Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Deep Eutectic Solvents
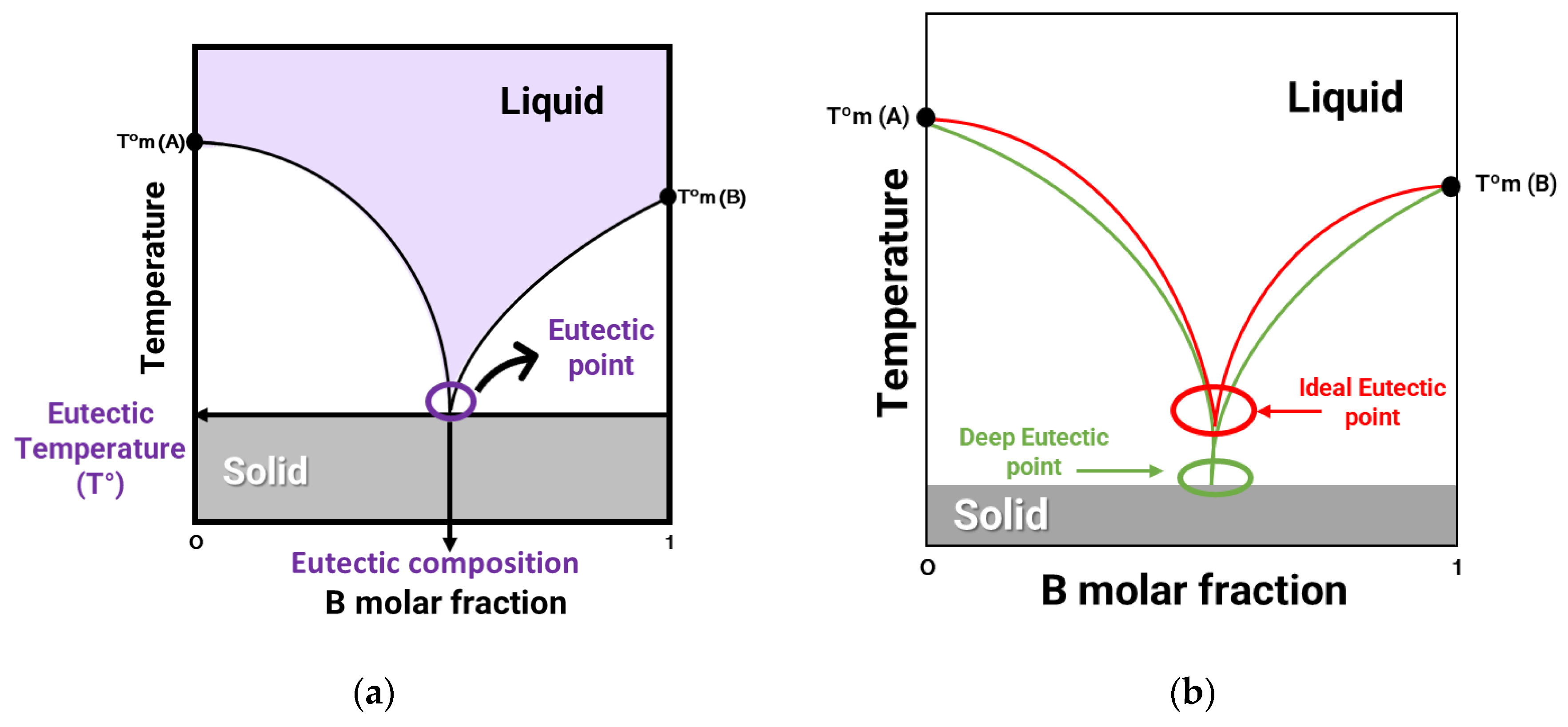
2.1. Deep Eutectic Solvents Classification
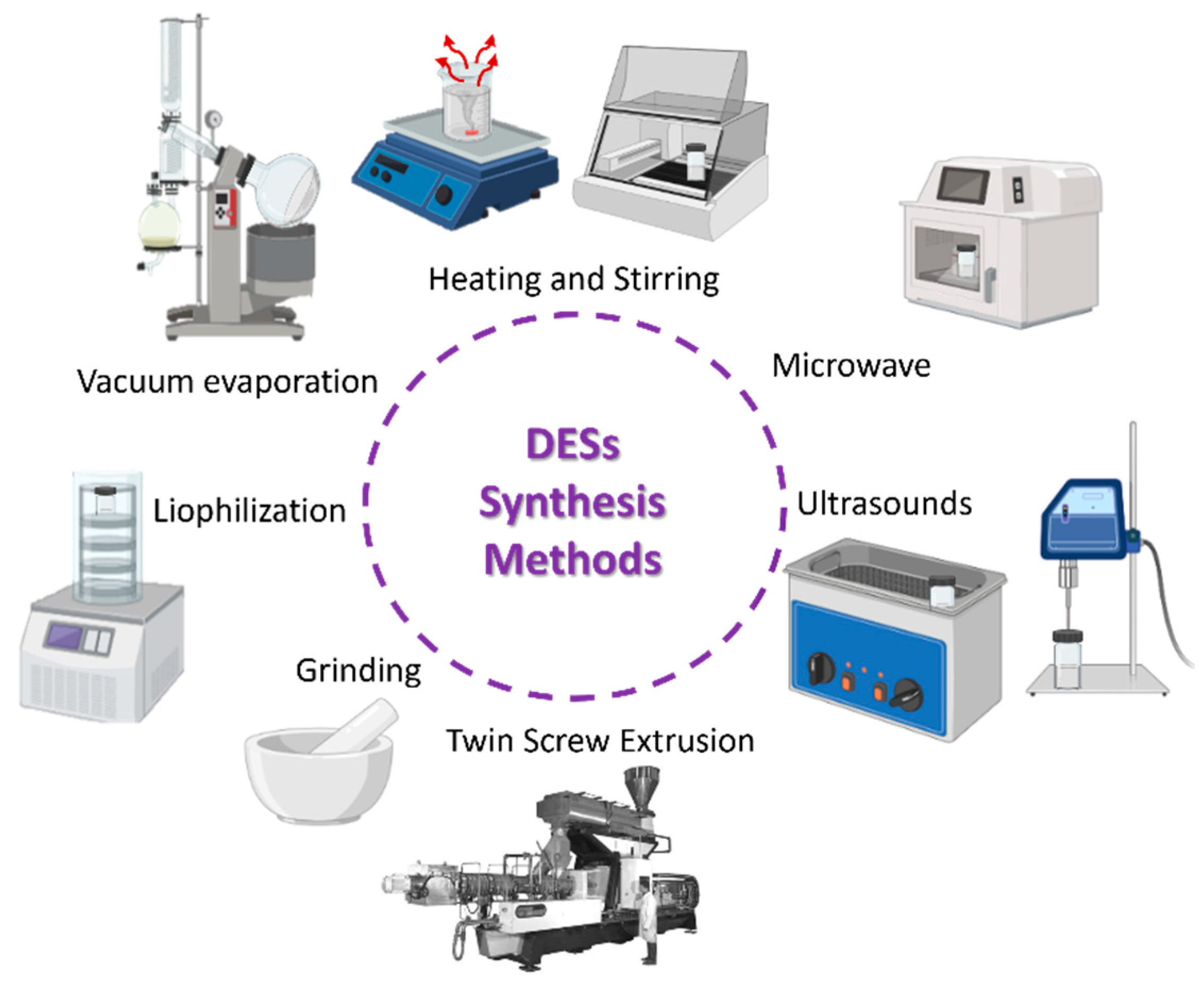
2.2. Deep Eutectic Solvents Synthesis
2.3. Deep Eutectic Solvents Properties
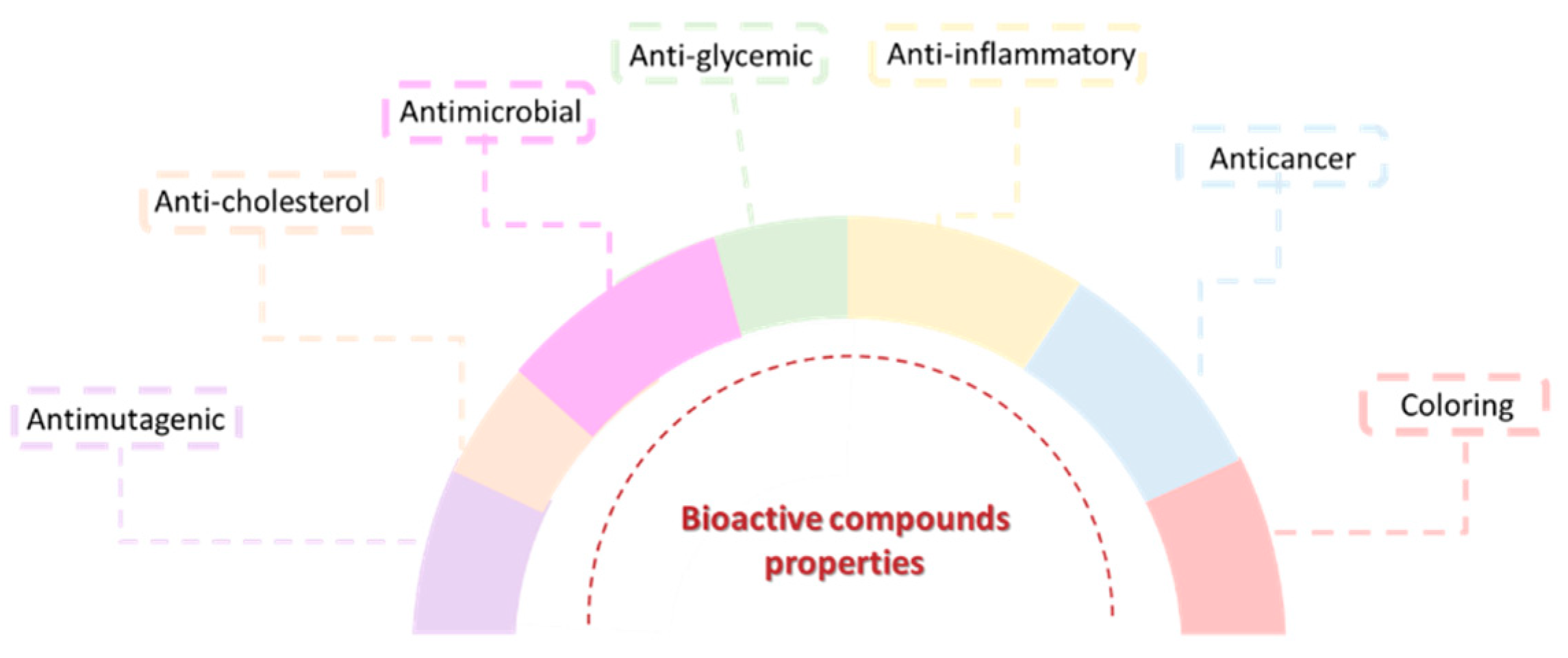
3. Bioactive Compounds
3.1. Extraction of Bioactive Compounds
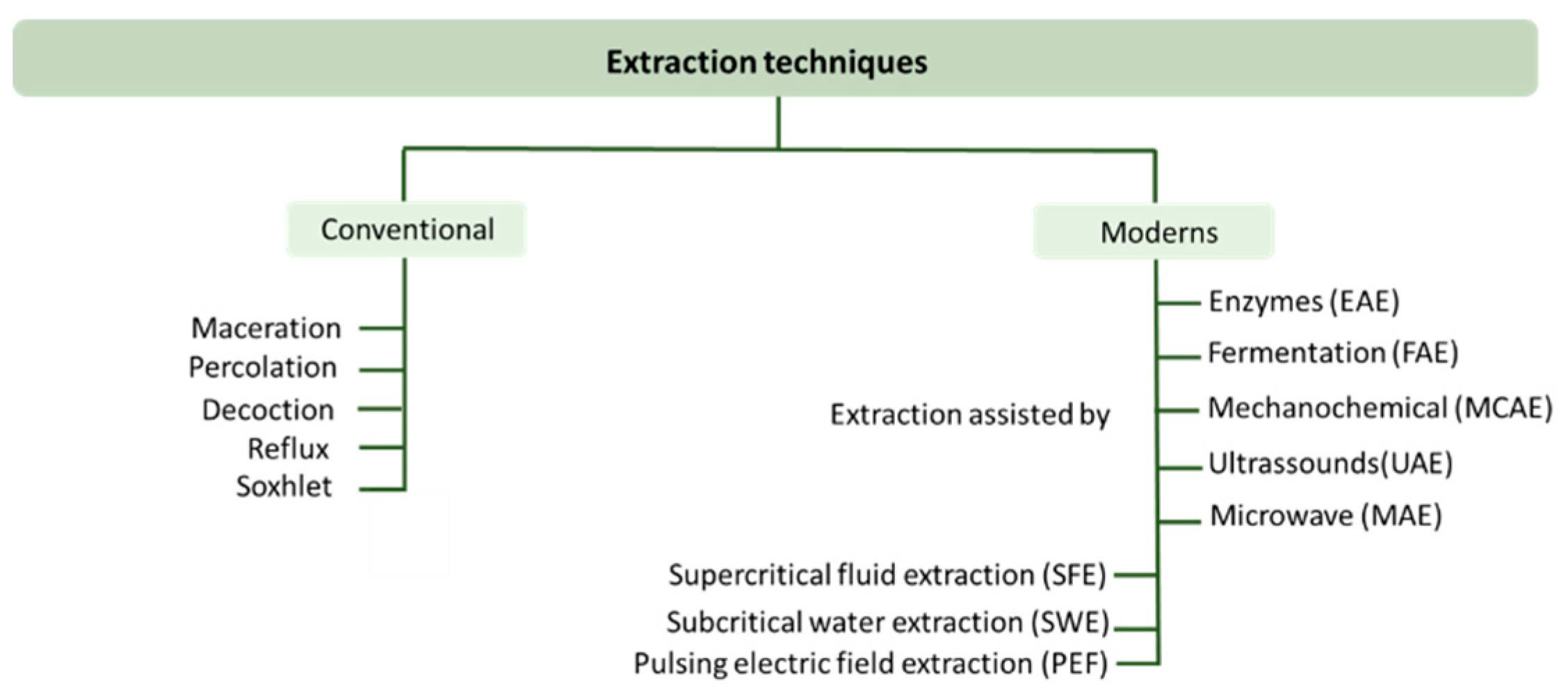
3.1.1. Extraction Techniques
4. Deep Eutectic Solvents for the Extraction of Bioactive Compounds
| Best DES(s) (molar ratio) | Natural source | Target compound | Extraction technique(s) | Reference |
|---|---|---|---|---|
| ChCl:PG (1:2) | Wolfberry | Rhamnogalacturonan-I (RG-I) pectin | CE | [179] |
| LA:water | Vine shoots | Phytochemicals (proanthocyanins, stilbenes, hydroxycinnamic acids, and flavonols) |
UAE/CE | [189] |
| ChChl:MA (1:1) ChChl:GLY (1:2) |
Orange | Bioactive compounds and ascorbic acid | CE | [190] |
| LA:GLY (1:2) | Evodia lepta | Alkaloids | MAE | [193] |
| ChChl:EG (1:3) | Abalone viscera | Polysaccharides | UAE | [198] |
| TET: LAU (1:1) | Lycium barbarum | Polysaccharides | UAE | [194] |
| Terpenoid-based | Rosmarinus officinalis L. | Bioactive oxidants | UAE | [181] |
| CAM:GLY (1:1) | Micromelum minutum | Polysaccharides | CE | [199] |
| ChChl:GLY (1:2) ChCl:LAC (1:3) ChCl:CA (1:1). |
Apple | Bioactive compounds | UAE | [169] |
| BetHCl:EG (1:10) | Kiwifruits | Antioxidants | CE | [167] |
| [N4444]Cl:AA (1:1). | --- | Aromatic amino acids | --- | [195] |
| TEA:4-MP (1:1) | Orange | Hesperidin | UAE | [174] |
| ChCl-ACA (1:2) | Peanut | Flavonoids | UAE | [183] |
| BET-LAC | Astragalus-Safflower | hydroxysafflor yellow A, anhydrosafflor yellow B, eleutheroside B, calycosin-7-O-glucoside, kaempferol-3-O-rutinoside, ononin, calycosin, astraganoside, carthamin |
UAE | [186] |
| LACT: NAACE:H2O (3:1:2) ChCl:OA (1:1) |
Blueberry | Phenolic compounds | UAE | [177] |
| ChCl:ACE (1:4) | Erigerontis Herb | Scutellarin | UAE | [171] |
| ChCl:CA (1:1) | Curcuma longa | Curcuminoids | MAE | [196] |
| BET:GLY (1:3) | Kale | Polyphenols | Solid/Liquid | [165] |
| BET:MA:PRO (1:1:1) | Propolis | Bioactive compunds | UAE | [200] |
| ChCl:Gly (1:2) ChCl:URE(1:2) |
Rhamnus alaternus | Polyphenols | CE | [184] |
| ChCl:EG(1:2) | Sophora japonica L. | Rutin | CE | [192] |
| BENZAC:FEN (1:4) | Artemisia annua L. | Artemisinin | CE | [192] |
| β-ALA:MA:H2O (1:1:3) | Mango | Phenolic compounds | CE/ UAE |
[176] |
| CA:MA:H2O (1:1:10) | Persimmon | Fibers/Antioxidants | UAE | [201] |
| ChCl:GLY (1:1) | Avocado | Phenolics/Carbohydrates | MAE | [202] |
| ChCl:GLY (1:2) | Kiwifruits | Polyphenols | UAE | [187] |
| ChCl:GLY (1:2) | Spice | Polyphenols | UAE | [203] |
| ChCl:ACA (1:4) | Tarragon | Bioactive compounds | CE | [204] |
| LAC:GLY (1:2) | Foxtail millet Husk | Bioactive compounds | UAE | [178] |
