Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General Workflow
2.2. Model Development
2.3. PBPK model development in LC patients
2.4. Criterion of the developed PBPK model.
3. Results
3.1. Collection of data and selection of the tested drugs
| No | Authors | Drug | Dose (mg) | Analytes | Subjects(n) | Ref |
| 1 | Ohnishi A et al. 1989 | enalapril maleate | 10, p.o | enalapril, enalaprilat | Healthy (7) | [14] |
| enalapril maleate | 10, p.o | enalapril, enalaprilat | CP-C (7) | |||
| 2 | Todd PA et al. 1986 | enalapril maleate | 10, p.o | enalapril, enalaprilat | Healthy(12) | [102] |
| 3 | Weisser K et al.1991 | enalapril maleate | 10, p.o | enalapril, enalaprilat | Healthy (8) | [103] |
| 4 | Dickstein K et al. 1987 | enalapril maleate | 10, p.o | enalapril, enalaprilat | Healthy(10) | [104] |
| 5 | Baba T et al. 1990 | enalapril maleate | 10 , p.o | enalapril, enalaprilat | CP-B (7) | [105] |
| 6 | Kaiser G et al. 1989 | benazepril.HC1 | 10, p.o | benazepril, benazeprilat | Healthy(59) | [106] |
| 7 | Schweizer C et al. 1993 | benazepril.HC1 | 10,p.o | benazepril, benazeprilat | Healthy(11) | [107] |
| 8 | Sioufi A et al.1994 | benazepril.HC1 | 20 , p.o | benazepril, benazeprilat | Healthy(24) | [108] |
| 9 | Waldmeier F et al. 1991 | benazepril.HC1 | 20, p.o | benazepril, benazeprilat | Healthy (4) | [109] |
| 10 | Kaiser G et al. 1990 | benazepril.HC1 | 20, p.o | benazepril, benazeprilat | CP-B(12) | [110] |
| 11 | Macdonald NJ et al.1993 | benazepril HCl | 10 , p.o | benazeprilat | Healthy(18) | [111] |
| 12 | Massarella J et al 1989 | cilazapril | 1.0,2.5,5, p.o | cilazapril, cilazaprilat | Healthy(24) | [65] |
| 13 | Williams PEO et al. 1990 | cilazapril | 2.5,p.o | cilazapril, cilazaprilat | Healthy(13) | [112] |
| 14 | Gross V et al.1993 | cilazapril | 1,p.o | cilazapril, cilazaprilat | Healthy(10) | [113] |
| cilazapril | 1,p.o | cilazapril, cilazaprilat | CP-B(9) | |||
| 15 | Williams PEO et al. 1989 | cilazapril | 1,p.o | cilazapril, cilazaprilat | Healthy(12) | [114] |
| 16 | Massarella JW et al. 1989 | cilazapril | 5.p.o | cilazapril, cilazaprilat | Healthy(16) | [115] |
| 17 | Francis RJ et al. 1987 | cilazapril | 1.25,2.5,5,10,p.o | cilazaprilat | Healthy(12) | [116] |
| 18 | Lecocq B et al. 1990 | perindoprila | 4,p.o | perindopril, perindoprilat | Healthy(12) | [117] |
| 19 | Tsai HH et al. 1989 | perindoprila | 8,p.o | perindopril, perindoprilat | CP-A(8) | [118] |
| 20 | Thiollet M et al. 1992 | perindoprila | 8,p.o | perindopril, perindoprilat | CP-B(10) | [119] |
| 21 | Lees KR et al. 1988 | perindoprila | 8,p.o | perindoprilat | Healthy(8) | [120] |
| 22 | Furuta S et al. 1993 | temocapril HCL | 1.p.o | temocapril, temocaprilat | Healthy(6) | [121] |
| temocapril HCL | 1,p.o | temocapril, temocaprilat | CP-C(7) | |||
| 23 | Abe M et al. 2006 | oseltamivirb | 75,p.o | oseltamivir,OC | Healthy(7) | [122] |
| 24 | Brewster M et al. 2006 | oseltamivirb | 75,p.o | oseltamivir,OC | Healthy(18) | [123] |
| 25 | Jittamala P et al. 2014 | oseltamivirb | 75,p.o | oseltamivir,OC | Healthy(12) | [124] |
| oseltamivirb | 150,p.o | oseltamivir,OC | Healthy(12) | |||
| 26 | Snell P et al. 2005 | oseltamivirb | 75, p.o | oseltamivir,OC | CP-B(11) | [15] |
| 27 | Amrei R et al. 1990 | flumazenil | 10mg,i.v. | flumazenil | Healthy(NA) | [125] |
| 28 | Breimer LTM et al. 1991 | flumazenil | 10/10 min,iv | flumazenil | Healthy(7) | [126] |
| 29 | Pomier-Layrargues G et al. 1989 | flumazenil | 2/5min,iv | flumazenil | CP-B(8) | [127] |
| flumazenil | 2/5min,iv | flumazenil | CP-C(8) | |||
| 30 | Klotz U, et al.1984 | flumazenil | 2.5,i.v | flumazenil | Healthy(6) | [82] |
| 31 | Janssen U,et al.1989 | flumazenil | 30 p.o | flumazenil | Healthy(8) | [128] |
| flumazenil | 2,i.v; 30 p.o | flumazenil | CP-C(8) | |||
| 32 | Verbeeck RK et al.1981 | pethidine HCL | 25,i.v | pethidine | Healthy(6) | [129] |
| pethidine HCL | 25,p.o | pethidine | Healthy(6) | |||
| 33 | Mather LE et al. 1975 | pethidine HCL | 50,i.v | pethidine | Healthy(4) | [130] |
| 34 | Kuhnert BR et al. 1980 | pethidine HCL | 50,i.v | pethidine | Healthy(7) | [131] |
| 35 | Guay DR et al. 1984 | pethidine HCL | 70,i.v | pethidine | Healthy(8) | [132] |
| 36 | Guay DR et al. 1985 | pethidine HCL | 70,i.v | pethidine | Healthy(8) | [133] |
| 37 | Pond SM et al. 1981 | pethidine HCL | 60, iv;112, po | pethidine | CP-A (5) | [134] |
| 38 | Pond SM et al. 1980 | pethidine HCL | 54.4, iv;108.8, po | pethidine | CP-B (4) | [135] |
| 39 | Mather LE et al. 1976 | pethidine HCL | 50, iv;100, po | pethidine | Healthy(4) | [136] |
| 40 | Klotz U et al. 1974 | pethidine HCL | 63.9,i.v | pethidine | Healthy(8) | [137] |
| pethidine HCL | 53.1,i.v | pethidine | CP-A(10) | |||
| 41 | Neal EA et al. 1979 | pethidine HCL | 56, iv; 56, po | pethidine | Healthy(4) | [138] |
| pethidine HCL | 56, iv; 56, po | pethidine | CP-A(8) | |||
| 42 | Sheng XY et al. 2020 | remimazolam besylate | 1.5425,3.315,i.v | remimazolam | Healthy(3) | [80] |
