1. Introduction
Watermelon(Citrullus lanatus)belongs to the Cucurbitaceaefamily is an herbaceous annual plant indigenous to Africa. The fruit of watermelon is rich in water and essential nutrients that are highly beneficial for human health [
1]. In 2021, the Food and Agriculture Organization (FAO) reported that the worldwide cultivation area of watermelon has surpassed 3 million hectares, representing approximately 7% of the planet’s total vegetable production area [
2]. Triploid seedless watermelon is a prime instance of artificially induced polyploid crops. Compared to diploid watermelon, triploid seedless watermelon offers numerous benefits, such as high yield, increased stress resistance, and seedlessness. Its cultivation area has been expanding annually and accounts for approximately 20% of the total watermelon area [
3].
Polyploidy stands out as a defining characteristic of genome evolution in higher plants, playing a crucial role in the evolutionary trajectory of organisms. Furthermore, homopolyploidy presents an ideal system for exploring ploidy variations [
4]. Homopolyploidy represents an inheritable system characterized by multiple gene repetitions, enhancing gene function conservation, markedly reducing the incidence of mutations and expression of latent genes, and establishing a stable ecological niche for competitive survival under challenging natural conditions [
5]. Doubling the genome of polyploid chromosomes can have multi-level effects on the morphological, developmental, and physiological functions of plants, in contrast to diploids [
6,
7]. Commercial watermelon varieties encompass seedless triploids achieved through the crossbreeding of femaletetraploidy with a male diploid. Tetraploid watermelon serves as a progenitor for triploid watermelon breeding and is typically obtained through diploid chromosome doubling [
8]. Typically, tetraploid watermelons are distinguished by their larger leaves, thicker vines and rinds, enlarged pistil and stamen floral organs, and bigger seeds [
9].
Fruit texture, is a sensory attribute perceived through vision, hearing, and touch [
10] and plays a crucial role in determining the sensory quality of watermelon. Fruit flesh texture serves as a vital indicator for assessing the fruit’s commercial viability [
11]. The characteristics of watermelon flesh texture influence the taste, flavor, and shelf-life of ripe watermelon [
12]. Alterations in fruit texture represent a complex physiological and biochemical process regulated by genetic background, cell wall structure and composition, cell wall degrading enzyme activities, cell size, hormones, and other factors [
13,
14]. Fruit softening typically involves water loss, reducedturgor, and degradation of pectin, cellulose, and hemicellulose [
14,
15,
16,
17,
18]. Variations in the flesh firmness of watermelon primarily resulted from changes in the structural components of the flesh cell wall. Cellulose, pectin, and hemicellulose constitute the major components of the cell wall, significantly influencing the flesh firmness and sensory quality of watermelon [
19,
20,
21,
22,
23,
24]. The size and arrangement of fruit cells also impacted fruit texture. Throughout the transition from fruit set to maturity, the flesh cells progressively enlarge, leading to a shift in flesh texture from firm to soft [
25]. The texture of watermelon fruits of different ploidy levels significantly influencesthe fruit taste. However, there are only limited studies on the factors affecting the watermelon fruit texture in different watermelon ploidy levels.
Previously, watermelon flesh texture evaluation primarily relied on sensory evaluation methods or the utilization of fruit hardness testers to measure fruit hardness.The sensory evaluation method encompasses various factors, including flesh water content, fiber content, flesh firmness, and flesh hardness. However, this method suffers from significant subjective bias, limited reproducibility, and the inability to quantitatively measure other sensory parameters that impact flesh texture, such as crispness and elasticity. In recent years, researchers, aimed to enhance the objectivity of fruit texture evaluation parameters. They have achieved this by employing instruments like texture meters, which find extensive application in assessing the quality of fruits and vegetables. Notably, these devices have previously been utilized in evaluating apple [
26], watermelon [
21], melon [
27], pear [
28], peach [
29], tomato [
30], and other fruit crops .
This study focused on examining watermelon fruits of varying ploidy levels within the same genotype at different developmental stages. Weuseda texture analyzer to assess the changes in flesh texture parameters during fruit developmental stages and conducted a comparative analysis of the polysaccharide content of flesh cell walls and cell microstructure. Current study aimed to identify the primary factors contributing to the textural differences in watermelon fruits with different ploidy levels. Additionally, our objective was to establish a foundation for exploring the intrinsic regulators and molecular mechanisms underlying the texture changes in polyploid watermelon fruits, as well as to offer insights for selecting superior polyploid watermelon varieties in future breeding programs.
2. Materials and Methods
2.1. Plant materials and growth conditions
For this study, the materials used were ’Yi Xuan,’ a watermelon inbred line obtained from the Polyploidy Watermelon Genetic Breeding Project at Zhengzhou Fruit Research Institute, Chinese Academy of Agricultural Sciences. The diploid inbred line (2n=2X=22, 2X) was induced to autotetraploidy (4n=4X=44, 4X) using 0.1% colchicine during the cotyledon spreading stage. The success of tetraploid mutagenesis was confirmed through physiological phenotype and chromosome observation. The plants were then self-crossed for 7 generations to restore fertility. It was then crossbred with its diploid counterpart to generate autotriploid watermelon (3n=3X=33, 3X). The appearance of diploid, triploid, and tetraploid plants was similar, differing only in ploidy level. ’Yi Xuan’ belongs to the cultivar type and is commonly utilized as the maternal variety for Asian watermelon cultivars, the fruit is globular with green rind, pink flesh, weighs 3.5kg, has TSS content of 11.5%, and rind thickness of 0.7cm.
In spring 2020, seeds were sown on seed trays in a greenhouse located in Xinxiang City, Henan Province, China (35.16° N; 113.80° E). The seedlings were transplanted to a greenhouse and maintained plant spacing of 50 cm and a row spacing of 2.5 m, while retaining three vines with regular agronomic practices. One fruit from the third female flower of the primary vine was preserved and manually pollinated at anthesis, with the pollination date being recorded. Watermelon fruits were harvested at 8, 16, 24, and 32 days after pollination (DAP) for physiological and biochemical index and cell microstructure analysis. Three fruits with normal growth potential and similar fruiting nodes were selected for each measurement.
Figure 1.
The Fruits of different ploidy Watermelon.
Figure 1.
The Fruits of different ploidy Watermelon.
