Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Indoles
2.1. Bisindoles
2.1.1. Staurosporines
2.1.2. Halogenated bisindoles
2.2. Indole sesquiterpenoids
2.3. Other indoles
3. Pyrroles
3.1. Pyrrolones and pyrrolidones
3.2. Pyrrolobenzodiazepines
3.3. Ansamycins
3.4. Other pyrroles
4. Oxazoles and thiazoles
5. Pyridines
5.1. Piericidins
5.2. Quinolines
5.3. Other pyridines
6. Pyrazines and piperazines
6.1. Pyrazines
6.2. Diketopiperazines
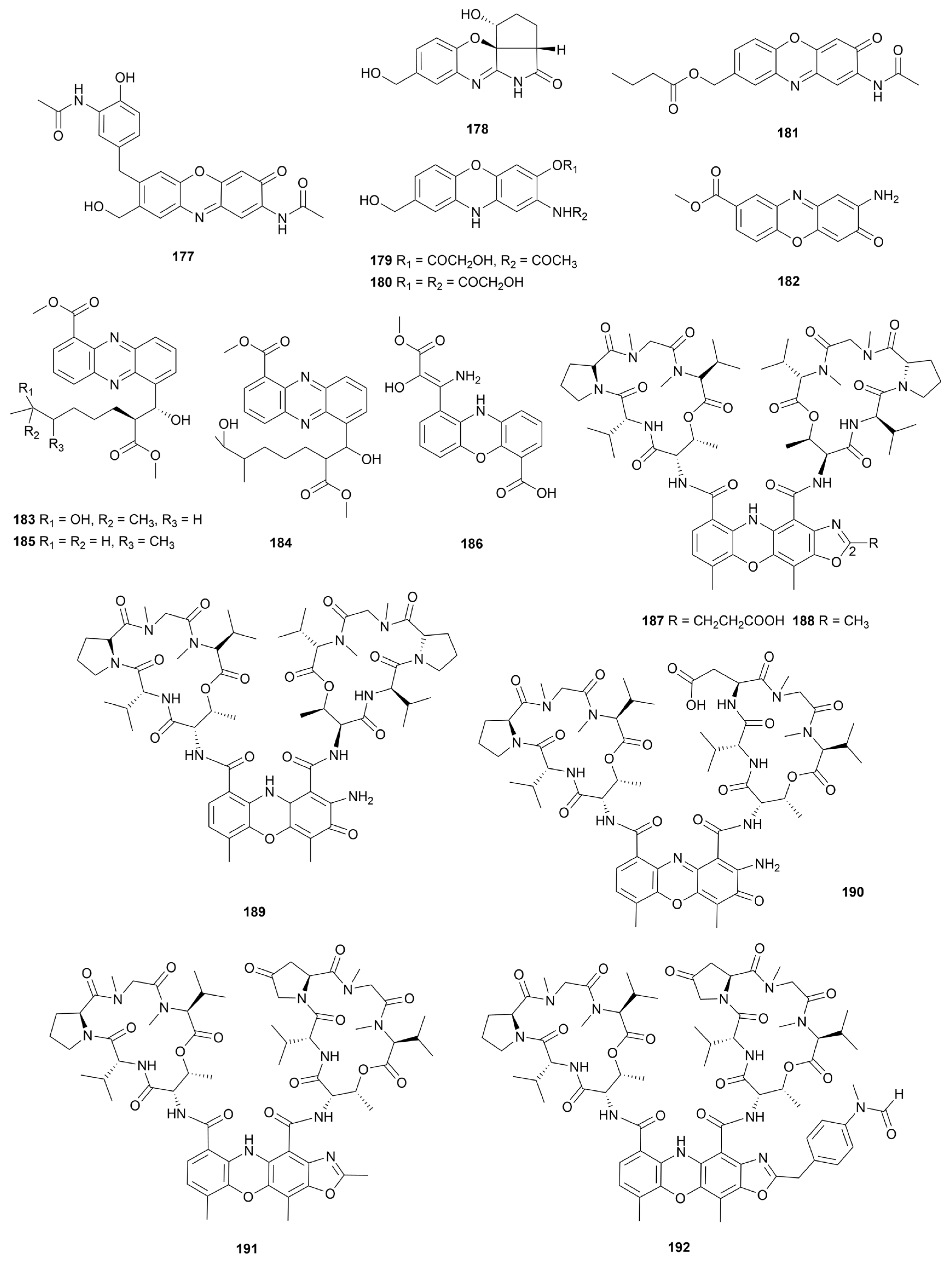
7. Phenazines and phenoxazines
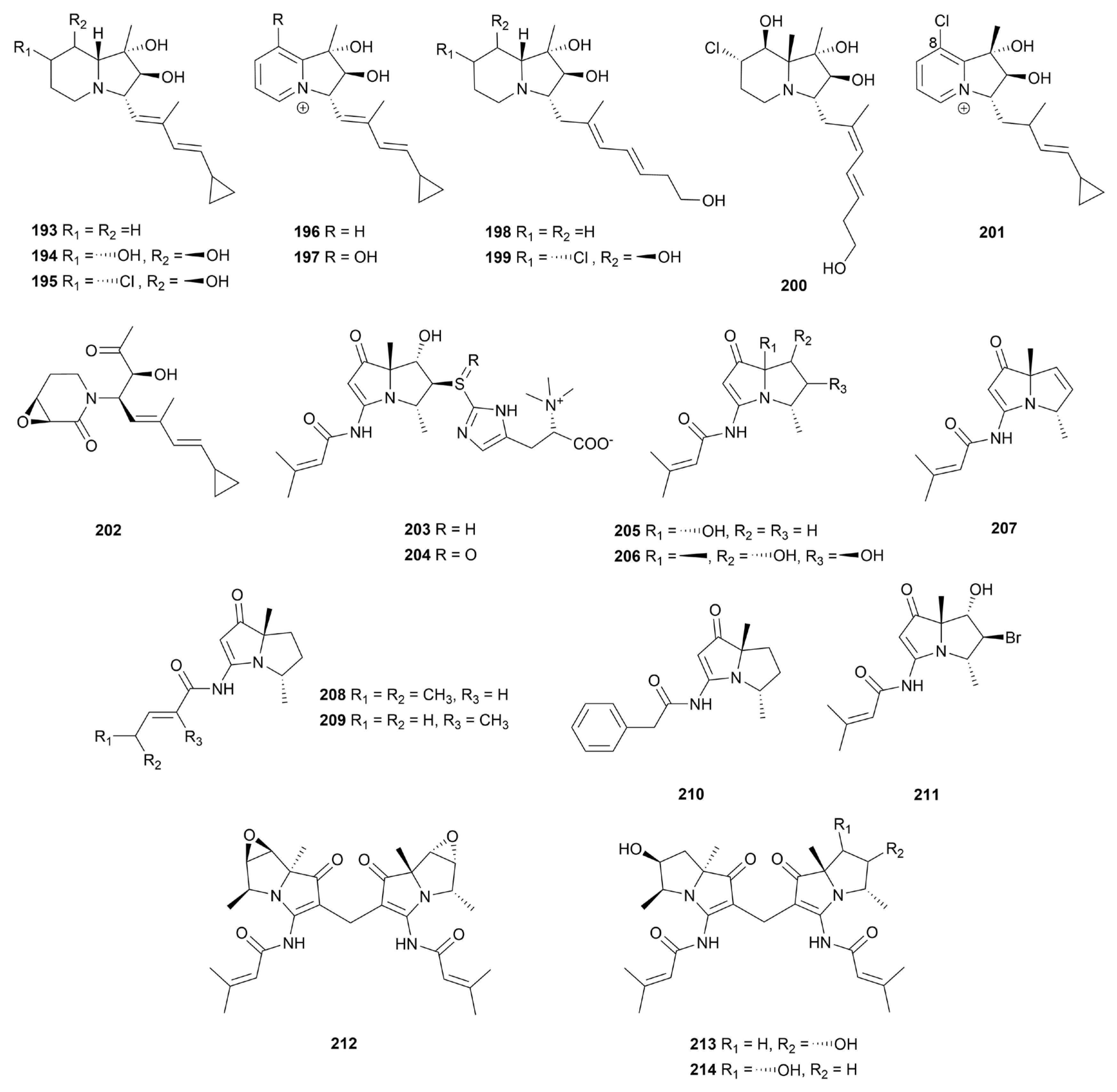
8. Indolizidines and pyrrolizidines
8.1. Indolizidines
8.2. Pyrrolizidines
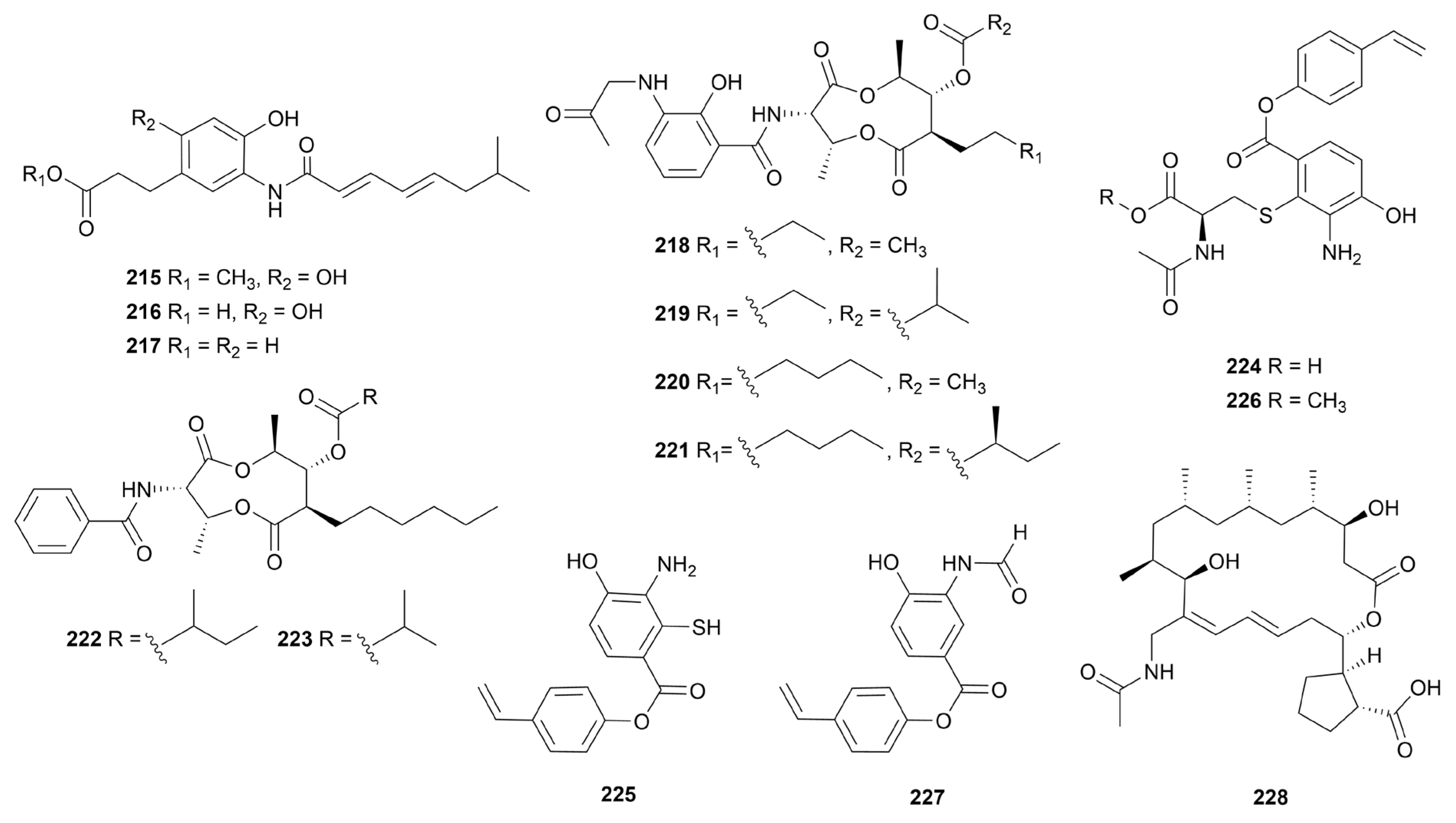
9. Amides
9.1. Linear amides
9.2. Macrolactams
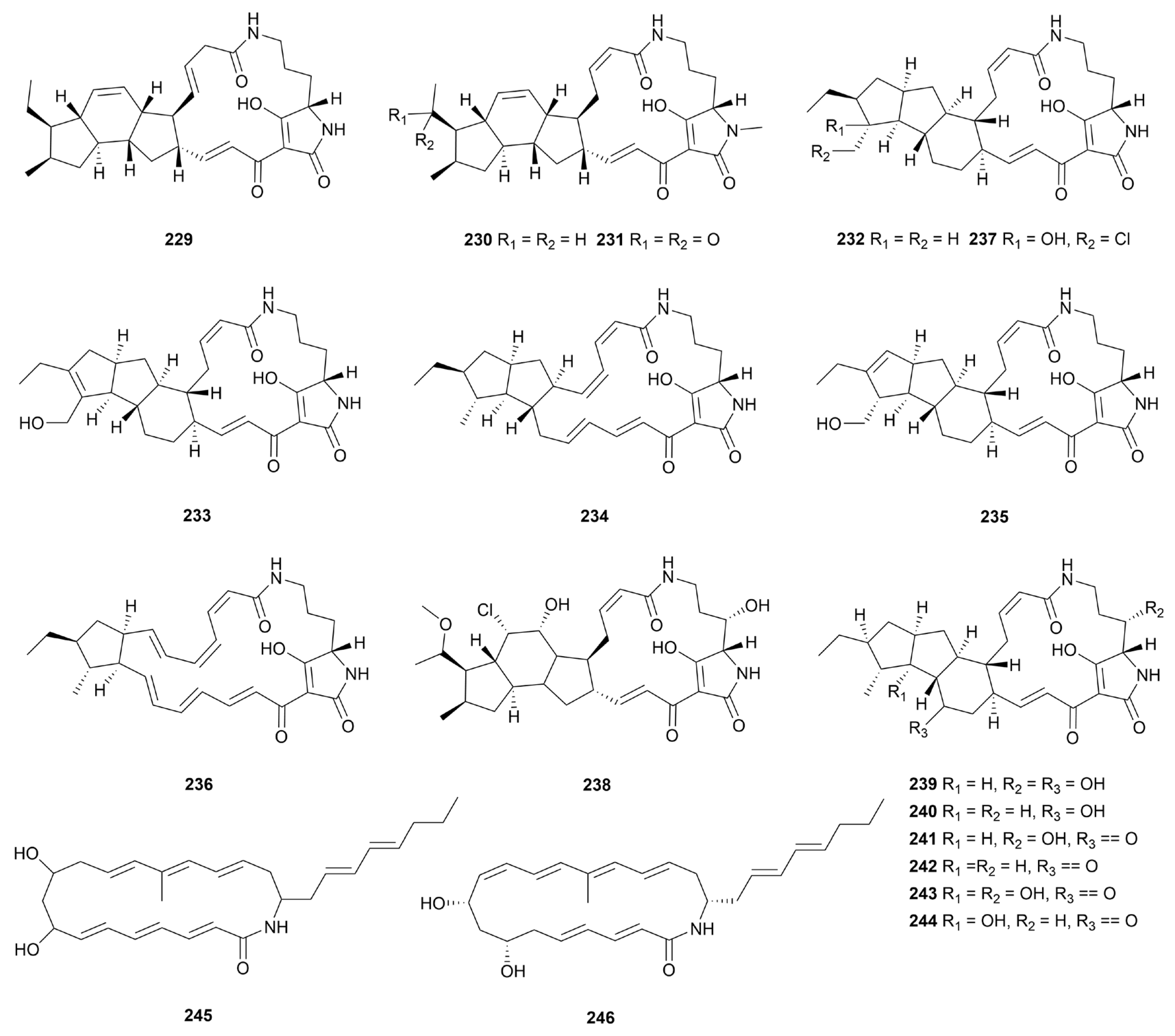
10. Miscellaneous alkaloids
11. Conclusion and future perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Konig, G. M.; Kehraus, S.; Seibert, S. F.; Abdel-Lateff, A.; Muller, D. Natural products from marine organisms and their associated microbes. Chembiochem 2006, 7, 229–38. [Google Scholar] [CrossRef]
- Yang, Z.; He, J.; Wei, X.; Ju, J.; Ma, J. Exploration and genome mining of natural products from marine Streptomyces. Appl. Microbiol. Biotechnol. 2020, 104, 67–76. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, S.; Yang, F.; Dong, S. Marine indole alkaloids—isolation, structure and bioactivities. Mar. Drugs 2021, 19, 658. [Google Scholar] [CrossRef]
- Bérdy, J. Thoughts and facts about antibiotics: where we are now and where we are heading. J. Antibiot. (Tokyo) 2012, 65, 385–395. [Google Scholar] [CrossRef]
- Barka, E. A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J. P.; Klenk, H. P.; Clement, C.; Ouhdouch, Y.; van Wezel, G. P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Ngamcharungchit, C.; Chaimusik, N.; Panbangred, W.; Euanorasetr, J.; Intra, B. Bioactive metabolites from terrestrial and marine actinomycetes. Molecules 2023, 28, 5915. [Google Scholar] [CrossRef]
- Donald, L.; Pipite, A.; Subramani, R.; Owen, J.; Keyzers, R. A.; Taufa, T. Streptomyces: Still the biggest producer of new natural secondary metabolites, a current perspective. Microbiol. Res. 2022, 13, 418–465. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Zhu, J.; Lu, Q.; Cryle, M. J.; Zhang, Y.; Yan, F. Structural diversity, biosynthesis, and biological functions of lipopeptides from Streptomyces. Nat. Prod. Rep. 2023, 40, 557–594. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhou, Z.; Liu, Q.; Wang, X.; Guo, W.; Lin, S. Biosynthetic mechanisms of alkaloids from actinomycetes. Prog. Chem. 2018, 30, 692–702. [Google Scholar]
- Netz, N.; Opatz, T. Marine indole alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef] [PubMed]
- Rosales, P. F.; Bordin, G. S.; Gower, A. E.; Moura, S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia 2020, 143, 104558. [Google Scholar] [CrossRef]
- Holland, D. C.; Carroll, A. R. Marine indole alkaloid diversity and bioactivity. What do we know and what are we missing? Nat. Prod. Rep. 2023, 40, 1595–1607. [Google Scholar] [CrossRef]
- Xu, M.; Peng, R.; Min, Q.; Hui, S.; Chen, X.; Yang, G.; Qin, S. Bisindole natural products: A vital source for the development of new anticancer drugs. Eur. J. Med. Chem. 2022, 243, 114748. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaf, L. M.; Du, Y. L.; Ryan, K. S. Synthetic biology approaches to new bisindoles. Methods Enzymol. 2016, 575, 21–37. [Google Scholar] [PubMed]
- Gani, O. A.; Engh, R. A. Protein kinase inhibition of clinically important staurosporine analogues. Nat. Prod. Rep. 2010, 27, 489–98. [Google Scholar] [CrossRef] [PubMed]
- Park, B. S.; Abdel-Azeem, A. Z.; Al-Sanea, M. M.; Yoo, K. H.; Tae, J. S.; Lee, S. H. Staurosporine analogues from microbial and synthetic sources and their biological activities. Curr. Med. Chem. 2003, 20, 3872–3902. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Saini, A.; Sharma, D. Indispensable role of microbes in anticancer drugs and discovery trends. Appl. Microbiol. Biotechnol. 2022, 106, 4885–4906. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, B.; Liu, H.; Huo, C.; Ding, W. One new indolocarbazole alkaloid from the Streptomyces sp. A22. Nat. Prod. Res. 2018, 32, 2583–2588. [Google Scholar] [CrossRef]
