1. Introduction
Sea urchins can evolve adaptive survival mechanisms over time in response to their environment. Environmental factors such as light are essential in the biological growth, development, reproduction, and behavior of marine organisms. The color, intensity, and photoperiod of light can significantly impact these factors [
1,
2,
3]. The absorption of light color in water is correlated with the wavelength and water depth [
4,
5]. Furthermore, differing light color environments can have inhibitory or promotional effects on the growth of aquatic life [
6]. Domestic and international scholars have verified that the color of light impacts the growth and development of organisms. When
Cyprinus carpio is raised under a red light environment, its feed conversion rate, growth performance, and survival rate are significantly increased [
7]. Blue and green light promote endocrine secretion of
Heterosomata with a higher growth rate [
8], and green light increases the hatchability and production of
Haliotis discus hannai, which increases commercial benefits [
9]. However, while
Strongylocentrotus intermedius grow better under blue light, the foraging behavior of sea urchins is reduced after prolonged periods [
10]. These results highlight that light color is species-specific for aquatic organisms.
When aquatic organisms undergo stress responses from external environmental factors, their different enzyme activities are changed, which impacts the regular physiological functions and activities of these organisms [
11]. The investigation of digestive enzymes in the intestinal tract of organisms is a crucial element in comprehending the digestive physiological functions of sea urchins. This can offer insights into the fundamental characteristics of digestive physiology in aquatic animals [
12]. The digestive enzyme activities of
Oreochromis niloticus were significantly increased under red and white light conditions [
13], whereas the trypsin activity of
Lateolabrax maculatus was significantly decreased under red light [
14]. Organisms respond to environmental changes and possess a well-developed antioxidant system to maintain free radical balance and reduce oxidative damage [
15]. Studies have demonstrated that alterations in suitable light color greatly impact the antioxidant properties and metabolism of
Litopenaeus vannamei [
16]. In a blue-light environment, the succinate dehydrogenase activity of
Haliotis discus hannai was significantly higher than that of the red and white light groups, whereas the lactate dehydrogenase activity was significantly lower than that of the red and white light groups [
9]. The results demonstrate that light color substantially impacts digestive and antioxidant enzyme activities in aquatic organisms, highlighting the crucial regulatory role of light in their physiological metabolism [
4]. Current studies on sea urchin digestive and antioxidant enzymes have focused on assessing the effects of bait [
17] and environmental factors [
18,
19] (salinity, pH, temperature), and less research has been carried out on the effects of light color on sea urchin digestive and antioxidant enzymes.
Light color is a crucial factor in shallow waters, affecting the feeding, growth, and development of sea urchins [
20].
T. gratilla is among the few edible sea urchins that exist in tropical regions [
21]. They inhabit sandy-bottom areas in shallow waters where seagrasses are abundant and feed on macroalgae and seagrasses [
22,
23,
24] and are widely distributed in the shallow waters of Hainan, Taiwan, and Sansha, China [
25]. In this study,
T. gratilla was selected as the research subject because it has excellent characteristics, such as full gonads [
26], a closed life cycle [
27], a high growth rate [
28]) and high market value [
26], among other excellent characteristics. Current studies on
T. gratilla have focused on the effects of different baits on the growth and reproduction of sea urchins [
29,
30,
31], feeding preferences of sea urchins on algae [
26,
32] and sea urchin population growth patterns and distribution [
33,
34]. There are few studies on the effect of light color on the growth and development and digestive antioxidant physiological mechanisms of
T. gratilla, and how light color affects its growth and development remains unclear. Therefore, there is a need to study the effects of light color on
T. gratilla.
In this study, we designed five light and dark groups to analyze the effects of light on the growth, development, feeding, digestion, and antioxidant activity of T. gratilla in an indoor mariculture environment. Our objective was to determine the optimal light color composition to promote the growth and development of T. gratilla and to provide fundamental theories for the large-scale cultivation of these organisms in China. Studying the impact of light color on the digestive and antioxidant processes of T. gratilla is essential to decrease physiological stress and enhance the wellbeing of farmed sea urchins.
2. Materials and Methods
2.1. Sea urchins
The study was executed during April and May 2023 at the Tropical Development Centre of the South China Sea Fisheries Research Institute of the Chinese Academy of Fisheries Sciences. The sea urchins utilized for the study were procured from the sea vicinity close to Xincun Town, Lingshui Lizu Autonomous County, Hainan Province. The sea water was maintained at a temperature of 29.5±0.72°C, with a salinity level of 31.4±0.86 and pH of 8.11±0.24, while ensuring adherence to natural light cycles. Sufficient dissolved oxygen was provided, and fresh seaweeds were provided during the transient incubation period. Daily bottom suction was carried out, or as needed, and the water was changed every two days.
2.2. Experimental design
Six experimental groups were set up: Blue light (B, λ450nm, 201lux), yellow light (Y, λ585-590nm, 202lux), red light (R, λ640nm, 198lux), green light (G, λ510nm, 199lux), white light group (W, λ400-780nm, 206lux), and the dark group (H). Each LED light was 10 W, the light source was 15 cm from the water surface, and the photoperiod was 12 L:12D.
We randomly selected 168 healthy sea urchins and weighed their body measurements, with an average test diameter of 82.27±4.36 mm, an average test height of 55.44±5.47 mm, and an average wet body weight of 142.45±4.36 g. The sea urchins were randomly divided into six groups (three parallels in each group) and placed into a recirculating aquaculture tank (length 60*width 60*height 40 cm), with eight urchins in each tank. The tanks were fully oxygenated, with a temperature at 29.5±0.43°C, salinity at 31.2±0.92, and pH at 8.11±0.38. The urchins of each group were cultivated for 30 d under the same cultivation conditions.
2.3. Sample collection
Samples were collected on the 7th, 15th and 30th days after the test, and three sea urchins with good vitality were randomly selected from each group. The body size data of each sea urchin were measured and placed on an ice box for vivisection. Approximately 2 mL of body cavity fluid was extracted from each sea urchin and placed in a centrifuge tube, and the supernatant was centrifuged (3500 rpm) for 10 min and then transferred to a new centrifuge tube. Immediately after extraction of the luminal fluid, the gut tissue was clipped and frozen in liquid nitrogen, and the same centrifuged luminal fluid was placed at -80℃ for storage.
2.4. Measurement and analysis of samples
2.4.1. Feeding performance measurement
During the experimental period, kelp was provided every 2 days. The mass of dried kelp (W0) was weighed before each feeding, and after 48 hours, the unfed kelp was removed and dried at 40°C, and the weight of the remaining kelp (W1) was weighed.
Daily food intake and daily food intake rate were calculated as:
where
R is the daily intake [g. (pcs-d)
-1] of sea urchins,
N is the number of sea urchins (pcs),
A is the number of feeding days (d),
F is the daily intake rate (%) of purple sea urchins, and
Wm is the average body mass of sea urchins (g).
