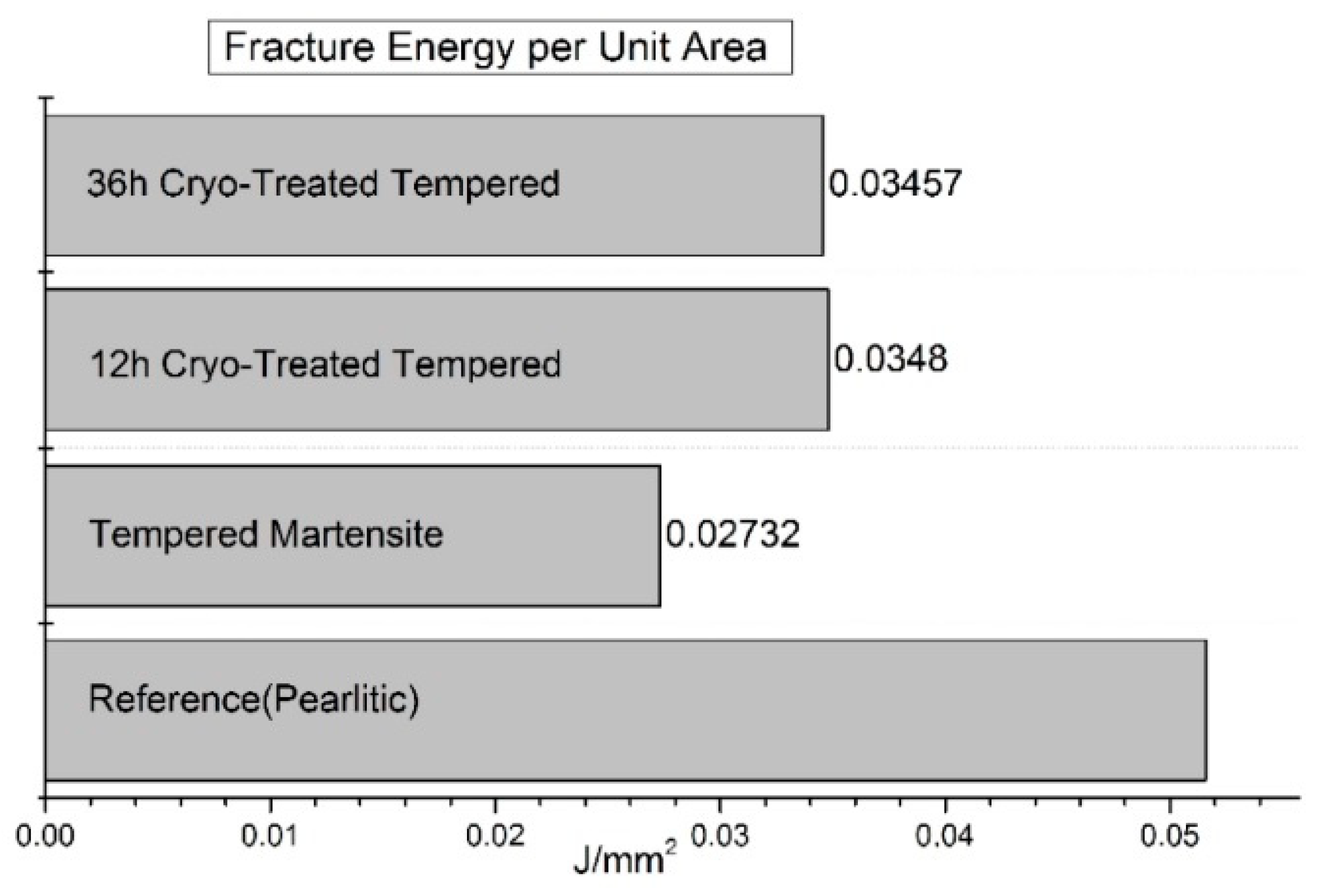
Compared to CHT, the CVN impact toughness of near-eutectoid steel (0.86 wt.% C) was improved by almost 27% after CT at -190 °C for 12 or 36 h,
Figure 31 [
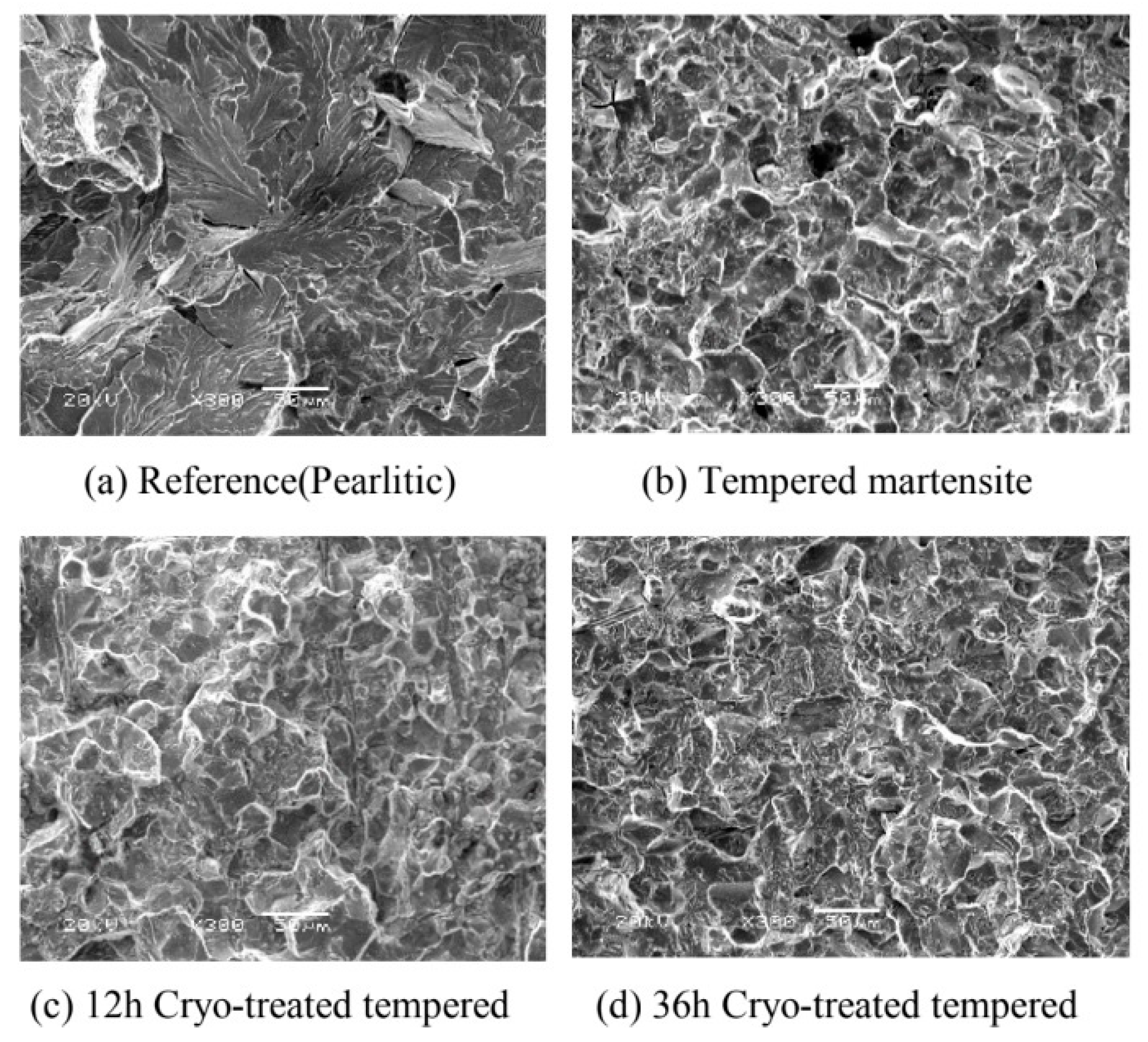
151]. Changes in CVN impact toughness are reflected in appearance of fractured surfaces,
Figure 32. SEM image of initial (pearlitic) structure shows ductile-brittle fracture, while for quenched samples, the fracture model was more likely brittle fracture. Cryo-treated samples have more microcracks which was a sign of improved toughness. Cryogenic treatment forms more likely higher amount of additional SGCs, which may act as a plastic deformation preventive barrier points. The crack cannot propagate through carbides but only at the carbide/matrix interfaces, which increases the plastic deformation energy until the fracture. One can summarize that cryogenic treatment helps to improve fracture toughness at some level.
In other work [
182] Widiantara et al. have reported that retained austenite reduction by half in 0.86 %C containing steel leads to hardness increase by 2 HRC, and to increase in fracture toughness from 29 MPa·m
1/2 after CHT to 43 MPa·m
1/2 after CT (at -40 °C for 24 h, Sequence
A). These results indicate that it is possible to increase the material toughness without sacrificing hardness by CT, owing to overall microstructural refinement (martensitic domains and η-carbide precipitates. Nevertheless, Karaca and Kumruoğlu [
274] reported opposite results. The CVN impact toughness of AISI 52100 steel notably decreased with the application of CT at -120 °C for 2 h. This is rather surprising at first glance, but fractographic analyses revealed completely brittle fractures of cryogenically treated samples. One of the basic reasons for this behaviour is the reduction of retained austenite (soft and ductile phase). In various steels, this effect is more or less counterbalanced by the general refinement of the microstructure, the presence of a large number of additional small globular carbides, and the enhanced precipitation of transient carbides. However, these phenomena become active only after a much longer hold at cryo-temperatures, usually 24 hours or longer [
173,
178,
180], while the duration of CT in this particular case was only 2 hours.
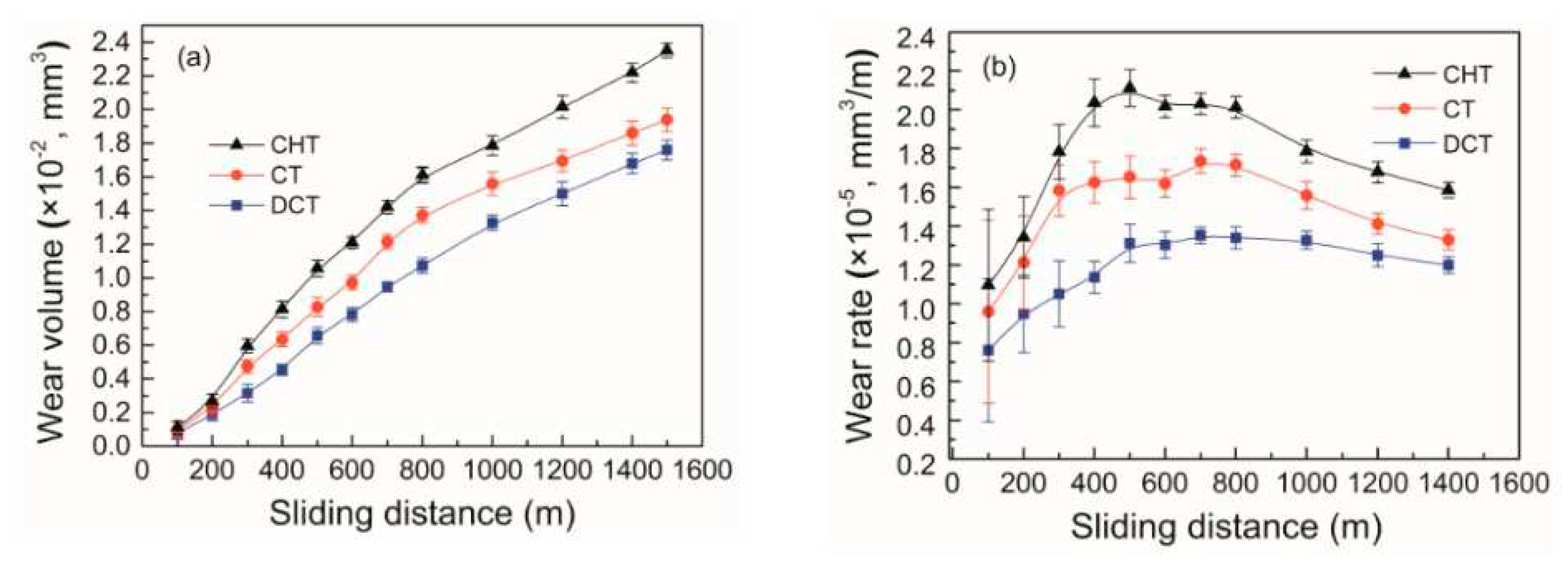
Enhancement of hardness and toughness (when treated at -196 °C) through cryogenic treatment improves wear resistance, a crucial factor for the service life of bearings. For AISI 52100 steel exposed to CT at either -185 °C for 24 h or -195 °C for 36 h (following Sequence
A), a 37%–50% (or even slightly more) reduction in wear rate was found [
24,
290,
291]. For steel subjected to CT at -196 °C for 24 h, this reduction was slightly higher (60%) [
292]. In other work [
228], Karaca et al. reported that an application of -100 °C cryogenic treatment for 3.5 h improved wear performance by only 10%, but the treatment at -120 °C gave a 40% improvement in wear rate [
274]. A CT duration of 24–36 h (at -185 or -196 °C) was recommended as optimal to achieve the best wear performance of bearing steels [
24,
151,
290]. Note, however, that the test conditions play an important role in the extent of wear performance. According to Paydar et al. [
173], the wear rate of AISI 52100 decreased by about 50% at a sliding speed of 0.05 ms
-1 but only 25% at a sliding speed of 0.15 ms
-1. Also, the wear performance of near-eutectoid 80CrMo12 5 steel was improved after CT at -196 °C for 0–168 h or -80 °C for 24 h (Sequence
A) due to a considerable retained austenite reduction and formation of additional carbides [
80]. The maximum improvement in wear behaviour occurred after a 48 h treatment at -196 °C; it coincided with the maximum number of additional carbides and the maximum hardness.
