1. Introduction
Campylobacter spp. is the most common etiological agent of bacterial gastroenteritis in Europe, with
Campylobacter jejuni as the main cause of campylobacteriosis, followed by
Campylobacter coli.
Campylobacter is a commensal microorganism of the gastrointestinal tract of many wild and domestic animals that serve as reservoirs for transmission. Most of the infections manifest as sporadic cases with unknown sources, often arising from the consumption of contaminated food or water, mishandling of contaminated food, interaction with domestic and farm animals, or exposure to contaminated environments [
1].
Campylobacter infection typically manifests with symptoms ranging from mild watery diarrhea to severe inflammatory bloody diarrhea, accompanied by abdominal pain, headache, nausea, and fever. More rarely, the infection can result in autoimmune complications such as Guillain-Barré and Miller-Fisher Syndromes. The onset of symptoms generally occurs between two to five days post-infection, with a duration of up to 10 days. The majority of cases of enteric campylobacteriosis follow a self-limiting course, thereby infrequently necessitating antimicrobial intervention [
2]. However, in cases where the condition worsens, particularly in immunocompromised individuals, antibiotic therapy may become imperative, thereby highlighting the increasing threat of antibiotic-resistant
Campylobacter strains [
2,
3]. The surveillance of such resistance stands as a pivotal parameter in combatting campylobacteriosis.
In Europe, the surveillance of campylobacteriosis is under the oversight of the European Center for Disease Prevention and Control (ECDC). This entity undertakes the coordination, analysis, and dissemination of surveillance data collected from countries within the European Union (EU) and the European Economic Area (EEA) [
4]. However, since the data is obtained individually at the national level, the accuracy of the analyses depends largely on the effectiveness and implementation of programs within each respective country [
5].
In the context of Portugal, limited published data exists concerning the epidemiology of campylobacteriosis, and the only comprehensive study addressing the epidemiological aspects of human infection dates back to 1992 [
6]. Subsequent investigations have been limited in scope and with reduced datasets, primarily focusing on the genotypic and phenotypic attributes, particularly those associated with antimicrobial susceptibility of
Campylobacter isolates sourced from animals, food, or humans [
7,
8,
9,
10,
11,
12].
The present study aims to bridge the information gap by presenting up-to-date epidemiological data on Campylobacter spp. infection in Portugal. Employing a sentinel laboratory-based surveillance approach, the study covers the nation's three most densely populated regions, from January 2009 to December 2021. The primary objective is to provide a current overview of campylobacteriosis in Portugal, delineate temporal trends, estimate disease burden, and monitor antimicrobial-resistant infections.
2. Materials and Methods
2.1. Study Design
This is a retrospective study carried out in Campylobacter spp. isolates from patients with diarrhea and/or other gastrointestinal symptomatology, collected from different Portuguese hospitals, from January 2009 to December 2021. The epidemiological, sociodemographic, microbiological, and clinical data were collected for positive C. jejuni and C. coli cases, from the clinical laboratories and from the National Reference Laboratory (NRL). As part of the routine surveillance, data from patients’ medical records were provided from the primary laboratories (clinical specimen, age and gender, symptoms, time (days) from onset of symptoms to initial medical visit, and epidemiological information) while microbiological data (species identification/confirmation and antimicrobial susceptibility) was collected from the NRL reports.
2.2. Statistical analyses
Categorical variables were presented with their relative and absolute values while quantitative ones were expressed as mean and standard deviation, or median and interquartile range. Regarding patients’ age, and depending on the analysis, patients were either defined as paediatric (age ≤15 years) or adult population, or stratified in seven age groups (<1, 1-4, 5-14, 15-24, 25-44, 45-64 and 65+ years). For statistical analysis, Student’s t-test, Mann–Whitney, Chi-square, and Fisher’s exact tests were applied using the statistical software package SPSS 28.0 for Windows (SPSS. Chicago. IL. USA). A binary logistic regression analysis was performed to determine independent risk factors for resistance to different antibiotics. P<0.05 indicates a statistically significant test result.
3. Results
3.1. Data from the surveillance network
Campylobacteriosis is a notifiable disease in Portugal since 2015. Cases are reported by clinicians in an electronic platform called SINAVE, and since 2017, laboratory notification using the same platform is also mandatory. In parallel, a sentinel laboratory-based surveillance of this disease has been in place since 2009, whose networks comprises primary laboratories mainly from hospitals, both public and private, which adhered on a voluntary basis. These laboratories send all
Campylobacterspp. isolates, collected throughout the year, to the NRL located at the National Institute of Health Doutor Ricardo Jorge. For each isolate, anonymized epidemiological, demographic, microbiological, and clinical data are also requested. Currently, the sentinel laboratory network comprises nine public hospital centers and three public hospitals from the national health system, from the three most populated geographical areas of Portugal, representing three among five regions from Mainland Portugal, from the second tier of Nomenclature of Territorial Units for Statistics level 2 (NUTSII): North (6 hospital centers, 1 hospital), Centre (1 hospital center), and Lisbon and Tagus Valley (2 hospitals centers, 2 hospitals), with a total catchment population of ~ 9,300,000. In addition, two private networks of clinical analysis and medical diagnosis laboratories, both covering private primary laboratories at the hospital and community level operating in mainland Portugal, were also included. At the NRL, species identification was confirmed or determined by a real-time PCR specific for
C. jejuni and
C. coli [
13], targeting the gyrA gene, or by MALDI-TOF (VITEK® MS, bioMérieux).
At the NRL, antimicrobial susceptibility testing (AST) was implemented in the processing of samples in 2013. Antimicrobial resistance (AMR) against the four priority antimicrobials, ciprofloxacin (CIP), erythromycin (ERY), tetracycline (TCY), and gentamicin (GEN) (cut-offs of EUCAST [
14,
15]), is analyzed, as well as against the optional antibiotics, ampicillin (AMP), amoxicillin-clavulanic acid (AMC), by disk diffusion, and for ertapenem (ETP) by E-test (cut-offs of the Comité de l’antibiogramme de la Société Française de Microbiologie [
16]).
The NRL's inability to keep pace, in terms of sample processing, with the exponentially increased number of isolates received, forced, as of 2019, the random selection of around 40% of samples for species confirmation/determination and AST.
3.2. Temporal distribution of cases of Campylobacter infection received from 2009 to 2021
Accompanying the expansion of the laboratory network, the number of isolates sent to the NRL has been increasing over time, with a mean number of 263 isolates, from 2009 to 2012, ≈465 isolates from 2013-2016, and 751 from 2017-2021, with its highest number reached in 2019 with a total of 916 isolates received (
Table 1). Simultaneously, the number of laboratory-notified cases of
Campylobacter infections at a national level, in SINAVE, increased as well, from 596 to 973 cases in the period 2017-2021 [
1]. However, neither the number of annual
Campylobacter infections nor their increasing trend can be accurately estimated with the available data, as the number of isolates received in the NRL is higher than the cases notified in SINAVE (except in 2021), and around 50% of those are not notified. Indeed, notification to SINAVE is no better for the laboratories participating in sentinel surveillance, with a relevant heterogeneity in the notification rates per 100 000 population among the three regions, 9.1, 7.1 and 3.9, for North, Centre and Lisbon and Tagus Valley, respectively.
