1. Introduction
Metapneumovirus (MPV), a negative-sense and single-stranded RNA virus, is classified into two different types, human metapneumovirus (hMPV) and avian metapneumovirus (aMPV) (subfamily Pneumovirinae, family Paramyxoviridae), which exhibits a propensity for interspecies transmission [
1,
2,
3]. aMPV is a major cause of acute rhinotracheitis in turkeys and is associated with swollen head syndrome in chickens and turkeys [
4], and can infect other avian species, such as ducks, sparrows, and pigeons [
5,
6,
7,
8]. Based on its genetic and antigenic properties, aMPV can be divided into four subgroups: A, B, C, and D [
9]. aMPV subgroup C (aMPV/C) was first identified in turkeys in the United States [
10,
11] and was subsequently detected in other avian species in other countries, such as France [
12], Korea [
13], China [
9,
13], and Italy [
14]. A recent study found that human metapneumovirus (hMPV) is closely associated with aMPV/C, suggesting that hMPV may have evolved from avian metapneumovirus via zoonotic transmission [
15]. In addition, aMPV/C has been shown to infect mice, where it can replicate in the lungs and cause pathological changes, including an increased number of inflammatory cells [
16]. These findings may implicate aMPV/C as a potential zoonotic pathogen, possessing a public health significance.
aMPV/C genome encodes eight proteins: nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), second matrix protein (M2), small hydrophobic protein (SH), attachment glycoprotein (G), and RNA-dependent RNA polymerase (L) [
4]. The virus entering the cell represents the beginning of infection. Paramyxovirus entry into host cells involves the fusion of viral envelope with the cell membrane [
17]. Membrane fusion is induced by G protein on the surface of the virus and also requires the fusion (F) protein [
18]. The F protein is synthesized from the precursor protein F0, which is processed into two disulfide-linked F1 and F2 subunits immediately after activation [
18]. It is generally acknowledged that binding of fusion proteins to cell surface receptor(s) mediates conformational changes in the F protein, which subsequently triggers membrane fusion [
18,
19,
20,
21].
Integrins, the major receptors, involve in adhesion of cell to extracellular matrix proteins and contribute crucially to vertebrate cell-cell adhesion [
22]. In addition, integrins induce the connections between transmembrane and the cytoskeleton for activating intracellular signals. Integrins are receptors for various viruses and bacteria [
22,
23]. For example, integrins modulate aMPV/B and hMPV by facilitating protein-induced cell-cell fusion and viral infection, in which F proteins of aMPV/B and hMPV can induce membrane fusion requiring the binding of Arg-Asp-Asp (RDD) or Arg-Gly-Asp (RGD) motifs to integrin [
24,
25]. Furthermore, the Arg-Ser-Asp (RSD) motif mediates membrane fusion by binding to integrins in the case of viral entry [
26]. The high expression of integrins on the cell membrane surface, high conservation of integrins among different species, and presence of an RSD motif in aMPV/C indicate that integrins might be potential receptors for aMPV/C infection.
In the present study, we verified the hypothesis that integrins act as functional receptors for aMPV/C. The divalent cation chelator ethylenediaminetetraacetic acid (EDTA), which decreases integrin-ligand binding, diminishes aMPV/C infectivity. Functional blocking of integrin β1 (ITGB1) specific antibodies inhibit aMPV/C infection. Transfection of avian-derived ITGB1 cDNA into poorly permissive cells conferred aMPV/C infectivity, and aMPV/C used ITGB1 to enhance its ability to infect human lung cells. Downregulation of ITGB1 expression by siRNA diminished aMPV/C infection. Collectively, these data provide strong evidence that aMPV/C can use ITGB1 as a cellular receptor to mediate viral entry into avian cells and that aMPV/C can also infect human lung cells via binding to human-derived ITGB1.
2. Results
2.1. aMPV/C F protein contains a highly conserved RSD motif
We compared the sequences of full-length F gene in all aMPV/C isolates collected from the NCBI GenBank database (
https://www.ncbi.nlm.nih.gov/). An Arg-Ser-Asp (RSD) motif at residues 329–331 was strictly conserved in all aMPV/C F proteins, (
Figure S1). To determine whether RSD motif exposure was lateral to the F protein, its structure was constructed using AlphaFold2 (
Figure S2). The resolved F protein structure of aMPV/C showed that the F protein comprised 527 amino acids, with a molecular formula of C2568H4150N690O783S26, and contained 8,217 atoms. The relative molecular mass was 58,052.91, the theoretical isoelectric point was 6.84, and the instability coefficient of the F protein was 36.34. The aliphatic coefficient was 97.09, and the average hydrophilicity was 0.060. The secondary structure of the F protein mainly consisted of 205 α helices (h, 38.18%), 129 extensions (e, 24.02%), 60 β turns (t, 11.17%), and 143 random helices (c, 26.63%). Meanwhile, a library comprising more than 30,000 protein receptors (
www.bindingdb.org) is used to simulate molecular docking with the aMPV/C F protein RSD motif, the results indicated that the RSD can bind to integrins, leading to the hypothesis that integrins may function as receptors for aMPV/C.
2.2. EDTA and integrin β1-specific antibodies reduce aMPV/C infectivity
Divalent cations, such as Ca2+ and Mg2+ [
23], are required for integrin–ligand interactions, and previous studies have indicated that EDTA is a divalent cation chelator that can inhibit such interactions [
25]. Firstly, the cell viability in EDTA-treated cells was analyzed using an MTT assay. The results showed that the EDTA treatment did not affect the cell viability up to the 2.5 mM
(Figure 1A). Subsequently, To verify the effec of EDTA on aMPV/C infection,the DF-1 cells treated with EDTA were infected with aMPV/C and the aMPV/C replication was analyzed by western blotting, viral titers, and viral genome copy numbers. The results showed that EDTA inhibited aMPV/C replication in a dose-dependent manner, which was characterized by the reduction of VP1 proteins, viral titers, and viral genome copy numbers (
Figure 1B–D). Moreover, Further validation was performed using an indirect immunofluorescence assay. The results showed that the numbers of positive cells with fluorescence decreased with increasing EDTA concentration (
Figure 1E). Thus, we confirmed that EDTA exerts an inhibitory effect on aMPV/C replication, supporting the positively regulatory role of integrins in aMPV/C infection.
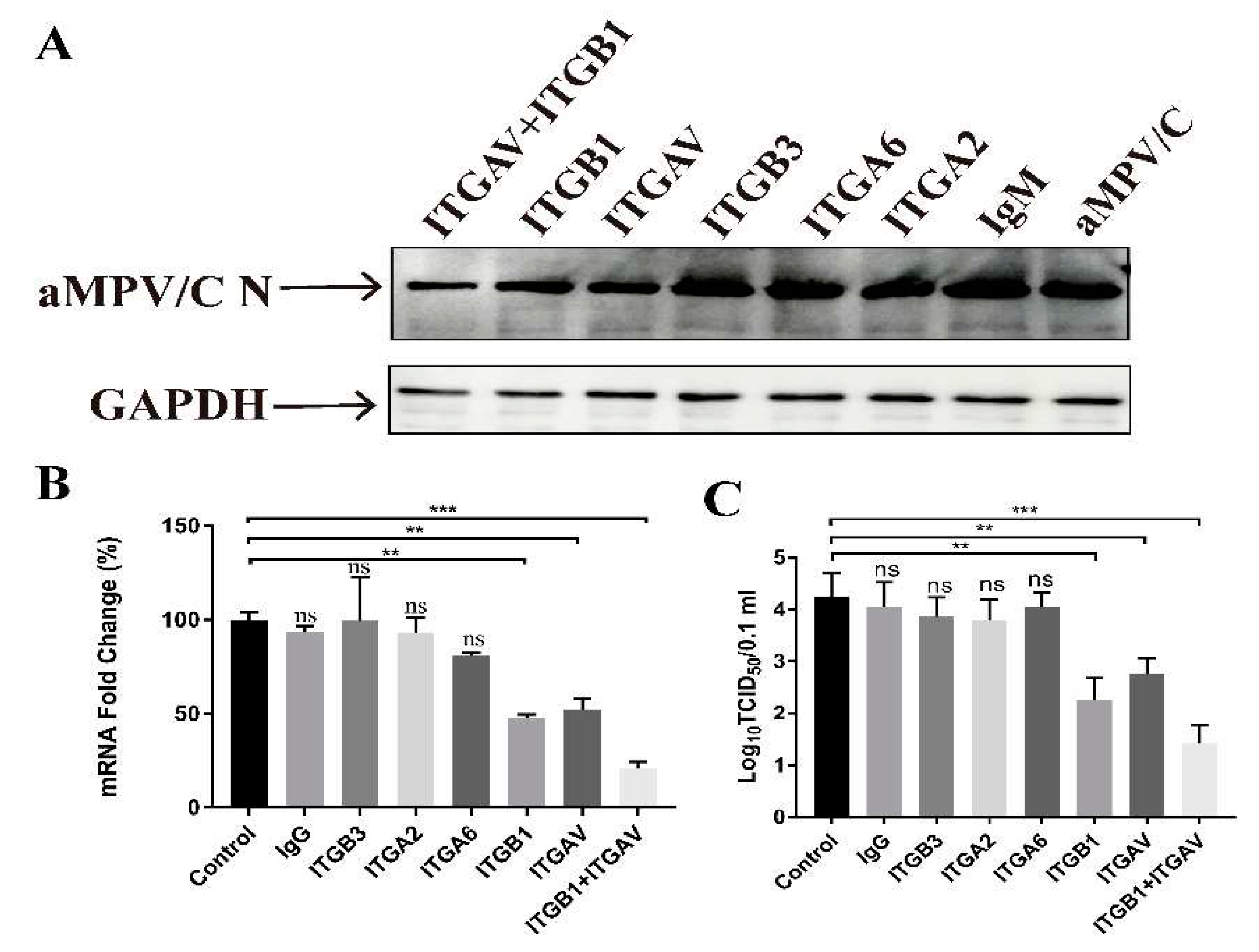
To identify whether the integrins were required for aMPV/C infection, DF-1 cells were incubated with anti-integrin antibodies for blocking the binding of RSD and integrins, followed by aMPV/C infection, and the F protein expression, virus replication, and viral titer were analyzed. The results showed that specific antibodies against αv and β1 integrins separately caused the significantly blockage for aMPV/C replication compared to the other antibodies (anti-α6, α2, and β3 integrins antibodies), exhibited approximate 40% blockage rates for aMPV/C replication and the combination anti-αv with anti-β1 integrin antibodies resulted in at least 60% blockage rates, whereas specific antibodies against α6, α2, and β3 integrins showed a minimal inhibitoryeffect, suggesting that anti-αv and/or anti-β1 integrin antibodies caused the significantly blockage for aMPV/C replication compared to the other antibodies (anti-α6, α2, and β3 integrins antibodies) (
Figure 2). Thus, these data indicated thatαvβ1 integrin was involved in the infection of aMPV/C.
