Submitted:
14 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Microalgae
2.1. Nutritional Value
2.2. The Use of Spirulina in Pig Diet
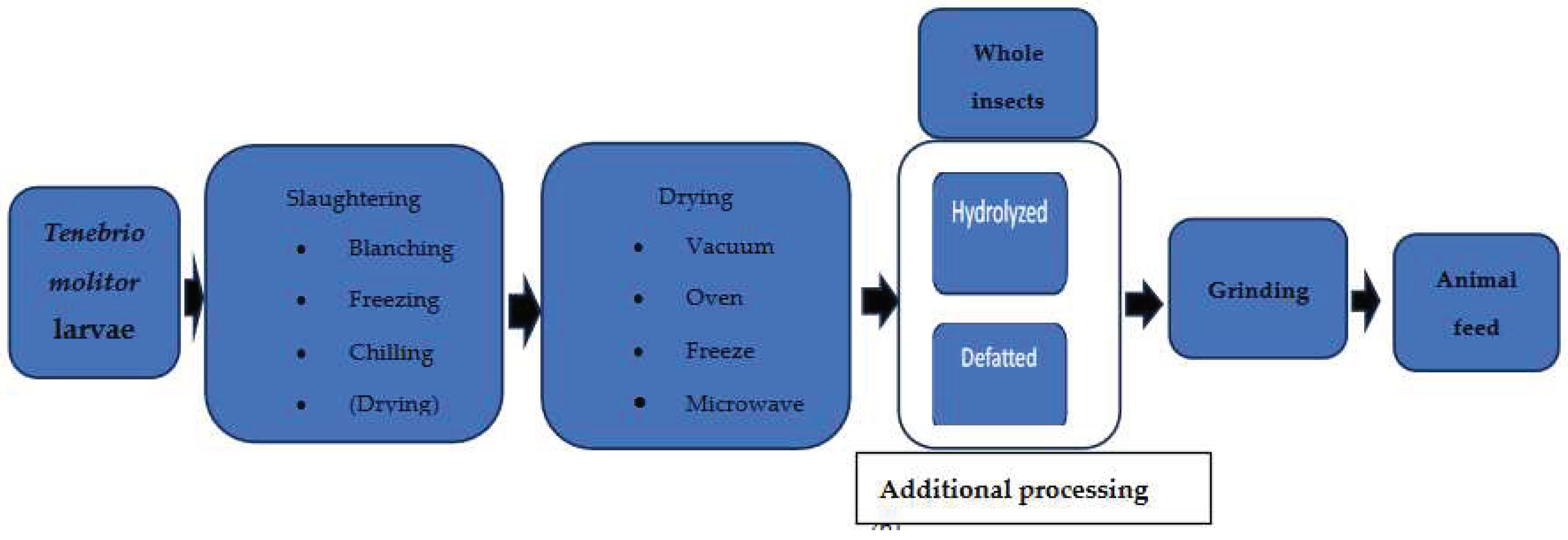
3. Insect Larvae
3.1. Nutritional Value of Insect Larvae
3.2. Safety Issues
3.3. Edible Insects in Studies of Swine
4. Rapeseed Meal and Legume Grains
4.1. Nutritional Value
4.2. The Use of Rapeseed Meal and Legume Grains in Pig Diets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). World Livestock 2011—Livestock in Food Security; FAO: Rome, Italy, 2011. [Google Scholar]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor Larvae) as an Alternative Protein Source for Monogastric Animal: A Review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef] [PubMed]
- Zmudzińska, A.; Bigorowski, B.; Banaszak, M.; Roślewska, A.; Adamski, M.; Hejdysz, M. The Effect of Diet Based on Legume Seeds and Rapeseed Meal on Pig Performance and Meat Quality. Animals 2020, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Grabež, V.; Egelandsdal, B.; Kjos, N.P.; Håkenåsen, I.M.; Mydland, L.T.; Vik, J.O.; Hallenstvedt, E.; Devle, H.; Øverland, M. Replacing soybean meal with rapeseed meal and faba beans in a growingfinishing pig diet: Effect on growth performance, meat quality and metabolite changes. Meat Sci. 2020, 166, 108134. [Google Scholar] [CrossRef] [PubMed]
- Dei, H.K. Soybean as a Feed Ingredient for Livestock and Poultry. In Recent Trends for Enhancing the Diversity and Quality of Soybean Products; Krezhova, D., Ed.; InTech. [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2012, 97, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Poppi, D.P.; McLennan, S.R. Nutritional research to meet future challenges. Anim. Prod. Sci. 2010, 50, 329–338. [Google Scholar] [CrossRef]
- Martins, C.F.; Pestana Assunção, J.; Ribeiro Santos, D.M.; Morgado dos S. Madeira, M.S.; Riscado Pereira Mateus Alfaia, C.M.; Brás Lopes, P.A.A.; Coelho, D.F.M.; Cardoso Lemos, J.P.; de Almeida, A.M.; Mestre Prates, J.A.; Bengala Freire, J.P. Effect of dietary inclusion of Spirulina on production performance, nutrient digestibility and meat quality traits in post-weaning piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 247–259. [Google Scholar] [CrossRef]
- Manceron, S.; Ben-Ari, T.; Dumas, P. Feeding proteins to livestock: global land use and food vs. feed competition. OCL 2014, 21, D408. [Google Scholar] [CrossRef]
- Al-Yahyaey, F.; Al-Marzooqi, W.; Shaat, I.; Smith, M.A.; Al-Sabahi, J.; Melak, S.; Russell, D.B. Effect of Spirulina platensis Supplementation on Carcass Characteristics, Fatty Acid Profile, and Meat Quality of Omani Goats. Animals 2023, 13, 2976. [Google Scholar] [CrossRef]
- Crosbie, M.; Zhu, C.; Shoveller, A.K.; Huber, L.-A. Standardized ileal digestible amino acids and net energy contents in full fat and defatted black soldier fly larvae meals (Hermetia illucens) fed to growing pigs. Transl. Anim. Sci. 2020, 4, txaa104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed. Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2016, 8, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Nedeva, R.; Jordanova, G.; Kistanova, E.; Shumkov, K.; Georgiev, B.; Abadgieva, D.; Kacheva, D.; Shimkus, A.; Shimkine, A. Effect of the addition of Spirulina platensis on the productivity and some blood parameters on growing pigs. Bulg. J. Agric. Sci. 2014, 20, 680–684. [Google Scholar]
