1. Introduction
With 17.9 million deaths per year, cardiovascular disease (CVD) is the leading cause of mortality worldwide [
1]. CVD is a broad term that refers to any illness of the cardiovascular system. According to the World Health Organization (WHO), heart disease accounts for 80% of these fatalities [
2]. Furthermore, socioeconomic factors such as jobs and money have an influence on the death rate via the impact on risk factors connected to lifestyle before and after cardiac disease [
3]. The best way to decrease these deaths is to detect them immediately. The ability to predict the existence of HD is critical for administering the necessary therapy on time. The incidence of HD is predicted to quadruple by the year 2020, and it is expected that in 2050, one person will develop the disease every 30 seconds [
4]. The symptoms and occurrence of heart disease vary according to the lifestyle of humans. It generally comprises pain in the chest, jaw ache, neck pain, back pain, belly issues, fingers and shoulder pains and significant shortness of breath [
5]. The various types of heart diseases are shown in
Table 1 below [
6].
As a result, it has become critical for precise and accurate prognosis of heart-related disorders. Many academics from all around the world began researching predicting cardiac problems by analyzing enormous databases for this purpose [
7]. Various deep-learning approaches have the capability of working on enormous datasets and drawing relevant results [
8]. Deep learning models are based on numerous algorithms, and so these algorithms have become significant in properly predicting the presence or absence of cardiac ailments. Different researchers all over the world worked on heart disease prediction using various techniques of machine learning, deep learning, and fuzzy logic [
9,
10], but still, there were some shortcomings, which are given below:
Almost all the research work with the target of predicting heart disease by techniques of machine learning, deep learning and fuzzy logic [
11] have been carried out using various parameters, but still, there is inadequate parameter tuning and parameter evaluation.
Lack of use of different discretization techniques, multiple classifiers, techniques of voting and other decision tree algorithms (Gini index, Gini ratio).
The technical issues with regard to overfitting.
Selection and usage of proper tools, proper pre-processing of datasets and use of advanced machine learning algorithms to reduce time complexity should be incorporated.
1.1. Socio-Economic Impact of Heart Diseases
In practically all Western nations, socioeconomic disparities in the frequency and occurrence of CVD fatalities have been documented [
1]. When compared to other countries in the same region, India has a much greater prevalence of heart disease. A prevalence rate of 11% is concerning [
2] despite the fact that India has a very large population. This is due to the fact that India has a very high population density. The prevalence rate sheds light on emerging patterns of sickness occurrence. It is estimated that cardiovascular disease, stroke, and diabetes would cost 1% of world GDP, which is equivalent to USD 236.6 billion, between the years 2005 and 2015 [
3]. It was projected in the year 2000 that adult Indians had lost a total of 9.2 billion years of productive life, which contributed to the overall economic loss. According to research conducted in 2015, the average lifespan of an Indian man is estimated to be 67.3 years, whilst the average lifespan of an Indian woman is estimated to be 69.9 years [
4]. It was projected in the year 2000 that adult Indians had lost a total of 9.2 billion years of productive life, which contributed to the overall economic loss. As a result, India contributes to the growing population of people who are affected by heart disease as a result of the increased number of elderly people who are prone to developing the condition. An essential medications list (EML) is a project that the World Health Organization (WHO) is working on to enhance healthcare systems in countries with low and intermediate incomes.
Age, modifications in lifestyle and diet, and rapidly expanding social issues, such as hospitalization, all have an influence on the likelihood of acquiring heart-related disorders and the advancement of preexisting conditions. Research indicates that heart disease is responsible for 80% of fatalities and 85% of disabilities in countries with poor and intermediate incomes [
5]. The forecasts of total deaths attributable to cardiovascular disease that are shown in
Table 2 include the period between the years 2000 and 2030. The treatment of expensive illnesses like heart disease takes up a significant portion of a family’s total income and may be rather pricey. As a result, hospitalization is often necessary since the patients need extensive therapy for their condition.
1.2. Literature review
Numerous studies have been conducted using machine learning, deep learning, fuzzy logic and data mining tools and methodologies to predict cardiac disease. Researchers have employed a variety of datasets, algorithms, and procedures [
7]; the findings they have seen thus far and future work will be used to determine the most effective techniques for diagnosing cardiovascular disease. The literature review has been divided into three categories on the basis of techniques including Deep learning, Machine learning and Ensemble learning.
1.2.1. Machine Learning Techniques
The major goal of this study is to create a predictive model with a small chance of success for people with heart disease. Weka was used for the experiment, and the Cleave Land UCI dataset underwent the following processing steps during training: pre-processing, classification, regression, clustering, association, and visualization. WEKA’s Explorer mode is employed to try out classification tools. For the analysis, decision tree classifiers, including J48, the Logistic Model Tree Algorithm, and Ran-dom Forest, were used, along with 10-fold cross-validation and reduced error trimming. The j48 algorithm with decreased error pruning has the highest accuracy overall. If alternative discretization approaches, multiple classifiers, voting strategies, and additional decision tree algorithms (Gain ratio, Gini index) were used, the accuracy might be increased [
8]. Taylan et al. proposed the prediction of heart disease using Na-ïve Bayes and k-mean clustering. K-mean clustering has been used to improve the efficiency of the desired output and used for grouping of attributes, followed by the Naïve Bayes algorithm for prediction of the disease. Naïve Bayes is mainly used when the inputs are generally high but gives no absurdity when the inputs are low. Moreover, it answered complex queries with respect to interpretation and accuracy [
12]. Usama et al. [
13] introduced a major purpose to automate a method for diagnosing heart disease using historical data and information. The purpose was to discuss numerous knowledge abstraction methodologies leveraging data mining techniques, as well as their advantages and disadvantages. Data mining makes use of the Decision Tree Algorithm, the Neural Network Algorithm, and the Naive Bayes Algorithm. By calculating Shannon entropy, the ID3 Algorithm builds trees of decisions. Due to the constraints of building a short tree decision tree from set-off learning data, we use Quinlan’s C4.5 and C5.O calculations to flow the data. Nave Bayes surpasses the aforementioned strategies since it is not dependent on any input attributes. Future studies and implementation of other ways to alleviate the problem of high overfitting are possible. In [
14] investigation, the subject of restricting and summarising the many data mining approaches that are used in the field of medical prediction is investigated. The dataset was analyzed using the naive Bayes, KNN, and decision tree algorithms, which are all different supervised machine learning methods. The Tangra tool and 10-fold cross-validation are used in this step, and afterwards, the findings are compared in order to accomplish the categorization of the dataset. Thirteen different characteristics were employed for the comparison and contrast. The accuracy of the decision tree is the highest at 99.2%, followed by the accuracy of the Nave Bayes method at 96.5% and the accuracy of the classification using clustering at 8.83% [
14].
The major goal of another study [
15] was to create a prediction model for the detection of heart disease using transthoracic echocardiography using data mining techniques. In the research, 7,339 cases were extracted and pre-processed using data gathered at PGI, Chandigarh, between 2008 and 2011. Using the Weka 3.6.4 machine learning software, three unique supervised machine learning techniques were applied to a model of a pre-processed Transthoracic echocardiography dataset. These methods are named J48 Classifier, Nave Bayes, and Multilayer Perception. The distribution frequency was used to check for noise, inconsistency, and missing data, while box plots were used to locate outliers. The effectiveness of the models was evaluated using standard metrics, including accuracy, precision, recall, and F-measure. Random selection of training and test data samples was performed using ten cross-validations. With a classification accuracy of 95.56%, the J48 classifier is the most successful in predicting heart disease based on the specified parameters. To enhance classification accuracy and forecast particular kinds of heart disease, researchers will need to conduct additional tests with a larger number of data sets in the future [
15]. The research entailed predicting cardiovascular disease using a different method. In order to forecast heart attacks successfully using data mining, the focus of this study is on the use of a wide variety of approaches, as well as combinations of a large number of target criteria.
A number of supervised machine learning strategies, such as Naive Bayes and Neural Network, as well as weighted association A prior algorithm and Decision algorithm, have been utilized for the purpose of conducting an analysis of the dataset. These strategies were used in order to accomplish this goal. For the purpose of another investigation [
16], the data mining program Weka, version 3.6.6, was used. All of the tools that were necessary for the pre-processing of data, as well as classification, regression, clustering, association rules, and visualization, are linked to WEKA. The Decision Tree algorithm has outperformed with an accuracy of 99.62% while using 15 criteria. In addition, the accuracy of the Decision Tree and Bayesian Classification increases even further when the genetic algorithm is used to minimize the actual data size in order to get the ideal subset of characteristics that are required for predicting heart disease. This is because the genetic algorithm is able to find the ideal subset of attributes that are necessary for making the prediction. This helps to ensure that the Decision Tree and Bayesian Classification produce the most accurate results possible. They used the Association Classification approach in addition to the prior algorithm and the Mafia algorithm [
16].
Recently, Paladino et al. [
17] proposed evaluating three AutoML tools (PyCaret, AutoGluon, and AutoKeras) on three datasets, comparing their performance with ten traditionally developed ML models. Traditionally designed models achieved 55%-60% accuracy, while AutoML tools outperformed them. AutoGluon consistently achieved 78%-86% accuracy, making it the top tool. PyCaret’s performance varied (65%-83%), and AutoKeras showed fluctuating results (54%-83%). AutoML tools simplify ML model creation, potentially surpassing traditional methods.
1.2.2. Deep Learning Techniques
For the development of effective heart disease prediction [
18], Multilayer Perceptron with back propagation neural network is employed. The dataset contains 303 records, of which 40% is used for training the Multilayer Perceptron neural network, and the remaining is used for testing using the backpropagation algorithm. The experiment was conducted using Weka 3.6.11. Based on the actual desired output obtained from the Learning Algorithm, in each neuron, the backpropagation algorithm calculates the error followed by the calculation of the output for each neuron, which in turn results in improved weights during the whole processing. The whole system developed shows an accuracy of 100% [
18]. The reference [
19] provided a novel idea to recognize individuals with a heart attack in colour photos by identifying heart attack-related postures. Images of persons in infarction-free conditions and those who may be experiencing one were combined to create a data set. Convolutional neural networks are used in the process of recognizing infarcts. A total of 1520 photos, 760 of class "Infarct" and 760 of class "No Infarct," were included in the first image data collection. These have been practised on a series of specifically created photos that feature people acting out heart attacks. The classification of infarcts shows encouraging results with 91.75% accuracy and 92.85% sensitivity.