2.3. RSD-specific peptide reduces aMPV/C infection
The previous study found that the RSD motif in the F protein was a key motif for binding integrin [
27]. To further evaluate the role of the RSD motif in virus replication, the RSD or Arg-Ser-Glu (RGE) peptides were synthesized to analyze blocking effect. Firstly, the cell viability of the RSD or Arg-Ser-Glu (RGE) peptides was analyzed by MTT assays. The results did not show significant cytotoxicity in the cells treated with the RSD or Arg-Ser-Glu (RGE) peptides (
Figure 3A). Subsequently, the DF-1 cells were incubated with the GRSDSP (integrin-binding peptide) or GRGESP (the control peptide), followed by aMPV/C infection. The results showed that GRSDSP treatment inhibited aMPV/C infection with a dose-dependent manner, whereas the GRGESP peptide had no significantly inhibitory effect (
Figure 3B-3E), suggesting that the RSD motif was required for aMPV/C infection. The inhibitory effect of the RSD peptide was modest, but the blockade magnitude of the infectivity was similar to that of other viruses engaging integrins [
28].
2.4. aMPV/C can use avian ITGB1 as a receptor for cell entry
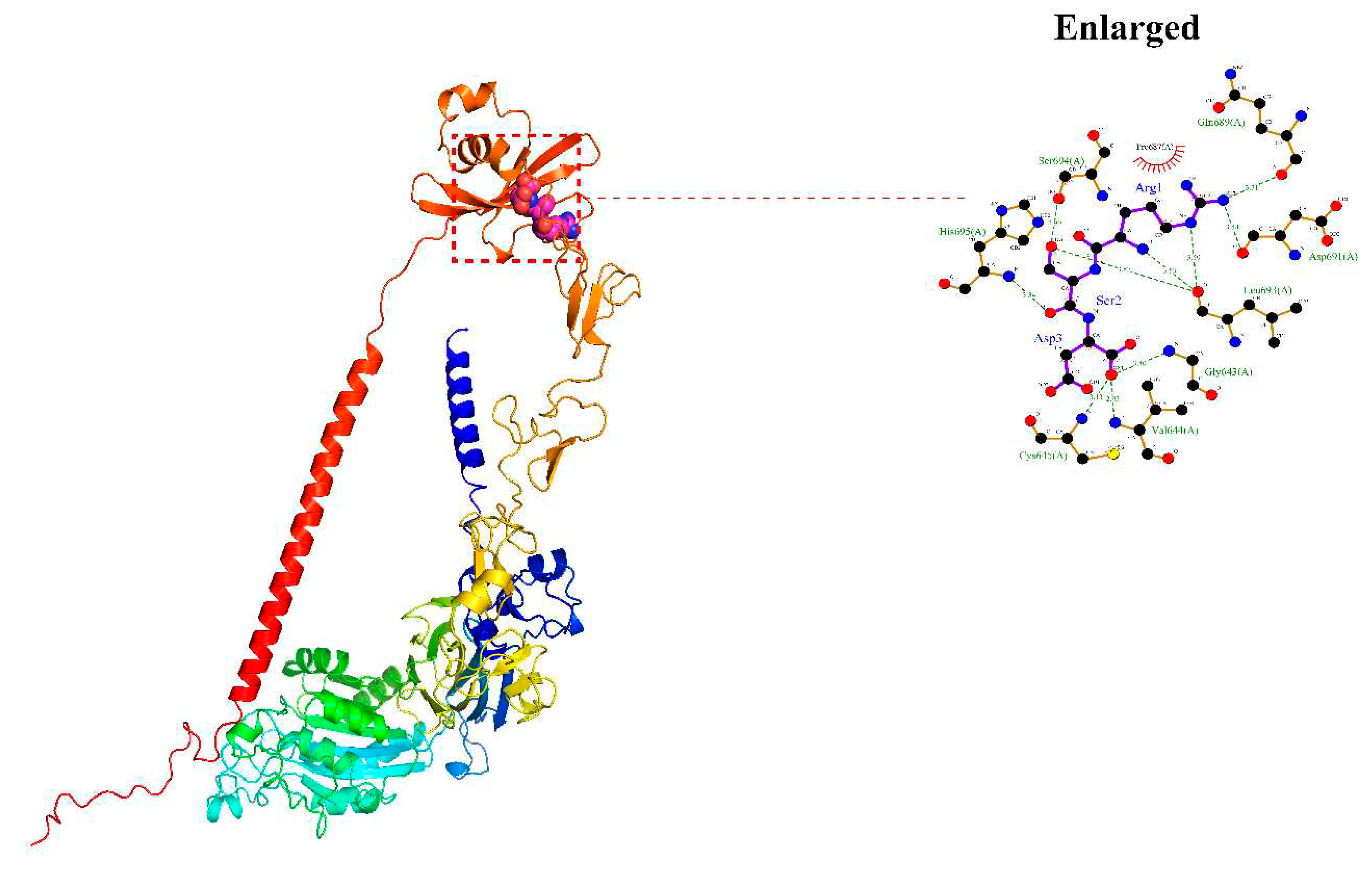
To investigate the role of the ITGB1 interaction with aMPV/C, molecular docking methods were used to simulate the binding between chicken ITGB1 (cITGB1) and the aMPV/C F protein. As shown in
Figure 4, the binding energy of the RSD motif to cITGB1 was -5.5 kcal/mol and the formation of hydrogen bonds and hydrophobic forces benefited for interaction between the RSD motif and ITGB1, indicating that the F protein was able to bind to cITGB1. During this interaction process, Arg (R) formed hydrogen bonds with Leu693, Asp691, and Gln689, with lengths of 3.53 Å, 3.59 Å, 3.44 Å, 3.71 Å, respectively; Ser (S) formed hydrogen bonds with Leu693 and Ser694 with lengths of 3.87 Å and 2.90 Å, respectively; and Asp (D) formed hydrogen bonds with His695, Cys645, Val644, and Gly643, with lengths of 3.34 Å, 3.13 Å, 2.95 Å, and 3.40 Å, respectively (
Figure 4). The formation of a hydrophobic interaction between RSD motif and Pro687 increased the ability of F protein binding to cITGB1 (Figure 4), suggesting that these residues may be the key active sites for peptides to act on the F protein.
To investigate the effect of integrin expression on aMPV/C infection, we transfected DF-1 cells with cITGB1 plasmids. The overexpression of cITGB1 increased the replication of aMPV/C (
Figure 5A-5C). The intestinal porcine epithelial (DQ) and feline kidney (CRFK) cell lines were poorly permissive to aMPV/C infection (
Figure 5D). Both of the DQ cells and CRFK cells transfected with cITGB1 plasmids were infected with aMPV/C, followed by fluorescence analysis. The results showed that the specific fluorescence signals were observed in the poorly permissive cells for aMPV/C replication, suggesting that cITGB1 expression substantially facilitated aMPV/C infection (
Figure 5D-5F). The enhanced efficiency of aMPV/C infection in chicken β1 integrin-transfected DQ and CRFK cells was inhibited by preincubation with blocking antibodies for β1 integrin (
Figure 5D-5F). These results demonstrated that the overexpression of cITGB1 promoted aMPV/C infection.
To determine whether the reduced cITGB1 expression limits aMPV/C infection, DF-1 cells were transfected with siRNAs targeting cITGB1. Transfection with cITGB1 siRNA reduced the cell surface expression of cITGB1 in most cells, whereas nonspecific siRNA transfection did not diminish the expression of cITGB1 (
Figure 6A). significantly down regulated aMPV/C replication compared to the control group (
Figure 6B). These results indicated that cITGB1 expression facilitated to the aMPV/C replication.
Based on ITGB1, as a receptor, located in the surface of cells [
22,
23], we speculated whether cITGB1 affected aMPV/C attachment or entry and finally regulated aMPV/C replication. To confirm this hypothesis, the treatment of chemical reagent, RSD peptide, or cITGB1 siRNA were used to analyze the effect of cITGB1 on aMPV/C attachment or entry.DF-1 cells treated with EDTA, GSD peptide, or cITGB1 siRNA were infected with aMPV/C at 4°C for analyzing viral attachment, followed by shifting to 37°C for analyzing viral entry. The results showed that EDTA, GSD peptide, or cITGB1 siRNA not only significantly decreased aMPV/C attachment, but also aMPV/C entry. (
Figure 7). Therefore, our results demonstrated that cITGB1 was identified as a cell receptor for aMPV/C infection and played a crucial role in the attachment process of aMPV/C.
2.5. aMPV/C can infect A549 cells
The genetic relationship between hMPV and aMPV/C and the presumed origin of hMPV in avian species [
15] allowed us to investigate whether hMPV-permissive cells, A549, could be infected with aMPV/C. Firstly, the F protein structures between aMPV/C and hMPV were compared. We found that the structures between RSD motif in aMPV F proteins and RGD motif in hMPV F proteins were highly similar and they are exposed to the surface of F proteins (
Figure S3). The previous study found that the RSD motif can also bind to integrin and mediate membrane fusion [
26]. To determine whether aMPV/C can infect A549 cells, we employed molecular docking to measure the binding energy between aMPV/C F protein and human ITGB1 (hITGB1). The simulation analysis results indicated that aMPV/C F protein can bind to hITGB1 with a binding energy of -5.2 kcal/mol, accompanied by the formation of hydrogen bonds and hydrophobic forces between RSD peptides and hITGB1 (
Figure S4). For example, Arg (R) formed hydrogen bonds with Asp622 and Thr621 with lengths of 3.48 Å and 3.63 Å, respectively; Ser (S) formed hydrogen bonds with Leu637, with lengths of 3.29 Å and 3.03 Å, respectively; and the RSD exhibited hydrophobic interactions with Arg610, Asn710, Asn711, Val639, and Cys636 (
Figure S4). Therefore, these results suggested that the hITGB1 could recognize the RSD motif.We performed aMPV/C infection experiments in A549 celles to confirm the above results. Remarkably, A549 cells were susceptible to aMPV/C infection without any treatment (
Figure 8). Moreover, to determine whether ITGB1 served as a receptor for aMPV/C infection in A549 cells, we transfected human and avian ITGB1 plasmids into A549 cells. The results showed that expression of cITGB1 and hITGB1 in A549 cells substantially enhanced the efficiency of aMPV/C infection (
Figure 8). These results indicate that aMPV/C can also use human ITGB1 molecule as a receptor to infect cells of human origin.