3.3. Seasonal variation in the number of Campylobacter infection cases
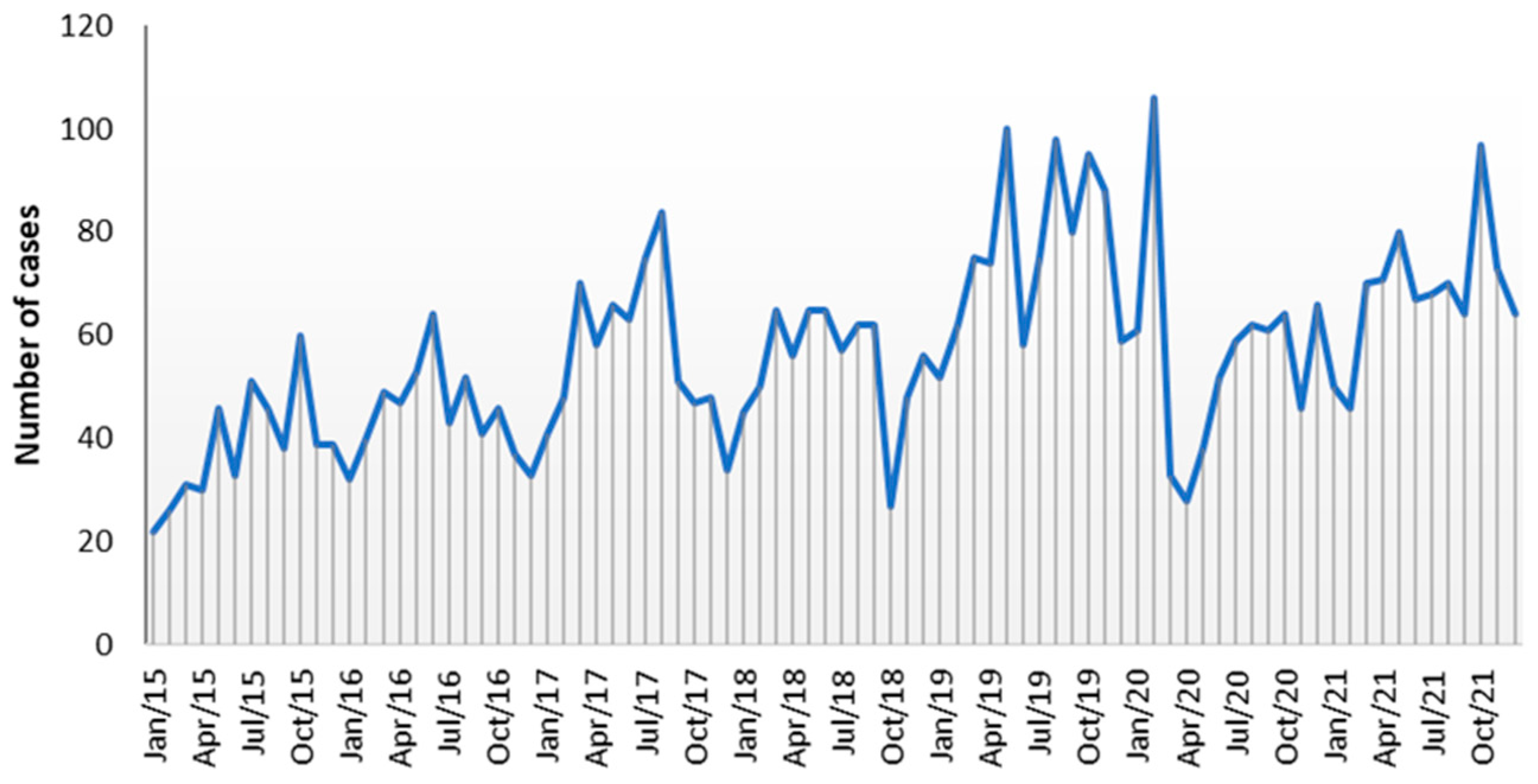
The graph of the number of cases received per month does not show a clear seasonal regularity over the years (
Figure 1). Despite this, a slight seasonal recrudescence of cases of
Campylobacter infections was noted during the mid-spring/summer period, with May and August as the months with the highest cumulative number of
Campylobacter cases. With the opposite trend, a small decay in the monthly prevalence of
Campylobacter infections was observed in the winter months, with January and December reporting the lowest numbers of cumulative cases of infection. Outside the mid-spring/summer period, three peaks of infection, associated mainly with C. jejuni, were noticed, occurring in October 2019 (95 cases), October 2021 (97 cases), and in February 2020 (106 cases), corresponding to 1.6 and 2.0 times more cases than the mean number for those months (62 and 54, respectively) (
Figure 1).
3.4. Demographic and clinical data
In the following analysis, the 5205 Campylobacter spp. isolates from human infections (one per patient, not travel-related) processed by the NRL in the period spanning from 2009 to 2021 were considered, being 4590 C. jejuni and 615 C. coli. Most of the cases reported the isolation of Campylobacter spp. from stool samples (5157, 99.08%), followed by blood samples (46, 0.88%). The isolation of C. jejuni from a gastric biopsy and of C. coli from a bile sample was also reported.
Overall, during the studied period,
C. jejuni accounted for 83.9% to 92.3% of
Campylobacter infections and was significantly more frequent than
C. coli, representing 7.7% to 16.1% of the infections (
Table 2). In the period 2009-2016, a progressive but not significant increase in the number of
C. jejuni was noted from year to year. In contrast, in the time period 2017-2020, there was a decrease in the number of
C. jejuni isolates. The number of
C. coli infections remained relatively stable during the study period, except for a relevant increase in the number of
C. coli infections from 2013 to 2015 (
Table 2).
The patients' mean age was 12.79 years (n=5120) (ranging from 1 day to 96 years). The majority of the cases (77.7%; 3979/5120) were from the paediatric population and 60.2% of the patients were male gender (3096/5144).
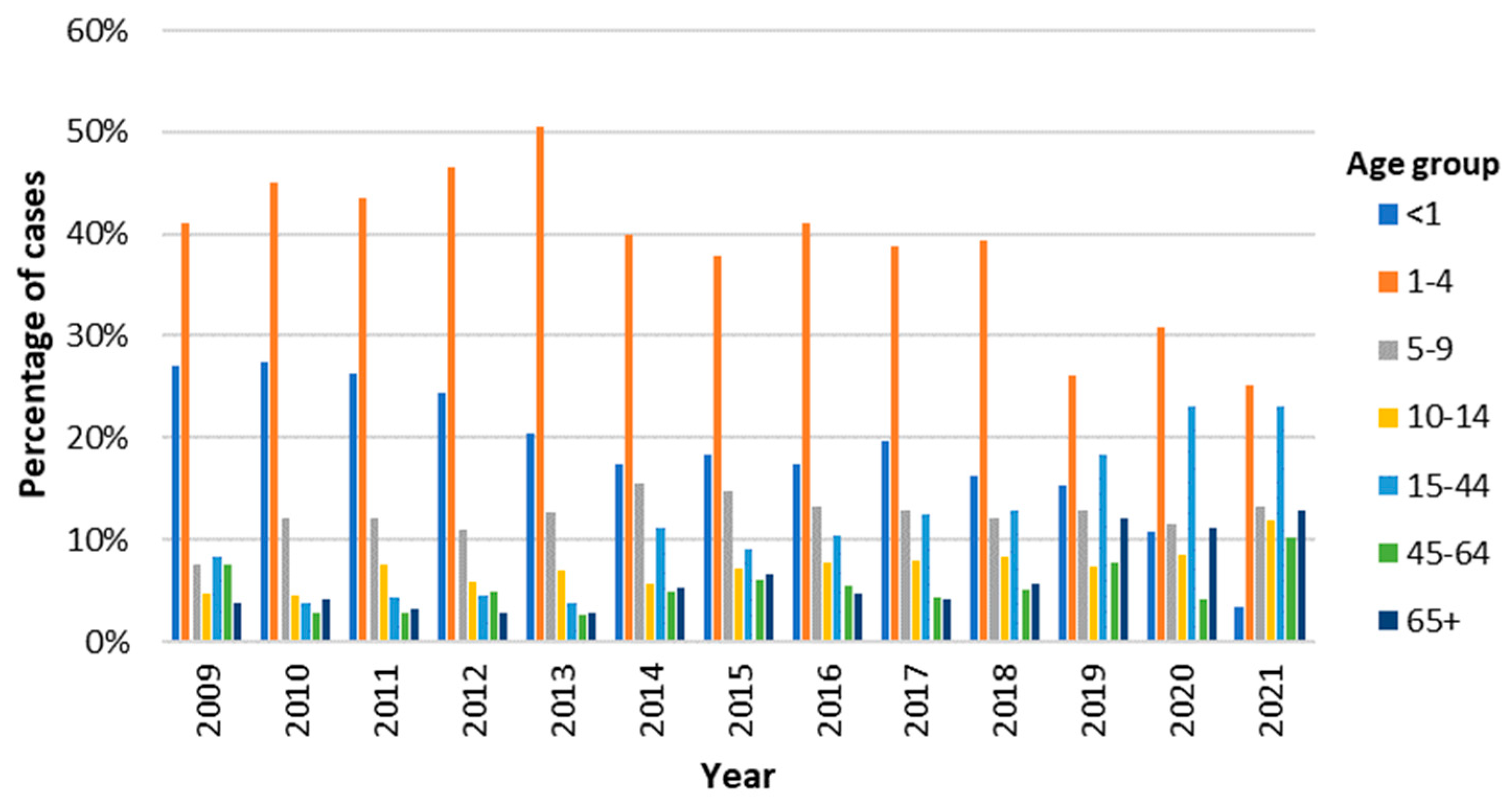
Considering the temporal distribution of cases in each age group, children from 1-4 years old represented the group with the highest percentage of cases every year (
Figure 2). However, after reaching its highest value in 2013 (50.5%), this percentage has been decreasing, only accounting for 25.2% of the cases in 2021. In another hand, the percentage of cases in the adult population has been increasing, with a particular notability in the group of younger adults (15-44 years), which in 2020 and 2021 accounted for 23.1% of
Campylobacter infections each (
Figure 2).
