Submitted:
12 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kashi, E.; Habibpour, R.; Gorzin, H.; Maleki, A. Solvent extraction and separation of light rare earth elements(La, Pr and Nd) in the presence of lactic acid as a complexing agent by Cyanex 272 in kerosene and the effect of citric acid, acetic acid and Titriplex III as auxiliary agents. J. Rare Earths 2018, 36, 317–323. [Google Scholar] [CrossRef]
- Banda, R.; Jeon, H.S.; Lee, M.S. Solvent extraction separation of La from chloride solution containing Pr and Nd with Cyanex 272. Hydrometallurgy 2012, 121-124, 74–80. [Google Scholar] [CrossRef]
- Yin, S.; Wu, W.; Bian, X.; Zhang, F. Effect of complexing agent lactic acid on the extraction and separation of Pr(III)/Ce(III) with di-(2-ethylhexyl) phosphoric acid. Hydrometallurgy 2013, 131–132, 133–137. [Google Scholar] [CrossRef]
- Michelsen, O.D.; Smutz, M. Separation of yttrium, holmium and herbium with di-2-ethylhexyl-phosphoric acid in chloride and nitrate systems. J. Inorg. Nucl. Chem. 1971, 33, 265–278. [Google Scholar] [CrossRef]
- Kao, H.C.; Yen, P.S.; Juang, R.S. Solvent extraction of La(III) and Nd(III) from nitrate solutions with 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester. Chem. Eng. J. 2006, 119, 167–174. [Google Scholar] [CrossRef]
- Radhika, S.; Kumar, B.N.; Kantam, M.L.; Reddy, B.R. Liquid-liquid extraction and separation possibilities of heavy and light rare-earths from phosphoric acid solutions with acidic organophosphorus reagents. Sep. Purif. Technol. 2010, 75, 295–302. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.; Dai, J.; Bian, X. Extraction and separation of Pr(III)/Ce(III) from chloride medium by 2-ethylhexylphosphonic acid mono-(2-ethylhexyl) ester in the presence of two complexing agents. Sep. Purif. Technol. 2016, 51, 778–783. [Google Scholar] [CrossRef]
- Quinn, J.E.; Soldenhoff, K.H.; Stevens, G.W.; Lengkeek, N.A. Solvent extraction of rare earth elements using phosphonic/phosphinic acid mixtures. Hydrometallurgy 2015, 157, 298–305. [Google Scholar] [CrossRef]
- Fontana, D.; Pietrelli, L. Separation of middle rare earths by solvent extraction using 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester as an extractant. J. Rare Earth 2009, 27, 830–833. [Google Scholar] [CrossRef]