2.4.2. Growth performance measurement
The weight growth rate and specific growth rate were calculated as:
where
WGR is the weight growth rate (%) of the sea urchin,
Wi is the initial body weight (g) of each urchin,
Wf is the final body weight (g) of each urchin,
SGR is the specific growth rate (%) of the sea urchin,
Xf is the final value of each stage of the trait (test diam and weight),
Xi is the initial value of the trait, and T is the duration of the stage (d).
2.4.3. Enzyme activity measurement
At different sampling times, the intestinal tissues of three rows of white spiny sea urchins were collected, and the samples were ground with 0.2 mol·L-1 saline at a ratio of 1:9 by mass volume. The ground solution was centrifuged at 4°C and 5000 r·min-1 for 10 min, and 1 mL of the supernatant was collected in a clean EP tube and placed at 80°C until measurement. Enzyme activities were determined using the appropriate kits (Nanjing Jiancheng Bioengineering Institute), the activities of α-amylase (AMS) and lipase (LPS) were measured in the intestinal tract, and the activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) and catalase (CAT) were measured in the body cavity supernatant, with three parallels in each group.
2.5. Data analysis
Excel 2019 was used to process the data, Origin 2022 was used for graphing, and SPSS 27.0 software was used to statistically analyze the experimental data. The T test and one-way ANOVA were used to determine whether there was a significant difference between the treatment groups, and the significance of the differences between the means (P<0.05, indicating that the differences were significant; P<0.01, indicating a highly significant difference) was compared using Duncan's method.
3. Results
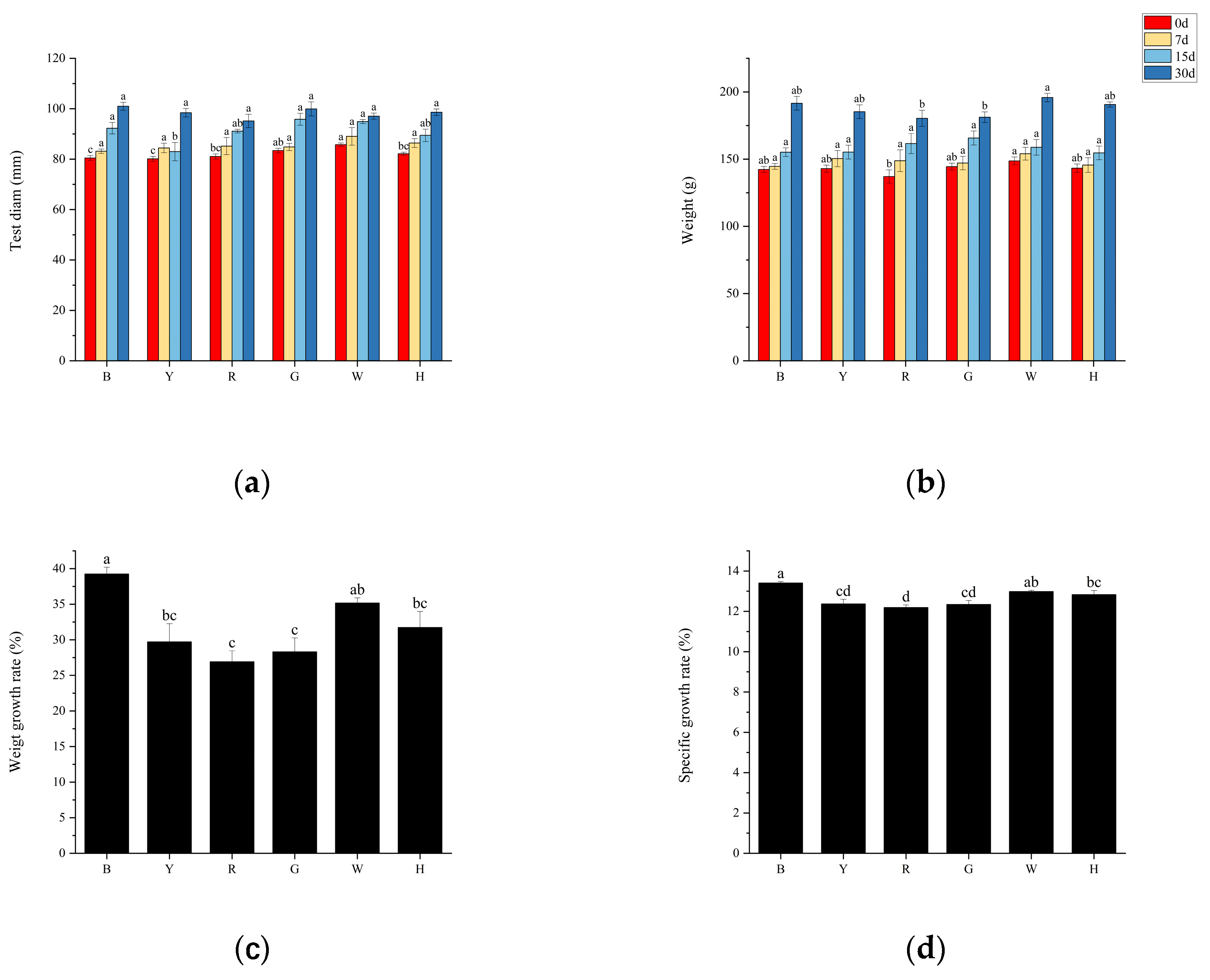
3.1. Growth performance of sea urchins under different light colors
Different light colors significantly affected the growth of
T. gratilla, and the test diam and body weight of sea urchins in all groups showed a gradual increase, with significant differences between the initial and final body weights (
P<0.05), and the highest weight gain of sea urchins in group B was 49.21±2.31 g. In the first stage (0~7 d), the body weights of the different groups did not show any significant differences (
P>0.05), but the test diam of groups W and H were significantly higher than those of the other groups (
P<0.01); in the second stage (7~15 d) and third stage (15-30 d), there was no significant difference (
P>0.05) in shell diameter and body weight between the groups (
Figure 1 a, b). The trends of weight gain rate (WGR) and specific growth rate (SGR) were consistent between the groups with different light color treatments: The WGR and SGR of sea urchins in group B were the highest, 39.26% and 13.41%, respectively, which were significantly higher than those of the other groups (
P<0.05), and those of sea urchins in group R were the lowest, 26.93% and 12.19%, respectively, which were significantly lower than those of groups B and W (
P<0.05) (
Figure 1 c, d). No sea urchins died during the experiment.