- Wang, J. N.; Zhang, H. J.; Li, J. Q.; Ding, W. J.; Ma, Z. J. Bioactive indolocarbazoles from the marine-derived Streptomyces sp. DT-A61. J. Nat. Prod. 2018, 81, 949-956.
- Onaka, H.; Asamizu, S.; Igarashi, Y.; Yoshida, R.; Furumai, T. Cytochrome P450 homolog is responsible for C-N bond formation between aglycone and deoxysugar in the staurosporine biosynthesis of Streptomyces sp. TP-A0274. Biosci. Biotechnol. Biochem. 2005, 69, 1753–1759. [Google Scholar] [CrossRef]
- Qin, L.; Zhou, B.; Ding, W.; Ma, Z. Bioactive metabolites from marine-derived Streptomyces sp. A68 and its Rifampicin resistant mutant strain R-M1. Phytochem. Lett. 2018, 23, 46–51. [Google Scholar] [CrossRef]
- Zhou, B.; Hu, Z. J.; Zhang, H. J.; Li, J. Q.; Ding, W. J.; Ma, Z. J. Bioactive staurosporine derivatives from the Streptomyces sp. NB-A13. Bioorg. Chem. 2019, 82, 33–40. [CrossRef] [PubMed]
- Zhou, B.; Qin, L.; Ding, W.; Ma, Z. Cytotoxic indolocarbazoles alkaloids from the Streptomyces sp. A65. Tetrahedron 2018, 74, 726–730. [Google Scholar] [CrossRef]
- Wang, C.; Monger, A.; Wang, L.; Fu, P.; Piyachaturawat, P.; Chairoungdua, A.; Zhu, W. Precursor-directed generation of indolocarbazoles with topoisomerase IIα inhibitory activity. Mar. Drugs 2018, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Lin, S.; Wang, Z.; Fu, P.; Wang, C.; Zhu, W. Cytotoxic indolocarbazoles from a marine-derived Streptomyces sp. OUCMDZ-5380. Front. Microbiol. 2022, 13, 957473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, L.; Li, S.; Liu, Z.; Chen, Y.; Zhang, H.; Zhang, G.; Zhang, Q.; Tian, X.; Yuan, C.; Zhang, S.; Zhang, W.; Zhang, C. Indimicins A–E, bisindole alkaloids from the deep-sea-derived Streptomyces sp. SCSIO 03032. J. Nat. Prod. 2014, 77, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, L.; Zhang, L.; Zhang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Zhang, C. Functional characterization of the halogenase SpmH and discovery of new deschloro-tryptophan dimers. Org. Biomol. Chem. 2019, 17, 1053–1057. [Google Scholar] [CrossRef]
- Paulus, C.; Rebets, Y.; Tokovenko, B.; Nadmid, S.; Terekhova, L. P.; Myronovskyi, M.; Zotchev, S. B.; Ruckert, C.; Braig, S.; Zahler, S.; Kalinowski, J.; Luzhetskyy, A. New natural products identified by combined genomics-metabolomics profiling of marine Streptomyces sp. MP131-18. Sci. Rep. 2017, 7, 42382. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Yu, J.; Li, J.; Yuan, J.; Wong, N. K.; Ju, J. Chlorinated bis-indole alkaloids from deep-sea derived Streptomyces sp. SCSIO 11791 with antibacterial and cytotoxic activities. J. Antibiot. (Tokyo) 2020, 73, 542–547. [Google Scholar] [CrossRef]
- Ding, L.; Munch, J.; Goerls, H.; Maier, A.; Fiebig, H. H.; Lin, W. H.; Hertweck, C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg. Med. Chem. Lett. 2010, 20, 6685–7. [Google Scholar] [CrossRef]
- Munda, M.; Nandi, R.; Gavit, V. R.; Kundu, S.; Niyogi, S.; Bisai, A. Total syntheses of naturally occurring antiviral indolosesquiterpene alkaloids, xiamycins C-F via Csp(3)-H functionalization. Chem. Sci. 2022, 13, 11666–11671. [Google Scholar] [CrossRef]
- Kim, S. H.; Ha, T. K.; Oh, W. K.; Shin, J.; Oh, D. C. Antiviral indolosesquiterpenoid xiamycins C-E from a halophilic actinomycete. J. Nat. Prod. 2016, 79, 51–8. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, H. S.; Moon, D. H.; Lee, D.; Kal, Y.; Cha, S.; Lee, S. K.; Yoon, Y. J.; Oh, D.-C. Discovery of new indolosesquiterpenoids bearing a N-O linkage by overexpression of LuxR regulator in a marine bacterium Streptomyces sp. Front. Mar. Sci. 2023, 10, 1140516. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Guo, L.; Zhang, Y.; Feng, L.; Zhou, L.; Yang, S.; Yao, Q.; Pescitelli, G.; Xie, Z. Isolation, structure elucidation and racemization of (+)- and (-)-pratensilins A-C: unprecedented spiro indolinone-naphthofuran alkaloids from a marine Streptomyces sp. Chem. Commun. (Camb.) 2017, 53, 10066–10069. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, L.; Yang, Q.; Xu, B.; Fu, X.; Liu, M.; Li, Z.; Zhang, S.; Xie, Z. Antibacterial and cytotoxic bridged and ring cleavage angucyclinones from a marine Streptomyces sp. Front. Chem. 2020, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.; Qiao, L.; Han, X.; Liu, Y.; Wang, W.; Gu, Q.; Zhu, T.; Li, D. Anthranosides A-C, anthranilate derivatives from a sponge-derived Streptomyces sp. CMN-62. Org. Lett. 2018, 20, 5466–5469. [Google Scholar] [CrossRef]