- Kolarik, Z. Review: dissociation, self-association, and partition of monoacidic organophosphorus extractants. Solvent Extr. Ion Exch. 2010, 28, 707–763. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liao, W.P.; Li, D.Q. A solvent extraction process with mixture of CA12 and Cyanex 272 for the preparation of high purity yttrium oxide from rare earth ores. Sep. Purif. Technol. 2011, 82, 197–201. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M.S. Solvent extraction of Pr and Nd from chloride solution by the mixtures of Cyanex 272 and amine extractants. Hydrometallurgy 2014, 150, 61–67. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M.S. Separation of Pr and Nd from La in chloride solution by extraction with a mixture of Cyanex 272 and Alamine 336. Met. Mater. Int. 2015, 21, 944–949. [Google Scholar] [CrossRef]
- Wang, X.L.; Du, M.; Liu, H. Synergistic extraction study of samarium(III) from chloride medium by mixtures of bis(2,4,4-trimethylpentyl)phosphinic acid and 8-hydroxyquinoline mixtures. Sep. Purif. Technol. 2012, 93, 48–51. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Li, D.; Li, H. Synergistic extraction of rare earths by mixture of bis(2,4,4-trimethylpentyl)phosphinic acid and sec-nonylphenoxy acetic acid. Sep. Purif. Technol. 2006, 50, 30–34. [Google Scholar] [CrossRef]
- Zaheri, P.; Abolghasemi, H.; Maraghe, M.G.; Mohammadi, T. Intensification of europium extraction through a supported liquid membrane using mixture of D2EHPA and Cyanex 272 as carrier. Chem. Eng. Process. 2015, 92, 18–24. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Li, D. Extraction and stripping of rare earths using mixtures of acidic phosphorus based reagents. J. Rare Earths 2011, 29, 413–415. [Google Scholar] [CrossRef]
- Kuang, S.; Zhang, Z.; Li, Y.; Wei, H.; Liao, W. Synergistic extraction and separation of rare earths from chloride medium by the mixture of HEHAPP and D2EHPA. Hydrometallurgy 2017, 174, 78–83. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, X.; Li, D. Synergistic extraction and separation of heavy lanthanide bymixtures of bis(2,4,4-trimethylpentyl)phosphinic acid and 2-ethylhexyl phosphinic acid mono-2-ethylhexyl ester. Sep. Sci. Technol. 2005, 40, 2325–2336. [Google Scholar] [CrossRef]
- Wang, Y. The novel extraction process based on Cyanex 572 for separating heavy rare earths from ion-adsorbed deposit. Sep. Purif. Technol. 2015, 151, 303–308.20. [Google Scholar] [CrossRef]
- Kolar, E.; Catthoor, R.P.R.; Kriel, F.H.; Sedev, R.; Middlemas, S.; Klier, E.; Hatch, G.; Priest, C. Microfluidic solvent extraction of rare earth elements from a mixed oxide concentrate leach solution using Cyanex 572. Chem. Eng. Sci. 2016, 148, 212–218. [Google Scholar] [CrossRef]
