Submitted:
13 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. A brief history of the genus Populus and a list of poplar pathogens
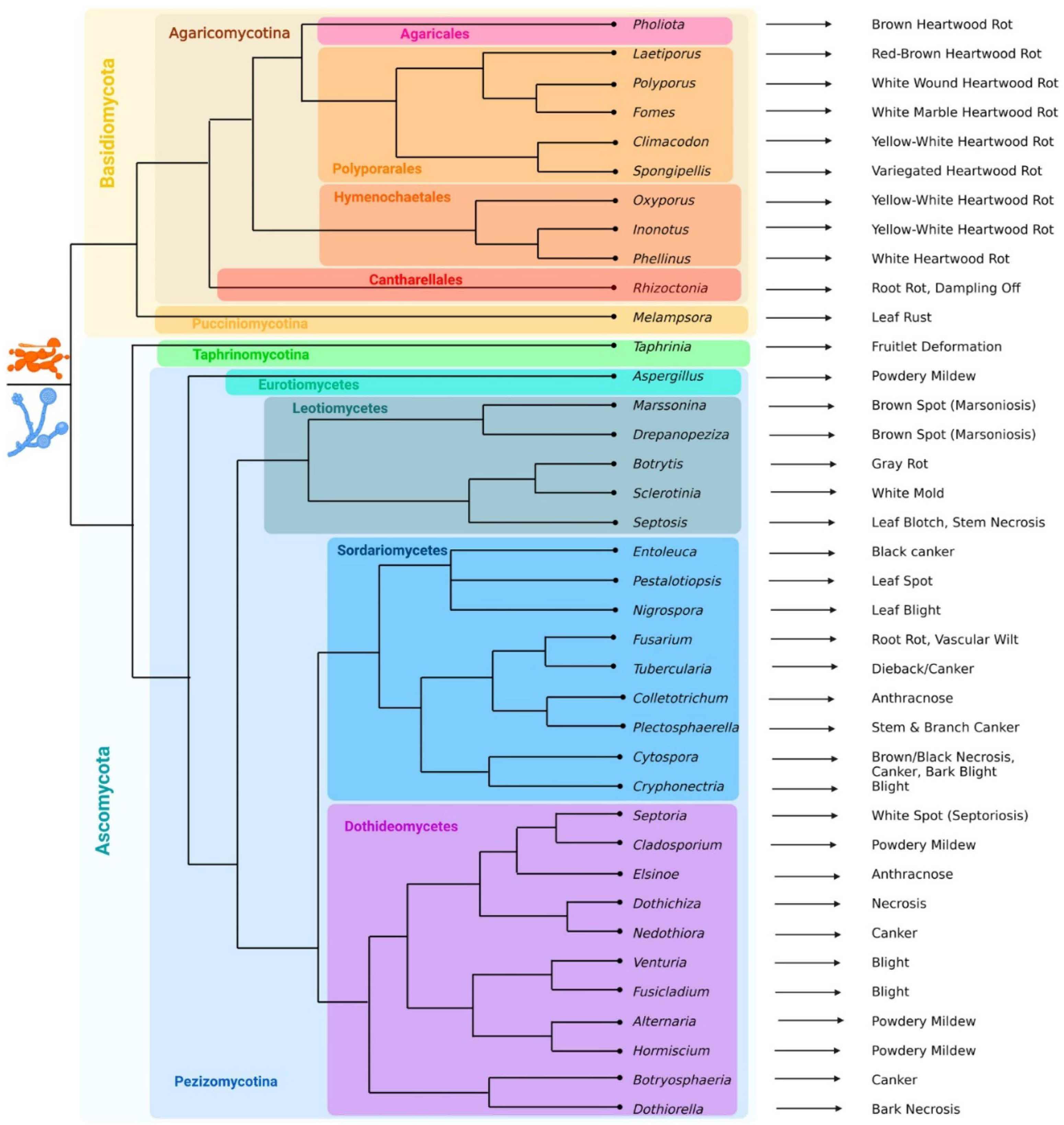
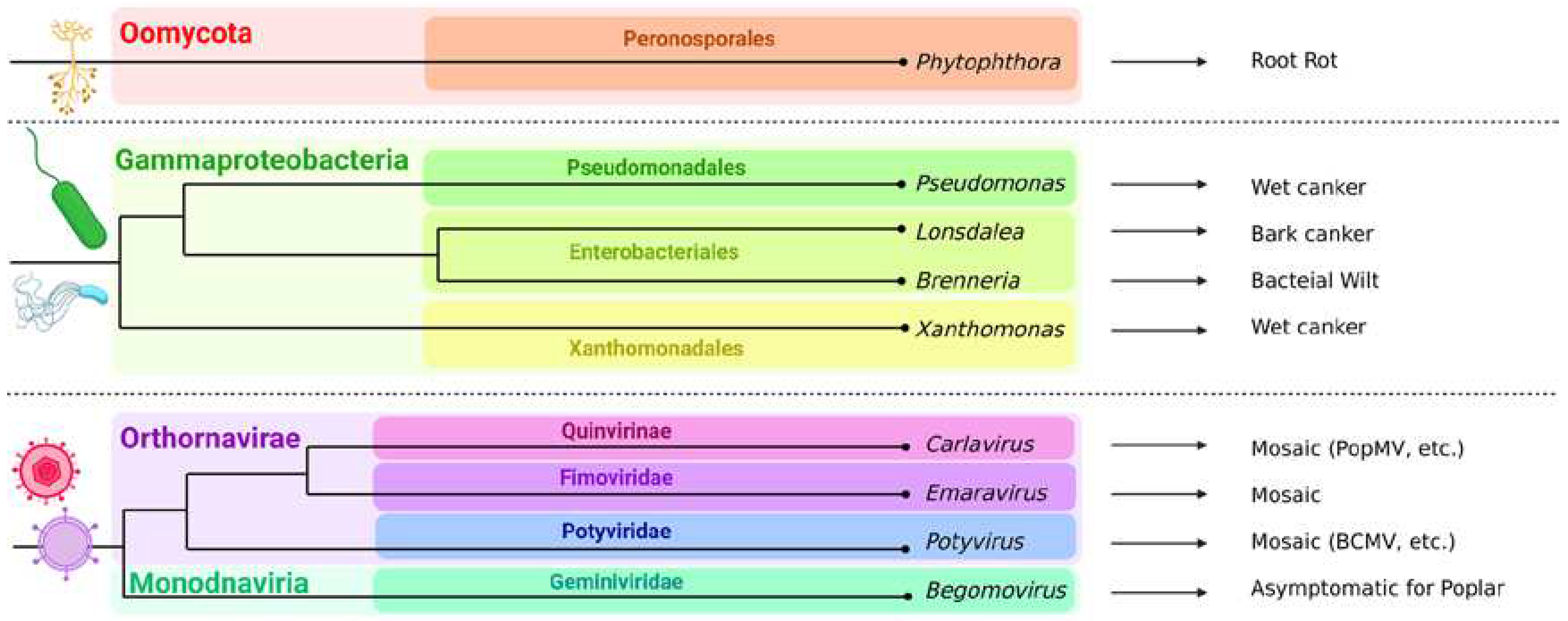
3. The Immunity of Poplars
3.1. A Brief Overview of the Major Plant Receptors and Signaling Pathways
3.2. Role of Primary and Secondary Metabolism in Poplar Disease Resistance
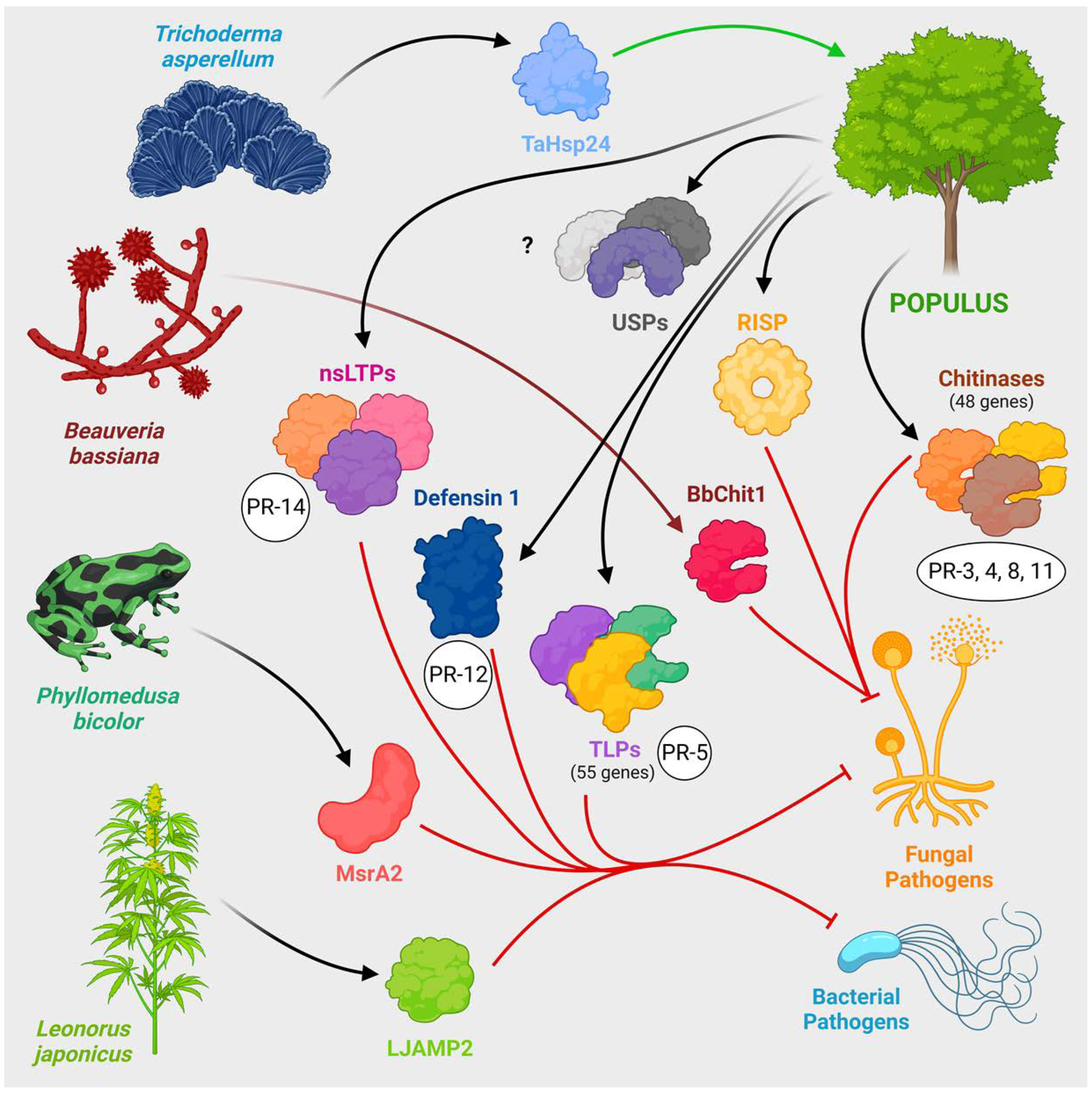
3.3. PRs and Related Defensive Peptides and Proteins in Poplars
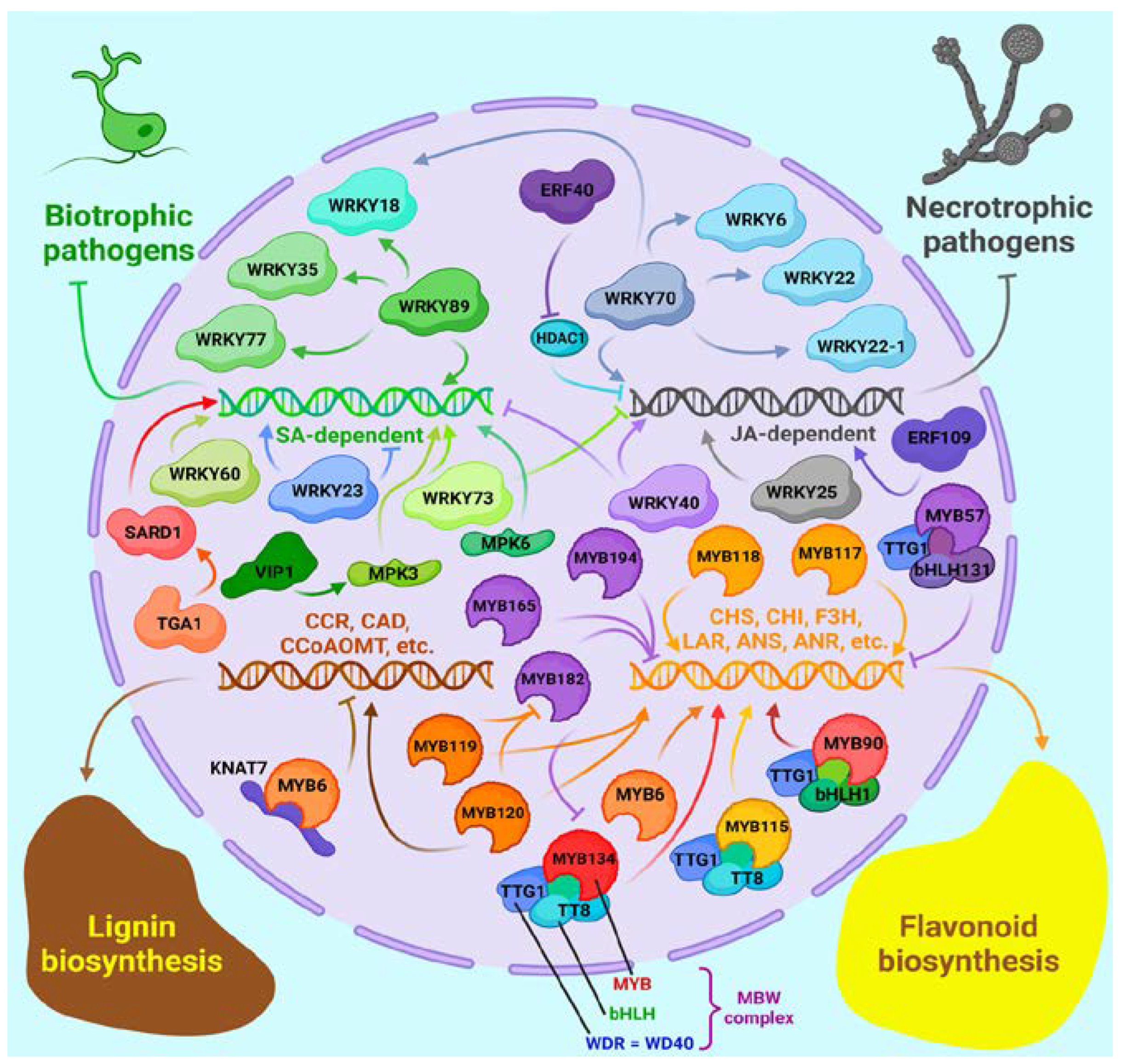
3.4. Transcription Factors Regulating the Immune Response
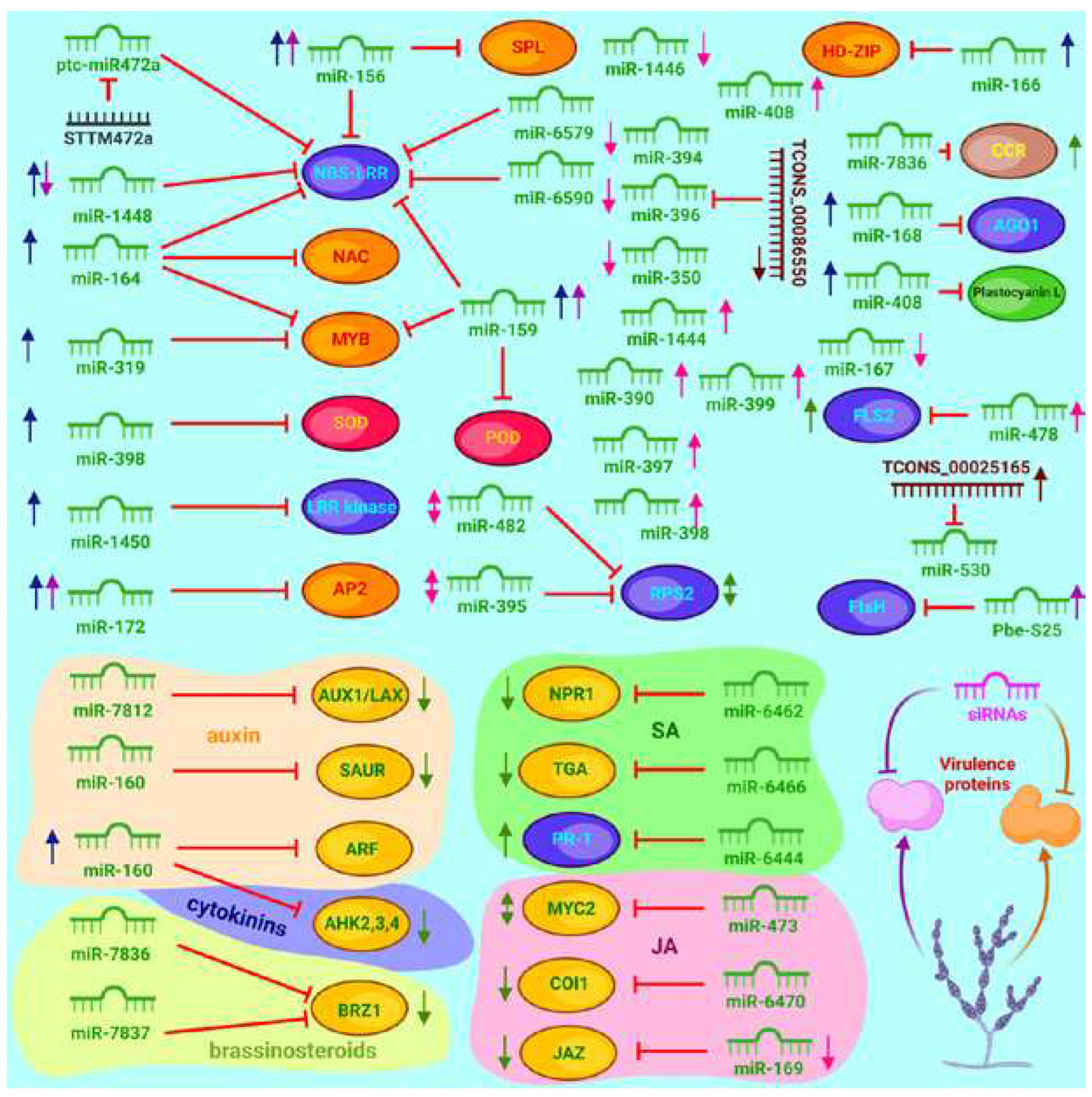
3.5. Contribution of microRNAs to the Regulation of Defense Responses
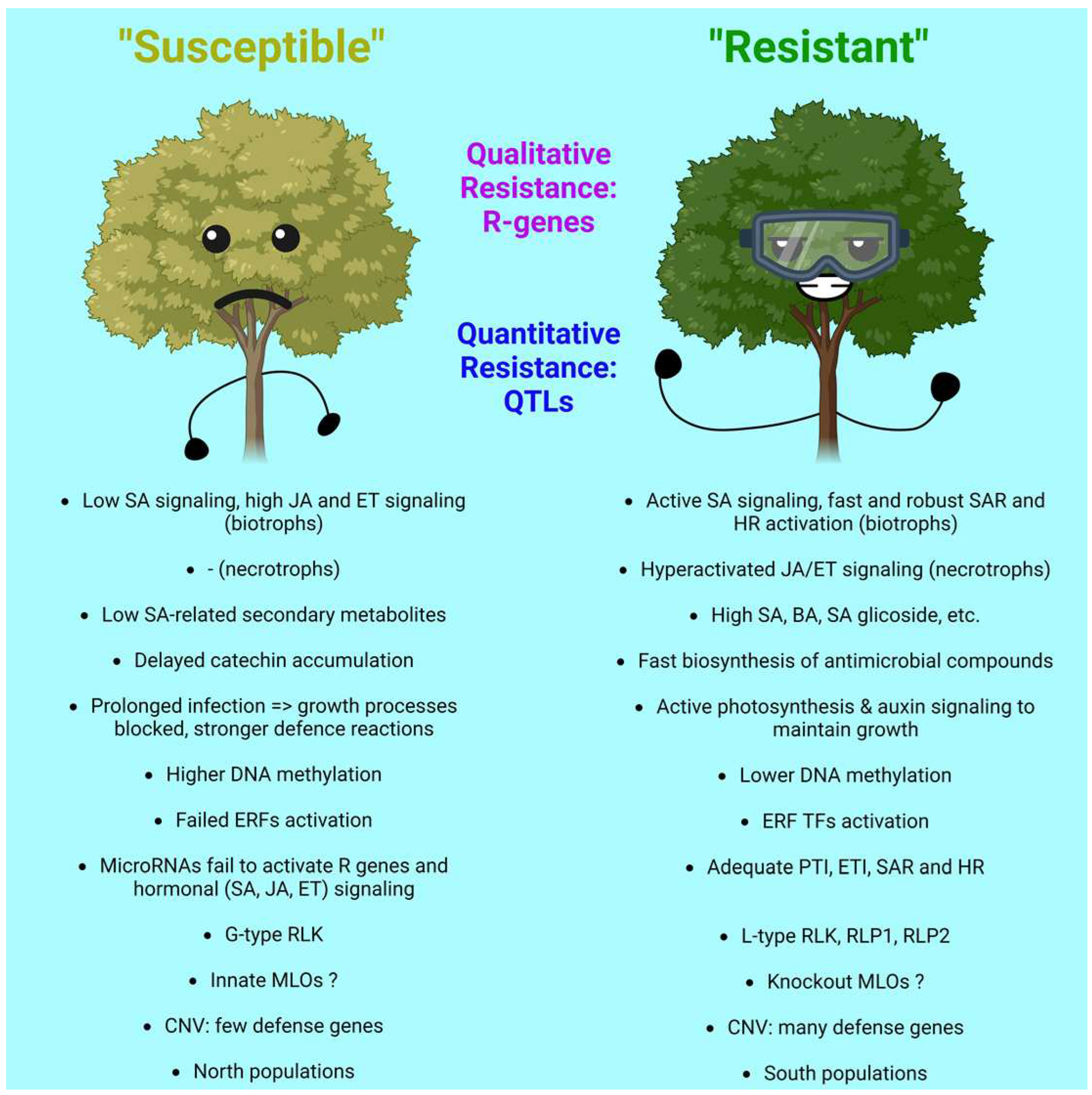
3.6. Intra-Population Differences in Resilience and Related Molecular Mechanisms
4. Environmental Factors Protecting Poplar from Infections: Endophytes, Phytophages and Chemical Elements
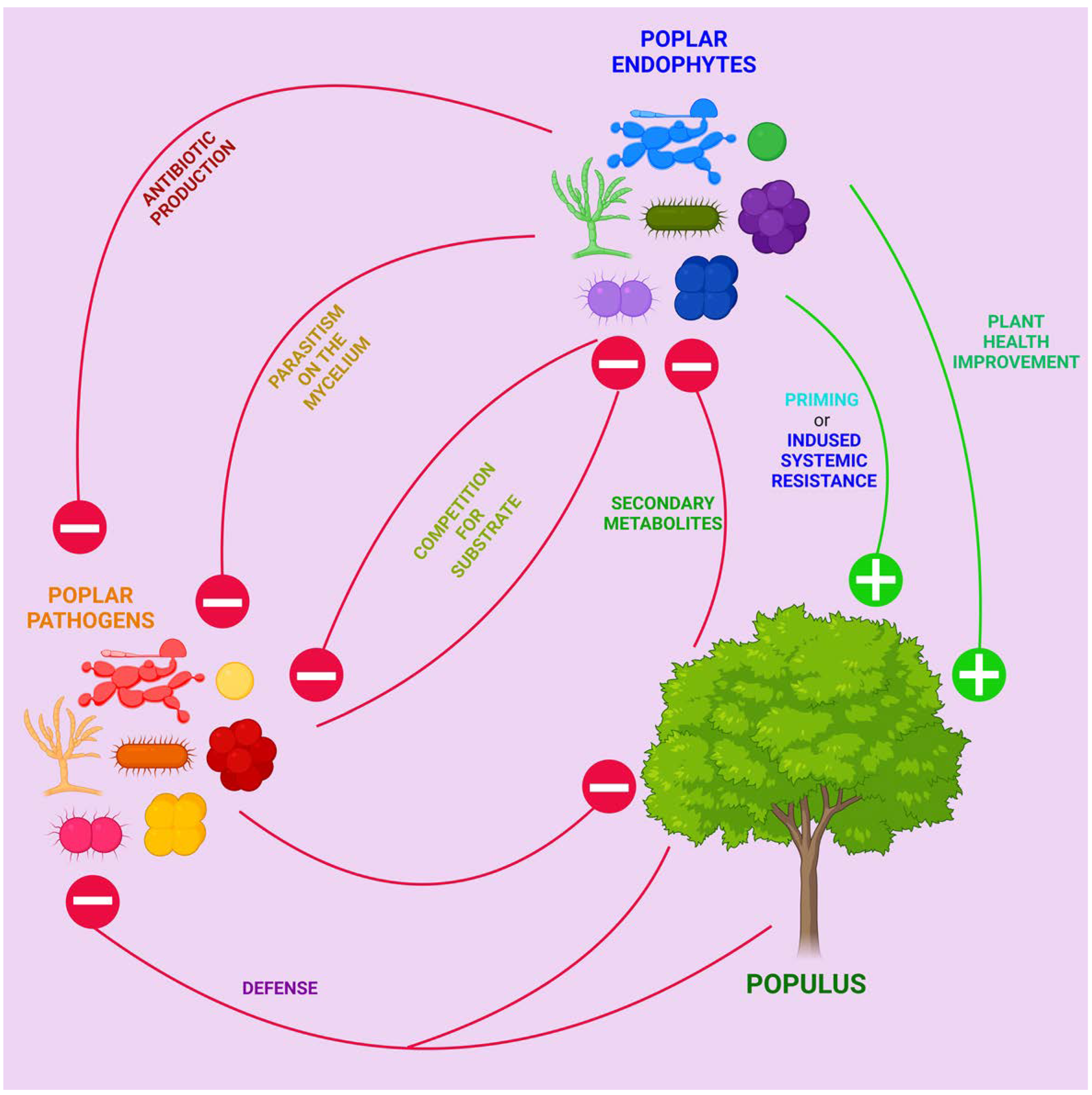
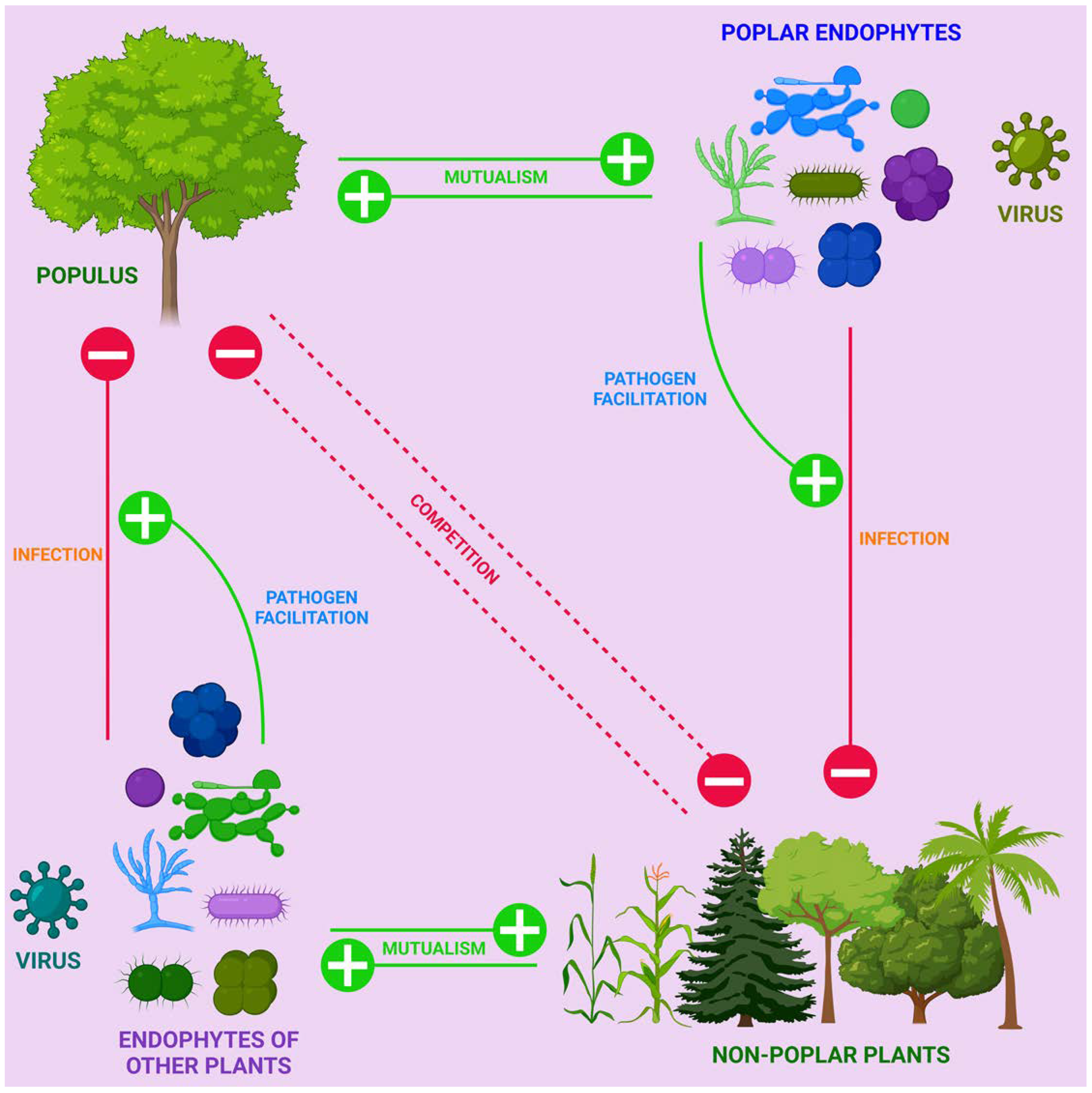
4.1. Endophytes
4.2. Phytophages
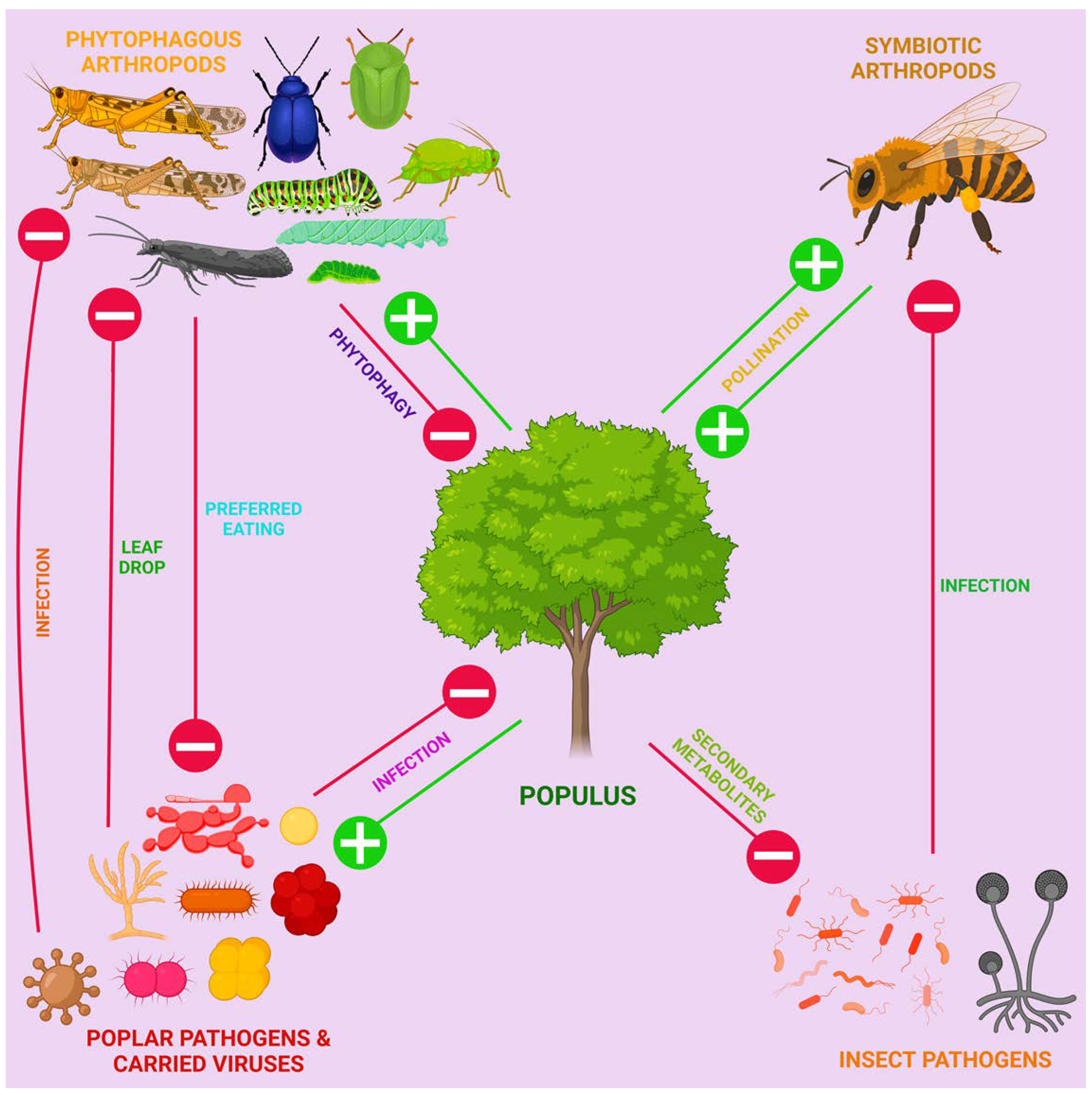
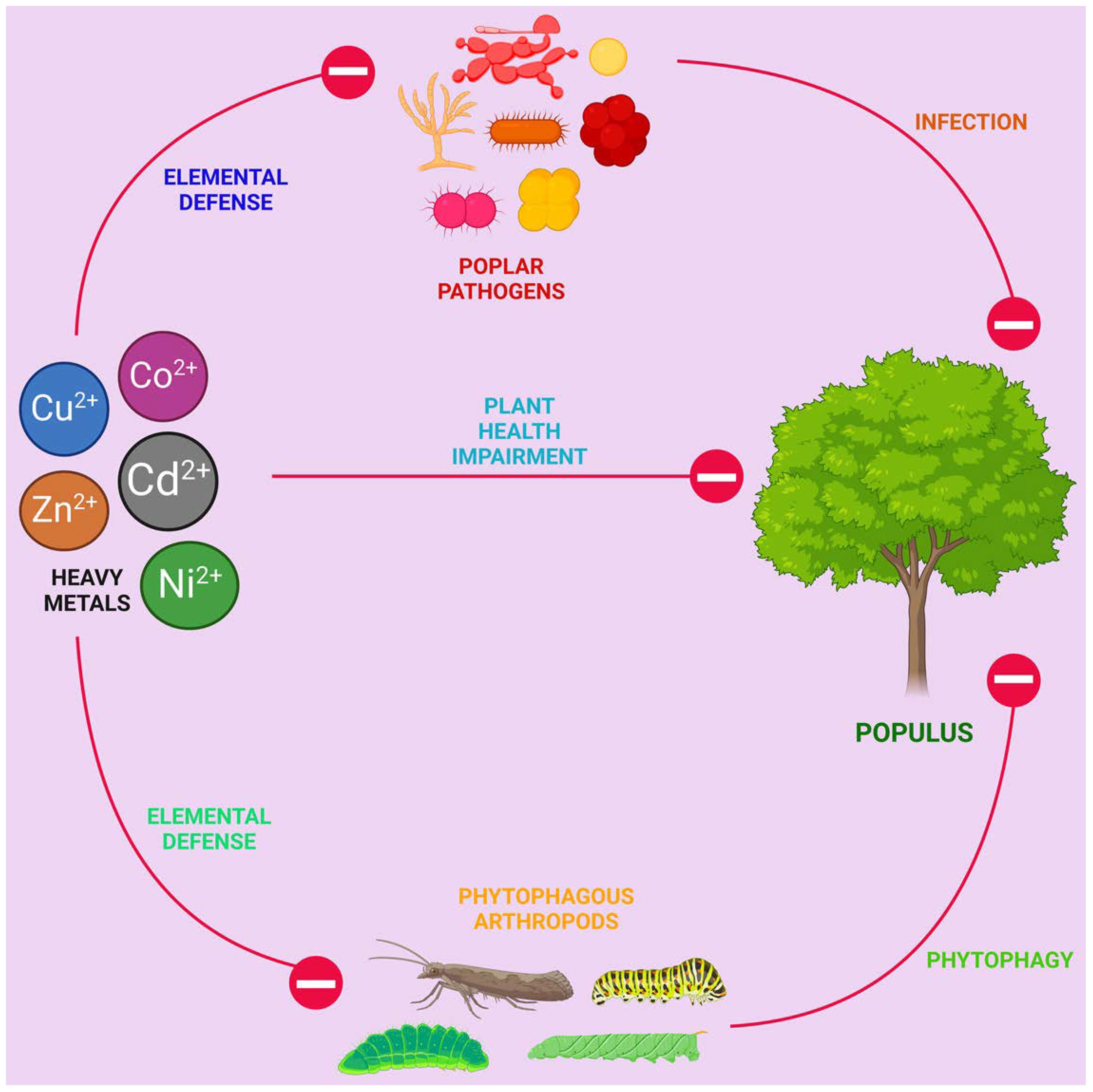
4.3. Elemental Defense Hypothesis
5. The Sex of the Tree - as a Factor in Determining the Effectiveness of Plant Protection
- To introduce a fundamentally new receptor (e.g. AtRLP23) or defence protein (BbChit1, LJAMP2, MsrA2, etc.) into the genome, the same can be achieved by overexpression of the native defensin gene (PtDef). This approach is