Submitted:
17 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
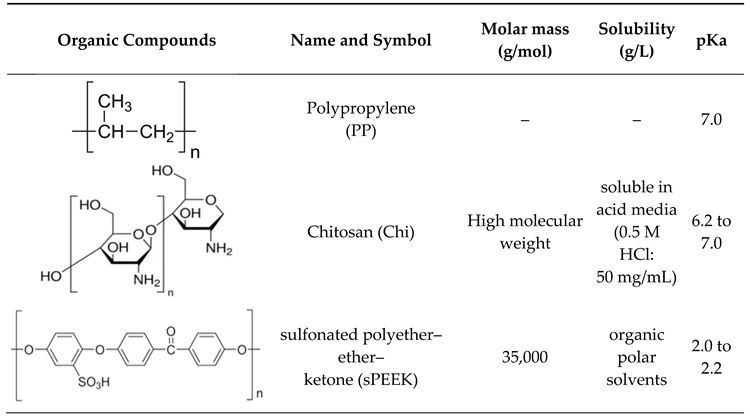
2.1. Reagents and Materials
2.2. Procedures and methods
2.2.1. Preparation of sPEEK–M membranes from PEEK solution in sulfuric acid
- undulation of the membrane;
- the occurrence of defects (microcracks, holes);
- local re-solubilizations (transparencies, gelations).
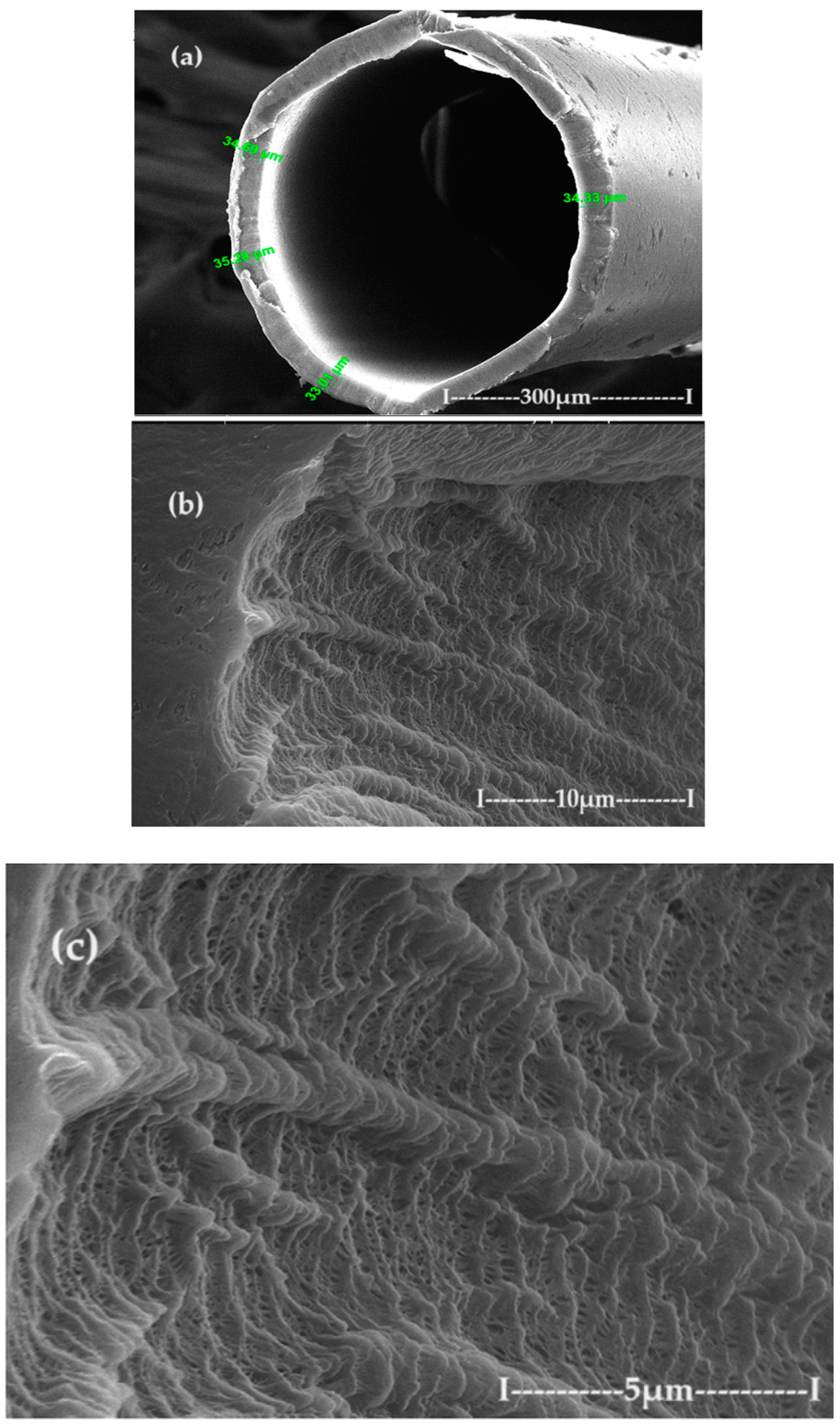
2.2.2. Preparation of Chitosan–Polypropylene Hollow Fibers membranes (C–PHF–M)
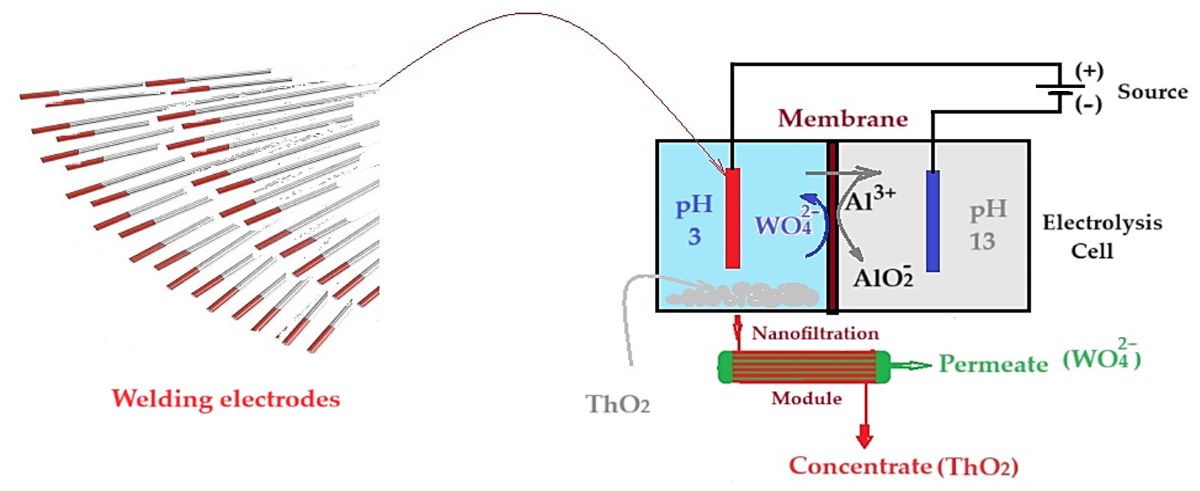
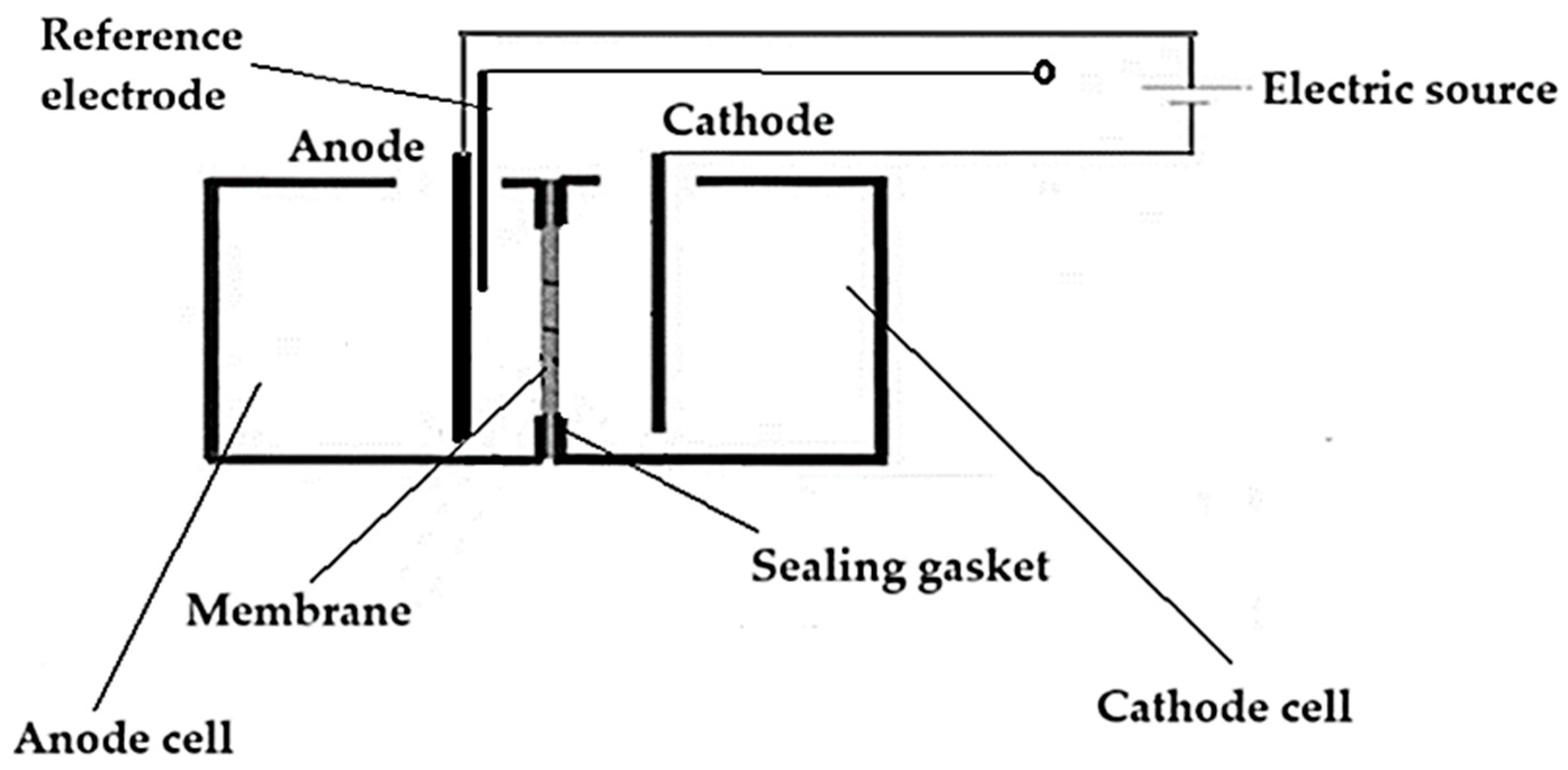
2.2.3. Electrolysis of tungsten and thorium-based electrodes in acidic aqueous solution
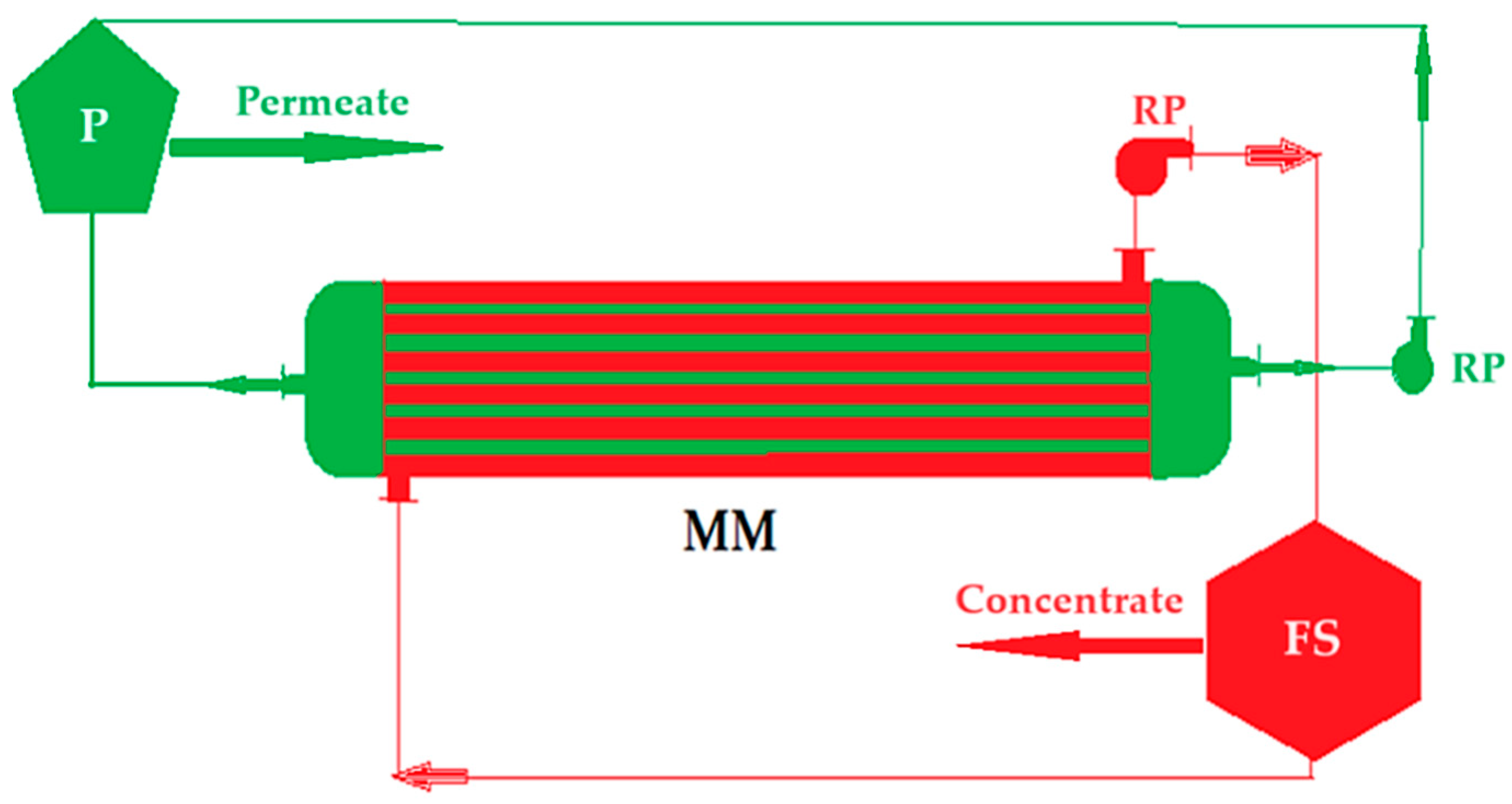
2.3. Equipment