Recently, Rani et al. [
20] suggested a method dubbed Cardio help that uses convolutional neural networks, a type of deep learning algorithm, to predict the likelihood that a patient would have a cardiovascular illness (CNN). The suggested approach focused on modelling temporal data while applying CNN for early-stage HF prediction. They employed convolutional neural networks and a cutting-edge dataset from the UCI library to predict a potential cardiac ailment (CNN). Some heart test parameters, as well as typical human behaviours, were included in the dataset. According to experimental findings, the proposed method performed better than the current methods in terms of performance evaluation measures. The proposed method’s attained accuracy is 97%. The application of deep learning models to segment the left ventricle using magnetic resonance data. The results were very close to the actual situation. They suggested a different approach for automatically segmenting the left ventricle using magnetic resonance data and employed a deep learning strategy together with a level-set method. UNET was recommended by researchers [
21] for segmenting biological images. To identify and differentiate boundaries in UNET, classification was done on each individual pixel. In order to improve segmentation in medical imaging, the Fully Convolutional Network was modified and utilized as the basic architecture for the U-Net design. A technique incorporating a deep learning architecture has been proposed [
22].
Another recent research [
23] endeavour introduces a methodology utilizing a CNN model that effectively combines the strengths inherent in both dense and residual blocks. By capitalizing on the benefits of residual and dense connections, this model is able to enhance the flow of information, propagate gradients more effectively, and facilitate the reuse of features, ultimately resulting in improved performance. The proposed model was composed of interleaved residual-dense blocks, with the option of incorporating pooling layers for downsampling. Heartbeat classification into five distinct classes is accomplished by utilising a linear support vector machine (LSVM), which simplifies the feature learning and representation derived from ECG signals. In order to address various issues such as baseline drift, power line interference, and motion noise, the initially collected ECG data is subjected to a denoising procedure. Subsequently, resampling techniques are employed to mitigate the impact of class imbalance. The proposed method underwent rigorous evaluation through extensive simulations conducted on well-established benchmarked datasets, with a wide array of performance measures being utilized. On average, the proposed approach achieved an accuracy rate of 98.5%, a sensitivity rate of 97.6%, a specificity rate of 96.8%, and an AUC (Area Under the Curve) value of 0.99.
1.2.3. Ensemble Learning Techniques
Yang et al. [
24] introduced a smote-XGboost-based approach to cardiovascular disease prediction in this study. A method was presented for feature selection that takes advantage of information gain; next, they used the hybrid Smote-Enn algorithm to handle imbalanced datasets. Lastly, the model was trained using the processed HDD dataset. XGboost was tested experimentally in comparison to five baseline algorithms. The results showed that the proposed model does very well across all four assessment metrics, with a prediction accuracy of 91.44%. Importantly, for the prediction of heart disease, they further quantify the feature relevance of the chosen algorithm [
24]. To enhance the effectiveness of machine learning classifiers in predicting the risk of heart disease, the work used three attribute evaluator approaches to choose key features from the Cleveland heart dataset. By using the chi-squared attribute assessment approach, the SMO classifier accomplished an impressive feat. Over time, they found that by carefully selecting attributes and fine-tuning the classifiers’ hyperparameters, the prediction performance was much enhanced. The study used a smaller dataset of 303 instances, three feature selection techniques, and ten machine learning classifiers. However, the classifiers’ performance is sufficient.
Numerous machine learning algorithms and feature selection approaches have a great deal of untapped potential. Reddy et al. [
25] aimed to merge datasets in the future to increase the number of observations and run additional tests to enhance the classifier’s predicting performance by choosing the right characteristics. The study created data mining algorithms with an 81.82% accuracy. They employed C4.5, CART, and RIPPER for the rule basis, compared the three fuzzy rule-based strategies in this research and deployed their system on 411 data sets [
21]. The major goal of the research was to use a real-world dataset and several methods to categorise cardiac diseases. Predicting the existence of cardiac disease was done using the k-mode clustering technique on a dataset of patients. In order to prepare the dataset for analysis, the age attribute was converted to years and then divided into 5-year intervals. Similarly, the diastolic and systolic blood pressure values were divided into 10-interval bins. To account for the different features and development of heart disease in men and women, the dataset was additionally divided according to gender [
26].
2. Technical details of dataset
The heart disease dataset [
27] offers an extensive range of attributes pertaining to cardiovascular health and lifestyle decisions, including specific patient information such as age, gender, cholesterol levels, blood pressure, heart rate, and factors such as diabetes, family history, smoking habits, obesity, and alcohol consumption. In addition, lifestyle variables such as the number of hours spent exercising, food habits, stress levels, and sedentary hours are taken into account. Medical factors such as prior cardiac issues, drug use, and triglyceride levels are taken into account. Factors such as income and geographical features, including nation, continent, and hemisphere, are also taken into account. The dataset contains 8763 patient records from various locations worldwide. It includes a vital binary classification feature that indicates the existence or absence of a heart attack risk. This dataset serves as a valuable resource for predictive analysis and research in the field of cardiovascular health.
Table 3.
Description of heart disease dataset.
Table 3.
Description of heart disease dataset.
| 1 |
Patient ID |
Features |
Value type |
| 2 |
Age |
Age of the patient |
Numerical |
| 3 |
Sex |
Gender of the patient |
(Male/Female) |
| 4 |
Cholesterol |
Cholesterol levels of the patient |
Numerical |
| 5 |
Blood Pressure |
Blood pressure of the patient (systolic/diastolic) |
Numerical |
| 6 |
Heart Rate |
Heart rate of the patient |
Numerical |
| 7 |
Diabetes |
Whether the patient has diabetes |
(Yes/No) |
| 8 |
Family History |
Family history of heart-related problems |
(1: Yes, 0: No) |
| 10 |
Obesity |
Obesity status of the patient |
(1: Obese, 0: Not obese) |
| 11 |
Alcohol Consumption |
Level of alcohol consumption by the patient |
(None/ Light/ Moderate/ Heavy) |
| 12 |
Exercise Hours Per Week |
Number of exercise hours per week |
Numerical |
| 13 |
Diet |
Dietary habits of the patient |
(Healthy/ Average/ Unhealthy) |
| 14 |
Previous Heart Problems |
Previous heart problems of the patient |
(1: Yes, 0: No) |
| 15 |
Medication Use |
Medication usage by the patient |
(1: Yes, 0: No) |
| 16 |
Stress Level |
Stress level reported by the patient |
(1-10) |
| 17 |
Sedentary Hours Per Day |
Hours of sedentary activity per day |
Numerical |
| 18 |
Income |
Income level of the patient |
Numerical |
| 19 |
BMI |
Body Mass Index (BMI) of the patient |
Numerical |
| 20 |
Triglycerides |
Triglyceride levels of the patient |
Numerical |
| 21 |
Physical Activity Days Per Week |
Days of physical activity per week |
Numerical |
| 22 |
Sleep Hours Per Day |
Hours of sleep per day |
Numerical |
| 23 |
Country |
Country of the patient |
Numerical |
| 24 |
Continent |
Continent where the patient resides |
Numerical |
| 25 |
Hemisphere |
Hemisphere where the patient resides |
Numerical |
| 26 |
Heart Attack Risk |
Presence of heart attack risk |
(1: Yes, 0: No) |
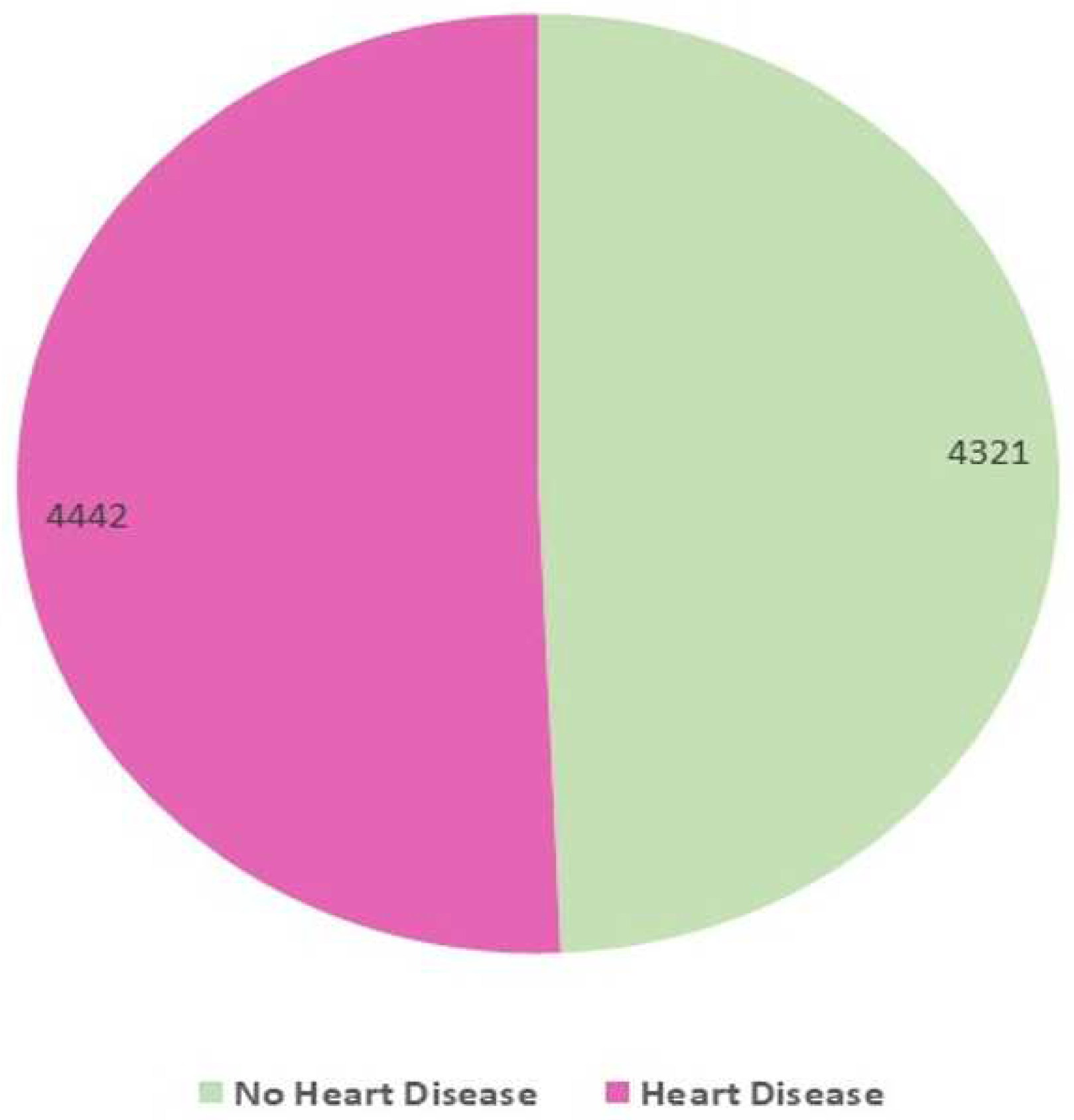
Frequency Distribution
This experiment’s dataset has a good mix of classes, with class 1 representing heart disease (4442 instances) and class 0 representing no heart disease (4321 instances), as shown in
Figure 1. If the dataset is not balanced for the problem statement, machine learning and ensemble learning models will produce poor results. Sampling techniques can be used in certain situations to create a balanced dataset.
