Submitted:
14 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Metabolomic Study
2.1.1. T0 vs T28 paired analysis
2.1.2. T0 vs T56 paired analysis
2.1.3. T0 vs T84 paired analysis
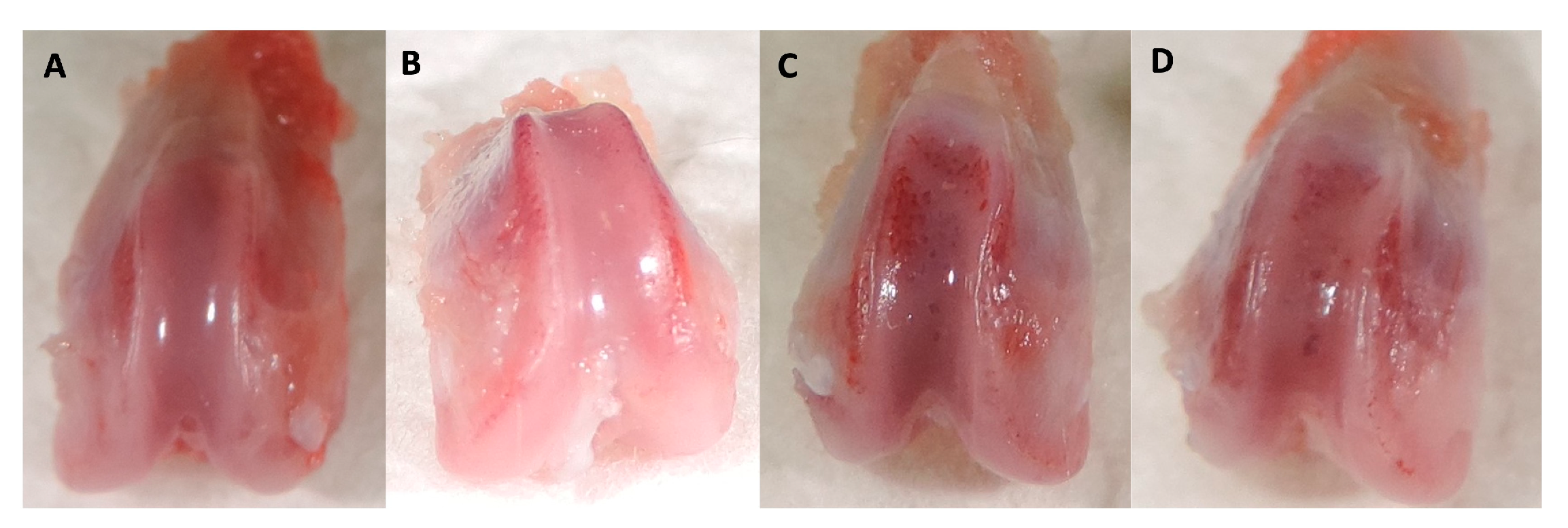
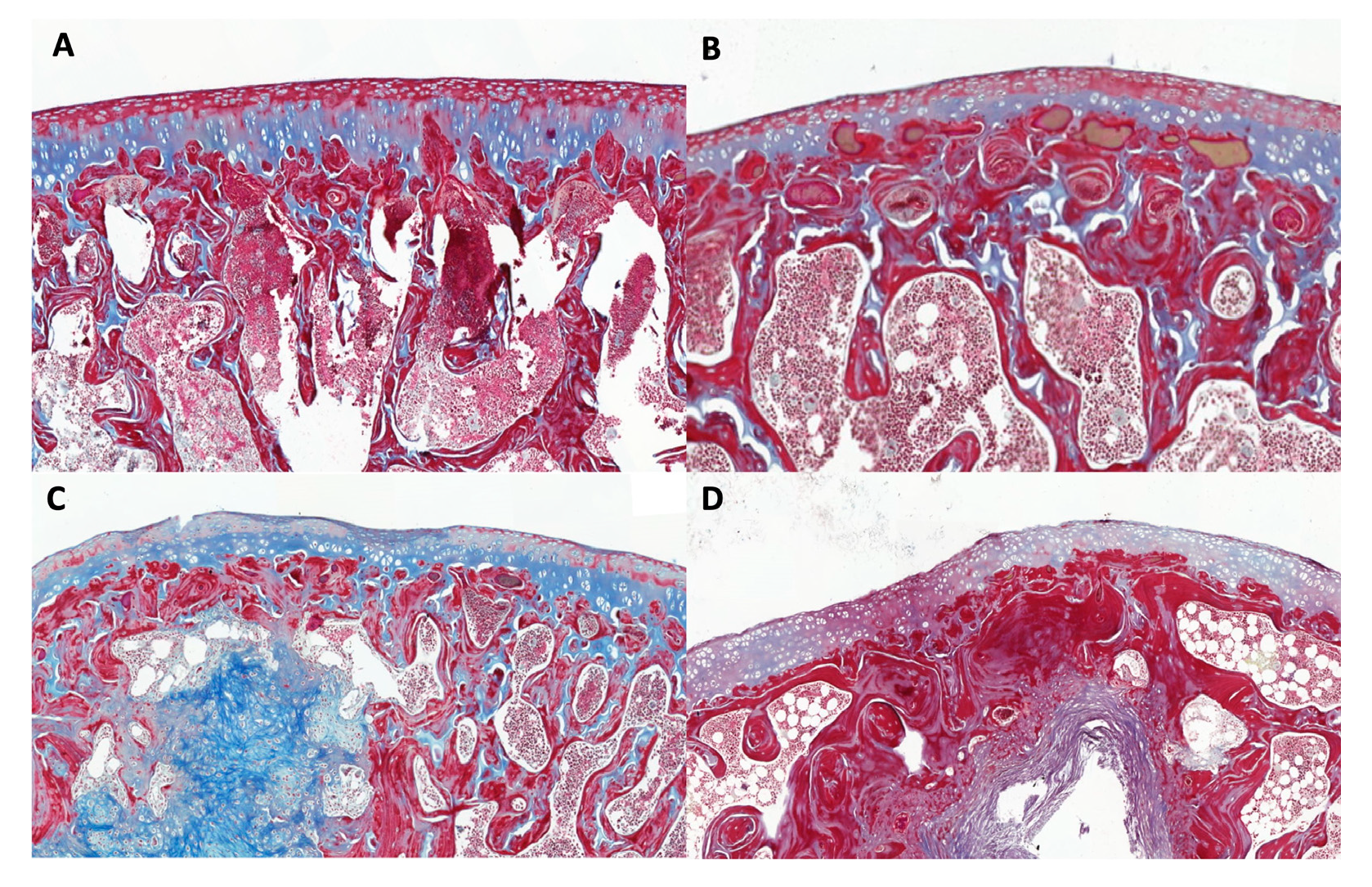
2.2. Histologic study
3. Discussion
4. Materials and Methods
4.1. Experimental model:
4.1.1. Experimental design:
4.1.2. Experimental trial:
4.2. Obtaining the metabolomic results:
4.2.1. Sample preparation:
4.2.2. Sample analysis:
4.2.3. Ultra-performance liquid chromatography, time-of-flight, mass spectrometry (UPLC-ToF-MS) method
4.3. Data analysis of metabolomic results:
4.3.1. Pre-processing of the metabolome data:
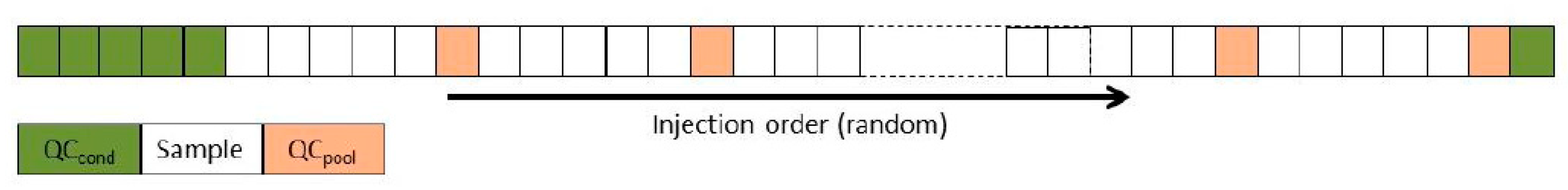
4.3.2. Analysis of the quality of the metabolome results:
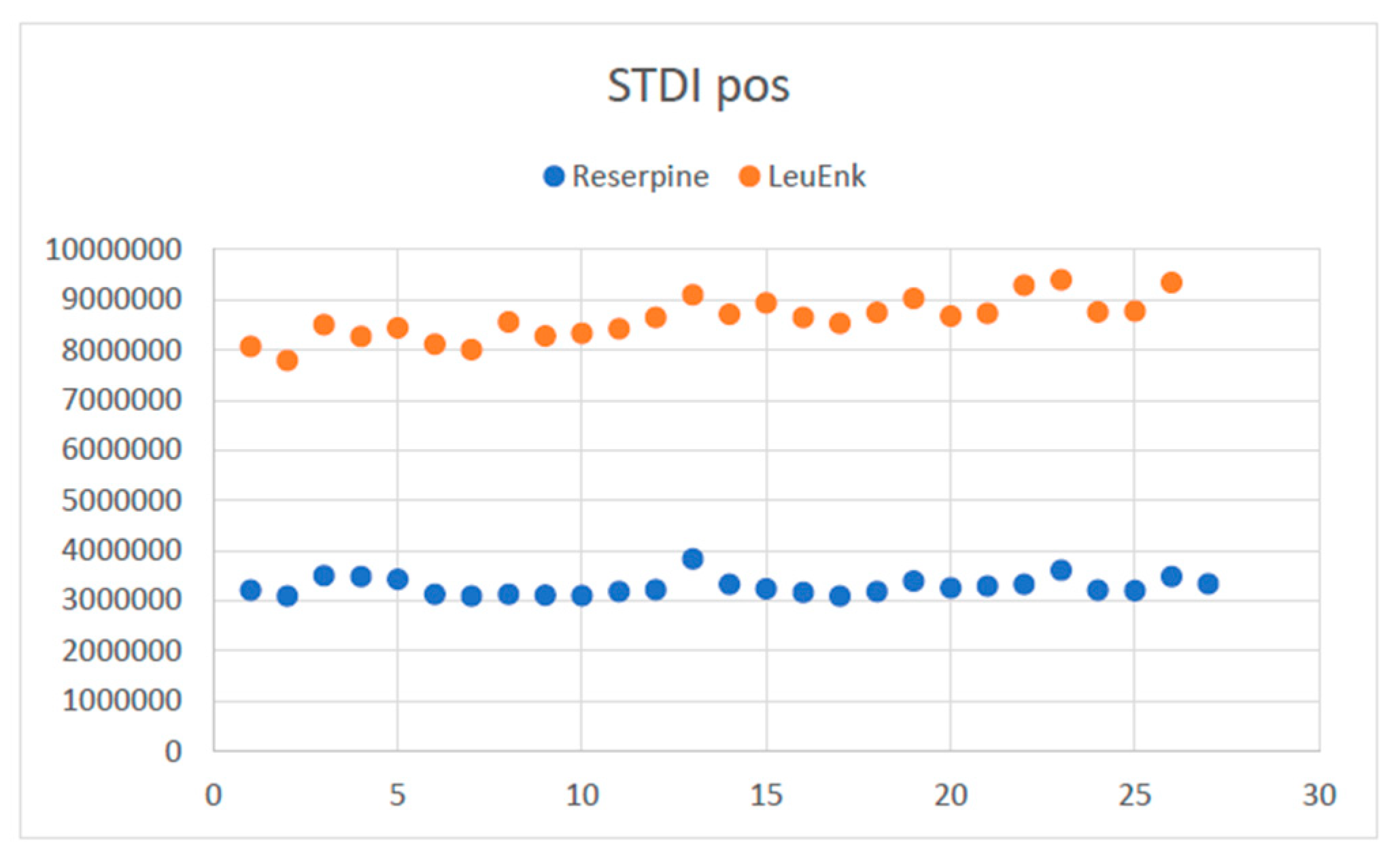
4.3.2.1. Evaluation of the response of internal standards
4.3.2.2. QC evaluation
4.4. Histological evaluation
4.4.1. Analysis of the Histological results.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chen, D.; Su, X.; Wang, N.; Li, Y.; Yin, H.; Li, L.; Li, L. Chemical Isotope Labeling LC-MS for Monitoring Disease Progression and Treatment in Animal Models: Plasma Metabolomics Study of Osteoarthritis Rat Model. Sci Rep 2017, 7, 40543. [Google Scholar] [CrossRef]
- Shi, X.; Wu, P.; Jie, L.; Zhang, L.; Mao, J.; Yin, S. Integrated Serum Metabolomics and Network Pharmacology to Reveal the Interventional Effects of Quzhi Decoction against Osteoarthritis Pain. Int J Anal Chem 2022, 2022, 9116175. [Google Scholar] [CrossRef]
- Zhai, G.; Randell, E.W.; Rahman, P. Metabolomics of Osteoarthritis: Emerging Novel Markers and Their Potential Clinical Utility. Rheumatology (Oxford) 2018, 57, 2087–2095. [Google Scholar] [CrossRef]
- Zhang, W.; Likhodii, S.; Aref-Eshghi, E.; Zhang, Y.; Harper, P.E.; Randell, E.; Green, R.; Martin, G.; Furey, A.; Sun, G.; et al. Relationship between Blood Plasma and Synovial Fluid Metabolite Concentrations in Patients with Osteoarthritis. J Rheumatol 2015, 42, 859–865. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Tobias, K.M.; Johnston, S.A. Veterinary Surgery: Small Animal; Elsevier: St. Louis, Mo, 2012; ISBN 978-1-4377-0746-5. [Google Scholar]