| ChCl:GLY (1:1) | Violet Potato | Bioactive compounds | UAE/MAE | [185] |
| BET:TEG (1:2) ChCl:PROP (1:2) |
Coffee ground | Polyphenols | CE | [170] |
| BET: FRU (1:1) | Dark Chocolate | Bioactive compounds | UAE | [172] |
| ACA:GLU (2:1) ACA:GLY (2:!) |
Tea | Tannins/Flavonoids/Terpenoids | UAE/EAE | [188] |
| MEN:LID (1:1) |
Perna canaliculus |
Omega-3 | CE | [133] |
| DDBAC:LA (1:3) | Gardenia | Bioactive Compounds | CE | [205] |
| [N1 1 16 (2OH)+][Br−]:THY (1:2) | Cannabis sativa L. | Phytochemicals | Microextraction | [166] |
| ChCl:BUT (1:4) | C. vulgaris | Bioactive compounds | CE | [173] |
| ChCl:MA (1:1) ChCl:LAC (1:3) |
Aralia elata | Triterpene Saponins |
CE | [206] |
| CA:GLY:H2O (1:4:10/15/20) | Chamaenerion angustifolium (L.) Scop. | Bioactive compounds | UAE | [207] |
| CA:MAL (1:2) | Nettle | Bioactive Compounds | UAE | [208] |
| ChCl:LAC (1:1) | Iris sibirica L. | Bioactive compounds | CE | [209] |
| ChCl:LAC (1:2) | Edible Feijoa | Flavonoids | CE | [210] |
| ChCl:LAC (1:4) | Mexican Oregano | Flavonoids | UAE | [211] |
| ChCl-PHE (1:3) | Hop | Polyphenols | CE/UAE/UHE | [212] |
| ChCl:GLU (1:0.8) | Capsicum chinense | Polyphenols | UAE | [175] |
| ChCl:LAC (1:1) | Glycyrrhiza glabra | Glycyrrhizic acid | MAE | [175] |
| ChCl:MA (1:2) ChCl:CA (1:2) ChCl:4BUT (1:2) |
Fenugreek | Flavonoids | UAE | [213] |
| ChCl:LAC (1:2) | black mulberry | Flavonoids/Phenolics | UAE | [182] |
| ChCl:4BUT (1:2) | Tea | Flavonoids/Alkaloids/Catechins | MCAE | [197] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation List
| Abbreviations | Name |
| BUT | 1,2-Butanodiol |
| PROP | 1,2-Propanediol |
| 4BUT | 1,4-Butanodiol |
| 4-MP | 4-Methoxyphenol |
| ACE | Acetamide |
| ACA | Acetic Acid |
| AA | Acrylic Acid |
| API | Active Pharmaceutical Ingredient |
| BENZAC | Benzoic acid |
| BET | Betaine |
| BetHCl | Betaine hydrochloride |
| [N1 1 16 (2OH)+][Br−] | Choline Bromide Salt |
| ChC l | Choline chloride |
| CA | Citric Acid |
| CAM | Citric Acid Monohydrate |
| COSMOS-RS | Conductor like Screening Model for Real Solvents |
| CE | Conventional Extraction |
| DES | Deep Eutectic Solvent |
| DESs | Deep Eutectic Solvents |
| GLU | D-Glucose |
| DDBAC | dodecyldimethylbenzylammonium chloride |
| EAE | Enzymatic Assisted Extraction |
| EG | Ethylene glycol |
| FEN | Fenchyl alcohol |
| FAE | Fermentation Assisted Extraction |
| FA | Formic acid |
| FRU | Frutose |
| Tg | Glass Transition Temperature |
| GLY | Glycerol |
| HBA | Hydrogen Bond Acceptor |
| HBAs | Hydrogen Bond Acceptors |
| HBD | Hydrogen Bond Donor |
| HBDs | Hydrogen Bond Donors |
| IUPAC | International Union of Pure and Applied Chemistry |
| Ils | Ionic Liquids |
| LACT | Lactacte |
| LAC | Lactic Acid |
| LAU | Lauric acid |
| LA | Levulinic acid |
| LID | Lidocaine |
| MA | Malic acid |
| MAL | Maltose |
| MCAE | Mechanochemical Extraction |