almost guaranteed to increase plant protection with the minimal tradeoffs, so we consider it to be the most simple and reliable. The result can be achieved using agrobacterial editing, but it is better to use CRISPR/Cas for more precise introduction of transgenes.
- To influence the activity of transcription factors, mainly from the MYB and WRKY families. Overexpression of MYB factors that activate the biosynthesis of secondary metabolites can be used to increase the concentration of flavonoids in the plant, this can be achieved by Agrobacterium-mediated transformation. Knockout of MYBs that inhibit the biosynthesis of secondary metabolites can be accomplished using CRISPR/Cas. Overexpression of WRKYs from different families may increase disease resistance in poplar, and although there is evidence that such overexpression in Arabidopsis may increase susceptibility to some pathogens due to SA-JA antagonism, this may not be an issue for poplar because SA and JA probably have a positive feedback in poplar.
- MicroRNA editing. It can be carried out by overexpression or STTM-mediated knockdown. Transgenic constructs can be introduced using agrobacteria. This field is just developing now and there are only a few examples of work.
- MLO gene knockout. There are 4 candidate genes whose knockout can provide poplar resistance to powdery mildew: PtMLO17, 18, 19 and 24. Agrobacterium must be used for delivery, and the editing itself is performed using the CRISPR/Cas system.
