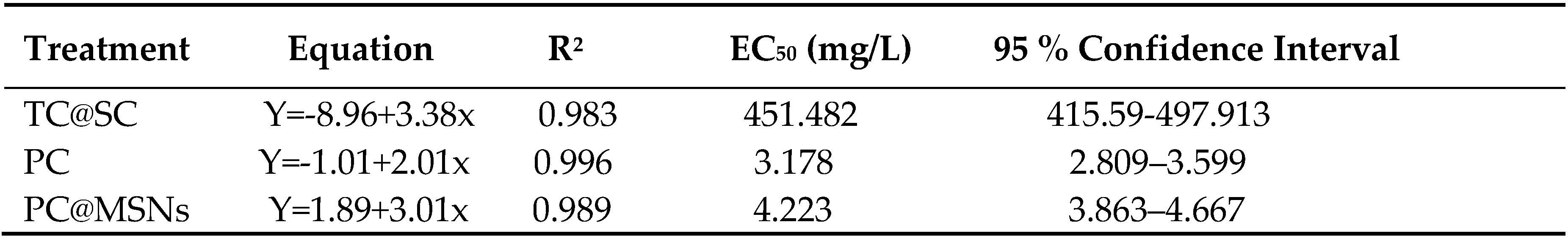1. Introduction
Pesticides are essential in modern agriculture and greatly contribute to the quality and quantity of food [
1,
2,
3]. However, more than 90 % of the pesticides applied in the field are eventually lost because of low utilization rates, instability, poor dispersibility, and environmental factors such as wind, rain, and photolysis, and only 0.1 % of the active ingredients reach the target organisms [
4,
5,
6,
7]. Furthermore, the excessive and unscientific use of pesticides severely threatens ecosystems and human health. Traditional pesticide formulations have drawbacks, including the presence of toxic organic solvents, coarse particles, and poor dispersion, which result in low biological activity, short duration of efficacy, and low utilization, leading to serious contamination and damage to non-target organisms [
8,
9,
10]. The development of innovative approaches to mitigate pesticide losses and enhance the efficacy of pesticide utilization has been necessitated by relevant factors, such as resistance, eutrophication of water bodies, short-term plant protection, and bioaccumulation [
11,
12].
Controlled-release formulations (CRFs) have become a research hotspot in the field of pesticide formulations [
13,
14,
15,
16]. CRFs have various advantages such as protecting pesticides from external factors, shielding irritating odors, reducing volatilization and toxicity to non-target organisms, controlling pesticide release, and improving pesticide adhesion and transportation capabilities within the target [
17,
18,
19,
20]. In recent years, nanopesticide formulations have emerged as a research focus in this field, providing the capability to improve utilization, reduce non-target toxicity, and achieve a sustained release of pesticides [
21,
22,
23,
24,
25]. Mesoporous silica nanoparticles (MSNs) have several advantages such as large specific surface area, adjustable pore size, uniform particle size, easy surface modification, and excellent biocompatibility [
26,
27,
28,
29]. Due to these advantages, they are widely used as carrier materials for pesticides. Incorporating pesticides into MSNs not only extends their effectiveness period but also reduces toxicity and environmental impacts on non-target organisms [
30,
31].
Rice is an important food crop cultivated worldwide, , feeding more than half the global population [
32]. Rice bacterial blight caused by
Xanthomonas oryzae pv. Oryzae(
X. oryzae) is a bacterial disease that seriously affects rice yield and quality [
33]. Currently, copper fungicides are the most commonly used pesticides for controlling bacterial blight. The extensive use of copper fungicides not only leads to pathogen resistance but also poses a significant threat to ecosystems and human health. Therefore, the pesticide, p-cymene (PC), was filtered to control bacterial blight and yielded satisfactory results.
PC is an aromatic terpene that shows a range of biological activities, including anti-inflammatory, anxiolytic, antinociceptive, antioxidant, anticancer, and antimicrobial effects, and is found in over 100 plant species (
Figure 1) [
34,
35,
36,
37]. PC is mainly used in medicine and rarely for crop disease control. The result of fungicidal activity (EC
50=3.178 mg/L) showed that PC exhibited excellent fungicidal activity against
X. oryzae. However, PC undergoes oxidation upon prolonged exposure to oxygen. The field environment is simultaneously complex, and the efficacy of PC applied in the field cannot be guaranteed. Additionally, PC can contaminate soil and groundwater and increase the risk of food safety hazards when present at high levels in food products [
36]. Thus, the popularization and application of PC in agriculture is limited. Processing PC into CRFs can reduce the required dosage and frequency of PC, improve its stability and safety, and address the shortcomings of traditional formulations.
Therefore, in the present study, mesoporous silica nanoparticles loaded with PC-loaded MSNs (PC@MSNs) were prepared using an impregnation method designed for the sustained release of the active ingredient. The physicochemical characteristics of the PC@MSNs were characterized. Additionally, the loading capacity, in vitro release behavior, antifungal activity, acute toxicity to zebrafish, and biosafety in rice growth were also evaluated.
2. Materials and Methods
2.1. Materials
X. oryzae was obtained from the Laboratory of Plant Disease Control and Utilization at Hunan Agricultural University, Hunan Province, China. Hexadecyl trimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), and PC were procured from Macklin Biochemical Co., Ltd. (Shanghai, China). N, N-Dimethylformamide (DMF), acetic acid, sodium hydroxide (NaOH), sodium chloride (NaCl), methanol, and ethyl alcohol were supplied by Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai).
2.2. Synthesis of MSNs
MSNs were synthesized according to a previously reported method with some modifications [
38]. First, 1 g of CTAB and 3.5 mL of NaOH aqueous solution (2mol/L) were added to 480 ml of pure water in a 500 mL conical flask. Then, 5 mL of TEOS was slowly added to the mixture with vigorous stirring The mixture was stirred at 80 °C for 4 h. After being stirred the precipitate was collected after filtration and washed with methyl alcohol, pure water, and dried under vacuum. Second, 0.1 g of the precipitate was added to a flask containing 50 mL of NaCl methanol solution, and the mixture was stirred at 70 °C for 3 h, which was repeated three times. The precipitate was collected after filtration, washed with methyl alcohol and pure water, and dried under vacuum.
2.3. Synthesis of PC@MSNs
The PC@MSNs were prepared by impregnation. First, 1 g of PC was dissolved in 50 mL of methyl alcohol in a 100 mL conical flask, and then 1 g of the MSNs was added to the solution, containing the PC, and slowly stirred for 24 h. The resultant PC@MSNs were washed with methyl alcohol and dried under a vacuum.
2.4. Characterization
The functional groups of the specimens were analyzed by Fourier transform infrared spectroscopy (FTIR, Nicolet-IS 5, Thermo, United States), using the KBr disc method with scanning from 4000 to 400 cm−1. The average particle size and zeta potential were measured using dynamic light scattering (DLS; Zetasizer Nano ZS90, Malvern, United Kingdom). The surface composition of the samples was analyzed using X-ray photoelectron spectroscopy (XPS, ESCALAB 250XI, Thermo, United States). The Brunauer Emmett-Teller (BET) surface areas, pore volumes, and pore-size distribution of the specimens were measured using N2 adsorption–desorption isotherms.
2.5. Microscopic Morphology Observation
The internal and external morphologies of MSNs and PC@MSNs were determined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM images were obtained using a Sigma 300 instrument (ZEISS, Germany) at an acceleration voltage of 25 kV. The TEM imageswere obtained using a Tecnai F20 microscope (FEI, United States) at an acceleration voltage of 200 kV.
