1. Introduction
The dystonias are a group of disorders characterized by excessive muscle contractions leading to involuntary postures and movements with a repetitive quality, resulting in jerky oscillations [
1,
2]. Dystonia affects 3 million people worldwide and is third most common movement disorder following tremor and Parkinson’s disease. Tremors are defined by regular oscillations of a body region, typically with a sinusoidal pattern [
3,
4]. Tremor is the most common of all movement disorders, affecting >20 million people worldwide affecting approximately 3% of the general population. Tremor is a progressive disease leading to more disability at older age. Like the dystonias, tremors are chronic, and patients face a lifetime of overt stigmatizing disability. Although dystonia and tremor are considered distinct disorders, they are closely related. Epidemiologically, many patients diagnosed with dystonia also have tremor. The reported prevalence of clinically apparent tremor among patients diagnosed with dystonia varies from 10-90%, with an overall weighted average of 35.1% [
5] Conversely, the patients diagnosed with essential tremor also have dystonia, with reported co-prevalence rates of up to 21% [
5].
Dystonia and tremor, although traditionally considered disorder of motor system, also share non-motor features. The non-motor features in essential tremor include deficits in executive function, attention, concentration, and memory. The essential tremor patients also present with impairments in special senses, in the form of impaired color vision, olfaction, and hearing abnormalities [
6,
7,
8]. Sleep dysregulation and social anxiety is also reported in essential tremor [
9]. The neuropsychological deficit in essential tremor is thought to be related to cerebellar cognitive affective [
10]. Among the non-motor deficits observed in cervical dystonia (CD) patients is the inability to mentally manipulate their surrounding space [
11]. Furthermore, CD patients experience difficulties in complex movement planning, motor dexterity, visual-spatial working memory, and tactile object recognition [
11,
12].
Balance dysfunction is not uncommon in essential tremor and dystonia, often it is attributed to abnormal cerebellar function. Cervical vestibular-evoked myogenic potentials and ocular vestibular-evoked myogenic potentials – examining the vestibulo-collic and vestibulo-ocular reflex have increased amplitude but shortened latency in essential tremor compared to healthy controls [
13]. Abnormal vestibulo-colic reflex in essential tremor suggests putative dysfunction in regions that process central vestibular information, such as the cerebellum [
14]. Balance dysfunction is commonly seen in cerebellar predominant form of essential tremor where action tremor is much more robust, and it is often associated with dysarthria in this subset of patients [
15]. Cerebellar predominant essential tremor also manifests with dysfunction of higher-order processing of vestibulo-ocular reflex that localize to caudal vermis [
16]. The visuospatial skills are notably abnormal in CD, as evidenced by impaired performance in tasks such as line bisection, spatial quadrant selection, subjective perception of verticality, and drawing [
17,
18,
19]. Additionally, approximately one-third of CD patients lack awareness of their own neck posture and exhibit proprioceptive dysfunction, along with an increased threshold of tactile temporal discrimination [
20]. Despite the prevalence of these visuospatial dysfunctions in CD, the underlying biological mechanisms remain largely unknown [
21].
Heading perception is a non-motor skill crucial for functional spatial navigation and encompasses the ability to perceive one's own motion in relation to their immediate environment while relying on sensations from the vision and vestibular systems. Our recent investigation of spatial navigation performance involving linear-translational heading in CD challenged the idea that CD-related vestibular dysfunction is due to peripheral vestibular deficits. Our study showed abnormal proprioceptive input from neck muscle inducing the noise into the central mechanism, integrating visual, vestibular, and proprioceptive signals. We found that heading perception thresholds are elevated in CD, and the worsening of threshold correlates with the severity of CD. The fundamental question remains is that whether impaired vestibular and visual heading perception in CD is dependent upon presence (or absence) of associated tremor, and that whether such performance is different if the participant does not have any other movement disorder but isolated tremor.
Here we examine the degree of heading perception performance for vestibular and visual heading, while interpreting threshold and biases for isolated CD without tremor (or any other movement disorder), CD with tremor, and isolated tremor without any dystonia (or other movement disorder). The hypothesis is that the visual heading perception is better than vestibular perception in isolated CD, isolated tremor, and CD with tremor. The vestibular heading perception is comparable in isolated tremor and CD with head tremor, while both conditions would differ from isolated CD.
2. Materials and Methods
Participants
Observations in the performance of visual and vestibular discrimination were made for 19 participants (6 with isolated CD, 3 with CD and tremor; 5 with isolated tremor, and 5 healthy controls). The tremor was defined as to-and fro regular rhythmic oscillations that approximates to a sinusoidal waveform [
22]. The oscillatory movements with jerky, coarse, irregular features, combined with drifts and corrective movements were not considered as tremor; when present they were considered dystonia [
23]. The tremor patients considered in this study did not have any other movement disorders. We also excluded those with dementia, clinically significant depression or anxiety, focal brain lesions, intracranial surgery (except deep brain stimulation) history, atypical parkinsonism or Parkinson's disease, and generalized, multifocal, or tardive dystonia were excluded. All CD participants were successfully treated with botulinum toxin, while one participant was implanted with a pallidal deep brain stimulator. The patients were adequately treated with pharmacotherapy or deep brain stimulation. In those who had DBS, the stimulator was turned off prior to measuring the performance.
