Submitted:
13 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
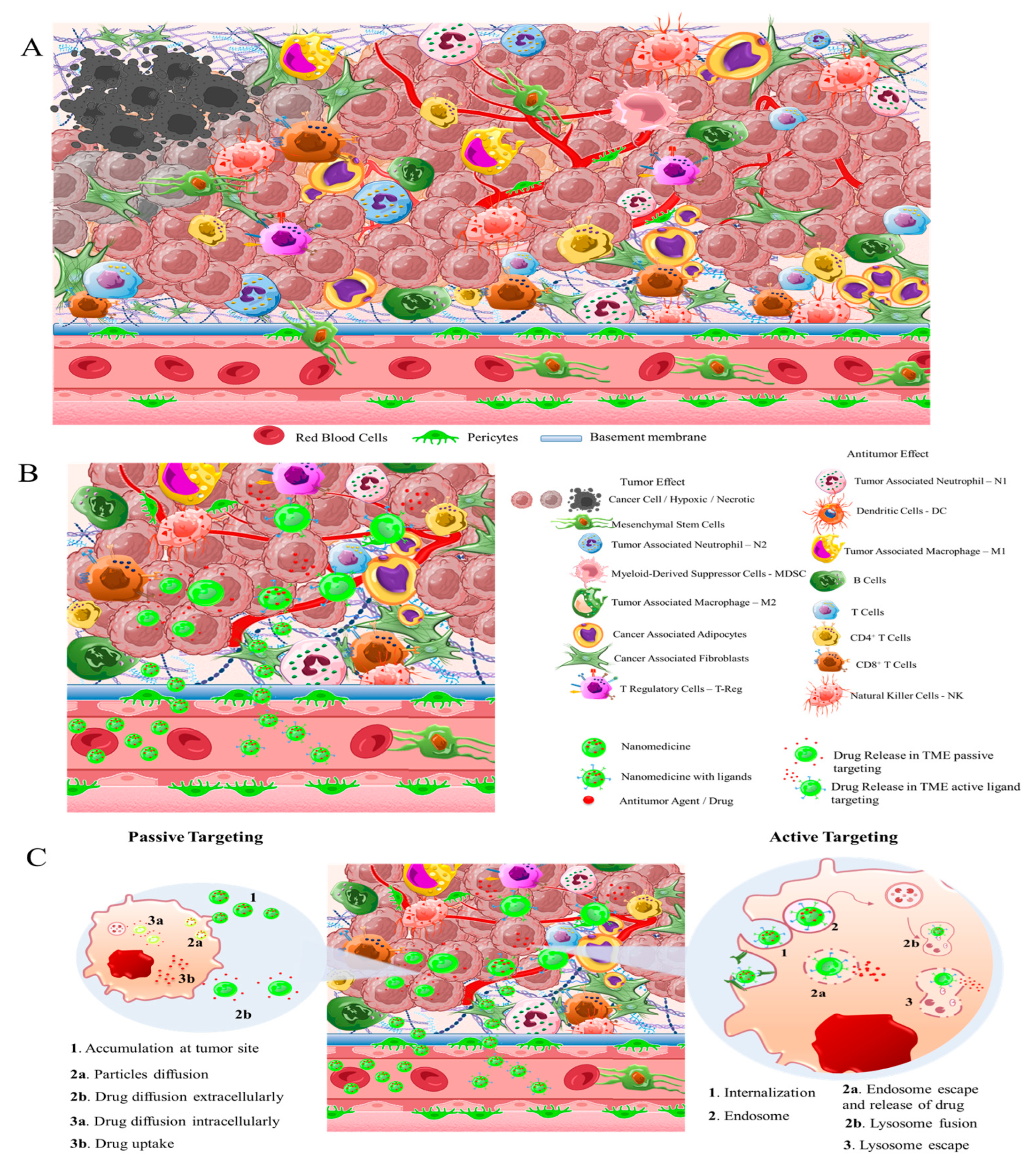
2. Solid Tumor Nanomedicine: Distribution in Tumor Microenvironment
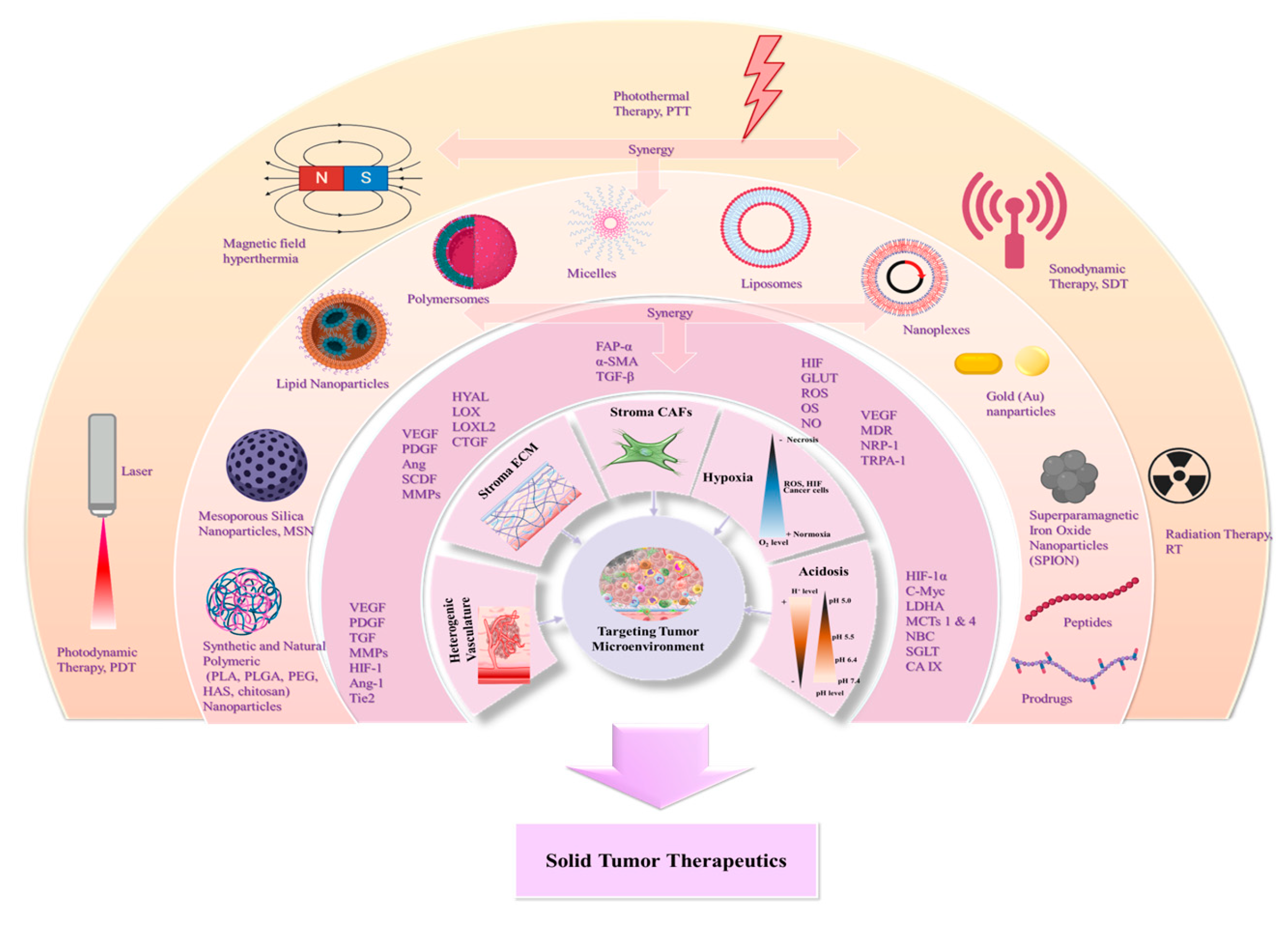
3. Nanomedicines for targeting TME: Application of Natural and Synthetic Biomaterials
3.1. The heterogenic vasculature
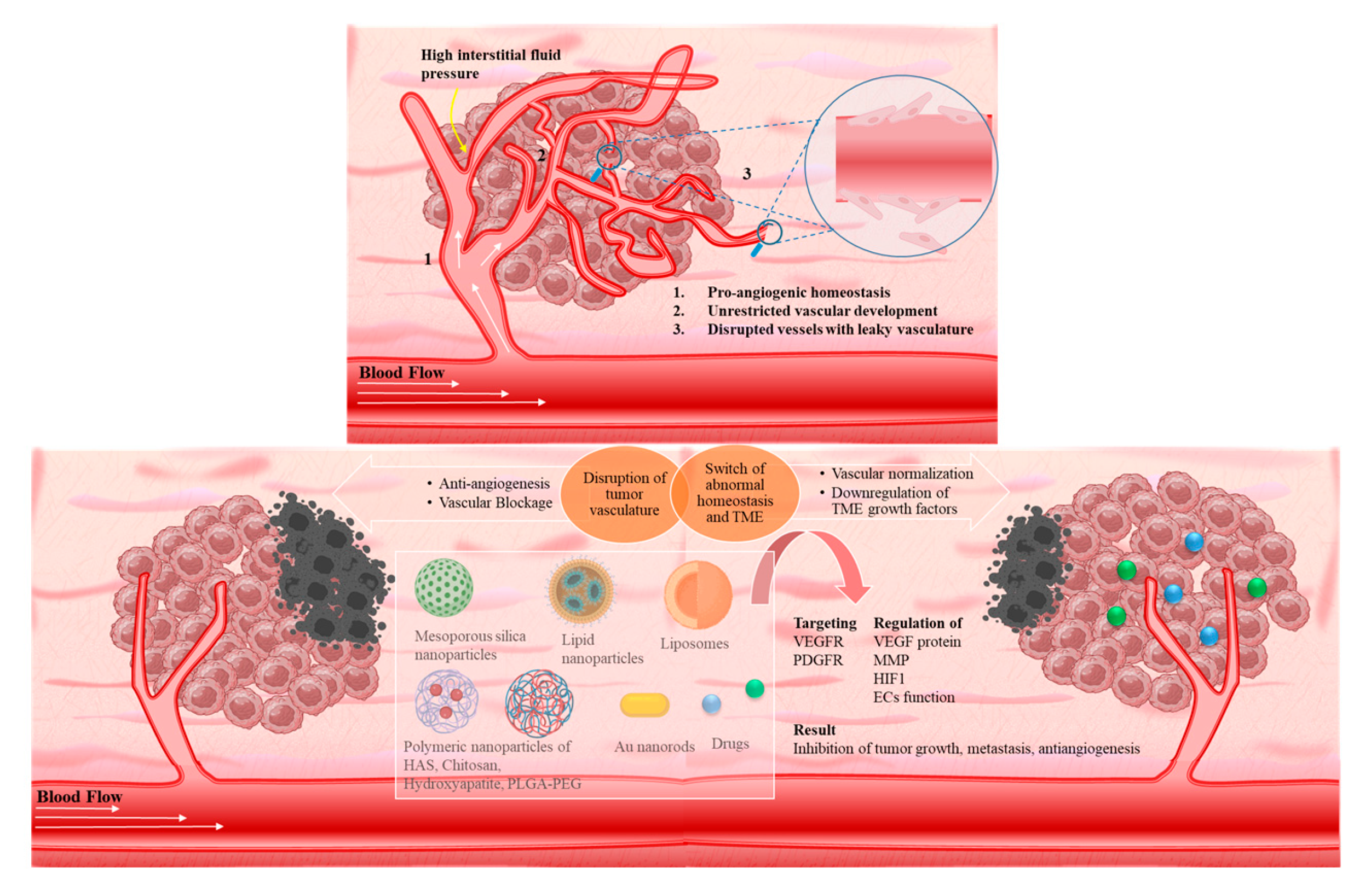
3.1.1. VEGF therapeutic targeting
3.1.2. Targeting molecular markers for vasculature regulation
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| VEGF | Hydroxyapatite (HA) | Sulfated s-HA | Non-selective binding of VEGF165a | [75] |
| Chitosan (CS) | Sulfated s-CS | Inhibition of VEGF/VEGFR2 signaling pathway | [76] | |
| CS/siRNA nanoplexes | siRNA | Silencing effect of siVEGF-A, siVEGFR-1, siVEGFR-2, NRP-1 inhibiting proliferation with improved immune functions | [77] | |
| carboxymethyl chitosan (CMCS) | CMCS | Regulate expression of VEGF levels, MMP-1 and CD34 and promoted inhibition of angiogenesis | [78] | |
| Endothelial Cell Regulation | HA-P123/F127 Polymeric nanoparticles |
thymoquinone | Modulating expression of miR-362/Rac1/RhoA and miR-361/VEGF-A pathways for inhibiting angiogenesis | [85] |
| CS nanoplexes | siRNA | Targeting PDGF-D and PDGFR-β expressions | [86] | |
| hydroxyapatite nanoparticles (HANP) | HANP | Regulating ECs function by the PI3K/Akt/eNOS signaling pathway | [87] | |
| hydroxyapatite nanoparticles | p53 plasmid and candesartan | Downregulation of VEGF protein secretion and functional microvessel density | [88] | |
| PLGA nanoparticles | P28 peptide and gefitinib | Inhibit tumor angiogenesis, primary tumor growth and metastasis | [89] | |
| FDA-Approved Drugs | Mesoporous silica nanoparticles (PEG-MSNs) | Sunitinib (anti-VEGFR) | Increased VEGFR targeting specificity, efficient inhibition of angiogenesis | [93] |
| Lipid-chitosan nanoparticles | Bevacizumab (VEGF-A antibody) | Suppressing proliferation and endothelial cells angiogenesis | [94] | |
| PLGA-PEG nanoparticles | Bevacizumab | higher internalization and bevacizumab delivery into CD44v6+ ECs | [95] | |
| human serum albumin nanoparticles | Bevacizumab | Decreased glycolysis and metabolic tumor volume, inhibition of tumor growth | [96] | |
| chitosan nanoparticles | Sorafenib (Tie2 inhibitor) | Superior antitumor activity | [97] | |
| Combinational Therapies | PEG-PCL-PAEA-SA nanoparticles | gambogenic acid / charge-reversible effect | Suppressed tumor angiogenesis, very little to no vascular tubes inside tumor models | [99] |
| PLGA nanoparticles | Sorafenib / Sunitinib / siRNA | Synergistic effect inhibiting cell proliferation | [101] | |
| PLGA-PEG nanoparticles | Anlotinib / pH-sensitivity | Inhibited tumor growth and metastasis suppressing lymphangiogenesis | [102] | |
| polycation liposomes | siRNA / calcium phosphate particles | Suppressed tumor growth and angiogenesis | [103] | |
| PEG-liposomes | doxorubicin / curcumin | Suppressed tumor growth, invasion and metastasis | [104] | |
| Au nanorods | NRP-1 peptide / PDT | inhibition of angiogenesis | [108] |
3.1.3. Targeting formulation based on FDA-approved drugs
3.1.4. Responsive targeting and combinational therapies
3.2. The tumor stroma extracellular matrix
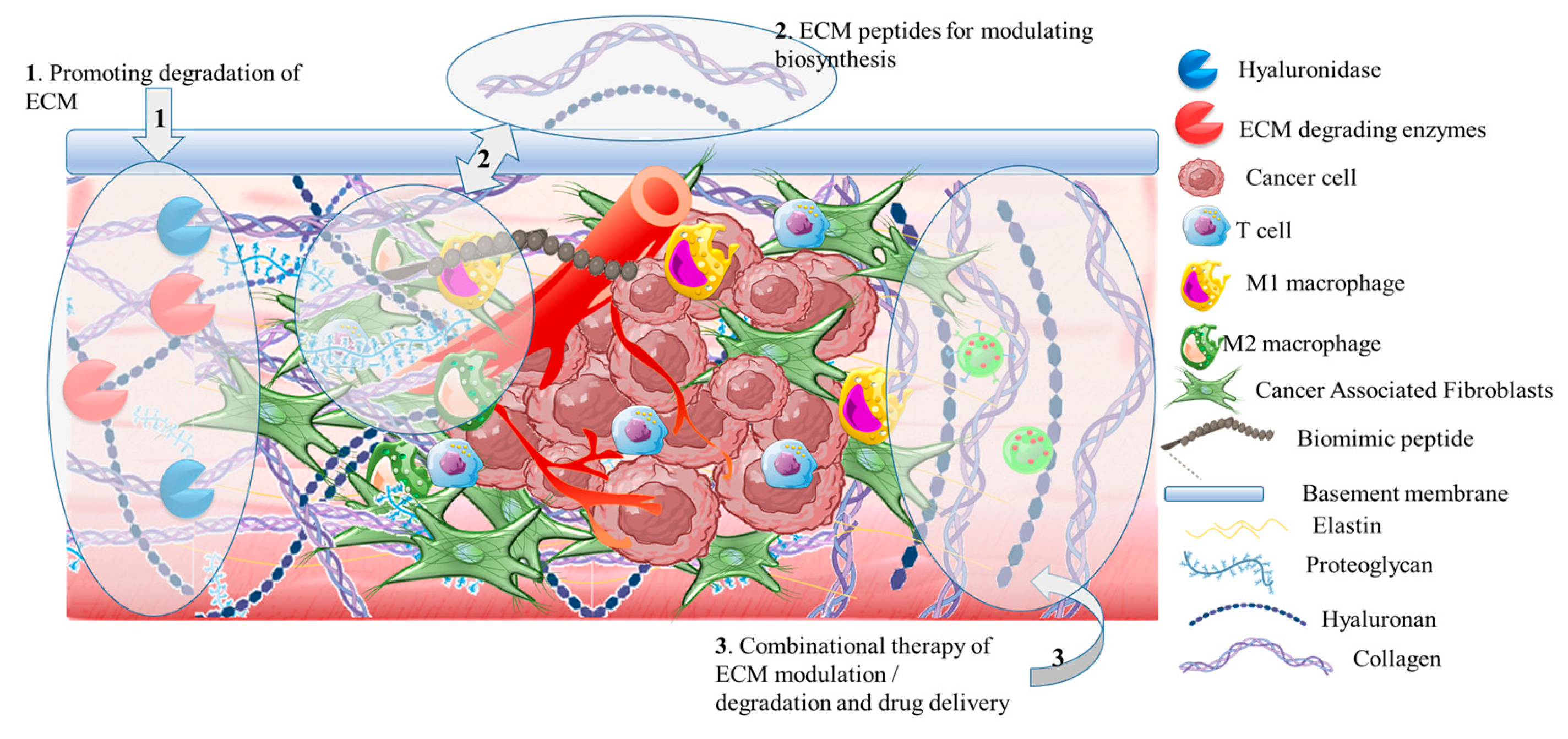
3.2.1. Targeting biomaterial formulations of hyaluronidase under clinical trials
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| Hyaluronidase | PEGPH20 | PEGPH20/ gemcitabine / nab-paclitaxel | Phase III trial (HALO-109-301) | [117] |
| PLGA-PEG nanoparticles | rHUPH20 / doxorubicin | Effective tumor accumulation enhanced antitumor effect | [118] | |
| ECM Degradation | Doxorubicin liposome (Doxosome) | Bromelain / Hyaluronic acid linked collagen type IV-binding peptide | Decayed the density of collagen fibers and advanced the tumor distribution | [119] |
| PLGA- polydopamine-PEG nanoparticles | Collagenase I / Doxorubicin | Degradation, enhanced the intratumoral distribution, and enhanced antitumor immunity | [120] | |
| LOXL2 antibody | LOXL2 antibody | Control of collagen assembly in ECM, potentially control tumor progression | [123] | |
| PLGA-PEG-PLGA thermosensitive hydrogel | Trastuzumab (Herceptin) / collagenase | degradation of intratumoral collagen promoting the antibody effect | [124] | |
| mPEG-PLGA nanoparticles | LOL2 and DDR1 inhibitors / Nab-paclitaxel | enhanced penetration and accumulation in tumor | [125] | |
| ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles | MMP9-sensitive peptide / Doxorubicin | effective bioimaging and synergistic chemo-photothermal antitumor effect | [126] | |
