Submitted:
14 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
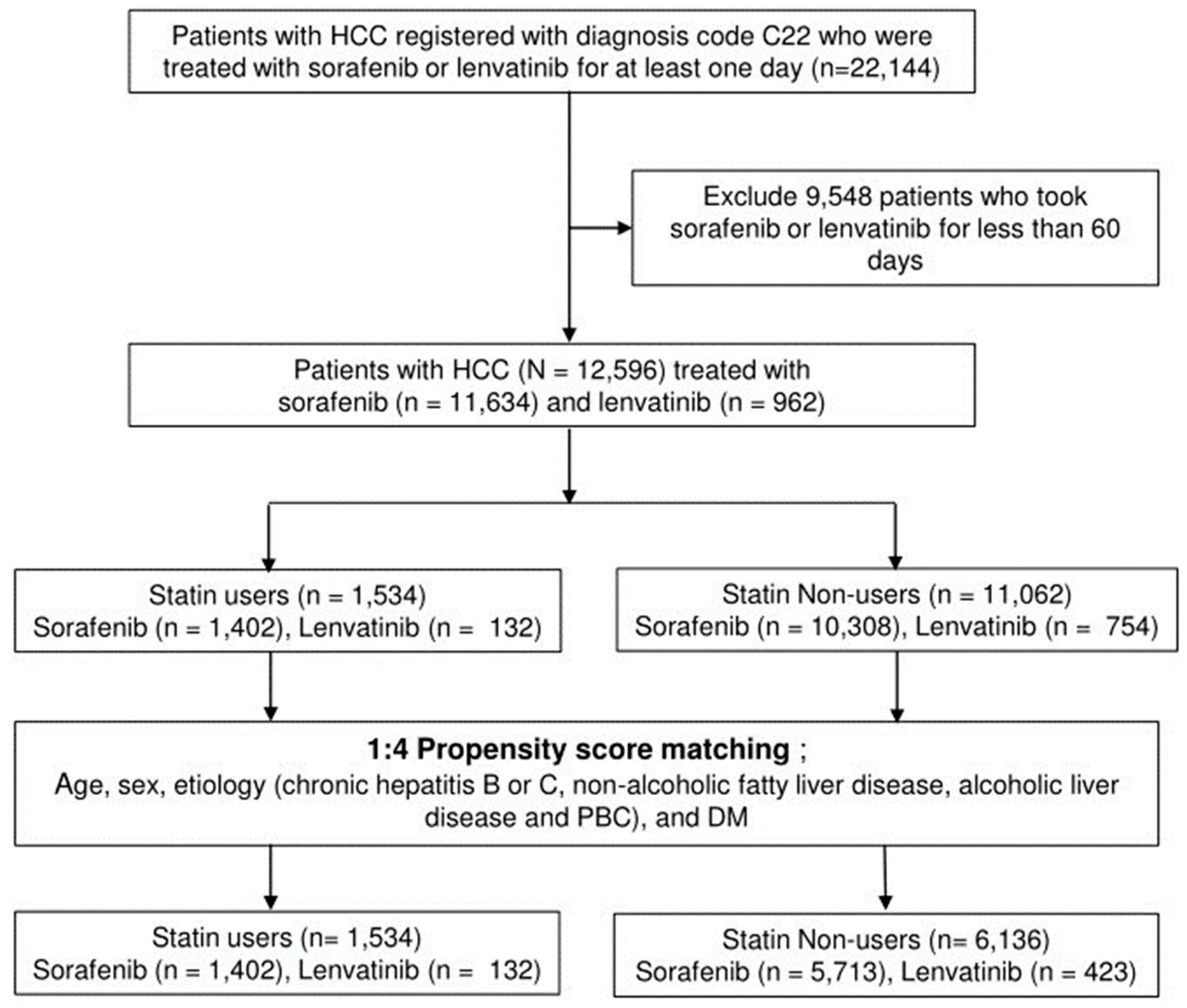
2.1. Data Source
2.2. Study Population and Definition of Terms
2.3. Data collection
2.4. Outcomes
2.5. Statistical analysis
3. Results
3.1. Comparing Baseline Characteristics between Statin Users and Nonusers in Unmatched and PS-matched Cohorts
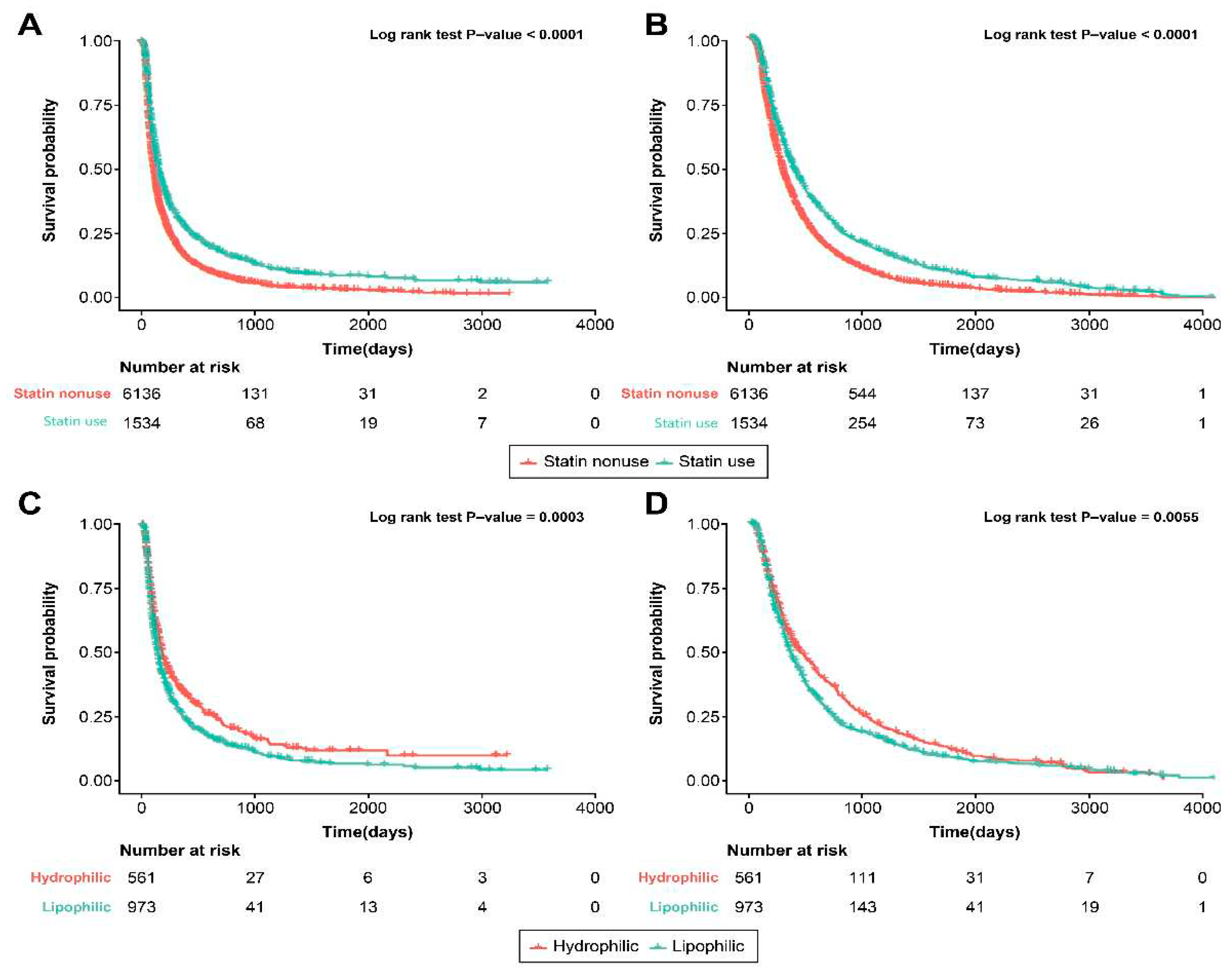
3.2. Statin Use and Survival Outcome
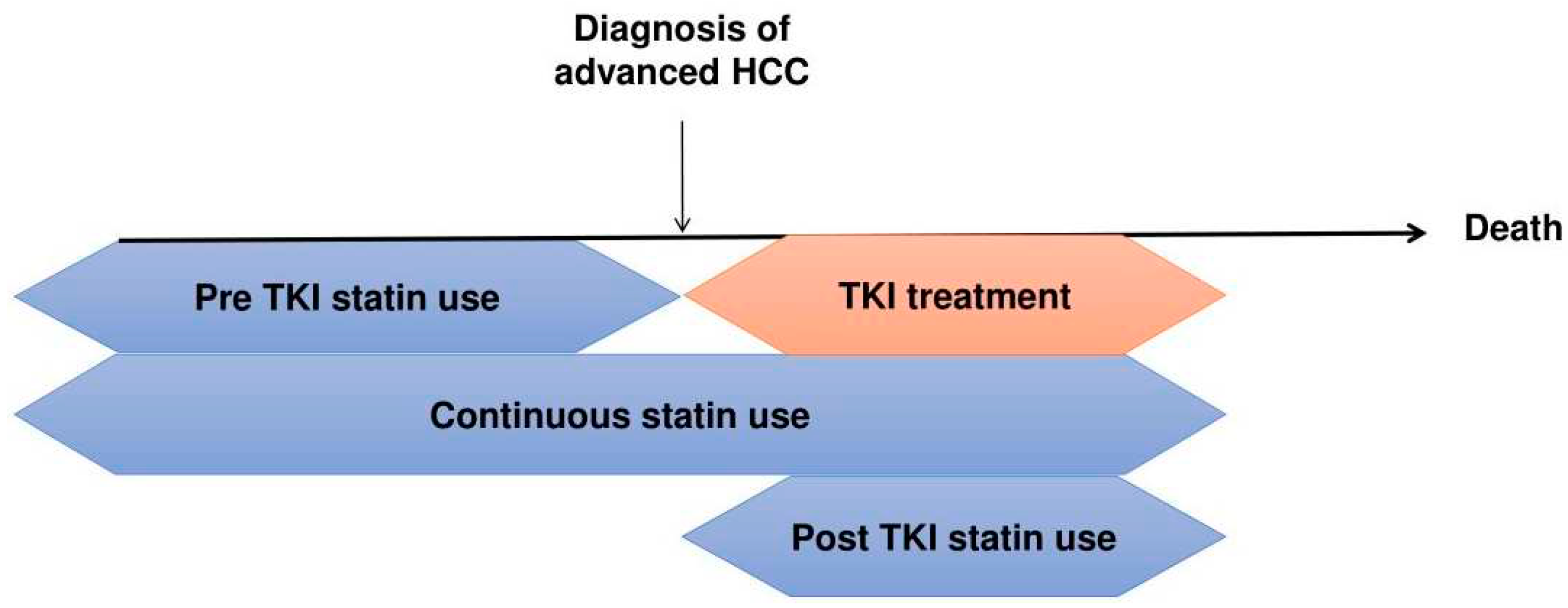
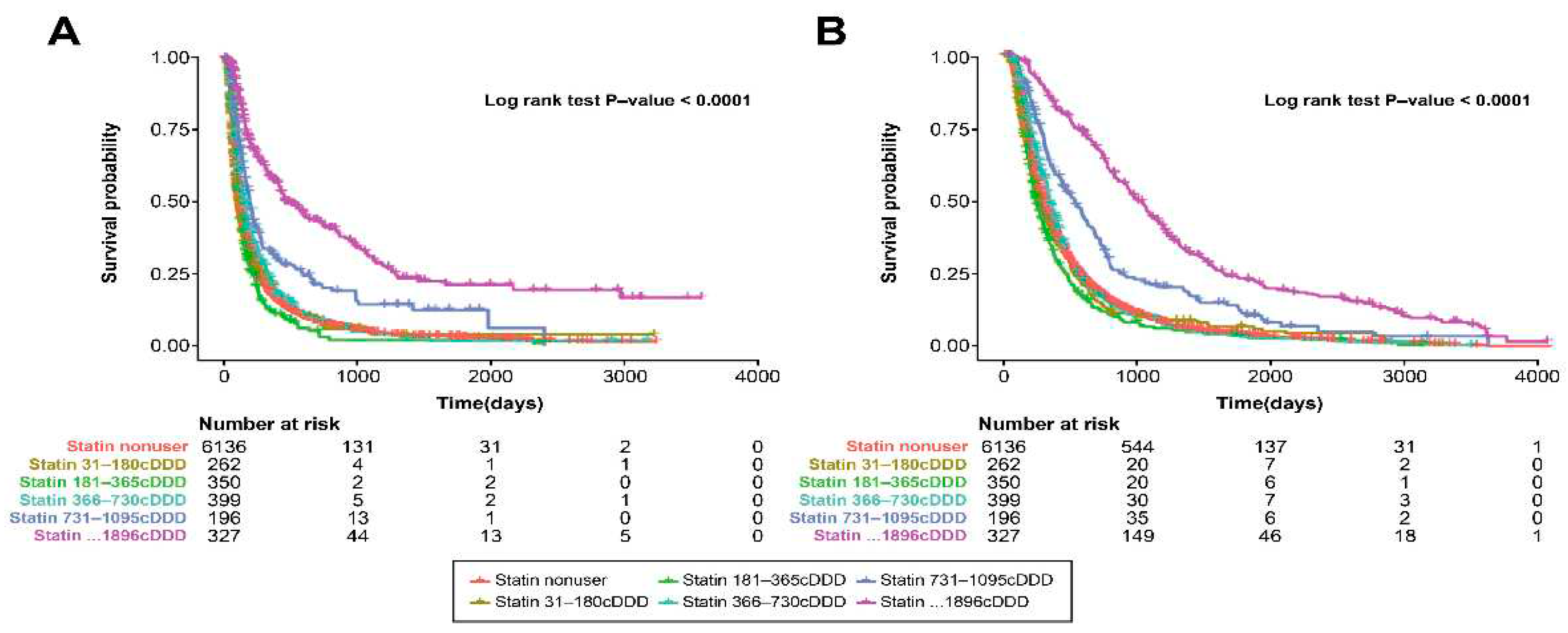
3.3. Timing of Statin Use and Survival Outcome
3.4. Statin Type and Survival Outcome
3.5. Statin Dose and Survival Outcome
3.6. Multivariate Stratified Analysis
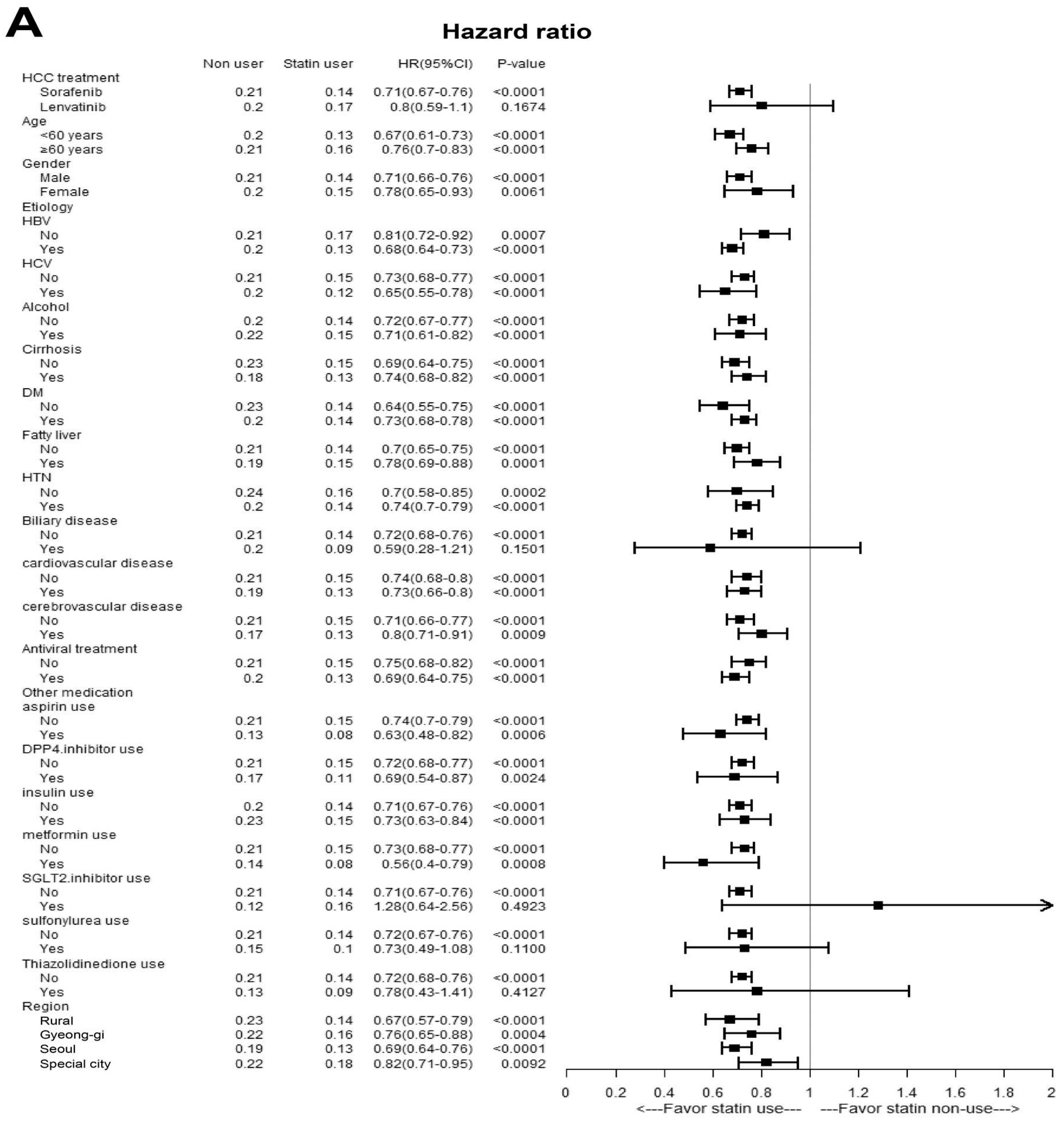
3.7. Subgroup Analysis According to Sorafenib or Lenvatinib Treatment
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Statin use pattern | ||||||||
| Non-user | reference | reference | ||||||
| pre-TKI use | 1.53 | 1.33–1.77 | <0.001 | 1.56 | 1.35–1.80 | <0.001 | ||
| continuous use from TKI treatment | 0.86 | 0.80–0.93 | <0.001 | 0.94 | 0.87–1.02 | 0.122 | ||
| post-TKI use | 0.38 | 0.34–0.43 | <0.001 | 0.42 | 0.38–0.48 | <0.001 | ||
| Age ≥60 yr | 1.04 | 0.99–1.09 | 0.156 | 1.00 | 1.00–1.01 | 0.032 | ||
| Sex, female | 0.98 | 0.91–1.06 | 0.603 | 1.02 | 0.95–1.10 | 0.571 | ||
| Region, urban | 0.88 | 0.82–0.94 | <0.001 | 0.86 | 0.80–0.92 | <0.001 | ||
| DM, yes | 0.91 | 0.85–0.98 | 0.007 | 0.92 | 0.86–0.99 | 0.023 | ||
| Fatty liver, yes | 0.94 | 0.89–1.00 | 0.054 | |||||
| Cirrhosis, yes | 0.82 | 0.79-0.86 | <0.001 | |||||
| HTN, yes | 0.80 | 0.75–0.84 | <0.001 | 0.86 | 0.81–0.91 | <0.001 | ||
| cardiovascular disease, yes | 0.84 | 0.79–0.88 | <0.001 | 0.91 | 0.86–0.96 | 0.001 | ||
| cerebrovascular disease, yes | 0.81 | 0.76–0.87 | <0.001 | 0.91 | 0.85–0.98 | 0.012 | ||
| Aspirin use, yes | 0.57 | 0.50–0.65 | <0.001 | 0.68 | 0.59–0.77 | <0.001 | ||
| DPP-4 inhibitor use, yes | 0.78 | 0.70–0.88 | <0.001 | 0.88 | 0.78–0.99 | 0.035 | ||
| Insulin use, yes | 1.13 | 1.06–1.20 | <0.001 | 1.25 | 1.17–1.33 | <0.001 | ||
| Metformin use, yes | 0.68 | 0.59–0.78 | <0.001 | 0.75 | 0.64–0.87 | <0.001 | ||
| SGLT-2 inhibitor use, yes | 0.70 | 0.50–0.98 | 0.038 | 0.74 | 0.53–1.03 | 0.077 | ||
| Sulfonylurea use, yes | 0.73 | 0.61–0.86 | <0.001 | 0.81 | 0.67–0.97 | 0.022 | ||
| Thiazolidinedione use, yes | 0.64 | 0.49–0.85 | 0.002 | |||||
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Blanco, C.; Fondevila, F.; García-Palomo, A.; González-Gallego, J.; Mauriz, J.L. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Zinna, V.M.; Dell’Endice, S.; Jaschke, N.; Kuhlmann, J.D.; Wimberger, P.; Rachner, T.D. Anti-tumor effects of mevalonate pathway inhibition in ovarian cancer. BMC Cancer 2020, 20, 703. [Google Scholar] [CrossRef]
