1. INTRODUCTION
The history of oil operations in Ogoniland is indeed significant. In 1957, Shell discovered oil in Bomu, Ogoniland, and subsequently proceeded to extract oil from 96 oil wells, establishing 9 oil fields in the region. This extraction continued from 1958 until late 1993 when protests by the Ogoni people against Shell's practices led to the company ceasing its operations in Ogoniland. Despite Shell's presence in Ogoniland for 35 years, the oil production from this region accounted for only a small fraction, approximately 3%, of their overall production. However, during the period of 1976 to 1991, there were approximately 3,000 oil spills reported in Ogoniland alone. These spills have had a detrimental impact on the environment, affecting the land, water, and livelihoods of the local communities. Addressing the environmental consequences of these oil spills and working towards the remediation and restoration of Ogoniland is crucial for the well-being of the affected communities. By conducting research and implementing innovative solutions like bio-remediation, we hope to contribute to the healing and recovery of Ogoniland, fostering a sustainable and resilient future for the region.
The high number of oil spills in Ogoniland is indeed alarming. In the 15-year period from 1976 to 1991, Ogoniland alone accounted for approximately 3,000 oil spills, which represents a staggering 40% of the total spills by Shell in its global operations. It is important to note that these spills occurred within an area of approximately 1,050 square kilometers. This concentration of spills within such a relatively small region highlights the significant environmental impact and challenges faced by the communities in Ogoniland. Addressing these environmental issues and promoting sustainable practices is crucial for the well-being and future of the affected communities.
"In response to the Ogoni people's court case against the Shell petroleum development company (SPDC) in the Ogoni community, there has been an increase in efforts to find effective technologies for soil remediation. The oil spillage has resulted in the introduction of foreign substances such as olefins, bituminous materials, heavy metals, and other Sulphuric compounds into the soil."The contamination of the soil in Ogoni land has had detrimental effects on farming activities, rendering it unsuitable for agricultural purposes. This issue has also been exacerbated by erosion, which has further spread the contamination and made the soil unusable. If prompt action is not taken, the consequences will be severe: the land in Ogoni will remain infertile for an estimated period of 500 years, if not indefinitely. This poses a significant threat to not only the current Ogoni population but also their future generations, their economy, and the sustainable use of their natural resources.
Successfully achieving the aim and objectives of this study will provide valuable information to institutions regarding the optimization of bio-remedial activity using bitter-leaf and other bio-remediation. This knowledge will bring hope to not only the Ogoni people but also to other areas grappling with polluted soils. Moreover, it will stimulate further research and development in this field by contributing to the existing knowledge base. By advancing our understanding of bio-remediation, we can work towards creating a cleaner and healthier environment for all.
Eco-Friendly Innovation: Harnessing the Remarkable Bio-Remediation Potential of Vernonia spp. for Sustainable Restoration of Hydrocarbon-Polluted Clay Soil in Ogoni Land, Nigeria. Alignment with UN SDGs:
Goal 3: Good Health and Well-being: By utilizing Vernonia spp. for bio-remediation, the research contributes to improving the health and well-being of the communities in Ogoni Land by reducing the harmful effects of hydrocarbon pollution on the environment and human health.
Goal 6: Clean Water and Sanitation: Restoring hydrocarbon-polluted clay soil through bio-remediation helps protect water sources from contamination, ensuring clean and sustainable water supply for the community.