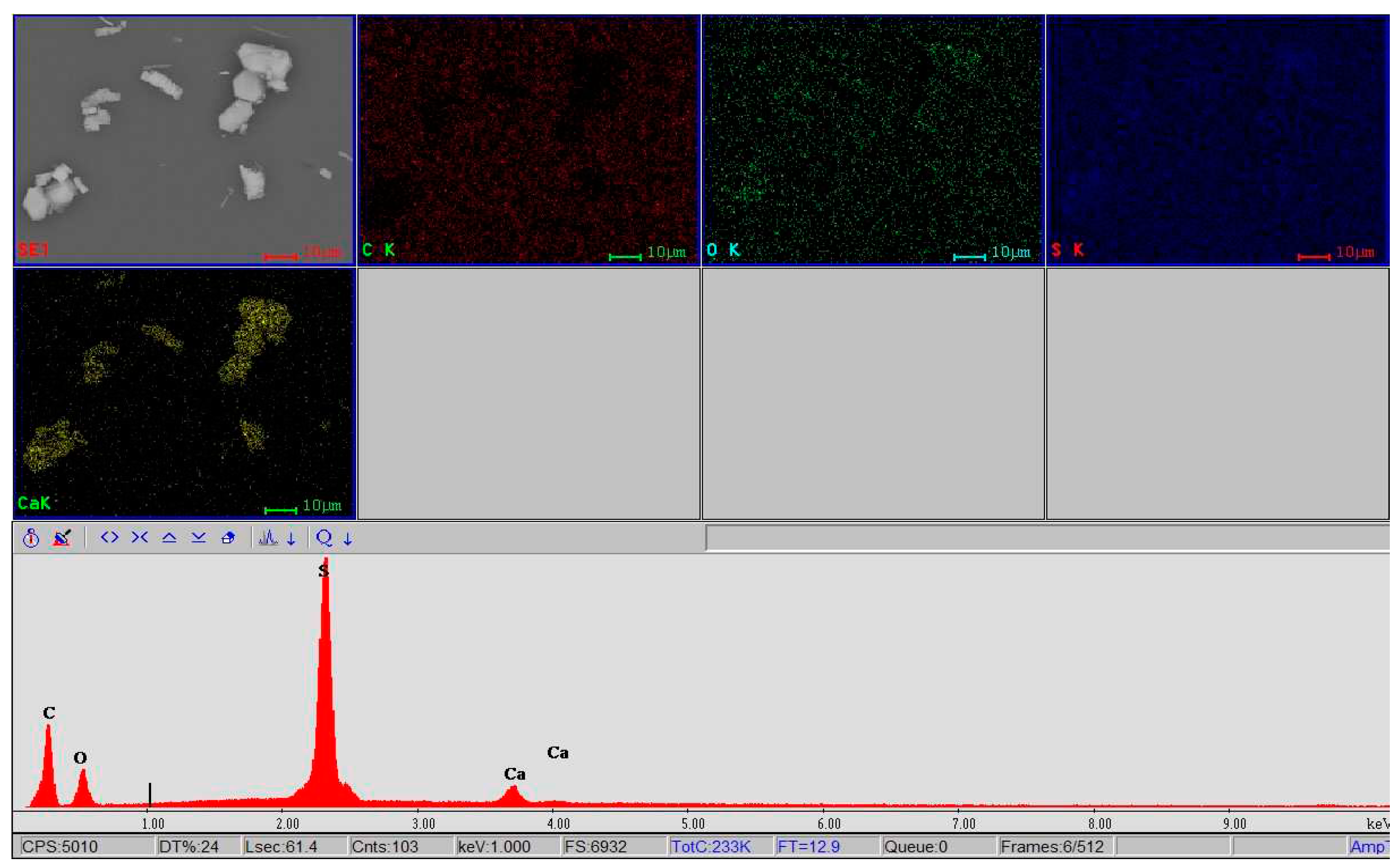
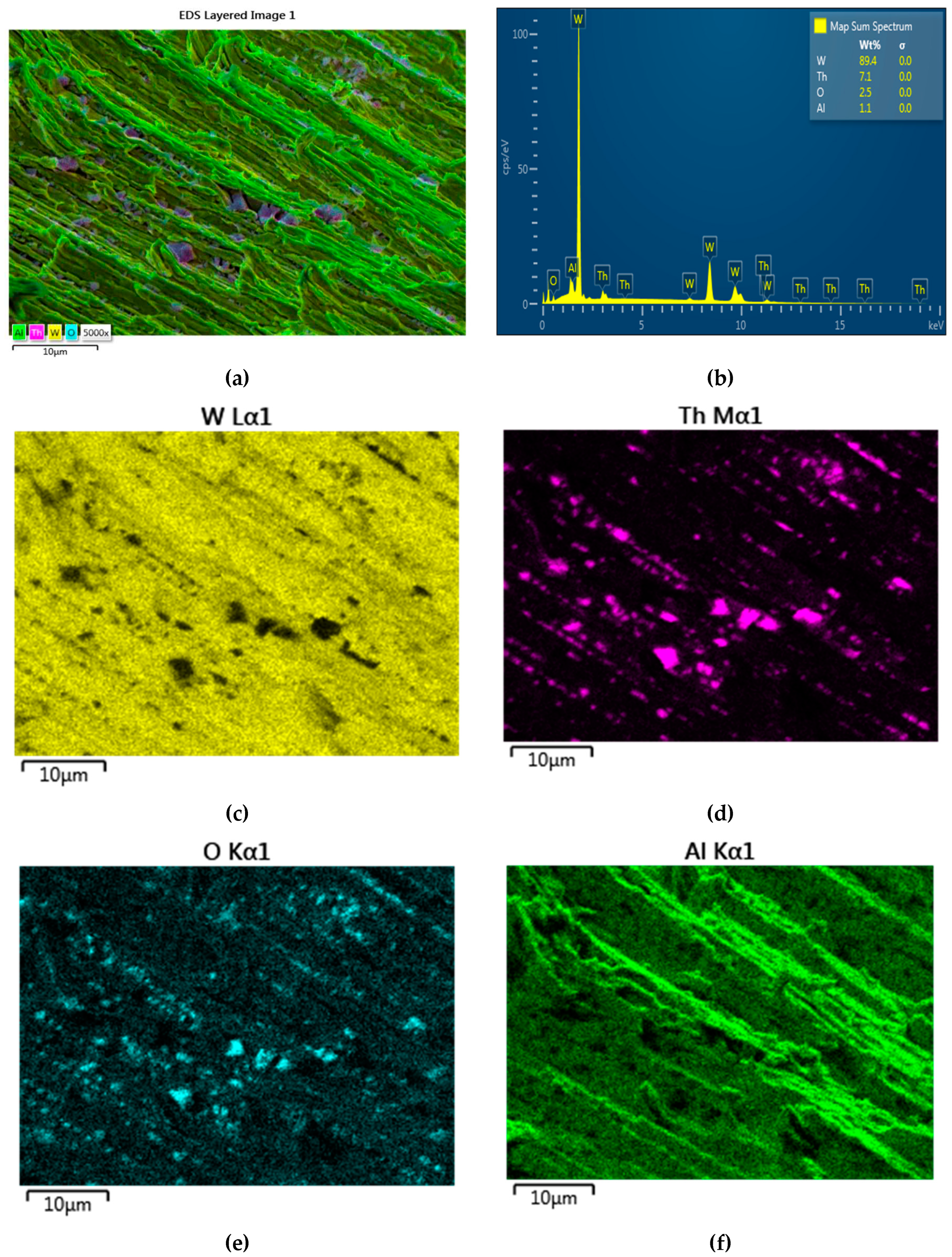
- semi-quantitative spot analyses located at certain intervals in the same micro-area, for the distribution of elements from the point of view of composition on the surface of the material as well as the variational verification of the composition of the investigated micro-area with the points from which the respective spectra were acquired;
- elemental mapping analyses, i.e. obtaining spectral images – where the distribution of the elements on the surface swept by the electron beam is superimposed and possible compositional differences are highlighted – increasing the area of the present element or the appearance/disappearance of an analyzed and identified element.