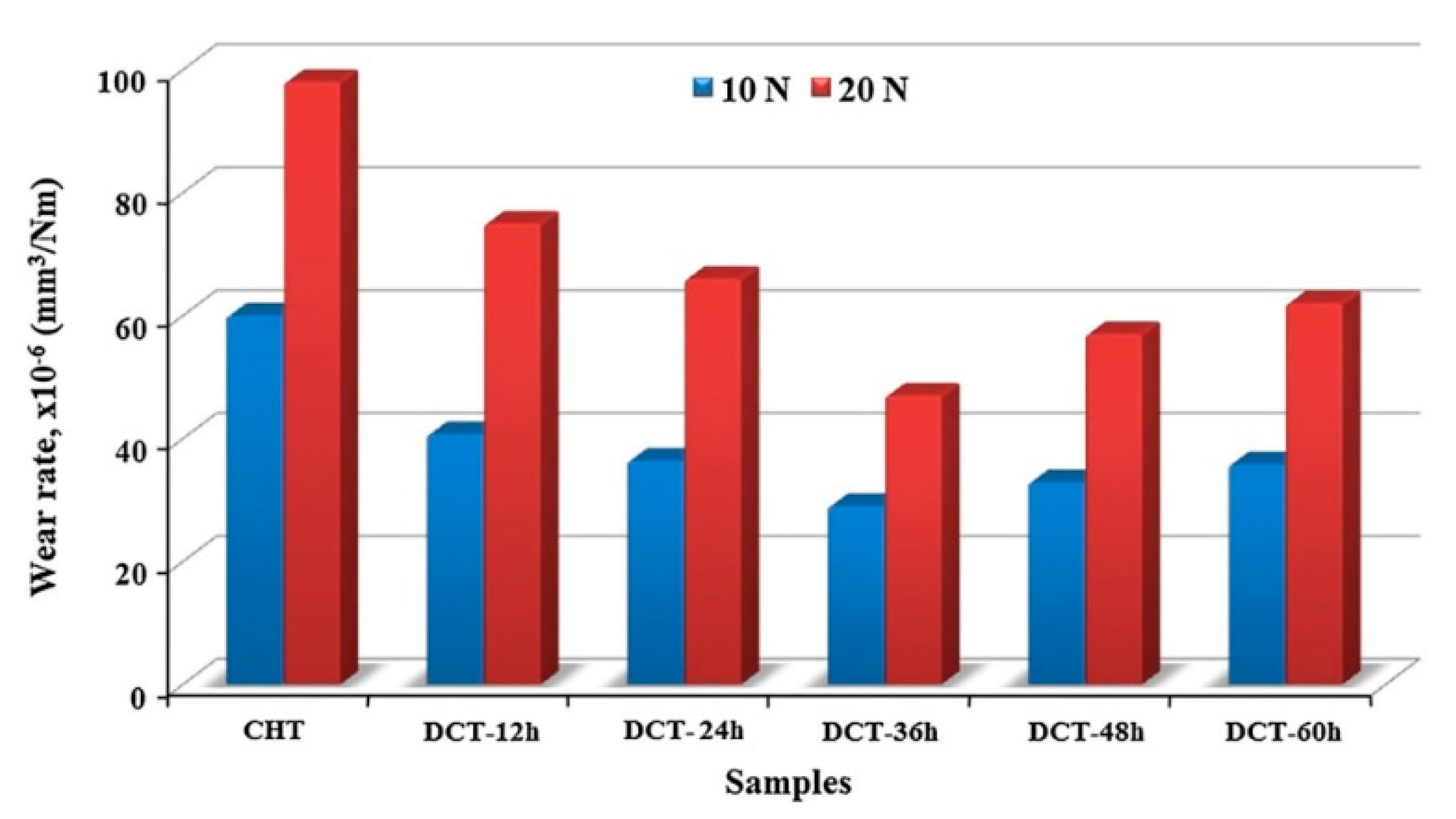
Although Sequence
E is less efficient in increasing the hardness of AISI 52100 steel, there was a relatively large improvement in wear resistance after CT at -145 °C for 12–60 was applied to pre-tempered steel. The decrease in wear rate was greatest (50%) after 36 h treatment,
Figure 33, and the friction coefficient reached the lowest value for the same treatment duration [
45].
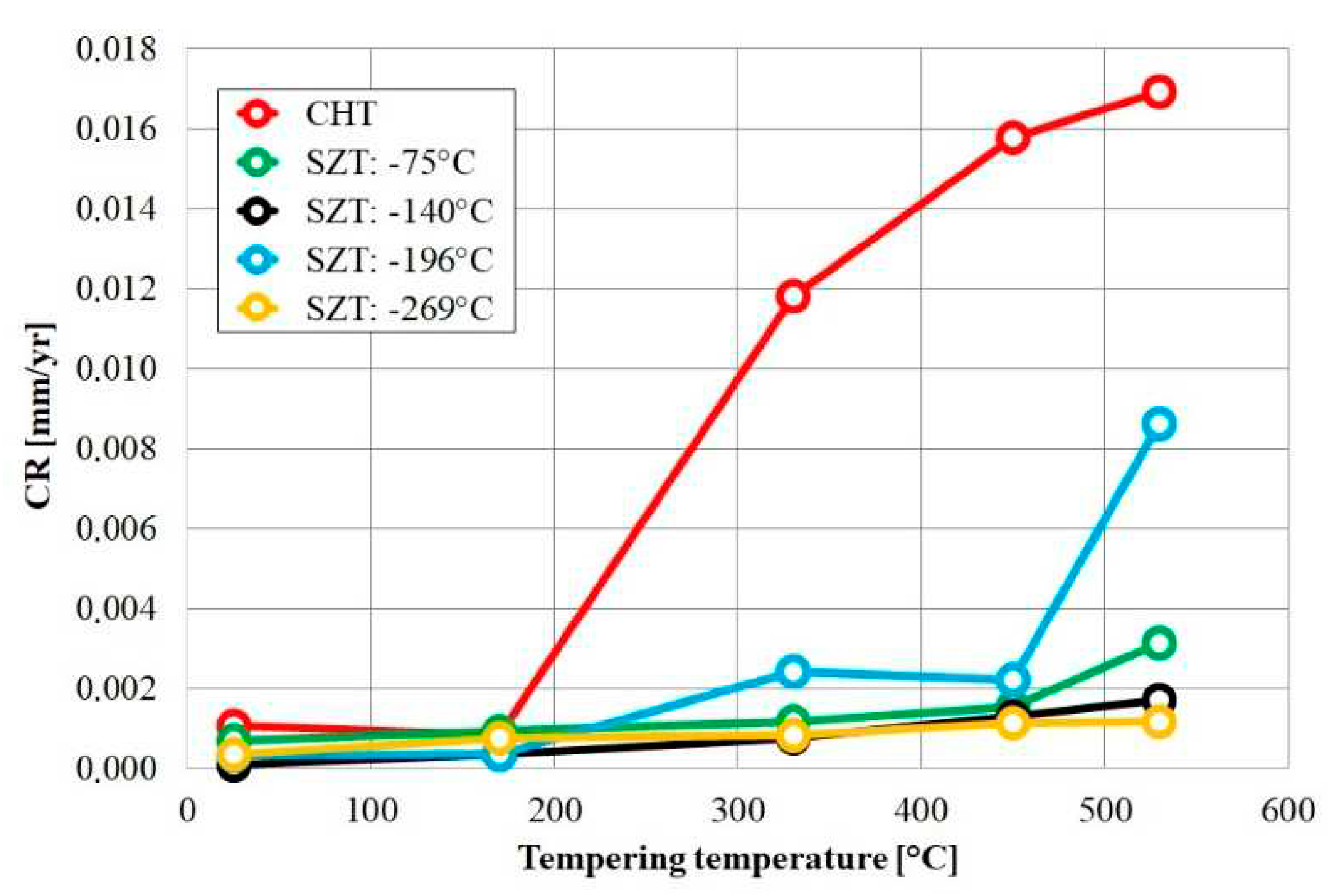
Corrosion resistance can be of some importance when bearings are operated in harsh environments containing acids, seawater, or other chemical substances. For AISI 52100 steel cryogenically treated at -185 °C for 24 h (following Sequence
A), corrosion resistance was tested in salt spray; cryogenic treatment improved the corrosion resistance by about 50% [
24]. This change could be attributed to the transformation of most retained austenite to martensite (an 11% reduction in RA) and the precipitation of fine carbides in the cryogenically treated steels. For the same material cryogenically treated at -196 °C for 24 h, the corrosion resistance was tested in a borate buffer (alkaline environment) using potentiodynamic measurements [
293]. The average corrosion rate of the CT samples was 65% lower than that of the CHT samples. The reason for this change was thought to be a lower carbide-to-carbide (interparticle) distance in the cryogenically treated steels. However, rather opposite results were obtained by Wang et al. [
210] when tested using a potentiodynamic method in a 3.5% aqueous NaCl solution. Almost no effect on the corrosion resistance was reported in this study for treatment at -196 °C for 24.
Concluding remarks: A hardness increase is an undeniable benefit of using the cryogenic treatments on ball bearing steels; Sequence A with treatments at -196 °C for 12-36 h can be recommended to achieve the highest hardness values. The increase in hardness generally results in better wear performance of the cryogenically treated steels, with maximum wear resistance achieved after CT at -196 °C for 24-36 h. This is due to sustained retained austenite, presumably greater additional carbides formation, and enhanced precipitation of nano-sized carbides. Unlike the carburised class of steels, cryogenic treatments may not necessarily reduce toughness, probably due to the general refinement of the microstructure. The effects of cryogenic treatments on corrosion resistance are still unclear.
4.3. Hot work tool steels
For hot work tool steels,
Table 3, bulk hardness is a crucial parameter as it controls the wear performance and thermal fatigue. However, the increase in hardness should not be at the expense of toughness. Hot work tool steels are medium-carbon steels. Therefore, they cannot retain a high amount of retained austenite after quenching to room temperature. They are also subjected to high-temperature tempering (570–620 °C) in CHT, which almost completely eliminates retained austenite [
239,
294]. For treatment of these steels, cryogenic treatment was inserted after performing some (tempering) ‘pre-treatments’ involving heating to temperatures above 500 °C (after CT without tempering [Sequence
E] or followed by high-temperature tempering [Sequence
F]) [
19,
22,
49,
51,
122,
199,
295,
296,
297]. In these cases, there was a minimal effect on the hardness of AISI H13 steel [
199,
296] or a marginal increase of 1–1.5 HRC for AISI H11 steel treated at -184 °C for 16–24 h [
22,
121,
295,
297]. When AISI H13 steel was tempered at a low temperature (100–110 °C) after CT at -185 °C for 8–32 h, there was a greater hardness increase: 5 HRC after 16 h [
19] or 2.2 HRC after 32 h [
298].
Diagram in
Figure 34 shows changes in hardness of differently cryogenically treated (at -154 or -184 °C, and for 6, 21 or 36 h) AISI H11 steel following the Sequence
F [
22]. It is seen that the treatments for 6 or 21 h increase the hardness moderately while 36-h treatments rather deteriorate this property. Since the retained austenite was stabilized by pre-tempering treatment (it is worth to note that diffraction peaks of this phase appeared in X-ray profiles of each specimen, according to [
22]), the hardness variations can be attributed mainly to precipitation of carbides; treatments for 6 or 21 h produce fine and uniformly distributed precipitates while 36-h treatments gave non-uniformly distributed coarser particles.
Tempering must be applied after CT (Sequence
A) to improve hardness more remarkably. For AISI H13 steel treated at -196 °C for 24-35 h, the hardness increase was 3-3.2 HRC [
50,
125,
245,
299]. For H11 steel treated at either -80 or -196 °C (each for 24 h), there was a 2 and 4.5 HRC hardness increase, respectively [
153]. A special hot stamping steel CR7V was treated at -196 °C for 3–12 h, and after 6-h treatment, there was a maximum hardness increase of 80 HV [
300].