2.6. Pesticide Loading Content
A Jupiter thermogravimetric analyzer (TGA, Mettler Toledo Co., Switzerland) was used to measure the thermogravimetric losses of MSNs and PC@MSNs. Under N2 protection, the temperature in the analyzer was increased from 30 to 800 °C at a rate of 10 °C min−1. The PC loading content of PC@MSNs was then calculated.
2.7. In Vitro Release of PC@MSNs
To evaluate the release behavior of PC from the PC@MSNs, controlled-release experiments were conducted. In the standard procedure, PC@MSNs were enclosed in a dialysis bag (MWCO 3500 Da) and immersed in 200 mL of the release medium (methanol/water, 7:3 v/v). A total of 1 mL of the solution was withdrawn at specific intervals and analyzed using high performance liquid chromatography. Following each sampling, an equivalent volume of fresh release medium was added to maintain a consistent volume throughout predetermined time intervals.
2.8. In Vitro Fungicidal Activity
The in vitro fungicidal activity of the PC@MSNs against X. oryzae was assessed using the turbidity method. Specifically, bacterial fluid of X. oryzae (50 μL) was inoculated to Nutrient Broth fluid medium (50 mL) containing different concentrations (1, 2, 3, 4, 5, and 6 mg/L) of PC and PC@MSNs respectively. The test tubes were then incubated on rotary shakers at 28 ± 1 °C for 48 h. After incubation, the absorbance of the medium was measured using an ultraviolet spectrophotometer. The fluid medium with only PC and PC@MSNs served as blank controls, and all experiments were conducted in triplicate. The concentration for 50 % of the maximal effect (EC50) was calculated using SPSS software.
2.9. Acute Toxicity of PC and PC@MSNs to Zebrafish
In this study, the acute toxicity of PC and PC@MSNs in zebrafish was assessed using a semi-static toxicity test. Zebrafish were acquired from an online marketplace and maintained under laboratory conditions. Zebrafish had an average length of 2.0 ± 0.1 cm. Before the experiment, they were acclimatized to laboratory conditions for over seven days, with feeding halted 24 h before and throughout the experiment. The concentration series was established based on a preliminary experiment with PC. Seven zebrafish were randomly placed into tanks containing 2 L of aerated water with varying concentrations of PC and PC@MSNs. Each treatment was conducted in triplicates. The control groups containing only water and carriers were established under identical conditions. Symptoms of intoxication and mortality were monitored and recorded at 24, 48, 72, and 96 h after the initiation of the test, and the deceased fish were promptly removed. The criterion for determining mortality was the absence of a response when the tail of the fish was prodded with a glass rod. Throughout the 96-h exposure, LC50 values and their 95 % confidence intervals were calculated using the SPSS software.
2.10. Safety Evaluation of Rice
First, the effect of the PC@MSNs on the germination of rice seeds was evaluated. For the germination assay, nine seeds were disinfected and placed on filter paper in a Petri dish (diameter, 9 cm). Five milliliters of water at concentrations of 50, 100, and 500 mg/L were introduced into the Petri dish to moisten the filter paper (three replicates per concentration). A treatment group containing pure water was used as the control. All rice seeds were incubated in an artificial climate chamber at 28 °C with a relative humidity of 95 % and a 12/12 h light/dark cycle for 7 days. The number of germinated seeds was determined daily, and the germination rates and potential were calculated using the number of rice seeds [
39].
A moderate quantity of rice seeds was placed on filter paper in a Petri dish for germination. Following germination, the seeds were transferred to pots containing 400 g of cultivated substrate soil, with an appropriate volume of water added to maintain a humidity level of approximately 80 %. After seven days, PC@MSNs containing 100 mg of PC were applied to the soil in each pot, and pure water served as a blank control. After 21 d of treatment, the fresh weight (mg), stem length (cm), and root length (cm) were measured to assess the effects of PC@MSNs on rice growth.
3. Results
3.1. SEM and TEM Analysis
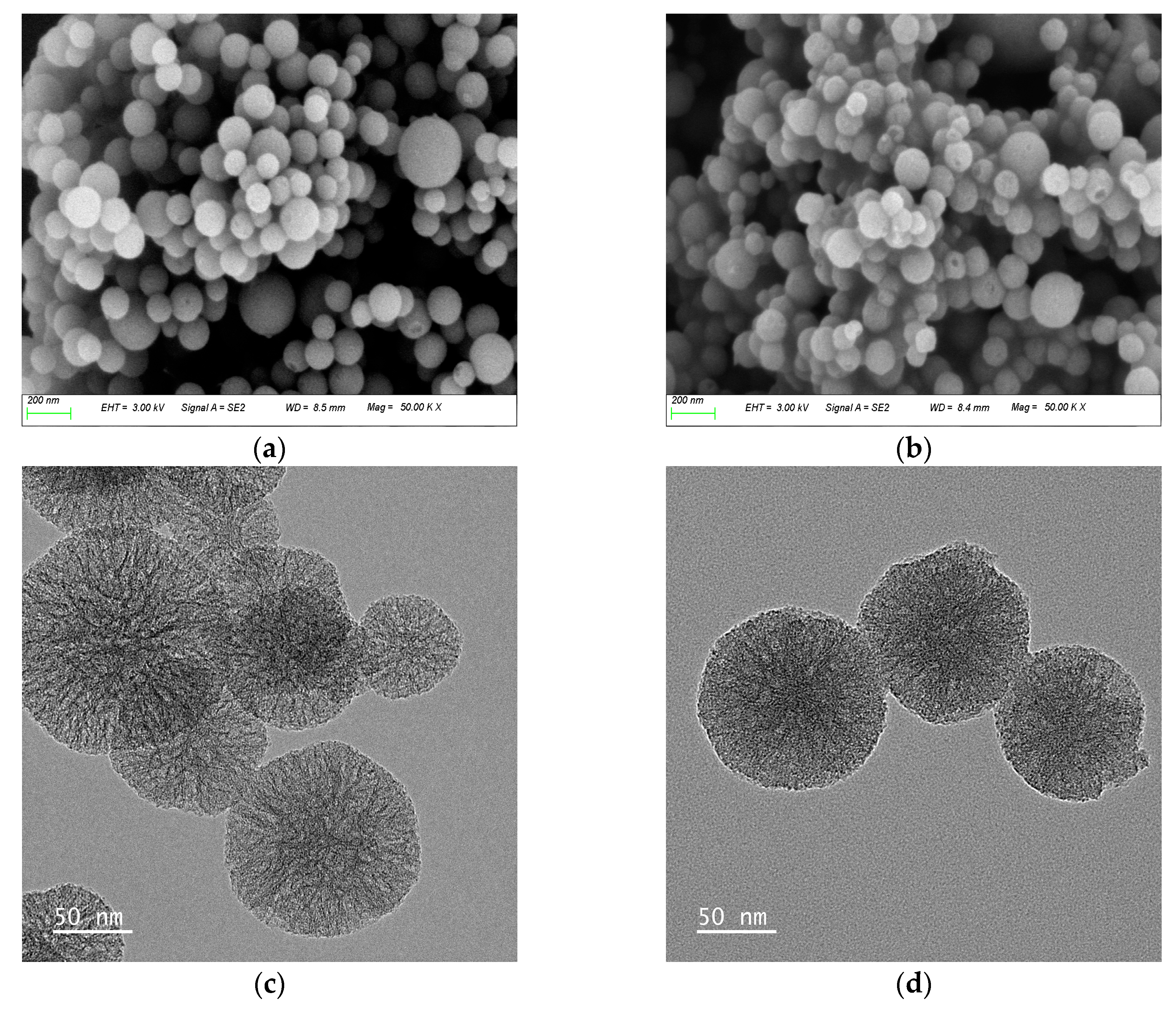
SEM and TEM were used to examine the morphology and mesoporous structure of MSNs and PC@MSNs. As shown in
Figure 2a,b, the MSNs had a spherical structure, with an average particle size of approximately 150 nm. The PC@MSNs also had a spherical structure and an increased particle size of 260 nm. In addition, the internal morphology of MSNs presented scattered star-shaped ordered wormhole structures, whereas scattered star-shaped ordered wormhole structures could not be observed on PC@MSNs, indicating that PC was embedded in the mesoporous structures of the MSNs (
Figure 2c,d).