- ly, M.S.; Amber, S.G.; El-Sayed, M.K. Production and application of Spirulina platensis rich in fatty acids, and vitamins. J. Am. Sci. 2011, 7, 36–45. [Google Scholar]
- Grinstead, G.S.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Nelssen, J.L. Effects of Spirulina platensis on growth performance of weanling pigs. Anim. Feed. Sci. Technol. 2000, 83, 237–247. [Google Scholar] [CrossRef]
- Belay, A.; Yoshimichi, O.; Miyakawa, K.; Shimamatsu, H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993, 5, 235–241. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.K.; Kim, J.; Lei, X.G. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J. Anim. Sci. Biotechnol. 2013, 21, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, P.; Meineri, G. Effects of diets with increasing levels of Spirulina platensis on the carcass characteristics, meat quality and fatty acid composition of growing rabbits. Livest. Sci. 2011, 140, 218–224. [Google Scholar] [CrossRef]
- Mustafa, A.F.; Thacker, P.A.; McKinnon, J.J.; Christensen, D.A.; Racz, V.J. Nutritional value of feed grade chickpeas for ruminants and pigs. J. Sci. Food Agric. 2000, 80, 1581–1588. [Google Scholar] [CrossRef]
- Lestingi, A.; Facciolongo, A.M.; Jambrenghi, A.C.; Ragni, M.; Toteda, F. The use of peas and sweet lupin seeds alone or in association for fattening lambs: Effects on performance, blood parameters and meat quality. Small Rumin. Res. 2016, 143, 15–23. [Google Scholar] [CrossRef]
- Lestingi, A.; Facciolongo, A.M.; De Marzo, D.; Nicastro, F.; Toteda, F. The use of faba bean and sweet lupin seeds in fattening lamb feed. 2. Effects on meat quality and fatty acid composition. Small Rumin. Res. 2015, 131, 2–5. [Google Scholar] [CrossRef]
- Lestingi, A.; Toteda, F.; Vicenti, A.; De Marzo, D.; Facciolongo, A.M. The Use of Faba Bean and Sweet Lupin Seeds Alone or in Combination for Growing Lambs. 1. Effects on Growth Performance, Carcass Traits, and Blood Parameters. Pak. J. Zool. 2015, 47, 989–996. [Google Scholar]
- He, Z.; Zhan, X.; Cao, S.; Wen, X.; Hou, L.; Liu, S.; Zheng, H.; Gao, K.; Yang, X.; Jiang, Z.; et al. Effect of Miscellaneous Meal Replacements for Soybean Meal on Growth Performance, Serum Biochemical Parameters, and Gut Microbiota of 50–75 kg Growing Pigs. Animals 2023, 13, 3499. [Google Scholar] [CrossRef] [PubMed]
- Skugor, A.; Kjos, N.P.; Sundaram, A.Y.M.; Mydland, L.T.; Ånestad, R.; Tauson, A.-H.; Øverland, M. Effects of long-term feeding of rapeseed meal on skeletal muscle transcriptome, production efficiency and meat quality traits in Norwegian Landrace growing-finishing pigs. PLOS ONE 2019, 14, e0220441. [Google Scholar] [CrossRef]
- Chen, C.; de Nanclares, M.P.; Kurtz, J.F.; Trudeau, M.P.; Wang, L.; Yao, D.; Saqui-Salces, M.; E Urriola, P.; Mydland, L.T.; Shurson, G.C.; et al. Identification of redox imbalance as a prominent metabolic response elicited by rapeseed feeding in swine metabolome1. J. Anim. Sci. 2018, 96, 1757–1768. [Google Scholar] [CrossRef]
- Jezierny, D.; Mosenthin, R.; Bauer, E. The use of grain legumes as a protein source in pig nutrition: A review. Anim. Feed. Sci. Technol. 2010, 157, 111–128. [Google Scholar] [CrossRef]
- Vadivel, V.; Pugalenthi, M. Effect of various processing methods on the levels of antinutritional constituents and protein digestibility of Mucuna pruriens (L.) DC. var. utilis (Wall. ex Wight) Baker ex Burck (velvet bean) seeds. J. Food Biochem. 2008, 32, 795–812. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Swiatkiewicz, M. Legume seeds and rapeseed press cake as substitutes for soybean meal in sow and piglet feed. Agric. Food Sci. 2013, 22, 435–444. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Świątkiewicz, M. Legume Seeds and Rapeseed Press Cake as Replacers of Soybean Meal in Feed for Fattening Pigs. Ann. Anim. Sci. 2014, 14, 921–934. [Google Scholar] [CrossRef]