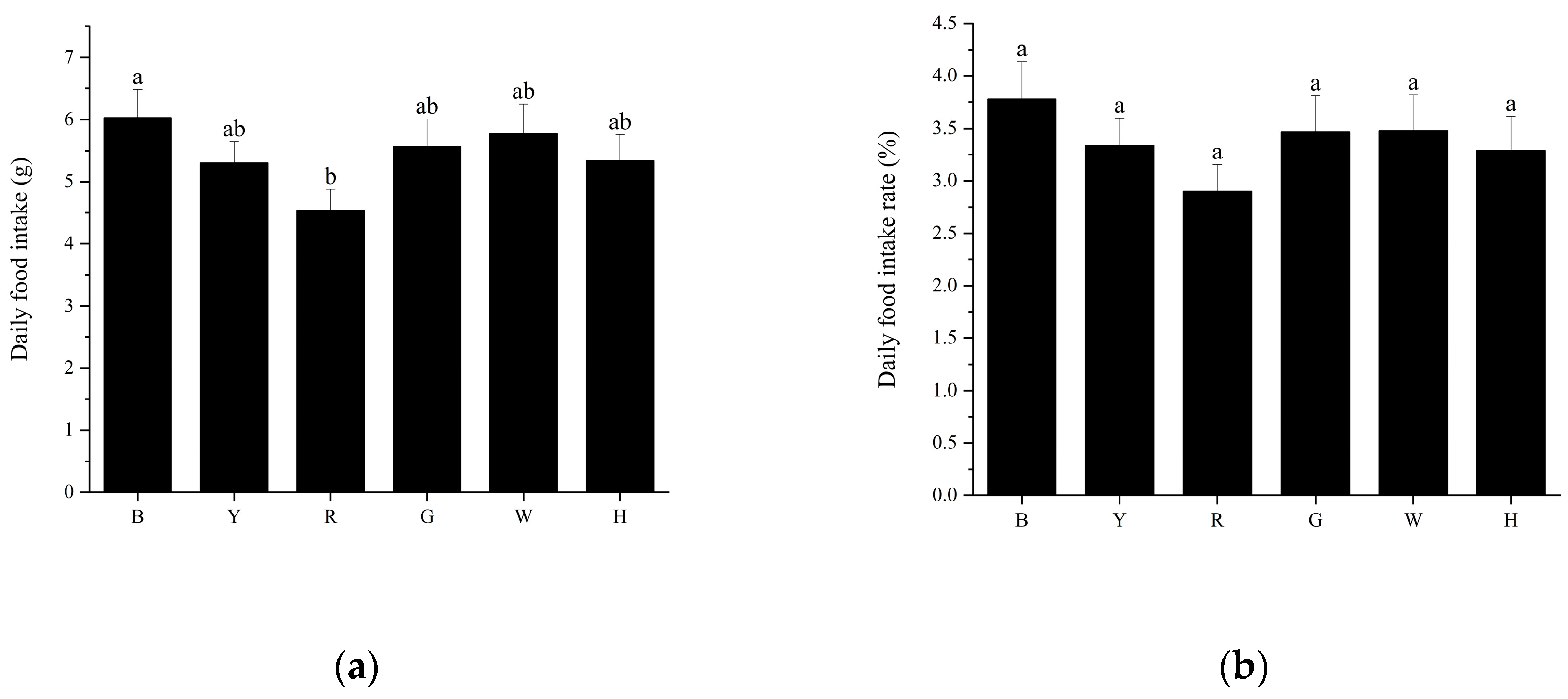
3.2. Feeding performance of sea urchins under different light colors
The trend of daily food intake and daily feeding rate per sea urchin was consistent among the different treatment groups (
Figure 2 a, b), with group B (6.03±1.69 g) consuming the most, followed by groups W (5.77±1.78 g), G (5.57±1.65 g), H (5.33±1.59 g), Y (5.30±1.30 g), and R (4.54±1.26 g). There was a significant difference in the daily food intake of sea urchins between groups B and R (
P<0.05). There was a significant difference (P<0.05) in the daily intake of sea urchins between groups B and R. There was no significant difference (
P>0.05) in the daily intake of sea urchins and daily intake rate between the remaining groups.
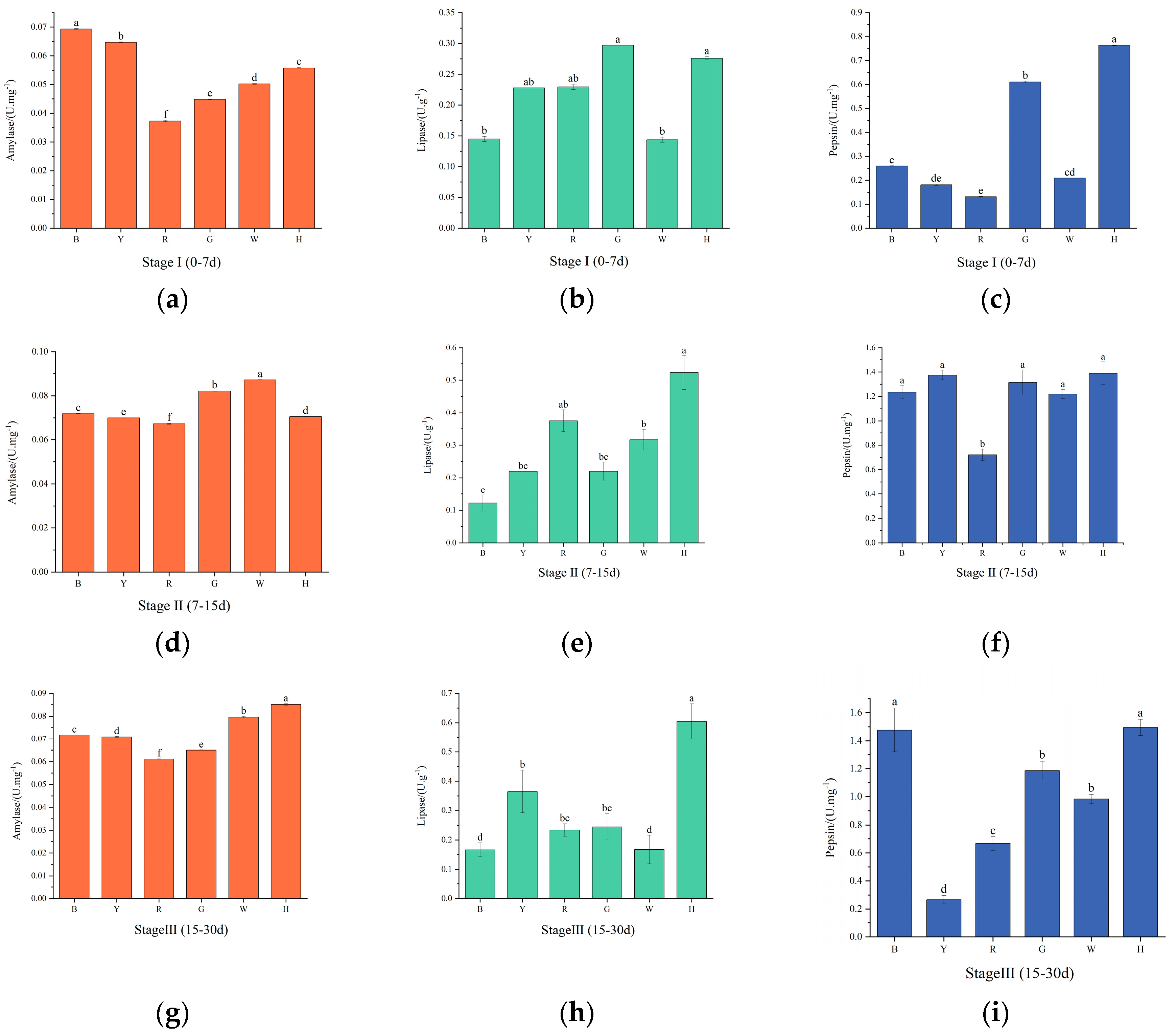
3.3. Changes in sea urchin digestive enzyme activities under different light colors
As shown in
Figure 3 (a, d, g), the AMS activity of each group showed a trend of increasing and then decreasing with increasing test time. In the first stage, there was a significant difference in AMS activity between groups (
P<0.01), with the highest activity in group B, followed by groups Y, H, W, G and R. In the second stage, AMS activity increased in all groups compared to the first stage, and there was no significant difference (
P>0.05) between groups except for group H. Group W had the highest activity, and group R had the lowest activity. In the third stage, AMS activity decreased compared to the second stage, and the difference between groups was significant (
P<0.01), with the highest activity in group H and the lowest activity remaining in group R.