3.2. FTIR Analysis
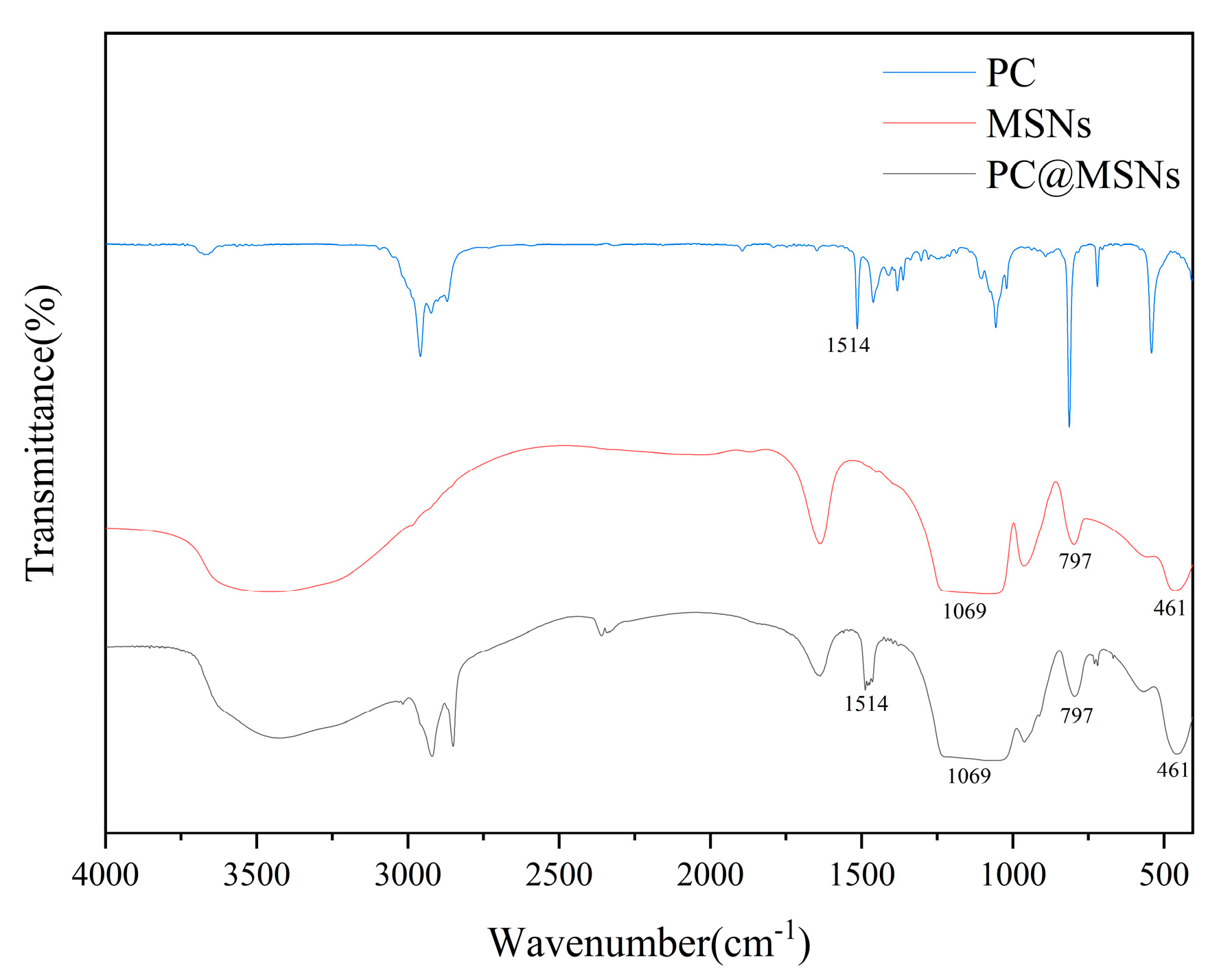
The FTIR spectra of PC, MSNs, and PC@MSNs are shown in
Figure 3. The spectrum of PC showed strong absorption bands at 1514 cm
−1, which was attributed to the vibration of carbonyl groups and benzene ring skeletons in PC [
40]. For MSNs, the peaks at 1069, 797, and 461 cm
−1 were attributed to the antisymmetric stretching, symmetric stretching, and bending vibrations in Si-O-Si, respectively [
41]. The FTIR spectrum of PC@MSNs had a stretching vibration at 1069,797 and 461 cm
-1, which was caused by Si–O–Si and the strong band absorption of the benzene ring appeared at 1514 cm
−1 which was also seen in PC. The attenuation of characteristic peaks of benzene ring skeleton vibrations at 1514 cm
−1 further proved that PC was successfully coated with MSNs.
3.3. DLS and XPS Analysis
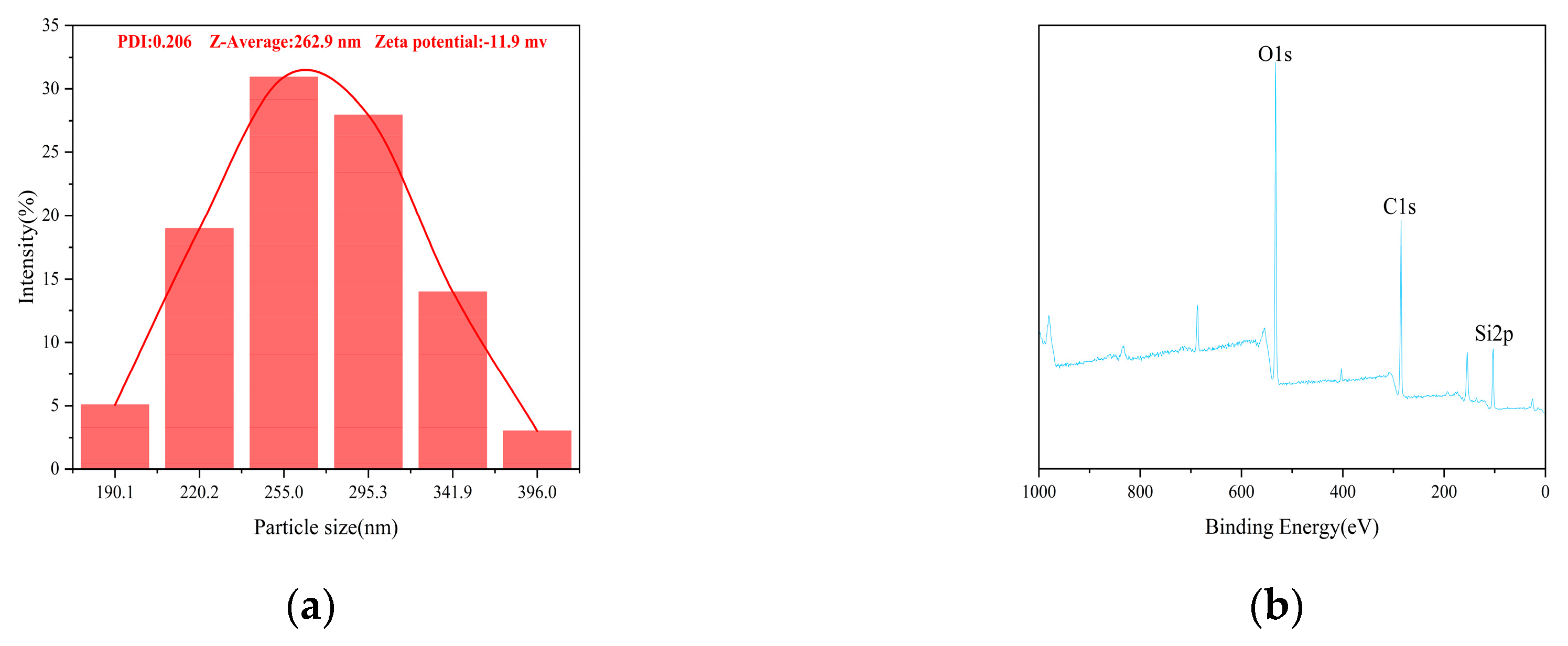
The particle size distribution and zeta potential of the PC@MSNs are shown in
Figure 4a. The average particle size and Zeta potential of PC@MSNs were 262.9 nm and -11.9 mv, respectively. In addition, the constituent elements of the PC@MSNs were determined using XPS. As shown in
Figure 4b, C, O, and Si were detected in the PC@MSNs, which proved that the PC was successfully loaded onto the MSNs.