- Wei, Z.; Dong, C.; Guan, L.; Wang, Y.; Huang, J.; Wen, X. A Metabolic Exploration of the Protective Effect of Ligusticum Wallichii on IL-1β-Injured Mouse Chondrocytes. Chin Med 2020, 15, 12. [Google Scholar] [CrossRef]
- Wu, P.; Huang, Z.; Shan, J.; Luo, Z.; Zhang, N.; Yin, S.; Shen, C.; Xing, R.; Mei, W.; Xiao, Y.; et al. Interventional Effects of the Direct Application of “Sanse Powder” on Knee Osteoarthritis in Rats as Determined from Lipidomics via UPLC-Q-Exactive Orbitrap MS. Chin Med 2020, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, M.; Shi, T.; Gao, M.; Lv, Y.; Zhao, Y.; Li, J.; Zhang, M.; Zhang, H.; Guan, F.; et al. Analysis of Serum Metabolomics in Rats with Osteoarthritis by Mass Spectrometry. Molecules 2021, 26, 7181. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, W.; Fan, Z.; Sun, G.; Likhodi, S.; Randell, E.; Zhai, G. METABOLOMICS DIFFERENTIAL CORRELATION NETWORK ANALYSIS OF OSTEOARTHRITIS. Pac Symp Biocomput 2016, 21, 120–131. [Google Scholar] [PubMed]
- Kwok, A.T.; Mohamed, N.S.; Plate, J.F.; Yammani, R.R.; Rosas, S.; Bateman, T.A.; Livingston, E.; Moore, J.E.; Kerr, B.A.; Lee, J.; et al. Spaceflight and Hind Limb Unloading Induces an Arthritic Phenotype in Knee Articular Cartilage and Menisci of Rodents. Sci Rep 2021, 11, 10469. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Hong, W.; Qian, D.; Peng, F.; Li, H.; Liang, C.; Du, M.; Gu, J.; Mai, J.; Bai, B.; et al. Quercetin Modulates the Gut Microbiota as Well as the Metabolome in a Rat Model of Osteoarthritis. Bioengineered 2021, 12, 6240–6250. [Google Scholar] [CrossRef]
- Guma, M.; Tiziani, S.; Firestein, G.S. Metabolomics in Rheumatic Diseases: Desperately Seeking Biomarkers. Nat Rev Rheumatol 2016, 12, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Maerz, T.; Sherman, E.; Newton, M.; Yilmaz, A.; Kumar, P.; Graham, S.F.; Baker, K.C. Metabolomic Serum Profiling after ACL Injury in Rats: A Pilot Study Implicating Inflammation and Immune Dysregulation in Post-Traumatic Osteoarthritis. J Orthop Res 2018, 36, 1969–1979. [Google Scholar] [CrossRef]