As shown in
Figure 3 (b, e, h), in the first stage, there was no significant difference (
P>0.05) between the LPS activity of groups Y, R and the other groups, with the highest LPS activity in group G and the lowest activity in group B. In the second stage, compared with the first stage, the LPS activity of groups R, H and W increased and the activity of the other groups decreased, and there was a significant difference in the LPS activity of groups B and H (
P<0.01). In the third phase, LPS activity increased in all groups except group R. There was a significant difference in LPS activity between group H and the other groups (
P<0.01).
As shown in
Figure 3 (c, f, i), the pepsin activity of each group tended to increase and then decrease with increasing test time. In the first stage, pepsin activity was higher in groups H and G, and there was a significant difference in pepsin activity between them and the other groups (
P<0.01). In the second stage, pepsin activity increased in all groups compared to the first stage, with the lowest activity in group R, which significantly differed from the remaining groups (
P<0.01), and in the third stage, pepsin activity increased in groups B and H, which significantly differed from the remaining groups (
P<0.01), and the lowest pepsin activity was found in group Y.
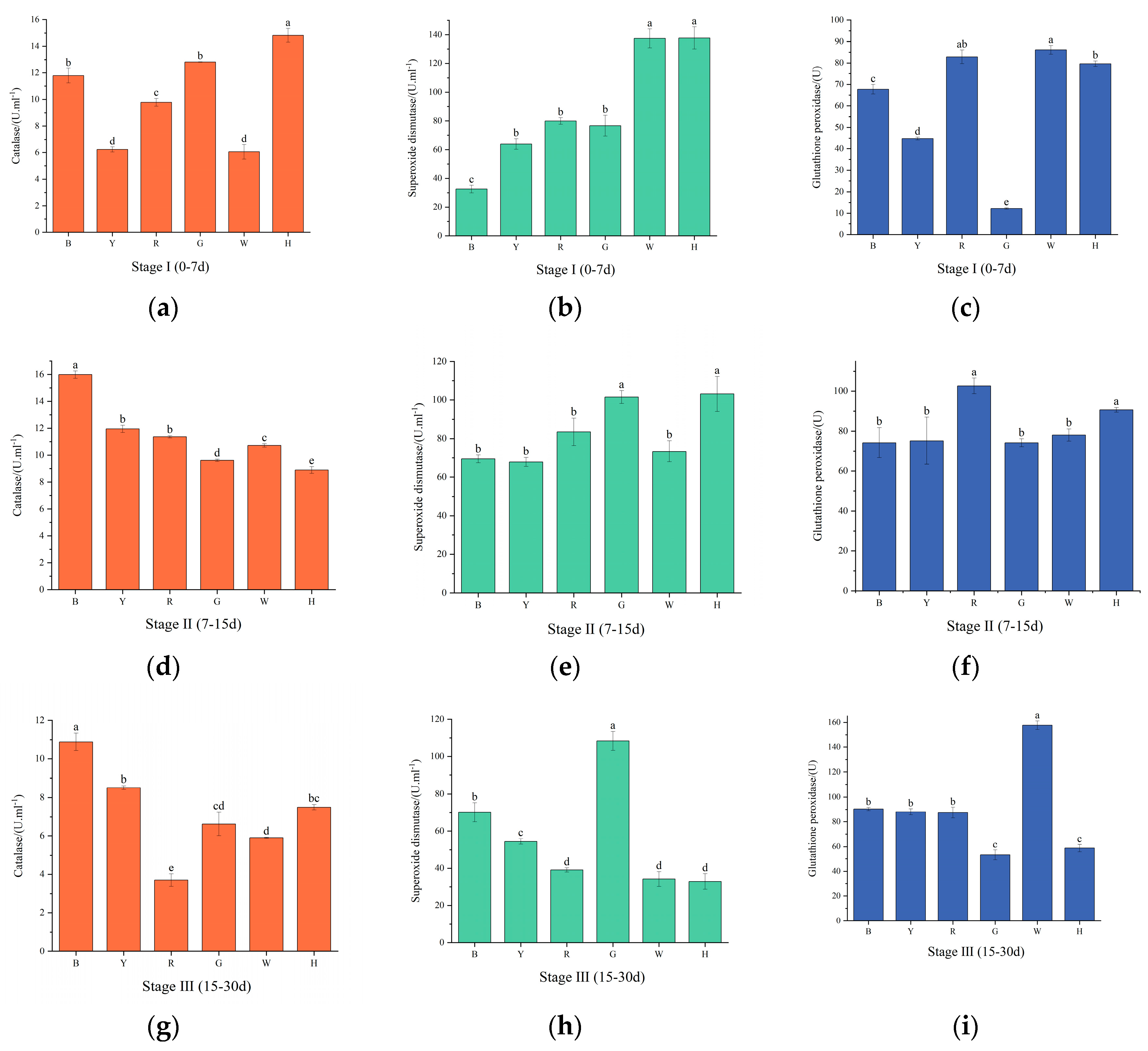
3.4. Changes in sea urchin antioxidant enzyme activities under different light colors
As shown in
Figure 4 (a, d, g), with increasing test time, CAT activity was highest in group H in the first phase, which significantly differed from the remaining groups (
P<0.01). In the second stage, CAT activity decreased in groups G and H and increased in the remaining groups, with the lowest CAT activity in group H, which significantly differed from the remaining groups (
P<0.01). In the third stage, CAT activity decreased in all groups, and the highest CAT activity was found in group B and the lowest in group R. There was a significant difference (
P<0.01) between the groups.