| MEN | Menthol |
| MAE | Microwave assisted extraction |
| NADESs | Natural Deep Eutectic Solvents |
| OA | Oxalic acid |
| PHE | Phenol |
| PDESs | Polymeric Deep Eutectic Solvents |
| PQDESs | Poly-Quasi Deep Eutectic Solvents |
| PRO | Proline |
| PG | Propylene Glycol |
| NAACE | Sodium Acetate |
| SWE | Subcfritical Water Extraction |
| SFE | Supercritical Fluid Extraction |
| [N4444]Cl | Tetrabutylammonium chloride |
| TET | Tetracaíne |
| THEDESs | Therapeutic Deep Eutectic Solvents |
| THY | Thymol |
| TEA | Triethanolamine |
| TEG | Triethylene glycol |
| UHE | Ultrasonic Homogenizer Extraction |
| URE | Urea |
| UAE | Ultrasound liquid extraction |
| β-ALA | β-Alanine |
References
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Devi, B.K.; Naraparaju, S.; Soujanya, C.; Gupta, S.D. Green Chemistry and Green Solvents: An Overview. Current Green Chemistry 2020, 7, 314–325. [Google Scholar] [CrossRef]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: PRODUCTIVELY. Green Chemistry 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Tundo, P.; Anastas, P.; Black, D.S.; Breen, J.; Collins, T.; Memoli, S.; Miyamoto, J.; Polyakoff, M.; Tumas, W. Synthetic pathways and processes in green chemistry. Introductory overview. Pure Appl. Chem. 2000, 72, 1207–1228. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W.; Qasem Ali, A.; O. Errayes, A. Green Chemistry: Principles, Applications, and Disadvantages. Chemical Methodologies 2020, 4, 408–423. [Google Scholar] [CrossRef]
- Warner, J.C.; Cannon, A.S.; Dye, K.M. Green chemistry. Environmental Impact Assessment Review 2004, 24, 775–799. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice. Oxford University Press:: New York, 1998; p. p.30.
- Bonventre, J.A. Solvents. In Encyclopedia of Toxicology (Third Edition); Wexler, P., Ed.; Academic Press: Oxford, 2014; pp. 356–357. [Google Scholar]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. Journal of Pharmacy and Bioallied Sciences 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Fayaz, U.; Shams, R.; Srivastava, S.; Singh, R. Deep eutectic solvents for extraction of functional components from plant-based products: A promising approach. Sustainable Chemistry and Pharmacy 2023, 33, 101102. [Google Scholar] [CrossRef]
- Grodowska, K.; Parczewski, A. Organic Solvents in the Pharmaceutical Industry. Acta Poloniae Pharmaceutica 2010, 67, 3–12. [Google Scholar]
- Hessel, V.; Tran, N.N.; Asrami, M.R.; Tran, Q.D.; Long, N.V.; Escriba-Gelonch, M.; Tejada, J.O.; Linke, S.; Sundmacher, K. Sustainability of green solvents - review and perspective. Green Chemistry 2022, 24, 410–437. [Google Scholar] [CrossRef]
- Horváth, I.T. Solvents from nature. Green Chemistry 2008, 10, 1024–1028. [Google Scholar] [CrossRef]
- Kerton, F. Alternative Solvents for Green Chemistry; The Royal Society of Chemistry: 2009.