Goal 9: Industry, Innovation, and Infrastructure: The research presents an eco-friendly innovation that harnesses the bio-remediation potential of Vernonia spp., providing a sustainable solution for restoring polluted soil and promoting sustainable practices in the industry.
Goal 13: Climate Action: By addressing hydrocarbon pollution and restoring the clay soil, the research contributes to climate action by mitigating the negative environmental impacts and promoting sustainable land use practices.
Goal 15: Life on Land: The research focuses on restoring the clay soil in Ogoni Land, which is essential for preserving biodiversity, promoting sustainable agriculture, and ensuring the long-term health of ecosystems in the area. Significance:
The research holds significant importance in several ways:
Environmental Restoration: By harnessing the bio-remediation potential of Vernonia spp., the research offers a sustainable and eco-friendly approach to restoring hydrocarbon-polluted clay soil. This has long-term benefits for the environment and ecosystems in Ogoni Land.
Health and Well-being: The restoration of hydrocarbon-polluted soil helps protect human health by reducing the exposure to harmful pollutants and toxins. This contributes to the overall well-being and quality of life for the communities living in the affected area.
Sustainable Development: The research aligns with the principles of sustainable development by providing a solution that balances environmental, social, and economic aspects. It promotes sustainable land use practices and contributes to the achievement of multiple UN SDGs.
Community Empowerment: Implementing bio-remediation techniques using Vernonia spp. can create opportunities for local communities to actively participate in the restoration process, fostering community engagement, and promoting sustainable development at the grassroots level. In summary, the research on harnessing the bio-remediation potential of Vernonia spp. for restoring hydrocarbon-polluted clay soil in Ogoni Land, Nigeria, aligns with various UN SDGs. It carries significant implications for environmental restoration, human health, sustainable development, and community empowerment.
2. LABORATORY ANALYSIS:
2.1. Clay Soil Bio Remedial Analysis
The study involved collecting clay soil samples and mixing them with bonny light crude oil to simulate the conditions found in the study area. We then measured the changes in pH, hydrocarbonbon content, and metal levels by comparing the initial and final values before and after the addition of crude oil.
Table 1 provides a record of the initial and final conditions of these factors
To conduct the experiment, we will be using a total of 30 batch reactors. These reactors will enable us to make various observations throughout the experiment.
2.1. pH Analysis
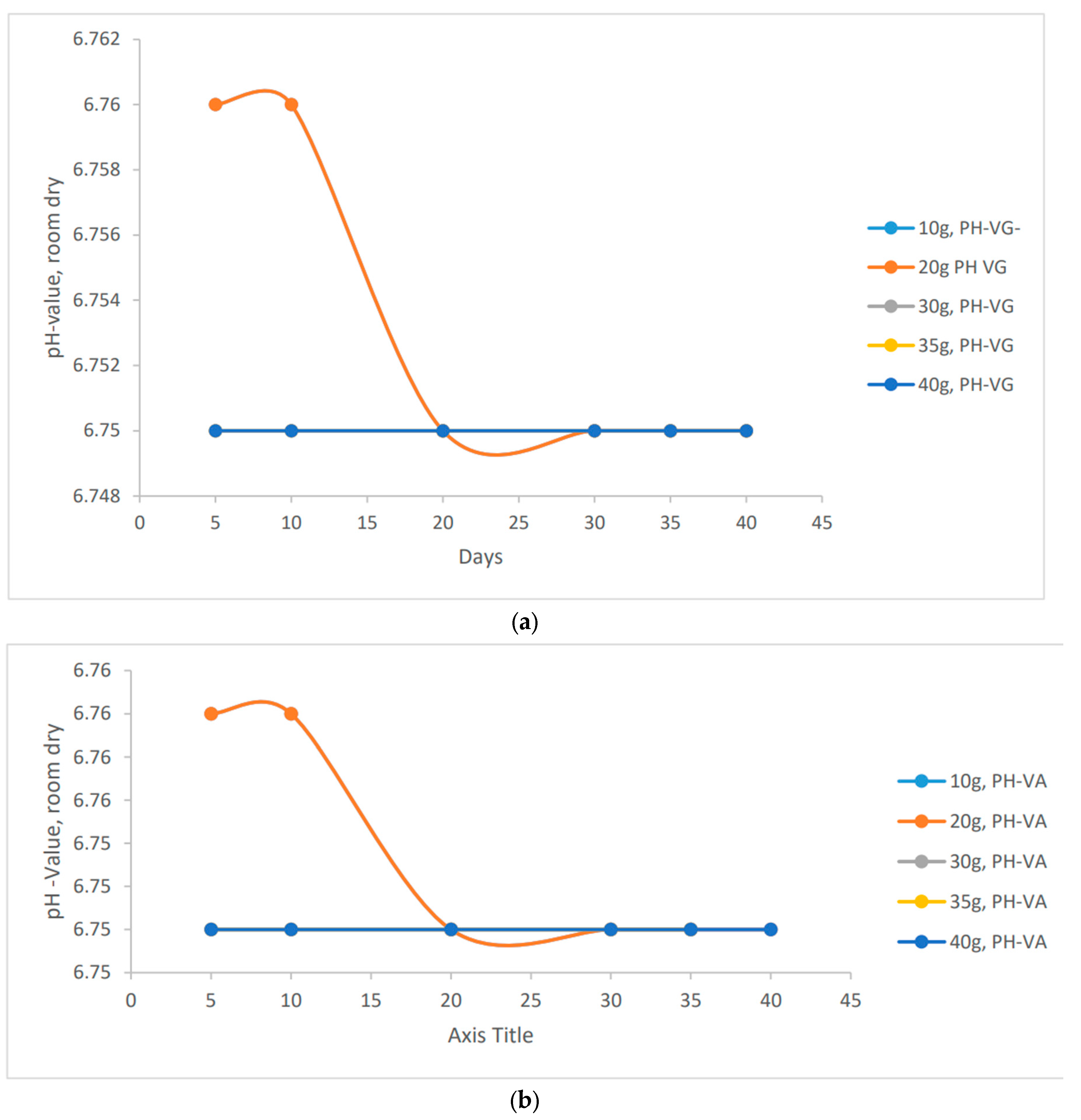
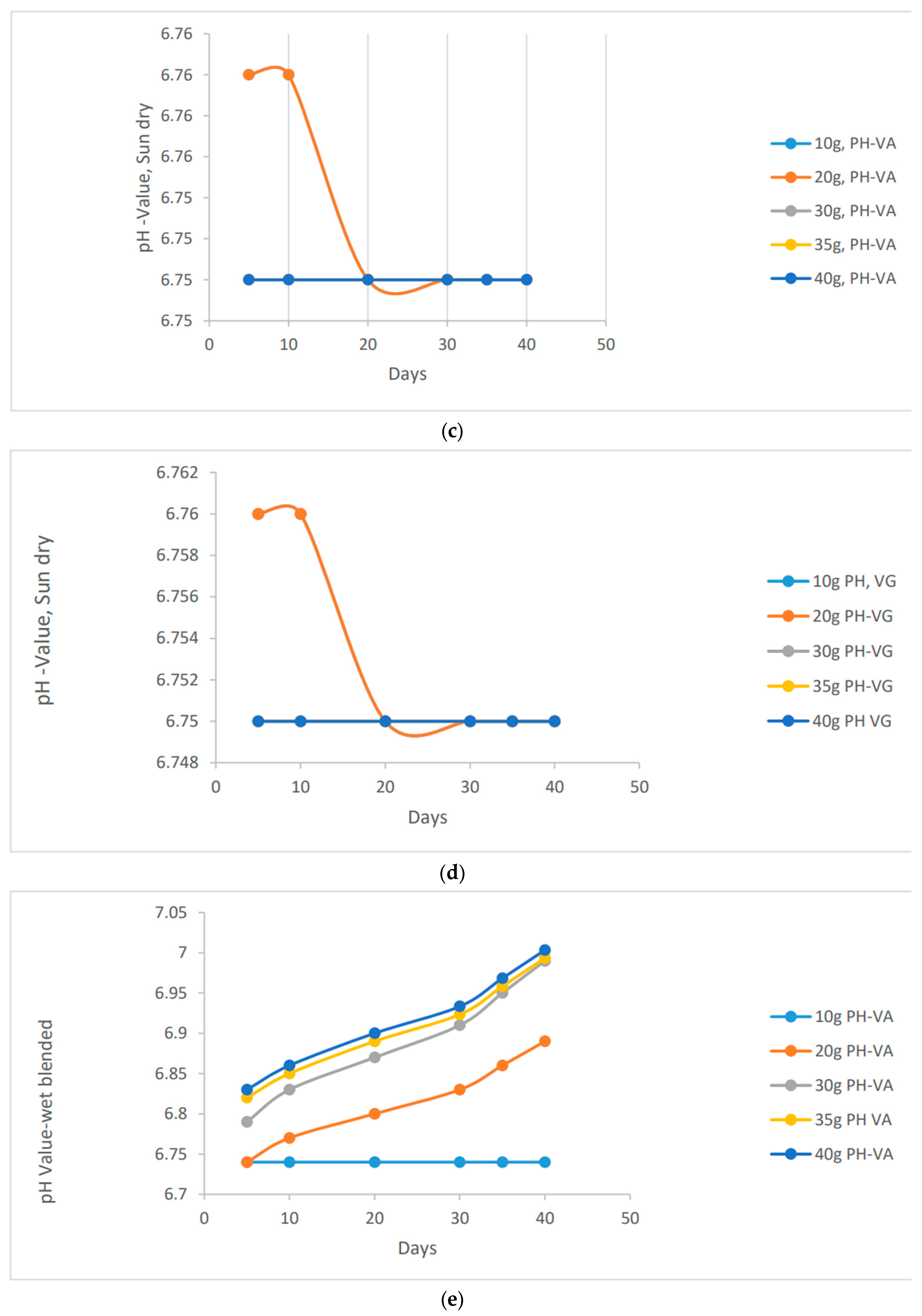
"To assess the stability or instability of pH in clay soil, it is necessary to analyze each scenario and determine whether the varying quantities of species have an impact on the pH value. This analysis aims to investigate how different masses of species may influence the stability of pH in the soil.
Figure 1.
a, b, c, d, e, f: pH behavioural characteristics of the vernonia extracts using different application methods in clay soil.
Figure 1.
a, b, c, d, e, f: pH behavioural characteristics of the vernonia extracts using different application methods in clay soil.
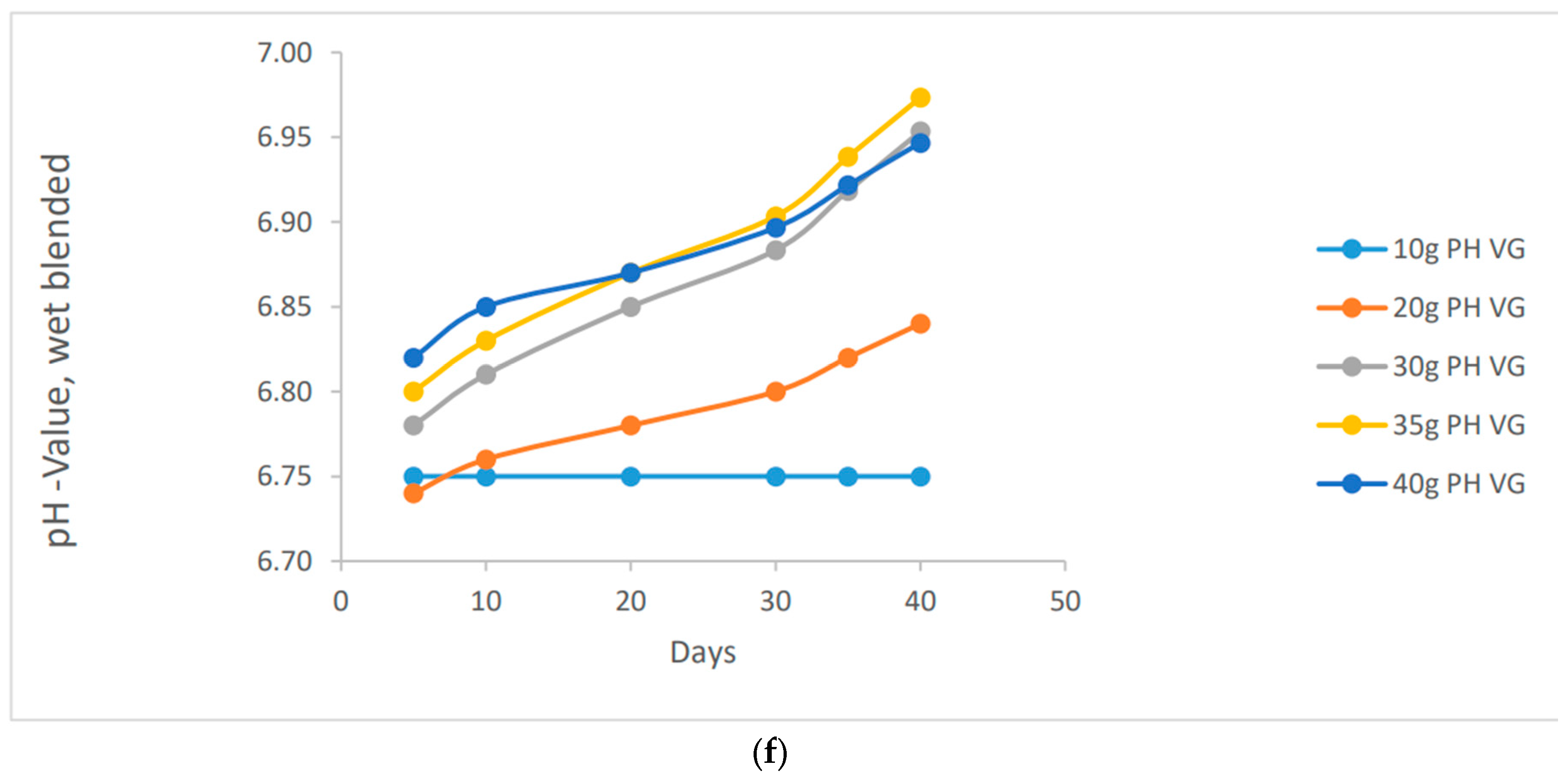
It's fascinating to observe the stability of pH values in various methods of preparation. In contrast to the other methods, the wet blended vernonia species showed an interesting trend. As the wet blended vernonia species interacted with the soil, it effectively remediated the excess metals present. Consequently, the pH gradually increased, transitioning from acidic to normal to alkaline. Specifically, after 40 days, the pH of the clay soil reached a value of 6.97 for 40 grams of vernonia Galamensis and 7.00 for vernonia amygdalina. This indicates the positive impact of these vernonia species in optimizing soil conditions.