As shown in
Figure 4 (b, e, h), SOD activity gradually decreased in groups H and W with increasing test time and increased and then decreased in the remaining groups. The highest SOD activity was observed in group H and the lowest in group B in the first stage, which significantly differed from the other groups (
P<0.01). In the second stage, SOD activity was not significantly different (
P>0.05) between the groups, and the highest SOD activity was found in group H. In the third stage, SOD activity of group G was significantly higher than all other groups (
P<0.01).
As shown in
Figure 4 (c, f, i), GSH-PX activity increased and then decreased in groups R, G and H and gradually increased in the other groups. Group G was significantly lower than the other groups in the first stage, and there was a significant difference between the groups (
P<0.01). GSH-PX activity was highest in group R in the second stage, and there was a significant difference between it and the other groups (
P<0.01). GSH-PX activity was significantly increased in group W at the third stage (
P<0.01).
4. Discussion
4.1. The effect of light color on the feeding of Tripneustes gratilla
In this experiment,
T. gratitla had a higher feeding rate under blue light. There were some differences in the order of group G, group Y and group H in weight gain rate and feeding rate, which could be caused by the different bait conversion rates of sea urchins after feeding under different light colors. The results suggested that blue light (short wavelength light) is more conducive to sea urchin feeding, while red light (long wavelength light) may increase the adaptive stress of sea urchins in the environment. We believe that due to the high density of phytoplankton and dissolved organic matter [
35], light (especially blue light) has a reduced ability to be projected in intertidal and shallow subtidal waters, thus increasing the range of distribution of long wavelength light (red and yellow light) [
20,
36].
Macroalgae, as a bait source for sea urchins, directly affect the growth of sea urchin feeding [
10], and light color is one of the important factors for the growth and distribution of macroalgae [
37,
38,
39]. Algae absorb wavelengths of light differently, such as brown algae having a greater use of blue‒green light [
40], and blue light can enhance the growth and reproduction of
Saccharina japonica [
41,
42]. Scott Seymour [
26] found that
T. gratitla prefers to feed on brown algae; therefore, we suggest that blue light can promote the growth of brown algae in the field, thus providing more food for
T. gratitla. Furthermore, blue light not only promotes sea urchin feeding but is also important for macroalgal growth, whereas sea urchins under red light have less bait and poorer feeding performance. Light color may affect not only the feeding and prey source of sea urchins but also the feed conversion rate, and it is necessary to further study such phenomena based on energy metabolism.
4.2. The effect of light color on the growth of Tripneustes gratilla
The effects of light color composition on marine organisms vary according to the species and their growth and development, and SGR and WGR, in particular, are important reference indices to evaluate the growth of organisms and are the most intuitive indicators [
43]. For example,
Dicentrarchus labrax showed higher SGR and WGR under red light [
1], and Korean rockfish showed better growth and feeding performance under green, blue and white light [
4]. In this study, all growth parameters of sea urchins in the B light group were significantly higher than those of other treatment groups, and the highest growth weight gain (55.89 ± 2.32 g) was observed in the B group, followed by W, H, Y, and G, and the lowest growth weight gain (41.06 ± 4.94 g) was observed in the R group. These results indicate that the overall growth of
T. gratilla, as measured by various growth indices, was good under the blue light environment, in contrast to the sea urchins under red light, which had the lowest weight gain and specific growth rate and was significantly lower than the other groups (
P<0.01). This is consistent with the study by Yang [
5,
10] on the effect of light color on
S. intermedius, which found that short wavelengths (blue, green) promoted the growth and foraging of
S. intermedius, but long-term exposure to blue light gradually reduced the foraging of sea urchins, and long-term exposure to red light inhibited the growth and development of sea urchins. Thus, the results are consistent.
T. gratitla lives under a shallow sandy seabed where red light attenuation is more severe. We hypothesize that the positive effect of blue light on sea urchin feeding is due to short light color, which is similar to the light color composition of sea urchins in their natural habitat and that sea urchins can better adapt to such light conditions, which can lead to better feeding and promote individual growth and development. Therefore, there may be a positive correlation between blue light and the growth performance of white-spined trilobite sea urchins. Green light is a short wavelength, and the growth of white spiny three-row sea urchins was slower in this experiment, probably due to the larger initial sea urchin size and the faster growth of small-sized sea urchins [
44]. However, the higher WGR in the white group with a larger initial size of sea urchins was considered because the white light was closer to the growth environment of
T. gratitla during transient incubation, thus reducing the aquaculture process for better development and growth.
4.3. The effect of light color on the digestive enzyme activities of Tripneustes gratilla
Digestive enzyme activity is an important indicator for assessing the digestive capacity of aquatic organisms and can directly reflect the development and nutritional status of the digestive tract of organisms [
45]. Changes in light color cause the body to lose more energy and require digestive enzymes to be more active in digesting food to ensure proper energy supply [
46]. Currently, there are fewer studies on the effect of light color on the digestive enzyme activities of sea urchins. Chen Jisheng [
47] reported that optimal digestive enzyme activities could be maintained in
Anthocidaris crassispina planktonic larvae under 500 lx light intensity. Shih Jiageng [
48] investigated the effects of the mixing ratios of different algae,
Apostichopus japonicu and
Hemicentrotus pulcherrimus, on the digestive enzyme activities of
H. pulcherrimus. Their results showed that in the three stages, the AMS activity of the B group changed nonsignificantly (
P>0.05), while the pepsin activity increased significantly (
P<0.01) and the LPS activity gradually decreased, and the changes in the W group remained almost the same as those in the B group. The AMS and pepsin activity of the R group continued to be at a lower level, and the LPS activity was significantly higher than that of the B group in all three stages (
P<0.01).
Lipase level is an important indicator to assess the maturity and function of the digestive system of sea urchins [
49]. The gradual decrease in LPS viability in group B may be due to the faster growth of sea urchins in the blue light environment, which inhibited the LPS activity in the body of sea urchins, resulting in a gradual decrease in its activity. The higher viability of LPS in group R may be because sea urchins in the red group environment have a strong ability to use fat [
50] to counteract the effects of environmental stress. Sea urchins in blue light are less stressed by external environmental changes and have higher overall digestive enzyme activity, which enhances their digestive ability and thus promotes feeding and growth. In addition, sea urchins have higher viable AMS and pepsin, suggesting that a high proportion of protein and starch in the bait is relevant [
51] .The mechanism by which bait composition affects the digestive enzymes of sea urchins in different light colors needs further investigation.