- Singh, M.B.; Kumar, V.S.; Chaudhary, M.; Singh, P. A mini review on synthesis, properties and applications of deep eutectic solvents. Journal of the Indian Chemical Society 2021, 98, 100210. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidovic, S.; Redovnikovic, I.R.; Jokic, S. Green solvents for green technologies. Journal of Chemical Technology and Biotechnology 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Hartonen, K.; Riekkola, M.-L. Chapter 2 - Water as the First Choice Green Solvent. In The Application of Green Solvents in Separation Processes, Pena-Pereira, F., Tobiszewski, M., Eds.; Elsevier: 2017; pp. 19–55.
- Kerton, F. Water. In Alternative Solvents for Green Chemistry; The Royal Society of Chemistry: 2009; p. 0.
- Nelson, W.M. Green solvents for chemistry: perspectives and practice. 2003.
- Sekharan, T.R.; Katari, O.; Ruhina Rahman, S.N.; Pawde, D.M.; Goswami, A.; Chandira, R.M.; Shunmugaperumal, T. Neoteric solvents for the pharmaceutical industry: an update. Drug Discov. Today 2021, 26, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Andre, V.; Duarte, M.T.; Gomes, C.S.B.; Sarraguca, M.C. Mechanochemistry in Portugal-A Step towards Sustainable Chemical Synthesis. Molecules 2022, 27, 241. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Baron, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Marechal, E.; Mormann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials - (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Obst, M.; Konig, B. Organic Synthesis without Conventional Solvents. European J. Org. Chem. 2018, 2018, 4213–4232. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chemistry 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Current Opinion in Food Science 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Häckl, K.; Kunz, W. Some aspects of green solvents. Comptes Rendus Chimie 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green Chemistry 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Shaibuna, M.; Theresa, L.V.; Sreekumar, K. Neoteric deep eutectic solvents: history, recent developments, and catalytic applications. Soft Matter 2022, 18, 2695–2721. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends in Analytical Chemistry 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. Acs Sustain Chem Eng 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends in Analytical Chemistry 2021, 134, 116108. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. Journal of Chromatography A 2019, 1598, 1–19. [Google Scholar] [CrossRef]
- Yang, T.-X.; Zhao, L.-Q.; Wang, J.; Song, G.-L.; Liu, H.-M.; Cheng, H.; Yang, Z. Improving Whole-Cell Biocatalysis by Addition of Deep Eutectic Solvents and Natural Deep Eutectic Solvents. Acs Sustain Chem Eng 2017, 5, 5713–5722. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: a review. Environmental Chemistry Letters 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Velez, C.; Acevedo, O. Simulation of deep eutectic solvents: Progress to promises. Wiley Interdisciplinary Reviews-Computational Molecular Science 2022, 12, e1598. [Google Scholar] [CrossRef]
- Álvarez, M.S.; Zhang, Y. Sketching neoteric solvents for boosting drugs bioavailability. J. Control. Release 2019, 311-312, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Santos, L.B.; Assis, R.S.; Barreto, J.A.; Bezerra, M.A.; Novaes, C.G.; Lemos, V.A. Deep eutectic solvents in liquid-phase microextraction: Contribution to green chemistry. TrAC Trends in Analytical Chemistry 2022, 146, 116478. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep Eutectic Solvents as Extraction Media for Valuable Flavonoids from Natural Sources. Applied Sciences-Basel 2019, 9, 4169. [Google Scholar] [CrossRef]
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. In Application of Ionic Liquids in Biotechnology; Itoh, T., Koo, Y.-M., Eds.; Springer International Publishing: Cham, 2019; pp. 31–59. [Google Scholar]
- Dai, Y.; Row, K.H. Application of Natural Deep Eutectic Solvents in the Extraction of Quercetin from Vegetables. Molecules 2019, 24, 2300. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. Journal of Separation Science 2015, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, D.C.; Monteiro, S.A.; Merib, J. A review on recent applications of deep eutectic solvents in microextraction techniques for the analysis of biological matrices. Advances in Sample Preparation 2022, 1, 100007. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. Journal of Agricultural and Food Chemistry 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Gurkan, B.; Squire, H.; Pentzer, E. Metal-Free Deep Eutectic Solvents: Preparation, Physical Properties, and Significance. The Journal of Physical Chemistry Letters 2019, 10, 7956–7964. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef]
- Ali, M.C.; Liu, R.; Chen, J.; Cai, T.; Zhang, H.; Li, Z.; Zhai, H.; Qiu, H. New deep eutectic solvents composed of crown ether, hydroxide and polyethylene glycol for extraction of non-basic N-compounds. Chinese Chemical Letters 2019, 30, 871–874. [Google Scholar] [CrossRef]
- Tan, T.; Zhang, M.; Wan, Y.; Qiu, H. Utilization of deep eutectic solvents as novel mobile phase additives for improving the separation of bioactive quaternary alkaloids. Talanta 2016, 149, 85–90. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Oktaviyanti, N.D.; Kartini; Mun’im, A. Application and optimization of ultrasound-assisted deep eutectic solvent for the extraction of new skin-lightening cosmetic materials from Ixora javanica flower. Heliyon 2019, 5, e02950. [Google Scholar] [CrossRef]
- Vorobyova, V.; Vasyliev, G.; Skiba, M.; Frolenkova, S.; Zaporozhets, J.; Gnatko, O.; Linyucheva, O. Green extraction of phenolic compounds from grape pomace by deep eutectic solvent extraction: physicochemical properties, antioxidant capacity. Chemical Papers 2023, 77, 2447–2458. [Google Scholar] [CrossRef]
- Vasyliev, G.; Lyudmyla, K.; Hladun, K.; Skiba, M.; Vorobyova, V. Valorization of tomato pomace: extraction of value-added components by deep eutectic solvents and their application in the formulation of cosmetic emulsions. Biomass Conversion and Biorefinery 2022, 12, S95–S111. [Google Scholar] [CrossRef]
- Boisset, A.; Menne, S.; Jacquemin, J.; Balducci, A.; Anouti, M. Deep eutectic solvents based on N-methylacetamide and a lithium salt as suitable electrolytes for lithium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 20054–20063. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.; Hashim, M.A. Potential applications of deep eutectic solvents in nanotechnology. Chemical Engineering Journal 2015, 273, 551–567. [Google Scholar] [CrossRef]
- Ma, C.; Sarmad, S.; Mikkola, J.-P.; Ji, X. Development of Low-Cost Deep Eutectic Solvents for CO2 Capture. Energy Procedia 2017, 142, 3320–3325. [Google Scholar] [CrossRef]
- Carriazo, D.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Resorcinol-Based Deep Eutectic Solvents as Both Carbonaceous Precursors and Templating Agents in the Synthesis of Hierarchical Porous Carbon Monoliths. Chemistry of Materials 2010, 22, 6146–6152. [Google Scholar] [CrossRef]
- Patiño, J.; Gutiérrez, M.C.; Carriazo, D.; Ania, C.O.; Fierro, J.L.G.; Ferrer, M.L.; del Monte, F. DES assisted synthesis of hierarchical nitrogen-doped carbon molecular sieves for selective CO2versus N2 adsorption. Journal of Materials Chemistry A 2014, 2, 8719–8729. [Google Scholar] [CrossRef]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Applied Materials Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Cai, T.; Qiu, H. Application of deep eutectic solvents in chromatography: A review. TrAC Trends in Analytical Chemistry 2019, 120, 115623. [Google Scholar] [CrossRef]
- Brett, C.M.A. Deep eutectic solvents and applications in electrochemical sensing. Current Opinion in Electrochemistry 2018, 10, 143–148. [Google Scholar] [CrossRef]
- Shekaari, H.; Mokhtarpour, M.; Mokhtarpour, F.; Faraji, S.; Martinez, F.; Zafarani-Moattar, M.T. Significant Increase in the Solubility of Celecoxib in Presence of Some Deep Eutectic Solvents as Novel Sustainable Solvents and the Thermodynamic Analysis of These Systems. Pharm Sci 2020, 26, 423–433. [Google Scholar] [CrossRef]
- Hayyan, M.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. A novel technique for separating glycerine from palm oil-based biodiesel using ionic liquids. Fuel Processing Technology 2010, 91, 116–120. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep eutectic solvents: Recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef]
- Sarraguca, M.C.; Ribeiro, P.R.S.; Nunes, C.; Seabra, C.L. Solids Turn into Liquids-Liquid Eutectic Systems of Pharmaceutics to Improve Drug Solubility. Pharmaceuticals 2022, 15, 279. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Atilhan, M.; Aparicio, S. Theoretical Study on Deep Eutectic Solvents as Vehicles for the Delivery of Anesthetics. The Journal of Physical Chemistry B 2020, 124, 1794–1805. [Google Scholar] [CrossRef]