"Increasing the amount of vernonia species in the clay soil will gradually raise its alkalinity
2.1.2. HC analysis
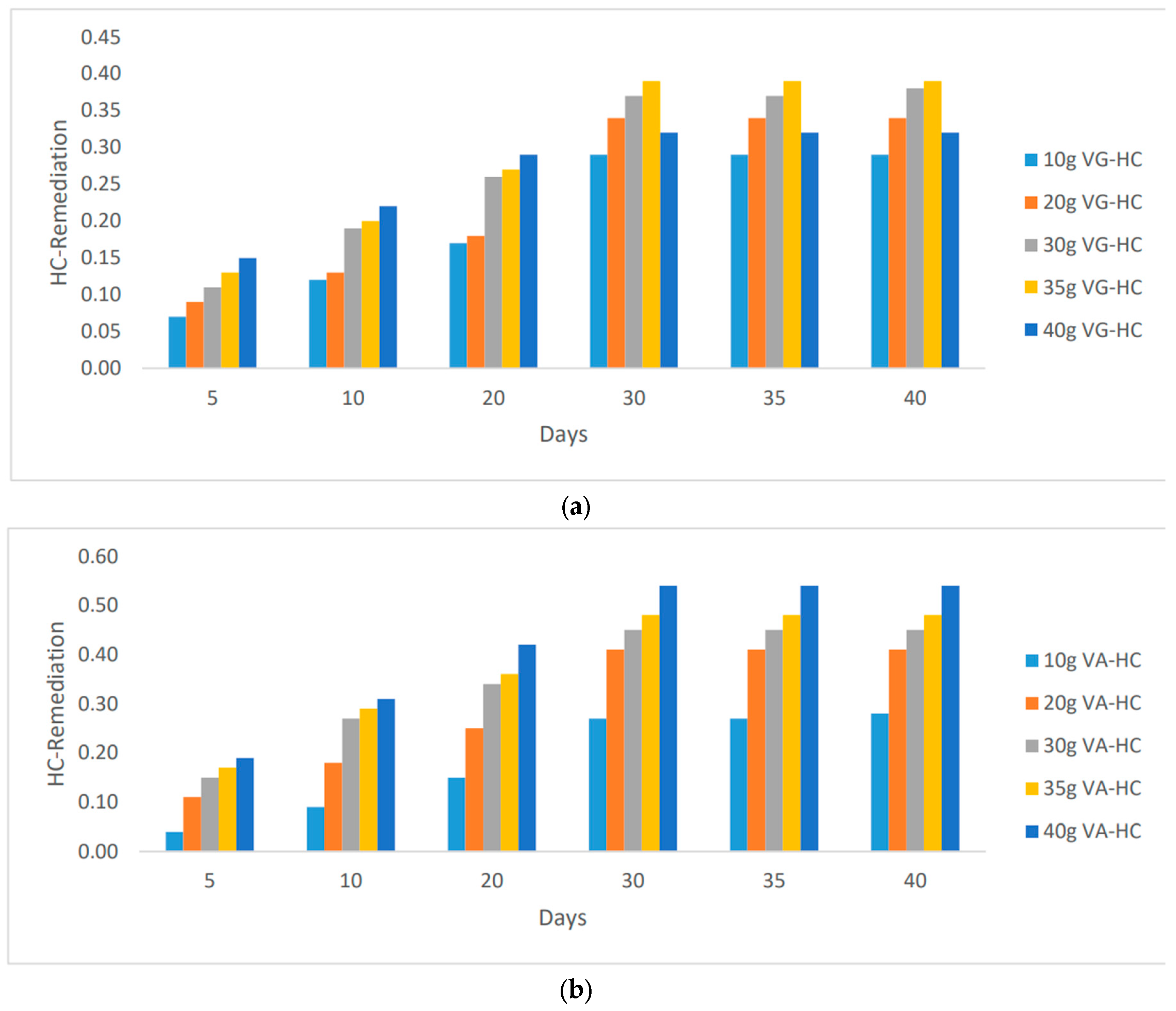
"Furthermore, there was a noticeable decrease in hydrocarbon content, indicating successful remediation, as the mass of vernonia species increased. However, it is worth noting that the remediation effects reached a plateau and remained constant after the 35th day. Specifically, while the addition of more mass led to a decrease in HC remediation for vernonia Galamensis, vernonia Amygdalina continued to exhibit an increase. These observations are illustrated in
Figure 2, which demonstrates the limiting values of hydrocarbon content remediation.""The most significant remediation values were achieved using 35g and 40g of vernonia Galamensis and vernonia Amygdalina, respectively. This emphasizes the importance of determining the most effective approach to maximize the remediation effects. Let's explore further to identify the optimal strategy.
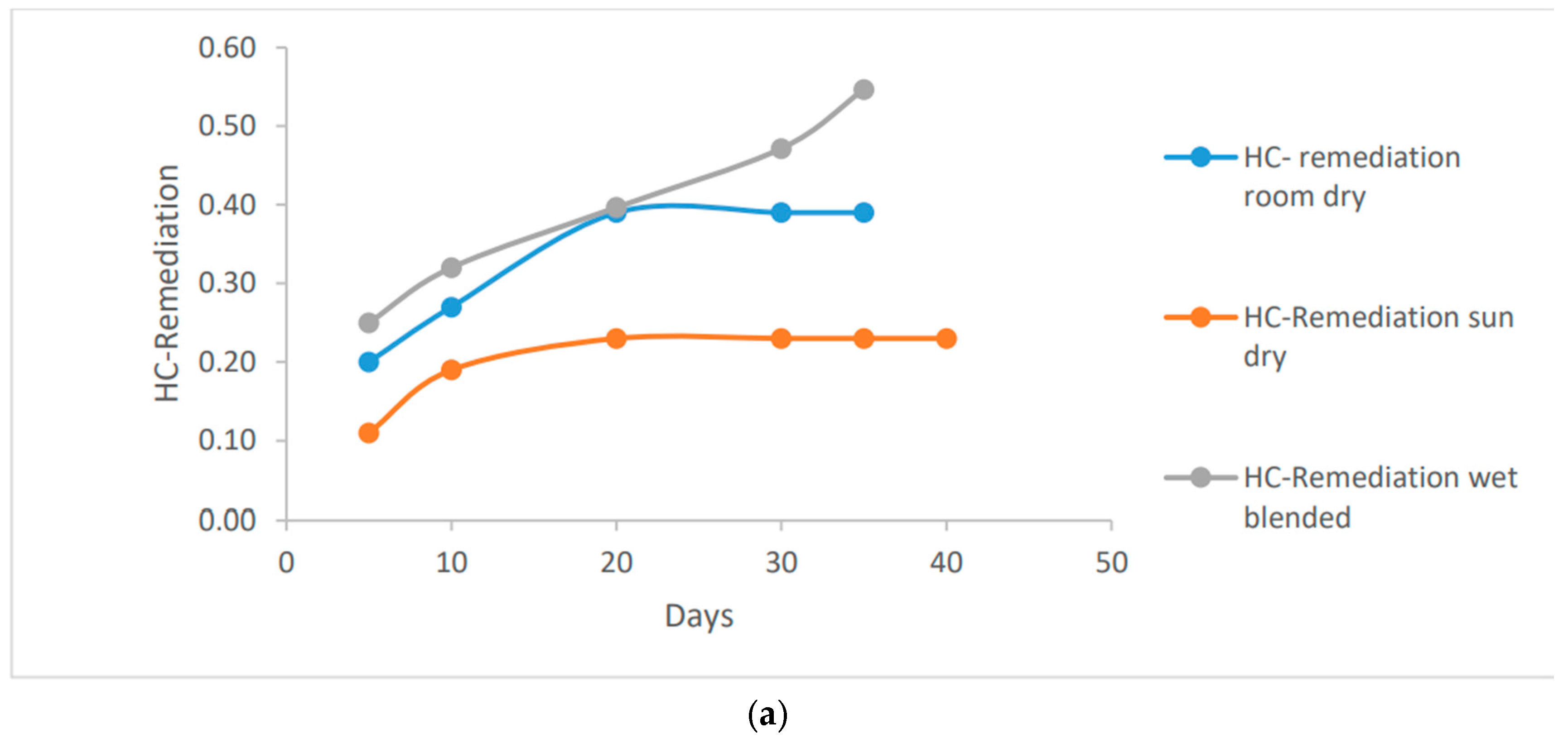
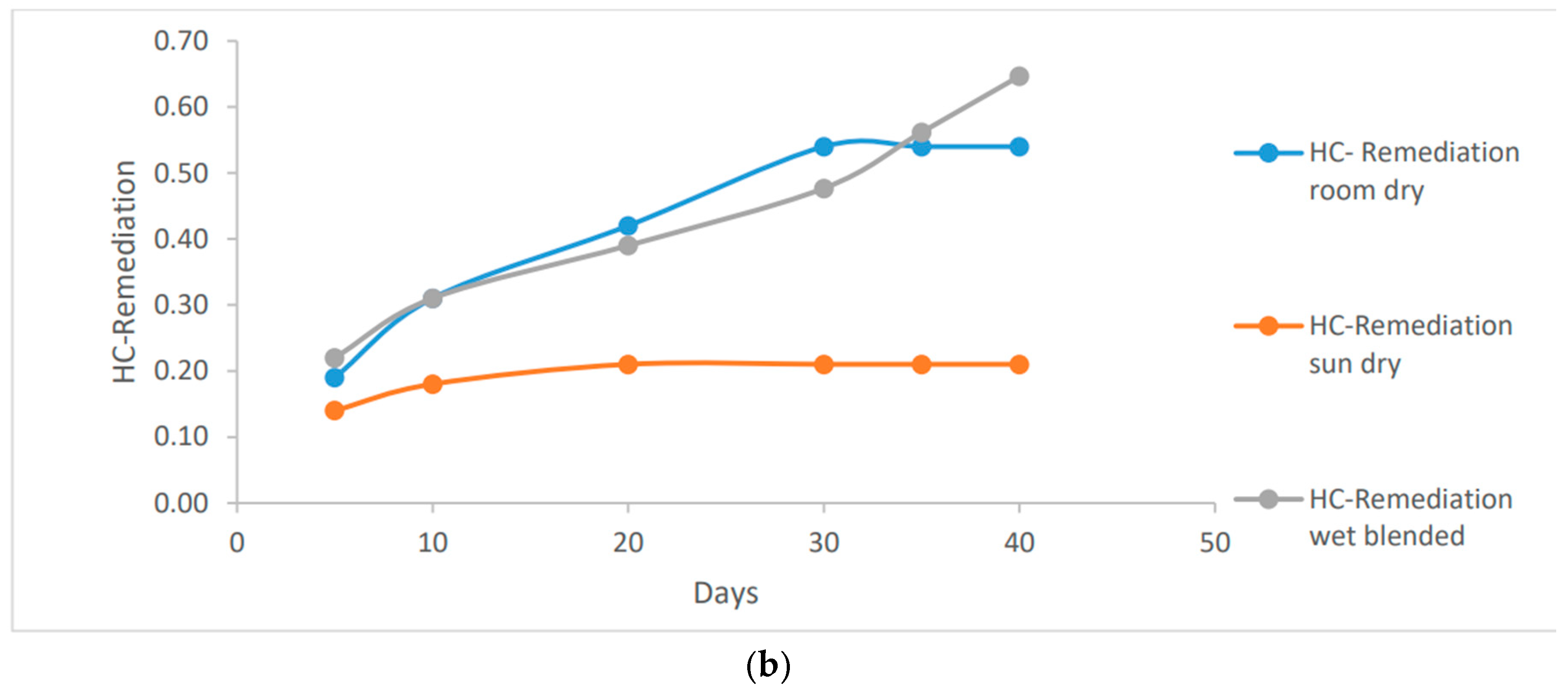
Based on the data presented in