- Knockout of genes from the SWEET, LOB, etc. families discussed in this chapter or already carried out on other plants. But to do this, it is necessary to find exactly those homologues in the poplar genome whose knockout will provide it with resistance to certain diseases. The protocol will be similar to that used for MLOs: transformation using agrobacteria and CRISPR/Cas-mediated knockout.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Zhang, L.; Zhang, Z.; Li, M.; Wang, D.; Zhang, X.; Xi, Z.; Keefover-Ring, K.; Smart, L.B.; DiFazio, S.P.; et al. Phylogenomics of the Genus Populus Reveals Extensive Interspecific Gene Flow and Balancing Selection. New Phytologist 2020, 225, 1370–1382. [Google Scholar] [CrossRef]
- Fuertes, A.; Oliveira, N.; Cañellas, I.; Sixto, H.; Rodríguez-Soalleiro, R. An Economic Overview of Populus Spp. in Short Rotation Coppice Systems under Mediterranean Conditions: An Assessment Tool for Decision-Making. Renewable and Sustainable Energy Reviews 2021, 151, 111577. [Google Scholar] [CrossRef]
- Palmer, M.A.; Bernhardt, E.S.; Chornesky, E.A.; Collins, S.L.; Dobson, A.P.; Duke, C.S.; Gold, B.D.; Jacobson, R.B.; Kingsland, S.E.; Kranz, R.H.; et al. Ecological Science and Sustainability for the 21st Century. Frontiers in Ecology and the Environment 2005, 3, 4–11. [Google Scholar] [CrossRef]
- Wang, Z.; MacFarlane, D.W. Evaluating the Biomass Production of Coppiced Willow and Poplar Clones in Michigan, USA, over Multiple Rotations and Different Growing Conditions. Biomass and Bioenergy 2012, 46, 380–388. [Google Scholar] [CrossRef]
- Quiroga, A.; Marzocchi, V.; Rintoul, I. Influence of Wood Treatments on Mechanical Properties of Wood–Cement Composites and of Populus Euroamericana Wood Fibers. Composites Part B: Engineering 2016, 84, 25–32. [Google Scholar] [CrossRef]
- Rostampour Haftkhani, A.; Hematabadi, H. Effect of Layer Arrangement on Bending Strength of Cross-Laminated Timber (CLT) Manufactured from Poplar (Populus Deltoides L.). Buildings 2022, 12, 608. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Chen, Y.; Li, R.; Wei, J. Lignin Modification Improves the Biofuel Production Potential in Transgenic Populus Tomentosa. Industrial Crops and Products 2012, 37, 170–177. [Google Scholar] [CrossRef]
- Biswal, A.K.; Hao, Z.; Pattathil, S.; Yang, X.; Winkeler, K.; Collins, C.; Mohanty, S.S.; Richardson, E.A.; Gelineo-Albersheim, I.; Hunt, K.; et al. Downregulation of GAUT12 in Populus Deltoides by RNA Silencing Results in Reduced Recalcitrance, Increased Growth and Reduced Xylan and Pectin in a Woody Biofuel Feedstock. Biotechnol Biofuels 2015, 8, 41. [Google Scholar] [CrossRef]
- Macaya-Sanz, D.; Chen, J.; Kalluri, U.C.; Muchero, W.; Tschaplinski, T.J.; Gunter, L.E.; Simon, S.J.; Biswal, A.K.; Bryan, A.C.; Payyavula, R.; et al. Agronomic Performance of Populus Deltoides Trees Engineered for Biofuel Production. Biotechnol Biofuels 2017, 10, 253. [Google Scholar] [CrossRef]
- Porth, I.; El-Kassaby, Y.A. Using Populus as a Lignocellulosic Feedstock for Bioethanol. Biotechnology Journal 2015, 10, 510–524. [Google Scholar] [CrossRef]
- Liberloo, M.; Calfapietra, C.; Lukac, M.; Godbold, D.; Luo, Z.; Polle, A.; Hoosbeek, M.R.; Kull, O.; Marek, M.; Raines, C.; et al. Woody Biomass Production during the Second Rotation of a Bio-energy Populus Plantation Increases in a Future High CO 2 World. Global Change Biology 2006, 12, 1094–1106. [Google Scholar] [CrossRef]
- Gao, S.; Chen, J.; Tang, Y.; Xie, J.; Zhang, R.; Tang, J.; Zhang, X. Ecosystem Carbon (CO2 and CH4) Fluxes of a Populus Dettoides Plantation in Subtropical China during and Post Clear-Cutting. Forest Ecology and Management 2015, 357, 206–219. [Google Scholar] [CrossRef]
- Berlizov, A.N.; Blum, O.B.; Filby, R.H.; Malyuk, I.A.; Tryshyn, V.V. Testing Applicability of Black Poplar (Populus Nigra L.) Bark to Heavy Metal Air Pollution Monitoring in Urban and Industrial Regions. Science of The Total Environment 2007, 372, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Muftakhova, S.I.; Blonskaya, L.N.; Sabirzyanov, I.G.; Konashova, S.I.; Timeryanov, A. Age Dynamics of Growth and Development of Populus Pyramidalis in City Planting. International Journal of Environmental Studies 2021, 78, 77–86. [Google Scholar] [CrossRef]
- Tőzsér, D.; Horváth, R.; Simon, E.; Magura, T. Heavy Metal Uptake by Plant Parts of Populus Species: A Meta-Analysis. Environ Sci Pollut Res 2023, 30, 69416–69430. [Google Scholar] [CrossRef] [PubMed]
- Di Lonardo, S.; Capuana, M.; Arnetoli, M.; Gabbrielli, R.; Gonnelli, C. Exploring the Metal Phytoremediation Potential of Three Populus Alba L. Clones Using an in Vitro Screening. Environ Sci Pollut Res 2011, 18, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rafati, M.; Khorasani, N.; Moattar, F.; Shirvany, A.; Moraghebi, F.; Hosseinzadeh, S. Phytoremediation Potential of Populus Alba and Morus Alba for Cadmium, Chromuim and Nickel Absorption from Polluted Soil. International Journal of Environmental Research 2011, 5. [Google Scholar] [CrossRef]
- Guleria, I.; Kumari, A.; Lacaille-Dubois, M.-A.; Nishant; Kumar, V.; Saini, A.K.; Dhatwalia, J.; Lal, S. A Review on the Genus Populus: A Potential Source of Biologically Active Compounds. Phytochem Rev 2022, 21, 987–1046. [CrossRef]
- Tebbi, S.O.; Debbache-Benaida, N. Phytochemistry, Chemical Composition and Therapeutic Uses of Populus Nigra L. Aerial Parts from 1991-2021 Onwards: An Overview. Sustainable Chemistry and Pharmacy 2022, 30, 100880. [Google Scholar] [CrossRef]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The Genome of Black Cottonwood, Populus Trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef]
- Taylor, G. Populus: Arabidopsis for Forestry. Do We Need a Model Tree? Annals of Botany 2002, 90, 681–689. [Google Scholar] [CrossRef]
- Ellis, B.; Jansson, S.; Strauss, S.H.; Tuskan, G.A. Why and How Populus Became a “Model Tree.” In Genetics and Genomics of Populus; Jansson, S., Bhalerao, R., Groover, A., Eds.; Springer New York: New York, NY, 2010; pp. 3–14. ISBN 978-1-4419-1540-5.
- Douglas, C.J. Populus as a Model Tree. In Comparative and Evolutionary Genomics of Angiosperm Trees; Groover, A., Cronk, Q., Eds.; Plant Genetics and Genomics: Crops and Models; Springer International Publishing: Cham, 2017; Volume 21, pp. 61–84. ISBN 978-3-319-49327-5. [Google Scholar]
- Zeng, Y.; Song, H.; Xia, L.; Yang, L.; Zhang, S. The Responses of Poplars to Fungal Pathogens: A Review of the Defensive Pathway. Front. Plant Sci. 2023, 14, 1107583. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G. A Review of Exapted Resistance to Diseases of Populus. European Journal of Forest Pathology 1998, 28, 209–216. [Google Scholar] [CrossRef]
- Rossetti, M.F.; Stoker, C.; Ramos, J.G. Agrochemicals and Neurogenesis. Molecular and Cellular Endocrinology 2020, 510, 110820. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, J.; Gustavsson, M.; Dekker, S.C.; Van Wezel, A.P. Towards ‘One Substance – One Assessment’: An Analysis of EU Chemical Registration and Aquatic Risk Assessment Frameworks. Journal of Environmental Management 2021, 280, 111692. [Google Scholar] [CrossRef] [PubMed]
- Sundhar, S.; Shakila, R.J.; Jeyasekaran, G.; Aanand, S.; Shalini, R.; Arisekar, U.; Surya, T.; Malini, N.A.H.; Boda, S. Risk Assessment of Organochlorine Pesticides in Seaweeds along the Gulf of Mannar, Southeast India. Marine Pollution Bulletin 2020, 161, 111709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xi, Z.; Wang, M.; Guo, X.; Ma, T. Plastome Phylogeny and Lineage Diversification of Salicaceae with Focus on Poplars and Willows. Ecology and Evolution 2018, 8, 7817–7823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-S.; Zeng, Q.-Y.; Liu, Y.-J. Frequent Ploidy Changes in Salicaceae Indicates Widespread Sharing of the Salicoid Whole Genome Duplication by the Relatives of Populus L. and Salix L. BMC Plant Biol 2021, 21, 535. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Zhang, Z.; Li, M.; Wang, D.; Zhang, X.; Xi, Z.; Keefover-Ring, K.; Smart, L.B.; DiFazio, S.P.; et al. Phylogenomics of the Genus Populus Reveals Extensive Interspecific Gene Flow and Balancing Selection. New Phytologist 2020, 225, 1370–1382. [Google Scholar] [CrossRef]
- Rösch, P.H.; Rösch, P. Chinese Wood Sculptures of the 11th to 13th Centuries: Images of Water-Moon Guanyin in Northern Chinese Temples and Western Collections; Ibidem-Verl: Stuttgart, 2007; ISBN 978-3-89821-662-3. [Google Scholar]
- DESTINATION/ROMANIA: Iasi - the county of centuries-old trees. September 4 2014 .
- Pourtet, J. Unasylva. 1951.
- Section 4 - Poplar (POPULUS, L. ). In Safety Assessment of Transgenic Organisms, Volume 2; Harmonisation of Regulatory Oversight in Biotechnology; OECD, 2006; pp. 100–135. ISBN 978-92-64-09539-7.
- Zhang, B.; Zhu, W.; Diao, S.; Wu, X.; Lu, J.; Ding, C.; Su, X. The Poplar Pangenome Provides Insights into the Evolutionary History of the Genus. Commun Biol 2019, 2, 215. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant–Pathogen Interactions. Nat Rev Genet 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Petrýdesová, J.; Bacigálová, K.; Sulo, P. The Reassignment of Three ‘Lost’ Taphrina Species (Taphrina Bullata, Taphrina Insititiae and Taphrina Rhizophora) Supported by the Divergence of Nuclear and Mitochondrial DNA. International Journal of Systematic and Evolutionary Microbiology 2013, 63, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Spiers, A.G. Comparative Studies of Host Specificity and Symptoms Exhibited by Poplars Infected with Marssonina Brunnea, Marssonina Castagnei and Marssonina Populi. European Journal of Forest Pathology 1984, 14, 202–218. [Google Scholar] [CrossRef]
- Newcombe, G. First Report of Pestalotiopsis Populi-Nigrae on Poplar in North America. Plant Disease 2000, 84, 595–595. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Movahedi, A.; Xu, C.; Sun, W.; Li, L.; Li, D.; Zhuge, Q. Characterization, Expression Profiling, and Functional Analysis of a Populus Trichocarpa Defensin Gene and Its Potential as an Anti-Agrobacterium Rooting Medium Additive. Sci Rep 2019, 9, 15359. [Google Scholar] [CrossRef] [PubMed]
- Chudinova, E.M.; Elansky, S.N. First Report of Septotinia Populiperda on Potato Tubers in Russia. J Plant Pathol 2021, 103, 665–665. [Google Scholar] [CrossRef]
- Bakhshi, M.; Arzanlou, M.; Zare, R.; Groenewald, J.Z.; Crous, P.W. New Species of Septoria Associated with Leaf Spot Diseases in Iran. Mycologia 2019, 111, 1056–1071. [Google Scholar] [CrossRef]
- Newcombe, G. Native Venturia Inopina Sp. Nov., Specific to Populus Trichocarpa and Its Hybrids. Mycological Research 2003, 107, 108–116. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria Parasitica, the Causal Agent of Chestnut Blight: Invasion History, Population Biology and Disease Control. Molecular Plant Pathology 2018, 19, 7–20. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria Parasitica, the Causal Agent of Chestnut Blight: Invasion History, Population Biology and Disease Control. Molecular Plant Pathology 2018, 19, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Ruess, R.W.; Winton, L.M.; Adams, G.C. Widespread Mortality of Trembling Aspen (Populus Tremuloides) throughout Interior Alaskan Boreal Forests Resulting from a Novel Canker Disease. PLoS ONE 2021, 16, e0250078. [Google Scholar] [CrossRef] [PubMed]
- Winton, L.M.; Adams, G.C.; Ruess, R.W. Determining the Novel Pathogen Neodothiora Populina as the Causal Agent of the Aspen Running Canker Disease in Alaska. Canadian Journal of Plant Pathology 2022, 44, 103–114. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Sun, N.; Wang, X.; Feng, Y.; Zhang, X. Identification and Functional Verification of Differences in Phenolic Compounds Between Resistant and Susceptible Populus Species. Phytopathology® 2020, 110, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Unsicker, S.B.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Accumulation of Catechin and Proanthocyanidins in Black Poplar Stems After Infection by Plectosphaerella Populi: Hormonal Regulation, Biosynthesis and Antifungal Activity. Front. Plant Sci. 2019, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Ruess, R.W.; Winton, L.M.; Adams, G.C. Widespread Mortality of Trembling Aspen (Populus Tremuloides) throughout Interior Alaskan Boreal Forests Resulting from a Novel Canker Disease. PLoS ONE 2021, 16, e0250078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cheng, Q. Heterologous Expression of Arabidopsis Pattern Recognition Receptor RLP23 Increases Broad-spectrum Resistance in Poplar to Fungal Pathogens. Molecular Plant Pathology 2023, 24, 80–86. [Google Scholar] [CrossRef]
- Dawwam, G.E.; Sehim, A.E. Promising Biological Agents Represented in Bacillus Velezensis 33RB and Aspergillus Niger 46SF Endophytic Isolates for Controlling Populus Tomentosa Wilt and Anthracnose Diseases. Egypt J Biol Pest Control 2022, 32, 144. [Google Scholar] [CrossRef]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The Transcription Factor MYB115 Contributes to the Regulation of Proanthocyanidin Biosynthesis and Enhances Fungal Resistance in Poplar. New Phytologist 2017, 215, 351–367. [Google Scholar] [CrossRef]
- Su, T.; Zhou, B.; Cao, D.; Pan, Y.; Hu, M.; Zhang, M.; Wei, H.; Han, M. Transcriptomic Profiling of Populus Roots Challenged with Fusarium Reveals Differential Responsive Patterns of Invertase and Invertase Inhibitor-Like Families within Carbohydrate Metabolism. JoF 2021, 7, 89. [Google Scholar] [CrossRef]
- Newcombe, G.; Fraser, S.J.; Ridout, M.; Busby, P.E. Leaf Endophytes of Populus Trichocarpa Act as Pathogens of Neighboring Plant Species. Front. Microbiol. 2020, 11, 573056. [Google Scholar] [CrossRef]
- Walker, P.L.; Ziegler, D.J.; Giesbrecht, S.; McLoughlin, A.; Wan, J.; Khan, D.; Hoi, V.; Whyard, S.; Belmonte, M.F. Control of White Mold (Sclerotinia Sclerotiorum) through Plant-Mediated RNA Interference. Sci Rep 2023, 13, 6477. [Google Scholar] [CrossRef] [PubMed]
- Vialle, A.; Frey, P.; Hambleton, S.; Bernier, L.; Hamelin, R.C. Poplar Rust Systematics and Refinement of Melampsora Species Delineation. Fungal Diversity 2011, 50, 227–248. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, H.; Ng, T.B. Isolation and Purification of Polysaccharides with Anti-Tumor Activity from Pholiota Adiposa (Batsch) P. Kumm. (Higher Basidiomycetes). Int J Med Mushr 2012, 14, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, R.; Yan, J.; Xu, K.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Purification and Characterization of Inonotus Hispidus Exopolysaccharide and Its Protective Effect on Acute Alcoholic Liver Injury in Mice. International Journal of Biological Macromolecules 2019, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Striganavičiūtė, G.; Žiauka, J.; Sirgedaitė-Šėžienė, V.; Vaitiekūnaitė, D. Impact of Plant-Associated Bacteria on the In Vitro Growth and Pathogenic Resistance against Phellinus Tremulae of Different Aspen (Populus) Genotypes. Microorganisms 2021, 9, 1901. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-W.; Vlasák, J.; Qin, W.-M.; Dai, Y.-C. Global Diversity and Phylogeny of the Phellinus Igniarius Complex (Hymenochaetales, Basidiomycota) with the Description of Five New Species. Mycologia 2016, 108, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Vasaitis, R.; Menkis, A.; Lim, Y.W.; Seok, S.; Tomsovsky, M.; Jankovsky, L.; Lygis, V.; Slippers, B.; Stenlid, J. Genetic Variation and Relationships in Laetiporus Sulphureus s. Lat., as Determined by ITS rDNA Sequences and in Vitro Growth Rate. Mycological Research 2009, 113, 326–336. [Google Scholar] [CrossRef]
- Minter, D.W.; Soliman, G.S. Laetiporus Sulphureus. [Descriptions of Fungi and Bacteria]. Descriptions of Fungi and Bacteria 2022, 2297. [CrossRef]
- Schubert, M.; Fink, S.; Schwarze, F.W.M.R. Evaluation of Trichoderma Spp. as a Biocontrol Agent against Wood Decay Fungi in Urban Trees. Biological Control 2008, 45, 111–123. [Google Scholar] [CrossRef]
- Badalyan, S.; Zhuykova, E.; Mukhin, V. The Phylogenetic Analysis of Armenian Collections of Medicinal Tinder Polypore Fomes Fomentarius (Agaricomycetes, Polyporaceae). Italian Journal of Mycology 2022, 23–33. [Google Scholar] [CrossRef]
- Szczepkowski, A.; Kowalczuk, W. Current Conservation Status of the Fungus Spongipellis Spumeus in Poland Revised Based on New Data. Polish Journal of Ecology 2020, 68, 1. [Google Scholar] [CrossRef]
- Doty, S.L.; Joubert, P.M.; Firrincieli, A.; Sher, A.W.; Tournay, R.; Kill, C.; Parikh, S.S.; Okubara, P. Potential Biocontrol Activities of Populus Endophytes against Several Plant Pathogens Using Different Inhibitory Mechanisms. Pathogens 2022, 12, 13. [Google Scholar] [CrossRef]
- Justo, A.; Miettinen, O.; Floudas, D.; Ortiz-Santana, B.; Sjökvist, E.; Lindner, D.; Nakasone, K.; Niemelä, T.; Larsson, K.-H.; Ryvarden, L.; et al. A Revised Family-Level Classification of the Polyporales (Basidiomycota). Fungal Biology 2017, 121, 798–824. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Matheny, P.B. The Relative Ages of Ectomycorrhizal Mushrooms and Their Plant Hosts Estimated Using Bayesian Relaxed Molecular Clock Analyses. BMC Biol 2009, 7, 13. [Google Scholar] [CrossRef]
- Ekanayaka, A. Preliminary Classification of Leotiomycetes. Mycosphere 2019, 10, 310–489. [Google Scholar] [CrossRef]
- Baral, H.-O.; Rönsch, P.; Richter, U.; Urban, A.; Kruse, J.; Bemmann, M.; Kummer, V.; Valencia, F.J.; Huth, W. Schroeteria Decaisneana, S. Poeltii, and Ciboria Ploettneriana (Sclerotiniaceae, Helotiales, Ascomycota), Three Parasites on Veronica Seeds: First Report of Teleomorphs in Schroeteria. Mycol Progress 2022, 21, 359–407. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Groenewald, J.Z.; Coetzee, M.P.A.; Wingfield, M.J.; Crous, P.W. Evolution of Lifestyles in Capnodiales. Studies in Mycology 2020, 95, 381–414. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.I.; Batzer, J.C.; Harrington, T.C.; Crous, P.W.; Lavrov, D.V.; Li, H.; Gleason, M.L. Ancestral State Reconstruction Infers Phytopathogenic Origins of Sooty Blotch and Flyspeck Fungi on Apple. Mycologia 2016, 108, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Lackus, N.D.; Morawetz, J.; Xu, H.; Gershenzon, J.; Dickschat, J.S.; Köllner, T.G. The Sesquiterpene Synthase PtTPS5 Produces (1S,5S,7R,10R)-Guaia-4(15)-En-11-Ol and (1S,7R,10R)-Guaia-4-En-11-Ol in Oomycete-Infected Poplar Roots. Molecules 2021, 26, 555. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, Y.; Taguchi, F.; Mukaihara, T. Pathogenicity and Virulence Factors of Pseudomonas Syringae. J Gen Plant Pathol 2013, 79, 285–296. [Google Scholar] [CrossRef]