Experimental Setup
All participants provided informed written consent per the Declaration of Helsinki and the Institutional Review Boards of the Louis Stokes Cleveland VA Medical Center.
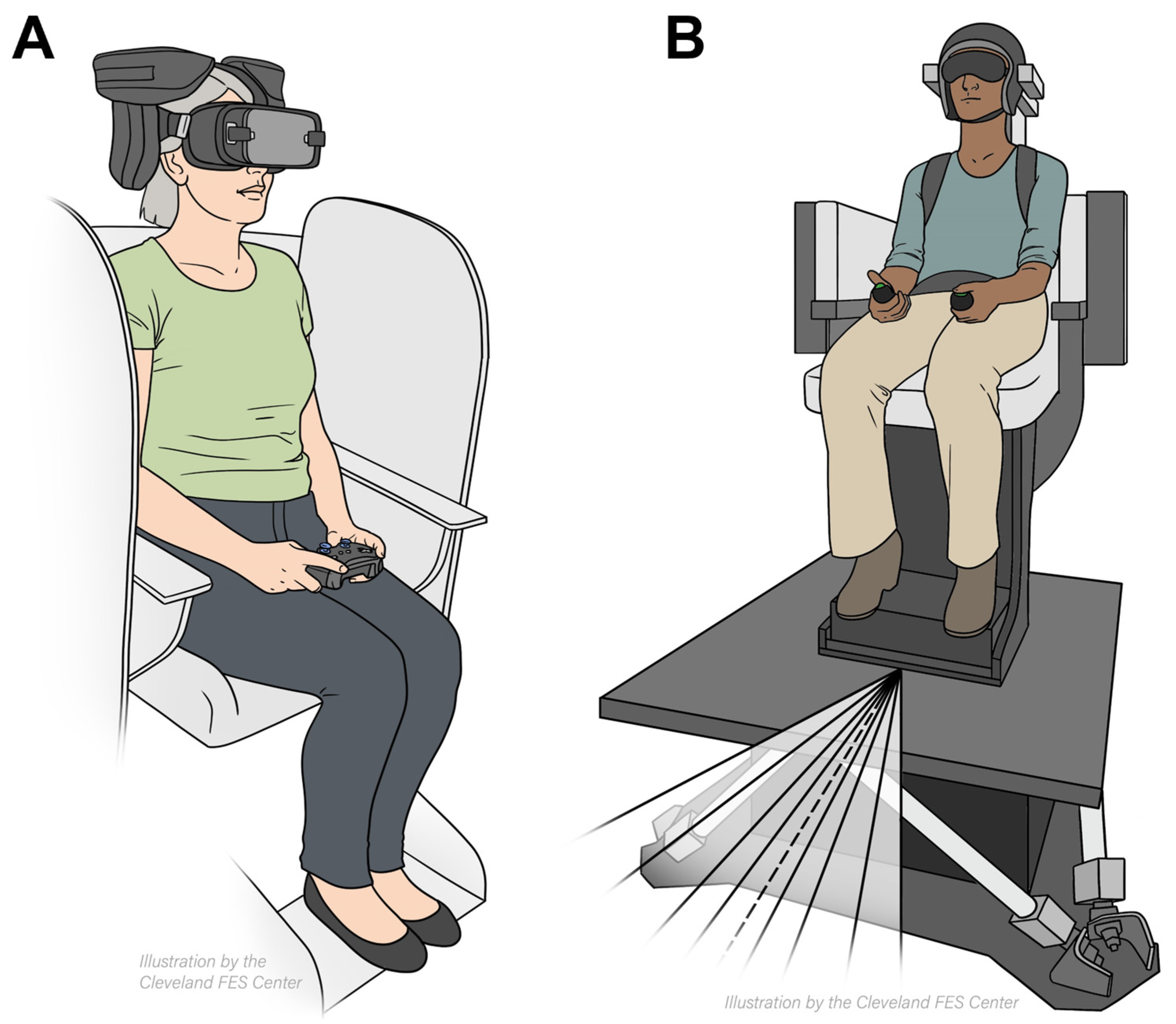
Vestibular Heading Discrimination was assessed using a two-alternative forced-choice task with Meta's Oculus Controllers (Oculus Rift, Menlo Park, California, USA). Participants were secured in a six-degree-of-freedom motion platform (hexapod) from Moog (Aurora, New York, USA), which enabled movement in three-dimensional space. Seated in the hexapod harness, participants had cushioned helmets immobilize their heads (
Figure 1A). The experiment took place in a dark room to eliminate external cues. Across three blocks, there were 99 trials with randomized forward motion angles at 0°, 5°, 10°, 20°, and 30° to the left or right (
Figure 1A). The hexapod displaced participants 0.4 meters over 1.5 seconds, surpassing vestibular thresholds, then paused for 3 seconds. During this pause, participants utilized the handheld controllers to indicate direction of movement.