The main reported symptom was diarrhea, including bloody diarrhea, affecting 3405 out of 3673 (92.7 %) of the patients. Other reported symptoms included abdominal pain, fever, and vomiting, to a lesser extent (
Table 3).
Bloody diarrhea was significantly more likely in the paediatric than in the adult population (OR=4.7367; 95% CI: 0.5399-0.8368), while abdominal pain (OR=0.5018; 95% CI: 0.4038-0.6236) and fever (OR=0.6721; 95% CI: 0.5399-0.8368) were less likely to occur in the paediatric than in the adult population (
Table 4).
The distribution of the main symptoms according to the seven defined age groups corroborates the former associations, showing that bloody diarrhea was significantly associated with younger children (≤4 years), while in contrast, it is a rare condition in patients aged 65+ years (
Table S1).
In addition to the main symptoms associated with Campylobacter infection, several patients described underlying medical conditions: 43 patients presented immunosuppression, 37 had colitis and 15 showed symptoms of infection other than Campylobacter. Concerning extra gastrointestinal conditions that can be associated with the infection, three patients reported the neurologic Guillain-Barré/Miller Fisher syndromes. Patients with immunosuppression were significantly more prone to develop bacteremia (positive blood culture) (58.1% vs. 41.9%; p=0.03).
3.5. Distribution of cases of Campylobacter infection according to age, gender and geographic region
To evaluate if the prevalence of
Campylobacter infection was associated with age, gender, and geographic region of sample provenience, a comparison of the distribution of positive samples according to these three criteria was performed (
Table 5). The association between campylobacteriosis and gender was statistically significant, with 3096 male (60.2%, 95% CI: 58.8-61.5) and 2048 female (39.8%, 95% CI: 38.5-41.2) patients reported as positive for
Campylobacter spp. isolation. Opposed to this trend, the risk of infection was significantly lower in males than in females for the age group 15-44 years old (RR 0.799; 95%CI 0.684-0.933; p=0.0045), while for the remaining age groups, the difference was not statistically significant (
Table S2).
When considering the distribution of
Campylobacter infection cases by age groups (
Table 5), the highest rate was observed in young children, with 2001 cases (39.1%) in 1-4 years old infants, followed by children aged < 1 year (934, 18.2%). By species, the highest number of cases of
C. jejuni infection was observed for the age groups from 1-4 years (40.0%) and 0-1 years (18.4%), the same being observed for
C. coli infections, 1-4 years (32.1%) followed by the age group 0-1 years (17.0%). However, considering the distribution of species per age group, it is shown that the prevalence of
C. jejuni infection is significantly higher than that of
C. coli in children aged 1-4 years old, while from the age of 15 years, the burden of
C. coli infection is significantly higher (P<0.05) (
Table 5). The distribution of
Campylobacter species for paediatric and adult populations per year also corroborates this trend, with the rate of
C. coli being around 2-fold higher in adults than in children, except in the year 2013 (
Table S3).
The distribution of cases according to the hospital’s geographical origin shows was higher in the North of the country (44.7%) than in Lisbon and Tagus Valley (35.1%) and in the Centre region (15.2%) (
Table 5). However, these numbers are influenced by the number and size of the participating hospitals, affecting the number of samples submitted to the NRL.
3.6. Other epidemiological data
Of all the considered cases, only 40 (0.768%) reported a possible transmission route: 42.5% indicated the consumption of untreated water from private drinking water wells, 17.5% a possible foodborne association, and 22.5% reported a traveling history soon before the infection. This contrasts with the notified data from SINAVE, retrieved from the epidemiological surveys, for which food was the main vehicle reported (68.7%), followed by person-to-person (19.0%), water (8.0%), and animal transmission (4.3%). According to records from the primary laboratories, only a small proportion of cases reported association with outbreaks (43 of 5203 cases).
The median (interquartile range) time elapsed from the onset of symptoms to the initial medical visit was three days (IQR: 2-5 days), but it could be extended beyond 16 days. The median delay was shorter for men [3 (IQR: 2-5) days] than for women [4 (IQR: 2-6) days] (p < 0.05). The time interval from the first symptoms to the initial medical visit was not significantly different in the seven age groups considered (p=0.896) (
Table S4).
3.7. Antimicrobial resistance of Campylobacter spp. strains
Antimicrobial susceptibility was determined for 2174
Campylobacter spp. isolates, 1807 (83.1%)
C. jejuni and 367 (16.9%)
C. coli, and the results are summarized in
Table 6. The
Campylobacter spp. isolates were analyzed for AMR against seven antibiotics, priority or optional for treatment of campylobacteriosis: CIP, ERY, TCY, and GEN from 2013 to 2021, AMC and ETP, from 2017-2021, and AMP from 2018-2021.