- Shekaari, H.; Mokhtarpour, M.; Faraji, S.; Zafarani-Moattar, M.T. Enhancement of curcumin solubility by some choline chloride-based deep eutectic solvents at different temperatures. Fluid Phase Equilibria 2021, 532, 112917. [Google Scholar] [CrossRef]
- Marei, H.F.; Arafa, M.F.; Essa, E.A.; El Maghraby, G.M. Lidocaine as eutectic forming drug for enhanced transdermal delivery of nonsteroidal anti-inflammatory drugs. Journal of Drug Delivery Science and Technology 2021, 61, 102338. [Google Scholar] [CrossRef]
- Vaidya, A.; Mitragotri, S. Ionic liquid-mediated delivery of insulin to buccal mucosa. J. Control. Release 2020, 327, 26–34. [Google Scholar] [CrossRef]
- Mondal, D.; Sharma, M.; Mukesh, C.; Gupta, V.; Prasad, K. Improved solubility of DNA in recyclable and reusable bio-based deep eutectic solvents with long-term structural and chemical stability. Chem. Commun. 2013, 49, 9606–9608. [Google Scholar] [CrossRef]
- Yadav, N.; Bhakuni, K.; Bisht, M.; Bahadur, I.; Venkatesu, P. Expanding the Potential Role of Deep Eutectic Solvents toward Facilitating the Structural and Thermal Stability of α-Chymotrypsin. Acs Sustain Chem Eng 2020, 8, 10151–10160. [Google Scholar] [CrossRef]
- Aroso, I.M.; Craveiro, R.; Rocha, Â.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES—Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79. [Google Scholar] [CrossRef]
- Pradeepkumar, P.; Sangeetha, R.; Gunaseelan, S.; Varalakshmi, P.; Chuturgoon, A.A.; Rajan, M. Folic Acid Conjugated Polyglutamic Acid Drug Vehicle Synthesis through Deep Eutectic Solvent for Targeted Release of Paclitaxel. Chemistryselect 2019, 4, 10225–10235. [Google Scholar] [CrossRef]
- Makoś, P.; Słupek, E.; Gębicki, J. Hydrophobic deep eutectic solvents in microextraction techniques–A review. Microchemical Journal 2020, 152, 104384. [Google Scholar] [CrossRef]
- Shishov, A.; Pochivalov, A.; Nugbienyo, L.; Andruch, V.; Bulatov, A. Deep eutectic solvents are not only effective extractants. TrAC Trends in Analytical Chemistry 2020, 129, 115956. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilibria 2017, 448, 135–142. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Ibrahim, M.; Hashim, M.A. Allyl triphenyl phosphonium bromide based DES-functionalized carbon nanotubes for the removal of mercury from water. Chemosphere 2017, 167, 44–52. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Ibrahim, R.K.; Hashim, M.A. Lead removal from water by choline chloride based deep eutectic solvents functionalized carbon nanotubes. J. Mol. Liq. 2016, 222, 883–894. [Google Scholar] [CrossRef]
- Zubeir, L.F.; van Osch, D.J.G.P.; Rocha, M.A.A.; Banat, F.; Kroon, M.C. Carbon Dioxide Solubilities in Decanoic Acid-Based Hydrophobic Deep Eutectic Solvents. Journal of Chemical & Engineering Data 2018, 63, 913–919. [Google Scholar] [CrossRef]
- Boldrini, C.L.; Manfredi, N.; Perna, F.M.; Capriati, V.; Abbotto, A. Designing Eco-Sustainable Dye-Sensitized Solar Cells by the Use of a Menthol-Based Hydrophobic Eutectic Solvent as an Effective Electrolyte Medium. Chem.-Eur. J. 2018, 24, 17656–17659. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-Designed Hydrophobic Deep Eutectic Solvents As Green and Efficient Media for the Extraction of Artemisinin from Artemisia annua Leaves. Acs Sustain Chem Eng 2017, 5, 3270–3278. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Tailor-made hydrophobic deep eutectic solvents for cleaner extraction of polyprenyl acetates from Ginkgo biloba leaves. J. Clean Prod. 2017, 152, 399–405. [Google Scholar] [CrossRef]
- Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules 2021, 26, 6556. [Google Scholar] [CrossRef]
- Dai, Y.; Jin, R.; Verpoorte, R.; Lam, W.; Cheng, Y.-C.; Xiao, Y.; Xu, J.; Zhang, L.; Qin, X.-M.; Chen, S. Natural deep eutectic characteristics of honey improve the bioactivity and safety of traditional medicines. Journal of Ethnopharmacology 2020, 250, 112460. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microcalorimetric investigation on the antimicrobial activity of honey of the stingless bee Trigona spp. and comparison of some parameters with those obtained with standard methods. Thermochim. Acta 2004, 415, 99–106. [Google Scholar] [CrossRef]
- Molan, P.C.; Smith, I.M.; Reid, G.M. A Comparison of the Antibacterial Activities of Some new Zealand Honeys. J. Apicult. Res. 1988, 27, 252–256. [Google Scholar] [CrossRef]
- Daneshjou, S.; Khodaverdian, S.; Dabirmanesh, B.; Rahimi, F.; Daneshjoo, S.; Ghazi, F.; Khajeh, K. Improvement of chondroitinases ABCI stability in natural deep eutectic solvents. J. Mol. Liq. 2017, 227, 21–25. [Google Scholar] [CrossRef]
- Fernández, M.d.l.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food. Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of natural deep eutectic solvents for extraction and determination of phenolics in Cajanus cajan leaves by ultra performance liquid chromatography. Separation and Purification Technology 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): screening as solvents for lignocellulosic biomass processing. Green Chemistry 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environmental Science and Pollution Research 2016, 23, 9265–9275. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Spisso, A.; Silva, M.F. Pencil graphite electrodes for improved electrochemical detection of oleuropein by the combination of Natural Deep Eutectic Solvents and graphene oxide. Electrophoresis 2017, 38, 2704–2711. [Google Scholar] [CrossRef]
- Shamseddin, A.; Crauste, C.; Durand, E.; Villeneuve, P.; Dubois, G.; Durand, T.; Vercauteren, J.; Veas, F. Resveratrol formulated with a natural deep eutectic solvent inhibits active matrix metalloprotease-9 in hormetic conditions. European Journal of Lipid Science and Technology 2017, 119, 1700171. [Google Scholar] [CrossRef]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: enhanced permeation of a model drug, ibuprofen. J. Control. Release 1998, 50, 297–308. [Google Scholar] [CrossRef]
- Duarte, A.R.; Ferreira, A.S.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur J Pharm Biopharm 2017, 114, 296–304. [Google Scholar] [CrossRef]
- Shi, D.D.; Dong, C.M.; Ho, L.C.; Lam, C.T.W.; Zhou, X.D.; Wu, E.X.; Zhou, Z.J.; Wang, X.M.; Zhang, Z.J. Resveratrol, a natural polyphenol, prevents chemotherapy-induced cognitive impairment: Involvement of cytokine modulation and neuroprotection. Neurobiology of Disease 2018, 114, 164–173. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, structure, and applications of therapeutic and amino acid-based deep eutectic solvents: An overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Saha, M.; Rahman, M.S.; Hossain, M.N.; Raynie, D.E.; Halim, M.A. Molecular and Spectroscopic Insights of a Choline Chloride Based Therapeutic Deep Eutectic Solvent. J. Phys. Chem. A 2020, 124, 4690–4699. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, F.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C. Untangling the bioactive properties of therapeutic deep eutectic solvents based on natural terpenes. Current Research in Chemical Biology 2021, 1, 100003. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur J Pharm Biopharm 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Ishaq, M.; Gilani, M.A.; Afzal, Z.M.; Bilad, M.R.; Nizami, A.-S.; Rehan, M.; Tahir, E.; Khan, A.L. Novel Poly Deep Eutectic Solvents Based Supported Liquid Membranes for CO2 Capture. Frontiers in Energy Research 2020, 8. [Google Scholar] [CrossRef]
- Ren’ai, L.; Zhang, K.; Chen, G.; Su, B.; Tian, J.; He, M.; Lu, F. Green polymerizable deep eutectic solvent (PDES) type conductive paper for origami 3D circuits. Chem. Commun. 2018, 54, 2304–2307. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, G.; Li, R.a.; Zhao, K.; Shen, J.; Tian, J.; He, M. Facile Preparation of Highly Transparent Conducting Nanopaper with Electrical Robustness. Acs Sustain Chem Eng 2020, 8, 5132–5139. [Google Scholar] [CrossRef]
- Wang, R.; Mao, Z.; Chen, Z. Monolithic column with polymeric deep eutectic solvent as stationary phase for capillary electrochromatography. Journal of Chromatography A 2018, 1577, 66–71. [Google Scholar] [CrossRef]
- Ghorpade, P.; Gadilohar, B.; Pinjari, D.; Shinde, Y.; Shankarling, G. Concentrated solar radiation enhanced one pot synthesis of DES and Phenyl phthalimide. Solar Energy 2015, 122, 1354–1361. [Google Scholar] [CrossRef]