Visual Heading Discrimination task the participants observed a 3D optical-flow pattern through Oculus Rift VR headsets (Menlo Park, California, USA) (
Figure 1B). There were 169 trials per 12-minute block. The trials featured star clouds expanding radially left or right at angles of 0°, 5°, 10°, 15°, 20°, 25°, or 30° (
Figure 1B). To respond, participants used a handheld button.
Data Analysis
We analyzed the button clicks during the vestibular and visual heading tasks that participants used to report perceived motion direction. This allowed us to quantify the perception threshold and direction perception bias for each participant. We determined these parameters by fitting a Gaussian cumulative distribution psychometric function to the percentage of rightward decisions at different motion direction levels. The motion direction levels were derived from the binary left/right responses. We defined the vestibular and visual thresholds (σ) as the standard deviation of the Gaussian fit, where lower threshold values indicated heightened sensitivity in detecting subtle heading changes. We gauged direction perception biases (μ) by linearly translating the psychometric function along the x-axis, denoting the heading angle equally likely to elicit left or right responses. This reflected asymmetry in perceiving straight motion.
3. Results
The overarching goal of this study was to examine the hypothesis that the visual heading perception is better than vestibular perception in isolated CD, isolated tremor, and CD with tremor. The vestibular heading perception is comparable in isolated tremor and CD with head tremor, while both conditions would differ from isolated CD. This study directly examined the hypothesis and compared a total of 19 human participants: 6 were isolated CD, 3 had CD with head tremor, 5 had isolated tremor, and 5 matched healthy controls. A two-alternative forced-choice task assessed perceived movement direction during passive forward motion (vestibular heading perception) and perceived direction of optic flow across different angular deviations from center in virtual reality (visual heading perception). As anticipated, larger eccentric angles correlated with more accurate direction identification responses, but the accuracy reduced as the direction of heading approached close to straight ahead. Straight-ahead motion was associated with random report of right-ward versus left-ward motion.
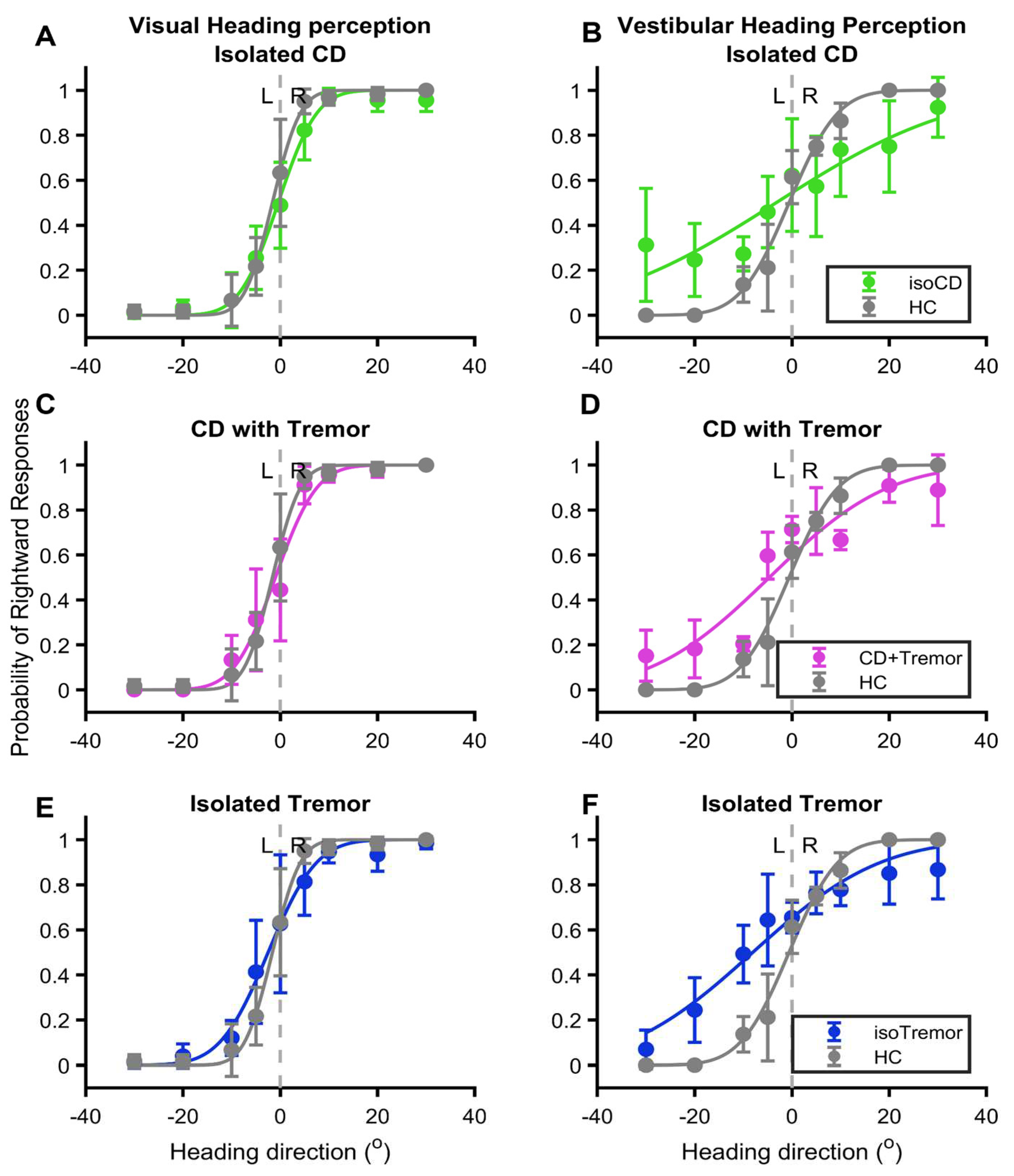