Figure 3, it is evident that both room dry and wet blended methods were highly effective in bio-remediating the hydrocarbon content in the clay soil. The use of Vernonia Galamensis resulted in a significant reduction of up to 0.55 ug/ml, while Vernonia Amygdalina achieved an even higher reduction of up to 0.67 ug/ml. These impressive results were observed at 35g and 40g respectively on the wet blended basis.
2.1.3. Metal Analysis
The increase in pH value towards the alkaline state in the clay soil can be attributed to the reduction of metals present. Hence, it is important to evaluate the potential of metal reduction in the soil. The data analysis indicates that a higher mass of vernonia species leads to a greater remediation of metals in the clay soil. This suggests that increasing the mass of vernonia species can enhance the effectiveness of metal reduction in the soil
2.1.4. Pb Remediating Response
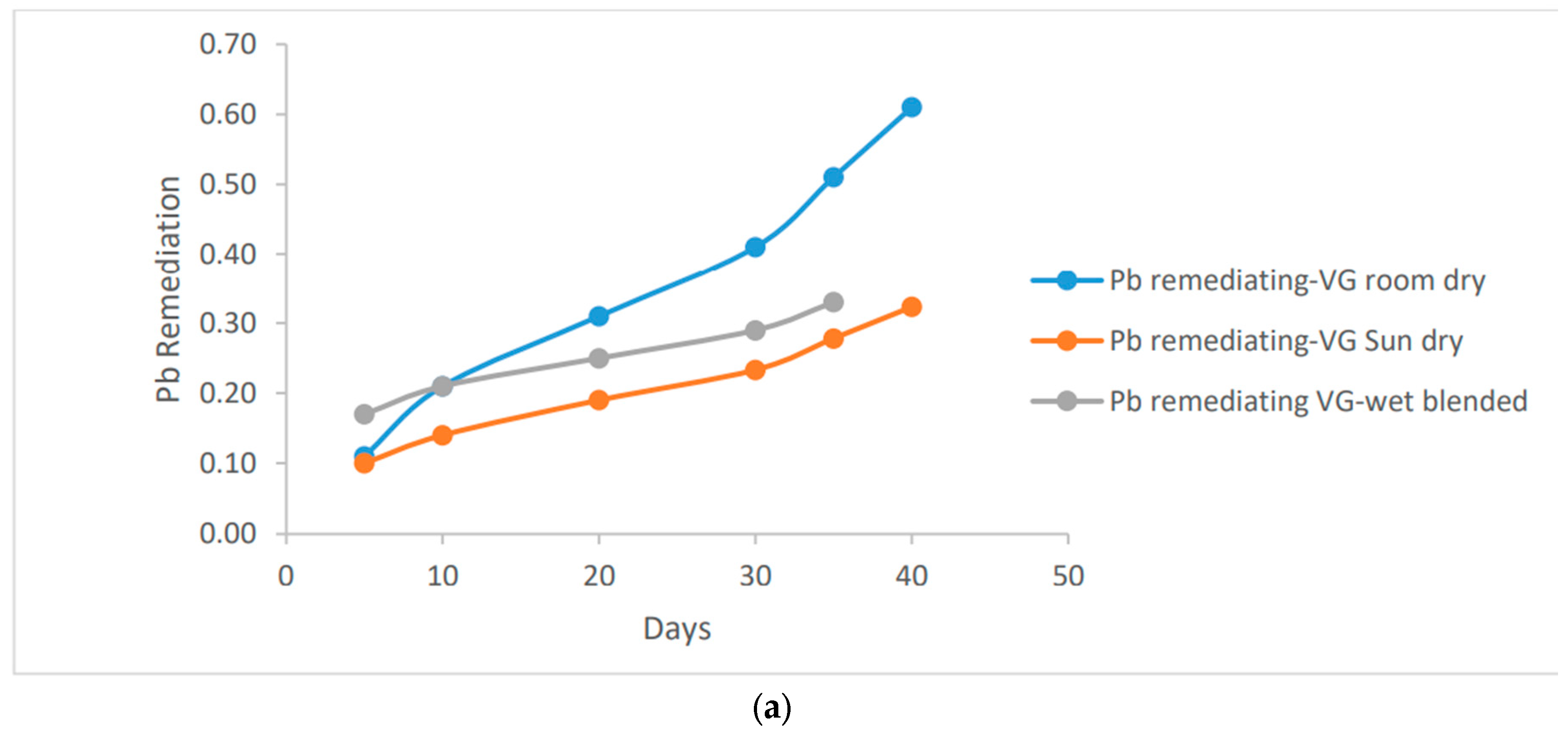
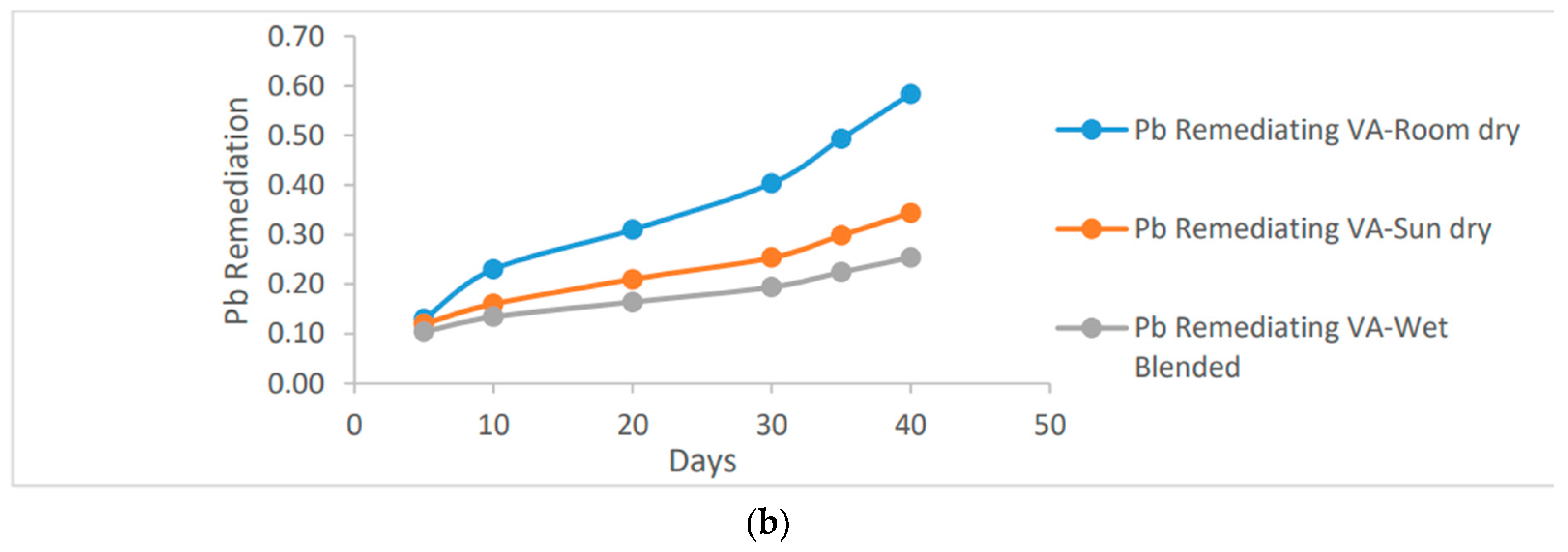
The positive impact of reducing pH concentration in the soil can be observed in the increased remediation effect. Specifically, as the mass of the vernonia species increases, there is a notable enhancement in the reduction of Pb (lead) in the soil. This indicates that a larger quantity of vernonia species contributes to a more significant reduction of lead contamination in the soil, thereby improving its overall quality.