3. Discussion
The virus must rely on the assistance of receptors to enter cells, but the receptor for aMPV/C is currently unclear. In this study, simulation of the aMPV/C F protein using an artificial intelligence algorithm (AlphaFold2) revealed the presence of an RSD motif on the outside of the F protein, indicating that the RSD motif may facilitate receptor binding. Peptide-blocking experiments showed that the RSD motif reduced the aMPV/C infection. Molecular dynamics was also used to simulate the combination of the aMPV/C F protein and ITGB1, and the results indicated that the RSD motif of the F protein interacted with ITGB1 via hydrogen bonds and hydrophobic forces. Further studies showed that ITGB1 plays an important role in aMPV/C infection at the early stage (attachment and internalization). In addition, aMPV/C can infect efficiently human A549 cells, which may be related to sharing the similar structures of F protein-specific motifs (RSD for aMPV/C and RGD for hMPV) recognized by ITGB1. Overall, our results indicate that ITGB1 serves as a functional receptor benefiting for aMPV/C infection by recognizing of the RSD motif of the aMPV/C F protein.
Viral attachment to a receptor is a prerequisite for viral infection. We used the CRISPR Cas9 system to knock out ITGB1 in DF-1 cells to investigate the role of ITGB1 in aMPV/C infection. The results found that the ITGB1-knocked-out cells were unable to survive, which is in agreement with that in a previous report [
29]. The integrin can combine with ligands for mediating viral attachment and entry such as aMPV/B, Kaposi’s sarcoma-associated herpesvirus, Epstein-Barr virus, and reovirus attachment [
30,
31,
32,
33]. Integrin-mediated viral attachment and entry is not only associated with RGD motif, but also bind to RDD and RSD motif [
26]. Fortunately, a highly conserved RSD motif (329RSD331) was found in the F protein of aMPV/C. aMPV/C F protein induces cell-cell fusion and virus infection [
34,
35], promoting us to explore the role of RSD motif in viral attachment and entry. RSD and RGD motif were not only play important in other pathogens infection, such as group A Streptococcus which can promote binds to human integrins alphavbeta3 and alphaIIbbeta3, Equine herpesvirus 1 entry via endocytosis is facilitated by alphaV integrins and an RSD motif in glycoprotein D [
36,
37], but also involved in cell adhesion and viability [
38,
39]. Molecular docking, which can well simulate the binding and force between proteins, proteins and peptides [
40,
41], was used to simulate the binding of the viral F protein to ITGB1, indicating that the aMPV/C F can tightly bind to ITGB1 via hydrogen bonds and hydrophobic forces, thereby promoting aMPV/C infection. ITGB1 can also cooperate with other integrins to promote Ebolavirus infection [
33]. In addition, peptide-drugs have been widely used in central nervous system diseases and infectious disease, and GRGDSP peptide was used as an alternative to vaccines to prevent Rabies virus infection [
42,
43]. Since there are currently no drugs targeting aMPV/C infection, peptide-drugs could become an alternative. However, the safety and efficacy of RSD peptide against aMPV/C infection need to be further monitored before being recommend for utilization.
Viruses often use conserved receptors like integrin to infect different species. Conserved receptors may provide viruses with an evolutionary advantage to explore alternative hosts, thereby leading to host switching and viral speciation [
44]. A previous study which using a reverse genetics system to rescue aMPV/C, researchers found that GFP-expressing aMPV/C and GFP-expressing hMPV could be recovered using the support plasmids of either virus, denoting that the genome promoters are conserved between the two metapneumoviruses and can be cross-recognized by the polymerase complex proteins of either virus [
45]. In addition, some research demonstrated integrin αVβ1 which was composed of integrin αV and ITGB1 as a functional receptor that can promote hMPV infection [
25]. Avians have been shown to act as intermediate hosts of some zoonotic viruses [
46,
47]. Considering the presumed avian origin of metapneumoviruses, the viral functional engagement of conserved receptors may offer an explanation for the mechanism that enables a virus ancestral to metapneumoviruses to break the barrier between birds and humans. However, ITGB1 as a receptor for a potential cross-species transmission of aMPV/C infection requires further study.
In summary, this study showed that ITGB1 is essential for efficient aMPV/C infection and that the blockade of cell surface ITGB1 inhibits aMPV/C infectivity. The aMPV/C F protein binds to target cells, and mutation of the RSD motif abolishes this binding. These findings demonstrate the role of ITGB as a receptor for aMPV/C infection and provide a potential therapeutic target and specific small molecule drugs for aMPV/C prevention and treatment.
4. Materials and Methods
Cells, viruses, antibodies, and peptides
DF-1 and A549 cells were gained from the American Type Culture Collection, maintained in our laboratory, and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, NY, USA) with 10% fetal bovine serum (FBS) (Gibco) supplemented with 1% penicillin and streptomycin. CRFK cells were gifted by Dr. Yuanhong Wang of the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, NY, USA) with 10% fetal bovine serum (FBS) (Gibco, NY, USA) supplemented with 1% penicillin and streptomycin. IPEC-DQ (DQ) cells, a subclone of the porcine intestinal epithelial cell line IPEC-J2, was a gift from Dr. Dongwan Yoo of the College of Veterinary Medicine, University of Illinois, Urbana-Champaign, Urbana, Illinois, USA. The cells were cultured in RPMI 1640 medium (Gibco, NY, USA) containing 10% FBS (Gibco) supplemented with 1% penicillin and 1% streptomycin at 37°C in a 5% CO2 incubator.
aMPV/C strain JC was isolated from Chinese native meat-type chickens with acute respiratory diseases and maintained in our laboratory (14). All viruses were stored at -80°C prior to use.
The RSD and control peptides were synthesized by Nanjing Genscript and stored at -20°C until use. The commercial antibodies were used as follows: integrin αV (MAB2021) and β1 (MAB1959) were purchased from Millipore; integrin α6 (100497-T08) and β3 (101954-T32) were purchased from Sino biological; integrin α2 (MAB228Hu22) was purchased from USCN Life Science; and horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (AS014), HRP-conjugated anti-mouse secondary antibody (AS003), and mouse anti-GAPDH antibody (AC001) were purchased from ABclone. The Alexa Fluor 488-conjugated goat anti-mouse antibody (A11001) was purchased from Sigma-Aldrich. Mouse antiviral N-polyclonal antibody was prepared and stored at our laboratory.
Cell viability
Cell Counting Kit-8 (Sigma Aldrich, USA) was used to determine cell viability. DF-1 cells in 96-well plates were incubated with 100 μl of Cell Counting reagent (Sigma Aldrich, USA) per well for 2 h on a shaker to mediate cell lysis. Luminescence was examined using Tecan Infinite M Plex (Tecan, Switzerland).
Transfection of chicken and human integrin cDNAs
Full-length cITGB1 and hITGB1 were isolated from DF-1 and A549 cells, respectively, and cloned into the pCMV-Flag-N vector. All constructed plasmids were confirmed by DNA sequencing, and all primers used for plasmid construction are displayed in below, Chicken-ITGB1 (sense, CGCGAATTCGGatggccgagactaatttaacattgctca; antisense, CGCGGTACCtcattttccctcatatttaggattgacca), Human-ITGB1 (sense, CCGGAATTCGGatgaatttacaaccaattttctggattgg; antisense, CGGGGTACCtcattttccctcatacttcggattgacca). Cells grown in six-well plates (Nest) were transfected with either control plasmids or plasmids encoding chicken and human integrin constructs using Lipofectamine™ 2000 reagent (11668030, Invitrogen). The cells were incubated for 24 h to allow integrin expression before adsorption with aMPV/C for infectivity studies.
Knockdown of integrin expression with siRNA
siRNA targeting chicken integrin β1 was synthesized by GenePharma, and the sequences are listed are as follows: SiITGB1, sense, GCGAUCGAUCAAACGGUUUTT, antisense, AAACCGUUUGAUCGAUCGCTT. DF-1 cells grown in six-well plates to reach 90% confluence were transfected with control or integrin-specific siRNAs using the Lipofectamine RNAi MAX reagent (13778150, Invitrogen). After incubation for 24 h, the cells were collected for the determination of integrin expression using western blotting.
Quantitative real-time reverse transcription PCR (RT-qPCR)
Total RNA was extracted from cells using TRIzol reagent (Pufei, China), and the extracted RNA was reverse-transcribed using the TransScript cDNA Synthesis Kit (TransScript, China) to generate cDNAs. The generated cDNA was analyzed via RT-qPCR using SuperReal Premix Color SYBR Green I mix (Tiangen, China) and quantified using a Light Cycler 96 system (Roche, Switzerland). The quantitative RT-qPCR method was developed in our laboratory and all samples were analyzed in triplicate. All RT-qPCR primers utilized in this study are displayed in below, aMPV/C-M-F, GTCAATTCAGCCAAGGCAGT; aMPV/C-M-R, GGGGCAATCCTAGCTTGAGT.
Virus attachment and entry assays
For the attachment assay, DF-1 cells were inoculated with aMPV/C at a MOI for 2 in the presence of the inhibitors at 4°C for 1 h. After the incubation period, the inoculum was removed, and the cells were washed three times with cold phosphate-buffered saline (PBS) and then harvested by three freeze-thaw cycles and subjected to viral RNA extraction for quantification of viral load via real-time RT-PCR. For the entry assay, after the binding period, cells treated with inhibitors or transfected with siRNAs were incubated with medium for 1 h at 37°C and washed with cold PBS, and then harvested and subjected to viral RNA extraction for quantification of viral load via real-time PCR (RT-PCR).
