Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
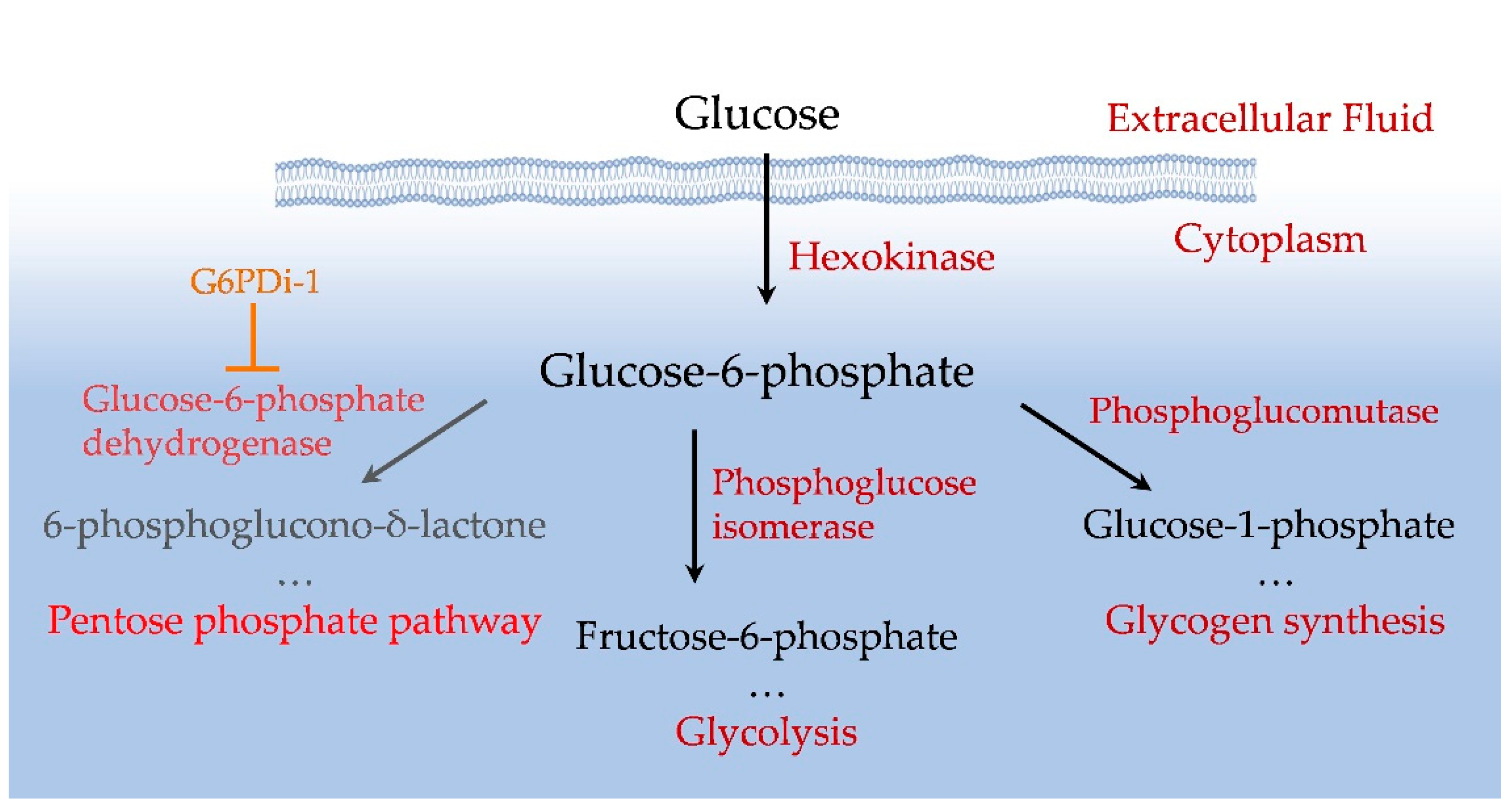
2. Results
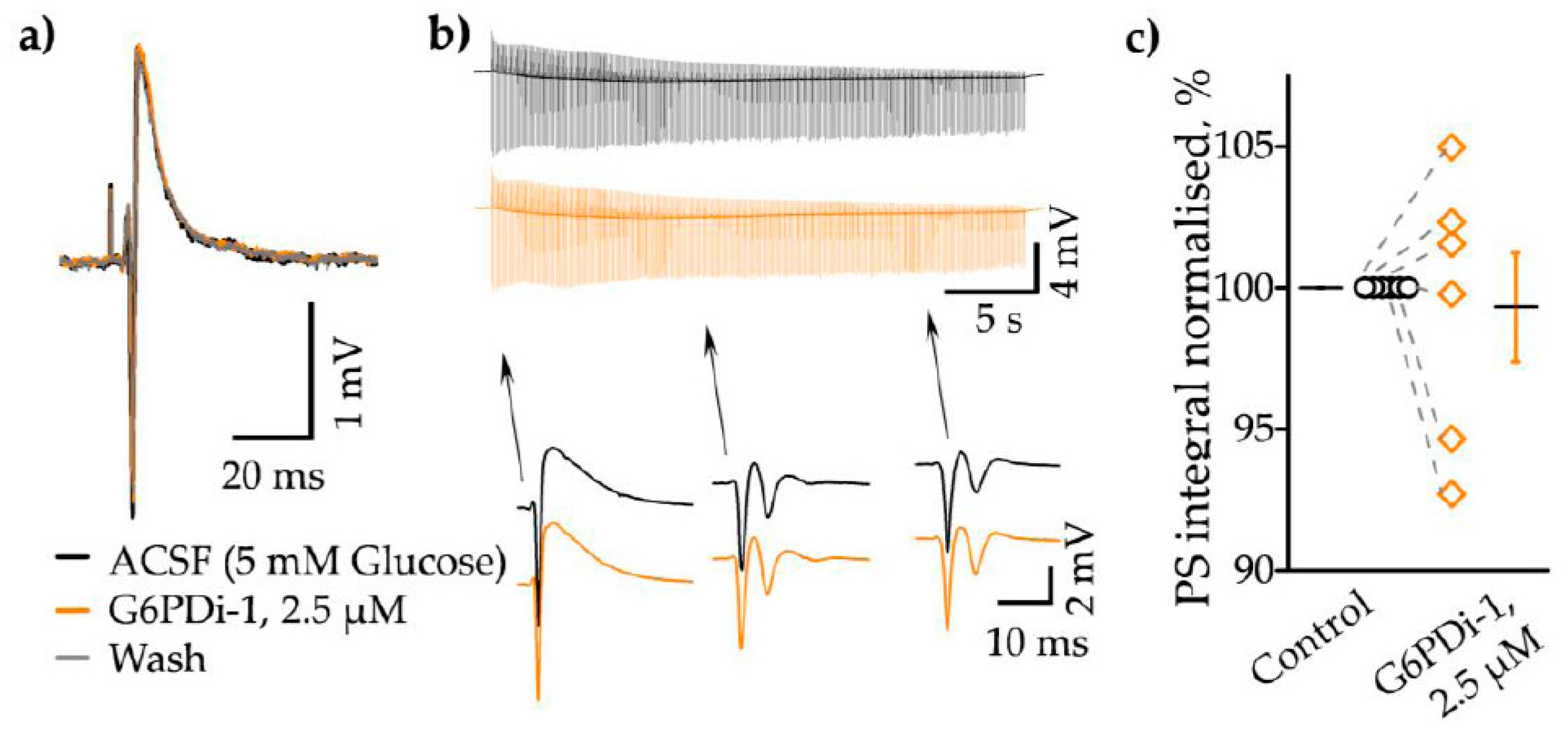
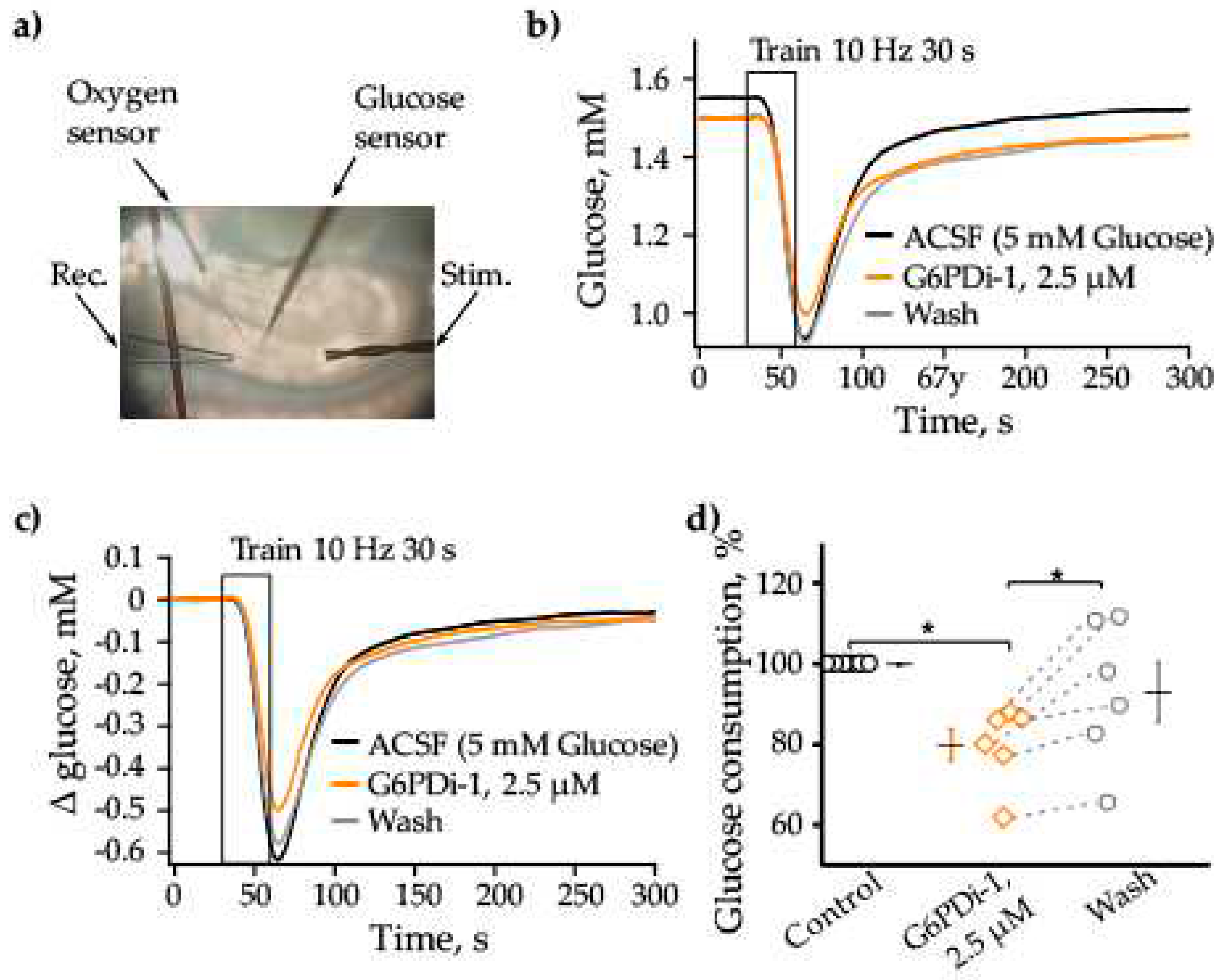
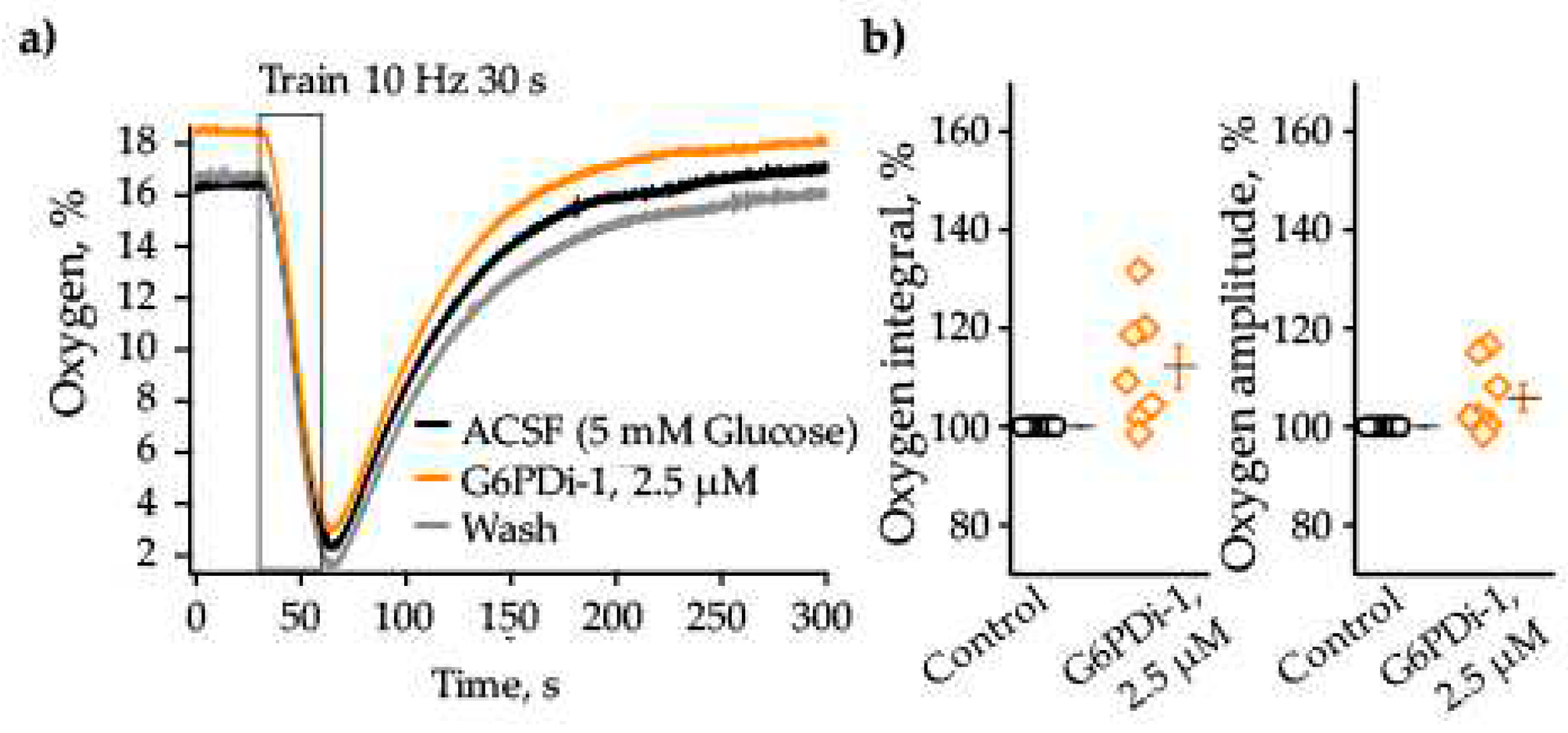
2.1. Effect of G6PDi-1 on consumption of glucose and oxygen in hippocampal slices
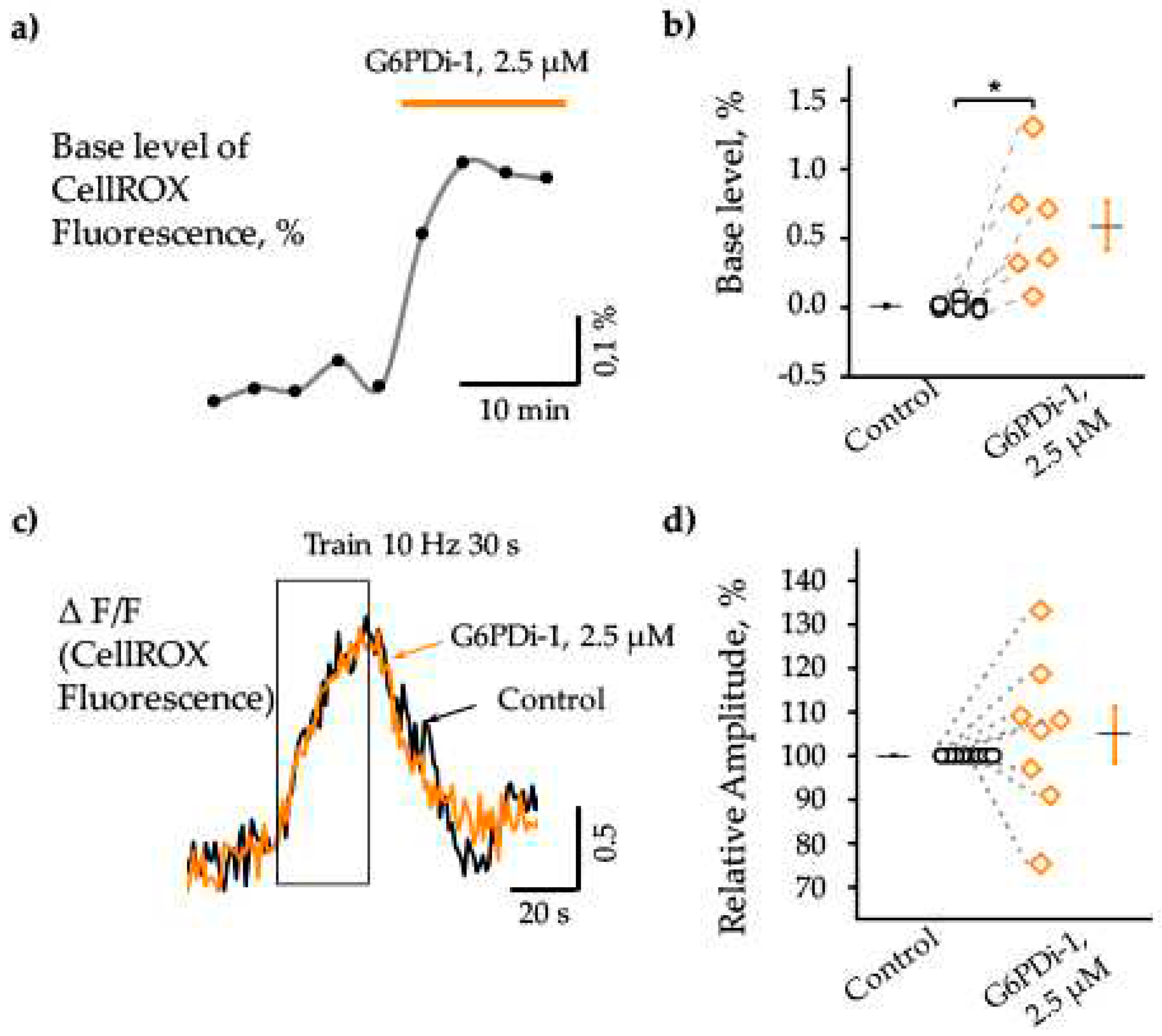
2.2. Effect G6PDi-1 on basic and synaptically induced changes of intracellular ROS levels
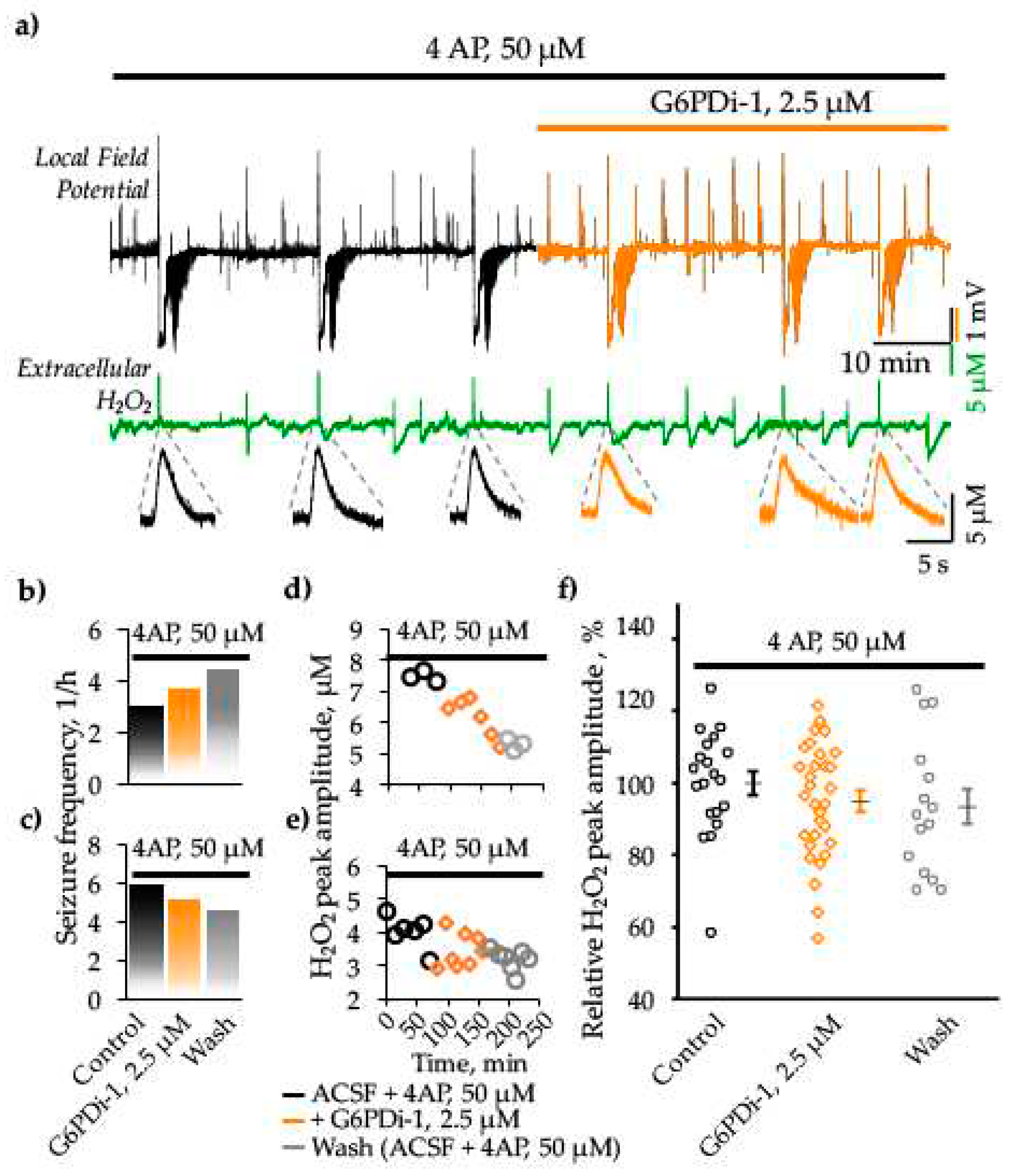