| ECM Biomolecules | Peptide nanoparticles | laminin (LN) mimic peptide | increased distribution in the tumor site and simultaneous transformation into nanofibers surrounding the tumor site | [128] |
| Hyaluronic acid mesoporous silica nanoparticles | siRNA suppressing CTGF expression / Doxorubicin | Inhibition of multidrug resistance and increased susceptibility of tumor cells to drug-induced apoptosis | [131] |
3.2.2. Targeting the extracellular matrix degradation
3.2.3. Targeting biomolecules for extracellular matrix
3.3. The tumor stroma cancer associated fibroblasts
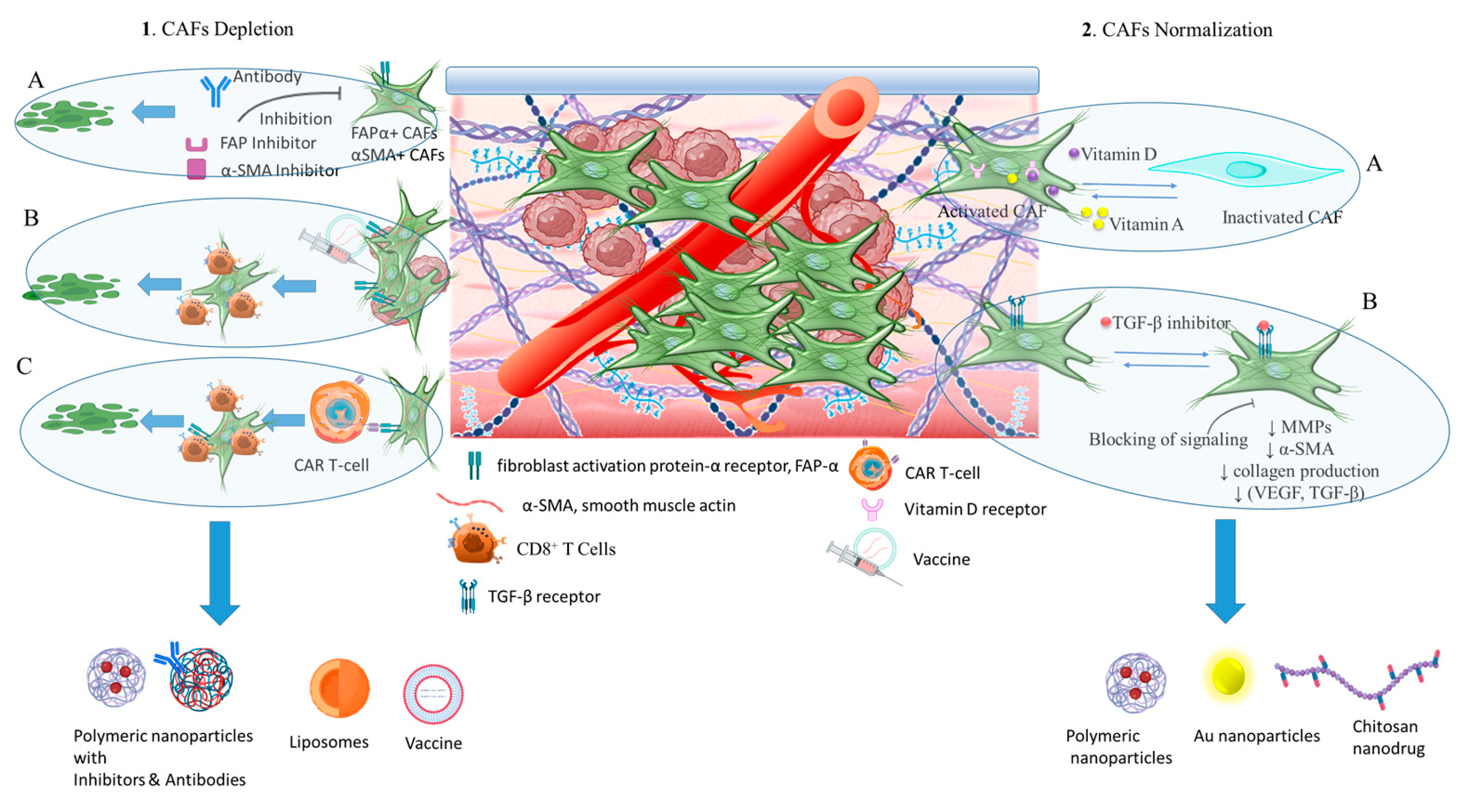
3.3.1. Targeting nanomedicine for CAFs depletion
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| CAFs depletion | anti-FAP-IL liposomes | single-chain Fv fragments against FAP (scFv’FAP) | Specifically and efficiently respond to FAP-α cell surface biomarker | [139] |
| cleavable amphiphilic peptide (CAP) nanoparticles | Doxorubicin / CAP | Disturbed the stromal barrier and increased drug intratumoral accumulation | [140] | |
| thermosensitive liposomes (CAP-TSL) | IR-780 photothermal agent / Paaclitaxel / Human serum albumin | increased cells apoptosis, expanded tumor interstitial space, promoted deep tumor penetration | [141] | |
| poly(amidoamine) (PAMAM) hyaluronic acid nanoparticles | Doxorubicin / CAP peptide | Deep intratumoral penetration, suppression of TGF-β, α-SMA, and FAP-α, degradation of tumor fibrotic stroma | [143] | |
| Vaccines | FAP targeting | synergistic antitumor immunity effect | [136,146,147] | |
| Synergistic inactivation | glycol chitosan – DEAP nanodrug | Methotrexate / quercetin | inhibition of pre-metastatic initiation, downregulation of metastasis promoting factors inactivation of CAFs | [144] |
| hydroxyethyl starch PLA nanoparticles | Doxorubicin / TGF-β receptor inhibitor | suppression of tumor growth and metastasis | [145] | |
| Au nanoparticles | Photodynamic therapy | inhibit the expression of pro-fibrotic signaling via Akt pathway | [154] |
3.3.2. Synergistic inactivation with antitumor targeting
3.4. The tumor hypoxia
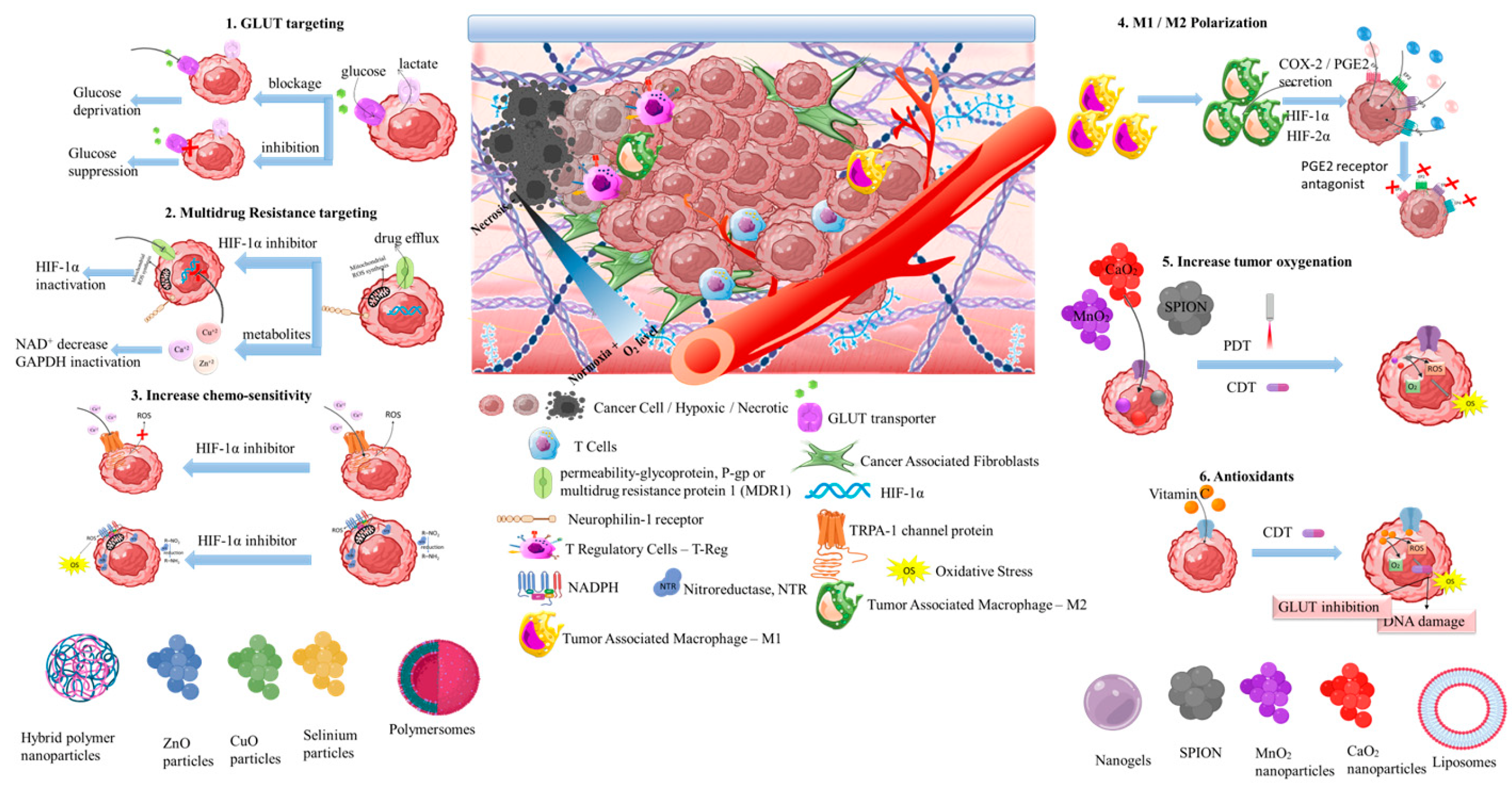
3.4.1. GLUT targeting nanomedicines
3.4.2. Multidrug resistance targeting therapeutics
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| GLUT | PLGA-chitosan particles | GLUT-1 | Glucose deprivation, increased apoptotic enzymes expression | [161] |
| Glucose-Methacrylate-OEGMA nanoparticles | Interferon-α | Tumor targeting and antitumor immunity | [162] | |
| Cu particles / tumor cell membrane coating | HIF-1α inhibitor / disulfiram | Enhanced tumor sensitivity | [167] | |
| Zn-imidazole – hyaluronic acid particles | DNAzymes | Antitumor effects inhibiting glucose energy | [168] | |
| Nanopipette sensors | Glucose Oxidase | Identification of intracellular glucose level | [169] | |
| Multidrug Resistance | Se / chitosan nanoparticles | Cisplatin | Suppressed ROS formation, inhibited HIF-1α, MDR-2, P-gp | [175] |
| Organosilica particles | Cisplatin / Acriflavine | Inhibition of tumor growth and metastasis | [176] | |
| Silk fibroin particles | Doxorubicin / PX478 HIF inhibitor | Downregulation of MDR1 and P-gp | [177] | |
| PLA-diazobenzene-PEG polymersomes | iRGD peptide / Doxorubicin | Increased accumulation, inhibition of tumor growth | [179] | |
| Chemo-Sensitivity | Hyaluronic acid nanogels / DSPE-PEG nano-micelles | Doxorubicin / TRPA-1 inhibitor | Enhanced tumors sensitivity, antitumor and antimetastatic effects | [183] |
| Chitosan-FA particles | Nitroreductase / Doxorubicin | Hypoxia triggered effective antitumor action | [184] | |
| CM-chitosan-maleimide particles | Dihydroartemicin / PDT | Suppression of HIF-1α and VEGF, inhibition of tumor metastasis | [185] | |
| M1/M2 polarization | Iron oxide-hyaluronic acid-chitosan nanoparticle | HIF-1α siRNA / PGE2 receptor antagonist | Suppression of proliferation, migration, angiogenesis, decreased protein levels, | [189] |
| Combinational | MnO2 – hyaluronic acid nanoparticles | Doxorubicin | Inhibiting tumor growth and cell proliferation | [193] |
| human serum albumin MnO2 nanoparticles | chlorin e6 / PDT | Tumor targeting ability, increased accumulation, elevated oxygen levels, tumor necrosis and apoptosis | [194] | |
| MnO2 – albumin nanoparticles | indocyanine green / PDT | Enhanced oxygen production, antitumor effect | [195] | |
| DSPE-PEG liposomes / MnO2-BSA nanoparticles | Atovaquone / hypericin / PDT | Suppressing hypoxia, increased antitumor effect | [196] | |
| lipid-PLGA-MnO2 particles | Sorafenib | Hypoxia suppression, inhibited tumor cells proliferation, suppressed angiogenesis and metastasis, | [198] | |
| solid lipid calcium peroxide (CaO2) nanocarriers | Doxorubicin / iron-oleate / Chemodynamic theapy | Oxidative damage to tumor tissues | [199] | |