- Newaz, A. W.; Yong, K.; Lian, X.; Zhang, Z. Streptoindoles A–D, novel antimicrobial indole alkaloids from the marine-associated actinomycete Streptomyces sp. ZZ1118. Tetrahedron 2022, 104, 132598. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, S.; Zhang, M.; Wu, Y. Research progress in biosynthesis of five-membered heterocyclic rings in natural products. J. Microbiol. 2019, 39, 1–12. [Google Scholar]
- Seipp, K.; Geske, L.; Opatz, T. Marine pyrrole alkaloids. Mar. Drugs 2021, 19, 514. [Google Scholar] [CrossRef]
- Asif, M.; Alghamdi, S. An overview on biological importance of pyrrolone and pyrrolidinone derivatives as promising Sscaffolds. Russ. J. Org. Chem. 2021, 57, 1700–1718. [Google Scholar] [CrossRef]
- Mo, X.; Wang, Z.; Wang, B.; Ma, J.; Huang, H.; Tian, X.; Zhang, S.; Zhang, C.; Ju, J. Cloning and characterization of the biosynthetic gene cluster of the bacterial RNA polymerase inhibitor tirandamycin from marine-derived Streptomyces sp. SCSIO1666. Biochem. Biophys. Res. Commun. 2011, 406, 341–7. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Du, L.; Chlipala, G. E.; Lopez, P. C.; Zhang, W.; Sherman, D. H.; Li, S. Identification of an unexpected shunt pathway product provides new insights into tirandamycin biosynthesis. Tetrahedron Lett. 2016, 57, 5919–5923. [Google Scholar] [CrossRef]
- Cong, Z.; Huang, X.; Liu, Y.; Liu, Y.; Wang, P.; Liao, S.; Yang, B.; Zhou, X.; Huang, D.; Wang, J. Cytotoxic anthracycline and antibacterial tirandamycin analogues from a marine-derived Streptomyces sp. SCSIO 41399. J. Antibiot. (Tokyo) 2019, 72, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Hu, J.; Zhang, X.; Xu, W.; Yang, J.; Li, S.; Xu, X. Unique cyclized thiolopyrrolones from the marine-derived Streptomyces sp. BTBU20218885. Mar. Drugs 2022, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.; Hwang, J. Y.; Park, S. C.; Kwon, O. S.; Cho, E.; Lee, J.; Lee, H. S.; Oh, D. C.; Shin, J.; Oh, K. B. Inhibitory effects of nitrogenous metabolites from a marine-derived Streptomyces bacillaris on isocitrate lyase of Candida albicans. Mar. Drugs 2022, 20, 138. [Google Scholar] [CrossRef]
- Kim, M. C.; Lee, J. H.; Shin, B.; Subedi, L.; Cha, J. W.; Park, J. S.; Oh, D. C.; Kim, S. Y.; Kwon, H. C. Salinazinones A and B: Pyrrolidinyl-oxazinones from solar saltern-derived Streptomyces sp. KMF-004. Org. Lett. 2015, 17, 5024-7.
- Zhang, Y. M.; Liu, B. L.; Zheng, X. H.; Huang, X. J.; Li, H. Y.; Zhang, Y.; Zhang, T. T.; Sun, D. Y.; Lin, B. R.; Zhou, G. X. Anandins A and B, two rare steroidal alkaloids from a marine Streptomyces anandii H41-59. Mar. Drugs 2017, 15, 355. [Google Scholar] [CrossRef] [PubMed]
- Lim, H. J.; An, J. S.; Bae, E. S.; Cho, E.; Hwang, S.; Nam, S. J.; Oh, K. B.; Lee, S. K.; Oh, D. C. Ligiamycins A and B, decalin-amino-maleimides from the co-culture of Streptomyces sp. and Achromobacter sp. Isolated from the marine wharf roach, Ligia exotica. Mar. Drugs 2022, 20, 83.
- Varvounis, G. An update on the synthesis of pyrrolo[1,4]benzodiazepines. Molecules 2016, 21, 154. [Google Scholar] [CrossRef]
- Pavlikova, M.; Kamenik, Z.; Janata, J.; Kadlcik, S.; Kuzma, M.; Najmanova, L. Novel pathway of 3-hydroxyanthranilic acid formation in limazepine biosynthesis reveals evolutionary relation between phenazines and pyrrolobenzodiazepines. Sci. Rep. 2018, 8, 7810. [Google Scholar] [CrossRef]
- Han, Y.; Li, Y. Y.; Shen, Y.; Li, J.; Li, W. J.; Shen, Y. M. Oxoprothracarcin, a novel pyrrolo[1,4]benzodiazepine antibiotic from marine Streptomyces sp. M10946. Drug Discov. Ther. 2013, 7, 243–7. [Google Scholar] [CrossRef]
- Çetinel Aksoy, S.; Küçüksolak, M.; Uze, A.; Bedir, E. Benzodiazepine derivatives from marine-derived Streptomyces cacaoi 14CM034. Rec. Nat. Prod. 2021, 15, 602–607. [Google Scholar] [CrossRef]
- Kang, Q.; Shen, Y.; Bai, L. Biosynthesis of 3,5-AHBA-derived natural products. Nat. Prod. Rep. 2012, 29, 243–63. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Li, Y.; Deng, J.; Lu, C.; Shen, Y.; Shen, Y. Biosynthesis of hygrocins, antitumor naphthoquinone ansamycins produced by Streptomyces sp. LZ35. Chembiochem 2014, 15, 94–102. [Google Scholar] [CrossRef]