2.3. Analysis of the G6PDi-1 action on the production of hydrogen peroxide and seizure-like phenomena in the 4AP model of epilepsy
3. Discussion
3.1. Effect of G6PDi-1 on glucose consumption in hippocampal slices
3.2. Effect G6PDi-1 on basic and synaptically induced changes of intracellular ROS levels
3.3 Effect G6PDi-1 on hydrogen peroxide (H2O2) production and epileptiform activity
4. Materials and Methods
4.1. Animals
4.2. Brain Slices Preparation
4.3. LFP recording and synaptic stimulation
4.4. Glucose and H2O2 measurement
4.5. Oxygen measurement
4.6. Pharmacology
4.7. Fluorescence monitoring of intracellular Reactive Oxygen Species (ROS)
4.8. Data analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgements
Conflicts of Interest
References
- Fioramonti, X.; Pénicaud, L.; Fioramonti, X.; Pénicaud, L. Carbohydrates and the Brain: Roles and Impact. Feed Your Mind - How Does Nutr. Modul. Brain Funct. throughout Life? 2019. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Chakravarthy, H.; Devanathan, V. Glucose metabolism in the brain: An update. Recent Dev. Appl. Microbiol. Biochem. Vol. 2 2021, 77–88. [Google Scholar] [CrossRef]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci. 2001, 26, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Hanczko, R.; Telarico, T.; Oaks, Z.; Landas, S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol. Med. 2011, 17, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Neuroprotection by glucose-6-phosphate dehydrogenase and the pentose phosphate pathway. J. Cell. Biochem. 2019, 120, 14285–14295. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, G.; Masullo, U.; Passarelli, S.; Filosa, S. Glucose-6-phosphate dehydrogenase deficiency: disadvantages and possible benefits. Cardiovasc. Hematol. Disord. Drug Targets 2013, 13, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Vanegas, O.C.; Patel, M.; Rosti, V.; Li, H.; Waka, J.; Merghoub, T.; Pandolfi, P.P.; Notaro, R.; Manova, K.; et al. Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO J. 2002, 21, 4229–4239. [Google Scholar] [CrossRef]
- Gupte, S.A. Glucose-6-phosphate dehydrogenase: a novel therapeutic target in cardiovascular diseases. Curr. Opin. Investig. Drugs 2008, 9, 993–1000. [Google Scholar]
- Boros, L.; Puigjaner, J.; Cascante, M.; research, W.L.-C. undefined Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. AACRLG Boros, J Puigjaner, M Cascante, WNP Lee, JL Brand. S Bassilian, FI YusufCancer Res. 1997. AACR.
