Submitted:
12 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
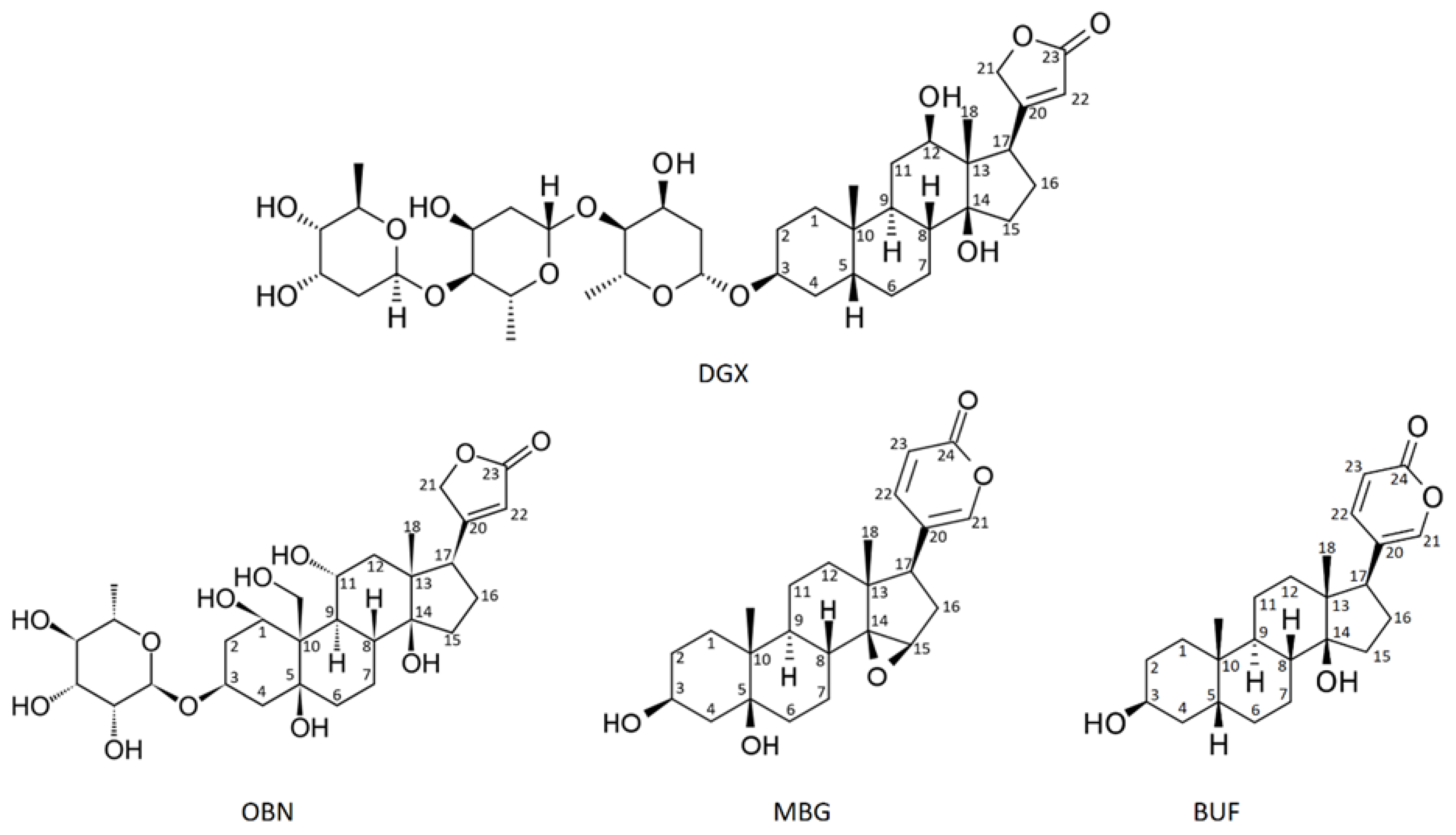
2. Cardiotonic steroids. Mechanism of action.
2.1. A brief history of CTS physiological effects studies
2.2. Current view of the mechanisms involved in CTS effects
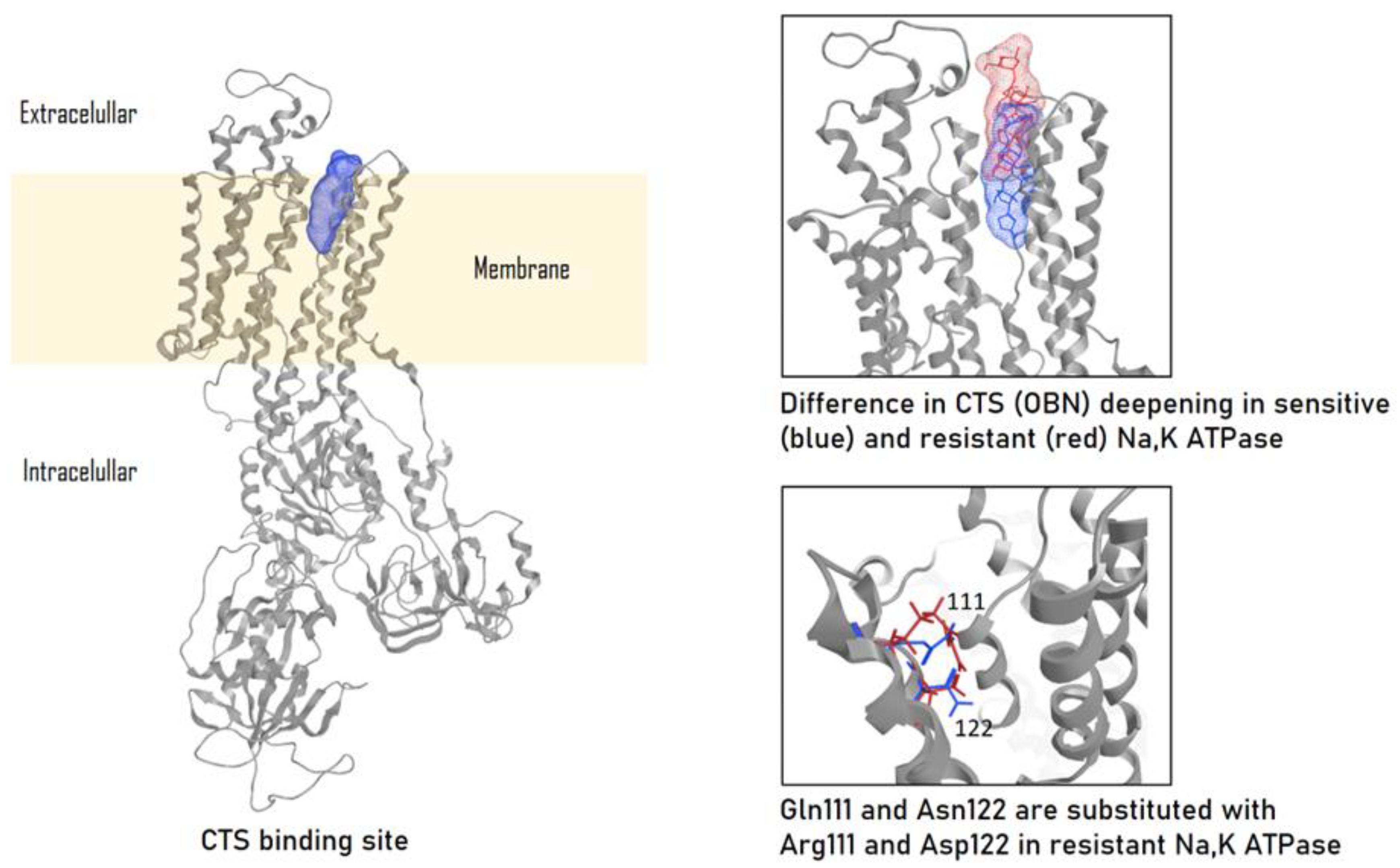
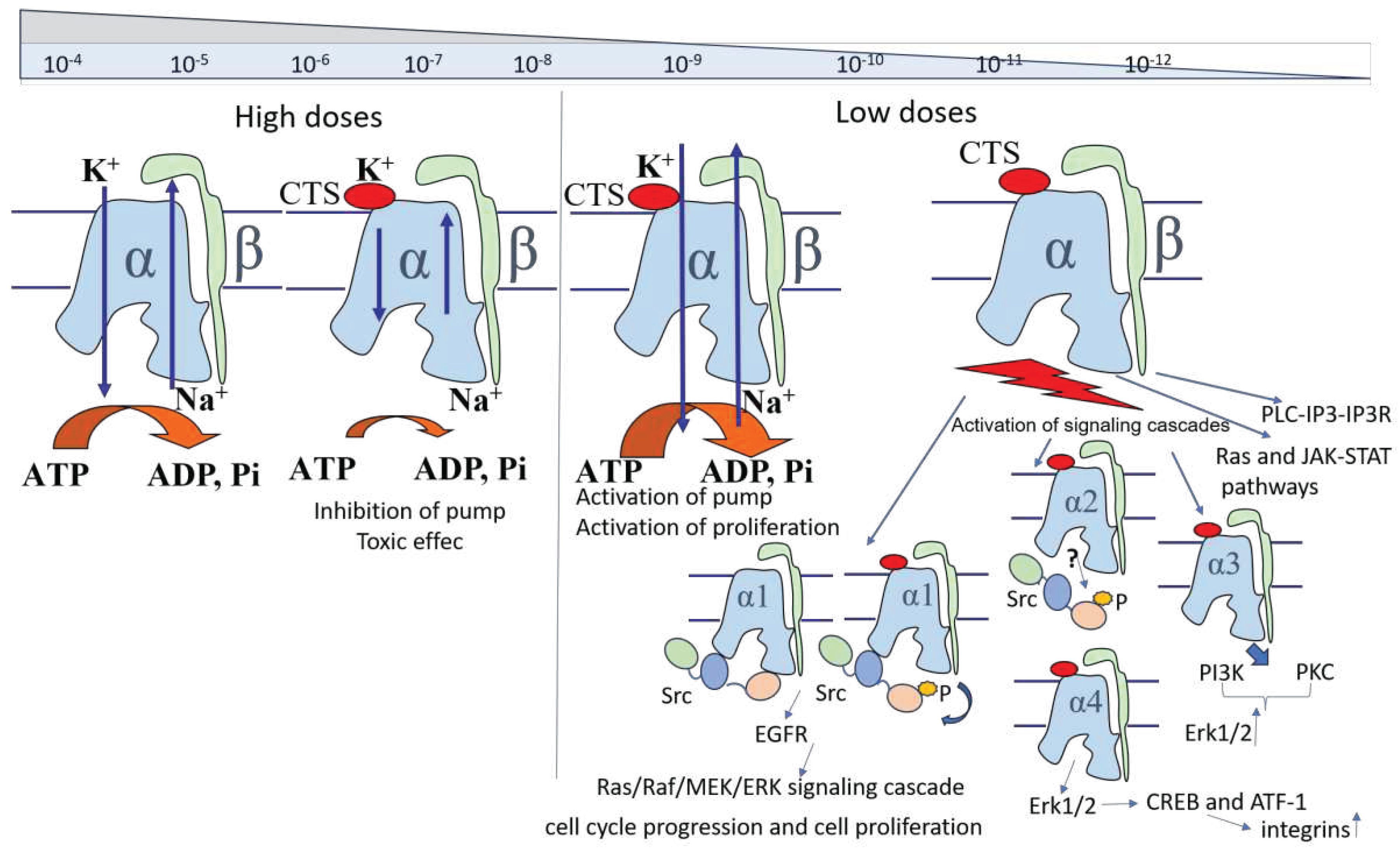
2.2.1. CTS binding to Na,K-ATPase provides its inhibition and activation of signaling cascades
2.2.2. CTS affinity to different isoforms of Na,K-ATPase
2.2.3. Endogenous CTS
2.2.4. Activating effect of CTS on Na,K-ATPase
2.2.5. Signaling cascades
3. Effect of CTS on blood cells
3.1. Distribution of Na,K-ATPase isoforms in blood cells
3.2. CTS effects on some immune diseases and blood immune cells
3.2.1. Leukocytes
3.2.1.1. Mononuclear cells/Macrophages
3.2.1.2. Neutrophils
3.2.1.3. Eosinophils
3.2.1.4. Lymphocytes3.2.1.4.1. NK-cells
3.2.1.4.2. T-helpers
3.2.1.4.3. Regulatory T-cells
3.2.1.4.4. T- cells CD8+ (cytotoxic T lymphocytes)
3.2.1.4.5. B cells
3.3. Red blood cells
3.4. Clotting and platelet dependent effects
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Orlov, S.N.; Tverskoi, A.M.; Sidorenko, S. V; Smolyaninova, L. V; Lopina, O.D.; Dulin, N.O.; Klimanova, E.A. Na, K-ATPase as a target for endogenous cardiotonic steroids: What’s the evidence? Genes Dis. 2021, 8, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; Dufrasne, F.; Kiss, R. Cardiotonic steroids-mediated targeting of the Na(+)/K(+)-ATPase to combat chemoresistant cancers. Curr. Med. Chem. 2012, 19, 627–646. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; Kiss, R. Cardiotonic steroids-mediated Na+/K+-ATPase targeting could circumvent various chemoresistance pathways. Planta Med. 2013, 79, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; Van Quaquebeke, E.; Delest, B.; Debeir, O.; Darro, F.; Kiss, R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim. Biophys. Acta 2007, 1776, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Triana-Martínez, F.; Picallos-Rabina, P.; Da Silva-Álvarez, S.; Pietrocola, F.; Llanos, S.; Rodilla, V.; Soprano, E.; Pedrosa, P.; Ferreirós, A.; Barradas, M.; et al. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Herranz, N.; Sun, B.; Wagner, V.; Gallage, S.; Guiho, R.; Wolter, K.; Pombo, J.; Irvine, E.E.; Innes, A.J.; et al. Cardiac glycosides are broad-spectrum senolytics. Nat. Metab. 2019, 1, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Hollman, A. Plants and cardiac glycosides. Br. Heart J. 1985, 54, 258. [Google Scholar] [CrossRef] [PubMed]
- Lichtstein, D.; Gati, I.; Ovadia, H. Digitalis-like compounds in the toad Bufo viridis: interactions with plasma proteins. J. Cardiovasc. Pharmacol. 1993, 22, S102–S105. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, J.M.; Blaustein, M.P.; Bova, S.; DuCharme, D.W.; Harris, D.W.; Mandel, F.; Mathews, W.R.; Ludens, J.H. Identification and characterization of a ouabain-like compound from human plasma. Proc. Natl. Acad. Sci. 1991, 88, 6259–6263. [Google Scholar] [CrossRef] [PubMed]
- Kieval, R.S.; Butler Jr, V.P.; Derguini, F.; Bruening, R.C.; Rosen, M.R. Cellular electrophysiologic effects of vertebrate digitalis-like substances. J. Am. Coll. Cardiol. 1988, 11, 637–643. [Google Scholar] [CrossRef]
- Kaplan, J.H. Biochemistry of na, K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M. V; Hilbers, F.; Poulsen, H. The structure and function of the Na, K-ATPase isoforms in health and disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.C.; Forte, J.G. Functional significance of the beta-subunit for heterodimeric P-type ATPases. J. Exp. Biol. 1995, 198, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.J.; Bijlani, S.; de Sautu, M.; Spontarelli, K.; Young, V.C.; Gatto, C.; Artigas, P. FXYD protein isoforms differentially modulate human Na/K pump function. J. Gen. Physiol. 2020, 152, e202012660. [Google Scholar] [CrossRef] [PubMed]
- Krupinski, T.; Beitel, G.J. Unexpected roles of the Na-K-ATPase and other ion transporters in cell junctions and tubulogenesis. Physiology 2009, 24, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Contreras, R.G.; Shoshani, L.; Larre, I. The Na+-K+-ATPase as self-adhesion molecule and hormone receptor. Am. J. Physiol. Physiol. 2012, 302, C473–C481. [Google Scholar] [CrossRef] [PubMed]
- Vagin, O.; Dada, L.A.; Tokhtaeva, E.; Sachs, G. The Na-K-ATPase α1β1 heterodimer as a cell adhesion molecule in epithelia. Am. J. Physiol. Physiol. 2012, 302, C1271–C1281. [Google Scholar] [CrossRef] [PubMed]
- Larre, I.; Ponce, A.; Franco, M.; Cereijido, M. The emergence of the concept of tight junctions and physiological regulation by ouabain. In Proceedings of the Seminars in cell & developmental biology; Elsevier, 2014; Vol. 36; pp. 149–156. [Google Scholar]
- Vagin, O.; Tokhtaeva, E.; Sachs, G. The role of the β1 subunit of the Na, K-ATPase and its glycosylation in cell-cell adhesion. J. Biol. Chem. 2006, 281, 39573–39587. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, J.; Kennedy, D.J. Regulation of cardiac remodeling by cardiac Na+/K+-ATPase isoforms. Front. Physiol. 2016, 7, 382. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.X.; Li, X.; Xie, Z. Regulation of renal function and structure by the signaling Na/K-ATPase. IUBMB Life 2013, 65, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Klimanova, E.A.; Petrushanko, I.Y.; Mitkevich, V.A.; Anashkina, A.A.; Orlov, S.N.; Makarov, A.A.; Lopina, O.D. Binding of ouabain and marinobufagenin leads to different structural changes in Na, K-ATPase and depends on the enzyme conformation. FEBS Lett. 2015, 589, 2668–2674. [Google Scholar] [CrossRef]