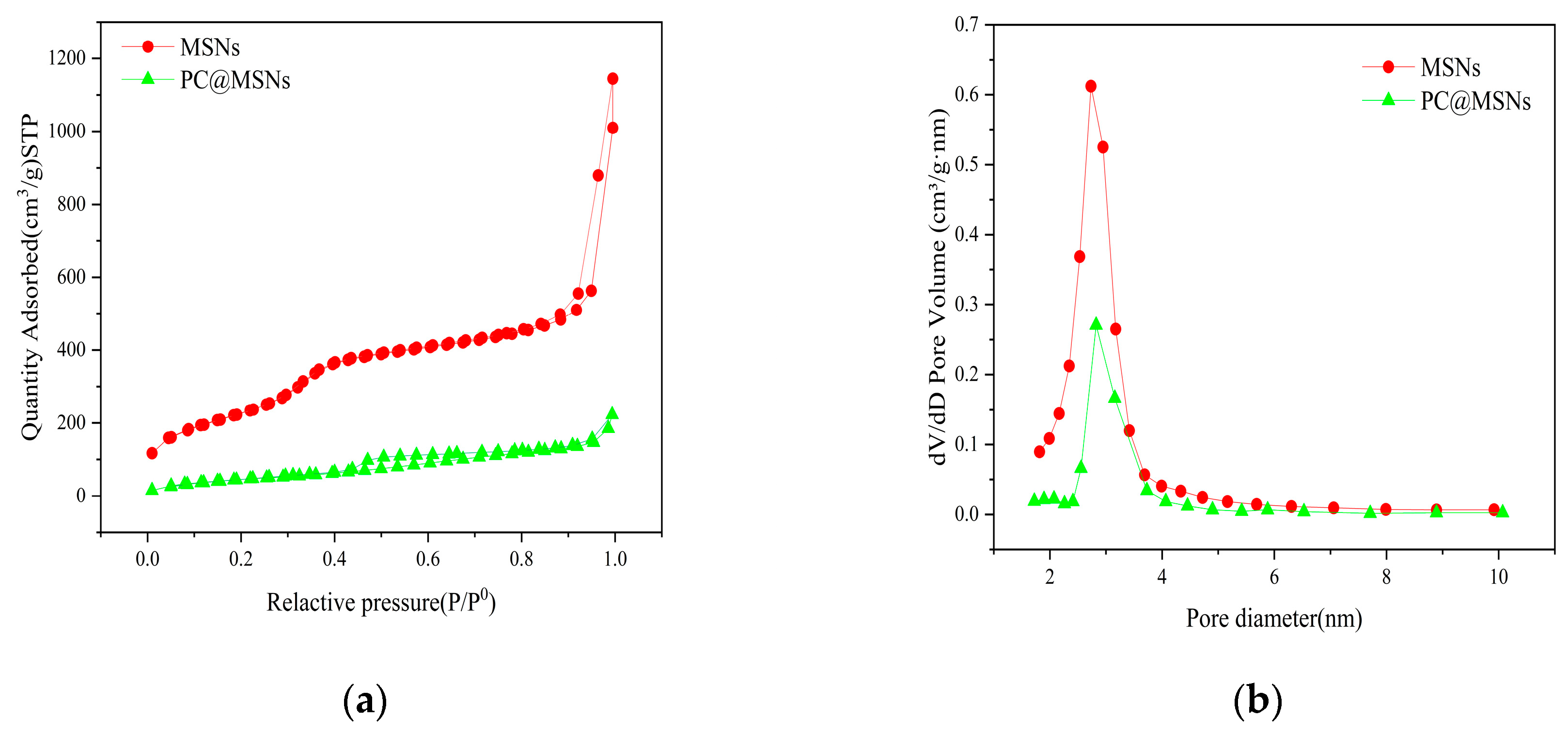
3.4. Nitrogen Adsorption-Desorption Isotherms
The nitrogen adsorption–desorption curves and pore size distributions are shown in
Figure 5. It can be seen from the figure that the N
2 adsorption isotherm is a type IV isotherm, and the type IV curve indicates that the MSNs and PC@MSNs have mesoporous structures (
Figure 5a).
Table 1 lists the specific surface areas, pore volumes, and pore sizes of MSNs and PC@MSNs. The specific surface area, pore volume, and pore size of MSNs were 926.0210 m
2/g, 1.771295 cm
3/g, and 8.4104 nm, respectively. After the MSNs were loaded with PC, the N
2 adsorption capacity of the PC@MSNs was significantly reduced. The specific surface pore volumes and sizes are 176.6145 m
2/g, 0.344577 cm
3/g, and 7.8040 nm, respectively.
Figure 2b shows the overall decrease in the curve after drug loading, the decrease was most significant at 2–3 nm. These results indicate that PC was loaded onto the MSNs and occupied their pores.