- Goychuk, A.; Kulbanska, I.; Shvets, M.; Pasichnyk, L.; Patyka, V.; Kalinichenko, A.; Degtyareva, L. Bacterial Diseases of Bioenergy Woody Plants in Ukraine. Sustainability 2023, 15, 4189. [Google Scholar] [CrossRef]
- Nesme, X. Differential Host-Pathogen Interactions Among Clones of Poplar and Strains of Xanthomonas Populi Pv. Populi. Phytopathology 1994, 84, 101. [Google Scholar] [CrossRef]
- Li, A.; He, W. Molecular Aspects of an Emerging Poplar Canker Caused by Lonsdalea Populi. Front. Microbiol. 2019, 10, 2496. [Google Scholar] [CrossRef]
- Zlatković, M.; Tenorio-Baigorria, I.; Lakatos, T.; Tóth, T.; Koltay, A.; Pap, P.; Marković, M.; Orlović, S. Bacterial Canker Disease on Populus × Euramericana Caused by Lonsdalea Populi in Serbia. Forests 2020, 11, 1080. [Google Scholar] [CrossRef]
- Tóth, T.; Lakatos, T.; Koltay, A. Lonsdalea Quercina Subsp. Populi Subsp. Nov., Isolated from Bark Canker of Poplar Trees. International Journal of Systematic and Evolutionary Microbiology 2013, 63, 2309–2313. [Google Scholar] [CrossRef] [PubMed]
- Abelleira, A.; Moura, L.; Aguín, O.; Salinero, C. First Report of Lonsdalea Populi Causing Bark Canker Disease on Poplar in Portugal. Plant Disease 2019, 103, 2121. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Ren, F.; Guo, L.; Chang, J.; Cleenwerck, I.; Ma, Y.; Wang, H. A Canker Disease of Populus × Euramericana in China Caused by Lonsdalea Quercina Subsp. Populi. Plant Disease 2014, 98, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Wang, M.; Cheng, Q. Cloning and Characterization of the PtVIP1 Gene in Populus. J. For. Res. 2019, 30, 2259–2266. [Google Scholar] [CrossRef]
- Naylor, M.; Reeves, J.; Cooper, J.I.; Edwards, M.-L.; Wang, H. Construction and Properties of a Gene-Silencing Vector Based on Poplar Mosaic Virus (Genus Carlavirus). Journal of Virological Methods 2005, 124, 27–36. [Google Scholar] [CrossRef]
- Smith, C.M.; Campbell, M.M. Populus Genotypes Differ in Infection by, and Systemic Spread of, Poplar Mosaic Virus. Plant Pathology 2004, 53, 780–787. [Google Scholar] [CrossRef]
- Von Bargen, S.; Al Kubrusli, R.; Gaskin, T.; Fürl, S.; Hüttner, F.; Blystad, D.; Karlin, D.G.; Jalkanen, R.; Büttner, C. Characterisation of a Novel Emaravirus Identified in Mosaic-diseased Eurasian Aspen ( POPULUS TREMULA ). Annals of Applied Biology 2020, 176, 210–222. [Google Scholar] [CrossRef]
- Hibben, C.R.; Bozarth, R.F.; Reese, J. Identification of Tobacco Necrosis Virus in Deteriorating Clones of Aspen. Forest Science 1979, 25, 557–567. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Gautam, S.; Rasmussen, D.A.; Srinivasan, R. Aphid Transmission of Potyvirus: The Largest Plant-Infecting RNA Virus Genus. Viruses 2020, 12, 773. [Google Scholar] [CrossRef]
- Martin, R.R. Isolation of a Potyvirus from Declining Clones of Populus. Phytopathology 1982, 72, 1158. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Shen, W.; Li, M.; Fu, Y.; Li, Z.; Li, J.; Liu, H.; Su, X.; Zhang, B.; et al. Integrated Transcriptome and microRNA Sequencing Analyses Reveal Gene Responses in Poplar Leaves Infected by the Novel Pathogen Bean Common Mosaic Virus (BCMV). Front. Plant Sci. 2023, 14, 1163232. [Google Scholar] [CrossRef]
- Mustafa, R.; Hamza, M.; Rehman, A.U.; Kamal, H.; Tahir, M.N.; Mansoor, S.; Scheffler, B.E.; Briddon, R.W.; Amin, I. Asymptomatic Populus Alba: A Tree Serving as a Reservoir of Begomoviruses and Associated Satellites. Australasian Plant Pathol. 2022, 51, 577–586. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Hwang, S.-Y.; Osier, T.L. Phytochemical Variation in Quaking Aspen: Effects on Gypsy Moth Susceptibility to Nuclear Polyhedrosis Virus. Journal of Chemical Ecology 1999, 25, 1331–1341. [Google Scholar] [CrossRef]
- Lo, C.-C.; Bonner, C.A.; Xie, G.; D’Souza, M.; Jensen, R.A. Cohesion Group Approach for Evolutionary Analysis of Aspartokinase, an Enzyme That Feeds a Branched Network of Many Biochemical Pathways. Microbiol Mol Biol Rev 2009, 73, 594–651. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.I.; Silas, S.; Wang, Y.; Wu, S.; Bocek, M.; Kazlauskas, D.; Krupovic, M.; Fire, A.; Dolja, V.V.; Koonin, E.V. Doubling of the Known Set of RNA Viruses by Metagenomic Analysis of an Aquatic Virome. Nat Microbiol 2020, 5, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Su, X.H. Patterns of Molecular Evolution and Predicted Function in Thaumatin-like Proteins of Populus Trichocarpa. Planta 2010, 232, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Li, Y.; Chen, Y.; Zhang, X.; Guan, H.; Yin, T. Melampsora Larici-Populina, the Main Rust Pathogen, Causes Loss in Biomass Production of Black Cottonwood Plantations in the South of China. Phytoparasitica 2013, 41, 337–344. [Google Scholar] [CrossRef]
- Biselli, C.; Vietto, L.; Rosso, L.; Cattivelli, L.; Nervo, G.; Fricano, A. Advanced Breeding for Biotic Stress Resistance in Poplar. Plants 2022, 11, 2032. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Petre, B.; Hacquard, S.; Duplessis, S.; Rouhier, N. Genome Analysis of Poplar LRR-RLP Gene Clusters Reveals RISP, a Defense-Related Gene Coding a Candidate Endogenous Peptide Elicitor. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.R.; Prates, E.T.; Miller, J.I.; Demerdash, O.N.A.; Shah, M.; Kainer, D.; Cliff, A.; Sullivan, K.A.; Cashman, M.; Lane, M.; et al. Exploring the Role of Plant Lysin Motif Receptor-like Kinases in Regulating Plant-Microbe Interactions in the Bioenergy Crop Populus. Computational and Structural Biotechnology Journal 2023, 21, 1122–1139. [Google Scholar] [CrossRef]
- Muhr, M. Characterization of Populus x Canescens LysM Receptor-Like Kinases CERK1-1 and CERK1-2 and Their Role in Chitin Signaling, Georg-August-University Göttingen, 2022.
- Zhao, Y.; Zheng, X.; Tabima, J.F.; Sondreli, K.; Zhu, S.; Hundley, H.; Bauer, D.; Barry, K.; Zhang, Y.; Schmutz, J.; et al. Secreted Effector Proteins of the Poplar Leaf Spot and Stem Canker Pathogen Sphaerulina Musiva Manipulate Plant Immunity and Contribute to Virulence in Diverse Ways. MPMI 2023, MPMI-07-23-0091-R. [CrossRef]
- Germain, H.; Séguin, A. Innate Immunity: Has Poplar Made Its BED? New Phytologist 2011, 189, 678–687. [Google Scholar] [CrossRef]
- Marchal, C.; Zhang, J.; Zhang, P.; Fenwick, P.; Steuernagel, B.; Adamski, N.M.; Boyd, L.; McIntosh, R.; Wulff, B.B.H.; Berry, S.; et al. BED-Domain-Containing Immune Receptors Confer Diverse Resistance Spectra to Yellow Rust. Nature Plants 2018, 4, 662–668. [Google Scholar] [CrossRef]
- Plett, J.M.; Daguerre, Y.; Wittulsky, S.; Vayssières, A.; Deveau, A.; Melton, S.J.; Kohler, A.; Morrell-Falvey, J.L.; Brun, A.; Veneault-Fourrey, C.; et al. Effector MiSSP7 of the Mutualistic Fungus Laccaria Bicolor Stabilizes the Populus JAZ6 Protein and Represses Jasmonic Acid (JA) Responsive Genes. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 8299–8304. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Kumar, D. Salicylic Acid Signaling in Disease Resistance. Plant Science 2014, 228, 127–134. [Google Scholar] [CrossRef]
- Ullah, C.; Chen, Y.-H.; Ortega, M.A.; Tsai, C.-J. The Diversity of Salicylic Acid Biosynthesis and Defense Signaling in Plants: Knowledge Gaps and Future Opportunities. Current Opinion in Plant Biology 2023, 72, 102349. [Google Scholar] [CrossRef]
- Li, M.; Yu, G.; Cao, C.; Liu, P. Metabolism, Signaling, and Transport of Jasmonates. Plant Communications 2021, 2, 100231. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate Action in Plant Growth and Development. Journal of Experimental Botany 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Dubois, M.; Van Den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends in Plant Science 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van De Poel, B. The Regulation of Ethylene Biosynthesis: A Complex Multilevel Control Circuitry. New Phytologist 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene Signaling in Plants. Journal of Biological Chemistry 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Yang, F.; Chai, S.; Wang, L.; De Dios, V.R.; Tan, W.; Yao, Y. Ethylene Activates Poplar Defense against Dothiorella Gregaria Sacc by Regulating Reactive Oxygen Species Accumulation. Physiologia Plantarum 2022, 174, e13726. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? IJMS 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Tsai, C.; Unsicker, S.B.; Xue, L.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Salicylic Acid Activates Poplar Defense against the Biotrophic Rust Fungus Melampsora Larici-populina via Increased Biosynthesis of Catechin and Proanthocyanidins. New Phytologist 2019, 221, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Schmidt, A.; Reichelt, M.; Tsai, C.; Gershenzon, J. Lack of Antagonism between Salicylic Acid and Jasmonate Signalling Pathways in Poplar. New Phytologist 2022, 235, 701–717. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic Acquired Resistance and Salicylic Acid: Past, Present, and Future. MPMI 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The Plant Hypersensitive Response: Concepts, Control and Consequences. Molecular Plant Pathology 2019, 20, 1163–1178. [Google Scholar] [CrossRef]
- Dalio, R.J.D.; Paschoal, D.; Arena, G.D.; Magalhães, D.M.; Oliveira, T.S.; Merfa, M.V.; Maximo, H.J.; Machado, M.A. Hypersensitive Response: From NLR Pathogen Recognition to Cell Death Response. Annals of Applied Biology 2021, 178, 268–280. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, M.; Zhou, H.; Zhou, X.; Wang, Y. Metabolite Profiles of Populus in Response to Pathogen Stress. Biochemical and Biophysical Research Communications 2015, 465, 421–426. [Google Scholar] [CrossRef]
- Su, T.; Han, M.; Min, J.; Zhou, H.; Zhang, Q.; Zhao, J.; Fang, Y. Functional Characterization of Invertase Inhibitors PtC/VIF1 and 2 Revealed Their Involvements in the Defense Response to Fungal Pathogen in Populus Trichocarpa. Front. Plant Sci. 2020, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Siemens, J.; González, M.; Wolf, S.; Hofmann, C.; Greiner, S.; Du, Y.; Rausch, T.; Roitsch, T.; Ludwig-Müller, J. Extracellular Invertase Is Involved in the Regulation of Clubroot Disease in Arabidopsis Thaliana. Molecular Plant Pathology 2011, 12, 247–262. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and Invertases, a Part of the Plant Defense Response to the Biotic Stresses. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Veillet, F.; Gaillard, C.; Coutos-Thévenot, P.; La Camera, S. Targeting the AtCWIN1 Gene to Explore the Role of Invertases in Sucrose Transport in Roots and during Botrytis Cinerea Infection. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Han, M.; Min, J.; Chen, P.; Mao, Y.; Huang, Q.; Tong, Q.; Liu, Q.; Fang, Y. Genome-Wide Survey of Invertase Encoding Genes and Functional Characterization of an Extracellular Fungal Pathogen-Responsive Invertase in Glycine Max. IJMS 2018, 19, 2395. [Google Scholar] [CrossRef]
- Chang, Q.; Liu, J.; Lin, X.; Hu, S.; Yang, Y.; Li, D.; Chen, L.; Huai, B.; Huang, L.; Voegele, R.T.; et al. A Unique Invertase Is Important for Sugar Absorption of an Obligate Biotrophic Pathogen during Infection. New Phytologist 2017, 215, 1548–1561. [Google Scholar] [CrossRef]
- Han, M.; Xu, X.; Xiong, Y.; Wei, H.; Yao, K.; Huang, T.; Long, Y.; Su, T. Genome-Wide Survey and Expression Analyses of Hexokinase Family in Poplar (Populus Trichocarpa). Plants 2022, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, W.; Zhang, Y.; Xing, J.; Li, J.; Feng, J.; Su, X.; Zhao, J. Fungal Canker Pathogens Trigger Carbon Starvation by Inhibiting Carbon Metabolism in Poplar Stems. Sci Rep 2019, 9, 10111. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Li, P.; Zhang, Y.; Li, J.; Liu, Y.; Lachenbruch, B.; Su, X.; Zhao, J. Fungal Pathogens of Canker Disease Trigger Canopy Dieback in Poplar Saplings by Inducing Functional Failure of the Phloem and Cambium and Carbon Starvation in the Xylem. Physiological and Molecular Plant Pathology 2020, 112, 101523. [Google Scholar] [CrossRef]
- Han, M.; Xu, X.; Li, X.; Xu, M.; Hu, M.; Xiong, Y.; Feng, J.; Wu, H.; Zhu, H.; Su, T. New Insight into Aspartate Metabolic Pathways in Populus: Linking the Root Responsive Isoenzymes with Amino Acid Biosynthesis during Incompatible Interactions of Fusarium Solani. IJMS 2022, 23, 6368. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microbial Pathogenesis 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Movahedi, A.; Almasi Zadeh Yaghuti, A.; Wei, H.; Rutland, P.; Sun, W.; Mousavi, M.; Li, D.; Zhuge, Q. Plant Secondary Metabolites with an Overview of Populus. IJMS 2021, 22, 6890. [Google Scholar] [CrossRef]
- Weng, J.; Chapple, C. The Origin and Evolution of Lignin Biosynthesis. New Phytologist 2010, 187, 273–285. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.-G.; Muchero, W. Regulation of Lignin Biosynthesis and Its Role in Growth-Defense Tradeoffs. Front. Plant Sci. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Barros, J.; Escamilla-Trevino, L.; Song, L.; Rao, X.; Serrani-Yarce, J.C.; Palacios, M.D.; Engle, N.; Choudhury, F.K.; Tschaplinski, T.J.; Venables, B.J.; et al. 4-Coumarate 3-Hydroxylase in the Lignin Biosynthesis Pathway Is a Cytosolic Ascorbate Peroxidase. Nat Commun 2019, 10, 1994. [Google Scholar] [CrossRef]
- Dixon, R.A.; Barros, J. Lignin Biosynthesis: Old Roads Revisited and New Roads Explored. Open Biol. 2019, 9, 190215. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kamimura, N.; Tokue, Y.; Nakata, M.T.; Yamamoto, M.; Hu, S.; Masai, E.; Mitsuda, N.; Kajita, S. Identification of Enzymatic Genes with the Potential to Reduce Biomass Recalcitrance through Lignin Manipulation in Arabidopsis. Biotechnol Biofuels 2020, 13, 97. [Google Scholar] [CrossRef]
- Bagniewska-Zadworna, A.; Barakat, A.; Łakomy, P.; Smoliński, D.J.; Zadworny, M. Lignin and Lignans in Plant Defence: Insight from Expression Profiling of Cinnamyl Alcohol Dehydrogenase Genes during Development and Following Fungal Infection in Populus. Plant Science 2014, 229, 111–121. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Zheng, K.; Xie, M.; Feng, K.; Jawdy, S.S.; Gunter, L.E.; Ranjan, P.; Singan, V.R.; Engle, N.; et al. GENOME‐WIDE ASSOCIATION STUDIES and Expression-based Quantitative Trait Loci Analyses Reveal Roles of HCT 2 in Caffeoylquinic Acid Biosynthesis and Its Regulation by Defense-responsive Transcription Factors in Populus. New Phytologist 2018, 220, 502–516. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustainable Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Liu, H.; Zhang, D.; Shi, Q.-S.; Zhong, X.-Q.; Guo, Y.; Xie, X.-B. High Value Valorization of Lignin as Environmental Benign Antimicrobial. Materials Today Bio 2023, 18, 100520. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Wang, P.; Li, H.; Rehman, S.; Li, D.; Zhuge, Q. Characterization, Expression, and Functional Analysis of the Pathogenesis-Related Gene PtDIR11 in Transgenic Poplar. International Journal of Biological Macromolecules 2022, 210, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Zhou, P.; Yang, X.; Tan, W.; Sun, W.; Zhuge, Q. Cloning and Expression Analysis of PeDIR19 Gene in Poplar. pgt 2023. [Google Scholar] [CrossRef]
- Liang, J.; Huang, X.; Ma, G. Antimicrobial Activities and Mechanisms of Extract and Components of Herbs in East Asia. RSC Adv. 2022, 12, 29197–29213. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Dong, H.; Liu, Z.; Ma, J.; Zhang, X. Salicylic Acid in Populus Tomentosa Is a Remote Signalling Molecule Induced by Botryosphaeria Dothidea Infection. Sci Rep 2018, 8, 14059. [Google Scholar] [CrossRef]
- Palma Ferreira, S. Populus Euphratica: An Incompatible Host for Biotrophic Pathogens? Molecular Plant Pathology 2016, 17, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, X.; Meng, Z.; Liu, Y. Efficacy of Topical 30% Salicylic Acid in Combination with Minocycline in Moderate to Severe Acne. Pak J Pharm Sci 2023, 36, 607–611. [Google Scholar]
- Cooper, B. The Detriment of Salicylic Acid to the Pseudomonas Savastanoi Pv. Phaseolicola Proteome. MPMI 2022, 35, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha Neto, A.C.; Maraschin, M.; Di Piero, R.M. Antifungal Activity of Salicylic Acid against Penicillium Expansum and Its Possible Mechanisms of Action. International Journal of Food Microbiology 2015, 215, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, T.; Song, Y.; Feng, L.; Kear, P.J.; Riseh, R.S.; Sitohy, M.; Datla, R.; Ren, M. Salicylic Acid Fights against Fusarium Wilt by Inhibiting Target of Rapamycin Signaling Pathway in Fusarium Oxysporum. Journal of Advanced Research 2022, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salgado Salomón, M.E.; Dresch, P.; Horak, E.; Galleguillos, F.; Barroetaveña, C.; Peintner, U. The Enigmatic Cortinarius Magellanicus Complex Occurring in Nothofagaceae Forests of the Southern Hemisphere. Fungal Biology 2018, 122, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Molla, K.A. CRISPR-Cas9 Helps Solve a Piece of the Puzzle of the Biosynthesis of Salicinoids and Suggests a Role in the Growth-Defense Trade-off in Poplar. Plant Cell 2022, 34, 2819–2820. [Google Scholar] [CrossRef]
- Ullah, C.; Tsai, C.; Unsicker, S.B.; Xue, L.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Salicylic Acid Activates Poplar Defense against the Biotrophic Rust Fungus Melampsora Larici-populina via Increased Biosynthesis of Catechin and Proanthocyanidins. New Phytologist 2019, 221, 960–975. [Google Scholar] [CrossRef]
- Simard, F.; Gauthier, C.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Pichette, A. Structure Elucidation of Anti-Methicillin Resistant Staphylococcus Aureus (MRSA) Flavonoids from Balsam Poplar Buds. Bioorganic & Medicinal Chemistry 2016, 24, 4188–4198. [Google Scholar] [CrossRef]
- Wang, Z.; Nur, F.A.; Ma, J.; Wang, J.; Cao, C. Effects of Poplar Secondary Metabolites on Performance and Detoxification Enzyme Activity of Lymantria Dispar. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2019, 225, 108587. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. IJMS 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, M.; Lam, P.-Y.; Dini-Andreote, F.; Dai, L.; Wei, Z. Multifaceted Roles of Flavonoids Mediating Plant-Microbe Interactions. Microbiome 2022, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Yan, J.; Fu, Z.; Yang, T.; Ziemah, J.; Ullrich, M.S.; Kuhnert, N.; Zeng, H. Lignin and Its Pathway-Associated Phytoalexins Modulate Plant Defense against Fungi. JoF 2022, 9, 52. [Google Scholar] [CrossRef]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide Analysis of Phenylpropanoid Defence Pathways. Molecular Plant Pathology 2010, 11, 829–846. [Google Scholar] [CrossRef]
- St-Pierre, A.; Blondeau, D.; Lajeunesse, A.; Bley, J.; Bourdeau, N.; Desgagné-Penix, I. Phytochemical Screening of Quaking Aspen (Populus Tremuloides) Extracts by UPLC-QTOF-MS and Evaluation of Their Antimicrobial Activity. Molecules 2018, 23, 1739. [Google Scholar] [CrossRef]