2.2. Physiological and biochemical indicators and measurement methods
2.2.1. Evaluation of fruit weight, rind hardness and flesh TSS content
All phenotypic traits were measured in triplicate following the protocol recommended by Saminathan [
8]. Fruit weight was determined using electronic scales, while rind hardness was assessed at the equatorial line of the fruit using an FT011 fruit hardness tester (Italy). Additionally, the total soluble solids (TSS) content was measured from the fruit center’s using an Atago PAL-2 digital refractometer (Japan). Each measurement was performed three times.
2.2.2. Flesh texture detection
Texture profile analysis (TPA) was conducted using the TA.XT.Plus Physical Property Analyzer (Stable Micro System, UK). Watermelon fruits were longitudinally divided into two halves , and various sections of the flesh were selected and cubed into 1cm×1 cm×1 cm pieces, as illustrated in
Figure 2. These samples were positioned on a TPA test plate, and texture analysis was performed using a P/100 probe (φ100 mm platen). The pre-test speed, test speed, and upward speed after the test were set at 2.0 mm/s, 1.0 mm/s, and 1.0 mm/s, respectively. The compression deformation rate of the flesh was 70%, with a compression stopping time of 5.0 s and a trigger force of 10.0 g. The resulting texture characteristic curve provided information on hardness, fracturability, springiness, adhesiveness, chewiness, and resilience.
2.2.3. Determination of cell wall polysaccharide content
Crude fiber content was determined based on the method described by Oyeyinka [
31] in which acid-base digestion was employed to remove other substances successively with sulphuric acid and sodium hydroxide. The method proposed by Gao [
21] was followed for measuring the pectin, cellulose, and hemicellulose contents. Pectin content was determined by the carbazole method, hemicellulose content by the anthrone method, and cellulose content by gravimetric analysis. Each treatment was repeated thrice to ensure accuracy.
2.2.4. Observation of flesh cell microstructure
The flesh tissue from the fruit center was chosen and cut into 0.5 cm×0.5 cm×0.5 cm pieces. These pieces were fixed with FAA fixative (70% ethanol), dehydrated through an alcohol gradient, embedded in paraffin wax after being dipped in wax and stained with toluidine blue. The cellular structure of the tissue was observed after sectioning. The target area of the tissue was selected for 100×imaging using an Eclipse Ci-L photomicrographic microscope. Image-Pro Plus 6.0 analysis software was used to assess the total cell area in each image, along with the total number of cells, the average area of individual cells, and cell density using millimeters as the standard unit of measurement.
2.3. Statistical analysis of data
The experimental data were analyzed using SPSS 27 software. To evaluate the difference between means, analysis of variance (ANOVA) and correlation analysis were conducted. Specifically, a single-factor analysis of variance was used to assess the difference between the means, and post-hoc testing with Bonferroni’s test was performed to determine significant differences between groups. The results were presented as mean±standard deviation (SD), and a significance level of P < 0.05 was applied.
3. Results
3.1. Differences in fruit weight, rind hardness and TSS content among different ploidy of watermelon
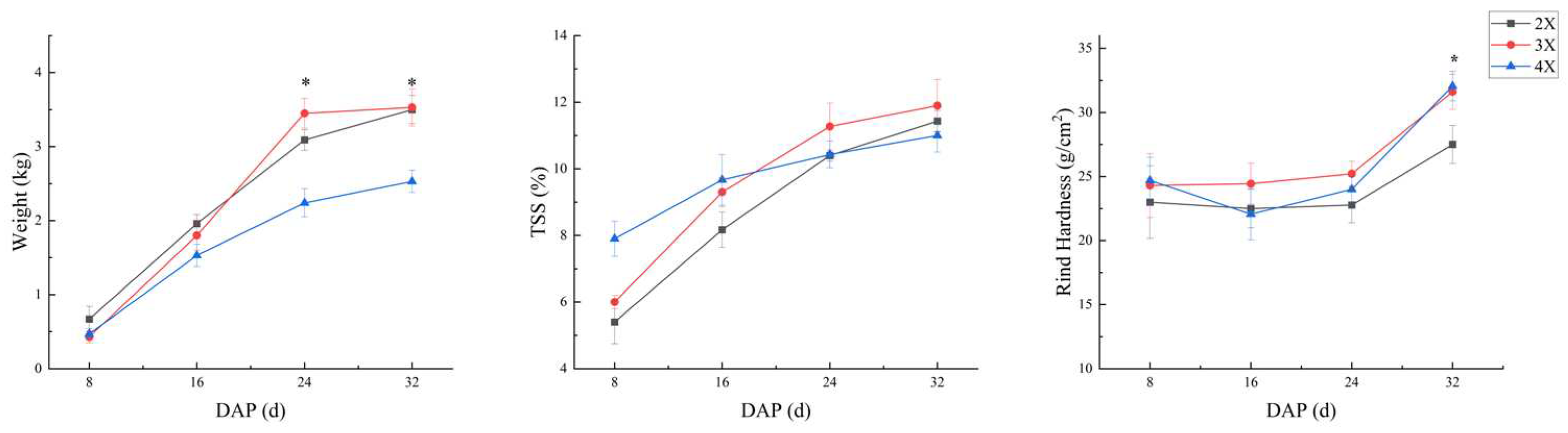
Fruit weight and TSS content responded to watermelon fruit development. As depicted in
Figure 3, all watermelon fruits exhibited an increase in both weight and TSS content as the days of development progressed. However, fruit weight plateaued after 24 days, showing a significant difference between 24 days and 32 days for 2X (3.09± 0.24 kg; 3.5±0.69 kg) and 3X (3.45±0.2 kg; 3.53±0.25 kg) compared to 4X (2.24±0.29 kg; 2.53±0.51 kg). The TSS content peaked at 32 days after pollination, with no statistically significant difference among 3X (11.9±0.73%), 2X (11.43±0.32%), and 4X (11.0±0.5%). Additionally, there were no significant differences in TSS content among different ploidy at other developmental stages. Fruit rind hardness was associated with fruit maturity, as indicated in
Figure 3. During the early stages of fruit development, the changes in rind hardness were relatively gradual, and the differences among ploidy were not significant. However, upon reaching the 32d maturity stage, the rind hardness increases rapidly, with the rind hardness of 3X and 4X being significantly higher than that of 2X.