- Farooq, M.Q.; Abbasi, N.M.; Anderson, J.L. Deep eutectic solvents in separations: Methods of preparation, polarity, and applications in extractions and capillary electrochromatography. Journal of Chromatography A 2020, 1633, 461613. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Vatanpour, V.; Ganjali, M.R.; Saeb, M.R. Deep eutectic solvents in membrane science and technology: Fundamental, preparation, application, and future perspective. Separation and Purification Technology 2021, 258, 118015. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, R.; Han, H.; Wu, K.; Wang, B.; Liu, Y.; Zhu, Y.; Lu, H.; Liang, B. Preparation strategy and stability of deep eutectic solvents: A case study based on choline chloride-carboxylic acid. J. Clean Prod. 2022, 345, 131028. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep eutectic solvents as new media for green extraction of food proteins: Opportunity and challenges. Food. Res. Int. 2022, 161, 111842. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food. Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. Journal of Chromatography A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Dheyab, A.S.; Abu Bakar, M.F.; AlOmar, M.; Sabran, S.F.; Muhamad Hanafi, A.F.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations 2021, 8, 176. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Larriba, M.; Navarro, P.; Rigual, V.; Ayuso, M.; García, J.; Rodríguez, F. Thermal stability of choline chloride deep eutectic solvents by TGA/FTIR-ATR analysis. J. Mol. Liq. 2018, 260, 37–43. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- González-Rivera, J.; Pelosi, C.; Pulidori, E.; Duce, C.; Tiné, M.R.; Ciancaleoni, G.; Bernazzani, L. Guidelines for a correct evaluation of Deep Eutectic Solvents thermal stability. Current Research in Green and Sustainable Chemistry 2022, 5, 100333. [Google Scholar] [CrossRef]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: glyceline, ethaline, and reline. Environmental Science and Pollution Research 2021, 28, 8812–8821. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environmental Science and Pollution Research 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Radojčić Redovniković, I. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicology and Environmental Safety 2015, 112, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Juneidi, I.; Hayyan, M.; Mohd Ali, O. Toxicity profile of choline chloride-based deep eutectic solvents for fungi and Cyprinus carpio fish. Environmental Science and Pollution Research 2016, 23, 7648–7659. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.L.C.; Wicińska, A.; Sarraguça, M.; Saraiva, M.L.M.F.S. An automatic approach for the evaluation of the effect of ionic liquids and deep eutectic solvents on elastase. J. Mol. Liq. 2023, 373, 121240. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. Acs Sustain Chem Eng 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J.; Shan, S.; Cao, Y. High toxicity of amino acid-based deep eutectic solvents. J. Mol. Liq. 2023, 370, 121044. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals. 1992, 301, 1–62.
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. Acs Sustain Chem Eng 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Ijardar, S.P.; Singh, V.; Gardas, R.L. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules 2022, 27, 1368. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Anstiss, L.; Weber, C.C.; Baroutian, S.; Shahbaz, K. Menthol-based deep eutectic solvents as green extractants for the isolation of omega-3 polyunsaturated fatty acids from Perna canaliculus. Journal of Chemical Technology and Biotechnology 2023, 98, 1791–1802. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: current industrial applications, limitations and future challenges. Food & Function 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Pai, S.; Hebbar, A.; Selvaraj, S. A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector. Environmental Science and Pollution Research 2022, 29, 35518–35541. [Google Scholar] [CrossRef]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Advances in Nutrition 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2021, 10, 37. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2 - Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds, Campos, M.R.S., Ed.; Woodhead Publishing: 2019; pp. 33–50.
- Slawinska, N.; Olas, B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients 2023, 15, 187. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Song, F.; Yao, Y.; Ya, F.; Li, D.; Ling, W.; Yang, Y. Effects of purified anthocyanin supplementation on platelet chemokines in hypocholesterolemic individuals: a randomized controlled trial. Nutrition & Metabolism 2016, 13, 86. [Google Scholar] [CrossRef]
- Chen, L.-C.; Huang, H.-L.; HuangFu, W.-C.; Yen, S.-C.; Ngo, S.-T.; Wu, Y.-W.; Lin, T.E.; Sung, T.-Y.; Lien, S.-T.; Tseng, H.-J.; et al. Biological Evaluation of Selected Flavonoids as Inhibitors of MNKs Targeting Acute Myeloid Leukemia. Journal of Natural Products 2020, 83, 2967–2975. [Google Scholar] [CrossRef]
- Kamma, M.; Lin, W.C.; Lau, S.C.; Chansakaow, S.; Leelapornpisid, P. Anti-aging Cosmeceutical Product Containing of Nymphaea rubra Roxb. ex Andrews Extract. Chiang Mai Journal of Science 2019, 46, 1143–1160. [Google Scholar]
- Mota, M.D.; da Boa Morte, A.N.; Silva, L.C.R.C.e.; Chinalia, F.A. Sunscreen protection factor enhancement through supplementation with Rambutan (Nephelium lappaceum L) ethanolic extract. Journal of Photochemistry and Photobiology B: Biology 2020, 205, 111837. [Google Scholar] [CrossRef]
- Kwon, O.S.; Han, J.H.; Yoo, H.G.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine 2007, 14, 551–555. [Google Scholar] [CrossRef]
- Thomas, R.; Williams, M.; Cauchi, M.; Berkovitz, S.; Smith, S.A. A double-blind, randomised trial of a polyphenolic-rich nail bed balm for chemotherapy-induced onycholysis: the UK polybalm study. Breast Cancer Research and Treatment 2018, 171, 103–110. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends in Analytical Chemistry 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. International Journal of Molecular Sciences 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chemistry 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Fan, L.; Fan, W.; Mei, Y.; Liu, L.; Li, L.; Wang, Z.; Yang, L. Mechanochemical assisted extraction as a green approach in preparation of bioactive components extraction from natural products - A review. Trends in Food Science & Technology 2022, 129, 98–110. [Google Scholar] [CrossRef]
- Ivanović, M.; Islamčević Razboršek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medicine 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends in Food Science & Technology 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques — a comparative perspective. Current Opinion in Food Science 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Korolev, K.G.; Lomovskii, O.I.; Rozhanskaya, O.A.; Vasil’ev, V.G. Mechanochemical preparation of water-soluble forms of triterpene acids. Chemistry of Natural Compounds 2003, 39, 366–372. [Google Scholar] [CrossRef]
- Kaoui, S.; Chebli, B.; Zaidouni, s.; Basaid, K.; Mir, Y. Deep eutectic solvents as sustainable extraction media for plants and food samples: A review. Sustainable Chemistry and Pharmacy 2023, 31, 100937. [Google Scholar] [CrossRef]
- Wu, K.; Ju, T.; Deng, Y.; Xi, J. Mechanochemical assisted extraction: A novel, efficient, eco-friendly technology. Trends in Food Science & Technology 2017, 66, 166–175. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Frontiers in Nutrition 2020, 7, 60. [Google Scholar] [CrossRef]
- Chang, Y.; Shi, X.; He, F.; Wu, T.; Jiang, L.; Normakhamatov, N.; Sharipov, A.; Wang, T.; Wen, M.; Aisa, H.A. Valorization of Food Processing Waste to Produce Valuable Polyphenolics. Journal of Agricultural and Food Chemistry 2022, 70, 8855–8870. [Google Scholar] [CrossRef]
- Hessel, V.; Tran, N.N.; Asrami, M.R.; Tran, Q.D.; Van Duc Long, N.; Escribà-Gelonch, M.; Tejada, J.O.; Linke, S.; Sundmacher, K. Sustainability of green solvents – review and perspective. Green Chemistry 2022, 24, 410–437. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food. Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Ko, M.-J.; Nam, H.-H.; Chung, M.-S. Subcritical water extraction of bioactive compounds from Orostachys japonicus A. Berger (Crassulaceae). Sci. Rep. 2020, 10, 10890. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends in Food Science & Technology 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food. Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Lee, S.Y.; Liang, Y.N.; Stuckey, D.C.; Hu, X. Single-step extraction of bioactive compounds from cruciferous vegetable (kale) waste using natural deep eutectic solvents. Separation and Purification Technology 2023, 317, 123677. [Google Scholar] [CrossRef]
- Mastellone, G.; Abbasi, N.M.; Cagliero, C.; Anderson, J.L. New Class of Tunable Choline Bromide-Based Hydrophobic Deep Eutectic Solvents for the Extraction of Bioactive Compounds of Varying Polarity from a Plant Matrix. Acs Sustain Chem Eng 2023, 11, 6665–6675. [Google Scholar] [CrossRef] [PubMed]
- Chagnoleau, J.B.; Ferreira, A.M.; Coutinho, J.A.P.; Fernandez, X.; Azoulay, S.; Papaiconomou, N. Sustainable extraction of antioxidants from out-of-caliber kiwifruits. Food. Chem. 2023, 401, 133992. [Google Scholar] [CrossRef]
- Benoit, C.; Virginie, C.; Boris, V. Chapter Twelve - The use of NADES to support innovation in the cosmetic industry. In Advances in Botanical Research, Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Academic Press: 2021; Volume 97, pp. 309–332.