- Abou Baker, D.H. An Ethnopharmacological Review on the Therapeutical Properties of Flavonoids and Their Mechanisms of Actions: A Comprehensive Review Based on up to Date Knowledge. Toxicology Reports 2022, 9, 445–469. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem Rev 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Wu, S.-C.; Yang, Z.-Q.; Liu, F.; Peng, W.-J.; Qu, S.-Q.; Li, Q.; Song, X.-B.; Zhu, K.; Shen, J.-Z. Antibacterial Effect and Mode of Action of Flavonoids From Licorice Against Methicillin-Resistant Staphylococcus Aureus. Front. Microbiol. 2019, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial Flavonoids as a Potential Substitute for Overcoming Antimicrobial Resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Ullah, C.; Unsicker, S.B.; Fellenberg, C.; Constabel, C.P.; Schmidt, A.; Gershenzon, J.; Hammerbacher, A. Flavan-3-Ols Are an Effective Chemical Defense against Rust Infection. Plant Physiol. 2017, 175, 1560–1578. [Google Scholar] [CrossRef]
- Bryant, N.; Muchero, W.; Weber, R.A.; Barros, J.; Chen, J.-G.; Tschaplinski, T.J.; Pu, Y.; Ragauskas, A.J. Cell Wall Response of Field Grown Populus to Septoria Infection. Front. Plant Sci. 2023, 14, 1089011. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, L.; Han, Z.; Jiang, Y.; Zhao, L.; Liu, H.; Yang, L.; Luo, K. Molecular Cloning and Characterization of PtrLAR3, a Gene Encoding Leucoanthocyanidin Reductase from Populus Trichocarpa, and Its Constitutive Expression Enhances Fungal Resistance in Transgenic Plants. Journal of Experimental Botany 2012, 63, 2513–2524. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant Pathogenesis-Related (PR) Proteins: A Focus on PR Peptides. Plant Physiology and Biochemistry 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Xu, C.; Sun, W.; Wang, P.; Li, D.; Yin, T.; Zhuge, Q. Characterization, Expression Profiling, and Functional Analysis of PtDef, a Defensin-Encoding Gene From Populus Trichocarpa. Front. Microbiol. 2020, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Movahedi, A.; Liu, G.; Zhu, S.; Chen, Y.; Yu, C.; Zhong, F.; Zhang, J. Characteristics, Expression Profile, and Function of Non-Specific Lipid Transfer Proteins of Populus Trichocarpa. International Journal of Biological Macromolecules 2022, 202, 468–481. [Google Scholar] [CrossRef]
- Jia, Z.; Gou, J.; Sun, Y.; Yuan, L.; Tang, Q.; Yang, X.; Pei, Y.; Luo, K. Enhanced Resistance to Fungal Pathogens in Transgenic Populus Tomentosa Carr. by Overexpression of an nsLTP-like Antimicrobial Protein Gene from Motherwort (Leonurus Japonicus). Tree Physiology 2010, 30, 1599–1605. [Google Scholar] [CrossRef]
- Rinaldi, C.; Kohler, A.; Frey, P.; Duchaussoy, F.; Ningre, N.; Couloux, A.; Wincker, P.; Le Thiec, D.; Fluch, S.; Martin, F.; et al. Transcript Profiling of Poplar Leaves upon Infection with Compatible and Incompatible Strains of the Foliar Rust Melampsora Larici-Populina. Plant Physiology 2007, 144, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Petre, B.; Hecker, A.; Germain, H.; Tsan, P.; Sklenar, J.; Pelletier, G.; Séguin, A.; Duplessis, S.; Rouhier, N. The Poplar Rust-Induced Secreted Protein (RISP) Inhibits the Growth of the Leaf Rust Pathogen Melampsora Larici-Populina and Triggers Cell Culture Alkalinisation. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Yevtushenko, D.P.; Misra, S. Enhancing Disease Resistance in Poplar through Modification of Its Natural Defense Pathway. Plant Mol Biol 2019, 100, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Song, J.; Huang, R.; Huang, M.; Xu, L. Cloning and Expression Analysis of Chitinase Genes from Populus Canadensis. Russ J Plant Physiol 2013, 60, 396–403. [Google Scholar] [CrossRef]
- Jia, Z.; Sun, Y.; Yuan, L.; Tian, Q.; Luo, K. The Chitinase Gene (Bbchit1) from Beauveria Bassiana Enhances Resistance to Cytospora Chrysosperma in Populus Tomentosa Carr. Biotechnol Lett 2010, 32, 1325–1332. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Jia, Z.; Fang, Q.; Luo, K. Combined Expression of Antimicrobial Genes (Bbchit1 and LJAMP2) in Transgenic Poplar Enhances Resistance to Fungal Pathogens. Tree Physiology 2012, 32, 1313–1320. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Y.; Movahedi, A.; Wei, H.; Zhuge, Q. Thaumatin-like Protein(Pe-TLP)Acts as a Positive Factor in Transgenic Poplars Enhanced Resistance to Spots Disease. Physiological and Molecular Plant Pathology 2020, 112, 101512. [Google Scholar] [CrossRef]
- Diao, J.; Gu, W.; Jiang, Z.; Wang, J.; Zou, H.; Zong, C.; Ma, L. Comprehensive Analysis of Universal Stress Protein Family Genes and Their Expression in Fusarium Oxysporum Response of Populus Davidiana × P. Alba Var. Pyramidalis Louche Based on the Transcriptome. IJMS 2023, 24, 5405. [Google Scholar] [CrossRef]
- Chi, Y.H.; Koo, S.S.; Oh, H.T.; Lee, E.S.; Park, J.H.; Phan, K.A.T.; Wi, S.D.; Bae, S.B.; Paeng, S.K.; Chae, H.B.; et al. The Physiological Functions of Universal Stress Proteins and Their Molecular Mechanism to Protect Plants From Environmental Stresses. Front. Plant Sci. 2019, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.D.; Wang, Z.Y.; Fan, H.J.; Zhang, R.S.; Yu, Z.Y.; Wang, J.J.; Liu, Z.H. Heterologous Expression of the Hsp24 from Trichoderma Asperellum Improves Antifungal Ability of Populus Transformant Pdpap-Hsp24 s to Cytospora Chrysosperma and Alternaria Alternate. J Plant Res 2016, 129, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Zhang, Y.; Huang, L.; Wang, N. Uncovering the Role of PdePrx12 Peroxidase in Enhancing Disease Resistance in Poplar Trees. JoF 2023, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Ji, L.; Wang, J.; Chen, Z.; Ye, M.; Ma, H.; An, X. Identification of Glutathione S-Transferase Genes Responding to Pathogen Infestation in Populus Tomentosa. Funct Integr Genomics 2014, 14, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Duan, Y.; Yin, J.; Ye, S.; Zhu, J.; Zhang, F.; Lu, W.; Fan, D.; Luo, K. Genome-Wide Identification and Characterization of the Populus WRKY Transcription Factor Family and Analysis of Their Expression in Response to Biotic and Abiotic Stresses. Journal of Experimental Botany 2014, 65, 6629–6644. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY Transcription Factors and Plant Defense Responses: Latest Discoveries and Future Prospects. Plant Cell Rep 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Zhu, Y.; Li, Y.; Yan, H.; Xiang, Y. Comparative Genomic Analysis of the WRKY III Gene Family in Populus, Grape, Arabidopsis and Rice. Biol Direct 2015, 10, 48. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, L.; Ma, X.; Zhao, X.; Jiao, B.; Li, C.; Luo, K. The WRKY Transcription Factors PtrWRKY18 and PtrWRKY35 Promote Melampsora Resistance in Populus. Tree Physiology 2017, 37, 665–675. [Google Scholar] [CrossRef]
- Wang, W.; Bai, X.-D.; Chen, K.; Gu, C.-R.; Yu, Q.-B.; Jiang, J.; Liu, G.-F. Role of PsnWRKY70 in Regulatory Network Response to Infection with Alternaria Alternata (Fr.) Keissl in Populus. IJMS 2022, 23, 7537. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Jiang, Y.; Guo, L.; Ling, Z.; Ye, S.; Duan, Y.; Li, C.; Luo, K. Isolation and Characterization of a Subgroup IIa WRKY Transcription Factor PtrWRKY40 from Populus Trichocarpa. Tree Physiol 2015, 35, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Jiang, Y.; Duan, Y.; Karim, A.; Fan, D.; Yang, L.; Zhao, X.; Yin, J.; Luo, K.; Li, C. Constitutive Expression of the Poplar WRKY Transcription Factor PtoWRKY60 Enhances Resistance to Dothiorella Gregaria Sacc. in Transgenic Plants. Tree Physiology 2014, 34, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Jiang, Y.; Ye, S.; Karim, A.; Ling, Z.; He, Y.; Yang, S.; Luo, K. PtrWRKY73, a Salicylic Acid-Inducible Poplar WRKY Transcription Factor, Is Involved in Disease Resistance in Arabidopsis Thaliana. Plant Cell Rep 2015, 34, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Levée, V.; Major, I.; Levasseur, C.; Tremblay, L.; MacKay, J.; Séguin, A. Expression Profiling and Functional Analysis of Populus WRKY23 Reveals a Regulatory Role in Defense. New Phytologist 2009, 184, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Wu, R.; Song, Z.; Dong, B.; Chen, T.; Wang, M.; Cao, H.; Du, T.; Wang, S.; Li, N.; et al. Sorbitol Reduces Sensitivity to Alternaria by Promoting Ceramide Kinases (CERK) Expression through Transcription Factor Pswrky25 in Populus (Populus Simonii Carr.). Genes 2022, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lu, B.; Dong, Y.; Li, Y.; Yang, M. Cloning and Functional Identification of PeWRKY41 from Populus × Euramericana. Industrial Crops and Products 2022, 175, 114279. [Google Scholar] [CrossRef]
- Shen, Z.; Yao, J.; Sun, J.; Chang, L.; Wang, S.; Ding, M.; Qian, Z.; Zhang, H.; Zhao, N.; Sa, G.; et al. Populus Euphratica HSF Binds the Promoter of WRKY1 to Enhance Salt Tolerance. Plant Science 2015, 235, 89–100. [Google Scholar] [CrossRef]
- Tan, L.; Salih, H.; Htet, N.N.W.; Azeem, F.; Zhan, R. Genomic Analysis of WD40 Protein Family in the Mango Reveals a TTG1 Protein Enhances Root Growth and Abiotic Tolerance in Arabidopsis. Sci Rep 2021, 11, 2266. [Google Scholar] [CrossRef]
- Yoshida, K.; Ma, D.; Constabel, C.P. The MYB182 Protein Down-Regulates Proanthocyanidin and Anthocyanin Biosynthesis in Poplar by Repressing Both Structural and Regulatory Flavonoid Genes. Plant Physiology 2015, 167, 693–710. [Google Scholar] [CrossRef]
- Ma, D.; Reichelt, M.; Yoshida, K.; Gershenzon, J.; Constabel, C.P. Two R2R3- MYB Proteins Are Broad Repressors of Flavonoid and Phenylpropanoid Metabolism in Poplar. The Plant Journal 2018, 96, 949–965. [Google Scholar] [CrossRef]
- Wan, S.; Li, C.; Ma, X.; Luo, K. PtrMYB57 Contributes to the Negative Regulation of Anthocyanin and Proanthocyanidin Biosynthesis in Poplar. Plant Cell Rep 2017, 36, 1263–1276. [Google Scholar] [CrossRef]
- Mellway, R.D.; Tran, L.T.; Prouse, M.B.; Campbell, M.M.; Constabel, C.P. The Wound-, Pathogen-, and Ultraviolet B-Responsive MYB134 Gene Encodes an R2R3 MYB Transcription Factor That Regulates Proanthocyanidin Synthesis in Poplar. Plant Physiology 2009, 150, 924–941. [Google Scholar] [CrossRef]
- Bai, Q.; Duan, B.; Ma, J.; Fen, Y.; Sun, S.; Long, Q.; Lv, J.; Wan, D. Coexpression of PalbHLH1 and PalMYB90 Genes From Populus Alba Enhances Pathogen Resistance in Poplar by Increasing the Flavonoid Content. Front. Plant Sci. 2020, 10, 1772. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-S.; Nguyen, V.P.; Jeon, H.-W.; Kim, M.-H.; Eom, S.H.; Lim, Y.J.; Kim, W.-C.; Park, E.-J.; Choi, Y.-I.; Ko, J.-H. Overexpression of PtrMYB119, a R2R3-MYB Transcription Factor from Populus Trichocarpa, Promotes Anthocyanin Production in Hybrid Poplar. Tree Physiol 2016, 36, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Cho, J.-S.; Bae, E.-K.; Choi, Y.-I.; Eom, S.H.; Lim, Y.J.; Lee, H.; Park, E.-J.; Ko, J.-H. PtrMYB120 Functions as a Positive Regulator of Both Anthocyanin and Lignin Biosynthetic Pathway in a Hybrid Poplar. Tree Physiology 2021, 41, 2409–2423. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, W.; Ran, L.; Dou, L.; Yao, S.; Hu, J.; Fan, D.; Li, C.; Luo, K. R2R3- MYB Transcription Factor MYB 6 Promotes Anthocyanin and Proanthocyanidin Biosynthesis but Inhibits Secondary Cell Wall Formation in Populus Tomentosa. The Plant Journal 2019, 99, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Yu, C.; Wang, C.; Jin, Y.; Zhang, H. MYB Transcription Factor PdMYB118 Directly Interacts with bHLH Transcription Factor PdTT8 to Regulate Wound-Induced Anthocyanin Biosynthesis in Poplar. BMC Plant Biol 2020, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Tang, H.; Reichelt, M.; Piirtola, E.-M.; Salminen, J.-P.; Gershenzon, J.; Constabel, C.P. Poplar MYB117 Promotes Anthocyanin Synthesis and Enhances Flavonoid B-Ring Hydroxylation by up-Regulating the Flavonoid 3′,5′-Hydroxylase Gene. Journal of Experimental Botany 2021, 72, 3864–3880. [Google Scholar] [CrossRef]
- Song, Q.; Kong, L.; Yang, X.; Jiao, B.; Hu, J.; Zhang, Z.; Xu, C.; Luo, K. PtoMYB142, a Poplar R2R3-MYB Transcription Factor, Contributes to Drought Tolerance by Regulating Wax Biosynthesis. Tree Physiology 2022, tpac060. [Google Scholar] [CrossRef] [PubMed]
- Yu; Shen; Newcombe; Fan; Chen Leaf Cuticle Can Contribute to Non-Host Resistance to Poplar Leaf Rust. Forests 2019, 10, 870. [CrossRef]
- Wei, M.; Xu, X.; Li, C. Identification and Expression of CAMTA Genes in Populus Trichocarpa under Biotic and Abiotic Stress. Sci Rep 2017, 7, 17910. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional Factors Regulate Plant Stress Responses Through Mediating Secondary Metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.; Zhang, Y.; Zhao, Y.; Xu, W.; Li, Y.; Xie, J. Genome-Wide Analysis of Coding and Non-Coding RNA Reveals a Conserved miR164–NAC–mRNA Regulatory Pathway for Disease Defense in Populus. Front. Genet. 2021, 12, 668940. [Google Scholar] [CrossRef]
- Pan, J.; Wang, H.; Cheng, Q. Cloning and Functional Analysis of PtATAF11 Transcription Factor Gene of Populus Trichocarpa. tgmb 2022. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Li, D.; Wang, F.; Zhang, R.; Wang, J. Molecular Characterization of the ERF Family in Susceptible Poplar Infected by Virulent Melampsora Larici-Populina. Physiological and Molecular Plant Pathology 2019, 108, 101437. [Google Scholar] [CrossRef]
- Diao, J.; Li, M.; Zhang, P.; Zong, C.; Ma, W.; Ma, L. Overexpression of the PdpapERF109 Gene Enhances Resistance of Populus Davidiana × P. Alba Var. Pyramidalis to Fusarium Oxysporum Infection. J. For. Res. 2022, 33, 1925–1937. [Google Scholar] [CrossRef]
- Ward, D. Agrobacterium VirE2 Gets the VIP1 Treatment in Plant Nuclear Import. Trends in Plant Science 2002, 7, 1–3. [Google Scholar] [CrossRef]
- Lang, J.; Genot, B.; Bigeard, J.; Colcombet, J. MPK3 and MPK6 Control Salicylic Acid Signaling by Up-Regulating NLR Receptors during Pattern- and Effector-Triggered Immunity. Journal of Experimental Botany 2022, 73, 2190–2205. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Tian, C. Mitogen-Activated Protein Kinase Cascade CgSte50-Ste11-Ste7-Mk1 Regulates Infection-Related Morphogenesis in the Poplar Anthracnose Fungus Colletotrichum Gloeosporioides. Microbiological Research 2021, 248, 126748. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, H.-G.; Liu, M.; Wang, H.-L.; Yang, Q.; Yan, D.-H.; Zhang, Y.; Li, Z.; Feng, C.-H.; Niu, M.; et al. PeTGA1 Enhances Disease Resistance against Colletotrichum Gloeosporioides through Directly Regulating PeSARD1 in Poplar. International Journal of Biological Macromolecules 2022, 214, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lie, J.; Cui, K.; Yang, J.; Baloch, A.M.; Liu, S.; Zhang, Y.; Baloch, A.W.; Zhang, R. Expression Profile of PdpapHB12 Gene in Response to Stress for Populus Davidana × P. Alba Var. Pyramidlis. PAK. J. BOT. 2024, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Wang, H.; Zhang, H.; Xu, X.; Li, C.; Yang, C. Comprehensive Analysis of Trihelix Genes and Their Expression under Biotic and Abiotic Stresses in Populus Trichocarpa. Sci Rep 2016, 6, 36274. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhou, K.; Yang, X.; Yang, Y.; Ma, Y.; Wang, Y. Crosstalk of DNA Methylation Triggered by Pathogen in Poplars With Different Resistances. Front. Microbiol. 2021, 12, 750089. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yang, M.; Le, B.H.; He, W.; Hou, Y. The Master Role of SIRNAS in Plant Immunity. Molecular Plant Pathology 2022, 23, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, S.; Zhou, Y.; Bai, J.; Huang, G.; Liu, X.; Zhang, Y.; Tang, D.; Lu, D. Transcriptional Regulation of the Immune Receptor FLS2 Controls the Ontogeny of Plant Innate Immunity. Plant Cell 2018, 30, 2779–2794. [Google Scholar] [CrossRef]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D.G. A Plant miRNA Contributes to Antibacterial Resistance by Repressing Auxin Signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.-L.; Zhao, J.-H.; Wang, S.; Jin, Y.; Chen, Z.-Q.; Fang, Y.-Y.; Hua, C.-L.; Ding, S.-W.; Guo, H.-S. Cotton Plants Export microRNAs to Inhibit Virulence Gene Expression in a Fungal Pathogen. Nature Plants 2016, 2, 16153. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Wafula, E.K.; Honaas, L.A.; Ralph, P.E.; Jones, S.; Clarke, C.R.; Liu, S.; Su, C.; Zhang, H.; et al. Horizontal Gene Transfer Is More Frequent with Increased Heterotrophy and Contributes to Parasite Adaptation. Proc. Natl. Acad. Sci. U.S.A. 2016, 113. [Google Scholar] [CrossRef]
- Boccara, M.; Sarazin, A.; Thiébeauld, O.; Jay, F.; Voinnet, O.; Navarro, L.; Colot, V. The Arabidopsis miR472-RDR6 Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-Transcriptional Control of Disease Resistance Genes. PLoS Pathog 2014, 10, e1003883. [Google Scholar] [CrossRef]
- Cai, Q.; Liang, C.; Wang, S.; Hou, Y.; Gao, L.; Liu, L.; He, W.; Ma, W.; Mo, B.; Chen, X. The Disease Resistance Protein SNC1 Represses the Biogenesis of microRNAs and Phased siRNAs. Nat Commun 2018, 9, 5080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, Z.; Wu, K.; Yang, L.; Li, Y.; Yang, Z.; Shi, S.; Liu, X.; Zhao, S.; Yang, Z.; et al. Suppression of Jasmonic Acid-Mediated Defense by Viral-Inducible MicroRNA319 Facilitates Virus Infection in Rice. Molecular Plant 2016, 9, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Z.; Wang, Y.; Zheng, L.; Ye, R.; Ji, Y.; Zhao, S.; Ji, S.; Liu, R.; Xu, L.; et al. Viral-Inducible Argonaute18 Confers Broad-Spectrum Virus Resistance in Rice by Sequestering a Host microRNA. eLife 2015, 4, e05733. [Google Scholar] [CrossRef]
- Zhao, J.-P.; Jiang, X.-L.; Zhang, B.-Y.; Su, X.-H. Involvement of microRNA-Mediated Gene Expression Regulation in the Pathological Development of Stem Canker Disease in Populus Trichocarpa. PLoS ONE 2012, 7, e44968. [Google Scholar] [CrossRef]
- Gou, J.-Y.; Felippes, F.F.; Liu, C.-J.; Weigel, D.; Wang, J.-W. Negative Regulation of Anthocyanin Biosynthesis in Arabidopsis by a miR156-Targeted SPL Transcription Factor. The Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Chen, L.; Ren, Y.; Zhang, Y.; Xu, J.; Zhang, Z.; Wang, Y. Genome-Wide Profiling of Novel and Conserved Populus microRNAs Involved in Pathogen Stress Response by Deep Sequencing. Planta 2012, 235, 873–883. [Google Scholar] [CrossRef]
- Gupta, O.P.; Sharma, P.; Gupta, R.K.; Sharma, I. Current Status on Role of miRNAs during Plant–Fungus Interaction. Physiological and Molecular Plant Pathology 2014, 85, 1–7. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Zhao, Y.; Xie, J. Transposon-Associated Small RNAs Involved in Plant Defense in Poplar; In Review, 2021.