3.2. Variations in the flesh texture of watermelon fruits with different ploidy
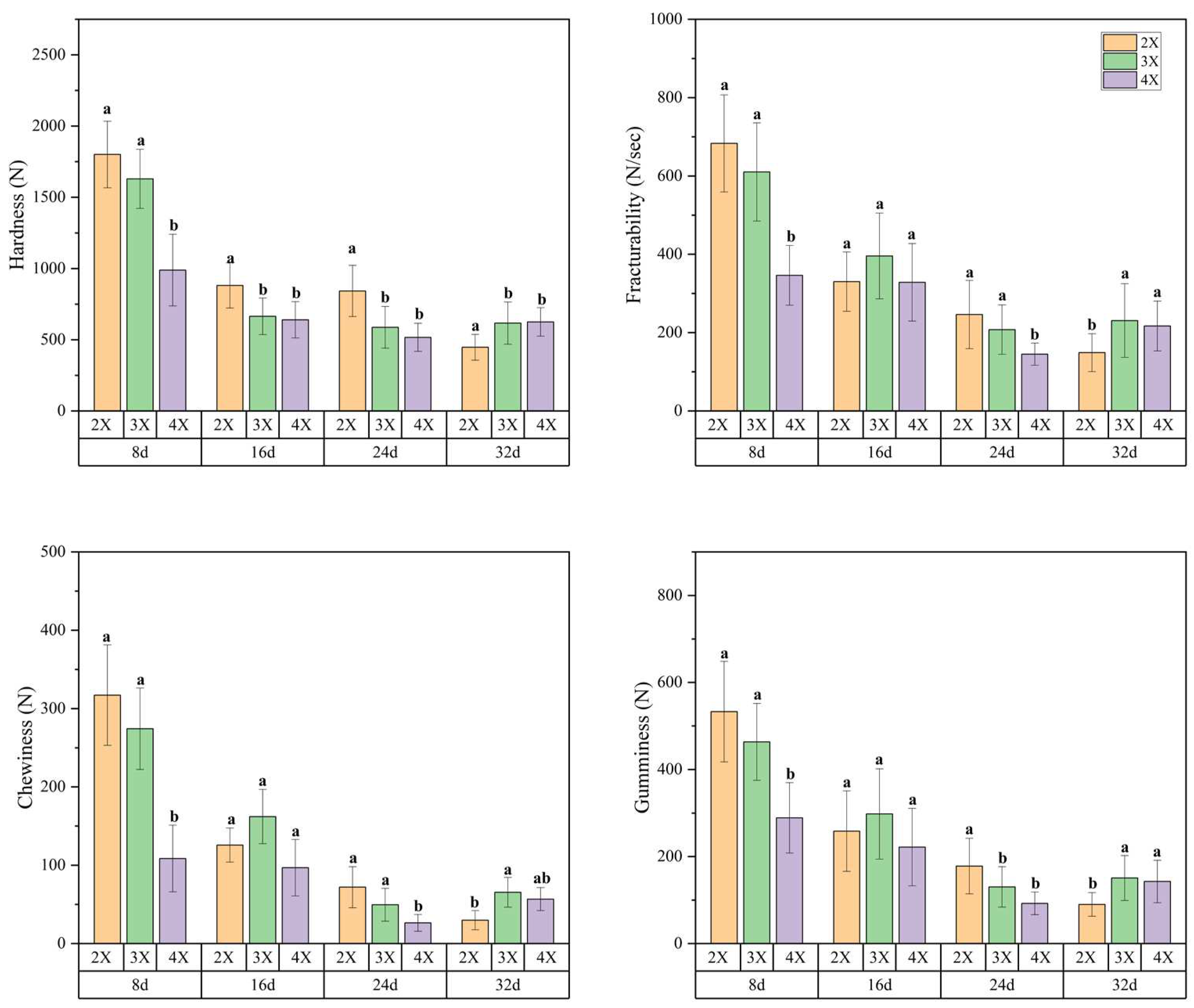
The indices of flesh hardness, fracturability, gumminess, and chewiness obtained from the TPA test provided a comprehensive characterization of watermelon flesh texture.
Figure 4 illustrates that texture indices such as hardness, fracturability, gumminess, and chewiness exhibit a significant decreasing trend as fruit development time increases. Additionally, the changes in these indices for 4X watermelon demonstrate a comparatively flatter trend compared to 2X and 3X.
Hardness: At 8 days after pollination, fruit flesh hardness was significantly higher in 2X (1800±234N) and 3X (1629±207N) than in 4X (989±251N). Similarly, at 16 and 24 days of fruit development, fruit flesh hardness was significantly higher in 2X (881±159N and 842±179N) than in 3X (664±128N and 587±146N) and 4X (639±127N and 517±98.6N). At 32 days, the fruit flesh hardness of 2X (447±90.6N) decreased faster and significantly lower compared to both 3X (616±148N) and 4X (624±99.6N).
Fracturability: The trend of flesh fracturability across different ploidy levels followed a similar pattern to that of hardness. Flesh fracturability was significantly higher in 2X (683±124N/sec) and 3X (610±125N/sec) compared to 4X (346±76N/sec) at 8 days of fruit development. At 16 days, there were no significant differences in flesh fracturability among the ploidy levels: 2X (330±75.6N/sec), 3X (395±109N/sec), and 4X (328±99N/sec). However, at 24 days, 2X (246±87N/sec) exhibited a significantly higher level of fracturability compared to both 3X (207±63N/sec) and 4X (145±28N/sec). At 32 days, there were no significant differences in flesh fracturability among the ploidy levels: 2X (330±75.6N/sec), 3X (395±109N/sec), and 4X (328±99N/sec). Interestingly, the decline in 2X flesh fracturability (148±48N/sec) was significantly faster and lower than that of 3X (231±94N/sec) and 4X (216±64N/sec).
Chewiness: The trend of watermelon flesh chewiness exhibited a similar pattern to that of hardness and fracturability across different ploidy levels. At 8 days of fruit development, watermelon flesh chewiness was significantly higher in 2X (317±64N) and 3X (274±52N) compared to 4X (108±42N). There were no significant differences in fruit flesh chewiness among the ploidy levels at 16 days: 2X (125±22N), 3X (162±35N), and 4X (97±36N). However, at 24 days, 2X (72±26N) exhibited a significantly higher level of chewiness compared to both 3X (49±21N) and 4X (26±10N). At 32 days, the chewiness of 2X (30±12N) declined faster and was significantly lower than 3X (65±19N) and 4X (57±15N).
Gumminess: The trend of fruit flesh gumminess across different ploidy levels exhibited a similar pattern to that of hardness, fracturability, and chewiness indexes. At 8 days of fruit development, both 2X (533±115N) and 3X (463±88N) were significantly higher than 4X (288±81N). At 16 days, there were no significant differences in flesh chewiness among the various ploidy levels of watermelon: 2X (258±92N), 3X (297±103N), and 4X (221±89N). However, at 24 days, 2X (178±64N) exhibited a significantly higher level of flesh gumminess compared to both 3X (130±46N) and 4X (92±26N). At 32 days, the flesh gumminess of 2X (30±12N) declined rapidly and was significantly lower than 3X (65±19N) and 4X (57±15N).