Virus titer determination
Supernatants from aMPV/C-infected cells were collected and stored at -80°C before analysis. To determine the aMPV/C titer, the supernatant samples were 10-fold serially diluted and cultured with DF-1 cells in 96-well plates. Virus titers were determined as previously described (13) and expressed as 50% tissue culture infective dose (TCID50) per 0.1 ml.
Western blotting
Proteins were extracted from whole cells using a radioimmunoprecipitation assay (RIPA) and NP40 lysis buffer (Beyotime, China) containing 1 mM phenylmethanesulfonyl fluoride (PMSF) (Beyotime, China). The extracted proteins were quantified using the BCA kit (Beyotime, China). The cell lysates were quantified, resolved by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted onto nitrocellulose membranes (Pall, NY, USA). The membranes were blocked with 5% non-fat milk, incubated with the corresponding primary and secondary antibodies. Immunoreactive bands were visualized using the AMERSHAM ImageQuant800 chemiluminescence imaging system (GE, USA).
Immunostaining assay of viral infection
After being washed with PBS, cells were fixed with 4% paraformaldehyde (PFA), blocked by PBS with 5% nonfat milk, subsequently incubated with the corresponding primary and secondary antibodies (Alexa Fluor 488-conjugated goat α-mouse antibody), followed by incubation with 4’,6-diamidino-2-phenylindole (DAPI) (Sigma Aldrich, USA). The cells were examined under an inverted fluorescence microscope (Olympus Corporation, JAPAN).
Artificial intelligence predicts protein structure and molecular docking
The structures of the aMPV/C F protein and chicken and human ITGB1 were predicted using the artificial intelligence algorithm AlphaFold2 deployed on a supercomputer (BKUNYUN CLOUD, China). For molecular docking, the RSD peptide structure was first mapped using ChemBioDraw Ultra 14.0, and the mapped peptide structures were imported into ChemBio3D Ultra 14.0 for energy minimization. The RMS gradient limit was established 0.001 and the small molecules were saved in the format mol2. The optimized small molecules were entered AutodockTools (v1.5.6) for hydrogenation, charge distribution, charge calculation, and rotatable bond setting, and saved in the format pdbqt. The prepared ITGB1 protein was imported into Pymol2.3.0 to delete the original ligand and protein crystal water, and the protein structure was entered AutoDocktools for hydrogenation, charge assignment, charge calculation, atom type specification, and saving in the format pdbqt. POCASA 1.1 was utilized to predict binding sites, and AutoDock Vina1.1.2 was utilized for docking. Center-x = 35.1, center-y = 58.0, center-z = -52.5. Search space: size-x:80, size-y:80, size-z:80 (the spacing of each grid point is 0.375 Å), exhaustiveness:10. All other parameters were set by default. PyMOL 2.3.0 and LIGPLOT (v2.2.4) were utilized to analyze the interaction manner of the docking results.
Phylogenetic analysis
A maximum-likelihood tree was constructed based on different host species of the F gene using IQtree software with the GTRGAMMAI model and 1,000 replicates.
Statistical analysis
Data are expressed as means ± standard deviation (SD). Differences were determined utilizing one-way analysis of variance or Student's t-test utilizing the GraphPad Prism 9.0 software (GraphPad Software, CA, USA). P value < 0.05 was considered statistically significant.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/, Figure S1: Relationship between different metapneumovirus was deduced using the F genes; Figure S2: F protein structure of aMPV/C predicted using AlphaFold2; Figure S3: F protein structures of hMPV and aMPV/C; Figure S4: aMPV/C F protein can bind with hITGB1.
Author Contributions
Conceptualization, JUE LIU; Data curation, JUE LIU; Formal analysis, Yongqiu Cui, Weiyin Xu, Jiali Xie and Jianwei Zhou; Methodology, Yongqiu Cui, Dedong Wang and Jinshuo Guo; Software, Yongqiu Cui, Siting Li and Xufei Feng; Validation, Yeqiu Li; Writing – original draft, Yongqiu Cui; Writing – review & editing, Yongqiu Cui, Lei Hou and JUE LIU.
Funding
This study was supported by grants from the National Natural Science Foundation (31830095), 111 Project D18007, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
The authors greatly appreciate Dongwan Yoo (College of Veterinary Medicine, University of Illinois at Urbana-Champaign, Urbana, Illinois, USA) and Yuanhong Wang (Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences ) for providing the IPEC-DQ and CRFK cell lines.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El Najjar, F.; Cifuentes-Munoz, N.; Chen, J.; Zhu, H.; Buchholz, U. J.; Moncman, C. L.; Dutch, R. E. , Human metapneumovirus induces reorganization of the actin cytoskeleton for direct cell-to-cell spread. PLoS Pathog 2016, 12, (9), e1005922. [Google Scholar] [CrossRef] [PubMed]
- Broor, S.; Bharaj, P. , Avian and human metapneumovirus. Ann N Y Acad Sci 2007, 1102, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Mohakud, N. K.; Pena, L.; Kumar, S. , Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis 2014, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, L.; Wei, L.; Yan, X.; Zhu, S.; Quan, R.; Li, Z.; Wang, D.; Jiang, H.; Song, J.; Cui, Y.; Liu, J. , Characterization of avain metapneumovirus subgroup C isolated from chickens in Beijing, China. Poult Sci 2023, 102, (1), 102250. [Google Scholar] [CrossRef]
- Franzo, G.; Legnardi, M.; Mescolini, G.; Tucciarone, C. M.; Lupini, C.; Quaglia, G.; Catelli, E.; Cecchinato, M. , Avian metapneumovirus subtype B around Europe: a phylodynamic reconstruction. Vet Res 2020, 51, (1), 88. [Google Scholar] [CrossRef]
- Catelli, E.; De Marco, M. A.; Delogu, M.; Terregino, C.; Guberti, V. , Serological evidence of avian pneumovirus infection in reared and free-living pheasants. Vet Rec 2001, 149, (2), 56–8. [Google Scholar] [CrossRef]
- Toquin, D.; Bayon-Auboyer, M. H.; Eterradossi, N.; Jestin, V.; Morin, H. , Isolation of a pneumovirus from a Muscovy duck. Vet Rec 1999, 145, (23), 680. [Google Scholar]
- Gharaibeh, S.; Shamoun, M. , Avian metapneumovirus subtype B experimental infection and tissue distribution in chickens, sparrows, and pigeons. Vet Pathol 2012, 49, (4), 704–9. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, S.; Yan, X.; Wang, J.; Zhang, C.; Liu, S.; She, R.; Hu, F.; Quan, R.; Liu, J. , Avian metapneumovirus subgroup C infection in chickens, China. Emerg Infect Dis 2013, 19, (7), 1092–4. [Google Scholar] [CrossRef]
- Seal, B. S. , Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains. Virus Res 1998, 58, (1-2), 45–52. [Google Scholar] [CrossRef] [PubMed]
- Cook, J. K.; Huggins, M. B.; Orbell, S. J.; Senne, D. A. , Preliminary antigenic characterization of an avian pneumovirus isolated from commercial turkeys in Colorado, USA. Avian Pathol 1999, 28, (6), 607–617. [Google Scholar] [CrossRef]
- Toquin, D.; Guionie, O.; Jestin, V.; Zwingelstein, F.; Allee, C.; Eterradossi, N. , European and American subgroup C isolates of avian metapneumovirus belong to different genetic lineages. Virus Genes 2006, 32, (1), 97–103. [Google Scholar] [CrossRef]
- Lee, E.; Song, M. S.; Shin, J. Y.; Lee, Y. M.; Kim, C. J.; Lee, Y. S.; Kim, H.; Choi, Y. K. , Genetic characterization of avian metapneumovirus subtype C isolated from pheasants in a live bird market. Virus Res 2007, 128, (1), 18–25. [Google Scholar] [CrossRef] [PubMed]
- Legnardi, M.; Allee, C.; Franzo, G.; Cecchinato, M.; Brown, P. , Research Note: Detection of avian metapneumovirus subgroup C specific antibodies in a mallard flock in Italy. Poult Sci 2021, 100, (7), 101186. [Google Scholar] [CrossRef]
- Jesse, S. T.; Ludlow, M.; Osterhaus, A. , Zoonotic origins of human metapneumovirus: a journey from birds to humans. Viruses 2022, 14, (4). [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhu, S.; She, R.; Hu, F.; Wang, J.; Yan, X.; Zhang, C.; Liu, S.; Quan, R.; Li, Z.; Du, F.; Wei, T.; Liu, J. , Viral replication and lung lesions in BALB/c mice experimentally inoculated with avian metapneumovirus subgroup C isolated from chickens. PLoS One 2014, 9, (3), e92136. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, Y.; Cai, H.; Mirza, A. M.; Iorio, R. M.; Peeples, M. E.; Niewiesk, S.; Li, J. , Roles of the putative integrin-binding motif of the human metapneumovirus fusion (f) protein in cell-cell fusion, viral infectivity, and pathogenesis. J Virol 2014, 88, (8), 4338–52. [Google Scholar] [CrossRef]
- Lamb, R. A. , Paramyxovirus fusion: a hypothesis for changes. Virology 1993, 197, (1), 1–11. [Google Scholar] [CrossRef]
- Lamb, R. A.; Jardetzky, T. S. , Structural basis of viral invasion: lessons from paramyxovirus F. Curr Opin Struct Biol 2007, 17, (4), 427–36. [Google Scholar] [CrossRef]
- Bose, S.; Heath, C. M.; Shah, P. A.; Alayyoubi, M.; Jardetzky, T. S.; Lamb, R. A. , Mutations in the parainfluenza virus 5 fusion protein reveal domains important for fusion triggering and metastability. J Virol 2013, 87, (24), 13520–31. [Google Scholar] [CrossRef]
- Smith, E. C.; Smith, S. E.; Carter, J. R.; Webb, S. R.; Gibson, K. M.; Hellman, L. M.; Fried, M. G.; Dutch, R. E. , Trimeric transmembrane domain interactions in paramyxovirus fusion proteins: roles in protein folding, stability, and function. J Biol Chem 2013, 288, (50), 35726–35. [Google Scholar] [CrossRef]
- Hynes, R. O. , Integrins: a family of cell surface receptors. Cell 1987, 48, (4), 549–54. [Google Scholar] [CrossRef]
- Hynes, R. O. , Integrins: bidirectional, allosteric signaling machines. Cell 2002, 110, (6), 673–87. [Google Scholar] [CrossRef]
- Yun, B. L.; Guan, X. L.; Liu, Y. Z.; Zhang, Y.; Wang, Y. Q.; Qi, X. L.; Cui, H. Y.; Liu, C. J.; Zhang, Y. P.; Gao, H. L.; Gao, L.; Li, K.; Gao, Y. L.; Wang, X. M. , Integrin alphavbeta1 modulation affects subtype B avian metapneumovirus fusion protein-mediated cell-cell fusion and virus infection. J Biol Chem 2016, 291, (28), 14815–25. [Google Scholar] [CrossRef]
- Cseke, G.; Maginnis, M. S.; Cox, R. G.; Tollefson, S. J.; Podsiad, A. B.; Wright, D. W.; Dermody, T. S.; Williams, J. V. , Integrin alphavbeta1 promotes infection by human metapneumovirus. Proc Natl Acad Sci U S A 2009, 106, (5), 1566–71. [Google Scholar] [CrossRef]
- Li, P.; Lu, Z.; Bao, H.; Li, D.; King, D. P.; Sun, P.; Bai, X.; Cao, W.; Gubbins, S.; Chen, Y.; Xie, B.; Guo, J.; Yin, H.; Liu, Z. , In-vitro and in-vivo phenotype of type Asia 1 foot-and-mouth disease viruses utilizing two non-RGD receptor recognition sites. BMC Microbiol 2011, 11, 154. [Google Scholar] [CrossRef]
- Williams, C. H.; Kajander, T.; Hyypia, T.; Jackson, T.; Sheppard, D.; Stanway, G. , Integrin alpha v beta 6 is an RGD-dependent receptor for coxsackievirus A9. J Virol 2004, 78, (13), 6967–73. [Google Scholar] [CrossRef]
- Bergelson, J. M.; Chan, B. M.; Finberg, R. W.; Hemler, M. E. , The integrin VLA-2 binds echovirus 1 and extracellular matrix ligands by different mechanisms. J Clin Invest 1993, 92, (1), 232–9. [Google Scholar] [CrossRef]
- Fassler R, Meyer M. consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995. 1;9(15):1896-908.