- El-Hefny, N.E.; Gasser, M.S.; Emam, S.S.; Mahmoud, W.H.; Aly, H.F. Comparative studies on Y(III) and Dy(III) extraction from hydrochloric and nitric acids by Cyanex 572 as a novel extractant. J. Rare Earths 2018, 36, 1342–1350. [Google Scholar] [CrossRef]
- El-Hefny, N.E.; Gasser, M.S.; Emam, Sh.Sh.; Mahmoud, W.H.; Aly, H.F. Comparative studies on Y(III) and Dy(III) extraction from hydrochloric and nitric acids by Cyanex 572 as a novel extractant. J. Rare Earths 2018, 36, 1342–1350. [Google Scholar] [CrossRef]
- Pavon, S.; Kutucu, M.; Coll, M.T.; Fortunya, A.; Sastre, A.M. Comparison of Cyanex 272 and Cyanex 572 for the separation of neodymium from a Nd/Tb/Dy mixture by pertraction. J. Chem. Technol. Biotechnol. 2017, 2152–2159. [Google Scholar] [CrossRef]
- Belova, V.V.; Petyaeva, M.M.; Kostanyan, A.E. Extraction of lanthanides from chloride solutions in hexane–isopropanol–water systems using Cyanex 572. Theor. Found. Chem. Eng. 2022, 56, 595–599. [Google Scholar] [CrossRef]
| CREE(init.). mol/L |
Distribution coefficients | |||||||||||
| Ce | Nd | Sm | Gd | Tb | Dy | Ho | Er | Tm | Yb | Y | ∑REE | |
| 0.050 | - | - | 0.160 | - | - | 0.180 | 0.460 | 0.770 | - | - | 0.470 | 0.340 |
| 0.100 | - | 0.120 | - | - | - | 0.081 | 0.220 | 0.360 | 1.44 | 4.21 | 0.220 | 0.130 |
| 0.160 | - | - | - | - | - | 0.048 | 0.130 | 0.220 | 0.930 | 2.50 | 0.130 | 0.083 |
| 0.210 | 0.089 | 0.035 | - | - | - | 0.040 | 0.100 | 0.160 | 0.630 | 1.93 | 0.100 | 0.068 |
| 0.330 | 0.068 | 0.078 | 0.0095 | - | 0.235 | 0.024 | 0.063 | 0.100 | 0.410 | 1.11 | 0.062 | 0.046 |
| 0.410 | - | - | 0.0016 | - | - | 0.019 | 0.049 | 0.081 | 0.300 | 0.860 | 0.048 | 0.032 |
| 0.520 | 0.020 | 0.015 | 0.0028 | - | - | 0.017 | 0.041 | 0.065 | 0.230 | 0.620 | 0.036 | 0.026 |
| 0.630 | - | - | 0.0018 | 0.0026 | - | 0.015 | 0.035 | 0.054 | 0.190 | 0.580 | 0.036 | 0.024 |
| 0.990 | 0.047 | 0.012 | 0.0025 | 0.0024 | - | 0.015 | 0.027 | 0.043 | 0.130 | 0.350 | 0.026 | 0.020 |
| CREE(init.). mol/L |
Distribution coefficients | |||||||||||
| Ce | Nd | Sm | Gd | Tb | Dy | Ho | Er | Tm | Yb | Y | ∑REE | |
| 0.050 | 0.190 | 0.160 | 0.048 | 0.150 | 0.790 | 2.24 | - | 12.6 | - | - | 7.82 | 1.29 |
| 0.110 | 0.074 | - | 0.008 | 0.025 | 0.130 | 0.320 | 0.590 | 1.55 | - | - | 1.01 | 0.430 |
| 0.120 | 0.370 | - | 0.008 | 0.025 | 0.130 | 0.300 | 0.530 | 1.54 | - | - | 0.990 | 0.430 |
| 0.220 | 0.120 | - | 0.003 | 0.0090 | 0.044 | 0.110 | 0.190 | 0.530 | 1.27 | 6.03 | 0.340 | 0.190 |
| 0.340 | 0.088 | - | 0.002 | 0.0058 | 0.026 | 0.060 | 0.100 | 0.280 | 0.760 | 3.14 | 0.190 | 0.110 |