Overall, both species presented extremely high (>93%) level of resistance to CIP, while for TCY,
C. jejuni presented very high level of resistance (≥80%), and
C. coli extremely high level (>93%). For both antibiotics, resistance was significantly higher for
C. coli than for
C. jejuni (P=0.043 for CIP and P<0.001 for TCY) (
Table 6). Regarding ERY,
C. coli presented high level of resistance (52.3%) contrasting to the low level determined for
C. jejuni (3.3%) (P<0.001). The level of resistance to GEN was overall low, however the difference was also significant when comparing
C. coli (2.5%) and
C. jejuni (0.1%) (P<0.001) (
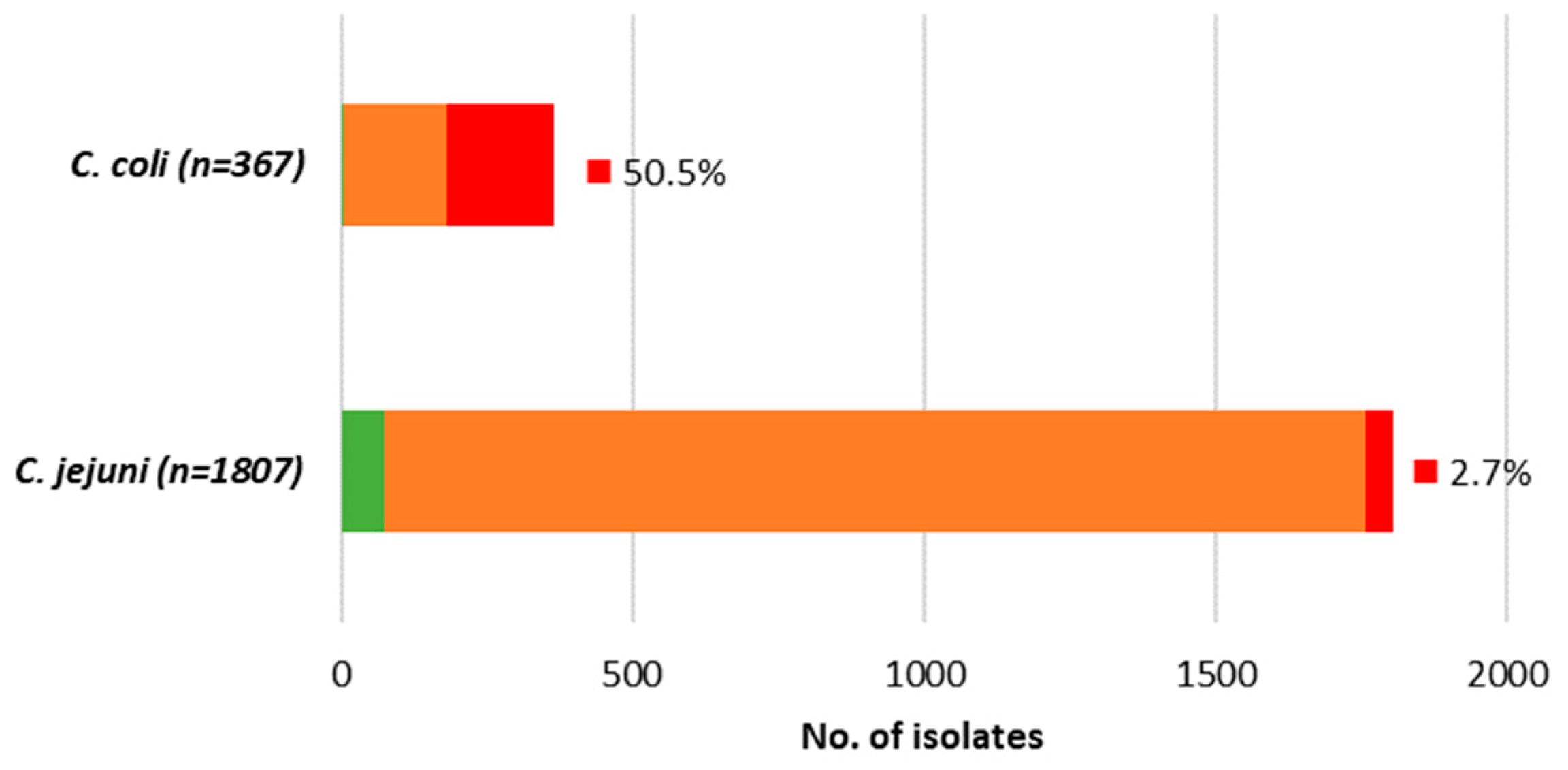
Table 6). Generally, a very low level of
Campylobacter strains circulating was fully susceptible (<2% in
C. coli, ~4% in
C. jejuni), while multidrug resistance (MDR), here defined as combined resistance to CIP, ERY, and TCY, was frequently found in
C. coli (50.5%) and was rare in
C. jejuni (2.7%) (
Figure 3).
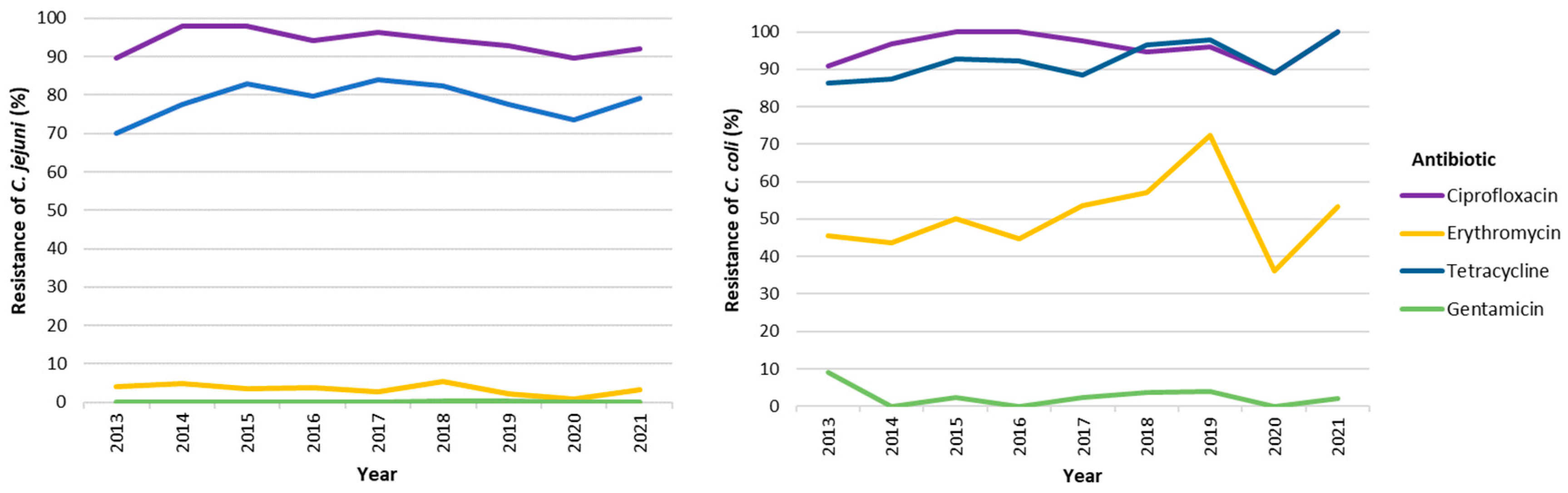
The evolution of resistance to antimicrobial agents from 2013 to 2021 is summarized in
Figure 4 and
Table S5 and shows that, for both species, the level of resistance to the four priority antimicrobials has remained relatively constant over the studied years, the only exception being the decrease of resistance to ERY for
C. coli in 2020 (36.1%), which returned to the higher rates in 2021 (
Figure 4 and
Table S5). Regarding the optional antibiotics tested, most of the strains were resistant to AMP (>75%), being this trend stable in the period covered, 2018-2021. In contrast, for ETP, and considering the minimal inhibitory concentration (MIC) breakpoint > 1mg/L, a very low to low proportion of strains presented resistance, although again, significantly higher in
C. coli than in
C. jejuni (6.5% vs 0.7%, P<0.001) (
Table 6). The MIC variation for susceptible isolates was 0.016-0.75 mg/L, while for non-susceptible isolates ranged from 2.0->32 mg/L for
C. coli and 1.5-4 mg/L for
C. jejuni. Regarding AMC, a very low to low proportion of the strains (0.2% in
C. jejuni, 3.9% in
C. coli; P<0.001) showed decreased susceptibility, with inhibition zone diameter between 14-19 mm, corresponding to MICs varying between 4 and 8 mg/L. This decreased susceptibility was only perceived in the years 2017 and 2018 (
Table S5).
Demographic features and species associated with resistance were explored through a univariate logistic regression (
Table S6), showing that resistance to CIP, TCY, and AMP was significantly less likely to occur in the age groups 15-44 and 45+ compared to the paediatric population. As previously observed,
C. coli strains were significantly more likely to have resistance to ERY and AMC compared to
C. jejuni strains. Compared to the Lisbon and Tagus Valley region, ETP resistance was less likely to occur in the regions Centre and North (
Table S6).
Variables from the univariate analysis (
Table S5) were subsequently included in a multivariate analysis (
Table 7) confirming that age significantly decreases the odds of resistance to AMP, CIP, and TCY, and that
C. coli strains were significantly more likely than C. jejuni strains to resist to all tested antibiotics, except to CIP and AMP. Accordingly,
C. coli isolates had significantly minor inhibition diameter zones than
C. jejuni, except for AMP.
4. Discussion
This study examined the laboratory surveillance data on campylobacteriosis in Portugal for a 13-year period from January 2009 to December 2021. The findings shed light on several key aspects of this disease, including notification rates, demographic trends, seasonal variation, and AMR patterns.