Compared to hardness, the response of hot work tool steel to mechanical loading in tension and flexure is more susceptible to the stress-strain state; therefore, cryogenic treatment has a different effect on this property. Cryogenic treatment had almost no effect on the ultimate tensile strength of AISI H13 steel treated in liquid nitrogen for 12 h (following Sequence
A). The yield strength deteriorated slightly (by 40–50 MPa). The ductility was reduced when the material was gas quenched but improved when oil quenched [
118,
243]. However, when the H13 steel was cryogenically treated after pre-tempering at 560 °C for 2 h (following Sequence
F), the tensile strength was increased: 1640 and 1720 MPa for CT at -72 and -196 °C, respectively, compared to 1580 MPa for steel subjected to CHT [
155]. The use of Sequence
F for treatment of AISI H11 and H13 steels at either -154 or -184 °C for 6–36 h resulted in a slight deterioration in the ultimate tensile strength (by 5–12%) of the steel alongside significantly improved ductility (up to 50% improvement) [
22,
301]. Han et al. [
302] examined tensile properties of selectively laser-printed H13 steel after cryogenic treatment at -196 °C for 24 h followed by 200 °C tempering in one cycle (Sequence
A) and established by 70 MPa enhanced ultimate tensile strength, at almost doubled ductility.
The rotating bending fatigue endurance was also investigated for cryogenically treated (at -185 °C for 16 h, following Sequence
F) hot work tool H13 steel [
52]. For a 660 MPa stress cycle, the steel completed 263,362 cycles before failure after CHT. After cryogenic treatment, the steel achieved more than 24 million cycles before failure in the high-cycle fatigue regime. There was a similar study for AISI H21 steel after CT at -185 °C for 24 h, according to Sequence
A or
F [
303]. The rotating fatigue limits of AISI H21 steel (at 1×10
7 cycles) were 555, 648, and 740 MPa for the specimens subjected to CHT, Sequence
A, and Sequence
F, respectively. These values indicate a 17–33% improvement in the rotating fatigue limit.
Toughness and fracture toughness parameters reflect the effects of the applied CT sequence in a way similar to strength and ductility. Katoch et al. [
22,
23,
301] treated H11 and H13 steels cryogenically in cold nitrogen gas at -154 or -184 °C for 6–36 h, following Sequence
F. For both steels, CVN impact energy was improved by cryogenic treatment. The improvement was moderate (up to 44%) for CT at -154 °C, while cryogenic treatment at -184 °C tended to slightly increase toughness [
22,
23,
301],
Figure 35.
In another study [
155], it was found that cryogenic treatment at -72 or -196 °C for 8 h, following Sequence
F, slightly improved CVN impact toughness of H13 steel (by 1–2 J), with increased material hardness (by 6 HRC). It is worth noting that in [
22,
23,
301] the steels were tempered at 600 °C after cryogenic treatment, while in [
155], they were tempered at 560 °C after cryogenic treatment. Application of Sequence
E (CT at -185 °C for 35 h) produced only negligible changes in CVN impact toughness, while Sequence
A (with the cryogenic temperatures -185 or -196 °C and durations in the range of 24-35 h) reduced this property [
50,
125]. Note that Sequence
E led to an increase in hardness (by 1.5–3 HRC) without affecting toughness (by a 0.2 J increase). Furthermore, due to the simultaneous effect of quantitatively different strengthening mechanisms, no clear rules can be generalised for cryogenically treated hot work tool steels in terms of CVN impact toughness and fracture toughness. CVN impact toughness increased by more than 50% for cryogenically treated (at -196 °C for 6 h, by Sequence
A) hot stamping CR7V steel, besides a remarkable hardness increase of 80 HV [
300]. The result of cryogenically treated AISI H21 steel (at -185 °C for 6–30 h, following Sequence
F) was the opposite [
304]. Cryogenic treatment led to a general toughness decrease, with the lowest value recorded after 24-h treatment. To explain the contrasting results, it should be noted that the pre-tempering before CT left a certain (unspecified) amount of retained austenite in the microstructure [
303]. This austenite transformed into martensite during CT, which increased the hardness of the steel. Subsequent “soft tempering” at 100 °C is not sufficient to reduce the brittleness of the newly formed martensite; therefore, reduced toughness could be a logical consequence.
The application of CT generally improves the fracture toughness of hot work tool steels. Using Sequence
A to treat H13 steel improved K
IC by either 22–24% (CT at -196 °C for 12 h) [
118] or 6% (CT at -185 °C for 35 h) [
50]. A direct comparison of the impacts of different sequences (
A vs
E) showed that Sequence
E improved K
IC more effectively (15%) than Sequence
A (6%) [
50].
Fractographic observations of CVN impact toughness specimens of H11 or H13 steel [
23,
301] revealed that CHT materials mainly exhibited cleavage facets and microcracks along the cleavage facets, while cryogenically treated CT specimens (Sequence
F) showed dimples of different sizes and small zones of microvoid coalescence during crack propagation. The mentioned morphology of the fracture surfaces indicates better ductility of CT and post-CT high-temperature tempered steels, which was reflected in generally higher impact toughness. On the other hand, the generally lower CVN impact toughness of the specimens treated following Sequence
A could be related to less retained austenite content as this sequence is more effective in reducing this phase.
When examining the wear performance of hot work tool steels, most authors have used heat treatment with tempering before and after CT (Sequence
F). Despite considerable inconsistencies in the test conditions (counterpart nature, load, sliding speed, and distance), some general outcomes can be derived. For AISI H11, H13, and H21 steels, a 16–24 h treatment at -184 °C (or -185 °C) was recommended to achieve the lowest wear rate against steel counterparts [
19,
49,
51,
122,
199,
200,
246,
295,
296,
297,
304]. However, when hard alumina counterparts were used for wear performance examination, then after CT at -180 °C for 32 h, there was only a 12% wear performance improvement of H13 steel [
305]. Application of CT at -185 °C for 32 h also had a beneficial effect on the hot wear resistance (testing at 400 °C) of AISI H13 steel [
298]. Beneficial effect of cryogenic treatments on wear performance is demonstrated in two diagrams (
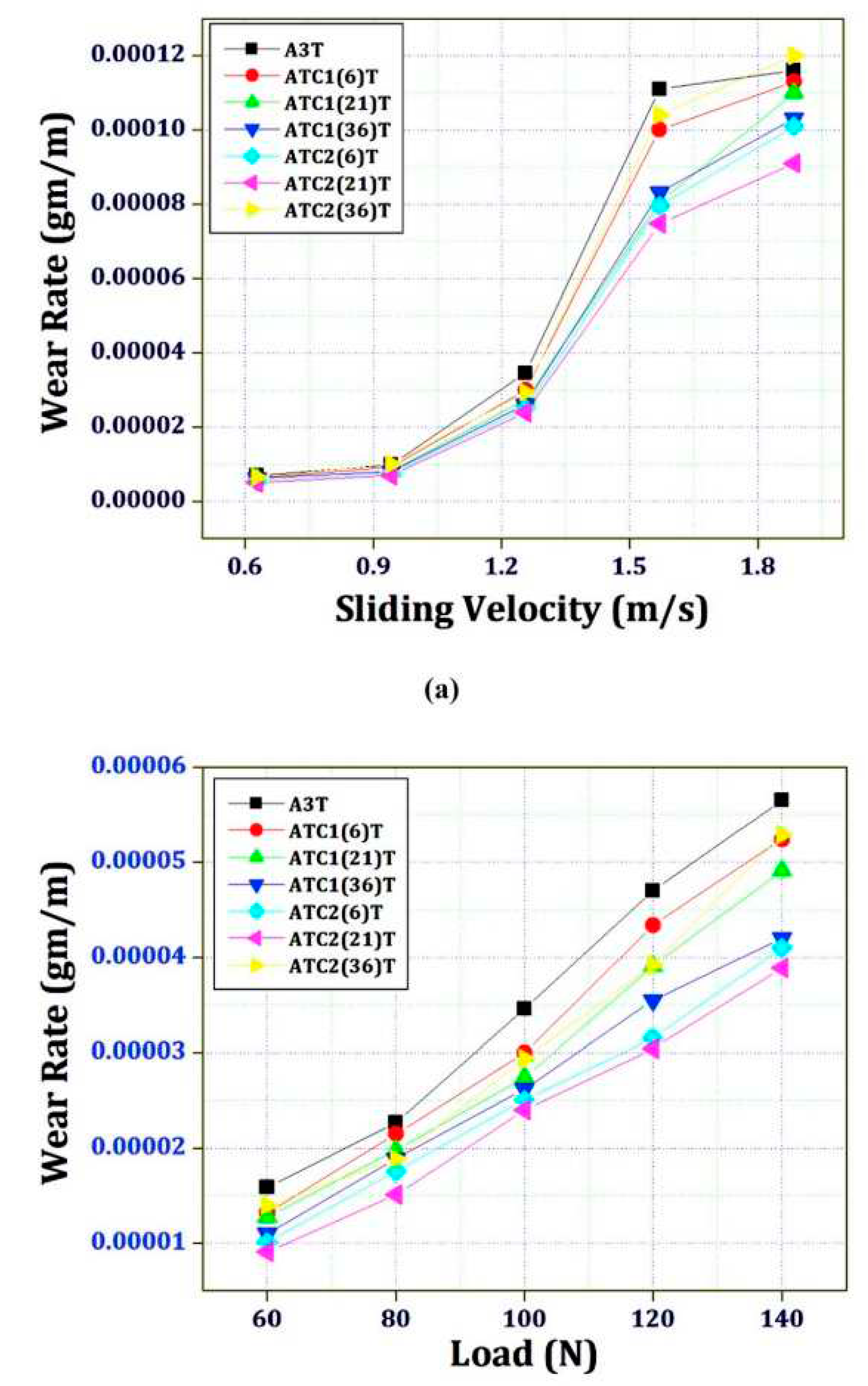
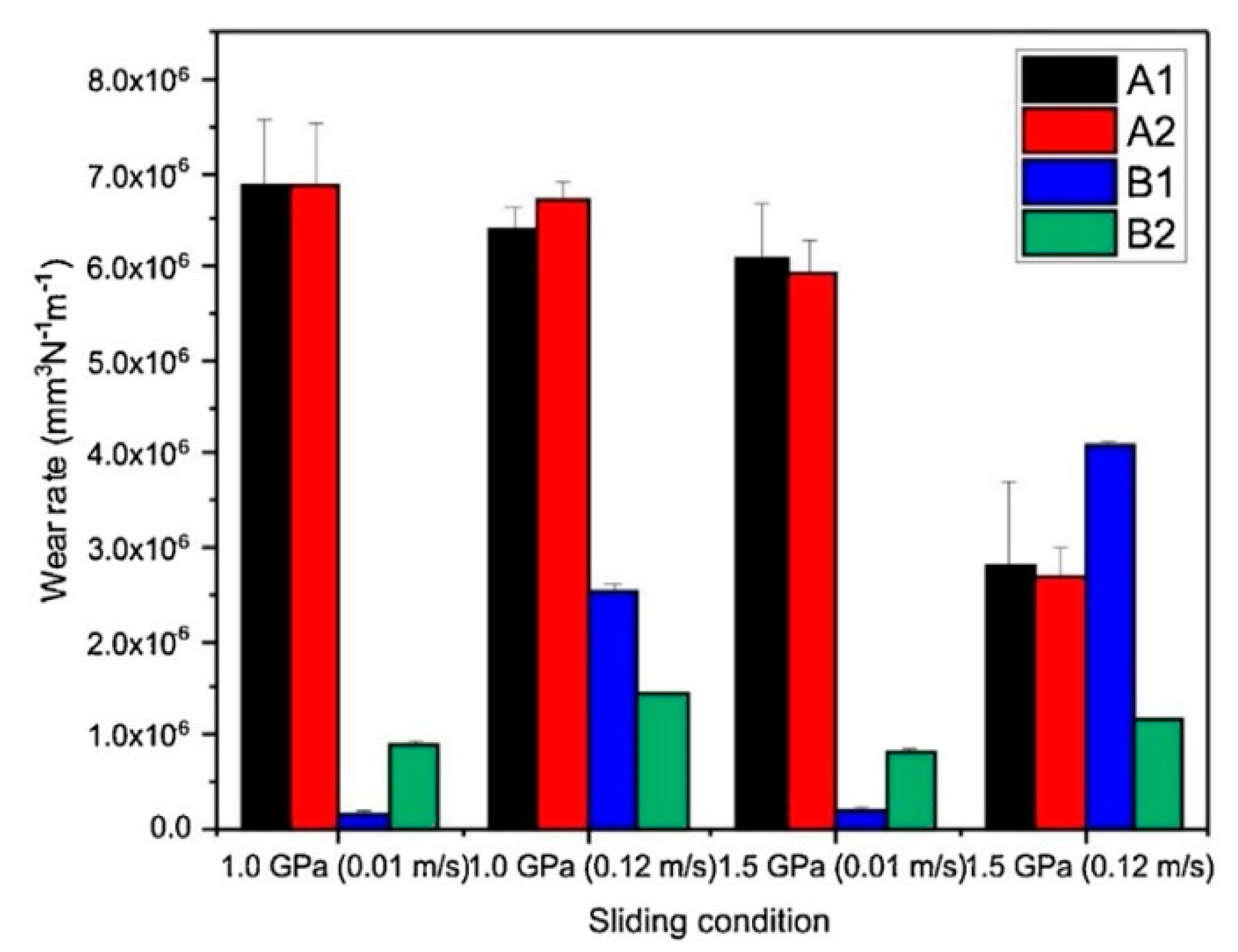
Figure 36) where the wear rate (WR) is plotted against either sliding velocity (a) or applied load (b) [
246]. A general trend of wear rate reduction with application of cryogenic treatments is apparently shown. The minimum wear rate (maximum wear performance) is obtained by applying a -185 °C cryogenic treatment for 21 h.