- Wang, F. Z.; Akula, S. M.; Sharma-Walia, N.; Zeng, L.; Chandran, B. , Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J Virol 2003, 77, (5), 3131–47. [Google Scholar] [CrossRef]
- Akula, S. M.; Pramod, N. P.; Wang, F. Z.; Chandran, B. , Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 2002, 108, (3), 407–19. [Google Scholar] [CrossRef]
- Tugizov, S. M.; Berline, J. W.; Palefsky, J. M. , Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med 2003, 9, (3), 307–14. [Google Scholar] [CrossRef]
- Schornberg, K. L.; Shoemaker, C. J.; Dube, D.; Abshire, M. Y.; Delos, S. E.; Bouton, A. H.; White, J. M. , Alpha5beta1-integrin controls ebolavirus entry by regulating endosomal cathepsins. Proc Natl Acad Sci U S A 2009, 106, (19), 8003–8. [Google Scholar] [CrossRef]
- Yun, B.; Gao, Y.; Liu, Y.; Guan, X.; Wang, Y.; Qi, X.; Gao, H.; Liu, C.; Cui, H.; Zhang, Y.; Gao, Y.; Wang, X. , Effect of amino acid sequence variations at position 149 on the fusogenic activity of the subtype B avian metapneumovirus fusion protein. Arch Virol 2015, 160, (10), 2445–53. [Google Scholar] [CrossRef]
- Yun, B.; Guan, X.; Liu, Y.; Gao, Y.; Wang, Y.; Qi, X.; Cui, H.; Liu, C.; Zhang, Y.; Gao, L.; Li, K.; Gao, H.; Gao, Y.; Wang, X. , Trypsin- and low pH-mediated fusogenicity of avian metapneumovirus fusion proteins is determined by residues at positions 100, 101 and 294. Sci Rep 2015, 5, 15584. [Google Scholar] [CrossRef] [PubMed]
- Stockbauer, K. E.; Magoun, L.; Liu, M. Y.; Burns, E. H.; Gubba, S.; Renish, S.; Pan, X.; Bodary, S. C.; Baker, E.; Coburn, J.; Leong, J. M.; Musser, J. M. , A natural variant of the cysteine protease virulence factor of group A with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins α and β. P Natl Acad Sci USA 1999, 96, (1), 242–247. [Google Scholar] [CrossRef]
- Van de Walle, G. R.; Peters, S. T.; VanderVen, B. C.; O'Callaghan, D. J.; Osterrieder, N. , Equine herpesvirus 1 entry via endocytosis is facilitated by αV integrins and an RSD motif in glycoprotein D. J Virol 2008, 82, (23), 11859–11868. [Google Scholar] [CrossRef]
- Tjong, W. Y.; Lin, H. H. , The RGD motif is involved in CD97/ADGRE5-promoted cell adhesion and viability of HT1080 cells. Sci Rep-Uk 2019, 9.
- Qiu, Z. Q.; Park, A.; Wang, L. Z. J.; Wilsey, R.; Lee, M. , The RGD (Arg-Gly-Asp) is a potential cell-binding motif of UNC-52/PERLECAN. Biochem Bioph Res Co 2022, 586, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Rastelli, G. , Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci 2019, 20, (18). [Google Scholar] [CrossRef]
- Ferreira, L. G.; Dos Santos, R. N.; Oliva, G.; Andricopulo, A. D. , Molecular docking and structure-based drug design strategies. Molecules 2015, 20, (7), 13384–421. [Google Scholar] [CrossRef]
- Shuai, L.; Wang, J.; Zhao, D.; Wen, Z.; Ge, J.; He, X.; Wang, X.; Bu, Z. , Integrin beta1 Promotes Peripheral Entry by Rabies Virus. J Virol 2020, 94, (2). [Google Scholar] [CrossRef]
- Zhou, X.; Smith, Q. R.; Liu, X. L. , Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wires Nanomed Nanobi 2021, 13, (4). [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.; Duffy, S.; Breitbart, M. , A field guide to eukaryotic circular single-stranded DNA viruses: insights gained from metagenomics. Arch Virol 2012, 157, (10), 1851–71. [Google Scholar]
- Govindarajan, D.; Buchholz, U. J.; Samal, S. K. , Recovery of avian metapneumovirus subgroup C from cDNA: cross-recognition of avian and human metapneumovirus support proteins. J Virol 2006, 80, (12), 5790–7. [Google Scholar] [CrossRef]
- Cui, P.; Shi, J.; Wang, C.; Zhang, Y.; Xing, X.; Kong, H.; Yan, C.; Zeng, X.; Liu, L.; Tian, G.; Li, C.; Deng, G.; Chen, H. Global dissemination of H5N1 influenza viruses bearing the clade 2.3.4.4b HA gene and biologic
analysis of the ones detected in China. Emerg Microbes Infect 2022, 1-41.
- Stohr, K. , Avian influenza and pandemics--research needs and opportunities. N Engl J Med 2005, 352, (4), 405–7. [Google Scholar] [CrossRef]
Figure 1.
EDTA effectively inhibits infection by aMPV/C in cultured DF-1 cells. (A) Cell viability was analyzed after treatment with different concentrations of EDTA (0.3, 0.6, 1.25, 2.5, 5, and 10 mM) using an MTT assay. (B) DF-1 cells were treated by different concentrations of EDTA (0.3, 0.6, 1.25, and 2.5 mM) at 4°C for 1 h before viral adsorption; then, infected cells were incubated in a medium supplemented with 2% FBS for 72 h. Western blotting was used to quantify aMPV/C N protein expression, and GAPDH was used as a control. (C) RT-qPCR was used to determine the relative expression level of aMPV/C RNA in DF-1 cells after EDTA treatment. Expression was normalized to GAPDH mRNA. (D) Viral titers in the supernatants of aMPV/C-infected cells with different concentrations of EDTA treatment were determined 72 h post-infection using a TCID50 assay. (E) DF-1 cells were infected with aMPV/C after EDTA treatment. The cells were incubated with antibodies corresponding to aMPV/C N protein followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG antibody (green), and nuclei were incubated with DAPI (blue). The cells were examined under an inverted fluorescence microscope. Data are expressed as means ± SD from triplicate independent experiments (ns, not significant; **, P<0.01; ***, P<0.001; ****, P<0.0001).
Figure 1.
EDTA effectively inhibits infection by aMPV/C in cultured DF-1 cells. (A) Cell viability was analyzed after treatment with different concentrations of EDTA (0.3, 0.6, 1.25, 2.5, 5, and 10 mM) using an MTT assay. (B) DF-1 cells were treated by different concentrations of EDTA (0.3, 0.6, 1.25, and 2.5 mM) at 4°C for 1 h before viral adsorption; then, infected cells were incubated in a medium supplemented with 2% FBS for 72 h. Western blotting was used to quantify aMPV/C N protein expression, and GAPDH was used as a control. (C) RT-qPCR was used to determine the relative expression level of aMPV/C RNA in DF-1 cells after EDTA treatment. Expression was normalized to GAPDH mRNA. (D) Viral titers in the supernatants of aMPV/C-infected cells with different concentrations of EDTA treatment were determined 72 h post-infection using a TCID50 assay. (E) DF-1 cells were infected with aMPV/C after EDTA treatment. The cells were incubated with antibodies corresponding to aMPV/C N protein followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG antibody (green), and nuclei were incubated with DAPI (blue). The cells were examined under an inverted fluorescence microscope. Data are expressed as means ± SD from triplicate independent experiments (ns, not significant; **, P<0.01; ***, P<0.001; ****, P<0.0001).