| remimazolam besylate | 4.8675,6.18,i.v | remimazolam | Healthy(7) | |||
| remimazolam besylate | 13.26,24.6,i.v | remimazolam | Healthy(8) | |||
| remimazolam besylate | 18.3,i.v | remimazolam | Healthy(10) | |||
| 43 | Stohr T et al. 2021 | remimazolam besylate | 10.4,i.v | remimazolam | CP-B(8) | [139] |
| remimazolam besylate | 8.2,i.v | remimazolam | CP-C(3) |
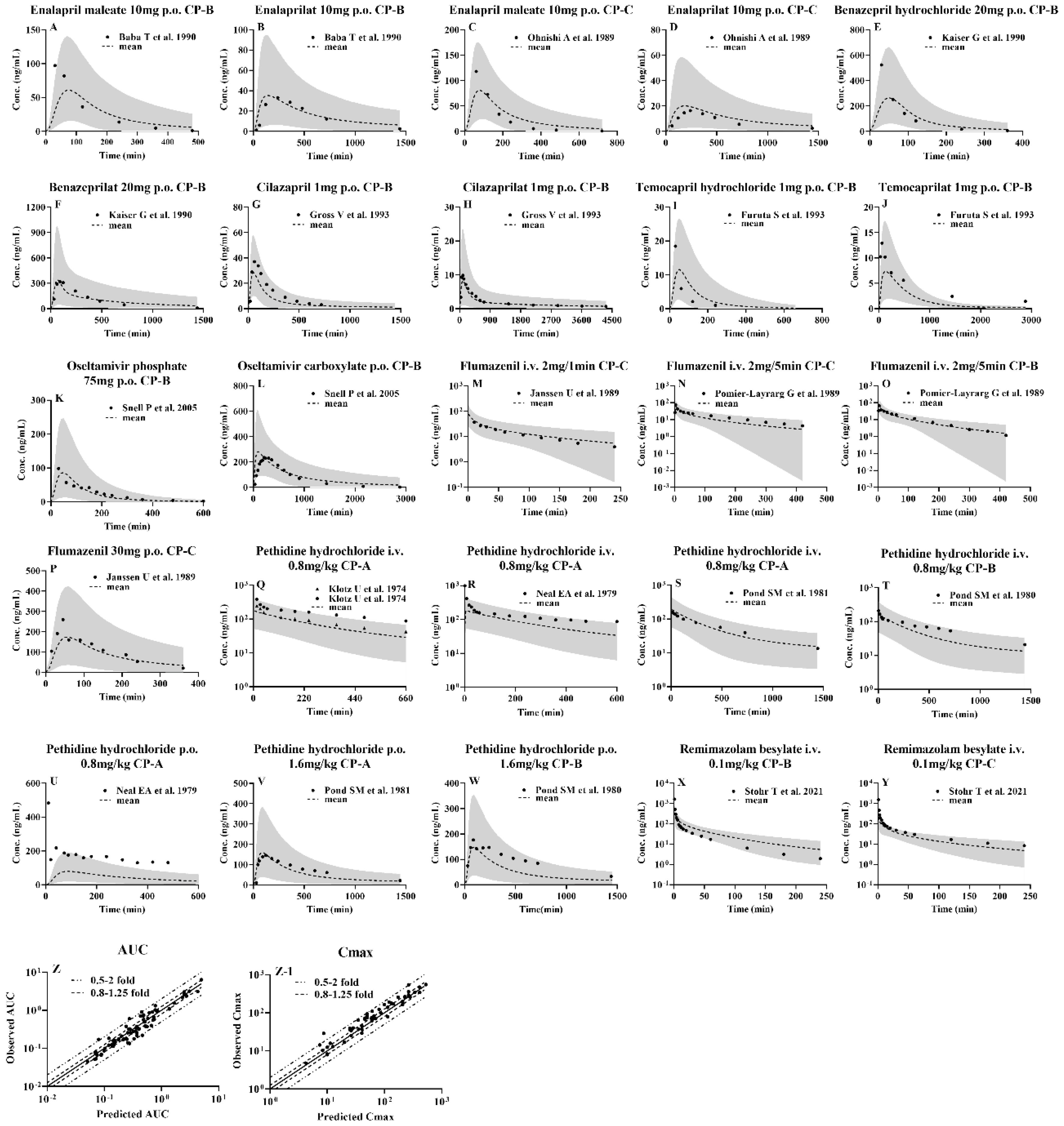
3.2. Development of PBPK model and validation using pharmacokinetic parameters from healthy subjects following i.v. or oral administrations
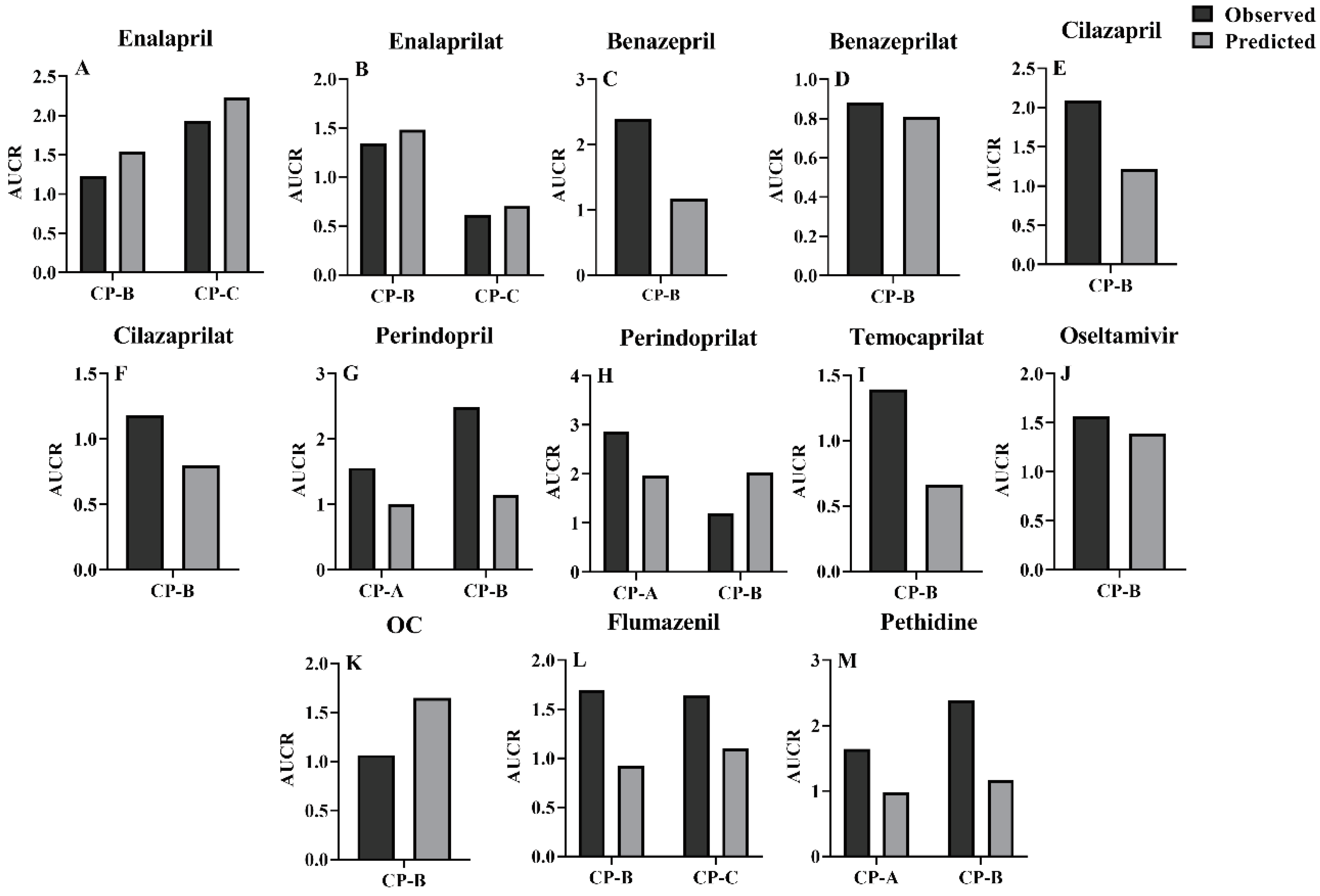
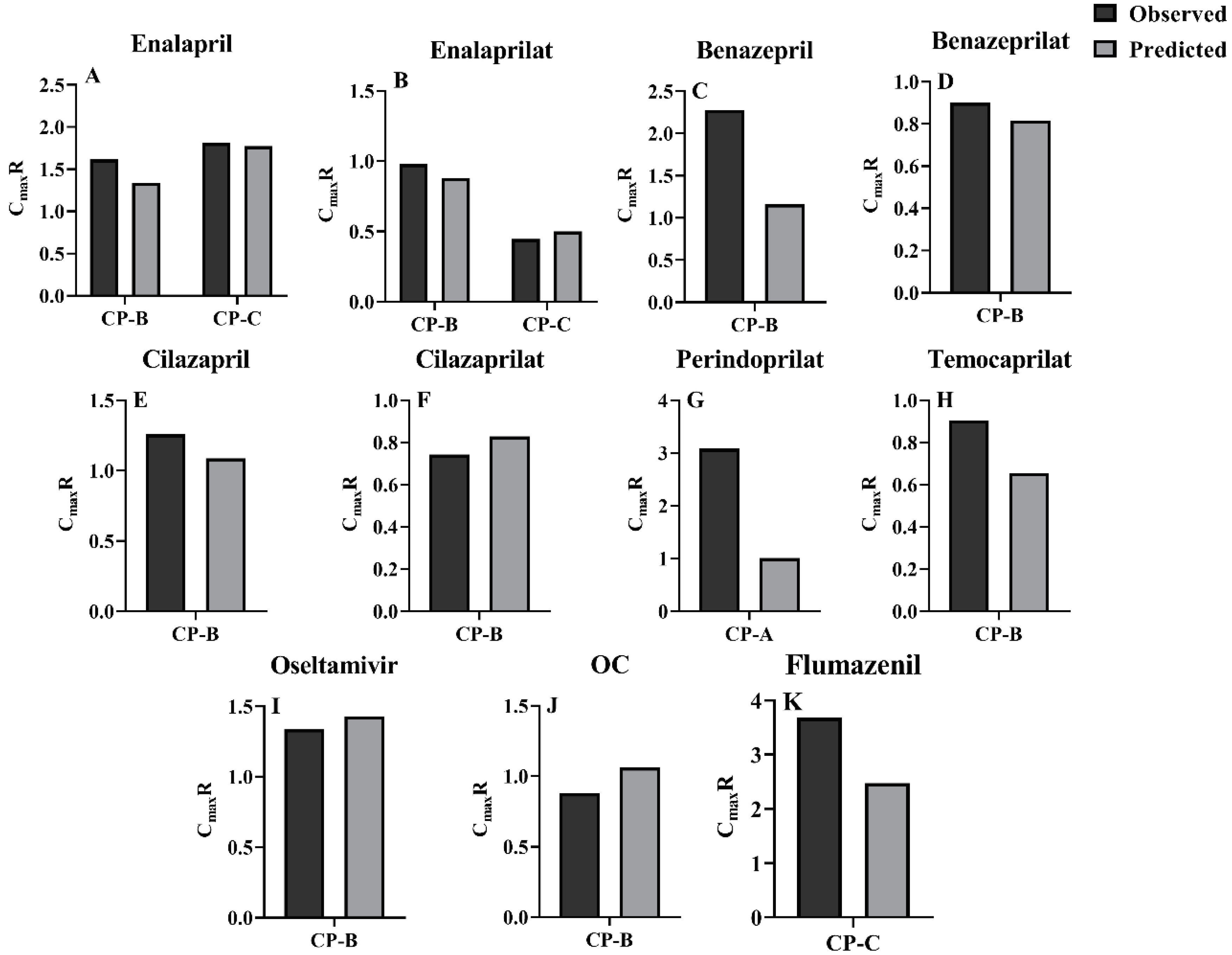
3.3. Prediction of pharmacokinetic profiles for CES1 substrates and their active metabolites following i.v. or oral administration to LC patients using the developed PBPK model
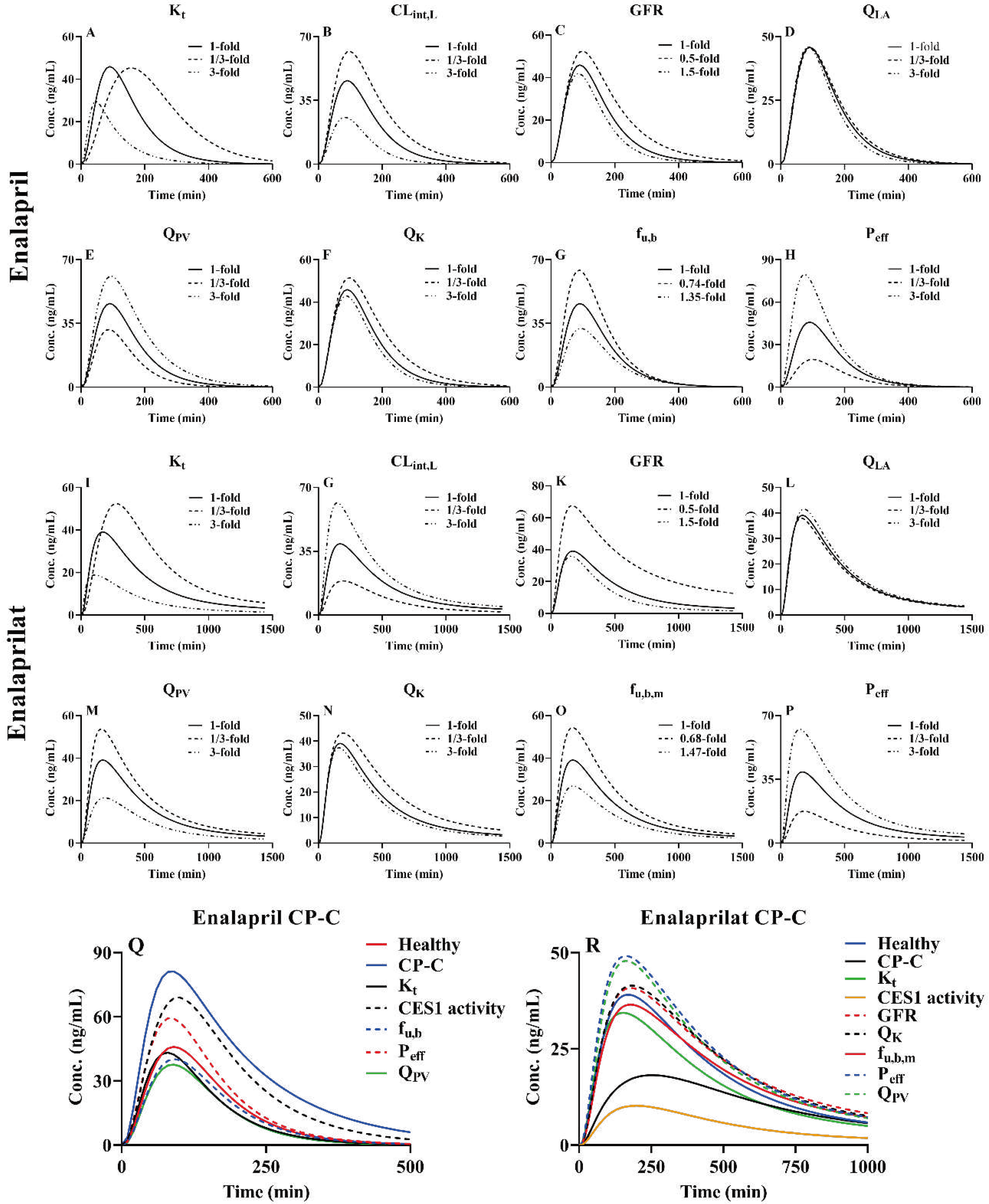
3.4. Sensitivity analysis of model parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gines, P.; Krag, A.; Abraldes, J.G.; Sola, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Lee, N.Y.; Suk, K.T. The Role of the Gut Microbiome in Liver Cirrhosis Treatment. Int J Mol Sci 2020, 22. [Google Scholar] [CrossRef]
- El-Khateeb, E.; Darwich, A.S.; Achour, B.; Athwal, V.; Rostami-Hodjegan, A. Review article: time to revisit Child-Pugh score as the basis for predicting drug clearance in hepatic impairment. Aliment Pharmacol Ther 2021, 54, 388–401. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Weersink, R.A.; Burger, D.M.; Hayward, K.L.; Taxis, K.; Drenth, J.P.H.; Borgsteede, S.D. Safe use of medication in patients with cirrhosis: pharmacokinetic and pharmacodynamic considerations. Expert Opin Drug Metab Toxicol 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Duthaler, U.; Bachmann, F.; Suenderhauf, C.; Grandinetti, T.; Pfefferkorn, F.; Haschke, M.; Hruz, P.; Bouitbir, J.; Krahenbuhl, S. Liver Cirrhosis Affects the Pharmacokinetics of the Six Substrates of the Basel Phenotyping Cocktail Differently. Clin Pharmacokinet 2022, 61, 1039–1055. [Google Scholar] [CrossRef]
- Villeneuve, J.P.; Verbeeck, R.K.; Wilkinson, G.R.; Branch, R.A. Furosemide kinetics and dynamics in patients with cirrhosis. Clin Pharmacol Ther 1986, 40, 14–20. [Google Scholar] [CrossRef]
- Thakkar, N.; Slizgi, J.R.; Brouwer, K.L.R. Effect of Liver Disease on Hepatic Transporter Expression and Function. J Pharm Sci 2017, 106, 2282–2294. [Google Scholar] [CrossRef]
- Chen, Y.; Ke, M.; Xu, J.; Lin, C. Simulation of the Pharmacokinetics of Oseltamivir and Its Active Metabolite in Normal Populations and Patients with Hepatic Cirrhosis Using Physiologically Based Pharmacokinetic Modeling. AAPS PharmSciTech 2020, 21, 98. [Google Scholar] [CrossRef]
- Her, L.; Zhu, H.J. Carboxylesterase 1 and Precision Pharmacotherapy: Pharmacogenetics and Nongenetic Regulators. Drug Metab Dispos 2020, 48, 230–244. [Google Scholar] [CrossRef]
- Hosokawa, M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules 2008, 13, 412–431. [Google Scholar] [CrossRef]
- Laizure, S.C.; Herring, V.; Hu, Z.; Witbrodt, K.; Parker, R.B. The role of human carboxylesterases in drug metabolism: have we overlooked their importance? Pharmacotherapy 2013, 33, 210–222. [Google Scholar] [CrossRef]
- Ross, M.K.; Streit, T.M.; Herring, K.L. Carboxylesterases: Dual roles in lipid and pesticide metabolism. J Pestic Sci 2010, 35, 257–264. [Google Scholar] [CrossRef]
- Ohnishi, A.; Tsuboi, Y.; Ishizaki, T.; Kubota, K.; Ohno, T.; Yoshida, H.; Kanezaki, A.; Tanaka, T. Kinetics and dynamics of enalapril in patients with liver cirrhosis. Clin Pharmacol Ther 1989, 45, 657–665. [Google Scholar] [CrossRef]
- Snell, P.; Dave, N.; Wilson, K.; Rowell, L.; Weil, A.; Galitz, L.; Robson, R. Lack of effect of moderate hepatic impairment on the pharmacokinetics of oral oseltamivir and its metabolite oseltamivir carboxylate. Br J Clin Pharmacol 2005, 59, 598–601. [Google Scholar] [CrossRef]
- Qian, C.Q.; Zhao, K.J.; Chen, Y.; Liu, L.; Liu, X.D. Simultaneously predict pharmacokinetic interaction of rifampicin with oral versus intravenous substrates of cytochrome P450 3A/P-glycoprotein to healthy human using a semi-physiologically based pharmacokinetic model involving both enzyme and transporter turnover. Eur J Pharm Sci 2019, 134, 194–204. [Google Scholar] [CrossRef]
- Edginton, A.N.; Willmann, S. Physiology-based simulations of a pathological condition: prediction of pharmacokinetics in patients with liver cirrhosis. Clin Pharmacokinet 2008, 47, 743–752. [Google Scholar] [CrossRef]
- Guo, H.; Liu, C.; Li, J.; Zhang, M.; Hu, M.; Xu, P.; Liu, L.; Liu, X. A mechanistic physiologically based pharmacokinetic-enzyme turnover model involving both intestine and liver to predict CYP3A induction-mediated drug-drug interactions. J Pharm Sci 2013, 102, 2819–2836. [Google Scholar] [CrossRef]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm Res 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- Li, R.; Barton, H.A.; Maurer, T.S. A Mechanistic Pharmacokinetic Model for Liver Transporter Substrates Under Liver Cirrhosis Conditions. CPT Pharmacometrics Syst Pharmacol 2015, 4, 338–349. [Google Scholar] [CrossRef]