- Cai, P.; Kong, F.; Ruppen, M. E.; Glasier, G. Hygrocins A and B, naphthoquinone macrolides from Streptomyces hygroscopicus. J. Nat. Prod. 2005, 68, 1736–1742. [Google Scholar] [CrossRef]
- Lu, C.; Li, Y.; Deng, J.; Li, S.; Shen, Y.; Wang, H.; Shen, Y. Hygrocins C-G, cytotoxic naphthoquinone ansamycins from gdmAI-disrupted Streptomyces sp. LZ35. J. Nat. Prod. 2013, 76, 2175-9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, C.; Ou, J.; Deng, J.; Shen, Y. Overexpression of hgc1 increases the production and diversity of hygrocins in Streptomyces sp. LZ35. RSC Adv. 2015, 5, 83843–83846. [Google Scholar] [CrossRef]
- Yi, W.; Newaz, A. W.; Yong, K.; Ma, M.; Lian, X. Y.; Zhang, Z. New Hygrocins K-U and streptophenylpropanamide A and bioactive compounds from the marine-associated Streptomyces sp. ZZ1956. Antibiotics-Basel 2022, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Le, T. C.; Yang, I.; Yoon, Y. J.; Nam, S. J.; Fenical, W. Ansalactams B-D illustrate further biosynthetic plasticity within the ansamycin pathway. Org. Lett. 2016, 18, 2256–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. M.; Kong, F. D.; Xie, Q. Y.; Ma, Q. Y.; Hu, Z.; Zhao, Y. X.; Luo, D. Q. Divergolides T-W with apoptosis-inducing activity from the mangrove-derived actinomycete Streptomyces sp. KFD18. Mar. Drugs 2019, 17, 219. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mico, X.; Jensen, P. R.; Fenical, W.; Hughes, C. C. Chlorizidine, a cytotoxic 5H-pyrrolo[2,1-a]isoindol-5-one-containing alkaloid from a marine Streptomyces sp. Org. Lett. 2013, 15, 988–991. [Google Scholar] [CrossRef]
- Heo, C. S.; Kang, J. S.; Kwon, J. H.; Anh, C. V.; Shin, H. J. Pyrrole-containing alkaloids from a marine-derived actinobacterium Streptomyces zhaozhouensis and their antimicrobial and cytotoxic activities. Mar. Drugs 2023, 21, 167. [Google Scholar] [CrossRef]
- Lian, X. Y.; Zhang, Z. Indanomycin-related antibiotics from marine Streptomyces antibioticus PTZ0016. Nat. Prod. Res. 2013, 27, 2161–7. [Google Scholar] [CrossRef]
- Zhou, B.; Huang, Y.; Zhang, H.; Li, J.; Ding, W. Nitricquinomycins A-C, uncommon naphthopyrrolediones from the Streptomyces sp. ZS-A45. Tetrahedron 2019, 75, 3958–3961. [Google Scholar] [CrossRef]
- Ko, K.; Kim, S. H.; Park, S.; Han, H. S.; Lee, J. K.; Cha, J. W.; Hwang, S.; Choi, K. Y.; Song, Y. J.; Nam, S. J.; Shin, J.; Nam, S. I.; Kwon, H. C.; Park, J. S.; Oh, D. C. Discovery and photoisomerization of new pyrrolosesquiterpenoids glaciapyrroles D and E, from deep-sea sediment Streptomyces sp. Mar. Drugs 2022, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Pepinosa, N. Y.; Cardona-Trujillo, M. C.; Garzon-Castano, S. C.; Veloza, L. A.; Sepulveda-Arias, J. C. Antiproliferative activity of thiazole and oxazole derivatives: A systematic review of in vitro and in vivo studies. Biomed. Pharmacother. 2021, 138, 111495. [Google Scholar] [CrossRef]
- Davyt, D.; Serra, G. Thiazole and oxazole alkaloids: isolation and synthesis. Mar. Drugs 2010, 8, 2755–2780. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, L.; Deng, Y.; Wang, X.; Cui, W.; He, S. Feature-based molecular networking-guided discovery of siderophores from a marine mesophotic zone Axinellida sponge-associated actinomycete Streptomyces diastaticus NBU2966. Phytochemistry 2022, 196, 113078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tao, F.; Cui, T.; Luo, C.; Zhou, Z.; Huang, Y.; Tan, L.; Peng, W.; Wu, C. Sources, transformations, syntheses, and bioactivities of monoterpene pyridine alkaloids and cyclopenta[c]pyridine derivatives. Molecules 2022, 27, 7187. [Google Scholar] [CrossRef]
- Zhou, X.; Fenical, W. The unique chemistry and biology of the piericidins. J. Antibiot. (Tokyo) 2016, 69, 582–93. [Google Scholar] [CrossRef]