- El-Mallakh, R.S.; Brar, K.S.; Yeruva, R.R. Cardiac glycosides in human physiology and disease: update for entomologists. Insects 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Tverskoi, A.M.; Poluektov, Y.M.; Klimanova, E.A.; Mitkevich, V.A.; Makarov, A.A.; Orlov, S.N.; Petrushanko, I.Y.; Lopina, O.D. Depth of the steroid core location determines the mode of Na, K-ATPase inhibition by cardiotonic steroids. Int. J. Mol. Sci. 2021, 22, 13268. [Google Scholar] [CrossRef] [PubMed]
- Lingrel, J.B.; Croyle, M.L.; Woo, A.L.; Argüello, J.M. Ligand binding sites of Na, K-ATPase. Acta Physiol. Scand. Suppl. 1998, 643, 69–77. [Google Scholar] [PubMed]
- Akimova, O.A.; Bagrov, A.Y.; Lopina, O.D.; Kamernitsky, A. V; Tremblay, J.; Hamet, P.; Orlov, S.N. Cardiotonic steroids differentially affect intracellular Na+ and [Na+] i/[K+] i-independent signaling in C7-MDCK cells. J. Biol. Chem. 2005, 280, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Maixent, J.M.; Charlemagne, D.; De La Chapelle, B.; Lelievre, L.G. Two Na, K-ATPase isoenzymes in canine cardiac myocytes. Molecular basis of inotropic and toxic effects of digitalis. J. Biol. Chem. 1987, 262, 6842–6848. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Melton, R.J.; Sánchez, G.; Mercer, R.W. Functional Characterization of a Testes-Specific α-Subunit Isoform of the Sodium/Potassium Adenosinetriphosphatase†. Biochemistry 1999, 38, 13661–13669. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Avila, J.; Cózar-Castellano, I.; Brownleader, M.D.; Trevan, M.; Francis, M.J.O.; Lamb, J.F.; Martín-Vasallo, P. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci. Rep. 2000, 20, 51–91. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Blaustein, M.P. Distinct Distribution of Different Na+ Pump α Subunit Isoforms in Plasmalemma: Physiological Implications a. Ann. N. Y. Acad. Sci. 1997, 834, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Crambert, G.; Hasler, U.; Beggah, A.T.; Yu, C.; Modyanov, N.N.; Horisberger, J.-D.; Lelievre, L.; Geering, K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J. Biol. Chem. 2000, 275, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.R.; Assentoft, M.; Cotrina, M.L.; Hua, S.Z.; Nedergaard, M.; Kaila, K.; Voipio, J.; MacAulay, N. Contributions of the Na+/K+-ATPase, NKCC1, and Kir4. 1 to hippocampal K+ clearance and volume responses. Glia 2014, 62, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Azarias, G.; Kruusmägi, M.; Connor, S.; Akkuratov, E.E.; Liu, X.-L.; Lyons, D.; Brismar, H.; Broberger, C.; Aperia, A. A specific and essential role for Na, K-ATPase α3 in neurons co-expressing α1 and α3. J. Biol. Chem. 2013, 288, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Zahler, R.; Zhang, Z.-T.; Manor, M.; Boron, W.F. Sodium kinetics of Na, K-ATPase α isoforms in intact transfected HeLa cells. J. Gen. Physiol. 1997, 110, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am. J. Physiol. - Ren. Physiol. 1998, 275, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Petrushanko, I.Y.; Yakushev, S.; Mitkevich, V. a.; Kamanina, Y. V.; Ziganshin, R.H.; Meng, X.; Anashkina, A. a.; Makhro, A.; Lopina, O.D.; Gassmann, M.; et al. S-glutathionylation of the Na,K-ATPase catalytic α subunit is a determinant of the enzyme redox sensitivity. J. Biol. Chem. 2012, 287, 32195–32205. [Google Scholar] [CrossRef] [PubMed]
- Xianyu, M.; Petrushanko, I.Y.; Klimanova, E.A.; Dergousova, E.A.; Lopina, O.D. Glutathionylation of the alpha-subunit of Na, K-ATPase from rat heart by oxidized glutathione inhibits the enzyme. Biochem. 2014, 79, 158–164. [Google Scholar] [CrossRef]
- Bogdanova, A.; Petrushanko, I.; Boldyrev, A.; Gassmann, M. Oxygen- and redox-induced regulaton of the Na/K ATPase. Curr. Enzym. Inhib. 2006, 2, 37–59. [Google Scholar] [CrossRef]
- Segall, L.; Javaid, Z.Z.; Carl, S.L.; Lane, L.K.; Blostein, R. Structural basis for α1 versus α2 isoform-distinct behavior of the Na, K-ATPase. J. Biol. Chem. 2003, 278, 9027–9034. [Google Scholar] [CrossRef]
- Poluektov, Y.M.; Dergousova, E.A.; Lopina, O.D.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Na,K-ATPase α-subunit conformation determines glutathionylation efficiency. Biochem. Biophys. Res. Commun. 2019, 510. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Mascarenhas, S.; De Oliveira, A.D.S.; Amoedo, N.D.; Affonso-Mitidieri, O.R.; Rumjanek, F.D.; Rumjanek, V.M. Modulation of the immune system by ouabain. Ann. N. Y. Acad. Sci. 2009, 1153, 153–163. [Google Scholar] [CrossRef]
- Segall, L.; Lane, L.K.; Blostein, R. Insights into the structural basis for modulation of E1↔E2transitions by cytoplasmic domains of the Na,K-ATPase α subunit. In Proceedings of the Annals of the New York Academy of Sciences; 2003; Vol. 986; pp. 58–62. [Google Scholar]