- Su, Y.; Li, H.-G.; Wang, Y.; Li, S.; Wang, H.-L.; Yu, L.; He, F.; Yang, Y.; Feng, C.-H.; Shuai, P.; et al. Poplar miR472a Targeting NBS-LRRs Is Involved in Effective Defence against the Necrotrophic Fungus Cytospora Chrysosperma. Journal of Experimental Botany 2018. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Zhou, X.; Cao, Z. Identification of Novel miRNAs and Their Target Genes from Populus Szechuanica Infected with Melampsora Larici-Populina. Mol Biol Rep 2019, 46, 3083–3092. [Google Scholar] [CrossRef]
- Chen, M.; Cao, Z. Genome-Wide Expression Profiling of microRNAs in Poplar upon Infection with the Foliar Rust Fungus Melampsora Larici-Populina. BMC Genomics 2015, 16, 696. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, F.; Wang, C.; Zou, L.; Wang, Z.; Chen, Q.; Niu, C.; Zhang, R.; Ling, Y.; Wang, B. MicroRNA-Mediated Susceptible Poplar Gene Expression Regulation Associated with the Infection of Virulent Melampsora Larici-Populina. BMC Genomics 2016, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Yin, Y.; Wang, J.; Wang, Y. Identification and Validation of miRNA Reference Genes in Poplar under Pathogen Stress. Mol Biol Rep 2021, 48, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chang, F.; Huo, X.; Zhou, K.; Wang, Y. Prediction and Analysis of miRNA Targets in Poplar in Response to the Infection of Lonsdalea Quercina Subsp Populi. tgmb 2022. [Google Scholar] [CrossRef]
- Tian, W.; Ge, Y.; Liu, X.; Dou, G.; Ma, Y. Identification and Characterization of Populus microRNAs in Response to Plant Growth-Promoting Endophytic Streptomyces Sp. SSD49. World J Microbiol Biotechnol 2019, 35, 97. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S. Do microRNA156a/168a Regulate Mycorrhizal Formation in Populus Tomentosa? 2023.
- Wang, N.; Cao, P.; Xia, W.; Fang, L.; Yu, H. Identification and Characterization of Long Non-Coding RNAs in Response to Early Infection by Melampsora Larici-Populina Using Genome-Wide High-Throughput RNA Sequencing. Tree Genetics & Genomes 2017, 13, 34. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Y.; Ren, Y.; Wang, S.; Yang, M. Conjoint Analysis of Genome-Wide lncRNA and mRNA Expression during the Salicylic Acid Response in Populus × Euramericana. Plants 2023, 12, 1377. [Google Scholar] [CrossRef]
- Filiz, E.; Vatansever, R. Genome-Wide Identification of Mildew Resistance Locus O (MLO) Genes in Tree Model Poplar (Populus Trichocarpa): Powdery Mildew Management in Woody Plants. Eur J Plant Pathol 2018, 152, 95–109. [Google Scholar] [CrossRef]
- Kohler, A.; Rinaldi, C.; Duplessis, S.; Baucher, M.; Geelen, D.; Duchaussoy, F.; Meyers, B.C.; Boerjan, W.; Martin, F. Genome-Wide Identification of NBS Resistance Genes in Populus Trichocarpa. Plant Mol Biol 2008, 66, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Bresson, A.; Jorge, V.; Dowkiw, A.; Guerin, V.; Bourgait, I.; Tuskan, G.A.; Schmutz, J.; Chalhoub, B.; Bastien, C.; Faivre Rampant, P. Qualitative and Quantitative Resistances to Leaf Rust Finely Mapped within Two Nucleotide-binding Site Leucine-rich Repeat (NBS-LRR)-rich Genomic Regions of Chromosome 19 in Poplar. New Phytologist 2011, 192, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.J.; Tschaplinski, T.J.; M. LeBoldus, J.; Keefover-Ring, K.; Azeem, M.; Chen, J.; Macaya-Sanz, D.; MacDonald, W.L.; Muchero, W.; DiFazio, S.P. Host Plant Genetic Control of Associated Fungal and Insect Species in a Populus Hybrid Cross. Ecology and Evolution 2020, 10, 5119–5134. [Google Scholar] [CrossRef]
- La Mantia, J.; Klápště, J.; El-Kassaby, Y.A.; Azam, S.; Guy, R.D.; Douglas, C.J.; Mansfield, S.D.; Hamelin, R. Association Analysis Identifies Melampsora ×columbiana Poplar Leaf Rust Resistance SNPs. PLoS ONE 2013, 8, e78423. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wu, H.; Li, X.; Chen, Y.; Yang, Y.; Dai, M.; Yin, T. Identification of Genes Underlying the Resistance to Melampsora Larici-Populina in an R Gene Supercluster of the Populus Deltoides Genome. Plant Disease 2020, 104, 1133–1143. [Google Scholar] [CrossRef]
- Muchero, W.; Sondreli, K.L.; Chen, J.-G.; Urbanowicz, B.R.; Zhang, J.; Singan, V.; Yang, Y.; Brueggeman, R.S.; Franco-Coronado, J.; Abraham, N.; et al. Association Mapping, Transcriptomics, and Transient Expression Identify Candidate Genes Mediating Plant–Pathogen Interactions in a Tree. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 11573–11578. [Google Scholar] [CrossRef]
- Louet, C.; Saubin, M.; Andrieux, A.; Persoons, A.; Gorse, M.; Pétrowski, J.; Fabre, B.; De Mita, S.; Duplessis, S.; Frey, P.; et al. A Point Mutation and Large Deletion at the Candidate Avirulence Locus AvrMlp7 in the Poplar Rust Fungus Correlate with Poplar RMlp7 Resistance Breakdown. Molecular Ecology 2023, 32, 2472–2483. [Google Scholar] [CrossRef]
- Ren, F.; Yan, D.-H.; Wu, G.; Sun, X.; Song, X.; Li, R. Distinctive Gene Expression Profiles and Effectors Consistent With Host Specificity in Two Formae Speciales of Marssonina Brunnea. Front. Microbiol. 2020, 11, 276. [Google Scholar] [CrossRef]
- Lenz, R.R.; Shrestha, H.K.; Carrell, A.A.; Labbé, J.; Hettich, R.L.; Abraham, P.E.; LeBoldus, J.M. Proteomics Reveals Pathways Linked to Septoria Canker Resistance and Susceptibility in Populus Trichocarpa. Front. Anal. Sci. 2022, 2, 1020111. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Li, D.; Wang, F. Integrating Transcriptome and Coexpression Network Analyses to Characterize Salicylic Acid- and Jasmonic Acid-Related Genes in Tolerant Poplars Infected with Rust. IJMS 2021, 22, 5001. [Google Scholar] [CrossRef]
- La Mantia, J.; Unda, F.; Douglas, C.J.; Mansfield, S.D.; Hamelin, R. Overexpression of AtGolS3 and CsRFS in Poplar Enhances ROS Tolerance and Represses Defense Response to Leaf Rust Disease. Tree Physiology 2018, 38, 457–470. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, H.; Yue, Y.; Zhou, T.; Zhu, Z.; Wang, C. Integrated Transcriptomic and Transgenic Analyses Reveal Potential Mechanisms of Poplar Resistance to Alternaria Alternata Infection. BMC Plant Biol 2022, 22, 413. [Google Scholar] [CrossRef]
- Lenz, R.R.; Louie, K.B.; Søndreli, K.L.; Galanie, S.S.; Chen, J.-G.; Muchero, W.; Bowen, B.P.; Northen, T.R.; LeBoldus, J.M. Metabolomic Patterns of Septoria Canker Resistant and Susceptible Populus Trichocarpa Genotypes 24 Hours Postinoculation. Phytopathology® 2021, 111, 2052–2066. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Yu, R.; Zhang, L.; Yang, Y.; Xiao, D.; Li, A.; Wang, Y. Integrated Transcriptomic and Metabolomic Profiles Reveal Adaptive Responses of Three Poplar Varieties against the Bacterial Pathogen Lonsdalea Populi. Plant Cell & Environment 2023, 46, 306–321. [Google Scholar] [CrossRef]
- Prunier, J.; Giguère, I.; Ryan, N.; Guy, R.; Soolanayakanahally, R.; Isabel, N.; MacKay, J.; Porth, I. Gene Copy Number Variations Involved in Balsam Poplar ( Populus Balsamifera L.) Adaptive Variations. Molecular Ecology 2019, 28, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Hacquard, S.; Schadt, C.W. Towards a Holistic Understanding of the Beneficial Interactions across the Populus Microbiome. New Phytologist 2015, 205, 1424–1430. [Google Scholar] [CrossRef]
- Cregger, M.A.; Carper, D.L.; Christel, S.; Doktycz, M.J.; Labbé, J.; Michener, J.K.; Dove, N.C.; Johnston, E.R.; Moore, J.A.M.; Vélez, J.M.; et al. Plant–Microbe Interactions: From Genes to Ecosystems Using Populus as a Model System. Phytobiomes Journal 2021, 5, 29–38. [Google Scholar] [CrossRef]
- Dove, N.C.; Veach, A.M.; Muchero, W.; Wahl, T.; Stegen, J.C.; Schadt, C.W.; Cregger, M.A. Assembly of the Populus Microbiome Is Temporally Dynamic and Determined by Selective and Stochastic Factors. mSphere 2021, 6, e01316-20. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends in Plant Science 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Science 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Sachin, N.; Tsang, A.; Shaanker, R.U.; Dayanandan, S. Genome Sequence Resource of Bacillus Velezensis EB14, a Native Endophytic Bacterial Strain with Biocontrol Potential Against the Poplar Stem Canker Causative Pathogen, Sphaerulina Musiva. Phytopathology® 2021, 111, 890–892. [Google Scholar] [CrossRef]
- Naik, S.; Palys, S.; Di Falco, M.; Tsang, A.; Périnet, P.; Ramanan, U.S.; Dayanandan, S. Isolation and Characterization of Bacillus Velezensis EB14, an Endophytic Bacterial Strain Antagonistic to Poplar Stem Canker Pathogen Sphaerulina Musiva and Its Interactions with the Endophytic Fungal Microbiome in Poplar. PhytoFrontiersTM 2021, 1, 229–238. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Macaya-Sanz, D.; Witzell, J.; Martín, J.A. Enhancement of Populus Alba Tolerance to Venturia Tremulae upon Inoculation with Endophytes Showing in Vitro Biocontrol Potential. Eur J Plant Pathol 2019, 153, 1031–1042. [Google Scholar] [CrossRef]
- Tyagi, K.; Kumar, P.; Pandey, A.; Ginwal, H.S.; Barthwal, S.; Nautiyal, R.; Meena, R.K. First Record of Cladosporium Oxysporum as a Potential Novel Fungal Hyperparasite of Melampsora Medusae f. Sp. Deltoidae and Screening of Populus Deltoides Clones against Leaf Rust. 3 Biotech 2023, 13, 213. [Google Scholar] [CrossRef]
- Zhang, P.; Hao, H.; Wang, L.; Liu, Z.; Ma, L. Endophytes Bacillus Amyloliquefaciens AW3 (CGMCC1.16683) Improves the Growth of Populus Davidiana × Populus Bolleana (PdPap) and Induces Its Resistance to Wilt Disease by Fusarium Oxysporum Fox68 (CFCC86068). Eur J Plant Pathol 2022, 162, 1–17. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Y.; Tang, M. Study on the Molecular Mechanism of Laccaria Bicolor Helping Populus Trichocarpa to Resist the Infection of Botryosphaeria Dothidea. Journal of Applied Microbiology 2022, 132, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.K.H.; Newcombe, G. The Contribution of Foliar Endophytes to Quantitative Resistance to Melampsora Rust. New Phytologist 2013, 197, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, S.; Ding, C.; Ma, C.; Chen, X.; Wang, J.; Yang, M.; Su, X. Correlation Analysis of the Bacterial Community and Wood Properties of Populus × Euramericana Cv. “74/76” Wet Heartwood. Front. Microbiol. 2022, 13, 868078. [Google Scholar] [CrossRef]
- Xie, J.; Ma, Y.; Li, X.; Wu, J.; Martin, F.; Zhang, D. Multifeature Analysis of Age-related Microbiome Structures Reveals Defense Mechanisms of Populus Tomentosa Trees. New Phytologist 2023, 238, 1636–1650. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, F.; Ge, W.; Ren, Y.; Bao, H.; Tian, C. Effects of Colletotrichum Gloeosporioides and Poplar Secondary Metabolites on the Composition of Poplar Phyllosphere Microbial Communities. Microbiol Spectr 2023, 11, e04603–22. [Google Scholar] [CrossRef]
- Busby, P.E.; Peay, K.G.; Newcombe, G. Common Foliar Fungi of Populus Trichocarpa Modify Melampsora Rust Disease Severity. New Phytologist 2016, 209, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.; Newcombe, G. The Frequency of Modification of Dothistroma Pine Needle Blight Severity by Fungi within the Native Range. Forest Ecology and Management 2015, 337, 153–160. [Google Scholar] [CrossRef]
- Kwaśna, H.; Szewczyk, W.; Baranowska, M.; Gallas, E.; Wiśniewska, M.; Behnke-Borowczyk, J. Mycobiota Associated with the Vascular Wilt of Poplar. Plants 2021, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Eberl, F.; Fernandez De Bobadilla, M.; Reichelt, M.; Hammerbacher, A.; Gershenzon, J.; Unsicker, S.B. Herbivory Meets Fungivory: Insect Herbivores Feed on Plant Pathogenic Fungi for Their Own Benefit. Ecology Letters 2020, 23, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Busby, P.E.; Lamit, L.J.; Keith, A.R.; Newcombe, G.; Gehring, C.A.; Whitham, T.G.; Dirzo, R. Genetics-based Interactions among Plants, Pathogens, and Herbivores Define Arthropod Community Structure. Ecology 2015, 96, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.B.; Pawlus, A.D.; Brinkman, D.; Gardner, G.; Hegeman, A.D.; Spivak, M.; Cohen, J.D. 3-Acyl Dihydroflavonols from Poplar Resins Collected by Honey Bees Are Active against the Bee Pathogens Paenibacillus Larvae and Ascosphaera Apis. Phytochemistry 2017, 138, 83–92. [Google Scholar] [CrossRef]
- Lin, T.; Chen, J.; Zhou, S.; Yu, W.; Chen, G.; Chen, L.; Wang, X.; Shi, H.; Han, S.; Zhang, F. Testing the Elemental Defense Hypothesis with a Woody Plant Species: Cadmium Accumulation Protects Populus Yunnanensis from Leaf Herbivory and Pathogen Infection. Chemosphere 2020, 247, 125851. [Google Scholar] [CrossRef]
- Zhao, Z.; Zou, L.; Zhi, J.; Niu, Y.; Yan, S.; Yan, R.; Lin, T. Increased Soil Cadmium Concentrations Enhanced Poplar Defence against a Leaf Pathogenic Fungus Infection. Journal of Phytopathology 2023, 171, 401–408. [Google Scholar] [CrossRef]
- Chen, J.; Qin, S.; Tang, J.; Chen, G.; Xie, J.; Chen, L.; Han, S.; Wang, X.; Zhu, T.; Liu, Y.; et al. Exogenous Nitrogen Enhances Poplar Resistance to Leaf Herbivory and Pathogen Infection after Exposure to Soil Cadmium Stress. Ecotoxicology and Environmental Safety 2021, 208, 111688. [Google Scholar] [CrossRef]
- Lin, T.; Lu, Q.; Zheng, Z.; Li, S.; Li, S.; Liu, Y.; Zhu, T.; Chen, L.; Yang, C.; Han, S. Soil Cadmium Stress Affects the Phyllosphere Microbiome and Associated Pathogen Resistance Differently in Male and Female Poplars. Journal of Experimental Botany 2023, 74, 2188–2202. [Google Scholar] [CrossRef]