3. Results and discussion
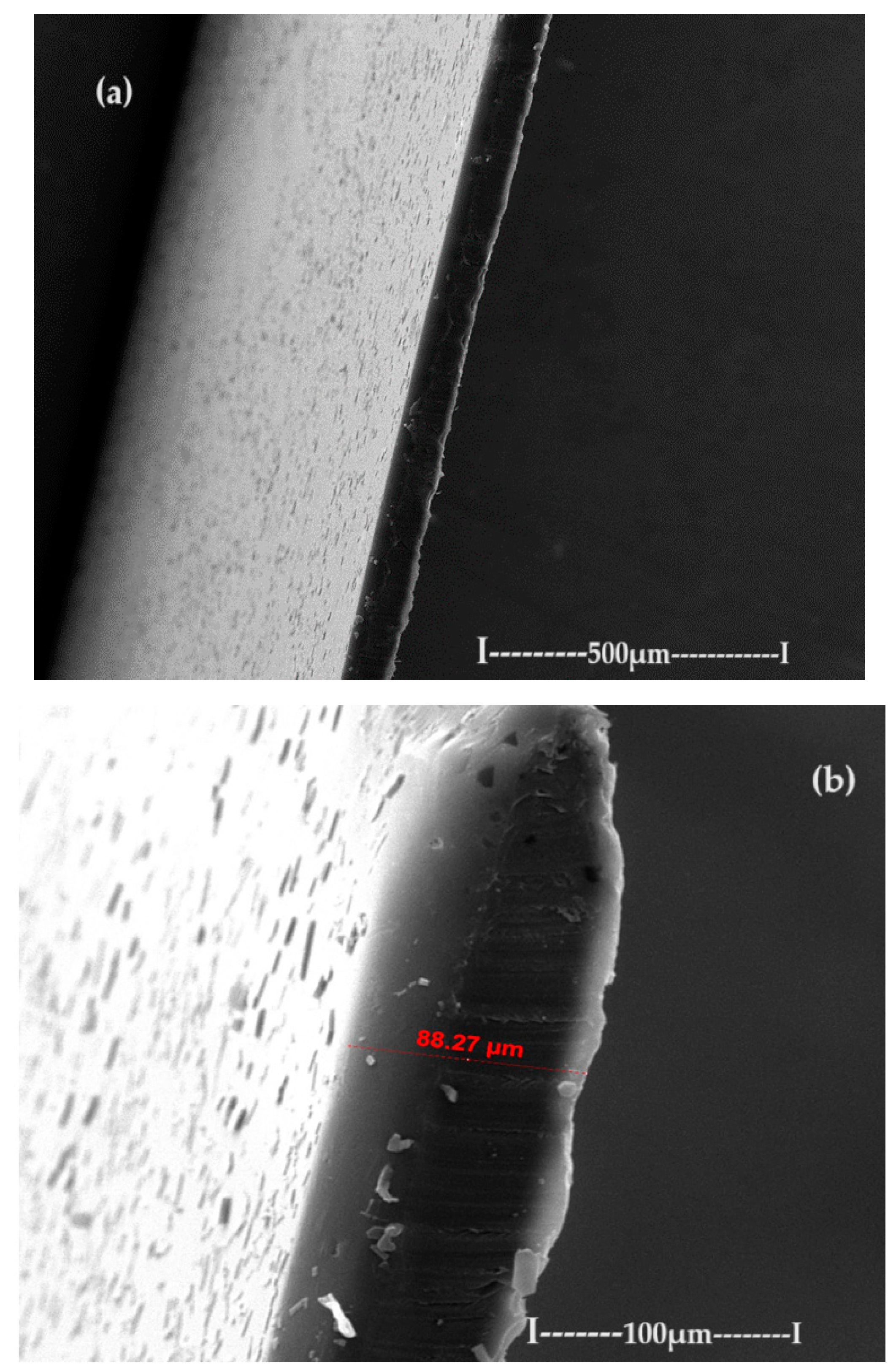
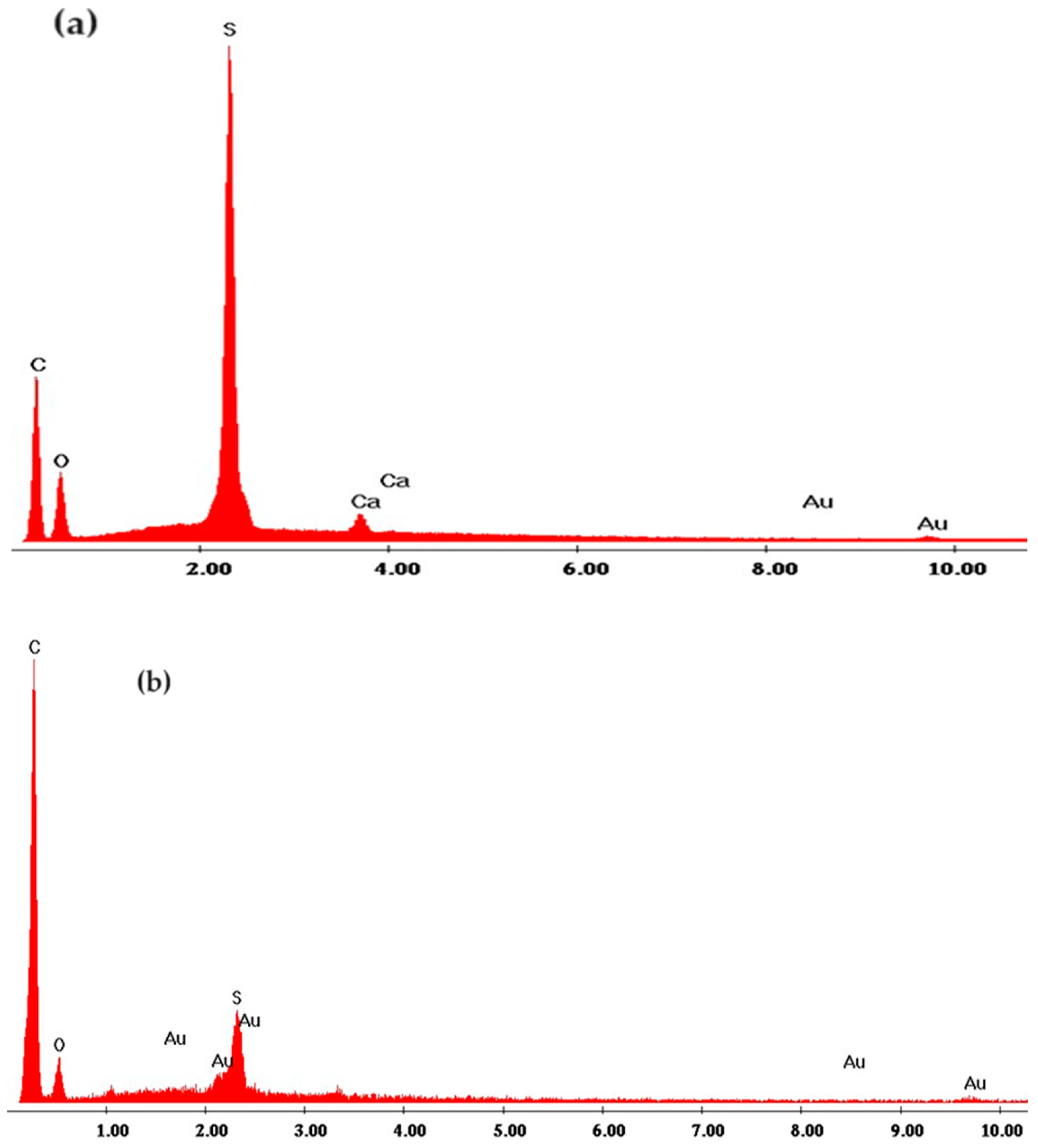
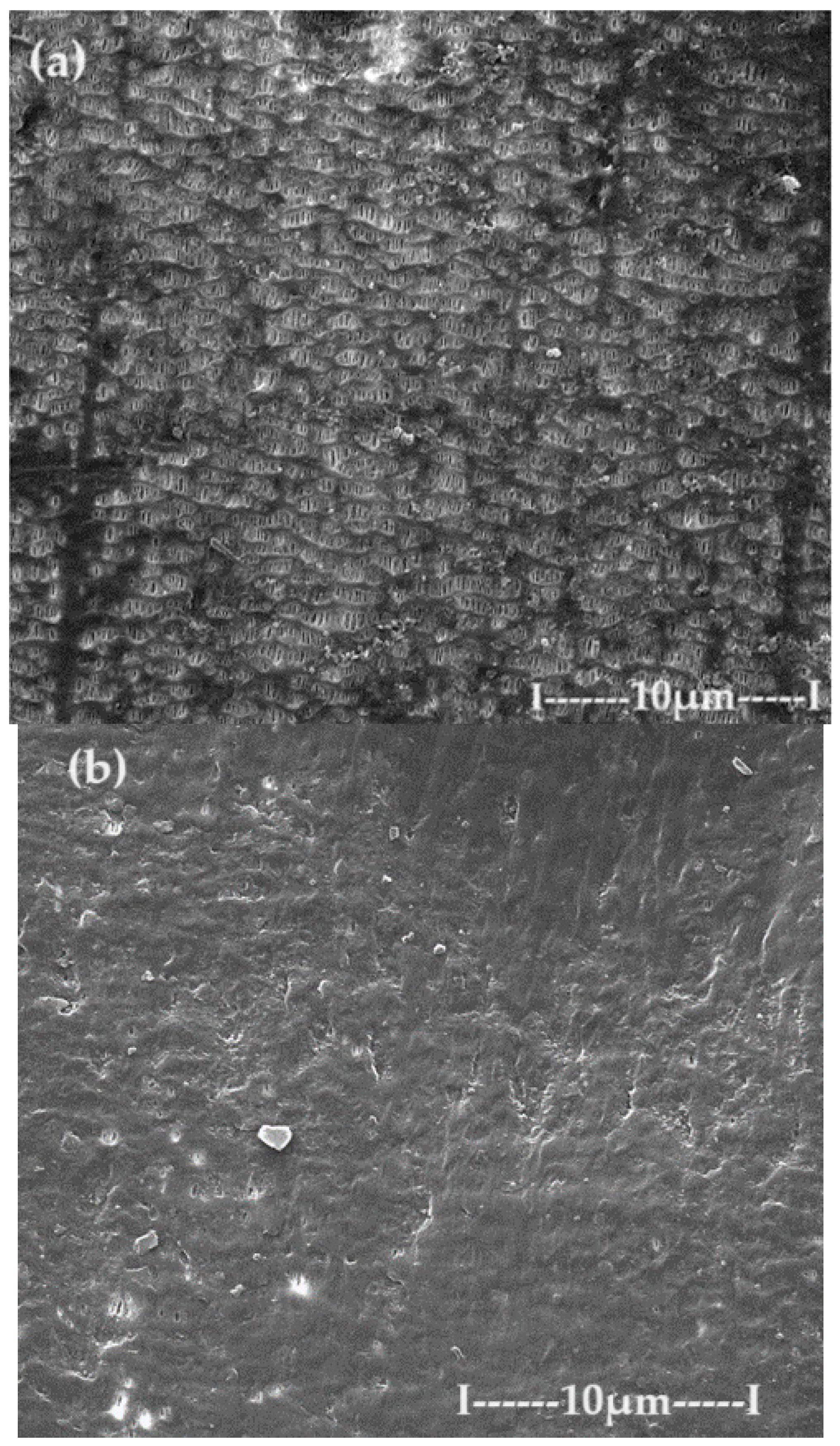
3.1. Characterization of the sPEEK–M membrane
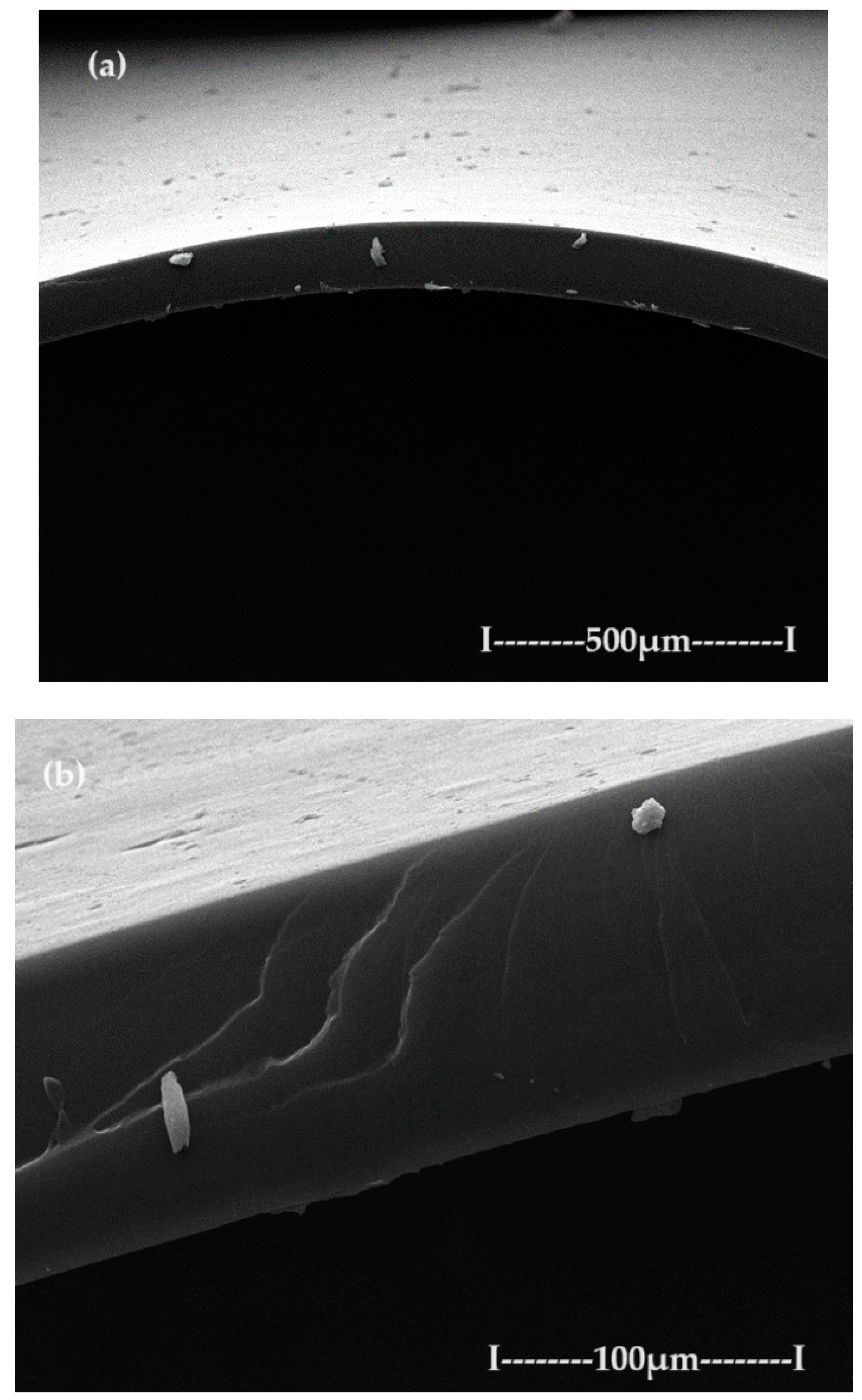
3.2. Characterization of C–PHF–M membrane
3.3. Electrolysis of tungsten electrodes for the recovery of thorium and tungsten
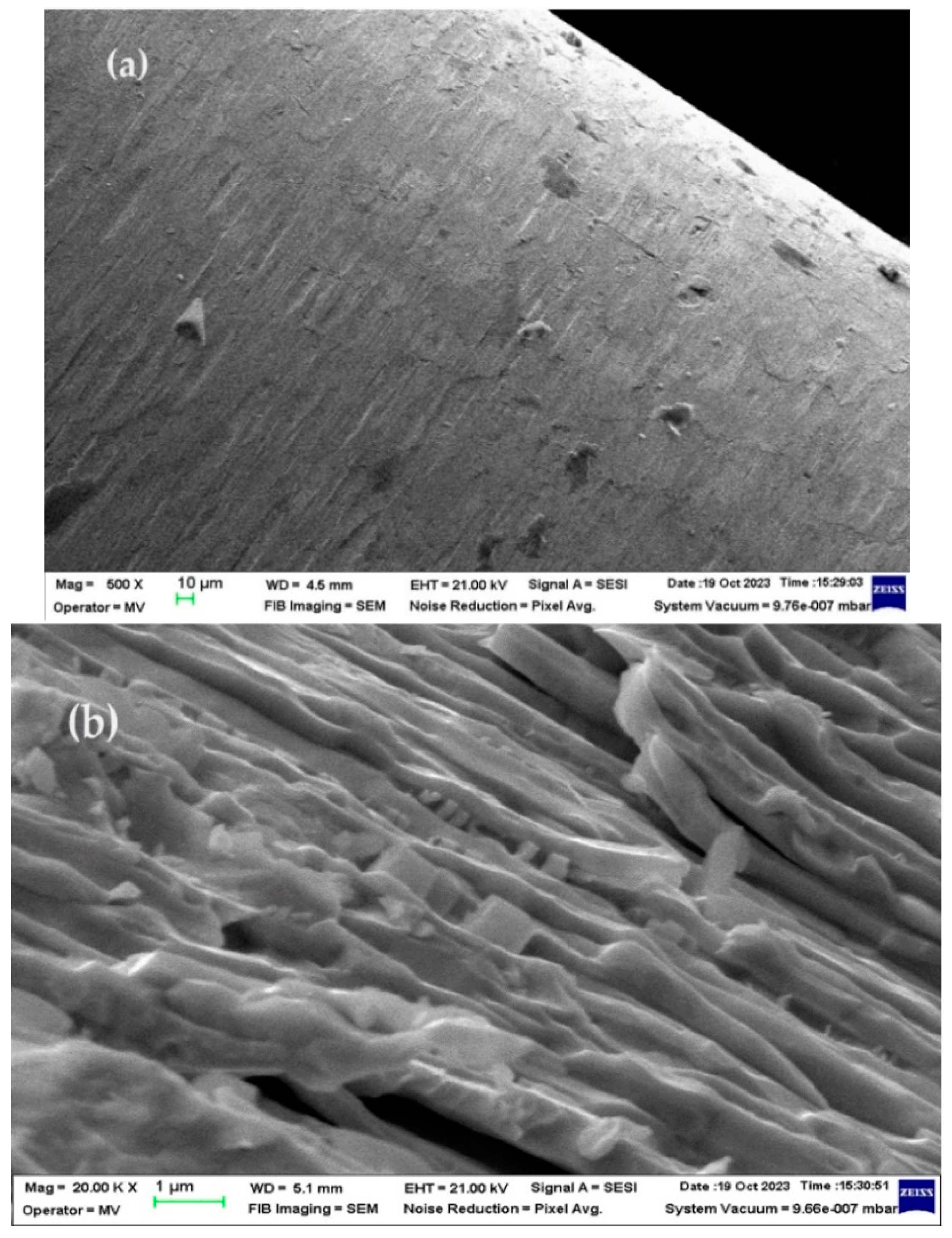
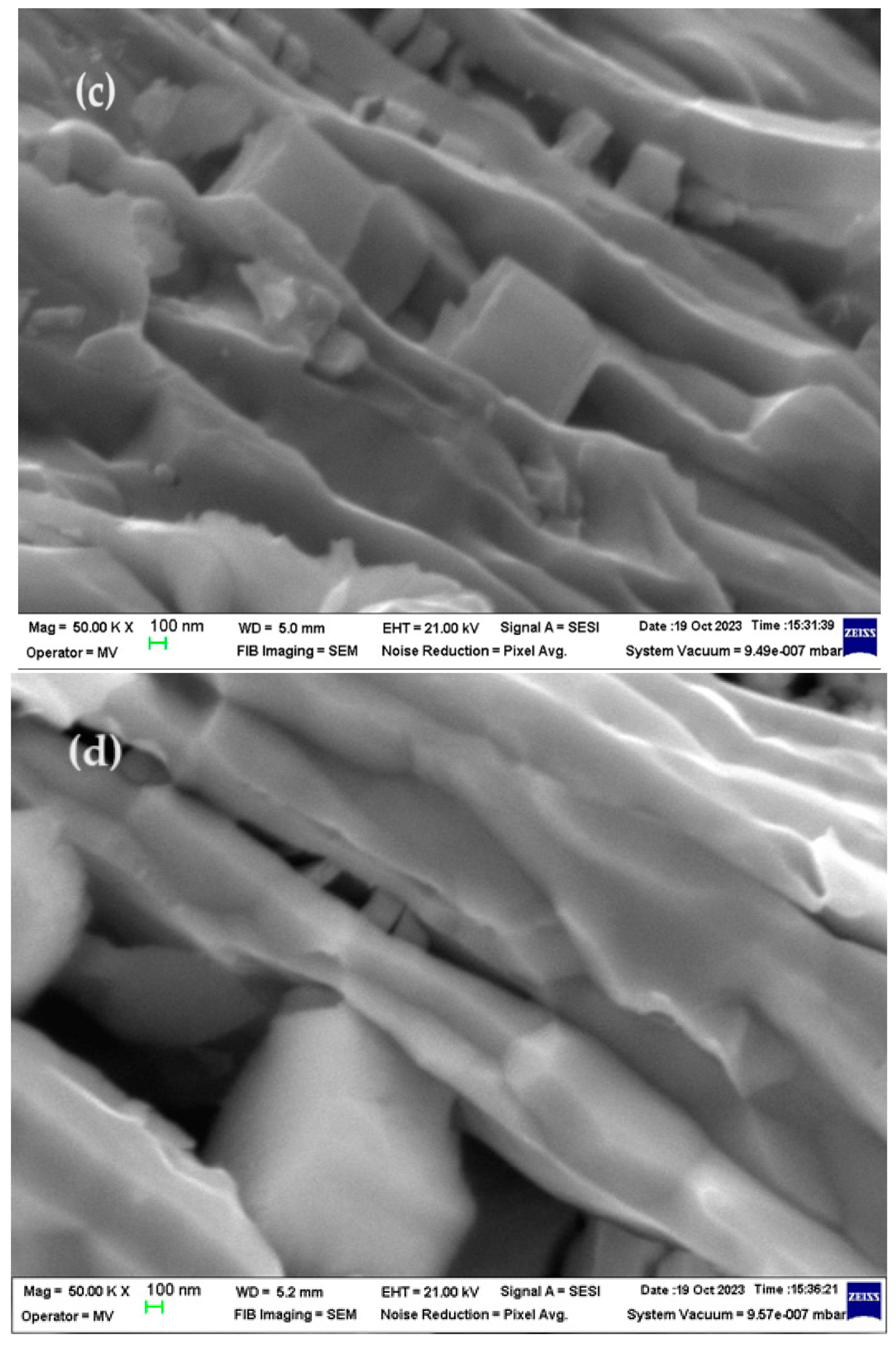
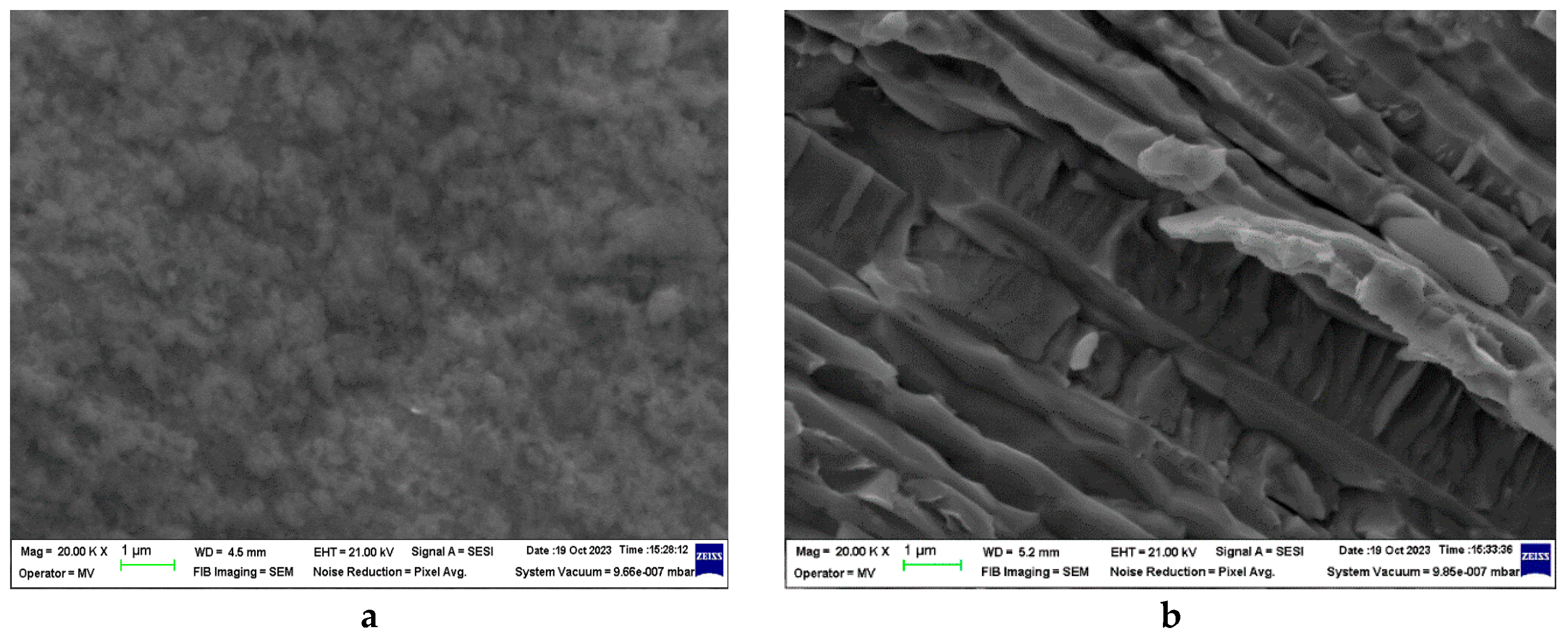
3.3.1. SEM and EDAX analysis of welding electrodes
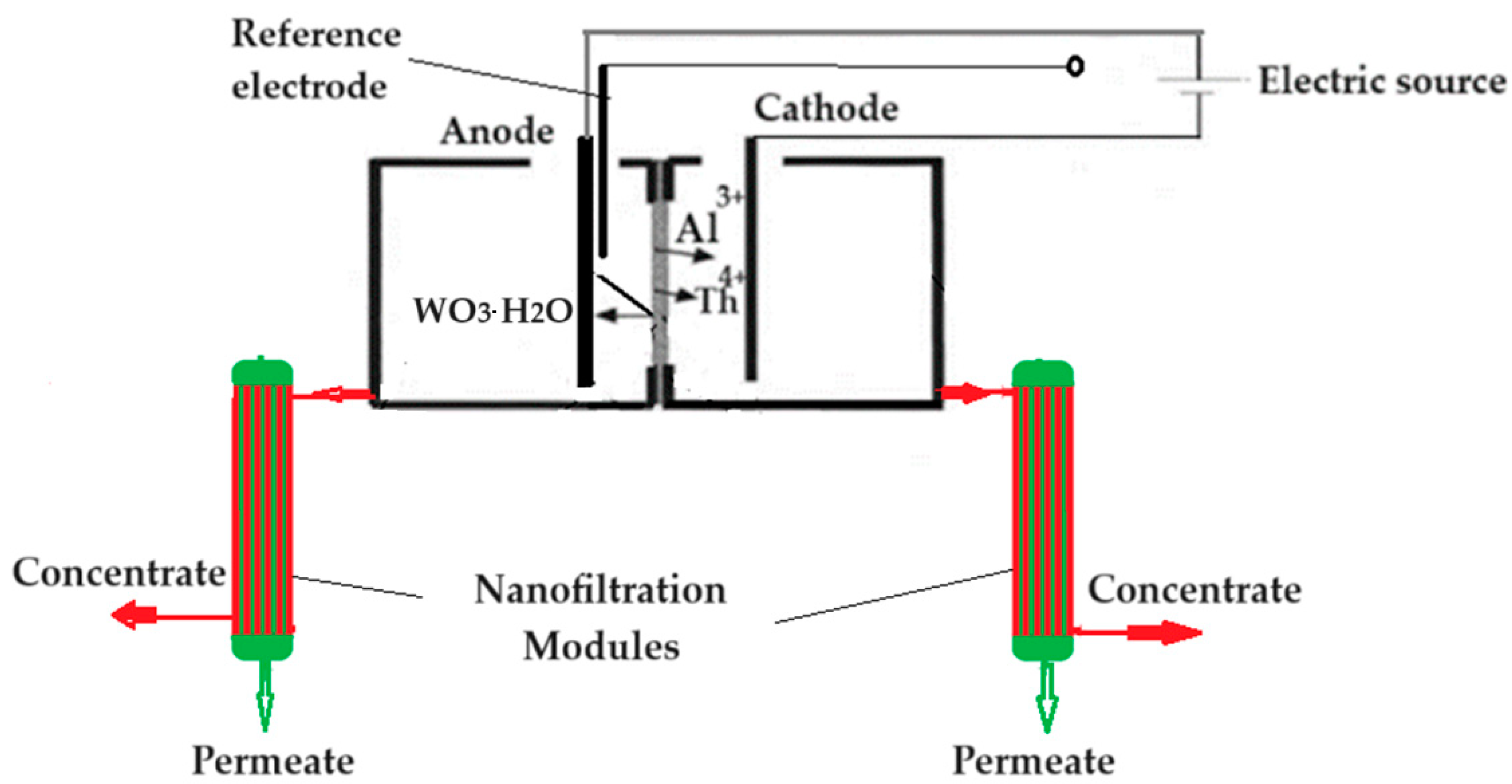
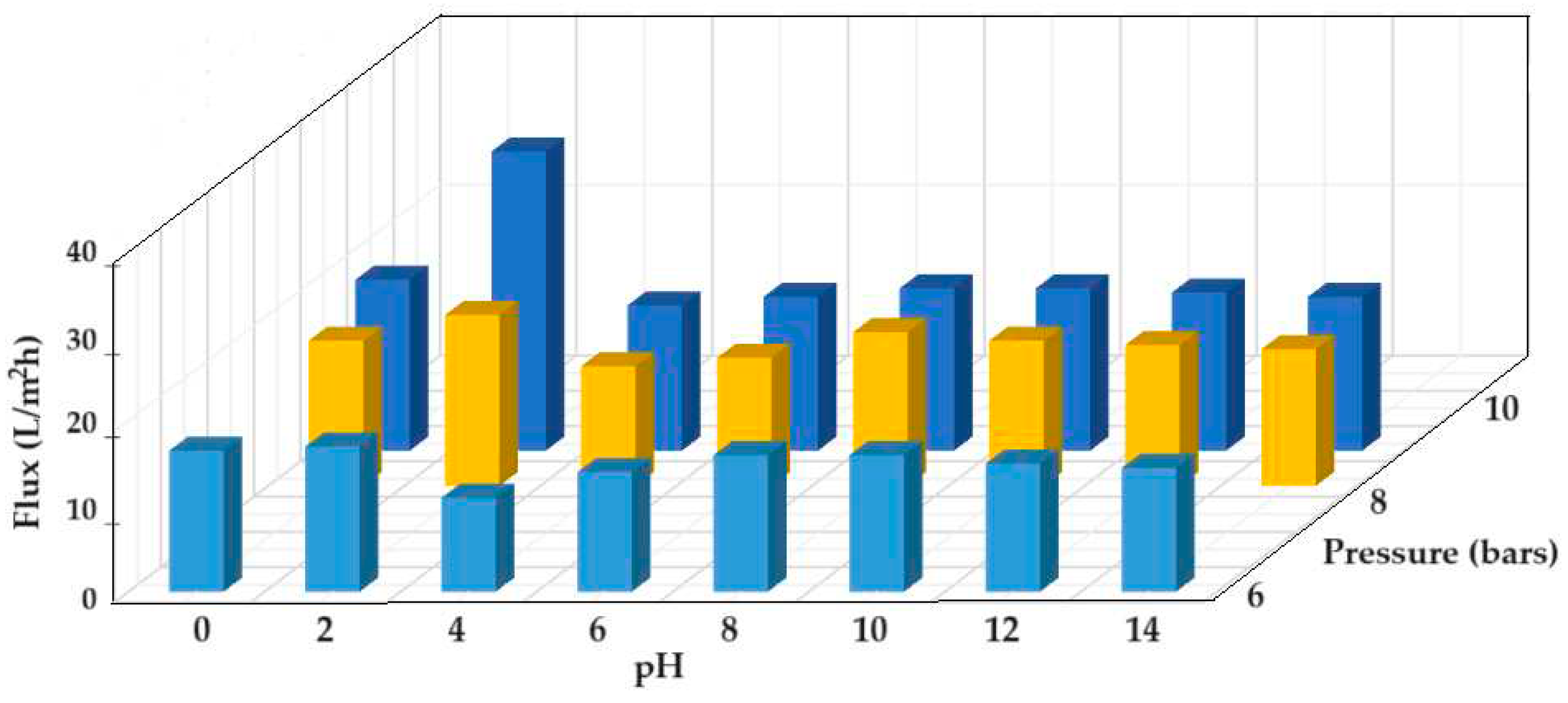
3.3.2. Membrane electrolysis of welding electrodes
- the first stage at the pH (6–12) from this space when a permeate, containing the soluble tungstate ion, and a concentrate containing thorium dioxide and aluminum hydroxide, are obtained;
- the second stage when the concentrate is mixed with the pH 13 solution from the cathodic space, when the aluminum is solubilized as aluminate, passing into the permeate, and thorium dioxide remains in the concentrate.
- the first stage at the pH in this space (above 12), when a permeate containing soluble tungstate and aluminate ions is obtained, and a concentrate containing thorium dioxide and aluminum hydroxide;
- the second stage when the permeate is brought to a pH between 6 and 9, when the aluminum precipitates as hydroxide and is separated from the tungstate ion by nanofiltration, in the second module.
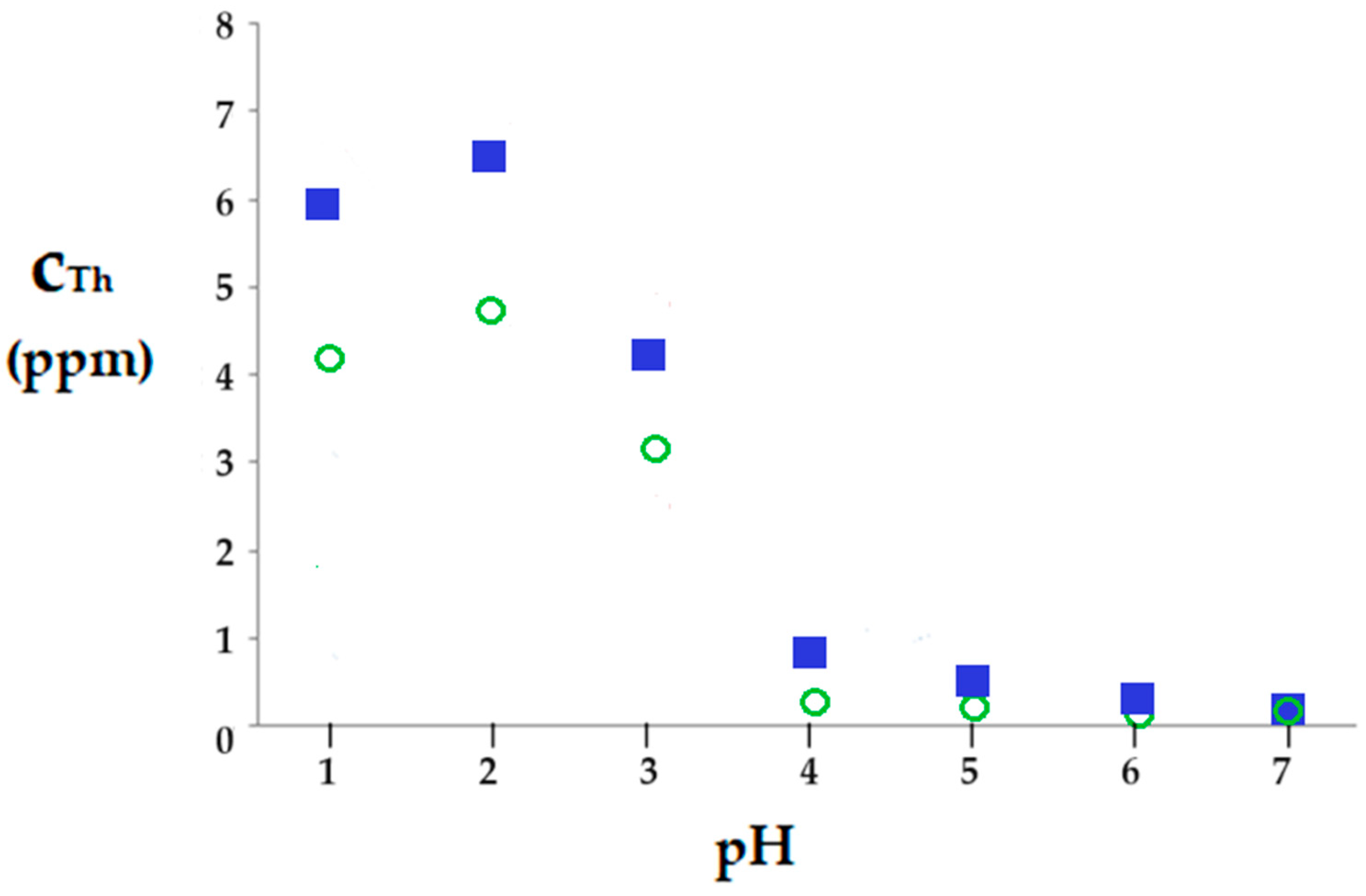
- the existence of thorium in ionic form and as thorium dioxide of different morphology (Figure 16);
- ionic charge of the nanofiltration membrane.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Deng, P.; Cheng, L.; Li, A.; Zeng, Z.; Liao, C. Recent Advances in the Utilization of Tungsten Residue: A Mini Review of China. Metals 2023, 13, 1481. [Google Scholar] [CrossRef]
- Huang. M.; Hu, K.; Li, X.; Wang, Y.; Ouyang, J, Zhou, L.; Liu, Z. Mineralogical Properties of a Refractory Tantalum-Niobium Slag and the Effect of Roasting on the Leaching of Uranium-Thorium. Toxics 2022; 10(8), 469. [CrossRef]
- Han, Z.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Mostafa, E. Nanofiltration Membranes for the Removal of Heavy Metals from Aqueous Solutions: Preparations and Applications. Membranes 2023, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Abdusamadzoda, D.; Abdushukurov, D.A.; Duliu, O.G.; Zinicovscaia, I. Assessment of the Toxic Metals Pollution of Soil and Sediment in Zarafshon Valley, Northwest Tajikistan (Part II). Toxics 2020, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Safiulina, A.M.; Lizunov, A.V.; Semenov, A.A.; Baulin, D.V.; Baulin, V.E.; Tsivadze, A.Y.; Aksenov, S.M.; Tananaev, I.G. Recovery of Uranium, Thorium, and Other Rare Metals from Eudialyte Concentrate by a Binary Extractant Based on 1,5-bis[2-(hydroxyethoxyphosphoryl)-4-ethylphenoxy]-3-oxapentane and Methyl Trioctylammonium Nitrate. Minerals 2022, 12, 1469. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Hong, Q.-J.; Gilbert, D.A.; Navrotsky, A.; Walle, A.v.d. Thorium and Rare Earth Monoxides and Related Phases. Materials 2023, 16, 1350. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, E.; Virtanen, E.J.; Moilanen, J.O. α-Aminophosphonates, -Phosphinates, and -Phosphine Oxides as Extraction and Precipitation Agents for Rare Earth Metals, Thorium, and Uranium: A Review. Molecules 2022, 27, 3465. [Google Scholar] [CrossRef] [PubMed]