- Xie, Z.; JackHays, M.; Wang, Y.; Periyasamy, S.M.; Blanco, G.; Huang, W.H.; Askari, A. Different oxidant sensitivities of the α1 and α2 isoforms of Na+/K+-ATPase expressed in baculovirus-infected insect cells. Biochem. Biophys. Res. Commun. 1995, 207, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Petrushanko, I.Y.; Yakushev, S.; Mitkevich, V.A.; Kamanina, Y. V.; Ziganshin, R.H.; Meng, X.; Anashkina, A.A.; Makhro, A.; Lopina, O.D.; Gassmann, M.; et al. S-glutathionylation of the Na,K-ATPase catalytic α subunit is a determinant of the enzyme redox sensitivity. J. Biol. Chem. 2012, 287, 32195–32205. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.L.; James, P.F.; Lingrel, J.B. Characterization of the fourth α isoform of the Na, K-ATPase. J. Membr. Biol. 1999, 169, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, K.; Sanchez, G.; Nguyen, A.N.; Enders, G.C.; Blanco, G. Different expression and activity of the α1 and α4 isoforms of the Na, K-ATPase during rat male germ cell ontogeny. Reproduction 2005, 130, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Lingrel, J.B. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na, K-ATPase. Annu. Rev. Physiol. 2010, 72, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Akimova, O.A.; Tverskoi, A.M.; Smolyaninova, L. V; Mongin, A.A.; Lopina, O.D.; La, J.; Dulin, N.O.; Orlov, S.N. Critical role of the α1-Na+, K+-ATPase subunit in insensitivity of rodent cells to cytotoxic action of ouabain. Apoptosis 2015, 20, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Ludens, J.H.; Clark, M.A.; DuCharme, D.W.; Harris, D.W.; Lutzke, B.S.; Mandel, F.; Mathews, W.R.; Sutter, D.M.; Hamlyn, J.M. Purification of an endogenous digitalislike factor from human plasma for structural analysis. Hypertension 1991, 17, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Fedorova, O. V; Dmitrieva, R.I.; Howald, W.N.; Hunter, A.P.; Kuznetsova, E.A.; Shpen, V.M. Characterization of a urinary bufodienolide Na+, K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension 1998, 31, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O. V; Doris, P.A.; Bagrov, A.Y. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin. Exp. Hypertens. 1998, 20, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Guo, J.; Itagaki, Y.; Bell, C.; Wang, Y.; Haupert Jr, G.T.; Magil, S.; Gallagher, R.T.; Berova, N.; Nakanishi, K. On the structure of endogenous ouabain. Proc. Natl. Acad. Sci. 1999, 96, 6654–6659. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Wray, V.; Nimtz, M.; Lehmann, W.D.; Kirch, U.; Antolovic, R.; Schoner, W. Bovine adrenals contain, in addition to ouabain, a second inhibitor of the sodium pump. J. Biol. Chem. 1998, 273, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Ishiguro, T.; Yamada, K.; Ishii, M.; Yoshioka, M.; Eguchi, C.; Shimora, M.; Sugimoto, T. Isolation of a urinary digitalis-like factor indistinguishable from digoxin. Biochem. Biophys. Res. Commun. 1990, 173, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Yoshika, M.; Komiyama, Y.; Konishi, M.; Akizawa, T.; Kobayashi, T.; Date, M.; Kobatake, S.; Masuda, M.; Masaki, H.; Takahashi, H. Novel digitalis-like factor, marinobufotoxin, isolated from cultured Y-1 cells, and its hypertensive effect in rats. Hypertension 2007, 49, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Fedorova, O. V Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na+, K+-pump in human mesenteric arteries. J. Hypertens. 1998, 16, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, Y.; Dong, X.H.; Nishimura, N.; Masaki, H.; Yoshika, M.; Masuda, M.; Takahashi, H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 2005, 38, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Haddy, F.J.; Overbeck, H.W. The role of humoral agents in volume expanded hypertension. Life Sci. 1976, 19, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, U.; Dolev, S.; Werber, M.M.; Shapiro, M.S.; Shilo, L.; Shenkman, L. Identification and preliminary characterization of two human digitalis-like substances that are structurally related to digoxin and ouabain. Biochem. Biophys. Res. Commun. 1992, 188, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.K.; Yandle, T.G.; Lewis, J.G.; Richards, A.M.; Pidgeon, G.B.; Kaaja, R.J.; Nicholls, M.G. Ouabain is not detectable in human plasma. Hypertension 1994, 24, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Manunta, P.; Hamlyn, J.M.; Pavan, E.; De Toni, R.; Semplicini, A.; Pessina, A.C. Immunoreactive endogenous ouabain primary aldosteronism and essential hypertension: relationship with plasma renin, aldosterone and blood pressure levels. J. Hypertens. 1995, 13, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.S.; Rogowski, A.C.; Weinberg, M.; Krichten, C.M.; Hamilton, B.P.; Hamlyn, J.M. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation 1992, 86, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Gonick, H.C.; Ding, Y.; Vaziri, N.D.; Bagrov, A.Y.; Fedorova, O. V Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin. Exp. Hypertens. 1998, 20, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, D.A.; Ailamazian, E.K.; Dmitrieva, R.I.; Shpen, V.M.; Fedorova, O. V; Doris, P.A.; Bagrov, A.Y. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J. Hypertens. 1999, 17, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Staessen, J.A.; Messaggio, E.; Nawrot, T.; Fagard, R.; Hamlyn, J.M.; Bianchi, G.; Manunta, P. Salt, endogenous ouabain and blood pressure interactions in the general population. J. Hypertens. 2003, 21, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Tverskoi, A.M.; Sidorenko, S. V; Klimanova, E.A.; Akimova, O.A.; Smolyaninova, L. V; Lopina, O.D.; Orlov, S.N. Effects of ouabain on proliferation of human endothelial cells correlate with Na+, K+-ATPase activity and intracellular ratio of Na+ and K+. Biochem. 2016, 81, 876–883. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, C.; Haupert Jr, G.T. Hypoxia triggers release of an endogenous inhibitor of Na+-K+-ATPase from midbrain and adrenal. Am. J. Physiol. Physiol. 