4.4. The effect of light color on the antioxidant enzyme activities of Tripneustes gratilla
The antioxidant system of aquatic animals removes reactive oxygen species generated during metabolism from the organism in a timely manner, and under environmental stress, a large amount of reactive oxygen species is generated in the organism, resulting in organismal damage [
52]. GSH, a tripeptide compound containing sulfhydryl groups, has strong reducing properties and is important in maintaining organismal homeostasis and preventing oxidative stress, whereas CAT can decompose H
2O
2 produced by SOD after the conversion of reactive oxygen species, thus reducing its toxicity. CAT, SOD and GSH-PX can effectively scavenge oxygen radicals, reduce oxidative damage in the body and increase the body's immunity [
53,
54].
In the present study, it was found that the antioxidant enzyme activities of sea urchins in different light colors changed with increasing test time in the body cavity fluid. In all three stages, CAT, SOD and GSH-PX activities in group R showed a trend of increasing and then decreasing. It was surmised that the sea urchin organism in group R was at a higher level of oxidative stress in the first stage, which led to a rapid increase in free radicals in cells and tissues, and the organism needed more antioxidant enzymes to cope with the excessive free radicals, thus increasing the antioxidant enzyme activities [
55]. In the third stage, the activity of CAT, SOD and GSH-PX in the R group decreased significantly (
P<0.01), which was presumed to be because the red light had already caused damage to the sea urchin and the antioxidant capacity had been weakened [
19]. Furthermore, the accumulation of reactive oxygen species in the body exceeded the scavenging capacity of the antioxidant enzymes, so that excessive toxic free radicals in the body had an inhibitory effect on the antioxidant enzyme activity [
56,
57]. The nonsignificant (
P>0.05) changes in antioxidant enzyme activity in the blue light group at all stages may be because sea urchins are better adapted to blue light, and the free radical content in the organism changes less.
5. Conclusion
In summary, different light colors had different effects on the growth, feeding and digestive metabolism of T. gratilla under a 12 L:12 D cycle and 200 lux light intensity. The sea urchins showed better growth and feeding performance under blue and green light conditions, where the sea urchin organism was in a better physiological condition under blue light. In contrast, red light had a significant inhibitory effect on the feeding and growth rate of sea urchins. Therefore, the results suggested that the effects of blue light on the feeding and nutrient metabolism of sea urchins are different from those of red light, and these differences may have differential effects on the development and reproduction of sea urchins, with T. gratilla being more suitable for growth and development in blue light, followed by white and green light, and not suitable for development and reproduction in red light. However, studies regarding the effects of light color on the growth and development of T. gratilla are limited, and the exact physiological mechanisms, including the effects of different sizes and larval development, are not fully understood. Additional studies regarding the effects of different light colors on sea urchin larval growth and development are needed to provide a theoretical basis for a more selective and suitable environment for T. gratilla Reproduction.
Author Contributions
Conceptualization, X.Z. and C.Q.; methodology, X.Z.; software, X.Z.; validation, X.Z., G.Y. and J.L.; formal analysis, X.Z.; investigation, Y.G.; resources, Y.G.; data curation, X.Z.; writing—original draft preparation, X.Z.; writing—review and editing, C.Q.; visualization, J.L.; supervision, Z.M.; G.Y. and C.Q.; project administration, Z.M.; funding acquisition, G.Y. and C.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (32160863), South Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (2023XK01) and Sanya Yazhou Bay Science and Technology City (SKJC-2022-PTDX-014).
Institutional Review Board Statement
The animal study protocol was approved by the Laboratory Animal Welfare and Ethics Committee of South China Sea Fisheries Research Institute (CAFS) (nhdf2023-14).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ren, J.; Wei, P.; Fei, F.; Dai, M.; Ma, H.; Gao, D.; Song, C.; Chen, T.; Liu, Y. Effects of LED spectroscopy on feeding, growth and energy partitioning in juvenile tongue-toothed perch (Lepomis macrocephalus). Journal of Fisheries of China 2019, 43, 1821–1829. [Google Scholar]
- Zhou, X.; Cuijuan, N.; Li, Q. Effects of light on feeding, growth and survival of aquatic animals. Acta Hydrobiologica Sinica 2000, 178–181. [Google Scholar]
- Villamizar, N.; Blanco-Vives, B.; Migaud, H.; Davie, A.; Carboni, S.; Sánchez-Vázquez, F.J. Effects of light during early larval development of some aquacultured teleosts: A review. Aquaculture 2011, 315, 86–94. [Google Scholar] [CrossRef]
- Liu, S.; Cai, H.; Liu, Y.; Zhang, Y.; Fang, Y.; Sun, F.; Wu, Y.; Li, X.; Lv, L.; Zhang, Q.; et al. Effects of LED spectra on the growth and physiological mechanism of juvenile Sebastes schlegelii. Part I: Growth, feeding and digestion and metabolism. Aquaculture 2023, 740295. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Z.; Hu, F.; Sun, J.; Ding, J.; Chang, Y.; Zhao, C. Light spectra regulated foraging and feeding behaviors shed light on stock enhancement of the sea urchin Strongylocentrotus intermedius. Aquaculture Reports 2020, 18, 100480. [Google Scholar] [CrossRef]
- Villamizar, N.; García-Alcazar, A.; Sánchez-Vázquez, F.J. Effect of light spectrum and photoperiod on the growth, development and survival of European sea bass (Dicentrarchus labrax) larvae. Aquaculture 2009, 292, 80–86. [Google Scholar] [CrossRef]
- Karakatsouli, N.; Papoutsoglou, E.S.; Sotiropoulos, N.; Mourtikas, D.; Stigen-Martinsen, T.; Papoutsoglou, S.E. Effects of light spectrum, rearing density and light intensity on growth performance of scaled and mirror common carp Cyprinus carpio reared under recirculating system conditions. Aquacultural Engineering 2010, 42, 121–127. [Google Scholar] [CrossRef]
- Takahashi, A.; Kasagi, S.; Murakami, N.; Furufuji, S.; Kikuchi, S.; Mizusawa, K.; Andoh, T. Chronic effects of light irradiated from LED on the growth performance and endocrine properties of barfin flounder Verasper moseri. General and Comparative Endocrinology 2016, 232, 101–108. [Google Scholar] [CrossRef]
- Xiaolong, G.; Mo, Z.; Xian, L.; Ce, S.; Changbin, S.; Ying, L. Effects of LED light quality on the growth, metabolism, and energy budgets of Haliotis discus discus. Aquaculture 2016, 453, 31–39. [Google Scholar] [CrossRef]
- Yang, M.; Hu, F.; Leng, X.; Chi, X.; Yin, D.; Ding, J.; Li, X.; Zuo, R.; Chang, Y.; Zhao, C. Long-term effects of light spectra on fitness related behaviors and growth of the sea urchin Strongylocentrotus intermedius. Aquaculture 2021, 537, 736518. [Google Scholar] [CrossRef]
- Zhao, W.; Wen, W.; Tan, C.; Huang, X.; Yang, R.; Chen, M.; Yang, Q.; Chen, X. Effects of starvation stress on the activities of immune enzymes in differenttissues of Harpiosquilla harpax. Acta Scientiarum Naturalium Universitatis Sunyatseni 2021, 60, 26–33. [Google Scholar] [CrossRef]
- Wang, M.; Lv, C.; Yang, D.; Zhao, J. Effects of Lactobacillus plantarum(LP HMX-3) on growth, digestion, immunityand intestinal flora of Apostichopus japonicus. ournal of Fisheries of China 2022, 1–11. [Google Scholar]
- Yi, M.; Zhai, W.L.; Wang, M.; Wang, H.; Liu, Z.; Gao, F.-y.; Ke, X.-l.; Song, C.; Cao, J.; Lu, M.-x. The Welfare of Nile Tilapia (Oreochromis niloticus, GIFT Strain) Juveniles Cultured in Different Light Spectra. In Proceedings of the Frontiers in Marine Science, 2022.