Distribution of Campylobacter infection cases
The number of
Campylobacter infections notified in the national platform SINAVE has been rising over time, contrary to what has been observed at the European level, where notification rates remained stable in the five-year period preceding the COVID-19 pandemic [
1]. Despite the notorious growth of notified confirmed cases (from 596 to 973 in the period 2017-2021), the notification rate remained far below expectations peaking at 9.4 cases per 100 000 population in 2021, less than a quarter of the European Union (EU)/ European Economic Area (EEA) overall notification rate of 44.5 cases per 100 000 population that year [
17]. Thus, neither the increase in notifications can be assumed as a direct increase in
Campylobacter infections, as there have been improvements in laboratory testing and reporting over time, nor the number of annual cases can be accurately estimated since there is evidence of a considerable rate of under-notification. As expected, the dynamics of the number of isolates sent to the NRL followed the same trend as the number of notifications, however, contrary to expectations, until 2021 the number of isolates received from the sentinel surveillance was almost as high as the cases notified to a national level, reinforcing the extent of under-notification of campylobacteriosis in Portugal. Due to the very high load of isolates received, a pre-selection step in the laboratory protocol was introduced, in 2019, resulting in a decrease in cases processed by the NRL, which was further noticed by the impact of the COVID-19 pandemic in 2020.
Seasonal variation of Campylobacter infection
The seasonal distribution of
Campylobacter infection cases exhibited a unique pattern, with a lack of clear summer peaks usually observed in the EU [
4], despite the highest cumulative number of cases occurring in August. Instead, a more random distribution throughout the year was noted, with slight decreases in the winter months. Reinforcing this random distribution was the occurrence of infection peaks in months with usually lower number of cases, such as October and February. Several studies have been carried out aiming to understand the underlying determinants of the marked campylobacteriosis seasonality [
18], however, epidemiological explanations remain uncertain [
19]. This highlights the potential of whole genome sequencing (WGS) to provide deeper insights into strain-specific dynamics and transmission patterns.
Antimicrobial resistance
Antibiotic resistance in
Campylobacter spp. is an increasingly serious threat due to its implications for public health and effective treatment, requiring coordinated actions to minimize the emergence and spread of antimicrobial-resistant strains. Ciprofloxacin and erythromycin had been considered the antibiotics of choice for treating
Campylobacter infections until very recently when an increasing trend in ciprofloxacin resistance has been observed [
2,
25]. In contrast, resistance to erythromycin and aminoglycosides like gentamicin remains very low [
1]. Thus, monitoring resistance levels in circulating isolates becomes one of the most relevant parameters for the surveillance of campylobacteriosis. Patterns of AMR in
Campylobacter isolates from Portugal revealed critical levels of resistance to priority antibiotics, particularly in
C. coli. This is in line with what has already been described in association with the cost-fitness that mutations associated with resistance, especially to macrolides, have in
C. jejuni and not in
C. coli [
26]. Overall,
C. coli presented extremely high level of resistance to both ciprofloxacin and tetracycline, and high level to erythromycin, while
C. jejuni presented extremely high level of resistance to ciprofloxacin and very high level to tetracycline only. Although rare in
C. jejuni (2.7%), MDR was frequently found in
C. coli isolates (50.5%) and is of concern for both species. Indeed, the occurrence of combined resistance to fluoroquinolones and macrolides in
Campylobacter spp. is considered of high public health relevance [
27]. These patterns of AMR observed both in
C. jejuni and in
C. coli human isolates, are of the highest among EU member states, according to the latest EU summary reports on AMR in zoonotic and indicator bacteria, and are in line with data from animal and food isolates [
27].
Temporal analysis of resistance to priority antibiotics demonstrated stability, with slight fluctuations, over the years, such as a momentary decrease in erythromycin resistance in 2020, potentially linked to COVID-19-related restrictions [
27]. However, given the fact that Portugal has come to be the European country with the highest levels of resistance to this antibiotic in both people and animals, it reduces the likelihood that the generally observed levels of resistance derive from travel-related cases. WGS analyses could be of great value in understanding the perpetuation of high levels of resistance over time. With the misuse of antibiotics in veterinary medicine pointed out as the main cause of the historical increase in the level of resistance, the banning and control of their use has not been accompanied by a decrease in the observed levels of AMR, which in part could be explained by the stability of the resistance phenotype that would remain even without antibiotic pressure, if not presenting biological costs [
28].
The monitoring of optional listed antibiotics is particularly relevant in countries with high rates of AMR, as is the case in Portugal, since they may represent the only treatment option in cases of infection with MDR strains [
29]. While most of the strains displayed resistance to ampicillin, only a very low to low proportion of strains displayed resistance to ertapenem and decreased susceptibility to amoxicillin-clavulanic acid. Resistance to carbapenems, despite their observed levels being low so far, represents a public health concern, as it is a recommended treatment option in more severe cases of systemic infections [
30,
31]. Thus, and taking into account the high levels of resistance recently observed in
C. coli isolated from animal sources, it becomes relevant to define the cut-off for ETP, as well as the harmonization of its surveillance both in
C. coli and in
C. jejuni [
27]. Again, WGS is of most value to unravel the mechanisms behind resistance to -lactams and carbapenems and monitor the evolution of resistant clones [
32].
Associations between demographic features and antimicrobial resistance were observed, particularly a reduced likelihood of resistance in the 15-44 years and 45+ age groups. Geographic distribution also showed relevance, with certain resistance patterns more prevalent in specific regions. These findings could reflect differences in exposure routes, emphasizing the importance of targeted interventions.
5. Conclusions
In conclusion, this study provides valuable insights into campylobacteriosis surveillance in Portugal, identifying trends, disparities, and areas of concern. While limitations in reporting and laboratory processing are evident, the data underscores the importance of sustained surveillance but also calls for a page turn, with the implementation of WGS-based surveillance for a more comprehensive understanding of disease dynamics and AMR evolution. The findings emphasize the urgent need to implement a One Health approach to effectively combat campylobacteriosis and its associated challenges in Portugal.
Supplementary Materials
Six supplementary tables are provided in ‘Supplementary Material’ to support additional findings produced from the present extensive analysis and the relevant contents of these tables are indicated in ‘Results’. Table S1: Distribution (odds ratio and confidence intervals) of a main symptom associated with Campylobacter infection by age group. Table S2: Risk ratio of cases of Campylobacter infection (male/female) by age group. Table S3: Distribution of Campylobacter species for paediatric (<15 years old) and adult population by year. Table S4. Time (in days) elapsed from onset of symptoms to the date of sample collection according to gender and age group. Table S5: Evolution of resistance (%) to different antimicrobial agents for Campylobacter jejuni and Campylobacter coli. Table S6: Univariate logistic regression analysis of the relationship between Campylobacter jejuni and Campylobacter coli resistance and sociodemographic features.
Author Contributions
For research Conceptualization: M.O.; Data curation: M.O., A.D., L.P.; Formal analysis: L.P., A.D.; Methodology: M.O., A.D., L.P. J.C.R., R.M.; Project administration: M.O.; Supervision: M.O.; Writing draft: M.O., A.D., L.P., M.L.L.; Writing – review & editing: M.O., A.D., L.P., M.L.L.
PTCampyNet work group:
Catarina Chaves, Henrique Oliveira - Centro Hospitalar e Universitário de Coimbra
Margarida Pinto, Cristina Marcelo, Fátima Meneses, João Marques, Vasco Mendes, Miguel Seruca - Centro Hospitalar e Universitário de Lisboa Central
Aurelia Selaru, Paulo Lopes - Centro Hospitalar de Vila Nova de Gaia/Espinho, E.P.E.
José Diogo, Filipa Alexandra Rocha Fortunato, Leonardo Goncalves Carneiro, José Manuel Carvalho Marta - Hospital Garcia de Orta
Alberta Faustino, Maria Carmen Iglésias, Glória Gonçalves, João Neves, Ana Raquel Vieira – Hospital de Braga
Elzara Aliyeva, Sandra Schafer, Maria Clara Portugal, Isabel Maria Machado Monge - Hospital Fernando da Fonseca
Adriana Pedrosa, Herminia Costa, Fátima Silva, Amélia Afonso, Ana Cristina Silva - Centro Hospitalar entre Douro e Vouga, E.P.E
Mariana Viana, Marvin Silva Oliveira, Hugo André Vasconcelos Macedo, Vanessa Pereira,
Paula Cristina Dias Ferreira Martins da Costa - Centro Hospitalar do Tâmega e Sousa, E.P.E
Ana Bento Pinto, Ana Fontes - Centro Hospitalar de Trás-os-Montes e Alto Douro, E.P.E
Manuela Ribeiro - Centro Hospitalar Universitário São João
Carla Leite, Centro Hospitalar Póvoa de Varzim-Vila do Conde, E.P.E
Ana Jesus, Ermelinda Luísa Pulso Teixeira - Centro Hospitalar Barreiro-Montijo, E.P.E.