- Ricco, N.; Flor, A.; Wolfgeher, D.; Efimova, E.V.; Ramamurthy, A.; Appelbe, O.K.; Brinkman, J.; Truman, A.W.; Spiotto, M.T.; Kron, S.J. Mevalonate pathway activity as a determinant of radiation sensitivity in head and neck cancer. Mol Oncol 2019, 13, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; E, J.Y.; Lin, Y.; Rebbeck, T.R.; Lu, S.E.; Shang, M.; Kelly, W.K.; D’Amico, A.; Stein, M.N.; Zhang, L.; et al. Individual and joint effects of metformin and statins on mortality among patients with high-risk prostate cancer. Cancer Med 2020, 9, 2379–2389. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.J.; Sinn, D.H.; Kim, S.; Woo, S.Y.; Cho, H.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; et al. Statin use and the risk of hepatocellular carcinoma in patients with chronic hepatitis B. Hepatology 2020, 71, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhu, G.Q.; Wang, Y.; Zheng, J.N.; Ruan, L.Y.; Cheng, Z.; Hu, B.; Fu, S.W.; Zheng, M.H. Systematic review with network meta-analysis: statins and risk of hepatocellular carcinoma. Oncotarget 2016, 7, 21753–21762. [Google Scholar] [CrossRef]
- Lai, S.W.; Liao, K.F.; Lai, H.C.; Muo, C.H.; Sung, F.C.; Chen, P.C. Statin use and risk of hepatocellular carcinoma. Eur J Epidemiol 2013, 28, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Joo, Y.S.; Kang, S.C.; Koh, H.B.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Association of statin treatment with hepatocellular carcinoma risk in end-stage kidney disease patients with chronic viral hepatitis. Sci Rep 2022, 12, 10807. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Hagstrom, H.; Nguyen, L.H.; Khalili, H.; Chung, R.T.; Ludvigsson, J.F. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med 2019, 171, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Yang, K.Y.; Huang, T.Y.; Hsu, C.C.; Chen, Y.M.; Yen, J.C.; Chou, Y.C.; Chang, Y.L.; He, C.H. The efficacy of first-line tyrosine kinase inhibitors combined with co-medications in Asian patients with EGFR mutation non-small cell lung cancer. Sci Rep 2020, 10, 14965. [Google Scholar] [CrossRef] [PubMed]
- Matusewicz, L.; Czogalla, A.; Sikorski, A.F. Attempts to use statins in cancer therapy: an update. Tumour Biol 2020, 42, 1010428320941760. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.; Joshi, S.; Viollet, B.; Hay, N.; Platanias, L.C. AMPK as a therapeutic target in renal cell carcinoma. Cancer Biol Ther 2010, 10, 1168–1177. [Google Scholar] [CrossRef]