The use of treatment Sequences
A and
C (cyclic CT) also leads to better wear performance of hot work tool steels compared to the post-CHT state. Typical examples are the 24% improvement in abrasive wear performance of AISI H13 steel after treatment at -145 °C for 24 h [
48], 30-70% improvements after treatments of the same steel at -196 °C for 18-24 h [
244,
245,
299] about 35% improvement in the same property of X37CrMoV5 steel after treatment at -160 °C for 12 h [
117], 20-30% improvement of AISI H13 steel after treatments at -80 or -185 °C (for 24 h each) [
244], 62% improvement of hot stamping CR7V steel after treatment at -196 °C for 3–12 h [
300], or 14% wear resistance increase of AISI A8 steel after a cyclic CT (five cycles with temperature changing between -172 and -73 °C) followed by two tempering cycles (at 500 °C for 2 h each) [
306]. The hot wear resistance of H11 steel improved by 30–40% (at a test temperature of 550 °C) when H11 steel was treated at either -80 or -196 °C (for 24 h) [
153].
Potentiodynamic corrosion tests of AISI H13 hot work tool steel after CT at -185 °C for 16 h (Sequence
F), on the other hand, indicated that this treatment did not benefit the corrosion resistance of the given steel [
307].
To clarify changes in mechanical properties, it should first be noted that all measurements were conducted after the steels had been high-temperature tempered, and the only difference between the processing methods used was whether the tempering occurred before CT (Sequence E or F) or after CT (Sequence A).
Based on the microstructural changes (described in
Section 3), the hardness variations in hot work tool steels are mainly related to alterations in the amount of retained austenite. Sequence
A, performed at or near -196 °C, reduces RA most effectively. The role of a possible increase in the number and population density of carbides as well as changes in the precipitation kinetics of nano-sized carbides is still unclear and deserves further careful investigation. One can only speculate whether applying Sequence
F, additional tempering (after CT), could induce a coarsening of precipitates already present (after pre-tempering before CT), which could have a slightly detrimental effect on hardness. On the other hand, no conclusive statement can be expressed with respect to the effect of CTs on the tensile properties of hot-work tool steels.
Variations in wear rate due to the application of cryogenic treatment could be attributed to the combined effects of greater RA-to-martensite transformation (although the extent of this can only be roughly estimated due to the ‘distortion’ caused by high-temperature tempering used in treatment sequences), more pronounced carbide precipitation, and martensite refinement [
308]. As harder and finer martensite forms along with a higher number and population density of nano-sized precipitates, the wear resistance of hot work tool steels is generally improved by CT. There have been opposite effects regarding corrosion resistance: The presence of carbide precipitates has a detrimental effect on corrosion resistance as microelectrochemical cells are formed at the carbide/matrix interfaces. This has been confirmed by many authors for ledeburitic steels containing lamellar eutectic mixtures [
309] or high-Cr white cast irons [
310,
311,
312,
313,
314]. Nano-sized precipitates in hot work tool steels are formed by diffusion processes at elevated temperatures. They differ chemically from the matrix and therefore form microcells at their interfaces with the matrix.
Concluding remarks: Most of the experimental work has been carried out using the Sequences E or F, and only a small proportion of experiments (especially the most recent) have used Sequence A for CT of hot work tool steels. From a detailed review, it appears that the use of Sequence A results in better hardness than the other sequences. The reason for this could be that in the quenched condition, more retained austenite is available for transformation during CT than in sequences with prior-to-CT tempering. Recommended parameters for CT are temperature -185 to -196 °C and duration 24 – 35 h. The changes in hardness are closely related to alterations in wear performance. If the aim is to enhance the wear resistance, then similar parameters of CT should be used. The results obtained for tensile strength show clear inconsistencies. Further systematic research on the effect of CT on this property is needed before drawing a decisive conclusion. It seems that CT has a beneficial effect on the fatigue resistance of hot-work tool steels. This could be due to the increased hardness that results from transforming retained austenite into martensite. A higher hardness would certainly delay the initiation of cracks, leading to an increased number of cycles to failure. On the other hand, the results indicate a deterioration of the corrosion resistance after CT due to the increased precipitation of nano-sized carbides during the subsequent high-temperature tempering.
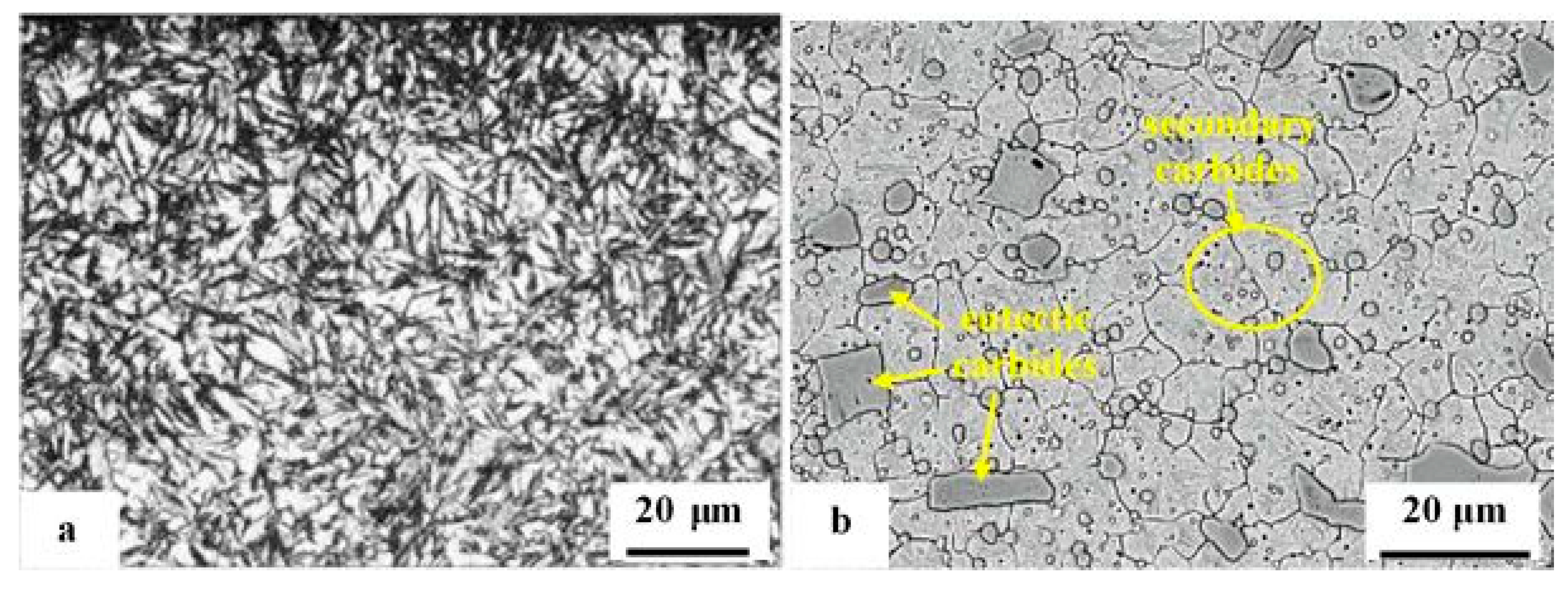
4.4. Ledeburitic steels and eutectic iron alloys
There are significant differences in the hardness of ledeburitic steels exposed to cryogenic treatment or conventional heat treatment,
Table 4. This is mainly observed in steel microstructures before tempering. For Cr-ledeburitic steels such as AISI D2, X290Cr12, and X210CrW12, an increase in hardness of 2–3 HRC was observed after CT in liquid nitrogen [
32,
78] or at -120 °C [
157]. Moreover, the extent of hardness increase was practically the same for short (15 min) or long (24 h) treatments. For white cast iron, there was a 4–9 HRC hardness increase (CT at -196 °C) [
315]. Vanadis 6 steel subjected to CHT had a hardness of 875 HV, but samples subjected to CT had a hardness of 920–950 HV. Treatment at -75, -140 or -196 °C for 17–24 h resulted in the greatest hardness [
15,
30,
57,
58,
59,
60]. The variations in hardness of cryogenically treated Vanadis 6 steel in an as-quenched state (before tempering) are summarised in
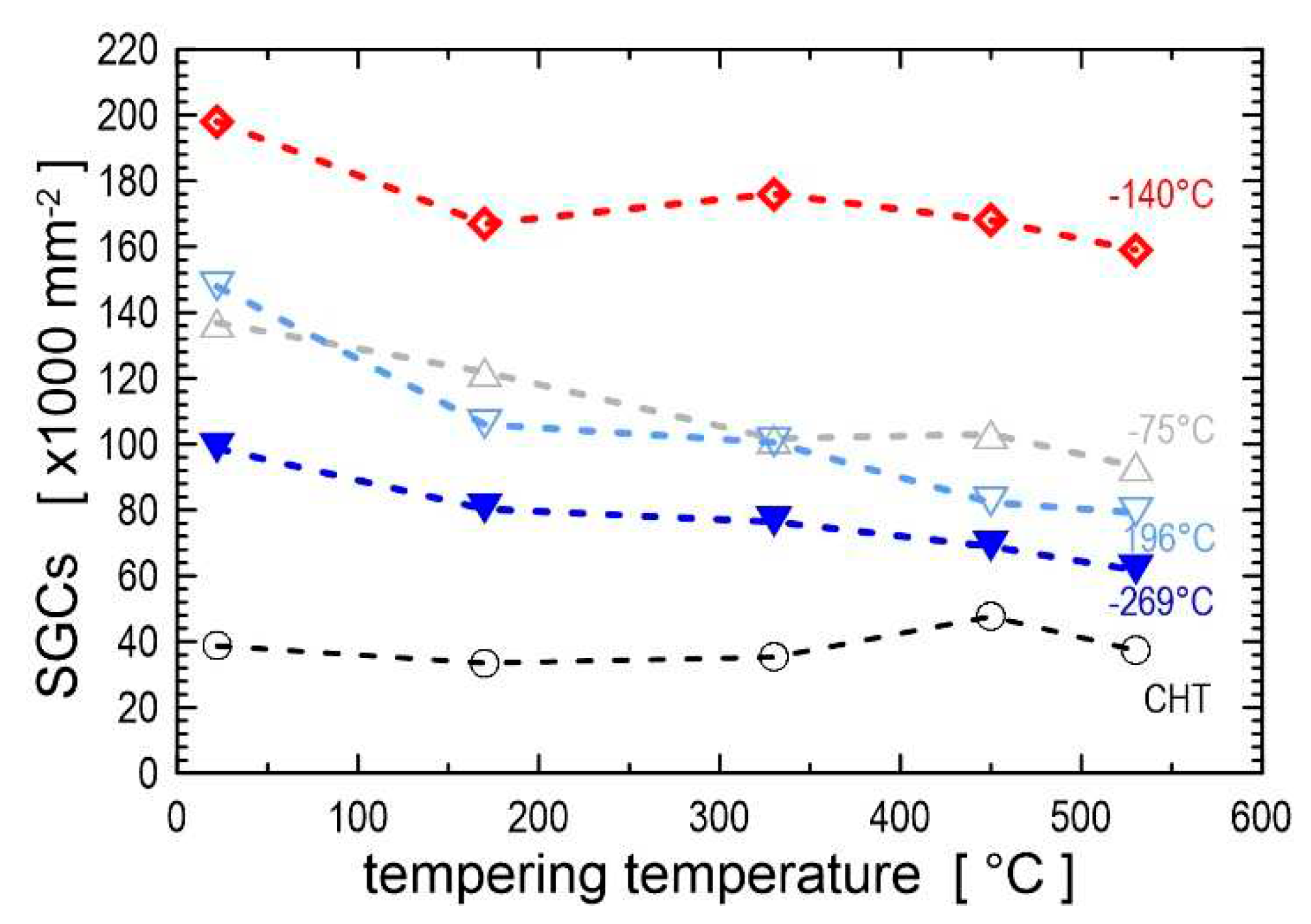
Figure 37. The curves show that the CT temperature affects the hardness level more markedly than the duration at CT temperature from a duration of about 5 h. Finally, the application of austenitising temperatures higher than those recommended by steel manufacturers before CT (e.g., 1100 or 1200 °C for Cr-ledeburitic steels) resulted in a much more pronounced hardness increase (by 15–20 HRC) [
32].