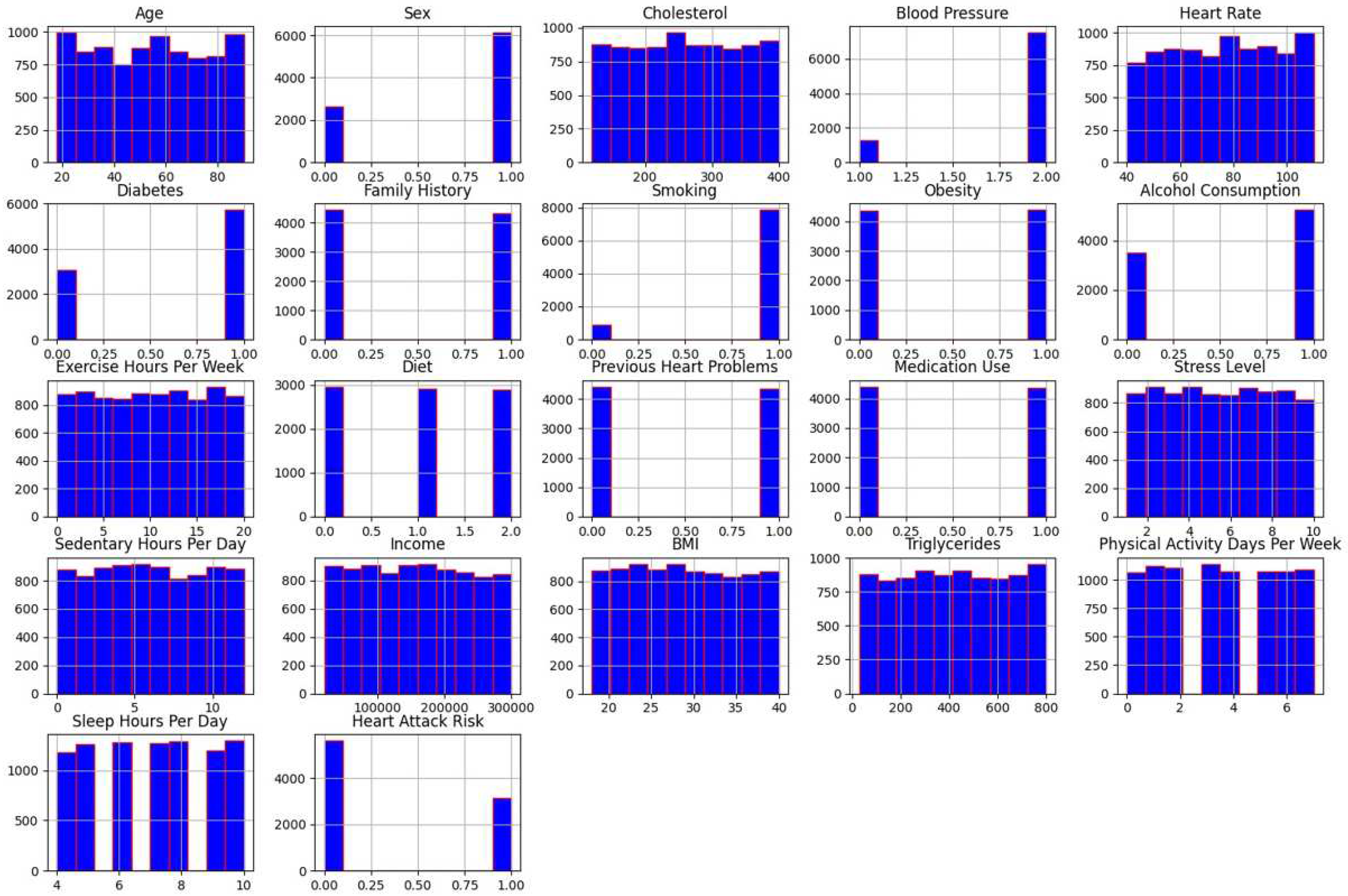
Histogram is a significant statistical analysis technique for data visualization. It depicts the continuous variable distribution for a given interval of time. Through a histogram, the data is plotted by dividing it into sections called bins. The main use of histogram is to inspect the underlying distribution of frequency as in the case of normal distribution, skewness, outliers and so on.
Figure 2 below represents the histogram for each attribute separately to analyze their distribution.
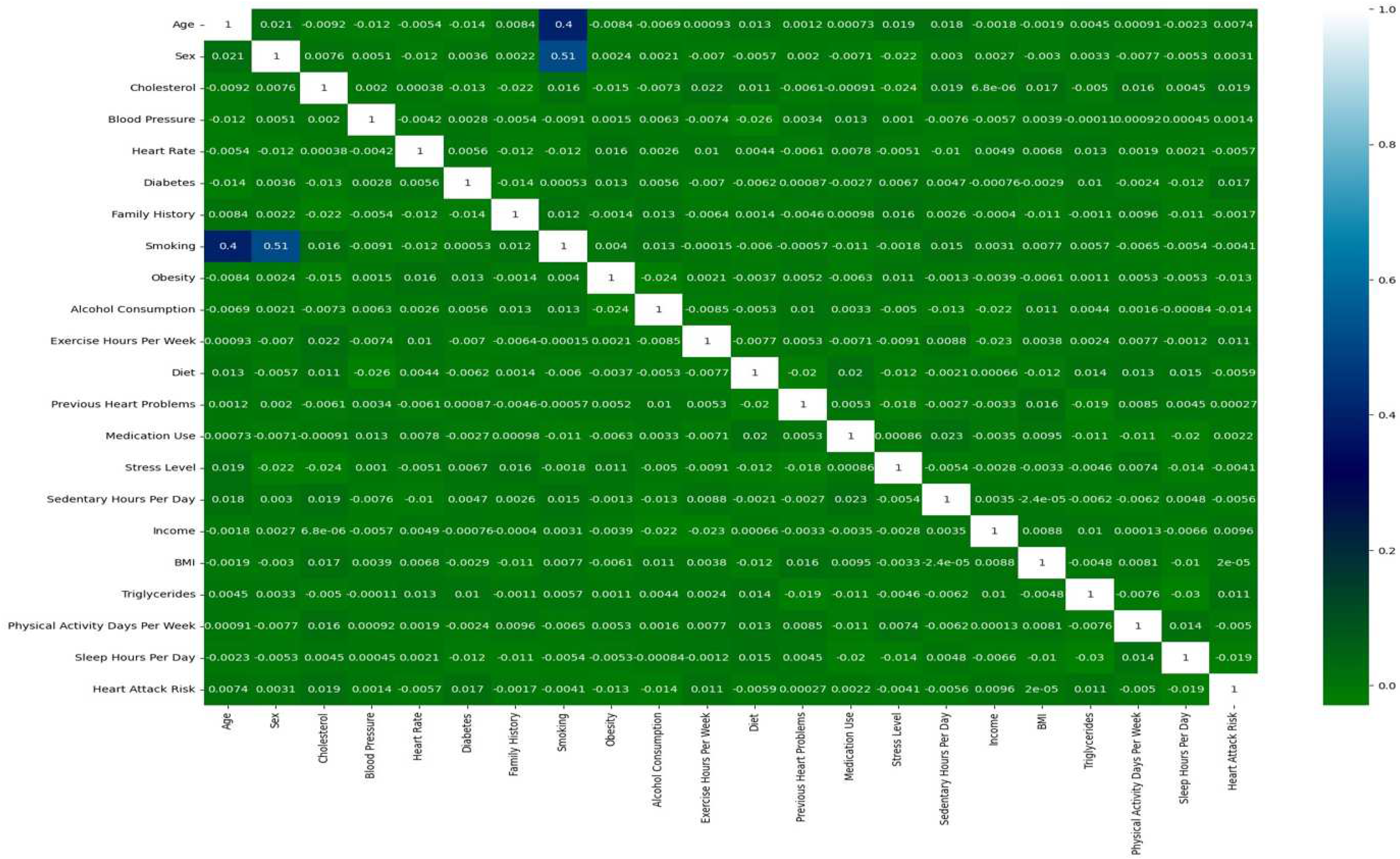
A map of the concentration of values between graphical figures, in which matrix values that are individual are presented as colours, is called a Heatmap. It is used in the visualization of two dimensions of the matrix shown in
Figure 3. It is also helpful in pattern finding and hence gives a perspective of depth. In Figure 7 below, we used the colour function in order to create colours of the heat map and added labels(row/column) to this. The Heatmap given below indicates how one attribute is co-related to another attribute, that is, whether it is negatively or positively correlated.
Descriptive statistics are crucial for defining the features of data. It condenses the facts to facilitate human comprehension and interpretation.
Table 4 provides an overview of the statistical measurements for the clinical characteristics, including the number of records, the lowest (min) value, the highest value, the maximum value, the mean, and the standard deviation (Std). As an example, the age property has a mean value of 53.84. The data set has a mean value and a standard deviation of 21.21. The highest and lowest age values in the set are 90, and the ages are 18 years individually. These statistical metrics are likewise computed for the other 22 properties.