- Jang, H.J.; Woo, Y.M.; Naka, K.; Park, J.H.; Han, H.J.; Kim, H.J.; Kim, S.H.; Ahn, J.S.; Kim, T.; Kimura, S.; et al. Statins enhance the molecular response in chronic myeloid leukemia when combined with tyrosine kinase inhibitors. Cancers (Basel) 2021, 13, 5543. [Google Scholar] [CrossRef]
- Hung, M.S.; Chen, I.C.; Lee, C.P.; Huang, R.J.; Chen, P.C.; Tsai, Y.H.; Yang, Y.H. Statin improves survival in patients with EGFR-TKI lung cancer: a nationwide population-based study. PLOS ONE 2017, 12, e0171137. [Google Scholar] [CrossRef]
- Lebo, N.L.; Griffiths, R.; Hall, S.; Dimitroulakos, J.; Johnson-Obaseki, S. Effect of statin use on oncologic outcomes in head and neck squamous cell carcinoma. Head Neck 2018, 40, 1697–1706. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.P.; Roberts, L.R.; Sanchez, W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2014, 11, 45–54. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wang, C.C.; Chen, K.D.; Liu, Y.W.; Lin, C.C.; Chuang, C.H.; Tsai, Y.C.; Yao, C.C.; Yen, Y.H.; Hsiao, C.C.; et al. Statin use is associated with a lower risk of recurrence after curative resection in BCLC stage 0-A hepatocellular carcinoma. BMC Cancer 2021, 21, 70. [Google Scholar] [CrossRef]
- Pose, E.; Trebicka, J.; Mookerjee, R.P.; Angeli, P.; Ginès, P. Statins: old drugs as new therapy for liver diseases? J Hepatol 2019, 70, 194–202. [Google Scholar] [CrossRef]
- Björnsson, E.; Jacobsen, E.I.; Kalaitzakis, E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol 2012, 56, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.E.; Serper, M.A.; Mehta, R.; Fox, R.; John, B.; Aytaman, A.; Baytarian, M.; Hunt, K.; Albrecht, J.; Njei, B.; et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology 2019, 156, 1693–1706.e12. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Villanueva, C.; Aracil, C.; Turnes, J.; Hernandez-Guerra, M.; Genesca, J.; Rodriguez, M.; Castellote, J.; García-Pagán, J.C.; Torres, F.; et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 2016, 150, 1160–1170.e3. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, N.; Chapin, S.; Goldberg, D.S.; Reddy, K.R.; Taddei, T.H.; Kaplan, D.E. Statin exposure is associated with reduced development of acute-on-chronic liver failure in a Veterans Affairs cohort. J Hepatol 2022, 76, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Chen, H.L.; Tai, W.T.; Feng, W.C.; Hsu, C.H.; Chen, P.J.; Cheng, A.L. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther 2011, 337, 155–161. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Zhu, X.; Wang, P.; Chi, H.; Meng, Z. Activation of phosphatidylinositol 3-kinase/Akt signaling mediates sorafenib-induced invasion and metastasis in hepatocellular carcinoma. Oncol Rep 2014, 32, 1465–1472. [Google Scholar] [CrossRef]