- Swank, K.R.; Furness, J.E.; Baker, E.A.; Gehrke, C.K.; Biebelhausen, S.P.; Baker, K.C. Metabolomic Profiling in the Characterization of Degenerative Bone and Joint Diseases. Metabolites 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; He, S.; Wu, S.; Zhang, T.; Gong, S.; Minjie, T.; Gao, Y. Diagnostic Biomarker Panels of Osteoarthritis: UPLC-QToF/MS-Based Serum Metabolic Profiling. PeerJ 2023, 11, e14563. [Google Scholar] [CrossRef] [PubMed]
- Jaggard, M.K.J.; Boulangé, C.L.; Graça, G.; Vaghela, U.; Akhbari, P.; Bhattacharya, R.; Williams, H.R.T.; Lindon, J.C.; Gupte, C.M. Can Metabolic Profiling Provide a New Description of Osteoarthritis and Enable a Personalised Medicine Approach? Clin Rheumatol 2020, 39, 3875–3882. [Google Scholar] [CrossRef] [PubMed]
- Tootsi, K.; Vilba, K.; Märtson, A.; Kals, J.; Paapstel, K.; Zilmer, M. Metabolomic Signature of Amino Acids, Biogenic Amines and Lipids in Blood Serum of Patients with Severe Osteoarthritis. Metabolites 2020, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Haartmans, M.J.J.; Emanuel, K.S.; Tuijthof, G.J.M.; Heeren, R.M.A.; Emans, P.J.; Cillero-Pastor, B. Mass Spectrometry-Based Biomarkers for Knee Osteoarthritis: A Systematic Review. Expert Rev Proteomics 2021, 18, 693–706. [Google Scholar] [CrossRef]
- The Human Metabolome Database (HMDB). Available online: https://hmdb.ca/ (accessed on 5 November 2023).
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res 2021, 50, D622–D631. [Google Scholar] [CrossRef]
- METLIN Gen2. Available online: https://metlin.scripps.edu/landing_page.php?pgcontent=mainPage (accessed on 5 November 2023).
- Zhai, G. Clinical Relevance of Biochemical and Metabolic Changes in Osteoarthritis. Adv Clin Chem 2021, 101, 95–120. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, H.; Chen, T.; Zhu, W.; Ding, S.; Xu, K.; Xu, Z.; Guo, Y.; Zhang, J. Metabolic Analysis of Osteoarthritis Subchondral Bone Based on UPLC/Q-TOF-MS. Anal Bioanal Chem 2016, 408, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J. Osteoarthritis Year in Review 2014: We Need More Biochemical Biomarkers in Qualification Phase. Osteoarthritis Cartilage 2014, 22, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- de Visser, H.M.; Mastbergen, S.C.; Ravipati, S.; Welsing, P.M.J.; Pinto, F.C.; Lafeber, F.P.J.G.; Chapman, V.; Barrett, D.A.; Weinans, H. Local and Systemic Inflammatory Lipid Profiling in a Rat Model of Osteoarthritis with Metabolic Dysregulation. PLoS One 2018, 13, e0196308. [Google Scholar] [CrossRef] [PubMed]