Figure 4 demonstrates that the room dried vernonia species exhibit higher Pb remediation activity compared to the wet basis vernonia species. This difference can be attributed to the reduced activity of micro-organisms and Phytochemicals responsible for Pb remediation under wet conditions. Both species of vernonia leaf, however, achieved approximately 0.60 ug/ml of Pb remediation, indicating that they are equally effective in reducing Pb contamination in the soil.
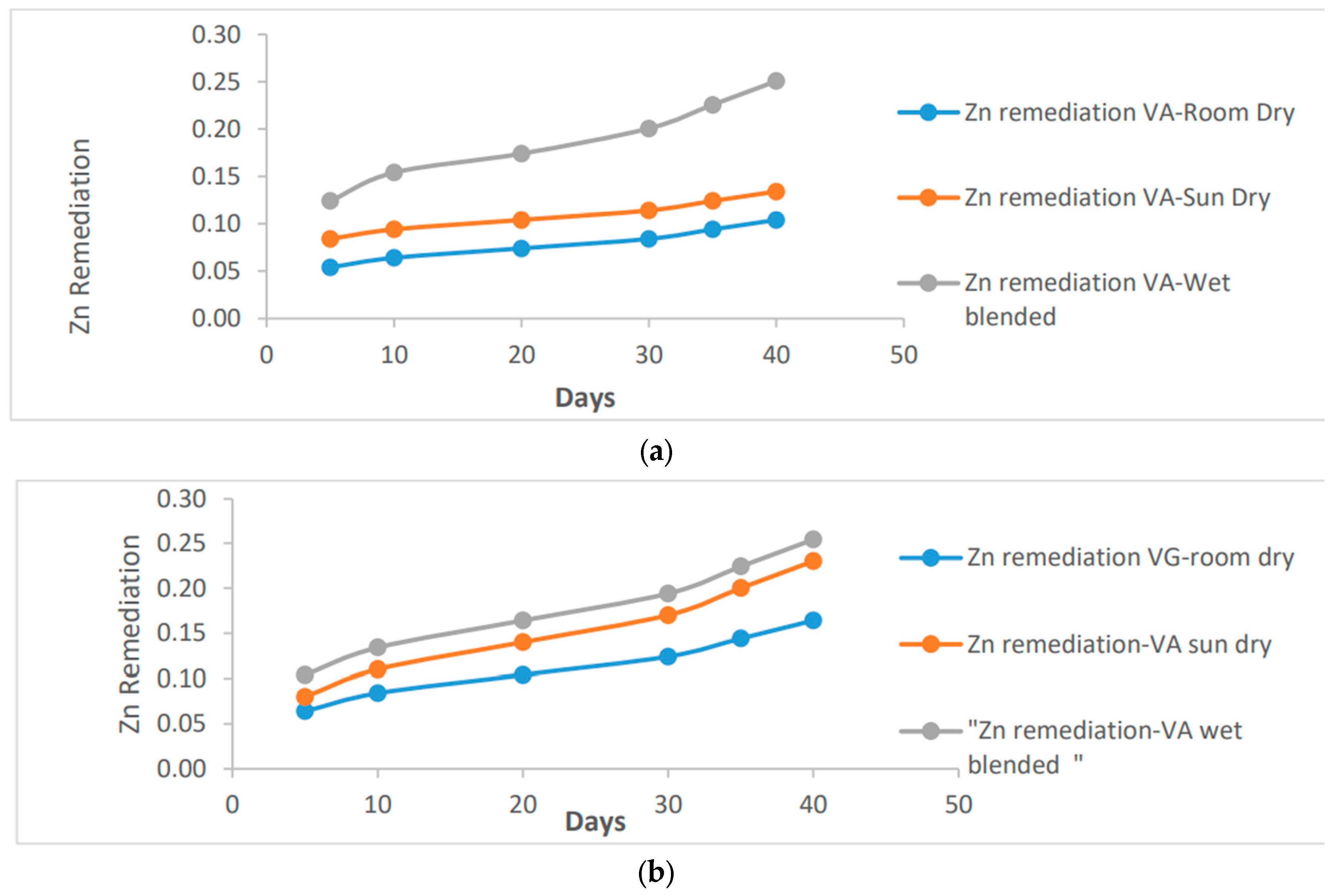
1.5. Zn Remediating Response
Similarly to Pb remediation, the presence of Zn in the clay soil can also be effectively remediated and reduced.
Figure 5 highlights that the remediating effects on Zn, compared to other metals, are relatively lower. Approximately 0.25 ug/ml of Zn was successfully remediated, primarily attributed to the wet blended preparation of vernonia species. On the other hand, the room dried vernonia species exhibited the lowest performance in soil remediation for Zn, removing only about 0.17 ug/ml and 0.10 ug/ml for Galamensis and Amygdalina, respectively.