- Liu, L.P.; Ho, R.L.; Chen, G.G.; Lai, P.B. Sorafenib inhibits hypoxia-inducible factor-1α synthesis: implications for antiangiogenic activity in hepatocellular carcinoma. Clin Cancer Res 2012, 18, 5662–5671. [Google Scholar] [CrossRef]
- Zhao, D.; Zhai, B.; He, C.; Tan, G.; Jiang, X.; Pan, S.; Dong, X.; Wei, Z.; Ma, L.; Qiao, H.; et al. Upregulation of HIF-2α induced by sorafenib contributes to the resistance by activating the TGF-α/EGFR pathway in hepatocellular carcinoma cells. Cell Signal 2014, 26, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Zou, X.; Song, Y.; Duan, Z.; Liu, L. PFKFB3/HIF-1α feedback loop modulates sorafenib resistance in hepatocellular carcinoma cells. Biochem Biophys Res Commun 2019, 513, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Dong, Z.; Cai, X.; Shen, J.; Xu, Y.; Zhang, M.; Li, H.; Yu, W.; Chen, W. Hypoxia induces sorafenib resistance mediated by autophagy via activating FOXO3a in hepatocellular carcinoma. Cell Death Dis 2020, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Blanc, J.F.; Khemissa, F.; Bronowicki, J.P.; Monterymard, C.; Perarnau, J.M.; Bourgeois, V.; Obled, S.; Abdelghani, M.B.; Mabile-Archambeaud, I.; Faroux, R.; et al. Phase 2 trial comparing sorafenib, pravastatin, their combination or supportive care in HCC with Child-Pugh B cirrhosis. Hepatol Int 2021, 15, 93–104. [Google Scholar] [CrossRef]
- Feng, J.; Dai, W.; Mao, Y.; Wu, L.; Li, J.; Chen, K.; Yu, Q.; Kong, R.; Li, S.; Zhang, J.; et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Cancer Res 2020, 39, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Doudican, N.A.; Quay, E.; Orlow, S.J. Fluvastatin enhances sorafenib cytotoxicity in melanoma cells via modulation of AKT and JNK signaling pathways. Anticancer Res 2011, 31, 3259–3265. [Google Scholar]
- Cheng, Y.; Luo, R.; Zheng, H.; Wang, B.; Liu, Y.; Liu, D.; Chen, J.; Xu, W.; Li, A.; Zhu, Y. Synergistic anti-tumor efficacy of sorafenib and fluvastatin in hepatocellular carcinoma. Oncotarget 2017, 8, 23265–23276. [Google Scholar] [CrossRef]
- Hisada, T.; Ayaori, M.; Ohrui, N.; Nakashima, H.; Nakaya, K.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Terao, Y.; Miyamoto, Y.; et al. Statin inhibits hypoxia-induced endothelin-1 via accelerated degradation of HIF-1α in vascular smooth muscle cells. Cardiovasc Res 2012, 95, 251–259. [Google Scholar] [CrossRef]