- Arabi, H.R.; Milani, S.A.; Abolghasemi, H; Zahakifar, F. Recovery and transport of thorium (IV) through polymer inclusion membrane with D2EHPA from nitric acid solutions. Journal of Radioanalytical and Nuclear Chemistry, 2021, 327, pp.653–665. [CrossRef]
- Houessionon, M.G.K.; Ouendo, E.-M.D.; Bouland, C.; Takyi, S.A.; Kedote, N.M.; Fayomi, B.; Fobil, J.N.; Basu, N. Environmental Heavy Metal Contamination from Electronic Waste (E-Waste) Recycling Activities Worldwide: A Systematic Review from 2005 to 2017. Int. J. Environ. Res. Public Health 2021, 18, 3517. [Google Scholar] [CrossRef]
- Lu, J.; He, K.; Wang, Y.; Chen, G.; Weng, H.; Lin, M. An Effective Process for the Separation of U(VI), Th(IV) from Rare Earth Elements by Using Ionic Liquid Cyphos IL 104. Chin. Chem. Lett. 2022, 33, 3422–3428. [Google Scholar] [CrossRef]
- Man, G.T.; Albu, P.C.; Nechifor, A.C.; Grosu, A.R.; Tanczos, S.-K.; Grosu, V.-A.; Ioan, M.-R.; Nechifor, G. Thorium Removal, Recovery and Recycling: A Membrane Challenge for Urban Mining. Membranes 2023, 13, 765. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y. Polymeric Materials for Rare Earth Elements Recovery. Gels 2023, 9, 775. [Google Scholar] [CrossRef]
- Pathapati, S.V.S.H.; Free, M.L.; Sarswat, P.K. A Comparative Study on Recent Developments for Individual Rare Earth Elements Separation. Processes 2023, 11, 2070. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Development and characterization of bentonite-gum arabic composite as novel highly-efficient adsorbent to remove thorium ions from aqueous media. Cellulose 2021, 28, 10321–10333. [Google Scholar] [CrossRef]
- Ault, T.; Krahn, S.; Croff, A. Comparing the environmental impacts of uranium- and thorium-based fuel cycles with different recycle options. Prog. Nucl. Energy 2017, 100, 114–134. [Google Scholar] [CrossRef]
- Elbshary, R.E.; Gouda, A.A.; El Sheikh, R.; Alqahtani, M.S.; Hanfi, M.Y.; Atia, B.M.; Sakr, A.K.; Gado, M.A. Recovery of W(VI) from Wolframite Ore Using New Synthetic Schiff Base Derivative. Int. J. Mol. Sci. 2023, 24, 7423. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, B. Extraction of Sodium Tungstate from Tungsten Ore by Pyrometallurgical Smelting. Metals 2023, 13, 312. [Google Scholar] [CrossRef]
- Kumar, R.; Kariminejad, A.; Antonov, M.; Goljandin, D.; Klimczyk, P.; Hussainova, I. Progress in Sustainable Recycling and Circular Economy of Tungsten Carbide Hard Metal Scraps for Industry 5.0 and Onwards. Sustainability 2023, 15, 12249. [Google Scholar] [CrossRef]