Our study also found that the differences in the cohesion, springiness, and reversibility indices of watermelon fruits of different ploidy at all developmental periods were insignificant.
3.3. Variations in the polysaccharide content within the cell walls of watermelon flesh across different ploidy levels
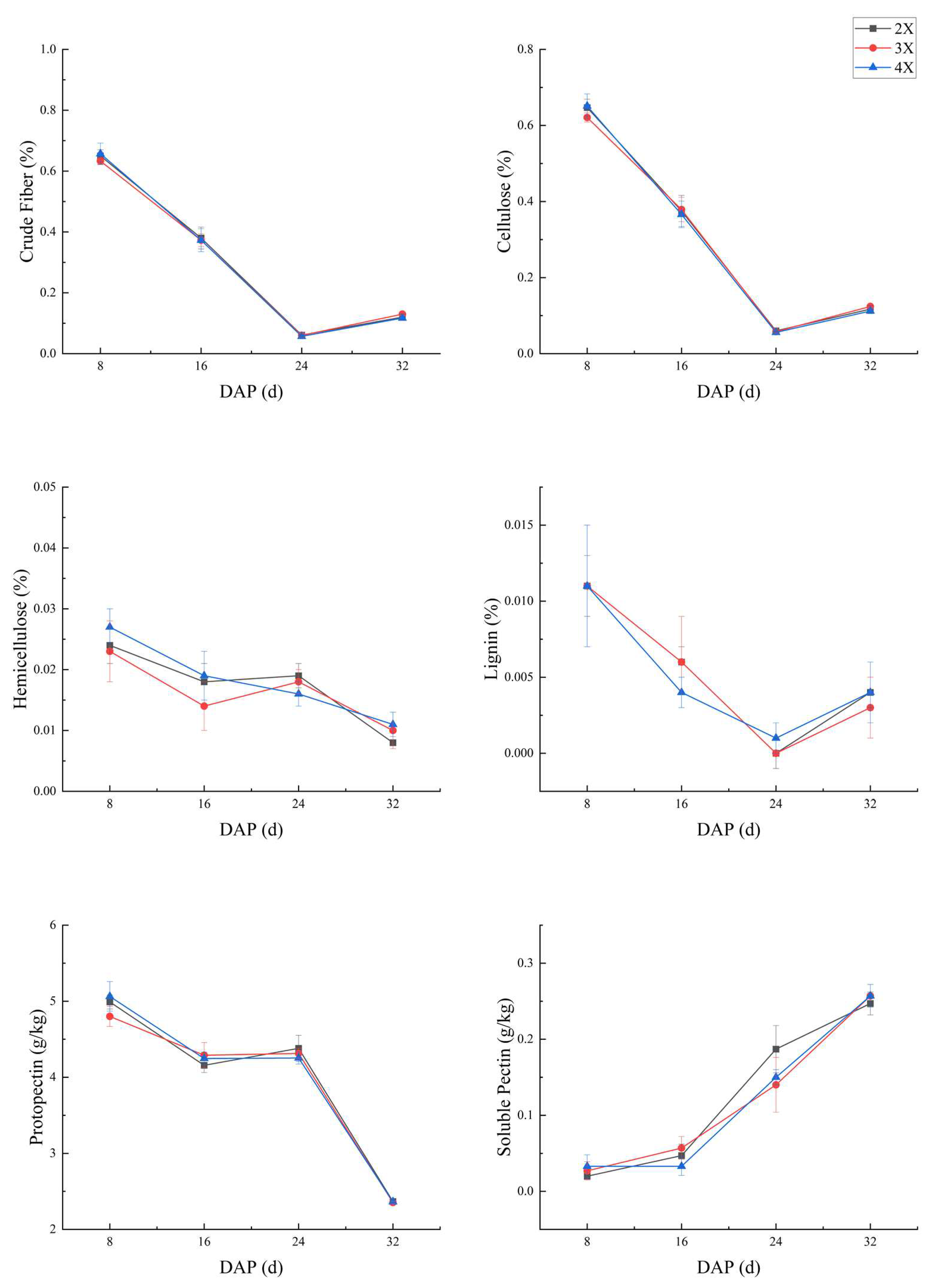
Polysaccharide fractions within the flesh’s cell wall, including crude fiber, cellulose, hemicellulose, lignin, protopectin, and soluble pectin, play a crucial role in determining fruit texture. As demonstrated in
Figure 5, crude fiber, cellulose, hemicellulose, and lignin levels in watermelon flesh gradually declined during fruit development, plateauing after 24 days. Furthermore, protopectin content remained stable during the initial three stages, followed by a significant decrease after 24 days. On the contrary, the soluble pectin content steadily increased throughout fruit development. Our analysis of the polysaccharide substances in the flesh cell wall of watermelon fruit with varying ploidy levels revealed no significant differences across all developmental periods.