3. Methods
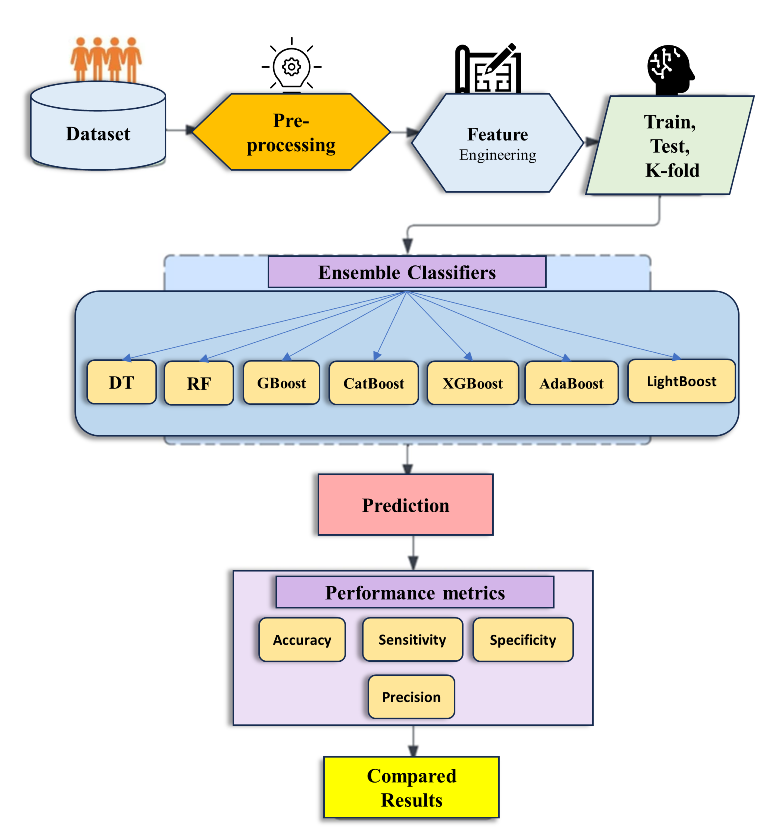
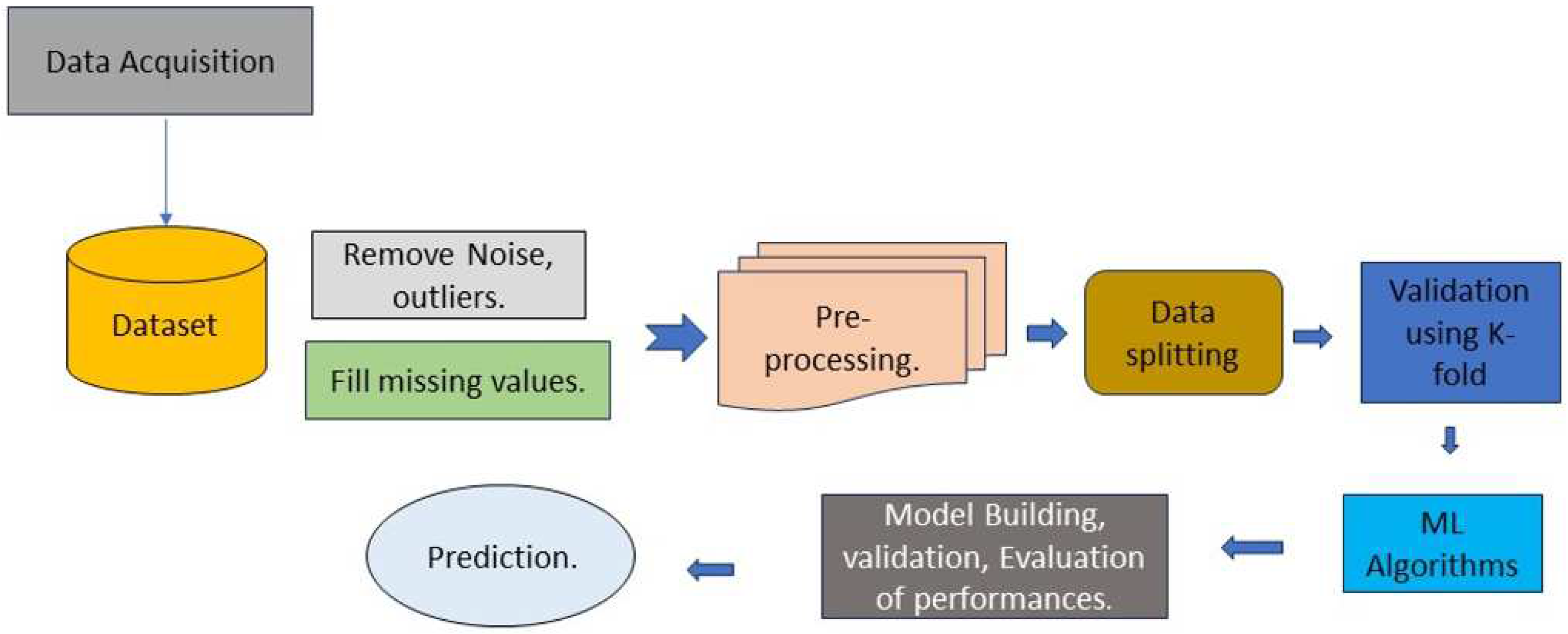
The whole procedure, from data collection through the production of useful outcomes, is shown in
Figure 4 below.
Algorithm A: Workflow of Methodology Employed:
Input: Dataset
Output: Prediction Model
BEGIN
Step 1; ingress dataset
Step 2: pre-process the dataset (data transformation, data cleaning)
Step 3: Xtrain, Ytrain...70% of dataset.
Step 4: Xtest, Ytest......30% of dataset.
Step 5: D1 models and their Algorithms
(DT, RF, Boosting techniques)
for do
Model.fit();
Model.predict();
Print (Accuracy(i), Confusion matrix, Roc curve)
End
Step 6: Placement of framework
STOP
3.1. Machine Learning framework
All experimental data was analyzed using Anaconda. Anaconda is a public and licensed accessible version of the popular Python and R language skills for scientific computing, designed to streamline deployment and package management (machine learning and data science applications, pre-processing of massive amounts of data, predictive analysis, etc.). Furthermore, we used TensorFlow to implement all ML models. TensorFlow is an open-source machine learning framework by Google. It provides tools for building and deploying machine learning models, including neural networks. TensorFlow supports distributed computing and offers high-level and low-level APIs. Keras is another Python library with a high-level neural networks API built on top of TensorFlow. It simplifies the process of creating and training deep learning models with an easy-to-use syntax. Keras includes pre-trained models and supports various neural network architectures. Finally, scikit-learn, which is a popular ML library for Python, is applied. It offers a wide range of algorithms and tools for tasks like classification, regression, and clustering. scikit-learn follows a modular design and provides utilities for data preprocessing and model evaluation.
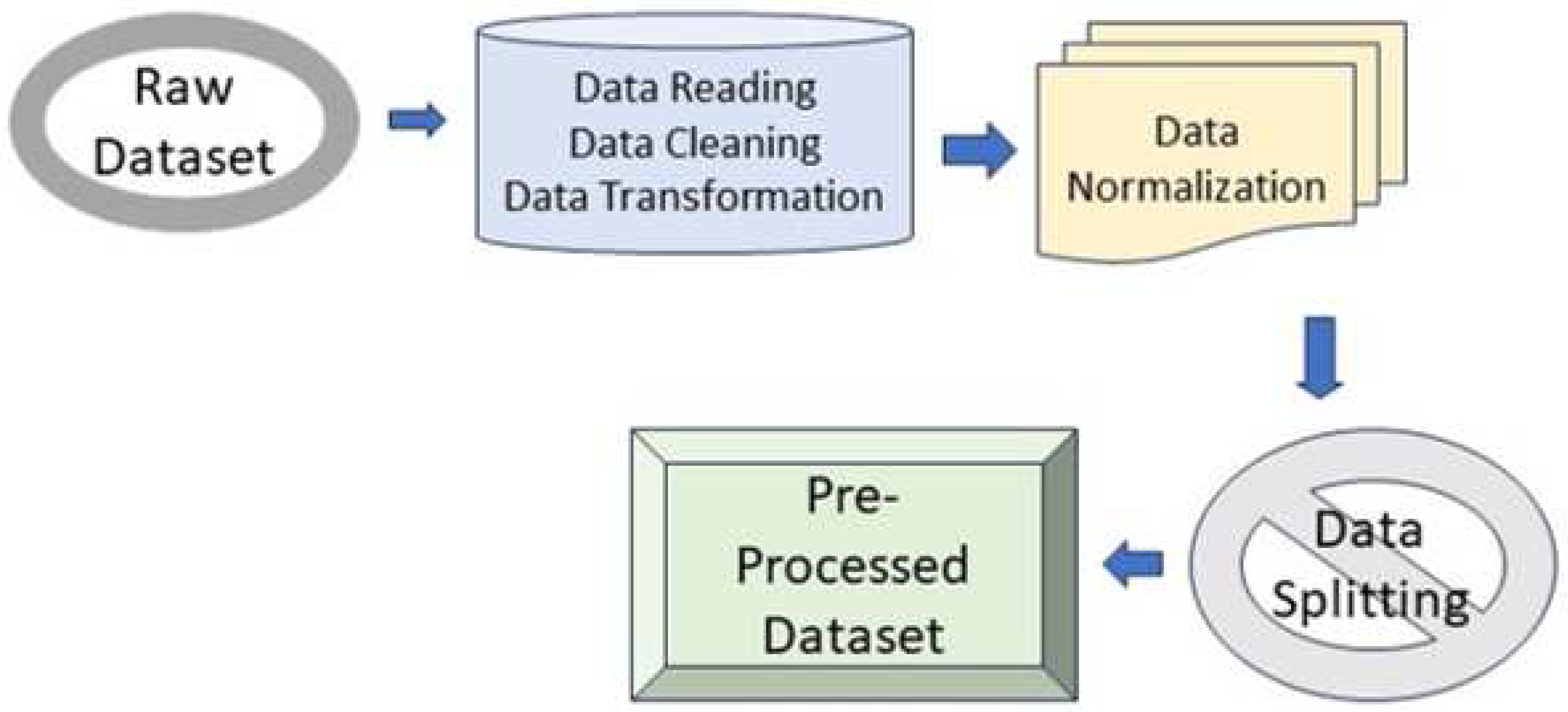
3.2. Pre-processing
The preprocessing of data is an integral step in achieving improved outcomes prior to the development of ensemble machine learning models. Through the utilization of techniques such as resampling and discretization, the acquired dataset underwent preprocessing using the Integrated Development Environment Spyder with Python (3.9.1) as the programming tool. The requisite libraries for evaluating data quality were imported, and missing values were imputed by averaging attribute values such as Age, Sex, cholesterol, triglycerides, and others, employing a data imputation approach. Outliers were detected using the boxplot method, with the interquartile range technique being employed to replace these outliers with suitable sample values. Nevertheless, out of the 26 attributes, attributes such as patient ID, Country Name, Continent Name, and Hemisphere were excluded. Prior to the creation of machine learning models, data transformation was performed to enhance data efficiency. The process of pre-processing is shown in
Figure 5 below.
The dataset splitting ratio has been chosen with care, taking into account aspects such as overfitting, model complexity, size of the dataset and the unique requirements of machine learning activity. The dataset is split into a 60:40 ratio. It means for every algorithm, 60% of the dataset is used for training, and the remaining 40% of the dataset is used for testing the algorithm. Through the train_test_split function, the implementation of the train test split evaluation procedure is done using the sci-kit learn library. The loaded dataset is taken by the function as the input, and then two subsets of this split dataset are returned. Ideally, we split the original dataset into input(x) and output(y) columns, followed by the function calling that passes both the arrays and splits them exactly into the train and test subsets separately. After the generation of results, 10-fold cross-validation will also be used to validate the results, and then all these results will be compared in terms of optimal accuracy.