- Johnson, T.N.; Boussery, K.; Rowland-Yeo, K.; Tucker, G.T.; Rostami-Hodjegan, A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet 2010, 49, 189–206. [Google Scholar] [CrossRef]
- Karlsen, S.; Fynne, L.; Gronbaek, H.; Krogh, K. Small intestinal transit in patients with liver cirrhosis and portal hypertension: a descriptive study. BMC Gastroenterol 2012, 12, 176. [Google Scholar] [CrossRef]
- Rodriquez, A.; Martin, A.; Oterino, J.A.; Blanco, I.; Jimenez, M.; Perez, A.; Novoa, J.M. Renal function in compensated hepatic cirrhosis: effects of an amino acid infusion and relationship with nitric acid. Dig Dis 1999, 17, 235–240. [Google Scholar] [CrossRef]
- Zuckerman, M.J.; Menzies, I.S.; Ho, H.; Gregory, G.G.; Casner, N.A.; Crane, R.S.; Hernandez, J.A. Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci 2004, 49, 621–626. [Google Scholar] [CrossRef]
- Jacobsen, A.C.; Nielsen, S.; Brandl, M.; Bauer-Brandl, A. Drug Permeability Profiling Using the Novel Permeapad(R) 96-Well Plate. Pharm Res 2020, 37, 93. [Google Scholar] [CrossRef]
- Shin, B.S.; Yoon, C.H.; Balthasar, J.P.; Choi, B.Y.; Hong, S.H.; Kim, H.J.; Lee, J.B.; Hwang, S.W.; Yoo, S.D. Prediction of drug bioavailability in humans using immobilized artificial membrane phosphatidylcholine column chromatography and in vitro hepatic metabolic clearance. Biomed Chromatogr 2009, 23, 764–769. [Google Scholar] [CrossRef]
- Claassen, K.; Willmann, S.; Eissing, T.; Preusser, T.; Block, M. A detailed physiologically based model to simulate the pharmacokinetics and hormonal pharmacodynamics of enalapril on the circulating endocrine Renin-Angiotensin-aldosterone system. Front Physiol 2013, 4, 4. [Google Scholar] [CrossRef]
- Jogiraju, V.K.; Avvari, S.; Gollen, R.; Taft, D.R. Application of physiologically based pharmacokinetic modeling to predict drug disposition in pregnant populations. Biopharm Drug Dispos 2017, 38, 426–438. [Google Scholar] [CrossRef]
- Remko, M. Acidity, lipophilicity, solubility, absorption, and polar surface area of some ACE inhibitors. Chemical Papers 2007, 61. [Google Scholar] [CrossRef]
- Nishimuta, H.; Houston, J.B.; Galetin, A. Hepatic, intestinal, renal, and plasma hydrolysis of prodrugs in human, cynomolgus monkey, dog, and rat: implications for in vitro-in vivo extrapolation of clearance of prodrugs. Drug Metab Dispos 2014, 42, 1522–1531. [Google Scholar] [CrossRef]
- Sun, J.X.; Cipriano, A.; Chan, K.; John, V.A. Pharmacokinetic interaction study between benazepril and amlodipine in healthy subjects. Eur J Clin Pharmacol 1994, 47, 285–289. [Google Scholar] [CrossRef]
- Navia, M.; Chaturvedi, P. Design principles for orally bioavailable drugs. Drug Discovery Today 1996, 1, 179–189. [Google Scholar] [CrossRef]
- Wu, L.P.; Cui, Y.; Xiong, M.J.; Wang, S.R.; Chen, C.; Ye, L.M. Mixed micellar liquid chromatography methods: modelling quantitative retention-activity relationships of angiotensin converting enzyme inhibitors. Biomed Chromatogr 2008, 22, 1243–1251. [Google Scholar] [CrossRef]
- Sugihara, M.; Takeuchi, S.; Sugita, M.; Higaki, K.; Kataoka, M.; Yamashita, S. Analysis of Intra- and Intersubject Variability in Oral Drug Absorption in Human Bioequivalence Studies of 113 Generic Products. Mol Pharm 2015, 12, 4405–4413. [Google Scholar] [CrossRef]
- Kitagawa, S.; Takeda, J.; Sato, S. pH-dependent inhibitory effects of angiotensin-converting enzyme inhibitors on cefroxadine uptake by rabbit small intestinal brush-border membrane vesicles and their relationship with hydrophobicity and the ratio of zwitterionic species. Biol Pharm Bull 1999, 22, 721–724. [Google Scholar] [CrossRef]
- Ohura, K. [Evaluation of the Oral Absorption of Ester-type Prodrugs]. Yakugaku Zasshi 2020, 140, 369–376. [Google Scholar] [CrossRef]
- Vistoli, G.; Pedretti, A.; Testa, B. Chemodiversity and molecular plasticity: recognition processes as explored by property spaces. Future Med Chem 2011, 3, 995–1010. [Google Scholar] [CrossRef]
- Shitara, Y.; Maeda, K.; Ikejiri, K.; Yoshida, K.; Horie, T.; Sugiyama, Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos 2013, 34, 45–78. [Google Scholar] [CrossRef]
- Helal, F.; Lane, M.E. Transdermal delivery of Angiotensin Converting Enzyme inhibitors. Eur J Pharm Biopharm 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Ono, A.; Tomono, T.; Ogihara, T.; Terada, K.; Sugano, K. Investigation of biopharmaceutical drug properties suitable for orally disintegrating tablets. ADMET and DMPK 2016, 4. [Google Scholar] [CrossRef]
- Sun, H. Capture hydrolysis signals in the microsomal stability assay: molecular mechanisms of the alkyl ester drug and prodrug metabolism. Bioorg Med Chem Lett 2012, 22, 989–995. [Google Scholar] [CrossRef]
- Hurst, M.; Jarvis, B. Perindopril: an updated review of its use in hypertension. Drugs 2001, 61, 867–896. [Google Scholar] [CrossRef]