- Hijona, E.; Banales, J.M.; Hijona, L.; Medina, J.F.; Arenas, J.; Herreros-Villanueva, M.; Aldazabal, P.; Bujanda, L. Pravastatin inhibits cell proliferation and increased MAT1A expression in hepatocarcinoma cells and in vivo models. Cancer Cell Int 2012, 12, 5. [Google Scholar] [CrossRef]
- Riaño, I.; Martín, L.; Varela, M.; Serrano, T.; Núñez, O.; Mínguez, B.; Rodrigues, P.M.; Perugorria, M.J.; Banales, J.M.; Arenas, J.I. Efficacy and safety of the combination of pravastatin and sorafenib for the treatment of advanced hepatocellular carcinoma (ESTAHEP clinical trial). Cancers (Basel) 2020, 12, 1900. [Google Scholar] [CrossRef] [PubMed]
- Jouve, J.L.; Lecomte, T.; Bouché, O.; Barbier, E.; Khemissa Akouz, F.; Riachi, G.; Nguyen Khac, E.; Ollivier-Hourmand, I.; Debette-Gratien, M.; Faroux, R.; et al. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J Hepatol 2019, 71, 516–522. [Google Scholar] [CrossRef]
- Kawata, S.; Yamasaki, E.; Nagase, T.; Inui, Y.; Ito, N.; Matsuda, Y.; Inada, M.; Tamura, S.; Noda, S.; Imai, Y.; et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer 2001, 84, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Natarajan, Y.; Liu, Y.; El-Serag, H.B. Statin use after diagnosis of hepatocellular carcinoma is associated with decreased mortality. Clin Gastroenterol Hepatol 2019, 17, 2117–2125.e3. [Google Scholar] [CrossRef] [PubMed]
- Lauschke, V.M.; Ingelman-Sundberg, M. The importance of patient-specific factors for hepatic drug response and toxicity. Int J Mol Sci 2016, 17, 1714. [Google Scholar] [CrossRef]
- Goh, M.J.; Sinn, D.H. Statin and aspirin for chemoprevention of hepatocellular carcinoma: time to use or wait further? Clin Mol Hepatol 2022, 28, 380–395. [Google Scholar] [CrossRef]
- Zeng, R.W.; Yong, J.N.; Tan, D.J.H.; Fu, C.E.; Lim, W.H.; Xiao, J.; Chan, K.E.; Tan, C.; Goh, X.L.; Chee, D.; et al. Meta-analysis: chemoprevention of hepatocellular carcinoma with statins, aspirin and metformin. Aliment Pharmacol Ther 2023, 57, 600–609. [Google Scholar] [CrossRef]
- Hsu, W.H.; Sue, S.P.; Liang, H.L.; Tseng, C.W.; Lin, H.C.; Wen, W.L.; Lee, M.Y. Dipeptidyl peptidase 4 inhibitors decrease the risk of hepatocellular carcinoma in patients with chronic hepatitis C infection and type 2 diabetes mellitus: a nationwide study in Taiwan. Front Public Health 2021, 9, 711723. [Google Scholar] [CrossRef]
- Nishina, S.; Yamauchi, A.; Kawaguchi, T.; Kaku, K.; Goto, M.; Sasaki, K.; Hara, Y.; Tomiyama, Y.; Kuribayashi, F.; Torimura, T.; et al. Dipeptidyl peptidase 4 inhibitors reduce hepatocellular carcinoma by activating lymphocyte chemotaxis in mice. Cell Mol Gastroenterol Hepatol 2019, 7, 115–134. [Google Scholar] [CrossRef]