- Azad, S. M.; Jin, Y.; Ser, H.-L.; Goh, B.-H.; Thawai, L.-H. I. C.; He, Y.-W. Biological insights into the piericidin family of microbial metabolites. J. Appl. Microbiol. 2022, 132, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, Z.; Zhang, Z.; Zhang, X.; Zhu, T.; Gu, Q.; Li, W.; Che, Q.; Li, D. Geranylpyrrol A and piericidin F from Streptomyces sp. CHQ-64 ΔrdmF. J. Nat. Prod. 2017, 80, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, Z.; Li, K.; Fang, W.; Tian, Y.; Luo, X.; Chen, Y.; Zhan, Z.; Zhang, T.; Liao, S.; Liu, S.; Liu, Y.; Fenical, W.; Tang, L. Exploring the natural piericidins as anti-renal cell carcinoma agents targeting peroxiredoxin 1. J. Med. Chem. 2019, 62, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, Q.; Jiang, X.; Ma, L.; Long, T.; Cheng, Z.; Zhang, C.; Zhu, Y. New piericidin derivatives from the marine-derived Streptomyces sp. SCSIO 40063 with cytotoxic activity. Nat. Prod. Res. 2022, 36, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M. P.; Chhabra, S. R.; Diggle, S. P.; Williams, P.; Camara, M. Quinolones: from antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–74. [Google Scholar] [CrossRef] [PubMed]
- Senerovic, L.; Opsenica, D.; Moric, I.; Aleksic, I.; Spasic, M.; Vasiljevic, B. Quinolines and quinolones as antibacterial, antifungal, anti-virulence, antiviral and anti-parasitic agents. Adv. Exp. Med. Biol. 2020, 1282, 37–69. [Google Scholar]
- Hassan, H. M.; Boonlarppradab, C.; Fenical, W. Actinoquinolines A and B, anti-inflammatory quinoline alkaloids from a marine-derived Streptomyces sp., strain CNP975. J. Antibiot. (Tokyo) 2016, 511-4–69. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lopez, F. J.; Alcalde, E.; Sarmiento-Vizcaino, A.; Diaz, C.; Cautain, B.; Garcia, L. A.; Blanco, G.; Reyes, F. New 3-hydroxyquinaldic acid derivatives from cultures of the marine derived actinomycete Streptomyces cyaneofuscatus M-157. Mar. Drugs 2018, 16, 371. [Google Scholar] [CrossRef]
- Mullowney, M. W.; E, O. h.; Shaikh, A.; Wei, X.; Tanouye, U.; Santarsiero, B. D.; Burdette, J. E.; Murphy, B. T. Diazaquinomycins E-G, novel diaza-anthracene analogs from a marine-derived Streptomyces sp. Mar. Drugs 2014, 12, 3574–3586. [Google Scholar] [CrossRef]
- Cheng, C.; Othman, E. M.; Reimer, A.; Grüne, M.; Kozjak-Pavlovic, V.; Stopper, H.; Hentschel, U.; Abdelmohsen, U. R. Ageloline A, new antioxidant and antichlamydial quinolone from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett. 2016, 57, 2786–2789. [Google Scholar] [CrossRef]
- Shaaban, M.; Shaaban, K. A.; Kelter, G.; Fiebig, H. H.; Laatsch, H. Mansouramycins E–G, cytotoxic isoquinolinequinones from marine Streptomycetes. Mar. Drugs 2021, 19, 715. [Google Scholar] [CrossRef]
- Yang, C. L.; Wang, Y. S.; Liu, C. L.; Zeng, Y. J.; Cheng, P.; Jiao, R. H.; Bao, S. X.; Huang, H. Q.; Tan, R. X.; Ge, H. M. Strepchazolins A and B: Two new alkaloids from a marine Streptomyces chartreusis NA02069. Mar. Drugs 2017, 15, 244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yi, W.; Ge, H.; Zhang, Z.; Wu, B. Bioactive streptoglutarimides A-J from the marine-derived Streptomyces sp. ZZ741. J. Nat. Prod. 2019, 82, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Dai, W.; Huang, H.; Liu, S.-L.; Liu, J.; Huang, L.-J.; Huang, X.-H.; Zeng, J.-L.; Gan, Z.-W.; Zhang, Z.-Y.; Lan, J.-X. Pharmacological activity and mechanism of pyrazines. Eur. J. Med. Chem. 2023, 258, 115544. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, M.; Zitko, J. Pyrazine derivatives: a patent review (June 2012-present). Expert Opin. Ther. Pat. 2015, 25, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, D. S.; Yoon, C. S.; Jung, Y. T.; Yoon, J. H.; Kim, Y. C.; Oh, H. Marine-derived secondary metabolite, griseusrazin A, suppresses inflammation through heme oxygenase-1 induction in activated RAW264.7 macrophages. J. Nat. Prod. 2016, 79, 1105–11. [Google Scholar] [CrossRef]
- Shaala, L. A.; Youssef, D. T.; Badr, J. M.; Harakeh, S. M. Bioactive 2(1H)-pyrazinones and diketopiperazine alkaloids from a tunicate-derived actinomycete Streptomyces sp. Molecules 2016, 21, 1116. [Google Scholar] [CrossRef]
- Chen, M.; Chai, W.; Zhu, R.; Song, T.; Zhang, Z.; Lian, X. Streptopyrazinones A-D, rare metabolites from marine-derived Streptomyces sp. ZZ446. Tetrahedron 2018, 74, 2100–2106. [Google Scholar] [CrossRef]
- Sano, S.; Nakao, M. Chemistry of 2,5-diketopiperazine and its bis-lactim ether: a brief review. Heterocycles 2015, 91, 1349. [Google Scholar] [CrossRef]
- Borthwick, A. D. 2,5-Diketopiperazines: synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–716. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y. X.; Huang, J. F.; Li, X. M.; Kang, Q. J.; Pan, Y. T. Three new 2,5-diketopiperazines from the fish intestinal Streptomyces sp. MNU FJ-36. Nat. Prod. Res. 2016, 30, 1771-5. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Qin, L.; Lian, X. Y.; Zhang, Z. New antifungal metabolites from the Mariana Trench sediment-associated actinomycete Streptomyces sp. SY196. Mar. Drugs 2020, 18, 385. [Google Scholar] [CrossRef] [PubMed]