| pH-sensitive methacrylate - CaO2 particles | CaO2 particles / PDT | Increased tumor oxygenation | [200] | |
| liposome nanoparticles | Cu-oleate / Acriflavine | Immunogenic cell death, combined antitumor immune responses | [201] | |
| Antioxidants | PEGylated liposomes | Palmitoyl ascorbate | Suppressed tumor growth | [206] |
| Liposomes | Doxorubicin / Palmitoyl ascorbate | Suppressed tumor growth | [207] |
3.4.3. Increasing chemo-sensitivity
3.4.4. Targeting M1 / M2 macrophage polarization
3.4.5. Combinational targeting for increasing tumor oxygenation
3.4.6. Synergistic targeting with antioxidants
3.5. The tumor acidosis
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| pH-sensitive peptides | Chitosan nanoparticles / cRGD peptide | Raloxifene | Increased accumulation, enhanced antitumor effect inhibiting angiogenesis and migration | [224] |
| Glycogen nanoparticles / hydrazine-based bond | Doxorubicin / β-galactose | Enhanced accumulation, inhibiting tumor growth | [225] | |
| PLGA – BSA particles ATRAM peptide | Doxorubicin / TPP | Enhanced mitochondria targeting, inhibited tumor volume and mass | [226] | |
| Hyaluronic acid nanogels E3/K3 peptides | Cytochrome C (CC) / saporin proteins | Inhibition of protein synthesis in the cytosol, efficient antitumor effect | [228] | |
|
Metals / Metal Oxides Chemo-Sensitivity |
Cerium oxide – glycol chitosan nanoparticles | CXCR4 antagonist / Doxorubicin | Elevated internalization, increased ROS production at acidic pH, tumor size suppression and reduced blood vessel leakage | [231] |
| PEG - MnO2 nanoparticles | Doxorubicin / Ce6 PDT | Tumor oxygenation, inhibition of tumor growth, elevated antitumor immune responses | [232] | |
| MnO2-coated mesoporous silicon nanoparticles | Metformin / fluvastatin sodium | Induced intracellular acidosis promoting tumor cell death, suppressed tumor growth and metastasis | [233] | |
| Au nanorods / P(Glu-co-Lys) polypetides | Au nanorods | Enhanced accumulation in tumors periphery and hypoxic core | [234] | |
| Iron oxide SPIONs / cystamine-dextran | Doxorubicin | Increased pH-triggered internalization, inhibition of tumor volume | [235] | |
| Iron oxide SPIONs / PMAA-g-PEGMA | Canagliflozin / Radiotherapy | Accumulation in tumor tissue, inhibition of tumor growth | [236] | |
| pH-sensitive Polymeric particles | carboxyethyl chitosan – PEGDA hydrogels | Doxorubicin | Self-healing properties, antitumor effect | [241] |
| Chitosan-PEG niosomes | Tamoxifen | Increased drug accumulation and antitumor efficacy | [242] | |
| Chitosan microformulations | - | Screening of tumor progression | [243] | |
| FA-PMgDP-PDPA-PDEMA particles | Doxorubicin / Galactose | Efficient internalization, increased toxicity and apoptosis | [245] | |
| PCL-b-PAEP-TMA-Cya/DMA micelles | Doxorubicin | Enhanced internalization, inhibition of tumor growth | [246] | |
| Iron oxide-PDPA particles | PEG-polycamptothecin prodrug | Effective antitumor activities, effective antitumor activities | [247] | |
| Graphene quantum dots-PLGA-BSA particles | Doxorubicin | Sufficient internalization and in vitro toxicity | [248] | |
| PLGA particles | Doxorubicin / sodium carbonate / liquid perfluorocarbon | Tumor accumulating ability, and inhibited tumor growth | [249] | |
| PEG-b-PHMA particles | Doxorubicin-P85 prodrug / iRGD peptide / Ce6 PDT | Elevated antitumor effect and complete suppression of tumor growth | [251] | |
| PCL-PEG particles | Paclitaxel / Acetazolamide | Inhibitory effect on tumor growth, increasing the survival rate | [252] | |
| DSPE-PEOz liposomes in platelet membrane particles | Doxorubicin | Enhanced antitumor effect | [253] | |
| Zeolitic imidazolate framework-8 nanoparticles | Doxorubicin / hemoglobin / LOX | Tumor targeting effect, suppressed tumor hypoxia, remodeled tumor acidity and inhibited tumor growth | [254] |
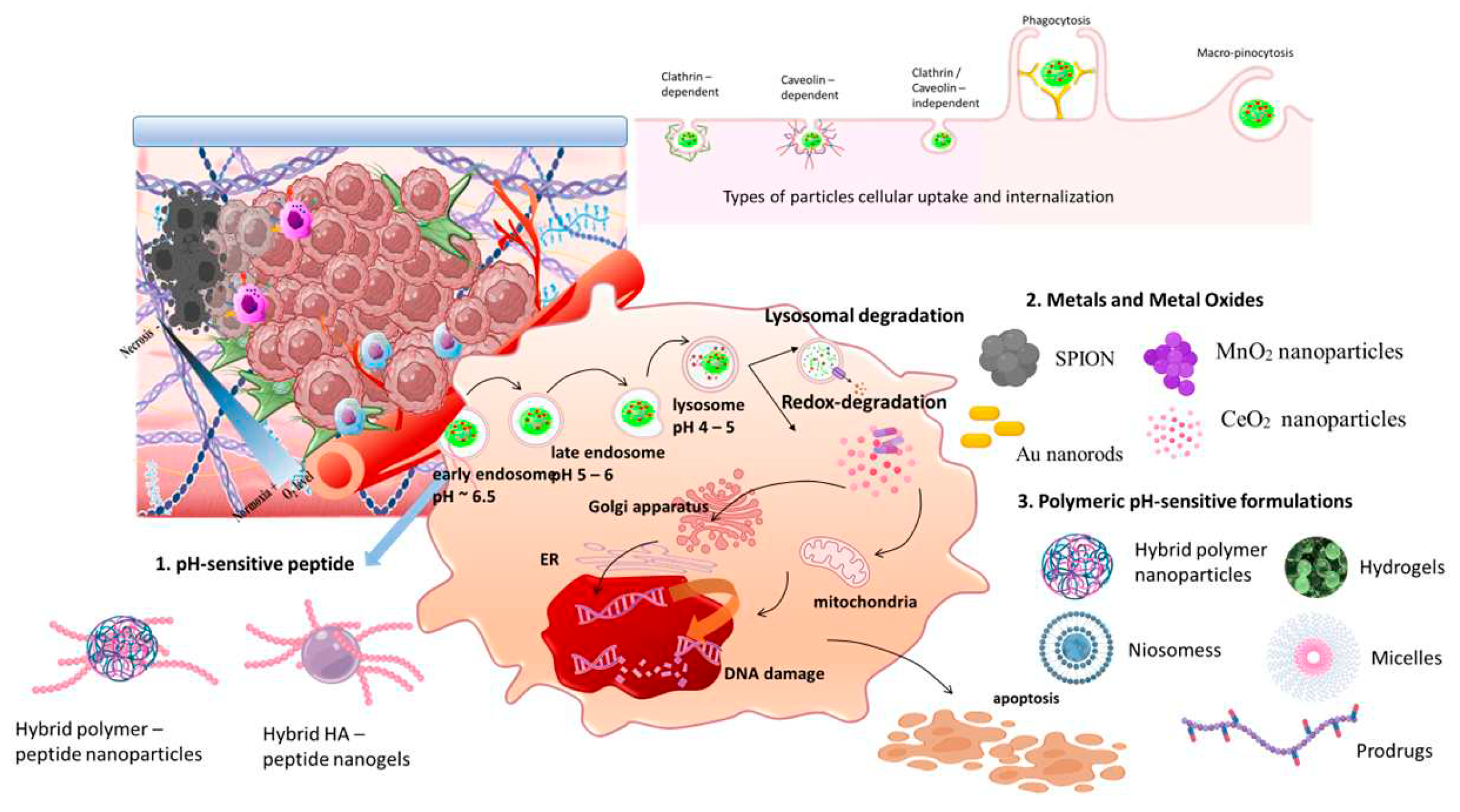
3.5.1. pH-sensitive peptides in acidic tumor targeting
3.5.2. Metals and Metal oxides in acidic tumor targeting
3.5.3. Biomaterial based polymeric nanomedicines in acidic tumor targeting
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Data Availability Statement
Conflicts of Interest
References
- Sung H.; Ferlay J.; Siegel R.L.; Laversanne M.; Soerjomataram I.; Jemal A.; Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209-249. [CrossRef]
- Siegel R.L.; Miller K.D.; Wagle N.S.; Jemal A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17-48. [CrossRef]
- World health statistics 2022: monitoring health for the SDGs, sustainable development goals., page 23, Geneva: World Health Organization. 2022. Licence: CC BY-NC-SA 3.0 IGO.