- Han, Z.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Randhawa, N.S. A comprehensive review on recycling methods for cemented tungsten carbide scraps highlighting the electrochemical techniques. Int. J. Refract. Met. Hard Mater. 2020, 90, 105251. [Google Scholar] [CrossRef]
- Das, S.K.; Nagesh, C.H.R.V.S.; Sreenivas, T.; Kundu, T.; Angadi, S.I. A treatise on occurrence, beneficiation and plant practices of tungsten-bearing ores. Powder Technology 2023, 429, 118938. [Google Scholar] [CrossRef]
- Sabbatovskii, K.G. The effect of the adsorption of multicharge cations on the selectivity of a nanofiltration membrane. Colloid J. 2003, 65, 237–243. [Google Scholar] [CrossRef]
- Kaptakov, V.O.; Milyutin, V.V.; Nekrasova, N.A.; Zelenin, P.G.; Kozlitin, E.A. Nanofiltration Extraction of Uranium and Thorium from Aqueous Solutions. Radiochemistry 2021, 63, 169–172. [Google Scholar] [CrossRef]
- Ilaiyaraja, P.; Deb, A.K.S.; Ponraju, D. Removal of uranium and thorium from aqueous solution by ultrafiltration (UF) and PAMAM dendrimer assisted ultrafiltration (DAUF). Journal of Radioanalytical and Nuclear Chemistry, 2015, 303, 441–450. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Randhawa, N.S.; Hait, J.; Jana, R.K.; Singh, K.K.; Mankhand, T.R. Anodic Dissolution Behaviour of Tungsten Carbide Scraps in Ammoniacal Media. Adv. Mater. Res. 2014, 828, 11–20. [Google Scholar] [CrossRef]
- Kuntyi, O.I.; Ivashkin, V.R.; Yavorskii, V.T.; Zozulya, G.I. Electrochemical processing of WC-Ni pseudoalloys in sulfuric acid solutions to ammonium paratungstate and nickel(II) sulfate. Russ. J. Appl. Chem. 2007, 80, 1856–1859. [Google Scholar] [CrossRef]
- Ubaldini, A.; Cicconi, F.; Rizzo, A.; Salvi, S.; Cuzzola, V.; Gennerini, F.; Bruni, S.; Marghella, G.; Gessi, A.; Falsini, N. Preparation and Characterization of Isostructural Na2MoO4 and Na2WO4 and a Study of the Composition of Their Mixed System. Molecules 2023, 28, 6602. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, H.; Nie, C.; Zhang, J.; Steenari, B.M.; Ekberg, C. Comprehensive treatments of tungsten slags in China: A critical review. Journal of Environmental Management, 2020, 270, p.110927. [CrossRef]
- Cao, Y.; Guo, Q.; Sun, W.; Chelnokov, G.A. Efficient and Fast Removal of Aqueous Tungstate by an Iron-Based LDH Delaminated in L-Asparagine. Int. J. Environ. Res. Public Health 2022, 19, 7280. [Google Scholar] [CrossRef]
- Leal Filho, W.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Aziman, E.S.; Mohd Salehuddin, A.H.J.; Ismail, A.F. Remediation of thorium (IV) from wastewater: Current status and way forward. Separation & Purification Reviews, 2021, 50(2), pp.177-202. [CrossRef]
- Gomaa, H.; Emran, M.Y.; Elsenety, M.M.; Abdel-Rahim, R.D.; Deng, Q.; Gadallah, M.I.; Saad, M.; ALMohiy, H.; Ezzeldien, M.; Seaf El-Nasr, T.A.; El-Gaby, M.S. Detection and Selective Removal Strategy of Thorium Ions Using a Novel Fluorescent Ligand and Hybrid Mesoporous γ-Al2O3-like Nanoneedles. ACS Sustainable Chemistry & Engineering 2023, 11, 2127–2138. [Google Scholar] [CrossRef]