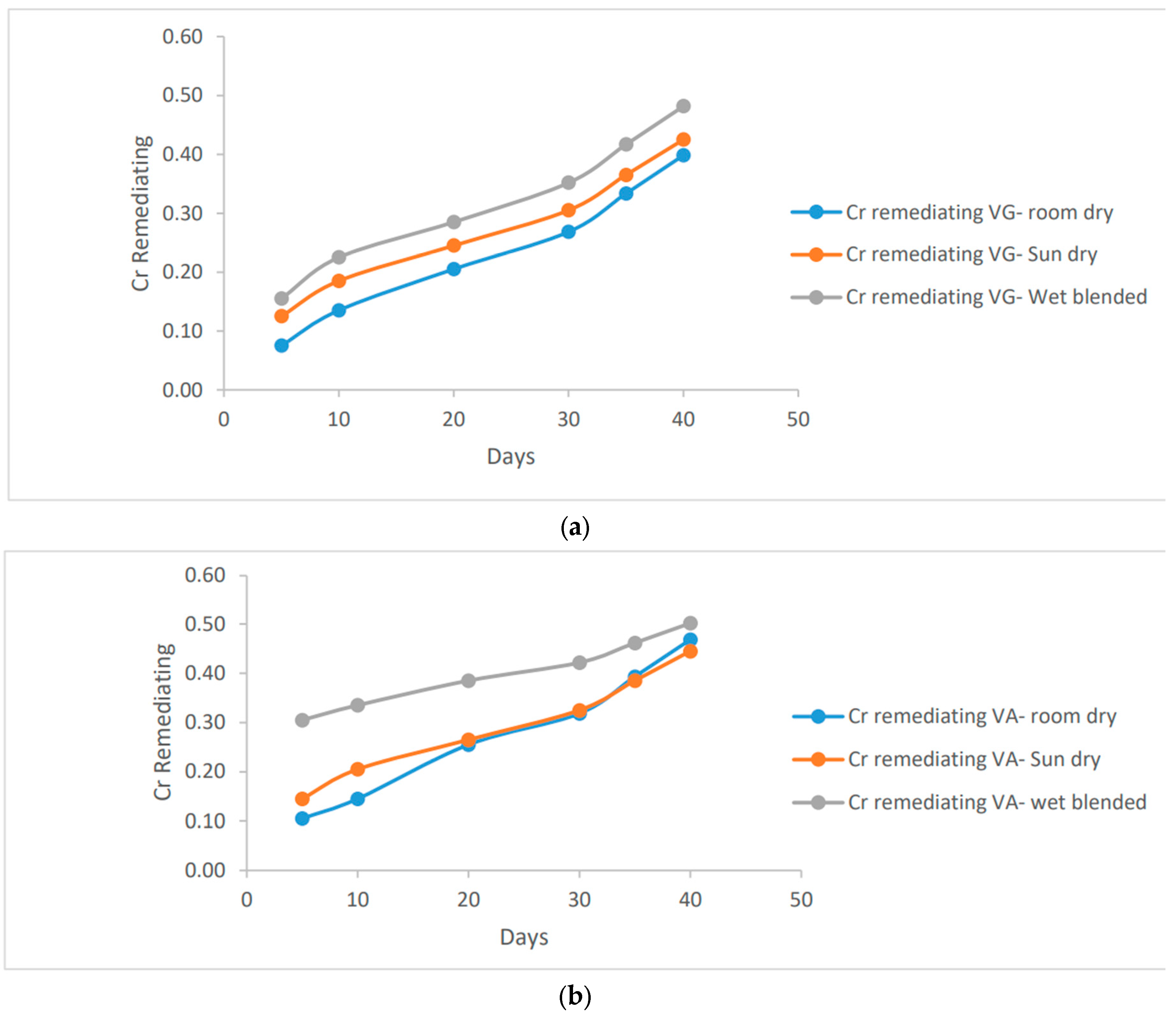
2.1.6. Cr remediating Response
When it comes to Chromium (Cr) remediation, the wet blended method exhibits slightly higher potential compared to other preparation methods. Although the difference is minimal, a remediation of 0.5 ug/ml of chromium was achieved using both forms of vernonia leaf extracts. The sun dry and room dry methods also demonstrated significant remediation potential, with values surpassing 0.4 ug/ml. Overall, all the methods show promise in effectively remediating chromium from the soil.
Figure 6.
a, b: Cr remediation method comparism using vernonia Galamensis and vernonia Amygdalina in clay soil.
Figure 6.
a, b: Cr remediation method comparism using vernonia Galamensis and vernonia Amygdalina in clay soil.
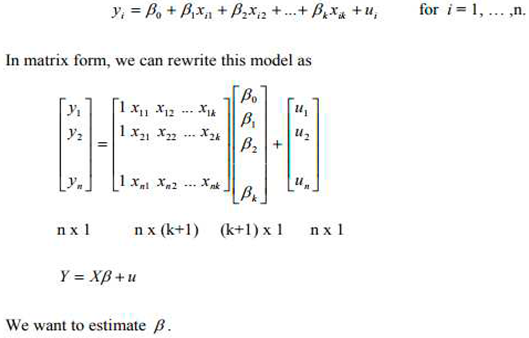
2.2 Developing a model to illustrate the individual remedial activity based on the data generated is a great idea. In this case, the measurable responses from the remediation process would be the concentrations of metals and hydrocarbon contents in the soil. The independent factors to consider would include the grams of Vernonia species applied and the duration of the remediation process (in days). Given that the wet blended method showed more promising remediation effects, you can focus on incorporating this approach into the model. By analyzing the data and considering the relationships between the independent factors (grams of Vernonia species and days taken) and the measurable responses (metal and hydrocarbon concentrations), you can develop a regression model or another appropriate statistical model to illustrate the individual remedial activity of the Vernonia extracts. Remember to consider the significance of the model, as indicated by the p-value, and the strength of the relationship, as indicated by the coefficient of determination (r2). These statistical measures will help assess the reliability and validity of the mode
Modal- Prediction Analysis
Performing a multiple regression analysis using the least square method with the Minitab software is a great approach to develop your model. By utilizing this statistical software, you can effectively analyze the relationships between the independent factors (grams of Vernonia species and days taken) and the measurable responses (metal and hydrocarbon concentrations) in the contaminated soil
2.2.1. Cr remediating Response
When it comes to the clay soil, a similar approach of conducting multiple regression analysis is performed using the Minitab software. (1) This analysis involves modelling Vernonia galamensis.
- A.
Regression Analysis: HC versus Time, Mass, pH
The regression equation is
HC = - 6.59 + 0.00538 Time + 0.00455 Mass + 0.970 pH
| Predictor |
Coef |
SE Coef |
T |
P |
| Constant |
-6.593 |
1.999 |
-3.30 |
0.003 |
| Time |
0.005381 |
0.001098 |
4.90 |
0.000 |
| Mass |
0.004554 |
0.001669 |
2.73 |
0.011 |
| PH |
0.9696 |
0.3016 |
3.22 |
0.003 |
S = 0.0422874 R-Sq = 93.5% R-Sq(adj) = 92.7%
Analysis of Variance
- B.
Regression Analysis: Pb versus Time, Mass, pH
The regression equation is
Pb = 2.96 + 0.00782 Time + 0.00324 Mass - 0.443 pH
| Predictor |
Coef |
SE Coef |
T |
P |
| Constant |
2.962 |
1.272 |
2.33 |
0.028 |
| Time |
0.0078210 |
0.0006984 |
11.20 |
0.000 |
| Mass |
0.003239 |
0.001061 |
3.05 |
0.005 |
| PH |
-0.4431 |
0.1918 |
-2.31 |
0.029 |
Analysis of Variance
S = 0.0268981 R-Sq = 91.9% R-Sq (adj) = 91.0%
- C.
Regression Analysis: Zn versus Time, Mass, pH
The regression equation is
Zn = - 2.67 + 0.00210 Time + 0.00169 Mass + 0.396 pH
| Predictor |
Coef |
SE Coef |
T |
P |
| Constant |
-2.6655 |
0.4158 |
-6.41 |
0.000 |
| Time |
0. 002099 |
0.0002283 |
9.20 |
0.000 |
| Mass |
0.0016856 |
0.0003470 |
4.86 |
0.000 |
| PH |
0.39558 |
0.06271 |
6.31 |
0.000 |
S = 0.0268981 R-Sq = 91.9% R-Sq(adj) = 91.0%
Analysis of Variance
- D.
