Submitted:
12 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- (1)
- biomass production (fresh weight, dry weight, fresh weight/dry weight ratio and dry weight yield);
- (2)
- phenotypic characteristics (index of compactness and number of dark glands per leaf);
- (3)
- production of total phenylpropanoids (phenolics, flavonoids, flavan-3-ols, condensed taninns, and phenolic acids);
- (4)
- identification and quantification of naphthodianthrones (hypericin, pseudohypericin, and protopseudohypericim) by UPLC analysis;
- (5)
- phenylalanine ammonia lyase and polyphenol oxidase activities;
- (6)
- non-enzymatic antioxidant capacity (cupric reducing antioxidant capacity; ferric ions reducing antioxidant power, ABTS radical scavenging activity, lipid peroxidation inhibition);
- (7)
- radical scavenging activity (hydrogen peroxide, superoxide anion and hydroxyl radical);
- (8)
- antioxidant enzyme activities (guaiacol peroxidase, ascorbate peroxidase, catalase and superoxide dismutase);
- (9)
- oxidative stress marker contents (hydrogen peroxide, superoxide anion and malondialdehyde).
2. Materials and Methods
2.1. Establishment of Hypericum perforatum Transgenic Shoots
2.2. Growth and Phenotypic Characteristics
2.3. Phenylpropanoid Production
2.4. UPLC-TUV Analysis of Naphthodianthrones
2.5. Phenylalanine Ammonia Lyase (PAL) and Polyphenol Oxidase (PPO) Activities
2.6. Antioxidant Activities
2.7. Radical Scavenging Activities
2.8. Antioxidant Enzymes
2.9. Oxidative Stress Markers
2.10. Statistical Analysis
3. Results
3.1. Establishment of Hypericum perforatum Transgenic Shoots
3.2. Morphological and Growth Characteristics of Transgenic Shoots
3.3. Phenylpropanoid Production in Transgenic Shoots
3.4. Naphthodianthrone Production in Transgenic Shoots
3.5. Antioxidant and Radical Scavenging Activities in Transgenic Shoots
3.6. Antioxidant Enzymes and Oxidative Stress Markers in Transgenic Shoots
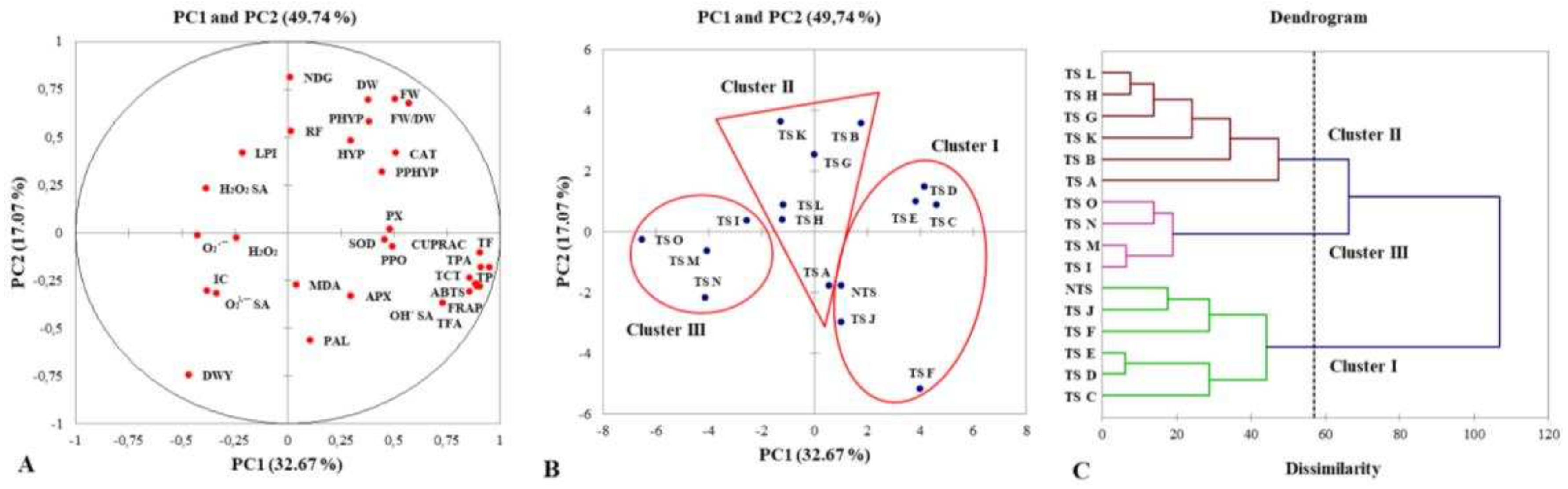
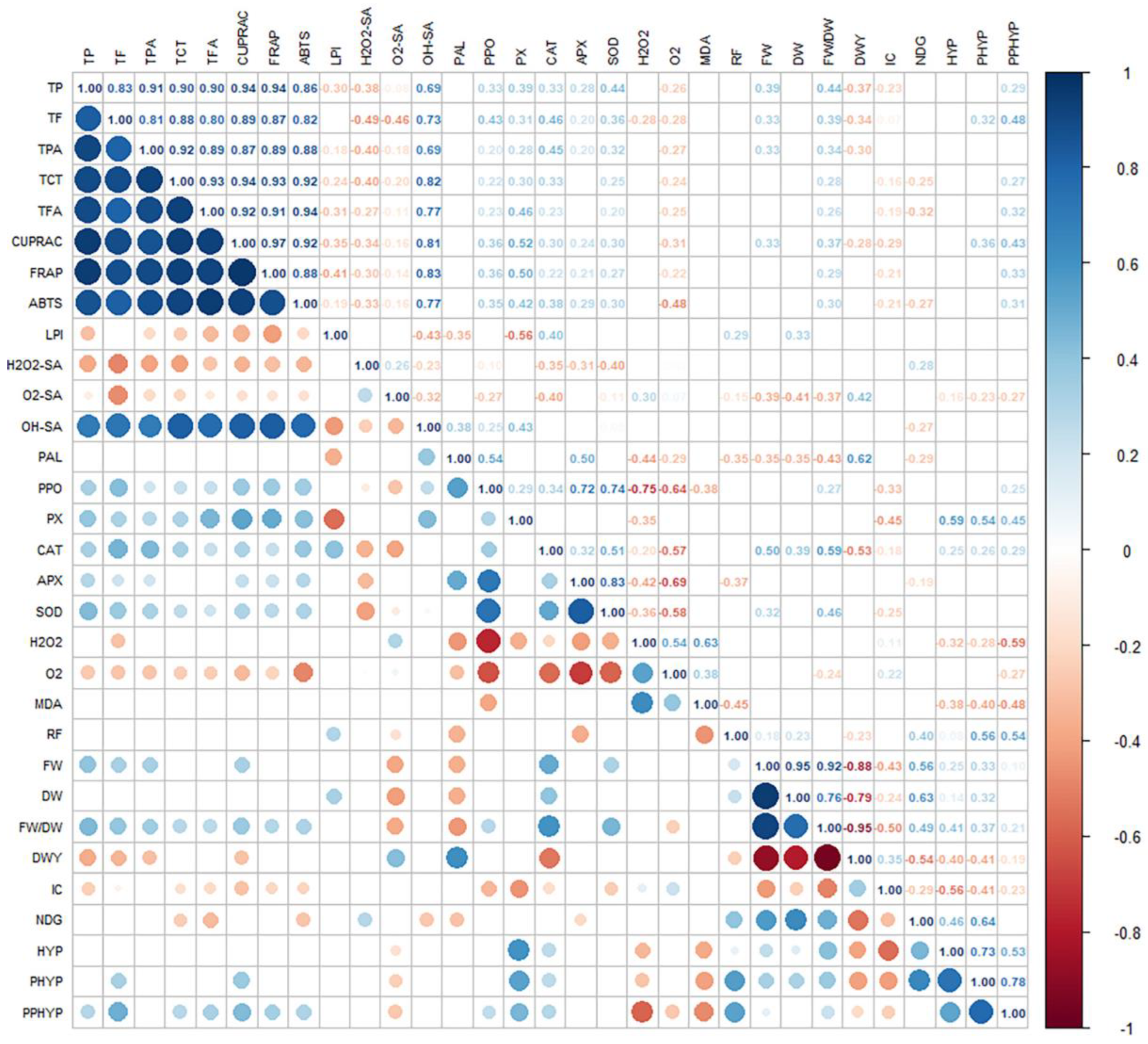
3.7. Principal Component Analysis and Hierarchical Agglomerative Clustering
4. Discussion
4.1. Growth Characteristics of Transgenic Shoots
4.2. Phenylpropanoid and Naphthodianthrone Production in Transgenic Shoots
4.3. Antioxidant Activity and Radical Scavenging Capacity in Transgenic Shoots
4.4. Antioxidant Enzymes and Oxidative Stress Markers in Transgenic Shoots
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mir, M.Y.; Hamid, S.; Kamili, A.N.; Hassan, Q.P. Sneak peek of Hypericum perforatum L.: phytochemistry, phytochemical efficacy and biotechnological interventions. J. Plant Biochem. Biotechnol. 2019, 28, 357–373. [Google Scholar] [CrossRef]
- Crockett, S.L.; Poller, B.; Tabanca, N.; Pferschy-Wenzig, E.M.; Kunert, O.; Wedge, D.E.; Bucar, F. Bioactive xanthones from the roots of Hypericum perforatum (common St John’s wort). J. Sci. Food Agric. 2011, 91, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Tusevski, O.; Krstikj, M.; Stanoeva, J.P.; Stefova, M.; Simic, S.G. Phenolic profile and biological activity of Hypericum perforatum L.: Can roots be considered as a new source of natural compounds? S. Afr. J. Bot. 2018, 117, 301–310. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Goto, E.; Kozai, T. Plant–environment interactions: accumulation of hypericin in dark glands of Hypericum perforatum. Ann. Bot. 2006, 98, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Theodossiou, T.A.; Olsen, C.E.; Jonsson, M.; Kubin, A.; Hothersall, J.S.; Berg, K. The diverse roles of glutathione-associated cell resistance against hypericin photodynamic therapy. Redox Biol. 2017, 12, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Velingkar, V.S.; Gupta, G.L.; Hegde, N.B. A current update on phytochemistry, pharmacology and herb–drug interactions of Hypericum perforatum. Phytochem. Rev. 2017, 16, 725–744. [Google Scholar] [CrossRef]