- Rahman, I.U.; Mohammed, H.J.; Siddique, M.F.; Ullah, M.; Bamasag, A.; Alqahtani, T; Algarni, S. Application of membrane technology in the treatment of waste liquid containing radioactive materials. Journal of Radioanalytical and Nuclear Chemistry, 2023, 1-14. [CrossRef]
- Xiong, L.; Lyu, K.; Zeng, Y.; Yang, C.; Chi, F.; Hu, S.; Long, X. Stable and high-flux polyacrylonitrile/hafnium phosphonate nanofibrous membranes for efficient removal of actinides from strong acidic solutions. Journal of Environmental Chemical Engineering, 2023,11(2), .109619. [CrossRef]
- Abbasizadeh, S.; Keshtkar, A.R.; Mousavian, M.A. Preparation of a novel electrospun polyvinyl alcohol/titanium oxide nanofiber adsorbent modified with mercapto groups for uranium (VI) and thorium (IV) removal from aqueous solution. Chem. Eng. J. 2013, 220, 161–171. [Google Scholar] [CrossRef]
- Tsezos, M.; Volesky, B. Biosorption of uranium and thorium. Biotechnol. Bioeng. 1981, 23, 583–604. [Google Scholar] [CrossRef]
- Hritcu, D.; Humelnicu, D.; Dodi, G.; Popa, M.I. Magnetic chitosan composite particles: Evaluation of thorium and uranyl ion adsorption from aqueous solutions. Carbohydr. Polym. 2012, 87, 1185–1191. [Google Scholar] [CrossRef]
- Zolfonoun, E.; Yousefi, S.R. Sorption and preconcentration of uranium and thorium from aqueous solutions using multi-walled carbon nanotubes decorated with magnetic nanoparticles. Radiochim. Acta 2015, 103, 835–841. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, G.; Păncescu, F.M.; Grosu, A.R.; Albu, P.C.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Pîrțac, A.; Nechifor, A.C. Osmium Nanoparticles-Polypropylene Hollow Fiber Membranes Applied in Redox Processes. Nanomaterials 2021, 11, 2526. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Parvulescu, V.; Anastasescu, M.; Petrescu, S.; Albu, C.; Nechifor, G.; Radu, G.L. New Hybrid Nanofiltration Membranes with Enhanced Flux and Separation Performances Based on Polyphenylene Ether-Ether-Sulfone/Polyacrylonitrile/SBA-15. Membranes 2022, 12, 689. [Google Scholar] [CrossRef]
- Cimbru, A.M.; Rikabi, A.A.K.K.; Oprea, O.; Grosu, A.R.; Tanczos, S.-K.; Simonescu, M.C.; Pașcu, D.; Grosu, V.-A.; Dumitru, F.; Nechifor, G. pH and pCl Operational Parameters in Some Metallic Ions Separation with Composite Chitosan/Sulfonated Polyether Ether Ketone/Polypropylene Hollow Fibers Membranes. Membranes 2022, 12, 833. [Google Scholar] [CrossRef]
- Păncescu, F.M.; Rikabi, A.A.K.K.; Oprea, O.C.; Grosu, A.R.; Nechifor, A.C.; Grosu, V.-A.; Tanczos, S.-K.; Dumitru, F.; Nechifor, G.; Bungău, S.G. Chitosan–sEPDM and Melatonin–Chitosan–sEPDM Composite Membranes for Melatonin Transport and Release. Membranes 2023, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Pașcu, D.; Nechifor, A.C.; Grosu, V.-A.; Oprea, O.C.; Tanczos, S.-K.; Man, G.T.; Dumitru, F.; Grosu, A.R.; Nechifor, G. Hydrogen Sulphide Sequestration with Metallic Ions in Acidic Media Based on Chitosan/sEPDM/Polypropylene Composites Hollow Fiber Membranes System. Membranes 2023, 13, 350. [Google Scholar] [CrossRef]