- Shan, Y.; Lu, C.; Wang, J.; Li, M.; Ye, S.; Wu, S.; Huang, J.; Bu, S.; Wang, F. IGF-1 contributes to liver cancer development in diabetes patients by promoting autophagy. Ann Hepatol 2022, 27, 100697. [Google Scholar] [CrossRef]
| Before PS-matching | After PS-matching | |||||
|---|---|---|---|---|---|---|
| Non-users (n=11062) | Statin users (n=1534) | P-value | Non-users (n=6136) | Statin users (n=1534) | P-value | |
| Age | 56.48 (8.28) | 59.87 (7.29) | <0.001 | 59.363 (7.013) | 59.870 (7.290) | 0.012 |
| Sex, male, No. (%) | 9652 (87.3%) | 1359 (88.6%) | 0.139 | 5439 (88.6%) | 1359 (88.6%) | 0.957 |
| Region, No. (%) | 0.033 | 0.004 | ||||
| Rural | 1542 (13.9%) | 202 (13.2%) | 912 (14.9%) | 202 (13.2%) | ||
| Gyeong-gi | 1721 (15.6%) | 242 (15.8%) | 906 (14.8%) | 242 (15.8%) | ||
| Seoul | 5590 (50.5%) | 826 (53.8%) | 3066 (50.0%) | 826 (53.8%) | ||
| Special city | 2209 (20.0%) | 264 (17.2%) | 1252 (20.4%) | 264 (17.2%) | ||
| HCC treatment, No. (%) | 0.010 | 0.021 | ||||
| Sorafenib | 10308 (93.2%) | 1402 (91.4%) | 5713 (93.1%) | 1402 (91.4%) | ||
| Lenvatinib | 754 (6.8%) | 132 (8.6%) | 423 (6.9%) | 132 (8.6%) | ||
| Statin use pattern, No. (%) | - | - | ||||
| pre-TKI use | - | 218 (14.2%) | - | 218 (14.2%) | ||
| continuous use from TKI treatment | - | 950 (61.9%) | - | 950 (61.9%) | ||
| post-TKI use | - | 366 (23.9%) | - | 366 (23.9%) | ||
| Etiology, No. (%) | ||||||
| HBV | 9363 (84.6%) | 1109 (72.3%) | <0.001 | 4818 (78.5%) | 1109 (72.3%) | <0.001 |
| HCV | 1368 (12.4%) | 185 (12.1%) | 0.732 | 793 (12.9%) | 185 (12.1%) | 0.364 |
| Alcoholic | 1472 (13.3%) | 263 (17.1%) | <0.001 | 972 (15.8%) | 263 (17.1%) | 0.214 |
| History of comorbidities | ||||||
| History of DM, No. (%) | 6413 (58.0%) | 1303 (84.9%) | <0.001 | 5163 (84.1%) | 1303 (84.9%) | 0.442 |
| History of fatty liver, No. (%) | 1735 (15.7%) | 415 (27.1%) | <0.001 | 1352 (22.0%) | 415 (27.1%) | <0.001 |
| History of cirrhosis, No. (%) | 4832 (43.7%) | 713 (46.5%) | 0.0385 | 2892(47.1%) | 713(46.5%) | 0.6473 |
| History of HTN, No. (%) | 7067 (63.9%) | 1397 (91.1%) | <0.001 | 4433 (72.2%) | 1397 (91.1%) | <0.001 |
| History of PBC, No. (%) | 52 (0.5%) | 17 (1.1%) | 0.002 | 45 (0.7%) | 17 (1.1%) | 0.143 |
| History of cardiovascular disease, No. (%) | 2448 (22.1%) | 717 (46.7%) | <0.001 | 1614 (26.3%) | 717 (46.7%) | <0.001 |
| History of cerebrovascular disease, No. (%) | 970 (8.8%) | 437 (28.5%) | <0.001 | 718 (11.7%) | 437 (28.5%) | <0.001 |
| Antiviral treatment, No. (%) | ||||||
| HBV treatment | 7752 (70.1%) | 842 (54.9%) | <0.001 | 3905 (63.6%) | 842 (54.9%) | <0.001 |
| HCV treatment | 248 (2.2%) | 47 (3.1%) | 0.046 | 793 (12.9%) | 185 (12.1%) | 0.364 |