- Hou, Z.-S.; Wen, H.-S.; Li, J.-F.; He, F.; Li, Y.; Qi, X.; Zhao, J.; Zhang, K.-Q.; Tao, Y.-X. Effects of photoperiod and light Spectrum on growth performance, digestive enzymes, hepatic biochemistry and peripheral hormones in spotted sea bass (Lateolabrax maculatus). Aquaculture 2019, 507, 419–427. [Google Scholar] [CrossRef]
- Yang, S.; Yan, T.; Wu, H.; Xiao, Q.; Fu, H.M.; Luo, J.; Zhou, J.; Zhao, L.L.; Wang, Y.; Yang, S.Y.; et al. Acute hypoxic stress: Effect on blood parameters, antioxidant enzymes, and expression of HIF-1alpha and GLUT-1 genes in largemouth bass (Micropterus salmoides). Fish & Shellfish Immunology 2017, 67, 449–458. [Google Scholar] [CrossRef]
- Guo, B.; Wang, F.; Dong, S.; Gao, Q. The effect of rhythmic light color fluctuation on the molting and growth of Litopenaeus vannamei. Aquaculture 2011, 314, 210–214. [Google Scholar] [CrossRef]
- Trenzado, C.E.; Hidalgo, F.; Villanueva, D.; Furné, M.; Díaz-Casado, M.E.; Merino, R.; Sanz, A. Study of the enzymatic digestive profile in three species of Mediterranean sea urchins. Aquaculture 2012, 344-349, 174–180. [Google Scholar] [CrossRef]
- Xi, S.; Qin, C.; Ma, Z.; Yu, G.; Sun, J.; Pan, W.; Zuo, T.; Ma, H.; Zhu, W. Effects of dietary microalgae on growth and survival of larval development of sea urchin(Anthocidaris crassispina). South China Fisheries Science 2020, 16, 115–120. [Google Scholar]
- Wang, W.; Han, L.; Zhang, X.; Liu, P.; Yang, X.; Wang, L.; Zhang, W.; Chang, Y.; Ding, J. Effects of Thermal Stress on the Activities of Antioxidant Enzymes andMitochondrial Structure and Function in Strongylocentrotus intermedius. Journal of Guangdong Ocean University 2022, 42, 42–48. [Google Scholar]
- Ackleson, S.G. Light in shallow waters: A brief research review. Limnology and Oceanography 2003, 48. [Google Scholar] [CrossRef]
- Lawrence, J.M. Chapter 2 - Sea Urchin Life History Strategies. In Developments in Aquaculture and Fisheries Science, Lawrence, J.M., Ed.; Elsevier: 2013; Volume 38, pp. 15–23.
- Brink, M.; Kuys, R.D.; Rhode, C.; Macey, B.M.; Christison, K.W.; Roodt-Wilding, R. Genetic diversity and population connectivity of the sea urchin Tripneustes gratilla along the South African coast. African Journal of Marine Science 2018, 40, 149–156. [Google Scholar] [CrossRef]
- Cyrus, M.; Bolton, J.; de Wet, L.; Macey, B. The development of a formulated feed containing Ulva (Chlorophyta) to promote rapid growth and enhanced production of high quality roe in the sea urchin Tripneustes gratilla (Linnaeus). Aquaculture Research 2012, 45. [Google Scholar] [CrossRef]
- Scholtz, R.; Bolton, J.; Macey, B. Effects of different microalgal feeds and their influence on larval development in the white-spined sea urchin Tripneustes gratilla. 2013, 35. [CrossRef]
- Zhang, T.; Li, X.; Cao, R.; Zhang, Q.; Qu, Y.; Wang, Q.; Dong, Z.; Zhao, J. Interactive effects of ocean acidification, ocean warming, and diurnal temperature cycling on antioxidant responses and energy budgets in two sea urchins Strongylocentrotus intermedius and Tripneustes gratilla from different latitudes. Science of The Total Environment 2022, 824, 153780. [Google Scholar] [CrossRef] [PubMed]
- Seymour, S.; Paul, N.A.; Dworjanyn, S.A.; de Nys, R. Feeding preference and performance in the tropical sea urchin Tripneustes gratilla. Aquaculture 2013, 400-401, 6–13. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; Pirozzi, I. Induction of settlement in the sea urchin Tripneustes gratilla by macroalgae, biofilms and conspecifics: A role for bacteria? Aquaculture 2008, 274, 268–274. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; Pirozzi, I.; Liu, W. The effect of the addition of algae feeding stimulants to artificial diets for the sea urchin Tripneustes gratilla. Aquaculture 2007, 273, 624–633. [Google Scholar] [CrossRef]
- Brink-Hull, M.; Cyrus, M.D.; Macey, B.M.; Rhode, C.; Hull, K.L.; Roodt-Wilding, R. Dietary effects on the reproductive performance of the sea urchin Tripneustes gratilla ll: Implications for offspring performance. Aquaculture 2022, 553, 738034. [Google Scholar] [CrossRef]
- Cyrus, M.D.; Bolton, J.J.; Macey, B.M. The role of the green seaweed Ulva as a dietary supplement for full life-cycle grow-out of Tripneustes gratilla. Aquaculture 2015, 446, 187–197. [Google Scholar] [CrossRef]
- Shpigel, M.; Erez, J. Effect of diets and light regimes on calcification and somatic growth of the sea urchin Tripneustes gratilla elatensis. Aquaculture 2020, 529, 735547. [Google Scholar] [CrossRef]
- Hernández-Almaraz, P.; Rivera, M.J.; Mazariegos-Villarreal, A.; Méndez-Rodríguez, L.C.; Serviere-Zaragoza, E. Macroalgae contribution to the diet of two sea urchins in Sargassum Beds: Tripneustes depressus (Camarodonta: Toxopneustidae) and Eucidaris thouarsii (Cidaroide: Cidaridae). Regional Studies in Marine Science 2022, 53, 102456. [Google Scholar] [CrossRef]
- Casilagan, I.L.N.; Juinio-Meñez, M.A.; Crandall, E.D. Genetic diversity, population structure, and demographic history of exploited sea urchin populations (Tripneustes gratilla) in the Philippines. Journal of Experimental Marine Biology and Ecology 2013, 449, 284–293. [Google Scholar] [CrossRef]
- Toha, A.H.A.; Sumitro, S.B.; Widodo; Hakim, L. Color diversity and distribution of sea urchin Tripneustes gratilla in Cenderawasih Bay ecoregion of Papua, Indonesia. The Egyptian Journal of Aquatic Research 2015, 41, 273–278. [Google Scholar] [CrossRef]
- Loew, E.R.; McFarland, W.N. The underwater visual environment. In The Visual System of Fish, Douglas, R., Djamgoz, M., Eds.; Springer Netherlands: Dordrecht, 1990; pp. 1–43.