- Buedenbender, L.; Grkovic, T.; Duffy, S.; Kurtböke, D. I.; Avery, V. M.; Carroll, A. R. Naseseazine C, a new anti-plasmodial dimeric diketopiperazine from a marine sediment derived Streptomyces sp. Tetrahedron Lett. 2016, 57, 5893–5895. [Google Scholar] [CrossRef]
- Shaala, L. A.; Youssef, D. T. A.; Badr, J. M.; Harakeh, S. M.; Genta-Jouve, G. Bioactive diketopiperazines and nucleoside derivatives from a sponge-derived Streptomyces species. Mar. Drugs 2019, 17, 584. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D.; Chen, M.; Zhang, Z.; Lian, X. Y. A rare diketopiperazine glycoside from marine-sourced Streptomyces sp. ZZ446. Nat. Prod. Res. 2020, 34, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Olyaei, A.; Sadeghpour, M. A review on lawsone-based benzo[a]phenazin-5-ol: synthetic approaches and reactions. RSC Adv. 2022, 12, 13837–13895. [Google Scholar] [CrossRef]
- Remali, J.; Mohamad Zin, N.; Ng, C. L.; Wan M. Aizat, W. M. A.; Tiong, J. J. L. Fenazin sebagai potensi antibiotik baru daripada Streptomyces kebangsaanensis. Sains Malays. 2019, 48, 543–553. [CrossRef]
- Ren, J.; Liu, D.; Tian, L.; Wei, Y.; Proksch, P.; Zeng, J.; Lin, W. Venezuelines A-G, new phenoxazine-based alkaloids and aminophenols from Streptomyces venezuelae and the regulation of gene target Nur77. Bioorg. Med. Chem. Lett. 2013, 23, 301–4. [Google Scholar] [CrossRef]
- Abdelfattah, M. S. A new bioactive aminophenoxazinone alkaloid from a marine-derived actinomycete. Nat. Prod. Res. 2013, 27, 2126–31. [CrossRef]
- Dharmaraj, S.; Sumantha, A. Bioactive potential of Streptomyces associated with marine sponges. World J. Microbiol. Biotechnol. 2009, 25, 1971–1979. [Google Scholar] [CrossRef]
- Kunz, A. L.; Labes, A.; Wiese, J.; Bruhn, T.; Bringmann, G.; Imhoff, J. F. Nature's lab for derivatization: new and revised structures of a variety of streptophenazines produced by a sponge-derived Streptomyces strain. Mar. Drugs 2014, 12, 1699–1714. [Google Scholar] [CrossRef]
- Cheng, C.; Othman, E. M.; Fekete, A.; Krischke, M. Strepoxazine A, a new cytotoxic phenoxazin from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett. 2016, 57, 4196–4199. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wang, M.; Tan, Y.; Hu, X.; He, H.; Xiao, C.; You, X.; Wang, Y.; Gan, M. Neo-actinomycins A and B, natural actinomycins bearing the 5H-oxazolo[4,5-b]phenoxazine chromophore, from the marine-derived Streptomyces sp. IMB094. Sci. Rep. 2017, 7, 3591. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, G.; Guo, L.; Wang, J.; Jing, C.; Liu, B.; Zhao, F.; Zhang, S.; Xie, Z. Asp-containing actinomycin and tetracyclic chromophoric analogues from the Streptomyces sp. strain S22. Org. Biomol. Chem. 2023, 21, 1737–1743. [Google Scholar] [CrossRef]
- Zhang, J.; Morris-Natschke, S. L.; Ma, D.; Shang, X. F.; Yang, C. J.; Liu, Y.-Q.; Lee, K.-H. Biologically active indolizidine alkaloids. Med. Res. Rev. 2020, 41, 928–960. [Google Scholar] [CrossRef]
- Michael, J. P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2002, 19, 719–41. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Li, J.-Q.; Zhang, H.-J.; Ding, W.-J.; Ma, Z.-J. Cyclizidine-type alkaloids from Streptomyces sp. HNA39. J. Nat. Prod. 2018, 81, 394–399. [Google Scholar] [CrossRef]
- Cheng, X. W.; Li, J. Q.; Jiang, Y. J.; Liu, H. Z.; Huo, C. A new indolizinium alkaloid from marine-derived Streptomyces sp. HNA39. J. Asian Nat. Prod. Res. 2021, 23, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhong, Y.; Shen, L.; Wu, X.; Ye, Y.; Chen, C.-T. A.; Wu, B. Stress-driven discovery of natural products from extreme marine environment- Kueishantao hydrothermal vent, a case study of Metal Switch Valve. Curr. Org. Chem. 2014, 18, 925–934. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, C.; Auckloo, B. N.; Chen, X.; Chen, C. A.; Wang, K.; Wu, X.; Ye, Y.; Wu, B. Stress-driven discovery of a cryptic antibiotic produced by Streptomyces sp. WU20 from Kueishantao hydrothermal vent with an integrated metabolomics strategy. Appl. Microbiol. Biotechnol. 2017, 101, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wu, L.; Urwald, C.; Mugat, M.; Wang, S.; Kyeremeh, K.; Philips, C.; Law, S.; Zhou, Y.; Deng, H. Genomic scanning enabling discovery of a new antibacterial bicyclic carbamate-containing alkaloid. Synth. Syst. Biotechnol. 2021, 6, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, Y.; Huang, Y. Discovery and biosynthesis of bacterial pyrrolizidine alkaloids. Microbiology China 2021, 48, 2437–2453. [Google Scholar]