3.5 Pesticide Loading Content
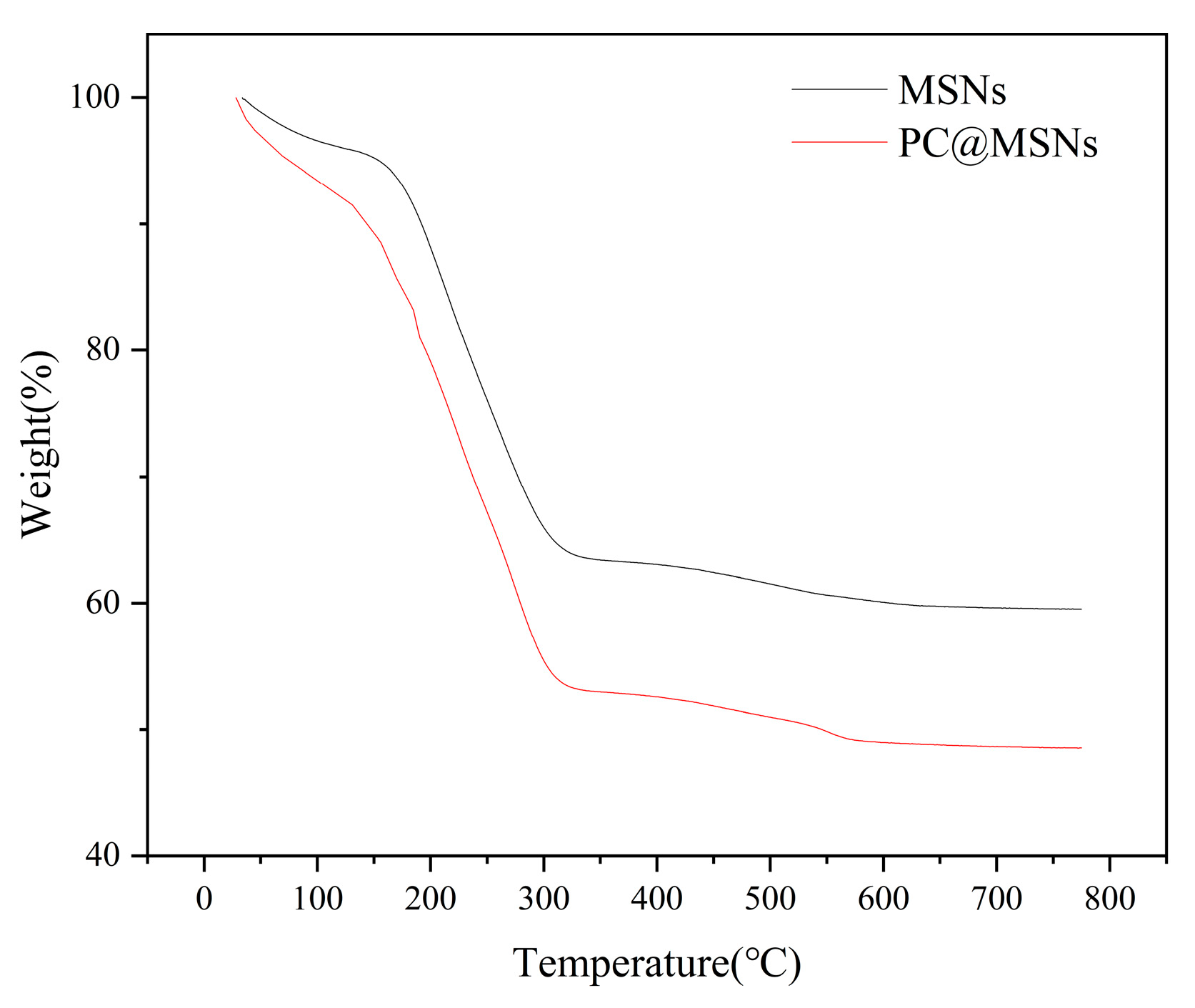
The TGA curves of the MSNs and PC@MSNs were obtained, and the results are presented in
Figure 6. When the treatment temperature reached 150 °C, the weight loss of MSNs and PC@MSNs were approximately 4.3 % and 6.1 %, respectively. This is due to the evaporation of water from the sample. When the treatment temperature increased from 150 °C to 800 °C, the weight loss of MSNs and PC@MSNs was 38.9 % and 47.5 %, respectively. The decomposition of the organic groups in the samples resulted in weight loss [
42,
43,
44,
45]. The loading content of PC onto MSNs was approximately 8.6 %.
3.6. Release Behavior of PC@MSNs
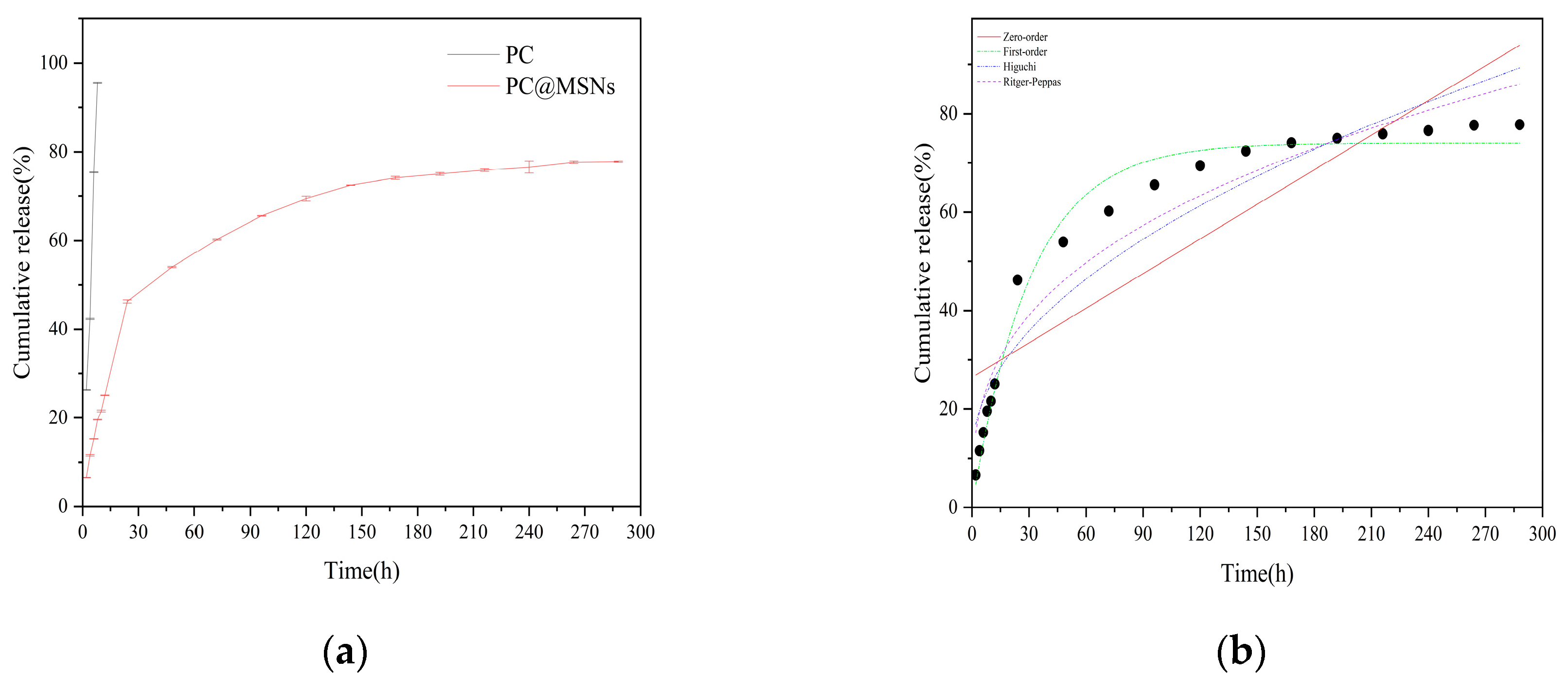
The release behavior of PC@MSNs was investigated using the dialysis bag method. The release profiles of the PC and PC@MSNs are graphically presented in
Figure 7a. The release rate of PC increased significantly, reaching 95.58 % after 8 h, which showed that the active component was precipitated in the medium through a concentration difference and was completely released within 24 h. In contrast, the release from PC@MSNs was slower, with cumulative release rates of 46.25 % and 53.98 % at 24 and 48 h, respectively. Subsequently, the release rate decreased, and the sustained release time exceeded 288 h, which may be because the drug adsorbed on the surface and at the outside diameter of the pores, which was released first, and then the drug was released slowly from the innermost layer.