3.3. Ensemble learning approaches
Ensemble learning approaches are widely investigated across several domains for the purpose of addressing practical challenges [
28]. These models have achieved substantial advancements in the accurate forecasting, identification, assessment, and prediction of many medical conditions. This research examined three ensemble-learning-based boosting algorithms for the purpose of predicting cardiac disease. The experiment made use of the algorithms listed below.
3.3.1. Decision Tree
When it comes to classification and regression, one non-parametric supervised learning approach that is often utilized is decision trees or DTs. The objective is to train a model to learn basic decision rules derived from data attributes in order to forecast the value of a target variable. A piecewise constant approximation is one way to look at a tree [
29].
3.3.2. Random Forest
Random forest algorithms consist of three primary hyperparameters that must be configured prior to the training process. These crucial parameters encompass the node size, the quantity of trees, and the number of features sampled. Once established, the random forest classifier becomes an invaluable tool to tackle both regression and classification problems. The underlying structure of the random forest algorithm is essentially a compilation of decision trees. Each individual tree within this ensemble is constructed by utilizing a data sample drawn from a training set with replacement, which is commonly referred to as the bootstrap sample. To evaluate the effectiveness of this algorithm, one-third of the training sample is reserved as test data, known as the out-of-bag (oob) sample. This oob sample will play a significant role in our subsequent analysis and exploration of the random forest algorithm [
30].
3.3.3. Gradient Boosting
Gradient Boosting is a machine-learning approach that belongs to the ensemble family. It is often used for both classification and regression applications. The algorithm constructs a prognostic model by assembling a collection of feeble learners, often decision trees, and then amalgamates them to generate a more robust and precise model. The fundamental concept behind gradient boosting is to iteratively train further models in order to rectify the inaccuracies of the preceding models. The mathematical intricacies entail the use of calculus, specifically the calculation of gradients and partial derivatives. The choice of loss function will dictate the mathematical formulations for these gradients. Typical loss functions used in machine learning include mean squared error for regression problems and cross-entropy for classification tasks. The intricacies might get intricate, but the underlying premise is iterative optimization to minimize the loss by modifying the model’s predictions [
31,
32].
3.3.4. CatBoost
CatBoost [
33] is a supervised machine learning technique used by the Train Using Auto ML program. It utilizes decision trees for both classification and regression tasks. Cat Boost is characterized by two primary attributes: its ability to handle categorical data and its use of gradient boosting. Gradient boosting is an iterative approach that involves constructing many decision trees. Each successive tree enhances the outcome of the preceding tree, resulting in superior outcomes. CatBoost enhances the original gradient boost technique to get a more efficient implementation. CatBoost addresses a constraint present in other decision tree-based techniques, where it is usually necessary to preprocess the input by converting category text variables into numerical values, such as one-hot encodings. This technique has the capability to directly handle a mixture of category and non-categorical explanatory factors without the need for preprocessing. Preprocessing is an integral aspect of the algorithm. Cat Boost employs an encoding technique known as ordered encoding to encapsulate category attributes [
34].
3.3.5. XGBoost
It employs an ensemble of diverse decision trees (weak learners) to compute similarity scores independently. To address the issue of overfitting during the training phase, the method adjusts the gradient descent and regularization procedure. You are able to control overfitting with the help of regularization, which is provided by XGBoost. This is accomplished by imposing L1/L2 penalties on the weights and biases of each tree [
35].
After performing optimization techniques, the objective function of XGBoost is composed of two distinct components that serve to capture both the model’s deviation and the regularization term, aiming to prevent over-fitting. Let us denote the dataset as
, which encompasses a comprehensive collection of
samples and
features. Within this dataset, the predictive variable can be visualized as an additive model, comprising a combination of
fundamental models [
36]. Upon conducting sample predictions, the outcome can be summarized as follows:
In the context where symbolizes the label prediction and stands for one of the samples, the predicted score is denoted by or the given sample. Moreover, is used to signify the set of regression trees, which essentially encapsulates the tree structure parameters of . Additionally, w serves as a representation of the weight of the leaves and the number of leaves.