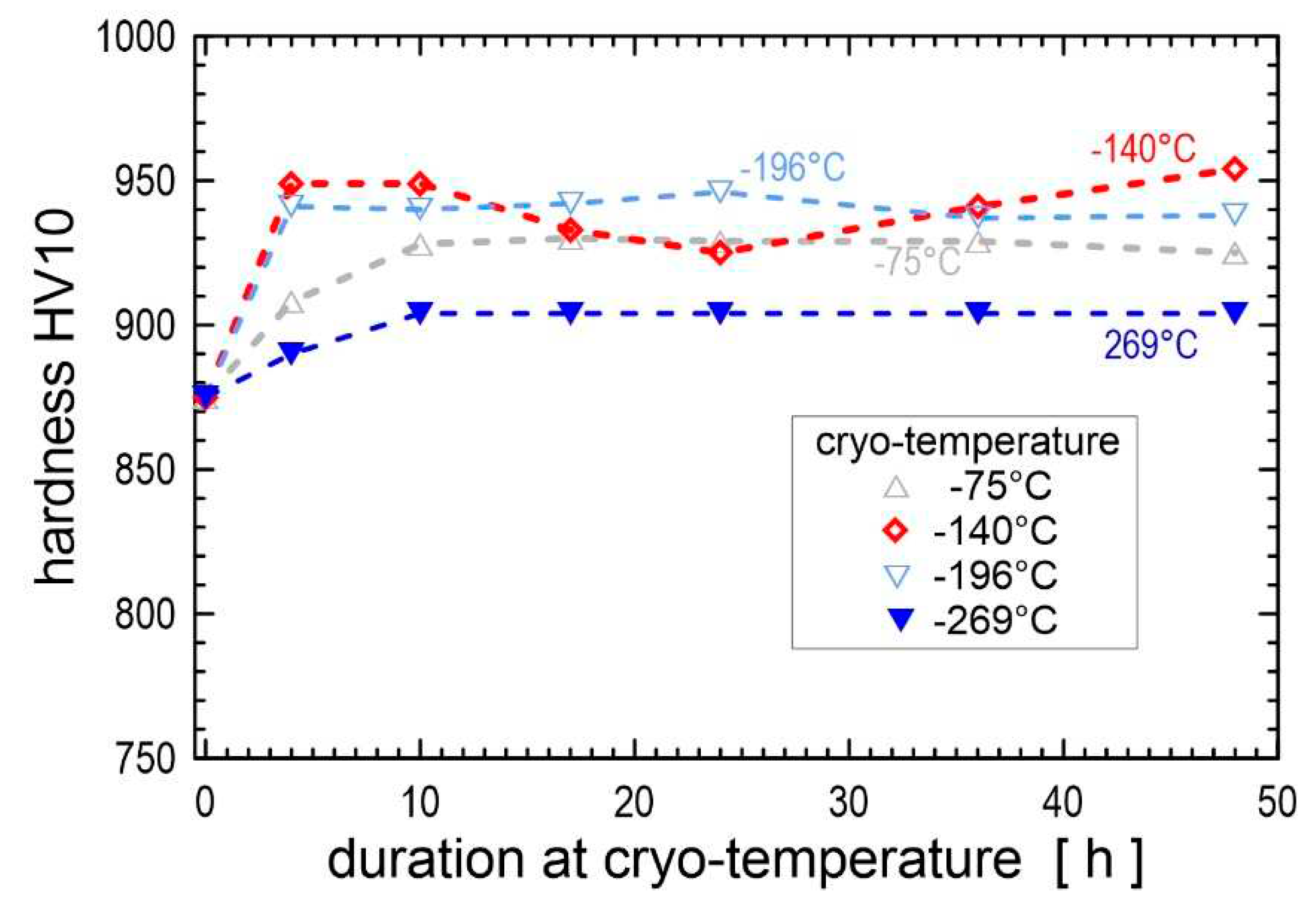
The higher hardness of cryogenically treated steels in the prior-to-tempered state could be simply related to a more complete RA-to-martensite transformation, additional small globular carbides, and accelerated precipitation of transient carbides. Both the extent of RA-to-martensite transformation and the amount of additional SGCs were the highest for the CT duration of 17-24 h. Therefore, the hardness of intrinsically nonhomogeneous steels, such as ledeburitic steels, is maximised by using a treatment duration that maximises the above microstructural changes.
The hardness variations due to tempering of cryogenically treated ledeburitic steels (and also high-speed steels, see section 4.5) are quite complex [
59]. According to modified Kulmburg’s consideration [
316], the final tempering curve consists of four components: (a) Martensite tempering, which reduces hardness. However, cryogenically treated steels contain more martensite, which can retard the decrease in overall hardness due to tempering. Therefore, low-temperature tempered CT steels have higher hardness than CHT steels. (b) Contribution of secondary retained austenite transformation to martensite. This leads to a significant increase in hardness during high-temperature tempering; however, CT steels contain considerably less retained austenite. Therefore, the contribution of the RA-to-martensite transformation to secondary hardening is small [
202]. (c) The presence of additional small globular carbides in cryogenically treated steels. These carbides have a positive effect on hardness, but their number decreases moderately with increasing tempering temperature [
15,
21,
116]. Therefore, the effect of these particles is more significant at lower tempering temperatures while it is suppressed after high-temperature tempering. (d) Precipitation of nano-sized carbides. At low tempering temperatures, the precipitation of transient carbides is accelerated [
21,
116,
179,
194,
201,
202,
203], and the contribution of these particles to the final hardness is positive. On the other hand, precipitation of stable carbides (at higher tempering temperatures) is suppressed due to CT [
21,
206]; therefore, the positive contribution of carbide precipitation at high temperatures is expected to be lower. More additional SGCs and martensite (in most cases) cannot fully compensate for the retained austenite reduction due to less intense precipitation of carbide nano-particles. Lower hardness (and loss of the secondary hardening) of cryogenically treated and high-temperature tempered ledeburitic steels is therefore logical.
Sequence
A has mostly been used to examine the impact of cryogenic treatment on the hardness of tempered ledeburitic steels, and this sequence increases hardness most effectively. For conditions after low-temperature tempering (180–210 °C), cryogenic treatment improved hardness compared to conventional heat treatment. The extent of improvement depended on the CT temperature. The maximum increase for D-class steels occurred after CT at -196 °C for 36–60 h. Such treatment improved the hardness by 30–55 HV compared to CHT [
12,
14,
25,
26,
29,
53,
54,
55,
78,
158,
160,
162,
212,
253,
264]. Considering for AISI D2 steel after CT (at -185 °C for 36 h) – as well as the effect of the number of tempering cycles (at 210 °C for 2 h each, Sequence
A) – the hardness after the first, second, and third tempering cycles increased by 3, 1.8, and 0.8 HRC, respectively, compared to CHT [
260]. Higher hardness after CT and low-temperature tempering, compared to CHT, was also reported for other ledeburitic and sub-ledeburitic steels that were also treated at a temperature above -196 °C: Vanadis 6 steel after CT at -75, -140, -196, or -269 °C (tempering in the range 170–450 °C) [
15,
58,
59,
317], sub-ledeburitic DC53 tool steel (tempered at 210 °C) [
203], or Uddeholm Sleipner after CT at either -80 or -180 °C [
318,
319]. On the other hand, the use of Sequence
E or
F resulted in almost no hardness improvement of D-class tool steels [
320].
For the states after high-temperature tempering (or tempering for secondary hardening), the hardness values of cryogenically treated ledeburitic steels are mostly lower than those conventionally quenched to room temperature. Moreover, the steels lose the secondary hardness peak, as demonstrated for AISI D2 and 190CrVMo20-4 steels treated at -120, -160, or -196 °C for ≥ 5 h [
156,
211,
321,
322,
323], HVAS steel [
324], or Vanadis 6 steel after CT at -75, -140, -196, or -269 °C [
57,
58,
59,
60,
325]. At a shorter CT duration, the secondary hardness peak does not disappear completely, but it shifts (by 20–30 °C) to lower than normally used (520-530 °C) tempering temperatures, as shown by the examples of three Cr-ledeburitic tool steels (X210CrW12, X165CrMoV12, and X155CrVMo12-1) [
32]. In one of the most recent works, Mochtar et al. [
326] treated AISI D2 steel at -196 °C for 5 min. and they arrived at a very similar finding. A small exception to the general trends mentioned is Vanadis 8 steel: hardness increased by 0.4 HRC after tempering cryogenically treated steel at 560 °C [
327]. Finally, CT of Vanadis 6 steel at -140 °C for 17 h resulted in the loss of the secondary hardness peak, but the hardness in the tempered state (at 530 °C) was higher than that after CHT [
59].
Vanadis 6 steel subjected to cryogenic treatments (at -75, -140 or -269 °C, following Sequence
A) showed slightly higher flexural strength compared to CHT, regardless of the tempering temperature (170–600 °C) [
15,
58,
59,
60,
317]. Conversely, CT at -196 °C for 17 or 24 h reduced the flexural strength [
28,
325], while a shorter treatment (up to 10 h) produced better values of this material property [
60]. For AISI D2 and 190CrVMo20-4 steels, CT followed by high-temperature tempering (following Sequence
A) had almost no effect on the flexural strength [
321].
A drastic reduction in CVN impact toughness of D-class ledeburitic steels as well as of sub-ledeburitic tool steels following application of CT followed by low-temperature tempering (Sequence
A) was demonstrated in many works, e.g. [
255,
263,
328,
329]. The application of Sequences
E or
F also had a detrimental effect on CVN impact toughness as reported by Li et al. for DC53 steel [
328]. The extent of the toughness decrease depends on the CT temperature. For example, the minimum toughness of AISI D2 steel occurred when treated at -70 °C, followed by a moderate increase in toughness when a lower cryogenic temperature was used [
81]. Furthermore, the reduction in toughness is more pronounced for longer CT durations. The only way to improve CVN toughness by CT, compared to CHT steels, is to temper the steels to the secondary hardness peak.
Trends in CVN toughness associated with cryogenic treatment are closely related to variations in fracture toughness [
330]. For AISI D2 steel, CT at -75, -125, or -196 °C (following Sequence
A, low-temperature tempering at 210 °C) resulted in a decrease in K
IC of 3.6, 7.7 and 2.5 MPa·m
1/2, respectively, compared to CHT [
253]. For CT at -196 °C for 4 h followed by tempering at 480 °C, the fracture toughness of the same steel grade was lower (22.7 MPa·m
1/2) than for CHT (25.4 MPa·m
1/2), a difference of about 8% [
256]. Cryogenic treatment CT also reduced the fracture toughness of the prior-to-tempered Vanadis 6 steel compared to the post-CHT state; this trend was maintained after tempering at 170, 330, or 450 °C [
15,
28,
58,
59,
317].