- Tocci, N.; Simonetti, G.; D’Auria, F.D.; Panella, S.; Palamara, A.T.; Ferrari, F.; Pasqua, G. Chemical composition and antifungal activity of Hypericum perforatum subsp. angustifolium roots from wild plants and plants grown under controlled conditions. Plant Biosyst. 2013, 147, 557–562. [Google Scholar] [CrossRef]
- Rafailovska, E.; Tushevski, O.; Shijakova, K.; Simic, S.G.; Kjovkarovska, S.D.; Miova, B. Hypericum perforatum L. extract exerts insulinotropic effects and inhibits gluconeogenesis in diabetic rats by regulating AMPK expression and PKCε concentration. J. Ethnopharmacol. 2023, 302, 115899. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. St. John’s wort (Hypericum perforatum L.): challenges and strategies for production of chemically-consistent plants. Can. J. Plant Sci. 2006, 86, 765–771. [Google Scholar] [CrossRef]
- Carrubba, A.; Lazzara, S.; Giovino, A.; Ruberto, G.; Napoli, E. Content variability of bioactive secondary metabolites in Hypericum perforatum L. Phytochem. Lett. 2021, 46, 71–78. [Google Scholar] [CrossRef]
- Murthy, H.N.; Kim, Y.S.; Park, S.Y.; Paek, K.Y. Hypericins: biotechnological production from cell and organ cultures. Appl. Microbiol. Biotechnol. 2014, 98, 9187–9198. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Pop, C.; Halmagyi, A.; Butiuc-Keul, A. Secondary Metabolites in Shoot Cultures of Hypericum. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Reference Series in, Phytochemistry, Ramawat, K., Ekiert, H., Goyal, S., Eds.; Springer Nature: Geneva, Switzerland, 2021; pp. 273–308. [Google Scholar]
- Kowalczyk, T.; Wieczfinska, J.; Skała, E.; Śliwiński, T.; Sitarek, P. Transgenesis as a tool for the efficient production of selected secondary metabolites from plant in vitro cultures. Plants 2020, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Shakya, P.; Franklin, G. A perspective on Hypericum perforatum genetic transformation. Front. Plant Sci. 2016, 7, 879. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Franklin, G.; Oliveira, M.; Dias, A.C.P. Production of transgenic Hypericum perforatum plants via particle bombardment-mediated transformation of novel organogenic cell suspension cultures. Plant Sci. 2007, 172, 1193–1203. [Google Scholar] [CrossRef]
- Franklin, G.; Conceição, L.F.; Kombrink, E.; Dias, A.C.P. Hypericum perforatum plant cells reduce Agrobacterium viability during co-cultivation. Planta 2008, 227, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Morey, K.J.; Peebles, C.A. Hairy roots: An untapped potential for production of plant products. Front. Plant Sci. 2022, 13, 937095. [Google Scholar] [CrossRef] [PubMed]
- Vinterhalter, B.; Zdravković-Korać, S.; Mitić, N.; Bohanec, B.; Vinterhalter, D.; Savić, J. Effect of sucrose on shoot regeneration in Agrobacterium transformed Hypericum perforatum L. roots. Acta Physiol. Plant. 2015, 37, 1–12. [Google Scholar] [CrossRef]
- Koperdáková, J.; Komarovská, H.; Košuth, J.; Giovannini, A.; Čellárová, E. Characterization of hairy root-phenotype in transgenic Hypericum perforatum L. clones. Acta Physiol. Plant. 2009, 31, 351–358. [Google Scholar] [CrossRef]
- Tusevski, O.; Stanoeva, J.P.; Stefova, M.; Kungulovski, D.; Pancevska, N.A.; Sekulovski, N.; Panov, S.; Simic, S.G. Hairy roots of Hypericum perforatum L.: a promising system for xanthone production. Cent. Eur. J. Biol. 2013, 8, 1010–1022. [Google Scholar] [CrossRef]