The relationship between the probability of correct responses and eccentricity of heading in visual or vestibular domain yields a sigmoid curve. Such sigmoid relationship is clearly evident in healthy controls, and it was much robust in the case of visual heading compared to vestibular in healthy participants (grey lines,
Figure 2A-F). The visual heading perception was least affected by any of the three conditions (green pink and blue lines in
Figure 2A,C,E) however, effects of CD with or without tremor and isolated tremor was much significant on vestibular perception (green pink and blue lines in
Figure 2B,D,F). The psychometric function curve depicting the vestibular heading perception almost had an oblique line instead of sigmoid fit in case of CD without tremor (green line
Figure 2B). The sigmoid fits of CD with tremor and isolated tremor groups were comparable to each other (pink and blue lines,
Figure 2D,F). Nevertheless, in all three disease types the vestibular heading perception psychometric function curve robustly differed from healthy controls.
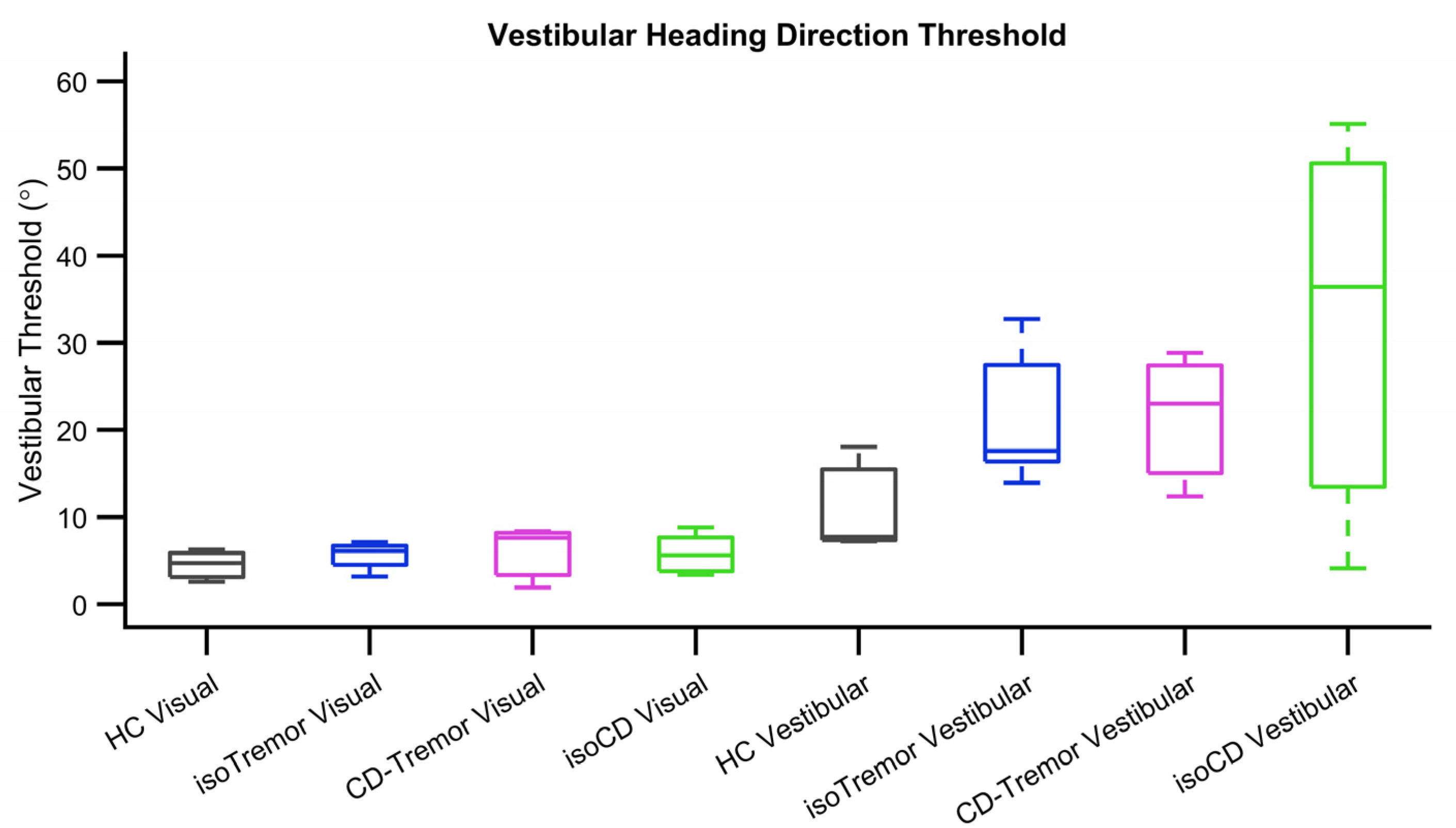
Further analysis examined the differences in sigmoid fit parameters, namely threshold and bias, in case of isolated CD, CD with tremor, and isolated tremor in compared them with healthy controls. The value of threshold, the measure of response precision (angle when 75% of correct responses are obtained) is much higher if perception is less precise. The visual heading threshold in case of healthy participants was 4.3°±1.9° and it was comparable to isolated CD 5.6°±2.1°, CD with tremor 7.6°±3.5°, and isolated tremor 5.1°±1.6°. The values of vestibular heading threshold in healthy participants (17.6°±6.1°) was lower compared to isolated CD (94.4°±21.6°). The threshold values in CD with tremor and isolated tremor were 33.5°±3.4° and 27.6°±7.7° respectively. They were better (smaller) compared to isolated CD, but comparable among CD with tremor and isolated tremor (
Figure 3). Given small number of samples, our study could only afford trend-wise comparison, skipping statistical analyses.
The lack of sigmoidal characteristics of vestibular heading perception is associated with unreliable measures of bias. The latter was however possible in the case of visual heading perception. The bias in visual heading perception was -2.5°±2.5° in patients with isolated CD, 5.6°±2.1° in CD with tremor, and -1.5°±3.6° in the case of isolated tremor; they were comparable to healthy controls -2.1°±4.6°.