3.4. Differences in the cellular structure of watermelon flesh of different ploid
The dimensions and organization of fruit flesh cells can provide insights into variations in fruit texture. We conducted observations and measurements on flesh cells of watermelons with different ploidy levels across distinct developmental stages. As illustrated in
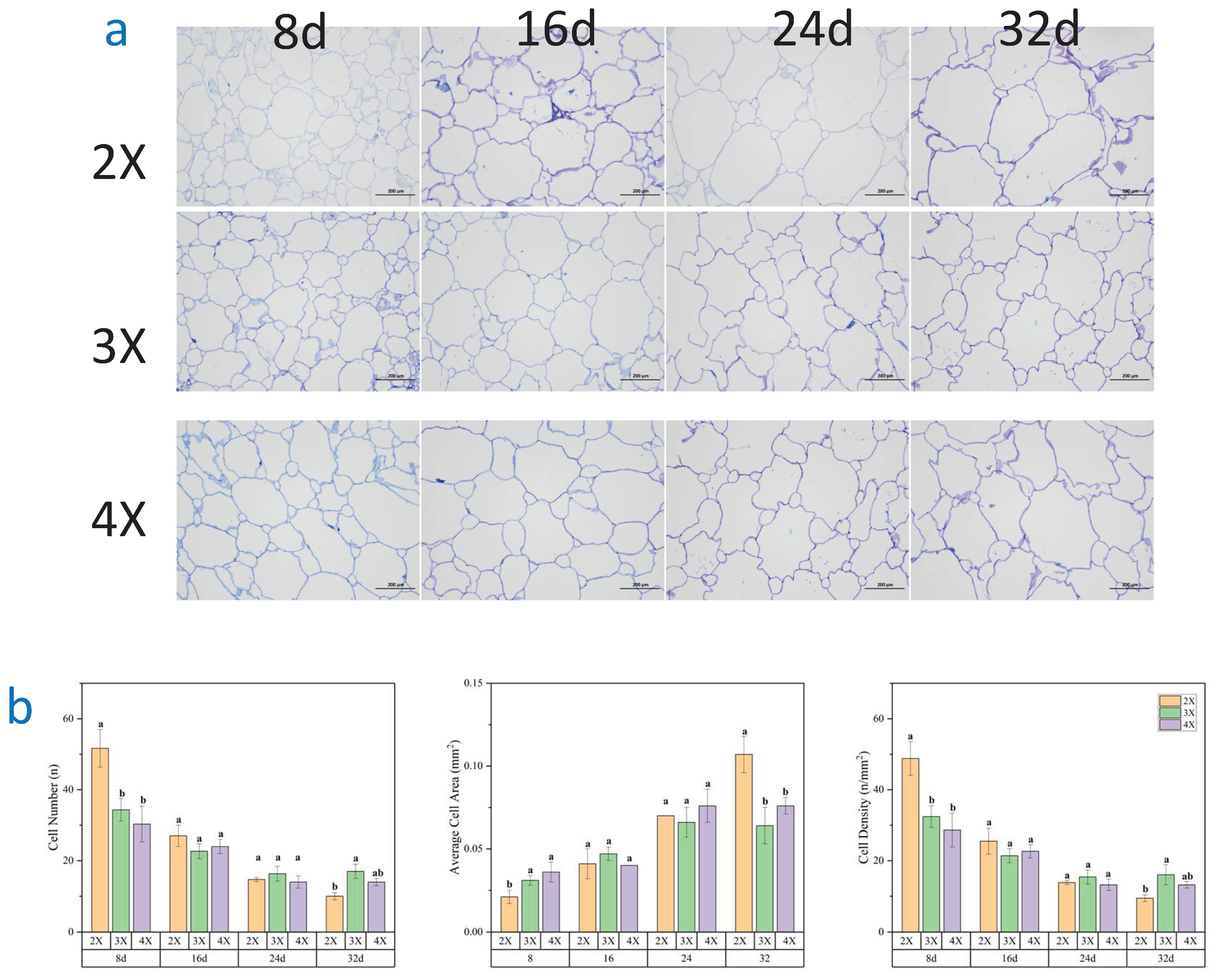
Figure 6a, the cellular microstructure of the fruit flesh reveals that during the initial 8d of fruit development, the flesh cells exhibited tight arrangement and regular shapes. However, as the fruit continued to develop, the flesh cells progressively enlarged, resulting in loose arrangement, irregular shapes, and increased intercellular gaps.
Cell size measurements indicated that the area of watermelon flesh cells increased gradually as they developed, whereas the number and density of flesh cells per unit area decreased. At the 8d, the number (51.7±5.29) and density (48.8±4.8) of 2X cells were significantly higher than those of 3X (34.3±3.2; 32.4±3) and 4X (30.3±5; 28.6±4.8) cells per unit area of the same view field (100X). No significant difference in flesh cell size was observed among ploidy levels at 16d and 24d. Similarly, the difference in flesh cell size between ploidy levels remained insignificant at 16d and 24d. At 34d, during fruit development and maturation, the number and density of flesh cells with 3X and 4X were significantly higher than those with 2X. The average cell area change trend was opposite to that of cell number and density (
Figure 6b).
3.5. Correlation analysis of flesh texture with cell microstructure and cell wall polysaccharide content in watermelon of different ploidy level
By conducting a thorough correlation analysis of data concerning the flesh texture of watermelon with varying ploidy levels at different developmental stages in
Table 1. We discovered highly significant correlations between the parameters of watermelon flesh texture (hardness, fracturability, gelatinousness, chewiness) the cell size and cell wall polysaccharide contents.
4. Discussion
4.1. Differences in fruit weight and TSS content among different ploidy of watermelon
Our findings indicate that as watermelon fruits developed, there was a corresponding increase in weight and TSS content, aligning with prior research [
32]. The weight of 2X and 3X fruits was notably more significant than that of 4X fruits at 24d and 32d. Our results was consistent with the findings of Henderson and Angela, who also noted that autotetraploid fruits tended to be significantly lighter than triploid fruits [
33,
34].
According to Henderson, tetraploid fruits generally possess higher TSS content than triploid fruits [
34], while Perkins-Veazie observed that triploid varieties typically have slightly higher TSS content than diploid varieties [
35]. Our results demonstrate that the TSS content was higher in 3X (10.67%) than in 2X and 4X fruits (9.7% and 9.2%, respectively). However, we found no significant differences in TSS content among different ploidy levels during different developmental stages. Similarly, Davis noticed that while tetraploid varieties exhibited higher TSS content than their diploid counterparts in six groups of experimental materials, only one group displayed a significant difference in TSS. Therefore, differences in TSS among ploidy levels were likely influenced by genotype, and watermelon TSS was less influenced by ploidy level. These studies reflect the intensive breeding pressure on triploid varieties to breed for high sugar content [
33]. To our knowledge, there were limited studies on the differences in watermelon rind hardness across ploidy levels. In our study, we found significant differences in watermelon rind hardness across ploidy at maturity, with polyploid rind hardness being notably higher than the 2X level. This difference in rind hardness may result in better storage tolerance in polyploidy than in diploid watermelon, as previous research has confirmed a correlation between rind hardness and storage tolerance [
36]
4.2. Differences in the texture among different ploidy of watermelon
The TPA testing method can objectively quantify various textural parameters in a single experimental process, greatly enhancing the data authenticity, reliability, and repeatability [
21,
37]. In this study, we discovered that the hardness of flesh firmness decreased significantly as watermelon fruit developed, which was consistent with the findings of Anees and Sun [
22,
33]. Previous studies on watermelon flesh texture have mainly focused on flesh hardness, with relatively few investigations into other texture indices. To date, no research has shown differences in the flesh texture of watermelon with different ploidy levels. Our study found that the fracturability, chewiness, and gelatinousness of the flesh also decreased gradually, consistent with the trend of hardness with fruit development. During flesh ripening, the hardness, crispness, chewiness, and adhesiveness of 2X flesh were significantly lower than those of 3X and 4X, aligning with the phenotypic differences in the oral feedback we usually observe in watermelon: polyploid watermelon is less delicate than diploid watermelon, and it requires more force to chew thandiploid watermelon . Therefore, watermelon flesh hardness, crunchiness, chewiness, and adhesive texture parameters can fully mimic the sensation of the human oral cavity, differentiating differences in flesh texture across different ploidy. Moreover, we observed an interesting phenomenon: the development rate of 2X flesh was higher than that of polyploid, and the texture parameters of 2X flesh were greater than those of polyploid at the early stage of fruit development. However, the texture of 2X flesh was lower than that of polyploid at the fruit ripening stage, possibly owing to ploidy’s impact on the fruit’s development rate.