- Komarovská, H.; Giovannini, A.; Košuth, J.; Čellárová, E. Agrobacterium rhizogenes-mediated transformation of Hypericum tomentosum L. and Hypericum tetrapterum Fries. Z. Naturforsch. C 2009, 64, 864–868. [Google Scholar] [CrossRef]
- Khlifa, H.D.; Klimek-Chodacka, M.; Baranski, R.; Combik, M.; Taha, H.S. Agrobacterium rhizogenes-mediated transformation of Hypericum sinaicum L. for the development of hairy roots containing hypericin. Braz. J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- Tusevski, O.; Vinterhalter, B.; Krstić Milošević, D.; Soković, M.; Ćirić, A.; Vinterhalter, D.; Zdravković Korać, S.; Petreska Stanoeva, J.; Stefova, M.; Gadzovska Simic, S. Production of phenolic compounds, antioxidant and antimicrobial activities in hairy root and shoot cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2017, 128, 589–605. [Google Scholar] [CrossRef]
- Tusevski, O.; Petreska Stanoeva, J.; Stefova, M.; Spasenoski, M.; Gadzovska Simic, S. State of antioxidant systems and phenolic compounds’ production in Hypericum perforatum L. hairy roots. Acta Physiol. Plant. 2019, 41, 1–15. [Google Scholar] [CrossRef]
- Tusevski, O.; Gadzovska Simic, S. Non-Enzymatic and Enzymatic Antioxidant Responses of Hypericum perforatum L. Hairy Roots upon Photooxidative Stress. Horticulturae 2023, 9, 581. [Google Scholar] [CrossRef]
- Tusevski, O.; Petreska Stanoeva, J.; Stefova, M.; Simic, S.G. Phenolic profile of dark-grown and photoperiod-exposed Hypericum perforatum L. hairy root cultures. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tusevski, O.; Todorovska, M.; Stanoeva, J.P.; Stefova, M.; Simic, S.G. In Vitro and in Silico Insights on the Biological Activities, Phenolic Compounds Composition of Hypericum perforatum L. Hairy Root Cultures. Phyton-Int. J. Exp. Bot. 2023, 92, 921–941. [Google Scholar] [CrossRef]
- Bertoli, A.; Giovannini, A.; Ruffoni, B.; Guardo, A.D.; Spinelli, G.; Mazzetti, M.; Pistelli, L. Bioactive constituent production in St. John’s wort in vitro hairy roots. Regenerated plant lines. J. Agric. Food Chem. 2008, 56, 5078–5082. [Google Scholar] [CrossRef] [PubMed]
- Tusevski, O.; Petreska Stanoeva, J.; Stefova, M.; Pavokovic, D.; Gadzovska Simic, S. Identification and quantification of phenolic compounds in Hypericum perforatum L. transgenic shoots. Acta Physiol. Plant. 2014, 36, 2555–2569. [Google Scholar] [CrossRef]
- Christey, M.C. Use of Ri-mediated transformation for production of transgenic plants. In Vitro Cell. Dev. Biol. - Plant 2001, 37, 687–700. [Google Scholar] [CrossRef]
- Komarovska, H.; Košuth, J.; Giovannini, A.; Smelcerovic, A.; Zuehlke, S.; Čellárová, E. Effect of the number of rol genes integrations on phenotypic variation in hairy root-derived Hypericum perforatum L. plants. Z. Naturforsc. C 2010, 65, 701–712. [Google Scholar] [CrossRef]
- Henzelyová, J.; Čellárová, E. Modulation of naphthodianthrone biosynthesis in hairy root-derived Hypericum tomentosum regenerants. Acta Physiol. Plant. 2018, 40, 1–12. [Google Scholar] [CrossRef]
- Gadzovska Simic, S.; Tusevski, O.; Maury, S.; Hano, C.; Delaunay, A.; Chabbert, B.; Lamblin, F.; Lainé, E.; Joseph, C.; Hagège, D. Fungal elicitor-mediated enhancement in phenylpropanoid and naphtodianthrone contents of Hypericum perforatum L. cell cultures. Plant Cell, Tiss. Organ Cult. 2015, 122, 213–226. [Google Scholar] [CrossRef]
- Gadzovska Simic, S.; Tusevski, O.; Maury, S.; Delaunay, A.; Lainé, E.; Joseph, C.; Hagège, D. Polysaccharide elicitors enhance phenylpropanoid and naphtodianthrone production in cell suspension cultures of Hypericum perforatum. Plant Cell, Tiss. Organ Cult., 2015, 122, 649–663. [Google Scholar] [CrossRef]