- Zhou, J.; Curd, L.; Lohmer, L.L.; Ossig, J.; Schippers, F.; Stoehr, T.; Schmith, V. Population Pharmacokinetics of Remimazolam in Procedural Sedation With Nonhomogeneously Mixed Arterial and Venous Concentrations. Clin Transl Sci 2021, 14, 326–334. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, L.; Chen, T.M.; Hwang, K.K. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur J Med Chem 2002, 37, 399–407. [Google Scholar] [CrossRef]
- Gottipati, G. Prediction of human systemic, biologically relevant pharmacokinetic (PK) properties using quantitative structure pharmacokinetic relationships (QSPKR) and interspecies pharmacokinetic allometric scaling (PK-AS) approaches for four different pharmacological classes of compounds. Doctor, Virginia Commonwealth University, Virginia, 2014.
- Ellison, C.A. Structural and functional pharmacokinetic analogs for physiologically based pharmacokinetic (PBPK) model evaluation. Regul Toxicol Pharmacol 2018, 99, 61–77. [Google Scholar] [CrossRef]
- Ghafourian, T.; Barzegar-Jalali, M.; Hakimiha, N.; Cronin, M.T. Quantitative structure-pharmacokinetic relationship modelling: apparent volume of distribution. J Pharm Pharmacol 2004, 56, 339–350. [Google Scholar] [CrossRef]
- Luttrell, W.E.; Castle, M.C. Species differences in the hydrolysis of meperidine and its inhibition by organophosphate compounds. Fundam Appl Toxicol 1988, 11, 323–332. [Google Scholar] [CrossRef]
- Alsmadi, M.M.; Idkaidek, N. The Analysis of Pethidine Pharmacokinetics in Newborn Saliva, Plasma, and Brain Extracellular Fluid After Prenatal Intrauterine Exposure from Pregnant Mothers Receiving Intramuscular Dose Using PBPK Modeling. Eur J Drug Metab Pharmacokinet 2023, 48, 281–300. [Google Scholar] [CrossRef]
- Holford, N.H.G. Basic Principles. In Basic & Clinical Pharmacology, Twelfth ed.; Katzung, B.G., Masters, S.B., Trevor, A.J., Eds.; McGraw·Hill: 2012; p. 39.
- Dahlgren, D.; Roos, C.; Sjogren, E.; Lennernas, H. Direct In Vivo Human Intestinal Permeability (Peff ) Determined with Different Clinical Perfusion and Intubation Methods. J Pharm Sci 2015, 104, 2702–2726. [Google Scholar] [CrossRef]
- Tarkiainen, E.K.; Tornio, A.; Holmberg, M.T.; Launiainen, T.; Neuvonen, P.J.; Backman, J.T.; Niemi, M. Effect of carboxylesterase 1 c.428G > A single nucleotide variation on the pharmacokinetics of quinapril and enalapril. Br J Clin Pharmacol 2015, 80, 1131–1138. [Google Scholar] [CrossRef]
- Gangnus, T.; Burckhardt, B.B.; consortium, C. Low-volume LC-MS/MS method for the pharmacokinetic investigation of carvedilol, enalapril and their metabolites in whole blood and plasma: Application to a paediatric clinical trial. Drug Test Anal 2021, 13, 694–708. [Google Scholar] [CrossRef]
- Faisal, M.; Cawello, W.; Burckhardt, B.B.; de Hoon, J.; Laer, S.; Consortium, L. Simultaneous Semi-Mechanistic Population Pharmacokinetic Modeling Analysis of Enalapril and Enalaprilat Serum and Urine Concentrations From Child Appropriate Orodispersible Minitablets. Front Pediatr 2019, 7, 281. [Google Scholar] [CrossRef]
- Hockings, N.; Ajayi, A.A.; Reid, J.L. Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br J Clin Pharmacol 1986, 21, 341–348. [Google Scholar] [CrossRef]
- Jhee, S.S.; Yen, M.; Ereshefsky, L.; Leibowitz, M.; Schulte, M.; Kaeser, B.; Boak, L.; Patel, A.; Hoffmann, G.; Prinssen, E.P.; et al. Low penetration of oseltamivir and its carboxylate into cerebrospinal fluid in healthy Japanese and Caucasian volunteers. Antimicrob Agents Chemother 2008, 52, 3687–3693. [Google Scholar] [CrossRef]
- He, G.; Massarella, J.; Ward, P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet 1999, 37, 471–484. [Google Scholar] [CrossRef]
- Snell, P.; Oo, C.; Dorr, A.; Barrett, J. Lack of pharmacokinetic interaction between the oral anti-influenza neuraminidase inhibitor prodrug oseltamivir and antacids. Br J Clin Pharmacol 2002, 54, 372–377. [Google Scholar] [CrossRef]
- Oh, J.; Lee, S.; Lee, H.; Cho, J.Y.; Yoon, S.H.; Jang, I.J.; Yu, K.S.; Lim, K.S. The novel carboxylesterase 1 variant c.662A>G may decrease the bioactivation of oseltamivir in humans. PLoS One 2017, 12, e0176320. [Google Scholar] [CrossRef]
- Hsueh, C.H.; Hsu, V.; Zhao, P.; Zhang, L.; Giacomini, K.M.; Huang, S.M. PBPK Modeling of the Effect of Reduced Kidney Function on the Pharmacokinetics of Drugs Excreted Renally by Organic Anion Transporters. Clin Pharmacol Ther 2018, 103, 485–492. [Google Scholar] [CrossRef]
- Wang, X.D.; Chan, E.; Chen, X.; Liao, X.X.; Tang, C.; Zhou, Z.W.; Huang, M.; Zhou, S.F. Simultaneous and rapid quantitation of benazepril and benazeprilat in human plasma by high performance liquid chromatography with ultraviolet detection. J Pharm Biomed Anal 2007, 44, 224–230. [Google Scholar] [CrossRef]
- Gatarić, B.B. Primena tehnika za naprednu analizu podataka u biofarmaceutskoj karakterizaciji lekova: identifikacija, klasifikacija i predviđanje faktora koji utiču na intestinalnu apsorpciju lekovitih supstanci. Doctor, Belgrade Faculty, Belgrade, 2021.