The objective function of XGBoost encompasses not only the traditional loss function but also the model complexity, thus enabling its utility in evaluating the operational efficiency of the algorithm. Within the framework of Formula
3, the first term is indicative of the traditional loss function, whereas the second term pertains to the model’s complexity. Consequently, the comprehensive nature of the XGBoost objective function allows for a thorough assessment of both the predictive performance and the overall complexity of the model.
In the realm of these two exquisite formulas, the mystical entity denoted by the ethereal symbol i gracefully signifies the ethereal concept of the numerous samples that reside within the sacred dataset, while the enigmatic symbol m adorns itself with the mantle of representing the grandiose total amount of data that has been ceremoniously imported into the ethereal k th tree. As if imbued with arcane power, the enigmatic symbols and step forward to take on the audacious task of adjusting the very fabric of complexity within the ethereal tree. Behold, for in this realm, the very essence of regularization terms emerges as a mighty force, capable of adorning the final learning weight with a velvety smoothness and shielding it from the treacherous clutches of over-fitting, like a knight in shining armour.
3.4. AdaBoost
As an Ensemble Method, AdaBoost [
37] (or Adaptive Boosting) applies Machine Learning techniques. One-level decision trees, also known as decision trees with a single split, are the most used estimator in AdaBoost. Decision stumps are a common name for these trees. In this method, every piece of data is given the same weight while building a model. It then gives points that were wrongly classified as a higher weight. The following model gives more weight to all points that have higher weights. It won’t stop training models until the reported error is reduced [
38,
39].
Light GBM
Distributed systems are ideal for maximizing Light GBM’s performance [
40]. With Light GBM, you may train decision trees that expand "leaf wise," meaning that for any given circumstance, you only divide the tree once, based on the gain. Especially when working with smaller datasets, leaf-wise trees have the potential to overfit. Overfitting may be prevented by reducing the tree depth. Light GBM employs a histogram-based approach, which involves dividing data into bins based on the distribution’s histogram. Instead of using each data point, the bins are used for iteration, gain calculation, and data splitting [
41].
3.5. Performance Measures
The results yielded from the proposed algorithms may be evaluated by the following below-mentioned measures [
42]:
Accuracy is interrupted from the given formula:
In the equation, TP and TN stand for True Positive and True Negative, and FP and FN stand for False Positive and False Negative, respectively. TP+TN signifies the percentage of correctly classified instances, and TP+TN+FN+FP signifies the total number of correctly and incorrectly classified instances.
Precision is part of significant instances involving the retrieved instances. The equation for precision is given below:
Recall is a small portion of appropriate instances, which is retrieved over the total quantity of relevant instances. The equation for the Re-call is given below:
The following equation, which satisfies the definition of specificity, is given below:
F-measure is based on double the precision times Recall divided by the sum of precision and recall. The equation for F-Measure is given below:
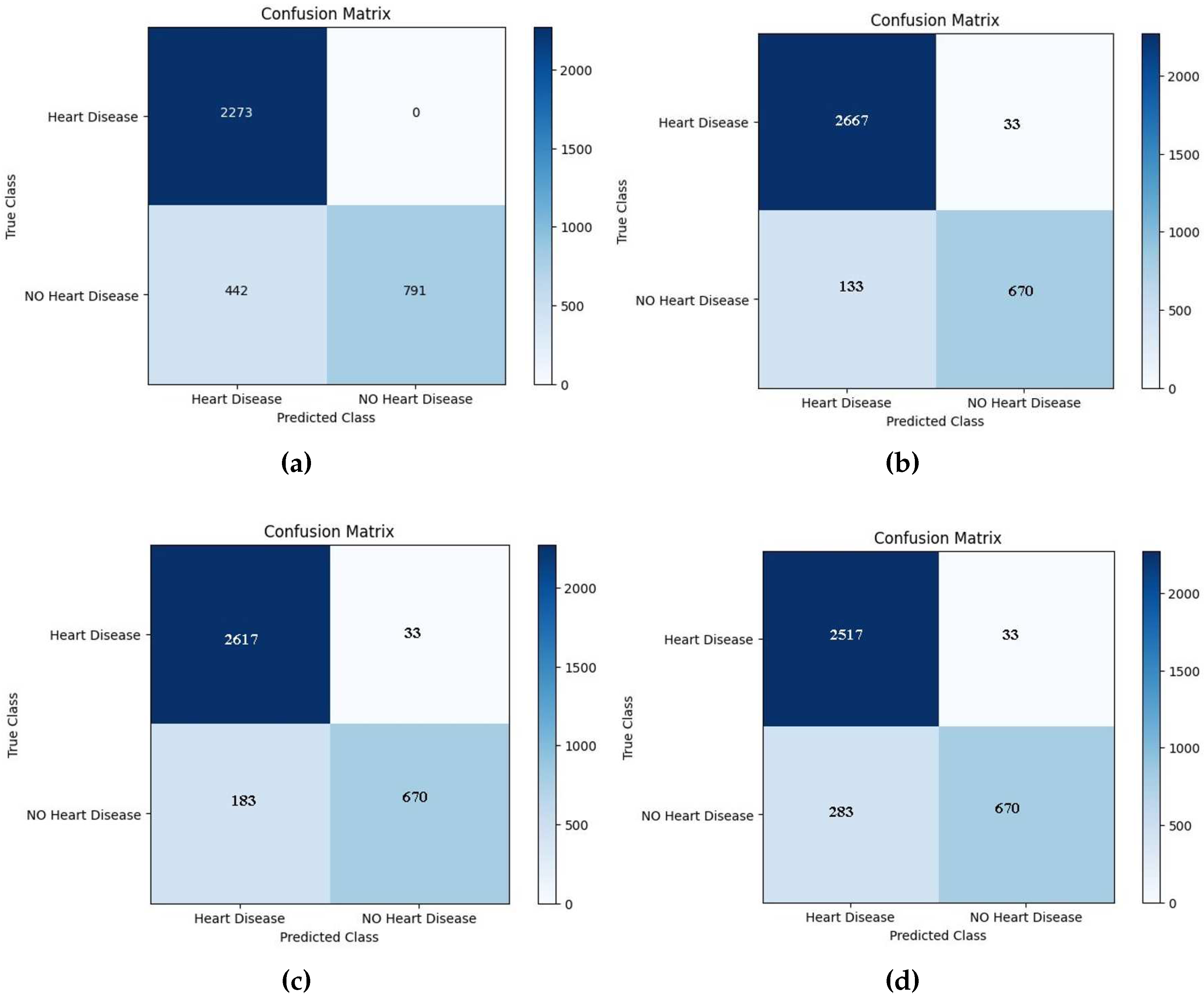
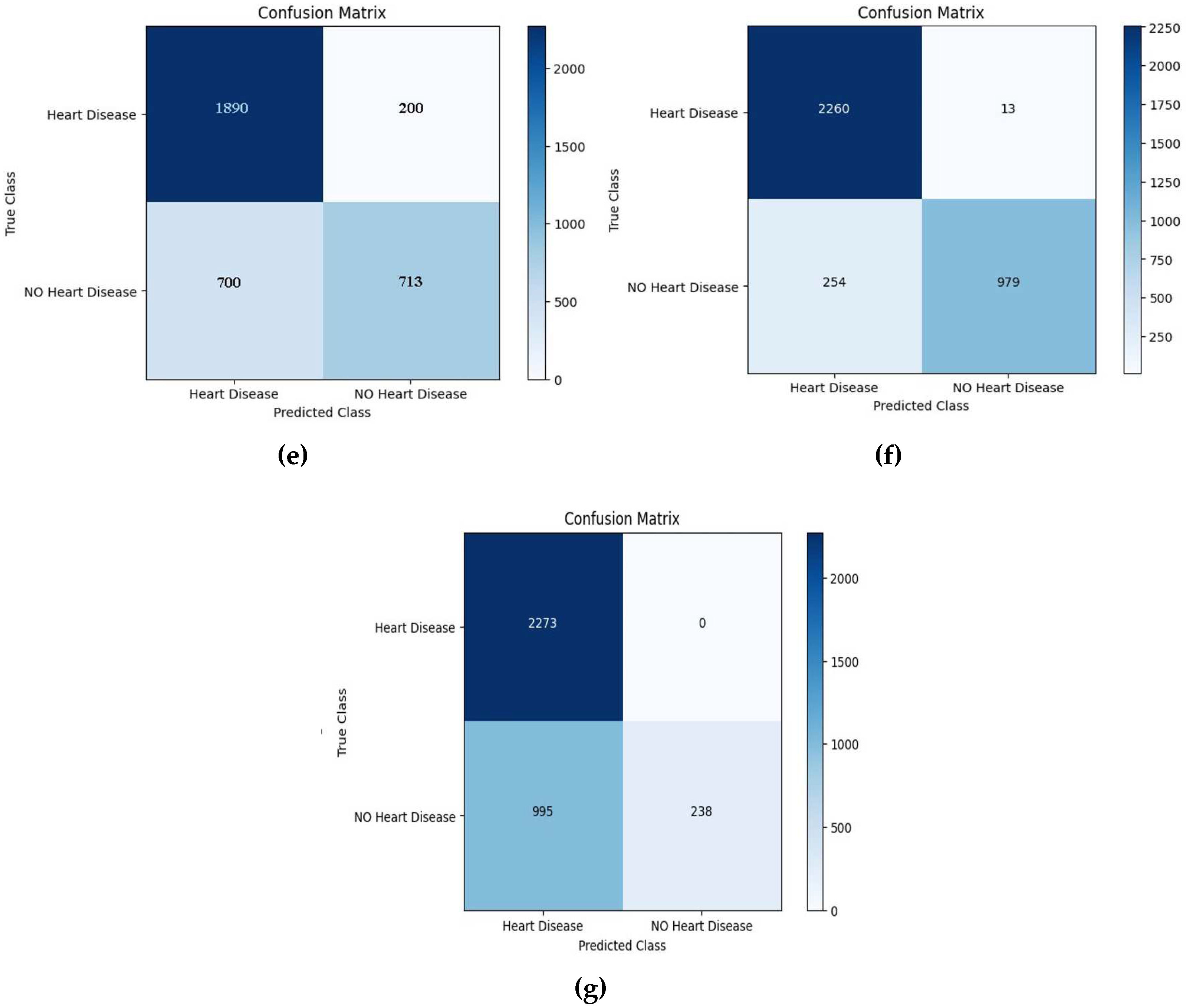
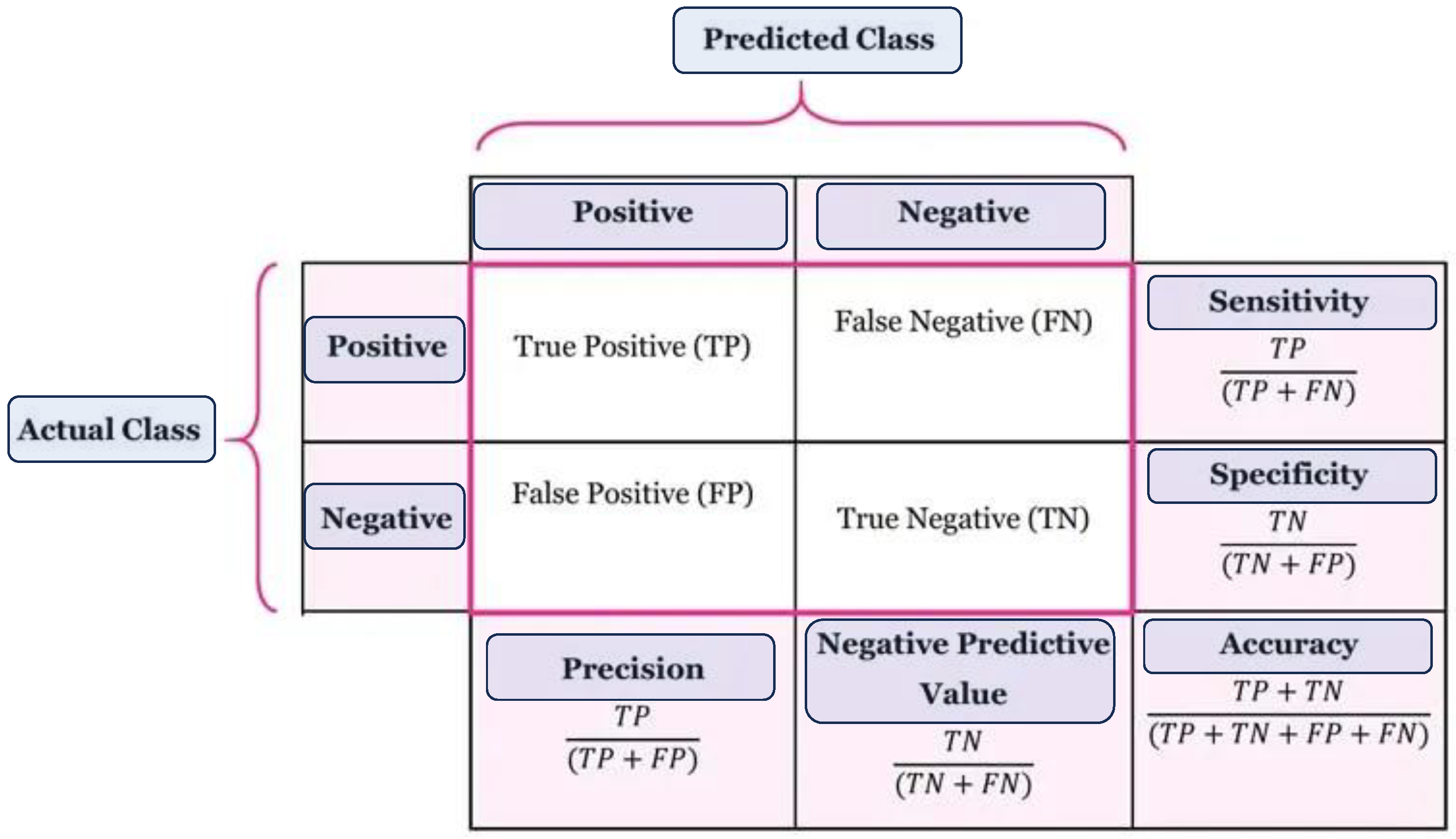
A confusion matrix, also referred to as an error matrix, is an incredibly useful tool in machine learning. It serves as a comprehensive and informative table that effectively summarizes the performance of a classification model. This matrix (See
Figure 6) is extensively utilized to evaluate the accuracy and effectiveness of a model’s predictions, providing valuable insights that aid in decision-making processes. Moreover, the confusion matrix acts as a visual representation that encompasses a wide range of information regarding how well a model classifies different classes. By comparing the predicted labels against the actual labels, it effectively showcases the model’s capabilities and limitations. Structured in a square matrix format, the rows and columns correspond to the true and predicted classes, allowing for a detailed analysis of the model’s performance. An essential aspect of the confusion matrix is its ability to facilitate the computation of various performance metrics. These metrics include accuracy, precision, recall, and F1 score, each offering unique insights into the model’s predictive capabilities. By providing a clear distribution of correct and incorrect predictions across different classes, the confusion matrix offers a comprehensive understanding of the model’s strengths and weaknesses. Overall, the confusion matrix serves as an invaluable tool in the field of machine learning. Its ability to summarize and visualize the performance of a classification model provides researchers and practitioners with the necessary information to make informed decisions. One can significantly enhance the accuracy and effectiveness of their models by harnessing the power of the confusion matrix.