- Huang, S.; Tabudravu, J.; Elsayed, S. S.; Travert, J.; Peace, D.; Tong, M. H.; Kyeremeh, K.; Kelly, S. M.; Trembleau, L.; Ebel, R.; Jaspars, M.; Yu, Y.; Deng, H. Discovery of a single monooxygenase that catalyzes carbamate formation and ring contraction in the biosynthesis of the legonmycins. Angew. Chem. Int. Ed. Engl. 2015, 54, 12697–701. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T. S.; Woolery, M.; Kauffman, C. A.; Jensen, P. R.; Fenical, W. Bohemamines from a marine-derived Streptomyces sp. J. Nat. Prod. 2006, 69, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; MacMillan, J. B. Spithioneines A and B, two new bohemamine derivatives possessing ergothioneine moiety from a marine-derived Streptomyces spinoverrucosus. Org. Lett. 2015, 17, 3046–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; La, S.; MacMillan, J. B. 1,3-oxazin-6-one derivatives and bohemamine-type pyrrolizidine alkaloids from a marine-derived Streptomyces spinoverrucosus. J. Nat. Prod. 2016, 79, 455–62. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Legako, A.; La, S.; MacMillan, J. B. Discovery, Characterization, and Analogue Synthesis of Bohemamine Dimers Generated by Non-enzymatic Biosynthesis. Chemistry 2016, 22, 3491–3495. [Google Scholar] [CrossRef]
- Fu, P.; Johnson, M.; Chen, H.; Posner, B. A.; MacMillan, J. B. Carpatamides A-C, cytotoxic arylamine derivatives from a marine-derived Streptomyces sp. J. Nat. Prod. 2014, 77, 1245–8. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, L.; Ito, T.; Qu, X.; Asakawa, Y.; Awakawa, T.; Abe, I.; Liu, W. Biosynthetic pathway for high structural diversity of a common dilactone core in antimycin production. Org. Lett. 2012, 14, 4142–4145. [Google Scholar] [CrossRef]
- Zhang, W.; Che, Q.; Tan, H.; Qi, X.; Li, J.; Li, D.; Gu, Q.; Zhu, T.; Liu, M. Marine Streptomyces sp. derived antimycin analogues suppress HeLa cells via depletion HPV E6/E7 mediated by ROS-dependent ubiquitin-proteasome system. Sci. Rep. 2017, 7, 42180. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, S. W.; Chen, F.; Zheng, X. H.; Shen, H. F.; Lin, B. R.; Zhou, G. X. Neoantimycins A and B, two unusual benzamido nine-membered dilactones from marine-derived Streptomyces antibioticus H12-15. Molecules 2017, 22, 557. [Google Scholar] [CrossRef]
- Bertasso, M.; Holzenkämpfer, M.; Zeeck, A.; Dall'Antonia, F. Bagremycin A and B, novel antibiotics from Streptomyces sp. Tü 4128. J. Antibiot. (Tokyo) 2001, 54, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chai, W.; Wang, W.; Song, T.; Lian, X. Y.; Zhang, Z. Cytotoxic bagremycins from mangrove-derived Streptomyces sp. Q22. J. Nat. Prod. 2017, 80, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shu, C.; Lian, X.; Zhang, Z. New antibacterial bagremycins F and G from the marine-derived Streptomyces sp. ZZ745. Mar. Drugs 2018, 16, 330. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Abdel-Razek, A. S.; Frese, M.; Wibberg, D.; El-Haddad, A. F.; Ibrahim, T. M. A.; Kalinowski, J.; Sewald, N.; Shaaban, M. N-Acetylborrelidin B: a new bioactive metabolite from Streptomyces mutabilis sp. MII. Z. Naturforsch. C 2018, 73, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Miyanaga, A.; Kudo, F.; Eguchi, T. Mechanisms of beta-amino acid incorporation in polyketide macrolactam biosynthesis. Curr. Opin. Chem. Biol. 2016, 35, 58–64. [Google Scholar] [CrossRef]
- Hugel, H. M.; Smith, A. T.; Rizzacasa, M. A. Macrolactam analogues of macrolide natural products. Org. Biomol. Chem. 2016, 14, 11301–11316. [Google Scholar] [CrossRef]
- Antosch, J.; Schaefers, F.; Gulder, T. A. Heterologous reconstitution of ikarugamycin biosynthesis in E. coli. Angew. Chem. Int. Ed. Engl. 2014, 53, 3011–4. [Google Scholar] [CrossRef]