- Rashid, R.; Wani, S.M.; Manzoor, S.; Masoodi, F.A.; Dar, M.M. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food. Chem. 2023, 398. [Google Scholar] [CrossRef]
- Garcia-Roldan, A.; Piriou, L.; Jauregi, P. Natural deep eutectic solvents as a green extraction of polyphenols from spent coffee ground with enhanced bioactivities. Frontiers in Plant Science 2023, 13, 1072592. [Google Scholar] [CrossRef]
- Liu, Y.J.; Lou, L.; Huang, Q.; Xu, W.; Li, H. Ultrasonic extraction and purification of scutellarin from Erigerontis Herba using deep eutectic solvent. Ultrason Sonochem 2023, 99, 106560. [Google Scholar] [CrossRef]
- Vinci, G.; Maddaloni, L.; Prencipe, S.A.; Orlandini, E.; Sambucci, M. Simple and reliable eco-extraction of bioactive compounds from dark chocolate by Deep Eutectic Solvents. A sustainable study. International Journal of Food Science and Technology 2023, 58, 4051–4065. [Google Scholar] [CrossRef]
- Dardavila, M.M.; Pappou, S.; Savvidou, M.G.; Louli, V.; Katapodis, P.; Stamatis, H.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from C. vulgaris Biomass Using Deep Eutectic Solvents. Molecules 2023, 28, 415. [Google Scholar] [CrossRef]
- Wang, S.; Lei, T.; Liu, L.; Tan, Z. CO(2)-responsive deep eutectic solvents for the enhanced extraction of hesperidin from Fertile orange peel. Food Chem 2024, 432, 137255. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Cauich-Rodríguez, J.V.; González-Ávila, M.; Scampicchio, M.; Morozova, K.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Natural Deep Eutectic Solvent Optimization to Obtain an Extract Rich in Polyphenols from Capsicum chinense Leaves Using an Ultrasonic Probe. Processes 2023, 11. [Google Scholar] [CrossRef]
- Ojeda, G.A.; Vallejos, M.M.; Sgroppo, S.C.; Sanchez-Moreno, C.; de Ancos, B. Enhanced extraction of phenolic compounds from mango by-products using deep eutectic solvents. Heliyon 2023, 9, e16912. [Google Scholar] [CrossRef]
- Santos-Martín, M.; Cubero-Cardoso, J.; González-Domínguez, R.; Cortés-Triviño, E.; Sayago, A.; Urbano, J.; Fernández-Recamales, Á. Ultrasound-assisted extraction of phenolic compounds from blueberry leaves using natural deep eutectic solvents (NADES) for the valorization of agrifood wastes. Biomass and Bioenergy 2023, 175. [Google Scholar] [CrossRef]
- Wang, C.Q.; Li, Z.Z.; Xiang, J.L.; Johnson, J.B.; Zheng, B.L.; Luo, L.; Beta, T. From Foxtail Millet Husk (Waste) to Bioactive Phenolic Extracts Using Deep Eutectic Solvent Extraction and Evaluation of Antioxidant, Acetylcholinesterase, and a-Glucosidase Inhibitory Activities. Foods 2023, 12, 1144. [Google Scholar] [CrossRef]
- Gu, J.; Lin, L.; Zhao, M. Demonstration of feasibility and effectiveness of deep eutectic solvent-water system extraction of RG-I type pectin from wolfberry based on target polysaccharide, solvent and their interactions. Food Hydrocolloids 2023, 144. [Google Scholar] [CrossRef]
- Fan, C.; Shan, Y.H.; Wen, L.J.; Cao, X.L. Extraction of artemisinin using natural deep eutectic solvent selected by COSMO-RS. Sustainable Chemistry and Pharmacy 2023, 33, 101096. [Google Scholar] [CrossRef]
- Ali, A.; Chua, B.L.; Chow, Y.H.; Chong, C.H. Development and characterisation of novel terpenoid-based hydrophobic deep eutectic solvents for sustainable extraction of bioactive antioxidants from Rosmarinus officinalis L. J. Mol. Liq. 2023, 388. [Google Scholar] [CrossRef]
- Vo, T.P.; Pham, T.V.; Weina, K.; Tran, T.N.H.; Vo, L.T.V.; Nguyen, P.T.; Bui, T.L.H.; Phan, T.H.; Nguyen, D.Q. Green extraction of phenolics and flavonoids from black mulberry fruit using natural deep eutectic solvents: optimization and surface morphology. BMC Chem 2023, 17, 119. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Cui, L.; Hussain, H.; Nadolnik, L.; Zhang, Z.; Zhao, Y.; Qin, X.; Li, J.; Park, J.H.; et al. Ultrasonic-assisted extraction of flavonoids from peanut leave and stem using deep eutectic solvents and its molecular mechanism. Food Chem 2024, 434, 137497. [Google Scholar] [CrossRef]
- Nekkaa, A.; Benaissa, A.; El Djalil Lalaouna, A.; Dupire, F.; Risler, A.; Mutelet, F.; Canabady-Rochelle, L. Green and innovative extraction of polyphenols from Rhamnus alaternus using natural deep eutectic solvents and evaluation of their bioactivities. Journal of Applied Research on Medicinal and Aromatic Plants 2023, 35. [Google Scholar] [CrossRef]
- Grillo, G.; Tabasso, S.; Capaldi, G.; Radosevic, K.; Radojcic-Redovnikovic, I.; Gunjevic, V.; Calcio Gaudino, E.; Cravotto, G. Food-Waste Valorisation: Synergistic Effects of Enabling Technologies and Eutectic Solvents on the Recovery of Bioactives from Violet Potato Peels. Foods 2023, 12. [Google Scholar] [CrossRef]
- Jin, L.; Jin, W.F.; Zeng, Q.; Yu, L.; Yang, J.H.; Wan, H.T.; He, Y. Optimization of green extraction process with natural deep eutectic solvent and comparative pharmacokinetics of bioactive compounds from Astragalus-Safflower pair. Phytomedicine 2023, 114, 154814. [Google Scholar] [CrossRef]
- Wu, D.T.; Deng, W.; Li, J.; Geng, J.L.; Hu, Y.C.; Zou, L.; Liu, Y.; Liu, H.Y.; Gan, R.Y. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Phenolic Compounds from Thinned Young Kiwifruits and Their Beneficial Effects. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Vo, T.P.; Tran, T.Q.D.; Phan, T.H.; Huynh, H.D.; Vo, T.T.Y.; Vo, N.M.K.; Ha, M.P.; Le, T.N.; Nguyen, D.Q. Ultrasonic-assisted and enzymatic-assisted extraction to recover tannins, flavonoids, and terpenoids from used tea leaves using natural deep eutectic solvents. International Journal of Food Science & Technology 2023, 58, 5855–5864. [Google Scholar] [CrossRef]
- Duarte, H.; Aliano-Gonzalez, M.J.; Cantos-Villar, E.; Faleiro, L.; Romano, A.; Medronho, B. Sustainable extraction of polyphenols from vine shoots using deep eutectic solvents: Influence of the solvent, Vitis sp., and extraction technique. Talanta 2024, 267, 125135. [Google Scholar] [CrossRef]
- Gómez-Urious, C.; Viñas-Ospino, A.; Puchades-Colera, P.; López-Malo, D.; Frígola, A.; Esteve, M.J. Choline chloride-based natural deep eutectic solvents for the extraction and stability of phenolic compounds, ascorbic acid, and antioxidant capacity from Citrus sinensis peel. Lwt-Food Science and Technology 2023, 177, 114595. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Rathod, V.K. Recovery and separation of glycyrrhizic acid from Natural Deep Eutectic Solvent (NADES) extract by macroporous resin: adsorption kinetics and isotherm studies. Prep Biochem Biotechnol 2023, 1–10. [Google Scholar] [CrossRef]