To elucidate the release kinetics mechanism of PC@MSNs, four kinetic models were employed to fit the in vitro release curves (
Figure 7b). The release curves correlated well with the first-order kinetic model, yielding an R
2 value of 0.9824 for the microspheres (
Table 2). These results suggest that the release of PC@MSNs adheres to the fundamental model of a sustained-release formulation. The Ritger–Peppas model, which is typically applied to erodible drug delivery systems, was used to describe the release curve. The resulting n value was less than 0.45, indicating that drug release belonged to Fickian diffusion.
3.7. Fungicidal Activity of PC@MSNs
To develop a more effective fungicide for the treatment of
X. oryzae, the fungicidal activities of PC and PC@MSNs were investigated. A Thiessen copper@suspension concentrate (TC@SC) was used as the control group. As depicted in
Table 3, the EC
50 value of TC@SC was determined to be 477.589 mg/L. The EC
50 value of PC was 3.178 mg/L, which is far below that of TC@SC. The fungicidal activity of the PC@MSNs was marginally lower than that of PC. This reduction was likely due to the time required for the PC@MSNs to release PC from the MSNs. These results indicate that PC and PC@MSNs had an excellent inhibitory effect against
X. oryzae compared to TC@SC.
3.8. Acute Toxicity to Zebrafish
PC is widely used in medicine but rarely used in agriculture. The application of PC to the control of rice bacterial blight requires an evaluation of its toxicity to aquatic organisms. Therefore, we assessed the toxicity of PC and PC@MSNs in zebrafish (
Table 4). Throughout the experimental period, no abnormal mortality was observed in the control or carrier-only group. The LC
50 (96 h) values for the PC treatment and PC@MSNs were 15.165 and 257.867 mg/L, respectively. The toxicity grades of PC and PC@MSNs for zebrafish were defined as slightly toxic (≥10 mg/L) based on Test Guidelines on Environmental Safety Assessment for Chemical Pesticides – Part 12: Zebrafish acute toxicity test (GB/T 31270.12–2014), according to the achieved LC
50 values. An increase in the LC
50 values indicated that PC and PC@MSNs were less toxic to aquatic organisms. The safety of PC@MSNs in zebrafish was markedly greater than that of PC, with an approximately 17-fold increase. Compared to direct environmental exposure to PC, PC@MSNs minimized direct contact with zebrafish owing to the loading carriers, thereby further improving the safety of PC for aquatic life. In summary, the PC@MSNs have low toxicity to aquatic organisms and can promote the large-scale use of PC.
3.9. Plant Safety
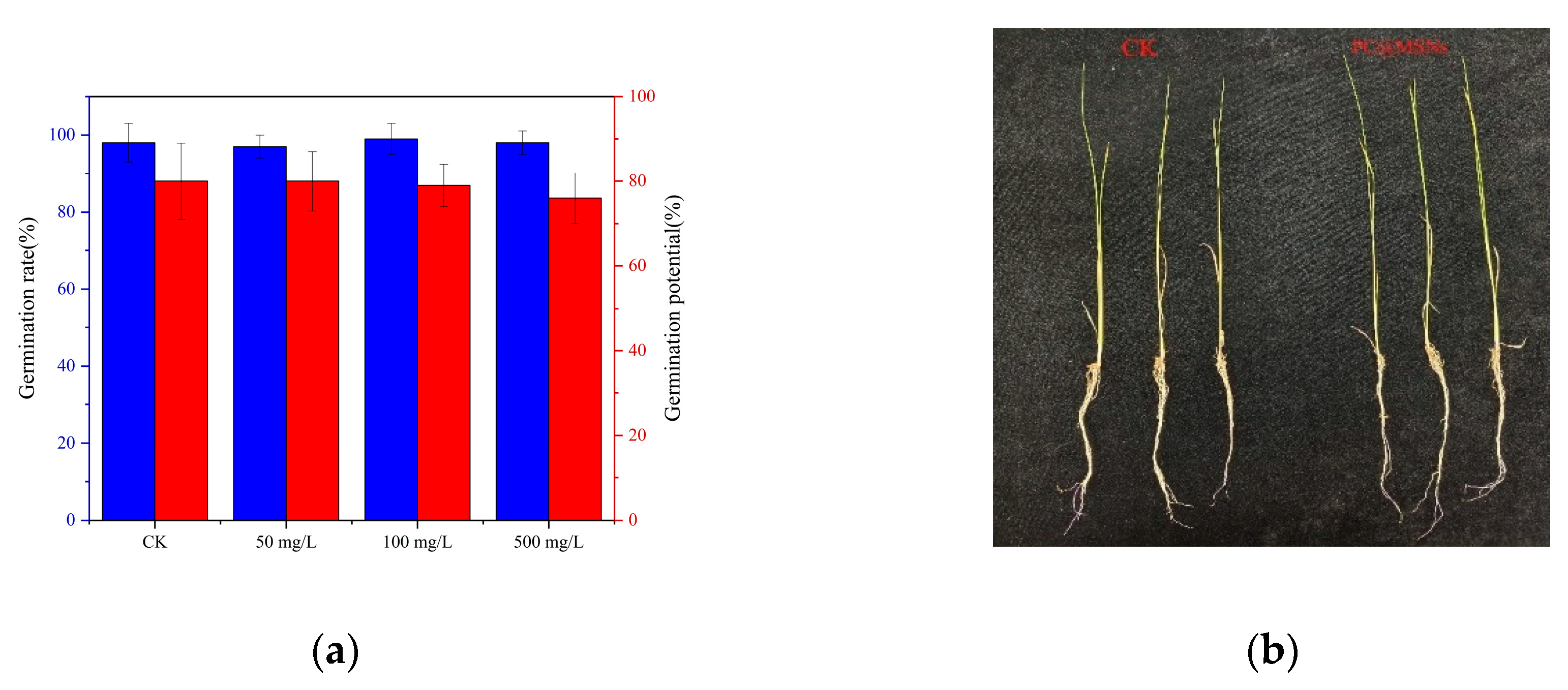
In the present study, PC@MSNs showed no adverse effects on rice seed germination. The germination rates of all groups treated with different concentrations were above 95 %, and there was no significant difference in the germination potential and germination rate among different groups (
Figure 8a).
As illustrated in
Figure 8b, there was no significant difference between the control group and the group treated with PC@MSNs. The results of the plant safety experiments are listed in
Table 5. The fresh weight, stem length, and root length of the control rice group were 330 mg, 30.0 cm, and 17.8 cm, respectively. In the treatment group, the fresh weight, stem length, and root length of the rice were 321 mg, 29.3 cm, and 17.2 cm, respectively. After 21 d, there were no significant differences between the PC@MSN treatment group and the control group, indicating that PC@MSNs are safe for rice plants.
4. Discussion
Rice is one of the most important food crops for mankind, and it is one of the most important crops in the world, providing the main food source for billions of people worldwide. Bacterial blight (X. oryzae) is a severe bacterial disease that affects rice yield and quality. Currently, chemical agents are the most effective agents for controlling bacterial blight in rice. Among these, copper fungicide is one of the most commonly used agents to effectively control the occurrence of bacterial blight in rice. However, the excessive use of copper fungicides leads to disease resistance and poses a serious threat to the ecological environment and human health. Many problems are associated with other pesticide formulations, including low biological activity, short duration of efficacy, and low utilization. Nanopesticide controlled-release formulations can effectively solve the problems associated with traditional pesticide formulations.