- Lin, T.; Tang, J.; He, F.; Chen, G.; Shi, Y.; Wang, X.; Han, S.; Li, S.; Zhu, T.; Chen, L. Sexual Differences in Above- and Belowground Herbivore Resistance between Male and Female Poplars as Affected by Soil Cadmium Stress. Science of The Total Environment 2022, 803, 150081. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, A.; Yan, S. Effects of Zn Exposure on Populus Simonii Seedling Growth and Its Resistance to Leaf Rust. Forests 2023, 14, 783. [Google Scholar] [CrossRef]
- Müller, N.A.; Kersten, B.; Leite Montalvão, A.P.; Mähler, N.; Bernhardsson, C.; Bräutigam, K.; Carracedo Lorenzo, Z.; Hoenicka, H.; Kumar, V.; Mader, M.; et al. A Single Gene Underlies the Dynamic Evolution of Poplar Sex Determination. Nat. Plants 2020, 6, 630–637. [Google Scholar] [CrossRef]
- Cronk, Q.; Müller, N.A. Default Sex and Single Gene Sex Determination in Dioecious Plants. Front. Plant Sci. 2020, 11, 1162. [Google Scholar] [CrossRef]
- Melnikova, N.V.; Borkhert, E.V.; Snezhkina, A.V.; Kudryavtseva, A.V.; Dmitriev, A.A. Sex-Specific Response to Stress in Populus. Front. Plant Sci. 2017, 8, 1827. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Wu, F.; Zhen, L. Sex-Specific Photosynthetic Capacity and Na+ Homeostasis in Populus Euphratica Exposed to NaCl Stress and AMF Inoculation. Front. Plant Sci. 2022, 13, 1066954. [Google Scholar] [CrossRef]
- Zhao, W.; Lin, X.; Wang, Y.; Yang, Q.; Liu, M. Nitrogen Level Induces Sex-Specific Cadmium Phloem Remobilization and Cell Wall Segregation in Populus Cathayana. Science of The Total Environment 2023, 890, 164184. [Google Scholar] [CrossRef] [PubMed]
- Randriamanana, T.R.; Nybakken, L.; Lavola, A.; Aphalo, P.J.; Nissinen, K.; Julkunen-Tiitto, R. Sex-Related Differences in Growth and Carbon Allocation to Defence in Populus Tremula as Explained by Current Plant Defence Theories. Tree Physiology 2014, 34, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Zhao, Y.; Korpelainen, H.; Li, C. Sex-Specific Nitrogen Allocation Tradeoffs in the Leaves of Populus Cathayana Cuttings under Salt and Drought Stress. Plant Physiology and Biochemistry 2022, 172, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, Y.; Liu, X.; Korpelainen, H.; Li, C. Ammonium and Nitrate Affect Sexually Different Responses to Salt Stress in Populus Cathayana. Physiologia Plantarum 2022, 174, e13626. [Google Scholar] [CrossRef] [PubMed]
- Kasper, K.; Abreu, I.N.; Feussner, K.; Zienkiewicz, K.; Herrfurth, C.; Ischebeck, T.; Janz, D.; Majcherczyk, A.; Schmitt, K.; Valerius, O.; et al. Multi-omics Analysis of Xylem Sap Uncovers Dynamic Modulation of Poplar Defenses by Ammonium and Nitrate. The Plant Journal 2022, 111, 282–303. [Google Scholar] [CrossRef]
- He, F.; Wu, Z.; Zhao, Z.; Chen, G.; Wang, X.; Cui, X.; Zhu, T.; Chen, L.; Yang, P.; Bi, L.; et al. Drought Stress Drives Sex-Specific Differences in Plant Resistance against Herbivores between Male and Female Poplars through Changes in Transcriptional and Metabolic Profiles. Science of The Total Environment 2022, 845, 157171. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Liu, J.; Korpelainen, H.; Li, C. Plant Sex Affects Plant-Microbiome Assemblies of Dioecious Populus Cathayana Trees under Different Soil Nitrogen Conditions. Microbiome 2022, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lu, L.; Li, H.; Meng, Z.; Dong, T.; Peng, C.; Xu, X. Divergence of Phyllosphere Microbial Communities Between Females and Males of the Dioecious Populus Cathayana. MPMI 2021, 34, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Tang, J.; Li, S.; Li, S.; Han, S.; Liu, Y.; Yang, C.; Chen, G.; Chen, L.; Zhu, T. Drought Stress-mediated Differences in Phyllosphere Microbiome and Associated Pathogen Resistance between Male and Female Poplars. The Plant Journal 2023, 115, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; He, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. Allelochemicals and Soil Microorganisms Jointly Mediate Sex-specific Belowground Interactions in Dioecious Populus Cathayana. New Phytologist 2023, 240, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Movahedi, A.; Xu, C.; Sun, W.; Wang, P.; Li, D.; Yin, T.; Zhuge, Q. Characterization, Expression Profiling, and Functional Analysis of PtDef, a Defensin-Encoding Gene From Populus Trichocarpa. Front. Microbiol. 2020, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Yuan, G.; Lu, H.; Liu, Y.; Zhang, J.; Tuskan, G.A.; Muchero, W.; Chen, J.-G.; Yang, X. CRISPR/Cas9-Based Gene Activation and Base Editing in Populus. Horticulture Research 2023, 10, uhad085. [Google Scholar] [CrossRef]
- Guo, J.; Morrell-Falvey, J.L.; Labbé, J.L.; Muchero, W.; Kalluri, U.C.; Tuskan, G.A.; Chen, J.-G. Highly Efficient Isolation of Populus Mesophyll Protoplasts and Its Application in Transient Expression Assays. PLoS ONE 2012, 7, e44908. [Google Scholar] [CrossRef]
- Qiao, J.; Kuroda, H.; Hayashi, T.; Sakai, F. Efficient Plantlet Regeneration from Protoplasts Isolated from Suspension Cultures of Poplar ( Populus Alba L.). Plant Cell Reports 1998, 17, 201–205. [Google Scholar] [CrossRef]
- Rahman, S.U.; Khan, M.O.; Ullah, R.; Ahmad, F.; Raza, G. Agrobacterium-Mediated Transformation for the Development of Transgenic Crops; Present and Future Prospects. Mol Biotechnol 2023. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; He, Y.; Li, S.; Yan, L.; Li, Y.; Zhu, Z.; Xia, L. Plant Base Editing and Prime Editing: The Current Status and Future Perspectives. JIPB 2023, 65, 444–467. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–Cas in Agriculture and Plant Biotechnology. Nat Rev Mol Cell Biol 2020, 21, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Illa-Berenguer, E.; LaFayette, P.R.; Parrott, W.A. Editing Efficiencies with Cas9 Orthologs, Cas12a Endonucleases, and Temperature in Rice. Front. Genome Ed. 2023, 5, 1074641. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Zhao, Y.; Zhang, R.; Tang, X.; Li, L.; Jia, X.; Guo, Y.; Wu, Y.; Han, Y.; et al. An Efficient CRISPR–Cas12a Promoter Editing System for Crop Improvement. Nat. Plants 2023, 9, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Sulis, D.B.; Jiang, X.; Yang, C.; Marques, B.M.; Matthews, M.L.; Miller, Z.; Lan, K.; Cofre-Vega, C.; Liu, B.; Sun, R.; et al. Multiplex CRISPR Editing of Wood for Sustainable Fiber Production. Science 2023, 381, 216–221. [Google Scholar] [CrossRef]
- Wienert, B.; Cromer, M.K. CRISPR Nuclease Off-Target Activity and Mitigation Strategies. Front. Genome Ed. 2022, 4, 1050507. [Google Scholar] [CrossRef]
- An, Y.; Geng, Y.; Yao, J.; Fu, C.; Lu, M.; Wang, C.; Du, J. Efficient Genome Editing in Populus Using CRISPR/Cas12a. Front. Plant Sci. 2020, 11, 593938. [Google Scholar] [CrossRef]
- Borrelli, V.M.G.; Brambilla, V.; Rogowsky, P.; Marocco, A.; Lanubile, A. The Enhancement of Plant Disease Resistance Using CRISPR/Cas9 Technology. Front. Plant Sci. 2018, 9, 1245. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Das, P.; Panda, D.; Xie, K.; Baig, M.J.; Molla, K.A. A Detailed Landscape of CRISPR-Cas-Mediated Plant Disease and Pest Management. Plant Science 2022, 323, 111376. [Google Scholar] [CrossRef]
- Khan, Z.A.; Kumar, R.; Dasgupta, I. CRISPR/Cas-Mediated Resistance against Viruses in Plants. IJMS 2022, 23, 2303. [Google Scholar] [CrossRef]
- Li, X.; Sandgrind, S.; Moss, O.; Guan, R.; Ivarson, E.; Wang, E.S.; Kanagarajan, S.; Zhu, L.-H. Efficient Protoplast Regeneration Protocol and CRISPR/Cas9-Mediated Editing of Glucosinolate Transporter (GTR) Genes in Rapeseed (Brassica Napus L.). Front. Plant Sci. 2021, 12, 680859. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.-L. Simultaneous Editing of Three Homoeoalleles in Hexaploid Bread Wheat Confers Heritable Resistance to Powdery Mildew. Nat Biotechnol 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid Generation of a Transgene-Free Powdery Mildew Resistant Tomato by Genome Deletion. Sci Rep 2017, 7, 482. [Google Scholar] [CrossRef]
- Malnoy, M.; Viola, R.; Jung, M.-H.; Koo, O.-J.; Kim, S.; Kim, J.-S.; Velasco, R.; Nagamangala Kanchiswamy, C. DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Brauer, E.K.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Schernthaner, J.; Subramaniam, R.; Ouellet, T. Genome Editing of a Deoxynivalenol-Induced Transcription Factor Confers Resistance to Fusarium Graminearum in Wheat. MPMI 2020, 33, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Bernhardsson, C.; Ingvarsson, P.K. Molecular Population Genetics of Elicitor-Induced Resistance Genes in European Aspen (Populus Tremula L., Salicaceae). PLoS ONE 2011, 6, e24867. [Google Scholar] [CrossRef] [PubMed]
- Pathi, K.M.; Rink, P.; Budhagatapalli, N.; Betz, R.; Saado, I.; Hiekel, S.; Becker, M.; Djamei, A.; Kumlehn, J. Engineering Smut Resistance in Maize by Site-Directed Mutagenesis of LIPOXYGENASE 3. Front. Plant Sci. 2020, 11, 543895. [Google Scholar] [CrossRef]
- Gautam, T.; Dutta, M.; Jaiswal, V.; Zinta, G.; Gahlaut, V.; Kumar, S. Emerging Roles of SWEET Sugar Transporters in Plant Development and Abiotic Stress Responses. Cells 2022, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Peng, Z.; Long, J.; Sosso, D.; Liu, B.; Eom, J.; Huang, S.; Liu, S.; Vera Cruz, C.; Frommer, W.B.; et al. Gene Targeting by the TAL Effector PthXo2 Reveals Cryptic Resistance Gene for Bacterial Blight of Rice. The Plant Journal 2015, 82, 632–643. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.-S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-Spectrum Resistance to Bacterial Blight in Rice Using Genome Editing. Nat Biotechnol 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Zhang, J.; Song, C.; Li, Y.; Li, J.; Lu, M. Expression and Localization of SWEETs in Populus and the Effect of SWEET7 Overexpression in Secondary Growth. Tree Physiology 2021, 41, 882–899. [Google Scholar] [CrossRef]
- Jia, H.; Orbovic, V.; Jones, J.B.; Wang, N. Modification of the PthA4 Effector Binding Elements in Type I Cs LOB 1 Promoter Using Cas9/Sg RNA to Produce Transgenic Duncan Grapefruit Alleviating XccΔpthA4:dCs LOB 1.3 Infection. Plant Biotechnology Journal 2016, 14, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering Canker-resistant Plants through CRISPR /Cas9-targeted Editing of the Susceptibility Gene Cs LOB 1 Promoter in Citrus. Plant Biotechnology Journal 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome Editing of the Disease Susceptibility Gene Cs LOB 1 in Citrus Confers Resistance to Citrus Canker. Plant Biotechnology Journal 2017, 15, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, Y.S.; Regan, S.; Busov, V. Members of the LATERAL ORGAN BOUNDARIES DOMAIN Transcription Factor Family Are Involved in the Regulation of Secondary Growth in Populus. The Plant Cell 2010, 22, 3662–3677. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Ali, Z.; Butt, H.; Mahas, A.; Aljedaani, F.; Khan, M.Z.; Ding, S.; Mahfouz, M. RNA Virus Interference via CRISPR/Cas13a System in Plants. Genome Biol 2018, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Shi, M.; Holmes, E.C. Using Metagenomics to Characterize an Expanding Virosphere. Cell 2018, 172, 1168–1172. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of Broad Virus Resistance in Non-transgenic Cucumber Using CRISPR/Cas9 Technology. Molecular Plant Pathology 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated Potyvirus Resistance in Transgene-free Arabidopsis Plants. Molecular Plant Pathology 2016, 17, 1276–1288. [Google Scholar] [CrossRef]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.; Chadha-Mohanty, P. Novel Alleles of Rice eIF4G Generated by CRISPR/Cas9-targeted Mutagenesis Confer Resistance to Rice Tungro Spherical Virus. Plant Biotechnology Journal 2018, 16, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.M.; Browning, K.S. The eIF4F and eIFiso4F Complexes of Plants: An Evolutionary Perspective. Comparative and Functional Genomics 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Arif, I.; Batool, M.; Schenk, P.M. Plant Microbiome Engineering: Expected Benefits for Improved Crop Growth and Resilience. Trends in Biotechnology 2020, 38, 1385–1396. [Google Scholar] [CrossRef]
- Doty, S.L.; Sher, A.W.; Fleck, N.D.; Khorasani, M.; Bumgarner, R.E.; Khan, Z.; Ko, A.W.K.; Kim, S.-H.; DeLuca, T.H. Variable Nitrogen Fixation in Wild Populus. PLoS ONE 2016, 11, e0155979. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Yu, Q.; Yang, K.; Yang, J.; Li, C.; Liu, X. Effects of Bacillus Subtilis T6-1 on the Rhizosphere Microbial Community Structure of Continuous Cropping Poplar. Biology 2022, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Yang, J.; Li, C.; Zhang, L.; Hua, X. Effects of a Microbial Restoration Substrate on Plant Growth and Rhizosphere Microbial Community in a Continuous Cropping Poplar. Microorganisms 2023, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Timm, C.M.; Pelletier, D.A.; Jawdy, S.S.; Gunter, L.E.; Henning, J.A.; Engle, N.; Aufrecht, J.; Gee, E.; Nookaew, I.; Yang, Z.; et al. Two Poplar-Associated Bacterial Isolates Induce Additive Favorable Responses in a Constructed Plant-Microbiome System. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Ciadamidaro, L.; Pfendler, S.; Girardclos, O.; Zappelini, C.; Binet, P.; Bert, V.; Khasa, D.; Blaudez, D.; Chalot, M. Mycorrhizal Inoculation Effects on Growth and the Mycobiome of Poplar on Two Phytomanaged Sites after 7-Year-Short Rotation Coppicing. Front. Plant Sci. 2022, 13, 993301. [Google Scholar] [CrossRef]