- Le, N.T.; Nguyen, T.P.D.; Ho, D.V.; Phung, H.T.; Nguyen, H.T. Green solvents-based rutin extraction from Sophora japonica L. Journal of Applied Research on Medicinal and Aromatic Plants 2023, 36. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, F.; Baroutian, S.; Zhang, Y.; Wang, Z.M.; Yuan, Z.H.; Chen, X.Y. A deep eutectic solvent binary-phase system for alkaloid extraction from Chinese herb residue and its mechanism. J. Clean Prod. 2023, 398, 136645. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, Y.; Cai, C.; Tan, Z. Extraction of Lycium barbarum polysaccharides using temperature-switchable deep eutectic solvents: A sustainable methodology for recycling and reuse of the extractant. J. Mol. Liq. 2023, 383. [Google Scholar] [CrossRef]
- Cai, C.; Ma, S.; Li, F.; Tan, Z. Aqueous two-phase system based on pH-responsive polymeric deep eutectic solvent for efficient extraction of aromatic amino acids. Food Chem 2024, 430, 137029. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Extraction and purification of curcuminoids from Curcuma longa using microwave assisted deep eutectic solvent based system and cost estimation. Process Biochemistry 2023, 126, 61–71. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhou, Y.; Zhang, M.; Xia, Q.; Bi, W.; Chen, D.D.Y. Ecofriendly Mechanochemical Extraction of Bioactive Compounds from Plants with Deep Eutectic Solvents. Acs Sustain Chem Eng 2017, 5, 6297–6303. [Google Scholar] [CrossRef]
- Qu, H.; Wu, Y.; Luo, Z.; Dong, Q.; Yang, H.; Dai, C. An efficient approach for extraction of polysaccharide from abalone (Haliotis Discus Hannai Ino) viscera by natural deep eutectic solvent. Int J Biol Macromol 2023, 244, 125336. [Google Scholar] [CrossRef]
- Yusoff, M.H.M.; Gan, C.Y.; Shafie, M.H. Characterization of citric acid monohydrate-glycerol based deep eutectic solvents which could be used as an extraction medium for hydrophilic bioactive components. J. Mol. Liq. 2023, 389, 122879. [Google Scholar] [CrossRef]
- Alpat, U.; Nar, T.; Karasu, S.; Sagdic, O. Optimization of propolis extraction with natural deep eutectic solvents using central composite design. Phytochemistry Letters 2023, 58, 49–59. [Google Scholar] [CrossRef]
- Salazar-Bermeo, J.; Moreno-Chamba, B.; Heredia-Hortigüela, R.; Lizama, V.; Martínez-Madrid, M.C.; Saura, D.; Valero, M.; Neacsu, M.; Martí, N. Green Technologies for Persimmon By-Products Revalorisation as Sustainable Sources of Dietary Fibre and Antioxidants for Functional Beverages Development. Antioxidants 2023, 12, 1085. [Google Scholar] [CrossRef]
- Del-Castillo-Llamosas, A.; Rodriguez-Rebelo, F.; Rodriguez-Martinez, B.; Mallo-Fraga, A.; Del-Rio, P.G.; Gullon, B. Valorization of Avocado Seed Wastes for Antioxidant Phenolics and Carbohydrates Recovery Using Deep Eutectic Solvents (DES). Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Tymczewska, A.; Klebba, J.; Szydłowska-Czerniak, A. Antioxidant Capacity and Total Phenolic Content of Spice Extracts Obtained by Ultrasound-Assisted Extraction Using Deep Eutectic and Conventional Solvents. Appl. Sci. 2023, 13. [Google Scholar] [CrossRef]
- Can Gercek, Y.; Kutlu, N.; Celik, S.; Gidik, B.; Bayram, S.; Bayram, N.E. Extraction of Functional Compounds from Tarragon (Artemisia dracunculus L.) by Deep Eutectic Solvents at Different Properties. Chem Biodivers 2023, 20, e202300417. [Google Scholar] [CrossRef]
- Wen, L.; Fan, C.; Zhao, X.; Cao, X. Rapid extraction of bioactive compounds from gardenia fruit using new and recyclable deep eutectic solvents. Journal of Separation Science 2023, 46, 2300163. [Google Scholar] [CrossRef]
- Petrochenko, A.A.; Orlova, A.; Frolova, N.; Serebryakov, E.B.; Soboleva, A.; Flisyuk, E.V.; Frolov, A.; Shikov, A.N. Natural Deep Eutectic Solvents for the Extraction of Triterpene Saponins from Aralia elata var. mandshurica (Rupr. & Maxim.) J. Wen. Molecules 2023, 28, 3614. [Google Scholar] [CrossRef]
- Koigerova, A.; Gosteva, A.; Samarov, A.; Tsvetov, N. Deep Eutectic Solvents Based on Carboxylic Acids and Glycerol or Propylene Glycol as Green Media for Extraction of Bioactive Substances from Chamaenerion angustifolium (L.) Scop. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Koraqi, H.; Qazimi, B.; Khalid, W.; Stanoeva, J.P.; Sehrish, A.; Siddique, F.; Çesko, C.; Ali Khan, K.; Rahim, M.A.; Hussain, I.; et al. Optimized conditions for extraction, quantification and detection of bioactive compound from Nettle (Urtica dioica L.) using the deep eutectic solvents, ultra-sonication and liquid chromatography-mass spectrometry (LC-DAD-ESI-MS/MS). International Journal of Food Properties 2023, 26, 2171–2185. [Google Scholar] [CrossRef]
- Karpitskiy, D.A.; Bessonova, E.A.; Shishov, A.Y.; Kartsova, L.A. Selective extraction of plant bioactive compounds with deep eutectic solvents: Iris sibirica L. as example. Phytochem Anal 2023. [Google Scholar] [CrossRef]
- Gil, K.A.; Jokic, S.; Cikos, A.M.; Banozic, M.; Kovac, M.J.; Fais, A.; Tuberoso, C.I.G. Comparison of Different Green Extraction Techniques Used for the Extraction of Targeted Flavonoids from Edible Feijoa(Acca sellowiana (O.Berg) Burret) Flowers. Plants-Basel 2023, 12, 1461. [Google Scholar] [CrossRef]
- Bernal-Millán, M.D.; Carrasco-Portugal, M.D.; Heredia, J.B.; Bastidas-Bastidas, P.D.; Gutierrez-Grijalva, E.P.; Leon-Felix, J.; Angulo-Escalante, M.A. Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids. Plants-Basel 2023, 12, 1692. [Google Scholar] [CrossRef]
- Metaj, I.; Hajdini, D.; Gliha, K.; Kosir, I.J.; Ocvirk, M.; Kolar, M.; Cerar, J. Extraction of Polyphenols from Slovenian Hop (Humulus lupulus L.) Aurora Variety Using Deep Eutectic Solvents: Choice of the Extraction Method vs. Structure of the Solvent. Plants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Ashraf, W.; Rehman, A.; Hussain, A.; Karim, A.; Sharif, H.R.; Siddiquy, M.; Lianfu, Z. Optimization of Extraction Process and Estimation of Flavonoids from Fenugreek Using Green Extracting Deep Eutectic Solvents Coupled with Ultrasonication. Food and Bioprocess Technology 2023. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