- Peng S.; Xiao F.; Chen M.; Gao H. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Immunotherapy. Adv. Sci. 2022, 9, 2103836. [CrossRef]
- Miao L.; Lin C. M.; Huang L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J. Contr. Rel. 2015, 219, 192–204. [CrossRef]
- Liu J.; Chen Q.; Feng L.; Liu Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today. 2018, 21, 55–73. [CrossRef]
- Yang S.; Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol. Res. 2017, 126, 97–108. [CrossRef]
- Das M.; Law S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int. J. Biochem. Cell Biol. 2018, 103, 115–124. [CrossRef]
- Ribeiro Franco P. I.; Perillo Rodrigues A.; Borges de Menezes L.; Pacheco Miguel M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729. [CrossRef]
- Wei R.; Liu S.; Zhang S.; Min L.; Zhu S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 6283796. [CrossRef]
- Gagliardi A.; Giuliano E.; Venkateswararao E.; Fresta M.; Bulotta S.; Awasthi V.; Cosco D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [CrossRef]
- Martin J.D.; Miyazaki T.; Cabral H. Remodeling tumor microenvironment with nanomedicines. WIREs Nanomed Nanobiotechnol. 2021, 13, 1730. [CrossRef]
- Saini H.; Eliato K.R; Veldhuizen J.; Zare A.; Allam M.; Silva C.; Kratz A.; Truong D.; Mouneimne G.; LaBaer J.; Ros R.; Nikkhah M. The role of tumor-stroma interactions on desmoplasia and tumorigenicity within a microengineered 3D platform. Biomaterials. 2020, 247, 119975. [CrossRef]
- Klemm F.; Joyce J. A. Microenvironmental regulation of therapeutic response in cancer. Trends in Cell Biol. 2015, 25, 198-213. [CrossRef]
- Deasy S. K.; Erez N. A glitch in the matrix: organ-specific matrisomes in metastatic niches. Trends in Cell Biol., 2022, 32, 110-123. [CrossRef]
- Lu P.; Weaver V.M.; Werb Z. The extracellular matrix: A dynamic niche in cancer progression, J. Cell Biol. 2012, 196, 395–406. www.jcb.org/cgi/doi/10.1083/jcb.201102147.
- Anand U.; Dey A.; Singh Chandel A. K.; Sanyal R.; Mishra A.; Kumar Pandey D.; De Falco V.; Upadhyay A.; Kandimalla R.; Chaudhary A.; Kaur Dhanjal J.; Dewanjee S.; Vallamkondu J.; Pérez de la Lastra J. M. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367-1401. [CrossRef]
- Kifle Z.D.; Tadele M.; Alemu E.; Gedamu T.; Ayele A.G. A recent development of new therapeutic agents and novel drug targets for cancer treatment. SAGE Open Med. 2021, 23, 1-12. [CrossRef]
- Boohaker R.J.; Lee M. W.; Vishnubhotla P.; Perez J. M.; Khaled A. R. The Use of Therapeutic Peptides to Target and to Kill Cancer Cells. Curr Med Chem. 2012, 19, 3794, . [CrossRef]
- Khongorzul P.; Ling C.J.; Khan F.U.; Ihsan A.U.; Zhang J. Antibody-drug conjugates: A comprehensive review. Mol Cancer Res. 2020, 18, 3–19. [CrossRef]
- Labrijn A.F.; Janmaat M.L.; Reichert J.M.; Parren P.W.H.I. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019, 18, 585-608. [CrossRef]
- Muthiah G.; Jaiswal A. Can the Union of Prodrug Therapy and Nanomedicine Lead to Better Cancer Management? Adv. NanoBiomed Res. 2022, 2, 2100074. [CrossRef]
- Kashkooli F. M.; Soltani M.; Hamedi M.-H. Drug delivery to solid tumors with heterogeneous microvascular networks: Novel insights from image-based numerical modeling. Europ. J. Pharmac. Scien. 2020, 151, 105399. [CrossRef]
- Wu M.; Frieboes H. B.; Chaplain M. A. J.; McDougall S. R.; Cristini V.; Lowengrub J. S. The effect of interstitial pressure on therapeutic agent transport: Coupling with the tumor blood and lymphatic vascular systems. J. Theor. Biol. 2014, 355, 194–207 . [CrossRef]
- Lim Z.-F.; Ma P. C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134. [CrossRef]
- Sarkar S.; Levi-Polyachenko N. Conjugated polymer nano-systems for hyperthermia, imaging and drug delivery. Adv. Drug Deliv. Rev. 2020, 163–164, 40–64. [CrossRef]
- Liao J.; Jia Y.; Wu Y.; Shi K.; Yang D.; Li P.; Qian Z. Physical-, chemical-, and biological-responsive nanomedicine for cancer therapy. WIREs Nanomed Nanobiotechnol. 2020, 12, e1581. [CrossRef]
- Granja A.; Pinheiro M.; Sousa C. T.; Reis S. Gold nanostructures as mediators of hyperthermia therapies in breast cancer. Biochem. Pharmacol. 2021, 190, 114639. [CrossRef]
- Angelopoulou A.; Voulgari E.; Kolokithas-Ntoukas A.; Bakandritsos A.; Avgoustakis K. Magnetic Nanoparticles for the Delivery of Dapagliflozin to Hypoxic Tumors: Physicochemical Characterization and Cell Studies. AAPS PharmSciTech,. 2018, 19, 2. [CrossRef]
- Seynhaeve A.L.B.; Amin M.; Haemmerich D.; van Rhoon G.C.; ten Hagen T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144. [CrossRef]
- Majumder N.; Das N. G.; Das S. K. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635. [CrossRef]
- Perez-Herrero E.; Fernandez-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Europ. J. Pharmac. Biopharmac. 2015, 93, 52–79. [CrossRef]
- Klein O.; Kee D.; Markman B.; Carlino M. S.; Underhill C.; Palmer J.; Power D.; Cebon J.; Behren A. Evaluation of TMB as a predictive biomarker in patients with solid cancers treated with anti-PD-1/CTLA-4 combination immunotherapy. Cancer Cell. 2021, 39, 592-593. [CrossRef]
- Marofi F.; Motavalli R.; Safonov V. A.; Thangavelu L.; Yumashev A. V.; Alexander M.; Shomali N.; Chartrand M. S.; Pathak Y.; Jarahian M.; Izadi S.; Hassanzadeh A.; Shirafkan N.; Tahmasebi S.; Khiavi F. M. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [CrossRef]
- Kirtane K.; Elmariah H.; Chung C. H.; Abate-Daga D. Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead. J. ImmunoTher. Cancer. 2021, 9, e002723. [CrossRef]
- Zhang X.; Zhang Y.; Zheng H.; He Y.; Jia H.; Zhang L.; Lin C.; Chen S.; Zheng J.; Yang Q.; Liu T.; Pan X.; Zhang H.; Wang C.; Ren L.; Shan W. In Situ biomimetic Nanoformulation for metastatic cancer immunotherapy. Acta Biomaterialia 2021, 134, 633–648. [CrossRef]
- Catalano V.; Turdo A.; Di Franco S.; Dieli F.; Todaro M.; Stassi G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23P, 522–532. [CrossRef]
- Ribatti D.; Pezzella F. Overview on the Different Patterns of Tumor Vascularization. Cells 2021, 10, 639. [CrossRef]
- Egeblad M.; Nakasone E. S.; Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010, 18, 884–901, . [CrossRef]
- Siemann D. W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat Rev. 2011, 37, 63–74. [CrossRef]
- Nagl L.; Horvath L.; Pircher A.; Wolf D. Tumor Endothelial Cells (TECs) as Potential Immune Directors of the Tumor Microenvironment – New Findings and Future Perspectives. Front. Cell Dev. Biol. 2020, 8, 766. [CrossRef]
- Salavati H.; Debbaut C.; Pullens P.; Ceelen W. Interstitial fluid pressure as an emerging biomarker in solid tumors. BBA – Rev. Cancer 2022, 1877, 188792. [CrossRef]
- Jiao D.; Cai Z.; Choksi S.; Ma D.; Choe M.; Kwon H.-J.; Baik J. Y.; Rowan B. G.; Liu C.; Liu Z.-G. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018, 28, 868–870. [CrossRef]
- Seok Youn Y.; Han Bae Y. Perspectives on the past, present, and future of cancer nanomedicine. Adv. Drug Deliv. Rev. 2018, 130, 3–11. [CrossRef]
- Al-Abd A.M.; Aljehani Z.K.; Gazzaz R.W.; Fakhri S.H.; Jabbad A.H.; Alahdal A.M.; Torchilin V.P. Pharmacokinetic strategies to improve drug penetration and entrapment within solid tumors. J. Control. Rel. 2015, 219, 269-277. [CrossRef]
- Lammers T.; Kiessling F.; Ashford M.; Hennink W.; Crommelin D.; Storm G. Cancer nanomedicine: is targeting our target? Nat Rev Mater 2016, 1, 16069. [CrossRef]
- Wu J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771 . [CrossRef]
- Nicolas-Boluda A.; Silva A. K.A.; Fournei S.; Gazeau F. Physical oncology: New targets for nanomedicine. Biomaterials. 2018, 150, 87-99. [CrossRef]
- Dhaliwal A.; Zheng G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics. 2019, 9, 8091-8108. [CrossRef]
- Dai W.; Wang X.; Song G.; Liu T.; He B.; Zhang H.; Wang X.; Zhang Q. Combination antitumor therapy with targeted dual-nanomedicines. Adv. Drug Deliv. Rev. 2017, 115, 23-45. [CrossRef]
- Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Contr. Rel. 2016, 244, 108-121. [CrossRef]
- Khot V. M.; Salunkhe A. B.; Pricl S.; Bauer J.; Thorat N. D.; Townley H. Nanomedicine-driven molecular targeting, drug delivery, and therapeutic approaches to cancer chemoresistance. Drug Disc. Tod. 2021, 26, 724-739. [CrossRef]
- Ang M.J.Y.; Chan S.Y.;Goh Y.-Y.; Luo Z.; Lau J.W.; Liu X. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv. Drug Deliv. Rev. 2021, 178, 113907. [CrossRef]
- Anjali Das C.G.; Ganesh Kumar V.; Stalin Dhas T.; Karthick V. Vineeth Kumar C.M. Nanomaterials in anticancer applications and their mechanism of action - A review. Nanomed. Nanothech., Biol., Med. 2023, 47, 102613. [CrossRef]
- Souri M.; Soltani M.; Kashkooli F.M.; Shahvandi M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J Contr. Rel. 2022, 341, 227-246. [CrossRef]
- Fulton M.D.; Najahi-Missaoui W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [CrossRef]
- Rodriguez F.; Caruana P.; De la Fuente N.; Espanol P.; Gamez M.; Balart J.; Llurba E.; Rovira R.; Ruiz R.; Martín-Lorente C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [CrossRef]
- Choo H.; Jeon S.I.; Ahn C.-H.; Shim M.K.; Kim K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728. [CrossRef]
- Pallerla S.; Abdul A.R.M.; Comeau J.; Jois S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [CrossRef]
- Micale N.; Molonia M.S.; Citarella A.; Cimino F.; Saija A.; Cristami M.; Speciale A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules 2021, 26, 4665. [CrossRef]
- George A.; Shah P.A.; Shrivastav P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharmac. 2019, 561, 244-264. [CrossRef]
- Tong X.; Pan W.; Su T.; Zhang M.;Dong W.; Qi X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [CrossRef]
- E van Vierken L.; Amiji M.M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006, 3, 205. Doi. 10.1517/17425247.3.2.205.