- Labbé, J.L.; Weston, D.J.; Dunkirk, N.; Pelletier, D.A.; Tuskan, G.A. Newly Identified Helper Bacteria Stimulate Ectomycorrhizal Formation in Populus. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Kandel, S.L.; Firrincieli, A.; Joubert, P.M.; Okubara, P.A.; Leston, N.D.; McGeorge, K.M.; Mugnozza, G.S.; Harfouche, A.; Kim, S.-H.; Doty, S.L. An In Vitro Study of Bio-Control and Plant Growth Promotion Potential of Salicaceae Endophytes. Front. Microbiol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Pfabel, C.; Eckhardt, K.-U.; Baum, C.; Struck, C.; Frey, P.; Weih, M. Impact of Ectomycorrhizal Colonization and Rust Infection on the Secondary Metabolism of Poplar (Populus Trichocarpa x Deltoides). Tree Physiology 2012, 32, 1357–1364. [Google Scholar] [CrossRef]
- Plett, J.M.; Khachane, A.; Ouassou, M.; Sundberg, B.; Kohler, A.; Martin, F. Ethylene and Jasmonic Acid Act as Negative Modulators during Mutualistic Symbiosis between L Accaria Bicolor and P Opulus Roots. New Phytologist 2014, 202, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; Kohler, A.; Miyauchi, S.; Singan, V.; Guinet, F.; Šimura, J.; Novák, O.; Barry, K.W.; Amirebrahimi, M.; Block, J.; et al. An Ectomycorrhizal Fungus Alters Sensitivity to Jasmonate, Salicylate, Gibberellin, and Ethylene in Host Roots. Plant Cell & Environment 2020, 43, 1047–1068. [Google Scholar] [CrossRef]
- Jiang, D.; Lin, R.; Tan, M.; Yan, J.; Yan, S. The Mycorrhizal-Induced Growth Promotion and Insect Resistance Reduction in Populus Alba × P. Berolinensis Seedlings: A Multi-Omics Study. Tree Physiology 2022, 42, 1059–1069. [Google Scholar] [CrossRef]
- Hussain, I.; Aleti, G.; Naidu, R.; Puschenreiter, M.; Mahmood, Q.; Rahman, M.M.; Wang, F.; Shaheen, S.; Syed, J.H.; Reichenauer, T.G. Microbe and Plant Assisted-Remediation of Organic Xenobiotics and Its Enhancement by Genetically Modified Organisms and Recombinant Technology: A Review. Science of The Total Environment 2018, 628–629, 1582–1599. [Google Scholar] [CrossRef]
- Mahaffee, W.F.; Kloepper, J.W. Bacterial communities of the rhizosphere and endorhiza associated with field-grown cucumber plants inoculated with a plant growth-promoting rhizobacterium or its genetically modified derivative. Can. J. Microbiol. 1997, 43, 344–353. [Google Scholar] [CrossRef] [PubMed]
- De Cárcer, D.A.; Martín, M.; Mackova, M.; Macek, T.; Karlson, U.; Rivilla, R. The Introduction of Genetically Modified Microorganisms Designed for Rhizoremediation Induces Changes on Native Bacteria in the Rhizosphere but Not in the Surrounding Soil. ISME J 2007, 1, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, L.; Ren, M.; Li, J.; Guan, X.; Tao, J. Comparison of Genetically Modified Insect-Resistant Maize and Non-Transgenic Maize Revealed Changes in Soil Metabolomes but Not in Rhizosphere Bacterial Community. GM Crops & Food 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Dias, A.C.F.; Dini-Andreote, F.; Hannula, S.E.; Andreote, F.D.; Pereira E Silva, M.D.C.; Salles, J.F.; De Boer, W.; Van Veen, J.; Van Elsas, J.D. Different Selective Effects on Rhizosphere Bacteria Exerted by Genetically Modified versus Conventional Potato Lines. PLoS ONE 2013, 8, e67948. [Google Scholar] [CrossRef]
- Zheng, Y.; Lv, G.B.; Chen, K.; Yu, Q.; Niu, B.; Jiang, J.; Liu, G. Impact of PaGLK Transgenic Poplar on Microbial Community and Soil Enzyme Activity in Rhizosphere Soil. Front. Bioeng. Biotechnol. 2022, 10, 965209. [Google Scholar] [CrossRef] [PubMed]
- Hily, J.; Demanèche, S.; Poulicard, N.; Tannières, M.; Djennane, S.; Beuve, M.; Vigne, E.; Demangeat, G.; Komar, V.; Gertz, C.; et al. Metagenomic-based Impact Study of Transgenic Grapevine Rootstock on Its Associated Virome and Soil Bacteriome. Plant Biotechnology Journal 2018, 16, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.M.B.; Näther, A.; Ortiz Cortes, V.; Mullins, E.; Kessel, G.J.T.; Lotz, L.A.P.; Tebbe, C.C. No Tangible Effects of Field-Grown Cisgenic Potatoes on Soil Microbial Communities. Front. Bioeng. Biotechnol. 2020, 8, 603145. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ye, S.; Chen, J.; Wang, L.; Li, Y.; Ge, L.; Wu, G.; Song, L.; Wang, C.; Sun, Y.; et al. Combined Metagenomic and Metabolomic Analyses Reveal That Bt Rice Planting Alters Soil C-N Metabolism. ISME COMMUN. 2023, 3, 4. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Nawaz, M.A.; Kouretas, D.; Balias, G.; Savolainen, K.; Tutelyan, V.A.; Golokhvast, K.S.; Lee, J.D.; Yang, S.H.; Chung, G. Environmental Impacts of Genetically Modified Plants: A Review. Environmental Research 2017, 156, 818–833. [Google Scholar] [CrossRef]
- Lottmann, J.; O’Callaghan, M.; Baird, D.; Walter, C. Bacterial and Fungal Communities in the Rhizosphere of Field-Grown Genetically Modified Pine Trees ( Pinus Radiata D.). Environ. Biosafety Res. 2010, 9, 25–40. [Google Scholar] [CrossRef]
- Danielsen, L.; Lohaus, G.; Sirrenberg, A.; Karlovsky, P.; Bastien, C.; Pilate, G.; Polle, A. Ectomycorrhizal Colonization and Diversity in Relation to Tree Biomass and Nutrition in a Plantation of Transgenic Poplars with Modified Lignin Biosynthesis. PLoS ONE 2013, 8, e59207. [Google Scholar] [CrossRef]
- Beckers, B.; Op De Beeck, M.; Weyens, N.; Van Acker, R.; Van Montagu, M.; Boerjan, W.; Vangronsveld, J. Lignin Engineering in Field-Grown Poplar Trees Affects the Endosphere Bacterial Microbiome. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 2312–2317. [Google Scholar] [CrossRef]
- Stefani, F.O.P.; Moncalvo, J.-M.; Séguin, A.; Bérubé, J.A.; Hamelin, R.C. Impact of an 8-Year-Old Transgenic Poplar Plantation on the Ectomycorrhizal Fungal Community. Appl Environ Microbiol 2009, 75, 7527–7536. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, L.; Thürmer, A.; Meinicke, P.; Buée, M.; Morin, E.; Martin, F.; Pilate, G.; Daniel, R.; Polle, A.; Reich, M. Fungal Soil Communities in a Young Transgenic Poplar Plantation Form a Rich Reservoir for Fungal Root Communities. Ecology and Evolution 2012, 2, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Ding, C.; Zhang, B.; Huang, Q.; Huang, R.; Su, X. Endophytic Communities of Transgenic Poplar Were Determined by the Environment and Niche Rather Than by Transgenic Events. Front. Microbiol. 2019, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Ge, L.; Hu, C.; Wu, G.; Sun, Y.; Song, L.; Wu, X.; Pan, A.; Xu, Q.; et al. Environmental Behaviors of Bacillus Thuringiensis (Bt) Insecticidal Proteins and Their Effects on Microbial Ecology. Plants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, J.; Chen, X.; Lv, J.; Jia, H.; Zhao, S.; Lu, M. An Empirical Assessment of Transgene Flow from a Bt Transgenic Poplar Plantation. PLoS ONE 2017, 12, e0170201. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Yang, R.; Zhen, Z.; Liu, J.; Huang, L.; Yang, M. A 5-Year Field Study Showed No Apparent Effect of the Bt Transgenic 741 Poplar on the Arthropod Community and Soil Bacterial Diversity. Sci Rep 2018, 8, 1956. [Google Scholar] [CrossRef]
- Wang, P.; Wei, H.; Sun, W.; Li, L.; Zhou, P.; Li, D.; Qiang, Z. Effects of Bt-Cry1Ah1 Transgenic Poplar on Target and Non-Target Pests and Their Parasitic Natural Enemy in Field and Laboratory Trials. Forests 2020, 11, 1255. [Google Scholar] [CrossRef]
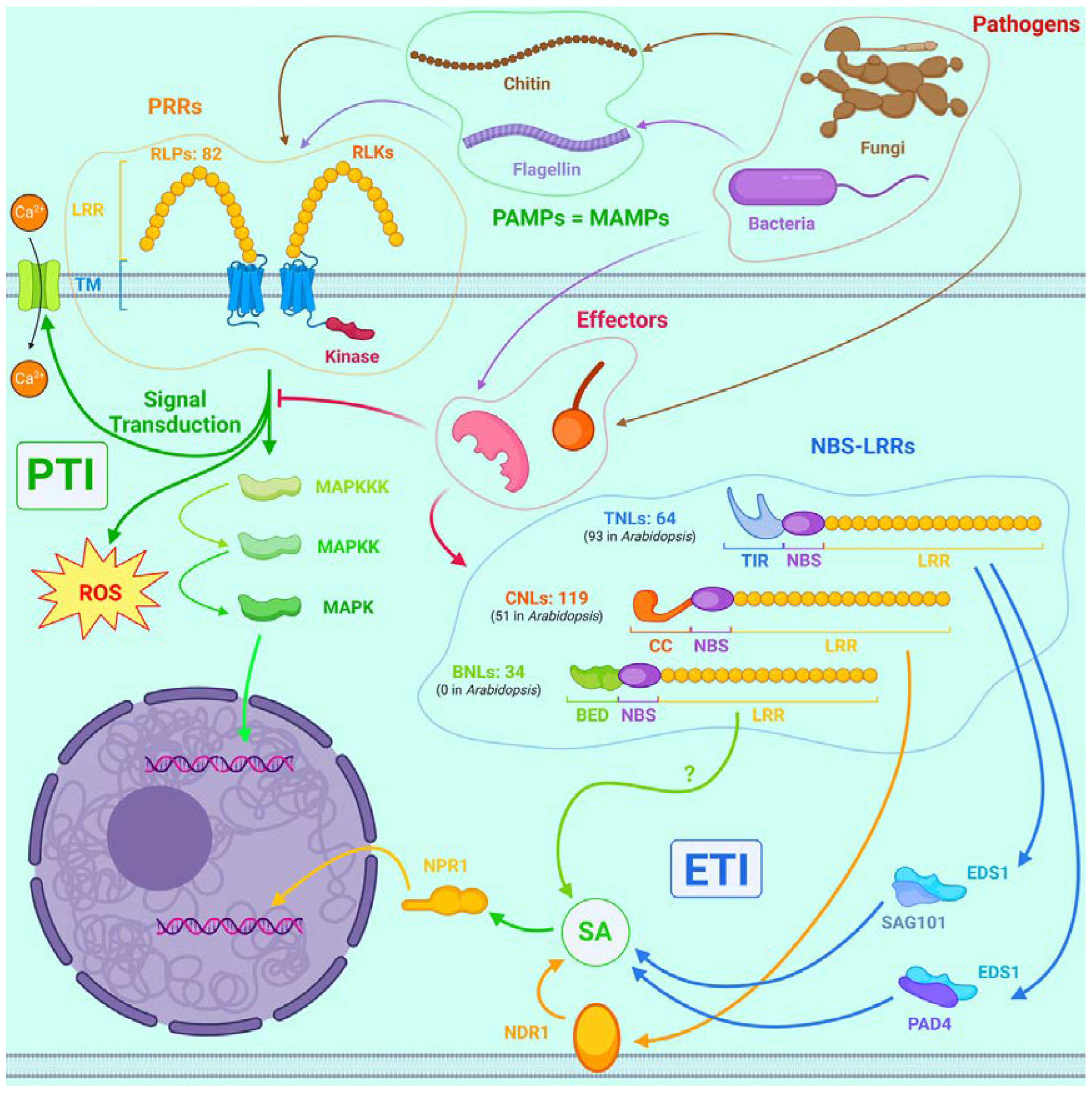
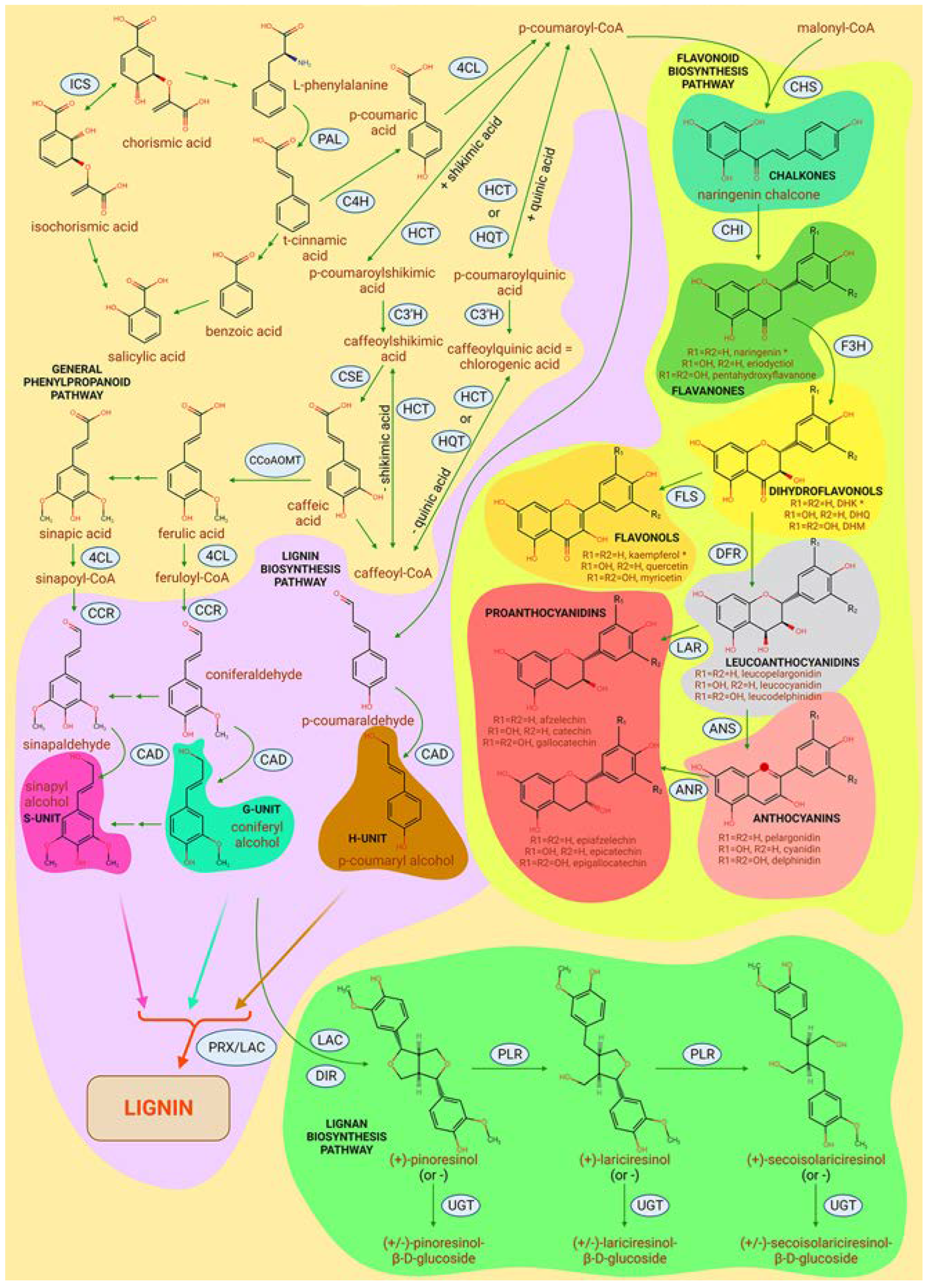
| Article № | Poplar species | Pathogen | Gene | Transgene | Method of Genetic Engineering | Change in expression | Change in resistance | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | P. davidiana × P. bolleana | M.brunnea | AtRLP23 | YES | A | ↑ | ↑ | Recognition of additional PAMP, which can not be detected by poplar receptors - NLP24 | [53] |
| E. australis | ↑ | ||||||||
| 2 | P. tomentosa | D. gregaria | PtoACO7 | NO | ↑ | ↑ | Increased ET biosynthesis, levels and signaling | [117] | |
| 3 | P. trichocarpa | S. populiperda | PtDIR11 | NO | ↑ | ↑ | Increased content of lignans and flavonoids, activation of JA and ET pathways | [147] | |
| 4 | P. tomentosa | M. brunnea | PtrLAR3 | NO* | A | ↑ | ↑ | Increased proanthocyanidin content | [172] |
| 5 | P. trichocarpa | S. populiperda | PtDef | NO | A | ↑ | ↑ | Defensin toxicity to fungus, increased SA and JA activity, increased PRs and nsLTPs accumulation, increased HR amplitude and H2O2 accumulation | [42] |
| 6, 7 | P. × euramericana | NO* | A | ↑ | ↑ | [308] | |||
| 8 | P. tomentosa | A. alternata | LJAMP2 | YES | A | ↑ | ↑ | LJAMP2 antifungal toxicity, maybe plant immunity activation as nsLTP | [176] |
| C. gloeosporioides | ↑ | ||||||||
| 9 | P. nigra × P. maximowiczii | S. musiva | MsrA2 | YES | A | ↑ | ↑ | MsrA2 (modified dermaseptin β1) is toxic to fungi | [179] |
| 10 | P. tomentosa | C. chrysosperma | BbChit1 | YES | A | ↑ | ↑ | Chitinase activity => fungal cell wall degradation | [181] |
| 11 | P. tomentosa | A. alternata | LJAMP2 or BbChit1 | YES | A | ↑ | ↑ | Same to article 10 or 11 | [182] |
| LJAMP2 + BbChit1 | A | ↑ + ↑ | ↑** | Combined action of LJAMP2 and BbChit1 | |||||
| 12 | P. deltoides × P. euramericana | M. brunnea | PeTLP | NO | A | ↑ | ↑ | Activation of other defense proteins | [183] |
| 13 | P. davidiana × P. alba | C. chrysosperma | TaHsp24 | YES | A | ↑ | ↑ | Increased activity of SA and JA pathways and PRs content | [186] |
| A. alternata | ↑ | ||||||||
| 14 | P. deltoides × P. euroamericana | B. dothidea or A. alternata | PdePrx12 | NO | A | ↑ | ↓ | H2O2 scavenging => H2O2 content reduced in overexpressor and increased in underexpressor line | [187] |
| A | ↓ | ↑ | |||||||
| 15 | P. tomentosa | D. gregaria | PtrWRKY89 | NO* | A | ↑ | ↑ | Activation of SA signaling and PR genes expression with no effect on JA signaling | [189] |
| 16 | P. trichocarpa | NO | A | ↑ | ↑ | [192] | |||
| M. brunnea | ↑ | ||||||||
| 17, 18 | P. simonii × P. nigra | A. alternata | PsnWRKY70 | NO | A | ↑ | ↑ | Activation of PTI, ETI and SA | [193] |
| 19 | P. tomentosa | D. gregaria | PtrWRKY40 | NO* | A | ↑ | ↓ | Activation of JA, but inactivation of SA signaling => increased resistance to necrotrophs, but decreased to biotrophs | [194] |
| Arabidopsis thaliana | B. cinerea | YES | A | ↑ | ↑ | ||||
| 20 | P. tomentosa | D. gregaria | PtoWRKY60 | NO | A | ↑ | ↑ | Activation of SA signaling, no effect on JA | [195] |
| 21 | Arabidopsis thaliana | P. syringae | PtrWRKY73 | YES | A | ↑ | ↑ | Activation of SA => increased resistance to biotrophs, but decreased to necrotrophs | [196] |
| B. cinerea | A | ↑ | ↓ | ||||||
| 22 | P. tremula × P. alba | Melampsora sp. | PtWRKY23 | NO | A | ↓ (RNAi) | ↓ | WRKY23 expression is at an optimal level in the WT plant and should be subject to adequate and flexible regulation? | [197] |
| A | ↑ | ||||||||
| 23 | P. simonii | A. alternata | PsnWRKY25 | NO | A | ↑ | ↑ | Activation of PsCERK1, PR1 and secondary metabolism | [198] |
| 24 | P. tremula × P. tremuloides | - | MYB182 | NO | A | ↑ | - | Repression of flavonoid biosynthesis pathway => decreased flavonoid content in overexpressors and increased in knockout plants*** | [202] |
| 25 | P. tremula × P. tremuloides | - | MYB165 | NO | A | ↑ | - | [203] | |
| MYB194 | NO | A | ↑ | - | |||||
| 26 | P. tomentosa | - | PtrMYB57 | NO* | A | ↑ | - | [204] | |
| NO | CRISPR/Cas9 | ↓ (knockout) | |||||||
| 27 | P. tremula × P. alba | M. larici-populina, M. aecidiodes | MYB134 | NO | A | ↑ | ↑ | Upregulation of flavonoid biosynthesis pathway => increased flavonoid content*** | [170] |
| 28 | P. tremula × P. alba | - | NO | A | ↑ | - | [205] | ||
| P. tremula × P. tremuloides | A | ||||||||
| 29 | P. tomentosa | D. gregaria | PtoMYB115 | NO | A | ↑ | ↑ | [55] | |
| Nicotiana benthamiana | - | PtoMYB115 + PtoTT8 + PtoTGA1 | YES | A | ↑ + ↑ + ↑ | - | |||
| 30 | P. alba | B. cinerea | PalMYB90 + PalbHLH1 | NO | A | ↑ + ↑ | ↑ | [206] | |
| ↑ | |||||||||
| 31 | P. alba × P. tremula | - | PtrMYB119 | NO* | A | ↑ | - | [207] | |
| PtrMYB120 | NO* | A | ↑ | ||||||
| 32 | P. alba × P. glandulosa | - | NO* | A | ↑ | - | [208] | ||
| A | ↓ | ||||||||
| 33 | P. tomentosa | - | PtoMYB6 | NO | A | ↑ | - | [209] | |
| 34 | P. deltoides. | - | PdeMYB118 | NO | A | ↑ | - | [210] | |
| 35 | P. tremula × P. tremuloides | - | MYB117 | NO | A | ↑ | - | [211] | |
| 36 | P. tomentosa | - | PtoMYB142 | NO | A | ↑ | - | Increased wax content*** | [212] |
| 37 | P. davidiana × P. alba | F. oxysporum | PdPapERF109 | NO | A | ↑ | ↑ | Increase of ROS-scavenging activity => more adequate immune response | [219] |
| 38 | P. trichocarpa | B. salicis | AtVIP1 | YES | A | ↑ | ↑ | PR1 activation; full molecular mechanism is unknown | [85] |
| PtVIP1 | NO | A | ↑ | ||||||
| 39 | P. tomentosa | C. gloeosporioides | PeTGA1 | NO* | ↑ | ↑ | PeSARD activation => upregulation of SA biosynthesis | [223] | |
| 40 | P. trichocarpa | C. gloeosporioides | ptc-miR472 | NO | A | ↑ | ↓ | Fail to activate ETI, NBS-LRRs, ROS => susceptible to biotrophs; active JA/ET signaling => resistant to necrotrophs | [241] |
| C. chrysosperma | ↑ | ||||||||
| C. gloeosporioides | A | ↓ (via STTM) | ↑ | Hyperactivated NBS-LRRs => quick and robust ETI and SA response | |||||
| C. chrysosperma | ~ unchanged | ||||||||
| 41 | P. alba × P. grandidentata | M. aecidiodes | AtGolS + CsRFS | YES | A | ↑ | ↓ | Suppressed SA, Ca2+, phosphatidic acid, activated JA signaling => failed ROS and PR1 accumulation => increased susceptibility to biotrophs (but resistance to necrotrophs?). | [262] |
| 42 | P. davidiana × P. bollena | A. alternata | PdbLOX2 | NO | A | ↑ | ↑ | Hyperaccumulation of JA => hyperresistance to necrotrophs | [263] |
| 43 | P. tremula x P. alba, P. deltoides | - | LecRLK-G, TPX2 | NO | CRISPRa (dCas9) | ↑ | - | More active immune responce*** | [309] |
| - | PLATZ | NO | CBE (nCas9) | ↓ | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