| Other medication, No. (%) | ||||||
| aspirin use | 264 (2.4%) | 106 (6.9%) | <0.001 | 158 (2.6%) | 106 (6.9%) | <0.001 |
| DPP-4 inhibitor use | 279 (2.5%) | 109 (7.1%) | <0.001 | 241 (3.9%) | 109 (7.1%) | <0.001 |
| insulin use | 1717 (15.5%) | 285 (18.6%) | 0.002 | 1010 (16.5%) | 285 (18.6%) | 0.048 |
| metformin use | 229 (2.1%) | 54 (3.5%) | <0.001 | 173 (2.8%) | 54 (3.5%) | 0.147 |
| SGLT-2 inhibitor use | 27 (0.2%) | 18 (1.2%) | <0.001 | 25 (0.4%) | 18 (1.2%) | <0.001 |
| sulfonylurea use | 131 (1.2%) | 38 (2.5%) | <0.001 | 109 (1.8%) | 38 (2.5%) | 0.073 |
| Thiazolidinedione use | 43 (0.4%) | 21 (1.4%) | <0.001 | 36 (0.6%) | 21 (1.4%) | 0.001 |
| Median treatment duration (days) | 260.00 (149.00, 498.00) | 337.00 (180.00, 708.00) | <0.001 | 269.00 (152.00, 512.00) | 337.00 (180.00, 708.00) | <0.001 |
| Median follow-up period (months) | 95.00 (56.00, 190.00) | 119.50 (63.00, 250.00) | <0.001 | 96.00 (56.00, 199.00) | 119.50 (63.00, 250.00) | <0.001 |
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Statin use, yes | 0.71 | 0.66–0.76 | <0.001 | 0.77 | 0.72–0.82 | <0.001 | |
| Age, ≥60 yr | 0.99 | 0.94–1.04 | 0.624 | 1.00 | 1.00–1.01 | 0.469 | |
| Sex, female | 0.93 | 0.86–1.01 | 0.080 | 0.95 | 0.88–1.03 | 0.205 | |
| Region, urban | 0.96 | 0.89–1.03 | 0.249 | ||||
| DM, yes | 0.92 | 0.86–0.99 | 0.022 | 0.94 | 0.87–1.01 | 0.086 | |
| HTN, yes | 0.80 | 0.75–0.85 | <0.001 | 0.87 | 0.82–0.92 | <0.001 | |
| Cardiovascular disease, yes | 0.86 | 0.81–0.91 | <0.001 | 0.93 | 0.88–0.98 | 0.010 | |
| Cerebrovascular disease, yes | 0.86 | 0.80–0.92 | <0.001 | ||||
| Fatty liver, yes | 0.90 | 0.84–0.96 | 0.001 | 0.95 | 0.89–1.01 | 0.081 | |
| Cirrhosis, yes | 0.80 | 0.76-0.84 | <0.001 | ||||
| Aspirin, yes | 0.61 | 0.53–0.70 | <0.001 | 0.64 | 0.55–0.74 | <0.001 | |
| DPP-4 inhibitor use, yes | 0.85 | 0.75–0.95 | 0.006 | ||||
| Insulin use, yes | 1.14 | 1.07–1.22 | <0.001 | 1.24 | 1.16–1.33 | <0.001 | |
| Metformin use, yes | 0.75 | 0.65–0.87 | <0.001 | 0.78 | 0.67–0.91 | 0.002 | |
| SGLT-2 inhibitor use, yes | 0.60 | 0.41–0.88 | 0.01 | 0.67 | 0.45–0.99 | 0.044 | |
| Sulfonylurea use, yes | 0.76 | 0.63–0.91 | 0.003 | 0.81 | 0.67–0.99 | 0.037 | |
| Thiazolidinedione use, yes | 0.65 | 0.47–0.89 | 0.007 | 0.79 | 0.57–1.09 | 0.150 | |
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Statin use, yes | 0.72 | 0.67–0.76 | <0.001 | 0.78 | 0.74–0.84 | <0.001 | |
| Age, ≥60 yr | 1.04 | 0.99–1.09 | 0.156 | 1.01 | 1.00–1.01 | 0.006 | |
| Sex, female | 0.98 | 0.91–1.06 | 0.603 | 1.01 | 0.94–1.09 | 0.825 | |
| Region, urban | 0.88 | 0.82–0.94 | <0.001 | 0.87 | 0.81–0.93 | <0.001 | |