In this study, we screened a new active component (PC) to control bacterial blight in rice. The EC50 value of PC against X. oryzae was 3.178 mg/L, which was markedly lower than that of the Cu fungicide (EC50=451.482 mg/L), indicating an approximately 142-fold decrease. PC is mainly used in medicine and rarely in agriculture. PC is easily volatilized and oxidized, and the field environment is complex, limiting its use in the field. Incorporating PC into nano carrier materials not only extends its effectiveness period but also assures its stability; hence, we prepared a nanopesticide formulation of PC, where a mesoporous silica nanoparticle served as the carrier and loaded PC as the active pesticidal agent.
The physicochemical characteristics of the PC@MSNs were characterized. Scanning electrons with SEM, particle size analysis, and drug-loading assessments revealed that the PC@MSNs maintained a spherical shape with a smooth surface. The average particle size of PC@MSNs was 262.9 nm, with a drug loading capacity of 8.6 %. The main peaks from the FTIR scan show similarities between the PC and the PC@MSN and the differences noted between the MSN and the PC@MSN.The decreased pore size of the PC@MSN in comparison to the MSN.The release results prove that the PC was loaded.
The fungicidal activity of the PC@MSNs was investigated, and the EC50 value of the PC@MSNs was determined to be 4.223 mg/L. The fungicidal activities of PC@MSNs against X. oryzae were similar to PC, which revealed that PC@MSNs have excellent control effects on rice bacterial blight. The result of release behavior showed that PC@MSNs had excellent slow control performance. In addition, PC is rarely used in agriculture and its toxicity to non-target organisms is unknown. Therefore, it is necessary to determine the toxicity of PC to aquatic organisms when used to control bacterial blight in rice. We assessed the toxicity of PC and PC@MSNs in zebrafish. The LC50 (96 h) values of PC and PC@MSNs were 15.165 and 257.867 mg/L, respectively. The toxicity grades of PC and PC@MSNs for zebrafish were defined as slightly toxic (≥10 mg/L). However, high doses of PC can harm aquatic organisms. The LC50 of PC@MSNs was higher than that of PC, which can promote the large-scale application of PC. It can also reduce the harm caused by PC to the ecosystem and human health. The plant safety evaluation of PC@MSNs indicates that PC@MSNs exhibit plant safety for rice. The PC release exhibited an instant bolus dose which contributes to the toxicity seen. However, the PC@MSN exhibited a slight initial bolus dose followed by a sustained controlled release which explains why the pesticide effect is seen, and sustained for a longer time overall with a lower toxicity. This release from the formulation proves that PC has great potential to be a pesticide once it is in the correct formulation, even though it has barely been used in agriculture.
This nanopesticide formulation is poised to broaden the application scope of PC for managing rice diseases and enhance its commercial viability, marking its development of considerable practical significance. However, there are still many problems with the application of PC in agriculture, such as toxicity to other non-target organisms, and further evaluation is needed to confirm whether PC can be truly applied to disease control.
5. Conclusions
This study introduced PC@MSNs, a nanopesticide formulation that has excellent inhibitory effects on rice bacterial blight. Studies on the in vitro release mechanism indicated that PC@MSNs exhibit commendable controlled release capabilities, with PC release modulated by drug diffusion. Moreover, in vitro fungicidal activity assays demonstrated that the PC@MSNs showed excellent efficacy against bacterial blight. The PC@MSNs did not impede the normal growth of rice plants. In biosafety evaluations, the PC@MSNs exhibited low toxicity to zebrafish after 96 h. The PC@MSNs, as a sustained-release nanopesticide formulation, have great application potential for managing rice bacterial blight. The findings from this study open new avenues for research incorporating PC into the agricultural field.
Author Contributions
Conceptualization, C.L. and X.L.; Methodology, C.L.; Software, C.L.; Validation, Y.M. and L.J.; Formal analysis, C.L.; Investigation, C.L.; Resources, X.L.; Data curation, C.L.; Writing—Original Draft Preparation, C.L.; Writing—Review and Editing, Y.M. and L.J.; Visualization, C.L.; Supervision, X.L.; Project Administration, C.L.; Funding Acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 32302335).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their thanks to the Testing Center of Hunan Agricultural University and Shiyanjia Lab (
www.shiyanjia.com).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. International Journal of Environmental Research and Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicology and Environmental Safety 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Fraceto, L.F.; Amorim, M.J.B.; Scott-Fordsmand, J.J.; Schoonjans, R.; Chaudhry, Q. Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. Journal of Hazardous Materials 2021, 404, 124148. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C.; et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nature Nanotechnology 2022, 17, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gao, Y.; Tang, G.; Tian, Y.; Li, Y.; Wang, H.; Li, X.; Yu, X.; Zhang, Z.; Li, Y.; et al. Facile preparation of pH/pectinase responsive microcapsules based on CaCO3 using fungicidal ionic liquid as a nucleating agent for sustainable disease. Chemical Engineering Journal 2022, 446, 137073. [Google Scholar] [CrossRef]
- Wang, H.; Tang, G.; Zhou, Z.; Chen, X.; Liu, Y.; Yan, G.; Zhang, X.; Li, X.; Huang, Y.; Wang, J.; et al. Stable Fluorescent Nanoparticles Based on Co-assembly of Acifluorfen and Poly(salicylic acid) for Enhancing Herbicidal Activity and Reducing Environmental Risks. Acs Applied Materials & Interfaces 2023, 15, 4303–4314. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current Challenges and Trends in the Discovery of Agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, Y.; Zhou, Z.; Tang, J.; Tang, G.; Niu, J.; Chen, X.; Tian, Y.; Li, Y.; Cao, Y. A simple and green preparation process for PRO@PIL-PHS-PEC microcapsules by using phosphonium ionic liquid as a multifunctional additive. Chemical Engineering Journal 2021, 424, 130371. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; Van Zwieten, L.; Vinu, A. Nanoporous materials for pesticide formulation and delivery in the agricultural sector. Journal of Controlled Release 2022, 343, 187–206. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nature Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Mattos, B.D.; Tardy, B.L.; Magalhaes, W.L.E.; Rojas, O.J. Controlled release for crop and wood protection: Recent progress toward sustainable and safe nanostructured biocidal systems. Journal of Controlled Release 2017, 262, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yu, N.; Jiang, X.; Han, Z.; Wang, S.; Zhang, X.; Wei, S.; Giesy, J.P.; Yu, H. Influence of blooms of phytoplankton on concentrations of hydrophobic organic chemicals in sediments and snails in a hyper-eutrophic, freshwater lake. Water Research 2017, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Armetta, F.; Spinella, A.; Chillura Martino, D.F.; Alduina, R.; Saladino, M.L. Formulation of Mesoporous Silica Nanoparticles for Controlled Release of Antimicrobials for Stone Preventive Conservation. Frontiers in Chemistry 2020, 8, 699. [Google Scholar] [CrossRef]