- Lacret, R.; Oves-Costales, D.; Gómez, C.; Díaz, C.; de la Cruz, M.; Pérez-Victoria, I.; Vicente, F.; Genilloud, O.; Reyes, F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar. Drugs 2014, 13, 128–140. [Google Scholar] [CrossRef]
- Saha, S.; Zhang, W.; Zhang, G.; Zhu, Y.; Chen, Y.; Liu, W.; Yuan, C.; Zhang, Q.; Zhang, H.; Zhang, L.; Zhang, W.; Zhang, C. Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams. Chem. Sci. 2017, 8, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Fukudaa, T.; Takahashia, M.; Kasaib, H.; Nagaia, K.; Tomodaa, H. Chlokamycin, a new chloride from the marine-derived Streptomyces sp. MA2-12. Nat. Prod. Commun. 2017, 12, 1223–1226. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, W.; Jin, H.; Zhang, Q.; Chen, Y.; Jiang, X.; Zhang, G.; Zhang, L.; Zhang, W.; She, Z.; Zhang, C. Genome mining of marine-derived Streptomyces sp. SCSIO 40010 leads to cytotoxic new polycyclic tetramate macrolactams. Mar. Drugs 2019, 17, 663. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Fujiwara, T.; Kagaya, N.; Shin-Ya, K. JBIR-150, a novel 20-membered polyene macrolactam from marine-derived Streptomyces sp. OPMA00071. J. Antibiot. (Tokyo) 2018, 71, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y. H.; Im, J. H.; Kang, I.; Kim, E.; Jang, S. C.; Cho, E.; Shin, D.; Hwang, S.; Du, Y. E.; Huynh, T. H.; Ko, K.; Ko, Y. J.; Nam, S. J.; Awakawa, T.; Lee, J.; Hong, S.; Abe, I.; Moore, B. S.; Fenical, W.; Yoon, Y. J.; Cho, J. C.; Lee, S. K.; Oh, K. B.; Oh, D. C. Genomic and spectroscopic signature-based discovery of natural macrolactams. J. Am. Chem. Soc. 2023, 145, 1886–1896. [Google Scholar] [CrossRef]
- Shen, J.; Wang, J.; Chen, H.; Wang, Y.; Zhu, W.; Fu, P. Cyclamenols E and F, two diastereoisomeric bicyclic macrolactams with a cyclopentane moiety from an Antarctic Streptomyces species. Org. Chem. Front. 2020, 7, 310–317. [Google Scholar] [CrossRef]
- Yang, F.; Sang, M.; Lu, J. R.; Zhao, H. M.; Zou, Y.; Wu, W.; Yu, Y.; Liu, Y. W.; Ma, W.; Zhang, Y.; Zhang, W.; Lin, H. W. Somalactams A-D: Anti-inflammatory macrolide lactams with unique ring systems from an Arctic actinomycete strain. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218085. [Google Scholar] [CrossRef]
- Nakayama, K.; Yamaguchi, T.; Doi, T.; Usuki, Y. Synergistic combination of direct plasma membrane damage and oxidative stress as a cause of antifungal activity of polyol macrolide niphimycin. J. Biosci. Bioeng. 2002, 94, 207–211. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, M.; Wu, C.; Tan, Y.; Li, J.; Hao, X.; Duan, Y.; Guan, Y.; Shang, X.; Wang, Y.; Xiao, C.; Gan, M. Identification and proposed relative and absolute configurations of niphimycins C-E from the marine-derived Streptomyces sp. IMB7-145 by genomic analysis. J. Nat. Prod. 2018, 81, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, Y.; Cai, B.; Zhou, D.; Qi, D.; Zhang, M.; Zhao, Y.; Li, K.; Wedge, D. E.; Pan, Z.; Xie, J.; Wang, W. Discovery of niphimycin C from Streptomyces yongxingensis sp. nov. as a promising agrochemical fungicide for controlling banana fusarium wilt by destroying the mitochondrial structure and function. J. Agric. Food Chem. 2022, 70, 12784–12795. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, W.; Huang, S.; Chu, J.; Liang, Y.; Tao, Z.; Wang, G.; Zhuang, J.; Zhang, Z.; Zhou, X.; Pan, X. Taxonomy and anticancer potential of Streptomyces niphimycinicus sp. nov. against nasopharyngeal carcinoma cells. Appl. Microbiol. Biotechnol. 2023, 107, 6325–6338. [Google Scholar] [CrossRef]
- Kim, D.; Lee, E. J.; Lee, J.; Leutou, A. S.; Shin, Y. H.; Choi, B.; Hwang, J. S.; Hahn, D.; Choi, H.; Chin, J.; Cho, S. J.; Hong, Y. D.; Ko, J.; Seong, C. N.; Maloney, K. N.; Oh, D. C.; Yang, I.; Hwang, H.; Nam, S. J. Antartin, a cytotoxic zizaane-type sesquiterpenoid from a Streptomyces sp. isolated from an Antarctic marine sediment. Mar. Drugs 2018, 16, 130. [Google Scholar] [CrossRef]
- Liu, W.; Ma, L.; Zhang, L.; Chen, Y.; Zhang, Q.; Zhang, H.; Zhang, W.; Zhang, C.; Zhang, W. Two new phenylhydrazone derivatives from the Pearl River Estuary sediment-derived Streptomyces sp. SCSIO 40020. Mar. Drugs 2022, 20, 449. [Google Scholar] [CrossRef] [PubMed]
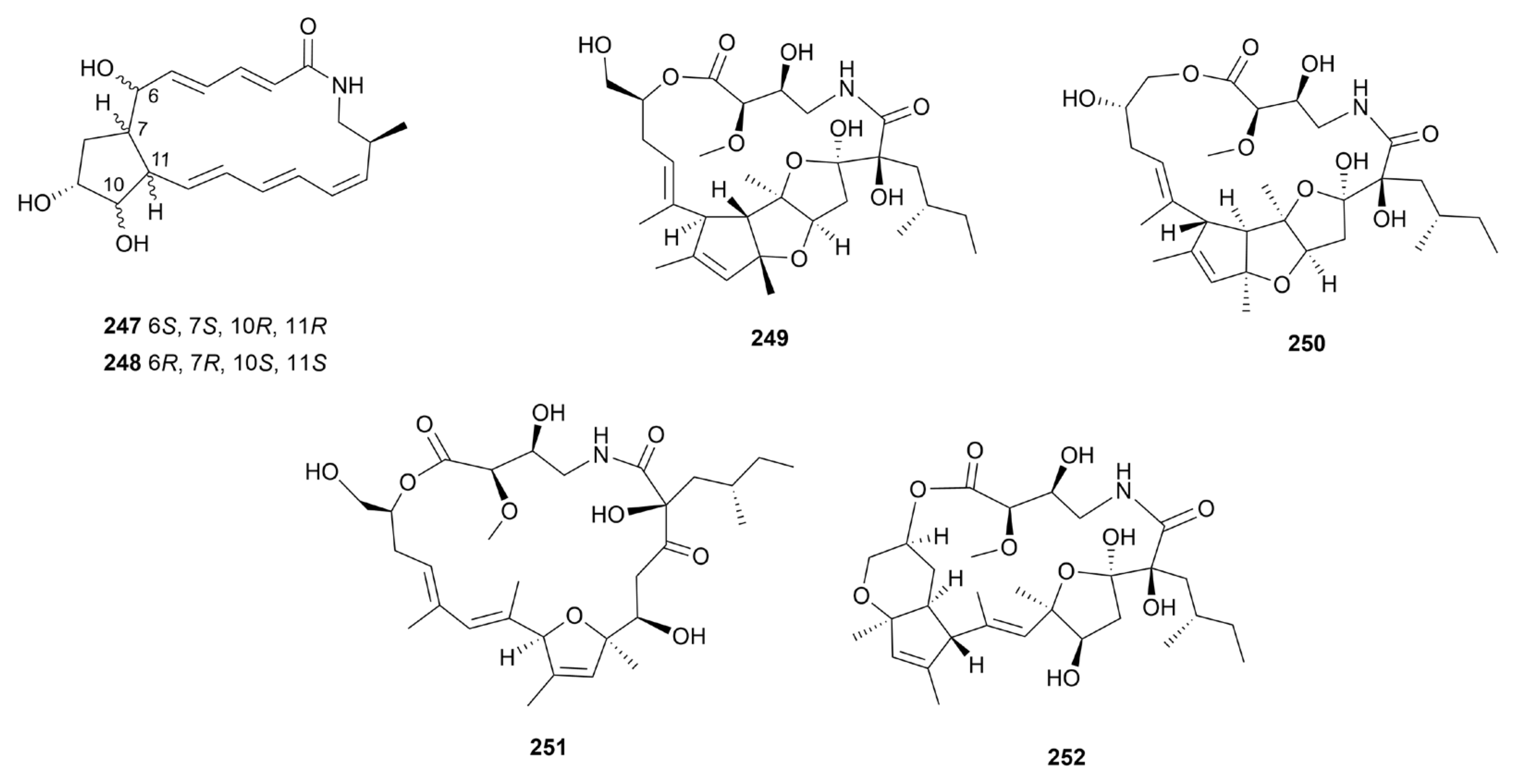
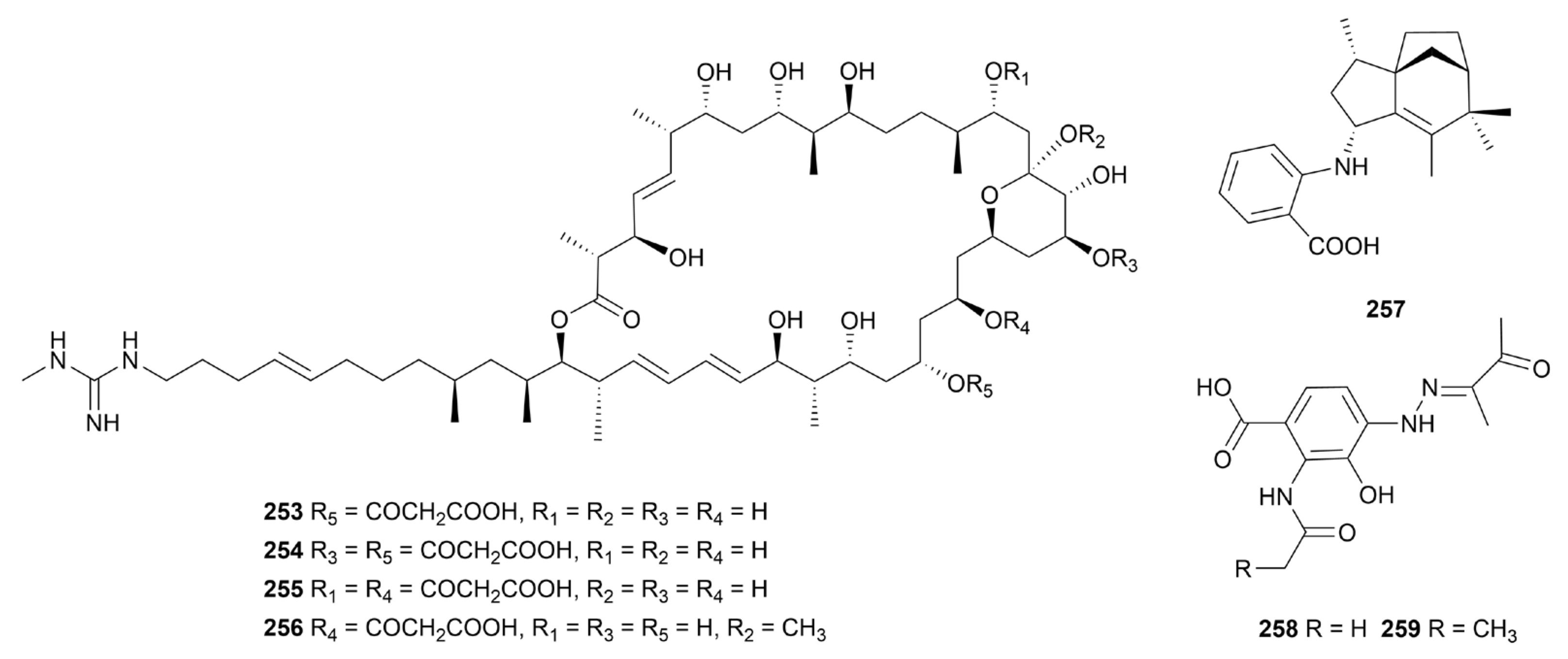
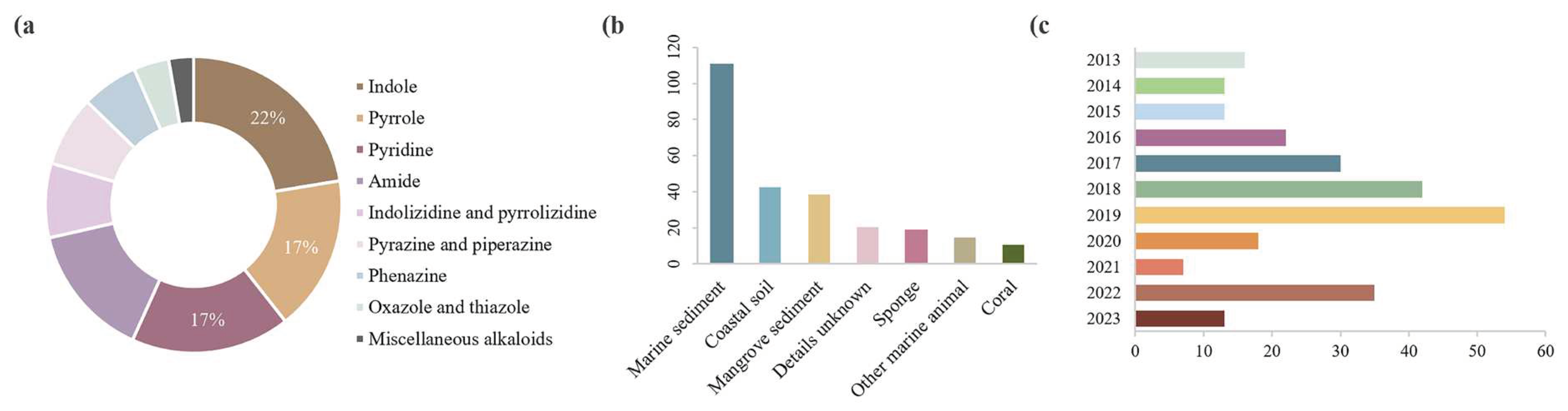
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