1998, 274, F182–F188. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am. J. Cardiovasc. drugs 2007, 7, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Haller, S.; Periyasamy, S.; Brewster, P.; Zhang, H.; Adlakha, S.; Fedorova, O. V; Xie, Z.; Bagrov, A.Y.; Shapiro, J.I. Renal ischemia regulates marinobufagenin release in humans. Hypertension 2010, 56, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Oselkin, M.; Tian, D.; Bergold, P.J. Low-dose cardiotonic steroids increase sodium–potassium ATPase activity that protects hippocampal slice cultures from experimental ischemia. Neurosci. Lett. 2010, 473, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Lakunina, V.A.; Burnysheva, K.M.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Changes in the receptor function of Na, K-ATPase during hypoxia and ischemia. Mol. Biol. 2017, 51, 148–154. [Google Scholar] [CrossRef]
- Petrushanko, I.Y.; Mitkevich, V.A.; Lakunina, V.A.; Anashkina, A.A.; Spirin, P. V; Rubtsov, P.M.; Prassolov, V.S.; Bogdanov, N.B.; Hänggi, P.; Fuller, W. Cysteine residues 244 and 458–459 within the catalytic subunit of Na, K-ATPase control the enzyme’s hydrolytic and signaling function under hypoxic conditions. Redox Biol. 2017, 13, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Duan, Q.; Xie, Z. SH2 ligand-like effects of second cytosolic domain of Na/K-ATPase α1 subunit on Src kinase. PLoS One 2015, 10, e0142119. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflügers Arch. J. Physiol. 2009, 457, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, L.; Tidow, H.; Clausen, M.J.; Nissen, P. Na+, K+-ATPase as a docking station: protein–protein complexes of the Na+, K+-ATPase. Cell. Mol. Life Sci. 2013, 70, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Karpova, L. V; Bulygina, E.R.; Boldyrev, A.A. Different neuronal Na+/K+-ATPase isoforms are involved in diverse signaling pathways. Cell Biochem. Funct. Cell. Biochem. its Modul. by Act. agents or Dis. 2010, 28, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Rognant, S.; Kravtsova, V. V.; Bouzinova, E. V.; Melnikova, E. V.; Krivoi, I.I.; Pierre, S. V.; Aalkjaer, C.; Jepps, T.A.; Matchkov, V. V. The microtubule network enables Src kinase interaction with the Na,K-ATPase to generate Ca2+ flashes in smooth muscle cells. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Tian, J.; Liu, L.; Pierre, S.; Liu, J.; Shapiro, J.; Xie, Z.J. Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 2007, 282, 10585–10593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Oh, D.; Dubey, A.K.; Yao, M.; Yang, B.; Groves, J.T.; Sheetz, M. EGFR family and Src family kinase interactions: mechanics matters? Curr. Opin. Cell Biol. 2018, 51, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Petrushanko, I.Y.; Tverskoi, A.M.; Barykin, E.P.; Petrovskaya, A. V; Strelkova, M.A.; Leonova, O.G.; Anashkina, A.A.; Tolstova, A.P.; Adzhubei, A.A.; Bogdanova, A.Y. Na, K-ATPase acts as a beta-amyloid receptor triggering src kinase activation. Cells 2022, 11, 2753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Yu, H.; Larre, I.; Dube, P.R.; Kennedy, D.J.; Wilson Tang, W.H.; Westfall, K.; Pierre, S. V.; Xie, Z.; et al. Regulation of Na/K-ATPase expression by cholesterol: isoform specificity and the molecular mechanism. Am. J. Physiol. Cell Physiol. 2020, 319, C1107–C1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Ouabain-induced endocytosis and signal transduction of the Na/K-ATPase. Front. Biosci. 2005, 10, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Sibarov, D.A.; Zhuravleva, Z.D.; Ilina, M.A.; Boikov, S.I.; Stepanenko, Y.D.; Karelina, T. V.; Antonov, S.M. Unveiling the Role of Cholesterol in Subnanomolar Ouabain Rescue of Cortical Neurons from Calcium Overload Caused by Excitotoxic Insults. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Akkuratov, E.E.; Westin, L.; Vazquez-Juarez, E.; de Marothy, M.; Melnikova, A.K.; Blom, H.; Lindskog, M.; Brismar, H.; Aperia, A. Ouabain Modulates the Functional Interaction Between Na,K-ATPase and NMDA Receptor. Mol. Neurobiol. 2020, 57, 4018–4030. [Google Scholar] [CrossRef] [PubMed]
- Madan, N.; Xu, Y.; Duan, Q.; Banerjee, M.; Larre, I.; Pierre, S. V; Xie, Z. Src-independent ERK signaling through the rat α3 isoform of Na/K-ATPase. Am. J. Physiol. Physiol. 2017, 312, C222–C232. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Akkuratov, E.E.; Bai, Y.; Gaskill, C.M.; Askari, A.; Liu, L. Cell signaling associated with Na(+)/K(+)-ATPase: activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry 2013, 52, 9059–9067. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Pinto, S.M.; Getnet, D.; Nirujogi, R.S.; Manda, S.S.; Chaerkady, R.; Madugundu, A.K.; Kelkar, D.S.; Isserlin, R.; Jain, S. A draft map of the human proteome. Nature 2014, 509, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A. Tissue-based map of the human proteome. Science 2015. [Google Scholar] [CrossRef] [PubMed]