- Kuang Y.; Zhai J.; Xiao Q.; Zhao S.; Li C. Polysaccharide/mesoporous silica nanoparticle-based drug delivery systems: A review. Int. J. Biol. Macromol. 2021, 193, 457. [CrossRef]
- Wu P.; Han J.; Gong Y.; Liu C.; Yu H.; Xie N. Nanoparticle-Based Drug Delivery Systems Targeting Tumor Microenvironment for Cancer Immunotherapy Resistance: Current Advances and Applications. Pharmaceutics 2022, 14, 1990. [CrossRef]
- Prajapati S. K.; Jain A.; Jain A.; Jain S. Biodegradable polymers and constructs: A novel approach in drug delivery. Europ. Polym. J. 2019, 120, 109191. [CrossRef]
- Fathi M.; Abdolahinia E.D.; Bara J.; Omidi Y. Smart stimuli-responsive biopolymeric nanomedicines for targeted therapy of solid tumors. Nanomedicine 2020, 15 . [CrossRef]
- Salavati H.; Soltani M.; Amanpour S. The pivotal role of angiogenesis in a multi-scale modeling of tumor growth exhibiting the avascular and vascular phases. Microvasc. Res. 2018, 119, 105–116. [CrossRef]
- Aguilar-Cazares D.; Chavez-Dominguez R.; Carlos-Reyes A.; Lopez-Camarillo C.; Hernadez de la Cruz O. N.; Lopez-Gonzalez J. S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [CrossRef]
- Al-Ostoot F. H.; Salah S.; Khamees H. A.; Khanum S. A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Canc. Treat. Res. Commun. 2021, 28, 100422. [CrossRef]
- Teleanu R. I.; Chircov C.; Grumezescu A. M.; Teleanu D. M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2020, 9, 84. [CrossRef]
- Fu L.-Q.; Du W.-L.; Cai M.-H.; Yao J.-Y.; Zhao Y.-Y.; Mou X.-Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immun. 2020, 353, 104119. [CrossRef]
- Vimalraj S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [CrossRef]
- Banik N.; Yang S.-B.; Kang T.-B.; Lim J.-H.; Park J. Heparin and Its Derivatives: Challenges and Advances in Therapeutic Biomolecules. Int. J. Mol. Sci. 2021, 22, 10524. [CrossRef]
- Lim D.-K.; Wylie R.G.; Lange R.; Kohane D.S. Selective binding of C-6 OH sulfated hyaluronic acid to the angiogenic isoform of VEGF165. Biomater. 2016, 77, 130. [CrossRef]
- Li Y.; Wang W.; Zhang Y.; Wang X.; Gao X.; Yuan.; Li Y. Chitosan sulfate inhibits angiogenesis via blocking the VEGF/VEGFR2 pathway and suppresses tumor growth in vivo. Biomater. Sci., 2019, 7, . [CrossRef]
- Salva E.; Kabasakai L.; Eren F.; Ozkan N.; Cakalagaoglu F.; Akbuga J. Local Delivery of Chitosan/VEGF siRNA Nanoplexes Reduces Angiogenesis and Growth of Breast Cancer In Vivo. Nucleic Acid Ther. 2012, 22, 40. [CrossRef]
- Jiang Z.; Han B.; Li H.; Yang Y.; Liu W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1. [CrossRef]
- Picoli C. C.; Goncalves B. O. P.; Santos G. S. P.; Rocha B. G. S.; Costa A. C.; Resende R. R.; Birbrair A. Pericytes cross-talks within the tumor microenvironment. BBA – Rev. on Cancer. 2021, 1876, 188608. [CrossRef]
- Ma X.; Geng Z.; Wang S.; Yu Z.; Liu T.; Guan S.; Du S.; Zhu C. The driving mechanism and targeting value of mimicry between vascular endothelial cells and tumor cells in tumor progression. Biomed. Pharmacoth. 2023, 165, 115029. [CrossRef]
- Zalpoor H.; Aziziyan F.; Liaghat M.; Bakhtiyari M.; Akbari A.; Nabi-Afjadi M.; Forghaniesfidvajani R.; Rezaei N. The roles of metabolic profiles and intracellular signaling pathways of tumor microenvironment cells in angiogenesis of solid tumors. Cell Commun Signal 2022, 20, 186. [CrossRef]
- Larionova I.; Kazakova E.; Gerashchenko T.; Kzhyshkowska J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers 2021, 13, 3253. [CrossRef]
- Seyyednia E.; Oroojalian F.; Baradaran B.; Mojarrad J.S.; Mokhtarzadeh A.; Valizadeh H. Nanoparticles modified with vasculature-homing peptides for targeted cancer therapy and angiogenesis imaging. J. Contr. Rel. 2021, 338, 367. [CrossRef]
- Kargozar S.; Baino F.; Hamzehiou S.; Hamblin M.R.; Mozafari M. Nanotechnology for angiogenesis: opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008. DOI . [CrossRef]
- Bhattacharya S.; Ghosh A.; Maiti S.; Ahir M.; Debnath G.H.; Gupta P.; Bhattacharjee M.; Chosh S.; Chattopadhyay S.; Mukherjee P.; Adhikary A.; Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Contr. Rel. 2020, 322, 357. [CrossRef]
- Salva E.; Ozbas S.; Alan S.; Ozkan N.; Ekentok-Atici C.; Kabasakal L.; Akbuga J. Combination therapy with chitosan/siRNA nanoplexes targeting PDGF-D and PDGFR-β reveals anticancer effect in breast cancer. J. Gene Med. 2023, 25, 3465. [CrossRef]
- Shi X.; Zhou K.; Huang F.; Wang C. Interaction of hydroxyapatite nanoparticles with endothelial cells: internalization and inhibition of angiogenesis in vitro through the PI3K/Akt pathway. Int. J. Nanomed. 2017, 12, 5781. [CrossRef]
- Zhao L.; Zhao W.; Liu Y.; Chen X.; Wang Y. Nano-Hydroxyapatite-Derived Drug and Gene Co-Delivery System for Anti-Angiogenesis Therapy of Breast Cancer. Med Sci Monit. 2017, 23, 4723. [CrossRef]
- Garizo A.R.; Castro F.; Martins C.; Almeida A.; Dias T.P.; Fernardes F.; Barrias C.C.; Bernardes N.; Fialho A.M.; Sarmento B. p28-functionalized PLGA nanoparticles loaded with gefitinib reduce tumor burden and metastases formation on lung cancer. J. Contr. Rel. 2021, 337, 329. [CrossRef]
- Micaily I.; Johnson J.; Argiris A. An update on angiogenesis targeting in head and neck squamous cell carcinoma. Cancers Head Neck 2020, 5, 5 . [CrossRef]
- Hu H.; Chen Y.; Tan S.; Wu S.; Huang Y.; Fu S.; Luo F.; He J. The Research Progress of Antiangiogenic Therapy, Immune Therapy and Tumor Microenvironment. Front. Immunol. 2022, 13, 802846. [CrossRef]
- Taylor M. H.; Schmidt E. V.; Dutcus C.; Pinheiro E. M.; Funahashi Y.; Lubiniecki G.; Rasco D. The LEAP program: lenvatinib plus pembrolizumab for the treatment of advanced solid tumors. Future Oncol. 2021, 17, 637–647. [CrossRef]
- Goel S.; Chen F.; Hong H.; Valdovinos H.F.; Hernandez R.; Shi S.; Barnhart T.E.; Cai W. VEGF121-Conjugated Mesoporous Silica Nanoparticle: A Tumor Targeted Drug Delivery System. Appl. Mater. Interfaces, 2014, 6, 21677. [CrossRef]
- Abdi F.; Arkan E.; Eidizadeh M.; Valipour E.; Naseriyeh T.; Gamizgy Y.H.; Mansouri K. The possibility of angiogenesis inhibition in cutaneous melanoma by bevacizumab-loaded lipid-chitosan nanoparticles. Drug Deliv. Transl. 2023, 13, 568. [CrossRef]
- Balao A.; Sousa F.; Oliveira V.; Oliveira C.; Sarmento B. Effective intracellular delivery of bevacizumab via PEGylated polymeric nanoparticles targeting the CD44v6 receptor in colon cancer cells. Biomater. Sci. 2020,8, 3720. [CrossRef]
- Luis de Redin I.; Exposito F.; Agueros M.; Collantes M.; Penuelas I.; Allemandi D.; Llabot J.M.; Calvo A.; Irache J.M. In vivo efficacy of bevacizumab-loaded albumin nanoparticles in the treatment of colorectal cancer. Drug Deliv. Transl. 2020, 10, 635. [CrossRef]
- Ruman U.; Buskaran K.; Pastorin G.; Masarudin M.J.; Fakurazi S.; Hussein M.Z. Synthesis and Characterization of Chitosan-Based Nanodelivery Systems to Enhance the Anticancer Effect of Sorafenib Drug in Hepatocellular Carcinoma and Colorectal Adenocarcinoma Cells. Nanomaterials 2021, 11, 497. [CrossRef]
- Wang, L.; Chen, M.; Ran, X.; Tang, H.; Cao, D. Sorafenib-Based Drug Delivery Systems: Applications and Perspectives. Polymers 2023, 15, 2638. [CrossRef]
- Du M.; Geng T.; Yu R.; Song G.; Cheng H.; Cao Y.; He W.; Haleem A.; Li Q.; Hu R.; Chen S. Smart anti-vascular nanoagent induces positive feedback loop for self-augmented tumor accumulation. J. Contr. Rel. 2023, 356, 595. [CrossRef]
- Zhang J.; Hu H.; Chen E. The combination of MnO2@Lipo-coated gefitinib and bevacizumab inhibits the development of non-small cell lung cancer. Drug Deliv. 2022, 29, 466. [CrossRef]
- Punuch K.; Wongwan C.; Jantana S.; Somboonyosdech C.; Rodponthukwaji K.; Kunwong N.; Nguyen K.T.; Sirivatanauksorn V.; Sirivatanauksorn Y.; Srisawat C.; Punnakitikashem P. Study of siRNA Delivery via Polymeric Nanoparticles in Combination with Angiogenesis Inhibitor for The Treatment of AFP-Related Liver Cancer. Int. J. Mol. Sci. 2022, 23, 12666. [CrossRef]
- Cong X.; Chen J.; Xu R. Tumor-Acidity Responsive Polymeric Nanoparticles for Targeting Delivery of Angiogenesis Inhibitor for Enhanced Antitumor Efficacy with Decreased Toxicity. Front. Bioeng. Biotechnol. 2021, 9, 664051. [CrossRef]
- Chen J.; Sun X.; Shao R.; Xu Y.; Gao J.; Liang W. VEG F siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017, 12, 6075. [CrossRef]
- Barui S.; Saha S.; Mondal G.; Haseena S.; Chaudhuri A. Simultaneous delivery of doxorubicin and curcumin encapsulated in liposomes of pegylated RGDK-lipopeptide to tumor vasculature. Biomaterials. 2014, 35, 1643. [CrossRef]
- Barui A.K.; Nethi S.K.; Haque S.; Basuthakur P.; Patra C.R. Recent Development of Metal Nanoparticles for Angiogenesis Study and Their Therapeutic Applications. ACS Appl. Bio Mater. 2019, 2, 5492. [CrossRef]
- Darweesh R.S.; Ayoub N.M.; Nazzal S. Gold nanoparticles and angiogenesis: molecular mechanisms and biomedical applications. Int. J. Nanomed. 2019, 14, 7643. [CrossRef]
- Roma-Rodrigues C.; Heuer-Jungemann A.; Fernandes A.R.; Kanaras A.G.; Baptista P.V. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int. J. Nanomed. 2016, 11, 2633. [CrossRef]
- Bartczak D.; Muskens O.L.; Nitti S.; Millar T.M.; Kanaras A.G. Nanoparticles for inhibition of in vitro tumour angiogenesis: synergistic actions of ligand function and laser irradiation. Biomater. Sci., 2015, 3, 733. [CrossRef]
- Ni Y.; Zhou X.; Yang J.; Shi H.; Li H.; Zhao X.; Ma X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 637675. [CrossRef]
- Xu M.; Zhang T.; Xia R.; Wei Y.; Wei X. Targeting the tumor stroma for cancer therapy. Molecular Cancer 2022, 21, 208. [CrossRef]
- Zhao X.; He Y.; Chen H. Autophagic tumor stroma: mechanisms and roles in tumor growth and progression. Int. J. Cancer. 2013, 132, 1–8. [CrossRef]
- Simsek H.; Klotzsch E. The solid tumor microenvironment Breaking the barrier for T cells How the solid tumor microenvironment influences T cells. BioEssays. 2022, 44, 2100285. [CrossRef]
- Gargalionis A. N.; Papavassiliou K. A.; Papavassiliou A. G. Mechanobiology of solid tumors. BBA – Molec. Basis of Dis. 2022, 1868, 166555. [CrossRef]
- Rianna C.; Kumar P.; Radmacher M. The role of the microenvironment in the biophysics of cancer. Semin. Cell Dev. Biol. 2018, 73, 107–114. [CrossRef]
- Abyaneh H. S.; Regenold M.; McKee T. D.; Allen C.; Gauthier M. A. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics. 2020, 10, 1960-1980, . [CrossRef]
- Henke E.; Nandigama R.; Ergun S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160 . [CrossRef]
- Doherty G.J.; Tempero M.; Corrie P.G. HALO-109–301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018, 14, 13. [CrossRef]
- Zhou H.; Fan Z.; Deng J.; Lemons P.K.; Arhontoulis D.C.; Bowne W.B.; Cheng H. Hyaluronidase Embedded in Nanocarrier PEG Shell for Enhanced Tumor Penetration and Highly Efficient Antitumor Efficacy. Nano Lett. 2016, 16, 3268. [CrossRef]
- Ikeda-Imafuku M.; Gao Y.; Shaha S.; Li-Weng Wang L.; Soo Park K.; Nakajima M.; Adebowale O.; Mitragotri S. Extracellular matrix degrading enzyme with stroma-targeting peptides enhance the penetration of liposomes into tumors. J. contr. Rel. 2022, 352, 1093. [CrossRef]
- Amoozgar Z.; Goldberg M.S. Surface modulation of polymeric nanocarriers enhances the stability and delivery of proteins and small molecules. Nanomed. 2017, 12, 729. [CrossRef]