Maria Favila Menezes, Bruno Miguel, Luís Nogueira Martins, Maria José Rego de Sousa - CML Germano de Sousa
Vitória Rodrigues, Manuela Azevedo – Synlab
Funding
This work was partly supported by funding from the European Union’s Horizon 2020 Research and Innovation programme under grant agreement No 773830: One Health European Joint Programme. Maria-Leonor Lemos is the recipient of a PhD grant (2022.10133.BD) from Fundação para a Ciência e a Tecnologia (FCT).
Data Availability Statement
The other data will be made available on request.
Acknowledgments
We thank all the clinical pathology laboratories of the PTCampyNet for providing the clinical isolates and metadata.
Conflicts of Interest
The authors declare that they have no conflict of interest in this research.
References
- European Centre for Disease Prevention and Control (ECDC). Campylobacteriosis. In: ECDC. Annual Epidemiological Report for 2021.; Stockholm, 2022.
- Rivera-Mendoza, D.; Martínez-Flores, I.; Santamaría, R.I.; Lozano, L.; Bustamante, V.H.; Pérez-Morales, D. Genomic Analysis Reveals the Genetic Determinants Associated With Antibiotic Resistance in the Zoonotic Pathogen Campylobacter Spp. Distributed Globally. Front. Microbiol. 2020, 11, 513070. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Fu, Q.; Wang, Y.; Li, X.; Wu, C.; Shen, Z.; Zhang, Q.; Qin, P.; Shen, J.; et al. Integrated Genomic and Proteomic Analyses of High-Level Chloramphenicol Resistance in Campylobacter jejuni. Sci. Rep. 2017, 7, 16973. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA), European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar] [CrossRef]
- Liu, F.; Lee, S.A.; Xue, J.; Riordan, S.M.; Zhang, L. Global Epidemiology of Campylobacteriosis and the Impact of COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 979055. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, J.; Pires, I.; Vlaes, L.; Coignau, H.; Levy, J.; Goossens, H.; Goncalves, A.P.; de Mol, P.; Butzler, J.P. Campylobacter enteritis in Portugal: Epidemiological Features and Biological Markers. Eur. J. Epidemiol. 1992, 8, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Clemente, L.; Rocha, T.; Tavares, A.; Geraldes, M.; Barahona, M.J.; Botelho, A.; Cunha, M. V. Comparative Genotypic and Antimicrobial Susceptibility Analysis of Zoonotic Campylobacter species Isolated from Broilers in a Nationwide Survey, Portugal. J. Food Prot. 2012, 75, 2100–2109. [Google Scholar] [CrossRef]
- Duarte, A.; Santos, A.; Manageiro, V.; Martins, A.; Fraqueza, M.J.; Caniça, M.; Domingues, F.C.; Oleastro, M. Human, Food and Animal Campylobacter spp. Isolated in Portugal: High Genetic Diversity and Antibiotic Resistance Rates. Int. J. Antimicrob. Agents 2014, 44, 306–313. [Google Scholar] [CrossRef]
- Lemos, M.L.; Nunes, A.; Ancora, M.; Cammà, C.; da Costa, P.M.; Oleastro, M. Campylobacter jejuni in Different Canine Populations: Characteristics and Zoonotic Potential. Microorganisms 2021, 9, 2231. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Martins, A.; Borges, A.C.; Fernandes, M.H.; Fernandes, M.J.; Vaz, Y.; Bessa, R.J.B.; Barreto, A.S. Antimicrobial Resistance among Campylobacter spp. Strains Isolated from Different Poultry Production Systems at Slaughterhouse Level. Poult. Sci. 2014, 93, 1578–1586. [Google Scholar] [CrossRef]
- Vicente, A.; Barros, R.; Florinda, A.; Silva, A.; Hanscheid, T. High Rates of Fluoroquinolone-Resistant Campylobacter in Portugal - Need for Surveillance. Euro Surveill. 2008, 13, 8031. [Google Scholar] [CrossRef]
- Barata, A.R.; Nunes, B.; Oliveira, R.; Guedes, H.; Almeida, C.; Saavedra, M.J.; da Silva, G.J.; Almeida, G. Occurrence and Seasonality of Campylobacter spp. in Portuguese Dairy Farms. Int. J. Food Microbiol. 2022, 383, 109961. [Google Scholar] [CrossRef]
- Ménard, A.; Dachet, F.; Prouzet-Mauleon, V.; Oleastro, M.; Mégraud, F. Development of a Real-Time Fluorescence Resonance Energy Transfer PCR to Identify the Main Pathogenic Campylobacter spp. Clin. Microbiol. Infect. 2005, 11, 281–287. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 12.0, 2022, http://www.eucast.org.
- European Centre for Disease Prevention and Control (ECDC). Annex 2. EUCAST Clinical Breakpoints and Epidemiological Cut-off Values for the Priority List of Antimicrobials to Be Tested for Campylobacter jejuni and C.coli as of 31 August 2021, 2021.
- Jehl F; Caron F; Cattoen C; Cattoir V; Dubreuil L; et al. Comité de l ’ Antibiogramme de La Société Française de Microbiologie Recommandations 2020. Com. L’Antibiogramme La Société Française Microbiol. 2020, 86, 1–181.
- European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Disease. Available online: www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases (accessed on 5 September 2023).