- Liguori, R.; Giannoccaro, M.P.; Pasini, E.; Riguzzi, P.; Valentino, M.L.; Comi, G. Pietro; Carelli, V.; Bresolin, N.; Michelucci, R. Acute rhabdomyolysis induced by tonic-clonic epileptic seizures in a patient with glucose-6-phosphate dehydrogenase deficiency. J. Neurol. 2013, 260, 2669–2671. [Google Scholar] [CrossRef]
- Abul, M.; Azad, K.; Nazmul, M.; Chowdhury, H.; Abdullah, M.; Hasan, A.; Masum Emran, M.; Das, P.; Akther, M.; Emran, M.M. Progressive Myoclonic Epilepsy: Lafora Disease - Clinical and Genetic Findings. http://www.sciencepublishinggroup.com 2022, 11, 126. [Google Scholar]
- Ulusu, N.N.; Sahilli, M.; Avci, A.; Canbolat, O.; Ozansoy, G.; Ari, N.; Bali, M.; Stefek, M.; Stolc, S.; Gajdosik, A.; et al. Pentose phosphate pathway, glutathione -dependent enzymes and antioxidant defense during oxidative stress in diabetic rodent brain and peripheral organs: Effects of stobadine and vitamin E. Neurochem. Res. 2003, 28, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.L.; Siedlak, S.L.; Raina, A.K.; Bautista, J.M.; Smith, M.A.; Perry, G. Increased neuronal glucose-6-phosphate dehydrogenase and sulfhydryl levels indicate reductive compensation to oxidative stress in Alzheimer disease. Arch. Biochem. Biophys. 1999, 370, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Ulusu, N.N. Glucose-6-phosphate dehydrogenase deficiency and Alzheimer’s disease: Partners in crime? The hypothesis. Med. Hypotheses 2015, 85, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Hippocampus, E.M. undefined Functional differentiation in the hippocampus. Wiley Online Libr. Moser, EI MoserHippocampus, 1998. Wiley Online Libr. 1998. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Brancati, G.E.; Rawas, C.; Ghestem, A.; Bernard, C.; Ivanov, A.I. Spatio-temporal heterogeneity in hippocampal metabolism in control and epilepsy conditions. Proc. Natl. Acad. Sci. U. S. A. 2021, 118. [Google Scholar] [CrossRef]
- Malkov, A.; Ivanov, A.I.; Latyshkova, A.; Bregestovski, P.; Zilberter, M.; Zilberter, Y. Activation of nicotinamide adenine dinucleotide phosphate oxidase is the primary trigger of epileptic seizures in rodent models. Ann. Neurol. 2019, 85, 907–920. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Ghergurovich, J.M.; García-Cañaveras, J.C.; Wang, J.; Schmidt, E.; Zhang, Z.; TeSlaa, T.; Patel, H.; Chen, L.; Britt, E.C.; Piqueras-Nebot, M.; et al. A small molecule G6PD inhibitor reveals immune dependence on pentose phosphate pathway. Nat. Chem. Biol. 2020, 16, 731. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.; Mukhtarov, M.; Bregestovski, P.; Zilberter, Y. Lactate Effectively Covers Energy Demands during Neuronal Network Activity in Neonatal Hippocampal Slices. Front. Neuroenergetics 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef] [PubMed]
- Malkov, A.; Ivanov, A.I.; Buldakova, S.; Waseem, T.; Popova, I.; Zilberter, M.; Zilberter, Y. Seizure-induced reduction in glucose utilization promotes brain hypometabolism during epileptogenesis. Neurobiol. Dis. 2018, 116, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Perreault, P.; Avoli, M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. 1991, 65, 771–785. [Google Scholar] [CrossRef]
- Smirnova, E.Y.; Chizhov, A. V.; Zaitsev, A. V. Presynaptic GABAB receptors underlie the antiepileptic effect of low-frequency electrical stimulation in the 4-aminopyridine model of epilepsy in brain slices of young rats. Brain Stimul. 2020, 13, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Angamo, E.A.; Haq, R.U.; Rösner, J.; Gabriel, S.; Gerevich, Z.; Heinemann, U.; Kovács, R. Contribution of Intrinsic Lactate to Maintenance of Seizure Activity in Neocortical Slices from Patients with Temporal Lobe Epilepsy and in Rat Entorhinal Cortex. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Pearson-Smith, J.N.; Patel, M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Pestana, R.R.F.; Kinjo, E.R.; Hernandes, M.S.; Britto, L.R.G. Reactive oxygen species generated by NADPH oxidase are involved in neurodegeneration in the pilocarpine model of temporal lobe epilepsy. Neurosci. Lett. 2010, 484, 187–191. [Google Scholar] [CrossRef]