4.3. Differences in the content of cell wall polysaccharides in the fruit flesh among different ploidy of watermelon
Fruit softening is closely associated with cell wall remodeling, particularly the degradation of pectin and other cell wall polysaccharides. Throughout fruit development, the levels of crude fiber, cellulose, hemicellulose, lignin, and protopectin progressively decrease in flesh cells while the level of soluble pectin content gradually increases. These findings are consistent with previous research [
19,
21,
23]. While there have been limited investigations on the quantity of cell wall polysaccharides in watermelon flesh with varying ploidy, our analysis did not reveal any significant differences among the different ploidy levels. Therefore, we hypothesize that changes in watermelon ploidy levels do not significantly impact the cell wall polysaccharide content in the fruit flesh.
4.4. Differences in cell structure among different ploidy of watermelon
In this study, we discovered that the development of watermelon flesh was accompanied by an increase in cell size, a gradual reduction in cell density, a loosening of cell arrangement, and an enlargement of cell gaps. Similar results were also observed in other fruits, such as in apple [
38], melon [
18], and kiwifruit [
39]. Our findings indicate that the average cell area of diploid watermelon flesh was significantly lower than that of polyploid at the early stage of fruit development (8 d), and the number of cells and density of flesh cells in 2X was significantly higher than 3X and 4X. This suggests that differences in the size and arrangement of flesh cells may be the primary contributing factor to the variations in watermelon flesh texture among different ploidy levels. Therefore, it is evident that the differences in cell size and arrangement play a crucial role in determining the taste differences in watermelon flesh with varying ploidy.
We also observed a highly significant correlation between flesh texture parameters, cell wall polysaccharide contents, and cell structure. This observation suggests that the feasibility of evaluating differences in watermelon flesh texture based on cell microstructure and cell wall polysaccharide content. While the content of cell wall polysaccharides did not exhibit significant differences across ploidy, the developmental pattern and trend of texture were entirely consistent. Hence, we can infer that the structural characteristics of flesh cells, determine the variations in watermelon texture among different ploidy levels to some extent.
5. Conclusions
In this study, we utilized homozygous diploid and its corresponding autotriploid and autotetraploid lines to investigate the developmental disparities in watermelon fruit texture. The findings demonstrated that fruit weight, rind hardness, flesh texture, and flesh cellular microstructure were all influenced by the fruit’s ploidy. Moreover, the developmental trends of flesh texture, cell wall polysaccharide content, and cell development were consistently aligned. Through comprehensive analysis, it can be concluded that the differences in fruit texture among different ploidy levels of watermelon are attributed to cell size and arrangement.
Author Contributions
Conceptualization, Xuqiang Lu, Wenge Liu and Hongju Zhu; methodology, Hongju Zhu and Xuqiang Lu; software, Xuqiang Lu; validation, Hongju Zhu; formal analysis, Xiaowen Luo; investigation, Dongdong Yang, Jiwen Zhang and Luming Yuan; resources, Nan He; data curation, Xuqiang Lu; writing—original draft preparation, Xuqiang Lu.; writing—review and editing, Wenge Liu, Hongju Zhu and Muhammad Anees; visualization, Xuqiang Lu; supervision, Wenge Liu and Hongju Zhu; project administration, Wenge Liu and Hongju Zhu; funding acquisition, Wenge Liu and Xuqiang Lu. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2021-ZFRI), the China Agriculture Research System of MOF and MARA, (CARS-25-03) and the Major Science and Technology Projects of Henan Province (221100110400).
Data Availability Statement
All relevant data can be found within this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nature Genetics 2019, 51, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- FAO. Available online: https://faostat.fao.org/ (accessed on 12 June 2023).
- Bharadwaj, D.N. Polyploidy in Crop Improvement and Evolution. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer India: New Delhi, 2015; pp. 619–638. [Google Scholar]
- Soltis, P.S.; Marchant, D.B.; Van de Peer, Y.; Soltis, D.E. Polyploidy and genome evolution in plants. Curr Opin Genet Dev 2015, 35, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Madlung, A. Polyploidy and its effect on evolutionary success: old questions revisited with new tools. Heredity 2013, 110, 99–104. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2015, 243, 281–296. [Google Scholar] [CrossRef]
- Cohen, H.; Fait, A.; Tel-Zur, N. Morphological, cytological and metabolic consequences of autopolyploidization in Hylocereus (Cactaceae) species. BMC Plant Biology 2013, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Saminathan, T.; Nimmakayala, P.; Manohar, S.; Malkaram, S.; Almeida, A.; Cantrell, R.; Tomason, Y.; Abburi, L.; Rahman, M.A.; Vajja, V.G.; et al. Differential gene expression and alternative splicing between diploid and tetraploid watermelon. Journal of Experimental Botany 2015, 66, 1369–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bao, Y.N.; Xie, Z.L.; Huang, X.; Sun, Y.H.; Feng, G.; Zeng, H.X.; Ren, J.; Li, Y.H.; Xiong, J.S.; et al. Efficient Characterization of Tetraploid Watermelon. Plants-Basel 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Contador, L.; Shinya, P.; Infante, R. Texture phenotyping in fresh fleshy fruit. Scientia Horticulturae 2015, 193, 40–46. [Google Scholar] [CrossRef]
- Predieri, S.; Ragazzini, P.; Rondelli, R. Sensory evaluation and peach fruit quality. Acta Hortic 2006, 429–434. [Google Scholar] [CrossRef]