3.6. Validation
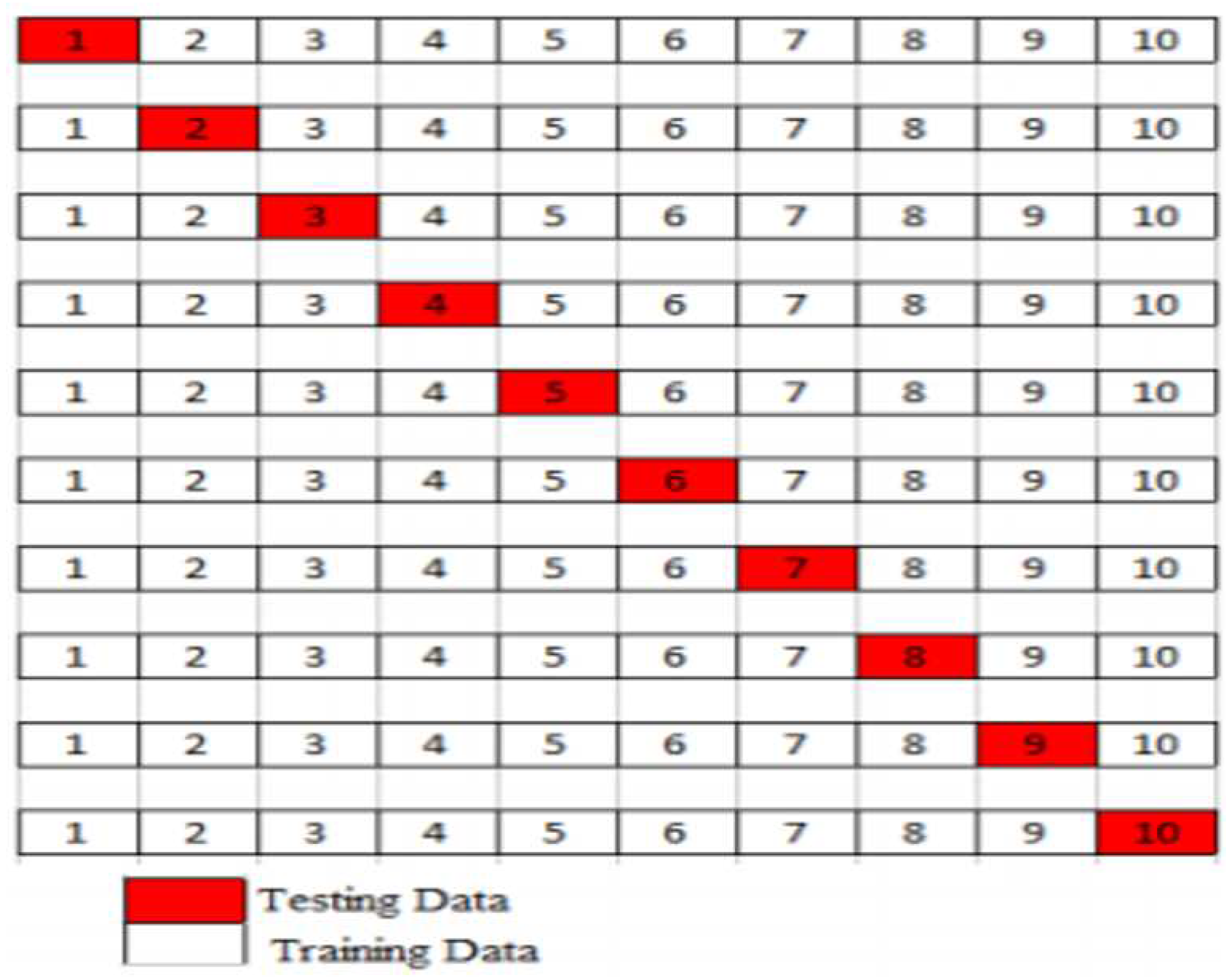
The next step is to randomize the supplied dataset, and for this, the k-fold cross-validation approach is utilized to test the data for assessment of various ensemble machine learning algorithms. The dataset is partitioned into k subgroups containing the same data. This search employs 10-fold. As a result, data is 10-folded, with each fold around the same size. As a result, for each of the ten subsets of data, this validation approach employs one-fold for testing and the remaining nine-fold for training, as seen in
Figure 7 below.
4. Experimental Results
The confusion matrix shown below is used to evaluate the performance of boosting models for identifying mislabeled/errors in predicting the cardiac illness. It compares actual results to projected values using four factors: True Positive (TP), True Negative (TN), False Positive (FP), and False Negative (FN) [
33]. The confusion matrix shown in Figures 7–13 below is used to evaluate the performance of boosting models for identifying mislabeled/errors in predicting heart illness. It compares actual results to projected values using four factors: True Positive (TP), True Negative (TN), False Positive (FP), and False Negative (FN).
Figure 8.
The testing confusion matrix of a) GBoost, b) AdaBoost, c) CatBoost, d) Light Boost, e) Random forest, f) XGBoost, and g) Decision Tree
Figure 8.
The testing confusion matrix of a) GBoost, b) AdaBoost, c) CatBoost, d) Light Boost, e) Random forest, f) XGBoost, and g) Decision Tree
Testing Performance of Algorithms Employed: The accuracies of algorithms are displayed in
Table 5 below. Before pre-processing, the algorithms achieved a testing accuracy of 60.73%, 63%, 67%, 68%, 71.21%, 71.54% and 72%. After pre-processing, the algorithms achieved an accuracy of 71%, 74%, 87%, 93%, 90%, 92.38% and 95% correspondingly.
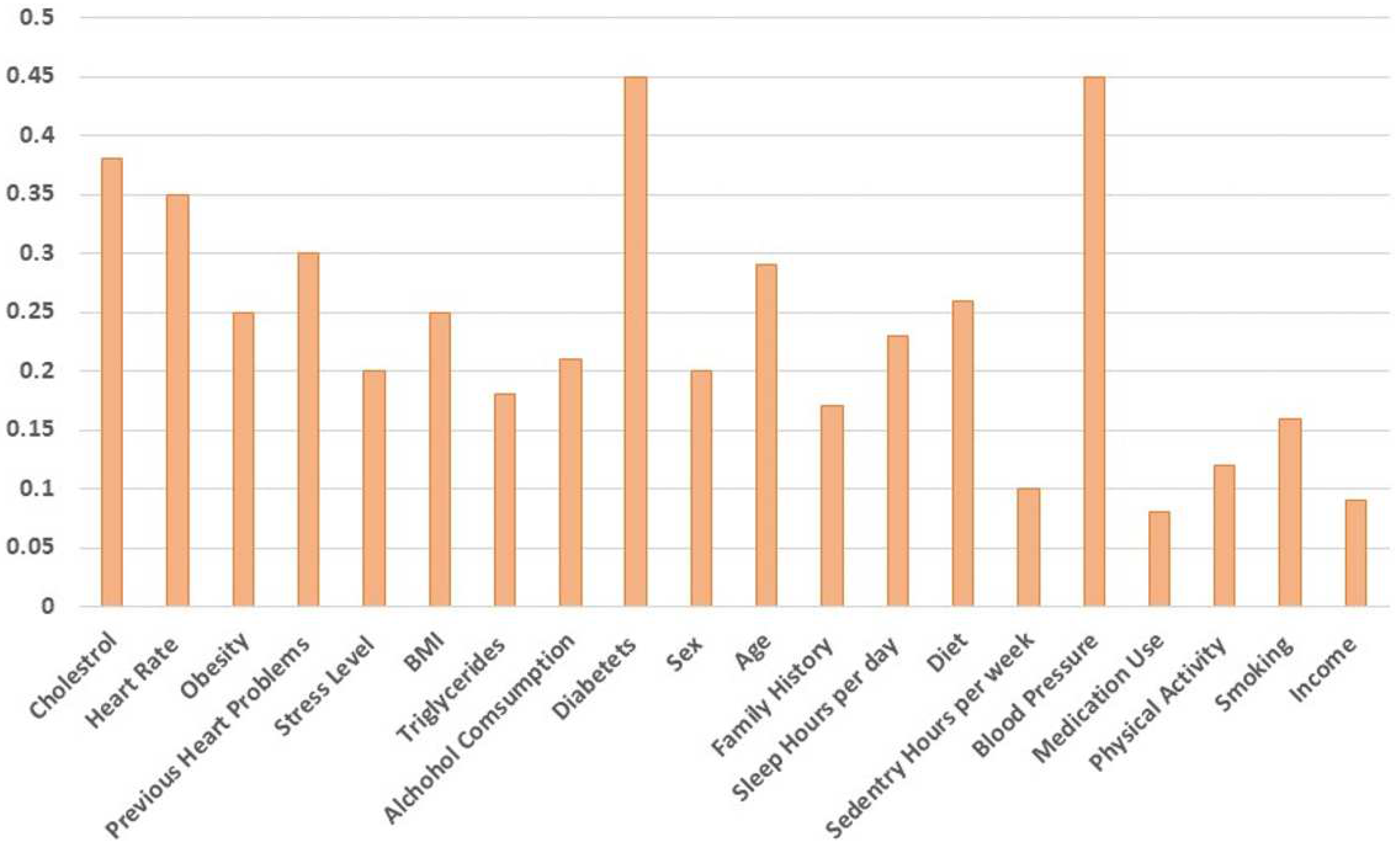
4.1. Feature importance for CNN
Feature importance is a technique used in machine learning to determine the most important features (i.e., variables or columns) in a dataset that contribute the most to the outcome of a model. When you set a threshold of 0.3 for feature importance, it means that only features with an importance score of 0.3 or higher will be considered significant and included in the final model. Any features with importance scores below this threshold will be excluded. The importance of the AdaBoost feature is shown in
Figure 9 below. Also, so far as the feature selection technique is concerned, we have used a correlation-based feature selection technique, and the results have been validated through five-fold and ten-fold cross-validation.
4.2. Comparative results
Table 6, presented below, serves as a means of conducting an analytical comparison between our proposed framework and the existing body of work. This comparison is rooted in the examination of various crucial aspects, including the methodology employed, the dataset utilized, and the analysis conducted. It is worth noting that the majority of lifestyle factors that were studied and explored in the context of our proposed framework are shared by all the studies that were conducted for the purpose of comparison. Through meticulous analysis, it was revealed that our proposed framework had exhibited remarkable performance, particularly in terms of various assessment criteria, with a notable focus on the accuracy of heart disease prediction. In order to surpass the achievements of previous relevant studies and to ensure the attainment of superior outcomes, a series of advanced procedures were implemented. These procedures encompassed techniques such as data imputation, which was employed to manage missing values effectively, as well as the identification and replacement of outliers through the utilization of the well-regarded boxplot method. Moreover, in order to enhance the reliability and validity of the findings, the data underwent a rigorous process of standardization and normalization, which was carried out using the transformation method. Furthermore, to further solidify the credibility of the results produced by our proposed framework, the K-fold cross-validation approach was meticulously employed during the development and implementation stages of the suggested framework. This approach ensures that the findings obtained are both robust and generalizable, thereby enhancing the overall validity of the framework’s predictions and conclusions.
5. Conclusions
A comparison of various ensemble machine learning with boosting classifiers for early CVD prediction was the key contribution of this paper. Pre-processing steps were employed to enhance the quality of the dataset. These approaches primarily focused on the management of corrupted and missing information, as well as the elimination of outliers. In order to predict the sickness, in addition to using a boosting algorithm, six other ensemble learning algorithms were used, and a range of statistical indicators were utilized to compare the findings. The results of the experiments show that the AdaBoost model has the highest accuracy of all the classifiers for our dataset. With a training set accuracy of 95.2% and a testing set accuracy of 95%, the AdaBoost model excels in terms of accuracy. The robustness validation of DT, RF, Cat Boost, GBoost and XGBoost, AdaBoost, and Light GM algorithms were validated by using five-fold and 10-fold cross-validation techniques. AdaBoost model performed well in terms of a range of performance criteria, including accuracy, recall, and f1-score, amongst others. Also, a correlation feature-based feature selection technique has been used in this study.
In order to regulate the boosting classifiers’ potential for improved heart disease prophecy, they will need to be trained and tested on big and primary datasets in the future. The present study still leaves room for additional research since data collection that results in visualization raises the calibre of the research. In future, this study can be extended by developing a web application based on boosting and other machine learning as well as deep learning models using primary and larger datasets to get optimal results that would help medicos while predicting heart disease in an efficient manner. Also, the existing work can be moved to other d leap learning techniques in which the images would be used as input instead of numerical or textual data.