4. Discussion
Accurate perception of one’s own motion, heading, is critical for maintenance of balance and postural stability [
23,
24,
25,
26,
27,
28]. The perception of verticality in the visual domain and awareness of one’s own posture and walking path when the visual information is compromised are common non-motor impairments in CD. Integration of vestibular, proprioceptive, and visual information via the cerebellum and basal ganglia, is critical for maintaining postural stability, balance, and gait [
29,
30,
31]. We recently discovered that CD patients have significant inaccuracy in discrimination of heading direction. The threshold to detect one’s own movement, the heading direction, is higher in CD; the severity is worse in vestibular domain compared to visual [
32]. These previous studies, in conjunction with other literature, suggest abnormal vestibular function and vestibulo-ocular reflex, and increased threshold to detect postural vertical in CD [
33,
34,
35,
36,
37,
38,
39]. Essential tremor is known to affect balance function. Studies have revealed impairment in vestibulo-ocular reflex, vestibulo-colic reflex, and overall balance impairments in patients with essential tremor [
13,
14,
15,
16]. Consistent with previous literature our experiments found impairment in vestibular heading perception in isolated CD, CD with tremor, and isolated tremor.
Why are vestibular and visual heading direction discrimination threshold elevated?
CD is increasingly thought to be a network level disorder involving the basal ganglia, cerebellum, brainstem and their connecting pathways [
40,
41,
42]. Likewise, tremor is also a network disorder involving the cerebellum and its connectivity with the thalamus. These regions are extensively connected with the areas responsible for motion perception. Impairment in cerebellar outflow, as predicted in CD or tremor, can influence the cerebellar-thalamic or vestibulo-thalamic projections that are critical for heading direction discrimination, at least in the vestibular [
43,
44,
45]. The vestibular-thalamic projections subsequently relay to the parieto-temporal cortex, playing an important role in vestibular motion perception and visuo-vestibular interaction in visual heading direction discrimination [
46,
47,
48]. Direct alteration of the cerebellar activity or the noise in the activity of the cerebellar projections to the thalamus can therefore adversely impact one's ability to perceive motion [
24,
25,
26,
27,
49,
50,
51].