- Risse, L.A.; Brecht, J.K.; Sargent, S.A.; Locascio, S.J.; Crall, J.M.; Elmstrom, G.W.; Maynard, D.N. Storage Characteristics of Small Watermelon Cultivars. Journal of the American Society for Horticultural Science 1990, 115, 440–443. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.C.; Gao, Y.H.; Zhang, L.J.; Zhou, Y.H. The plant cell wall: Biosynthesis, construction, and functions. Journal of Integrative Plant Biology 2021, 63, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.T.; Schröder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus x domestica) fruit growth. Bmc Plant Biology 2013, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.A.; Wang, W.; Zhang, Q.; Jia, W.S. Cell Wall Integrity Signaling in Fruit Ripening. International Journal of Molecular Sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A. Cell wall metabolism during maturation, ripening and senescence of peach fruit. Journal of Experimental Botany 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Guo, M.R.; Yang, W.T.; Liu, Y.X.; Wang, Y.; Chen, G.G. The Role of Cell Wall Polysaccharides Disassembly and Enzyme Activity Changes in the Softening Process of Hami Melon (L.). Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Soteriou, G.A.; Siomos, A.S.; Gerasopoulos, D.; Rouphael, Y.; Georgiadou, S.; Kyriacou, M.C. Biochemical and histological contributions to textural changes in watermelon fruit modulated by grafting. Food Chemistry 2017, 237, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Hu, Z.; Li, Y.; Hao, J.; Chen, S.; Xue, Q.; Ma, Y.; Zhang, K.; Mahmoud, A.; Ali, A.; et al. Ethylene-responsive factor 4is associated with the desirable rind hardness trait conferring cracking resistance in fresh fruits of watermelon. Plant Biotechnology Journal 2019, 18, 1066–1077. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, Y.; Su, Z.Y.; Yu, Y.; Zhu, Z.C.; Gao, P.; Wang, X.Z. Transcriptome analysis of genes related to fruit texture in watermelon. Scientia Horticulturae 2020, 262. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Cui, H.; Zhang, L.; Sha, T.; Wang, C.; Fan, C.; Luan, F.; Wang, X. Linkage Mapping and Comparative Transcriptome Analysis of Firmness in Watermelon (Citrullus lanatus). Frontiers in Plant Science 2020, 11. [Google Scholar] [CrossRef]
- Anees, M.; Gao, L.; Umer, M.J.; Yuan, P.L.; Zhu, H.J.; Lu, X.Q.; He, N.; Gong, C.S.; Kaseb, M.O.; Zhao, S.J.; et al. Identification of Key Gene Networks Associated With Cell Wall Components Leading to Flesh Firmness in Watermelon. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef]
- Anees, M.; Gao, L.; Gong, C.; Umer, M.J.; Yuan, P.; Zhu, H.; Lu, X.; He, N.; Kaseb, M.O.; Yang, D.; et al. Aux/IAA gene Cla004102, is involved in synergistic regulation of various endogenous hormones, regulating flesh firmness in watermelon. Scientia Horticulturae 2023, 310, 111719. [Google Scholar] [CrossRef]
- Poles, L.; Gentile, A.; Giuffrida, A.; Valentini, L.; Endrizzi, I.; Aprea, E.; Gasperi, F.; Distefano, G.; Artioli, G.; La Malfa, S.; et al. Role of fruit flesh cell morphology and MdPG1 allelotype in influencing juiciness and texture properties in apple. Postharvest Biology and Technology 2020, 164, 111161. [Google Scholar] [CrossRef]
- Kim, M.S.; Duizer, L.M.; Grygorczyk, A.J.P.B. Technology. Application of a Texture Analyzer friction rig to evaluate complex texture attributes in apples. Postharvest Biology and Technology 2022, 186, 111820. [Google Scholar] [CrossRef]
- Bianchi, T.; Guerrero, L.; Gratacós-Cubarsí, M.; Claret, A.; Argyris, J.; Garcia-Mas, J.; Hortós, M. Textural properties of different melon (Cucumis melo L.) fruit types: Sensory and physical-chemical evaluation. Scientia Horticulturae 2016, 201, 46–56. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, D.; Ying, Y. Nondestructive measurement of pear texture by acoustic vibration method. Postharvest Biology and Technology 2014, 96, 99–105. [Google Scholar] [CrossRef]
- Ciacciulli, A.; Cirilli, M.; Chiozzotto, R.; Attanasio, G.; Da Silva Linge, C.; Pacheco, I.; Rossini, L.; Bassi, D. Linkage and association mapping for the slow softening (SwS) trait in peach (P. persica L. Batsch) fruit. Tree Genetics & Genomes 2018, 14, 93. [Google Scholar] [CrossRef]
- Pieniazek, F.; Messina, V. Texture and color analysis of freeze-dried potato (cv. Spunta) using instrumental and image analysis techniques. International Journal of Food Properties 2016, 20, 1422–1431. [Google Scholar] [CrossRef]
- Oyeyinka, B.O.; Afolayan, A.J. Comparative Evaluation of the Nutritive, Mineral, and Antinutritive Composition of L. (Banana) and L. (Plantain) Fruit Compartments. Plants-Basel 2019, 8, 598. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, P.; Liu, S.; Zhu, Z.; Amanullah, S.; Davis, A.R.; Luan, F. Comparative transcriptome analysis of two contrasting watermelon genotypes during fruit development and ripening. BMC Genomics 2017, 18, 3. [Google Scholar] [CrossRef]
- Davis, A.; Webber, C.; Wenge, L.; Perkins, P.; Levi, A.; King, S. Watermelon Quality Traits as Affected by Ploidy. HortScience 2013, 48, 1113–1118. [Google Scholar] [CrossRef]
- Henderson, W. Effect of Cultivar, Polyploidy and “Reciprocal” Hybridization on Characters Important in Breeding Triploid Seedless Watermelon Hybrids1. Journal of the American Society for Horticultural Science 1977, 102, 293–297. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid content of 50 watermelon cultivars. Journal of agricultural and food chemistry 2006, 54, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Brashlyanovabr, B.; Ganeva, G. Texture quality of tomatoes as affected by different storage temperatures and growth habit. Emirates Journal of Food and Agriculture 2014, 23, 750. [Google Scholar] [CrossRef]
- Bianchi, T.; Guerrero, L.; Gratacós-Cubarsí, M.; Claret, A.; Argyris, J.; Garcia-Mas, J.; Hortós, M. Textural properties of different melon ( L.) fruit types: Sensory and physical-chemical evaluation. Scientia Horticulturae 2016, 201, 46–56. [Google Scholar] [CrossRef]
- Hou, J.; Sun, Y.; Chen, F.; Yu, L.; Mao, Q.; Wang, L.; Guo, X.; Liu, C. Analysis of microstructures and macrotextures for different apple cultivars based on parenchyma morphology. Microsc Res Tech 2016, 79, 304–312. [Google Scholar] [CrossRef]
- Li, H.; Pidakala, P.; Billing, D.; Burdon, J. Kiwifruit firmness: Measurement by penetrometer and non-destructive devices. Postharvest Biology and Technology 2016, 120, 127–137. [Google Scholar] [CrossRef]
Figure 2.