- Migaud, H.; Taylor, J.F.; Taranger, G.L.; Davie, A.; Cerdá-Reverter, J.M.; Carrillo, M.; Hansen, T.; Bromage, N.R. A comparative ex vivo and in vivo study of day and night perception in teleosts species using the melatonin rhythm. J Pineal Res 2006, 41, 42–52. [Google Scholar] [CrossRef]
- Zhou, B.; Zheng, S.; Zeng, C. Comparative study of the absorption spectra of several species of green, brown and red algae. Journal of Integrative Plant Biology 1974, 146–155. [Google Scholar]
- de Mooij, T.; de Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of light color on photobioreactor productivity. Algal Research 2016, 15, 32–42. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hanelt, D.; Wiencke, C. Growth and DNA damage in young Laminaria sporophytes exposed to ultraviolet radiation: implication for depth zonation of kelps on Helgoland (North Sea). Marine Biology 2006, 148, 1201–1211. [Google Scholar] [CrossRef]
- Cheng, X. Response of photosynthetic activity of macroalgae to different temperatures and light conditions. 硕士, 2019.
- Sui, X.; Ren, W.; Yan, W.; Liu, S.; Yang, N. Effects of light wavelength on growth and reproduction of the gametophytesof Laminaria japonica Aresch. Marine Sciences 2011, 35, 33–36. [Google Scholar]
- Mizuta, H.; Kai, T.; Tabuchi, K.; Yasui, H. Effects of light quality on the reproduction and morphology of sporophytes of Laminaria japonica (Phaeophyceae). Aquaculture Research 2007, 38, 1323–1329. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Zhang, H.; Fang, L.; Wang, Q.; Ruan, G. Effect of yeast hydrolysate on growth performance, digestion, antioxidant and immunity of crayfish (Litopenaeus vannamei). Acta Hydrobiologica Sinica, 1-12.
- Li, M.; Zhang, F.; Ding, J.; Zuo, R.; Chang, Y. Effects of lipid sources on the growth performance, gonad development, fatty acid profile and transcription of related genes in juvenile sea urchin (Strongylocentrotus intermedius). Aquaculture Nutrition 2021, 27, 28–38. [Google Scholar] [CrossRef]
- Moyano, F.J.; Díaz, M.; Alarcón, F.J.; Sarasquete, M.C. Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiology and Biochemistry 1996, 15, 121–130. [Google Scholar] [CrossRef]
- Ren, X. Influence of environmental factors and nutritional levels on digestive enzyme activities in half-smooth tongue sole (Solea solea). Master's degree, Ocean University of China, 2008.
- Chen, J.; Xi, S.; Qin, C.; Guo, Y.; Pan, W.; Shao, G. Effect of light intensity on the growth and digestive enzyme activities of purple sea urchin(Heliocidaris crassispina, Agassiz, 1864) planktonic larvae. Progress in Fishery Sciences 2021, 42, 125–131. [Google Scholar] [CrossRef]
- Shi, J.; Feng, Y.; Jiang, X.; Liu, X. Effects of different algae and polyculture ratio of sea cucumber Apostichopus japonicus and sea urchin Hemicentrotus pulcherrimus ongrowth, body composition and digestive enzyme activities in seacucumber and sea urchin. Journal of Dalian Ocean University 2020, 35, 509–515. [Google Scholar] [CrossRef]
- Zambonino Infante, J.L.; Cahu, C.L. Ontogeny of the gastrointestinal tract of marine fish larvae. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2001, 130, 477–487. [Google Scholar] [CrossRef]
- Zhao, C.; Feng, W.; Wei, J.; Zhang, L.; Sun, P.; Chang, Y. Effects of temperature and feeding regime on food consumption, growth, gonad production and quality of the sea urchin Strongylocentrotus intermedius. Journal of the Marine Biological Association of the United Kingdom 2016, 96, 185–195. [Google Scholar] [CrossRef]
- Zuo, R.; Hou, S.; Li, G.; Chang, Y. Study on the activities of digestive enzyme and anti-oxidative enzymes injuvenile sea urchin (Strongylocentrotus intermedius). Feed Industry 2016, 37, 26–29. [Google Scholar] [CrossRef]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental toxicity, oxidative stress and apoptosis: Ménage à Trois. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2009, 674, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 2003, 35, 236–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Li, S.; Guo, X.; Fu, Y.; He, N.; Ruan, G.; Wang, Q.; Gao, W.; Fang, L. Impact of nitrite exposure on oxidative stress and antioxidative-related genes responses in the gills of Procambarus clarkii. Fish & Shellfish Immunology 2022, 131, 624–630. [Google Scholar] [CrossRef]
- Yuan, X.; Jiang, F.; Shi, Y.; Xu, J.; Liu, Y.; Deng, P. Effects of high temperature stress on antioxidative and non-specific immunityindices of one-year-old Alosa sapidissima. Journal of Zhejiang University(Agriculture and Life Sciences) 2021, 47, 107–117. [Google Scholar]
- Wu, Z.; You, F.; Wang, Y.; Wen, A.; Ma, D.; Xu, Y.; Zhang, P. The effects of hypoxia and hyperoxia on nucleus anomaly,SOD,CAT activitiesand MDA content in juvenile turbot Scophthalmus maximus. Journal of Shanghai Ocean University 2011, 20, 808–813. [Google Scholar]
- Zhai, S.; Fu, H.; Qiao, H.; Zhang, W.; Jin, S.; Jiang, S.; Xiong, Y.; Xu, L.; Wang, Y.; Hu, Y.; et al. Effects of high temperature on heat shock proteins,antioxidant enzymeactivity,and histology of oriental river prawn Macrobrachium nipponense. Journal of Fishery Sciences of China 2022, 29, 684–695. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).