| DM, yes | 0.91 | 0.85–0.98 | 0.007 | 0.95 | 0.88–1.01 | 0.104 | |
| Fatty liver, yes | 0.94 | 0.89–1.00 | 0.054 | ||||
| Cirrhosis, yes | 0.82 | 0.79-0.86 | <0.001 | ||||
| HTN, yes | 0.80 | 0.75–0.84 | <0.001 | 0.86 | 0.81–0.91 | <0.001 | |
| Cardiovascular disease, yes | 0.84 | 0.79–0.88 | <0.001 | 0.90 | 0.86–0.96 | <0.001 | |
| Cerebrovascular disease, yes | 0.81 | 0.76–0.87 | <0.001 | 0.90 | 0.84–0.97 | 0.004 | |
| Aspirin use, yes | 0.57 | 0.50–0.65 | <0.001 | 0.63 | 0.55–0.73 | <0.001 | |
| DPP-4 inhibitor use, yes | 0.78 | 0.70–0.88 | <0.001 | 0.90 | 0.80–1.01 | 0.085 | |
| Insulin use, yes | 1.13 | 1.06–1.20 | <0.001 | 1.26 | 1.18–1.35 | <0.001 | |
| Metformin use, yes | 0.68 | 0.59–0.78 | <0.001 | 0.72 | 0.62–0.84 | <0.001 | |
| SGLT-2 inhibitor use, yes | 0.70 | 0.50–0.98 | 0.038 | 0.77 | 0.54–1.07 | 0.123 | |
| Sulfonylurea use, yes | 0.73 | 0.61–0.86 | <0.001 | 0.83 | 0.69–1.00 | 0.049 | |
| Thiazolidinedione use, yes | 0.64 | 0.49–0.85 | 0.002 | 0.80 | 0.60–1.06 | 0.114 | |
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Statin use pattern | ||||||||
| Non-user | reference | reference | ||||||
| pre-TKI use | 1.31 | 1.13–1.52 | <0.001 | 1.33 | 1.14–1.54 | <0.001 | ||
| continuous use from TKI treatment | 0.80 | 0.74–0.87 | <0.001 | 0.87 | 0.80–0.95 | 0.002 | ||
| post-TKI use | 0.40 | 0.35–0.46 | <0.001 | 0.43 | 0.38–0.50 | <0.001 | ||
| Age, ≥60 yr | 0.99 | 0.94–1.04 | 0.624 | 1.00 | 1.00–1.00 | 0.767 | ||
| Sex, female | 0.93 | 0.86–1.01 | 0.080 | 0.95 | 0.88–1.03 | 0.246 | ||
| Region, urban | 0.96 | 0.89–1.03 | 0.249 | |||||
| DM, yes | 0.92 | 0.86–0.99 | 0.022 | 0.93 | 0.87–1.00 | 0.048 | ||
| Fatty liver, yes | 0.90 | 0.84–0.96 | <0.001 | 0.94 | 0.88–1.00 | 0.050 | ||
| Cirrhosis, yes | 0.80 | 0.76-0.84 | <0.001 | |||||
| HTN, yes | 0.80 | 0.75–0.85 | <0.001 | 0.87 | 0.82–0.92 | <0.001 | ||
| Cardiovascular disease, yes | 0.86 | 0.81–0.91 | <0.001 | 0.93 | 0.88–0.99 | 0.016 | ||
| Cerebrovascular disease, yes | 0.86 | 0.80–0.92 | <0.001 | |||||
| Aspirin use, yes | 0.61 | 0.53–0.70 | <0.001 | 0.69 | 0.59–0.79 | <0.001 | ||
| DPP-4 inhibitor use, yes | 0.85 | 0.75–0.95 | 0.006 | 0.90 | 0.80–1.03 | 0.119 | ||
| Insulin use, yes | 1.14 | 1.07–1.22 | <0.001 | 1.23 | 1.15–1.32 | <0.001 | ||
| Metformin use, yes | 0.68 | 0.59–0.78 | <0.001 | 0.83 | 0.71–0.97 | 0.022 | ||
| SGLT-2 inhibitor use, yes | 0.60 | 0.41–0.88 | 0.010 | 0.66 | 0.45–0.98 | 0.038 | ||
| Sulfonylurea use, yes | 0.76 | 0.63–0.91 | 0.003 | 0.79 | 0.65–0.96 | 0.020 | ||
| Thiazolidinedione use, yes | 0.65 | 0.47–0.89 | 0.007 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