Schematic diagram of the texture detection site of watermelon fruit profile.
Figure 2.
Schematic diagram of the texture detection site of watermelon fruit profile.
Figure 3.
Illustrates the variances in fruit weight, TSS content, and rind hardness among different ploidy of watermelon during various developmental stages. * represents significant differences (P<0.05) among different ploidy at the same period. The numbers 8, 16, 24, and 32 correspond to the number of days of fruit development after pollination, and the terms 2X, 3X, and 4X represent diploid, triploid, and tetraploid watermelon, respectively.
Figure 3.
Illustrates the variances in fruit weight, TSS content, and rind hardness among different ploidy of watermelon during various developmental stages. * represents significant differences (P<0.05) among different ploidy at the same period. The numbers 8, 16, 24, and 32 correspond to the number of days of fruit development after pollination, and the terms 2X, 3X, and 4X represent diploid, triploid, and tetraploid watermelon, respectively.
Figure 4.
Variations in flesh texture among different ploidy of watermelon at various developmental stages. Letters ‘a’, ‘b’, and ‘ab’ denote significant differences (P< 0.05) between different ploidy within the same developmental stage. 8d, 16d, 24d, and 32d correspond to the number of days of fruit development after pollination, and the terms 2X, 3X, and 4X represent diploid, triploid, and tetraploid watermelon, respectively.
Figure 4.
Variations in flesh texture among different ploidy of watermelon at various developmental stages. Letters ‘a’, ‘b’, and ‘ab’ denote significant differences (P< 0.05) between different ploidy within the same developmental stage. 8d, 16d, 24d, and 32d correspond to the number of days of fruit development after pollination, and the terms 2X, 3X, and 4X represent diploid, triploid, and tetraploid watermelon, respectively.
Figure 5.
Variations in the polysaccharide content within the cell walls of watermelon flesh across different ploidy levels. The numbers 8, 16, 24, and 32 correspond to the number of days of fruit development after pollination, the terms 2X, 3X, and 4X represent diploid, triploid, and tetraploid watermelon, respectively.
Figure 5.
Variations in the polysaccharide content within the cell walls of watermelon flesh across different ploidy levels. The numbers 8, 16, 24, and 32 correspond to the number of days of fruit development after pollination, the terms 2X, 3X, and 4X represent diploid, triploid, and tetraploid watermelon, respectively.
Figure 6.
(a) Observation on the microstructure of flesh cells at different developmental stages of different ploidy of watermelon (b) Variations in the cellular structure of watermelon flesh at different developmental stages across different ploidy levels were observed. Letters ’a’, ’b’, and ’ab’ denote significant differences (P < 0.05) between different ploidy within the same developmental stage. The numbers 8, 16, 24, and 32 correspond to the number of days of fruit development after pollination, the terms 2X, 3X, and 4X represent diploid, triploid, and tetraploid watermelon, respectively.
Figure 6.
(a) Observation on the microstructure of flesh cells at different developmental stages of different ploidy of watermelon (b) Variations in the cellular structure of watermelon flesh at different developmental stages across different ploidy levels were observed. Letters ’a’, ’b’, and ’ab’ denote significant differences (P < 0.05) between different ploidy within the same developmental stage. The numbers 8, 16, 24, and 32 correspond to the number of days of fruit development after pollination, the terms 2X, 3X, and 4X represent diploid, triploid, and tetraploid watermelon, respectively.
Table 1.
Correlation analysis of flesh texture with cell microstructure and cell wall polysaccharide content in watermelon of different ploidy level.
Table 1.
Correlation analysis of flesh texture with cell microstructure and cell wall polysaccharide content in watermelon of different ploidy level.
| |
Hardness |
Fracturability |
Gumminess |
Chewiness |
Cell Number |
Average Cell Area |
Cell Density |
Crude Fiber |
Cellulose |
Hemicellulose |
Lignin |
Protopectin |
Soluble Pectin |
| Hardness |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
| Fracturability |
0.898**
|
1 |
|
|
|
|
|
|
|
|
|
|
|
| Gumminess |
0.907**
|
0.944**
|
1 |
|
|
|
|
|
|
|
|
|
|
| Chewiness |
0.882**
|
0.968**
|
0.968**
|
1 |
|
|
|
|
|
|
|
|
|
| Cell Number |
0.828**
|
0.868**
|
0.867**
|
0.850**
|
1 |
|
|
|
|
|
|
|
|
| Average Cell Area |
-0.687**
|
-0.779**
|
-0.756**
|
-0.726**
|
-0.876**
|
1 |
|
|
|
|
|
|
|
| Cell Density |
0.828**
|
0.868**
|
0.867**
|
0.850**
|
1.000**
|
-0.876**
|
1 |
|
|
|
|
|
|
| Crude Fiber |
0.736**
|
0.794**
|
0.762**
|
0.733**
|
0.859**
|
-0.841**
|
0.859**
|
1 |
|
|
|
|
|
| Cellulose |
0.731**
|
0.793**
|
0.762**
|
0.733**
|
0.861**
|
-0.843**
|
0.861**
|
1.000**
|
1 |
|
|
|
|
| Hemicellulose |
0.584**
|
0.581**
|
0.545**
|
0.520**
|
0.637**
|
-0.711**
|
0.637**
|
0.688**
|
0.688**
|
1 |
|
|
|
| Lignin |
0.642**
|
0.643**
|
0.612**
|
0.573**
|
0.710**
|
-0.647**
|
0.710**
|
0.889**
|
0.889**
|
0.460**
|
1 |
|
|
| Protopectin |
0.557**
|
0.576**
|
0.559**
|
0.518**
|
0.623**
|
-0.706**
|
0.623**
|
0.621**
|
0.625**
|
0.867**
|
0.396**
|
1 |
|
| Soluble Pectin |
-0.567**
|
-0.675**
|
-0.650**
|
-0.617**
|
-0.740**
|
0.839**
|
-0.740**
|
-0.818**
|
-0.821**
|
-0.755**
|
-0.608**
|
-0.860**
|
1 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).