- Ravindran, R.; Koopmans, S.; Sanders, J.P.M.; McMahon, H.; Gaffey, J. Production of Green Biorefinery Protein Concentrate Derived from Perennial Ryegrass as an Alternative Feed for Pigs. Clean Technol. 2021, 3, 656–669. [Google Scholar] [CrossRef]
- Kitada, K.; Machmudah, S.; Sasaki, M.; Goto, M.; Nakashima, Y.; Kumamoto, S.; Hasegawa, T. Antioxidant and Antibacterial Activity of Nutraceutical Compounds from Chlorella vulgaris Extracted in Hydrothermal Condition. Sep. Sci. Technol. 2009, 44, 1228–1239. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and Medical Applications of Spirulina Microalgae. Mini-Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Lugarà, R.; Realini, L.; Kreuzer, M.; Giller, K. Effects of maternal high-energy diet and spirulina supplementation in pregnant and lactating sows on performance, quality of carcass and meat, and its fatty acid profile in male and female offspring. Meat Sci. 2022, 187, 108769. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Navarro, N.; Yúfera, M.; García-Gallego, M. Use of freeze-dried microalgae for rearing gilthead seabream, Sparus aurata L., larvae. II. Biochemical composition. Hydrobiologia 2001, 452, 69–77. [Google Scholar] [CrossRef]
- Martínez-Fernández, E.; Acosta-Salmón, H.; Southgate, P.C. The nutritional value of seven species of tropical microalgae for black-lip pearl oyster (Pinctada margaritifera, L.) larvae. Aquaculture 2006, 257, 491–503. [Google Scholar] [CrossRef]
- Martínez-Fernández, E.; Southgate, P.C. Use of tropical microalgae as food for larvae of the black-lip pearl oyster Pinctada margaritifera. Aquaculture 2007, 263, 220–226. [Google Scholar] [CrossRef]
- Gutiérrez-Salmeán, G.; Fabila-Castillo, L.; Chamorro-Cevallos, G. Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. 2015, 32, 34–40. [Google Scholar] [PubMed]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kulpys, J.; Paulauskas, E.; Pilipavicius, V.; Stankevicius, R. Influence of cyanobacteria Arthrospira (Spirulina) platensis biomass additive towards the body condition of lactation cows and biochemical milk indexes. Agron. Res. 2009, 7, 823–835. [Google Scholar]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.B.; Sant’Anna, E.S. Cultivation of Arthrospira (Spirulina) platensis in desalinator wastewater and salinated synthetic medium: protein content and amino-acid profile. Braz. J. Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Siangdung, W.; Paithoonrangsarid, K.; Bunnag, B. Cultivation of Spirulina platensis using pig wastewater in a semi-continuous process. J. Microbiol. Biotechn. 2010, 20, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.A.; Richmond, A. Optimization of a growth medium for Spirulina based on cattle waste. Biol. Wastes 1988, 25, 41–50. [Google Scholar] [CrossRef]
- Hasdai, A.; Ben Ghedalia, D. Sewage-grown algae as a source of supplementary nitrogen for ruminants. J. Agric. Sci. 1981, 97, 533–537. [Google Scholar] [CrossRef]
- Gerken, H.G.; Donohoe, B.; Knoshaug, E.P. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 2012, 237, 239–253. [Google Scholar] [CrossRef]