Proprioception from the neck muscles have an important role in modulating the directional tuning patterns of deep cerebellar and vestibular neurons [
52]. It is therefore possible that abnormal activity of the dystonic neck muscles, especially in those with robust dystonia, even without presence of tremor, putatively distorts proprioceptive output, introducing the noise in vestibular and proprioceptive convergence neurons in deep cerebellar and vestibular nuclei. Such an effect impacts the directional tuning pattern of deep cerebellar and vestibular neurons and would affect further upstream function of heading direction discrimination. According to this mechanism, the effect of dystonia and tremor is more robust and direct on vestibular heading direction discrimination, but less so in visual heading direction discrimination.
All the experiments, in visual and vestibular heading direction discrimination tasks, were performed while participants' heads were immobilized in straight-ahead orientation. Such a setup excluded the possibility of asymmetrically stimulating the vestibular end-organs due to tonic head turning.
5. Conclusions
This report dually demonstrates shared deficits and distinctions in linear translational heading discrimination between individuals with isolated CD, CD with tremor, and isolated tremor. While all three groups had reduced heading perception compared to healthy controls, those with tremor (with or without CD) displayed less severe elevations in threshold detection. Collectively, the findings unveil overlapping navigation challenges, likely stemming from common cerebellar-brainstem dysfunctions, contrasted by nuances in impairment severity and characteristics across these movement disorders. This signifies fresh insights into altered spatial cognition with implications for individualized management, targeting core deficits. Additional inquiries across diverse patient cohorts with larger sample sizes and exploration of multi-faceted navigation will serve to enhance interventions that improve mobility and reduce associated fall risks in these cohorts.
References
- Evatt, M.L.; Freeman, A.; Factor, S. Adult-onset dystonia. Handb Clin Neurol 2011, 100, 481–511. [Google Scholar] [PubMed]
- Jinnah, H.A.; Berardelli, A.; Comella, C.; Defazio, G.; Delong, M.R.; Factor, S.; Galpern, W.R.; Hallett, M.; Ludlow, C.; Perlmutter, J.S.; et al. The focal dystonias: Current views and challenges for future research. Mov. Disord. 2013, 28, 926–943. [Google Scholar] [CrossRef] [PubMed]
- Elias, W.J.; Shah, B.B. Tremor. JAMA 2014, 311, 948–954. [Google Scholar] [CrossRef]
- Govert, F.; Deuschl, G. Tremor entities and their classification: an update. Curr Opin Neurol 2015, 28, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.G. , Beylergil, S.B.; Scorr, L.; Kilic-Berkmen, G.; Freeman, A.; Klein, C.; Junker, J.; Loens, S.; Bruggemann, N.; Munchau, A.; et al. Dystonia and Tremor: A Cross-Sectional Study of the Dystonia Coalition Cohort. Neurology 2021, 96, e563–e574. [Google Scholar] [PubMed]
- Jhunjhunwala, K.; George, L.; Kotikalapudi, R.; Gupta, P.K.; Lenka, A.; Stezin, A.; Naduthota, R.M.; Yadav, R.; Gupta, A.K.; Saini, J.; et al. A preliminary study of the neuroanatomical correlates of primary writing tremor: role of cerebellum. Neuroradiology 2016, 58, 827–836. [Google Scholar] [CrossRef]
- Louis, E.D. Non-motor symptoms in essential tremor: A review of the current data and state of the field. Park. Relat. Disord. 2015, 22 (Suppl. 1), S115–S118. [Google Scholar] [CrossRef]
- Shalash, A.S.; Mohamed, H.; Mansour, A.H.; Elkady, A.; Elrassas, H.; Hamid, E.; Elbalkimy, M.H. Clinical Profile of Non-Motor Symptoms in Patients with Essential Tremor: Impact on Quality of Life and Age-Related Differences. Tremor Other Hyperkinet Mov (N Y), 2019; 9. [Google Scholar]
- Hou, L.; Lei, X. Risk factors of social anxiety in patients with essential tremor. Front. Psychiatry 2023, 14, 1051290. [Google Scholar] [CrossRef] [PubMed]
- Destrebecq, V.; Naeije, G. Cognitive impairment in essential tremor assessed by the cerebellar cognitive affective syndrome scale. Front. Neurol. 2023, 14, 1224478. [Google Scholar] [CrossRef]
- Hinse, P.; Leplow, B.; Humbert, T.; Lamparter, U.; Junge, A.; Emskötter, T. Impairment of visuospatial function in idiopathic spasmodic torticollis. J. Neurol. 1995, 243, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Alemán, G.G.; de Erausquin, G.A.; Micheli, F. Cognitive disturbances in primary blepharospasm. Mov. Disord. 2009, 24, 2112–2120. [Google Scholar] [CrossRef]