- Gengo, F.M.; Brady, E. The pharmacokinetics of benazepril relative to other ACE inhibitors. Clin Cardiol 1991, 14, IV44–50. [Google Scholar] [CrossRef]
- Chan, K.K.; Buch, A.; Glazer, R.D.; John, V.A.; Barr, W.H. Site-differential gastrointestinal absorption of benazepril hydrochloride in healthy volunteers. Pharm Res 1994, 11, 432–437. [Google Scholar] [CrossRef]
- Massarella, J.; DeFeo, T.; Lin, A.; Limjuco, R.; Brown, A. The pharmacokinetics and dose proportionality of cilazapril. Br J Clin Pharmacol 1989, 27 Suppl 2, 199S–204S. [Google Scholar] [CrossRef]
- Fillastre, J.P.; Moulin, B.; Godin, M.; Williams, P.E.; Brown, A.N.; Francis, R.J.; Pinta, P.; Manfredi, R. Pharmacokinetics of cilazapril in patients with renal failure. Br J Clin Pharmacol 1989, 27 Suppl 2, 275S–282S. [Google Scholar] [CrossRef]
- Kleinbloesem, C.H.; van Brummelen, P.; Francis, R.J.; Wiegand, U.W. Clinical pharmacology of cilazapril. Drugs 1991, 41 Suppl 1, 3–10. [Google Scholar] [CrossRef]
- Williams, P.E.; Brown, A.N.; Rajaguru, S.; Francis, R.J.; Walters, G.E.; McEwen, J.; Durnin, C. The pharmacokinetics and bioavailability of cilazapril in normal man. Br J Clin Pharmacol 1989, 27 Suppl 2, 181S–188S. [Google Scholar] [CrossRef]
- Maeda, K.; Ieiri, I.; Yasuda, K.; Fujino, A.; Fujiwara, H.; Otsubo, K.; Hirano, M.; Watanabe, T.; Kitamura, Y.; Kusuhara, H.; et al. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharmacol Ther 2006, 79, 427–439. [Google Scholar] [CrossRef]
- Puchler, K.; Eckl, K.M.; Fritsche, L.; Renneisen, K.; Neumayer, H.H.; Sierakowski, B.; Lavrijssen, A.T.; Thomsen, T.; Roots, I. Pharmacokinetics of temocapril and temocaprilat after 14 once daily oral doses of temocapril in hypertensive patients with varying degrees of renal impairment. Br J Clin Pharmacol 1997, 44, 531–536. [Google Scholar] [CrossRef]
- Ohura, K.; Nozawa, T.; Murakami, K.; Imai, T. Evaluation of transport mechanism of prodrugs and parent drugs formed by intracellular metabolism in Caco-2 cells with modified carboxylesterase activity: temocapril as a model case. J Pharm Sci 2011, 100, 3985–3994. [Google Scholar] [CrossRef]
- Oguchi, H.; Miyasaka, M.; Koiwai, T.; Tokunaga, S.; Hora, K.; Sato, K.; Yoshie, T.; Shioya, H.; Furuta, S. Pharmacokinetics of temocapril and enalapril in patients with various degrees of renal insufficiency. Clin Pharmacokinet 1993, 24, 421–427. [Google Scholar] [CrossRef]
- Song, J.C.; White, C.M. Clinical pharmacokinetics and selective pharmacodynamics of new angiotensin converting enzyme inhibitors: an update. Clin Pharmacokinet 2002, 41, 207–224. [Google Scholar] [CrossRef]
- Suzuki, H.; Kawaratani, T.; Shioya, H.; Uji, Y.; Saruta, T. Study on pharmacokinetics of a new biliary excreted oral angiotensin converting enzyme inhibitor, temocapril (CS-622) in humans. Biopharm Drug Dispos 1993, 14, 41–50. [Google Scholar] [CrossRef]
- Devissaguet, J.P.; Ammoury, N.; Devissaguet, M.; Perret, L. Pharmacokinetics of perindopril and its metabolites in healthy volunteers. Fundam Clin Pharmacol 1990, 4, 175–189. [Google Scholar] [CrossRef]
- Vrhovac, B.; Sarapa, N.; Bakran, I.; Huic, M.; Macolic-Sarinic, V.; Francetic, I.; Wolf-Coporda, A.; Plavsic, F. Pharmacokinetic changes in patients with oedema. Clin Pharmacokinet 1995, 28, 405–418. [Google Scholar] [CrossRef]
- Ghiadoni, L. Perindopril for the treatment of hypertension. Expert Opin Pharmacother 2011, 12, 1633–1642. [Google Scholar] [CrossRef]
- Li, Q.; Hao, Z.; Yu, Y.; Tang, Y. Bioequivalence study of two perindopril tert-butylamine tablet formulations in healthy Chinese subjects under fasting and fed conditions: A randomized, open-label, single-dose, crossover trial. Biomed Pharmacother 2021, 135, 111221. [Google Scholar] [CrossRef]
- Ogawa, R.; Stachnik, J.M.; Echizen, H. Clinical pharmacokinetics of drugs in patients with heart failure: an update (part 2, drugs administered orally). Clin Pharmacokinet 2014, 53, 1083–1114. [Google Scholar] [CrossRef]
- Sheng, X.Y.; Liang, Y.; Yang, X.Y.; Li, L.E.; Ye, X.; Zhao, X.; Cui, Y.M. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol 2020, 76, 383–391. [Google Scholar] [CrossRef]
- Kim, K.M. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med (Seoul) 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Klotz, U.; Ziegler, G.; Reimann, I.W. Pharmacokinetics of the selective benzodiazepine antagonist Ro 15-1788 in man. European Journal of Clinical Pharmacology 1984, 27, 115–117. [Google Scholar] [CrossRef]
- Patel, R.D.; Kumar, S.P.; Patel, C.N.; Shankar, S.S.; Pandya, H.A.; Solanki, H.A. Parallel screening of drug-like natural compounds using Caco-2 cell permeability QSAR model with applicability domain, lipophilic ligand efficiency index and shape property: A case study of HIV-1 reverse transcriptase inhibitors. Journal of Molecular Structure 2017, 1146, 80–95. [Google Scholar] [CrossRef]
- Karavokiros, K.A.; Tsipis, G.B. Flumazenil: a benzodiazepine antagonist. DICP 1990, 24, 976–981. [Google Scholar] [CrossRef]
- Paixao, P.; Gouveia, L.F.; Morais, J.A. Prediction of the in vitro intrinsic clearance determined in suspensions of human hepatocytes by using artificial neural networks. Eur J Pharm Sci 2010, 39, 310–321. [Google Scholar] [CrossRef]
- Klotz, U.; Kanto, J. Pharmacokinetics and clinical use of flumazenil (Ro 15-1788). Clin Pharmacokinet 1988, 14, 1–12. [Google Scholar] [CrossRef]
- Pond, S.M.; Kretschzmar, K.M. Effect of phenytoin on meperidine clearance and normeperidine formation. Clin Pharmacol Ther 1981, 30, 680–686. [Google Scholar] [CrossRef]
- Chan, K.; Tse, J.; Jennings, F.; Orme, M.L. Pharmacokinetics of low-dose intravenous pethidine in patients with renal dysfunction. J Clin Pharmacol 1987, 27, 516–522. [Google Scholar] [CrossRef]
- Paixao, P.; Gouveia, L.F.; Morais, J.A. Prediction of the human oral bioavailability by using in vitro and in silico drug related parameters in a physiologically based absorption model. Int J Pharm 2012, 429, 84–98. [Google Scholar] [CrossRef]