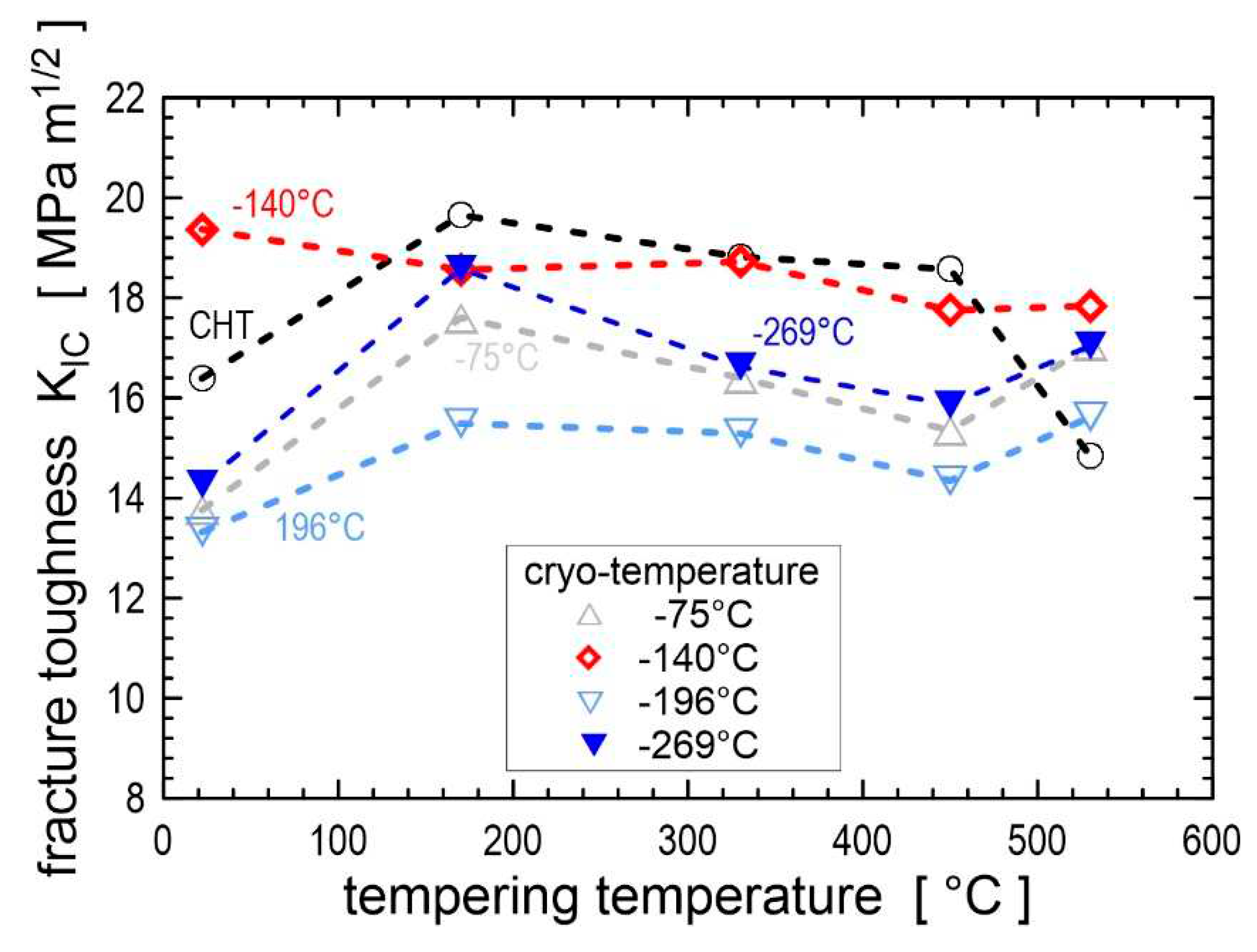
Figure 38 represents the fracture toughness values in the prior-to-tempered state and as a function of tempering temperature; only the state after CT at -140 °C shows K
IC values close to the conventional treatment. However, when tempered at 530 °C (secondary hardening peak), the cryogenically treated steel exhibited better fracture toughness than the steel after conventional quenching [
58,
59,
325]. Also, another general trend can be derived. While cryogenic treatments at -75, -196 or -269 °C combined with low-temperature tempering significantly reduced fracture toughness, there was only a slight decrease in fracture toughness after CT at -140 °C (
Figure 38).
The main explanation for the very low toughness and fracture toughness is that CT and low-temperature tempered steels contain less retained austenite and, accordingly, a higher portion of hard and brittle martensite [
282]. In addition, the martensite contains more nano-sized precipitates, which may tend to reduce its plasticity. More additional small globular carbides, which can essentially act as barriers to crack propagation, cannot fully compensate for the above microstructural phenomena. The possible increase in CVN impact toughness of CT and high-temperature tempered steels could be due to the fact that high-temperature tempering leads to significant martensite softening and thus, lower hardness. Furthermore, cryogenic treatment suppresses the precipitation of stable carbides at high tempering temperatures, thus enhancing the plasticity of the matrix compared to the same steel after CHT.
Thanks to specific microstructural changes, cryogenic treatment provides ledeburitic steels with the possibility of simultaneous increasing hardness (strength) and toughness, albeit only to a very limited extent and within a very narrow processing window. Ghasemi-Nanesa [
259] was the first to point out a simultaneous increase in the above mechanical properties, which are often in strong contradiction, in cryogenically treated AISI D2 steel. A very similar finding resulted from experimental works on cryogenically treated Vanadis 6 steel, as
Figure 37 illustrates.
Sequence
A with low-temperature tempering as the post-CT treatment has mostly been used to investigate the abrasive or adhesive wear resistance of cryogenically treated ledeburitic cold work tool steels. For AISI D2 steel, maximum abrasive wear resistance was achieved by a 36 h treatment in liquid nitrogen (followed by tempering at 210 °C for 2 h) [
27,
29,
54,
55]. A longer dwell time in liquid nitrogen did not provide additional benefits [
196]. Furthermore, CT at higher temperatures (e.g., -75 or -125 °C) led to less pronounced improvements in wear resistance. Improvements in wear resistance can be attributed to both the retained austenite reduction and the presence of more and a larger volume fraction of additional SGCs [
56,
331,
332,
333]. Moreover, variations in wear performance are associated with a change in the wear mechanism. At a low load, up to approximately 30 N, the wear resistance improvement was only 1.7 times, whereas the wear mechanism was identified as oxidative for both the CHT and CT steel specimens [
27]. However, at a higher load, up to 69 N, the wear mechanism for the CHT specimens (and also for the specimens exposed to CT at -75 °C) changed early to a delamination mechanism, resulting in a significant difference in wear performance between the CHT and CT samples, up to 82-fold. A further increase in the applied load led to a transition of the wear mechanism from light to heavy for the cryogenically treated specimens, which reduced the improvement in wear performance to 2-3.3 [
26,
27]. In the high load range, with a load of about 100 N, the average improvement was further reduced to about 85%, while it slightly decreased with increasing contact load [
55].
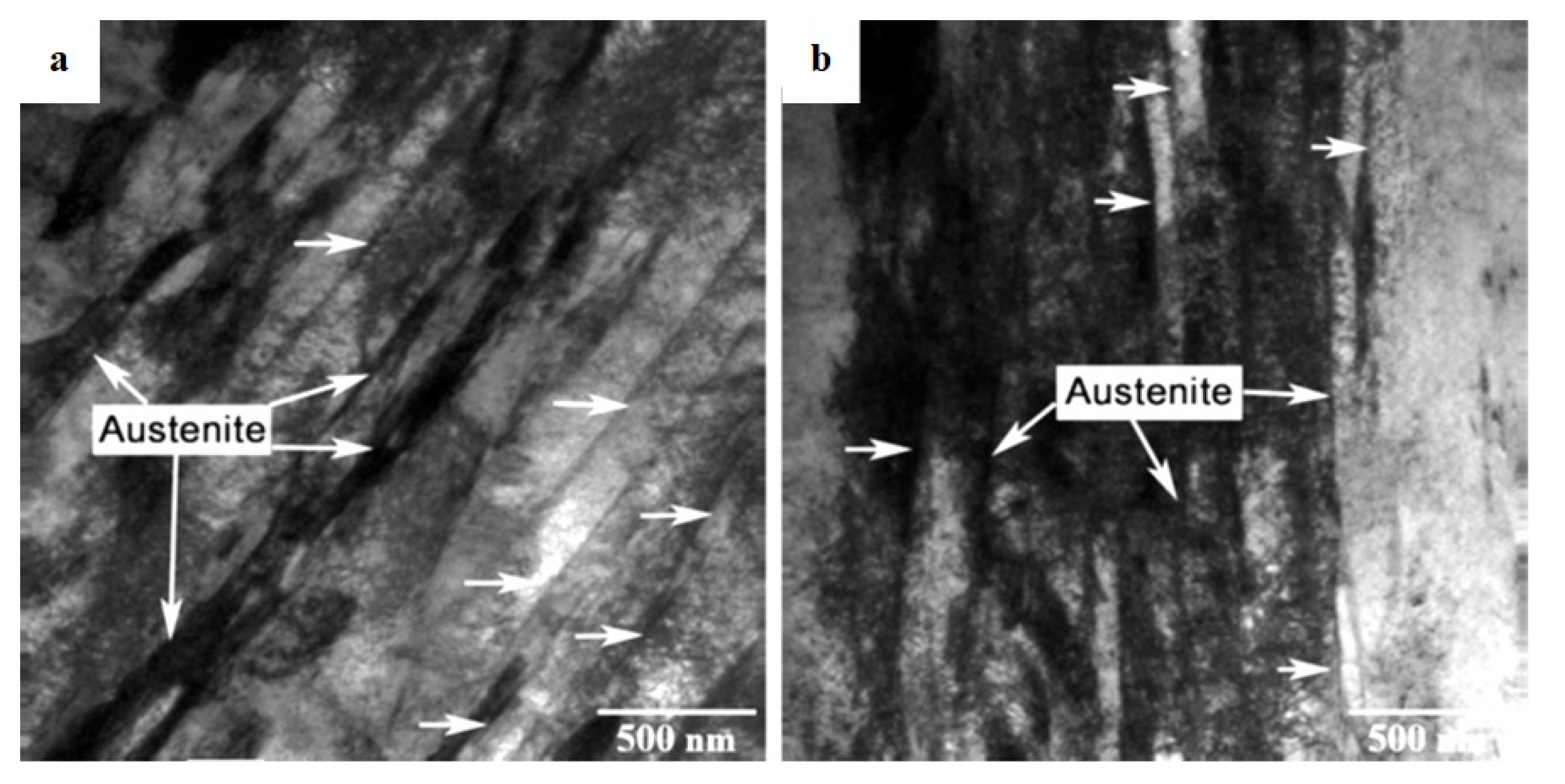
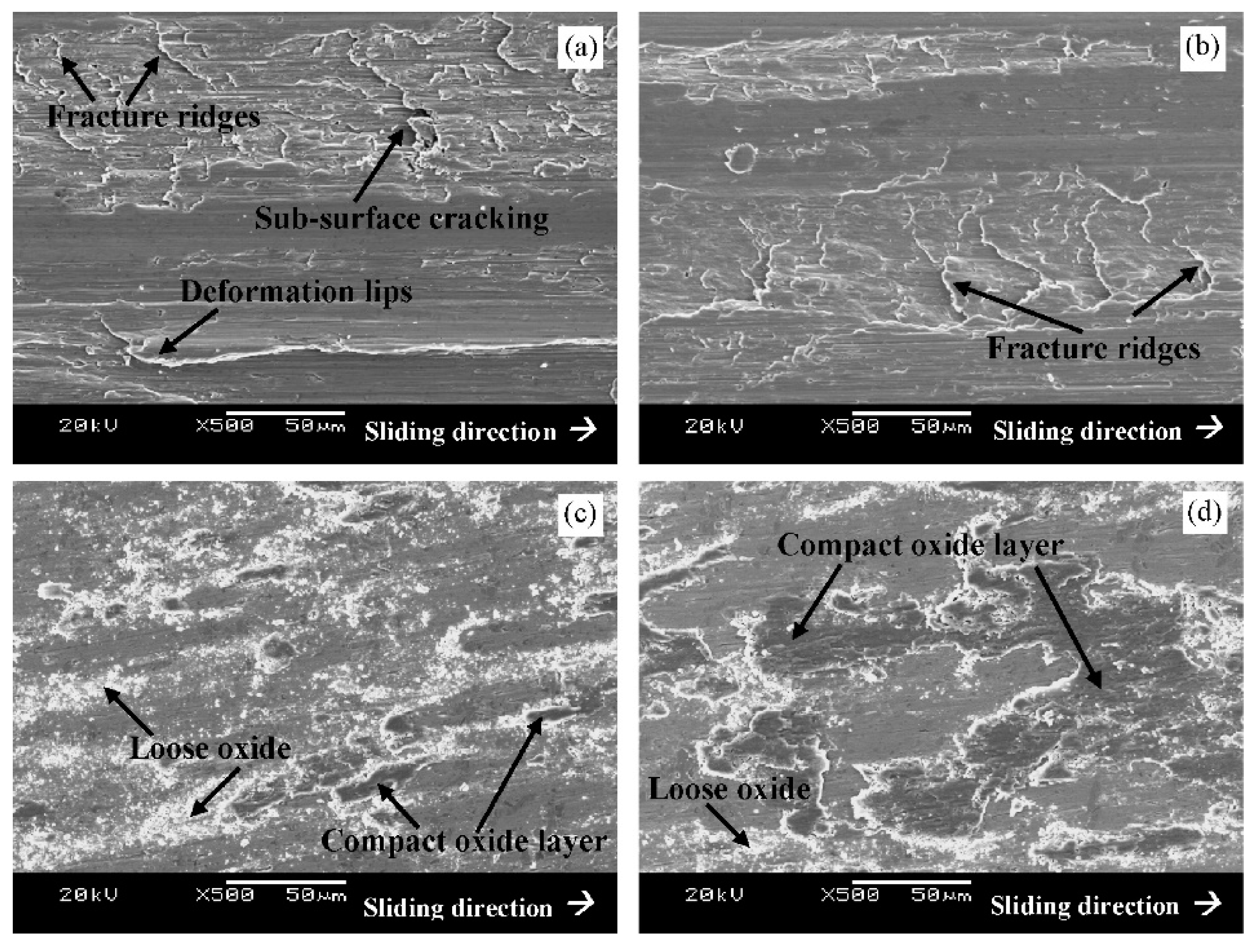
Figure 39 shows representative features on the worn surfaces of specimens subjected to CHT or CT at -75, -125, or -196 °C. The worn surface of the CHT steel (
Figure 39a) appears relatively rough and exhibits fracture ridges and deformation lips stretched parallel to the sliding direction. The presence of deformation lips suggests that the CHT specimen has undergone heavy plastic deformation during the wear test, accompanied by delamination of the deformed material. Similar features are also typical for specimens subjected to CT at -75 °C (
Figure 39b). In contrast, the worn surfaces of the other two specimens (
Figure 39c,d) are much smoother and manifest the presence of more or less compact oxides. The changes in the wear mechanisms are closely related to the morphology and composition of the produced wear debris. While the wear debris of CHT specimens and specimens subjected to CT at -75 °C is almost fully metallic (
Figure 40a,b,e), the wear debris of specimens subjected to CT at -125 or -196 °C is covered by oxides (
Figure 40c,d,f). In addition, the near-surface region of specimens subjected to CHT or CT at -75 °C exhibit heavy plastic deformation accompanied by cracking and delamination (
Figure 41a,b). On the other hand, the plastic deformation in near-surface regions of specimens subjected to CT at -125 or -196 °C is much lower (
Figure 41c,d), suggesting oxidative wear of the steels treated in this way.