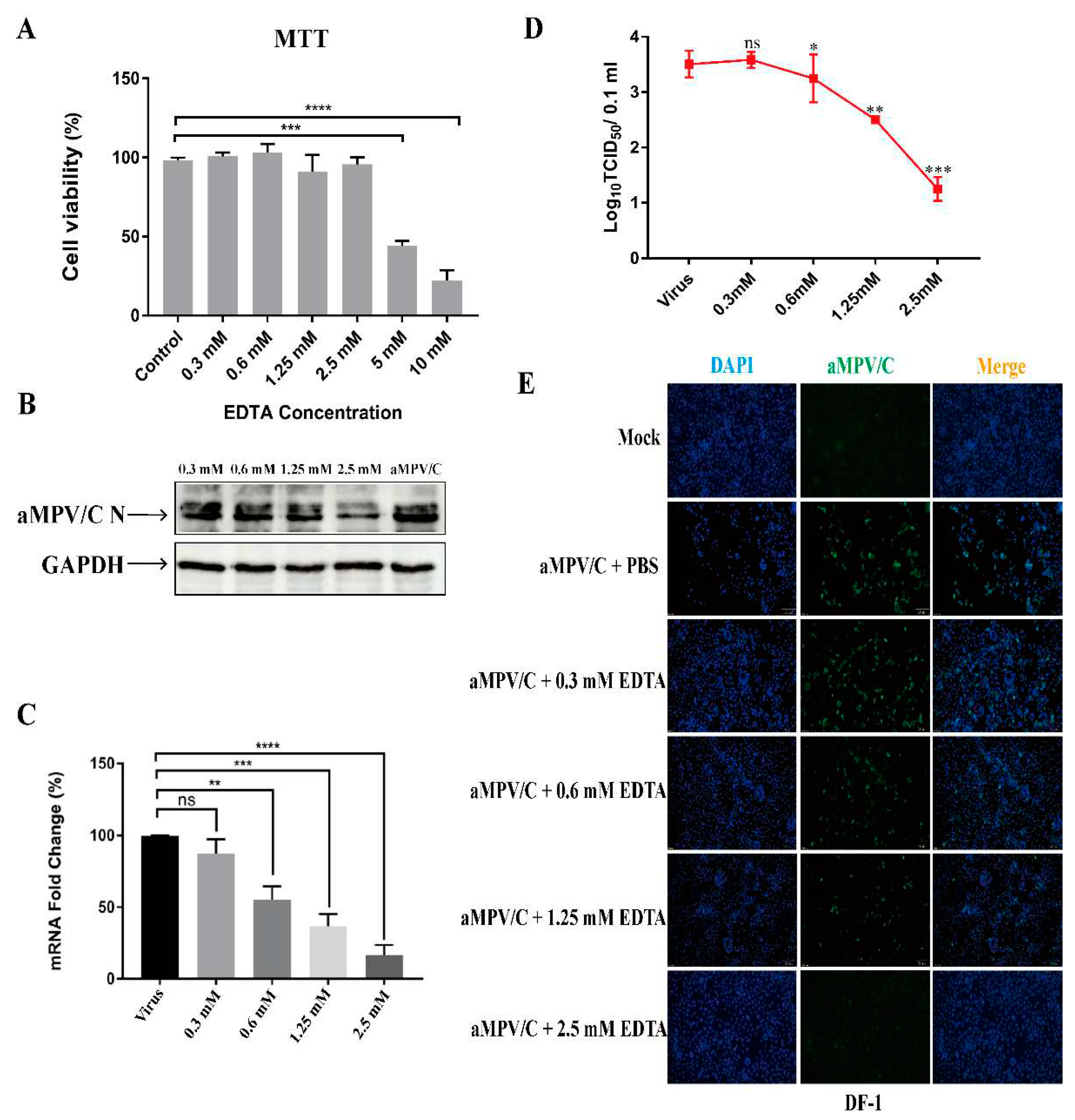
Figure 2.
Integrin function-blocking antibodies inhibit aMPV/C infection. (A) DF-1 cells were incubated with blocking antibodies to integrin or control IgG treatment before adsorption with aMPV/C and incubation in a medium supplemented with 2% FBS for 72 h. All antibodies were used at 20 μg/ml. Western blotting was utilized to quantify the expression level of the viral protein, and GAPDH was used as a control. (B) RT-qPCR was used to determine the aMPV/C M gene RNA level in DF-1 cells after antibody inhibition treatment. Expression was normalized to GAPDH mRNA. (C) Viral productions in the supernatants of aMPV/C-infected cells with different concentrations of EDTA treatment were determined 72 h post-infection using a TCID50 assay. Data are shown as means ± SD from triplicate independent experiments (**, P<0.01; ***, P<0.001).
Figure 2.
Integrin function-blocking antibodies inhibit aMPV/C infection. (A) DF-1 cells were incubated with blocking antibodies to integrin or control IgG treatment before adsorption with aMPV/C and incubation in a medium supplemented with 2% FBS for 72 h. All antibodies were used at 20 μg/ml. Western blotting was utilized to quantify the expression level of the viral protein, and GAPDH was used as a control. (B) RT-qPCR was used to determine the aMPV/C M gene RNA level in DF-1 cells after antibody inhibition treatment. Expression was normalized to GAPDH mRNA. (C) Viral productions in the supernatants of aMPV/C-infected cells with different concentrations of EDTA treatment were determined 72 h post-infection using a TCID50 assay. Data are shown as means ± SD from triplicate independent experiments (**, P<0.01; ***, P<0.001).
Figure 3.
RSD peptide effectively inhibits infection by aMPV/C in cultured DF-1 cells. (A) Cell viability was analyzed after treatment with different concentrations of the peptides GRGESP or GRSDSP using an MTT assay. (B) Different concentrations of GRSDSP (10, 20, and 40 μM) or the control peptide GRGESP (10, 20, and 40 μM) were mixed with aMPV/C and incubated at 4°C for 1 h; then, infected cells were incubated in a medium supplemented with 2% FBS for 72 h. Western blotting was used to quantify the protein expression level of aMPV/C N after GRSDSP and GRGESP peptide treatment, and GAPDH was used as a control. (C) RT-qPCR was used to analyze the aMPV/C RNA level in DF-1 cells after peptide treatment. Expression was normalized to GAPDH mRNA. (D) Viral titers in the supernatants of aMPV/C-infected cells under different peptide treatment were determined 72 h post-infection using a TCID50 assay. (E) Cells were infected with aMPV/C after GRSDSP and GRGESP peptide treatment and incubated in a medium supplemented with 2% FBS for 72 h. The cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were observed under an inverted fluorescence microscope. Data are expressed as means ± SD from triplicate independent experiments (ns, not significant; *, P<0.05; **, P<0.01; ***, P<0.001).
Figure 3.
RSD peptide effectively inhibits infection by aMPV/C in cultured DF-1 cells. (A) Cell viability was analyzed after treatment with different concentrations of the peptides GRGESP or GRSDSP using an MTT assay. (B) Different concentrations of GRSDSP (10, 20, and 40 μM) or the control peptide GRGESP (10, 20, and 40 μM) were mixed with aMPV/C and incubated at 4°C for 1 h; then, infected cells were incubated in a medium supplemented with 2% FBS for 72 h. Western blotting was used to quantify the protein expression level of aMPV/C N after GRSDSP and GRGESP peptide treatment, and GAPDH was used as a control. (C) RT-qPCR was used to analyze the aMPV/C RNA level in DF-1 cells after peptide treatment. Expression was normalized to GAPDH mRNA. (D) Viral titers in the supernatants of aMPV/C-infected cells under different peptide treatment were determined 72 h post-infection using a TCID50 assay. (E) Cells were infected with aMPV/C after GRSDSP and GRGESP peptide treatment and incubated in a medium supplemented with 2% FBS for 72 h. The cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were observed under an inverted fluorescence microscope. Data are expressed as means ± SD from triplicate independent experiments (ns, not significant; *, P<0.05; **, P<0.01; ***, P<0.001).
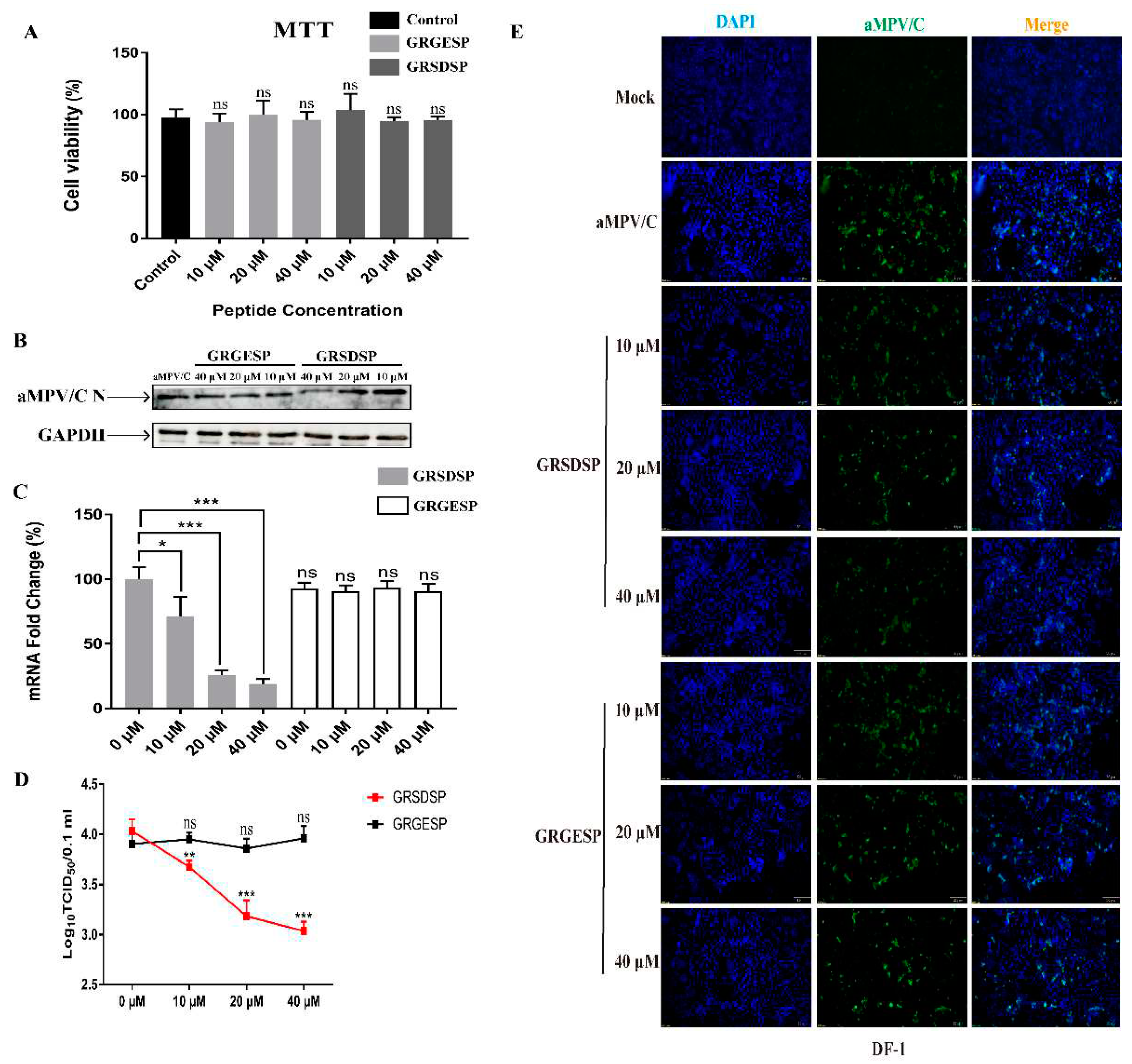
Figure 4.
aMPV/C F protein binds cITGB1. POCASA 1.1 was utilized to predict binding sites, and AutoDock Vina1.1.2 was utilized for docking. aMPV/C F protein could bind ITGB1, and its main binding region was located in the 643–695 region of ITGB1.