- Popper, Z.A.; Tuohy, M.G. Beyond the Green: Understanding the Evolutionary Puzzle of Plant and Algal Cell Walls. Plant Physiol. 2010, 153, 373–383. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Ashraf, S.; Hisaindee, S.; Darmaki, N.A.; Battah, S.; Svistunenko, D.; Reeder, B.; Stanway, G.; Chaudhary, A. Enzymatic pre-treatment of microalgae cells for enhanced extraction of proteins. Eng. Life Sci. 2016, 17, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Furbeyre, H.; Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of dietary supplementation with freshwater microalgae on growth performance, nutrient digestibility and gut health in weaned piglets. Animal 2017, 11, 183–192. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Boudry, G.; Favier, C.; Le Floc’h, N.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, K.; Chen, X.; Xu, J. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Saeid, A.; Chojnacka, K.; Korczyński, M.; Korniewicz, D.; Dobrzański, Z. Effect on supplementation of Spirulina maxima enriched with Cu on production performance, metabolical and physiological parameters in fattening pigs. J. Appl. Phycol. 2013, 25, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Simkus, A.; Simkiene, A.; Cernauskiene, J.; Kvietkute, N.; Cernauskas, A.; Paleckaitis, M.; Kerziene, S. The effect of blue algae Spirulina platensis on pig growth performance and carcass and meat quality. Vet. ir Zootech. 2013, 61, 70–74. [Google Scholar]
- Evans, A.M.; Smith, D.L.; Moritz, J.S. Effects of algae incorporation into broiler starter diet formulations on nutrient digestibility and 3 to 21 d bird performance. J. Appl. Poult. Res. 2015, 24, 206–214. [Google Scholar] [CrossRef]
- Lestingi, A. Use of Wild Boar (Sus scrofa) as a Sustainable Alternative in Pork Production: A Review. Animals 2023, 13, 2258. [Google Scholar] [CrossRef] [PubMed]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to Recycle Organic Wastes and as Feed for Broiler Chickens. J. Econ. Èntomol. 2002, 95, 214–220. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed. Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Yoo, J.S.; Cho, K.H.; Hong, J.S.; Jang, H.S.; Chung, Y.H.; Kwon, G.T.; Shin, D.G.; Kim, Y.Y. Nutrient ileal digestibility evaluation of dried mealworm (Tenebrio molitor) larvae compared to three animal protein by-products in growing pigs. Asian-Australasian J. Anim. Sci. 2019, 32, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kang, S.W.; Yoo, J.S.; Song, D.K.; Chung, Y.H.; Kwon, G.T.; Kim, Y.Y. Effects of mealworm (Tenebrio molitor) larvae hydrolysate on nutrient ileal digestibility in growing pigs compared to those of defatted mealworm larvae meal, fermented poultry by-product, and hydrolyzed fish soluble. Asian -Australas. J. Anim. Sci. 2019, 33, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.H.; Heo, P.S.; Hong, J.S.; Kim, N.J.; Kim, Y.Y. Supplementation of Dried Mealworm (Tenebrio molitor larva) on Growth Performance, Nutrient Digestibility and Blood Profiles in Weaning Pigs. Asian-Australasian J. Anim. Sci. 2016, 29, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Benzertiha, A.; Kiero ´nczyk, B.; Kołodziejski, P.; Pruszy ´nska–Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Parsa, S. Determination of yellow mealworm (Tenebrio molitor) nutritional value as an animal and human food supplementation. Arthropods 2018, 7, 94–102. [Google Scholar]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Hussain, I.; Khan, S.; Sultan, A.; Chand, N.; Khan, R.; Alam, W.; Ahmad, N. Meal worm (Tenebrio molitor) as potential alternative source of protein supplementation in broiler. Int. J. Biosci. 2017, 10, 255–262. [Google Scholar]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhoutc, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]