Regression Analysis: Cr versus Time, Mass, pH
The regression equation is
Cr = 1.71 + 0.00830 Time + 0.00608 Mass - 0.271 pH
| Predictor |
Coef |
SE Coef |
T |
P |
| Constant |
1.713 |
1.689 |
1.01 |
0.320 |
| Time |
0.008298 |
0.0009276 |
8.95 |
0.000 |
| Mass |
0.006083 |
0.001410 |
4.31 |
0.000 |
| PH |
-0.2708 |
0.2548 |
-1.06 |
0.298 |
S = 0.0357295 R-Sq = 91.4% R-Sq(adj) = 90.4%
Analysis of Variance
- 2.
Vernonia amygdalina Modelling
- A.
Regression Analysis: HC_1 versus Time_1, Mass_1, pH_1
The regression equation is
HC_1 = - 13.8 + 0.00243 Time_1 - 0.00250 Mass_1 + 2.06 pH_1
| Predictor |
Coef |
SE Coef |
T |
P |
| Constant |
-13.772 |
1.484 |
-9.28 |
0.000 |
| Time_1 |
0.0024258 |
0.0009678 |
2.51 |
0.019 |
| Mass_1 |
-0.002502 |
0.001491 |
-1.68 |
0.105 |
| pH_1 |
2.0617 |
0.2248 |
9.17 |
0.000 |
S = 0.0345595 R-Sq = 96.8% R-Sq(adj) = 96.5%
Analysis of Variance
- B.
Regression Analysis: Pb_1 versus Time_1, Mass_1, pH_1
The regression equation is
Pb_1 = 3.96 + 0.00844 Time_1 + 0.00589 Mass_1 - 0.598 pH_1
| Predictor |
Coef |
SE Coef |
T |
P |
| Constant |
3.962 |
1.697 |
2.33 |
0.028 |
| Time_1 |
0.008439 |
0.001107 |
7.62 |
0.000 |
| Mass_1 |
0.005892 |
0.001705 |
3.46 |
0.002 |
| pH_1 |
-0.5983 |
0.2571 |
-2.33 |
0.028 |
S = 0.0395213 R-Sq = 84.2% R-Sq(adj) = 82.4%
Analysis of Variance
The regression equation is
Zn_1 = - 1.63 + 0.00253 Time_1 + 0.00189 Mass_1 + 0.244 pH_
| Predictor |
Coef |
SE Coef |
T |
P |
| Constant |
-1.6346 |
0.3737 |
-4.37 |
0.000 |
| Time_1 |
0.0025315 |
0.0002436 |
10.39 |
0.000 |
| Mass_1 |
0.0018940 |
0.0003753 |
5.05 |
0.000 |
| pH_1 |
0.24446 |
0.05659 |
4.32 |
0.000 |
S = 0.00870020 R-Sq = 98.1% R-Sq(adj) = 97.8%
Analysis of Variance
- C.
Regression Analysis: Cr_1 versus Time_1, Mass_1, pH_1
The regression equation is
Cr_1 = 1.80 + 0.00685 Time_1 + 0.00940 Mass_1 - 0.279 pH_1
| Predictor |
Coef |
SE Coef |
T |
P |
| Constant |
1.801 |
1.196 |
1.51 |
0.144 |
| Time_1 |
0.0068464 |
0.0007800 |
8.78 |
0.000 |
| Mass_1 |
0.009396 |
0.001202 |
7.82 |
0.000 |
| pH_1 |
-0.2788 |
0.1812 |
-1.54 |
0.136 |
S = 0.0278546 R-Sq = 94.9% R-Sq(adj) = 94.3%
Analysis of Variance
In statistical analysis, the p‐value is a crucial factor in determining the significance of a model.
It represents the probability value and is used to assess whether the observed data supports the
hypothesis being tested. In this case, for the model to be considered statistically significant, the overall
p‐value should be less than 0.05. This means that if the p‐value is below 0.05, it suggests that the
modelʹs results are unlikely to occur by chance and therefore can be accepted with confidence. On
the other hand, the r2 value, also known as the coefficient of determination, measures the strength of
the relationship between variables in a model. It ranges from 0 to 1, with a higher value indicating a
stronger relationship. An r2 value closer to 100% suggests that the variables in the model explain a
larger proportion of the variation observed in the data, indicating a better fit of the model. Both the
p‐value and r2 value are important indicators in assessing the validity and reliability of a statistical
model.
CONCLUSION
The analysis conducted in this study has demonstrated the successful remediation of contaminated clay soil using Vernonia galamensis and Vernonia amygdalina. The contamination of the soil involved the introduction of hydrocarbons and metals. The microorganisms, namely Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli, along with the phytochemicals present in the leaf extracts, played a crucial role in degrading the metals and hydrocarbon contents present in the soil. Through their combined action, these microorganisms and phytochemicals facilitated the remediation process, effectively reducing the levels of contaminants in the clay soil. This finding highlights the potential of Vernonia galamensis and Vernonia amygdaline as eco-friendly solutions for soil remediation.
Indeed, the findings from the study indicate that the application of approximately 40g of both Vernonia extracts resulted in a reduction of more than 50% of the contaminants concentration within 40 days across all soil samples. This demonstrates the effectiveness of both Vernonia extracts as bio-remediating agents for polluted soil. The significant reduction in contaminants over the given time period suggests that these Vernonia extracts have the potential to be utilized in the remediation of various types of polluted soil. Their ability to effectively reduce the concentration of contaminants makes them promising options for eco-friendly and sustainable soil remediation practices.
Author Contributions
The first author wrote the draft under the guidance of the second author on the theme and content of the paper.
Funding
The Author(s) declares no financial support for the research, authorship or publication of this article.
Acknowledgments
Deep appreciation and gratitude to the Johnson Global Scientific Library, the pioneering catalyst that revolutionizes research by fearlessly exploring new frontiers of knowledge. Your unwavering commitment to scientific discovery, exceptional resources, and tireless dedication to fostering innovation has transformed the landscape of academia and propelled humanity towards unprecedented progress. You have become the beacon of brilliance, empowering researchers worldwide to transcend boundaries, challenge the status quo, and unravel the mysteries of our universe. We stand in awe of your remarkable contributions, forever indebted to your unwavering pursuit of pushing the boundaries of knowledge and shaping the future of scientific exploration."
Conflict of Interests
The Authors declare that they have no conflict of interest.
References
- Aldrett, S., Bonner, J.S., McDonalds, T.J., Mills, M.A., Autenrieth, R.L. (1997) Degradation of crude oil enhanced by commercial microbial cultures. Proceedings of 1997 International Oil Spill Conference. American Petroleum Institute, Washington DC, 995-996.
- Anderson, I: (2005). Niger River basin: A Vision for Sustainable Development, The World Bank Pp. 1-131.
- Atlas, R. M. , (1988). Biodegradation of hydrocarbons in the environment, In: Environmental Biotechnology Omenn, GS EdPlenum Press, New York.
- Atlas, R. M. , Microbial hydrocarbon degradation: bioremediation of oil spills. J. Chem. Technol. Biotechnol. 1991, 52, 149–156. [Google Scholar] [CrossRef]
- Azarowicz, R.M. (1973) Microbial degradation of petroleum. US Patent 3,769, 164.
- Baird, J. ,(2010). "Oil's Shame in Africa". Newsweek: 27.
- Banerjee, D. K. , Fedora, P. M., Hashimoto, A., Masliyah, J. H., Pickard, M.A. and Gray, M. R.. Monitoring the biological treatment of anthracite-contaminated soil in a rotating –drum bioreactor. Appl. Microbial. Biotechnol. 1995, 43, 521–528. [Google Scholar]
- Barbee, G. C. , Brown, K. W., Thomas, J. C., Donelley, K. C., Murray, H. E. Mutagenic activity (Ames test) of wood-preserving waste sludge applied to soil Bull. Environ Contam Toxicol. 1996, 57, 54–62. [Google Scholar]
- Bogumil T, (2014). Oil-Induced Displacement and Resettlement: Social Problem and Human Rights Issue, http://www.conflictrecovery.org/bin/Bogumil_Terminski-Oil.
- Bonaventura, C., Boneventura, J., Bodishbaugh, D. F., (1995). Environmental Bioremediation: Approaches and processes. In: Ecotoxicity and Human Health (Eds) de serres, F.J and Bloom, AD CRS Lewis Publ. Boca Raton, New York. 199-200.