Figure 4.
aMPV/C F protein binds cITGB1. POCASA 1.1 was utilized to predict binding sites, and AutoDock Vina1.1.2 was utilized for docking. aMPV/C F protein could bind ITGB1, and its main binding region was located in the 643–695 region of ITGB1.
Figure 5.
Overexpression of cITGB1 promotes aMPV/C infection in DF-1 cells. (A-B) Cells were transfected with recombinant plasmid cITGB1 for 24 h and subsequently infected with aMPV/C for 72 h. The aMPV/C replication levels were detected using western blotting (A) and TCID50 assay (B). (C) Cells were infected with aMPV/C after transfection with cITGB1. The cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were observed under an inverted fluorescence microscope. (D-E) CRFK and DQ cells were transfected with recombinant plasmid cITGB1 for 24 h, incubated with the integrin-specific antibody, and subsequently infected with aMPV/C for 72 h. aMPV/C replication levels were detected using western blotting (D) and RT-qPCR (E). (F) DQ and CRFK cells were infected with aMPV/C after transfection with cITGB1. The cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were examined under an inverted fluorescence microscope. Data are shown as means ± SD from triplicate independent experiments (*, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001).
Figure 5.
Overexpression of cITGB1 promotes aMPV/C infection in DF-1 cells. (A-B) Cells were transfected with recombinant plasmid cITGB1 for 24 h and subsequently infected with aMPV/C for 72 h. The aMPV/C replication levels were detected using western blotting (A) and TCID50 assay (B). (C) Cells were infected with aMPV/C after transfection with cITGB1. The cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were observed under an inverted fluorescence microscope. (D-E) CRFK and DQ cells were transfected with recombinant plasmid cITGB1 for 24 h, incubated with the integrin-specific antibody, and subsequently infected with aMPV/C for 72 h. aMPV/C replication levels were detected using western blotting (D) and RT-qPCR (E). (F) DQ and CRFK cells were infected with aMPV/C after transfection with cITGB1. The cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were examined under an inverted fluorescence microscope. Data are shown as means ± SD from triplicate independent experiments (*, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001).
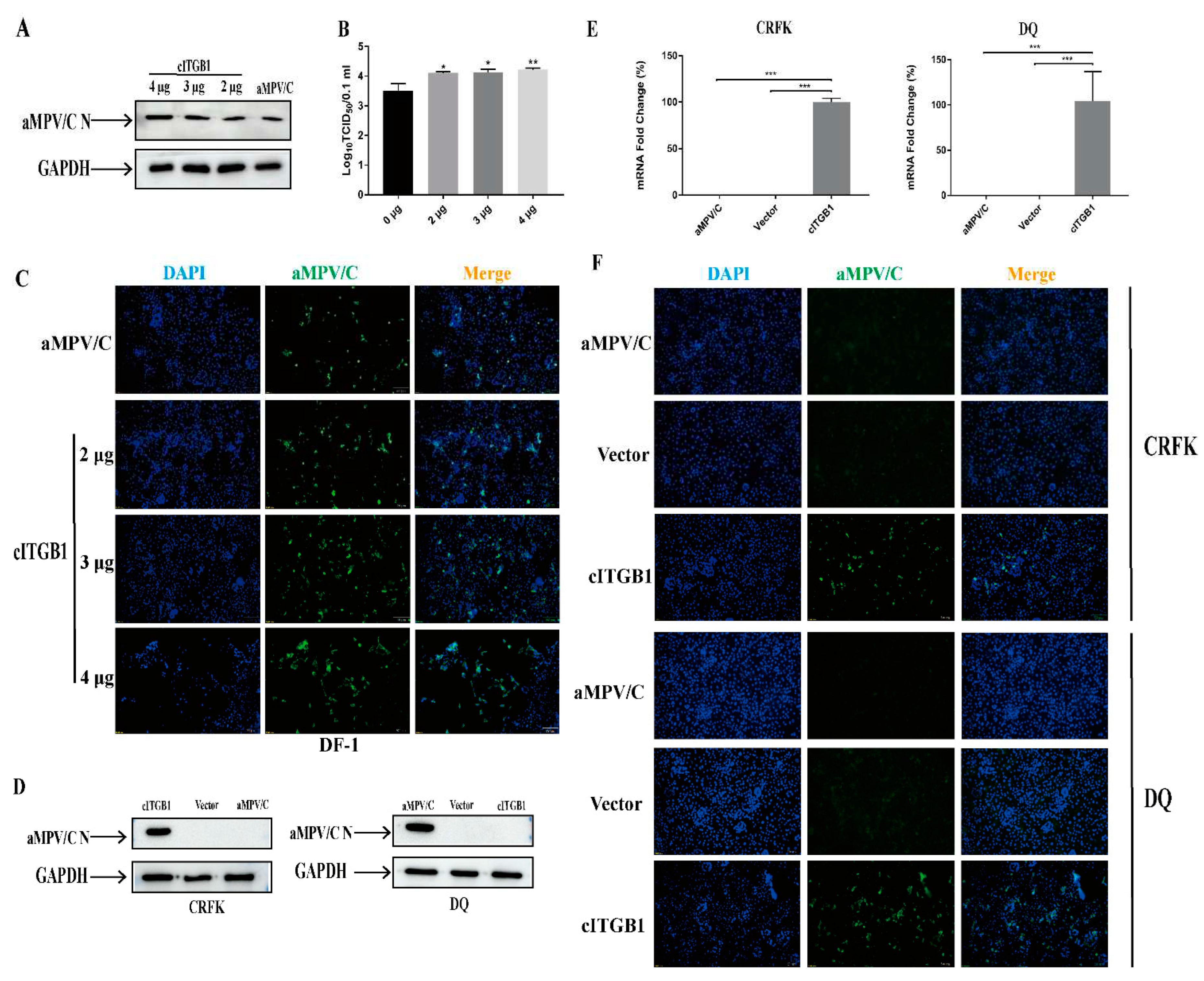
Figure 6.
Knockdown of cITGB1 expression inhibits aMPV/C infection. DF-1 cells were transfected with siRNA targeting cITGB1 or negative control siRNA for 24 h and subsequently infected with aMPV/C for 72 h. (A) Knockdown effects of different concentrations of siRNA on ITGB1. (B) Western blotting was used to analyze the viral protein expression level after siRNA knockdown, and GAPDH was used as a control. (C) Viral titers in the supernatants of aMPV/C-infected cells after siRNA knockdown were determined 72 h post-infection using TCID50 assay. (D) DF-1 cells were transfected with siRNA targeting cITGB1 or negative control siRNA and subsequently infected with aMPV/C for 72 h. The cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were examined under an inverted fluorescence microscope. Data are shown as means ± SD from triplicate independent experiments (***, P<0.001).
Figure 6.
Knockdown of cITGB1 expression inhibits aMPV/C infection. DF-1 cells were transfected with siRNA targeting cITGB1 or negative control siRNA for 24 h and subsequently infected with aMPV/C for 72 h. (A) Knockdown effects of different concentrations of siRNA on ITGB1. (B) Western blotting was used to analyze the viral protein expression level after siRNA knockdown, and GAPDH was used as a control. (C) Viral titers in the supernatants of aMPV/C-infected cells after siRNA knockdown were determined 72 h post-infection using TCID50 assay. (D) DF-1 cells were transfected with siRNA targeting cITGB1 or negative control siRNA and subsequently infected with aMPV/C for 72 h. The cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were examined under an inverted fluorescence microscope. Data are shown as means ± SD from triplicate independent experiments (***, P<0.001).