- Stein, H.H.; Adeola, O.; Cromwell, G.L.; Kim, S.W.; Mahan, D.C.; Miller, P.S. Concentration of dietary calcium supplied by calcium carbonate does not affect the apparent total tract digestibility of calcium, but decreases digestibility of phosphorus by growing pigs1. J. Anim. Sci. 2011, 89, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.A.; Neumann, C.; Rothstein, S.; Liebert, F.; Mörlein, D. Do dietary soy alternatives lead to pork quality improvements or drawbacks? A look into micro-alga and insect protein in swine diets. Meat Sci. 2019, 153, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Jonas-Levi, A.; Martinez, J.-J.I. The high level of protein content reported in insects for food and feed is overestimated. J. Food Compos. Anal. 2017, 62, 184–188. [Google Scholar] [CrossRef]
- Huang, S.X.; Sauer, W.C.; Marty, B. Ileal digestibilities of neutral detergent fiber, crude protein, and amino acids associated with neutral detergent fiber in wheat shorts for growing pigs. J. Anim. Sci. 2001, 79, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.H.; Vincken, J.P.; van den Brock, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J Agri Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Nery, J.; Gasco, L.; Dabbou, S.; Schiavone, A. Protein composition and digestibility of black soldier fly larvae in broiler chickens revisited according to the recent nitrogen-protein conversion ratio. J. Insects Food Feed. 2018, 4, 171–177. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Feng, W.; Xiong, J.; Wang, T.; Wang, W.; Wang, C.; Yang, F. Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens L.) larvae: amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. Eur. Food Res. Technol. 2018, 245, 11–21. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Lee, J.-Y.; Hartl, D.; Elias, J.A. Chitin regulation of immune responses: an old molecule with new roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: a review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Kramer, K.J.; Hopkins, T.L.; Schaefer, J. Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem. Mol. Biol. 1995, 25, 1067–1080. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Wang, Z.Q.; Wang, Y.L.; Yan, S.M.; Shi, B.L. Effects of chitosan as growth promoter on diarrhea, nutrient apparent digestibility, fecal microbiota and immune response in weaned piglets. J. Appl. Anim. Res. 2018, 46, 1437–1442. [Google Scholar] [CrossRef]
- Valdés, F.; Villanueva, V.; Durán, E.; Campos, F.; Avendaño, C.; Sánchez, M.; Domingoz-Araujo, C.; Valenzuela, C. Insects as Feed for Companion and Exotic Pets: A Current Trend. Animals 2022, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Zuk-Gołaszewska, K.; Gał ˛ecki, R.; Obremski, K.; Smetana, S.; Figiel, S.; Gołaszewski, J. Edible Insect Farming in the Context of ˙ the EU Regulations and Marketing—An Overview. Insects 2022, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A. Insects as food and feed, a new emerging agricultural sector: a review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Attygalle, A.B.; Blankespoor, C.L.; Meinwald, J.; Eisner, T. Defensive secretion of Tenebrio molitor (Coleoptera: Tenebrionidae). J. Chem. Ecol. 1991, 17, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; Milanovi´c, V.; Garofalo, C.; Osimani, A.; Clementi, F.; Van Campenhout, L.; Aquilanti, L. Real-time PCR detection and quantification of selected transferable antibiotic resistance genes in fresh edible insects from Belgium and the Netherlands. Int. J. Food. Microbiol. 