- Bal, N.; Şengül, Y.; Behmen, M.B.; Powell, A.; Louis, E.D. Vestibular reflexes in essential tremor: abnormalities of ocular and cervical vestibular-evoked myogenic potentials are associated with the cerebellum and brainstem involvement. J. Neural Transm. 2023, 130, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Uyaroglu, F.G.; Ucar, R.; Acarer, A.; Celebisoy, N. What might cervical vestibular–evoked myogenic potential abnormalities mean in essential tremor? Neurol. Sci. 2021, 42, 5271–5276. [Google Scholar] [CrossRef] [PubMed]
- Kronenbuerger, M.; Konczak, J.; Ziegler, W.; Buderath, P.; Frank, B.; Coenen, V.A.; Kiening, K.; Reinacher, P.; Noth, J.; Timmann, D. Balance and Motor Speech Impairment in Essential Tremor. Cerebellum 2009, 8, 389–398. [Google Scholar] [CrossRef]
- Helmchen, C.; Hagenow, A.; Miesner, J.; Sprenger, A.; Rambold, H.; Wenzelburger, R.; Heide, W.; Deuschl, G. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain 2003, 126, 1319–1332. [Google Scholar] [CrossRef]
- Chillemi, G.; Formica, C.; Salatino, A.; Calamuneri, A.; Girlanda, P.; Morgante, F.; Milardi, D.; Terranova, C.; Cacciola, A.; Quartarone, A. Biased Visuospatial Attention in Cervical Dystonia. J. Int. Neuropsychol. Soc. 2017, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Chillemi, G.; Formica, C.; Salatino, A.; Calamuneri, A.; Girlanda, P.; Morgante, F.; Milardi, D.; Terranova, C.; Cacciola, A.; Quartarone, A.; et al. Biased Visuospatial Attention in Cervical Dystonia. J. Int. Neuropsychol. Soc. 2017, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Leplow, B.; Tübinger, C.S. Visuospatial Functions in Patients with Spasmodic Torticollis. Percept. Mot. Ski. 1994, 78, 1363–1375. [Google Scholar] [CrossRef]
- Ferrazzano, G.; Berardelli, I.; Belvisi, D.; De Bartolo, M.I.; Di Vita, A.; Conte, A.; Fabbrini, G. Awareness of Dystonic Posture in Patients With Cervical Dystonia. Front. Psychol. 2020, 11, 1434. [Google Scholar] [CrossRef]
- Avanzino, L.; Cherif, A.; Crisafulli, O.; Carbone, F.; Zenzeri, J.; Morasso, P.; Abbruzzese, G.; Pelosin, E.; Konczak, J. Tactile and proprioceptive dysfunction differentiates cervical dystonia with and without tremor. Neurology 2020, 94, e639–e650. [Google Scholar] [CrossRef]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; Parkinson Tremor Task Force of the International, and Society Movement Disorder. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018, 33, 75–87. [Google Scholar] [CrossRef]
- Panyakaew, P.; Jinnah, H.A.; Shaikh, A.G. Clinical features, pathophysiology, treatment, and controversies of tremor in dystonia. J Neurol Sci 2022, 435, 120199. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Ramat, S.; Bockisch, C.J.; Marti, S.; Straumann, D.; Palla, A. Is Vestibular Self-Motion Perception Controlled by the Velocity Storage? Insights from Patients with Chronic Degeneration of the Vestibulo-Cerebellum. PLOS ONE 2012, 7, e36763. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Wicki, A.; Baumann, C.R.; Straumann, D.; Palla, A. Impaired Tilt Perception in Parkinson’s Disease: A Central Vestibular Integration Failure. PLOS ONE 2015, 10, e0124253. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.G.; Palla, A.; Marti, S.; Olasagasti, I.; Optican, L.M.; Zee, D.S.; Straumann, D. Role of cerebellum in motion perception and vestibulo-ocular reflex-similarities and disparities. Cerebellum 2013, 12, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.G.; Straumann, D.; Palla, A. Motion Illusion—Evidence towards Human Vestibulo-Thalamic Projections. Cerebellum 2017, 16, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Barnett-Cowan, M.; Dyde, R.T.; Fox, S.H.; Moro, E.; Hutchison, W.D.; Harris, L.R. Multisensory determinants of orientation perception in Parkinson's disease. Neuroscience 2010, 167, 1138–1150. [Google Scholar] [CrossRef]
- Maschke, M.; Gomez, C.M.; Tuite, P.J.; Konczak, J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 2003, 126, 2312–1222. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.G.; Gurfinkel, V.; King, L.; Horak, F. Parkinson's disease shows perceptuomotor asymmetry unrelated to motor symptoms. Neurosci Lett 2007, 417, 10–15. [Google Scholar] [CrossRef]
- Konczak, J.; Krawczewski, K.; Tuite, P.; Maschke, M. The perception of passive motion in Parkinson's disease. J Neurol 2007, 254, 655–663. [Google Scholar] [CrossRef]