- Piscitelli, S.C.; Kress, D.R.; Bertz, R.J.; Pau, A.; Davey, R. The effect of ritonavir on the pharmacokinetics of meperidine and normeperidine. Pharmacotherapy 2000, 20, 549–553. [Google Scholar] [CrossRef]
- Toutain, P.L.; Lefebvre, H.P.; King, J.N. Benazeprilat disposition and effect in dogs revisited with a pharmacokinetic/pharmacodynamic modeling approach. J Pharmacol Exp Ther 2000, 280, 1087–1093. [Google Scholar]
- Pan, D.Q.; Jiang, M.; Liu, T.T.; Wang, Q.; Shi, J.H. Combined spectroscopies and molecular docking approach to characterizing the binding interaction of enalapril with bovine serum albumin. Luminescence 2017, 32, 481–490. [Google Scholar] [CrossRef]
- Lee, A.; Shirley, M. Remimazolam: A Review in Procedural Sedation. Drugs 2021, 81, 1193–1201. [Google Scholar] [CrossRef]
- Blei, A.T. Albumin dialysis for the treatment of hepatic encephalopathy. Journal of Gastroenterology and Hepatology 2004, 19, S224–S228. [Google Scholar] [CrossRef]
- Nafisi, S.; Vishkaee, T.S. Study on the interaction of tamiflu and oseltamivir carboxylate with human serum albumin. J Photochem Photobiol B 2011, 105, 34–39. [Google Scholar] [CrossRef]
- Obradovic, D.; Radan, M.; Dikic, T.; Nikolic, M.P.; Oljacic, S.; Nikolic, K. The evaluation of drug-plasma protein binding interaction on immobilized human serum albumin stationary phase, aided by different computational approaches. J Pharm Biomed Anal 2022, 211, 114593. [Google Scholar] [CrossRef]
- Anderson, P.J.; Critchley, J.A.; Tomlinson, B.; Resplandy, G. Comparison of the pharmacokinetics and pharmacodynamics of oral doses of perindopril in normotensive Chinese and Caucasian volunteers. Br J Clin Pharmacol 1995, 39, 361–368. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, B.; Parker, R.B.; Laizure, S.C. Clinical implications of genetic variation in carboxylesterase drug metabolism. Expert Opin Drug Metab Toxicol 2018, 14, 131–142. [Google Scholar] [CrossRef]
- Gomez, H.J.; Cirillo, V.J.; Irvin, J.D. Enalapril: a review of human pharmacology. Drugs 1985, 30 Suppl 1, 13–24. [Google Scholar] [CrossRef]
- Grislain, L.; Mocquard, M.T.; Dabe, J.F.; Bertrand, M.; Luijten, W.; Marchand, B.; Resplandy, G.; Devissaguet, M. Interspecies comparison of the metabolic pathways of perindopril, a new angiotensin-converting enzyme (ACE) inhibitor. Xenobiotica 1990, 20, 787–800. [Google Scholar] [CrossRef]
- Duthaler, U.; Bachmann, F.; Ozbey, A.C.; Umehara, K.; Parrott, N.; Fowler, S.; Krahenbuhl, S. The Activity of Members of the UDP-Glucuronosyltransferase Subfamilies UGT1A and UGT2B is Impaired in Patients with Liver Cirrhosis. Clin Pharmacokinet 2023, 62, 1141–1155. [Google Scholar] [CrossRef]
- Todd, P.A.; Heel, R.C. Enalapril. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension and congestive heart failure. Drugs 1986, 31, 198–248. [Google Scholar] [CrossRef]
- Weisser, K.; Schloos, J.; Lehmann, K.; Dusing, R.; Vetter, H.; Mutschler, E. Pharmacokinetics and converting enzyme inhibition after morning and evening administration of oral enalapril to healthy subjects. Eur J Clin Pharmacol 1991, 40, 95–99. [Google Scholar] [CrossRef]
- Dickstein, K.; Till, A.E.; Aarsland, T.; Tjelta, K.; Abrahamsen, A.M.; Kristianson, K.; Gomez, H.J.; Gregg, H.; Hichens, M. The pharmacokinetics of enalapril in hospitalized patients with congestive heart failure. Br J Clin Pharmacol 1987, 23, 403–410. [Google Scholar] [CrossRef]
- Baba, T.; Murabayashi, S.; Tomiyama, T.; Takebe, K. The pharmacokinetics of enalapril in patients with compensated liver cirrhosis. Br J Clin Pharmacol 1990, 29, 766–769. [Google Scholar] [CrossRef]
- Kaiser, G.; Ackermann, R.; Brechbuhler, S.; Dieterle, W. Pharmacokinetics of the angiotensin converting enzyme inhibitor benazepril.HCl (CGS 14 824 A) in healthy volunteers after single and repeated administration. Biopharm Drug Dispos 1989, 10, 365–376. [Google Scholar] [CrossRef]
- Schweizer, C.; Kaiser, G.; Dieterle, W.; Mann, J. Pharmacokinetics and pharmacodynamics of benazepril hydrochloride in patients with major proteinuria. Eur J Clin Pharmacol 1993, 44, 463–466. [Google Scholar] [CrossRef]
- Sioufi, A.; Pommier, F.; Gauducheau, N.; Godbillon, J.; Choi, L.; John, V. The absence of a pharmacokinetic interaction between aspirin and the angiotensin-converting enzyme inhibitor benazepril in healthy volunteers. Biopharm Drug Dispos 1994, 15, 451–461. [Google Scholar] [CrossRef]
- Waldmeier, F.; Kaiser, G.; Ackermann, R.; Faigle, J.W.; Wagner, J.; Barner, A.; Lasseter, K.C. The disposition of [14C]-labelled benazepril HCl in normal adult volunteers after single and repeated oral dose. Xenobiotica 1991, 21, 251–261. [Google Scholar] [CrossRef]
- Kaiser, G.; Ackermann, R.; Gschwind, H.P.; James, I.M.; Sprengers, D.; McIntyre, N.; Defalco, A.; Holmes, I.B. The influence of hepatic cirrhosis on the pharmacokinetics of benazepril hydrochloride. Biopharm Drug Dispos 1990, 11, 753–764. [Google Scholar] [CrossRef]
- Macdonald, N.J.; Sioufi, A.; Howie, C.A.; Wade, J.R.; Elliott, H.L. The effects of age on the pharmacokinetics and pharmacodynamics of single oral doses of benazepril and enalapril. Br J Clin Pharmacol 1993, 36, 205–209. [Google Scholar] [CrossRef]
- Williams, P.E.; Brown, A.N.; Rajaguru, S.; Francis, R.J.; Bell, A.J.; Dewland, P.M. Pharmacokinetics of cilazapril during repeated oral dosing in healthy young volunteers. Eur J Drug Metab Pharmacokinet 1990, 15, 63–67. [Google Scholar] [CrossRef]
- Gross, V.; Treher, E.; Haag, K.; Neis, W.; Wiegand, U.; Scholmerich, J. Angiotensin-converting enzyme (ACE)-inhibition in cirrhosis. Pharmacokinetics and dynamics of the ACE-inhibitor cilazapril (Ro 31-2848). J Hepatol 1993, 17, 40–47. [Google Scholar] [CrossRef]
- Williams, P.E.; Brown, A.N.; Rajaguru, S.; Walters, G.E.; McEwen, J.; Durnin, C. A pharmacokinetic study of cilazapril in elderly and young volunteers. Br J Clin Pharmacol 1989, 27 Suppl 2, 211S–215S. [Google Scholar] [CrossRef]
- Massarella, J.W.; DeFeo, T.M.; Brown, A.N.; Lin, A.; Wills, R.J. The influence of food on the pharmacokinetics and ACE inhibition of cilazapril. Br J Clin Pharmacol 1989, 27 Suppl 2, 205S–209S. [Google Scholar] [CrossRef]