- Lamers, R.J. a. N.; van Nesselrooij, J.H.J.; Kraus, V.B.; Jordan, J.M.; Renner, J.B.; Dragomir, A.D.; Luta, G.; van der Greef, J.; DeGroot, J. Identification of an Urinary Metabolite Profile Associated with Osteoarthritis. Osteoarthritis Cartilage 2005, 13, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Wang-Sattler, R.; Hart, D.J.; Arden, N.K.; Hakim, A.J.; Illig, T.; Spector, T.D. Serum Branched-Chain Amino Acid to Histidine Ratio: A Novel Metabolomic Biomarker of Knee Osteoarthritis. Ann Rheum Dis 2010, 69, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.J.; Anderson, J.R.; Peffers, M.J. Nuclear Magnetic Resonance Spectroscopy of Biofluids for Osteoarthritis. Br Med Bull 2021, 137, 28–41. [Google Scholar] [CrossRef]
- Costello, C.A.; Hu, T.; Liu, M.; Zhang, W.; Furey, A.; Fan, Z.; Rahman, P.; Randell, E.W.; Zhai, G. Differential Correlation Network Analysis Identified Novel Metabolomics Signatures for Non-Responders to Total Joint Replacement in Primary Osteoarthritis Patients. Metabolomics 2020, 16, 61. [Google Scholar] [CrossRef]
- Anderson, J.R.; Chokesuwattanaskul, S.; Phelan, M.M.; Welting, T.J.M.; Lian, L.-Y.; Peffers, M.J.; Wright, H.L. 1H NMR Metabolomics Identifies Underlying Inflammatory Pathology in Osteoarthritis and Rheumatoid Arthritis Synovial Joints. J Proteome Res 2018, 17, 3780–3790. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Qiu, Y.; Zhao, T.; Chen, T.; Su, M.; Chu, L.; Lv, A.; Liu, P.; Jia, W. Urinary Metabolomics as a Potentially Novel Diagnostic and Stratification Tool for Knee Osteoarthritis. Metabolomics 2010, 6, 109–118. [Google Scholar] [CrossRef]
- Zhai, G. Alteration of Metabolic Pathways in Osteoarthritis. Metabolites 2019, 9, 11. [Google Scholar] [CrossRef]
- Mickiewicz, B.; Kelly, J.J.; Ludwig, T.E.; Weljie, A.M.; Wiley, J.P.; Schmidt, T.A.; Vogel, H.J. Metabolic Analysis of Knee Synovial Fluid as a Potential Diagnostic Approach for Osteoarthritis. J Orthop Res 2015, 33, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Castro-Perez, J.M.; Kamphorst, J.; DeGroot, J.; Lafeber, F.; Goshawk, J.; Yu, K.; Shockcor, J.P.; Vreeken, R.J.; Hankemeier, T. Comprehensive