- Kondělková, K.; Vokurková, D.; Krejsek, J.; Borská, L.; Fiala, Z.; Ctirad, A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta medica (Hradec Kral. 2010, 53, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, G.; Fei, J.; Wu, Y.; Yan, J. Bufotalin ameliorates experimental Sjögren’s syndrome development by inhibiting Th17 generation. Naunyn. Schmiedebergs. Arch. Pharmacol. 2020, 393, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.L.; Leite, J.A.; Alves, A.K.A.; Rodrigues, Y.K.S.; Amorim, F.M.; Néris, P.L.N.; Oliveira, M.R.; Rodrigues-Mascarenhas, S. Immunomodulatory activity of ouabain in Leishmania leishmania amazonensis-infected Swiss mice. Parasitol. Res. 2013, 112, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.C.M.; Cavalcante-Silva, L.H.A.; De A Lima, É.; Galvão, J.G.F.M.; De A Alves, A.K.; Feijó, P.R.O.; Quintas, L.E.M.; Rodrigues-Mascarenhas, S. Marinobufagenin Inhibits Neutrophil Migration and Proinflammatory Cytokines. J. Immunol. Res. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.P.; Rumjanek, V.M. Ouabain affects the expression of activation markers, cytokine production, and endocytosis of human monocytes. Mediators Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F.; Ramsdell, F.; Alderson, M.R. The activation antigen CD69. Stem Cells 1994, 12, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, I.M.; Tiniakos, D.; Kouloulias, V.; Zygogianni, A. The molecular basis of immuno-radiotherapy. Int. J. Radiat. Biol. 2023, 99, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Olona, A.; Hateley, C.; Guerrero, A.; Ko, J.H.; Johnson, M.R.; Anand, P.K.; Thomas, D.; Gil, J.; Behmoaras, J. Cardiac glycosides cause cytotoxicity in human macrophages and ameliorate white adipose tissue homeostasis. Br. J. Pharmacol. 2022, 179, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Aminuddin, A.; Kado, T.; Takikawa, A.; Yamamoto, S.; Tsuneyama, K.; Igarashi, Y.; Ikutani, M.; Nishida, Y.; Nagai, Y. CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 2017, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Nawaz, A.; Kado, T.; Bilal, M.; Kuwano, T.; Yamamoto, S.; Sasahara, M.; Jiuxiang, X.; Inujima, A.; Koizumi, K. Partial depletion of CD206-positive M2-like macrophages induces proliferation of beige progenitors and enhances browning after cold stimulation. Sci. Rep. 2018, 8, 14567. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; He, J.; Kisoh, K.; Hayashi, H.; Tanaka, S.; Si, N.; Zhao, H.Y.; Hirano, T.; Bian, B.; Takagi, N. Effects of active bufadienolide compounds on human cancer cells and CD4+CD25+Foxp3+ regulatory T cells in mitogen-activated human peripheral blood mononuclear cells. Oncol. Rep. 2016, 36, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Raviprakash, N.; Manna, S.K. Short-term exposure to oleandrin enhances responses to IL-8 by increasing cell surface IL-8 receptors. Br. J. Pharmacol. 2014, 171, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, F.K.; Dube, P.; Kleinhenz, A.L.; Malhotra, D.; Gohara, A.; Drummond, C.A.; Tian, J.; Haller, S.T.; Xie, Z.; Kennedy, D.J. Proinflammatory effects of cardiotonic steroids mediated by NKA α-1 (Na+/K+-ATPase α-1)/Src complex in renal epithelial cells and immune cells. Hypertension 2019, 74, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; de Almeida Lima, É.; Rodrigues-Mascarenhas, S. Ouabain inhibits p38 activation in mice neutrophils. Inflammopharmacology 2021, 29, 1829–1833. [Google Scholar] [CrossRef]
- Vieira, L.; Saldanha, A.A.; Moraes, A.M.; Oliveira, F.M. de; Lopes, D.O.; Barbosa, L.A. de O.; Ribeiro, R.I.M. de A.; Thomé, R.G.; Santos, H.B. dos; Villar, J.A.F.P.; et al. 21-Benzylidene digoxin, a novel digoxin hemi-synthetic derivative, presents an anti-inflammatory activity through inhibition of edema, tumour necrosis factor alpha production, inducible nitric oxide synthase expression and leucocyte migration. Int. Immunopharmacol. 2018, 65, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zhakeer, Z.; Hadeer, M.; Tuerxun, Z.; Tuerxun, K. Bufalin Inhibits the Inflammatory Effects in Asthmatic Mice through the Suppression of Nuclear Factor-Kappa B Activity. Pharmacology 2017, 99, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Yu, F.; Wu, W.; Liu, J.; Li, J.; Guo, F.; Xu, L.; Wang, F.; Cui, X. Bufalin enhances the killing efficacy of NK cells against hepatocellular carcinoma by inhibiting MICA shedding. Int. Immunopharmacol. 2021, 101. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Fu, B.D.; Shen, H.Q.; Yi, P.F.; Zhang, L.Y.; Lv, S.; Guo, X.; Xia, F.; Wu, Y.L.; Wei, X. Bin Telocinobufagin enhances the Th1 immune response and protects against Salmonella typhimurium infection. Int. Immunopharmacol. 2015, 25, 353–362. [Google Scholar] [CrossRef]
- Da Silva, J.M.C.; Azevedo, A.D.N.; Barbosa, R.P.D.S.; Teixeira, M.P.; Vianna, T.A.G.; Fittipaldi, J.; Cabral, V.R.; De Paiva, L.S. Ouabain Decreases Regulatory T Cell Number in Mice by Reducing IL-2 Secretion. Neuroimmunomodulation 2019, 26, 188–197. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.M.C.; Campos, M.L.A.; Teixeira, M.P.; da Silva Faustino, R.; Aleixo, R.C.; Cavalcante, F.J.P.; Gomes, L.R.O.; de Albuquerque, L.Z.; das Neves Azevedo, A.; Cabral, V.R.; et al. Ouabain pre-treatment modulates B and T lymphocytes and improves survival of melanoma-bearing animals. Int. Immunopharmacol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Baek, S.; Lee, J.; Lee, J.; Lee, D.G.; Park, M.K.; Cho, M. La; Park, S.H.; Kwok, S.K. Digoxin ameliorates autoimmune arthritis via suppression of Th17 differentiation. Int. Immunopharmacol. 2015, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.R.; Leung, M.W.L.; Huang, P.; Ryan, D.A.; Krout, M.R.; Malapaka, R.R.V.; Chow, J.; Manel, N.; Ciofani, M.; Kim, S. V.; et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 2011, 472, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kitani, A.; Fuss, I.; Strober, W. Cutting edge: regulatory T cells induce CD4+ CD25− Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J. Immunol. 2007, 178, 6725–6729. [Google Scholar] [CrossRef]