2019, 290, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional Value of Mealworm, Tenebrio molitor as Food Source. Int. J. Ind. Èntomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Gutierrez, J.M.; De Rijk, T.C.; De Nijs, W.C.; Van Loon, J.J. Degradation and excretion of the Fusarium toxin deoxynivalenol by an edible insect, the Yellow mealworm (Tenebrio molitor L.). World Mycotoxin J. 2017, 10, 163–169. [Google Scholar] [CrossRef]
- Camenzuli, L.; Van Dam, R.; De Rijk, T.; Andriessen, R.; Van Schelt, J.; der Fels-Klerx, V. Tolerance and excretion of the mycotoxins aflatoxin B1, zearalenone, deoxynivalenol, and ochratoxin A by Alphitobius diaperinus and Hermetia illucens from contaminated substrates. Toxins 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- AAFCO Association of American feed control officials. In: Proceedings of the AAFCO Annual Meeting Agenda and Committee Reports, Pittsburgh, PA, 31 July–3 August 2016. AAFCO: Pittsburgh, PA. p. 112.
- Cutrignelli, M.I.; Messina, M.; Tulli, F.; Randazzo, B.; Olivotto, I.; Gasco, L.; et al. Evaluation of an insect meal of the black soldier Fly (Hermetia illucens) as soybean substitute: intestinal morphometry, enzymatic and microbial activity in laying hens. Res. Ve.t Sci. 2018, 117, 209–15. [Google Scholar] [CrossRef] [PubMed]
- Maurer, V.; Holinger, M.; Amsler, Z.; Früh, B.; Wohlfahrt, J.; Stamer, A.; Leiber, F. Replacement of soybean cake by Hermetia illucens meal in diets for layers. J. Insects Food Feed. 2016, 2, 83–90. [Google Scholar] [CrossRef]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Renna, M.; Gai, F.; Dabbou, S.; Meneguz, M.; Perona, G.; Martinez, S.; Lajusticia, A.C.B.; Bergagna, S.; Sardi, L.; et al. Partially defatted black soldier fly larva meal inclusion in piglet diets: effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.; Tanga, C.; Osuga, I.; Alaru, A.; Mwangi, D.; Githinji, M.; Dubois, T.; Ekesi, S.; Van Loon, J.; Dicke, M. Black soldier fly larval meal in feed enhances growth performance, carcass yield and meat quality of finishing pigs. J. Insects Food Feed. 2021, 7, 433–447. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Li, J.; Ma, X. Use of Hermetia illucens larvae as a dietary protein source: Effects on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 2019, 158, 107837. [Google Scholar] [CrossRef] [PubMed]
- Ipema, A.F.; Gerrits, W.J.; Bokkers, E.A.; Kemp, B.; Bolhuis, J.E. Live black soldier fly larvae (Hermetia illucens) provisioning is a promising environmental enrichment for pigs as indicated by feed- and enrichment-preference tests. Appl. Anim. Behav. Sci. 2021, 244, 105481. [Google Scholar] [CrossRef]
- Cevolani, D. Prontuario degli alimenti per il suino. 75 Schede per valutare le materie prime; Edagricole: Bologna, Italy, 2004; pp. 308–310. [Google Scholar]
- Schöne, F.; Jahreis, G.; Lange, R.; Seffner, W.; Groppel, B.; Hennig, A. Effect of varying glucosinolate and iodine intake via rapeseed meal diets on serum thyroid hormone level and total iodine in the thyroid in growing pigs. Endocrinol. Exp. 1990, 24, 415–27.