- Shin, E.J.; Jeong, J.H.; Chung, Y.H.; Kim, W.K.; Ko, K.H.; Bach, J.H.; Hong, J.S.; Yoneda, Y.; Kim, H.C. Role of oxidative stress in epileptic seizures. Neurochem. Int. 2011, 59, 122–137. [Google Scholar] [CrossRef] [PubMed]
- McElroy, P.B.; Liang, L.P.; Day, B.J.; Patel, M. Scavenging reactive oxygen species inhibits status epilepticus-induced neuroinflammation. Exp. Neurol. 2017, 298, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Puttachary, S.; Sharma, S.; Stark, S.; Thippeswamy, T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Shekh-Ahmad, T.; Kovac, S.; Abramov, A.Y.; Walker, M.C. Reactive oxygen species in status epilepticus. Epilepsy Behav. 2019, 101. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A.; Banks, J. Inhibition of mammalian glucose-6-phosphate dehydrogenase by steroids. Proc. Natl. Acad. Sci. U. S. A. 1960, 46, 447–452. [Google Scholar] [CrossRef]
- Monaco, M. Di; Pizzini, A.; Gatto, V.; … L.L.-B. journal of; 1997, undefined Role of glucose-6-phosphate dehydrogenase inhibition in the antiproliferative effects of dehydroepiandrosterone on human breast cancer cells. nature.comM Di Monaco, A Pizzini, V Gatto, L Leonardi, M Gall. E Brignardello, G BoccuzziBritish J. cancer, 1997. nature.com. [CrossRef]
- Girón, R.A.; Montaño, L.F.; Escobar, M.L.; López-Marure, R. Dehydroepiandrosterone inhibits the proliferation and induces the death of HPV-positive and HPV-negative cervical cancer cells through an androgen- and estrogen-receptor independent mechanism. FEBS J. 2009, 276, 5598–5609. [Google Scholar] [CrossRef] [PubMed]
- Prough, R.A.; Clark, B.J.; Klinge, C.M. Novel mechanisms for DHEA action. J. Mol. Endocrinol. 2016, 56, R139–R155. [Google Scholar] [CrossRef]
- Malkov, A.; Ivanov, A.I.; Popova, I.; Mukhtarov, M.; Gubkina, O.; Waseem, T.; Bregestovski, P.; Zilberter, Y. Reactive oxygen species initiate a metabolic collapse in hippocampal slices: Potential trigger of cortical spreading depression. J. Cereb. Blood Flow Metab. 2014, 34, 1540–1549. [Google Scholar] [CrossRef]
- Demirgören, S.; Majewska, M.D.; Spivak, C.E.; London, E.D. Receptor binding and electrophysiological effects of Dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience 1991, 45, 127–135. [Google Scholar] [CrossRef]
- Dong, L.Y.; Cheng, Z.X.; Fu, Y.M.; Wang, Z.M.; Zhu, Y.H.; Sun, J.L.; Dong, Y.; Zheng, P. Neurosteroid dehydroepiandrosterone sulfate enhances spontaneous glutamate release in rat prelimbic cortex through activation of dopamine D1 and sigma-1 receptor. Neuropharmacology 2007, 52, 966–974. [Google Scholar] [CrossRef]
- Hostetler, K.Y.; Landau, B.R.; White, R.J.; Albin, M.S.; Yashon, D. Contribution of the pentose cycle to the metabolism of glucose in the isolated, perfused brain of the monkey. J. Neurochem. 1970, 17, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, P.; Fernandez, E.; Bolaños, J.P. Underestimation of the pentose–phosphate pathway in intact primary neurons as revealed by metabolic flux analysis. J. Cereb. Blood Flow Metab. 2013, 33, 1843. [Google Scholar] [CrossRef] [PubMed]
- Jekabsons, M.B.; Gebril, H.M.; Wang, Y.H.; Avula, B.; Khan, I.A. Updates to a 13C metabolic flux analysis model for evaluating energy metabolism in cultured cerebellar granule neurons from neonatal rats. Neurochem. Int. 2017, 109, 54. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.L.; Pathak, S.D.; Jeromin, A.; Ng, L.L.; MacPherson, C.R.; Mortrud, M.T.; Cusick, A.; Riley, Z.L.; Sunkin, S.M.; Bernard, A.; et al. Genomic anatomy of the hippocampus. Neuron 2008, 60, 1010–1021. [Google Scholar] [CrossRef]
- Dong, H.W.; Swanson, L.W.; Chen, L.; Fanselow, M.S.; Toga, A.W. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 11794–11799. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Dougherty, K.A.; Parikh, K.; Byrne, C.; Johnston, D. Mapping the electrophysiological and morphological properties of ca 1 pyramidal neurons along the longitudinal hippocampal axis. Wiley Online Libr. Malik, KA Dougherty, K Parikh, C Byrne, D JohnstonHippocampus, 2016. Wiley Online Libr. 2016, 26, 341–361. [Google Scholar] [CrossRef]
- Isaeva, E.; Romanov, A.; Holmes, G.L.; Isaev, D. Status epilepticus results in region-specific alterations in seizure susceptibility along the hippocampal longitudinal axis. Epilepsy Res. 2015, 110, 166–170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