- Tusevski, O.; Stanoeva, J.P.; Markoska, E.; Brndevska, N.; Stefova, M.; Gadzovska Simic, S. Callus cultures of Hypericum perforatum L. a novel and efficient source for xanthone production. Plant Cell Tiss. Org. Cult. 2016, 125, 309–319. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hemeda, H.M.; Klein, B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Sergiev, I.; Alexieva, V.; Karanov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Comptes Rendus de l ’Academie Bulg. des Sci. 1997, 51, 121–124. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Franklin, G.; Dias, A.C.P. Organogenesis and embryogenesis in several Hypericum perforatum genotypes. In Vitro Cell. Dev. Biol. Plant 2006, 42, 324–330. [Google Scholar] [CrossRef]
- Goel, M.K.; Kukreja, A.K.; Bisht, N.S. In vitro manipulations in St. John’s wort (Hypericum perforatum L.) for incessant and scale up micropropagation using adventitious roots in liquid medium and assessment of clonal fidelity using RAPD analysis. Plant Cell Tiss. Org. Cult. 2009, 96, 1–9. [Google Scholar] [CrossRef]
- Di Guardo, A.; Cellarova, E.; Koperdáková, J.; Pistelli, L.; Ruffoni, B.; Allavena, A.; Giovannini, A. Hairy root induction and plant regeneration in Hypericum perforatum L. J. Genet. Breed. 2003, 57, 269–278. [Google Scholar]
- Vinterhalter, B.; Ninković, S.; Cingel, A.; Vinterhalter, D. Shoot and root culture of Hypericum perforatum L. transformed with Agrobacterium rhizogenes A4M70GUS. Biol. Plant. 2006, 50, 767–770. [Google Scholar] [CrossRef]
- Damgaard, O.; Rasmussen, O. Direct regeneration of transformed shoots in Brassica napus from hypocotyl infections with Agrobacterium rhizogenes. Plant Mol. Biol. 1991, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Goel, M.K.; Rahman, L.U.; Kukreja, A.K. Molecular and chemical characterization of plants regenerated from Ri-mediated hairy root cultures of Rauwolfia serpentina. Plant Cell Tiss. Org. Cult. 2013, 114, 31–38. [Google Scholar] [CrossRef]
- Piątczak, E.; Kuźma, Ł.; Skała, E.; Żebrowska, M.; Balcerczak, E.; Wysokińska, H. Iridoid and phenylethanoid glycoside production and phenotypical changes in plants regenerated from hairy roots of Rehmannia glutinosa Libosch. Plant Cell Tiss. Org. Cult. 2015, 122, 259–266. [Google Scholar] [CrossRef]
- Largia, M.J.V.; Satish, L.; Johnsi, R.; Shilpha, J.; Ramesh, M. Analysis of propagation of Bacopa monnieri (L.) from hairy roots, elicitation and Bacoside A contents of Ri transformed plants. World J. Microbiol. Biotechnol. 2016, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Casanova, E.; Trillas, M.I.; Moysset, L.; Vainstein, A. Influence of rol genes in floriculture. Biotechnol. Adv. 2005, 23, 3–39. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Vlase, L.; Halmagyi, A.; Deliu, C.; Coldea, G. Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tiss. Org. Cult. 2011, 106, 279–288. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R.J.; Weidner, D.A. Genetic transformation and gene silencing mediated by multiple copies of a transgene in eastern white pine. J. Exp. Bot. 2007, 58, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Sinkar, V.P.; Pythoud, F.; White, F.F.; Nester, E.W.; Gordon, M.P. rolA locus of the Ri plasmid directs developmental abnormalities in transgenic tobacco plants. Genes Dev. 2, 1101. [Google Scholar]
- Bettini, P.; Baraldi, R.; Rapparini, F.; Melani, L.; Mauro, M. L.; Bindi, D.; Buiatti, M. The insertion of the Agrobacterium rhizogenes rolC gene in tomato (Solanum lycopersicum L.) affects plant architecture and endogenous auxin and abscisic acid levels. Sci Hortic. 2010, 123, 323–328. [Google Scholar] [CrossRef]