- Mejicanos, G.; Sanjayan, N.; Kim, I.H.; Nyachoti, C.M. Recent advances in canola meal utilization in swine nutrition. J. Anim. Sci. Technol. 2016, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jansman, A.J.; Verstegen, M.W.A.; Huisman, J.; van den Berg, J.W. Effects of hulls of faba beans (Vicia faba L.) with a low or high content of condensed tannins on the apparent ileal and fecal digestibility of nutrients and the excretion of endogenous protein in ileal digesta and feces of pigs. J. Anim. Sci. 1995, 73, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Svetina, A.; Jerković, I.; Vrabac, L.; Ćurić, S. Thyroid function, metabolic indices and growth performance in pigs fed 00-rapeseed meal. Acta Veter- Hung. 2003, 51, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Lestingi, A.; Colonna, M.A.; Marsico, G.; Tarricone, S.; Facciolongo, A.M. Effects of legume seeds and processing treatment on growth, carcass traits and blood constituents of fattening lambs. S. Afr. J. Anim. Sci. 2019, 49, 799–809. [Google Scholar] [CrossRef]
- Batterham, E.S.; Saini, H.S.; Andersen, L.M.; Baigent, R.D. Tolerance of growing pigs to trypsin and chymotrypsin inhibitors in chickpeas (Cicer arietinum) and pigeonpeas (Cajanus cajan). J. Sci. Food Agric. 1993, 61, 211–216. [Google Scholar] [CrossRef]
- Jansman, A.J.M.; Huisman, J.; van der Poel, A.F.B. Ileal and faecal digestibility in piglets of field beans (Vicia faba L.) varying in tannin content. Anim. Feed. Sci. Technol. 1993, 42, 83–96. [Google Scholar] [CrossRef]
- Flis, M.; Sobotka, W.; Purwin, C.; Zduńczyk, Z. Nutritional value of diets containing field bean (Vicia faba L.) seeds with high or low proanthocyanidin levels for pig. J. Anim. Feed. Sci. 1999, 8, 171–180. [Google Scholar] [CrossRef]
- Dunshea, F.R.; Gannon, N.J.; van Barneveld, R.J.; Mullan, B.P.; Campbell, R.G.; King, R.H. Dietary lupins (Lupinus angustifolius and Lupinus albus) can increase digesta retention in the gastrointestinal tract of pigs. Aust. J. Agric. Res. 2001, 52, 593–602. [Google Scholar] [CrossRef]
- Stein, H.H.; Bohlke, R.A. The effects of thermal treatment of field peas (Pisum sativum L.) on nutrient and energy digestibility by growing pigs1. J. Anim. Sci. 2007, 85, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Asiedu, A.; Baidoo, S.K.; Nyachoti, C.M. Effect of heat processing on nutrient digestibility in pea and supplementing amylase and xylanase to raw, extruded or micronized pea-based diets on performance of early-weaned pigs. Can. J. Anim. Sci. 2002, 82, 367–374. [Google Scholar] [CrossRef]
- O’doherty, J.V.; Keady, U. The effect of expander processing and extrusion on the nutritive value of peas for pigs. Anim. Sci. 2001, 72, 43–53. [Google Scholar] [CrossRef]