- Dimulescu, I.A.; Nechifor, A.C.; Bǎrdacǎ, C.; Oprea, O.; Paşcu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungău, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, G.; Păncescu, F.M.; Grosu, A.R.; Albu, P.C.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Pîrțac, A.; Nechifor, A.C. Osmium Nanoparticles-Polypropylene Hollow Fiber Membranes Applied in Redox Processes. Nanomaterials 2021, 11, 2526. [Google Scholar] [CrossRef] [PubMed]
- Bărdacă Urducea, C.; Nechifor, A.C.; Dimulescu, I.A.; Oprea, O.; Nechifor, G.; Totu, E.E.; Isildak, I.; Albu, P.C.; Bungău, S.G. Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method. Nanomaterials 2020, 10, 2349. [Google Scholar] [CrossRef]
- Pavel, E.; Prodan, G.; Marinescu, V.; Trusca, R. Recent advances in 3- to 10-nm quantum optical lithography. Journal of Micro/Nanolithography, MEMS, and MOEMS 2019,18(2), 020501 (7 May). [CrossRef]
- Prioteasa, P.; Marinescu, V.; Bara, A.; Iordoc, M.; Teisanu, A.; Banciu, C.; Meltzer, V. Electrodeposition of Polypyrrole on Carbon Nanotubes/Si in the Presence of Fe Catalyst for Application in Supercapacitors. Rev. Chim. 2015, 66, 820–824. [Google Scholar]
- Zamfir, L.-G.; Rotariu, L.; Marinescu, V. E.; Simelane, X.T.; Baker, P.G.L.; Iwuoha, E. I.; Bala, C. Non-enzymatic polyamic acid sensors for hydrogen peroxide detection. Sensors and Actuators B: Chemical, 2016, 226, 525–533. [Google Scholar] [CrossRef]
- Cazalaà, J.B. Radium and thorium applications for the general public: unexpected consequences of the discovery from Pierre and Marie Curie. The Journal of the American Society of Anesthesiologists 2012, 117(6), 1202–1202. [Google Scholar] [CrossRef]
- Pohjalainen, I.; Moore, I.D.; Geldhof, S.; Rosecker, V.; Sterba, J.; Schumm, T. Gas cell studies of thorium using filament dispensers at IGISOL. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 2020, 484, 59–70. [Google Scholar] [CrossRef]
- Takaki, N.; Mardiansah, D. Core Design and Deployment Strategy of Heavy Water Cooled Sustainable Thorium Reactor. Sustainability 2012, 4, 1933–1945. [Google Scholar] [CrossRef]
- Sidik, P.; Takaki, N.; Sekimoto, H. Feasible region of design parameters for water cooled thorium breeder reactor. J. Nucl. Sci. Technol. 2007, 44, 946–957. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Alhalafi, M.H.; Emam, E.-A.M.; Ibrahim, H.; Mosaad, R.M. A Review of Chitosan and Chitosan Nanofiber: Preparation, Characterization, and Its Potential Applications. Polymers 2023, 15, 2820. [Google Scholar] [CrossRef]
- Radu, E.-R.; Pandele, A.M.; Tuncel, C.; Miculescu, F.; Voicu, S.I. Preparation and Characterization of Chitosan/LDH Composite Membranes for Drug Delivery Application. Membranes 2023, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Nave, M.I.; Kornev, K.G. Complexity of Products of Tungsten Corrosion: Comparison of the 3D Pourbaix Diagrams with the Experimental Data. Metall. Mater. Trans. A 2017, 48, 1414–1424. [Google Scholar] [CrossRef]