LC-MS E Lipidomic Analysis Using a Shotgun Approach and Its Application to Biomarker Detection and Identification in Osteoarthritis Patients. J Proteome Res 2010, 9, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Pousinis, P.; Gowler, P.R.W.; Burston, J.J.; Ortori, C.A.; Chapman, V.; Barrett, D.A. Lipidomic Identification of Plasma Lipids Associated with Pain Behaviour and Pathology in a Mouse Model of Osteoarthritis. Metabolomics 2020, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A Lipidomic Study of Phospholipid Classes and Species in Human Synovial Fluid. Arthritis Rheum 2013, 65, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. Sphingolipids in Human Synovial Fluid - A Lipidomic Study. PLOS ONE 2014, 9, e91769. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, M.K.; Mastbergen, S.C.; Liebisch, G.; Wilhelm, J.; Dettmeyer, R.B.; Ishaque, B.; Rickert, M.; Schmitz, G.; Lafeber, F.P.; Steinmeyer, J. Comparative Lipidomic Analysis of Synovial Fluid in Human and Canine Osteoarthritis. Osteoarthritis Cartilage 2016, 24, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Martano, C.; Mugoni, V.; Dal Bello, F.; Santoro, M.M.; Medana, C. Rapid High Performance Liquid Chromatography-High Resolution Mass Spectrometry Methodology for Multiple Prenol Lipids Analysis in Zebrafish Embryos. J Chromatogr A 2015, 1412, 59–66. [Google Scholar] [CrossRef]
- Neogi, T.; Booth, S.L.; Zhang, Y.Q.; Jacques, P.F.; Terkeltaub, R.; Aliabadi, P.; Felson, D.T. Low Vitamin K Status Is Associated with Osteoarthritis in the Hand and Knee. Arthritis Rheum 2006, 54, 1255–1261. [Google Scholar] [CrossRef]
- Kidd, P.M. Vitamins D and K as Pleiotropic Nutrients: Clinical Importance to the Skeletal and Cardiovascular Systems and Preliminary Evidence for Synergy. Altern Med Rev 2010, 15, 199–222. [Google Scholar]
- Vermeer, C.; Theuwissen, E. Vitamin K, Osteoporosis and Degenerative Diseases of Ageing. Menopause Int 2011, 17, 19–23. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free Radic Biol Med 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.B.; Praticò, D. Vitamin E in Aging, Dementia, and Alzheimer’s Disease. Biofactors 2012, 38, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Werdyani, S.; Liu, M.; Zhang, H.; Sun, G.; Furey, A.; Randell, E.W.; Rahman, P.; Zhai, G. Endotypes of Primary Osteoarthritis Identified by Plasma Metabolomics Analysis. Rheumatology (Oxford) 2021, 60, 2735–2744. [Google Scholar] [CrossRef]