- Louis, V.R.; Gillespie, I.A.; O’Brien, S.J.; Russek-Cohen, E.; Pearson, A.D.; Colwell, R.R. Temperature-Driven Campylobacter Seasonality in England and Wales. Appl. Environ. Microbiol. 2005, 71, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lake, I.R.; Colón-González, F.J.; Takkinen, J.; Rossi, M.; Sudre, B.; Gomes Dias, J.; Tavoschi, L.; Joshi, A.; Semenza, J.C.; Nichols, G. Exploring Campylobacter Seasonality across Europe Using the European Surveillance System (TESSy), 2008 to 2016. Eurosurveillance 2019, 24, 1800028. [Google Scholar] [CrossRef] [PubMed]
- Luber, P.; Brynestad, S.; Topsch, D.; Scherer, K.; Bartelt, E. Quantification of Campylobacter species Cross-Contamination during Handling of Contaminated Fresh Chicken Parts in Kitchens. Appl. Environ. Microbiol. 2006, 72, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.J.; Ferreira, V.; Truninger, M.; Maia, R.; Teixeira, P. Cross-Contamination Events of Campylobacter spp. in Domestic Kitchens Associated with Consumer Handling Practices of Raw Poultry. Int. J. Food Microbiol. 2021, 338, 108984. [Google Scholar] [CrossRef] [PubMed]
- Mylius, S.D.; Nauta, M.J.; Havelaar, A.H. Cross-Contamination during Food Preparation: A Mechanistic Model Applied to Chicken-Borne Campylobacter. Risk Anal. 2007, 27, 803–813. [Google Scholar] [CrossRef]
- Levesque, S.; Fournier, E.; Carrier, N.; Frost, E.; D. Arbeit, R.; Michaud, S. Campylobacteriosis in Urban versus Rural Areas: A Case-Case Study Integrated with Molecular Typing to Validate Risk Factors and to Attribute Sources of Infection. PLoS One 2013, 8, 17–20. [Google Scholar] [CrossRef]
- Murray, R.T.; Cruz-Cano, R.; Nasko, D.; Blythe, D.; Ryan, P.; Boyle, M.M.; Wilson, S.M.; Sapkota, A.R. Association between Private Drinking Water Wells and the Incidence of Campylobacteriosis in Maryland: An Ecological Analysis Using Foodborne Diseases Active Surveillance Network (FoodNet) Data (2007–2016). Environ. Res. 2020, 188, 109773. [Google Scholar] [CrossRef]
- Ge, B.; Wang, F.; Sjölund-Karlsson, M.; McDermott, P.F. Antimicrobial Resistance in Campylobacter: Susceptibility Testing Methods and Resistance Trends. J. Microbiol. Methods 2013, 95, 57–67. [Google Scholar] [CrossRef]
- Bolinger, H.; Kathariou, S. The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms. Appl. Environ. Microbiol. 2017, 83, e00416–17. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA), European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2020/2021. EFSA J. 2023, 21, 7867. [Google Scholar] [CrossRef]
- Tang, Y.; Fang, L.; Xu, C.; Zhang, Q. Antibiotic Resistance Trends and Mechanisms in the Foodborne Pathogen, Campylobacter. Anim. Heal. Res. Rev. 2017, 18, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Sifré, E.; Salha, B.A.; Ducournau, A.; Floch, P.; Chardon, H.; Mégraud, F.; Lehours, P. EUCAST Recommendations for Antimicrobial Susceptibility Testing Applied to the Three Main Campylobacter Species Isolated in Humans. J. Microbiol. Methods 2015, 119, 206–213. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Aerts, M.; Battisti, A.; Hendriksen, R.; Kempf, I.; Teale, C.; Tenhagen, B.A.; Veldman, K.; Wasyl, D.; Guerra, B.; et al. Technical Specifications on Harmonised Monitoring of Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Food-Producing Animals and Food. EFSA J. 2019, 17, 5709. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and Alternative Strategies for the Prevention, Control, and Treatment of Antibiotic-Resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Nunes, A.; Oleastro, M.; Alves, F.; Liassine, N.; Lowe, D.M.; Benejat, L.; Ducounau, A.; Jehanne, Q.; Borges, V.; Gomes, J.P.; et al. Recurrent Campylobacter jejuni Infections with In Vivo Selection of Resistance to Macrolides and Carbapenems: Molecular Characterization of Resistance Determinants. Microbiol. Spectr. 2023, 11, e0107023. [Google Scholar] [CrossRef]
Figure 1.
Distribution of the number of cases of Campylobacter infections according to the month of sampling, considering the total of cases received in the NRL in the most representative period spanning from 2015 to 2021.
Figure 1.
Distribution of the number of cases of Campylobacter infections according to the month of sampling, considering the total of cases received in the NRL in the most representative period spanning from 2015 to 2021.
Figure 2.
Percentage of cases of Campylobacter infections by age group in the time period spanning from 2009-2021.
Figure 2.
Percentage of cases of Campylobacter infections by age group in the time period spanning from 2009-2021.
Figure 3.
Proportion of Campylobacter spp. isolates that are multidrug resistant (MDR), resistant to one and/or two antimicrobials and completely susceptible, considering the four priority antimicrobials (CIP, ERY, TET, GEN).
Figure 3.
Proportion of Campylobacter spp. isolates that are multidrug resistant (MDR), resistant to one and/or two antimicrobials and completely susceptible, considering the four priority antimicrobials (CIP, ERY, TET, GEN).
Figure 4.
Evolution of the resistance rate for Campylobacter jejuni (at the left) and Campylobacter coli (at the right) to the four priority antimicrobials (CIP, ERY, TET, GEN), from 2013 to 2021.
Figure 4.
Evolution of the resistance rate for Campylobacter jejuni (at the left) and Campylobacter coli (at the right) to the four priority antimicrobials (CIP, ERY, TET, GEN), from 2013 to 2021.
Table 1.
Total number of cases of Campylobacter infections received in the National Reference Laboratory, and total number of non-duplicated isolates processed, including with antimicrobial susceptibility testing performed.
Table 1.
Total number of cases of Campylobacter infections received in the National Reference Laboratory, and total number of non-duplicated isolates processed, including with antimicrobial susceptibility testing performed.
| |
Year |
Overall |
| 2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
| Total of cases received in the NRL |
129 |
248 |
347 |
328 |
426 |
437 |
461 |
537 |
685 |
658 |
916 |
676 |
820 |
6668 |
| Isolates processed by the NRL |
129 |
248 |
347 |
328 |
426 |
437 |
461 |
537 |
685 |
658 |
364 |
260 |
325 |
5205 |
| Isolates with ATB results |
0 |
0 |
0 |
0 |
119 |
135 |
182 |
191 |
289 |
336 |
349 |
248 |
325 |
2174 |
Table 2.
Distribution of Campylobacter species by year.
Table 2.
Distribution of Campylobacter species by year.
| |
Year |
Overall |
| 2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
C. jejuni
n (%)
|
112 (86.8) |
208 (83.9) |
300 (86.5) |
294
(89.6) |
393
(92.3) |
388
(88.8) |
394
(85.5) |
474
(88.3) |
626
(91.4) |
591
(89.8) |
311
(85.4) |
221
(85.0) |
278
(85.5) |
4590
(88.2) |
C. coli
n (%)
|
17 (13.2) |
40 (16.1) |
47
(13.5) |
34
(10.4) |
33
(7.7) |
49
(11.2) |
67
(14.5) |
63
(11.7) |
59
(8.6) |
67
(10.2) |
53
(14.6) |
39
(15.0) |
47
(14.5) |
615
(11.8) |
| Total |
129 |
248 |
347 |
328 |
426 |
437 |
461 |
537 |
685 |
658 |
364 |
260 |
325 |
5205 |
Table 3.
Distribution of the main symptoms associated with Campylobacter infections.
Table 3.
Distribution of the main symptoms associated with Campylobacter infections.
| Reported symptoms |
With diarrhea
(Including bloody diarrhea)
n=3405
n (% within group) |
Without diarrhea n=268
n (% within group) |
| Abdominal pain |
299 (8.8) |
65 (24.3) |
| Fever |
316 (9.3) |
58 (21.6) |
| Vomiting |
102 (3.0) |
12 (4.5) |
| Abdominal pain + fever |
61 (1.8) |
14 (5.2) |
| Abdominal pain + vomiting |
27 (0.8) |
7 (2.6) |
| Abdominal pain + fever + vomiting |
12 (0.4) |
2 (0.7) |
| Fever + vomiting |
68 (2.0) |
2 (0.7) |
| No other reported symptoms |
2520 (74.0) |
-- |
Table 4.
Distribution of the main symptoms among paediatric and adult population.
Table 4.
Distribution of the main symptoms among paediatric and adult population.
| Main symptom |
Paediatric population
(n=2982)
n (% within group) |
Adult population
(n=677)
n (% within group) |
Total
(n=3659)
n (% of total) |
OR (95%CI) |
P |
| Bloody Diarrhea |
1353 (45.4) |
101 (14.9) |
1454 (39.7) |
4.7367 (3.788-5.922) |
<0.001 |
| Non-bloody diarrhea |
1492 (48.4) |
449 (66.3) |
1941 (53.0) |
0.5085 (0.427-0.606) |
<0.001 |
| Abdominal pain |
345 (11.6) |
140 (20.7) |
485 (13.3) |
0.5018 (0.404-0.624) |
<0.001 |
| Fever |
404 (13.5) |
128 (18.9) |
592 (16.2) |
0.6721 (0.540-0.837) |
<0.001 |
| Vomiting |
180 (7.3) |
52 (7.7) |
232 (6.3) |
0.7721 (0.560-1.064) |
0.114 |
Table 5.
Number of Campylobacter infections in the studied population according to age, gender and geographic region.
Table 5.