- Martin, K.P.; Sabovljevic, A.; Madassery, J. High-frequency transgenic plant regeneration and plumbagin production through methyl jasmonate elicitation from hairy roots of Plumbago indica L. J. Crop Sci. Biotechnol. 2011, 14, 205–212. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Chakraborty, D.; Bhattacharyya, S.; Bhattacharya, S. Regeneration of transformed plants from hairy roots of Plumbago indica. Plant Cell Tiss. Org. Cult. 2010, 102, 109–114. [Google Scholar] [CrossRef]
- Behera, P.R.; Jena, R.C.; Das, A.; Thirunavoukkarasu, M.; Chand, P.K. Genetic stability and coumarin content of transformed rhizoclones and regenerated plants of a multi-medicinal herb, Hybanthus enneaspermus (L.) F. Muell. Plant Growth Regul. 2016, 80, 103–114. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Ounnar, S.; Righezza, M.; Kascakova, S.; Refregiers, M.; Spasenoski, M.; Joseph, C.; Hagège, D. Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol. Biochem. 2005, 43, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.N.; Zhang, X.Q.; Sun, J.S. Effects of cytokinins and elicitors on the production of hypericins and hyperforin metabolites in Hypericum sampsonii and Hypericum perforatum. Plant Growth Regul. 2007, 53, 207–214. [Google Scholar] [CrossRef]
- Bulgakov, V.P. Functions of rol genes in plant secondary metabolism. Biotechnol. Adv. 2008, 26, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Garai, S.; Jha, S. Genetic transformation of Bacopa monnieri by wild type strains of Agrobacterium rhizogenes stimulates production of bacopa saponins in transformed calli and plants. Plant Cell Rep. 2011, 30, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Yan, Q.; Shi, M.; Ng, J.; Wu, J.Y. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Sci. 2006, 170, 853–858. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Joseph, C.; Hagege, D. Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tiss Org Cult. 2007, 89, 1–13. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Hagège, D.; Courtois, D.; Joseph, C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2013, 113, 25–39. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Savio, L.E.B.; Astarita, L.V.; Santarém, E.R. Secondary metabolism in micropropagated Hypericum perforatum L. grown in non-aerated liquid medium. Plant Cell Tiss. Org. Cult. 2012, 108, 465–472. [Google Scholar] [CrossRef]
- Dilshad, E.; Ismail, H.; Haq, I.U.; Cusido, R.M.; Palazon, J.; Ramirez-Estrada, K.; Mirza, B. Rol genes enhance the biosynthesis of antioxidants in Artemisia carvifolia Buch. BMC Plant Biol. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Božin, B.; Kladar, N.; Grujić, N.; Anačkov, G.; Samojlik, I.; Gavarić, N.; Čonić, B.S. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’s wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Cornea, C.P.; Dragos, S.; Rosu, A.; Guidea, S.; Israel, F. Peroxidase production in armoracia sp. transformed hairy roots. ROM. BIOTECHNOL. LETT. 2006, 11, 2619. [Google Scholar]
- Nikravesh, F.; Khavari-Nejad, R.A.; Rahimian, H.; Fahimi, H. Study of antioxidant enzymes activity and isozymes pattern in hairy roots and regenerated plants in Nicotiana tabacum. Acta Physiol. Plant. 2012, 34, 419–427. [Google Scholar] [CrossRef]
- Veena, V.; Taylor, C.G. Agrobacterium rhizogenes: recent developments and promising applications. In Vitro Cell. Dev. Biol. Plant 2007, 43, 383–403. [Google Scholar] [CrossRef]