- Froidmont, E.; Wathelet, B.; Beckers, Y.; Romnée, J.M.; Dehareng, F.; Wavreille, J.; Schoeling, O.; Decauwert, V.; Bartiaux-Thill, N. Improvement of lupin seed valorisation by the pig with the addition of -galactosidase in the feed and the choice of a suited variety. Biotechnol. Agron. Soc. Environ. 2005, 9, 225–235. [Google Scholar]
- Christodoulou, V.; Ambrosiadis, J.; Sossidou, E.; Bampidis, V.; Arkoudilos, J.; Hucko, B.; Iliadis, C. Effect of replacing soybean meal by extruded chickpeas in the diets of growing–finishing pigs on meat quality. Meat Sci. 2006, 73, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Partanen, K.; Alaviuhkola, T.; Siljander-Rasi, H.; Suomi, K. Faba beans in diets for growing-finishing pigs. Agric. Food Sci. 2003, 12, 35–47. [Google Scholar] [CrossRef]
- Skoufos, I.; Tzora, A.; Giannenas, I.; Bonos, E.; Papagiannis, N.; Tsinas, A.; Christaki, E.; Florou-Paneri, P. Dietary Inclusion of Rapeseed Meal as Soybean Meal Substitute on Growth Performance, Gut Microbiota, Oxidative Stability and Fatty Acid Profile in Growing-Fattening Pigs. Asian J. Anim. Veter- Adv. 2016, 11, 89–97. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Øverland, M.; Skrede, A.; Anderson, D.M.; Collins, S.A. A meta-analysis of the effects of dietary canola / double low rapeseed meal on growth performance of weanling and growing-finishing pigs. Anim. Feed. Sci. Technol. 2020, 259, 114302. [Google Scholar] [CrossRef]
| Parameter | C (n = 16) | HI (n = 15) | SP (n = 16) |
| Carcass weight (kg)1 | 95.08 (1.17) | 97.99 (1.21) | 93.11 (1.17) |
| Lean meat yield (%)1 | 59.52 (0.45) | 58.77 (0.46) | 59.05 (0.45) |
| GM pH45min | 6.08 (0.05) | 6.24 (0.05) | 5.97 (0.05) |
| LTL pH45min | 5.91 (0.08) | 6.05 (0.09) | 6.04 (0.09) |
| LTL pHu | 5.41 (0.02) | 5.40 (0.03) | 5.43 (0.03) |
| Cooking loss (%) | 32.4 (0.30) | 31.3 (0.30) | 32.4 (0.30) |
| Instrumental tenderness (N) | 10.78 (0.27) | 10.49 (0.30) | 10.51 (0.29) |
| Protein (%) | 23.10 (0.12) | 23.05 (0.13) | 22.93 (0.13) |
| Intramuscular fat (%) | 2.96 (0.19) | 3.27 (0.21) | 3.04 (0.20) |
| Water content (%) | 72.60 (0.16) | 72.02 (0.18) | 72.44 (0.17) |
| Backfat L* | 79.09a (0.27) | 78.17b (0.30) | 78.72ab (0.29) |
| Backfata * | 4.09 (0.18) | 4.26 (0.19) | 4.02 (0.19) |
| Backfatb * | 4.85 (0.20) | 5.15 (0.22) | 5.52 (0.21) |
| Lean colour L* | 63.18 (0.89) | 61.62 (0.97) | 62.96 (0.93) |
| Lean coloura * | 2.74 (0.23) | 3.56 (0.25) | 2.94 (0.24) |
| Lean colourb * | 13.84 (0.30) | 13.77 (0.33) | 13.71 (0.32) |
| TBARS (μg/g) | 0.357 (0.043) | 0.399 (0.047) | 0.470 (0.047)2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