- Karaś, K.; Sałkowska, A.; Walczak-Drzewiecka, A.; Ryba, K.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. The cardenolides strophanthidin, digoxigenin and dihydroouabain act as activators of the human RORγ/RORγT receptors. Toxicol. Lett. 2018, 295, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, J.; Chen, S.; Meng, F.; Ning, J.; Sun, S. Oleandrin Induces Immunogenic Cell Death Via the PERK/elF2α/ATF4/CHOP Pathway in Breast Cancer. 2020.
- Rodrigues-Mascarenhas, S.; Dos Santos, N.F.; Rumjanek, V.M. Synergistic effect between ouabain and glucocorticoids for the induction of thymic atrophy. Biosci. Rep. 2006, 26, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Smolarczyk, R.; Cichoń, T.; Pilny, E.; Jarosz-Biej, M.; Poczkaj, A.; Kułach, N.; Szala, S. Combination of anti-vascular agent-DMXAA and HIF-1α inhibitor-digoxin inhibits the growth of melanoma tumors. Sci. Rep. 2018, 8, 7355. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Chen, L.; Li, L.; Huang, Y. Restoration and enhancement of immunogenic cell death of cisplatin by coadministration with digoxin and conjugation to HPMA copolymer. ACS Appl. Mater. Interfaces 2019, 12, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, L.S.; da Costa, K.M.; do Canto, F.B.; Cabral, V.R.; Fucs, R.; Nobrega, A.; Rumjanek, V.M. Modulation of mature B cells in mice following treatment with ouabain. Immunobiology 2011, 216, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.M.C.; das Neves Azevedo, A.; dos Santos Barbosa, R.P.; Vianna, T.A.G.; Fittipaldi, J.; Teixeira, M.P.; do Canto, F.B.; da Costa, K.M.; Pozzatti, R.R.; Cabral, V.R. Dynamics of murine B lymphocytes is modulated by in vivo treatment with steroid ouabain. Immunobiology 2016, 221, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-L.; Chou, J.-S.; Chen, Y.-L.; Hsueh, S.-C.; Chung, H.-Y.; Lee, M.-H.; Chen, C.-P.; Lee, M.-Z.; Hou, H.-T.; Lu, H.-F. Bufalin enhances immune responses in leukemic mice through enhancing phagocytosis of macrophage in vivo. In Vivo (Brooklyn). 2018, 32, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, L.; Smith, C.I.E.; Persson, U. Functional Characterization of Lanatoside-C-Responsive Cells. Scand. J. Immunol. 1978, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Radosinska, J.; Vrbjar, N. Erythrocyte deformability and Na, K-ATPase activity in various pathophysiological situations and their protection by selected nutritional antioxidants in humans. Int. J. Mol. Sci. 2021, 22, 11924. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, E.; Hasse, W. Quantitative aspects of ouabain binding to human erythrocyte and cardiac membranes. J. Physiol. 1975, 251, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Balzan, S.; D’Urso, G.; Nicolini, G.; Forini, F.; Pellegrino, M.; Montali, U. Erythrocyte sodium pump stimulation by ouabain and an endogenous ouabain-like factor. Cell Biochem. Funct. 2007, 25, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Lopina, O.D.; Tverskoi, A.M.; Klimanova, E.A.; Sidorenko, S.V.; Orlov, S.N. Ouabain-induced cell death and survival. Role of α1-Na, K-ATPase-mediated signaling and [Na+] i/[K+] i-dependent gene expression. Front. Physiol. 2020, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-D.; Ma, Y.-J.; Liu, Q.-F.; Ye, T.-Z.; Meng, F.-Y.; Zhou, Y.-W.; Yu, G.-P.; Yang, J.-P.; Jiang, H.; Wang, Q.-S. Human erythrocyte lifespan measured by Levitt’s CO breath test with newly developed automatic instrument. J. Breath Res. 2018, 12, 36003. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.D.; Chuang, J.; Chaudhry, M.; Nie, Y.; Bai, F.; Sodhi, K.; Liu, J.; Shapiro, J.I. The potential role of Na-K-ATPase and its signaling in the development of anemia in chronic kidney disease. Am. J. Physiol. Physiol. 2021, 320, F234–F242. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Mallozzi, C.; Di Stasi, A.M.M.; Minetti, M. Peroxynitrite-dependent upregulation of SRC kinases in red blood cells: strategies to study the activation mechanisms. Methods Enzymol. 2005, 396, 215–229. [Google Scholar] [PubMed]
- Hazegh, K.; Fang, F.; Kelly, K.; Sinchar, D.; Wang, L.; Zuchelkowski, B.E.; Ufelle, A.C.; Esparza, O.; Davizon-Castillo, P.; Page, G.P. Erythrocyte mitogen-activated protein kinases mediate hemolytic events under osmotic and oxidative stress and in hemolytic diseases. Cell. Signal. 2022, 99, 110450. [Google Scholar] [CrossRef] [PubMed]
- Fenk, S.; Melnikova, E. V; Anashkina, A.A.; Poluektov, Y.M.; Zaripov, P.I.; Mitkevich, V.A.; Tkachev, Y. V; Kaestner, L.; Minetti, G.; Mairbäurl, H. Hemoglobin is an oxygen-dependent glutathione buffer adapting the intracellular reduced glutathione levels to oxygen availability. Redox Biol. 2022, 58, 102535. [Google Scholar] [CrossRef] [PubMed]
- Zaripov, P.I.; Kuleshova, I.D.; Poluektov, Y.M.; Sidorenko, S. V; Kvan, O.K.; Maksimov, G. V; Mitckevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Metabolic Stress of Red Blood Cells Induces Hemoglobin Glutathionylation. Mol. Biol. 2023, 6, 1188–1198. [Google Scholar] [CrossRef]
- Emelyanov, I. V.; Konradi, A.O.; Lakatta, E.G.; Fedorova, O. V.; Bagrov, A.Y. Acute salt loading and cardiotonic steroids in resistant hypertension. Curr. Top. Membr. 2019, 83, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O. V; Fadeev, A. V; Grigorova, Y.N.; Marshall, C.A.; Zernetkina, V.; Kolodkin, N.I.; Agalakova, N.I.; Konradi, A.O.; Lakatta, E.G.; Bagrov, A.Y. Cardiotonic Steroids Induce Vascular Fibrosis Via Pressure-Independent Mechanism in NaCl-Loaded Diabetic Rats. J. Cardiovasc. Pharmacol. 2019, 74, 436. [Google Scholar] [CrossRef]