- Tyburski, J.; Dunajska, K.; Mazurek, P.; Piotrowska, B.; Tretyn, A. Exogenous auxin regulates H2O2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol. Plant. 2009, 31, 249–260. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Bulgakov, V.P.; Gorpenchenko, T.Y.; Aminin, D.L.; Zhuravlev, Y.N. Decreased ROS level and activation of antioxidant gene expression in Agrobacterium rhizogenes pRiA4-transformed calli of Rubia cordifolia. Planta 2010, 232, 1023–1032. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Gorpenchenko, T.Y.; Veremeichik, G.N.; Shkryl, Y.N.; Tchernoded, G.K.; Bulgakov, D.V.; Aminin, D.L.; Zhuravlev, Y.N. The rolB gene suppresses reactive oxygen species in transformed plant cells through the sustained activation of antioxidant defense. Plant Physiol. 2012, 158, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Qu, G.Z.; Li, H.Y.; Wu, Y.J.; Wang, C.; Liu, G.F.; Yang, C.P. Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii. Mol. Biol. Rep. 2010, 37, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Masuta, Y.; Saito, K.; Murayama, S.; Ozawa, K. Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep. 2011, 30, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Franklin, G.; Conceição, L.F.; Kombrink, E.; Dias, A.C. Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry 2009, 70, 60–68. [Google Scholar] [CrossRef] [PubMed]

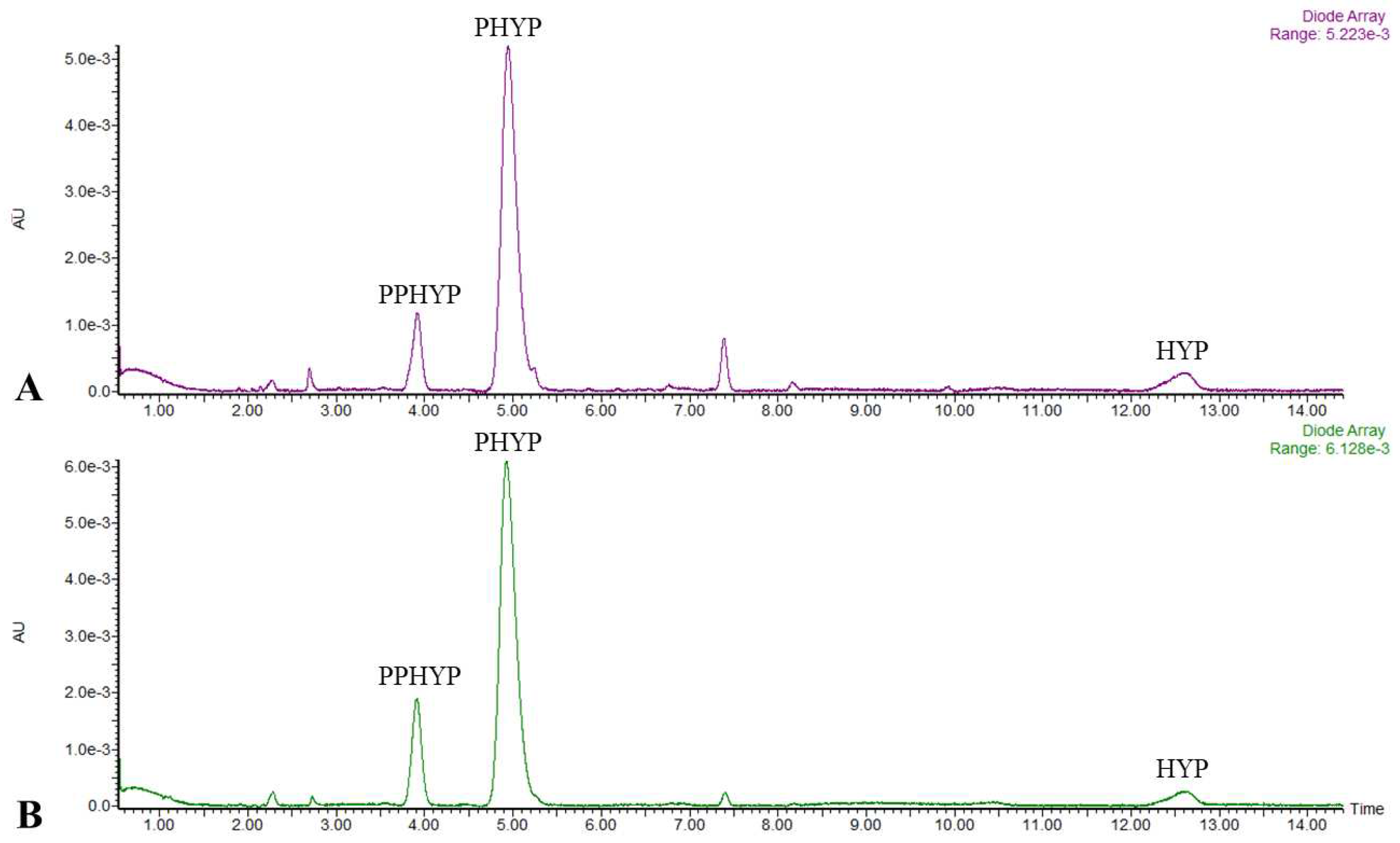
| Compounds | UV (nm) | [M-H]‒ (m/z) |
-MS2 [M-H] ‒ (m/z) |
|---|---|---|---|
| PPHYP | 285, 375, 550 | 521 | 423 |
| PHYP | 288, 325, 465, 580 | 519 | 487, 421 |
| HYP | 288, 325, 465, 580 | 503 | 405 |
| Compound | Test range (μg·mL-1) | Linear regression equation |
R2 | LOD (μg·mL-1) |
LOQ (μg·mL-1) |
Spiked conc. (μg·mL-1) |
Recovery (%) |
Average Recovery (%) |
RSD (%) |
|---|---|---|---|---|---|---|---|---|---|
| PHYP | 0.1-3 | y=334.76x-26.28 | 0.997 | 0.0086 | 0.0260 | 1.534 | 114.98 | 103.15 | 1.27 |
| 1.918 | 97.68 | ||||||||
| 2.302 | 96.79 | ||||||||
| HYP | 0.02-0.1 | y=788x-7.96 | 0.998 | 0.0005 | 0.0016 | 0.020 | 90 | 97.77 | 1.40 |
| 0.025 | 100 | ||||||||
| 0.030 | 103.30 |
| Root cultures | RF (%) | Shoot cultures | FW (g) | DW (g) | FW/DW | DWY (%) | IC (NLS/SL) | NDG |
|---|---|---|---|---|---|---|---|---|
| NTR | 20.00±1.50 b-e | NTS | 5.28±0.59 bc | 0.34±0.02 d | 15.48±1.24 e | 6.46±0.32 de | 3.73±0.33 cdg | 9.00±1.83 bc |
| HR A | 26.60±0.80 g | TS A | 2.41±0.33 a | 0.27±0.03 bc | 8.83±0.38 a | 11.33±1.00 n | 3.43±0.39 bd | 9.75±0.50 be |
| HR B | 20.00±2.00 b-df | TS B | 13.70±1.23 e | 0.63±0.01 fg | 21.81±1.57 gh | 4.58±0.20 ac | 2.40±0.11 a | 15.67±3.21 lm |
| HR C | 12.50±1.20 a | TS C | 17.47±2.51 f | 0.70±0.04 i | 25.06±1.45 h | 3.99±0.11 a | 3.31±0.14 bc | 10.75±1.50 bh |
| HR D | 45.00±2.10 hi | TS D | 18.60±3.45 f | 0.77±0.05 j | 24.10±1.31 h | 4.15±0.30 ab | 3.11±0.16 be | 10.67±1.15 bg |
| HR E | 50.00±3.80 i | TS E | 14.17±1.66 e | 0.76±0.02 j | 18.58±1.29 f | 5.38±0.19 cd | 3.68±0.17 cd | 12.50±2.08 c-k |
| HR F | 15.00±0.70 ac | TS F | 2.16±0.22 a | 0.21±0.01 a | 10.31±1.04 ab | 9.69±0.09 lm | 4.23±0.41 f-k | 6.00±1.00 a |
| HR G | 78.00±5.60 k | TS G | 8.57±0.14 d | 0.59±0.03 f | 14.61±1.23 de | 6.85±0.50 eh | 4.72±0.03 kl | 14.00±1.63 i-km |
| HR H | 44.00±2.40 h | TS H | 4.05±0.28 ab | 0.32±0.01 d | 12.78±0.73 cd | 7.82±0.31 f-jl | 3.93±0.20 dj | 8.60±0.89 ab |
| HR I | 16.70±0.90 ad | TS I | 10.03±1.10 d | 0.68±0.02 g-i | 14.71±1.13 de | 6.80±0.49 eg | 5.11±0.68 lm | 12.00±2.45 c-k |
| HR J | 12.50±0.40 a | TS J | 9.17±0.56 d | 0.63±0.02 fh | 14.56±1.27 de | 6.87±0.62 ei | 3.83±0.18 cdi | 11.40±0.89 bj |
| HR K | 65.00±4.40 j | TS K | 18.68±2.20 f | 0.98±0.07 k | 19.10±1.86 fg | 5.24±0.10 bc | 3.00±0.10 b | 13.33±1.15 g-l |
| HR L | 82.00±7.90 k | TS L | 4.06±0.88 ac | 0.27±0.01 bc | 14.86±1.33 de | 6.73±0.42 ef | 3.81±0.29 cdh | 11.67±0.58 bk |
| HR M | 22.20±3.40 dg | TS M | 5.17±0.19 bc | 0.42±0.02 e | 12.28±1.21 cd | 8.14±0.81 jk | 5.27±0.15 m | 9.75±0.96 bf |