- Ding M.; Zhang Y.; Li J.; Pu K. Bioenzyme-based nanomedicines for enhanced cancer therapy. Nano Converg. 2022, 9, 7. [CrossRef]
- Setargew Y. F. I.; Wyllie K.; Grant R. D.; Chitty J. L.; Cox T. R. Targeting Lysyl Oxidase Family Meditated Matrix Cross-Linking as an Anti-Stromal Therapy in Solid Tumours. Cancers 2021, 13, 491. [CrossRef]
- Grossman M.; Ben-Chetrit N.; Zhuravlev A.; Afik R.; Bassat E.; Solomonov I.; Yarden Y.; Sagi I. Tumor Cell Invasion Can Be Blocked by Modulators of Collagen Fibril Alignment That Control Assembly of the Extracellular Matrix. Cancer Res. 2016, 76, 4249. [CrossRef]
- Pan A.; Wang Z.; Chen B.; Dai W.; Zhang H.; He B.; Wang X.; Wang Y.; Zhang Q. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018, 25, 1495. [CrossRef]
- Wei D.; Cheng X.; Du C.; Wang Y.; Sun J.; Li C.; Wu J.; Tian X.; Zhao Y.; Nie G.; Yang Y. Stroma-targeted nanoparticles that remodel stromal alignment to enhance drug delivery and improve the antitumor efficacy of Nab-paclitaxel in pancreatic ductal adenocarcinoma models. Nano Today. 2022, 45, 101533. [CrossRef]
- Chen A.; Lu H.; Cao R.; Zhu Y.; Li Y.; Ge R.; Zhang S.; Li Y.; Xiao L.; Su L.; Zhao J.; Hu H.; Wang Z. A novel MMP-responsive nanoplatform with transformable magnetic resonance property for quantitative tumor bioimaging and synergetic chemo-photothermal therapy. Nano Today. 2022, 45, 101524. [CrossRef]
- Guo Z.; Hu K.; Sun J.; Zhang T.; Zhang Q.; Song L.; Zhang X.; Gu N. Fabrication of Hydrogel with Cell Adhesive Micropatterns for Mimicking the Oriented Tumor-Associated Extracellular Matrix. ACS Appl. Mater. Interfaces. 2014, 6, 10963. [CrossRef]
- Hu X.-X.; He P.-P.; Qi G.-B.; Gao Y.-J.; Lin Y.-X.; Yang C.; Yang P.-P.; Hao H.; Wang L.; Wang H. Transformable Nanomaterials as an Artificial Extracellular Matrix for Inhibiting Tumor Invasion and Metastasis. ACS Nano 2017, 11, 4086. [CrossRef]
- Hellmud K.S.; Koksch B. Self-Assembling Peptides as Extracellular Matrix Mimics to Influence Stem Cell's Fate. Front. Chem. 2019, 7, 172. [CrossRef]
- Richeldi L.; Perez E. R.; Costabel U.; Albera C.; Lederer D. J; Flaherty K. R; Ettinger N.; Perez R.; Scholand M. B.; Goldin J.; Peony Yu K.-H.; Neff T.; Porter S.; Zhong M.; Gorina E.; Kouchakji E.; Raghu G. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE):a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2020, 8, 25–33. [CrossRef]
- Ding J.; Liang T.; Zhou Y.; He Z.; Min Q.; Jiang L.; Zhu J. Hyaluronidase-triggered anticancer drug and siRNA delivery from cascaded targeting nanoparticles for drug resistant breast cancer therapy. Nano Res. 2017, 10, 690 . [CrossRef]
- Cheng B.; Yu Q.; Wang W. Intimate communications within the tumor microenvironment: stromal factors function as an orchestra. J. Biomed. Sci. 2023, 30, 1 – 17. [CrossRef]
- Li Z.; Zheng Z.; Li C.; Li Z.; Wu J.; Zhang B. Therapeutic drugs and drug delivery systems targeting stromal cells for cancer therapy: A review. J. Drug Deliv. 2020, 714-726. [CrossRef]
- Hurtado P.; Martinez-Pena I.; Pineiro R. Dangerous Liaisons: Circulating Tumor Cells (CTCs) and Cancer-Associated Fibroblasts (CAFs). Cancers 2020, 12, 2861. [CrossRef]
- Chen X.; Song E. Turning foes to friends: targeting cancer- associated fibroblasts, Nat Rev Drug Discov 2019, 18, 99–115. [CrossRef]
- Shahvali S.; Rahiman N.; Jaafari M. R.; Arabi L. Targeting fibroblast activation protein (FAP): advances in CAR-T cell, antibody, and vaccine in cancer immunotherapy. Drug Deliv. and Transl. Res. 2023, 13, 2041–2056. [CrossRef]
- Mao Y.; Keller E. T.; Garfield D. H.; Shen K.; Wang J. Stromal cells in tumor microenvironment and breast cancer, Cancer Metastasis Rev 2013, 32, 303–315. [CrossRef]
- Fanfan L.; Shuping Z.; Cheng W.; Yaodi H.; Tianlong X.; Xueyi X.; Tingwei Z.; Liting S.; Shanwen K.; Jiang Z.; Xiaojun X.; Yue G.; Ai Z.; Jimin G. Development of Nectin4/FAP-targeted CAR-T cells secreting IL-7, CCL19, and IL-12 for malignant solid tumors. Front. Immunol. 2022, 13, 958082. [CrossRef]
- Ruger R.; Tansi F.L.; Rabenhold M.; Steiniger F.; Kontermann R.E.; Fahr A.; Hilger I. In vivo near-infrared fluorescence imaging of FAP-expressing tumors with activatable FAP-targeted, single-chain Fv-immunoliposomes. J. Contr. Rel. 2014, 186, 1. [CrossRef]
- Ji T.; Zhao Y.; Ding Y.; Wang J.; Zhao R.; Lang J.; Qin H.; Liu X.; Shi J.; Tao N.; Qin Z.; Nie G.; Zhao Y. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew. Chem. Int. Ed. 2016, 55, 1050. [CrossRef]
- Yu Q.; Qiu Y.; Li J.; Tang X.; Wang X.; Cun X.; Xu S.; Liu Y.; Li M.; Zhang Z.; He Q. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J Contr. Rel. 2020, 321, 564. [CrossRef]
- Liu H.; Shi Y.; Qian F. Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv. Drug Deliv. Rev. 2021, 172, 37. [CrossRef]
- Hou L.; Chen D.; Hao L.; Tian C.; Yan Y.; Zhu L.; Zhang H.; Zhang Y.; Zhang Z. Transformable nanoparticles triggered by cancer associated fibroblasts for improving drug permeability and efficacy in desmoplastic tumors. Nanoscale, 2019, 11, 20030. [CrossRef]
- Huo M.; Zhou J.; Wang H.; Zheng Y.; Tong Y.; Zhou J.; Liu J.; Yin T. A pHe sensitive nanodrug for collaborative penetration and inhibition of metastatic tumors. J. Contr. Rel. 2022, 352, 893. [CrossRef]
- Zhou Q.; Li Y.; Zhu Y.; Yu C.; Jia H.; Bao B.; Hu H.; Xiao C.; Zhang J.; Zeng X.; Wan Y.; Xu H.; Li Z.; Yang X. Co-delivery nanoparticle to overcome metastasis promoted by insufficient chemotherapy. J. Contr. Rel. 2018, 275, 67. [CrossRef]
- Chen M.; Xiang R.; Wen Y.; Xu G.; Wang C.; Luo S.; Yin T.; Wei X.; Shao B.; Liu N.; Guo F.; Li M.; Zhang S.; Li M.; Ren K.; Wang Y.; Wei Y. A whole-cell tumor vaccine modified to express fibroblast activation protein induces antitumor immunity against both tumor cells and cancer-associated fibroblasts. Sci Rep 2015, 5, 14421. [CrossRef]
- Chintala N. K.; Restle D.; Quach H; Saini J; Bellis R; Offin M; Beattie J; Adusumilli P.S. CAR T-cell therapy for pleural mesothelioma: Rationale, preclinical development, and clinical trials. Lung Cancer. 2021, 157, 48-59, . [CrossRef]
- Li J.; Luco A.-L.; Camirand A.; St-Arnaud R.; Kremer R.; Vitamin D regulates CXCL12/CXCR4 and epithelial-to-mesenchymal transition in a model of breast cancer metastasis to lung, Endocrinol. 2021, 162, 1-15. [CrossRef]
- Gorchs L.; Ahmed S.; Mayer C.; Knauf A; Fernández Moro C.; Svensson M.; Heuchel R.; Rangelova E.; Bergman P.; Kaipe H. The vitamin D analogue calcipotriol promotes an anti-tumorigenic phenotype of human pancreatic CAFs but reduces T cell mediated immunity. Sci Rep 2020, 10, 17444. [CrossRef]
- Han X.; Xu Y.; Geranpayehvaghei M.; Anderson G. J.; Li Y.; Nie G. Emerging nanomedicines for anti-stromal therapy against desmoplastic tumors. Biomaterials 2020, 232, 119745 . [CrossRef]
- Mortezaee K.; Majidpoor J. Key promoters of tumor hallmarks., Int. J. Clin. Oncol. 2022, 27, 45-58. [CrossRef]
- Meng H.; Nel A.E. Use of nano engineered approaches to overcome the stromal barrier in pancreatic cancer. Adv. Drug Del. Rev. 2018, 130, 50. [CrossRef]
- Guo J.; Zeng H.; Chen Y. Emerging Nano Drug Delivery Systems Targeting Cancer-Associated Fibroblasts for Improved Antitumor Effect and Tumor Drug Penetration. Mol. Pharmaceutics 2020, 17, 1028. [CrossRef]
- Zhao X.; Pan J.; Li W.; Yang W.; Qin L.; Pan Y. Gold nanoparticles enhance cisplatin delivery and potentiate chemotherapy by decompressing colorectal cancer vessels. Int. J. Nanomed. 2018, 13, 6207. [CrossRef]
- Garcia-Bermudez J.; Williams R. T.; Guarecuco R.; Birsoy K. Targeting extracellular nutrient dependencies of cancer cells. Molecular Metabolism 2020, 33, 67-82. [CrossRef]
- Horsman M. R.; Sorensen B. S.; Busk M.; Siemann D. W. Therapeutic Modification of Hypoxia. Clinic. Oncol. 2021, 33, e492-e509. [CrossRef]
- McAleese C. E.; Choudhury C.; Butcher N. J.; Minchin R. F. Hypoxia-mediated drug resistance in breast cancers. Cancer Letters 2021, 502, 189–199. [CrossRef]
- Khoshinani H. M.; Afshar S.; Najafi R. Hypoxia: A Double-Edged Sword in Cancer Therapy. Canc. Investig. 2016, 34, 536-545. [CrossRef]
- Wiechec E.; Matic N.; Ali A.; Roberg K. Hypoxia induces radioresistance, epithelial-mesenchymal transition, cancer stem cell-like phenotype and changes in genes possessing multiple biological functions in head and neck squamous cell carcinoma. Oncology Reports 2022, 47, 58. [CrossRef]
- Zeng W.; Liu P.; Pan W.; Singh S. R.; Wei Y. Hypoxia and hypoxia inducible factors in tumor metabolism. Canc. Let. 2015, 356, 263-267, . [CrossRef]
- Abolhasani A.; Biria D.; Abolhasani]H.; Zarrabi A.; Komeili T. Investigation of the Role of Glucose Decorated Chitosan and PLGA Nanoparticles as Blocking Agents to Glucose Transporters of Tumor Cells. Int. J. Nanomed. 2019, 14, 9535. [CrossRef]
- Sun J.; Guo J.; Zhang L.; Gong L.; Sun Y.; Deng X.; Gao W. Active-targeting long-acting protein-glycopolymer conjugates for selective cancer therapy. J. Control. Release. 2023, 356, 175. [CrossRef]
- Torres-Perez S.A.; Torres-Perez C.E.; Pedraza-Escalona M.; Perez-Tapia S.M.; Ramon-Gallegos E. Glycosylated Nanoparticles for Cancer-Targeted Drug Delivery. Front. Oncol. 2020, 10, . [CrossRef]
- Geng C.; Pang S.; Ye R.; Shi J.; Yang Q.; Chen C.; Wang W. Glycolysis-based drug delivery nanosystems for therapeutic use in tumors and applications. Biomed. Pharmacother. 2023, 165, 115009. [CrossRef]
- Heddleston J.M.; Li Z.; Lathia J.D.; Bao S.; Hjelmeland A.B.; Rich J.N. Hypoxia inducible factors in cancer stem cells. British J. Cancer. 2010, 102, 789. [CrossRef]
- Shibuya K.; Okada M.; Suzuki S.; Seino M.; Seino S.; Takeda H.; Kitanaka C. Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget. 2015, 6, 651. [CrossRef]
- Yang S.; Han Y.; Bao B.; Hu C.; Li Z. Boosting the anti-tumor performance of disulfiram against glioblastoma by using ultrasmall nanoparticles and HIF-1α inhibitor. Compos. B: Eng. 2022, 243, 110117. [CrossRef]
- Wu S.; Zhang K.; Liang Y.; Wei Y.; An J.; Wang Y.; Yang J.; Zhang H.; Zhang Z.; Liu J.; Shi J. Nano-enabled Tumor Systematic Energy Exhaustion via Zinc (II) Interference Mediated Glycolysis Inhibition and Specific GLUT1 Depletion. Adv. Sci. 2022, 9, 2103534. [CrossRef]
- Nascimento R.A.S.; Ozel R.E.; Han Mak W.; Mulato M.; Singaram B.; Pourmand N. Single Cell “Glucose Nanosensor” Verifies Elevated Glucose Levels in Individual Cancer Cells. Nano Lett. 2016, 16, 1194. [CrossRef]
- Zhang Y.; Coleman M.; Brekken R.A. Perspectives on Hypoxia Signaling in Tumor Stroma. Cancers 2021, 13, 3070. [CrossRef]
- Shirai, Y.; Chow, C.C.T.; Kambe, G.; Suwa, T.; Kobayashi, M.; Takahashi, I.; Harada, H.; Nam, J.-M. An Overview of the Recent Development of Anticancer Agents Targeting the HIF-1 Transcription Factor. Cancers 2021, 13, 2813. [CrossRef]
- Xu X.-X.; Chen S.-Y.; Yi N.-B.; Li X.; Chen S.-L.; Lei Z.; Cheng D.-B.; Sun T. Research progress on tumor hypoxia-associative nanomedicine. J. Contr. Rel. 2022, 350, 829-840. [CrossRef]
- Zhang X.; He C.; Xiang G. Engineering nanomedicines to inhibit hypoxia-inducible Factor-1 for cancer therapy. Cancer Let. 2022, 530, 110. [CrossRef]
- Bukowski K.; Kciuk M.; Kontek R.; Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [CrossRef]
- Zhang X.; He C.; Yan R.; Chen Y.; Zhao P.; Li M.; Fan T.; Yang T.; Lu Y.; Luo J.; Ma X.; Xiang G. HIF-1 dependent reversal of cisplatin resistance via anti-oxidative nano selenium for effective cancer therapy. Chem. Eng. J. 2020, 380, 122540. [CrossRef]