Number of Campylobacter infections in the studied population according to age, gender and geographic region.
| |
No. positive samples (% within group) (% of total) |
Total |
| |
Campylobacter jejuni |
Campylobacter coli |
| Gender (n=5144) |
|
|
|
| Female |
1791 (39.5 a) (34.8) |
257 (42.3 a) (5.0) |
2048 (39.8) |
| Male |
2745 (60.5 a) (53.4) |
351 (57.7 a) (6.8) |
3096 (60.2) |
| Total |
4536 (88.2) |
608 (11.8) |
5144 (100) |
| Age group (n=5120) |
|
|
|
| <1 |
831 (18.4 a) (16.2) |
103 (17.0 a) (2.0) |
934 (18.2) |
| 1-4 |
1807 (40.0 a) (35.3) |
194 (32.1 b) (3.8) |
2001 (39.1) |
| 5-9 |
589 (13.0 a) (11.5) |
68 (11.2 a) (1.3) |
657 (12.8) |
| 10-14 |
348 (7.7 a) (6.8) |
36 (6.0 b) (0.7) |
384 (7.5) |
| 15-44 |
482 (10.7 a) (9.4) |
95 (15.7 b) (1.9) |
577 (11.3) |
| 45-64 |
212 (4.7 a) (4.1) |
52 (8.6 b) (1.0) |
264 (5.2) |
| + 65 |
246(5.4 a) (4.8) |
57 (9.4 b) (1.1) |
303 (5.9) |
| Total |
4515 (88.2 a) |
605 (11.8 b) |
5120 (100) |
| Region (n=5203) |
|
|
|
| North |
2317 (50.5 a) (45.0) |
271(44.1 b) (5.2) |
2588 (44.7) |
| Centre |
711 (15.5 a) (13.9) |
78 (12.7 a) (1.5) |
789 (15.2) |
| Lisbon and Tagus Valley |
1560 (34.0 a) (29.5) |
241 (43.3 b) (5.1) |
1826 (35.1) |
| Total |
4588 (88.2) |
615 (11.8) |
5203 (100) |
Table 6.
Resistance profile to seven antimicrobial agents of Campylobacter jejuni and Campylobacter coli strains.
Table 6.
Resistance profile to seven antimicrobial agents of Campylobacter jejuni and Campylobacter coli strains.
| Antimicrobial category |
Antimicrobial agent |
Mean inhibition diameter zone (in mm) (SD) |
P |
% Resistance (n/n total) |
P |
| Total |
C. jejuni |
C. coli |
Total
(n=2174) |
C. jejuni (n=1807) |
C. coli
(n=367) |
| Fluoroquinolones |
Ciprofloxacin (CIP) |
9.35 (7.07) |
10.54 (8.70) |
8.66 (579) |
0.002 |
94.2 (2048) |
93.7 (1694) |
96.5 (354) |
0.043 |
| Macrolides |
Erythromycin (ERY) |
26.31 (8.02) |
28.12 (5.44) |
15.85 (11.06) |
<0.001 |
11.8 (252) |
3.3 (60) |
52.3 (192) |
<0.001 |
| Tetracyclines |
Tetracycline (TCY) |
14.36 (11.53) |
16.66 (12.78) |
10.02 (7.97) |
<0.001 |
81.6 (1773) |
79.2 (1431) |
93.2 (342) |
<0.001 |
| Aminoglycosides |
Gentamicin (GEN) |
28.40 (3.95) |
28.47 (3.78) |
26.69 (4.21) |
<0.001 |
0.5 (11) |
0.1 (2) |
2.5 (9) |
<0.001 |
| Penicillins+β-lactamase inhibitors |
Amoxicillin-clavulanic acid (AMC)* |
28.66 (4.71) |
28.76 (4.34) |
23.95 (5.79) |
<0.001 |
0.8 (12/1547) |
0.2 (3/1314) |
3.9 (9/233) |
<0.001 |
| Carbapenems |
Ertapenem (ETP)** |
0.125 (0.019) |
0.19 (0.034) |
0.5 (0.075) |
<0.001 |
1.6 (24/1543) |
0.7 (9/1311) |
6.5 (15/232) |
<0.001 |
| Penicillins |
Ampicillin (AMP) |
10.42 (7.22) |
11.56 (7.64) |
10.69 (7.47) |
0.348 |
76.3 (944/1238) |
75.5 (794/1051) |
80.2 (150/187) |
0.192 |
Table 7.
Multivariate logistic regression analysis of the relationship between Campylobacter jejuni and Campylobacter coli resistance and sociodemographic features.
Table 7.
Multivariate logistic regression analysis of the relationship between Campylobacter jejuni and Campylobacter coli resistance and sociodemographic features.
| |
Amoxicillin |
Ampicillin |
Ciprofloxacin |
Erythromycin |
Ertapenem |
Gentamicin |
Tetracycline |
| |
R |
S |
P value
OR (95%CI) |
R |
S |
P value
OR (95%CI) |
R |
S |
P value
OR (95%CI) |
R |
S |
P value
OR (95%CI) |
R |
S |
P value
OR (95%CI) |
R |
S |
P value
OR (95%CI) |
R |
S |
P value
OR (95%CI) |
Gender
Female
Male |
6
6 |
614
916
|
0.697
Ref
0.793
(0.247-2.547) |
372
568
|
127
167 |
0.279
Ref
1.160
(0.887-1.515) |
810
1225
|
48
78 |
0.734
Ref
0.937
(0.645-1.363) |
112
138 |
1185
746
|
0.959
Ref
0.991
(0.714-1.377) |
11
13 |
605
909
|
0.662
Ref
0.831
(0.362-1.907) |
6
5 |
852
1298
|
0.470
Ref
0.640
(0.190-2.150) |
708
1055
|
150
248 |
0.509
Ref
0.926
(0.736-1.164) |
Age
<15
15-44
45+ |
8
2
2 |
960
302
267
|
0.825
Ref
0.658
(0.134-3.234)
0.700
(0.143-3.425) |
589
184
168 |
160
67
67 |
0.022
Ref
0.714
(0.510-1.000)
0.660
(0.472-0.923) |
1344
355
337 |
63
30
33 |
0.001
Ref.
0.531
(0.335-0.841)
0.456
(0.293-0.709) |
148
44
57 |
1259
341
313 |
0.183
Ref.
1.002
(0.646-1.553)
1.458
(0.965-2.202) |
16
5
3 |
948
299
267 |
0.564
Ref.
0822
(0.291-2.322)
0.506
(0.143-1.790) |
7
2
2 |
1400
383
368 |
0.949
Ref.
0.850
(0.171-4.230)
0.787
(0.158-3.916) |
1178
308
278 |
229
77
92 |
<0.001
Ref.
0.778
(0.579-1.046)
0.571
(0.431-0.756) |
Species
C. jejuni
C. coli
|
3
9
|
1311
224 |
<0.001
Ref.
17.987
(4.765-67.90) |
794
150 |
257
37 |
0.123
Ref.
1.360
(0.920-2.010) |
1694
354
|
113
13 |
0.041
Ref.
1.850
(1.026-3.336) |
60
192 |
1747
175 |
<0.001
Ref.
31.088
(22.27-43.40) |
9
15 |
1302
217 |
<0.001
Ref.
9.775
(4.193-22.79) |
2
9 |
1805
358 |
<0.001
Ref.
20.989
(4.48-98.33) |
1431
342 |
376
25 |
<0.001
Ref.
3.705
(2.423-5.666) |
Region
North
Center
Lisbon and TV |
5
3
4 |
690
176
668 |
0.390
Ref.
1.994
(0.447-8.901)
0.680
(0.177-2.614) |
403
112
428 |
136
32
126 |
0.421
Ref.
1.206
(0.773-1.882)
1.195
(0.901-1.585) |
940
227
880 |
59
20
47 |
0.166
Ref.
0.767
(0.449-1.310)
1.276
(0.854-1.908) |
98
35
119 |
901
212
808 |
0.698
Ref.
1.205
(0.714-2.034)
0.966
(0.680-1.373) |
6
2
16 |
686
177
655 |
0.135
Ref.
1.081
(0.21-5.556)
2.480
(0.945-6.513) |
3
1
7 |
996
246
920 |
0.574
Ref.
1.151
(0.116-11.47)
2.023
(0.507-8.066) |
828
203
741 |
171
44
186 |
0.215
Ref.
1.077
(0.741-1.564)
1.236
(0.974-1.568) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).