- Pang, L.; Gao, Z.D.; Feng, H.J.; Wang, S.Y.; Wang, Q.Y. Cellulose based materials for controlled release formulations of agrochemicals: A review of modifications and applications. Journal of Controlled Release 2019, 316, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development Strategies and Prospects of Nano-based Smart Pesticide Formulation. Journal of Agricultural and Food Chemistry 2018, 66, 6504–6512. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Hoffmann, N.; Tortella, G.; Ruiz, A.; Cornejo, P.; Diez, M.C.; Seabra, A.B.; Benavides-Mendoza, A.; Rubilar, O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials 2023, 13, 1978. [Google Scholar] [CrossRef]
- Nong, W.; Wu, J.; Ghiladi, R.A.; Guan, Y. The structural appeal of metal-organic frameworks in antimicrobial applications. Coordination Chemistry Reviews 2021, 442, 214007. [Google Scholar] [CrossRef]
- Hou, R.; Zhou, J.; Song, Z.; Zhang, N.; Huang, S.; Kaziem, A.E.; Zhao, C.; Zhang, Z. pH-responsive λ-cyhalothrin nanopesticides for effective pest control and reduced toxicity to Harmonia axyridis. Carbohydrate Polymers 2023, 302, 120373. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Chu, X.; Feng, W.; Li, J.; Huang, X.; Zhou, N.; Shen, J. A new temperature-responsive controlled-release pesticide formulation - poly(N-isopropylacrylamide) modified graphene oxide as the nanocarrier for lambda-cyhalothrin delivery and their application in pesticide transportation. Colloids and Surfaces a-Physicochemical and Engineering Aspects 2021, 612, 125987. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Qin, X.; Guo, Z.; Li, D.; Li, C.; Wan, H.; Zhu, F.; Li, J.; Zhang, Z.; et al. Dual stimuli-responsive fungicide carrier based on hollow mesoporous silica/hydroxypropyl cellulose hybrid nanoparticles. Journal of Hazardous Materials 2021, 414, 125513. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patriaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. Journal of Hazardous Materials 2020, 385, 121525. [Google Scholar] [CrossRef]
- Sharma, S.; Sahu, B.K.; Cao, L.; Bindra, P.; Kaur, K.; Chandel, M.; Koratkar, N.; Huang, Q.; Shanmugam, V. Porous nanomaterials: Main vein of agricultural nanotechnology. Progress in Materials Science 2021, 121, 100812. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environmental Science-Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Yan, S.; Gu, N.; Peng, M.; Jiang, Q.; Liu, E.; Li, Z.; Yin, M.; Shen, J.; Du, X.; Dong, M. A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies. Nanomaterials 2022, 12, 2419. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, X.; Shangguan, S.; Zhao, L.; Fang, X.; Huang, Y.; Hermanowicz, S.W. Public Perceptions and Willingness-to-Pay for Nanopesticides. Nanomaterials 2022, 12, 1292. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, H.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. Coordination bonding-based polydopamine-modified mesoporous silica for sustained avermectin release. Materials Science and Engineering C-Materials for Biological Applications 2019, 105, 110073. [Google Scholar] [CrossRef]
- Xu, C.; Shan, Y.; Bilal, M.; Xu, B.; Cao, L.; Huang, Q. Copper ions chelated mesoporous silica nanoparticles via dopamine chemistry for controlled pesticide release regulated by coordination bonding. Chemical Engineering Journal 2020, 395, 125093. [Google Scholar] [CrossRef]
- Kaziem, A.E.; Yang, L.P.; Lin, Y.G.; Song, Z.X.; Xu, H.H.; Zhang, Z.X. Efficiency of mesoporous silica/carboxymethyl β-glucan as a fungicide nano-delivery system for improving chlorothalonil bioactivity and reduce biotoxicity. Chemosphere 2022, 287, 131902. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Cao, C.; Zhang, J.; Li, F.; Huang, Q. Quaternized Chitosan-Capped Mesoporous Silica Nanoparticles as Nanocarriers for Controlled Pesticide Release. Nanomaterials 2016, 6, 126. [Google Scholar] [CrossRef]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery - current status and perspective of MSNs drug carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, X.; Cao, L.; Cao, C.; Li, F.; Chen, C.; Xu, C.; Huang, Q.; Du, F. Uptake and Distribution of Fenoxanil-Loaded Mesoporous Silica Nanoparticles in Rice Plants. International Journal of Molecular Sciences 2018, 19, 2854. [Google Scholar] [CrossRef]
- Wang, Y.; Dimkpa, C.; Deng, C.; Elmer, W.H.; Gardea-Torresdey, J.; White, J.C. Impact of engineered nanomaterials on rice (Oryza sativa L.): A critical review of current knowledge. Environmental Pollution 2022, 297, 118738. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ming, H.; Shanshan, C.; Anqun, H.; Shimao, L.; Haiya, H.; Guangtao, L.; Lisha, Z.; Jianuan, Z. Enterobacter asburiae and Pantoea ananatis causing rice bacterial blight in China. Plant Disease 2021, 105, 2078–2088. [Google Scholar] [CrossRef]
- Agulló, L.; Romero-Silva, M.J.; Domenech, M.; Seeger, M. p-Cymene Promotes Its Catabolism through the p-Cymene and the p-Cumate Pathways, Activates a Stress Response and Reduces the Biofilm Formation in Burkholderia xenovorans LB400. PLoS ONE 2017, 12, e0169544. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Sokeng, A.J.T.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Xu-Liang, C.; Sparling, M.; Dabeka, R. p-Cymene, a natural antioxidant, in Canadian total diet foods: Occurrence and dietary exposures. Journal of the Science of Food and Agriculture 2019, 99, 5606–5609. [Google Scholar] [CrossRef]
- Cao, X.L.; Sparling, M.; Dabeka, R. p-Cymene, a natural antioxidant, in Canadian total diet foods: Occurrence and dietary exposures. Journal of the Science of Food and Agriculture 2019, 99, 5606–5609. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Zhou, Z.; Xu, C.; Shan, Y.; Lin, Y.; Huang, Q. Fluorophore-free luminescent double-shelled hollow mesoporous silica nanoparticles as pesticide delivery vehicles. Nanoscale 2018, 10, 20354–20365. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Li, H.; Qu, X.; Yang, S.-T.; Chang, X.-L. Bioaccumulation and Toxicity of 13C-Skeleton Labeled Graphene Oxide in Wheat. Environmental Science & Technology 2017, 51, 10146–10153. [Google Scholar] [CrossRef]
- Wang, A.; Cui, J.; Wang, Y.; Zhu, H.; Li, N.; Wang, C.; Shen, Y.; Liu, P.; Cui, B.; Sun, C.; et al. Preparation and characterization of a novel controlled-release nano-delivery system loaded with pyraclostrobin via high-pressure homogenization. Pest Management Science 2020, 76, 2829–2837. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Y.; Mao, K.; Qin, X.; Zhang, Y.; Li, D.; Zhang, Y.; Li, J.; Wan, H.; He, S. Thermoresponsive polymer-encapsulated hollow mesoporous silica nanoparticles and their application in insecticide delivery. Chemical Engineering Journal 2020, 383, 123169. [Google Scholar] [CrossRef]
- Ding, G.; Li, D.; Liu, Y.; Guo, M.; Duan, Y.; Li, J.; Cao, Y. Preparation and characterization of kasuga-silica-conjugated nanospheres for sustained antimicrobial activity. Journal of Nanoparticle Research 2014, 16, 2671. [Google Scholar] [CrossRef]
- Qian, K.; Shi, T.; He, S.; Luo, L.; Liu, X.; Cao, Y. Release kinetics of tebuconazole from porous hollow silica nanospheres prepared by miniemulsion method. Microporous And Mesoporous Materials 2013, 169, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Li, L.; Li, W.; Yang, C. Dual Functional N-Doped TiO2-Carbon Composite Fibers for Efficient Removal of Water Pollutants. Acs Sustainable Chemistry & Engineering 2018, 6, 12893–12905. [Google Scholar] [CrossRef]
- Zhang, W.; He, S.; Liu, Y.; Geng, Q.; Ding, G.; Guo, M.; Deng, Y.; Zhu, J.; Li, J.; Cao, Y. Preparation and Characterization of Novel Functionalized Prochloraz Microcapsules Using Silica-Alginate-Elements as Controlled Release Carrier Materials. Acs Applied Materials & Interfaces 2014, 6, 11783–11790. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).