| 0.430 | - | - | 0.003 | 0.0059 | 0.023 | 0.047 | 0.082 | 0.220 | 0.570 | 2.41 | 0.140 | 0.085 |
| 0.550 | - | - | 0.003 | 0.0061 | 0.020 | 0.041 | 0.067 | 0.170 | 0.450 | 1.78 | 0.110 | 0.069 |
| 0.640 | - | 0.007 | 0.004 | 0.0063 | 0.020 | 0.036 | 0.055 | 0.140 | 0.360 | 1.44 | 0.093 | 0.060 |
| 0.960 | - | - | 0.005 | 0.0080 | 0.023 | 0.036 | 0.052 | 0.110 | 0.260 | 0.920 | 0.074 | 0.049 |
| CREE(init.). mol/L |
Distribution coefficients | |||||||||||||
| Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | ∑REE | |
| 0.050 | 0.990 | 0.180 | 1.31 | 3.33 | 5.10 | - | 83.6 | - | - | - | - | - | - | 6.04 |
| 0.090 | 0.560 | 0.020 | 0.021 | 0.070 | 0.110 | 0.540 | 1.65 | 2.97 | 9.36 | 19.4 | 98.5 | - | 5.18 | 1.14 |
| 0.130 | 0.880 | 0.022 | 0.017 | - | 0.040 | 0.160 | 0.470 | 0.790 | 2.52 | 6.88 | 24.2 | - | 1.52 | 0.590 |
| 0.180 | 0.520 | 0.017 | 0.011 | - | 0.023 | 0.100 | 0.300 | 0.530 | 1.51 | 4.31 | 15.5 | 9.90 | 0.950 | 0.430 |
| 0.270 | 0.330 | - | 0.008 | - | 0.013 | 0.040 | 0.150 | 0.250 | 0.700 | 2.39 | 8.87 | 6.63 | 0.450 | 0.240 |
| 0.370 | 0.220 | - | 0.006 | - | 0.011 | 0.040 | 0.110 | 0.160 | 0.490 | 1.44 | 4.78 | 4.50 | 0.310 | 0.180 |
| 0.450 | 0.230 | 0.010 | 0.006 | - | 0.010 | 0.030 | 0.080 | 0.120 | 0.370 | 1.06 | 3.49 | 3.50 | 0.250 | 0.140 |
| 0.530 | 0.190 | 0.006 | 0.008 | - | 0.011 | 0.030 | 0.080 | 0.110 | 0.310 | 0.900 | 2.95 | 3.19 | 0.200 | 0.120 |
| 0.810 | 0.140 | 0.006 | 0.009 | 0.010 | 0.013 | 0.040 | 0.070 | 0.080 | 0.210 | 0.490 | 1.61 | 1.92 | 0.130 | 0.090 |
| CREE(init.). mol/L |
Distribution coefficients | |||||||||||||
| Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | ∑REE | |
| 0.090 | 1.83 | 0.075 | 0.340 | 0.800 | 1.21 | 8.31 | 24.5 | - | - | - | - | - | 86.7 | - |
| 0.140 | 0.920 | 0.027 | 0.029 | 0.085 | 0.130 | 0.600 | 1.79 | 3.15 | 9.60 | 19.2 | 93.6 | - | 5.74 | 2.99 |
| 0.170 | 0.830 | 0.023 | 0.021 | 0.033 | 0.051 | 0.220 | 0.660 | 1.20 | 3.42 | 9.90 | 33.4 | - | 2.14 | 1.23 |
| 0.260 | 0.410 | 0.015 | 0.011 | - | 0.024 | 0.120 | 0.290 | 0.480 | 1.40 | 3.97 | 13.4 | 8.80 | 0.920 | 0.740 |
| 0.340 | 0.260 | 0.010 | 0.009 | - | 0.017 | 0.063 | 0.170 | 0.260 | 0.770 | 2.20 | 7.54 | 5.97 | 0.540 | 0.420 |
| 0.450 | 0.260 | 0.008 | 0.010 | 0.013 | 0.017 | 0.063 | 0.150 | 0.220 | 0.600 | 1.79 | 5.71 | 4.99 | 0.430 | 0.270 |
| 0.520 | 0.190 | 0.039 | 0.010 | 0.013 | 0.016 | 0.051 | 0.110 | 0.160 | 0.450 | 1.20 | 3.96 | 3.83 | 0.300 | 0.240 |
| 0.800 | 0.120 | 0.011 | 0.011 | 0.016 | 0.019 | 0.047 | 0.094 | 0.120 | 0.270 | 0.690 | 2.16 | 2.39 | 0.190 | - |