- Raccah, D.; Dadoun, F.; Coste, T.; Vague, P. Decreased Na/K ATPase ouabain binding sites in red blood cells of patients with insulin-dependent diabetes and healthy north African control subjects: relationship with diabetic neuropathy. Horm. Metab. Res. 1996, 28, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Castrellon, A.; Zambrano, J.P.; Jimenez, J.J.; Jy, W.; Horstman, L.L.; Willens, H.J.; Castellanos, A.; Myerburg, R.J.; Ahn, Y.S. Digoxin use is associated with increased platelet and endothelial cell activation in patients with nonvalvular atrial fibrillation. Hear. Rhythm 2005, 2, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Carnevale, R.; Nocella, C.; Bartimoccia, S.; Novo, M.; Cammisotto, V.; Piconese, S.; Santulli, M.; Vasaturo, F.; Violi, F.; et al. Digoxin and Platelet Activation in Patients With Atrial Fibrillation: In Vivo and In Vitro Study. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.; Hagberg, I.; Lyberg, T.; Gjesdal, K. Do cardiac glycosides affect platelet function? A flow cytometric study in healthy volunteers. Eur. J. Clin. Pharmacol. 2002, 58, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tomasiak, M.; Stelmach, H.; Rusak, T.; Ciborowski, M.; Radziwon, P. The involvement of Na+/K(+)-ATPase in the development of platelet procoagulant response. Acta Biochim. Pol. 2007, 54, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.D.; Brickman, C.R.; Cottrill, C.L.; Shapiro, J.I.; Liu, J. The Na/K-ATPase signaling: from specific ligands to general reactive oxygen species. Int. J. Mol. Sci. 2018, 19, 2600. [Google Scholar] [CrossRef] [PubMed]
- Paula, S.; Tabet, M.R.; Ball, W.J. Interactions between cardiac glycosides and sodium/potassium-ATPase: three-dimensional structure− activity relationship models for ligand binding to the E2-pi form of the enzyme versus activity inhibition. Biochemistry 2005, 44, 498–510. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.; Rimpelová, S. Cardiac glycosides as immune system modulators. Biomolecules 2021, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Upmanyu, N.; Dietze, R.; Kirch, U.; Scheiner-Bobis, G. Ouabain interactions with the α4 isoform of the sodium pump trigger non-classical steroid hormone signaling and integrin expression in spermatogenic cells. Biochim. Biophys. Acta (BBA)-Molecular Cell Res. 2016, 1863, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Rakhmetova, V.S.; Kapanova, G.; Tashenova, G.; Tulebayeva, A.; Akhenbekova, A.; Ibekenov, O.; Turgambayeva, A.; Xu, B. Bufalin-Mediated Regulation of Cell Signaling Pathways in Different Cancers: Spotlight on JAK/STAT, Wnt/β-Catenin, mTOR, TRAIL/TRAIL-R, and Non-Coding RNAs. Molecules 2023, 28, 2231. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cai, T. Na+-K+–ATPase-mediated signal transduction: from protein interaction to cellular function. Mol. Interv. 2003, 3, 157. [Google Scholar] [CrossRef] [PubMed]
- Poluektov, Y.M.; Petrushanko, I.Y.; Undrovinas, N.A.; Lakunina, V.A.; Khapchaev, A.Y.; Kapelko, V.I.; Abramov, A.A.; Lakomkin, V.L.; Novikov, M.S.; Shirinsky, V.P.; et al. Glutathione-related substances maintain cardiomyocyte contractile function in hypoxic conditions. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O. V.; Ishkaraeva, V. V.; Grigorova, Y.N.; Reznik, V.A.; Kolodkin, N.I.; Zazerskaya, I.E.; Zernetkina, V.; Agalakova, N.I.; Tapilskaya, N.I.; Adair, C.D.; et al. Antibody to Marinobufagenin Reverses Placenta-Induced Fibrosis of Umbilical Arteries in Preeclampsia. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O. V.; Emelianov, I. V.; Bagrov, K.A.; Grigorova, Y.N.; Wei, W.; Juhasz, O.; Frolova, E. V.; Marshall, C.A.; Lakatta, E.G.; Konradi, A.O.; et al. Marinobufagenin-induced vascular fibrosis is a likely target for mineralocorticoid antagonists. J. Hypertens. 2015, 33, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Espada, J.; Martín-Pérez, J. An update on Src family of nonreceptor tyrosine kinases biology. Int. Rev. Cell Mol. Biol. 2017, 331, 83–122. [Google Scholar] [PubMed]
- De Paiva, L.S.; Costa, K.M. da; Canto, F.B. do; Cabral, V.R.; Fucs, R.; Nobrega, A.; Rumjanek, V.M. Modulation of mature B cells in mice following treatment with ouabain. Immunobiology 2011, 216, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.M.C.; Campos, M.L.A.; Teixeira, M.P.; da Silva Faustino, R.; Aleixo, R.C.; Cavalcante, F.J.P.; Gomes, L.R.O.; de Albuquerque, L.Z.; das Neves Azevedo, A.; Cabral, V.R. Ouabain pre-treatment modulates B and T lymphocytes and improves survival of melanoma-bearing animals. Int. Immunopharmacol. 2020, 86, 106772. [Google Scholar] [CrossRef] [PubMed]
| Isoforms | Ouabain Sensitivity [29,41] |
K+ K 0.5 mM [31,32] |
Na+ K 0.5 mM [31,34,35] | Oxidation Sensitivity [37,38,42,43,44] |
|---|---|---|---|---|
| α 1 | low | 1 | 10 | |
| low | ||||
| α 2 | 3 | 10 | high | |
| intermediate[29,41]/high [29,41] | ||||
| α 3 | 1 | 25-50 | high | |
| high | ||||
| α 4 | 2.14 ±1.14*[45] | 9.13 ± 1.81*[45] | ||
| Low (300 нМ Кd) [45] Ki 1.6 ± 1 × 10− 9 M [46] |
5.9 +/- 1.1 [28] |
13.5 +/- 1.3 [28] |
| Cells/Alpha subunit | α1 | α2 | α3 | α4 |
|---|---|---|---|---|
| Red blood cells | +++ [87] | - [87,88] | ++ [87] | - [88] |
| Leucocytes | ||||
| Mononucleacytes/macrophages | +++ [87,88] | ++ [87] | ++ [87] | + [87] |