| HR N | 23.30±1.60 e-g | TS N | 2.19±0.10 a | 0.23±0.01 ac | 9.53±0.75 ab | 10.49±1.20 mn | 3.62±0.05 cde | 11.00±2.31 bi |
| HR O | 14.80±2.20 ab | TS O | 2.59±0.21 a | 0.23±0.01 ab | 11.38±0.94 bc | 8.79±0.97 kl | 3.71±0.56 cdf | 9.40±1.14 bd |
| TP (mg GA·g-1 DW) |
TF (mg C·g-1 DW) |
TFA (mg C·g-1 DW) |
TPA (mg P·g-1 DW) |
TCT (mg CG·g-1 DW) |
PAL (pkat·mg-1 P) |
PPO (nkat·mg-1 P) |
|
|---|---|---|---|---|---|---|---|
| NTS | 45.06±1.40 f | 19.77±2.00 eg | 5.16±0.21 f | 3.60±0.02 h | 3.30±0.02 hj | 0.72±0.10 fg | 5.60±0.26 a |
| TS A | 38.95±2.30 e | 22.26±0.91 h | 4.14±0.25 d | 2.44±0.08 ef | 2.85±0.14 g | 2.80±0.30 i | 51.39±3.01 l |
| TS B | 42.13±1.63 ef | 18.09±1.09 de | 4.64±0.01 e | 2.38±0.18 ef | 2.41±0.03 cf | 0.63±0.25 eg | 24.45±1.60 j |
| TS C | 61.05±3.29 h | 26.14±1.38 i | 5.80±0.20 g | 3.61±0.16 h | 3.17±0.16 i-k | 0.30±0.11 b-d | 51.17±2.44 l |
| TS D | 51.69±0.90 g | 26.32±1.53 i | 7.08±0.13 h | 3.64±0.03 h | 3.35±0.13 hk | 0.70±0.19 fg | 27.74±1.02 k |
| TS E | 62.03±0.74 h | 22.86±1.03 h | 7.56±0.11 i | 3.85±0.28 i | 3.47±0.23 h | 0.16±0.01 ab | 17.65±1.85 gh |
| TS F | 61.63±4.28 h | 27.82±0.45 i | 10.44±0.37 j | 4.35±0.13 j | 4.20±0.13 l | 1.00±0.10 h | 23.13±2.60 ij |
| TS G | 40.70±1.57 e | 22.80±1.84 h | 4.18±0.16 d | 3.22±0.07 g | 2.82±0.20 fg | 0.24±0.00 ac | 14.26±2.14 bf |
| TS H | 35.12±1.48 bd | 19.26±0.97 ef | 4.13±0.11 d | 2.30±0.14 df | 2.51±0.04 cd | 0.35±0.02 b-d | 13.61±0.42 bd |
| TS I | 32.09±0.16 b | 16.95±1.03 cd | 2.50±0.16 b | 2.42±0.05 ef | 2.06±0.09 b | 0.59±0.06 ef | 20.57±3.27 i |
| TS J | 54.22±3.44 g | 23.53±1.89 h | 5.42±0.22 f | 3.49±0.02 h | 3.28±0.11 hi | 0.77±0.09 fg | 11.89±0.06 b |
| TS K | 35.62±1.23 cd | 15.18±0.19 bc | 3.30±0.26 c | 2.27±0.06 de | 2.72±0.25 d-g | 0.29±0.14 ad | 15.32±0.76 c-fh |
| TS L | 39.36±4.03 e | 20.64±1.48 fg | 3.53±0.24 c | 1.88±0.09 c | 2.53±0.18 ce | 0.10±0.01 a | 15.18±0.37 c-g |
| TS M | 32.13±2.34 b | 14.80±0.78 b | 2.48±0.14 b | 1.53±0.01 b | 2.03±0.03 b | 0.31±0.05 b-d | 11.49±3.14 b |
| TS N | 35.17±0.08 bc | 8.73±0.19 a | 2.61±0.16 b | 2.20±0.10 d | 1.92±0.06 b | 0.97±0.07 h | 13.50±1.10 bc |
| TS O | 23.26±0.99 a | 7.36±0.96 a | 1.85±0.03 a | 1.29±0.18 a | 1.37±0.08 a | 0.45±0.04 de | 14.22±0.01 be |
| HYP (µg·g-1 DW) |
PHYP (µg·g-1 DW) |
PPHYP (µg·g-1 DW) |
|
|---|---|---|---|
| NTS | 28.55±1.45 g | 660.64±35.33 b | 91.92±1.12 a |
| TS A | 24.33±1.19 f | 1101.39±20.57 e | 366.51±11.85 f |
| TS B | 69.41±2.54 i | 1915.34±56.88 g | 477.90±3.41 h |
| TS C | 24.14±1.01 f | 755.99±19.09 c | 356.37±5.67 ef |
| TS D | 21.15±1.35 e | 1096.43±35.96 de | 364.90±10.08 f |
| TS E | 27.13±1.09 g | 1543.25±43.12 f | 441.51±8.61 g |
| TS F | 19.18±1.27 e | 758.68±62.04 c | 348.99±3.32 e |
| TS G | 23.98±0.95 f | 1495.50±26.04 f | 511.09±9.89 i |
| TS H | 34.63±1.11 h | 1178.44±64.78 e | 578.80±12.22 j |
| TS I | 13.25±0.56 b | 626.12± 21.77 b | 142.61±7.54 b |
| TS J | 14.16±1.22 bc | 1062.12±9.88 d | 220.77±3.12 d |
| TS K | 23.53±1.85 f | 1074.68±12.99 de | 198.98±6.09 c |
| TS L | 26.67±1.18 | 1463.86±38.31 f | 506.73±12.49 i |
| TS M | 16.97±0.72 d | 652.18±8.76 b | 147.43±2.67 b |
| TS N | 11.11±0.86 a | 537.09±18.71 a | 190.03±7.91 c |
| TS O | 15.87±0.58 cd | 647.69±11.67 b | 146.94±1.20 b |
| CUPRAC (μМ Т·g-1 DW) |
FRAP (μМ Fe2+·g-1 DW) |
ABTS (μМ Т·g-1 DW) |
LPI (%) |
H2O2 SA (%) |
O2•- SA (%) |
OH• SA (mM M·g-1 DW) |
|
|---|---|---|---|---|---|---|---|
| NTS | 178.27±2.90 e-g | 432.11±0.74 fg | 123.51±2.90 i | 74.10±2.40 dg | 18.47±0.92 a | 34.68±1.15 df | 1.63±0.02 i |
| TS A | 188.20±4.83 g | 492.19±14.87 h | 113.54±4.05 h | 66.53±1.92 c | 26.36±0.64 dg | 30.95±0.78 c | 1.71±0.03 i |
| TS B | 186.66±4.11 g | 440.00±21.21 g | 100.89±1.89 g | 60.60±0.32 b | 28.87±2.30 hikl | 38.15±0.48 gj | 1.53±0.05 d-h |