- Moon, S.-J.; Jeong, J.-H.; Jhun, J.Y.; Yang, E.J.; Min, J.-K.; Choi, J.Y.; Cho, M.-L. Ursodeoxycholic Acid Ameliorates Pain Severity and Cartilage Degeneration in Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Immune Netw 2014, 14, 45–53. [Google Scholar] [CrossRef]
- Carlson, A.K.; Rawle, R.A.; Wallace, C.W.; Adams, E.; Greenwood, M.C.; Bothner, B.; June, R.K. Global Metabolomic Profiling of Human Synovial Fluid for Rheumatoid Arthritis Biomarkers. Clin Exp Rheumatol 2019, 37, 393–399. [Google Scholar]
- Attur, M.; Krasnokutsky, S.; Statnikov, A.; Samuels, J.; Li, Z.; Friese, O.; Hellio Le Graverand-Gastineau, M.-P.; Rybak, L.; Kraus, V.B.; Jordan, J.M.; et al. Low-Grade Inflammation in Symptomatic Knee Osteoarthritis: Prognostic Value of Inflammatory Plasma Lipids and Peripheral Blood Leukocyte Biomarkers. Arthritis Rheumatol 2015, 67, 2905–2915. [Google Scholar] [CrossRef]
- Gierman, L.M.; Wopereis, S.; van El, B.; Verheij, E.R.; Werff-van der Vat, B.J.C.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J.V.M.; Kloppenburg, M.; Stojanovic-Susulic, V.; Huizinga, T.W.J.; et al. Metabolic Profiling Reveals Differences in Concentrations of Oxylipins and Fatty Acids Secreted by the Infrapatellar Fat Pad of Donors with End-Stage Osteoarthritis and Normal Donors. Arthritis Rheum 2013, 65, 2606–2614. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Aitken, D.; Liu, M.; Lei, G.; Jones, G.; Cicuttini, F.; Zhai, G. Serum Metabolomic Signatures for Knee Cartilage Volume Loss over 10 Years in Community-Dwelling Older Adults. Life (Basel) 2022, 12, 869. [Google Scholar] [CrossRef] [PubMed]
- Laverty, S.; Girard, C.A.; Williams, J.; Hunziker, E.; Pritzker, K. The OARSI Histopathology Initiative – Recommendations for Histological Assessments of Osteoarthritis in the Rabbit. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2010, 18 Suppl 3, S53-65. [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI Histopathology Initiative - Recommendations for Histological Assessments of Osteoarthritis in the Rat. Osteoarthritis Cartilage 2010, 18 Suppl 3, S24–34. [Google Scholar] [CrossRef]