- Francis, R.J.; Brown, A.N.; Kler, L.; Fasanella d'Amore, T.; Nussberger, J.; Waeber, B.; Brunner, H.R. Pharmacokinetics of the converting enzyme inhibitor cilazapril in normal volunteers and the relationship to enzyme inhibition: development of a mathematical model. J Cardiovasc Pharmacol 1987, 9, 32–38. [Google Scholar]
- Lecocq, B.; Funck-Brentano, C.; Lecocq, V.; Ferry, A.; Gardin, M.E.; Devissaguet, M.; Jaillon, P. Influence of food on the pharmacokinetics of perindopril and the time course of angiotensin-converting enzyme inhibition in serum. Clin Pharmacol Ther 1990, 47, 397–402. [Google Scholar] [CrossRef]
- Tsai, H.H.; Lees, K.R.; Howden, C.W.; Reid, J.L. The pharmacokinetics and pharmacodynamics of perindopril in patients with hepatic cirrhosis. Br J Clin Pharmacol 1989, 28, 53–59. [Google Scholar] [CrossRef]
- Thiollet, M.; Funck-Brentano, C.; Grange, J.D.; Midavaine, M.; Resplandy, G.; Jaillon, P. The pharmacokinetics of perindopril in patients with liver cirrhosis. Br J Clin Pharmacol 1992, 33, 326–328. [Google Scholar] [CrossRef]
- Lees, K.R.; Green, S.T.; Reid, J.L. Influence of age on the pharmacokinetics and pharmacodynamics of perindopril. Clin Pharmacol Ther 1988, 44, 418–425. [Google Scholar] [CrossRef]
- Furuta, S.; Kiyosawa, K.; Higuchi, M.; Kasahara, H.; Saito, H.; Shioya, H.; Oguchi, H. Pharmacokinetics of temocapril, an ACE inhibitor with preferential biliary excretion, in patients with impaired liver function. Eur J Clin Pharmacol 1993, 44, 383–385. [Google Scholar] [CrossRef]
- Abe, M.; Smith, J.; Urae, A.; Barrett, J.; Kinoshita, H.; Rayner, C.R. Pharmacokinetics of oseltamivir in young and very elderly subjects. Ann Pharmacother 2006, 40, 1724–1730. [Google Scholar] [CrossRef]
- Brewster, M.; Smith, J.R.; Dutkowski, R.; Robson, R. Active metabolite from Tamiflu solution is bioequivalent to that from capsule delivery in healthy volunteers: a cross-over, randomised, open-label study. Vaccine 2006, 24, 6660–6663. [Google Scholar] [CrossRef]
- Jittamala, P.; Pukrittayakamee, S.; Tarning, J.; Lindegardh, N.; Hanpithakpong, W.; Taylor, W.R.; Lawpoolsri, S.; Charunwattana, P.; Panapipat, S.; White, N.J.; et al. Pharmacokinetics of orally administered oseltamivir in healthy obese and nonobese Thai subjects. Antimicrob Agents Chemother 2014, 58, 1615–1621. [Google Scholar] [CrossRef]
- Amrein, R.; Hetzel, W. Pharmacology of Dormicum (Midazolam) and Anexate (Flumazenil). Acta Anaesth Scand 1990, 34, 6–15. [Google Scholar] [CrossRef]
- Breimer, L.T.; Hennis, P.J.; Burm, A.G.; Danhof, M.; Bovill, J.G.; Spierdijk, J.; Vletter, A.A. Pharmacokinetics and EEG effects of flumazenil in volunteers. Clin Pharmacokinet 1991, 20, 491–496. [Google Scholar] [CrossRef]
- Pomier-Layrargues, G.; Giguere, J.F.; Lavoie, J.; Willems, B.; Butterworth, R.F. Pharmacokinetics of benzodiazepine antagonist Ro 15-1788 in cirrhotic patients with moderate or severe liver dysfunction. Hepatology 1989, 10, 969–972. [Google Scholar] [CrossRef]
- Janssen, U.; Walker, S.; Maier, K.; von Gaisberg, U.; Klotz, U. Flumazenil disposition and elimination in cirrhosis. Clin Pharmacol Ther 1989, 46, 317–323. [Google Scholar] [CrossRef]
- Verbeeck, R.K.; Branch, R.A.; Wilkinson, G.R. Meperidine disposition in man: influence of urinary pH and route of administration. Clin Pharmacol Ther 1981, 30, 619–628. [Google Scholar] [CrossRef]
- Mather, L.E.; Tucker, G.T.; Pflug, A.E.; Lindop, M.J.; Wilkerson, C. Meperidine kinetics in man. Intravenous injection in surgical patients and volunteers. Clin Pharmacol Ther 1975, 17, 21–30. [Google Scholar] [CrossRef]
- Kuhnert, B.R.; Kuhnert, P.M.; Prochaska, A.L.; Sokol, R.J. Meperidine disposition in mother, neonate, and nonpregnant females. Clin Pharmacol Ther 1980, 27, 486–491. [Google Scholar] [CrossRef]
- Guay, D.R.; Meatherall, R.C.; Chalmers, J.L.; Grahame, G.R. Cimetidine alters pethidine disposition in man. Br J Clin Pharmacol 1984, 18, 907–914. [Google Scholar] [CrossRef]
- Guay, D.R.; Meatherall, R.C.; Chalmers, J.L.; Grahame, G.R.; Hudson, R.J. Ranitidine does not alter pethidine disposition in man. Br J Clin Pharmacol 1985, 20, 55–59. [Google Scholar] [CrossRef]
- Pond, S.M.; Tong, T.; Benowitz, N.L.; Jacob, P.; Rigod, J. Presystemic metabolism of meperidine to normeperidine in normal and cirrhotic subjects. Clin Pharmacol Ther 1981, 30, 183–188. [Google Scholar] [CrossRef]
- Pond, S.M.; Tong, T.; Benowitz, N.L.; Jacob, P. Enhanced bioavailability of pethidine and pentazocine in patients with cirrhosis of the liver. Aust N Z J Med 1980, 10, 515–519. [Google Scholar] [CrossRef]
- Mather, L.E.; Tucker, G.T. Systemic availability of orally administered meperidine. Clin Pharmacol Ther 1976, 20, 535–540. [Google Scholar] [CrossRef]
- Klotz, U.; McHorse, T.S.; Wilkinson, G.R.; Schenker, S. The effect of cirrhosis on the disposition and elimination of meperidine in man. Clin Pharmacol Ther 1974, 16, 667–675. [Google Scholar] [CrossRef]
- Neal, E.A.; Meffin, P.J.; Gregory, P.B.; Blaschke, T.F. Enhanced Bioavailability and Decreased Clearance of Analgesics in Patients with Cirrhosis. Gastroenterology 1979, 77, 96–102. [Google Scholar] [CrossRef]
- Stohr, T.; Colin, P.J.; Ossig, J.; Pesic, M.; Borkett, K.; Winkle, P.; Struys, M.; Schippers, F. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth 2021, 127, 415–423. [Google Scholar] [CrossRef]
- Ishizuka, H.; Konno, K.; Naganuma, H.; Sasahara, K.; Kawahara, Y.; Niinuma, K.; Suzuki, H.; Sugiyama, Y. Temocaprilat, a novel angiotensin-converting enzyme inhibitor, is excreted in bile via an ATP-dependent active transporter (cMOAT) that is deficient in Eisai hyperbilirubinemic mutant rats (EHBR). J Pharmacol Exp Ther 1997, 280, 1304–1311. [Google Scholar]
- Gao, G.; Law, F.; Wong, R.N.S.; Mak, N.K.; Yang, M.S.M. A physiologically-based pharmacokinetic model of oseltamivir phosphate and its carboxylate metabolite for rats and humans. ADMET DMPK 2019, 7, 22–43. [Google Scholar] [CrossRef]