| TS C | 210.44±10.62 h | 530.79±6.39 i | 123.21±2.53 i | 84.72±0.52 ij | 22.42±1.50 bc | 33.42±1.40 d | 1.44±0.00 cd |
| TS D | 212.95±11.95 h | 550.13±28.47 i | 144.42±2.21 j | 75.89±4.12 e-g | 25.56±1.30 de | 27.27±0.80 b | 1.70±0.05 i |
| TS E | 236.27±10.40 i | 595.79±20.97 j | 142.19±1.58 j | 71.97±4.12 d | 27.86±0.64 dh-jmn | 33.39±2.43 d | 1.71±0.01 i |
| TS F | 250.81±14.52 i | 649.91±37.46 k | 168.75±0.00 k | 63.97±4.08 c | 27.46±1.06 e-gi | 41.94±2.23 k | 1.85±0.05 j |
| TS G | 166.52±8.81 de | 431.05±9.86 g | 98.66±2.23 e-g | 84.97±0.48 ij | 27.11±2.00 e-h | 34.41±0.36 de | 1.27±0.06 b |
| TS H | 157.86±3.44 cd | 390.79±14.89 ce | 88.69±6.70 d | 82.69±1.29 hi | 25.58±0.31 df | 26.60±0.10 b | 1.45±0.04 cf |
| TS I | 126.78±7.50 b | 366.93±20.15 c | 78.87±7.16 bc | 79.97±0.77 h | 28.76±2.19 gjk | 24.20±3.51 a | 1.50±0.00 ch |
| TS J | 202.22±4.36 h | 586.18±1.67 j | 92.19±10.00 df | 51.50±2.61 a | 24.46±1.77 cd | 37.94±1.52 gi | 1.73±0.03 i |
| TS K | 152.95±4.85 c | 380.26±8.66 cd | 84.97±5.65 cd | 91.33±3.01 k | 33.15±0.35 o | 36.12±0.57 e-g | 1.48±0.01 cg |
| TS L | 168.69±4.36 df | 401.32±49.87 d-f | 90.63±1.89 de | 63.76±3.13 c | 21.92±1.13 b | 38.11±0.12 gh | 1.39±0.10 c |
| TS M | 131.96±1.88 b | 328.42±11.16 b | 76.12±4.10 b | 83.00±4.73 hj | 30.00±0.71 k-m | 40.17±0.39 hjk | 1.45±0.03 ce |
| TS N | 133.68±9.34 b | 368.68±13.40 c | 83.63±0.68 bd | 74.04±1.43 df | 30.10±0.42 kln | 57.94±1.33 l | 1.24±0.09 b |
| TS O | 79.56±6.05 a | 230.88±13.41 a | 48.66±0.63 a | 73.03±1.17 de | 28.81±0.44 gjl | 39.67±2.53 h-j | 1.14±0.02 a |
| PX (nkat·mg-1 P) |
АPX (pkat·mg-1 P) |
CAT (nkat·mg-1 P) |
SOD (U·mg-1 P) |
H2O2 (μM·g-1 FW) |
O2•- (nM·min-1·g-1 FW) |
MDA (nM·g-1 FW) |
|
|---|---|---|---|---|---|---|---|
| NTS | 0.03±0.00 a | 66.15±10.54 j | 0.80±0.10 j | 2.05±0.20 j | 1.34±0.04 j | 0.64±0.10 efh | 1.42±0.08 j |
| TS A | 0.14±0.02 gk | 104.96±7.42 k | 0.48±0.00 h | 2.44±0.16 k | 0.38±0.06 a | 0.35±0.04 b | 0.41±0.02 a |
| TS B | 0.29±0.01 m | 34.08±2.71 d-h | 0.55±0.01 i | 1.19±0.23 hi | 0.69±0.02 b | 0.67±0.01 ghj | 0.82±0.02 bd |
| TS C | 0.09±0.02 bd | 124.69±12.61 l | 1.02±0.02 k | 7.51±0.47 l | 0.63±0.03 b | 0.24±0.02 a | 1.18±0.02 g-i |
| TS D | 0.14±0.03 fgi | 28.32±2.06 cg | 0.87±0.02 j | 1.32±0.32 i | 0.81±0.08 c | 0.44±0.04 c | 1.07±0.01 fg |
| TS E | 0.15±0.00 h-k | 32.08±1.45 d-g | 0.51±0.07 hi | 0.84±0.10 b-df | 1.04±0.08 h | 0.59±0.08 dfi | 1.37±0.14 j |
| TS F | 0.20±0.03 l | 25.96±3.66 cd | 0.19±0.03 c | 1.28±0.15 i | 0.92±0.06 d-f | 0.57±0.02 de | 1.10±0.05 fi |
| TS G | 0.08±0.01 bc | 41.52±5.05 hi | 1.17±0.10 l | 0.75±0.07 b-d | 0.87±0.08 ce | 0.55±0.02 d | 0.74±0.09 b |
| TS H | 0.10±0.03 be | 17.10±3.68 ab | 0.33±0.03 f | 0.47±0.15 a | 0.64±0.02 b | 0.62±0.03 e-g | 0.90±0.01 c-e |
| TS I | 0.11±0.00 deg | 27.93±0.26 cf | 0.27±0.02 de | 0.84±0.05 b-dg | 0.95±0.05 e-g | 0.75±0.06 k | 1.45±0.01 j |
| TS J | 0.13±0.02 f-h | 20.05±0.78 ac | 0.11±0.00 a | 0.60±0.04 ab | 1.18±0.06 i | 1.22±0.03 n | 1.75±0.10 k |
| TS K | 0.08±0.02 b | 21.73±3.46 bc | 0.40±0.01 g | 0.73±0.03 b-d | 1.17±0.01 i | 0.65±0.04 g-i | 0.77±0.03 bc |
| TS L | 0.11±0.01 c-f | 26.73±1.30 ce | 0.18±0.02 bc | 0.69±0.02 ad | 0.88±0.07 cf | 0.83±0.06 l | 0.85±0.04 be |
| TS M | 0.05±0.01 a | 45.48±3.36 i | 0.23±0.05 ce | 0.80±0.00 b-e | 0.84±0.08 cd | 0.73±0.03 jk | 1.08±0.05 fh |
| TS N | 0.14±0.01 fgj | 73.75±0.67 j | 0.12±0.01 ab | 1.02±0.06 e-h | 1.02±0.06 gh | 0.45±0.08 c | 1.39±0.10 j |
| TS O | 0.04±0.01 a | 13.06±2.41 a | 0.22±0.00 cd | 0.66±0.02 ac | 1.06±0.03 h | 0.95±0.07 m | 0.97±0.10 ef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