| Granular leucocytes | +? [88] | +- [88] | +- [88] | - [88] |
| Lymphocytes | ||||
| NK-cells | ++ [87] | + [87] | + [87] | + [87] |
| CD4 Cells | ++ [87] | ++ [87] | ++ [87] | + [87] |
| CD8 Cells | ++ [87] | ++ [87] | ++ [87] | + [87] |
| T-reg | +? [88] | +- [88] | +? [88] | +- [88] |
| Platelets | ++ [87] | + [87] | + [87] | + [87] |
| Cell type | CTS | Cellular and systematic effects |
|---|---|---|
| Erythrocytes | OBN | (1) Play an important role in the development of anemia in chronic kidney disease [126] through the increase in ROS levels, impaired erythrocyte deformability and lifespan reduction |
| MBG | (1) Released in response of hypervolemia [132] (2) An increase in endogenous marinobufagenin in rats with inducted 2 diabetes mellitus leads to a 30% decrease in erythrocyte Na,K-ATPase activity [132] |
|
| DGX | (1) Did not stimulate the Na,K-ATPase activity [123] | |
| Neutrophils |
OBN | (1) Inhibiting migration [97] (2) Reducing chemotaxis induced by chemotactic peptide fMLP [102] |
| MBG | (1) Inhibiting migration [92] | |
| 21-Benzylidene digoxin | (1) Inhibiting of TNF–α production release (anti-inflammatory and edema inhibiting effects) [103]. Decrease in the inducible nitric oxide synthase (iNOS) expression in the paw pads of mice | |
| BUF | (1) Attenuation of hyperresponsiveness. Decreased total number of inflammatory cells [104] | |
| Eosinophils | BUF | (1) Attenuation of hyperresponsiveness. Decreased total number of inflammatory cells [104] |
| Macrophages |
OBN | (1) Reduced TNF-α and IFN-γ levels [91] (2) Cell death of monocyte-derived macrophages [100] (dose-dependent toxic effect on human macrophages) (3) Produced higher levels of IL-1 β and TNF- α, IL-10 and VEGF. Increased expression of surface activation markers [99] (4) Decrease of macrophage mannose receptor CD206 (marker for adipose tissue macrophages) [97] |
| MBG | (1) Attenuation of proinflammatory cytokines [92] | |
| Oleandrin | (1) Enhanced biological responses to IL-8, without cytoxicity [102] | |
| Telocinobufagin | (1) Induce oxidative burst and enhanced NF-KB activation. [101] | |
| Monocytes | MBG | (1) Reduced levels of proinflammatory cytokines IL-1β, IL-6, and TNF- α [92]. |
| NK-cells | Oleandrin | (1) Balancing stimulating and inhibitory receptors on the surface of NK cells and indirectly activates NK cells by inhibiting MICA shedding [105] |
| B-lymphocytes | OBN | (1) Decrease in the bone marrow cellularity with diminution of the mature B cell subpopulation while the other B cell subpopulations were preserved [148] (2) Maintenance of the number and percentage of B lymphocytes in peripheral organs of melanoma-injected mice [108] |
| T-killers | OBN | (1) Did not alter the percentage and absolute numbers of CD8+T lymphocytes [108] |
| T-helpers | OBN | (1) Reduced number of CD4+ T-lymphocytes in the spleen [107] (2) Did not alter the percentage and absolute numbers of CD4+T lymphocytes [108] |
| Telocinobufagin | (1) Enhancing a Th1 immune response to control intracellular infections [106] | |
| T-regs | OBN | (1) Reduced number by decreased Il-2 production by T-lymphocytes [107] (2) Did not alter the percentage and absolute numbers of CD4+T lymphocytes [108] (3) |
| DihydroOBN | (1) Upregulation of IL17A and IL17F expression and enhanced IL17 secretion [112] | |
| DGX | (1) Reduced expression of proinflammatory cytokines. Can regulate Th17 and reciprocally promote Treg cells and suppress joint inflammation and bone erosion in CIA. [109] (2) Reduced in vitro differentiation and LPS-stimulated IgG production. Suppression of joint inflammation and bone erosion in CIA [109] (3) Upregulation of IL17A and IL17F expression and enhanced IL17 secretion [112] (4) Inhibiting RORγt translational activity. [110] |
|
| BUF | (1) Inhibiting polarization [90] (2) Inhibiting secretion of cytokines IL-17 and IFN-γ [90] |
|
| Strophantin | (1) Upregulation of IL17A and IL17F expression and enhanced IL17 secretion [112] | |
| Gamabufotalin | (1) Downregulation of the percentages of CD4+CD25+Foxp3+ Treg cells in mitogen-activated PBMCs [99] | |
| CD8+ cells | OBN | (1) Induce the death of immature double positive lymphocytes (CD4+CD8+) [114]. |
| DGX | (1) Inhibition of the growth of melanoma tumors in murine model [115] (2) Reversed the inability of Cisplatin to trigger calreticulin exposure, and HPMA copolymer-amplified Cisplatin-induced ATP release in melanoma mice model [116] |
|
| Oleandrin | (1) Inhibite tumor growth and increase tumor-infiltrating lymphocytes including dendritic cells and T cells [113] | |
| B cells | OBN | (1) Decrease the level of B-cells in bone marrow, spleen and peripheral blood in 24 hours Immunobiology. [117] (2) Regulate the dynamic of В-lymphocyte settling in peripheral organs. [118]. (3) Pre-treatment modulates B lymphocytes and improves survival of melanoma-bearing animals.[149] |
| BUF | (1) Increased B-cell proliferation from leukemic BALB/c mice [119,120] | |
| Platelets | OBN | (1) Rise in membrane curvature leading to the generation of a procoagulant activity [137] (due to inefficiently operating Na+/K(+)-ATPase and increased expression of phosphatidylserine) |
| DGX | (1) Activation of platelets in thrombosis-prone patients with heart failure and/or atrial fibrillation [136] (2) Induced calcium mobilization [134] (3) Increased levels of endothelial and platelet activation [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