| CREE(init.). mol/L |
Separation factor | |||||||
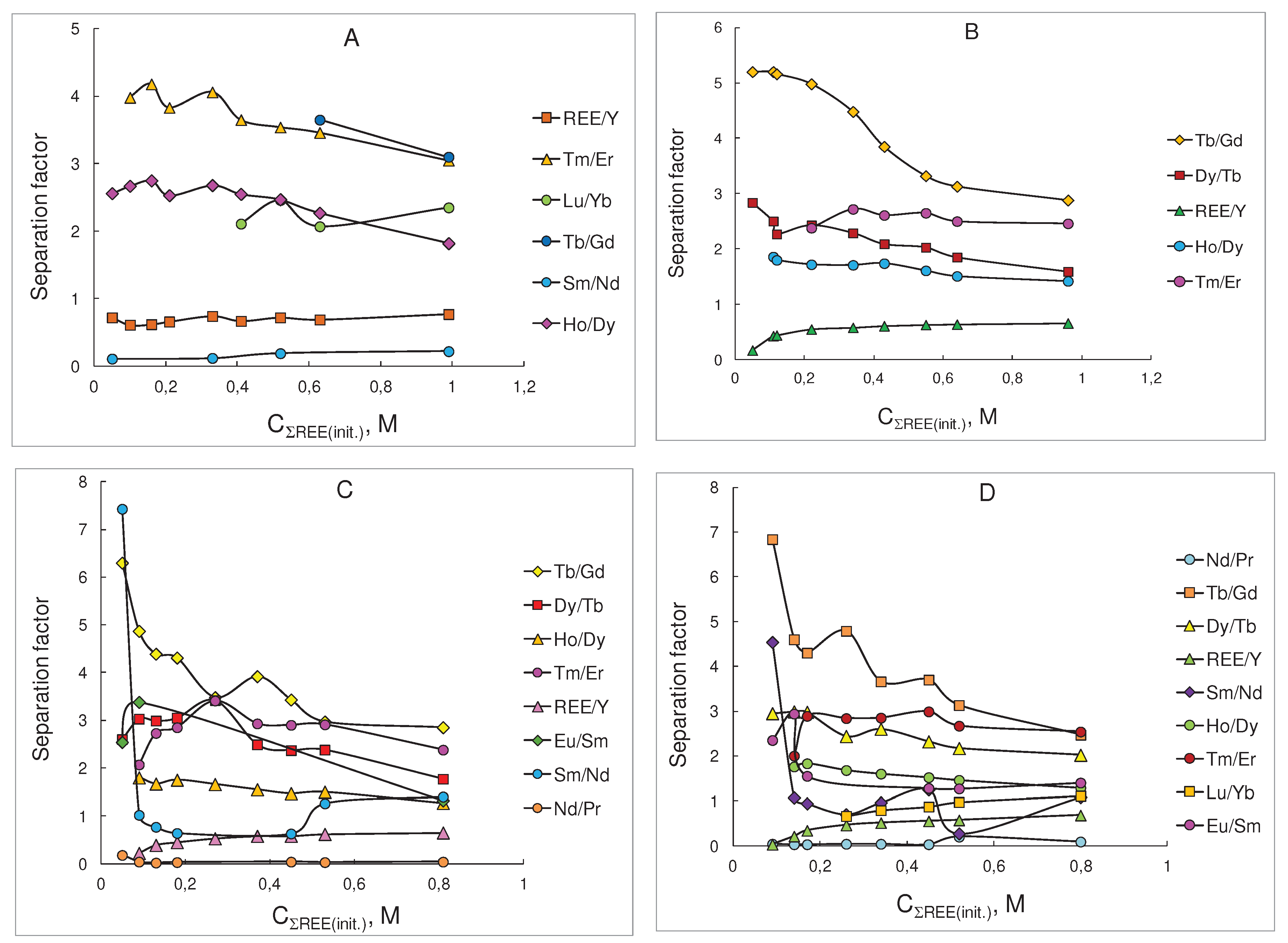
| Sm/Nd | Tb/Gd | Dy/Tb | Ho/Dy | Tm/Er | Lu/Yb | ∑REE/Y | ||
| 0.050 | 0.110 | - | 1.58 | 2.56 | - | - | - | |
| 0.100 | - | - | - | 2.67 | 3.98 | - | 0.610 | |
| 0.160 | - | - | - | 2.75 | 4.18 | - | 0.620 | |
| 0.210 | - | - | - | 2.53 | 3.83 | - | 0.660 | |
| 0.330 | 0.120 | - | - | 2.68 | 4.06 | - | 0.740 | |
| 0.410 | - | - | - | 2.55 | 3.65 | 2.11 | 0.670 | |
| 0.520 | 0.190 | - | - | 2.47 | 3.54 | 2.46 | 0.720 | |
| 0.630 | - | 3.65 | 1.64 | 2.27 | 3.46 | 2.07 | 0.690 | |
| 0.990 | 0.220 | 3.10 | 2.00 | 1.82 | 3.05 | 2.35 | 0.770 | |
| CREE(init.). mol/L |
Separation factor | ||||||
| Sm/Nd | Tb/Gd | Dy/Tb | Ho/Dy | Tm/Er | ∑REE/Y | ||
| 0.050 | 0.300 | 5.20 | 2.84 | - | - | 0.170 | |
| 0.110 | - | 5.20 | 2.50 | 1.86 | - | 0.430 | |
| 0.120 | - | 5.16 | 2.27 | 1.80 | - | 0.440 | |
| 0.220 | - | 4.98 | 2.43 | 1.72 | 2.38 | 0.550 | |
| 0.340 | - | 4.48 | 2.29 | 1.71 | 2.72 | 0.580 | |
| 0.430 | - | 3.85 | 2.09 | 1.74 | 2.61 | 0.610 | |
| 0.550 | - | 3.32 | 2.03 | 1.61 | 2.65 | 0.630 | |
| 0.640 | 0.530 | 3.13 | 1.85 | 1.51 | 2.50 | 0.640 | |
| 0.960 | - | 2.88 | 1.59 | 1.42 | 2.46 | 0.660 | |
| CREE(init.). mol/L |
Separation factor | ||||||
| Sm/Nd | Tb/Gd | Dy/Tb | Ho/Dy | Tm/Er | ∑REE/Y | ||
| 0.050 | 7.43 | 6.30 | 2.60 | - | - | - | |
| 0.090 | 1.02 | 4.87 | 3.03 | 1.80 | 2.07 | 0.220 | |
| 0.130 | 0.770 | 4.39 | 2.99 | 1.67 | 2.73 | 0.390 | |
| 0.180 | 0.640 | 4.31 | 3.05 | 1.75 | 2.85 | 0.450 | |
| 0.270 | - | 3.48 | 3.42 | 1.66 | 3.41 | 0.530 | |
| 0.370 | - | 3.92 | 2.49 | 1.55 | 2.93 | 0.580 | |
| 0.450 | 0.630 | 3.43 | 2.37 | 1.47 | 2.90 | 0.580 | |
| 0.530 | 1.26 | 2.97 | 2.38 | 1.50 | 2.91 | 0.620 | |
| 0.810 | 1.44 | 2.85 | 1.77 | 1.27 | 2.38 | 0.650 | |
| CREE(init.). mol/L |
Separation factor | |||||||
| Sm/Nd | Eu/Sm | Tb/Gd | Dy/Tb | Ho/Dy | Tm/Er | Lu/Yb | ∑REE/Y | |
| 0.040 | - | - | - | - | - | - | - | - |
| 0.090 | 4.54 | 2.35 | 6.84 | 2.95 | - | - | - | - |
| 0.140 | 1.07 | 2.94 | 4.60 | 2.99 | 1.76 | 2.00 | - | 0.210 |
| 0.170 | 0.940 | 1.55 | 4.30 | 2.98 | 1.83 | 2.89 | - | 0.340 |
| 0.260 | 0.700 | - | 4.79 | 2.44 | 1.68 | 2.84 | 0.660 | 0.460 |
| 0.340 | 0.960 | - | 3.66 | 2.60 | 1.60 | 2.85 | 0.790 | 0.510 |
| 0.450 | 1.25 | 1.28 | 3.70 | 2.32 | 1.52 | 2.99 | 0.870 | 0.550 |
| 0.520 | 0.270 | 1.27 | 3.13 | 2.17 | 1.46 | 2.67 | 0.970 | 0.570 |
| 0.800 | 1.08 | 1.40 | 2.47 | 2.02 | 1.29 | 2.54 | 1.11 | 0.680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