- Kleingeist, B.; Bocker, R.; Geisslinger, G.; Brugger, R. Isolation and pharmacological characterization of microsomal human liver flumazenil carboxylesterase. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques 1998, 1, 38–46. [Google Scholar]
- Tegeder, I.; L??tsch, J.r.; Geisslinger, G. Pharmacokinetics of Opioids in Liver Disease. Clinical Pharmacokinetics 1999, 37, 17–40. [Google Scholar] [CrossRef]
- Riccardi, K.; Cawley, S.; Yates, P.D.; Chang, C.; Funk, C.; Niosi, M.; Lin, J.; Di, L. Plasma Protein Binding of Challenging Compounds. J Pharm Sci 2015, 104, 2627–2636. [Google Scholar] [CrossRef]
- Turpeinen, M.; Zanger, U.M. Cytochrome P450 2B6: function, genetics, and clinical relevance. Drug Metabol Drug Interact 2012, 27, 185–197. [Google Scholar] [CrossRef]
- Prasad, B.; Bhatt, D.K.; Johnson, K.; Chapa, R.; Chu, X.; Salphati, L.; Xiao, G.; Lee, C.; Hop, C.; Mathias, A.; et al. Abundance of Phase 1 and 2 Drug-Metabolizing Enzymes in Alcoholic and Hepatitis C Cirrhotic Livers: A Quantitative Targeted Proteomics Study. Drug Metab Dispos 2018, 46, 943–952. [Google Scholar] [CrossRef]
- Kapczinski, F.; Sherman, D.; Williams, R.; Lader, M.; Curran, V. Differential effects of flumazenil in alcoholic and nonalcoholic cirrhotic patients. Psychopharmacology 1995, 120, 220–226. [Google Scholar] [CrossRef]
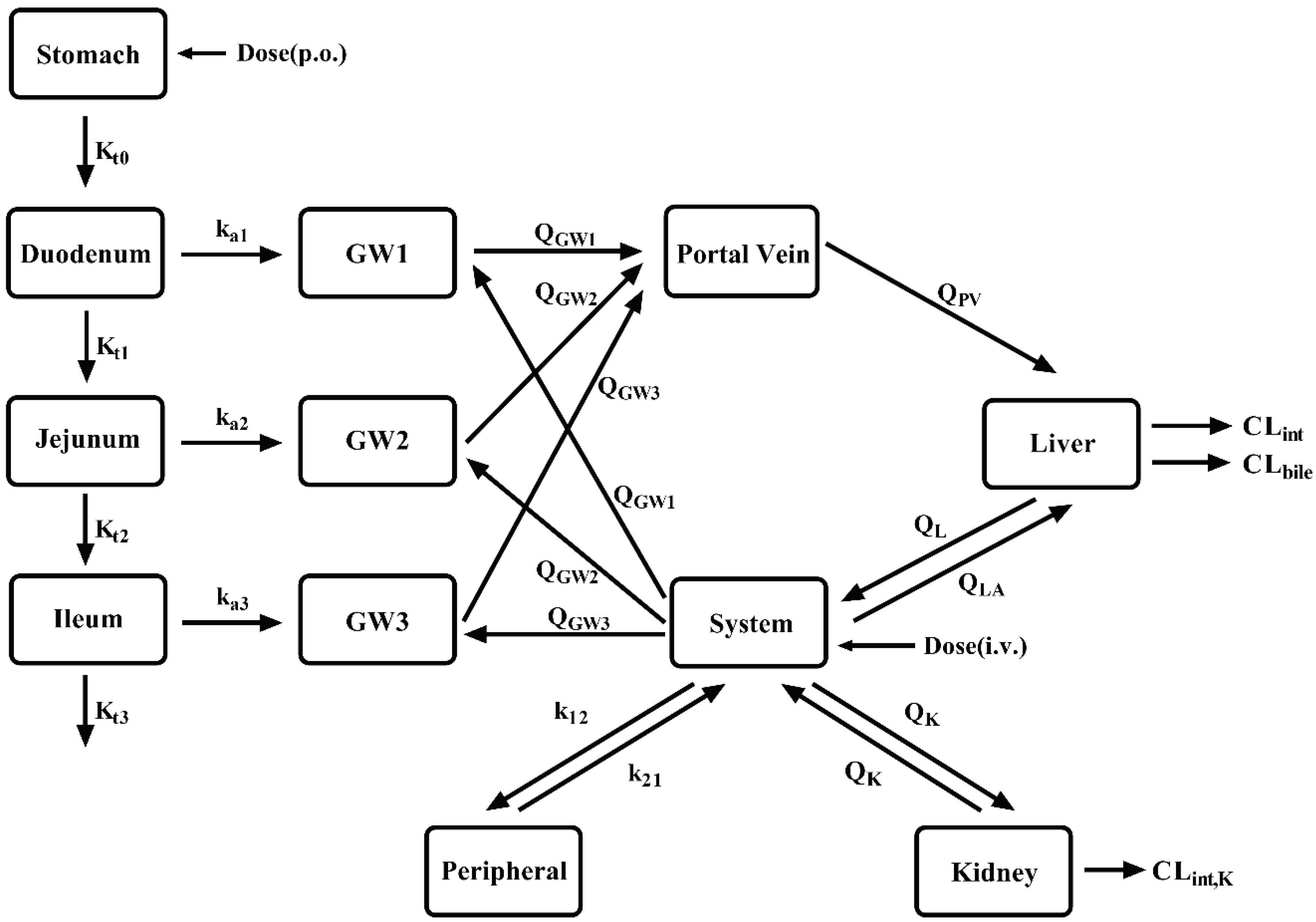

| Normal | Child-Pugh class | Units | |||
| A | B | C | |||
| Blood flow rates | |||||
| Liver | 1450 | 1436.5 | 1176.9 | 1656.3 | mL/min |
| Hepatic arterial | 300 | 390[17] | 486.9[9] | 1020[17] | mL/min |
| Portal vein | 1150 | 1046.5[9] | 690[20] | 636.3[9] | mL/min |
| Kidney | 1240 | 1091.2[17] | 806[17] | 595.2[17] | mL/min |
| Duodenum | 45 | 45 | 45 | 45 | mL/min |
| Jejunum | 173 | 173 | 173 | 173 | mL/min |
| Ileum | 102 | 102 | 102 | 102 | mL/min |
| Volume | |||||
| Liver | 1690 | 1368.9[21] | 1098.5[21] | 895.7[21] | mL |
| Portal vein | 70 | 70 | 70 | 70 | mL |
| Kidney | 280 | 280 | 280 | 280 | mL |
| Duodenum | 21 | 21 | 21 | 21 | mL |
| Jejunum | 63 | 63 | 63 | 63 | mL |
| Ileum | 42 | 42 | 42 | 42 | mL |
| Transit rates | |||||
| Stomach | 0.04 | 0.0504[22] | 0.0504[22] | 0.0504[22] | min-1 |
| Duodenum | 0.07 | 0.0889[22] | 0.0889[22] | 0.0889[22] | min-1 |
| Jejunum | 0.03 | 0.0381[22] | 0.0381[22] | 0.0381[22] | min-1 |
| Ileum | 0.04 | 0.0508[22] | 0.0508[22] | 0.0508[22] | min-1 |
| Gut radius | |||||
| r1 | 2 | 2 | 2 | 2 | cm |
| r2 | 1.63 | 1.63 | 1.63 | 1.63 | cm |
| r3 | 1.45 | 1.45 | 1.45 | 1.45 | cm |
| Glomerular filtration rate | 105 | 82[23] | 82[23] | 82[23] | mL/min |
| Albumin | 44.7 | 36.2[17] | 30.4[17] | 26.3[9] | g/L |
| α1-acid glycoprotein | 0.8 | 0.57[21] | 0.52[21] | 0.46[21] | g/L |
| CES1 | 2.45 | 2.45[9] | 1.715[9] | 0.735[9] | mg/g Liver |
| CYP2B6 | 17 | 17[21] | 15.3[21] | 13.6[21] | pmol/mg |
| Lactulose/Rhamnose ratio | 0.037 | 0.046[24] | 0.052[24] | 0.057[24] | / |
| MRP2 ratio | 1 | 0.54[20] | 0.54[20] | 0.54[20] | / |
| Drug | logP | pka | CLint | Vmax | Km | KL;Pd | KG;Pd | KK;Pd | CLb |
| mL/min | nmoL/min/mg protein | μmol/L | mL/min | ||||||
| Enalapril | 0.59[25] | 5.20[25] | 784[26] | / | / | 1.66 | 2.29 | 1.79 | / |
| Enalaprilat | -0.74[27] | 2.03[27] | / | / | / | 1.12 | 1.04 | 1.25 | / |
| Oseltamivir | 0.36[28] | 7.7[28] | 20255.4[28] | / | / | 1.19 | 1.12 | 1.29 | / |
| OC | -1.3a | 4.19a | / | / | / | 1.71 | 1.89 | 1.91 | / |
| Benazepril | 1.11[29] | 4.74[29] | 6696[30] | / | / | 0.087 | 0.122 | 0.088 | 385.8[31] g |
| Benazeprilat | 0.56[29] | 1.97[29] | / | / | / | 0.093 | 0.088 | 0.101 | / |
| Cilazapril | 0.55[32] | 3.3[33] | 199.7 c | / | / | 1.32 | 1.31 | 1.43 | 205a |
| Cilazaprilat | -0.48a | 3.17 a | / | / | / | 1.28 | 1.22 | 1.42 | / |
| Temocapril | 2.102[34] | 2.8[35] | 5359.7[36] | / | / | 2.82 | 3.17 | 2.47 | / |
| Temocaprilat | 2.215[37] | 2.09[38] | / | / | / | 0.289 | 0.322 | 0.251 | / |
| Perindopril | -1.31[39] | 3.2[40] | 1011.15[41]156.47[42]c, j | / | / | 0.665 | 0.633 | 0.742 | / |
| Perindoprilat | -0.08a | 3.08a | / | / | / | 1.45 | 1.38 | 1.61 | / |
| Remimazolam | 3.68a | 5.99a | 79212.96c | / | / | 36.34 | 63.19 | 31.2 | 1180[43] |
| Flumazenil | 1.64[44] | 0.86[45] | 8169.9c | / | / | 2.57 | 2.71 | 2.41 | 1120[46] |
| Pethidine | 2.35[47] | 8.7[47] | / | 1.56[48] h5.382[49] i | 261[48] h356[49] i | 14.82 | 4.18 | 12.02 | / |
| Drug | Vsys | K12 | K21 | Peff,A-B | CLint,K | Rb | fu,b | F | ka |
| L | min-1 | min-1 | 10-4cm/s | mL/min | 1/min | ||||
| Enalapril | 40[50] | / | / | 1.60[51] | 624.6[52] | 0.74[53] | 0.74[27] | / | |
| Enalaprilat | 46.1[54] | 0.001[54] | 0.0009[54] | / | 186.4[55] | 0.73[53] | 0.68[27] | / | |
| Oseltamivir | 61.289[56] f | / | / | / | 1357.95[57] | 1e | 0.58[28] | / | 0.061[58] g |
| OC | 160.729[59] f | / | / | / | 438.5[60] | 1e | 0.97[28] | / | |
| Benazepril | 4.8[61] g | 0.0215[61] g | 0.0238[61] g | 1.21[62] | 8391.6c | 1e | 0.03[63] | 0.35[50] | |
| Benazeprilat | 1.204[64] f | 0.0438[64] f | 0.00837[64] f | / | 447.9[63] | 1e | 0.05[63] | / | |
| Cilazapril | 18.23[65] f | 0.00325[65] f | 0.00155[65] f | / | 118.095[66] | 1e | 0.7[32] | / | 0.099[67] g |
| Cilazaprilat | 10.3517[65] f | 0.00084[65] f | 0.008[65] f | / | 75.48[66] | 1e | 0.76[68] | / | / |
| Temocapril | 15.398[69] g | / | / | / | 110.2[70] | 1e | 0.3[38] | 0.65[71] | 0.065[69] g |
| Temocaprilat | 58.535[72] f | 0.00184[72] f | 0.000078[72] f | / | 949.84[73]1899.68[74] b | 1e | 0.025[38] | / | / |
| Perindopril | 13.119[75] g | 0.0028[75] g | 0.0024[75] g | 1.34[30] | 130.2[76] | 1e | 0.4[77] | 0.66[73] | / |
| Perindoprilat | 53.44[78] f | 0.271[78] f | 0.0996[78] f | / | 231.78[79] | 1e | 0.85[77] | / | / |
| Remimazolam | 15.0768[80] | 0.01638[80]0.3117[80](K13) | 0.000476[80]0.5057[80](K31) | / | / | 1e | 0.08[81] | / | |
| Flumazenil | 24.054[82] g | 0.0376[82] g | 0.0427[82] g | 3.78[83] | 1.67[84] | 1[85] | 0.6[86] | / | |
| Pethidine | 328.676[87] f | 0.002224[87] f | 0.0003697[87] f | / | 58.78[88] | 0.87[89] | 0.48[49] | / | 0.117[90] g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