- Berlanga-Labari, C.; Biezma-Moraleda, M.V.; Rivero, P.J. Corrosion of Cast Aluminum Alloys: A Review. Metals. 2020, 10(10), 1384. [Google Scholar] [CrossRef]
- Han, K.N. Characteristics of Precipitation of Rare Earth Elements with Various Precipitants. Minerals 2020, 10, 178. [Google Scholar] [CrossRef]
- Ma, H.; Shen, M.; Tong, Y.; Wang, X. Radioactive Wastewater Treatment Technologies: A Review. Molecules 2023, 28, 1935. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A. Characteristics of Some Selected Methods of Rare Earth Elements Recovery from Coal Fly Ashes. Metals 2021, 11, 142. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, G. Separation Techniques for the Efficient and Green Recovery of Metal Minerals. Separations 2023, 10, 520. [Google Scholar] [CrossRef]
- Nisbet, H.; Migdisov, A.; Xu, H.; Guo, X., van Hinsberg, V.; Williams-Jones, A.E.; Boukhalfa, H.; Roback, R. An experimental study of the solubility and speciation of thorium in chloride-bearing aqueous solutions at temperatures up to 250 C. Geochimica et Cosmochimica Acta 2018, 239, 363–373. [CrossRef]
- Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Daňo, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, M. Thenoyltrifluoroacetone: Preferable Molecule for Solvent Extraction of Metals—Ancient Twists to New Approaches. Separations 2022, 9, 154. [Google Scholar] [CrossRef]
- Peng, C.; Ma, Y.; Ding, Y.; He, X.; Zhang, P.; Lan, T.; Wang, D.; Zhang, Z.; Zhang, Z. Influence of Speciation of Thorium on Toxic Effects to Green Algae Chlorella pyrenoidosa. Int. J. Mol. Sci. 2017, 18, 795. [Google Scholar] [CrossRef]
- Vakanjac, B.; Naunovic, Z.; Ristić Vakanjac, V.; Đumić, T.; Bakrač, S.; Štrbački, J.; Gajić, V.; Alzarog, T.M. Distribution of Potentially Toxic Elements in the City of Zintan and Its Surroundings (Northwestern Libya) by Surface Soil Sampling. Minerals 2023, 13, 1048. [Google Scholar] [CrossRef]
- García, A.C.; Latifi, M.; Amini, A.; Chaouki, J. Separation of Radioactive Elements from Rare Earth Element-Bearing Minerals. Metals 2020, 10, 1524. [Google Scholar] [CrossRef]
- Kaptakova, V.O.; Milyutina, V.V.; Nekrasovaa, N.A.; Zelenina, P.G.; Kozlitina, E.A. Nanofiltration Extraction of Uranium and Thoriumfrom Aqueous Solutions. Radiochemistry, 2021, 63(2),169–172. ISSN 1066-3622, © Pleiades Publishing, Inc., 2021. Russian Text © The Author(s), 2021, published in Radiokhimiya, 2021, 63(2), 139–142.

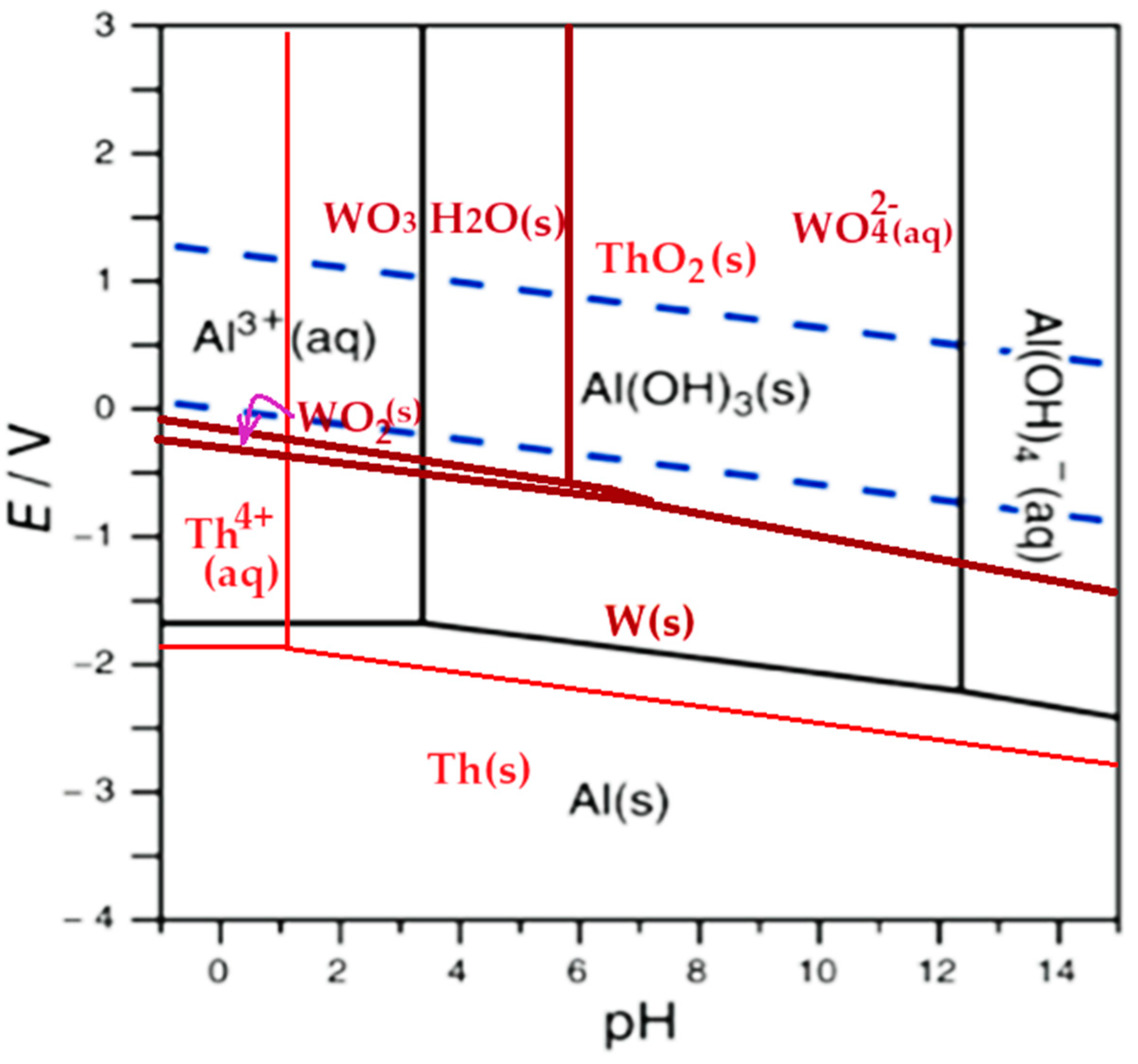
| Metallic element or membrane reactive group |
Metal element speciation at variable pH in the anodic space, at pH 13 in the cathodic space and 20.0 V anodic potential |
||||
| 0–1 | 1–3 | 3–6 | 6–12 | >12 | |
| Wolfram | WO3·H2O(s) | WO3·H2O(s) | WO3·H2O(s) | WO42⁻(aq) | WO42⁻(aq) |
| Thorium | Th4+(aq) | ThO2(s) | ThO2(s) | ThO2(s) | ThO2(s) |
| Aluminum | Al3+(aq) | Al3+(aq) | Al(OH)3(s) | Al(OH)3(s) | AlO2⁻(aq) |
| sPEEK–M *) | HSO3–Ar | HSO3–Ar | ⁻SO3–Ar | ⁻SO3–Ar | ⁻SO3–Ar |
| C–PHF–M **) | +H3N–R | +H3N–R | +H3N–R | H2N–R | H2N–R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).