Other cryogenically treated (at about -196 °C) and low-temperature tempered D-class ledeburitic tool steels such as AISI D6 [
13,
78,
158,
334], AISI D5 [
335] or AISI D3 [
159,
161,
261,
262] were also found to show a significant improvement in wear performance (up to 68–80%). The optimum dwell time was found to be 24–48 h. Examination of the effect of the number of tempering cycles at 150 °C on the wear resistance of AISI D3 steel revealed a 93% improvement after the first tempering cycle; additional tempering cycles reduced the extent of wear resistance improvement. Investigation of the effect of treatment sequences (
A vs
F) on the wear performance of AISI D2 steel (CT at -185 °C for 36 h) showed that Sequence
A provides better wear performance than Sequence
F [
260]. Alternatively, the application of cryogenic treatments at either -80 or -180 °C, both for 12–36 h and followed by 200 °C tempering (Sequence
A) did not provide the Sleipner sub-ledeburitic tool steel with any benefit with respect to the abrasive wear resistance [
319].
When high-temperature tempering was applied in treatment schedules, cryogenic treatment resulted in significantly less abrasive wear resistance improvement (up to 30%) for AISI D2 grade steel [
258,
324]. Marginal or no effects from CT followed by high-temperature tempering were also found if abrasive wear occurs (when hard counterparts such as alumina are used) in the cases of 190CrVMo20-4 and Vanadis 6 steels [
320,
336]. Alternatively, standardised (according to [
337]) pin-on-disc tests of cryogenically treated Vanadis 6 steel (at -90 °C for 4 h, -196 °C for 4 h, or -196 °C for 10 h) tempered at 530 °C showed better resistance to adhesive wear against 100Cr6 steel or bronze counterparts. In two recent papers, Yarasu et al. [
338,
339] studied mixed abrasive-adhesive (against 100Cr6 ball) and anti-galling (against CuSn6 bronze) properties of different cryogenic treatments (-75, -140, and -196 °C) of Vanadis 6 steel followed by low- or high-temperature tempering. They recommended using -196 °C cryogenic treatment followed by 530 °C to maximise the galling resistance of the examined tool steel. In contrast, cryogenic treatment at -140 °C with 170 °C tempering provided the steel with the best abrasive/adhesive wear resistance.
For ledeburitic steel tools operating in harsh corrosive environments, corrosion resistance is a key parameter that determines their durability. Carbides are known to exhibit much more noble behaviour in a variety of corrosive environments and can effectively protect metallic surfaces from corrosion [
340]. Cryogenically treated ledeburitic steels contain more carbides; therefore, a larger area fraction of their exposed surfaces is covered with phases that are comparatively more noble than ferrite or austenite. The corrosion resistance of ledeburitic steel could thus be improved by CT. This assumption was confirmed for X190CrVMo 20-4 steel subjected to CT (-196 °C for 15 min, Sequence
A, and tempering at 200 or 540 °C) and tested in a 0.5 M sulphuric acid solution [
341]. Cryogenic treatment also improved the corrosion resistance of Vanadis 6 steel (in a 3.5% aqueous NaCl solution). When the steel was low-temperature tempered after CT, the improvement was most pronounced after treatment at -140 °C. On the other hand, CT in liquid helium gave the best corrosion resistance for high-temperature tempered steel (
Figure 42) [
36]. By contrast, the corrosion resistance of 1.2080 steel grade (AISI D3) in 3.5% NaCl solution was worse after CT in liquid nitrogen for 24–48 h [
254,
342]. This phenomenon was attributed to an increased carbide content [
254], which reduces the number of dissolved Cr atoms in martensite and increases the number of martensite/carbide interfaces (galvanic cell areas). However, an opposite result has also been reported: > 50% improvement in the corrosion resistance of AISI D3 steel in borate buffer (pH 10) [
293].
Based on published data, there is disagreement about the change in corrosion resistance in response to CT. So far, there is no general explanation for this phenomenon. It appears that only steels produced by powder metallurgy (X190CrVMo 20-4 or Vanadis 6) show improved corrosion resistance after CT. The effect of CT on the corrosion resistance of wrought steels (such as AISI D3) remains unclear.
Concluding remarks: Cryogenically treated and low-temperature tempered ledeburitic steels invariably exhibit a great increase in hardness due to a much more complete transformation of austenite to martensite, increased precipitation of transient carbides, and the formation of a large number of additional small globular carbides. To maximise hardness, treatment in the range of -140 to -196 °C for 17– 36 h is the best choice. In this case, however, deterioration of toughness is inevitable; it is more pronounced in cast and wrought steels (D-class) while acceptable toughness can be achieved in newly developed PM grades. High-temperature tempering of cryogenically treated steel often results in the loss of the secondary hardness peak and thus in somewhat lower hardness than CHT steels. In this case, improved toughness is one of advantages of this type of treatment. Wear resistance can be extremely improved by cryogenic treatments (at -140 to -196 °C for 17–60 h, depending on the steel grade) followed by low-temperature tempering. Cryogenically treated and high-temperature tempered steels also have better wear resistance than conventionally treated steels, but the extent of wear resistance improvement is relatively small. It appears that corrosion resistance can be improved by CT of PM grades with fine and more uniformly distributed carbides. On the other hand, there are unclear results when cast and wrought grades have been cryogenically treated.
4.5. High-speed steels
High-speed steels,
Table 5, like ledeburitic cold work tool steels, are high alloy and intrinsically nonhomogeneous steels. They also contain martensite, retained austenite, and carbides in their as-quenched microstructures. It can be assumed that the application of cryogenic treatment to high-speed steels has effects similar to ledeburitic tool steels. High-speed steels are usually tempered at > 500 °C to achieve so-called secondary hardness [
343]. Therefore, unless otherwise stated, the experimental results described below refer to high-temperature tempering after CT (Sequence
A).
Tempering high-speed steels after cryogenic treatment (at temperatures close to -196 °C) reduced the maximum secondary hardness peak temperature by 15–30 °C [
121] in a way similar to chromium ledeburitic steels as reported by Berns [
32]. Diagrams in
Figure 43 show the extent of the shift of maximum secondary hardness peak temperature to lower tempering temperatures as well as the fact that the maximum achievable hardness can be lower after crygenic treatments for some high-speed steels grades [
121]. However, opinions on the effect of post-CT tempering on the resulting hardness are different and often contradict one another although obtained by examination of the same steel grade. A reduction of the maximum achievable hardness was experimentally demonstrated for AISI M2 or HS 6-5-3 (AISI M3:2) in the works [
63,
168]. No effect of CT at -196 °C on the hardness of AISI M35 and experimental (low alloyed HSS with 2.8 % Mo, 2.55 % W, 2.1 % V and 4.5 % Co) steels in the tempered condition was found in the works [
63,
164,
168]. However, most of the experimental works led to hardness increments of various high speed steels due to cryogenic treatment. This concerns the AISI M2 [
50,
62,
64,
65,
115,
344], AISI W9 [
10], AISI M35 [
170,
345], HS6-5-3-8 [
63,
168], and S390 Microclean grades [
166,
167]. The extent of this hardness increase seems to depend on the CT temperature. For AISI M2 and AISI W9 steels, hardness increased as the CT temperature decreased from -80 to -196 °C [
10,
65]. The cryogenic treatment duration also affected the hardness, with a maximum value at a 24 h treatment (+3.8 HRC compared to CHT) for AISI M2 steel [
65]. Xu et al. [
186] pointed out that the hardness of AISI M35 steel increases after up to 5 h of CT at -196 °C, and further duration of CT does not have a practical effect on hardness. There are also convincing experimental results that Sequence
A has a greater effect on increasing hardness than Sequence
E or
F for AISI W9 [
10], M2, M35, or T1 steels [
62,
64,
170,
344]. The effectiveness of Sequence
C should not be neglected. This sequence increased hardness the most for both AISI M2 and T1 steels [
64,
344]. Interesting results were achieved by CT (at -196 °C for 16 or 24 h) of AISI M2 steels produced by different methods. While the treatments of cast steel had no effect on the steel hardness, this property was increased by 27 and 40 HV, respectively, after 16 and 24 hour treatments of PM steel [
346].
The effect of prior-to-CT treatment (especially the austenitisation temperature) was also examined. For AISI M2, M3:2, and M35 steels, the hardness increased due to CT when austenitising temperatures were lower than those recommended by the steel suppliers, but hardness tended to decrease when the steels were austenitised at higher temperatures [
67,
347]. PM steel S390 Microclean was treated at -196 °C for either 25 or 40 h according to Sequence
A [
166,
167]. CT after austenitising at 1130 °C did not change hardness, but austenitising at 1230 °C increased the hardness by 1.5–2 HRC.
Tempering at low temperatures as a final treatment step also increases hardness after cryogenic treatment. For AISI M2 steel, the hardness increased from 62.2 HRC (after CHT) to 67 and 68.2 HRC for treatments at -110 °C for 18 h and -196 °C for 38 h, respectively (following Sequence
A) [
115].
In most papers dealing with the effects of CT on the toughness of high-speed steels (irrespective of the method used, e.g., CVN impact toughness, toughness measured on unnotched specimens, flexural strength, etc.), CT increased this property [
50,
62,
64,
68,
114,
186]. This change is due to the fact that the steels were tempered to their secondary hardness. High-temperature tempering induces martensite softening, making this phase more amenable to storing plastic deformation energy at the crack tip during crack propagation. In addition, more additional SGCs provide serious barriers to crack propagation, similar to ledeburitic steels subjected to CT. And finally, toughness improvement (with no hardness sacrifice at the same time) can be referred to as overall microstructural refinement, as Xu et al. have reported in their two recent works [
186,
345].
The effect of pre-treatment prior to CT, represented by the austenitisation temperature level, may also play a certain role in toughness variations. Unfortunately, the obtained results are quite contradictory. Fantinelli et al. [
62], for instance, have reported that if the AISI M2 steel was austenitised at 1170 °C before the cryogenic treatment at -190 °C for 24 h then the toughness was improved while austenitising at 1230 °C decreased this property. On the other hand, thorough investigations of effects of austenitising temperatures and CT (-196 °C for 24 h) on Charpy V-notch (CVN) impact toughness of three steel grades (AISI M2, AISI M3:2, AISI M35) resulted in opposite results [
67]. Better toughness (by 7-12%) was obtained for AISI M2 and AISI M3:2 steels after austenitising at higher temperatures, while the use of lower austenitising temperatures manifested almost no effect for these two grades. And finally, the toughness is always slightly reduced in the case of AISI M35 steel. The results obtained are summarised in
Figure 44 [
67]. Another parameter investigated in some research articles is the effect of pre-tempering prior to cryogenic treatment on toughness. It was found that this kind of treatment (Sequence
E or
F) produces better toughness as compared with post-CT tempering (Sequence
A) for AISI M2 steel [
62,
66]. Very promising results in terms of toughness increase (measured by CVN or flexural strength methods) were also obtained by cyclic CT (-180 °C or -196 °C) combined with tempering (Sequences
C or
G) for the treatment of AISI M2 or AISI T1 high-speed steels [
64,
66,
114]. However, a slight toughness reduction due to CT (by Sequences
A or
F) at -84 or -196 °C for 24 or 36 h has been reported for cobalt-containing wrought M35 steel [
67,
170].
Regarding the fracture toughness, a short treatment at -196 °C (1 h, Sequence
A) of AISI M2 steel had a detrimental effect on this property [
63], but the K
IC was improved by 10% after a 24 h treatment at the same temperature (
Figure 45) [
67,
168]. Conversely, other researchers observed only improvement in K
IC in AISI M2 steel; this improvement was more pronounced when a higher austenitisation temperature was used [
164]. For AISI M35 steel, the effect of austenitisation temperature prior to CT (Sequence
A) at -196 °C for 6 or 20 h on K
IC was investigated. Increases of 60%, 20%, and 15% were found for austenitising at 1070, 1100 and 1130 °C, respectively [
164]. On the other hand, CT at -196 °C for 24 h deteriorated the fracture toughness of M3:2 and M35 steels; using a lower austenitisation temperature reduced K
IC more significantly than using a higher austenitisation temperature (
Figure 45) [
67].