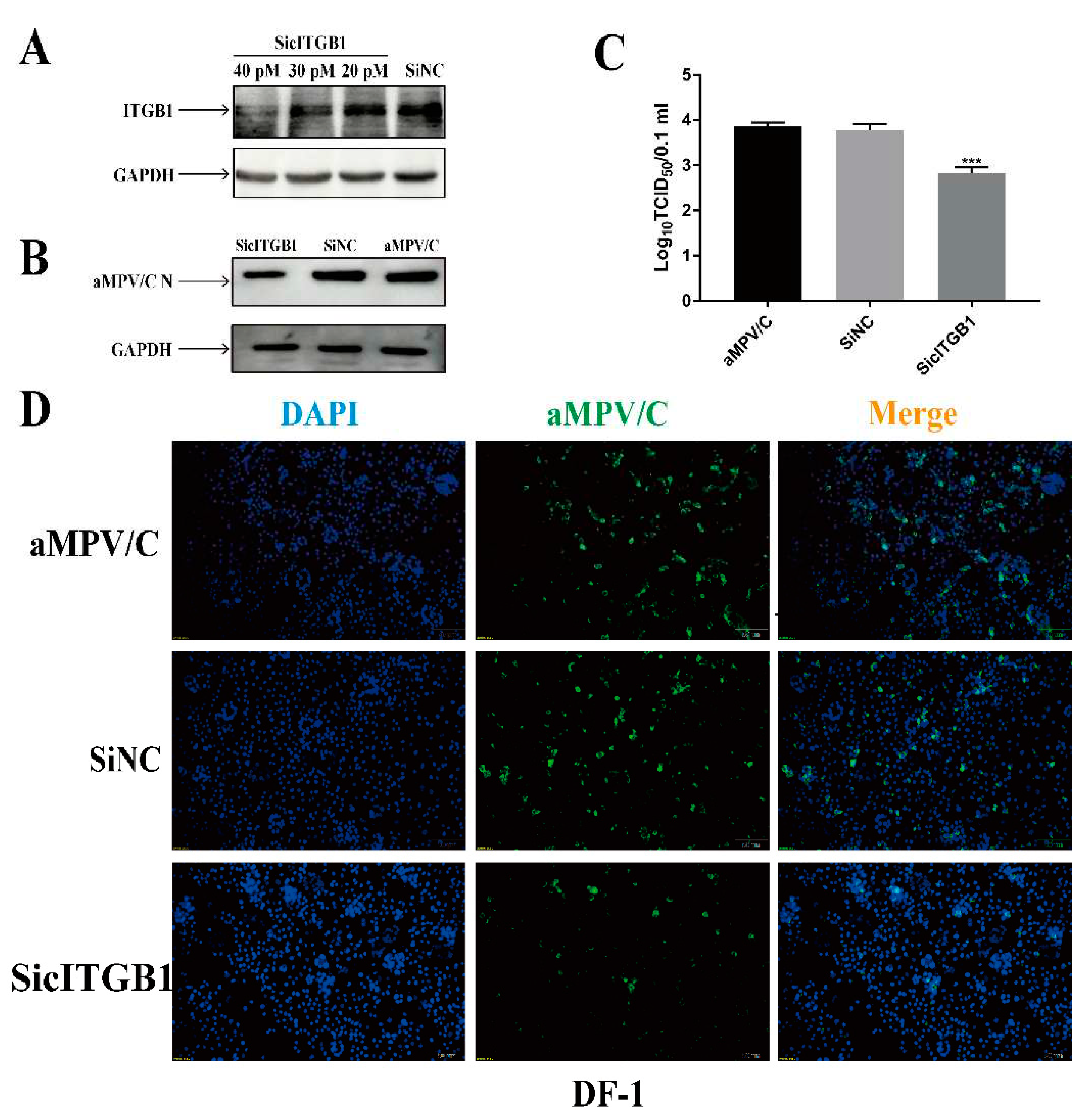
Figure 7.
aMPV/C relies on ITGB1 adsorption on cell surface. (A-B) EDTA inhibited aMPV/C attachment. DF-1 cells were treated by EDTA (2.5 mM) at 4°C for 1 h before viral adsorption. After that, the EDTA treated DF-1 cells were infected by aMPV/C strain JC for 1 h at 4°C (virus attachment), followed by removal of unbound viruses, then incubated at 37°C for 1 h (virus entry), and detected viral load by RT-qPCR. (C-F) RSD peptide (GRSDSP) blocked aMPV/C attachment in DF-1 cells. GRSDSP peptide or control peptide (40 μM) were mixed with aMPV/C at an MOI of 2 at 4°C for 1 h. And, DF-1 cells were infected with mixed peptide and aMPV/C for 1 h at 4°C (virus attachment) (C and E), followed by removal of unbound viruses, then incubated at 37°C for 1 h (virus entry) (D and F). (G- H) Knockdown the ITGB1 expression can inhibit aMPV/C attachment. DF-1 cells were transfected with siRNA targeting cITGB1 or negative control siRNA for 24 h, after that, the cells were infected with aMPV/C for 1 h at 4°C for detecting virus attachment level (G), followed by removal of unbound viruses, then incubated at 37°C for 1 h for detecting virus entry level (H) by RT-qPCR (**, P<0.01; ***, P<0.001).
Figure 7.
aMPV/C relies on ITGB1 adsorption on cell surface. (A-B) EDTA inhibited aMPV/C attachment. DF-1 cells were treated by EDTA (2.5 mM) at 4°C for 1 h before viral adsorption. After that, the EDTA treated DF-1 cells were infected by aMPV/C strain JC for 1 h at 4°C (virus attachment), followed by removal of unbound viruses, then incubated at 37°C for 1 h (virus entry), and detected viral load by RT-qPCR. (C-F) RSD peptide (GRSDSP) blocked aMPV/C attachment in DF-1 cells. GRSDSP peptide or control peptide (40 μM) were mixed with aMPV/C at an MOI of 2 at 4°C for 1 h. And, DF-1 cells were infected with mixed peptide and aMPV/C for 1 h at 4°C (virus attachment) (C and E), followed by removal of unbound viruses, then incubated at 37°C for 1 h (virus entry) (D and F). (G- H) Knockdown the ITGB1 expression can inhibit aMPV/C attachment. DF-1 cells were transfected with siRNA targeting cITGB1 or negative control siRNA for 24 h, after that, the cells were infected with aMPV/C for 1 h at 4°C for detecting virus attachment level (G), followed by removal of unbound viruses, then incubated at 37°C for 1 h for detecting virus entry level (H) by RT-qPCR (**, P<0.01; ***, P<0.001).
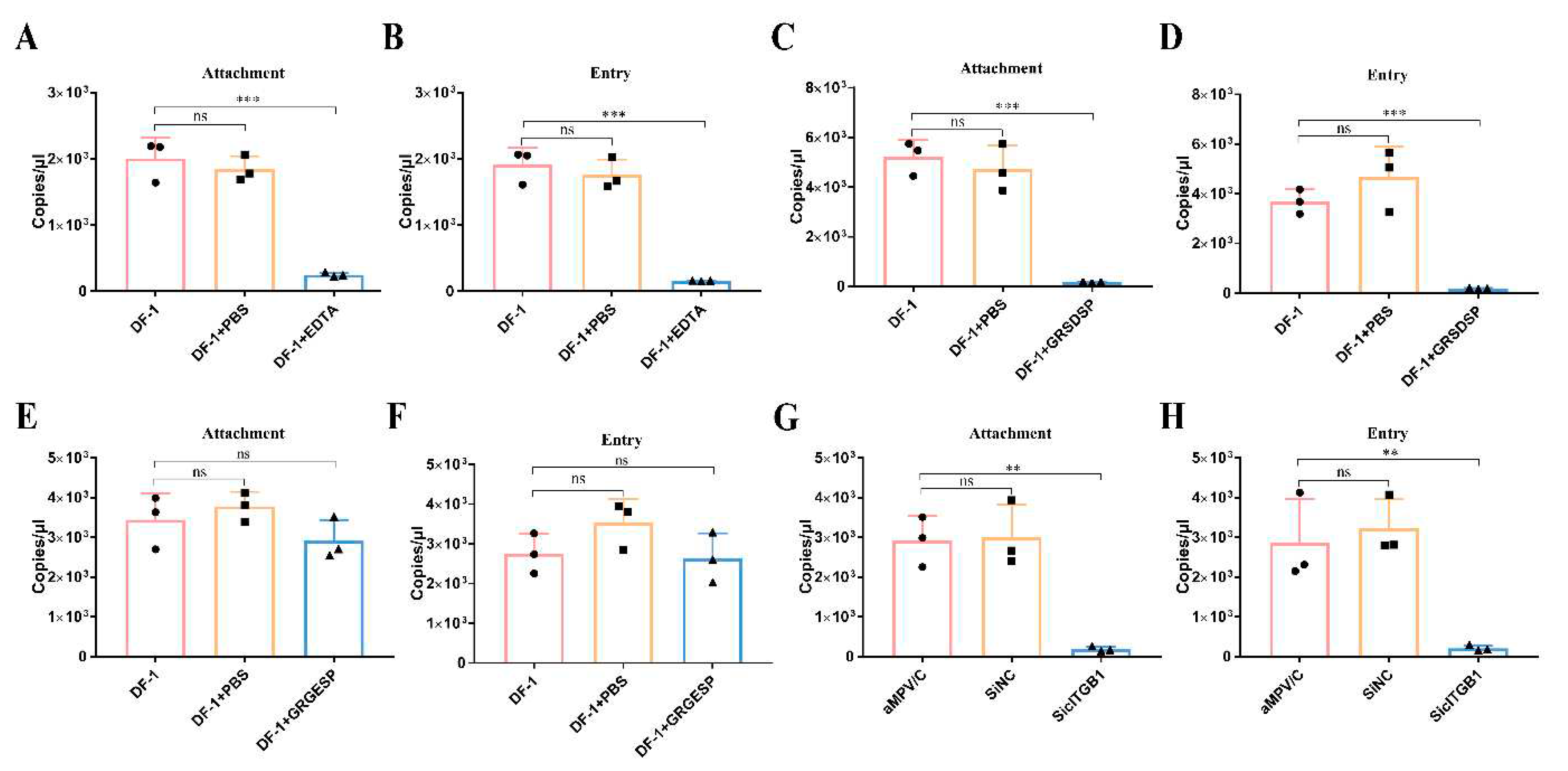
Figure 8.
ITGB1 promotes aMPV/C infection in human cells. A549 cells were transfected with recombinant plasmid cITGB1 or hITGB1 for 24 h and subsequently infected with aMPV/C for 72 h. (A) Western blotting was used to analyze the viral protein expression level after transfection with cITGB1 or hITGB1 in A549 cells, and GAPDH was used as a control. (B) RT-qPCR was used to analyze the relative aMPV/C RNA levels in A549 cells after transfection with cITGB1 or hITGB1. Expression was normalized to GAPDH mRNA. (C) After transfection with the expression plasmid cITGB1 or hITGB1, A549 cells were infected with aMPV/C for 72 h. Subsequently, the cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were examined under an inverted fluorescence microscope.
Figure 8.
ITGB1 promotes aMPV/C infection in human cells. A549 cells were transfected with recombinant plasmid cITGB1 or hITGB1 for 24 h and subsequently infected with aMPV/C for 72 h. (A) Western blotting was used to analyze the viral protein expression level after transfection with cITGB1 or hITGB1 in A549 cells, and GAPDH was used as a control. (B) RT-qPCR was used to analyze the relative aMPV/C RNA levels in A549 cells after transfection with cITGB1 or hITGB1. Expression was normalized to GAPDH mRNA. (C) After transfection with the expression plasmid cITGB1 or hITGB1, A549 cells were infected with aMPV/C for 72 h. Subsequently, the cells were incubated with antibodies against aMPV/C N protein, followed by incubation with FITC-conjugated goat anti-mouse IgG (green) secondary antibodies. Nuclei were incubated with DAPI (blue) and the cells were examined under an inverted fluorescence microscope.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).