- Zhang X.; He C.; Liu X.; Zhao P.; Chen C.; Yan R.; Li M.; Fan T.; Altine B.; Yang T.; Lu Y.; Lee R.J.; Gai Y.; Xiang G. One-pot synthesis of a microporous organosilica-coated cisplatin nanoplatform for HIF-1–targeted combination cancer therapy. Theranostics 2020, 10, 2918. [CrossRef]
- Li Z.; Cheng G.; Zhang Q.; Wu W.; Zhang Y.; Wu B.; Liu Z.; Tong X.; Xiao B.; Cheng L.; Dai F. PX478-loaded silk fibroin nanoparticles reverse multidrug resistance by inhibiting the hypoxia-inducible factor. Int. J. Biol. Macromol. 2022, 222, 2309. [CrossRef]
- Fernandez-Palanca P.; Payo-Serafin T.; San-Miguel B.; Mendez-Blanco C.; Tunon M.J.; Gonzalez-Gallego J.; Mauriz J.L.; Hepatocellular carcinoma cells loss lenvatinib efficacy in vitro through autophagy and hypoxia response-derived neuropilin-1 degradation. Acta Pharmacol. Sin. 2022, 44, 1066. [CrossRef]
- Mamnoon B.; Loganathan J.; Confeld MI.; De Fonseka N.; Feng L.; Froberg J.; Choi Y.; Tuvin D.M.; Sathish V.; Mallik S. Targeted Polymeric Nanoparticles for Drug Delivery to Hypoxic, Triple-Negative Breast Tumors. ACS Appl. Bio Mater. 2021, 4, 1450. [CrossRef]
- Telarovic I.; Wenger R.H.; Pruschy M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [CrossRef]
- Gong L.; Zhang Y.; Liu C.; Zhang M.; Han S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083-1102. [CrossRef]
- Faris P.; Rumolo A.; Pellavio G.; Tanzi M.; Vismara M.; Berra-Romani R.; Gerbino A.; Corallo S.; Pedrazzoli P.; Laforenza U.; Montagna D.; Moccia F. Transient receptor potential ankyrin 1 (TRPA1) mediates reactive oxygen species-induced Ca2+ entry, mitochondrial dysfunction, and caspase-3/7 activation in primary cultures of metastatic colorectal carcinoma cells. Cell Death Discov. 2023, 9, 213. [CrossRef]
- Wang Y.; Yin S.; Mei L.; Yang Y.; Xu S.; He X.; Wang M.; Li M.; Zhang Z.; He Q. A dual receptors-targeting and size-switchable “cluster bomb” co-loading chemotherapeutic and transient receptor potential ankyrin 1 (TRPA-1) inhibitor for treatment of triple negative breast cancer. J. Control. Release. 2020, 321, 71. [CrossRef]
- Jang E.H.; Shim M.K.; Kim G.L.; Kim S.-H.; Kang H.; Kim J.-H. Hypoxia-responsive folic acid conjugated glycol chitosan nanoparticle for enhanced tumor targeting treatment. Int. J. Pharm. 2020, 580, 119237. [CrossRef]
- Luo R.; Zhang Z.; Han L.; Xue Z.; Zhang K.; Liu F.; Feng F.; Xue J.; Liu W.; Qu W. An albumin-binding dimeric prodrug nanoparticle with long blood circulation and light-triggered drug release for chemo-photodynamic combination therapy against hypoxia-induced metastasis of lung cancer. Biomater. Sci., 2021, 9, 3718. [CrossRef]
- Chen Y.; Song Y.; Du W.; Gong L.; Chang H.; Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019, 26, 78. [CrossRef]
- Cebola I.; Custodio J.; Munoz M.; Diez-Villanueva A.; Pare L.; Prieto P.; Ausso S.; Coll-Mulet L.; Bosca L.; Moreno V.; Peinado M.A. Epigenetics override pro-inflammatory PTGS transcriptomic signature towards selective hyperactivation of PGE2 in colorectal cancer. Clin. Epigenetics. 2015, 7, 74. [CrossRef]
- Jun J.C.; Rathore A.; Younas H.; Gilkes D.; Polotsky V. Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1. [CrossRef]
- Karpisheh V.; Afjadi J.F.; Afjadi M.N.; Haeri M.S.; Sough T.S.A.; Asl S.H.; Edalati M.; Atyabi F.; Masjedi A.; Hajizadeh F.; Izadi S.; Tekie F.S.M.; Hajiramezanali M.; Sojoodi M.; Jadidi-Niaragh F. Inhibition of HIF-1α/EP4 axis by hyaluronate-trimethyl chitosan-SPION nanoparticles markedly suppresses the growth and development of cancer cells. Int. J. Biol. Macromol. 2021, 167, 1006. [CrossRef]
- Hughes V.S.; Wiggins J.M.; Siemann D.W. Tumor oxygenation and cancer therapy—then and now. Br J Radiol 2019, 92 20170955. [CrossRef]
- Moen I.; Stuhr L.E.B. Hyperbaric oxygen therapy and cancer-a review. Targ Oncol. 2012, 7, 233. [CrossRef]
- Wang X.; Ye N.; Xu C.; Xiao C.; Zhang Z.; Deng Q.; Li S.; Li J.; Li Z.; Yang X. Hyperbaric oxygen regulates tumor mechanics and augments Abraxane and gemcitabine antitumor effects against pancreatic ductal adenocarcinoma by inhibiting cancer-associated fibroblasts. Nano Today 2022, 44, 101458. [CrossRef]
- Song M.; Liu T.; Shi C.; Zhang X.; Chen X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS Nano. 2016, 10, 633. [CrossRef]
- Lin T.; Zhao X.; Zhao S.; Yu H.; Cao W.; Chen W.; Wei H.; Guo H. O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics. 2018, 8, 990 . [CrossRef]
- Jiang N.; Zhou Z.; Xiong W.; Chen J.; Shen J.; Li R.; Ye R. Tumor microenvironment triggered local oxygen generation and photosensitizer release from manganese dioxide mineralized albumin-ICG nanocomplex to amplify photodynamic immunotherapy efficacy. Chin Chem Lett. 2021, 32, 3948. [CrossRef]
- Ren C.; Xu X.; Yan D.; Gu M.; Zhang J.; Zhang H.; Han C.; Kong L. Dual-action nanoplatform with a synergetic strategy to promote oxygen accumulation for enhanced photodynamic therapy against hypoxic tumors. Acta Biomater. 2022, 146, 465. [CrossRef]
- Zhou R.; Zeng X.; Zhao H.; Chen Q.; Wu P. Combating the hypoxia limit of photodynamic therapy through reversing the survival-related pathways of cancer cells. Coord. Chem. Rev. 2022, 452, 214306. [CrossRef]
- Chang C.-C.; Dinh T.K.; Lee Y.-A.; Wang F.-N.; Sung Y.-C.; Yu P.-L.; Chiu S.-C.; Shih Y.-C.; Wu C.-Y.; Huang Y.-D.; Wang J.; Lu T.-T.; Wan D.; Chen Y. Nanoparticle Delivery of MnO2 and Anti-angiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces. 2020, 12, 44407. [CrossRef]
- He C.; Zhang X.; Chen C.; Liu X.; Chen Y.; Yan R.; Fan T.; Gai Y.; Lee R.J.; Ma X.; Luo J.; Lu Y.; Yang T.; Xiang G. A solid lipid coated calcium peroxide nanocarrier enables combined cancer chemo/chemodynamic therapy with O2/H2O2 self-sufficiency. Acta Biomater. 2021, 122, 354. [CrossRef]
- Sheng Y.; Nesbitt H.; Callan B.; Taylor M.A.; Love M.; McHale A.P.; Callan J.F. Oxygen generating nanoparticles for improved photodynamic therapy of hypoxic tumours. J. Control. Release. 2017, 264, 333. [CrossRef]
- Zhang X.; He C.; He X.; Fan S.; Ding B.; Lu Y.; Xiang G. HIF-1 inhibitor-based one-stone-two-birds strategy for enhanced cancer chemodynamic-immunotherapy. J. Contr. Rel. 2023, 356, 649. [CrossRef]
- Li Y.; Jeon J.; Park J.H. Hypoxia-responsive nanoparticles for tumor-targeted drug delivery. Cancer Let. 2020, 490, 31. [CrossRef]
- Wang H.; Li J.; Wang Y.; Gong X.; Xu X.; Wang J.; Li Y.; Sha X.; Zhang Z. Nanoparticles-mediated reoxygenation strategy relieves tumor hypoxia for enhanced cancer therapy. J. Control. Releas. 2020, 319, 25. [CrossRef]
- Patel A.; Sant S. Hypoxic tumor microenvironment: Opportunities to develop targeted therapies. Biotechnol. Adv. 2016, 34, 803. [CrossRef]
- Liu Z.; Ren Z.; Zhang J.; Chuang C.-C.; Kandaswamy E.; Zhou T.; Zuo L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Rhysiol. 2018, 9, 477. [CrossRef]
- Sawant R.R.; Vaze O.S.; Wang T.; D’Souza G.G.M.; Rockwell K.; Gada K.; Khaw B.-A.; Torchilin V.P. Palmitoyl Ascorbate Liposomes and Free Ascorbic Acid: Comparison of Anticancer Therapeutic Effects Upon Parenteral Administration. Pharm Res. 2012, 29, 375. [CrossRef]
- Yang Y.; Lu X.; Liu Q.; Dai Y.; Zhu X.; Wen Y.; Xu J.; Lu J.; Lu Y.; Zhao D.; Chen X.; Li N. Palmitoyl ascorbate and doxorubicin co-encapsulated liposome for synergistic anticancer therapy. Eur. J. Pharm. Sci. 2017, 105, 219. [CrossRef]
- Razi S.; Haghparast A.; Khameneh S.C.; Sadrabadi A.E.; Aziziyan F.; Bakhtiyari M.; Nabi-Afjadi M.; Tarhriz V.; Jalili A.; Zalpoor H.; The role of tumor microenvironment on cancer stem cell fate in solid tumors. Cell Commun Signal. 2023, 21, 143. [CrossRef]
- Vaupel P.; Multhoff G. Fatal Alliance of Hypoxia-/HIF-1α-Driven Microenvironmental Traits Promoting Cancer Progression. In: Ryu, PD., LaManna, J., Harrison, D., Lee, SS. (eds) Oxygen Transport to Tissue XLI. Advances in Experimental Medicine and Biology, Springer, Cham. 2020 vol 1232. [CrossRef]
- Kes M.M.G.; Van den Bossche J.; Griffioen A.W.; Huijbers E.J.M. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. BBA – Rev. Cancer. 2020, 1874, 188427 . [CrossRef]
- Singh L.; Nair L.; Kumar D.; Arora M.K.; Bajaj S.; Gadewar M.; Mishra S.S.; Rath S.K.; Dubey A.K.; Kaithwas G.; Choudhary M.; Singh M. Hypoxia induced lactate acidosis modulates tumor microenvironment and lipid reprogramming to sustain the cancer cell survival. Front. Oncol. 2023, 13, 1034205. [CrossRef]
- Wang J.; Matisevic S. Functional and metabolic targeting of natural killer cells to solid tumors. Cell Oncol. 2020, 43, 577–600. [CrossRef]
- Chen C.; Wang Z.; Ding Y.; Qin Y. Manipulating T-cell metabolism to enhance immunotherapy in solid tumor. Front. Immunol. 2022, 13, 1090429. [CrossRef]
- Goswami K.K.; Banerjee S.; Bose A.; Baral R. Lactic acid in alternative polarization and function of macrophages in tumor microenvironment. Human Immunol. 2022, 83, 409-417. [CrossRef]
- Koltai T. The complex relationship between multiple drug resistance and the tumor pH gradient: a review. Cancer Drug Resist. 2022, 5, 277. [CrossRef]
- Vaidya F.U.; Chhipa A.S.; Mishra V.; Gupta V.K.; Rawat S.G.; Kumar A.; Pathak C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Reports. 2022, 5, e1291. [CrossRef]
- AlSawaftah N.M.; Awad N.S.; Pitt W.G.; Husseini G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers. 2022, 14, 936. [CrossRef]
- Zhou W.; Jia Y.; Liu Y.; Chen Y.; Zhao P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics 2022, 14, 2346. [CrossRef]
- Yan Y.; Ding H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [CrossRef]
- Lim E.-K.; Chung B.H.; Chung S. Recent Advances in pH-Sensitive Polymeric Nanoparticles for Smart Drug Delivery in Cancer Therapy. Current Drug Targets 2018, 19, 300. [CrossRef]
- Sethuraman V.; Janakiraman K.; Krishnaswami V.; Kandasamy R. Recent Progress in Stimuli-Responsive Intelligent Nano Scale Drug Delivery Systems: A Special Focus Towards pH-Sensitive Systems. Current Drug Targets. 2021, 22, 947. [CrossRef]
- Corbet C.; Feron O. Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer. 2017, 17, 577. [CrossRef]
- Wang C.; Ding S.; Wang S.; Shi Z.; Pandey N.K.; Chudal L.; Wang L.; Zhang Z.; Wen Y.; Yao H.; Lin L.; Chen W.; Xiong L. Endogenous tumor microenvironment-responsive multifunctional nanoplatforms for precision cancer theranostics. Coord. Chem. Rev. 2021, 426, 213529. [CrossRef]
- Yadav A.S.; Venkata Radharani N.N.; Gorain M.; Bulbule A.; Shetti D.; Roy G.; Baby T.; Kundu G.C. RGD functionalized chitosan nanoparticle mediated targeted delivery of raloxifene selectively suppresses angiogenesis and tumor growth in breast cancer. Nanoscale 2020, 12, 10664. [CrossRef]
- Han Y.; Hu B.; Wang M.; Yang Y.; Zhang L.; Zhou J.; Chen J. pH-Sensitive tumor-targeted hyperbranched system based on glycogen nanoparticles for liver cancer therapy. Appl. Mater. Today. 2020, 18, 100521. [CrossRef]
- Palanikumar L.; Al-Hosani S.; Kalmouni M.; Nguyen V.P.; Ali L.; Pasricha R.; Barrera F.N.; Magzoub M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [CrossRef]
- Li Z.; Huang J.; Wu J. pH-Sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 2021, 9, 574. [CrossRef]