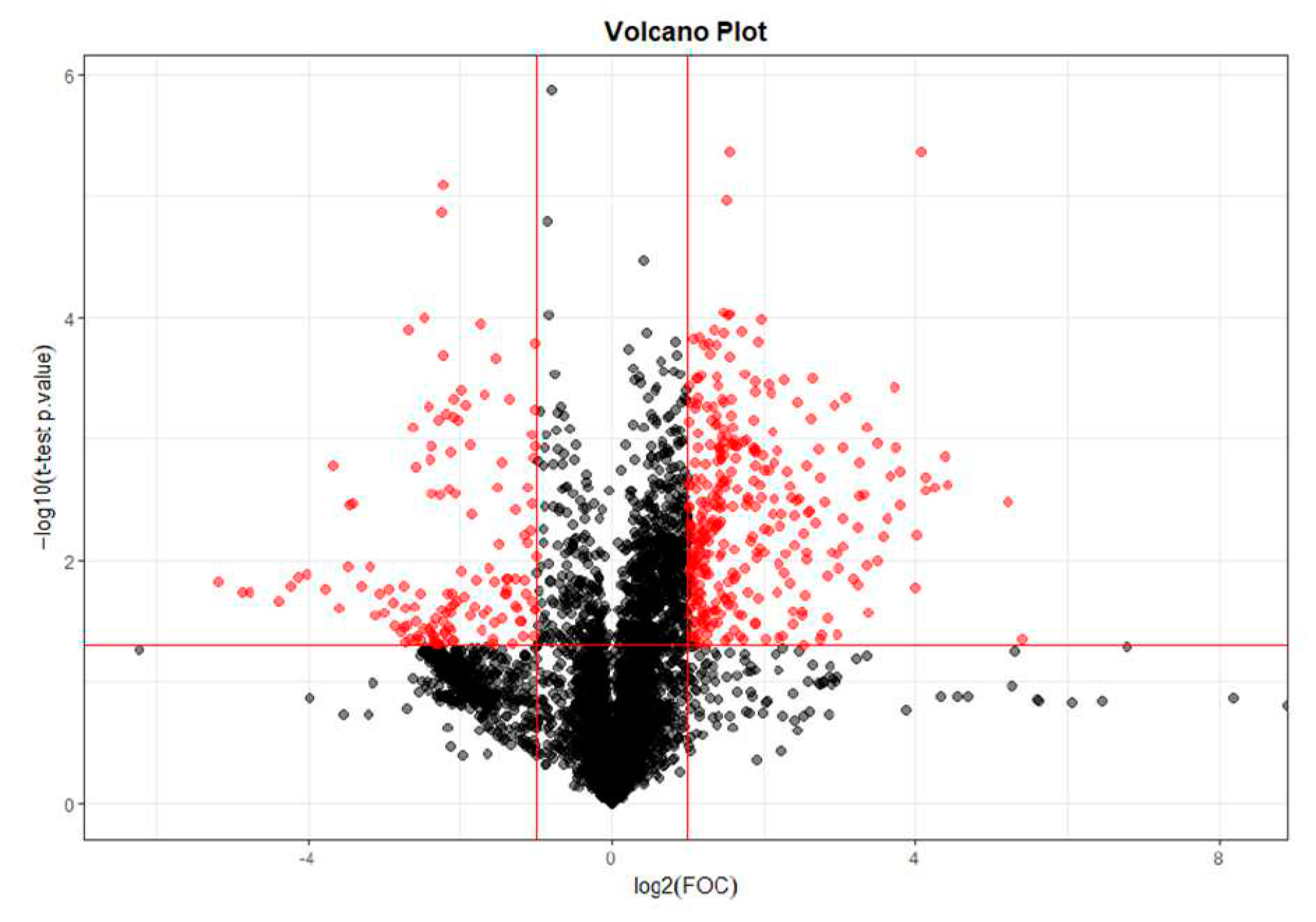
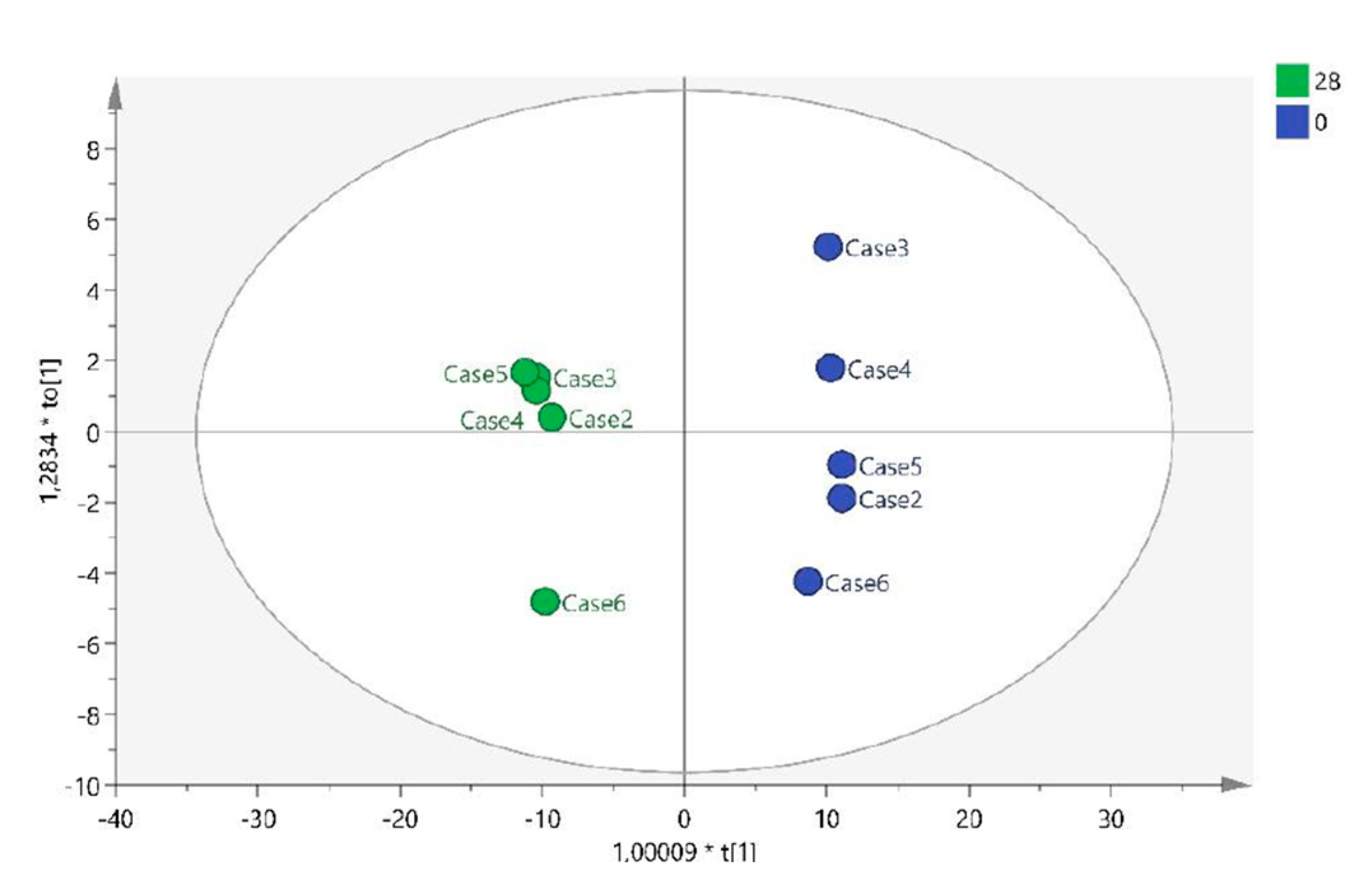
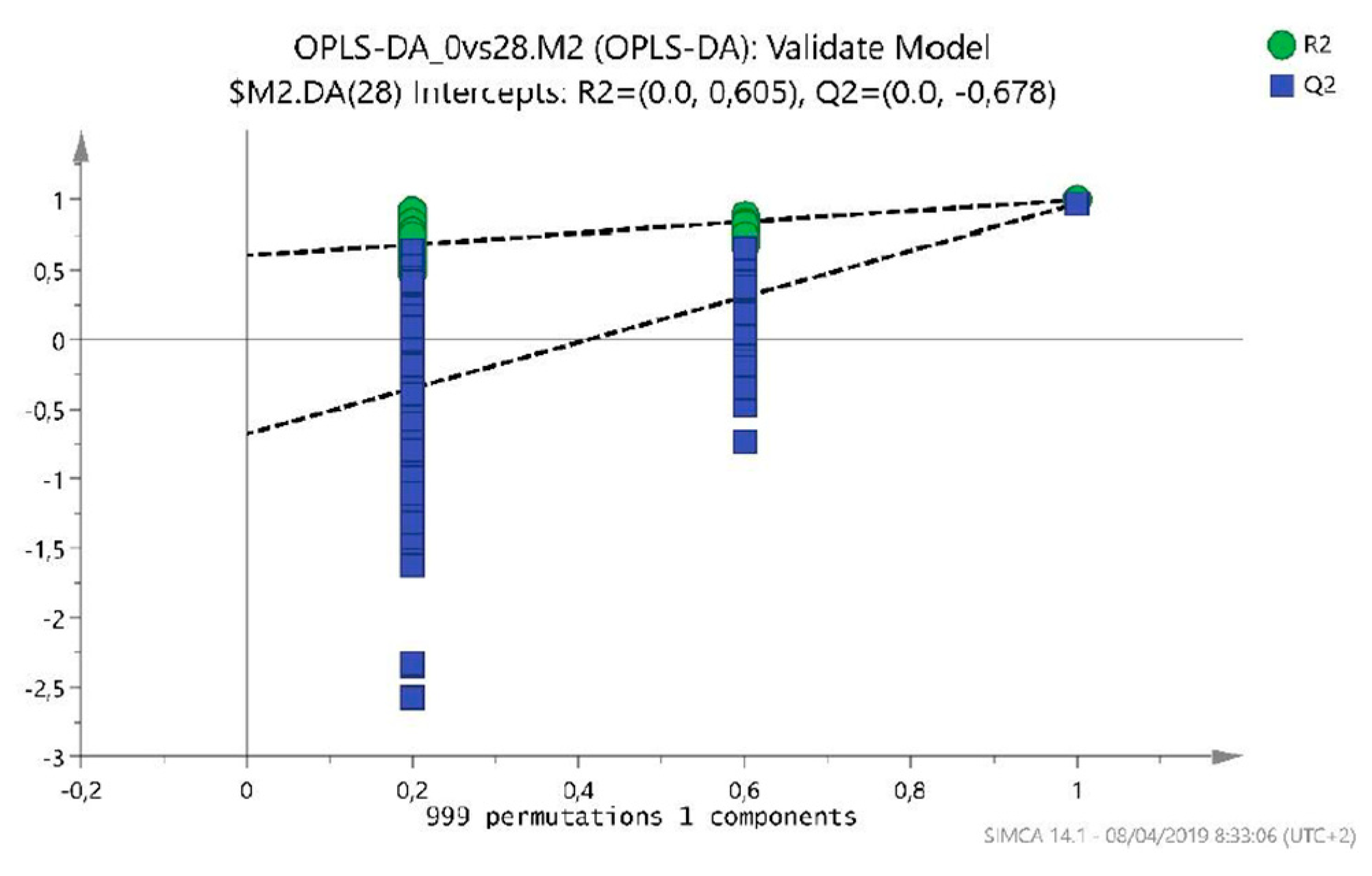
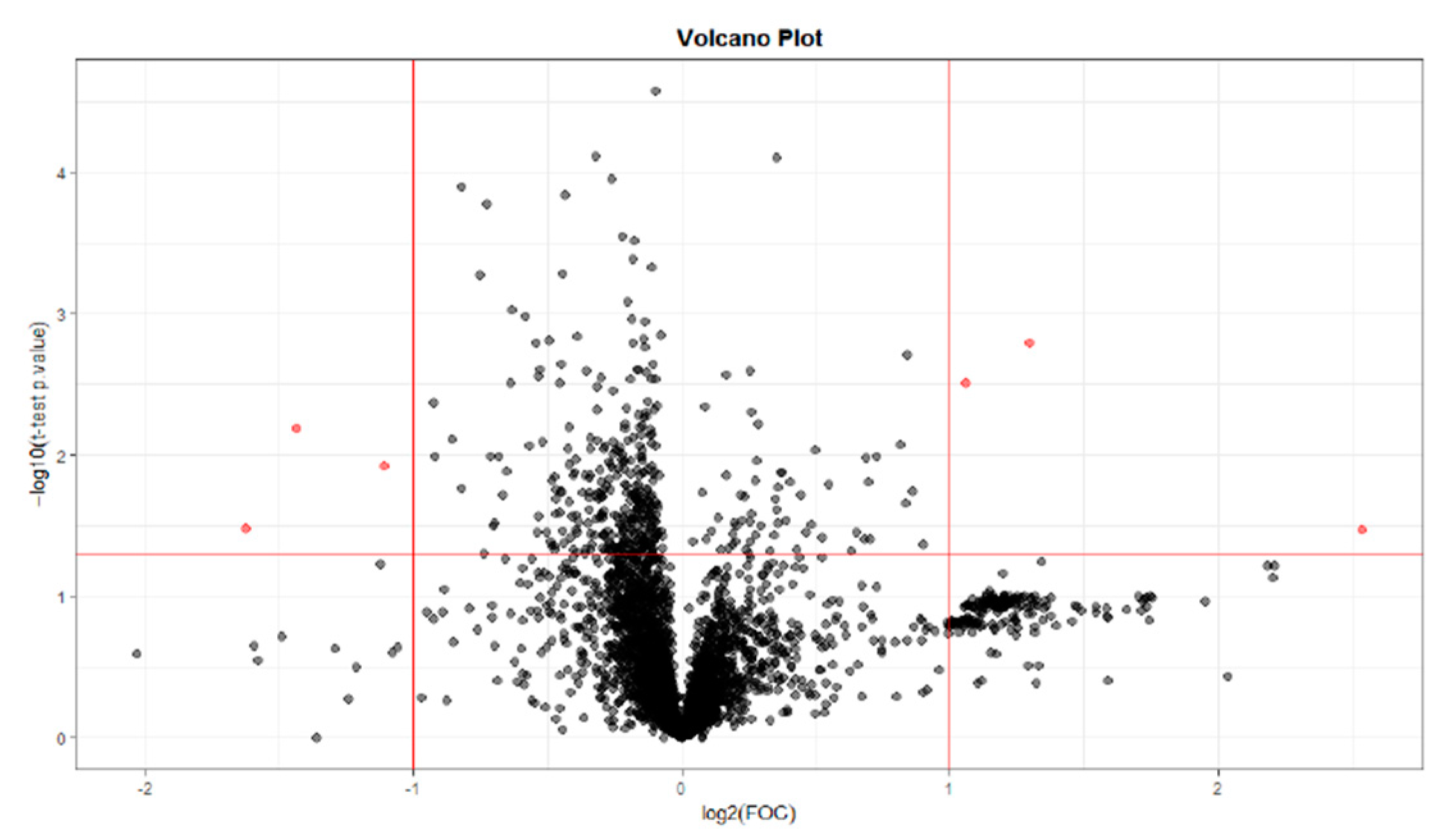

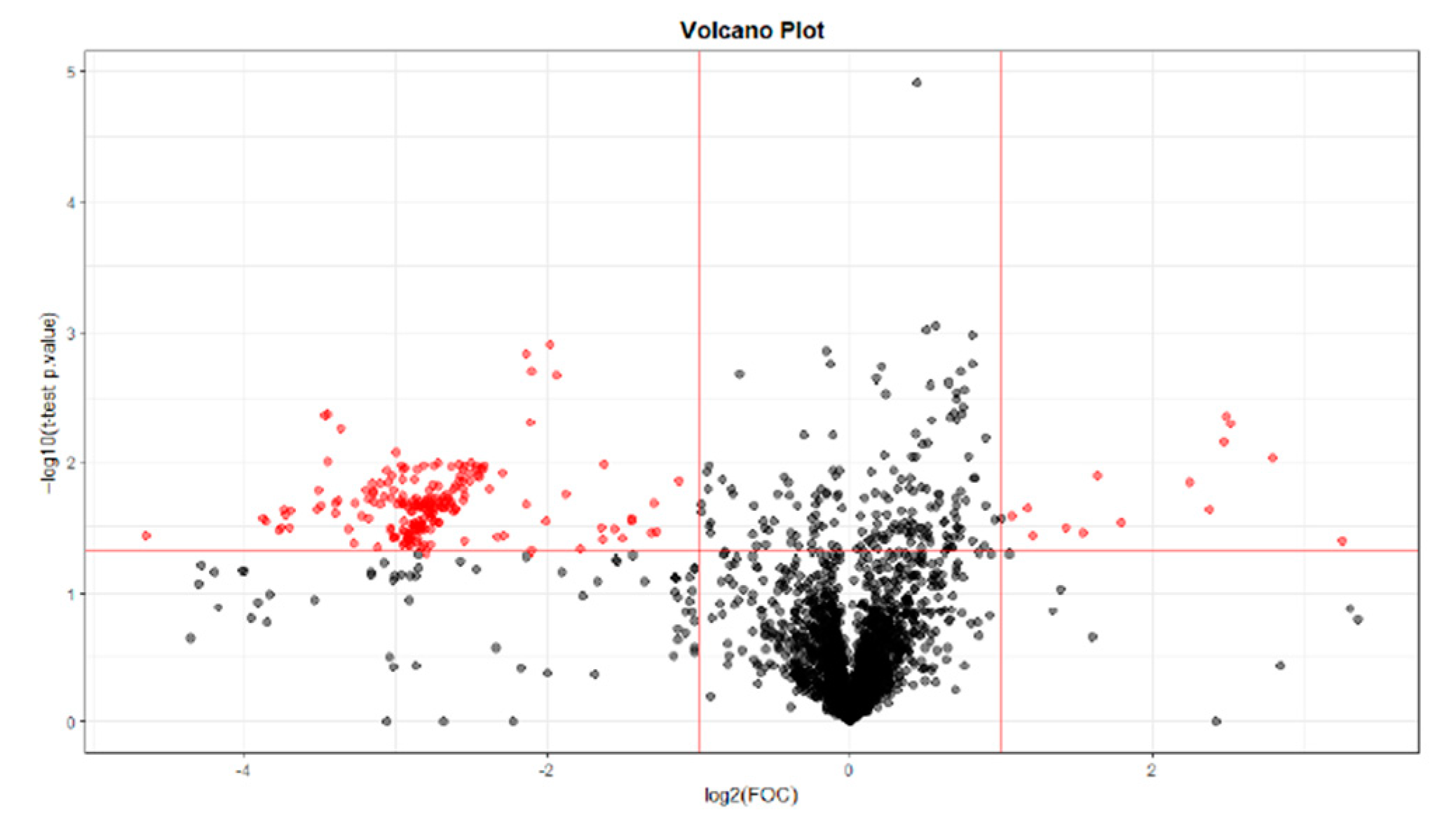
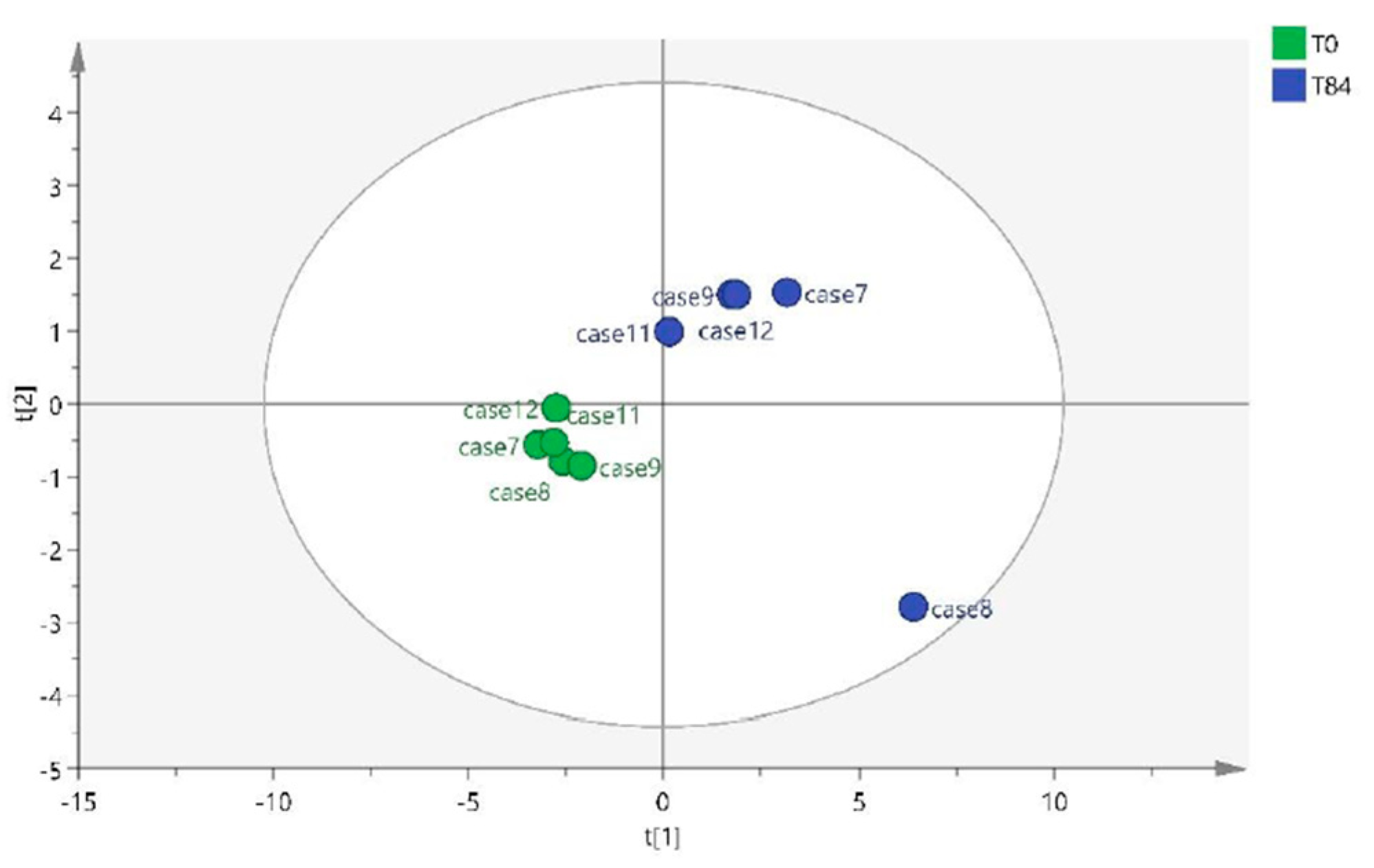
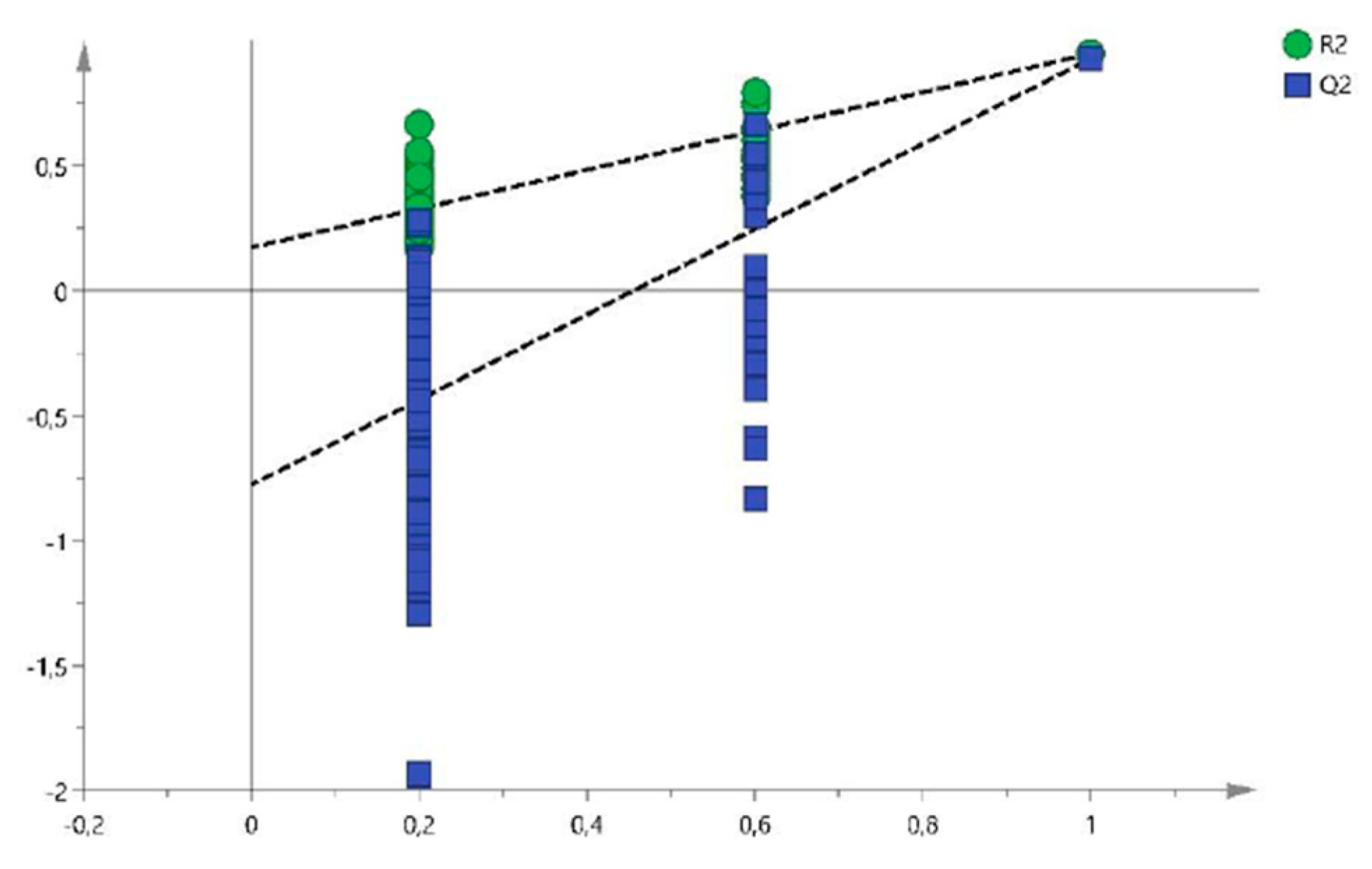
| Variable (mz/rt) | ESI | VIP score |
|---|---|---|
| 377.1360487:8.543 | pos | 5.547 |
| 103.0543706:1.108 | pos | 4.738 |
| 395.1254806:8.062 | pos | 4.337 |
| 166.0864745:1.108 | pos | 4.189 |
| 120.0810237:1.109 | pos | 3.800 |
| 220.1461317:1.105 | pos | 2.808 |
| 171.9808059:0.632 | pos | 2.491 |
| 1145.217439:9.503 | pos | 2.402 |
| 352.3057184:8.762 | pos | 2.314 |
| 529.3525685:9.107 | pos | 2.292 |
| 148.0038081:0.632 | pos | 2.127 |
| 1274.179758:9.5 | pos | 2.112 |
| 335.2787707:8.765 | pos | 2.046 |
| 1307.668417:9.502 | pos | 1.990 |
| 1200.745803:0.579 | pos | 1.957 |
| 541.3706018:8.817 | pos | 1.926 |
| 1423.929102:9.5 | pos | 1.921 |
| 1240.189772:9.501 | pos | 1.905 |
| 1239.680395:9.501 | pos | 1.890 |
| 1501.870123:9.502 | pos | 1.877 |
| 1429.869007:9.499 | pos | 1.860 |
| 126.0220994:0.635 | pos | 1.695 |
| 1173.204232:9.499 | pos | 1.584 |
| 1208.700279:9.501 | pos | 1.575 |
| Metabolites | Theory (m/z) (HMDB) | Observed (m/z) | Observed retention time (min) | Sub class (HMDB) | Class (HMDB) | Super class (HMDB) |
|---|---|---|---|---|---|---|
| Lactacystin | 376.42 | 377.1360 | 8.543 | Amino acids, peptides and analogues | Carboxylic acid and derivatives | Organic acids and derivatives |
| Taurine | 125.147 | 148.0038 | 0.632 | Organosulfonic acids and derivatives | Organic sulfonic acids and derivatives | |
| 126.0220 | 0.635 | |||||
| Styrene Oxide | 120.151 | 103.0543 | 1.108 | -- | Benzene and substituted derivatives | Benzenoids |
| Tyramine | 137.179 | 120.0810 | 1.109 | Phenethylamines | ||
| Setanaxib | 394.86 | 395.1254 | 8.062 | Phenylpyridines | Pyridines and derivatives | Organoheterocyclic compounds |
| Norsalsolinol | 165.1891 | 166.0864 | 1.108 | -- | Tetrahydroisoquinolines | |
| Ganoderic acid V | 528.7199 | 529.3525 | 9.107 | Triterpenoids | Prenol lipids | Lipids and lipid-like molecules |