- Cheesbrough, M. , 2002. District Laboratory Practice in Tropical Countries, Part 2. Cambridge University Press, UK., pp: 135-162.
- Compeau, G. C., Mahaffey, W. D., Patras, L. Full scale bioremediation of contaminated soil and water.
In : Environmental biotechnology for waste treatment. Eds. GS Sayler, R. Fox, JW Blackbourn. Plenum Press,
New York and London. Environ. Sci. Res. 1991, 25, 91–109.
- Forsyth, J.V. , Tsao, Y.M., Blem, R.D. (1995) Bioremediation: when is augmentation needed? In Hinchee, R.E. et al. (eds) Bio augmentation for Site Remediation. Battelle Press, Columbus, OH, pp1 14.
- Goldstein, R.M., Mallory, L.M., Alexander, M. (1985) Reasons for possible failure of inoculation to enhance
biodegradation. Applied and Environmental Microbiology 1985, 50, 977–983. [CrossRef] [PubMed]
- Gray, M. R., Banerjee, D. K., Fedora, K. P. M., Habhimoto,A., Maslryah,J. H., Pickard, M.A., (1994). Biological
remediation of anthracene‐contaminated soil in rotating bioreactors. Appl. Microbial Biotechnol. 1994, 40, 933–944. [CrossRef]
- Hozumi, T., Tsutsumi, H. and Kono, M. (2000) Bioremediation on the shore after an oil spill from the Nakhodka
in the Sea of Japan. I. Chemistry and characteristics of the heavy oil loaded on the Nakhodka and biodegradation
tests on oil by a bioremediation agent with microbial cultures in the laboratory. Marine Pollution Bulletin 2000, 40, 308–314. [CrossRef]
- Huan Jing Ke Xue (February 2007). "Chemical fixation of metals in soil using bone char and assessment of the soil genotoxicity". Huan Jing Ke Xue. 28: 232–7.
- Hupe, K. , Luth, J. C., Heerenklage, J., Stegmann, R., (1995). Blade-milking reactors in the biological treatment of contaminated soils, in Biological unit processes for HazardousWasteTreatment. Ed by HuncheeRE, Skeen RS, and Sayles GD, Battele Press, Columbus. 153-159.
- Jenkins, K. D. , Sanders, B. M., (1992). Biomonitoring with biomarkers :A multi-tiered framework for conducting the ecological impact of contaminants. In: Ecological indicators. Mekenzie, D., Hyatt, E., McDonald, J. Eds. Elsever, NewYork.
- Le Floch, S., Merlin, F.X., Guillerme, M., Dalmazzone, C., and Le Corre, P. A field experimentation on
bioremediation:. Bioren. Environmental Technology 1999, 20, 897–907. [CrossRef]
- Le Floch, S. , Merlin, F.X., Guillerme, M., Tozzolino, P., Ballerini, D., Dalmazzone, C., and Lundh, T. (1997) Bioren: recent experiment on oil polluted shoreline in temperate climate. In: In-Situ and On-Site Bioremediation: Volume 4, Battelle Press, Columbus, OH, pp. 411-417.
- Leahy, J. A., Colwell, R. R., Microbial degradation of hydrocarbons in the environment. Microbiological Reviews 1990, 54, 305–315. [CrossRef]
- Lovley, D. R. Cleaning up with genomics: applying molecular biology to bioremediation. Nature Reviews/Microbiology 2003, 1, 35–44. [Google Scholar] [CrossRef]
- Mauro, G. and Wynne, III, B.J. (1990) Mega Borg oil spill: an open water bioremediation test. Texas General Land Office, Austin, Texas.
- Meagher, RB. Phytoremediation of toxic elemental and organic pollutants. Current Opinion in Plant Biology 2000, 3, 153–162. [Google Scholar] [CrossRef]
- Microbiological cultures for the treatment of an oil spill. Marine Pollution Bulletin, 40, 320 324.
- Moffat, F and Linden, K., (2009). Perception and Reality: Assessing Priorities for Sustainable Development in the Niger River Delta.
- Mohan, R.R., Byrd, G.H., Nixon, J., and Bucker, E.R. (1975) Use of microorganisms to disperse and degrade oil
spills. US Patent 3,871,957.
- National Research Council (NRC), (1985). Oil in the sea; inputs, fates and effects. National Academy Press, Washington, DC.
- Olapade, OA; Ronk, AJ. Isolation, Characterization and Community Diversity of Indigenous Putative Toluene-Degrading Bacterial Populations with Catechol-2,3-Dioxygenase Genes in Contaminated Soils. Microbial Ecology. Microbial Ecology. 2014, 69, 59–65.
- O'Loughlin, E. J; Traina, S. J.; Sims, G. K. Effects of sorption on the biodegradation of 2 methylpyridine in aqueous suspensions of reference clay minerals. Environ. Toxicol. And Chem. 2000, 19, 2168–2174. [Google Scholar]
- Olson, B. H. , Tsai, Y., (1992). Molecular approaches to environmental management. In: Environmental Microbiology ed Ralph Mitchell. John Wiley and sons Inc. Pub. New York,239-263.
- Onwurah, I. N. E. Restoring the crop sustaining potential of crude oil polluted soil by means of Azotobacter inoculation. Plant Prod. Res, J. 1999, 4, 6–16. [Google Scholar]
- Onwurah, I. N. E., (2000). A Perspective of Industrial and Environmental Biotechnology. Snaap Press / Publishers Enugu, Nigeria, 148.
- Paerl, H., Piehler, M., Swistak, J., (1996). Coastal diesel fuel pollution effects on the native microbial community.
Poster presentedat the Meeting of the American Society of Microbiology, New Orleans May 19‐.
- Prince, P. C. , (1992). Bioremediation of oil spills with particular reference to the spill from Exxon Valdez. Microbial Control of Pollution. (Eds. Fry Jc, Gadd GM Herbert RA, Jones CW and Watson-Craik 1A.) Society for General Microbiology symposium 48.
- Ritter, W. F., Scarborough, R. W. A review of bioremediation of contaminated soils and ground water. J. Environ Sci Health. A. 1995, 30, 323–330.
- Robson, B.D (2003) : ‘Phytoremediation of hydrocarbon-contaminated soil using plants adapted to the western Canadian climate’, Department of Soil Science, University of Saskatchewan, Saskatoon.
- Rosenberg, E. and Ron, E.Z (1996) Bioremediation of petroleum contamination, In R.L. Crawford and D.L. Crawford (Eds.), Bioremediation: principles and Applications, Cambridge University Press, UK, 100-124.
- Shell International Petroleum Company, Developments in Nigeria (London: March 1995).
- Simon, M. , Autenrieth, R.L., McDonald, T.J., Bonner, J.S.(1999) Evaluation of bioaugmentation for remediation of petroleum in a wetland. Proceedings of 1999 International Oil Spill Conference. American Petroleum Institute, Washington DC.
- Sims, G.K. "Nitrogen Starvation Promotes Biodegradation of N-Heterocyclic Compounds in Soil". Soil Biology & Biochemistry. 2006, 38, 2478–2480. [Google Scholar]
- Stroo, H. F. Biotechnology and hazardous waste treatment. J Environ. Qual. 1992, 21, 167. [Google Scholar] [CrossRef]
- Swannell, R.P.J., Lee, K., and McDonagh, M. Field evaluations of marine oil spill bioremediation. Microbiological Reviews 1996, 60, 342–365. [CrossRef]
- The Daily Independent Lagos (2010)."Shell And The N15bn Oil Spill Judgement Debt". . 2010-07 19. Retrieved 27 July 2010.
- Tsutsumi, H., Kono, M., Takai, K., Manabe, T. (2000) Bioremediation on the shore after an oil spill from the Nakhodka in the Sea of Japan. III. Field test of a bioremediation agent with.
- UNDP.( 2006 )"Niger Delta Human Development Report". . p. 76. Retrieved 19 June 2011.
- Venosa, A. D., Lee, K., Suidan, M. T., Garcia‐Blanco, S., Cobanli, S., Moteleb, M., Haines, J.R., Tremblay, G., and
Hazelwood, M. (2002) Bioremediation and biorestoration of a crude oilcontaminated freshwater wetland on the
St. Lawrence River. Bioremediation Journal, 6.
- Vidal, J., (2010). "Nigeria's agony dwarfs the Gulf oil spill. The US and Europe ignore it". The Observer. Retrieved 27 July 2010. government's national oil spill detection and response agency (Nosdra) says that between 1976 and 1996 alone, more than 2.4m barrels.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).