- Ding L.; Jiang Y.; Zhang J.; Klok H.-A.; Zhong Z. pH-Sensitive Coiled-Coil Peptide-Cross-Linked Hyaluronic Acid Nanogels: Synthesis and Targeted Intracellular Protein Delivery to CD44 Positive Cancer Cells. Biomacromolecules 2018, 19, 555. [CrossRef]
- Vinardell M.P.; Mitjans M. (2018). Metal/Metal Oxide Nanoparticles for Cancer Therapy. In: Gonçalves, G., Tobias, G. (eds) Nanooncology. Nanomedicine and Nanotoxicology. Springer, Cham. [CrossRef]
- Subhan MA. Advances with metal oxide-based nanoparticles as MDR metastatic breast cancer therapeutics and diagnostics. RSC Adv. 2022, 12, 32956. [CrossRef]
- Gao R.; Mitra R.N.; Zheng M.; Wang K.; Dahringer J.C.; Han Z. Developing Nanoceria-Based pH-Dependent Cancer-Directed Drug Delivery System for Retinoblastoma, Adv. Funct. Mater. 2018, 28, 1806248. [CrossRef]
- Yang G.; Xu L.; Chao Y.; Xu J.; Sun X.; Wu Y.; Peng R.; Liu Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [CrossRef]
- Chen Z.-X.; Liu M.-D.; Guo D.-K.; Zou M.-Z.; Wang S.-B.; Cheng H.; Zhong Z.; Zhang X.-Z. A MSN-Based Tumor-Targeted Nanoplatform to Interfere with Lactate Metabolism to Induce Tumor Cell Acidosis for Tumor Suppression and Anti-Metastasis, Nanoscale, 2020, 12, 2966. [CrossRef]
- Rauta P.R.; Mackeyev Y.; Sanders K.; Kim J.B.K.; Gonzalez V.V.; Zahra Y.; Shohayeb M.A.; Abousaida B.; Vijay G.V.; Tezcan O.; Derry P.; Liopo A.V.; Zubarev E.R.; Carter R.; Singh P.; Krishnan S. Pancreatic tumor microenvironmental acidosis and hypoxia transform gold nanorods into cell-penetrant particles for potent radiosensitization. Sci. Adv. 2022, 8, eabm9729. [CrossRef]
- Xue Y.; Tao L.; Zhou Y.; Liu J.; Yu B.; Long S.; Huang S.; Yu F. Enhanced Targeted Delivery of Doxorubicin Based on Acid Induced Charge Reversal and Combinational Stimuli-Responsive Nanocarrier. Adv. Eng. Mater. 2018, 20, 1701151. [CrossRef]
- Angelopoulou A.; Kolokithas-Ntoukas A.; Papaioannou L.; Kakazanis Z.; Khoury N.; Zoumpourlis V.; Papatheodorou S.; Kardamakis D.; Bakandritsos A.; Hatziantoniou S.; Avgoustakis K. Canagliflozin-loaded magnetic nanoparticles as potential treatment of hypoxic tumors in combination with radiotherapy. Nanomed. 2018, 13, 2435. [CrossRef]
- Ma Q.; Li Q.; Cai X.; Zhou P.; Wu Z.; Wang B.; Ma W.; Fu S. Injectable hydrogels as drug delivery platform for in-situ treatment of malignant tumor. J. Drug. Deliv. Sci. Technol. 2022, 76, 103817. [CrossRef]
- Deirram N.; Zhang C.; Kermaniyan S.S.; Johnston A.P.R.; Such G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [CrossRef]
- Sim T.M. Nanoparticle-assisted targeting of the tumour microenvironment, OpenNano. 2022, 8, 100097. [CrossRef]
- Wang Q.; Atluri K.; Tiwari A.K.; Babu R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [CrossRef]
- Qu J.; Zhao X.; Ma P.X.; Guo B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168. [CrossRef]
- Megahed M.A.; El-Sawy H.S.; Reda A.M.; Abd-Allah F.I.; Abu Elyazid S.K.; Lila A.E.; Ismael H.R.; El-Say H.M. Effect of nanovesicular surface-functionalization via chitosan and/or PEGylation on cytotoxicity of tamoxifen in induced-breast cancer model. Life Sci. 2022, 307, 120908. [CrossRef]
- Ivanova, D.; Tacheva, T.; Semkova, S.; Panovska, R.; Yaneva, Z. In Vitro Model for Evaluation of Cancer Cell Proliferative Activity under Simulated Acidosis and Using Chitosan Microparticles. Appl. Sci. 2022, 12, 12029. [CrossRef]
- Zhou T.; Lu W.; Mezhuev Y.; Lan M.; Li L.; Liu F.; Cai T.; Wu X.; Cai Y. A review of nanoparticle drug delivery systems responsive to endogenous breast cancer microenvironment. Eur. J. Pharm. Biopharm. 2021, 166, 30. [CrossRef]
- Zhao J.; Yan C.; Chen Z.; Liu J.; Song H.; Wang W.; Liu J.; Yang N.; Zhao Y.; Chen L. Dual-targeting nanoparticles with core-crosslinked and pH/redox bioresponsive properties for enhanced intracellular drug delivery. J. Colloid Interface Sci. 2019, 540, 66. [CrossRef]
- Yuan Y.-Y.; Mao C.-Q.; Du X.-J.; Du J.-Z.; Wang F.; Wang J. Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24, 5476. [CrossRef]
- Wang S.; Wang Z.; Yu G.; Zhou Z.; Jacobson O.; Liu Y.; Ma Y.; Zhang F.; Chen Z.-Y.; Chen X. Tumor-Specific Drug Release and Reactive Oxygen Species Generation for Cancer Chemo/Chemodynamic Combination Therapy. Adv. Sci. 2019, 6, 1801986. [CrossRef]
- Liang J.; Huang Q.; Hua C.; Hu J.; Chen B.; Wan J.; Hu Z.; Wang B. pH-Responsive Nanoparticles Loaded with Graphene Quantum Dots and Doxorubicin for Intracellular Imaging, Drug Delivery and Efficient Cancer Therapy. Chemistry Select 2019, 4, 6004. [CrossRef]
- Meng X.; Xu Y.; Lu Q.; Sun L.; An X.; Zhang J.; Chen J.; Dao Y.; Zhang Y.; Ning X. Ultrasound-responsive alkaline nanorobots for the treatment of lactic acidosis-mediated doxorubicin resistance. Nanoscale, 2020,12, 13801. [CrossRef]
- Shi Z.; Li Q.; Mei L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin. Chem. Lett. 2020, 31, 1345. [CrossRef]
- Wang T.; Wang D.; Liu J.; Feng B.; Zhou F.; Zhang H.; Zhou L.; Yin Q.; Zhang Z.; Cao Z.; Yu H.; Li Y. Acidity-Triggered Ligand-Presenting Nanoparticles To Overcome Sequential Drug Delivery Barriers to Tumors. Nano Lett. 2017, 17, 5429. [CrossRef]
- Liu S.; Luo X.; Liu S.; Xu P.; Wang J.; Hu Y. Acetazolamide-Loaded pH-Responsive Nanoparticles Alleviating Tumor Acidosis to Enhance Chemotherapy Effects. Macromol. Biosci. 2019, 19, 1800366. [CrossRef]
- Liu G.; Zhao X.; Zhang Y.; Xu J.; Xu J.; Li Y.; Min H.; Shi J.; Zhao Y.; Wei j.; Wang J.; Nie G. Engineering Biomimetic Platesomes for pH-Responsive Drug Delivery and Enhanced Antitumor Activity. Adv. Mater. 2019, 31, 1900795. [CrossRef]
- Luo X.; Cao J.; Yu J.; Dai D.; Jiang W.; Feng Y.; Hu Y. Regulating Acidosis and Relieving Hypoxia by Platelet Membrane-Coated Nanoparticle for Enhancing Tumor Chemotherapy. Front. Bioeng. Biotechnol. 2022, 10, 885105. [CrossRef]
- Overchuk M.; Zheng G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials. 2018, 156, 217. [CrossRef]
- Li Y.: Tang K.; Zhang X.; Pan W.; Li N.; Tang B. Tumor microenvironment responsive nanocarriers for gene therapy. Chem Commun. 2022, 58, 8754. [CrossRef]
- Lee S.H.; Park O.K.; Kim J.; Shin K.; Pack C.G.; Kim K.; Ko G.; Lee N.; Kwon S.-H.; Hyeon T. Deep Tumor Penetration of Drug-Loaded Nanoparticles by Click Reaction-Assisted Immune Cell Targeting Strategy. J. Am. Chem. Soc. 2019, 141, 13829. [CrossRef]
- Chen R.; Ma Z.; Xiang Z.; Xia Y.; Shi Q.; Wong S.-C.; Yin J. Hydrogen Peroxide and Glutathione Dual Redox-Responsive Nanoparticles for Controlled DOX Release. Macromol. Biosci. 2020, 20, 1900331. [CrossRef]
- Chen X.; Jia F.; Li Y.; Deng Y.; Huang Y.; Liu W.; Jin Q.; Ji J. Nitric oxide-induced stromal depletion for improved nanoparticle penetration in pancreatic cancer treatment. Biomaterials. 2020, 246, 119999. [CrossRef]
- Dabaghi M.; Rasa S.M.M.; Cirri E.; Ori A.; Neri F.; Quaas R.; Hilger I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress, and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics 2021, 13, 1625. [CrossRef]
- Paholak H.J.; Stevers N.O.; Chen H.; Burnett J.P.; He M.; Korkaya H.; McDermott S.P.; Deol Y.; Clouthier S.G.; Luther T.; Li Q.; Wicha M.S.; Sun D. Elimination of epithelial-like and mesenchymal-like breast cancer stem cells to inhibit metastasis following nanoparticle-mediated photothermal therapy. Biomaterials. 2016, 104, 145. [CrossRef]
- Tan T.; Wang Y.; Wang J.; Wang Z, Wang H.; Cao H.; Li J.; Zhang Z.; Wang S. Targeting peptide-decorated biomimetic lipoproteins improve deep penetration and cancer cells accessibility in solid tumor. Acta Pharm. Sin. B. 2020, 10, 529. [CrossRef]
- Kola P.; Nagesh P.K.B.; Roy P.K.; Deepak K.; Reis R.L.; Kundu S.C.; Mandal M. Innovative nanotheranostics: Smart nanoparticles based approach to overcome breast cancer stem cells mediated chemo- and radioresistances. Wiley Interdiscip. Rev.: Nanomed. 2023, 15, e1876. [CrossRef]
- Ruan S.; Huang Y.; He M.; Gao H. Advanced Biomaterials for Cell-Specific Modulation and Restore of Cancer Immunotherapy. Adv. Sci. 2022, 9, 2200027. [CrossRef]
- Cai L.; Xu J.; Yang Z.; Tong R.; Dong Z.; Wang C.; Leong K.W. Engineered biomaterials for cancer immunotherapy. Med. Comm. 2020, 1, 35. [CrossRef]
- Xie Y.-Q.; Wei L.; Tang L. Immunoengineering with biomaterials for enhanced cancer immunotherapy. WIREs Nanomed Nanobiotechnol. 2018, 10, e1506. [CrossRef]
- Bo Y.; Wang H. Biomaterial-Based In Situ Cancer Vaccines. Adv. Mater. 2023, 2210452, . [CrossRef]
- Abdou P.; Wang Z.; Chen Q.; Chan A.; Zhou D.R.; Gunadhi V.; Gu Z. Advances in engineering local drug delivery systems for cancer immunotherapy. WIREs Nanomed Nanobiotechnol. 2020, 12, e1632. [CrossRef]
- Hu L.; Cao Z.; Ma L.; Liu Z.; Liao G.; Wang J.; Shen S.; Li D.; Yang X. The potentiated checkpoint blockade immunotherapy by ROS-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials, 2019, 223, 119469. [CrossRef]
- Wang C.; Ye Y.; Hu Q.; Bellotti A.; Gu Z. Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Adv. Mater. 2017, 29, 1606036. [CrossRef]
- Rosalia R.A.; Cruz L.J.; van Duikeren S.; Tromp A.T.; Silva A.L.; Jiskoot W.; de Gruijl T.; Lowik C.; Oostendorp J.; van der Burg A.; Ossendorp F. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015, 40, 88. [CrossRef]
- Wang C.; Li P.; Liu L.I.; Pan H.; Li H.; Cai L.; Ma Y. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials. 2016, 79, 88. [CrossRef]
- Yan S.; Luo Z.; Li Z.; Wang Y.; Tao J.; Gong C.; Liu X. Improving Cancer Immunotherapy Outcomes Using Biomaterials. Angew. Chem. 2020, 132, 17484. [CrossRef]
- Yang J.; Zhang C. Regulation of cancer-immunity cycle and tumor microenvironment by nanobiomaterials to enhance tumor immunotherapy. WIREs Nanomed Nanobiotechnol. 2020, 12, e1612. [CrossRef]
| Carrier Type | Product Name | Therapeutic Agent | Cancer Type | Stage | Ref. |
|---|---|---|---|---|---|
| Liposomes | Zolsketil® | Doxorubicin | Metastatic breast cancer, advanced ovarian cancer, multiple myeloma, AIDS-related Kaposi’s sarcoma | Approved (EMA, 2022) | [56,57] |
| Vyxeos® | Cytarabine: daunorubicin |
Newly diagnosed therapy–related acute myeloid leukemia, acute myeloid leukemia with myelodysplasia related changes | Approved (EMA, 2018) (FDA, 2017) | [56,57] | |
| Onivyde® / CPX-351 | Irinotecan | Pancreatic cancer | Approved (EMA, 2016) (FDA, 1996) | [56,57] | |
| Mepact® | Mifamurtide | Osteosarcoma | Approved (EMA, 2009) | [56,57] | |
| Ameluz® | 5-aminolevulinic acid | Superficial and/or nodular basal cell carcinoma | Approved (EMA, 2011) | [56,57] | |
| DaunoXome® | Daunorubicin | Kaposi’s sarcoma | Approved (FDA 1996) | [56,57] | |
| Iron Oxide nanoparticles | NanoTherm® | Fe2O3 | Glioblastoma, prostate, and pancreatic cancer | Approved (EMA, 2013) | [57] |
| Albumin nanoparticles | Abraxane® | Paclitaxel | Metastatic breast cancer, locally advanced or metastatic non-small cell lung cancer, Metastatic adenocarcinoma of the pancreas |
Approved (EMA 2008) (FDA 2005) |
[57,58] |
| Pazenir® | Paclitaxel | Metastatic breast cancer, metastatic adenocarcinoma of the pancreas, non-small cell lung cancer | Approved (EMA 2019) | [58] | |
| Vaccines | Adstiladrin® | adenoviral vector-based gene therapy | Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors | Approved (FDA 2022) | [59] |
| Provenge® | autologous peripheral-blood mononuclear cells | metastatic castration-resistant prostate cancer (mCRPC) | Approved (EMA 2013) (FDA 2019) | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