Apparent fracture toughness (also known as K
a) was studied for AISI M35 after cryogenic treatment at -180 °C for 24 h (Sequence
A or
E) [
344]. Only a marginal (1.4%) improvement in apparent fracture toughness was found for Sequence
E, while K
a increases of 3.5% and 4.6% were obtained for Sequence
A, with triple and double tempering, respectively. In another study [
121], the effects of the same heat treatment schedules on the apparent fracture toughness of four different high-speed steels were examined–namely, M2 and M35 wrought steels and M3:2 and S6-5-3-8 PM steels. The wrought steels exhibited lower apparent fracture toughness than the PM steels at a certain hardness due to the non-uniform distribution of carbides arranged in strings. Cryogenic treatment caused an overall increase in toughness in Co-free grades (M2 or M3:2); a decrease was always observed for the two Co-containing grades.
When evaluating the wear resistance of high-speed steels, the specimens were subjected to high-temperature tempering (up to or around the secondary hardness temperature) unless otherwise designated. Among the investigated materials, the AISI M2 grade has been the most popular.
Leskovšek et al. [
63] reported improved adhesive/abrasive wear resistance of AISI M2 steel after CT at -196 °C for 24 h and after tempering at 500, 550 or 600 °C, but tempering at 540 °C gave opposite results. This was related to the combined effect of hardness and fracture toughness, namely that the steel should have the highest possible fracture toughness (fulfilled after tempering at 550 and 600 °C) with sufficiently high hardness (64 HRC or more). Molinari et al. [
50] and Li et al. [
65] recommended CT at -196 °C for 35 or 24 h to achieve the best abrasive wear performance of AISI M2. Da Silva [
114] and Jovičevič-Klug et al. [
168] tested CHT and cryogenically treated (at -196 °C for 24 or 48 h) AISI M2 against alumina abrasives and found no effect of CT on abrasive wear resistance, as
Figure 46 demonstrates. They noted that the reduction of retained austenite by CT may not provide any benefit to wear performance under the given conditions, as retained austenite in CHT specimens may transform to martensite during testing. This could offset the improvement in wear performance due to more carbides in the cryogenically treated samples. Other works have used treatments at -180 or -190 °C (24 h) for CT, with moderately positive results [
62,
121,
344]. The extent of the changes in wear performance can also be affected by the manufacturing route applied for steel preparation. Savas et al. [
346] found only a small positive effect of cryogenic treatments at -196 °C on the wear resistance of cast AISI M2, while the same treatments on PM steel resulted in a significant reduction in wear rate.
Low-temperature (150 °C) tempered AISI M2 steel subjected to cryogenic treatments showed significantly enhanced abrasive wear performance, up to 40% after CT at -110 °C for 18 h or up to 58% after CT at -196 °C for 38 h [
115]. In another study, an optimisation experiment led to the final recommendation that 24 h treatment at -195 °C should be used to maximise the abrasive/adhesive wear performance of the given high-speed steel [
11].
For other Co-free high-speed steels such as AISI W9 [
10] and PM AISI M3:2 [
121,
168], cryo-temperatures of -180 to -196 °C were recommended to obtain better adhesive/abrasive wear performance. However, researchers also found that the effect of CT on wear performance is load-dependent; it is negative at low loads but exerts a strong positive influence at higher loads (> 40%),
Figure 46. Moreover, CT at -196 °C for 25 or 40 h (Sequence
A) had no effect on the abrasive wear resistance of S390 Microclean steel, but CT at -196 °C for 40 h after austenitising at 1130 °C (lower than the manufacturer’s recommended temperature) led to maximum anti-galling performance of the given steel, as more undissolved carbides had been maintained in its microstructure [
166,
167]. Similarly, the anti-galling performance was also improved for AISI M3:2 steel [
348].
For AISI M2, M3:2, and M35 high-speed steels, there was a moderate improvement in anti-galling performance after CT at -196 °C for 24 h (following Sequence
A) [
121,
348]. CT was not recommended for Co-containing high-speed steels because it reduced their wear performance [
121]. However, this statement is not consistent with the results obtained by other investigators when testing Co-containing steel grades such as AISI T42 (at -185 °C for 8-24 h) or AISI M35. In these cases a 38%–50% adhesive/abrasive wear resistance improvement [
10,
170,
267], and anti-galling performance improvement (for AISI M35 after CT at -196 °C for 24 h) were reported.
Examination of the effects of different CT sequences has led to the conclusion that Sequence
A is most effective in the wear performance enhancement of most high-speed steels tested [
11,
62,
115,
121,
348]. However, in selected trials, Sequence
E, with triple tempering at 570 °C before CT at -196 °C for 24 h, has also given promising results [
170,
267].
The effect of austenitisation temperature on the effectiveness of subsequent CT has also been evaluated. Pellizzari et al. [
66] concluded that using an austenitisation temperature higher than that recommended by the steel manufacturer does not improve wear performance. Fantinelli et al. [
62] found that for AISI M2 steel austenitised at either 1170 or 1230 °C before quenching and CT, Sequence
E, gave the best wear performance, and Sequence
A resulted in the best wear performance when the samples were austenitised at 1200 °C. Voglar et al. [
165] examined the corrosion resistance of three high-speed steel grades (AISI M2, AISI M3:2 and AISI M35) in different corrosive environments. They established that cast and wrought grades (AISI M2, M35) did not respond to corrosive environments favourably after cryogenic treatment at -196 °C for 24 h, whereas the powder metallurgy produced AISI M3:2 high-speed steel with a very fine microstructure showed promising results.
Concluding remarks: The evaluation of the mechanical properties and wear behaviour of cryogenically treated high-speed steels has led to contradictory results. For example, an increase in hardness has been reported by some authors after CT (with the maximum at -196 °C for 24-40 h), but others have demonstrated no effect or a hardness decrease, even in analogous steels due to CT. Improved toughness and fracture toughness were also observed in most cryogenically treated and high-temperature tempered steels, with the exception of the cobalt-containing grades. The sequence with tempering after CT (A) has a more effective influence on hardness than the sequences with pre-tempering (E or F). However, a rather opposite tendency was observed for the effect of these sequences on toughness. This is due to the fact that more austenite is subjected to CT in Sequence A, in which it is transformed into martensite. This martensite contains more lattice defects and produces a greater number of nano-sized precipitates during subsequent tempering. Finally, the effect of additional small globular carbides formation should not be ignored. On the contrary, CT of steels before tempering can only affect a small part of the retained austenite (which remains in as-tempered steel microstructures). This must inevitably reduce the positive effect on hardness but have a negative effect on toughness. In some cases, the use of multiple CT (Sequences C or G) gave promising results in terms of mechanical properties. However, further research is needed to clarify this issue. So far, the effect of the austenitisation temperature on the mechanical properties of cryogenically treated high-speed steels is unclear. It can also be suggested that there is a 'processing window' (a combination of austenitising temperature, CT temperature, and dwell time at that temperature) where hardness and toughness can be improved simultaneously, albeit to a limited extent. A typical example is AISI M2 steel. In this steel, the use of austenitising at 1170 °C, followed by CT at -190 °C/24 h and tempering, resulted in an increase in the hardness of several HRCs and a simultaneous increase in CVN toughness.
The final wear performance of high-speed steels is affected by the content of the martensite, retained austenite, and carbides in their microstructure. Since the high-speed steels studied are mostly high temperature tempered, they contain very little RA. Therefore, the effect of RA on their wear behaviour is minimal. The quenched and low-temperature tempered steels are an exception. In these cases, large improvements in abrasive/adhesive wear performance can be attributed to the reduction of RA by CT. The amount of carbides plays a crucial role in both abrasive/adhesive wear performance and anti-galling properties. It is noteworthy that the amount of additional SGCs in most high-speed steels reached a maximum after CT at -196 °C (or close to this temperature) for 24 h by Sequence
A [
61,
67,
115,
348]. Bergmann et al. and Badisch and Mitterer [
349,
350] reported that it is desirable to maximise the amount of hard carbides in steels if the highest possible wear performance is to be achieved. Therefore, treatment at -196 °C for 24 h resulted in the best wear performance of Co-free steels. In contrast, CT gave contradictory results for Co-containing HSS; therefore, this issue deserves further careful consideration. Finally, it appears that powder metallurgy produced HSS reacts much better to corrosion than forged grades after applying the same CT strategies.
4.6. Martensitic stainless steels
Martensitic stainless steels,
Table 6, are mostly alloys with a high content of Cr and other alloying elements. Therefore, they tend to retain high amounts of retained austenite in their microstructures when quenched from temperatures higher than the austenitising temperature recommended by the manufacturer. Some martensitic stainless steels exhibit a secondary hardness peak when tempered at around 500 °C. This is due to the complementary effect of the secondary RA-to-martensite transformation and carbide precipitation. However, to obtain high hardness in the tempered state, the amount of retained austenite should be carefully controlled before the steels are tempered. The application of cryogenic treatment is one way to regulate the amount of retained austenite in the steel before tempering.
For X30 CrMoN 15 1 steel, CT (at -198 °C for 24 h, Sequence
A) reduced the temperature of the secondary hardness peak by 70-100 °C after quenching in two different media – air or an aqueous solution of polyoxyethylene glycol [
71]. However, the hardness of samples subjected to CT increased by 15–20 HRC compared to CHT when austenitised at 1100–1200 °C. A similar hardness increase was also found for cryogenically treated (-196 °C/24 h, Sequence
A) AISI 420 steel [
210]. The use of a quenching medium had almost no effect on the final steel hardness, probably due to the excellent hardenability of the steel. The hardness of 0.15% C–14% Cr–13% Co–4.8% Mo–2.4% Ni steel austenitized at 1020 °C increased by 7 HRC by subjecting it to CT at -196 °C for 2 or 10 h (following Sequence
A) [
69,
70]. Very consistent results were obtained by cryogenic treatments of 0.17% C, 15% Cr, 11% Co, 3.3% Mo, 2.5% Ni, 2% W steel at -196 °C for 20 h [
272]. Cryogenic treatment at -196 °C for 10 h increased the hardness by 12 HRC after hardening from 1050 °C, indicating that the given CT effectively reduced RA [
69]. Furthermore, Sequence
A increased the hardness more effectively than post-tempering CT according to Sequence
E (a 3 HRC increase). Yildiz et al. [
351] have tried to treat the AISI 431 steel following the Sequence
F. They have found only very limited (by 17 HV) hardness increase due to cryogenic treatment at -180 °C for 6 h. The effect of cryogenic treatment temperature on hardness of AISI 420 grade was investigated by Prieto et al. [
68]. Cryogenic treatment at -196 °C for 2 h (following Sequence
A) increased the hardness from 560 HV10 (after CHT) to 585 HV10, but treatments at -40 or -80 °C did not affect hardness. For martensitic steel AISI 440C, CT at -80 °C for 5 h increased the final hardness by 2 HRC; cryogenic treatment at -196 °C resulted in a hardness increase of 4 HRC compared to the state after CHT [
16]. The results on hardness variations indicate that temperatures above -80 °C are insufficient to transform most retained austenite to martensite. Thus, much lower temperatures, close to boiling point of liquid nitrogen, are required to produce the maximum possible hardness increase.
Yildiz et al. [
351] have also examined the tensile properties of the AISI 431 steel treated at -180 °C for 6 h, following the Sequence
F. They have found that this kind of treatment leads to only negligible ultimate tensile strength increase (20 MPa) but more pronounced yield strength increase (by 70 MPa). On the other hand, however, a 50% reduction in wear rate has been reported in the same work, for the same steel treated by the same way. Yang et al. [
352] have reported by almost 300 MPa yield strength increase for Ferrium S53 ultra-high strength stainless steel after CT by Sequence
A.
Retained austenite reduction has a detrimental effect on the toughness of martensitic stainless steels. For example, CVN impact toughness of AISI 420 steel after CT showed a general decrease in toughness, except for CT at -196 °C for 2 h, where there was a 4 J increase in CVN toughness [
68]. For martensitic steel AISI 440C, CT at -196 °C for 24 h considerably reduced CVN impact toughness compared with CHT [
16]. There were similar results on impact toughness for 13% Cr–12% Co–4% Mo–1.7% Ni–0.15% C martensitic stainless steel after CT at -75 °C (following Sequence
A or
G) [
17].
Potentiodynamic tests of cryogenically treated (-196 °C/24 h, Sequence
A) AISI 420 steel [
210] showed almost no effect on corrosion resistance.
Concluding remarks: Overall, the results show that it is possible to increase the hardness of martensitic steels by cryogenic treatments. The maximum hardness increase can be obtained by CT at -196 °C, by using the Sequence A, while higher temperatures have limited effects on this property. In most cases, however, the increase in hardness is accompanied by a significant loss in toughness. These changes are primarily due to a significant RA reduction. This reduction can be as much as 90% of the original amount of retained austenite obtained in conventional room temperature quenching (see subsection 3.6). The use of Sequences E or F should generally be avoided in heat treatment strategy since they have brought only negligible changes in hardness and tensile properties of martensitic stainless steels.