Author Contributions
The authors’ contributions are as follows. “Conceptualization, N.N., S.J. and M.N.; methodology, N.N., S.J.; software, N.N., and S.J.; validation, N.N., and S.J.; formal analysis, N.N., and S.J.; investigation, N.N., S.J., and M.N.; resources, N.N., and M.N.; data curation, N.N., and S.J.; writing—original draft preparation, N.N., and S.J.; writing—review and editing, N.N., S.J., and M.N.; visualization, N.N., and M.N.; supervision, S.J.; project administration, S.J.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petrelli, A.; Gnavi, R.; Marinacci, C.; Costa, G. Socioeconomic inequalities in coronary heart disease in Italy: a multilevel population-based study. Social science & medicine 2006, 63, 446–456. [Google Scholar]
- Sharma, H.; Rizvi, M. Prediction of heart disease using machine learning algorithms: A survey. International Journal on Recent and Innovation Trends in Computing and Communication 2017, 5, 99–104. [Google Scholar]
- Gheorghe, A.; Griffiths, U.; Murphy, A.; Legido-Quigley, H.; Lamptey, P.; Perel, P. The economic burden of cardiovascular disease and hypertension in low-and middle-income countries: a systematic review. BMC public health 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.N.; Ullah, S.; Algethami, A.; Fatima, H.; Akhter, S.M.H. Comparative study of optimum medical diagnosis of human heart disease using machine learning technique with and without sequential feature selection. ieee access 2022, 10, 23808–23828. [Google Scholar] [CrossRef]
- Mohammad, F.; Al-Ahmadi, S. WT-CNN: A Hybrid Machine Learning Model for Heart Disease Prediction. Mathematics 2023, 11, 4681. [Google Scholar] [CrossRef]
- Osisanwo, F.; Akinsola, J.; Awodele, O.; Hinmikaiye, J.; Olakanmi, O.; Akinjobi, J.; others. Supervised machine learning algorithms: classification and comparison. International Journal of Computer Trends and Technology (IJCTT) 2017, 48, 128–138. [Google Scholar]
- Rashid, Y.; Bhat, J.I. Topological to deep learning era for identifying influencers in online social networks: a systematic review. Multimedia Tools and Applications, 2023, pp. 1–44.
- Taylan, O.; Alkabaa, A.S.; Alqabbaa, H.S.; Pamukçu, E.; Leiva, V. Early prediction in classification of cardiovascular diseases with machine learning, neuro-fuzzy and statistical methods. Biology 2023, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Adeli, A.; Neshat, M. A fuzzy expert system for heart disease diagnosis. International Multi-Conference of Engineers and Computer Scientists, IMECS 2010. IEEE, 2010, pp. 1–6.
- Neshat, M.; Zadeh, A.E. Hopfield neural network and fuzzy Hopfield neural network for diagnosis of liver disorders. In 2010 5th IEEE International Conference Intelligent Systems. IEEE, 2010, pp. 162–167.
- Neshat, M.; Yaghobi, M.; Naghibi, M.B.; Zadeh, A.E. Fuzzy expert system design for diagnosis of liver disorders. International Symposium on Knowledge Acquisition and Modeling. IEEE, 2008, pp. 252–256.
- Li, X.; Zhao, Y.; Zhang, D.; Kuang, L.; Huang, H.; Chen, W.; Fu, X.; Wu, Y.; Li, T.; Zhang, J.; others. Development of an interpretable machine learning model associated with heavy metals’ exposure to identify coronary heart disease among US adults via SHAP: Findings of the US NHANES from 2003 to 2018. Chemosphere 2023, 311, 137039. [Google Scholar] [CrossRef] [PubMed]
- Usama, M.; Qadir, J.; Raza, A.; Arif, H.; Yau, K.L.A.; Elkhatib, Y.; Hussain, A.; Al-Fuqaha, A. Unsupervised machine learning for networking: Techniques, applications and research challenges. IEEE access 2019, 7, 65579–65615. [Google Scholar] [CrossRef]
- Ngiam, K.Y.; Khor, W. Big data and machine learning algorithms for health-care delivery. The Lancet Oncology 2019, 20, e262–e273. [Google Scholar] [CrossRef] [PubMed]
- Nissa, N.; Jamwal, S.; Mohammad, S. Early detection of cardiovascular disease using machine learning techniques an experimental study. Int. J. Recent Technol. Eng 2020, 9, 635–641. [Google Scholar] [CrossRef]
- Kecman, V. Support vector machines–an introduction. In Support vector machines: theory and applications; Springer, 2005; pp. 1–47.
- Paladino, L.M.; Hughes, A.; Perera, A.; Topsakal, O.; Akinci, T.C. Evaluating the Performance of Automated Machine Learning (AutoML) Tools for Heart Disease Diagnosis and Prediction. AI 2023, 4, 1036–1058. [Google Scholar] [CrossRef]
- Rojas-Albarracin, G.; Chaves, M.Á.; Fernandez-Caballero, A.; Lopez, M.T. Heart attack detection in colour images using convolutional neural networks. Applied Sciences 2019, 9, 5065. [Google Scholar] [CrossRef]
- Mehmood, A.; Iqbal, M.; Mehmood, Z.; Irtaza, A.; Nawaz, M.; Nazir, T.; Masood, M. Prediction of heart disease using deep convolutional neural networks. Arabian Journal for Science and Engineering 2021, 46, 3409–3422. [Google Scholar] [CrossRef]
- Rani, M.; Bakshi, A.; Gupta, A. Prediction of Heart Disease Using Naïve bayes and Image Processing. 2020 International Conference on Emerging Smart Computing and Informatics (ESCI). IEEE, 2020, pp. 215–219.
- Rairikar, A.; Kulkarni, V.; Sabale, V.; Kale, H.; Lamgunde, A. Heart disease prediction using data mining techniques. 2017 International conference on intelligent computing and control (I2C2). IEEE, 2017, pp. 1–8.
- Zakariah, M.; AlShalfan, K. Cardiovascular Disease Detection Using MRI Data with Deep Learning Approach. Int. J. Comp. Electr. Eng. 2020, 12, 72–82. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Abbas, Q.; Daadaa, Y.; Qureshi, I.; Perumal, G.; Ibrahim, M.E. A Residual-Dense-Based Convolutional Neural Network Architecture for Recognition of Cardiac Health Based on ECG Signals. Sensors 2023, 23, 7204. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guan, J. A heart disease prediction model based on feature optimization and smote-Xgboost algorithm. Information 2022, 13, 475. [Google Scholar] [CrossRef]
- Reddy, K.V.V.; Elamvazuthi, I.; Aziz, A.A.; Paramasivam, S.; Chua, H.N.; Pranavanand, S. Heart disease risk prediction using machine learning classifiers with attribute evaluators. Applied Sciences 2021, 11, 8352. [Google Scholar] [CrossRef]
- Kiran, P.; Swathi, A.; Sindhu, M.; Manikanta, Y.; others. Effective heart disease prediction using hybrid machine learning technique. South Asian Journal of Engineering and Technology 2022, 12, 123–130. [Google Scholar] [CrossRef]
- Banerjee, S. Heart Attack Risk Prediction Dataset. https://www.kaggle.com/datasets/iamsouravbanerjee/heart-attack-prediction-dataset, 2023. Accessed: 12/2023.
- Hassan, C.A.u.; Iqbal, J.; Irfan, R.; Hussain, S.; Algarni, A.D.; Bukhari, S.S.H.; Alturki, N.; Ullah, S.S. Effectively predicting the presence of coronary heart disease using machine learning classifiers. Sensors 2022, 22, 7227. [Google Scholar] [CrossRef] [PubMed]
- Tayefi, M.; Tajfard, M.; Saffar, S.; Hanachi, P.; Amirabadizadeh, A.R.; Esmaeily, H.; Taghipour, A.; Ferns, G.A.; Moohebati, M.; Ghayour-Mobarhan, M. hs-CRP is strongly associated with coronary heart disease (CHD): A data mining approach using decision tree algorithm. Computer methods and programs in biomedicine 2017, 141, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Thirumalai, C.; Srivastava, G. Effective heart disease prediction using hybrid machine learning techniques. IEEE access 2019, 7, 81542–81554. [Google Scholar] [CrossRef]
- Kubat, M.; Kubat, J. An introduction to machine learning; Vol. 2, Springer, 2017.
- Graczyk, M.; Lasota, T.; Trawiński, B.; Trawiński, K. Comparison of bagging, boosting and stacking ensembles applied to real estate appraisal. Intelligent Information and Database Systems: Second International Conference, ACIIDS, Hue City, Vietnam, March 24-26, 2010. Proceedings, Part II 2. Springer, 2010, pp. 340–350.
- Prokhorenkova, L.; Gusev, G.; Vorobev, A.; Dorogush, A.V.; Gulin, A. CatBoost: unbiased boosting with categorical features. Advances in neural information processing systems 2018, 31. [Google Scholar]
- Hancock, J.T.; Khoshgoftaar, T.M. CatBoost for big data: an interdisciplinary review. Journal of big data 2020, 7, 1–45. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining, 2016, pp. 785–794.
- Qiu, Y.; Zhou, J.; Khandelwal, M.; Yang, H.; Yang, P.; Li, C. Performance evaluation of hybrid WOA-XGBoost, GWO-XGBoost and BO-XGBoost models to predict blast-induced ground vibration. Engineering with Computers, 2021, pp. 1–18.
- Freund, Y.; Schapire, R.E. ; others. Experiments with a new boosting algorithm. icml. Citeseer, 1996, Vol. 96, pp. 148–156.
- Ganie, S.M.; Dutta Pramanik, P.K.; Mallik, S.; Zhao, Z. Chronic kidney disease prediction using boosting techniques based on clinical parameters. Plos one 2023, 18, e0295234. [Google Scholar] [CrossRef]
- Chandrasekhar, N.; Peddakrishna, S. Enhancing Heart Disease Prediction Accuracy through Machine Learning Techniques and Optimization. Processes 2023, 11, 1210. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. Lightgbm: A highly efficient gradient boosting decision tree. Advances in neural information processing systems 2017, 30. [Google Scholar]
- Ceylan, Z.; Bulkan, S.; Elevli, S. Prediction of medical waste generation using SVR, GM (1, 1) and ARIMA models: a case study for megacity Istanbul. Journal of Environmental Health Science and Engineering 2020, 18, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.; Bhavani, V.R.; Xu, A.Q.; Hossain, M. An artificial intelligence model for heart disease detection using machine learning algorithms. Healthcare Analytics 2022, 2, 100016. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).