- Agharazi, H.; Wang, A.; Guha, A.; Gupta, P.; Shaikh, A.G. Unraveling the Twist: Spatial Navigational Challenges in Cervical Dystonia. Mov. Disord. 2023, 38, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Stell, R.; Bronstein, A.M.; Marsden, C.D. Vestibulo-ocular abnormalities in spasmodic torticollis before and after botulinum toxin injections. J. Neurol. Neurosurg. Psychiatry 1989, 52, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Stell, R.; Gresty, M.; Metcalfe, T.; Bronstein, A.M. Cervico-ocular function in patients with spasmodic torticollis. J. Neurol. Neurosurg. Psychiatry 1991, 54, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.G.; Markham, C.H.; Baloh, R.W. Vestibular involvement in spasmodic torticollis: an old hypothesis with new data from otolith testing. Adv Otorhinolaryngol 1988, 42, 219–223. [Google Scholar] [PubMed]
- Anastasopoulos, D.; Bhatia, K.; Bisdorff, A.; Bronstein, A.M.; Gresty, M.A.; Marsden, C.D. Perception of spatial orientation in spasmodic torticollis. Part I: The postural vertical. Mov Disord 1997, 12, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Anastasopoulos, D.; Bhatia, K.; Bronstein, A.M.; Marsden, C.D.; Gresty, M.A. Perception of spatial orientation in spasmodic torticollis. Part 2: The visual vertical. Mov Disord 1997, 12, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Anastasopoulos, D.; Nasios, G.; Psilas, K.; Mergner, T.; Maurer, C.; Lücking, C.H. What is straight ahead to a patient with torticollis? Brain 1998, 121, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lekhel, H.; Popov, K.; Anastasopoulos, D.; Bronstein, A.; Bhatia, K.; Marsden, C.D.; Gresty, M. Postural responses to vibration of neck muscles in patients with idiopathic torticollis. Brain 1997, 120, 583–591. [Google Scholar] [CrossRef]
- Corp, D.T.; Joutsa, J.; Darby, R.R.; Delnooz, C.C.S.; van de Warrenburg, B.P.C.; Cooke, D.; Prudente, C.N.; Ren, J.; Reich, M.M.; Batla, A.; et al. Network localization of cervical dystonia based on causal brain lesions. Brain 2019, 142, 1660–1674. [Google Scholar] [CrossRef]
- Shakkottai, V.G.; Batla, A.; Bhatia, K.; Dauer, W.T.; Dresel, C.; Niethammer, M.; Eidelberg, D.; Raike, R.S.; Smith, Y.; Jinnah, H.A.; et al. Current Opinions and Areas of Consensus on the Role of the Cerebellum in Dystonia. Cerebellum 2016, 16, 577–594. [Google Scholar] [CrossRef]
- Neychev, V.K.; Fan, X.; Mitev, V.I.; Hess, E.J.; Jinnah, H.A. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain 2008, 131, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Liedgren, S.R.; Milne, A.C.; Schwarz, D.W.; Tomlinson, R.D. Representation of vestibular afferents in somatosensory thalamic nuclei of the squirrel monkey (Saimiri sciureus). J. Neurophysiol. 1976, 39, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, E.; Tremblay, L.; Féger, J.; Carras, P.L.; Strick, P.L. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 2005, 8, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Pinto, A.D.; Lang, A.E.; Chen, R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann. Neurol. 2010, 68, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, S.; Grüsser, O.; Guldin, W.O. Thalamic connections of the vestibular cortical fields in the squirrel monkey (Saimiri sciureus). J. Comp. Neurol. 1992, 326, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Bremmer, F.; Klam, F.; Duhamel, J.; Ben Hamed, S.; Graf, W. Visual–vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur. J. Neurosci. 2002, 16, 1569–1586. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; DeAngelis, G.C.; E Angelaki, D. A functional link between area MSTd and heading perception based on vestibular signals. Nat. Neurosci. 2007, 10, 1038–1047. [Google Scholar] [CrossRef]
- Lenz, F.; Tasker, R.; Kwan, H.; Schnider, S.; Kwong, R.; Murayama, Y.; Dostrovsky, J.; Murphy, J. Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic "tremor cells" with the 3-6 Hz component of parkinsonian tremor. J. Neurosci. 1988, 8, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Dahlem, K.; Valko, Y.; Schmahmann, J.D.; Lewis, R.F. Cerebellar contributions to self-motion perception: evidence from patients with congenital cerebellar agenesis. J. Neurophysiol. 2016, 115, 2280–2285. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Palla, A.; Marti, S.; Schuknecht, B.; Straumann, D. Hypertrophy of the Inferior Olivary Nucleus Impacts Perception of Gravity. Front. Neurol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Shaikh, A.G.; Meng, H.; Angelaki, D.E. Multiple Reference Frames for Motion in the Primate Cerebellum. J. Neurosci. 2004, 24, 4491–4497. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).