| Ganglioside GM1 (d18:0/16:0) | 1519.7974 | 1501.8701 | 9.502 | Glycosphingolipids | Sphingolipids |
| Variable (mz/rt) | ESI | VIP score |
|---|---|---|
| 203.2846; 6.00 | neg | 1.41 |
| 231.0456; 1.12 | neg | 0.66 |
| 279.0362; 0.80 | neg | 0.39 |
| 251.1023; 4.46 | pos | 0.12 |
| Metabolites | Theory (m/z) (HMDB) | Observed (m/z) | Observed retention time (min) | Sub class (HMDB) | Class (HMDB) | Super class (HMDB) |
|---|---|---|---|---|---|---|
| gamma-Glutamylcysteine | 250.272 | 231.0456 | 1.12 | Amino acids, peptides and analogues | Carboxylic acid and derivatives | Organic acids and derivatives |
| Tyrosyl-Serine | 268.2658 | 251.1023 | 4.46 | |||
| Acetyl citrate | 234.16 | 279.0362 | 0.80 | Tetracarboxylic acids and derivatives |
| Variable (mz/rt) | ESI | VIP score |
|---|---|---|
| 595.4212; 9.168 | pos | 2.269 |
| 573.4081; 9.183 | pos | 2.266 |
| 551.3949; 9.198 | pos | 2.232 |
| 617.4344; 9.154 | pos | 2.226 |
| 529.3819; 9.213 | pos | 2.139 |
| 639.4473; 9.141 | pos | 2.118 |
| 661.4605; 9.126 | pos | 2.019 |
| 507.3685; 9.229 | pos | 2.001 |
| 683.4734; 9.113 | pos | 1.87 |
| 485.3556; 9.245 | pos | 1.822 |
| 705.4863; 9.100 | pos | 1.662 |
| 463.3419; 9.260 | pos | 1.548 |
| 820.5975; 9.294 | pos | 1.427 |
| 727.4989; 9.087 | pos | 1.34 |
| 274.2747; 7.486 | pos | 1.326 |
| 437.2907; 7.905 | neg | 1.775 |
| 391.2852; 7.905 | neg | 1.822 |
| 391.2125: 6.377 | neg | 1.715 |
| Metabolites | Theory (m/z) (HMDB) | Observed (m/z) | Observed retention time (min) | Sub class (HMDB) | Class (HMDB) | Super class (HMDB) |
|---|---|---|---|---|---|---|
| Ginsenoside Rh1 | 638.8721 | 639.4473 | 9.141 | Triterpenoids | Prenol lipids | Lipids and lipid-like molecules |
| Theasapogenol A | 506.7144 | 507.3685 | 9.229 | |||
| Phosphatidic acid | 674.941 | 705.4863 | 9.100 | Glycerophosphates | Glycerophospholipids | |
| Ursodeoxycholic acid | 392.572 | 437.2907 | 7.905 | Bile acids, alcohols and derivatives | Steroids and steroid derivatives | |
| Polyporusterone F | 462.6618 | 463.3419 | 9.260 | |||
| Brassinolides | 480.6771 | Steroid lactones | ||||
| 10-Hydroperoxy-H4-neuroprostane | 392.492 | 391.2125 | 6.377 | Eicosanoids | Fatty acyls |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





